ABSTRACT
Background
Progression of coronary artery calcification is an important marker for cardiovascular morbidity in end-stage renal disease patients. Therefore, we reviewed the evidence on coronary artery calcification progression in different renal replacement therapies.
Methods
MEDLINE (PubMed), Embase and TRIP databases were searched from 1999 – 2016. Additionally, bibliographies were searched by hand and citation tracking of key publications was performed. Prospective studies were included that examined coronary artery calcification with two or more multislice computed tomography scans ≥6 months apart in patients 18–75 years old receiving any renal replacement therapy, including kidney transplantation. Reporting of separate scores for different modalities was required. Two researchers extracted data independently with pilot-tested forms and assessed the risk of bias using a validated tool.
Results
We identified 29 eligible studies that assessed coronary artery calcification progression in end-stage renal disease patients, of which 19 studies evaluated haemodialysis and 8 kidney transplantation. Evidence on progression in peritoneal dialysis (three studies) and nocturnal haemodialysis (one study) was limited. Meta-analysis was not possible due to diverse reporting methods of coronary artery calcification scores and definitions of progression. Median coronary artery calcification scores were considerably higher in haemodialysis cohorts at baseline, presumably due to a generally higher age and dialysis vintage. Median coronary artery calcification progressed universally. Visual inspection suggested the least progression in kidney transplant recipients.
Conclusions
There is insufficient evidence to compare the influence of renal replacement therapies on coronary artery calcification progression. We advocate the adoption of a standardized reporting method of coronary artery calcification progression.
Keywords: coronary artery calcification, haemodialysis, kidney transplantation, peritoneal dialysis, systematic review
Introduction
Cardiovascular disease is the leading cause of death in patients with end-stage renal disease (ESRD), accounting for >50% of deaths [1]. Often this cardiovascular disease burden is linked to the extensive vascular calcifications observed in ESRD patients. Contrasting with the general population, in which vascular calcifications are confined to atherosclerotic plaques in the intima, vascular calcifications also occur in the tunica media of the arterial wall in ESRD patients [2].
Calcifications of the coronary arteries (CAC) are highly prevalent in ESRD patients and are associated with clinically overt cardiovascular disease [3]. Although CAC has been established as a predictor of mortality [4], there is an ongoing debate on the implications of CAC progression in ESRD. It has been argued that vascular calcification is merely a healing process and as such does not play a causal role in cardiovascular disease in ESRD [5]. However, even though there is a dearth of evidence to confirm conclusively that CAC progression corresponds with clinical endpoints in ESRD, meta-analytical data on, for instance, phosphate binders suggest that attenuation of CAC progression is reflected by a reduction in mortality [6–9].
As for the mechanisms by which vascular calcifications may be linked to mortality, calcifications in the ESRD population presumably carry additional risks besides myocardial ischaemia through associated vascular stiffness [10, 11], progressive left ventricular hypertrophy, myocardial fibrosis [12] and conductive abnormalities [10]. Although the complex pathogenetic mechanisms have not been fully elucidated, it is tenable that CAC and CAC progression is a portentous sign in patients with ESRD.
Kidney transplantation is considered as the treatment of choice for ESRD, but recipients still suffer from a high cardiovascular risk [1] and CAC is highly prevalent in kidney transplant recipients [13]. Thus far it has not been delineated whether certain renal replacement therapies (e.g. haemodialysis, kidney transplantation, peritoneal dialysis or intensive forms of haemodialysis such as nocturnal haemodialysis) have different effects on the progression of CAC. Therefore, to examine the comparative influence of different renal replacement therapies on CAC progression, we systematically reviewed prospective studies that assessed CAC in patients treated with haemodialysis, peritoneal dialysis, nocturnal haemodialysis and kidney transplantation.
Materials and methods
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14] and a pre-specified protocol (CRD42016053649). In short, we included all English-, French-, German-, Dutch- and Spanish-language studies that performed two or more repeated CAC score measurements with a scanning interval of ≥6 months in patients receiving any form of renal replacement therapy, including kidney transplantation. Prospective studies (randomized clinical trials, observational cohorts) studying adult patients 18–75 years old with multislice computed tomography (MSCT) were included. Studies were excluded if CAC scores for different renal replacement therapy modalities were not provided separately.
We searched the MEDLINE, TRIP, and Embase databases for studies published from 1999 to 1 January 2017, as MSCT was introduced in 1999. The following terms were used as MeSH terms (shown in italics) and free text terms: (chronic kidney failure, renal replacement therapy, renal dialysis, hemodiafiltration, peritoneal dialysis, home haemodialysis, kidney transplantation, dialysis modality, coronary artery calcification, progression, advance, change, increase, decrease). The last search was run on 31 March 2017. A complete draft of the search strategy is available as Supplementary data. We also hand-searched bibliographies of relevant publications and used ISI Web of Science to track citations of relevant publications.
One investigator (T.J.) screened for eligibility based on titles and abstracts. We retrieved the full text of any potentially relevant study. Two investigators (T.J. and B.J.) reviewed full texts and independently assessed eligibility in a standardized manner, unblinded for author and journal. Each investigator extracted data using a pilot-tested form. Data on patient characteristics [including age, sex, dialysis vintage, history of cardiovascular disease, diabetes mellitus, phosphate, calcium, parathyroid hormone (PTH), C-reactive protein (CRP), creatinine, blood pressure and body mass index (BMI)], sample size (with second CAC score), type of renal replacement therapy, CAC scores (preferentially in Agatston units [15]) and follow-up duration were extracted. Whenever more than one study provided data from the same cohort population, we included the study with the most complete data. When data were reported in strata, data were pooled when possible or extracted in separate cohorts. Whenever CAC scores were available at more than one follow-up moment within the same cohort, we used the most complete data on the longest follow-up duration. Studies were excluded when neither CAC scores at follow-up nor other workable measures of CAC progression were provided. Disagreements were resolved by consensus. Twenty-eight study authors were contacted for further information, nine responded and seven provided data that had not been presented in the original publication.
Risk of bias was assessed with an adaption of the Quality in Prognostic Studies (QUIPS) tool [16]. The QUIPS tool evaluates six domains: study participation, study attrition, prognostic factor management, outcome measurement, study confounding and statistical analysis and reporting. A summary judgement of low, moderate or high risk of bias is made based on criteria scored for the domain concerned. For example, we considered risk of bias in study participation high when a highly selected patient group was enrolled as a result of in-/exclusion criteria, or when patient selection was not described at all. We considered risk of attrition bias high when >30% of patients whose CAC was measured at baseline did not undergo follow-up MSCT. We considered the risk of bias in statistical reporting high when CAC scores were reported as mean ± SD. Further criteria used in risk of bias assessment are available in the Supplementary data. Risk of bias assessment was performed in duplicate (T.J. and B.J.), with disagreements resolved by consensus.
To describe CAC progression, we report the median and (preferably interquartile) range of the CAC score at baseline and at follow-up, with follow-up duration. Because of the fundamentally skewed nature of CAC scores, and consequent heterogeneity in statistical reporting and analysis of CAC progression, it was not possible or appropriate to perform meta-analysis.
Results
Study characteristics
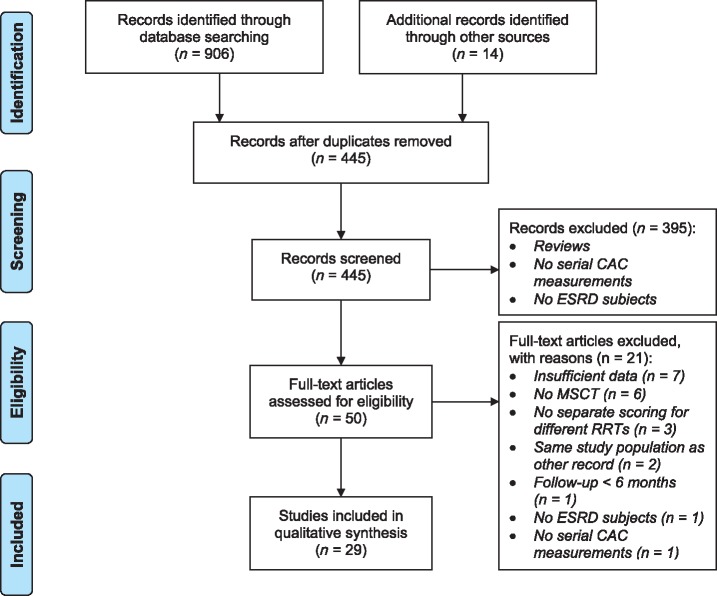
Our search yielded 445 individual citations after discarding duplicates, of which 391 were discarded after reviewing abstracts that made clear these citations did not meet eligibility criteria. An additional four citations were discarded because full texts were not available, and abstracts provided too little data. Full texts of the 50 remaining citations were examined in detail, 29 of which met inclusion criteria and were included in the systematic review. Citation tracking and hand-searching bibliographies of included publications did not bring forth citations that were unidentified by previous searches. Figure 1 displays the study screening and selection process.
Fig. 1.
Flowchart of the study screening and selection process. CAC: coronary artery calcification; ESRD: end-stage renal disease; MSCT: multi-slice computed tomography; RRT: renal replacement therapy.
Of the 29 included studies (32 unique cohorts), most focused on haemodialysis patients [10, 17–35] (20 studies, 1499 patients) or kidney transplant recipients [30, 33, 36–41] (8 studies, 649 patients), 2 of which investigated both haemodialysis patients and kidney transplant recipients [30, 33]. Three studies (92 patients) investigated peritoneal dialysis patients [29, 42, 43], one of which also investigated haemodialysis patients [29]. One study (38 patients) investigated nocturnal haemodialysis patients [44]. Six studies were randomized controlled trials [17, 22, 23, 32, 35, 36] (five evaluating pharmacological interventions, one evaluating dialysate calcium), two studies were non-randomized controlled trials [27, 34] (one of which incorporated a retrospective control group [34]) and one was a pilot study [25] (all three evaluating pharmacological interventions). The 20 other studies were observational cohort studies. Sample sizes ranged from 7 to 235 and follow-up durations ranged from 6 to 52.8 months. Study characteristics are shown in Table 1.
Table 1.
Characteristics of 29 included studies
| Author (year) | No. of patients with 2nd CAC | Follow-up (months) | CAC score at baseline (Agatston units) | CAC score at follow-up (Agatston units) | Age (years) | Male (%) | Diabetes (%) | Cardiovascular disease (%) | Dialysis vintage (months) | Phosphate (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Haemodialysis | ||||||||||
| Ok (2016) [17] | 224 | 24 | 97 (IQR 0–521) | 166 (IQR 5–800) | 60 ± 14 | 56 | 26 | 22 | 51 ± 42 | 1.41 ± 0.32 |
| Barros (2016) [18] | 37 | 23.9 ± 4.7 | 267 (IQR 15–1206) | 477 (IQR 33–1524) | 60 ± 15 | 46 | 30 | 32a | 45 (IQR 21–80) | 1.85 ± 0.55 |
| Wang (2015) [19] | 42 | 48 | 259 (IQR 48–1350) | 545 (IQR 110–1761) | 64 ± 14 | 50 | 27 | 38 | 63 ± 11 | 1.53 ± 0.39 |
| Malluche (2015) [20] | 122 | 12 | 353 (IQR 20–1186) | 552 (IQR 57–1608) | 53 ± 13 | 61 | 43 | 23a | 40 (IQR 3–292) | 1.84 ± 0.52 |
| Ozkok (2013) [21] | 74 | 12 | 52 (IQR 1–767) | 120 (IQR 1–796) | 52 ± 14 | 49 | − | − | 54 (IQR 23– 96) | 1.78 ± 0.49 |
| Ohtake (2013) [22] | 52 | 6 | 1020 (range 12–8462) | 1246 (range 24–7887) | 68 ± 6 | 60 | 43 | 26 | 124 ± 48 | 1.74 ± 0.48 |
| Di Iorio (2011) [10] | 132 | 12 | 290 (IQR 10–986) | 322 (IQR 20–1019) | 65 ± 17 | 64 | 24 | 34 | <4 | 1.58 ± 0.48 |
| Kakuta (2011) [23] | 163 | 12 | 875 ± 1262 | 1014 ± 1409 | 58 ± 12 | 54 | 21 | 7 | 112 ± 88 | 1.84 ± 0.22 |
| Kurnatowska (2011) [24] | 33 | 30 | 1037 ± 1571 | 1571 ± 1705 | 54 ± 9 | − | − | 0 | 51 ± 11 | 1.84 ± 0.50 |
| Raggi (2011) [35] | 235 | 12 | 695 (p10/p90 98–1959) [n = 115] | − | 62 ±13 | 58 | 43 | 28a | 38 (p10/p90 9–105) | 1.87 ± 0.58 |
| 590 (p10/p90 71–2583) [n = 120] | ||||||||||
| Cejka (2010) [25] | 7 | 6 | 361 (range 0–5197) | 543 (range 17–5254) | 61 ± 5 | 43 | − | − | 48 ± 10 | 1.78 ± 0.24 |
| Coen (2010) [26] | 81 | 12b | 481 (1783)c | 528 (12 406)b,c | 59 ± 11 | 67 | 10 | − | 45 (100)c | 1.74 ± 0.45 |
| Adirekkiat (2010) [27] | 32 | 8.4 ±1.4 | 1008 (IQR 537–1723) | 1075 (IQR 606–1955) | 60 ± 13 | 60 | 54 | 38a | 46 ± 31 | 1.60 ± 0.39 |
| Caro (2010) [28] | 33 | 12 | 1913 | 2235 | 68 ± 11 | 73 | 30 | 42a | 43 ± 37 | − |
| Lee (2010a) [29] | 18 | 12 | 110 (433)c | 175 (543)c | 57 (17)c | 61 | 22 | − | 48 (range 12–300) | 1.94 ± 0.10 |
| Mazzaferro (2009) [30] | 30 | 24 ± 3 | 239 (IQR 8–1109) | 318 (IQR 128–1648) | 51 ± 14 | 67 | 13 | 13 | 68 ± 65 | 1.78 ± 0.61 |
| Barreto (2008) [32] | 71 | 12 | 123 (IQR 0–823) | 175 (IQR 0–994) | 47 ± 13 | 68 | 14 | − | 37 ± 25 | 2.30 ± 0.61 |
| Jung (2006) [31] | 40 | 21.6 ±4.5 | 191 (range 0–2403)d | 253 (range 0 –2745)d | 56 ± 12 | 65 | 43 | 33 | 27 (range 1–111) | 1.64 ± 0.25 |
| Moe (2004)[33] | 17 | 13.2 ±1.0 | 22 (range 0–391)d | 154 (range 0–555)d | 51 ± 8 | − | 12 | − | 80 ± 77 | 1.87 ± 0.36 |
| Nitta (2004) [34] | 56 | 12 | 1409 (range 168–8768)d [n = 35] | 1333 (range 213–7348)d [n = 35] | 62 ± 8 | 80 | 17 | − | 88 ± 69 | 1.80 ± 0.27 |
| 1303 (range 231–3133)d [n = 21] | 1462 (range 220–3450)d [n = 21] | |||||||||
| Kidney transplantation | ||||||||||
| Yazbek (2016) [36] | 100 | 12 | 0 (IQR 0–71) | 0 (IQR 0–94) | 41 ± 10 | 56 | 7 | 0 | 28 (IQR 14–66) [n = 51] 18 (IQR 9–38) [n = 49] | 0.87 ± 0.25 |
| Seyahi (2012) [37] | 150 | 33.7 ± 4.7 | 0 (IQR 0–15) | 3 (IQR 0–46) | 39 ± 11 | 67 | 5 | 0 | 16 (range 0–114) | 1.04 ± 0.16 |
| Maréchal (2012) [38] | 197 | 52.8 ± 3.4 | 110 (IQR 1–582) | 202 (IQR 8–936) | 52 ± 12 | 57 | 13 | 25 | 24 ± 24 | 1.00 ± 0.30 |
| Bargnoux (2009) [39] | 76 | 12 | 54 (range 0–4897) | 84 (range 0–4192) | 51 (range 22–66) | 62 | 7 | 27 | 35 (range 1–267) | 1.45 (range 0.77–2.79) |
| Abedi (2009) [40] | 31 | 6 | 40 ± 63 | 24 ± 40 | 38 ± 14 | 55 | − | − | 20 ± 16 | − |
| Mazzaferro (2009) [30] | 41 | 25 ± 4 | 5 (IQR 0–300) | 12 (IQR 0 – 668) | 48 ±13 | 61 | 10 | 10 | 58± 52 | 1.07 ± 0.29 |
| Oschatz (2006)[41] | 31 | 12 | 250 (range 0–3152) | 366 (range 0–4460) | 52 ±12 | 78 | 15 | 29a | 38 ± 33 | 1.04 ± 0.21 |
| Moe (2004) [33] | 23 | 15 ± 1.9 | 19 (range 0–1764)d | 44 (range 0–2801)d | − | − | − | − | − | − |
| Peritoneal dialysis | ||||||||||
| Lee (2010) [29] | 15 | 12 | 3 (824)c | 76 (1386)c | 53 (6)c | 40 | 33 | − | 24 (range 5–96) | 1.94 ± 0.32 |
| Ammirati (2007) [42] | 30 | 12 | 8 (IQR 0–136) | 20 (IQR 0–263) | 52 (range 20–70) | 45 | 35 | 29a | 24 (range 3–120) | 1.55 (range 0.87–2.87) |
| Stompór (2004) [43] | 47 | 12 | 23 (range 0–5503) | 84 (range 0–5001) | 53 ±13 | 53 | − | − | 18 (range 1–96) | 1.74 ± 0.52 |
| Nocturnal haemodialysis | ||||||||||
| Yuen (2006) [44] | 38 | 12b | 0 (IQR 0–221) | 3 (IQR 0–336)b | 43 ± 2 | 55 | 18 | − | 45 ± 10 | 1.56 ± 0.08 |
Data are reported as mean ± SD, median (IQR) or median (range). Mean CAC scores are printed in italics to indicate the defective interpretability of this summary measure.
Coronary artery disease was reported instead of cardiovascular disease.
1-year standardized CAC score was reported.
Distance between 25th and 75th percentiles.
Volume scores (mm3) instead of Agatston units.
Risk of bias assessment
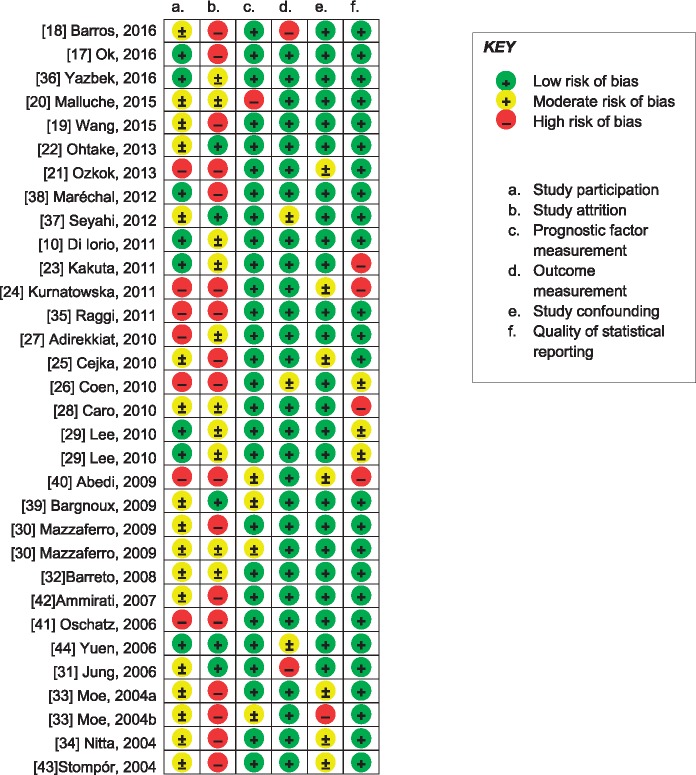
Results of the risk of bias assessment of included studies are summarized in Figure 2. Many studies did not report the patient recruitment process. Also, inherent to research in the dialysis setting, attrition rates were substantial in many studies. Frequently subjects that did not complete follow-up were different from subjects that did complete follow-up [17, 24, 32, 33, 36, 38, 41, 42] or were not described sufficiently [18, 21, 23, 25–30, 34, 36–40, 43]. In three publications, renal replacement therapy was described as ‘dialysis, not otherwise specified’. Authors of two of these publications confirmed that these cohorts concerned haemodialysis patients only [10, 30]. As the authors of the third publication did not respond, we assumed the third publication concerned haemodialysis patients exclusively as well but consequently adjudged this study a high risk of bias on the prognostic factor domain [20]. Four studies on kidney transplant recipients did not report transplant function [30, 33, 39, 40]. All but two studies reported relatively homogeneous follow-up durations [18, 31] and two studies reported CAC scores normalized for a 1-year interval assuming a linear increase in CAC [26, 44]. All studies reported CT scanning and CAC measurement procedures. CAC scores were reported in various ways, although most studies reported medians with (interquartile) ranges. Seven studies reported CAC scores as means only [23, 24, 28, 40], and upon request, authors of three of these provided median CAC scores [10, 30, 44].
Fig. 2.
Risk of bias in the 29 included studies.
CAC progression in different renal replacement therapies
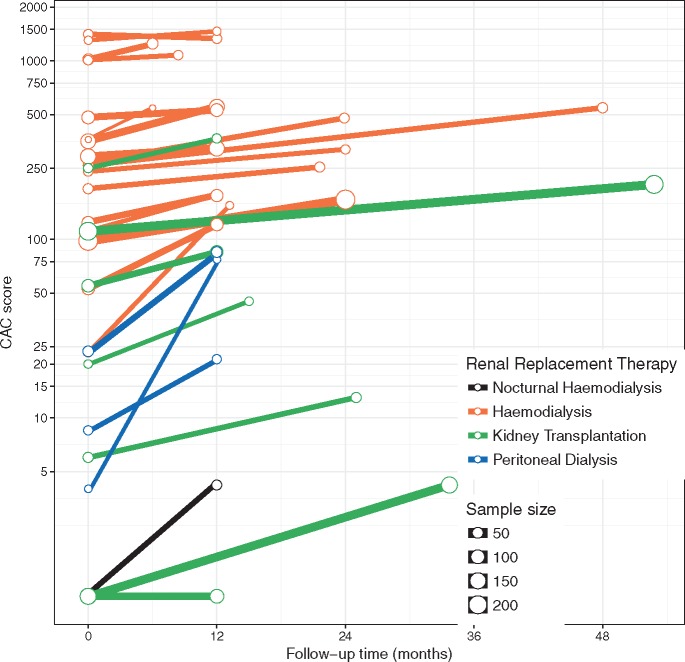
As can be seen from Figure 3, median CAC scores were high in the haemodialysis cohorts, ranging from 52 to 1409 at baseline, and progressed to a range of 120 to 1462 at follow-up. One large study on haemodialysis patients did not report CAC scores at follow-up, but reported a median increase of 94 and 149 in two strata (treatment with cinacalcet and low-dose vitamin D or flexible doses of vitamin D, respectively) over a 1-year time span [35]. Remarkably, median CAC scores regressed (1409 to 1333) in one cohort receiving bisphosphonate treatment [34]. Of note, two studies on haemodialysis included only patients with baseline CAC scores ≥30 [35] or ≥300 [27].
Fig. 3.
Median CAC score [logarithmic scale] at baseline and at follow-up in 24 studies on 18 haemodialysis cohorts (orange lines), 7 kidney transplant cohorts (green lines), 3 peritoneal dialysis cohorts (blue lines) and 1 nocturnal haemodialysis cohort (black line). CAC scores are in Agatston units (with the exception of volume scores in mm3 in three studies [31, 33, 34]). Note: As a trade-off for the logarithmic scale, slopes are not readily comparable when baseline CAC scores are disparate.
In kidney transplantation cohorts, median CAC scores were lower, ranging from 0 to 250 at baseline, and progressed slightly to a range of 0 to 366 at follow-up. However, patients in kidney transplantation cohorts were also younger (mean age per cohort ranging from 38 to 52 years) than patients in the haemodialysis cohorts (mean age per cohort ranging from 47 to 68 years) and had a shorter dialysis vintage (mean and median vintages per cohort ranging from 20 to 58 months and 16 to 35 months, respectively) than patients in the haemodialysis cohorts (mean and median vintages per cohort ranging from 3 to 124 and 27 to 54, respectively, disregarding one study on incident dialysis patients [10]). In addition, two studies on kidney transplantation exclusively included patients with no history of coronary artery disease [36, 37]. The two studies that compared CAC progression between haemodialysis and kidney transplantation reported greater CAC progression in the former group [30, 33]. Yet, haemodialysis patients and kidney transplant recipients were significantly different with regard to baseline CAC scores and other characteristics in these two studies.
In peritoneal dialysis cohorts, median CAC scores were also lower compared with haemodialysis, ranging from 3 to 23 at baseline and progressing to a range of 20 to 84 at follow-up, while median ages (range 52–53 years) and median dialysis vintages (range 18–24 months) were also low. At the same time, the one study that compared CAC progression between haemodialysis and peritoneal dialysis did not find significant differences in CAC progression [29].
Median CAC scores in the sole study on nocturnal haemodialysis increased from 0 at baseline to 3 at follow-up. Here, mean age was 43 years and mean dialysis vintage was 45 months.
Discussion
In this article we systematically reviewed the current literature on progression of CAC in different renal replacement therapies. CAC progression is observed in every study on patients with ESRD. Overall, the evidence is insufficient to determine the comparative influence of different renal replacement therapies on the progression of CAC. Although progression appears to be slower in kidney transplantation compared with haemodialysis, a proper comparison is hampered by important differences between these patient groups, i.e. lower age, shorter dialysis vintage and considerably lower baseline CAC scores in kidney transplantation cohorts. Moreover, meta-analysis was unworkable due to differences in the statistical reporting of CAC scores and progression. Based on the limited available studies, it is unclear whether CAC progression is different between peritoneal dialysis, nocturnal haemodialysis and haemodialysis.
A major limitation of this systematic review is that reporting methods of CAC scores and CAC progression are far from concordant across studies. CAC scores are highly skewed, while scores of zero are also frequent, limiting the usefulness of common transformations such as log transformation. Furthermore, a lack of consensus on the definition of CAC progression has led to various reporting methods and definitions of CAC progression, e.g. (normalized) absolute or percentage differences [17, 18, 22, 24, 25, 28, 29, 33–36, 38, 40, 41, 43], at times with varying cut-off values to define progression [10, 19, 21, 23, 37, 38, 42]; difference in square root transformed CAC [17, 20]; odds ratios of progression to higher quantiles of CAC scores [26]; the method described by Hokanson et al. [45] (change in √volume score ≥2.5 mm3) [27, 31, 37, 39] or the method described by Sevrukov et al. [46] (Agatston score change ≥4.93 × √baseline CAC score or Agatston score at follow up >11.6 when baseline CAC = 0) [30, 37]. Previous systematic reviews and meta-analyses comparing pharmacological interventions on CAC progression have used mean (percentage) annualized progression rates [47], or mean differences [9, 48], both of which yield biased results. Currently, direct comparisons of CAC scores and CAC progression across renal replacement therapies by meta-analysis are infeasible without individual participant data.
Another limitation of this review is the high risk of attrition bias in many included studies. The risk of attrition bias was high in 16 studies and moderate in 8. This possibly led to an underestimation of CAC progression since—as far as described—patients without a second CAC assessment were generally older and had higher CAC scores at baseline [24, 32, 33, 36, 38, 41, 42].
In many studies the scanning interval was ∼6–12 months. As incident haemodialysis patients with low or nil CAC develop minimal to no progression for up to 30 months [49], it is questionable if the 6–12 months follow-up time is enough to detect CAC progression effectively. Therefore we recommend adequate scanning intervals (>30 months) in future studies.
Considerable CAC progression in peritoneal dialysis was observed in three studies; then again, patients in the peritoneal dialysis cohorts had a lower age, shorter dialysis vintage and considerably lower baseline CAC scores than patients in the haemodialysis cohorts. As phosphate levels, associated with vascular calcification, are notoriously low in nocturnal haemodialysis, one would expect slow CAC progression in nocturnal haemodialysis. Indeed, the only publication on CAC progression in nocturnal haemodialysis found moderate CAC progression. All the same, the evidence to determine the comparative influence of peritoneal dialysis or nocturnal haemodialysis on CAC progression remains insufficient.
From the summary of study characteristics (Table 1) and Figure 3, it is apparent that CAC progressed remarkably more in some cohorts. Generally, mean/median dialysis vintages [21, 29, 33, 41] and mean phosphate levels [21, 29] were high in these cohorts. On the other hand, little to no progression was observed in two kidney transplantation cohorts, with low mean ages, low median dialysis vintages, low mean phosphate levels and a zero median CAC score at baseline [36, 37]. It is likely that dialysis vintage, age and phosphate levels are risk factors for the progression of CAC, which is endorsed by some of the included studies [20, 21, 33, 41–43].
Further research on the comparative influence of different renal replacement therapies on CAC progression is needed. A suitable design for future studies would be to measure CAC longitudinally in cohorts of different renal replacement therapies that are similar in characteristics such as age, sex and dialysis vintage. Furthermore, we advocate the adoption of a standardized manner of reporting CAC scores and progression. For instance, both the median (interquartile range) and quantiles should be reported for CAC scores and both the median (annualized) progression rates and odds ratios of progression to distinct categories should be reported for CAC progression.
In conclusion, CAC progresses in ESRD patients undergoing any form of renal replacement therapy. As CAC progression is a strong predictor of mortality, and likely has a causal role, it is of the utmost importance to identify interventions that can slow down CAC progression. Given the central role of phosphate in the development and progression of CAC, high-quality research is needed that compares the effects of treatments that can control phosphate levels, such as kidney transplantation, as well as intensive forms of haemodialysis, such as frequent or nocturnal haemodialysis.
Authors’ contributions
T.J., M.V. and B.J. conceived the study. T.J. and B.J. collected the data and drafted the manuscript. All authors provided critically important intellectual content and approved of the final version submitted.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Funding
T.J. is supported by a grant from the Wellerdieck-de Goede Foundation with mediation from Friends of the University Medical Centre Utrecht.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. United States Renal Data System. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2016; 67(Suppl 1): SA1–A8, S1–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moe SM, O'Neill KD, Duan D. et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int 2002; 61: 638–647 [DOI] [PubMed] [Google Scholar]
- 3. Raggi P, Boulay A, Chasan-Taber S. et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 2002; 39: 695–701 [DOI] [PubMed] [Google Scholar]
- 4. Block GA, Raggi P, Bellasi A. et al. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 2007; 71: 438–441 [DOI] [PubMed] [Google Scholar]
- 5. Zoccali C, London G.. Con: vascular calcification is a surrogate marker, but not the cause of ongoing vascular disease, and it is not a treatment target in chronic kidney disease. Nephrol Dial Transplant. 2015; 30: 352–357 [DOI] [PubMed] [Google Scholar]
- 6. Patel L, Bernard LM, Elder GJ.. Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2016;11: 232–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmer SC, Gardner S, Tonelli M. et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68: 691–702 [DOI] [PubMed] [Google Scholar]
- 8. Habbous S, Przech S, Acedillo R. et al. The efficacy and safety of sevelamer and lanthanum versus calcium-containing and iron-based binders in treating hyperphosphatemia in patients with chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant .2017; 32: 111–125 [DOI] [PubMed] [Google Scholar]
- 9. Jamal SA, Vandermeer B, Raggi P. et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet 2013; 382: 1268–1277 [DOI] [PubMed] [Google Scholar]
- 10. Di Iorio B, Nargi O, Cucciniello E. et al. Coronary artery calcification progression is associated with arterial stiffness and cardiac repolarization deterioration in hemodialysis patients. Kidney Blood Press Res 2011; 34: 180–187 [DOI] [PubMed] [Google Scholar]
- 11. Blacher J, Guerin AP, Pannier B. et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001; 38: 938–942 [DOI] [PubMed] [Google Scholar]
- 12. Nitta K, Akiba T, Uchida K. et al. Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens Res 2004; 27: 47–52 [DOI] [PubMed] [Google Scholar]
- 13. Roe P, Wolfe M, Joffe M. et al. Inflammation, coronary artery calcification and cardiovascular events in incident renal transplant recipients. Atherosclerosis 2010; 212: 589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agatston AS, Janowitz WR, Hildner FJ. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15: 827–832 [DOI] [PubMed] [Google Scholar]
- 16. Hayden JA, van der Windt DA, Cartwright JL. et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280–286 [DOI] [PubMed] [Google Scholar]
- 17. Ok E, Asci G, Bayraktaroglu S. et al. Reduction of dialysate calcium level reduces progression of coronary artery calcification and improves low bone turnover in patients on hemodialysis. J Am Soc Nephrol 2016; 27: 2475–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barros X, Dirrichs T, Koos R. et al. Epicardial adipose tissue in long-term hemodialysis patients: its association with vascular calcification and long-term development. J Nephrol 2016; 29: 241–250 [DOI] [PubMed] [Google Scholar]
- 19. Wang YN, Sun Y, Wang Y. et al. Serum S100A12 and progression of coronary artery calcification over 4 years in hemodialysis patients. Am J Nephrol 2015; 42: 4–13 [DOI] [PubMed] [Google Scholar]
- 20. Malluche HH, Blomquist G, Monier-Faugere MC. et al. High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis. J Am Soc Nephrol 2015; 26: 2534–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ozkok A, Kekik C, Karahan GE. et al. FGF-23 associated with the progression of coronary artery calcification in hemodialysis patients. BMC Nephrol 2013; 14: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohtake T, Kobayashi S, Oka M. et al. Lanthanum carbonate delays progression of coronary artery calcification compared with calcium-based phosphate binders in patients on hemodialysis: a pilot study. J Cardiovasc Pharmacol Ther 2013; 18: 439–446 [DOI] [PubMed] [Google Scholar]
- 23. Kakuta T, Tanaka R, Hyodo T. et al. Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis 2011; 57: 422–431 [DOI] [PubMed] [Google Scholar]
- 24. Kurnatowska I, Grzelak P, Kaczmarska M. et al. Serum osteoprotegerin is a predictor of progression of atherosclerosis and coronary calcification in hemodialysis patients. Nephron Clin Pract 2011; 117: c297–304 [DOI] [PubMed] [Google Scholar]
- 25. Cejka D, Kodras K, Bader T. et al. Treatment of hemodialysis-associated adynamic bone disease with teriparatide (PTH1-34): a pilot study. Kidney Blood Press Res 2010; 33: 221–226 [DOI] [PubMed] [Google Scholar]
- 26. Coen G, Pierantozzi A, Spizzichino D. et al. Risk factors of one year increment of coronary calcifications and survival in hemodialysis patients. BMC Nephrol 2010; 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adirekkiat S, Sumethkul V, Ingsathit A. et al. Sodium thiosulfate delays the progression of coronary artery calcification in haemodialysis patients. Nephrol Dial Transplant 2010; 25: 1923–1929 [DOI] [PubMed] [Google Scholar]
- 28. Caro P, Hernandez R, Delgado R.. Progression of coronary artery calcification using a multidetector CT on hemodialysis patients in one year. Dial Transplant 2010; 39: 27–32 [Google Scholar]
- 29. Lee CM, Chen PW, Leung TK. et al. Comparison of coronary artery calcification in peritoneal and hemodialysis patients. J Exp Clin Med 2011; 3: 89–92 [Google Scholar]
- 30. Mazzaferro S, Pasquali M, Taggi F. et al. Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin J Am Soc Nephrol 2009; 4: 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jung HH, Kim SW, Han H.. Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol Dial Transplant 2006; 21: 1915–1920 [DOI] [PubMed] [Google Scholar]
- 32. Barreto DV, Barreto Fde C, de Carvalho AB. et al. Phosphate binder impact on bone remodeling and coronary calcification–results from the BRiC study. Nephron Clin Pract 2008; 110: c273–283 [DOI] [PubMed] [Google Scholar]
- 33. Moe SM, O'Neill KD, Reslerova M et al.. Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant 2004; 19: 2387–2393 [DOI] [PubMed] [Google Scholar]
- 34. Nitta K, Akiba T, Suzuki K. et al. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis 2004; 44: 680–688 [PubMed] [Google Scholar]
- 35. Raggi P, Chertow GM, Torres PU. et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011; 26: 1327–1339 [DOI] [PubMed] [Google Scholar]
- 36. Yazbek DC, de Carvalho AB, Barros CS. et al. Effect of statins on the progression of coronary calcification in kidney transplant recipients. PLoS One 2016; 11: e0151797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seyahi N, Cebi D, Altiparmak MR. et al. Progression of coronary artery calcification in renal transplant recipients. Nephrol Dial Transplant 2012; 27: 2101–2107 [DOI] [PubMed] [Google Scholar]
- 38. Marechal C, Coche E, Goffin E. et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis 2012; 59: 258–269 [DOI] [PubMed] [Google Scholar]
- 39. Bargnoux AS, Dupuy AM, Garrigue V. et al. Evolution of coronary artery calcifications following kidney transplantation: relationship with osteoprotegerin levels. Am J Transplant 2009; 9: 2571–2579 [DOI] [PubMed] [Google Scholar]
- 40. Abedi SA, Tarzamni MK, Nakhjavani MR. et al. Effect of renal transplantation on coronary artery calcification in hemodialysis patients. Transplant Proc 2009; 41: 2829–2831 [DOI] [PubMed] [Google Scholar]
- 41. Oschatz E, Benesch T, Kodras K. et al. Changes of coronary calcification after kidney transplantation. Am J Kidney Dis 2006; 48: 307–313 [DOI] [PubMed] [Google Scholar]
- 42. Ammirati AL, Dalboni MA, Cendoroglo M. et al. The progression and impact of vascular calcification in peritoneal dialysis patients. Perit Dial Int 2007; 27: 340–346 [PubMed] [Google Scholar]
- 43. Stompor TP, Pasowicz M, Sulowicz W. et al. Trends and dynamics of changes in calcification score over the 1-year observation period in patients on peritoneal dialysis. Am J Kidney Dis. 2004; 44: 517–528 [PubMed] [Google Scholar]
- 44. Yuen D, Pierratos A, Richardson RM. et al. The natural history of coronary calcification progression in a cohort of nocturnal haemodialysis patients. Nephrol Dial Transplant 2006; 21: 1407–1412 [DOI] [PubMed] [Google Scholar]
- 45. Hokanson JE, MacKenzie T, Kinney G. et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol 2004; 182: 1327–1332 [DOI] [PubMed] [Google Scholar]
- 46. Sevrukov AB, Bland JM, Kondos GT.. Serial electron beam CT measurements of coronary artery calcium: Has your patient's calcium score actually changed? AJR Am J Roentgenol 2005; 185: 1546–1553 [DOI] [PubMed] [Google Scholar]
- 47. McCullough PA, Chinnaiyan KM.. Annual progression of coronary calcification in trials of preventive therapies: a systematic review. Arch Intern Med 2009; 169: 2064–2070 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Q, Li M, Lu Y. et al. Meta-analysis comparing sevelamer and calcium-based phosphate binders on cardiovascular calcification in hemodialysis patients. Nephron Clin Pract 2010; 115: c259–267 [DOI] [PubMed] [Google Scholar]
- 49. Bellasi A, Kooienga L, Block GA. et al. How long is the warranty period for nil or low coronary artery calcium in patients new to hemodialysis? J Nephrol 2009; 22: 255–262 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.