Abstract
Introduction:
Despite the theory that long-acting injectable (LAI) antipsychotics should be more likely to improve adherence, reduce gaps in therapy, and prevent relapse compared with oral antipsychotics, there is little published evidence on this issue, specifically in patients with early psychosis.
Methods:
Patients with a new diagnosis for a psychotic disorder between July 1, 2013, and August 31, 2014, were retrospectively evaluated during a 12-month duration. The primary outcomes were adherence and persistence. Adherence was determined by proportion of days with medication, and persistence was defined as zero gaps in medication therapy. The secondary outcome was the number of times a psychiatric acute care service was used. Patients were divided into 3 groups based on their antipsychotic prescription history: oral only, LAI only, or both formulations at separate times throughout the study period.
Results:
Forty-seven patients met inclusion criteria. The average proportions of days with medication were 32%, 76%, and 75% for the oral, LAI, and both formulations groups, respectively (P < .001). For medication persistence, there were 32 patients (91%), 3 patients (75%), and 5 patients (63%) with at least 1 gap in therapy for the oral, LAI, and both formulations groups, respectively (P = .098). For acute care services, there was a median number of zero acute care visits for each of the 3 groups (P = .179). A post hoc subgroup analysis found medication adherence to be statistically different between the oral and LAI groups.
Discussion:
Long-acting injectable antipsychotics were associated with better adherence compared with oral antipsychotics in patients with early psychosis.
Keywords: antipsychotic, adherence, persistence, acute care services, early psychosis, long-acting injectable antipsychotic
Introduction
Patients with psychotic illness cycle between periods of recovery and acute psychotic episodes.1 Effective early treatment with antipsychotic medication decreases relapse risk. Relapse prevention after early psychosis leads to better patient outcomes.2 Nonadherence to oral antipsychotic medications may inhibit achieving a significant time period without relapse.3 Long-acting injectable (LAI) antipsychotic development has provided a treatment that omits daily dosing in order to potentially improve treatment adherence and efficacy. Despite the theory that LAI antipsychotics should be more likely to prevent relapse compared with oral antipsychotics, there is little published evidence on this issue, specifically in patients with early episode psychosis.
Studies4-9 involving both early and chronic psychosis have focused on hospitalization rates and adverse effects associated with LAI antipsychotics. Clinical trials10-13 for patients with early psychosis have evaluated adherence to LAI antipsychotics without comparison to oral agents. In studies14,15 comparing the 2 formulations with respect to adherence, many include all patients with schizophrenia regardless of time since diagnosis. Studies13,16-21 including patients with early psychosis, comparing the 2 formulations, included only 1 antipsychotic or referred to all-cause discontinuation without specific parameters denoted. Previous literature3-9 suggests nonadherence or inconsistent administration with antipsychotics puts individuals at higher risk for needing acute care. This study aims to retrospectively compare adherence, persistence, and frequency of psychiatric acute care services in patients treated at an early psychosis outpatient clinic.
Methods
Study Design
This retrospective, single-center study involved patients receiving a new diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder, or psychosis not otherwise specified who were receiving outpatient treatment at Eskenazi Health's Prevention and Recovery Center for Early Psychosis (PARC) between July 1, 2013, and August 31, 2014. Clinic enrollment requires an age between 16 and 30 years and psychosis onset within 2 years prior. Each patient included was monitored for 1 year following his or her clinic enrollment date. Although the study took place during the Diagnostic and Statistical Manual of Mental Disorders, 5th edition release, documentation for psychosis not otherwise specified remained consistent throughout the study period. Specifiers defined by Diagnostic and Statistical Manual of Mental Disorders (5th edition) were not used for diagnosis. Electronic health records and prescription fill histories provided fill dates and quantities dispensed for oral antipsychotics, administration dates of LAI antipsychotics, and documentation of visits to Eskenazi Health acute care settings (crisis intervention unit, inpatient mental health unit, or the emergency department with a psychiatric-related emergency). All LAI injections given at PARC were documented by nursing staff in the electronic health record after each administration. Outside pharmacies were contacted to determine prescription fill histories. The only antipsychotic prescriptions not captured were those possibly administered at outside facilities, because information from outside hospitals was not gathered. Comorbidities and other psychiatric medications were not accounted for.
Each patient was placed in 1 of 3 groups dependent on the formulation of antipsychotic prescribed during the follow-up period: oral only, LAI only, or both formulations at separate times. All commercially available oral and LAI antipsychotics were eligible for study inclusion, with the exception of asenapine and iloperidone, which were nonformulary during the study. The Indiana University Institutional Review Board approved the protocol.
Study Population
For inclusion, at least 1 administered LAI or 1 filled oral antipsychotic prescription was necessary during the study period. Patients could have used antipsychotics prior to the study. Those with a wrong diagnosis, those with insufficient information available, or those requiring long-term simultaneous oral and LAI antipsychotics were excluded. Long-term simultaneous therapy involved a duration longer than manufacturer-recommended oral overlap when switching from oral to LAI therapy. Separate analyses were conducted, including and excluding patients on civil commitment at any point during the study. No PARC patients are involved in assertive community treatment teams.
Study Outcomes
The primary outcomes were adherence and persistence to medication therapy for 1 year following PARC enrollment. Adherence was defined as the proportion of days with medication based on total day supply of all prescribed antipsychotics during the study period. Patient-specific LAI dosing frequencies were used to calculate the proportion of days covered. Persistence was evaluated by gaps in medication therapy. If more time had elapsed than the quantity available from the previous oral antipsychotic prescription, or if there was no documented administration date within the patient's prescribed LAI dosing frequency (including manufacturer-recommended grace periods), then a gap in therapy was present.
The secondary outcome was frequency of psychiatric acute care services used. Patients presenting to Eskenazi Health psychiatric acute care settings as described in “Study Design” were considered to have required acute care.
Statistical Analysis
Data analysis was completed using SPSS 24.0 (SPSS Inc, Chicago, IL). A power calculation was not used. One-way analysis of variance was used for normally distributed data, whereas Kruskal-Wallis analysis of variance was used for nonparametric data. Nominal data were analyzed using the Fisher exact test. A multiple regression analysis was conducted to predict adherence from the other variables collected. A post hoc subgroup analysis was conducted evaluating the oral and LAI-only groups, excluding patients in the both formulations group. The t test was used for continuous data, whereas Mann-Whitney U test was employed for nonparametric data.
Results
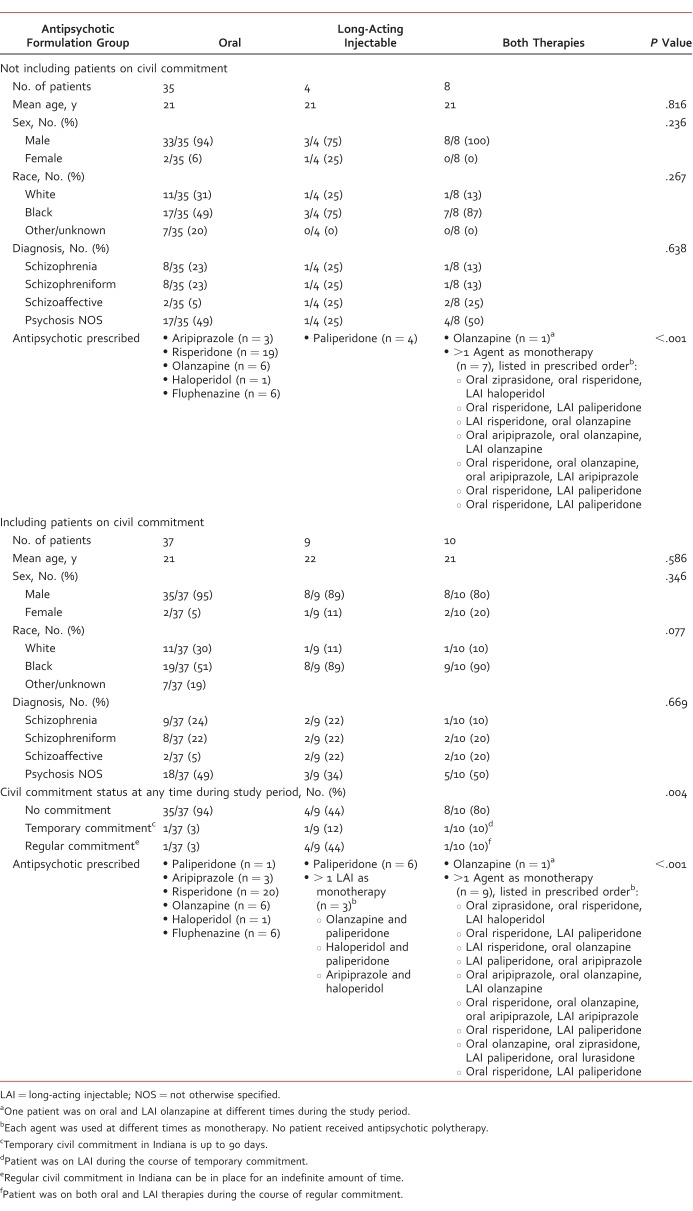
A total of 100 patients received a diagnosis in PARC during the study period. A total of 44 patients were excluded, including 15 with a wrong diagnosis (major depressive disorder with psychotic features, substance-induced psychosis), 21 who did not follow up in PARC after their intake appointment and were never prescribed antipsychotics from a PARC provider, 6 with insufficient information available in the electronic health record, and 2 who required long-term concomitant oral and LAI therapy. For the statistical analysis excluding patients on civil commitment at any point during the study period, an additional 9 patients were excluded, leaving 47 patients eligible for inclusion, including patients prescribed oral (n = 35), LAI (n = 4), and both formulations (n = 8). All 4 patients in the LAI group were prescribed paliperidone. There were no significant differences in baseline characteristics (Table 1). A separate statistical analysis for the 3 study outcomes was conducted including patients on civil commitment, increasing the inclusion population to 56 (n = 37, n = 9, and n = 10 for oral, LAI, and both formulations groups, respectively).
TABLE 1.
Baseline characteristics and antipsychotics prescribed to study patients
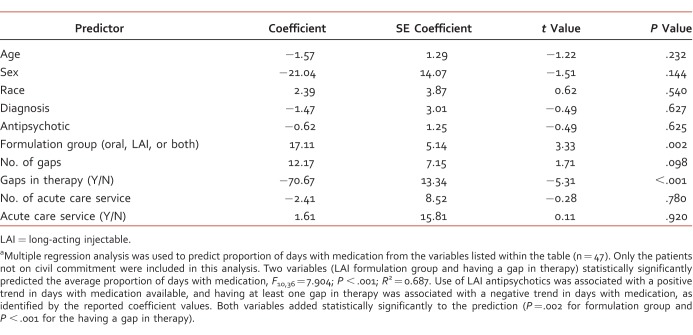
Table 1 shows antipsychotics prescribed in each treatment group. For the primary end point of adherence, the average proportion of days with medication was 32% (interquartile range [IQR], 41.7), 76% (IQR, 63.9), and 75% (IQR, 41.7) for the oral, LAI, and both formulations groups, respectively (Figure). This was found to be statistically significant (P < .001). Statistical analysis including patients on civil commitment determined statistically significant differences between groups, with an average proportion of days with medication of 17% (IQR, 41.7), 97% (IQR, 16.7), and 79% (IQR, 41.7) for the oral, LAI, and both formulations groups, respectively (P < .001). Multiple regression analysis showed that formulation group and having a gap in therapy statistically significantly predicted adherence. Use of LAI antipsychotics was associated with a positive trend in days with medication available, and having at least 1 gap in therapy was associated with a negative trend, as identified by the coefficient values (Table 2).
FIGURE.
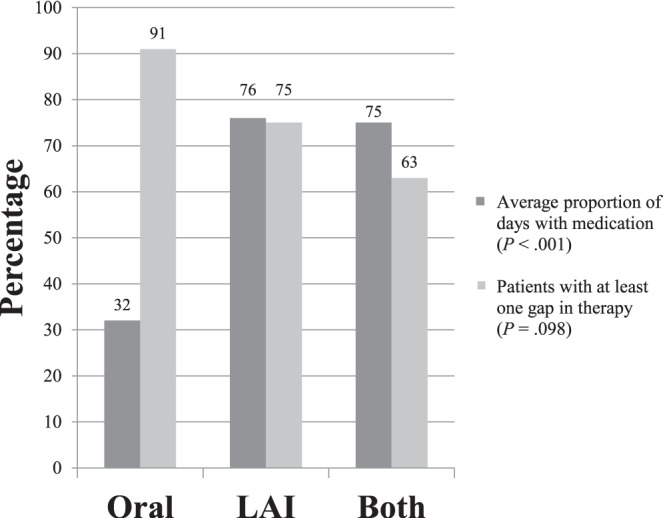
Primary outcome results: medication adherence and persistence; only the patients not on civil commitment were included in this analysis (LAI = long-acting injectable)
TABLE 2.
Logistic regression analysis for adherencea
For the second primary outcome of medication persistence, there were 32 patients (91%), 3 patients (75%), and 5 patients (63%) with at least 1 gap in therapy for the oral, LAI, and both formulations groups, respectively (Figure). This primary outcome was not statistically significant between treatment groups (P = .098). Statistical analysis including patients on civil commitment showed statistically significant differences between groups for persistence including 34 patients (92%), 5 patients (56%), and 7 patients (70%) for the oral, LAI, and both formulations groups, respectively (P = .021).
The secondary outcome of acute care services use resulted in a median number of zero visits for each of the 3 groups (IQRs, 0, 0.75, and 2.5 for the oral, LAI, and both formulations groups, respectively). The group prescribed both formulations had 3.5-fold more acute care visits, on average, because of 2 outliers seeking acute care 3 times during the study period; however, there was no statistically significant difference between groups (P = .179). All of these patients were prescribed oral antipsychotics during the times of the acute care visits.
The post hoc statistical comparison of oral and LAI treatment groups, which excluded patients on civil commitment, found statistical significance for the adherence primary outcome (P = .008) favoring LAI therapy. Statistically significant differences in persistence and use of acute care services between treatment groups were not found (P = .363 and P = .918, respectively). The post hoc statistical comparison including patients on civil commitment found statistical significance between groups for both primary outcomes, including average proportion of days with medication (P < .001) and a gap in therapy present (P = .02), favoring LAI therapy. The secondary outcome of acute care services was not statistically significant between treatment groups (P = .935).
Discussion
This study evaluated adherence, persistence, and rate of use of acute care services in patients with early episode psychosis prescribed oral and LAI antipsychotics. The results point to LAI antipsychotics being associated with better adherence. When patients on civil commitment were excluded, there was statistical significance for the primary outcome of adherence; however, there was no difference between groups for both the primary outcome of persistence or the secondary outcome involving rate of use of acute care services. The results of this study differ from other previous research including patients with early episode psychosis because the end points and definitions of adherence often differ among studies.
Limitations of this study include the sample size and its retrospective nature. The sample size had an impact on persistence of use and use of acute care services, with few patients able to be included in the LAI group. Additionally, in the statistical analysis including patients on civil commitment, all 4 patients in the LAI group were prescribed paliperidone, which does not allow for comparison to other LAI antipsychotics. The retrospective nature made complete data collection difficult for patients prescribed oral antipsychotics; the only information available regarding medication adherence and persistence was prescription fill history, which does not guarantee medication ingestion. It is difficult to determine the clinical significance of a short gap in LAI treatment (greater than the manufacturer-recommended grace period following a missed dose) because of scheduling issues or lack of transportation; having only retrospective data available limits investigators to gauge quality of life based on use of acute care visits, but it does not identify hardships due to lack of medication adherence or persistence. In addition, only the acute care services within Eskenazi Health were captured. One factor to consider is that patients who are more severely ill tend to be the patients considered for LAI antipsychotics. Investigators question whether this factor may have impacted the statistical significance for gaps in therapy and use of acute care services for those patients in the LAI and both formulations groups. Unfortunately, retrospective data do not provide much supporting evidence of this theory.
A strength of the present study is the inclusion of patients with early episode psychosis. Patients who have had a psychotic disorder for many years may not respond as well to treatment because of multiple relapses without full recovery to baseline.2 Inclusion of only patients with early episode psychosis allows us to gain a better picture of adherence and persistence in patients with fewer confounding factors. Another strength is inclusion of all commercially available antipsychotics except asenapine and iloperidone, which is more reflective of clinical practice; thus, it provides higher external validity compared with studies evaluating only 1 antipsychotic. An additional strength is the multiple regression analysis, which points to medication formulation as a specific predictor of adherence in this population. This study also includes statistical analysis including and excluding patients on a civil commitment at some point during the study period, which allows for a better understanding of adherence patterns between those with and without a civil commitment; as often observed in clinical practice, civil commitment does not translate to better adherence in all patients. Although this study does not show statistical significance for 2 study end points excluding patients on civil commitment, it does point to an important observation that patients with early psychosis have significant trouble with medication adherence while taking oral antipsychotics.
Conclusions
This study found that LAI antipsychotics were associated with better adherence compared with oral antipsychotics in patients with early psychosis but did not significantly decrease gaps in therapy or use of psychiatric acute care services. Large prospective studies may be beneficial for this unique population.
Acknowledgments
The authors would like to thank Todd Walroth, PharmD, BCCCP, for his contributions to the statistical analysis.
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1. Robinson D, Woerner MG, Alvir JMJ, Bilder R, Goldman R, Geisler S, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999; 56 3: 241- 7. DOI: 10.1001/archpsyc.56.3.241. PubMed PMID: 10078501. [DOI] [PubMed] [Google Scholar]
- 2. McGorry PD. . Early intervention in psychotic disorders: beyond debate to solving problems. Br J Psychiatry. 2005; 187 48: s108- 10. DOI: 10.1192/bjp.187.48.s108. PubMed PMID: 16055797. [DOI] [PubMed] [Google Scholar]
- 3. Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IPM, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008; 371 9618: 1085- 97. DOI: 10.1016/S0140-6736(08)60486-9. PubMed PMID: 18374841. [DOI] [PubMed] [Google Scholar]
- 4. Liu CC, Shan JC, Chiang CL, Hsieh MH, Liu CM, Chien YL, et al. Initiating long-acting injectable antipsychotics during acute admission for patients with schizophrenia--a 3-year follow-up. J Formos Med Assoc. 2015; 114 6: 539- 45. DOI: 10.1016/j.jfma.2013.01.004. PubMed PMID: 26062967. [DOI] [PubMed] [Google Scholar]
- 5. Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. . Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013; 74 10: 957- 65. DOI: 10.4088/JCP.13r08440. PubMed PMID: 24229745. [DOI] [PubMed] [Google Scholar]
- 6. Rosenheck RA, Krystal JH, Lew R, Barnett PG, Fiore L, Valley D, et al. Long-acting risperidone and oral antipsychotics in unstable schizophrenia. N Engl J Med. 2011; 364 9: 842- 51. DOI: 10.1056/NEJMoa1005987. PubMed PMID: 21366475. [DOI] [PubMed] [Google Scholar]
- 7. Emsley R, Oosthuizen P, Koen L, Niehaus DJH, Medori R, Rabinowitz J. . Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008; 30 12: 2378- 86. DOI: 10.1016/j.clinthera.2008.12.020. PubMed PMID: 19167596. [DOI] [PubMed] [Google Scholar]
- 8. Lafeuille MH, Dean J, Carter V, Duh MS, Fastenau J, Dirani R, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014; 30 8: 1643- 55. DOI: 10.1185/03007995.2014.915211. PubMed PMID: 24730586. [DOI] [PubMed] [Google Scholar]
- 9. Kamat S, Offord S, Docherty J, Lin J, Eramo A, Baker RA, et al. Reduction in inpatient resource utilization and costs associated with long-acting injectable antipsychotics across different age groups of Medicaid-insured schizophrenia patients. Drugs Context. 2015; 4: 1- 12. DOI: 10.7573/dic.212267. PubMed PMID: 25834621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McEvoy JP, Byerly M, Hamer RM, Dominik R, Swartz MS, Rosenheck RA, et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA. 2014; 311 19: 1978- 87. DOI: 10.1001/jama.2014.4310. PubMed PMID: 24846035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeong HG, Lee MS. . Long-acting injectable antipsychotics in first-episode schizophrenia. Clin Psychopharmacol Neurosci. 2013; 11 1: 1- 6. DOI: 10.9758/cpn.2013.11.1.1. PubMed PMID: 23678347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viala A, Cornic F, Vacheron MN. . Treatment adherence with early prescription of long-acting injectable antipsychotics in recent-onset schizophrenia. Schizophr Res Treat. 2012; 2012: 368687 DOI: 10.1155/2012/368687. PubMed PMID: 22966435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emsley R, Chiliza B, Asmal L, Mashile M, Fusar-Poli P. . Long-acting injectable antipsychotics in early psychosis: a literature review. Early Interv Psychiatry. 2013; 7 3: 247- 54. DOI: 10.1111/eip.12027. PubMed PMID: 23342964. [DOI] [PubMed] [Google Scholar]
- 14. Novick D, Haro JM, Bertsch J, Anand H, Jemiai N, Haddad PM. . Comparison of treatment discontinuation and hospitalization among nonadherent patients initiating depot or oral typical antipsychotic medications. Int Clin Psychopharmacol. 2012; 27 5: 275- 82. DOI: 10.1097/YIC.0b013e328354db12. PubMed PMID: 22699789. [DOI] [PubMed] [Google Scholar]
- 15. Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. . Oral versus depot antipsychotic drugs for schizophrenia--a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011; 127 1-3: 83- 92. DOI: 10.1016/j.schres.2010.11.020. PubMed PMID: 21257294. [DOI] [PubMed] [Google Scholar]
- 16. Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. . A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011; 168 6: 603- 9. DOI: 10.1176/appi.ajp.2011.10081224. PubMed PMID: 21362741. [DOI] [PubMed] [Google Scholar]
- 17. Weiden PJ, Schooler NR, Weedon JC, Elmouchtari A, Sunakawa A, Goldfinger SM. . A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009; 70 10: 1397- 406. DOI: 10.4088/JCP.09m05284yel. PubMed PMID: 19906343. [DOI] [PubMed] [Google Scholar]
- 18. Weiden PJ, Schooler NR, Weedon JC, Elmouchtari A, Sunakawa-McMillan A. . Maintenance treatment with long-acting injectable risperidone in first-episode schizophrenia: a randomized effectiveness study. J Clin Psychiatry. 2012; 73 9: 1224- 33. DOI: 10.4088/JCP.11m06905. PubMed PMID: 22938760. [DOI] [PubMed] [Google Scholar]
- 19. Heres S, Lambert M, Vauth R. . Treatment of early episode in patients with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry. 2014; 29 Suppl 2: 1409- 13. DOI: 10.1016/S0924-9338(14)70001-X. PubMed PMID: 25455704. [DOI] [PubMed] [Google Scholar]
- 20. Schreiner A, Aadamsoo K, Altamura AC, Franco M, Gorwood P, Neznanov NG, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015; 169 1-3: 393- 9. DOI: 10.1016/j.schres.2015.08.015. PubMed PMID: 26431793. [DOI] [PubMed] [Google Scholar]
- 21. Kim B, Lee SH, Choi TK, Suh SY, Kim YW, Lee EH, et al. Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008; 32 5: 1231- 5. DOI: 10.1016/j.pnpbp.2008.03.012. PubMed PMID: 18442879. [DOI] [PubMed] [Google Scholar]