Abstract
Background
End-of-life care for older adults with malignant brain tumors is poorly understood. The purpose of this study is to quantify end-of-life utilization of hospice care, cancer-directed therapy, and associated Medicare expenditures among older adults with malignant brain tumors.
Methods
This retrospective cohort study included deceased Medicare beneficiaries age ≥65 with primary malignant brain tumor (PMBT) or secondary MBT (SMBT) receiving care within a southeastern cancer community network including academic and community hospitals from 2012–2015. Utilization of hospice and cancer-directed therapy and total Medicare expenditures in the last 30 days of life were calculated using generalized linear and mixed effect models, respectively.
Results
Late (1–3 days prior to death) or no hospice care was received by 24% of PMBT (n = 383) and 32% of SMBT (n = 940) patients. SMBT patients received late hospice care more frequently than PMBT patients (10% vs 5%, P = 0.002). Cancer-directed therapy was administered to 18% of patients with PMBT versus 25% with SMBT (P = 0.003). Nonwhite race, male sex, and receipt of any hospital-based care in the final 30 days of life were associated with increased risk of late or no hospice care. The average decrease in Medicare expenditures associated with hospice utilization for patients with PMBT was $−12,138 (95% CI: $−18,065 to $−6210) and with SMBT was $−1,508 (95% CI: $−3,613 to $598).
Conclusions
Receiving late or no hospice care was common among older patients with malignant brain tumors and was significantly associated with increased total Medicare expenditures for patients with PMBT.
Keywords: brain metastasis, brain tumor, cancer elderly, cancer hospice, malignant brain neoplasm
Importance of the study
A quarter of all Medicare funds are spent in the final year of life. Previous analyses have attempted to detail end-of-life care and resource utilization for cancer patients at large. However, no prior studies have focused on utilization of cancer treatment and hospice care for the growing number of older patients dying with malignant brain tumors. This study shows that over 20–30% of primary and secondary malignant brain tumor patients receive late or no hospice care despite predictable mortality. Notably, receiving hospital-based care resulted in the highest relative risk of never or late hospice enrollment. In addition, disparities exist within this population, with men and minorities more likely to receive aggressive end-of-life care. This study highlights the need for further exploration of underlying reasons for aggressive care at the end of life and potential preemptive palliative interventions, especially for higher-risk populations.
Medical care for patients dying with terminal cancer is often aggressive, costly, and, most importantly, discordant with patient and caregiver preferences.1–5 Recognized indicators of aggressive end-of-life care include chemotherapy utilization within 30 days of death, late (1–3 days) hospice entry, and lack of hospice care.6 Health care costs in the last year of life account for 25% of Medicare expenditures, with nearly half of this amount spent on care received within the final 30 days.7 With the number of patients enrolled in Medicare expected to nearly double over the next 20 years, improving the quality and sustainability of end-of-life care for older patients with terminal diseases is of particular concern.8 Early access to hospice care is known to be associated with improved quality of care for terminally ill patients and their caregivers.9,10
If considered a collective entity, the malignant brain tumor incidence in the US would rival those of prostate, breast, and lung cancers. Over 200000 new cases of primary malignant brain tumor (PMBT) or secondary MBT (SMBT) are diagnosed each year.11–13 Malignant brain tumors are often terminal, with median survival in older adults on the order of 6–9 months. They also commonly inflict significant symptom burden that can have devastating effects on quality of life, which may not be improved with aggressive oncologic treatment.14–17 Patients with tumors affecting the brain are at heightened risk of early cognitive decline, making them more vulnerable than other cancer patients to receiving aggressive care that is discordant with their preferences.4,5,18–22 Unfortunately, there are limited data on end-of-life practice patterns in this population, including use of hospice. This analysis was performed to describe and compare utilization of hospice care and cancer-directed therapy, risk factors for receiving aggressive care, and impact on overall Medicare expenditures in the last 30 days of life for Medicare beneficiaries 65 years and older with PMBT and SMBT.
Methods
Study Design and Data Sources
This study was approved by the University of Alabama at Birmingham (UAB) institutional review board as a retrospective cohort study of Medicare administrative claims data for fee-for-service beneficiaries age 65 and older with Medicare Part A and B coverage in the UAB Health System Cancer Community Network (UAB CCN). The UAB CCN includes 2 academic and 10 community medical centers in Alabama, Florida, Georgia, Tennessee, and Mississippi. Patients with health maintenance organization (HMO) coverage were excluded. Decedents were identified from 2012 to 2015 with a diagnosis of PMBT (International Classification of Diseases, Ninth Revision [ICD-9], code 191.X) or SMBT (ICD-9 code 198.2). Decedents with diagnosis codes for both PMBT and SMBT (dual-coded cohort) were excluded from the primary analysis.
Patient characteristics were collected from claims data and local cancer registries from the UAB CCN sites and included age at death, race (white vs nonwhite), sex, Charlson comorbidity score (0 vs ≥1; excluding cancer diagnosis), and, in the case of SMBT, coexisting primary cancer site diagnosis (lung vs breast vs other). Comorbid conditions were abstracted from claims data from the entire time period (2012–2015) and classified based on the Klabunde modification for comorbidities as denoted by the reported Charlson score.23–26 Hospital-based care was defined to include: emergency department visits (Healthcare Common Procedure Coding System [HCPCS] code G0380), intensive care unit admissions (HCPCS G9657), and hospital admissions as determined by Centers for Medicare & Medicaid Services inpatient admission claims.27
Hospice Utilization, Cancer-Directed Therapy, and Medicare Expenditures
Hospice care, utilization of cancer-directed therapies, and total Medicare expenditures were examined in the 30 days prior to death based on National Quality Forum endorsed quality measures.6 Hospice care was defined as any use of outpatient, in-home, or inpatient hospice services (HCPCS Q50xx). Hospice utilization was evaluated prior to death and total duration from initiation of hospice utilization until death was calculated. Late hospice utilization was defined as enrollment 1–3 days prior to death in accordance with the National Quality Forum. Cancer-directed therapy was defined by two measures: systemic therapy, including chemotherapy and immunotherapy administration (HCPCS J9000-9280, J9999, G0355, Q0083-0085; ICD-9 V58.1x), and radiation therapy (ICD-9 92.3x, HCPCS G6003-6015; ICD-9 92.23, 92.24, 92.26). Medicare expenditures were defined as total reimbursements to providers for all services received within 30 days of death including inpatient, outpatient, physician visit, skilled nursing facility, home health, durable medical equipment, and hospice care.
Statistical Analysis
Descriptive statistics of baseline patient characteristics were calculated for each cohort and were compared using the independent sample t-test and the chi-square test. Measures of hospice utilization and cancer-directed therapy were compared for PMBT versus SMBT using the Mann–Whitney U-test (continuous variables) and chi-square test (categorical variables). For both PMBT and SMBT, relative risks and corresponding 95% confidence intervals (CI) were calculated for cancer-directed therapy utilization, no hospice utilization, and late hospice utilization using generalized linear models with a log link and Poisson distribution with robust variance estimates.28 Models included age, sex, race, comorbidity status, and hospital-based care (hospice models only) as predictors.
Linear mixed effect models accounting for random effects (intercept) with an unstructured covariance matrix were constructed to assess total expenditures to Medicare for patients with PMBT and SMBT. Beta coefficients and 95% CIs were estimated after including known risk factors: patient characteristics (age, sex, race, and comorbidity status), hospital-based care, cancer-directed therapy, and hospice utilization. All analyses were performed using SAS software, version 9.4. Results were considered statistically significant if the P-value was <0.05. A sensitivity analysis was performed to assess the effect of excluding the dual-coded cohort.
Results
Population Characteristics
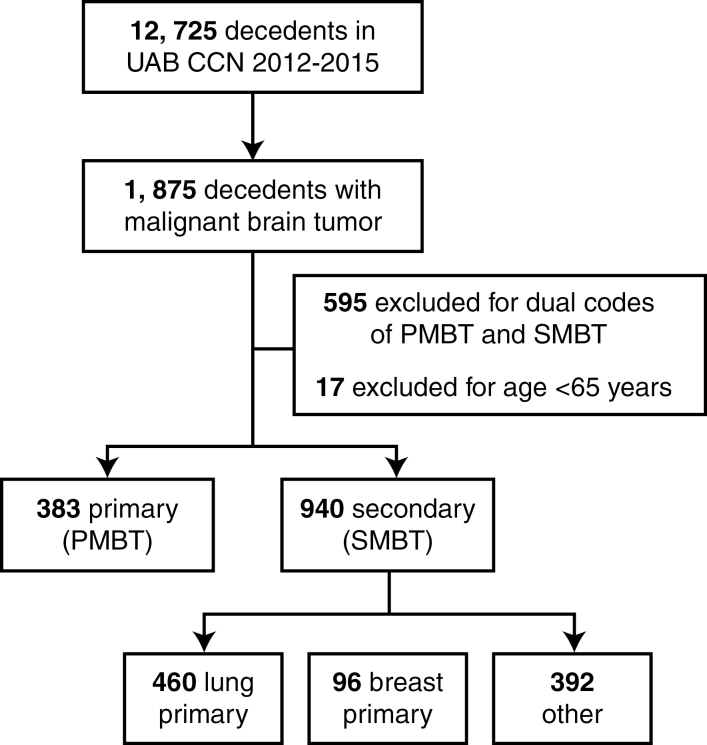
Of the 12725 decedents in the UAB CCN from 2012 to 2015 who died, 1323 (10%) had a diagnosis of either PMBT (n = 383) or SMBT (n = 940; Fig. 1). Patients with diagnosis codes for both PMBT and SMBT (n = 595) and who were <65 years of age (n = 17) were excluded, though sensitivity analyses that included the dual-coded cohort provided similar results for demographic characteristics. Baseline patient characteristics were similar between groups (Table 1). The most common primary cancer diagnosis in the SMBT cohort was lung cancer (49%).
Fig. 1.
Study schema.
Table 1.
Study population characteristics of decedents with PMBT or SMBT (n = 1323)
| Characteristics | PMBT, n = 383 | SMBT, n = 940 | P | |
|---|---|---|---|---|
| Age at death, median (interquartile range), y | 74 (69–79) | 73 (69–78) | 0.10 | |
| Sex, no. (%) | Male | 198 (52) | 495 (53) | 0.75 |
| Female | 185 (48) | 445 (47) | ||
| Race, no. (%) | White | 333 (87) | 792 (84) | 0.16 |
| Nonwhite | 48 (13) | 147 (16) | ||
| Charlson comorbidity score, no. (%) | 0 | 91 (26) | 183 (21) | 0.10 |
| ≥1 | 265 (74) | 677 (79) | ||
| Site of primary cancer, no. (%) | Lung | NA | 460 (49) | |
| Breast | NA | 96 (10) | ||
| Other | NA | 392 (42) |
Hospice Care
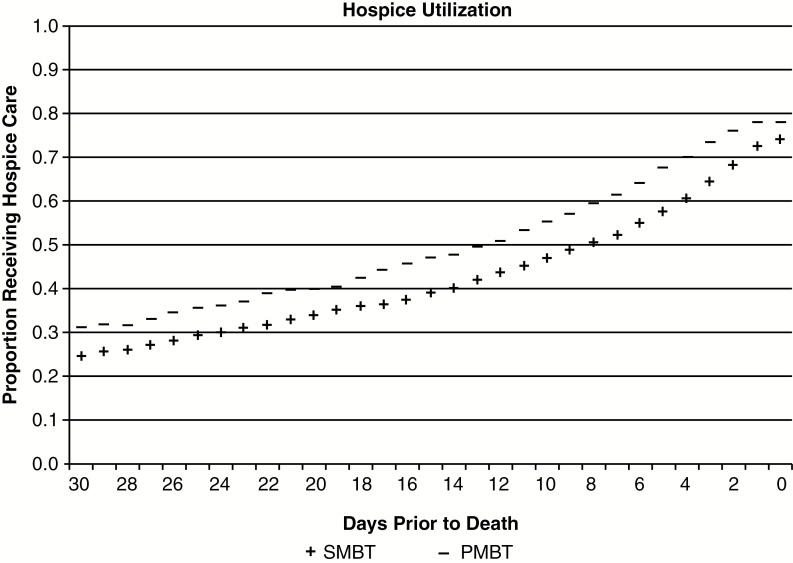
Fig. 2 presents the proportion of patients enrolled in hospice throughout the final 30 days of life. Among PMBT and SMBT patients, 31.3% and 24.9%, respectively, were enrolled in hospice care at 30 days prior to death. Sensitivity analyses that included the dual-coded cohort provided similar results for hospice care utilization. The majority of both PMBT and SMBT patients (81% vs. 78%, P = 0.19) utilized hospice care prior to death (Table 2). The median duration of hospice care was modestly shorter for those with SMBT compared with those with PMBT (15 vs 18 days, P = 0.004). Patients with SMBT had twice the frequency of late (1–3 days) hospice enrollment compared with those with PMBT (10% vs 5%, P = 0.002).
Fig. 2.
Proportion of patients with PMBT and SMBT receiving hospice care throughout the final 30 days of life.
Table 2.
Cancer-directed therapy, hospice utilization, and total Medicare expenditures in the last 30 days of life for decedents with PMBT and SMBT (n = 1323)
| PMBT, n = 383 | SMBT, n = 940 | P | ||
|---|---|---|---|---|
| Hospice care, no. (%) | Late (1–3 days prior to death) | 21 (5) | 97 (10) | 0.002 |
| None | 73 (19) | 210 (22) | 0.19 | |
| Duration in days, median (interquartile range [IQR]) | 18 (7–48) | 15 (5–40) | 0.004 | |
| Cancer therapy, no. (%) | Any | 68 (18) | 239 (25) | 0.003 |
| Systemic therapy | 44 (11) | 124 (13) | 0.40 | |
| Radiation therapy | 25 (7) | 142 (15) | <0.001 | |
| Total Medicare expenditures | US $, median (IQR) | 8592 (5406 – 19214) | 9964 (5593 – 17714) | 0.75 |
In the multivariable model, nonwhite race was associated with an increased risk of never enrolling in hospice for both PMBT (relative risk [RR] 1.59, 95% CI: 1.03–2.78; Table 3) and SMBT (RR 1.56, 95% CI: 1.17–2.09). Male sex was also associated with never enrolling in hospice among patients with SMBT (RR 1.33, 95% CI: 1.04–1.70). Hospital-based care resulted in the greatest relative risk of late or no hospice enrollment. Receipt of any hospital-based care by patients with PMBT or SMBT was associated with late (PMBT RR 5.22, 95% CI: 1.78–15.3 and SMBT RR 5.24, 95% CI: 3.00–9.13) and no (PMBT RR 6.99, 95% CI: 3.55–13.75 and SMBT RR 3.64, 95% CI: 2.55–5.21) hospice enrollment.
Table 3.
Risk factors for health care utilization and expenditures
| Variable | PMBT (n = 383) | SMBT (n = 940) | ||
|---|---|---|---|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Late Hospice Care a | ||||
| Age | 1.14 (0.77–1.68) | 0.52 | 1.06 (0.86–1.29) | 0.60 |
| Male | 2.53 (0.91–7.08) | 0.08 | 1.16 (0.79–1.69) | 0.45 |
| Nonwhite | 1.84 (0.60–5.69) | 0.29 | 0.80 (0.43–1.47) | 0.47 |
| Any comorbidity | 2.33 (0.54–9.96) | 0.25 | 1.21 (0.69–2.12) | 0.50 |
| Any hospital-based care | 5.22 (1.78–15.3) | 0.003 | 5.24 (3.00–9.13) | <0.001 |
| No Hospice Care | ||||
| Age | 1.01 (0.82–1.25) | 0.90 | 0.98 (0.85–1.12) | 0.72 |
| Male | 0.89 (0.59–1.33) | 0.56 | 1.33 (1.04–1.70) | 0.02 |
| Nonwhite | 1.59 (1.03–2.78) | 0.04 | 1.56 (1.17–2.09) | 0.003 |
| Any comorbidity | 0.87 (0.54–1.39) | 0.56 | 1.20 (0.83–1.73) | 0.34 |
| Any hospital-based care | 6.99 (3.55–13.75) | <0.001 | 3.64 (2.55–5.21) | <0.001 |
| Cancer Therapy | ||||
| Age | 0.85 (0.69–1.06) | 0.15 | 0.86 (0.75–0.98) | 0.03 |
| Male | 1.08 (0.69–1.71) | 0.73 | 1.08 (0.86–1.36) | 0.49 |
| Nonwhite | 0.48 (0.18–1.27) | 0.14 | 0.72 (0.49–1.02) | 0.07 |
| Any comorbidity | 1.16 (0.68–1.98) | 0.59 | 1.55 (1.11–2.15) | 0.009 |
| Total Medicare Expenditures b | Estimate (95% CI), US dollars | P-value | Estimate (95% CI), US dollars | P-value |
| Any cancer therapy | −3789 ( −9404 −1825) | 0.19 | 1672 ( −268–3613) | 0.09 |
| Any hospice care | −12138 ( −18065 to −6210) | <0.001 | −1508 ( −3613‒598) | 0.16 |
*Generalized linear models predicting risk for cancer-directed therapy (systemic therapy or radiation therapy), no hospice care, late (1–3 days prior to death) hospice care, and linear mixed effect models predicting total Medicare expenditures in the last 30 days of life for patients with PMBT and SMBT.
aModel of late hospice care excludes patients with no hospice care (PMBT, n = 310; SMBT, n = 730).
bModel also includes age, sex, race, any comorbidity, and any hospital-based care.
Cancer-Directed Therapy
Differences in cancer-directed therapy between decedents with PMBT and SMBT in the last 30 days of life are presented in Table 2. Sensitivity analyses that included the dual-coded cohort provided similar results for cancer-directed therapy utilization. Systemic therapy utilization at the end-of-life was similar for patients with PMBT and SMBT (11% vs 13%, P = 0.40). However, patients with SMBT had more than double the rate of radiation therapy utilization in the final 30 days of life compared with those with PMBT (15% vs 7%, P < 0.001). Among patients with SMBT, the presence of at least one comorbidity (RR 1.55, 95% CI: 1.11–2.15; Table 3) was associated with an increased risk for cancer therapy in the final 30 days of life, while increasing age (RR 0.86, 95% CI: 0.75–0.98) was associated with a decreased risk. Among patients with PMBT, no factors were associated with cancer therapy utilization.
Medicare Expenditures
Total Medicare expenditure in the final 30 days life was previously reported and was similar between patients with PMBT and SMBT ($8592 vs $9964, P = 0.75; Table 2).27 Sensitivity analyses that included the dual-coded cohort provided similar results for total Medicare expenditures. In the multivariable model, hospice utilization was associated with a decrease in total expenditure of $−12138 (95% CI: $ −18065 to $ −6210) in the last 30 days of life for patients with PMBT (Table 3) and $ −1508 (95% CI: $ −3613 to $598) for patients with SMBT. Cancer-directed therapy was not significantly associated with total Medicare expenditures in the last 30 days of life for PMBT or SMBT.
Discussion
To our knowledge, this is the largest study to date reporting trends in oncologic and hospice care for older patients with malignant brain tumors at the end of life. In this cohort of patients treated in the southeastern US, the rate of receiving no or late hospice care was over 30%. Overall rates of hospice use in PMBT patients in the last month of life were similar to those reported this year from a large academic cancer center.29 Diamond et al previously reported that among evaluated PMBT patients enrolled in hospice, 22.5% did so less than 7 days prior to death.21 Our data showed that 11.3% of all hospice enrollments for older patients with malignant brain tumors occurred within 3 days prior to death. Together these data highlight how dichotomous evaluations of whether or not a patient enters hospice prior to death may misrepresent the proportion of patients receiving meaningful hospice care.6
While late and no hospice care rates were high, a larger proportion of patients with malignant brain tumors were enrolled in hospice prior to death relative to reports from other cancer sites.30–33 Though hospice utilization in end-of-life cancer care has increased nationally over the past few decades, the duration of hospice care is often short and immediately preceded by aggressive care.19–22 In this setting, hospice care is often used to manage the dying process rather than improve quality of life. This pattern of care in advanced cancer patients is often attributed to patient or provider overestimation of life expectancy leading to a choice of aggressive care at a juncture not perceived to be the end of life.4,5,18,19,34,35 Although malignant brain tumors are often incurable, data are lacking on managing physicians’ abilities to estimate life expectancies of individual patients.
We found that late hospice care was twice as common in SMBT compared with PMBT patients, which may reflect less certain disease trajectories and a wider array of potential treatment options for SMBT. This explanation is supported by the increased risk of receiving cancer-directed therapy at the end of life in SMBT patients. Little data exist on patterns of end-of-life cancer-directed therapy in brain tumor patients.36–38 However, the rate of systemic therapy utilization in the last 30 days of life for the cohort in this study (12.7%) was congruent with the rate among patients older than 65 years with any cancer in the US in 2012 (10.6%) as reported by Bekelman et al.38 While billing data do not reliably delineate between chemotherapy and its more expensive counterpart immunotherapy, the systemic therapy for SMBT likely comprised a greater proportion of immunotherapy than that for PMBT, which may explain the nominally more expensive impact of cancer therapy at the end of life among SMBT patients. The increased utilization of radiation therapy for SMBT versus PMBT could represent end-of-life palliation of extracranial metastases not present in those with PMBT. Importantly, though, cancer therapy did not significantly contribute to increased Medicare expenditures.
Hospice care in PMBT patients was associated with a significant reduction in overall expenditures in the last month of life. Of note, receipt of hospital-based care in the final days of life had an increased relative risk for no or late hospice care, a perhaps unsurprising finding given the often diverging immediate goals of hospital-based care (to prolong life) and hospice care (to support quality of life). In our previous study, we reported that SMBT patients were more likely than PMBT patients to be hospitalized in the final month of life (50% vs 42%, P = 0.009), and hospitalizations were associated with a significant increase in Medicare expenditures.27 The transition to hospice in SMBT patients may be more likely to occur following hospitalizations, which would curtail any cost savings potentially associated with hospice in that population.
The receipt of aggressive care at the end of life is not specific to patients with malignant brain tumors compared with other cancer types and likely reflects patterns of end-of-life care for advanced cancer patients across the entire health care system.38 Therefore, interventions aimed at reducing nonbeneficial health care utilization could target processes within the health care system that have cross-cutting potential for quality improvement regardless of the underlying malignancy. Uniform, early integration of palliative services facilitates appropriate use of aggressive medical interventions at the end of life and improves outcomes for patients and caregivers, as outlined by several professional societies.39–47
This study was designed to broadly describe end-of-life cancer care and hospice utilization for patients with malignant brain tumors, and therefore it has important limitations. First, aggressive end-of-life care is not uniformly discordant with patient preferences. For example, male and nonwhite patients were at increased risk of receiving no hospice care in this study. These findings are consistent with previous studies reporting these characteristics to be risk factors for aggressive end-of-life care and lower hospice utilization.21,48–50 Some have argued that these trends represent different preferences as opposed to true inequities in the delivery of care.49 Medicare data do not include evaluation of patient preferences, which would be important to fully delineate appropriateness of aggressive end-of-life care.
Second, the population consisted of Medicare decedents with malignant brain tumors cared for at two academic and 10 community hospitals in the southeastern US, limiting generalizability to younger populations or different regions of the country.33 For example, primary cancer sites for SMBT patients may not reflect those of the population at large (eg, the proportion of patients with lung cancer primaries far outweigh breast in our cohort). Furthermore, several patients (n = 595) were excluded due to the presence of dual coding for both primary and secondary brain tumors—a much higher number than would be expected to concurrently harbor a primary brain malignancy and metastatic disease to the brain—which highlights the potential for human error in billing classifications. We felt that including only patients with either single code produced purer cohorts notwithstanding the introduction of potential sampling bias if an unrecognized characteristic was more commonly represented in the dual-coded group. However, sensitivity analyses with inclusion of these patients had no significant impact on results.
Third, limitations of ICD-9 coding prevented determination of extracranial disease status and molecular studies that confer differences in expected prognosis. It also limits a more comprehensive assessment of individual demographic factors further influencing rates of aggressive end-of-life care such as marital status and/or education and income levels. In that vein, expenditures measured in this study are only those incurred by Medicare through reimbursements to health care providers and do not represent the out-of-pocket financial burden of individual beneficiaries. There also remain significant controversies over the values attributed to various utilization metrics.51 Finally, we did not explore many potentially interesting clinical scenarios such as the impacts of immunotherapy versus cytotoxic chemotherapy or the sequences of utilization which merit thorough investigations beyond the scope of this study.
In conclusion, results of this analysis suggest that a substantial number of older patients with malignant brain tumors do not receive hospice care for greater than 3 days prior to death, contrary to national guidelines.6,46–47 In addition, disparities exist within this population, with men and minorities being more likely to receive aggressive care. Oncologists should consider early goals-of-care discussions and integration of palliative care to avoid undesired aggressive measures in the final days of life for their older patients with malignant brain tumors.
Funding
This project was supported by the University of Alabama at Birmingham Department of Radiation Oncology Intramural Pilot Grant Program.
Acknowledgments
Co-first authors Caleb Dulaney, MD and Laura Dover, MD contributed equally to the data synthesis and reporting of outcomes presented in this manuscript. The preliminary findings from this study have previously been presented in abstract form at the 2016 Society of Neuro-Oncology Annual Meeting in Phoenix, Arizona. An analysis of hospital-based care and its impact on overall Medicare expenditures in this cohort has been previously published online June 22, 2017 in a research letter to JAMA Oncology.
Conflict of interest statement
No disclosures.
References
- 1. IOM (Institute of Medicine). 2015. Dying in America: Improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 2. Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care. 2007;45(5):386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. N Engl J Med. 1996;335(3):172–178. [DOI] [PubMed] [Google Scholar]
- 4. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709–1714. [DOI] [PubMed] [Google Scholar]
- 5. Voogt E, van der Heide A, Rietjens JA, et al. Attitudes of patients with incurable cancer toward medical treatment in the last phase of life. J Clin Oncol. 2005;23(9):2012–2019. [DOI] [PubMed] [Google Scholar]
- 6. Palliative Care and End-of-Life Endorsement Maintenance Standards. National Quality Forum. Web 2012: http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0ahUKEwiau7_Li-3PAhVCNj4KHZKFD8AQFggiMAE&url=http%3A%2F%2Fwww.qualityforum.org%2FProjects%2Fn-r%2FPalliative_Care_and_End-of-Life_Care%2FTable_of_Measures.aspx&usg=AFQjCNEeTI5gsWRhgOO7zMZW7mjQnMXrpw&bvm=bv.136499718,d.cWw. Accessed December 12, 2017.
- 7. Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45(2):565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. 2013 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. Web. 31 May 2013: https://downloads.cms.gov/files/tr2013.pdf. Accessed December 12, 2017.
- 9. Wright AA, Keating NL, Ayanian JZ, et al. Family Perspectives on Aggressive Cancer Care Near the End of Life. JAMA. 2016;315(3):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDermott CL, Fedorenko C, Kreizenbeck K, et al. End-of-Life Services Among Patients With Cancer: Evidence From Cancer Registry Records Linked With Commercial Health Insurance Claims. J Oncol Pract. 2017:JOP2017021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 12. Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011;22(1):1–6, v. [DOI] [PubMed] [Google Scholar]
- 13. 2016 Fact Sheet. Central Brain Tumor Registry of the United States. Web 2016: http://cbtrus.org/factsheet/factsheet.html. Accessed December 12, 2017.
- 14. Prognostic factors for high-grade malignant glioma: development of a prognostic index. A Report of the Medical Research Council Brain Tumour Working Party. J Neurooncol. 1990;9(1):47–55. [DOI] [PubMed] [Google Scholar]
- 15. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sizoo EM, Braam L, Postma TJ, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010;12(11):1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stone PC, Lund S. Predicting prognosis in patients with advanced cancer. Ann Oncol. 2007;18(6):971–976. [DOI] [PubMed] [Google Scholar]
- 19. Daugherty CK. Examining ethical dilemmas as obstacles to hospice and palliative care for advanced cancer patients. Cancer Invest. 2004;22(1):123–131. [DOI] [PubMed] [Google Scholar]
- 20. Catt S, Chalmers A, Fallowfield L. Psychosocial and supportive-care needs in high-grade glioma. Lancet Oncol. 2008;9(9):884–891. [DOI] [PubMed] [Google Scholar]
- 21. Diamond EL, Russell D, Kryza-Lacombe M, et al. Rates and risks for late referral to hospice in patients with primary malignant brain tumors. Neuro Oncol. 2016;18(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pace A, Di Lorenzo C, Lorenzo CD, et al. End of life issues in brain tumor patients. J Neurooncol. 2009;91(1):39–43. [DOI] [PubMed] [Google Scholar]
- 23. Frenkel WJ, Jongerius EJ, Mandjes-van Uitert MJ, van Munster BC, de Rooij SE. Validation of the Charlson Comorbidity Index in acutely hospitalized elderly adults: a prospective cohort study. J Am Geriatr Soc. 2014;62(2):342–346. [DOI] [PubMed] [Google Scholar]
- 24. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 25. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 26. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 27. Dover LL, Dulaney CR, Fiveash JB, et al. Hospital-Based End-of-Life Care and Costs for Older Patients With Malignant Brain Tumors. JAMA Oncol. 2017;3(11):1581–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160(4):301–305. [DOI] [PubMed] [Google Scholar]
- 29. Kuchinad KE, Strowd R, Evans A, Riley WA, Smith TJ. End of life care for glioblastoma patients at a large academic cancer center. J Neurooncol. 2017;134(1):75–81. [DOI] [PubMed] [Google Scholar]
- 30. McCarthy EP, Burns RB, Ngo-Metzger Q, Davis RB, Phillips RS. Hospice use among Medicare managed care and fee-for-service patients dying with cancer. JAMA. 2003;289(17):2238–2245. [DOI] [PubMed] [Google Scholar]
- 31. Keating NL, Herrinton LJ, Zaslavsky AM, Liu L, Ayanian JZ. Variations in hospice use among cancer patients. J Natl Cancer Inst. 2006;98(15):1053–1059. [DOI] [PubMed] [Google Scholar]
- 32. Wennberg JE, Fisher ES, Stukel TA, Skinner JS, Sharp SM, Bronner KK. Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States. BMJ. 2004;328(7440):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang SY, Aldridge MD, Gross CP, et al. Geographic Variation of Hospice Use Patterns at the End of Life. J Palliat Med. 2015;18(9):771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gramling R, Fiscella K, Xing G, et al. Determinants of Patient-Oncologist Prognostic Discordance in Advanced Cancer. JAMA Oncol. 2016;2(11):1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Epstein AS, Prigerson HG, O’Reilly EM, Maciejewski PK. Discussions of Life Expectancy and Changes in Illness Understanding in Patients With Advanced Cancer. J Clin Oncol. 2016;34(20):2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li D, Prigerson HG, Kang J, Maciejewski PK. Impact of Radiation Therapy on Aggressive Care and Quality of Life Near Death. J Pain Symptom Manage. 2017;53(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol. 2011;104(3):639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of Site of Death, Health Care Utilization, and Hospital Expenditures for Patients Dying With Cancer in 7 Developed Countries. JAMA. 2016;315(3):272–283. [DOI] [PubMed] [Google Scholar]
- 39. Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30(4):394–400. [DOI] [PubMed] [Google Scholar]
- 40. Levy MH, Back A, Benedetti C, et al. NCCN clinical practice guidelines in oncology: palliative care. J Natl Compr Canc Netw. 2009;7(4):436–473. [DOI] [PubMed] [Google Scholar]
- 41. Emanuel EJ, Young-Xu Y, Levinsky NG, Gazelle G, Saynina O, Ash AS. Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med. 2003;138(8):639–643. [DOI] [PubMed] [Google Scholar]
- 42. Bakitas MA, Tosteson TD, Li Z, et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol. 2015;33(13):1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 44. Morita T, Akechi T, Ikenaga M, et al. Late referrals to specialized palliative care service in Japan. J Clin Oncol. 2005;23(12):2637–2644. [DOI] [PubMed] [Google Scholar]
- 45. Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA. 2008;299(14):1698–1709. [DOI] [PubMed] [Google Scholar]
- 46. Ferris FD, Bruera E, Cherny N, et al. Palliative cancer care a decade later: accomplishments, the need, next steps – from the American Society of Clinical Oncology. J Clin Oncol. 2009;27(18):3052–3058. [DOI] [PubMed] [Google Scholar]
- 47. Ferrell BR, Temel JS, Temin S, et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(1):96–112. [DOI] [PubMed] [Google Scholar]
- 48. Degenholtz HB, Thomas SB, Miller MJ. Race and the intensive care unit: disparities and preferences for end-of-life care. Crit Care Med. 2003;31(5 Suppl):S373–S378. [DOI] [PubMed] [Google Scholar]
- 49. Smith AK, Earle CC, McCarthy EP. Racial and ethnic differences in end-of-life care in fee-for-service Medicare beneficiaries with advanced cancer. J Am Geriatr Soc. 2009;57(1):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramey SJ, Chin SH. Disparity in hospice utilization by African American patients with cancer. Am J Hosp Palliat Care. 2012; 29(5):346–354. [DOI] [PubMed] [Google Scholar]
- 51. Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue?J Clin Oncol. 2008;26(23):3860–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]