Abstract
Background
Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting and disabling side effect of taxane anticancer agents. We prospectively evaluated the efficacy of cryotherapy for CIPN prevention.
Methods
Breast cancer patients treated weekly with paclitaxel (80 mg/m2 for one hour) wore frozen gloves and socks on the dominant side for 90 minutes, including the entire duration of drug infusion. Symptoms on the treated sides were compared with those on the untreated (nondominant) sides. The primary end point was CIPN incidence assessed by changes in tactile sensitivity from pretreatment baseline in a monofilament test at a cumulative dose of 960 mg/m2. We also assessed thermosensory deficits, subjective symptoms (Patient Neuropathy Questionnaire [PNQ]), manipulative dexterity, and the time to events and hazard ratio by PNQ. All statistical tests were two-sided.
Results
Among the 40 patients, four did not reach the cumulative dose (due to the occurrence of pneumonia, severe fatigue, severe liver dysfunction, and macular edema), leaving 36 patients for analysis. None dropped out due to cold intolerance. The incidence of objective and subjective CIPN signs was clinically and statistically significantly lower on the intervention side than on the control (hand: tactile sensitivity = 27.8% vs 80.6%, odds ratio [OR] = 20.00, 95% confidence interval [CI] = 3.20 to 828.96, P < .001; foot: tacile sensitivity = 25.0% vs 63.9%, OR = infinite, 95% CI = 3.32 to infinite, P < .001; hand: warm sense = 8.8% vs 32.4%, OR = 9.00, 95% CI = 1.25 to 394.48, P = .02; foot: warm sense: 33.4% vs 57.6%, OR = 5.00, 95% CI = 1.07 to 46.93, P = .04; hand: PNQ = 2.8% vs 41.7%, OR = infinite, 95% CI = 3.32 to infinite, P < .001; foot: PNQ = 2.8% vs 36.1%, OR = infinite, 95% CI = 2.78 to infinite, P < .001; hand: hazard ratio [HR] = 0.13, 95% CI = 0.05 to 0.34; foot: HR = 0.13, 95% CI = 0.04 to 0.38, dexterity mean delay = −2.5 seconds, SD = 12.0 seconds, vs + 8.6 seconds, SD = 25.8 seconds, P = .005).
Conclusions
Cryotherapy is useful for preventing both the objective and subjective symptoms of CIPN and resultant dysfunction.
Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent and disabling side effect of cancer treatment, primarily taxanes and platinum agents (1). CIPN reduces health-related quality of life (2) and often results in dose delay, dose reduction, or treatment discontinuation (3). A patient-reported outcome study found that CIPN numbness persisted in 67%–80% of patients for one year following the completion of paclitaxel therapy (4). Duloxetine was recommended for CIPN; however, it has limited efficacy for the amelioration of chemotherapy-induced pain, and none for numbness or functional disability (4,5). Furthermore, no established strategy exists for CIPN prevention (4).
Therapeutic regional hypothermia (cryotherapy) can reduce chemotherapy-induced complications by decreasing regional perfusion with acceptable tolerability (6). Frozen gloves and socks prevented docetaxel-induced nail and skin toxicity in prospective, self-controlled trials that compared the protected side with the nonprotected side (7,8). A retrospective study indicated that the occurrences of docetaxel-induced peripheral neuropathy was lower in the patients who used frozen gloves and socks compared to the patients who did not wear them (35% vs. 57%) (9).
Because CIPN symptoms are largely subjective and many clinicians underestimate their severity using the Common Terminology Criteria for Adverse Events v. 4.0 (CTCAE) (10,11), prospective trials with patient-reported outcomes may be superior for evaluating preventative efficacy; however, additional end points, including objective and functional assessments, are also needed to control for the placebo response bias of patient-reported outcomes. A self-controlled design can mitigate the effects of other confounders, including individual differences in sensory detection. Therefore, we investigated the effectiveness of cryotherapy against paclitaxel-induced peripheral neuropathy in a prospective self-controlled trial with multiple end points (e.g., objective, subjective, and functional assessments).
Methods
Study Design
This self-controlled clinical trial evaluated the preventive effects of cryotherapy for CIPN. As in previous cryotherapy studies (7,8), each patient wore frozen flexible gloves and socks (Elasto-Gel, 84400 APT Cedex, Akromed, France) on the dominant hand and foot from 15 minutes before paclitaxel administration to 15 minutes after the infusion was complete (90 minutes in total). Frozen gloves were replaced after the first 45 minutes. The nondominant side acted as the untreated control. Symptoms of CIPN were assessed before chemotherapy (baseline) and before every cycle of paclitaxel administration during outpatient care. We analyzed the time to events (the cumulative doses to subjective CIPN events [PNQ ≥ D]) and CIPN symptoms at the cumulative dose of 960 mg/m2, which is the recommended dose for neo-adjuvant and adjuvant weekly paclitaxel therapy (12).
To explore the risk factors for CIPN, we assessed the pharmacokinetics during the first administration of paclitaxel. Breast cancer patients were recruited from the Kyoto University Hospital (Kyoto, Japan) between May 2014 and August 2015 according to the following inclusion criteria: planned administration of weekly paclitaxel (80 mg/m2 for one hour) for at least 12 cycles (cumulative dose of 960 mg/m2), an Eastern Cooperative Oncology Group Performance Status of 0 or 1, and a provision of signed informed consent. The exclusion criteria were as follows: peripheral sensory/motor neuropathy (CTCAE grade ≥ 2); neuralgia or edema (CTCAE grade ≥ 2); tumor metastasis in bone, soft tissue, or skin of the hands or feet; the absence of one or more fingers or toes; Raynaud’s symptoms; peripheral arterial ischemia; hand-foot syndrome; and any other reasons based on the primary physician’s judgment.
This trial was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine (G638) in accordance with Helsinki guidelines and was registered with the University Hospital Medical Information Network in February 2014 (UMIN000013398).
Outcome Measures
Primary End Point Assessment: Tactile Disturbance
The primary end point was the incidence of CIPN (any grade), defined as a decline in tactile sensation from the pretreatment baseline as assessed by the Semmes-Weinstein monofilament test (NIHON MEDIX, Chiba, Japan), which is a validated measure of peripheral neuropathy (13). Patients were blinded and stimulated by 20 grades of nylon filaments. We set the Semmes-Weinstein monofilament test as the primary end point for two reasons: 1) it is a robust and patient-blinded assessment, which will decrease the effects of patient expectancy in this nonblinded intervention trial; and 2) patients suffer who undergo paclitaxel therapy from tactile problems more than other types of sensory or motor problems. (14). Patients experiencing at least a diminished sensation in response to light touch after a cumulative dose of 960 mg/m2 were counted as events in the primary end point assessment.
Thermosensory Disturbance (Objective Symptoms)
Thermosensory disturbance was assessed using a thermal stimulator (Yufu Itonaga, Tokyo, Japan) with 3 °C and 48 °C outputs. We stimulated the patients’ (with their eyes closed) hands or feet with hot or cold stimulation and assessed the sensation following the thermal stimulation (normal, delayed, or diminished compared with baseline). The delayed and diminished sensations at 12 cycles (cumulative dose, 960 mg/m2) compared with the pretreatment baseline were classified as events.
Vibration Perception (Objective Symptoms)
Vibration perception at the wrist and ankle was assessed by a C 128-Hz tuning fork (NITI-ON CO, LTD, Chiba, Japan). Patients no longer feeling vibrations within 10 seconds of application after 12 paclitaxel cycles were considered events. Patients who exhibited abnormal scores at baseline were excluded from the analysis.
Performance Speed (Objective Symptoms)
Manipulative dexterity was assessed using the grooved pegboard test (Lafayette Instrument Company, Lafayette IN), a validated sensory motor speed test (15). The pegboard has 25 holes, with randomly positioned slots for pegs and keys along one side. Each peg must be properly rotated to match the hole before it can be inserted. We measured the time (seconds) required for the insertion of 25 pegs by each hand (dominant vs nondominant) and calculated the mean difference from the baseline score.
Patient-Reported Assessment (Subjective Symptoms)
Subjective symptoms were assessed using the Japanese version of the PNQ, a validated patient-reported questionnaire on neuropathy and activities of daily living (ADL) that correlates with quality of life (11). The patient subjectively responded to each item, grading each as A (no neuropathy), B (mild neuropathy), C (moderate neuropathy that does not interfere with ADL), D (moderate neuropathy that interferes with ADL), or E (severe neuropathy that interferes with ADL) (11). We assessed the grades of CIPN, and patients having grades D or E, as severe as CTCAE grades 2 and 3 (11), were counted as severe CIPN events.
Cryotherapy Tolerability
Adherence to cryotherapy, pain, abnormality of sensation, and other discomforts due to cryotherapy were checked during every intervention.
Electrophysiological Signs
We measured the conduction velocity and action potential amplitude of the median nerve using Neuropack X1 (Nihon Kohden Corporation, Tokyo, Japan). The current perception thresholds on the hands and feet were also assessed using a Neurometer CPT (Neurotron, Towson, MD). Patients exhibiting abnormal values (16,17) were counted as events.
Pharmacokinetics
The pharmacokinetics of paclitaxel was assessed by plotting the area under the plasma concentration-time curve (AUC) for 24 hours, commencing immediately before administration (AUC0-24). Blood samples were obtained prior to infusion and immediately before and one, three, five, and 23 hours after the end of the infusion. The plasma drug concentration was measured by high-performance liquid chromatography with tandem mass spectrometry (18). Pharmacokinetic parameters were calculated according to the two-compartment model using the nonlinear least squares method in WinNonlin 6.4 (Pharsight, Inc., Mountain View, CA). Furthermore, we calculated the dose intensity (mg/m2/wk) of paclitaxel as the cumulative dose (mg/m2) divided by the administration period.
Statistical Analysis
The sample size was determined by referring to the sizes recorded in similar previous cryotherapy studies (8). CIPN symptoms are presented as the incidence rate, odds ratio (OR), and 95% confidence interval (CI) of the hands and feet. The time to subjective CIPN was analyzed using the Kaplan-Meier method and Cox regression analysis. Manipulative dexterity was presented as the mean time difference (SD). McNemar’s test (tactile, thermal, vibration perception, and subjective CIPN), log-rank test (time to CIPN events), and two-sided paired t test between intervention and control sides (manipulative dexterity) were used for statistical comparison. A P value of less than .05 was considered statistically significant. All analyses were performed using SAS v. 9.3 (SAS Institute Inc., Cary, USA) and R (R Foundation for Statistical Computing, Vienna, Austria), supervised by a statistician. Data quality was ensured by an independent data center (Medical Research Support Co., Ltd., Osaka, Japan). All statistical tests were two-sided.
Results
Patient Recruitment and Characteristics
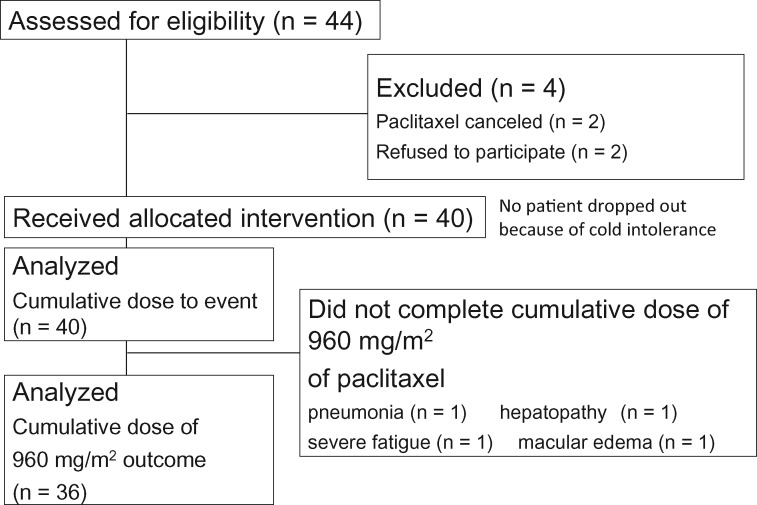
Among the 44 patients registered, four did not undergo any intervention. An additional four did not reach a cumulative paclitaxel dose of 960 mg/m2, leaving 36 patients for the analysis (Figure 1; Table 1). A total of 25 patients completed paclitaxel therapy at a cumulative dose of 960 mg/m2, and 11 underwent chemotherapy at a cumulative dose higher than 960 mg/m2 (maximum = 4080 mg/m2).
Figure 1.
Study flow diagram. We included 40 patients who received the allocated intervention and analyzed the cumulative dose for subjective chemotherapy-induced peripheral neuropathy (CIPN) events. All CIPN signs were analyzed in the 36 patients who reached a cumulative dose of 960 mg/m2. We compared the hands and feet of the intervention side to those of the control side.
Table 1.
Patient characteristics (n = 40)
| Characteristic | |
|---|---|
| Mean age (SD), y | 56.0 (13.8) |
| Mean weight (SD), kg | 55.6 (7.5) |
| Mean body mass index (SD), kg/m2 | 22.4 (4.3) |
| Mean area under the curve (SD), µg·h/mL | 7.5 (1.4) |
| Smoker, No. (%) | 3 (7.5) |
| Diabetes, No. (%) | 3 (7.5) |
| Left-handed, No. (%) | 3 (7.5) |
| Breast cancer, No. (%) | |
| Left | 19 (47.5) |
| Right | 18 (45.0) |
| Left and right | 3 (7.5) |
| Treatment, No. (%) | |
| Neo-adjuvant | 22 (27.5) |
| Adjuvant | 11 (55.0) |
| Palliative | 7 (17.5) |
Cryotherapy Tolerability
No patients dropped out due to cold intolerance. The most frequently reported adverse events (events / (person*cycle)) were pain (8.2%), sensory abnormalities (0.4%), and feeling cold (4.2%). The adverse events diminished immediately during or after cryotherapy intervention.
Primary End Point
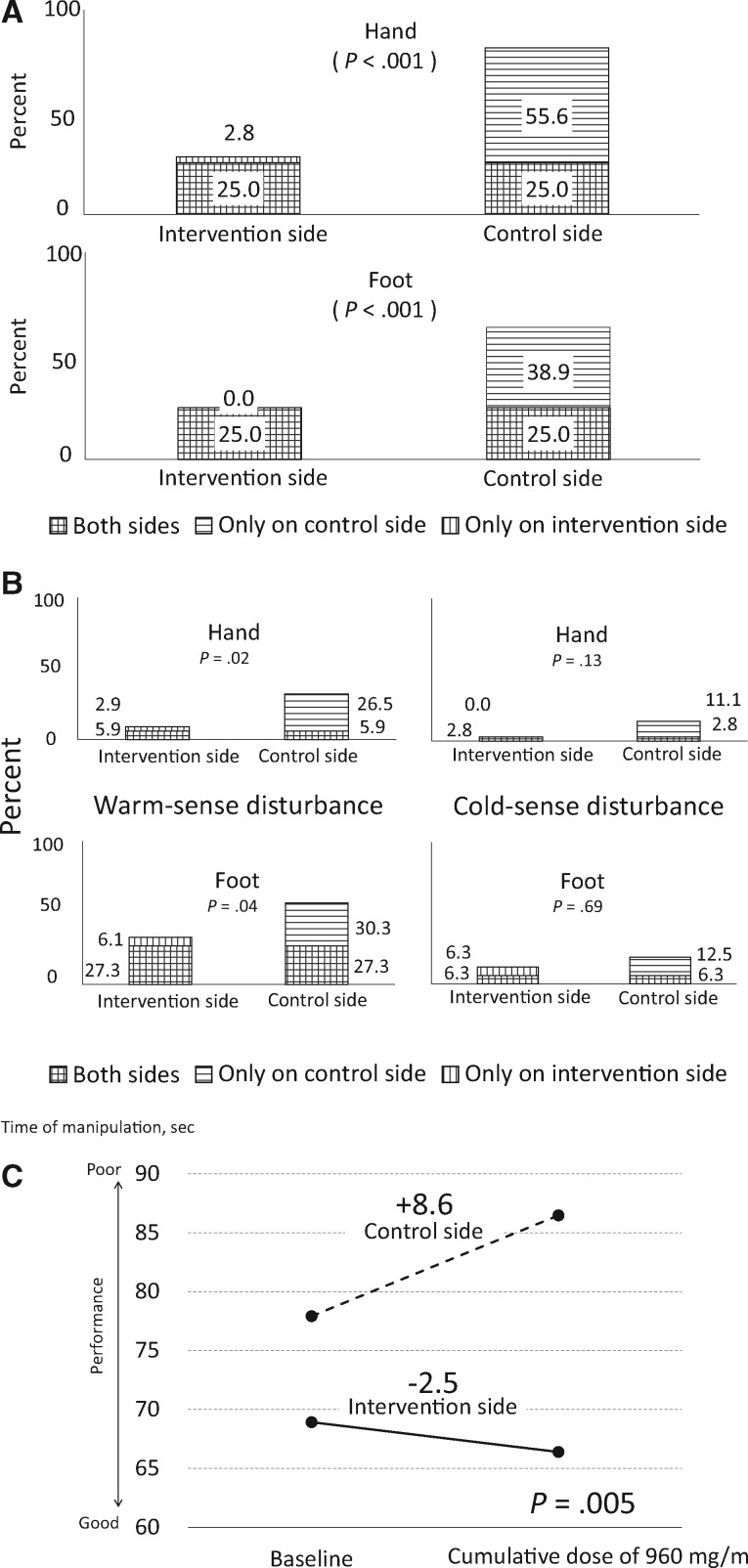
The proportion of hands and feet exhibiting tactile deterioration were clinically and statistically significantly lower for the intervention side than the control side (hand: 27.8% vs 80.6%, OR = 20.00, 95% CI = 3.20 to 828.96, P < .001; foot: 25.0% vs 63.9%, OR = infinite, 95% CI = 3.32 to infinite, P < .001). The proportions include the patients who experienced CIPN from both the intervention and control sides of the hand and foot (Figure 2A).
Figure 2.
Objective chemotherapy-induced peripheral neuropathy (CIPN) events at a cumulative dose of 960 mg/m2. A) The efficacy of cryotherapy for reducing the primary end point, incidence of CIPN, was assessed by tactile-sensory deficits on the monofilament test. Any tactile deterioration from the pretreatment baseline in an intervention or control side hand or foot at a cumulative dose of 960 mg/m2 was considered a CIPN event. The differential incidence between the intervention and control sides was evaluated using a two-sided McNemar’s test (n = 36). B) Treatment with cryotherapy reduced thermosensory dysfunction. Only patients who exhibited a normal sensory threshold at baseline were included (hand: warm, n = 34, cold, n = 36; foot: warm, n = 33, cold, n = 32). Any response delay, response reduction, or thermal analgesia at a cumulative dose of 960 mg/ m2 was considered a CIPN. C) This figure shows manipulative dexterity deficits. P values were determined by a two-sided paired t test between the intervention and control side. The solid line denotes the intervention side, and the dotted line indicates the control side (N = 36).
Secondary End Points
Objective End Points
Figure 2B presents the incidence of thermosensory deficits. Patients who exhibited an abnormal thermal sense at baseline (hands warmth, n = 2; feet warmth, n = 3; and feet cold, n = 4) were excluded from the analysis. The incidence of a reduced perception of warmth was clinically and statistically significantly lower on the intervention side (hand: 8.8% vs 32.4%, OR = 9.00, 95% CI = 1.25 to 394.48, P = .02; foot: 33.4% vs 57.6%, OR = 5.00, 95% CI = 1.07 to 46.93, P = .04). The proportions include the patients who experienced CIPN from both the intervention and control sides of the hands and feet). In contrast, cold-sense deficits also tended to be numerically lower on the intervention side but showed no statistical significance (hand: 2.8% vs 13.9%, OR = inifinite, 95% CI = 0.66 to infinite, P = .13; foot: 12.6% vs 18.8%, OR = 2.00, 95% CI = 0.29 to 22.11, P = .69). The proportions include the patients who experienced CIPN from both the intervention and control sides of the hands and feet). For the incidence of vibration perception deficits, patients exhibiting an abnormal sense at the pretreatment baseline (hand, n = 5; foot, n = 7) were excluded from the analysis; however, the incidences tended to be numerically lower on the intervention side but showed no statistically significant differences between the intervention and control sides (hand: 9.7% vs 12.9%, OR = inifinite, 95% CI = 0.03 to infinite, P = 1.00; foot: 13.8% vs 24.1%, OR = inifinite, 95% CI = 0.41 to infinite, P = .25). The proportions include the patients who experienced CIPN from both the intervention and control sides of the hands and feet). The performance speed compared with the baseline level exhibited a greater delay on the control side (−2.5-second delay, SD = 12.0 seconds, on the intervention side vs +8.6-second delay, SD = 25.8 seconds, on the control side, P = .005) (Figure 2C). Some patients showed abnormal scores, and there were no statistically significant differences in nerve degeneration in the electrophysiological signs. Supplementary Figure 1 (available online) shows the incidence overlaps of CIPN in the hands and feet.
Subjective End Point
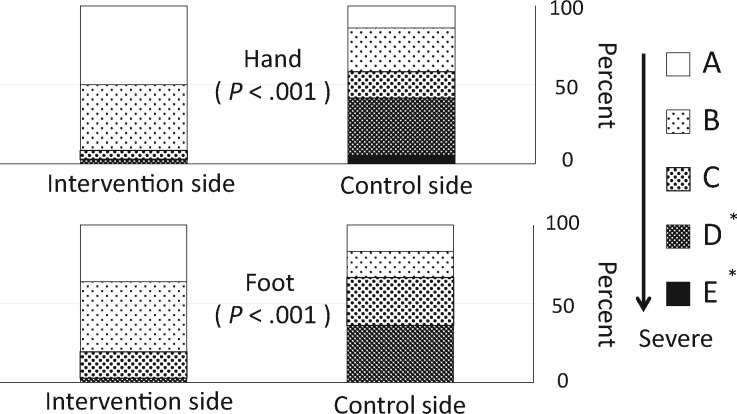
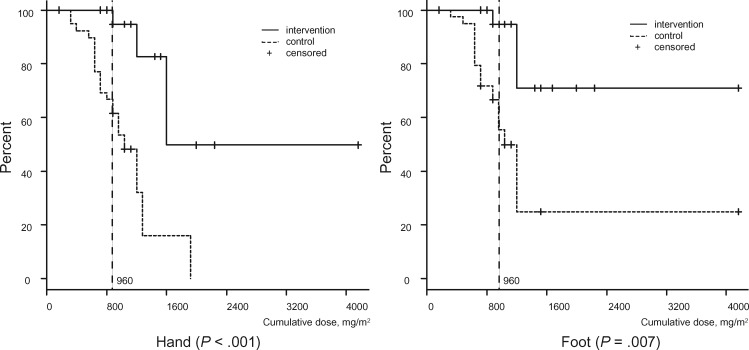
For sensory dysfunction, Figure 3 shows the subjective severity grades of CIPN at a cumulative dose of 960 mg/m2 (PNQ grades A–E). The occurrences of CIPN (PNQ grades C–E) were prevented by cryotherapy (severe CIPN with grades D or E; hand: 2.8% vs 41.7%, OR = infinite, 95% CI = 3.32 to infinite, P < .001; foot: 2.8% vs 36.1%, OR = infinite, 95% CI = 2.78 to infinite, P < .001). A log-rank analysis of Kaplan-Meier curves (Figure 4) revealed that CIPN also occurred faster on the control side than on the intervention side (hand: HR = 0.13, 95% CI = 0.05 to 0.34, P < .001; foot: HR = 0.13, 95% CI = 0.04 to 0.38, P = .007). Only two patients reported motor dysfunction, which lasted less than one week.
Figure 3.
Severity of subjective symptoms (at a cumulative dose of 960 mg/m2). The administration of cryotherapy also reduced the subjective symptoms based on the Patient Neuropathy Questionnaire responses (the secondary end point, subjective) at a cumulative dose of 960 mg/m2 (P values determined by the McNemar’s test, n = 36). The subjective responses to each item were graded from A (no neuropathy) to E (severe neuropathy) by the patient. A rank of D or E indicates impaired activities of daily living. *Activities of daily living were interfered with.
Figure 4.
The appearance of severe subjective neuropathy symptoms with cumulative dose. Severe subjective neuropathy symptoms (Patient Neuropathy Questionnaire ≥ D; moderate to severe tingling, pain, or numbness that interferes with activities of daily living) with cumulative dose were compared between the intervention and control sides using a log-rank test (n = 40). The solid line denotes the intervention side, and the dotted line indicates the control side. The dotted vertical line represents a cumulative dose of 960 mg/m2. We included four censored patients who did not complete cumulative dose of 960 mg/m2 paclitaxel due to pneumonia (n = 1), severe fatigue (n = 1), severe liver dysfunction (n = 1), and macular edema (n = 1). A two-sided log-rank test was used to calculate the P values.
Risk Factors of CIPN
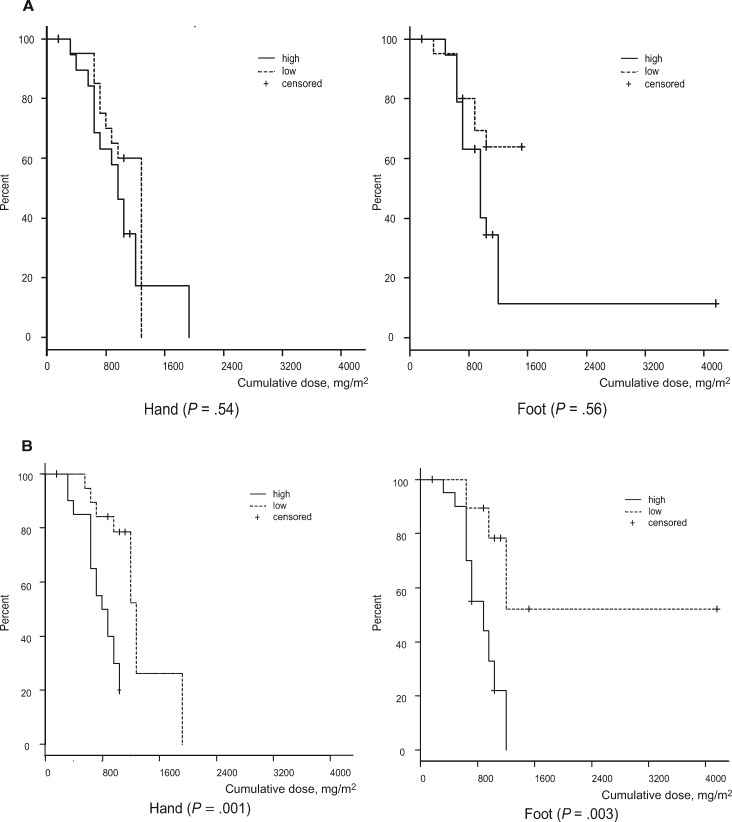
We analyzed the effect of clinical factors on the time to subjective CIPN events (PNQ ≥ D) on the control side. No statistically significant differences were found for the time to events between the low AUC0 − 24 group, with the low AUC means below the median value (group mean = 6.6 µg·h/mL, SD = 0.5 µg·h/mL; total cohort median = 7.2 µg·h/mL) and the high AUC0 − 24 group (mean = 8.3 µg·h/mL, SD = 1.4 µg·h/mL) (hand: P = .54, foot: P = .56) (Figure 5A). The dose intensity varied because of the results of temporary delays due to chemotherapy-induced neutropenia. Symptoms occurred statistically earlier in the high–dose intensity group (mean = 75.0 mg/m2/wk, SD = 4.8 mg/m2/wk) above the median (68.6 mg/m2/wk) than in the low–dose intensity group (mean = 56.6 mg/m2/wk, SD = 7.0 mg/m2/wk; hand: P = .001, foot: P = .003) (Figure 5B). We also examined the effects of other factors; however, none were statistically significant risk factors for CIPN (Supplementary Table 1, availale online).
Figure 5.
Cumulative dose to subjective symptoms (Patient Neuropathy Questionnaire rank ≥ D; moderate to severe tingling, pain, or numbness that interferes with activities of daily living [ADL]) on the control side to identify chemotherapy-induced peripheral neuropathy (CIPN) risk factors (n = 40). A) This figure presents the high/low area under the curve (AUC; µg·h/mL). The low-AUC group (mean = 6.6 µg·h/mL, SD = 0.5µg·h/mL) and high-AUC group (mean = 8.3 µg·h/mL, SD = 1.4 µg·h/mL) were divided by the median AUC (7.2 µg·h/mL). The solid line denotes the high-AUC group, and the dotted line indicates the low-AUC group. B) This figure presents high/low dose intensity (mg/m2/wk). The low–dose intensity group (mean = 56.6 mg/m2/wk, SD = 6.7 mg/m2/wk) and high–dose intensity group (mean = 75.0 mg/m2/wk, SD = 4.8 mg/m2/wk) were divided by the median dose intensity (68.6 mg/ m2/wk). The solid line represents the high–dose intensity group, and the dotted line denotes the low–dose intensity group. A two-sided log-rank test was used to calculate the P values.
Electrophysiological Signs
For the incidence of electrophysiological signs, patients exhibiting a normal sense at the pretreatment baseline were included in the analysis (median nerve conduction velocity, n = 18; median nerve action potential amplitude, n = 14; current perception thresholds, hands, n = 11, feet, n = 8); they showed no statistically significant differences between intervention and control sides (nerve conduction velocity: 5.5% vs 5.5%, OR = 1.00, 95% CI = 0.01 to 78.50, P = 1.00; action potential: 28.5% vs 28.5%, OR = 1.00, 95% CI = 0.01 to 78.50, P = 1.00; current perception threshold; hand: 18.1% vs 27.2%, OR = inifinite, 95% CI = 0.03 to inifinite, P = 1.00, foot: 25.0% vs 25.0%, OR = 1.00, 95% CI = 0.01 to 78.50, P = 1.00) . All P values were analyzed using McNemar’s test.
Discussion
Our findings support the efficacy of cryotherapy for CIPN prevention, as evidenced by a clinically and statistically significant reduction in patient-reported subjective symptoms, diminished objective signs (tactile and thermosensory), and prevention of manipulative dexterity. The development of subjective CIPN symptoms was clinically and statistically significantly delayed during the course of the paclitaxel treatment, the occurrence of subjective CIPN at a cumulative dose of 960 mg/m2 was almost completely prevented, and the CIPN incidence as assessed by other objective modalities tended to be lower on the intervention side. Because the self-controlled design can reduce the effects of unknown potential confounders to levels lower than expected in randomized clinical trials, data consistency among the multiple assessments and large effect size, as exampled by a small hazard ratio, support the robustness of our conclusions despite the limited sample size (19). Furthermore, no patients dropped out due to cold intolerance in response to cryotherapy.
Our study had several limitations. First, placebo effects are inevitable. To minimize differences in expectancy between the intervention and control sides, we supported a subjective symptom evaluation with objective measures. One potential confounder is that the control side may exhibit higher skin temperatures concomitant with a homeostatic whole-body temperature increase due to cooling on the intervention side; however, this influence was likely minimal because the incidence of CIPN symptoms on the control sides did not deviate substantially from that reported in previous studies (1,2). A comparison between patients with and without intervention would control for this physiological response. Second, the nondominant hand and foot always acted as the control, as in previous studies of cryotherapy (7,8). To the best of our knowledge, there have been no reports on bilateral differences in CIPN symptoms (either subjective or objective). Impairments in ADL are likely less severe when CIPN occurs in the nondominant hand due to easier compensation using the dominant hand. Third, we did not plan to follow the patients after the completion of paclitaxel treatment because postpaclitaxel therapy could impact the sensory status. In this study, patients underwent surgery (n = 10), radiotherapy (n = 8), hormonal therapy, and/or additional chemotherapy (n = 18) following paclitaxel therapy. The 30 patients who returned to our clinic within a median of 6.1 weeks (2 to 126 weeks) stated that there was no worsening of CIPN symptoms after the cessation of paclitaxel treatment. While previous studies have suggested that the development of additional CIPN signs or coasting is relatively rare after cessation of chemotherapy (20,21), long-term follow-up would reveal the effects of cryotherapy on the natural course of CIPN signs and symptoms.
Compression therapy using surgical gloves modestly prevents CTCAE grade 2 or higher sensory and motor peripheral neuropathy with four cycles of triweekly nanoparticle albumin-bound paclitaxel (22). Compression therapy and cryotherapy share an analogous mechanism of reduced drug exposure due to vasoconstriction during paclitaxel infusion. The low temperature associated with cryotherapy may also decrease the uptake of paclitaxel and damage of neurons or mechanotransductions, which might be related to decreased CIPN (20,23).
Total drug exposure may also enhance the risk of CIPN. In a previous study, the CIPN incidence increased with AUC and time above the paclitaxel concentration threshold (24). Although we found clinically and statistically significant differences in the cumulative dose to events between the high- and low-dose intensity groups, no differences were found between high- and low-AUC groups with a uniform dosage and relatively small variability in pharmacokinetics. Any other risk factor analyses have low power, and we could not identify any correlation between CIPN occurrence and the baseline assessments.
We conclude that cyrotherapy is a simple, safe, and effective strategy for the prevention of CIPN in patients with cancer undergoing paclitaxel treatment. Cyrotherapy could support the delivery of optimal chemotherapy by preventing a dose delay or reduction, as well as inhibiting the deterioration of quality of life in cancer patients during and after treatment.
Funding
This work was supported by the Japan Society for the Promotion of Science (grant No. DC1-6751 to AH) and the Promotion Plan for the Platform of Human Resource Development for Cancer administered by the Ministry of Education, Culture, Sports, Science and Technology in Japan (grant No. 12 to TT).
Notes
We gratefully acknowledge support for the data collection provided by H. Ishikawa, A. Mizushima, A. Yamaguchi, K. Maekawa, Y. Fukui, M. Nio, T. Nakakimura, Y. Nakayama, T. Kotake, S. Yanai, T. Hitomi, Y, Nakayama, M.Torii, K. Tsuji, M. Miyayama, and the medical staff (physicians, nurses, and pharmacists) of Kyoto University Hospital.
This material is based on work supported by the Japan Society for the Promotion of Science and the Promotion Plan for the Platform of Human Resource Development for Cancer administered by the Ministry of Education, Culture, Sports, Science and Technology in Japan. Neither funder had a role in the study design; collection, analysis, or interpretation of the data; the writing of the report; or the decision to submit the article for publication.
Parts of this manuscript have previously been presented at the American Society of Clinical Oncology (ASCO) 2016 Annual Meeting, June 3–7, 2016, Chicago, Illinois.
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Cavaletti G, Marmiroli P.. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6(12):657–666. [DOI] [PubMed] [Google Scholar]
- 2. Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L.. Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support Care Cancer. 2014;22(8):2261–2269. [DOI] [PubMed] [Google Scholar]
- 3. Speck RM, Sammel MD, Farrar JT, et al. . Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J Oncol Pract. 2013;9(5):e234–e240. [DOI] [PubMed] [Google Scholar]
- 4. Hershman DL, Lacchetti C, Dworkin RH, et al. . Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967. [DOI] [PubMed] [Google Scholar]
- 5. Smith EML, Pang H, Cirrincione C, et al. . Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA. 2013;309(13):1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kadakia KC, Rozell SA, Butala AA, Loprinzi CL.. Supportive cryotherapy: A review from head to toe. J Pain Symptom Manage. 2014;47(6):1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scotté F, Banu E, Medioni J, et al. . Matched case-control phase 2 study to evaluate the use of a frozen sock to prevent docetaxel-induced onycholysis and cutaneous toxicity of the foot. Cancer. 2008;112(7):1625–1631. [DOI] [PubMed] [Google Scholar]
- 8. Scotté F, Tourani J-M, Banu E, et al. . Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23(19):4424–4429. [DOI] [PubMed] [Google Scholar]
- 9. Eckhoff L, Knoop AS, Jensen M-B, Ejlertsen B, Ewertz M.. Risk of docetaxel-induced peripheral neuropathy among 1,725 Danish patients with early stage breast cancer. Breast Cancer Res Treat. 2013;142(1):109–118. [DOI] [PubMed] [Google Scholar]
- 10. Alberti P, Rossi E, Cornblath DR, et al. . Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: Two sides of the same coin. Ann Oncol. 2014;25(1):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimozuma K, Ohashi Y, Takeuchi A, et al. . Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer. 2009;17(12):1483–1491. [DOI] [PubMed] [Google Scholar]
- 12. Sparano JA, Wang M, Martino S, et al. . Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jayaprakash P, Bhansali A, Bhansali S, et al. . Validation of bedside methods in evaluation of diabetic peripheral neuropathy. Indian J Med Res. 2011;133(6):645–649. [PMC free article] [PubMed] [Google Scholar]
- 14. Dougherty PM, Cata JP, Cordella J V, Burton A, Weng H-R.. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109(1–2):132–142. [DOI] [PubMed] [Google Scholar]
- 15. Bryden PJ, Roy EA.. A new method of administering the Grooved Pegboard Test: Performance as a function of handedness and sex. Brain Cogn. 2005;58(3):258–268. [DOI] [PubMed] [Google Scholar]
- 16. Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. 4th ed.Oxford: Oxford University Press; 2013. [Google Scholar]
- 17. Neuval Database II–Normative Data. Neurometer CPT Sensory Nerve Conduction Threshold Current Perception Threshold Test (sNCT/CPT). 2004. http://www.neurotron.com. Accessed September 10, 2016.
- 18. Onoue H, Yano I, Tanaka A, et al. . Significant effect of age on docetaxel pharmacokinetics in Japanese female breast cancer patients by using the population modeling approach. Eur J Clin Pharmacol. 2016;72(6):703–710. [DOI] [PubMed] [Google Scholar]
- 19. Huizenga C, Zhang H, Arens E, Wang D.. Skin and core temperature response to partial- and whole-body heating and cooling. J Therm Biol. 2004;29(7):549–558. [Google Scholar]
- 20. Zhang J, Tuckett RP.. Comparison of paclitaxel and cisplatin effects on the slowly adapting type I mechanoreceptor. Brain Res. 2008;1214:50–57. [DOI] [PubMed] [Google Scholar]
- 21. Park SB, Goldstein D, Krishnan A V, et al. . Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J Clin. 2013;63(6):419–437. [DOI] [PubMed] [Google Scholar]
- 22. Tsuyuki S, Senda N, Kanng Y, et al. . Evaluation of the effect of compression therapy using surgical gloves on nanoparticle albumin-bound paclitaxel-induced peripheral neuropathy: A phase II multicenter study by the Kamigata Breast Cancer Study Group. Breast Cancer Res Treat. 2016;160(1):61–67. [DOI] [PubMed] [Google Scholar]
- 23. Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C.. Terminal arbor degeneration—a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci. 2011;33(9):1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mielke S, Sparreboom A, Steinberg SM, et al. . Association of paclitaxel pharmacokinetics with the development of peripheral neuropathy in patients with advanced cancer. Clin Cancer Res. 2005;11(13):4843–4850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.