Abstract
Background
We previously reported the unexpected finding of significantly improved survival for newly diagnosed glioblastoma in patients when radiation therapy (RT) was initiated later (>4 wk post-op) compared with earlier (≤2 wk post-op). In that analysis, data were analyzed from 2855 patients from 16 NRG Oncology/Radiotherapy Oncology Group (RTOG) trials conducted prior to the era of concurrent temozolomide (TMZ) with RT. We now report on 1395 newly diagnosed glioblastomas from 2 studies, treated with RT and concurrent TMZ followed by adjuvant TMZ. Our hypothesis was that concurrent TMZ has a synergistic/radiosensitizing mechanism, making RT timing less significant.
Methods
Data from patients treated with TMZ-based chemoradiation from NRG Oncology/RTOG 0525 and 0825 were analyzed. An analysis comparable to our prior study was performed to determine whether there was still an impact on survival by delaying RT. Overall survival (OS) was investigated using the Kaplan–Meier method and Cox proportional hazards model. Early progression (during time of diagnosis to 30 days after RT completion) was analyzed using the chi-square test.
Results
Given the small number of patients who started RT early following surgery, comparisons were made between >4 and ≤4 weeks delay of radiation from time of operation. There was no statistically significant difference in OS (hazard ratio = 0.93; P = 0.29; 95% CI: 0.80–1.07) after adjusting for known prognostic factors (recursive partitioning analysis and O6-methylguanine-DNA methyltransferase methylation status). Similarly, the rate of early progression did not differ significantly (P = 0.63).
Conclusions
We did not observe a significant prognostic influence of delaying radiation when given concurrently with TMZ for newly diagnosed glioblastoma. The effects of early (1–3 wk post-op) or late (>5 wk) initiation of radiation tested in our prior study could not be replicated.
Keywords: chemoradiation, delay, glioblastoma, survival, temozolomide
Importance of the study
Glioblastomas are rapidly growing neoplasms, and many patients and families assume that adjuvant therapy must begin immediately following surgery, with every day of delay detrimental to survival; this presumption is reinforced in some cases by the treating physician. Therefore, having relevant data available can be of great logistical help in treatment planning. Our present study of the impact of delay of initiation of treatment in the era of concurrent chemoradiation adds to our original findings for similar patients treated with radiation monotherapy, for whom delay (up to 6 wk from surgery) of initiating treatment was surprisingly beneficial. In this study, we found that a short delay in beginning chemoradiation was neither beneficial nor detrimental.
Until the mid-2000s, irradiation alone was the established standard for treatment of newly diagnosed glioblastoma (GBM). Clinical trials of patients receiving radiation therapy (RT) for GBM have led to a doubling of overall survival (OS) compared with patients who did not receive RT.1 Results of a phase III trial published by the European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada (EORTC/NCIC) in 20052 led to the uniformly adopted treatment standard for newly diagnosed GBM: addition of temozolomide (TMZ) to the front-line regimen concurrent with external beam conformal radiation, followed by 6 adjuvant 5-day monthly cycles of TMZ. While there remains a subset of patients whose tumors do not benefit from treatment with RT and who progress during the first-line RT/TMZ period, the benefit of radiation for the standard adult population in newly diagnosed GBM is not disputed.
Further prospective phase III trials (NRG Oncology/RTOG 0525 and 0825) have validated the impact of adding TMZ to first-line therapy for GBM, with progression-free survival (PFS) and OS data similar to those seen on the RT/TMZ arm of the initially published study (ie, superior to treatment with radiation alone).
Prior work by our group3 showed that a short delay in the initiation of radiation for treatment of newly diagnosed GBM was not detrimental. In fact, we observed an unexpected progressive-linear benefit in median OS for delaying radiation treatment from 0–2 weeks to 6 weeks postoperatively. While the phenomenon described was thought-provoking, our prior work was based on data from NRG Oncology/RTOG trials for newly diagnosed GBM with RT alone arms, before TMZ became commonly used as standard first-line therapy in the clinic and in research trials. Thus, it is unclear whether our previous conclusions are germane in an era where almost all patients diagnosed with GBM receive not only RT but also concurrent chemotherapy.
Our objective with this study was to examine the impact of short delay (up to 6 wk) of RT initiation on survival outcome, in patients with newly diagnosed GBM treated on the RT/TMZ arms in the context of 2 large NRG Oncology/RTOG phase III trials. Our working hypothesis was that addition of TMZ to radiation would lessen the impact of the timing of initiating first-line therapy after surgery.
Methods
Data were analyzed from patients with newly diagnosed GBM treated using standard chemoradiation (TMZ 75 mg/kg daily with 60 Gy external beam radiation). We utilized data obtained from both arms of RTOG 0525 and the placebo arm of RTOG 0825. In addition, the analyses included patients who were eligible for the respective studies, started the standard concurrent chemoradiation, but did not reach the randomization stage due to reasons such as early progression/death, consent withdrawal, etc. RTOG 0525 was a phase III trial which compared conventional adjuvant TMZ with dose-intensive TMZ in patients with newly diagnosed GBM.4 Patients were randomized after successful completion of concurrent chemoradiation, which was required to begin less than 5 weeks postoperatively. The clinical dataset used is from January 6, 2011. By that time, 80% of all the eligible patients had died, and 91% had either progressed or died without disease progression. RTOG 0825 was a phase III double-blind, placebo-controlled trial of conventional concurrent chemoradiation and adjuvant TMZ plus bevacizumab versus conventional concurrent chemoradiation and adjuvant TMZ in patients with newly diagnosed GBM.5 Patients were randomized by 10 days after start of radiation. Radiation was required to begin after 3 weeks and less than 5 weeks from the date of surgery. The clinical dataset is from December 17, 2012. By that time, 67% of all the eligible patients had died, and 81% had either progressed or died without disease progression.
We computed the time interval between surgery and initiation of RT. Patients were initially categorized into one of 3 groups: time interval ≤3 weeks, >3 and ≤4 weeks, or >4 weeks. Overall survival was defined as the interval from start of RT to death due to any cause or to the time when the patient was last reported alive. Frequency distributions of the patient pretreatment characteristics for the 3 groups of patients were compared using chi-square tests for categorical variables and Kruskal–Wallis tests for continuous variables. The Kaplan–Meier method was used to calculate the survival rates for each group of patients. Hazard ratios (HRs) on the timing of RT initiation were calculated using the stratified Cox proportional hazards model, with study (RTOG 0525 vs 0825) included as strata, and tested using log-rank tests.
Because the treatment start time was not a randomized event, specific host and tumor variables could have nonrandomly led to certain patients starting therapy earlier versus others starting later; to account for this disparity, multivariate analyses were conducted using the stratified Cox proportional hazards model, with patient pretreatment characteristics included as covariates (Supplementary Table S1). The time interval between surgery and the initiation of RT was also examined as a continuous variable. Early progression was defined as progression that occurred between start of RT and 30 days after RT completion, and rates of early progression versus progression after 30 days of RT completion were analyzed using the chi-square test.
Results
A retrospective analysis of 1463 patients (1125 from RTOG 0525 and 338 from RTOG 0825) comparable to our prior published study was performed to determine whether, with the addition of TMZ, there was still an impact on survival by delaying RT (+TMZ). Sixty-eight patients were excluded from the analysis due to no radiation (44) or chemotherapy received (21), or unknown surgery date (3), leaving 1395 patients with data available for analysis. Consort diagrams are shown for RTOG 0525 and 0825, respectively, in Supplementary Figures S1 and S2. The distribution of the time between surgery and the initiation of RT with the same grouping method applied as in the previous manuscript is listed in Table 1, segregated into 3 groups: chemo-RT initiated ≤3 weeks; >3–4 weeks; and >4 weeks. Overall, only 2.2% and 2.5% of the patients initiated RT ≤2 and >5 weeks after surgery, respectively. Seven patients began chemo-RT >6 weeks from the time of surgery.
Table 1.
Distribution of interval from surgery to RT start
| Interval | RTOG 0525 | RTOG 0825 | Total | |||
|---|---|---|---|---|---|---|
| n | % | N | % | n | % | |
| ≤3 wk | 173 | 16.0 | 13 | 4.1 | 186 | 13.3 |
| >3–4 wk | 345 | 32.0 | 119 | 37.7 | 464 | 33.3 |
| >4 wk | 561 | 52.0 | 184 | 58.2 | 745 | 53.4 |
| Total | 1079 | 100.0 | 316 | 100.0 | 1395 | 100.0 |
Shown in Table 2 are pretreatment characteristics (age, sex, Karnofsky performance status, prior surgery, neurologic function, recursive partitioning analysis [RPA], and O6-methylguanine-DNA methyltransferase [MGMT] status) and RT dose by time interval from surgery to initiation of RT. The median ages ranged from 57 to 59 among the 3 different time interval groups, and median RT dose was 60 Gy for each of the groups. RPA stage and MGMT status are balanced among the 3 groups of patients. As expected, almost all the patients who started RT ≤3 weeks after surgery came from RTOG 0525.
Table 2.
Pretreatment characteristics
| Patient or Tumor Characteristic | ≤3 wk | >3–4 wk | >4 wk | P-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Days from surgery to start of RT | |||||||
| Median | 19 | 26 | 33 | ||||
| Min–Max | 0–21 | 22–28 | 29–68 | ||||
| Q1–Q3 | 16–21 | 24–27 | 31–35 | ||||
| RT dose | 0.42** | ||||||
| Median | 60 | 60 | 60 | ||||
| Min–Max | 22–60.14 | 4–64 | 2–62 | ||||
| Q1–Q3 | 60–60 | 60–60 | 60–60 | ||||
| Age, y | 0.24** | ||||||
| Median | 59 | 58 | 57 | ||||
| Min–Max | 22–87 | 19–84 | 20–84 | ||||
| Q1–Q3 | 51–66 | 50–65 | 50–65 | ||||
| Sex | 0.21* | ||||||
| Male | 105 | 56.5 | 286 | 61.6 | 423 | 56.8 | |
| Female | 81 | 43.5 | 178 | 38.4 | 322 | 43.2 | |
| Karnofsky performance status | 0.32* | ||||||
| 60 | 11 | 5.9 | 17 | 3.7 | 33 | 4.4 | |
| 70 | 23 | 12.4 | 57 | 12.3 | 89 | 11.9 | |
| 80 | 37 | 19.9 | 115 | 24.8 | 152 | 20.4 | |
| 90 | 87 | 46.8 | 183 | 39.4 | 311 | 41.7 | |
| 100 | 28 | 15.1 | 92 | 19.8 | 160 | 21.5 | |
| Surgery | Partial vs total resection | 0.003* | |||||
| Partial resection | 82 | 44.1 | 171 | 36.9 | 346 | 46.4 | |
| Total resection | 89 | 47.8 | 277 | 59.7 | 373 | 50.1 | |
| Other | 15 | 8.1 | 16 | 3.4 | 26 | 3.5 | |
| Neurologic function | No vs minor vs moderate/severe symptoms | 0.04* | |||||
| No symptoms | 46 | 24.7 | 141 | 30.4 | 260 | 34.9 | |
| Minor symptoms | 101 | 54.3 | 218 | 47.0 | 320 | 43.0 | |
| Moderate symptoms | 39 | 21.0 | 103 | 22.2 | 161 | 21.6 | |
| Severe symptoms | 0 | 0.0 | 2 | 0.4 | 4 | 0.5 | |
| RPA | RPA III vs IV vs V | 0.53* | |||||
| III | 28 | 15.1 | 77 | 16.6 | 138 | 18.5 | |
| IV | 111 | 59.7 | 288 | 62.1 | 450 | 60.4 | |
| V | 47 | 25.3 | 96 | 20.7 | 152 | 20.4 | |
| Unknown | 0 | 0.0 | 3 | 0.6 | 5 | 0.7 | |
| MGMT status | Methylated vs unmethylated | 0.70* | |||||
| Methylated | 46 | 24.7 | 120 | 25.9 | 209 | 28.1 | |
| Unmethylated | 105 | 56.5 | 267 | 57.5 | 422 | 56.6 | |
| Unknown (indeterminate, invalid) | 16 | 8.6 | 31 | 6.7 | 49 | 6.6 | |
| Insufficient tissue | 18 | 9.7 | 40 | 8.6 | 57 | 7.7 | |
| Not done | 1 | 0.5 | 6 | 1.3 | 8 | 1.1 | |
| Radiotherapy delivery declared at registration | <0.001* | ||||||
| RTOG RT# | 178 | 95.7 | 438 | 94.4 | 618 | 83.0 | |
| EORTC RT## | 8 | 4.3 | 26 | 5.6 | 127 | 17.0 | |
| Study number | <0.001* | ||||||
| RTOG 0525 | 173 | 93.0 | 345 | 74.4 | 561 | 75.3 | |
| RTOG 0825 | 13 | 7.0 | 119 | 25.6 | 184 | 24.7 | |
| Total | 186 | 100.0 | 464 | 100.0 | 745 | 100.0 | |
*Chi-square test.
**Kruskal–Wallis test.
# RTOG RT: RTOG radiation guidelines assume a 2-phase plan (46 + 14 = 60 Gy), with a wider field in most cases, that includes the “edema”/infiltrative non-enhancing tumor: T2 area of hyperintensivity + T1 enhancing residual tumor + resection cavity + a 2 cm margin.
## EORTC RT: EORTC radiation guidelines assume a 1-phase planned clinical target volume to treat T1 enhancing residual tumor + resection cavity + a 2–3 cm margin.
Kaplan–Meier curves for OS between the 2 studies, RTOG 0525 and 0825, show similar survival results (Supplementary Figure S1), justifying combining patients from these 2 studies for the analysis.
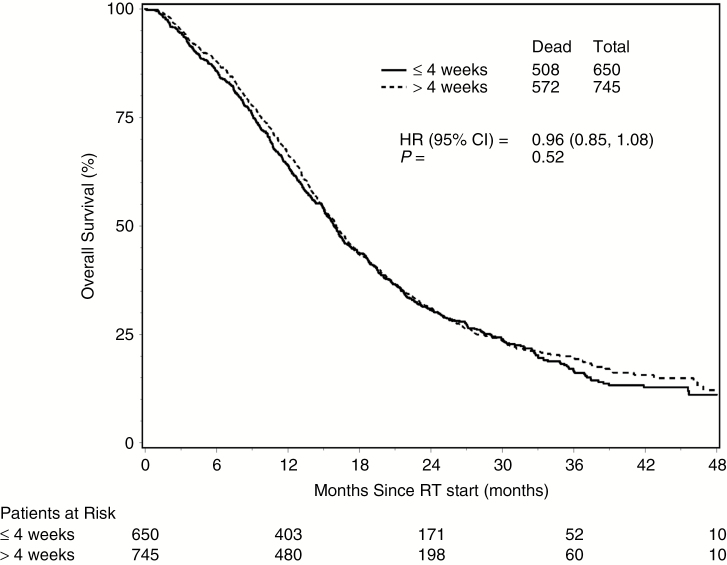
The median time from surgery to RT start was approximately 4 weeks. Given the relatively small number of patients who started RT early following surgery, we used 4 weeks as a cutoff to further define 2 groups. Comparisons were ultimately made between >4 and ≤4 weeks delay of radiation from time of surgery. Median survival times were 16.0 months (95% CI: 15.1–17.1) for the >4-week group and 15.9 months (95% CI: 15.0–16.7) for the ≤4-week group, with HR = 0.96 (P = 0.52; 95% CI: 0.85–1.08). Fig. 1 shows the Kaplan–Meier curves on OS for the 2 groups. There was no statistically significant difference in OS between the 2 groups (HR = 0.95; P = 0.49; 95% CI: 0.84–1.09) after adjusting for known prognostic factors (RPA and MGMT methylation status) and other pretreatment characteristics (Table 3). Among the 1395 patients, 1019 (73.0%) have experienced disease progression, and 18.1% of those events were categorized as early progression. Similar to OS, the rate of early progression did not differ significantly (P = 0.63) between the 2 groups. Comparable results were found regarding the effect (or lack of effect) of delaying RT, following subgroup analyses performed by RPA class and by MGMT methylation status.
Fig. 1.
Overall survival by 2 interval groups.
Table 3.
Cox proportional hazards model for OS (multivariate) using 2 interval groups
| Variable (bolded value has unfavorable outcome) | P-value | Hazard Ratio (95% CI) |
|---|---|---|
| Time from surgery to start of RT (>4 wk vs ≤4 wk) | 0.490 | 0.95 (0.84, 1.09) |
| RPA (IV vs III) | <0.001 | 1.65 (1.37, 1.99) |
| RPA (V vs III) | <0.001 | 2.91 (2.34, 3.61) |
| MGMT status (unmethylated vs methylated) | <0.001 | 1.72 (1.48, 2.00) |
| Sex (male vs female) | <0.001 | 1.31 (1.14, 1.50) |
Model derived from stepwise selection.
Overall survival comparisons were examined among 3 (smaller) different time interval groups (Fig. 2). Pairwise comparisons of OS between time intervals showed that there was no difference in each comparison in either univariate or multivariate analyses. No significant difference was found in the rate of early progression for the 3 group comparisons.
Fig. 2.
Overall survival by 3 interval groups.
Time from surgery to start of RT was not shown to have a significant effect on OS, when analyzed as a continuous variable.6 These data are shown in Supplementary Table S2.
Discussion
We previously reported the unexpected finding of significantly improved survival in patients with newly diagnosed GBM when radiation was initiated later (>4 wk post-op) compared with earlier (≤2 wk post-op).3 In a prior controlled experiment in rat models undergoing cranial irradiation, increased tissue damage was observed when radiation therapy was delivered closer to the time of a surgical procedure compared with a delay in radiation or with controls who did not receive radiation; it is speculatively possible that this observation may have implications relevant to our findings in the present analysis.7 Our working hypothesis for this specific analysis was that concurrent TMZ has an additive or synergistic/radiosensitizing mechanism, rendering RT timing less significant than was seen in the results of our initial work done in the radiation monotherapy era.3
Temozolomide has been shown to provide significant additive effects in combination with radiation when exposing glioblastoma cell lines to the treatments, specifically at subtherapeutic levels of TMZ.8
The combination of the 2 modalities has been shown to have radiosensitizing effect (beyond additive) in selected cell lines when clinically therapeutic doses of TMZ are used.9,10 Radiosensitization as measured by damage to the tumor cell DNA is significantly increased by combining TMZ and radiation compared with either modality alone. Inhibition of tumor growth is seen with an almost 3-fold increase of radiation dose enhancement in xenografts, with end results of tumor growth inhibition more than what would be expected by additive effects.11 Temozolomide enhances radiosensitivity in vitro and in vivo, not via apoptosis but most likely via interference of mechanisms of DNA double-strand break repair.
We speculate that the impact of timing of initiating (chemo)radiation is less important in light of radiosensitizing effect of TMZ compared with radiation treatment as monotherapy for newly diagnosed glioblastoma.
Other recently published, smaller retrospective reports have examined the effect of postsurgical therapy initiation for GBM in the TMZ era and concur with our findings of no significant benefit or detriment for delaying initiation of chemoradiation therapy within the guidelines of 6 weeks (a criterion typically required for participation in a prospective clinical trial for first-line therapy of GBM)12–17 (see Table 4).
Table 4.
Recent studies reporting impact of RT-delay for RT/chemo-RT in newly diagnosed glioblastoma (all studies other than Blumenthal 2009 involved chemo-RT)
| Author | Type of Study | No. of Pts | Delay | Multivariate Analysis Significant Factors for OS | MGMT | Impact of Delay |
|---|---|---|---|---|---|---|
| Current study, Blumenthal 2016 |
NRG Oncology/RTOG retrospective analysis of 2 studies in the RT/ TMZ era | 1395 | ≤4 vs >4 (up to 6) wk delay.* Most patients were treated between 3 and 5 wk |
RPA stage, MGMT status | Known in 80% of pts | None |
| Blumenthal 2009 | RTOG retrospective analysis 1974– 2003 of 16 single agent RT trials | 2855 | ≤2 vs >4 (up to 6) wk delay | RPA stage; RT delay >4 wk | Not known | Advantage (OS) for delaying RT up to 6 wk: 12.5 mo vs 9.2 mo (P < 0.0001) |
| Noel 2012 | French multicenter (18) 2006 | 282 | Median 41 d | Age, type of surgery | Not known | None |
| Spratt 2014 | Memorial Sloan- Kettering Cancer Center 2000–2012 | 345 | ≤2, 2–5, and ≥6 wk | Known in 45.8% | Detrimental to OS if delay >6 wk | |
| Hans 2015 | University of San Francisco: 4 clinical trials 2004–2010 | 198 | <30 d vs 30–34 d post-op | KPS, extent of resection | Not available | Advantage for PFS and OS if delayed 30–34 d (not >34 d) |
| Adeberg 2015 | Heidelberg, retrospective | 50 | While awaiting MGMT results (~1 wk) | MGMT | Known in all study cases, and 25% of the reference group | Decreased survival (PFS and OS) if treatment begun earlier than 24 d |
| Sun 2015 | The Cancer Genome Atlas | 218 | Median delay 27 d; examined per quartiles (<20; 21–27 d; 28–35; >36 d) | Age | Not known | None* (up to 6 wk; worse OS >6 wk) |
| Louvel 2016 | French multicenter | 692 | Median 1.5 mo | Female sex, total/subtotal resection; RPA stage | Not examined | None |
Only one study of 345 GBM patients treated at Memorial Sloan-Kettering Cancer Center showed discrepant findings: a multivariate analysis including MGMT status showed that delaying the onset of chemoradiation was associated with decreased OS, particularly when therapy was delayed beyond 6 weeks.18
While the bulk of the data suggest that delaying adjuvant therapy more than 6 weeks from the time of surgery may be detrimental,15 there appears to be no benefit or detriment to delaying standard chemoradiation therapy within the 6-week timeframe that is typically observed in most clinical trials.
Our present study is valuable as it analyzes patients eligible for treatment on large, randomized clinical trials in settings comparable to those in which patients received first-line radiation as monotherapy, reported in our first publication. The number of patients studied, 1395, comprises a much larger database than for any of the recent studies, which is relevant for the statistical conclusions that can be made from the analyses. Additionally, more than 80% of the patients in our analysis had MGMT status determined, which lends credence to the survival results. Tumors with methylation of the promoter region of MGMT (methylated MGMT) are known to have the greatest magnitude of benefit from the combined chemoradiation treatment, with an impressive 2-year survival rate of 50% for this molecularly sensitive group.19
A lower (more favorable) RPA class was found by multivariate analysis to be a predictive factor for improved survival in this current study, as was the case in our prior paper (Tables 3 and 4).
Pretreatment factors, including the volume of tumor treated, the exact dose delivered, and posttreatment sequelae/toxicities, were not analyzed and as such may limit the study results and implications; however, strengths of this analysis include central review of treatment fields and protocol dose conformity quality assurance, which were performed on the initial clinical study cohorts as required by RTOG/NRG standards.
Both of the randomized studies inherently restricted the window of treatment initiation: in RTOG 0525, treatment needed to be initiated by <5 weeks from the time of surgery, and in RTOG 0825, within the narrow window of 3–5 weeks postoperatively. Due to the limited number of outlying patients who were treated at <3 weeks or >5 weeks from the time of surgery, we changed our prospectively designed analysis post hoc, from 3 time intervals (planned to correspond more directly to our previously published work): ≤3 weeks, >3–4 weeks, and >4 weeks to 2 groups: >4 and ≤4 weeks, based on the median timepoint between surgery and initiation of chemoradiation of 4 weeks. As such, the implications for generalization to patients in the community starting earlier or later than this time period may be limited.
Conclusion
Delaying the initiation of concurrent TMZ and radiation for the treatment of newly diagnosed GBM is not detrimental within the accepted guidelines of the 5–6 weeks postoperative period.
Supplementary Material
Supplementary material is available at Neuro-Oncology online
Funding
This project was supported by grants U10CA180868 (NRG Oncology Operations) and U10CA180822 (NRG Oncology SDMC) from the National Cancer Institute (NCI) and Genentech. This project is also funded, in part, under a grant from the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Acknowledgment
We would like to acknowledge Sesalie L Smathers, MD of the Southeast Cancer Control Consortium, Inc, CCOP, Asheville, North Carolina, for her contribution to this study. This paper was presented in abstract form at SNO 2015, #0237
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1. Walker MD, Alexander E Jr, Hunt WE et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333–343. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Blumenthal DT, Won M, Mehta MP et al. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. 2009;27(5):733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gilbert MR, Wang M, Aldape KD et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park HS, Gross CP, Makarov DV, Yu JB. Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(5):1365–1373. [DOI] [PubMed] [Google Scholar]
- 7. Peker S, Abacioglu U, Sun I, Yuksel M, Pamir MN. Irradiation after surgically induced brain injury in the rat: timing in relation to severity of radiation damage. J Neurooncol. 2004;70(1):17–21. [DOI] [PubMed] [Google Scholar]
- 8. van Rijn J, Heimans JJ, van den Berg J, van der Valk P, Slotman BJ. Survival of human glioma cells treated with various combination of temozolomide and X-rays. Int J Radiat Oncol Biol Phys. 2000;47(3):779–784. [DOI] [PubMed] [Google Scholar]
- 9. van Nifterik KA, van den Berg J, Stalpers LJ et al. Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys. 2007;69(4):1246–1253. [DOI] [PubMed] [Google Scholar]
- 10. Wedge SR, Porteous JK, Glaser MG, Marcus K, Newlands ES. In vitro evaluation of temozolomide combined with X-irradiation. Anticancer Drugs. 1997;8(1):92–97. [DOI] [PubMed] [Google Scholar]
- 11. Kil WJ, Cerna D, Burgan WE et al. In vitro and in vivo radiosensitization induced by the DNA methylating agent temozolomide. Clin Cancer Res. 2008;14(3):931–938. [DOI] [PubMed] [Google Scholar]
- 12. Lawrence YR, Blumenthal DT, Matceyevsky D, Kanner AA, Bokstein F, Corn BW. Delayed initiation of radiotherapy for glioblastoma: how important is it to push to the front (or the back) of the line?J Neurooncol. 2011;105(1):1–7. [DOI] [PubMed] [Google Scholar]
- 13. Noel G, Huchet A, Feuvret L et al. Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: a French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J Neurooncol. 2012;109(1):167–175. [DOI] [PubMed] [Google Scholar]
- 14. Louvel G, Metellus P, Noel G et al. ; Club de Neuro-Oncologie of Société Française de Neurochirurgie Delaying standard combined chemoradiotherapy after surgical resection does not impact survival in newly diagnosed glioblastoma patients. Radiother Oncol. 2016;118(1):9–15. [DOI] [PubMed] [Google Scholar]
- 15. Sun MZ, Oh T, Ivan ME et al. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg. 2015;122(5):1144–1150. [DOI] [PubMed] [Google Scholar]
- 16. Adeberg S, Bostel T, Harrabi S et al. Impact of delays in initiating postoperative chemoradiation while determining the MGMT promoter-methylation statuses of patients with primary glioblastoma. BMC Cancer. 2015;15:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han SJ, Rutledge WC, Molinaro AM et al. The effect of timing of concurrent chemoradiation in patients with newly diagnosed glioblastoma. Neurosurgery. 2015;77(2):248–253; discussion 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spratt DE, Folkert M, Zumsteg ZS et al. Temporal relationship of post-operative radiotherapy with temozolomide and oncologic outcome for glioblastoma. J Neurooncol. 2014;116(2):357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.