Abstract
If schizotypy is a taxonic liability for schizophrenia with a general population prevalence of ~10%, it should also be taxonic among biological siblings of probands with schizophrenia. Moreover, assuming this is so, siblings’ schizotypy class membership should be predicted by probands’ familial load for psychotic disorder and clinical severity, consistent with a multifactorial polygenic threshold model of schizophrenia. We tested these hypotheses in the Genetic Risk and Outcome of Psychosis (GROUP) Study where siblings of probands (n = 792) and unaffected controls (n = 559) provided self-report ratings on the Community Assessment of Psychic Experiences (CAPE). Maximum covariance analyses of control group ratings led to the identification of CAPE items sensitive to nonredundant positive and negative schizotypy classes in the control group (prevalence = 7.9% and 11.1%, respectively). When the same taxonic solution was applied to siblings’ CAPE rating, taxometric analyses yielded evidence for larger positive and negative schizotypy classes among siblings (prevalence = 14.1% and 21.8%, respectively). Whereas probands’ familial loads for bipolar disorder or drug use disorders did not predict siblings’ membership in the schizotypy classes, probands’ familial load for psychotic disorder did. Siblings were more likely to be members of the positive schizotypy class where their probands were more severely affected. The pattern of findings is consistent with Meehl’s argument that schizotypy reflects liability for schizophrenia.
Keywords: schizotypal personality, schizophrenia, taxometrics, familial risk
Introduction
Meehl1–3 proposed a gene × environment model of liability for schizophrenia. Key propositions in this model are that: a heritable neurointegrative endophenotype, which is embodied in the schizotaxic brain, is inceptive in the development of schizotypy; the endophenotype has a taxonic distribution in the general population, with a prevalence of ~10%; and that those with the schizotaxia endophenotype all develop schizotypy. The cognitive, psychological, and behavioral expressions of schizotypy range from the clinical signs and symptoms seen in schizophrenia to hardly-discernable differences on objective performance measures.4 Consistent with these propositions, taxometric studies of schizotypy commonly reveal underlying taxonic population structures with risk classes comprising 8.5% to 10.5% of samples.5–23 In contrast, findings from a smaller number of studies suggest the underlying structure is dimensional (ie, non-taxonic)12,17,24–27 and in accordance with dimensional models, such as that of Claridge.28
There is variability in estimated prevalence rates of schizotypy risk classes. For example, there are small differences in rates obtained from analyses of the different facets of schizotypy (eg, positive vs negative features).13,15,23 When performance or observer-rated indices are used, rates are sometimes higher19,20 but not always,21 and rates are much higher for mixed psychiatric samples.5,18 To the extent that schizotypy is the disposition for schizophrenia, its prevalence should also vary with biological risk for schizophrenia in a manner consistent with findings from family, twin, and adoption studies29 as well as studies of genome-wide associations30 and copy number variants.31 In 2 studies, each with commingled samples comprising offspring of mothers with schizophrenia or no psychiatric disorder, schizotypy prevalence rates ranged from 18% to 48% and offspring of mothers with schizophrenia were overrepresented in schizotypy classes.20,21 Although these findings suggest that the prevalence of schizotypy may be higher in first-degree relatives, there is no direct evidence from relative-only samples that schizotypy is taxonic nor estimates of its prevalence.
Therefore, our aim was to test for direct evidence that schizotypy class structure varies with biological risk for schizophrenia. We analyzed self-report schizotypy ratings from siblings of probands with schizophrenia-related disorders and a comparable general population sample, who participated in the first wave of the multisite, longitudinal Genetic Risk and Outcome of Psychosis (GROUP) study.32 Our first goal was to identify in exploratory analyses whether schizotypy in the control group had an underlying taxonic structure. If identified, we would then test for the taxonicity of schizotypy among siblings in a confirmatory fashion, using analysis parameters obtained with the control group.
We examined the underlying structure of schizotypy using multivariate (coherent-cut kinetic) taxometric procedures applied to item-level ratings. Taxometric procedures contrast dimensional and taxonic formulations of the latent population structure. That is, unlike null hypothesis testing where nonsignificant findings are not evidence of the null hypothesis, results from taxometric procedures constitute evidence of taxonicity or evidence of dimensionality.33,34 Taxometric methods are also unlike factor and latent class analyses, which require the assumptions that sampling populations are non-taxonic and nondimensional, respectively, and do not have the interpretative limitations of factor-mixture hybrids (see34 for a discussion of these limitations).
Item-level data were used for 2 reasons. First, schizotypy is multifaceted, comprising positive, negative, and disorganized components that, in turn, are composed of a variety of specific phenotypes.35 Although its multifaceted nature does not determine the latent structure, the processes that give rise to the diverse phenotypes (eg,36) could have different latent structures14 or stem from structures that are overlapping but not redundant.13,15 Consequently, a priori composite scores will not necessarily represent the variability in the item-level phenotypes—referred to as the problem of item parceling.37–39 Second, Meehl’s3,40 view was that taxometric analyses should be used iteratively to refine the selection of indicators of the schizotaxia taxon. This approach to taxometric analysis was prominent in earlier studies of schizotypy (eg,5,41) but is pre-empted by item parceling.
We hypothesized that schizotypy would be taxonic (composed of schizotypy and complement classes) in the general population and in siblings of probands with schizophrenia, and that the class prevalence in the control sample would be ~10% (or slightly lower because of control group exclusion criteria32,34). We considered point-predictions for the rate of schizotypy among siblings given the multifactorial polygenic threshold (MPT) model of schizophrenia.29,42–44 However, research design attributes (eg, assessment age, clinical exclusion criteria such as diagnosis of psychotic disorder), lack of control over environmental risk exposures, and the interplay of variable expressivity with sensitivity of measurement may each diminish the observed prevalence among siblings.45 Therefore, without a means to estimate the influences of these factors, we did not test point-predictions but expected the prevalence of schizotypy among siblings would be higher than in the control sample.
Also, we used several consistency tests, premised on the MPT model, to corroborate the idea that schizotypy class membership is attributable to genetic risk. First, if the probability of schizotypy class membership is a function of genetic load, the probands’ familial load of psychotic disorder will predict siblings’ class membership whereas the familial load of bipolar disorder and drug abuse will not.29,45 Similarly, probands’ clinical severity will predict siblings’ class membership.45 Third, those with greater genetic risk are more likely to transition to schizophrenia than those with lesser genetic risk and, consequently, be excluded from the sibling group on the grounds of psychotic disorder. Given this, the sibling schizotypy class should have fewer older members, or be younger on average, than the complement.45 Finally, we estimated the degree to which schizotypy class membership reflects multifactorial effects using the phenotypic correlation in liability between relatives (PCLR).44
Method
Participants
In representative regions in the Netherlands and Belgium, investigators identified patients with nonaffective psychotic disorders by screening the caseloads of representative clinicians. Subsequently, investigators invited those patients presenting consecutively at these services to participate. Investigators assessed control participants and biological siblings of patients, the former selected through advertising and random mailings to addresses in cases’ catchment areas.32,46
Siblings were identified through probands. Probands were 15 to 57 years old, met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for nonaffective psychotic disorders, and were recruited through more than 75% of mental health institutions in the Netherlands. Siblings were 15 to 50 years old (n = 792 from 629 families) and, in this report, were restricted to those whose index proband had DSM-IV schizophrenia (n = 629), schizophreniform disorder (50), or schizoaffective disorder (113) (table 1). Siblings of probands with delusional disorder or other psychotic disorders (brief, drug-induced, due to general medical conditions, not otherwise specified) were excluded. Control participants (n = 559 from 467 families) were aged from 15 to 50 years, had no current or history of psychotic disorder, and had no first-degree relative with a lifetime psychotic disorder (table 1). All GROUP participants were fluent in Dutch and were able and willing to give informed consent.
Table 1.
Demographic Characteristics of Control and Sibling Groups
| Variable with M ± SD or n (%) | Control | Siblings | ||
|---|---|---|---|---|
| Male (n = 255) | Female (n = 304) | Male (n = 361) | Female (n = 431) | |
| Age at baseline (years) | 29.1 ± 10.3 | 31.0 ± 10.3 | 27.5 ± 7.7 | 28.0 ± 7.8 |
| WAIS-III IQ | 110.6 ± 15.4 | 108.6 ± 15.1 | 105.6 ± 16.1 | 100.8 ± 15.2 |
| Educational achievementa | 5.11 ± 1.82 | 5.67 ± 1.71 | 5.08 ± 2.12 | 5.22 ± 2.08 |
| Living alone | 61 (23.9) | 57 (18.8) | 80 (22.2) | 76 (17.6) |
| Married/living together | 83 (32.5) | 139 (45.7) | 128 (35.5) | 192 (44.5) |
| Ethnicity | ||||
| White | 226 (89.7) | 279 (93.6) | 313 (86.7) | 350 (81.2) |
| Moroccan | 5 (2.0) | — | 5 (1.4) | 13 (3.0) |
| Turkish | — | — | 5 (1.4) | 7 (1.6) |
| Surinamese or Caribbean | 1 (0.4) | 3 (1.0) | 8 (2.2) | 5 (1.2) |
| Asian | — | — | — | 2 (0.5) |
| Other, mixed or unknown | 20 (7.9) | 16 (5.4) | 30 (8.3) | 53 (12.3) |
| CAPE frequency scores | ||||
| Positive | 3.90 ± 3.35 | 3.75 ± 3.69 | 3.76 ± 3.58 | 4.11 ± 4.41 |
| Negative | 6.76 ± 4.55 | 6.75 ± 4.43 | 7.51 ± 5.07 | 8.00 ± 6.01 |
| Depression | 3.94 ± 2.53 | 5.24 ± 2.83 | 4.26 ± 2.82 | 5.68 ± 3.46 |
Note: CAPE, Community Assessment of Psychic Experiences; WAIS-III, Wechsler Adult Intelligence Scale, 3rd ed.
aTreated as an ordinal variable where 0 = no education, 1 = primary, 2 = lower secondary, 3 = higher secondary, 4 = lower high school, 5 = higher high school, 6 = lower vocational, 7 = higher vocational, 8 = university.
Most siblings had no DSM-IV diagnosis (n = 684); some met criteria for a history of (50) or current unipolar affective disorder (39); smaller numbers met criteria for bipolar disorder (6), adjustment disorder (2), Rett’s disorder (2), anorexia (1), autism (1), cannabis dependence (1), borderline personality disorder (1), and schizotypal personality disorder (1); 4 met criteria for psychotic features specifiers. Control group members mostly had no current or past mental disorder (n = 508); some had a history of major depressive disorder (41) or bipolar disorder (1); a few had current unipolar affective disorders (7), adjustment disorder (1), or obsessive-compulsive disorder (1); none met criteria for substance abuse or dependence.
The Ethical Review Board of the University Medical Centre Utrecht and local review boards of each participating centre (Amsterdam, Groningen, Maastricht) reviewed and approved the study protocol. After receiving full verbal and written information about the study, participants provided written informed consent.
Measures and Interviewer Training
DSM-IV diagnoses were determined using the Comprehensive Assessment of Symptoms and History47 at 3 sites and the Schedules for Clinical Assessment for Neuropsychiatry48 at one. All interviewers were trained in the use of one of these measures before undertaking any assessments.32 Reliability of DSM-IV diagnosis was assessed with a random sample (n = 65) of patients referred to the study by examining agreement between trained interviewers and the treating clinician. There was only one instance of disagreement, giving a total percent agreement of 98.5%.32
Self-reported schizotypy was assessed using the Community Assessment of Psychic Experiences (CAPE),49,50 a measure of lifetime subclinical features of schizophrenia. The CAPE contains 42 items relating to positive (20 items), negative (14), and depressive (8) experiences that are rated for frequency of occurrence (0 = never, 1 = sometimes, 2 = often, 3 = almost always) and, if present, distress caused (0 = no, 1 = little, 2 = moderate, 3 = lots). We are not aware of studies of the reliability or validity of item-level ratings on the CAPE. Psychometric studies of CAPE positive and negative frequency scores show modest internal consistency (mean meta-analytic α = .84 and .81, respectively).51 In a large Dutch community sample, the 7-month test-retest reliability coefficients for the positive and negative frequency scores were r = .71 and .78, respectively. These scores also showed convergent and discriminant validity, respectively, in correlations with like- vs different-content scores from the Structured Interview for Schizotypy–Revised (SIS-R) and Brief Psychiatric Rating Scale.52 Three factors load onto CAPE items.50,53 The CAPE discriminates schizophrenia, affective and anxiety disordered, and general population samples49; is sensitive to family-specific variation for positive and negative subclinical psychosis dimensions54; and generates scores that are stable over time and associated with interview measures of schizotypy.52
Consistency tests and PCLR coefficients were examined using data from the Family Interview for Genetic Studies (FIGS)55 and SIS-R.56 The FIGS was used to obtain information from which familial loads for psychotic, bipolar, and substance use disorders were estimated using the algorithm described by Derks et al.57 The probands’ parents were the primary informants and, if parents were not available, the siblings. The SIS-R was used to assess positive, disorganized, and negative signs and symptoms of schizotypy by semi-structured interview. The SIS-R comprises 31 items that are rated using 4-point scales; the SIS-R total score was obtained. Probands’ hospital admission details, Global Assessment of Functioning (GAF) symptom and disability ratings, course characteristics, and negative symptom counts were also used in consistency testing. Psychometric intelligence was assessed using a 4-test short-form of the Wechsler Adult Intelligence Scale–Third Edition58 comprising the Arithmetic, Digit-Symbol, Block Design, and Information subtests.
Statistical Analyses
Taxometric analyses were applied to CAPE positive and negative item-level occurrence ratings. Sporadic missing ratings (<5 missing ratings across 42 items) were estimated using prorated mean subscale ratings. Items with endorsement frequencies below 1% or that were strongly associated with sex were excluded from analyses. In order to increase the resolution of the ordering variable used in the subsequent taxometric analyses,59 ordinal item ratings were dichotomized with ≥ sometimes = 1 unless the resulting endorsement frequency was over 50%, in which case the threshold was > sometimes = 1. Tetrachoric correlations (ρ) were calculated to determine that items were monotonically related. Maximum covariance (MAXCOV) analysis was the primary taxometric method, with maximum eigenvalue (MAXEIG), and latent modes (LMODE) analyses used to corroborate MAXCOV results.33 These were applied without correction for nonindependent sampling because taxometric methods do not require independent observations (personal communication, Niels Waller, June 9, 2016). Taxometric analyses were completed using R60 code adapted from Grove61,62 and Waller and Meehl.33
MAXCOV was applied iteratively (slab n ≥ 20). If an item covariance curve was nontaxonic, the item was removed and MAXCOV was repeated. Taxonicity was judged on covariance curve form, variances of raw and loess-smoothed covariance estimates, and cusp number and location. After a final indicator set was obtained, the performance of excluded indicators was reviewed one by one by determining whether returning the item to the final indicator set improved mean indicator validity, covariance curves, or prevalence variance.
MAXCOV results were corroborated using the variance of prevalence estimates, distribution of membership probabilities, and peak mean covariance. Subsequently, MAXEIG was applied using the inchworm consistency test33 (from 5 windows, increasing until n per window was not less than 5 × the number of items; 90% window overlap; 20 randomized sequence replications for each indicator). LMODE was applied to whole samples and to restricted samples using the case-removal consistency method (nominal prevalence target was ≥20%).63 Available procedures for simulation and curve fit indices64 cannot be used with binary data.
A confirmatory approach was taken in the taxometric analyses of sibling data. That is, taxometric analyses of sibling data used those indicators that were retained in analyses of the control group. To test whether the retained indicators behaved consistently across the control and sibling groups, dichotomous differential item functioning was examined using a logistic regression method from the package difR65 in R. Hypotheses on probands’ familial load of psychotic disorder, probands’ clinical severity, and siblings’ ages were tested using logistic regression with SEs clustered in families to correct for nonindependent sampling.
Results
Table 1 reports CAPE frequency scores for the sibling and control groups. Fifty-four item ratings (0.23%) by 33 control participants and 93 ratings (0.28%) by 77 siblings were missing. One item (conversing voices) had an endorsement rate below 1%. Bonferroni-corrected analyses of the remaining 33 items identified 4 items that were associated with sex: poverty of affect, anergia, and 2 grandiose ideation items (for all, χ2[3, N = 559] > 22.0, P < .0001). The remaining 29 indicators were dichotomized. Of the 406 ρ coefficients, 12 were negative indicating non-monotonicity was present. All negative coefficients were for pairs including a positive and negative item. Given this and evidence suggesting positive and negative items do not identify redundant classes,13–15 taxometric analyses of positive and negative items proceeded separately.
Taxometric Analysis of the Control Group
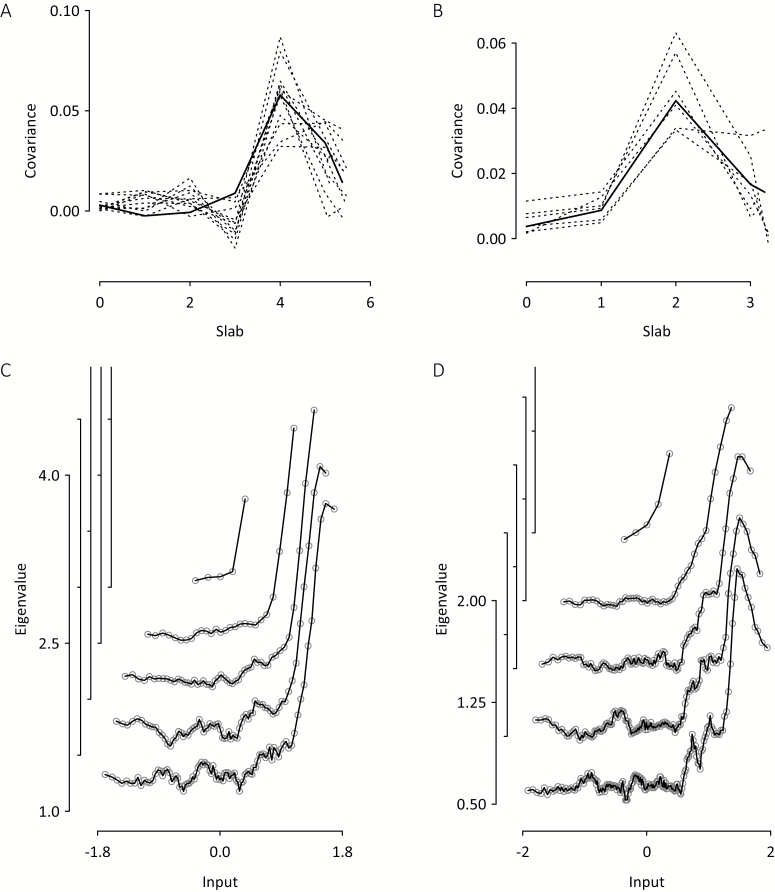
In the control group, MAXCOV analyses of positive items concluded after the removal of 6 items (visual hallucination, self-reference ideas, telepathy, thought withdrawal, hearing voices, Capgras delusion). With the 11 remaining items, the unsmoothed median covariance curve peaked at 0.058 with a cusp; all individual item covariance curves were also cusped (figure 1A). The SD of prevalence estimates was 11.0% and the distribution of posterior probabilities was consistent with a taxonic structure (supplementary figure S1). Mean indicator validity (between-group effect size) was low (M = 0.80, SD = 0.26). The mean within-class correlations were ρ = −.05 and ρ = .14 for the taxon and complement, respectively. No excluded item improved the final item set.
Fig. 1.
Taxometric results for the control group (n = 559). Final iteration maximum covariance (MAXCOV) curves for (A) 11 positive and (B) 6 negative Community Assessment of Psychic Experiences (CAPE) items. Dashed lines show unsmoothed mean covariance for individual items; solid lines show loess-smoothed median covariance for all items. MAXEIG unsmoothed median inchworm consistency curves for (C) 11 positive CAPE items using 5, 27, 50, 73, and 95 windows and (D) 6 negative CAPE items using 5, 49, 93, 136, and 180 windows. For clarity, maximum eigenvalue (MAXEIG) curve y-coordinates were shifted in steps of 0.5; the numbered axes relate to the lowest curves, which also have the most windows.
MAXCOV analyses of negative items concluded following the removal of 6 items (enthusiasm-expressivity, talkativeness, empty mind, inactivity, lack of spontaneity, blunted affect). The mean covariance curve for the remaining 6 items was cusped with a peak median unsmoothed covariance of 0.042 (figure 1B), suggesting a class structure. All individual item covariance curves were also cusped. Prevalence estimates were in a narrow range (SD = 3.6%); indicator validity was low (M = 0.90, SD = 0.32); the posterior probabilities had a taxonic distribution (supplementary figure S1). The mean within-class correlations for the taxon and complement were ρ = .16 and ρ = .40, respectively.
With 11 positive and 6 negative items, the maximum numbers of windows in the MAXEIG analyses were set at 95 and 180, respectively. MAXEIG and LMODE analyses of both item sets yielded results consistent with taxonic structures. MAXEIG inchworm consistency results showed rising peaks in eigenvalues as the numbers of windows increased (figures 1C and 1D), consistent with low prevalence taxonic structures. The LMODE analysis yielded skewed density plots (supplementary figure S2) and discrepant prevalence estimates (table 2).
Table 2.
Class Prevalence Estimates (%) for Control and Sibling Groups
| Estimation method | Control (n = 559) | Siblings (n = 792) | Difference (z) | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| MAXCOV, classification | 7.2 | 11.6 | 5.8 | 21.5 | 0.98 | 4.94*** |
| MAXCOV, estimated | 8.6 | 10.6 | 8.9 | 18.1 | 0.19 | 3.96*** |
| MAXEIG, estimated | 5.0 | 6.6 | 4.1 | 9.4 | 0.73 | 1.92* |
| LMODE, classification | 9.8 | 12.7 | 19.3 | 22.6 | 5.03*** | 4.48*** |
| LMODE, mode 1 estimate | 6.0 | 7.9 | 26.1 | 22.2 | 10.85*** | 7.68*** |
| LMODE, mode 2 estimate | 41.1 | 47.2 | 33.8 | 44.8 | 2.71** | 0.85 |
| Median rate | 7.9 | 11.1 | 14.1 | 21.8 | 3.71*** | 5.41*** |
Note: MAXCOV, maximum covariance; MAXEIG, maximum eigenvalue; LMODE, latent modes.
*P < .05, **P < .01, ***P < .001, all 1-tailed tests.
In the LMODE case-removal consistency test for positive items (supplementary figure S2), the score distribution necessitated the removal of 326 cases (expected prevalence = 23.6%). The observed prevalence in the restricted sample (20.6%) did not differ from expected (z = 1.13, P = .27). Similarly, for the negative CAPE items (supplementary figure S2), n = 247 cases were removed (expected prevalence = 22.8%) and the observed prevalence (20.2%) was not different from expected (z = 1.13, P = .26). Moreover, for positive and negative classes, the observed and expected rates were within the 5% range specified by Ruscio.63
MAXCOV analyses of the same case-removal consistency subsamples generated taxonic covariance curves for positive and negative items (supplementary figure S3). For the positive items, the restricted sample prevalence (21.6%) did not differ from expected (z < 1) and was within a 5% range whereas for the negative items, the restricted sample prevalence (14.2%) was lower than expected (z = 4.35, P < .001) and beyond a 5% range.
Taxometric Analysis of the Sibling Group
Taxometric analyses were applied to siblings’ ratings on the 11 positive and 6 negative CAPE items retained following MAXCOV analyses of control group data. Sibling data were monotonic. There was no evidence that the positive and negative item sets had differential item functioning (supplementary table S1).
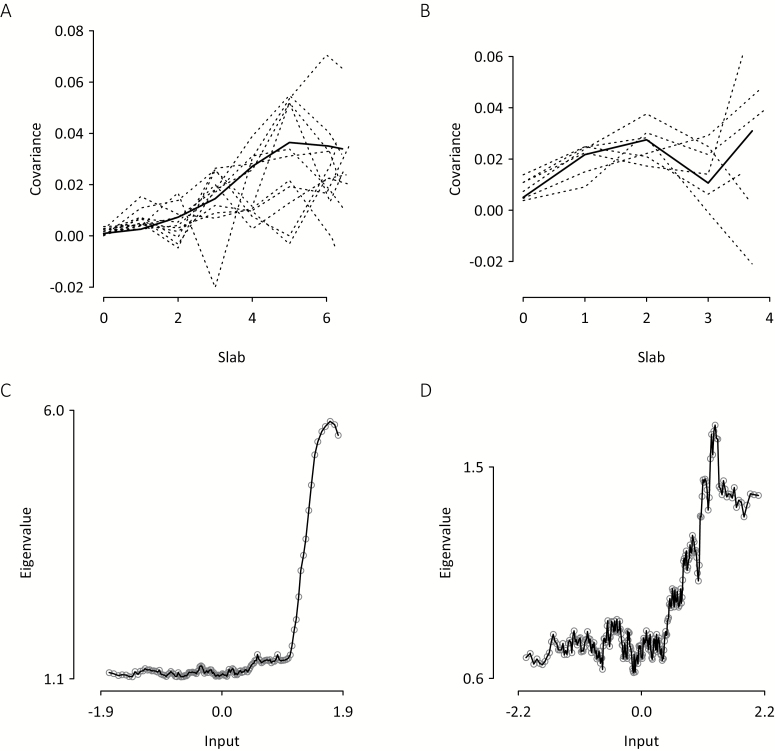
MAXCOV analysis of positive items yielded a cusped covariance curve with peak unsmoothed median covariance of 0.039 (figure 2). Bayesian posterior probabilities had a taxonic distribution (supplementary figure S1) and the SD of item prevalence estimates was 11.9%. Indicator validity was low (M = 0.81, SD = 0.26) and the mean within-taxon and -complement correlations among indicators were ρ = −.01 and ρ = .15, respectively. For the negative CAPE items, the covariance curve was generally lower (maximum unsmoothed median covariance = 0.031) and fluctuating, with a mid-range cusp and a right-end peak. Bayesian posterior probabilities had a taxonic distribution (supplementary figure S1); the prevalence rate SD = 12.3%. Indicator validity was low (M = 0.82, SD = 0.28) and the mean within-class correlations for the taxon and complement were both ρ = .41.
Fig. 2.
Taxometric results for siblings (n = 792) obtained from the 11 positive and 6 negative Community Assessment of Psychic Experiences (CAPE) items retained following analysis of control group data. Maximum covariance (MAXCOV) curves for (A) positive and (B) negative CAPE items. Dashed lines show unsmoothed mean covariance for individual items; solid lines show loess-smoothed median covariance for all items. Maximum eigenvalue (MAXEIG) unsmoothed median plots for (C) positive items using 135 windows and (D) negative items using 255 windows.
The MAXEIG curves for the positive and negative CAPE items were more clearly indicative of taxonicity (figures 2C and 2D) than the MAXCOV covariance curves, but yielded lower estimated prevalence rates (table 2). LMODE density plots (supplementary figure S2) were consistent with the presence of latent taxonic structures. The LMODE prevalence estimates for siblings were less discrepant than for the control group (table 2).
Class Prevalence Estimates, Class Independence, and Case Classification
Median prevalence estimates for the positive and negative classes were calculated from all available prevalence estimates (table 2). The median estimates for positive and negative schizotypy among siblings were significantly higher than those of the control group (table 2). Also, positive and negative MAXCOV class memberships were not independent for control group members (OR = 2.40, P = .030) or for siblings (OR = 9.02, P = .001; supplementary material).
For the analyses that follow, siblings and control participants were classified to schizotypy or complement classes using posterior membership probabilities from MAXCOV and LMODE. A sibling-proband family classification was also obtained. The supplementary material describes the handling of inconsistencies across MAXCOV and LMODE, and across siblings within families.
Corroborating Genetic Risk
Table 3 shows results of tests corroborating the notion of genetic risk. Siblings’ positive and negative CAPE classifications were predicted by probands’ familial load for psychotic disorder but not by familial loads for bipolar disorder or drug abuse. Siblings of probands with a greater number and frequency of negative symptoms, more severe GAF symptom ratings, and more frequent depressive symptoms were more likely to be positive class members. Unexpectedly, siblings’ positive class membership was associated with probands having fewer psychiatric hospital admissions. Probands’ CAPE positive and depression frequency ratings predicted siblings’ negative class membership. There was no evidence that other indices of probands’ clinical severity predicted siblings’ negative class membership.
Table 3.
ORs for Univariate Predictors of Membership in CAPE Taxa for Siblings
| Predictor | Positive CAPE Taxon | Negative CAPE Taxon | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Proband characteristics | ||||
| Family load, bipolar disorder | 0.01 | 0.00, 5.45 | 1.02 | 0.43, 2.43 |
| Family load, psychotic disorder | 1.99** | 1.29, 3.05 | 1.75** | 1.21, 2.55 |
| Family load, drug abuse | 0.56 | 0.04, 7.80 | 2.51† | 0.98, 6.42 |
| Months in hospital | 0.99 | 0.96, 1.02 | 1.00 | 0.98, 1.02 |
| Hospital admissions | 0.82* | 0.69, 0.98 | 1.00 | 0.93, 1.07 |
| GAF symptoms | 0.97** | 0.96, 0.99 | 0.99 | 0.98, 1.01 |
| GAF disability | 0.98† | 0.96, 1.00 | 1.00 | 0.98, 1.01 |
| Chronic course | 1.28 | 0.59, 2.81 | 1.09 | 0.64, 1.87 |
| Negative symptom count > 2 | 2.16* | 1.01, 4.61 | 1.44 | 0.91, 2.26 |
| CAPE frequency, positive | 1.67† | 0.98, 2.87 | 1.59* | 1.07, 2.37 |
| CAPE frequency, negative | 2.21*** | 1.36, 3.59 | 1.40† | 0.95, 2.05 |
| CAPE frequency, depression | 1.98** | 1.25, 3.13 | 1.57* | 1.10, 2.24 |
| Sibling characteristics | ||||
| Age | 0.96† | 0.92, 1.01 | 0.97* | 0.95, 1.00 |
Note: CAPE, Community Assessment of Psychic Experiences; GAF, Global Assessment of Functioning.
† P < .10, *P < .05, **P < .01, ***P < .001.
Negative CAPE class membership, but not positive CAPE class membership, was associated with younger age (table 3). Interestingly, younger age predicted positive but not negative class membership in the control group (supplementary tables S2 and S3).
Phenotypic Correlation in Liability Between Relatives
PCLR coefficients were obtained for positive and negative classes using deviation scores from the sums of the 11 positive and 6 negative CAPE items. As the PCLR is based on the assumption of a normal liability distribution, yet CAPE scores were significantly positively skewed (P < .001), a deviation score was also obtained using the SIS-R total, which was not skewed after Box-Cox transformation (P = .67). Prevalence estimates were the mean of the MAXCOV and LMODE classification rates (table 2). With CAPE deviation scores, PCLR coefficients for positive and negative schizotypy were rPCLR = .09 and rPCLR = .20, respectively, and with the SIS-R deviation score, rPCLR = .28 and rPCLR = .60, respectively.
Discussion
Nonredundant positive and negative schizotypy classes with median prevalence estimates of 8% and 11%, respectively, were identified in schizotypy ratings from a general population sample. When the class indicators obtained with the general population were applied to schizotypy ratings from biological siblings of probands with schizophrenia, evidence of positive and negative schizotypy taxa was also obtained. The positive and negative schizotypy classes were significantly larger in the siblings (14% and 22%, respectively) than in the control group.
Siblings’ membership of positive and negative schizotypy classes appeared to reflect liability for schizophrenia. First, probands’ familial load for psychotic disorder predicted siblings’ class membership whereas familial loads for bipolar disorder and drug use did not. Second, siblings of probands with more severe conditions were more likely to be class members. This was particularly so for probands’ negative symptoms and siblings’ membership in the positive class. Unexpectedly, siblings were also more likely to be classified in the positive class if their probands had fewer psychiatric hospital admissions. Third, siblings in the negative schizotypy class were younger than those in the complement group, consistent with a greater likelihood of transition to psychotic disorder where schizophrenia risk is greater.45 Finally, the PCLR coefficient range is comparable to PCLR reported for schizophrenia,44 although the degree of familial similarity may be greater with negative schizotypy than with positive schizotypy.
Whereas the MAXCOV, MAXEIG, and LMODE figures and key consistency tests (inchworm, case-removal) from the control group suggest taxonicity, several corroborating results prompt some caution. Class separations (validities or the K coefficients) were much lower than has typically been observed or recommended.33 For the negative schizotypy classes, the within-class correlations remained very high, suggesting that the assumption of conditional independence was not met. The LMODE prevalence estimates did not converge across modes, suggesting some underlying instability or that the correct modes were not identified. The MAXCOV and LMODE curves from the siblings were not unambiguously taxonic. Overall, the results for negative schizotypy in siblings appeared less compelling than results for positive schizotypy.
Several other limitations may affect interpretation of the results. First, a lack of difference in positive CAPE frequency scores between the control group and siblings (table 1) suggests siblings may have responded defensively on positive CAPE items. We examined this possibility in ad hoc analyses (supplementary material), finding that siblings’ positive CAPE frequency scores were lower (by an effect size of d = 0.15, P < .001) than would be expected given other CAPE ratings, demographic characteristics, and IQ. Whereas the overall endorsement rate of a set of items will not affect the prevalence of a class structure per se, non-uniform defensiveness across siblings may have obscured or diminished the positive schizotypy class size.66 Defensive responding could also account for the lower PCLR coefficients for positive vs negative schizotypy. Second, PCLR coefficients may be unreliable because of severe positive skewness in CAPE ratings and because the SIS-R was not used in case classification. Third, we note that many do not regard the CAPE as a measure of trait schizotypy or schizophrenia liability. Whereas there are important theoretical distinctions between psychosis experience and trait schizotypy, psychosis experiences have long been regarded as indicative of trait schizotypy.67–69 Nevertheless, the generalizability of our findings using alternative assessments of schizotypy should be addressed in future research. Fourth, whereas dichotomization of the CAPE ordinal frequency ratings was undertaken to improve the resolution of the ordering variable used in MAXCOV analyses, dichotomization does result in loss of information. Fifth, the application of diagnostic exclusion criteria affects the generalizability of findings to the sampling populations.34 Exclusion criteria were unavoidable but their presence likely reduced the prevalence estimates that we observed. Finally, our reliance on self-report has both strengths and weaknesses. For the latter, inattentive or biased responding was not assessed with any infrequency or social desirability scales. For the former, subjective experience can only be assessed via self-report, observer bias can affect latent structure analyses,4,34,66 and self-report may better capture underlying genetic and environmental processes than observer ratings.70
With these limitations in mind, findings show that positive and negative schizotypy, assessed with the CAPE, have nonindependent latent taxonic structures that are sensitive to risk for schizophrenia. Critically, this evidence supports the arguments that schizotypy is a taxonic liability state2,4,71 that reflects multifactorial polygenic risk for schizophrenia.29,43 The findings add to evidence that taxonic schizotypy in the general population is associated with schizophrenia among first-degree biological relatives19 and with MMP16, a single-nucleotide polymorphism associated with schizophrenia.22 Together, this body of evidence suggests that schizotypy is a useful phenotype for understanding the pathogenesis of schizophrenia. Moreover, as a discrete construct that can be classified without relying on arbitrary or nominal criteria, schizotypy may serve as a better criterion in the study of the pathogenesis of schizophrenia than the disease end-state itself.
Importantly, the notion that schizotypy is taxonic is entirely compatible with the idea that the psychosis phenotype is dimensional or continuous. We distinguish dimensionality or continuity within the population structure from dimensional variability in phenotype expression and temporal continuity.34,72 Here, our focus has been on the latent population structure and whether the population should be thought of as comprising a single group or 2 groups. At the same time, there is good reason to view the phenotype as continuous, including that experience appears graduated,73 the affordances of language allow the specification of phenomena that vary in seemingly graduated ways,74 and key drivers of the phenotype probably generate a net load that varies quantitatively too—although these need not be isomorphic.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
The infrastructure for the GROUP study is funded by the Geestkracht program of the Dutch Health Research Council (ZON-MW, grant 10-000-1002) and matching funds from participating universities and mental health care organizations (Site Amsterdam: Academic Psychiatric Centre AMC, Ingeest, Arkin, Dijk en Duin, Rivierduinen, Erasmus MC, GGZ Noord Holland Noord; Site Utrecht: University Medical Centre Utrecht, Altrecht, Symfora, Meerkanten, RIAGG Amersfoort, Delta; Site Groningen: University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGZ De Grote Rivieren and Parnassia Bavo Groep; Site Maastricht: Maastricht University Medical Center, GGZEindhoven en de Kempen,GGZMidden-Brabant,GGZOost-Brabant,GGZ Noord-en Midden-Limburg, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem). The research leading to these results has received funding from the European Community’s Seventh Framework Program under grant agreement No. HEALTH-F2-2009-241909 (Project EU-GEI).
Supplementary Material
Acknowledgments
GROUP Investigators: Berhooz Z. Alizadeh1, Agna A. Bartels-Velthuis1, Nico J. van Beveren2–4, Richard Bruggeman1, Wiepke Cahn5, Lieuwe de Haan6, Philippe Delespaul7, Carin J. Meijer6, Inez Myin-Germeys8, Rene S. Kahn5, Frederike Schirmbeck6, Claudia J.P. Simons7,9, Neeltje E. van Haren5, Jim van Os7,10, Ruud van Winkel7,8. Affiliations: 1University of Groningen, University Medical Center Groningen, University Center for Psychiatry, Groningen, The Netherlands; 2Antes Center for Mental Health Care, Rotterdam, The Netherlands; 3Erasmus MC, Department of Psychiatry, Rotterdam, The Netherlands; 4Erasmus MC, Department of Neuroscience, Rotterdam, The Netherlands; 5Department of Psychiatry, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, The Netherlands; 6Department of Psychiatry, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands; 7Department of Psychiatry and Psychology, School for Mental Health and Neuroscience, Maastricht University Medical Center, Maastricht, The Netherlands; 8KU Leuven, Department of Neuroscience, Research Group Psychiatry, Center for Contextual Psychiatry, Leuven, Belgium; 9GGzE, Institute for Mental Health Care Eindhoven and De Kempen, Eindhoven, The Netherlands; 10Department of Psychosis Studies, Institute of Psychiatry, King’s College London, King’s Health Partners, London, United Kingdom. We are grateful to the many volunteers who contributed to this research, the research staff involved in the project, and the many mental health services that enabled the GROUP project. The authors declare no conflicts of interest with respect to the authorship or publication of this article.
Contributor Information
GROUP (Genetic Risk and Outcome of Psychosis) Investigators:
Berhooz Z Alizadeh, Agna A Bartels-Velthuis, Nico J van Beveren, Richard Bruggeman, Wiepke Cahn, Lieuwe de Haan, Philippe Delespaul, Carin J Meijer, Inez Myin-Germeys, Rene S Kahn, Frederike Schirmbeck, Claudia J P Simons, Neeltje E van Haren, Jim van Os, and Ruud van Winkel
References
- 1. Meehl PE. Schizotaxia, schizotypy, schizophrenia. Am Psychol. 1962;17:827–838. [Google Scholar]
- 2. Meehl PE. Toward an integrated theory of schizotaxia, schizotypy, and schizophrenia. J Pers Disord. 1990;4:1–99. [Google Scholar]
- 3. Meehl PE. MAXCOV-HITMAX: a taxonomic search method for loose genetic syndromes. In: Meehl PE, ed. Psychodiagnosis: Selected Papers. Minneapolis, MN: University of Minnesota Press; 1973:200–224. [Google Scholar]
- 4. Lenzenweger MF. Schizotypy and Schizophrenia: The View From Experimental Psychopathology. New York: Guilford Press; 2010. [Google Scholar]
- 5. Golden RR, Meehl PE. Detection of the schizoid taxon with MMPI indicators. J Abnorm Psychol. 1979;88:217–233. [DOI] [PubMed] [Google Scholar]
- 6. Lenzenweger MF, Korfine L. Confirming the latent structure and base rate of schizotypy: a taxometric analysis. J Abnorm Psychol. 1992;101:567–571. [DOI] [PubMed] [Google Scholar]
- 7. Korfine L, Lenzenweger MF. The taxonicity of schizotypy: a replication. J Abnorm Psychol. 1995;104:26–31. [DOI] [PubMed] [Google Scholar]
- 8. Lenzenweger MF. Deeper into the schizotypy taxon: on the robust nature of maximum covariance analysis. J Abnorm Psychol. 1999;108:182–187. [DOI] [PubMed] [Google Scholar]
- 9. van Kampen D. Genetic and environmental influences on pre-schizophrenic personality: MAXCOV-HITMAX and LISREL analyses. Eur J Pers. 1999;13:63–80. [Google Scholar]
- 10. Blanchard JJ, Gangestad SW, Brown SA, Horan WP. Hedonic capacity and schizotypy revisited: a taxometric analysis of social anhedonia. J Abnorm Psychol. 2000;109:87–95. [DOI] [PubMed] [Google Scholar]
- 11. Meyer TD, Keller F. Exploring the latent structure of the Perceptual Aberration, Magical Ideation, and Physical Anhedonia Scales in a German sample. J Pers Disord. 2001;15:521–535. [DOI] [PubMed] [Google Scholar]
- 12. Keller F, Jahn T, Klein C. Anwendung von taxometrischen methoden und von mischverteilungsmodellen zur erfassung der schizotypie. In: Andresen B, Maß R, eds. Schizotypie: Psychometrische entwicklungen und biopsychologische forschungsansätze. Göttingen, Germany: Hogrefe; 2001:391–412. [Google Scholar]
- 13. Horan WP, Blanchard JJ, Gangestad SW, Kwapil TR. The psychometric detection of schizotypy: do putative schizotypy indicators identify the same latent class?J Abnorm Psychol. 2004;113:339–357. [DOI] [PubMed] [Google Scholar]
- 14. Linscott RJ. The latent structure and coincidence of hypohedonia and schizotypy and their validity as indices of psychometric risk for schizophrenia. J Pers Disord. 2007;21:225–242. [DOI] [PubMed] [Google Scholar]
- 15. Linscott RJ. The taxonicity of schizotypy: does the same taxonic class structure emerge from analyses of different attributes of schizotypy and from fundamentally different statistical methods?Psychiatry Res. 2013;210:414–421. [DOI] [PubMed] [Google Scholar]
- 16. Linscott RJ, Marie D, Arnott KL, Clarke BL. Over-representation of Maori New Zealanders among adolescents in a schizotypy taxon. Schizophr Res. 2006;84:289–296. [DOI] [PubMed] [Google Scholar]
- 17. Fossati A, Raine A, Borroni S, Maffei C. Taxonic structure of schizotypal personality in nonclinical subjects: issues of replicability and age consistency. Psychiatry Res. 2007;152:103–112. [DOI] [PubMed] [Google Scholar]
- 18. Everett KV, Linscott RJ. Dimensionality vs taxonicity of schizotypy: some new data and challenges ahead. Schizophr Bull. 2015;41(suppl 2):S465–S474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lenzenweger MF, McLachlan G, Rubin DB. Resolving the latent structure of schizophrenia endophenotypes using expectation-maximization-based finite mixture modeling. J Abnorm Psychol. 2007;116:16–29. [DOI] [PubMed] [Google Scholar]
- 20. Tyrka AR, Cannon TD, Haslam N, et al. The latent structure of schizotypy: I. Premorbid indicators of a taxon of individuals at risk for schizophrenia-spectrum disorders. J Abnorm Psychol. 1995;104:173–183. [DOI] [PubMed] [Google Scholar]
- 21. Erlenmeyer-Kimling L, Golden RR, Cornblatt BA. A taxometric analysis of cognitive and neuromotor variables in children at risk for schizophrenia. J Abnorm Psychol. 1989;98:203–208. [DOI] [PubMed] [Google Scholar]
- 22. Morton SE, O’Hare KJM, Maha JLK, et al. Testing the validity of taxonic schizotypy using genetic and environmental risk variables. Schizophr Bull. 2017;43:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linscott RJ, Lenzenweger MF, van Os J. Continua or classes? Vexed questions on the latent structure of schizophrenia. In: Gattaz WF, Busatto GF, eds. Advances in Schizophrenia Research 2009. New York, NY: Springer; 2010:333–355. [Google Scholar]
- 24. Rawlings D, Williams B, Haslam N, Claridge G. Taxometric analysis supports a dimensional latent structure for schizotypy. Pers Individ Dif. 2008;44:1640–1651. [Google Scholar]
- 25. Daneluzzo E, Stratta P, Di Tommaso S, Pacifico R, Riccardi I, Rossi A. Dimensional, non-taxonic latent structure of psychotic symptoms in a student sample. Soc Psychiatry Psychiatr Epidemiol. 2009;44:911–916. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed AO, Buckley PF, Mabe PA. Latent structure of psychotic experiences in the general population. Acta Psychiatr Scand. 2012;125:54–65. [DOI] [PubMed] [Google Scholar]
- 27. Ahmed AO, Green BA, Goodrum NM, Doane NJ, Birgenheir D, Buckley PF. Does a latent class underlie schizotypal personality disorder? Implications for schizophrenia. J Abnorm Psychol. 2013;122:475–491. [DOI] [PubMed] [Google Scholar]
- 28. Claridge G. Theoretical background and issues. In: Claridge G, ed. Schizotypy: Implications for illness and health. New York, NY: Oxford University Press; 1997:3–18. [Google Scholar]
- 29. Gottesman II, Shields J, Hanson DR.. Schizophrenia: The Epigenetic Puzzle. Cambridge, UK: Cambridge University Press; 1982. [Google Scholar]
- 30. International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Szatkiewicz JP, O’Dushlaine C, Chen G, et al. Copy number variation in schizophrenia in Sweden. Mol Psychiatry. 2014;19:762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L; GROUP investigators Genetic Risk and Outcome of Psychosis (GROUP), a multi-site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Methods Psychiatr Res. 2012;21:205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waller NG, Meehl PE.. Multivariate Taxometric Procedures: Distinguishing Types From Continua. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- 34. Linscott RJ, Allardyce J, van Os J. Seeking verisimilitude in a class: a systematic review of evidence that the criterial clinical symptoms of schizophrenia are taxonic. Schizophr Bull. 2010;36:811–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vollema MG, van den Bosch RJ. The multidimensionality of schizotypy. Schizophr Bull. 1995;21:19–31. [DOI] [PubMed] [Google Scholar]
- 36. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 37. Bandalos DL, Finney SJ. Item parceling issues in structural equation modeling. In: Marcoulides GA, Schumacker RE, eds. New Developments and Techniques in Structural Equation Modeling. Mahwah, NJ: Lawrence Erlbaum; 2001:269–296. [Google Scholar]
- 38. Little TD, Rhemtulla M, Gibson K, Schoemann AM. Why the items versus parcels controversy needn’t be one. Psychol Methods. 2013;18:285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marsh HW, Lüdtke O, Nagengast B, Morin AJ, Von Davier M. Why item parcels are (almost) never appropriate: two wrongs do not make a right–camouflaging misspecification with item parcels in CFA models. Psychol Methods. 2013;18:257–284. [DOI] [PubMed] [Google Scholar]
- 40. Meehl PE. Schizotaxia revisited. Arch Gen Psychiatry. 1989;46:935–944. [DOI] [PubMed] [Google Scholar]
- 41. Tyrka AR, Cannon TD, Haslam N, et al. The latent structure of schizotypy: I. Premorbid indicators of a taxon of individuals at risk for schizophrenia-spectrum disorders. J Abnorm Psychol. 1995;104:173–183. [DOI] [PubMed] [Google Scholar]
- 42. Gottesman II, McGue M. Mixed and mixed-up models for the transmission of schizophrenia. In: Grove WM, Cicchetti D, eds. Thinking Clearly About Psychology. Vol. 2: Personality and psychopathology. Essays in honor of Paul E. Meehl. Minneapolis, MN: University of Minnesota Press; 1991:295–312. [Google Scholar]
- 43. Gottesman II, Shields J. Schizophrenia and genetics: a twin study vantage point. New York, NY: Academic Press; 1972. [Google Scholar]
- 44. Reich R, Cloninger CR, Guze SB. The multifactorial model of disease transmission: I. Description of the model and its use in psychiatry. Br J Psychiatry. 1975;127:1–10. [DOI] [PubMed] [Google Scholar]
- 45. Hanson DR, Gottesman II, Meehl PE. Genetic theories and the validation of psychiatric diagnoses: implications for the study of children of schizophrenics. J Abnorm Psychol. 1977;86:575–588. [DOI] [PubMed] [Google Scholar]
- 46. GROUP Investigators. Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatry. 2011;68:138–147. [DOI] [PubMed] [Google Scholar]
- 47. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. [DOI] [PubMed] [Google Scholar]
- 48. Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. [DOI] [PubMed] [Google Scholar]
- 49. Hanssen M, Peeters F, Krabbendam L, Radstake S, Verdoux H, van Os J. How psychotic are individuals with non-psychotic disorders?Soc Psychiatry Psychiatr Epidemiol. 2003;38:149–154. [DOI] [PubMed] [Google Scholar]
- 50. Stefanis NC, Hanssen M, Smirnis NK, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–358. [DOI] [PubMed] [Google Scholar]
- 51. Mark W, Toulopoulou T. Psychometric properties of “Community Assessment of Psychic Experiences”: review and meta-analyses. Schizophr Bull. 2016;42:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Konings M, Bak M, Hanssen M, van Os J, Krabbendam L. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr Scand. 2006;114:55–61. [DOI] [PubMed] [Google Scholar]
- 53. Verdoux H, Sorbara F, Gindre C, Swendsen JD, van Os J. Cannabis use and dimensions of psychosis in a nonclinical population of female subjects. Schizophr Res. 2003;59:77–84. [DOI] [PubMed] [Google Scholar]
- 54. Hanssen M, Krabbendam L, Vollema M, Delespaul P, Van Os J. Evidence for instrument and family-specific variation of subclinical psychosis dimensions in the general population. J Abnorm Psychol. 2006;115:5–14. [DOI] [PubMed] [Google Scholar]
- 55. Maxwell ME. Manual for the FIGS. Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; 1992. [Google Scholar]
- 56. Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15:559–571. [DOI] [PubMed] [Google Scholar]
- 57. Derks EM, Verweij KH, Kahn RS, Cahn WC. The calculation of familial loading in schizophrenia. Schizophr Res. 2009;111:198–199. [DOI] [PubMed] [Google Scholar]
- 58. Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 59. Meehl PE. Clarifications about taxometric method. Appl Prev Psychol. 1999;8:165–174. [Google Scholar]
- 60. R Core Team. R: A language and environment for statistical computing [computer program]. Version 3.1.2 Pumpkin Helmet. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 61. Grove WM. MAXEIGwmg taxometric procedure [computer program]. Version June 29, 2003. Minneapolis, MN; 2003. www.psych.umn.edu/faculty/grove/. Accessed August 1, 2005. [Google Scholar]
- 62. Grove WM. MAXCOVwmg taxometric procedure [computer program]. Version February 17, 2004. Minneapolis, MN; 2004www.psych.umn.edu/faculty/grove/. Accessed August 1, 2005. [Google Scholar]
- 63. Ruscio J. Taxometric analysis with dichotomous indicators: the modified MAXCOV procedure and a case-removal consistency test. Psychol Rep. 2000;87:929–939. [DOI] [PubMed] [Google Scholar]
- 64. Ruscio J, Haslam N, Ruscio AM.. Introduction to the Taxometric Method: A Practical Guide. Mahwah, NJ: Lawrence Erlbaum; 2006. [Google Scholar]
- 65. Magis D, Beland S, Raiche G.. difR: Collection of Methods to Detect Dichotomous Differential Item Functioning (DIF) [computer program]. Version 4.7.Liege, Belgium: University of Liege; 2016. [Google Scholar]
- 66. Beauchaine TP, Waters E. Pseudotaxonicity in MAMBAC and MAXCOV analyses of rating-scale data: turning continua into classes by manipulating observer’s expectations. Psychol Methods. 2003;8:3–15. [DOI] [PubMed] [Google Scholar]
- 67. Claridge G, McCreery C, Mason O, et al. The factor structure of “schizotypal’ traits: a large replication study. Br J Clin Psychol. 1996;35(Pt 1):103–115. [DOI] [PubMed] [Google Scholar]
- 68. Rawlings D, Williams B, Haslam N, Claridge G. Is schizotypy taxonic? Response to Beauchaine, Lenzenweger, and Waller. Pers Individ Dif. 2008;44:1663–1672. [Google Scholar]
- 69. Kwapil TR, Gross GM, Silvia PJ, Raulin ML, Barrantes-Vidal N. Development and psychometric properties of the Multidimensional Schizotypy Scale: a new measure for assessing positive, negative, and disorganized schizotypy. Schizophr Res. In press. [DOI] [PubMed] [Google Scholar]
- 70. Kendler KS, Myers J, Torgersen S, Neale MC, Reichborn-Kjennerud T. The heritability of cluster A personality disorders assessed by both personal interview and questionnaire. Psychol Med. 2007;37:655–665. [DOI] [PubMed] [Google Scholar]
- 71. Lenzenweger MF. Thinking clearly about schizotypy: hewing to the schizophrenia liability core, considering interesting tangents, and avoiding conceptual quicksand. Schizophr Bull. 2015;41(suppl 2):S483–S491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 73. Strauss JS. Hallucinations and delusions as points on continua function. Rating scale evidence. Arch Gen Psychiatry. 1969;21:581–586. [DOI] [PubMed] [Google Scholar]
- 74. Linscott RJ, van Os J. Systematic reviews of categorical versus continuum models in psychosis: evidence for discontinuous subpopulations underlying a psychometric continuum. Implications for DSM-V, DSM-VI, and DSM-VII. Annu Rev Clin Psychol. 2010;6:391–419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.