Abstract
The use of antipsychotic medications has now expanded to multiple mental health conditions beyond schizophrenia. This has increased the overall population exposure to these medications, which have been associated with both metabolic changes and adverse cardiovascular effects. QTc prolongation, torsades de pointes, sudden cardiac death, myocarditis, and cardiomyopathy are all very real concerns that clinicians face on a regular basis. One must take these risks into consideration when selecting antipsychotic therapy and also when determining whether therapeutic changes and adjustments are necessary. This review examines a number of cardiac-associated concerns, the role that antipsychotics may play in contributing to these adverse events, and suggested management interventions.
Keywords: antipsychotic, cardiac, cardiomyopathy, sudden death, myocarditis, torsades de pointes, QTc prolongation
Take Home Points:
A prolonged QTc interval is a well-documented risk factor for torsades de pointes. However, not all medications that have demonstrated QTc prolongation have been associated with developing torsades de pointes.
When evaluating patients for the potential to experience QTc prolongation from medications, it is important to evaluate additional risk factors, including serum electrolytes, blood pressure, renal and hepatic function, advanced age, and female sex.
Myocarditis and cardiomyopathy both present similarly and are most commonly associated with the use of clozapine.
Introduction
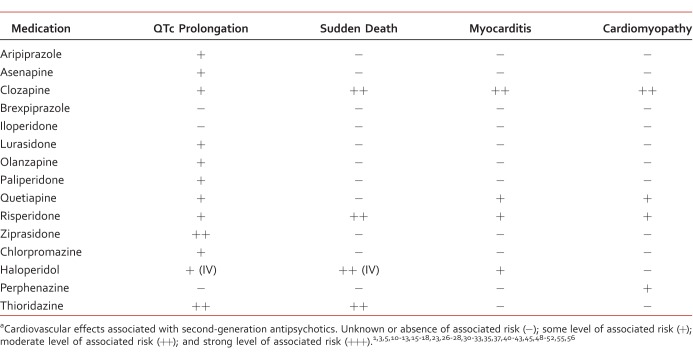
Antipsychotic medications are being more widely used because their approved indications have expanded beyond schizophrenia and include bipolar and major depressive disorder, delirium, dementia, and substance-induced psychosis. With broadened use comes the increased need for improved understanding of not only the illnesses they treat, but also their potential impact on co-occurring disorders. This is especially important for cardiovascular disorders (Table).
TABLE:
Cardiovascular-related antipsychotic side effectsa
The treatment of schizophrenia is particularly challenging because the disease state itself is associated with increased cardiovascular morbidity and mortality rates. The risk of sudden cardiac death is estimated to be 2 to 4 times greater than in the general population.1 An increased risk for coronary heart disease has been directly linked to schizophrenia (adjusted hazard ratio, 1.49).2 Patients with psychosis and bipolar disorder are already likely to have a shortened life expectancy by 15 to 25 years because of an increased presence of cardiovascular disease and an increased likelihood of sudden cardiac death (SCD).3-5 A primary concern when using antipsychotic medications is the possibility of potentially fatal cardiac-related adverse events. Although this risk is generally accepted, it remains unclear whether the psychiatric condition itself or the medications confer the greatest risk. Common risk factors for cardiovascular disease in the general population and in lower socioeconomic groups are also observed in patients with schizophrenia and bipolar disorder. These risk factors include sedentary lifestyles, increased tobacco use, poor dietary habits, lack of exercise, and alcohol abuse. In addition, many second-generation antipsychotics (SGAs) or atypical antipsychotics increase the risk of weight gain, as well as that of elevations in blood sugar and cholesterol concentrations, which further compounds the cardiovascular concerns related to their use.
The development of SGAs has created an increased risk for metabolic side effects, including obesity, diabetes mellitus, and dyslipidemia. The SGAs olanzapine and clozapine are associated with the greatest risk of weight gain. Ziprasidone, lurasidone, and aripiprazole confer a lower risk of metabolic side effects, specifically weight gain.6,7 When considering the increased cardiovascular risk related to mental health conditions alone, it is also expected that there is an increased risk of metabolic disorders as well as cardiovascular events in patients taking SGAs.8,9 Lastly, a US Food and Drug Administration boxed warning states that both first-generation antipsychotics (FGAs) and SGAs present an increased mortality rate when used in elderly patients with dementia. Risperidone and olanzapine were the first SGAs to be associated with increased mortality. Further study has implicated multiple antipsychotics across drug classes. The mortality increase is associated primarily with cerebrovascular-associated events, although a direct cardiac-related contribution must also be considered.10-12 The most recent data13 from clinical trials suggest that in patients older than 65 years who have dementia, there is a greater mortality risk associated with haloperidol, followed by risperidone, then olanzapine, and then quetiapine.
In addition to cardiometabolic concerns, other cardiovascular side effects from antipsychotic use may occur. Several antipsychotics antagonize α1 receptors, which may cause orthostatic hypotension and reflex tachycardia.14 A number of antipsychotics possess anticholinergic activity that is also associated with the development of tachycardia. These side effects are typically manageable with antipsychotic dose adjustments or treatment with medications to reduce heart rate when necessary. Cardiac side effects of a more severe nature that may result in sudden cardiac death include the development of ventricular tachycardia, torsades de pointes (TdP), delayed cardiac repolarization, myocarditis, myocardial infarction, and cardiomyopathy.5,15
A recent case-crossover study16 completed in Taiwan reviewed more than 17 000 cases of patients who developed ventricular arrhythmia (VA) or who experienced SCD between 2001 and 2009. Patients receiving antipsychotic treatment were at a 1.53-fold increased risk of developing VA or experiencing SCD.16 A higher risk was noted with FGAs compared with SGAs (adjusted odds ratio [AOR], 1.66 versus 1.36). Antipsychotics that produced blockade of the hERG potassium channel were associated with a higher VA/SCD risk.16 Individual agents approved for use in the United States that were statistically associated with a higher risk of VA/SCD included: haloperidol, prochlorperazine, thioridazine, quetiapine, and risperidone.16 Olanzapine also demonstrated an increased risk, with an AOR of 1.64; however, the confidence interval (CI) was 0.98 to 2.72. Aripiprazole (AOR, 0.94; CI, 0.41-3.15) and ziprasidone (AOR, 0.80; CI, 0.37-3.93) also conferred a lower risk of VA and SCD.16 A unique finding was that short-term antipsychotic use also served as a greater predictor of VA/SCD-related events (AOR, 2.11; CI, 1.70-2.61 for less than 7 cumulative days; AOR, 1.38; CI, 1.19-1.60 for 8-28 cumulative days; and AOR, 1.22; CI, 0.91-1.63 for 29 cumulative days or more).16
Case 1: QTc Prolongation Case
A 48-year-old male who received a diagnosis of schizophrenia presents for a regular 3-month appointment. The patient appears his stated age, is dressed appropriately, and demonstrates good grooming and hygiene. He reports a normal, positive mood, and affect is appropriate. He denies current suicidal or homicidal thoughts and is optimistic about the future and one day moving to an independent apartment. The patient denies current auditory or visual hallucinations and paranoid ideation. He reports getting along well in the boarding home and has had no conflicts with staff or peers. He describes a favorable energy level and getting 8 hours of uninterrupted sleep per night. Speech is regular rate and rhythm. Memory is good for immediate, short-term, and remote recall, with good insight and judgment.
Routine examination reveals the following:
Blood pressure: 156/94 mm Hg
Pulse: 74 beats per minute (bpm)
Respiratory rate: 20 breaths per minute
Weight: 112 kg (246 lbs)
Height: 178 cm (5′10”)
Temperature: 37°C (98.6°F)
Body mass index: 35.3 kg/m2
Laboratory data (normal ranges in parentheses):
Glucose: 122 mg/dL (70-110 mg/dL)
Low-density lipoprotein: 175 mg/dL (<130 mg/dL)
High-density lipoprotein: 24 mg/dL (>40 mg/dL)
Alanine aminotransferase: 42 international units per liter (0-30 units per liter)
Aspartate aminotransferase: 62 international units per liter (8-42 units per liter)
C-reactive protein: 0.2 mg/L (0-0.5 mg/L)
Troponin: 0.04 ng/mL (<0.06 ng/mL)
White blood cell count: 5.3 × 103 cells/mm3 (4.4 × 103 cells/mm3 to 11.3 × 103 cells/mm3)
Absolute neutrophil count: 2.05 × 103/μL
Platelet count: 253 000/mm3 (140 000/mm3 to 440 000/mm3)
Hemoglobin: 16.4 g/dL (14.0-17.5 g/dL)
Hematocrit: 44.2% (42%-50%)
Hepatitis C: positive
HIV: negative
Sodium: 137 mEq/L (135-145 mEq/L)
Potassium: 4.8 mEq/L (3.5-5.0 mEq/L)
Magnesium: 2.0 mEq/L (1.5-2.2 mEq/L)
Calcium: 8.6 mg/dL (8.5-10.8 mg/dL)
Blood urea nitrogen: 13 mg/dL (8-20 mg/dL)
Creatinine: 0.6 mg/dL (0.7-1.5 mg/dL)
Medical assessments:
Most recent electrocardiogram (ECG): QTc 470 ms
Baseline ECG prior to starting current antipsychotic: QTc 440 ms
Current medications:
Lurasidone 160 mg orally at bedtime (with a snack)
Divalproex sodium 1500 mg orally twice a day for mood stabilization
Carvedilol 25 mg orally twice a day for hypertension
Lisinopril 10 mg orally daily for hypertension
Metformin XR 1500 mg orally daily
Lorazepam 1 mg orally 3 times a day for anxiety
QTc Prolongation
The QT interval is defined as the time from the initiation of ventricular depolarization (intracellular movement of sodium) until repolarization (extracellular movement of potassium) is complete. The extracellular flow of potassium is regulated by 2 rectifying currents—rapid and slow. Blockade of either channel can prolong repolarization and lengthen the QT interval. The rapid current is regulated by the ether-a-go-go–related gene, and any dysregulation may prolong the QT interval.17,18 The QT interval is inversely proportional to heart rate and is referred to as the QTc interval when corrected for heart rate. The upper limit of normal QTc interval varies; although most consider a normal interval for men to be less than 450 ms and for women less than 460 ms, others consider greater than 440 ms as borderline abnormal or as cause for additional monitoring.19 In the clinical setting, an abnormally prolonged QTc interval is most often defined or recognized as greater than 470 ms in men and greater than 480 ms in women.
A prolonged QTc interval is a known risk factor for the development of the VA (polymorphic ventricular tachycardia) TdP, with the greatest risk occurring when the QTc interval exceeds 500 ms. The presence of TdP is mostly benign and self-limiting; however, it is potentially fatal. Estimates suggest that for every 10-ms increase in QTc interval, there is a 5% to 7% increase of developing TdP.20,21 Medications, when prescribed, should be reviewed for their QTc-prolonging potential; however, it is important to remember that not all medications associated with QTc interval prolongation have been associated with the development of TdP. Conversely, there are antipsychotics with known TdP risk that are not strongly associated with QTc prolongation (Table). The risk of TdP following the administration of medications that may prolong the QTc interval is greatest within the first 30 days of treatment; however, an estimated 40% of cases also occur after this time frame.22
Common risk factors for QTc prolongation include: female sex, increased age, long QT syndrome, hypokalemia, hypomagnesemia, hypocalcemia, anorexia nervosa, bradycardia, heart failure, hypertension, renal and hepatic dysfunction, diabetes, and obesity.17 When evaluating the presence of risk factors for QTc prolongation, one must also note that the QTc interval is susceptible to interindividual variability, diurnal variation, and changes in heart rate.23 Some of the most common noncardiac medications associated with QTc prolongation are the FGAs and SGAs. Therefore, increased emphasis has been placed on identifying risk factors and monitoring baseline or periodic ECGs when treating patients with antipsychotic medication. A long QT syndrome is commonly associated with different genetic mutations, with most patients exhibiting QTc prolongation at baseline. However, other patients with long QT syndrome may only display ECG changes after unmasking by antipsychotic medications.
The risk of QTc prolongation and subsequent development of TdP has been associated with increased doses of antipsychotic medication. Intravenous administration of medications with QTc-prolonging potential increases the risk of QTc prolongation and TdP due to increased drug concentrations and increased cardiovascular exposure.19 Additionally, there is an increased risk of QTc prolongation and TdP from increased drug concentrations caused by pharmacokinetic drug interactions.
A number of different medications and medication classes have been implicated in prolonging the QTc interval. These include, but are not limited to, antiarrhythmics, macrolide and quinolone antibiotics, and antifungals. Another important consideration in those with mental illness and those dually diagnosed with substance use disorders is that alcohol, cocaine, and other psychostimulants are associated with QTc prolongation. Both FGAs and SGAs have been associated with QTc prolongation. Thioridazine is the antipsychotic most likely to result in QTc prolongation, followed by ziprasidone, quetiapine, risperidone, olanzapine, haloperidol, and clozapine, in that order.24,25 The risk of haloperidol-associated QTc prolongation and the development of TdP is most often associated with intravenous administration.26 Other antipsychotics, such as iloperidone, paliperidone, asenapine, lurasidone, fluphenazine, pimozide, droperidol, and chlorpromazine, have been associated with the development of QTc prolongation, although the risk is considered to be less.27-30 A recent 7-year review from the US Food and Drug Administration reported cases of TdP most commonly with olanzapine, quetiapine, clozapine, ziprasidone, risperidone, haloperidol, droperidol, and amisulpride, and, to a lesser extent, aripiprazole.31 Aripiprazole's decreased likelihood to impact QTc has been further supported in other work comparing SGAs with the greatest risk of QTc prolongation and TdP in the absence of additional risk factors.32,33
Given this information and the varying effects on the QTc interval and development of ventricular tachycardia, concerns related to sudden cardiac death remain. One study suggested that FGAs and SGAs produce a 2-fold increased risk of sudden cardiac death. Specifically, this study examined cases involving thioridazine, haloperidol, olanzapine, quetiapine, risperidone, and clozapine. While the reported deaths were unable to be confirmed to be secondary to QTc prolongation, the association is likely.5
In managing the different clinical aspects associated with QTc prolongation, intervention is focused primarily on preventative measures. Trinkley and colleagues34 offered a suggested assessment and monitoring plan for reducing the risk of developing TdP from QTc-prolonging medications. First, it is important to identify existing risk factors for QTc prolongation. If risk factors are present, it is best to use antipsychotic medications with a lower potential for QTc prolongation. In some cases, risk factors may be modifiable and, if corrected, would not preclude one from using specific antipsychotics. Second, use the lowest effective dose when a QTc-prolonging medication must be used. Although specific QTc monitoring is not required for antipsychotic use, a conservative practice-based approach would be to obtain a baseline ECG prior to the initiation of antipsychotic, again once the drug is at a steady-state concentration, and then on a periodic basis thereafter (eg, monthly for 6 months and then every 6-12 months).34 Serum electrolyte concentrations should also be monitored. QTc-prolonging drugs should usually be avoided if the baseline QTc is greater than 480 ms in women and greater than 470 ms in men.34 If QTc is normal, but an increase greater than 60 ms is noted following the administration of an antipsychotic, consideration should be given to antipsychotic discontinuation.34
In the case of the 48-year-old male currently being treated with lurasidone, the patient should receive a complete and comprehensive evaluation. All indications suggest the patient is psychiatrically stable on lurasidone and has been demonstrating an increased ability to function in an independent manner. The patient possesses the following risk factors for QTc prolongation: hypertension, diabetes, and obesity. Additionally, some may be concerned with the recent QTc interval of 470 ms, which is at the cutoff of what is considered QTc prolongation in men. However, the patient's baseline QTc interval was 440 ms (below this threshold), and the QTc increase after the addition of lurasidone was less than 60 ms. Despite the fact that the patient is at this cutoff, this is not his baseline reading, he is asymptomatic, his psychiatric symptoms are well controlled, and lurasidone is not considered to be a high-risk agent for QTc prolongation; thus, lurasidone is reasonable to continue. The patient should be strongly encouraged to improve control of the modifiable disease states of hypertension, diabetes, and obesity.
Sudden Cardiac Death
There are many causes of sudden death, and not all cases are linked to cardiac-specific events. One study found acute coronary syndromes to be the most common identifiable cause of sudden death, followed by upper airway obstruction, pulmonary emboli, and thrombotic strokes. Other identifiable causes include myocarditis, diabetic ketoacidosis, and septic shock. Diabetes, arterial hypertension, cigarette smoking, and dyslipidemia are common contributing factors. Many unexplained sudden deaths occur in patients with coronary artery disease. Ventricular arrhythmia or TdP is commonly suspected in these cases, even though they are not definitively identified as the cause of death.35,36 The development of TdP following ventricular fibrillation has been estimated to occur in 1% to 17% of TdP cases resulting in sudden death.37 Dilated, hypertrophic, and right ventricular cardiomyopathies are some of the more common causes of sudden cardiac-related deaths.38,39 One early study40 found a risk of sudden cardiac death that was 2.39 times higher in patients receiving 100 mg of thioridazine or equivalent dosing of antipsychotics. The same study found sudden death incidence rate ratios of 1.99 and 2.26 with the use of FGAs and SGAs, respectively. In a recent meta-analysis41 of observational studies of sudden cardiac and unexpected death, higher odds ratios (ORs) were found with thioridazine (OR, 4.58), clozapine (OR, 3.67), risperidone (OR, 3.04), haloperidol (OR, 2.97), olanzapine (OR, 2.04), and quetiapine (OR, 1.72). Another review42 has suggested a low number of reported deaths related to cardiovascular events for risperidone and paliperidone. Another study43 examined ECGs for 6790 patients during a 5-year period upon admission to a public psychiatric hospital. They found 27.3% of admissions had abnormal ECG findings; 1.6% had long QTc intervals; and 0.9% were identified as having drug-induced QTc prolongation (≥500 ms). A total of 5 patients experienced sudden cardiac death, and 7 others received a diagnosis of TdP. The authors43 found that the risk of drug-induced QTc prolongation and arrhythmia significantly increases in the presence of hypokalemia, abnormal T-wave morphology, hepatitis C infection, and human immunodeficiency virus infection.
Sudden death is not always the result of a cardiac event and is often associated with different acute coronary syndromes. Additionally, common metabolic side effects associated with FGA and SGA use (eg, diabetes) may also play a contributing role. Although one cannot predict sudden death, consideration should be given to the use of agents less likely to contribute to metabolic abnormalities and with a lower reported association with sudden death events, especially when known risk factors are present.
Case 2: Myocarditis
A 47-year-old patient presents to the outpatient clinic with complaints of fatigue, muscle and joint pain, and shortness of breath. The patient's blood pressure is 90/64, pulse is 110 bpm and irregular, temperature is 39.1°C (102.4°F), and respiratory rate is 24 breaths per minute. The patient is currently taking clozapine 150 mg twice daily, started 3 weeks ago during an inpatient hospitalization for acute, decompensated schizophrenia. The patient has a history of a positive response to haloperidol decanoate, although it was discontinued because of the presence of early-onset tardive dyskinesia symptoms.
Myocarditis is an inflammation of the heart muscle thought to result from immunoglobulin E–mediated hypersensitivity. Patients experiencing myocarditis often present with no specific complaints. However, when symptomatic, the presentation often includes flulike symptoms, chest and joint pain, shortness of breath, fatigue, joint swelling, irregular pulse, leg swelling, and reduced urine output.44,45 Additionally, specific patients with clozapine-associated myocarditis have reported fever, vomiting, diarrhea, tachycardia, and dysuria.44 Laboratory changes seen with clozapine-induced myocarditis include peripheral eosinophilia, and elevations in concentrations of C-reactive protein (CRP), creatinine phosphokinase, and troponin (I and T).44-46 Patients may also experience a drop in systolic blood pressure, with nonspecific ECG changes. Impairment of left ventricular function may occur, although recovery typically occurs with the discontinuation of clozapine.44 Clozapine is the antipsychotic most commonly associated with myocarditis; however, additional reports are linked to haloperidol, quetiapine, fluphenazine, chlorpromazine, olanzapine, and risperidone.47-51 The estimated prevalence of clozapine-associated myocarditis is 0.7% to 1.2%. One review44 suggested that the onset is most often between days 14 and 21 after initiation of clozapine therapy, although another52 identified a median of 17 days of clozapine treatment. An absolute risk of 0.015% to 0.188% was found in another study.45 Although not considered standard clinical practice, there have been reports of successful rechallenge with clozapine following episodes of myocarditis.53,54
Limited information is available concerning monitoring for myocarditis; however, the authors44 of 1 review of 75 cases of clozapine-associated myocarditis provided recommendations. Prior to initiation of clozapine, they recommend obtaining baseline concentrations of troponin (I or T), CRP, and a baseline ECG. In addition, vital signs should be monitored at least every other day, and CRP and troponin concentrations monitored weekly for 4 weeks. If patients develop myocarditis-type symptoms (heart rate increases) or CRP increases above 50 mg/L, troponin and CRP should be checked daily, along with daily monitoring for myocarditis symptoms. If troponin concentrations are elevated, but less than twice the upper limit of normal (normal <0.06 ng/mL), and CRP is below 100 mg/L, clozapine may be continued. Clozapine should be discontinued if troponin becomes elevated beyond twice the normal limit or if CRP is above 100 mg/L.44
In the case of the 47-year-old patient receiving a diagnosis of schizophrenia, the presenting symptoms strongly suggest myocarditis. The first step is to discontinue clozapine and to obtain CRP and troponin concentrations immediately. Clozapine should be permanently discontinued if troponin concentrations are above 2 times normal or if CRP is above 100 mg/L. If troponin concentrations are not above 2 times normal and CRP is below 100 mg/L, consideration may be given to continuing clozapine; however, should symptoms of myocarditis continue, clozapine discontinuation is recommended. Even though this patient has responded in the past to haloperidol, its use should be avoided because of an increased association with tardive dyskinesia. As noted, myocarditis is most commonly associated with clozapine, but it has also been reported with haloperidol, fluphenazine, chlorpromazine, quetiapine, and risperidone, and these agents should be avoided. Additional agents that the patient has not been treated with or has not failed treatment with, may be considered. One agent, iloperidone, has not been associated with myocarditis and is associated with a lower risk of developing tardive dyskinesia, although the risk of orthostatic hypotension associated with iloperidone may make it difficult to use in a patient being taken off of clozapine abruptly.
Case 3: Cardiomyopathy
A 54-year-old patient presents to the emergency department with new-onset complaints of extreme shortness of breath, frequent coughing, fatigue, and mild chest pain. The patient takes clozapine 200 mg daily in the morning and 300 mg every evening, divalproex 1500 mg twice daily, and gabapentin 600 mg 3 times daily. The electronic medical record shows that clozapine was initiated 10 months ago during an inpatient hospitalization. The patient's history suggests failures of both FGAs and SGAs, although documentation describing the past treatment failures is limited. Blood pressure is 154/94, pulse is 110 bpm and irregular, patient is afebrile, respiratory rate is 28 breaths per minute, and O2 saturation is 93%. ECG shows T-wave abnormalities suggesting left ventricular hypertrophy, and echocardiogram shows an ejection fraction less than 30%.
Dilated cardiomyopathy accounts for an estimated 7% of sudden cardiac deaths. It is the result of chronic disease of the heart muscle with subsequent impairment of systolic function.38,39 Hypertrophic cardiomyopathy and right ventricular cardiomyopathy may also occur and contribute to sudden cardiac death. Cardiomyopathy is difficult to detect clinically because symptoms typically present in a subtle manner and primarily consist of complaints of shortness of breath and fatigue. If unrecognized and untreated, mortality rates are high.
Clozapine is the primary antipsychotic associated with the development of cardiomyopathy, although the mechanism is not understood.55 A recent systematic review56 analyzed various published case reports and clinical trials that identified cardiomyopathy in patients treated with clozapine. The average age of those presenting with symptoms was 33.5 years, and the mean dosage was 360 mg/d.56 The broad range of dosing at which cases have been reported suggests the phenomenon is not dose related. The primary presenting symptom was shortness of breath in 60% of reports; palpitations were the second most frequently reported symptom, in 36% of cases.56 Additional complaints included cough (16%), fatigue (16%), atypical symptoms (12%), and chest pain (8%).56 The average duration of treatment prior to onset was 14.4 months, with most patients presenting within 12 months; however, some cases presented as early as 3 weeks.56 Diagnosis can be confirmed via echocardiogram showing a reduced ejection fraction. Current estimates of the prevalence of clozapine-associated cardiomyopathy suggest a rate of approximately 8.9 per 100 000 person-years; the rate in the general population is estimated at 7.5 to 10 per 100 000 patient-years.56 It should be noted that clozapine-associated cardiomyopathy is likely to be underreported. Case reports also describe possible cardiomyopathy associated with perphenazine, risperidone, and quetiapine.57-59
The primary treatment for clozapine-associated cardiomyopathy is the immediate discontinuation of clozapine. The ejection faction at the time of diagnosis appears to be a reliable predictor of prognosis. Ejection fractions below 25% predict a poor prognosis and higher mortality rate. Ejection fractions above 40% predict a high likelihood of full recovery if usual heart failure treatment is initiated. Although there are case reports of successful rechallenge with clozapine after cardiomyopathy, clinical guidelines do not support rechallenge.56 Before starting clozapine, patients should have a baseline ECG and chest x-ray.56 Echocardiograms are also recommended to establish a baseline for future comparisons.56
In the case of the 54-year-old patient, all signs and symptoms suggest the patient is experiencing clozapine-associated cardiomyopathy. The time of onset is consistent with the onset of cardiomyopathy reported with clozapine. An accurate account of the patient's history of response to medication is critical given that clozapine is reserved for refractory cases. In this case, clozapine should be discontinued, and under no circumstances should this patient be rechallenged. In this case, one should also be prepared to intervene because patients who respond to clozapine often experience rapid psychiatric decompensations when clozapine is abruptly discontinued. Identification of any antipsychotics that have not been used, or a more clear assessment of whether previous treatment failures were related to nonadherence is an important determination to make and can be used to guide the next steps. Clozapine, quetiapine, risperidone, and perphenazine have all been associated with cardiomyopathy, often in case report form, although clozapine clearly possesses the strongest association. The onset of symptoms ranges from as early as 3 weeks to as late as slightly more than 1 year. In the event that patients report shortness of breath, cough, or fatigue, further evaluation is warranted to rule out cardiomyopathy. In patients with a history of cardiomyopathy, it may also be wise to avoid the use of quetiapine, risperidone, and perphenazine, given their reported association with cardiomyopathy. This patient is clearly at increased risk based on current symptoms, and ECG and echocardiogram findings and consideration should be given to the use of an antipsychotic not associated with the development of cardiomyopathy.
Footnotes
Disclosures: I have nothing personal to disclose. Psychopharmacology Pearls are review articles intended to highlight both the evidence base available and/or controversial areas of clinical care for psychiatric and neurologic conditions, as well as strategies of clinical decision-making used by expert clinicians. As pearls, articles reflect the views and practice of each author as substantiated with evidence-based facts as well as opinion and experience. Articles are edited by members of the Psychopharmacology Pearls Editorial Board and are peer reviewed by MHC reviewers. This article was developed as part of the 2017 Psychopharmacology Pearls product for BCPP recertification credit. The course information and testing center is at cpnp.org/322903.
References
- 1. Buckley NA, Sanders P. . Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000; 23 3: 215- 28. DOI: 10.2165/00002018-200023030-00004. PubMed PMID: 11005704. [DOI] [PubMed] [Google Scholar]
- 2. Gale CR, Batty GD, Osborn DPJ, Tynelius P, Rasmussen F. . Mental disorders across the adult life course and future coronary heart disease: evidence for general susceptibility. Circulation. 2014; 129: 186- 93. DOI: 10.1161/CIRCULATIONAHA.113.002065. PubMed PMID: 24190959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Correll CU, Nielsen J. . Antipsychotic-associated all-cause and cardiac mortality: what should we worry about and how should the risk be assessed? Acta Psychiatrica Scand. 2010; 122 5: 341- 4. DOI: 10.1111/j.1600-0447.2010.01610.x. PubMed PMID: 21029051. [DOI] [PubMed] [Google Scholar]
- 4. Laursen TM, Wahlbeck K, Hällgren J, Westman J, Ösby U, Alinaghizadeh H, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013; 8 6: e67133 DOI: 10.1371/journal.pone.0067133. PubMed PMID: 23826212; PubMed Central PMCID: PMC3691116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009; 360 3: 225- 35. DOI: 10.1056/NEJMoa0806994. PubMed PMID: 19144938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musil R, Obermeier M, Russ P, Hamerle M. . Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015; 14 1: 73- 96. DOI: 10.1517/14740338.2015.974549. PubMed PMID: 25400109. [DOI] [PubMed] [Google Scholar]
- 7. Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010; 123 2-3: 225- 33. DOI: 10.1016/j.schres.2010.07.012. PubMed PMID: 20692814; PubMed Central PMCID: PMC2957510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimmet P. . Epidemiology of diabetes mellitus and associated cardiovascular risk factors: Focus on human immunodeficiency virus and psychiatric disorders. Am J Med. 2005; 118 Suppl 2: 3- 8. DOI: 10.1016/j.amjmed.2005.01.044. PubMed PMID: 15903289. [DOI] [PubMed] [Google Scholar]
- 9. Schneider LS, Dagerman KS, Insel P. . Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005; 294 15: 1934- 43. DOI: 10.1001/jama.294.15.1934. PubMed PMID: 16234500. [DOI] [PubMed] [Google Scholar]
- 10. Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005; 353 22: 2335- 41. DOI: 10.1056/NEJMoa052827. PubMed PMID: 16319382. [DOI] [PubMed] [Google Scholar]
- 11. Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang PS. . Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Can Med Assoc J. 2007; 176 5: 627- 32. DOI: 10.1503/cmaj.061250. PubMed PMID: 17325327; PubMed Central PMCID: PMC1800321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015; 72 5: 438- 45. DOI: 10.1001/jamapsychiatry.2014.3018. PubMed PMID: 25786075; PubMed Central PMCID: PMC4439579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gugger JJ. . Antipsychotic pharmacotherapy and orthostatic hypotension. CNS Drugs. 2011; 25 8: 659- 71. DOI: 10.2165/11591710-000000000-00000. PubMed PMID: 21790209. [DOI] [PubMed] [Google Scholar]
- 14. Honkola J, Hookana E, Malinen S, Kaikkonen KS, Junttila MJ, Isohanni M, et al. Psychotropic medications and the risk of sudden cardiac death during an acute coronary event. Eur Heart J. 2012; 33 6: 745- 51. DOI: 10.1093/eurheartj/ehr368. PubMed PMID: 21920969. [DOI] [PubMed] [Google Scholar]
- 15. Wu CS, Tsai YT, Tsai HJ. . Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation-wide case-crossover study. J Am Heart Assoc. 2015; 4 2: e001568 DOI: 10.1161/JAHA.114.001568. PubMed PMID: 25713294; PubMed Central PMCID: PMC4345877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017; 135 10: e146- 603. DOI: 10.1161/CIR.000000000000485. PubMed PMID: 28122885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. . QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013; 54 1: 1- 13. DOI: 10.1016/j.psym.2012.11.001. PubMed PMID: 23295003. [DOI] [PubMed] [Google Scholar]
- 18. Polcwiartek C, Kragholm K, Schjerning O, Graff C, Nielsen J. . Cardiovascular safety of antipsychotics: a clinical overview. Expert Opin Drug Saf. 2016; 13: 1- 10. DOI: 10.1517/14740338.2016.1161021. PubMed PMID: 26934282. [DOI] [PubMed] [Google Scholar]
- 19. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010; 121 8: 1047- 60. DOI: 10.1161/CIRCULATIONAHA.109.192704. PubMed PMID: 20142454; PubMed Central PMCID: PMC3056123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, et al. The long QT syndrome: prospective longitudinal study of 328 families. Circulation. 1991; 84 3: 1136- 44. PubMed PMID: 1884444. [DOI] [PubMed] [Google Scholar]
- 21. Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, et al. Influence of the genotype on the clinical course of the long-QT syndrome. N Engl J Med. 1998; 339 14: 960- 5. DOI: 10.1056/NEJM199810013391404. PubMed PMID: 9753711. [DOI] [PubMed] [Google Scholar]
- 22. Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. . Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore). 2003; 82 4: 282- 90. DOI: 10.1097/01.md.0000085057.63483.9b. PubMed PMID: 12861106. [DOI] [PubMed] [Google Scholar]
- 23. Yap YG. . Drug induced QT prolongation and torsades de pointes. Heart. 2003; 89 11: 1363- 72. DOI: 10.1136/heart.89.11.1363. PubMed PMID: 14594906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010; 36 1: 71- 93. DOI: 10.1093/schbul/sbp116. PubMed PMID: 19955390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004; 161 8: 1334- 49. DOI: 10.1176/appi.ajp.161.8.1334. PubMed PMID: 15285957. [DOI] [PubMed] [Google Scholar]
- 26. US Food and Drug Administration. Haloperidol drug alert [updated 2013 Aug 14; cited 2007 Sept]. Available from: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm085203.htm
- 27. Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophrenia Res. 2008; 105 1-3: 175- 87. DOI: 10.1016/j.schres.2008.07.006. PubMed PMID: 18775645; PubMed Central PMCID: PMC2614656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Citrome L. . Iloperidone for schizophrenia: a review of the efficacy and safety profile for this newly commercialized second generation antipsychotic. Int J Clin Pract. 2009; 63 8: 1237- 48. DOI: 10.1111/j.1742-1241.2009.02142.x. PubMed PMID: 19624791. [DOI] [PubMed] [Google Scholar]
- 29. Citrome L. . Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic. Int J Clin Pract. 2009; 63 12: 1762- 84. DOI: 10.1111/j.1742-1241.2009.02228.x. PubMed PMID: 19840150. [DOI] [PubMed] [Google Scholar]
- 30. Nielsen J, Graff C, Kanters JK, Toft E, Taylor D, Meyer JM. . Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011; 25 6: 473- 90. DOI: 10.2165/11587800-000000000-00000. PubMed PMID: 21649448. [DOI] [PubMed] [Google Scholar]
- 31. Poluzzi E, Raschi E, Koci A, Moretti U, Spina E, Behr ER, et al. Antipsychotics and torsadogenic risk: signals emerging from the US FDA Adverse Event Reporting System Database. Drug Saf. 2013; 36 6: 467- 79. DOI: 10.1007/s40264-013-0032-z. PubMed PMID: 23553446; PubMed Central PMCID: PMC3664739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chung AK, Chua SE. . Effects on prolongation of Bazett's corrected QT interval of seven second-generation antipsychotics in the treatment of schizophrenia: a meta-analysis. J Psychopharmacol. 2011; 25 5: 646- 66. DOI: 10.1177/0269881110376685. PubMed PMID: 20826552. [DOI] [PubMed] [Google Scholar]
- 33. Polcwiartek C, Sneider B, Graff C, Taylor D, Meyer J, Kanters JK, et al. The cardiac safety of aripiprazole treatment in patients at high risk for torsade: a systematic review with a meta-analytic approach. Psychopharmacol (Berl). 2015; 232 18: 3297- 308. DOI: 10.1007/s00213-015-4024-9. PubMed PMID: 26231497. [DOI] [PubMed] [Google Scholar]
- 34. Trinkley KE, Page RL 2nd, Lien H, Yamanouye K, Tisdale JE. . QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013; 29 12: 1719- 26. DOI: 10.1185/03007995.2013.840568. PubMed PMID: 24020938. [DOI] [PubMed] [Google Scholar]
- 35. Manu P, Kane JM, Correll CU. . Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011; 72 07: 936- 41. DOI: 10.4088/JCP.10m06244gry. PubMed PMID: 21672496; PubMed Central PMCID: PMC3305794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beary M, Hodgson R, Wildgust HJ. . A critical review of major mortality risk factors for all-cause mortality in first-episode schizophrenia: clinical and research implications. J Psychopharmacol. 2012; 26 5 Suppl: 52- 61. DOI: 10.1177/0269881112440512. PubMed PMID: 22465947. [DOI] [PubMed] [Google Scholar]
- 37. Salle P, Rey JL, Bernasconi P, Quiret JC, Lombaert M. . [Torsades de pointe. Apropos of 60 cases]. Ann Cardiol Angeiol (Paris). 1985; 34 6: 381- 8. French. PubMed PMID: 4026164. [PubMed] [Google Scholar]
- 38. Priori S. . Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J. 2001; 22 16: 1374- 450. DOI: 10.1053/euhj.2001.2824. PubMed PMID: 11482917. [DOI] [PubMed] [Google Scholar]
- 39. Di Lenarda A, Secoli G, Perkan A, Gregori D, Lardieri G, Pinamonti B, et al. Changing mortality in dilated cardiomyopathy: the Heart Muscle Disease Study Group. Br Heart J. 1994; 72 6 Suppl: S46- 51. PubMed PMID: 7873326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. . Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001; 58 12: 1161- 7. DOI: 10.1001/archpsyc.58.12.1161. PubMed PMID: 11735845. [DOI] [PubMed] [Google Scholar]
- 41. Salvo F, Parient A, Shakir S, Robinson P, Arnaud M, Thomas SHL, et al. Sudden cardiac and sudden unexpected death related to antipsychotics: a meta-analysis of observational studies. Clin Pharmacol Ther. 2016; 99 3: 306- 14. DOI: 10.1002/cpt.250. PubMed PMID: 26272741. [DOI] [PubMed] [Google Scholar]
- 42. Gopal S, Hough D, Karcher K, Nuamah I, Palumbo J, Berlin JA, et al. Risk of cardiovascular morbidity with risperidone or paliperidone treatment: analysis of 64 randomized, double-blind trials. J Clin Psychopharmacol. 2013; 33 2: 157- 61. DOI: 10.1097/JCP.0b013e318283983f. PubMed PMID: 23422378. [DOI] [PubMed] [Google Scholar]
- 43. Girardin FR, Gex-Fabry M, Berney P, Shah D, Gaspoz JM, Dayer P. . Drug-induced long QT in adult psychiatric inpatients: the 5-year cross-sectional ECG Screening Outcome in Psychiatry study. Am J Psychiatry. 2013; 170 12: 1468- 76. DOI: 10.1176/appi.ajp.2013.12060860. PubMed PMID: 24306340. [DOI] [PubMed] [Google Scholar]
- 44. Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, McNeil JJ. . A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011; 45 6: 458- 65. DOI: 10.3109/00048674.2011.572852. PubMed PMID: 21524186. [DOI] [PubMed] [Google Scholar]
- 45. Merrill DB, Dec GW, Goff DC. . Adverse cardiac effects associated with clozapine. J Clin Psychopharmacol. 2005; 25 1: 32- 41. DOI: 10.1097/01.jcp.0000150217.51433.9f. PubMed PMID: 15643098. [DOI] [PubMed] [Google Scholar]
- 46. Nielsen J, Correll CU, Manu P, Kane JM. . Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013; 74 6: 603- 13; quiz 613. DOI: 10.4088/JCP.12r08064. PubMed PMID: 23842012. [DOI] [PubMed] [Google Scholar]
- 47. Leung JYT, Barr AM, Procyshyn RM, Honer WG, Pang CCY. . Cardiovascular side-effects of antipsychotic drugs: the role of the autonomic nervous system. Pharmacol Ther. 2012; 135 2: 113- 22. DOI: 10.1016/j.pharmthera.2012.04.003. PubMed PMID: 22565090. [DOI] [PubMed] [Google Scholar]
- 48. Wassef N, Khan N, Munir S. . Quetiapine-induced myocarditis presenting as acute STEMI. 2015; 2015. pii: bcr2014207151. DOI: 10.1136/bcr-2014-207151. PubMed PMID: 25576507; PubMed Central PMCID: PMC4289804. [DOI] [PMC free article] [PubMed]
- 49. Roesch-Ely D, Van Einsiedel R, Kathöfer S, Schwaninger M, Weisbrod M. . Myocarditis with quetiapine. Am J Psychiatry. 2002; 159 9: 1607- 8. DOI: 10.1176/appi.ajp.159.9.1607-a. PubMed PMID: 12202290. [DOI] [PubMed] [Google Scholar]
- 50. Lang UE, Willbring M, von Golitschek R, Schmeisser A, Matschke K, Tugtekin SM. . Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008; 22 5: 576- 80. DOI: 10.1177/0269881107082136. PubMed PMID: 18308817. [DOI] [PubMed] [Google Scholar]
- 51. Vang T, Rosenzweig M, Bruhn CH, Polcwiartek C, Kanters JK, Nielsen J. . Eosinophilic myocarditis during treatment with olanzapine--report of two possible cases. BMC Psychiatry. 2016; 16: 70 DOI: 10.1186/s12888-016-0776-y. PubMed PMID: 26988850; PubMed Central PMCID: PMC4794841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haas SJ, Hill R, Krum H, Liew D, Tonkin A, Demos L, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf. 2007; 30 1: 47- 57. DOI: 10.2165/00002018-200730010-00005. PubMed PMID: 17194170. [DOI] [PubMed] [Google Scholar]
- 53. Ittasakul P, Archer A, Kezman J, Atsariyasing W, Goldman MB. . Rapid rechallenge with clozapine following pronounced myocarditis in a treatment-resistant schizophrenia patient. Clin Schizophr Relat Psychoses. 2016; 10 2: 120- 2. DOI: 10.3371/1935-1232-10.2.120. PubMed PMID: 27440213. [PubMed] [Google Scholar]
- 54. Rosenfeld AJ, Gibbs T, Ivie R, Clarke L, Merrill DB. . Successful clozapine retrial after suspected myocarditis. Am J Psychiatry. 2010; 167 3: 350- 1. DOI: 10.1176/appi.ajp.2009.09081118. PubMed PMID: 20194490. [DOI] [PubMed] [Google Scholar]
- 55. Buckley NA, Sanders P. . Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000; 23 3: 215- 28. DOI: 10.2165/00002018-200023030-00004. PubMed PMID: 11005704. [DOI] [PubMed] [Google Scholar]
- 56. Alawami M, Wasywich C, Cicovic A, Kenedi C. . A systematic review of clozapine induced cardiomyopathy. Int J Cardiol. 2014; 176 2: 315- 20. DOI: 10.1016/j.ijcard.2014.07.103. PubMed PMID: 25131906. [DOI] [PubMed] [Google Scholar]
- 57. Coffey S, Williams M. . Quetiapine-associated cardiomyopathy. N Z Med J. 2011; 124 1337: 105- 7. PubMed PMID: 21946883. [PubMed] [Google Scholar]
- 58. Ansari A, Maron BJ, Berntson DG. . Drug-induced toxic myocarditis. Tex Heart Inst J. 2003; 30 1: 76- 9. PubMed PMID: 12638679. [PMC free article] [PubMed] [Google Scholar]
- 59. Marti V. . Sudden cardiac death due to risperidone therapy in a patient with possible hypertrophic cardiomyopathy. Ann Pharmacother. 2005; 39 5: 973 DOI: 10.1345/aph.1E539. PubMed PMID: 15827070. [DOI] [PubMed] [Google Scholar]