Abstract
Introduction:
Clozapine is an atypical antipsychotic medication approved for treatment-resistant schizophrenia and suicidal behavior in schizophrenia or schizoaffective disorders. Despite its therapeutic efficacy, clozapine is associated with several adverse effects, including agranulocytosis. In late 2015, the Food and Drug Administration updated the risk evaluation and mitigation strategy (REMS) for clozapine with new requirements for monitoring, prescribing, and dispensing. The purpose of this study was to evaluate clozapine prescribing practices at a Kentucky state psychiatric hospital before and after the implementation of the updated REMS program.
Methods:
The primary outcome of this study was to evaluate clozapine prescribing practices by identifying the number of patients on clozapine therapy in the 6 months pre and post updated REMS implementation. Included in the study were patients at a Kentucky state psychiatric hospital on clozapine therapy for the 24 months before the updated REMS implementation and in the 6-month study period after the implementation. The secondary objective of this study examined psychiatrist comfort level of prescribing clozapine.
Results:
Since the implementation of the updated REMS program, there has been an increased percentage of patients that were prescribed clozapine at a Kentucky state psychiatric hospital. This increase was not statistically significant (P = .2610).
Discussion:
The prescribing practices of clozapine within this facility did not differ significantly comparing pre- and post-REMS change in terms of number of patients prescribed clozapine, patient's dose, and therapy duration. Data from this study contributes to the body of knowledge evaluating this new standard of practice under the updated REMS.
Keywords: clozapine, REMS, Kentucky, state psychiatric hospital, prescribing practices
Introduction
Clozapine is an antipsychotic medicine used to treat schizophrenia. It is the only antipsychotic specifically indicated for treatment-resistant schizophrenia. Therefore, it is traditionally reserved for patients whose symptoms have not been controlled with other antipsychotic trials.1 It is also used to treat recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.2 However, clozapine does have several boxed warnings and is associated with several adverse effects, including but not limited to neutropenia and agranulocytosis.3
A risk evaluation and mitigation strategy (REMS) is a strategy to manage a known or potential serious risk associated with a drug or biological product. The Food and Drug Administration (FDA) determines if a REMS is necessary to assure the benefits of the drug or biological product outweigh its risks.4 Clozapine has had a REMS program in place since 2008 in order to monitor for the risk of agranulocytosis. However, in September 2015, the FDA proposed updates to the REMS for clozapine to include new requirements in order to monitor, prescribe, dispense, and receive the medication. All patients from existing registries for clozapine were combined into 1 registry. The updated monitoring requirements for clozapine include a lower absolute neutrophil count threshold with even lower thresholds approved for patients deemed to have benign ethnic neutropenia and the removal of the requirement to monitor white blood cell count.5 These changes had the potential to expand the pool of individuals who are candidates for clozapine therapy with the associated possibility for positive impact on their treatment outcomes. The objective of this observational study was to evaluate the prescribing practices of clozapine therapy before and after implementation of the updated REMS program, which went into effect on October 12, 2015.
The primary outcome of this study was to evaluate clozapine prescribing practices by identifying the number of patients on clozapine therapy in the 6 months pre and post updated REMS implementation. Included in the study were patients at a Kentucky state psychiatric hospital on clozapine therapy for the 24 months before the implementation of the updated REMS program in October 2015 and in the 6-month study period after the implementation. The additional pre-REMS data were collected in order to provide further baseline assessment of the prescribing practices.
The secondary outcome was to describe the prescriber's comfort level in prescribing clozapine. A questionnaire assessing prescriber comfort level in prescribing clozapine was implemented before the updated REMS. Additionally, all prescribers (n = 4) were educated by a pharmacist on the updated REMS requirements immediately after REMS implementation. Education was provided on an individualized basis and included verbal and visual information regarding changes to the program. All education was provided by the principal investigator of the study and was geared so prescribers could successfully complete the knowledge assessment required to become registered in the new REMS program.
Methods
Approval for this study was granted by the Commonwealth of Kentucky Cabinet for Health and Family Services Institutional Review Board. The first portion of the study was a retrospective, observational chart review of patients at an acute care, inpatient state psychiatric hospital who were prescribed clozapine therapy in the 24 months prior to the implementation of the updated REMS. Only adult (≥18 years old) psychiatric inpatients who were prescribed and administered at least 1 dose of clozapine per electronic medication administration record review were included in the study. Data collected included basic demographic information, such as age, sex, ethnicity, and diagnosis of the patient. Other data collected were related to the medication clozapine and included dosing regimen, duration of therapy, and required laboratory monitoring. The second portion of the study was a prospective evaluation of patients at the same facility who were prescribed clozapine in the 6 months following the updated REMS implementation. Charts were reviewed for those patients on clozapine therapy in the 6 months after the implementation of the updated REMS program. The same demographic data were collected as well as data related to the medication and required laboratory monitoring data.
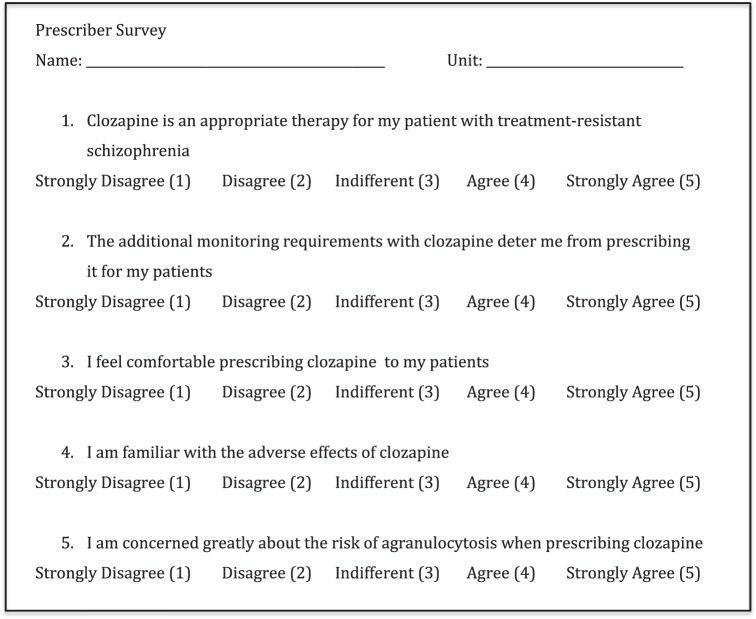
To describe the comfort level of those prescribing clozapine, a survey was administered to all prescribers prior to the implementation of the updated REMS. This 5-question survey, included as Figure 1, asked prescribers to rate their comfort level with the medication, its monitoring requirements, and the adverse effects on a 5-point Likert scale.
FIGURE 1.
Survey administered to prescribers at a Kentucky state psychiatric hospital
Data collection and statistical analysis was performed utilizing Microsoft Excel 2010 (Redmond, WA). A paired t test compared the proportion of patients at the study facility who were prescribed clozapine in the months before and after the implementation of the new REMS program. Descriptive statistics including mean, median, percentages, and other numerical values, such as standards of deviation, were utilized to display data.
Results
Most patients at the study facility prescribed clozapine were white (n = 16, 62%), male (n = 18, 69%), and had a diagnosis of schizophrenia (n = 19, 73%). The demographics were similar before and after the REMS implementation. In the 24 months prior to the implementation of the updated REMS, 18 patients at the study facility were identified as having received at least 1 dose of clozapine. In the months after the implementation of the REMS program in October 2015, until March of 2016, an additional 8 patients were identified as those who were prescribed and received at least 1 dose of clozapine for a total of 26 patients identified. In the 6 months prior to the implementation of the REMS program, there had been only 3 patients prescribed clozapine. After the implementation of the REMS program, a greater percentage of inpatients were receiving clozapine. The percentage of patients prescribed clozapine is illustrated in Figure 2. In the 6 months pre-REMS implementation, approximately 6% of patients had been prescribed clozapine. In the 6 months post-REMS implementation, the percentage of patients on clozapine therapy had increased to approximately 14% although this increase was not statistically significant (P = .2610).
FIGURE 2.
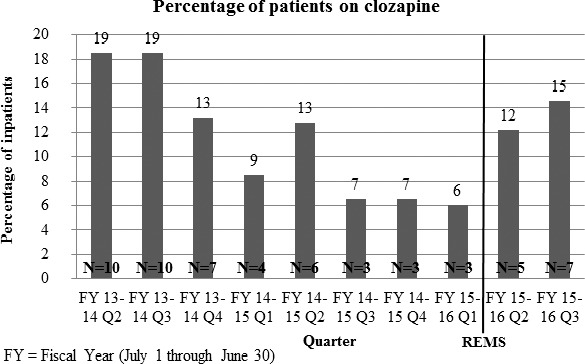
Percentage of patients at a Kentucky state psychiatric hospital prescribed clozapine (REMS = risk evaluation and mitigation strategy)
The dosages of clozapine prescribed for patients in the 24 months before and 6 months after the implementation of the REMS program were also collected. The average maximum daily dose of clozapine prescribed to patients is represented in Figure 3. In the months after the implementation of the REMS program, clozapine was prescribed at maximum daily doses ranging from 375 ± 156 to 450 ± 167 mg/d. In the 24 months prior to the implementation of the REMS program the maximum daily doses of clozapine had been steadily increasing from 300 ± 178 to 425 ± 161 mg/d.
FIGURE 3.
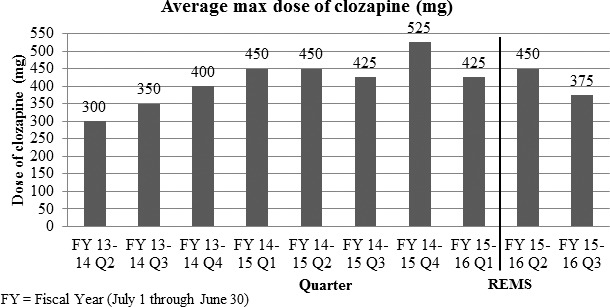
Average maximum daily doses of clozapine at a Kentucky state psychiatric hospital (REMS = risk evaluation and mitigation strategy)
Additionally, the duration of clozapine therapy was evaluated in these patients. Before the implementation of the REMS program, the average duration of clozapine therapy was 47 days. The average duration of clozapine therapy remained unchanged in the 6 months after the implementation of the REMS program with the average duration being 46 days. The patients prescribed clozapine also tended to have a longer-than-average length of stay compared to patients not on clozapine therapy. The average length of stay for patients on clozapine therapy was 95 days. The average length of stay for all patients hospitalized at this state psychiatric hospital, including those prescribed clozapine therapy, was 19 days during the study period.
The findings from the 5-question survey administered to prescribers found that all prescribers (n = 4, 100%) agreed they felt comfortable prescribing clozapine. However, with the additional monitoring requirements with clozapine, 2 of the 4 (50%) prescribers felt this deterred them from ordering the medication. Additionally, all prescribers (n = 4, 100%), agreed, but none strongly agreed, that clozapine is an appropriate therapy for patients with treatment-resistant schizophrenia and were familiar with the adverse effects of clozapine. Most prescribers (n = 3, 75%) were concerned with the risk of agranulocytosis when prescribing clozapine.
Discussion
The findings of this descriptive study aim to contribute to the general body of knowledge assessing the impact of the new standard of practice driven by the updated REMS program. The study was limited as a single-center study with small sample size. The findings illustrate that since the implementation of the updated REMS program, a larger percentage of patients had been prescribed clozapine at the study facility. There was a change in the prescribing practices of clinicians post-REMS implementation with more patients being prescribed clozapine than in the months prior to the implementation although this increase was not statistically significant. This non–statistically significant increase may be due to increased awareness of the agent following the prescriber education. Other potential contributing factors could include the possibility of prescribing to patients with lower absolute neutrophil count values. In the 6 months prior to the implementation, there had been a decrease in the prescribing of clozapine. The decrease in prescribing could be attributed to the personnel change in the prescribers as well as other unidentified secondary causative factors.
To fully evaluate the extent to which the impact of the implementation of the updated REMS program will have on prescribing practices, this observational study would need to be continued. The prescribing practices of clozapine were observed for 24 months prior to the implementation of the updated REMS program, which occurred in October 2015. For a full comparison of the prescribing practices of clozapine, the prescribing practices would need to be observed for the same time period of 24 months after the implementation of the updated REMS program. These additional data would be needed to evaluate whether the trend in the increase in the larger percentage of patients prescribed clozapine were to continue.
In the months after the implementation of the REMS program, not only was there a percentage increase in number of patients prescribed clozapine, but these patients appear to have been prescribed more appropriate doses of clozapine. In the months after the implementation of the REMS program, therapeutic doses were more often achieved as clozapine was prescribed at doses ranging from 350 to 450 mg/d. A maintenance dose of 300 to 600 mg/d is usually required for efficacy.3 In the months prior to the implementation of the REMS program, the maximum daily doses of clozapine had been steadily increasing from 300 to 425 mg/d.
The findings that those patients prescribed clozapine also tended to have a longer than average length of stay compared to patients not on clozapine therapy at the study facility are to be expected with the population for which clozapine is indicated and utilized. As clozapine is reserved for patients with treatment-resistant schizophrenia, who have failed trials of other antipsychotics, these patients are more debilitated by their mental illness and oftentimes will require longer hospitalizations. Additionally, clozapine must be titrated slowly due to the autonomic adverse effects of the medication.3 The longer average duration of therapy for patients on clozapine is to be expected as well with the slow titration of clozapine required to achieve maintenance doses greater than 300 mg/d.
Additionally, while prescribers at the study facility reported feeling comfortable prescribing clozapine to their patients, it would be necessary to reevaluate if this level of comfort has changed post-REMS implementation. There is the potential confounder of the initial technical issues with REMS program. This may influence responses on the subsequent questionnaire if administered. It is possible that prescribers might report less comfort prescribing clozapine due to the initial technological issues with accessing the REMS program.
It is important to note with this observational study that the prescribing of clozapine did not decrease after the implementation of the REMS program. The implementation of the updated REMS program for clozapine occurred only shortly after an announcement by the FDA of the changes and was plagued by many technical issues. Glitches and delays prevented many prescribers from accessing the new program and completing the registration process to effectively utilize the program. There was concern that these changes would cause a decrease in clozapine utilization as a result of the difficulties accessing the new program.6 The findings of this study do not support this concern as evidenced by a nonsignificant numerical increase in the number of patients prescribed clozapine after the implementation of the REMS program. These data suggest that, with support and education of prescribers, clozapine may continue to be appropriately utilized in the institutional setting.
Footnotes
Disclosures: The authors have nothing to disclose concerning possible financial or personal relationships with commercial entities or their competitors that may be referenced in this article.
References
- 1. Warnez S, Alessi-Severini S. . Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014; 14: 102 DOI: 10.1186/1471-244X-14-102. PubMed PMID: 24708834; PubMed Central PMCID: PMC3999500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A. . Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003; 60 1: 82- 91. PubMed PMID: 12511175. [DOI] [PubMed] [Google Scholar]
- 3. Clozaril (clozapine) tablets, 25 mg [package insert]. East Hanover (NJ): Novartis Pharmaceuticals Corp.; 2015. Sep. Distributed by Novartis Pharmaceuticals Corp. [Google Scholar]
- 4. Medication Guides: Distribution Requirements and Inclusion in Risk Evaluation and Mitigation Strategies (REMS) [Internet]. Silver Spring (MD): Food and Drug Administration; [updated 2011. Nov]. Available from: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM244570.pdf [Google Scholar]
- 5. FDA Drug Safety Communication: FDA Modifies Monitoring for Neutropenia Associated with Schizophrenia Medicine Clozapine; Approves New Shared REMS Program for All Clozapine Medicines [Internet]. Silver Spring (MD): Food and Drug Administration; ; [updated 2015. Sept 9]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm461853.htm [Google Scholar]
- 6. Smith J. . Clozapine REMS still plagued by problems. Clinical Psychiatry News. [Internet]. 2016. May 11 Available from: http://www.clinicalpsychiatrynews.com/specialty-focus/schizophrenia/single-article-page/clozapine-rems-still-plagued-by-problems/64821e9739a5a310ff21e81c6f6f3050.html