Abstract
Introduction: Abused women often report a wide range of physical and psychological symptoms that present challenges to providers. Specifically, injuries to the head or strangulation, may initiate neurological changes that contribute to central nervous system (CNS) symptoms. These symptoms are often attributed to mental health diagnoses in this population. The purpose of this analysis is to examine the prevalence of and associations between reported probable traumatic brain injury (TBI) and CNS symptoms in a sample of women of African descent.
Methods: A convenience sample of 901 women of African descent from Baltimore, MD and the US Virgin Islands, aged 18–55, was used to examine relationships among self-reported intimate partner violence (IPV), TBI, and CNS symptoms. Data were collected via Audio Computer-Assisted Self-Interview.
Results: Abused women who experienced a probable TBI were more likely to report CNS symptoms than those who did not. When controlling for demographics, IPV, and mental health symptoms, probable TBI was associated with a two point increase in CNS symptom frequency score (95% confidence interval: 1.55–2.93, p < 0.001).
Conclusions: Women who reported both probable TBI and IPV were more likely than their abused counterparts who reported no TBI to report CNS symptoms. This relationship held true even when controlling for symptoms of depression and post-traumatic stress disorder (PTSD). Clinicians working with women should be aware of TBI as a possible etiology for symptoms in abused women. Appropriate screening and treatment protocols should be designed and implemented across medical settings to improve outcomes for women who have experienced IPV and TBI.
Keywords: : intimate partner violence, traumatic brain injury, central nervous system symptoms
Introduction
Traumatic brain injury (TBI) has been defined as “an alteration in brain function, or other evidence of brain pathology, caused by an external force,” that may result in cognitive impairment.1 In the United States, there are ∼1.5 to 2 million persons that incur a TBI each year.2,3 TBIs are most often the result of falls or being struck by a person or object.4 TBI is most often categorized by severity of initial injury as measured by the Glasgow Coma Scale (GCS) into mild (GCS 13–15), moderate (GCS 9–12), and severe (GCS 3–8) subtypes.5,6 Mild TBI (mTBI) is the most underreported type of TBI as many people do not seek medical attention, often self-diagnosing with a concussion and making a false assumption that the condition will not be associated with further side effects.7 Although, attention has been given to increase the awareness and identification of mTBI, many patients are discharged from emergency department or primary care visits without adequate information regarding the risk for lasting neurologic symptoms such as headache, dizziness, or cognitive slowing. This symptom cluster along with difficulty in concentrating and emotional lability among other symptoms comprises postconcussive syndrome.8 When these symptoms do occur, referrals to appropriate resources such as neuropsychologists, neurologists, and occupational therapy may not be made.
One challenge with TBI literature is that it does not routinely differentiate the various mechanisms by which TBI can occur; specifically, physical abuse among victims of intimate partner violence (IPV) representing a subpopulation of individuals who often sustain multiple TBIs over time and reporting for many reasons may be reduced. Other populations, such as athletes and military personnel who sustain multiple mTBIs are shown to have a high risk for chronic neurological symptoms and deficits, whereas individuals with one or fewer TBIs tend to recover.9,10 Multiple mTBIs have been shown to be particularly dangerous in situations where the brain has not recovered from the first insult, and over time, the effects of cumulative mTBI has been termed chronic traumatic encephalopathy, which has been associated with depression, suicidality, and Alzheimer's-like syndromes.10 This leads us to question how mTBIs in abused women may contribute to many of the neurological symptoms that we observe in these better studied, multiple mTBI subpopulations.
IPV is a pattern of physical and/or sexual violence in the context of coercive control by an intimate or ex-intimate partner.11,12 Women may be at high risk for assault related mTBIs, as one in three women experience IPV during their lifetimes,11 and in women experiencing IPV, between 40% and 92% incur physical injuries to the head, and almost half report having experienced strangulation.13,14 For many abused women, head injuries occur multiple times, in an escalating pattern, and cognitive or psychological effects are often viewed within the context of abuse rather than as a specific medical injury.13,14 Damage to the structure and connectivity of neurons underlies functional impairments following an acute TBI. In chronic cases as has been shown in military and athlete subpopulations, it is likely that the secondary injury mechanisms, including the inflammatory response and disruption of neuronal electrophysiology result in cellular energy depletion and dysregulation of glucose metabolism that is related to long-term consequences.10,15 The cascade of secondary injury influences neuronal recovery as manifested by cognitive impairment, as well as psychological and physical health.10
While there has been evidence published, which shows associations between IPV and neurologic and neuropsychiatric symptoms consistent with postconcussive syndrome, limited work has examined this pathway through known or potential TBIs (inclusive of both blunt force injury to the head and strangulation events).14,16–18 As part of a case–control study examining the prevalence of IPV and its impact on health outcomes in women of African descent in Baltimore, MD and the US Virgin Islands (USVI), we aimed to examine the prevalence of probable TBIs and associations between probable TBIs and central nervous system (CNS) symptoms. Specific questions included examining differences in prevalence of probable TBIs and CNS symptoms between abused women with and without reported probable TBI, as well as control group reporting no lifetime IPV.
Methods
A comparative case–control study was conducted in Baltimore, MD and St. Thomas and St. Croix in the USVI to examine health outcomes of IPV. The protocol was approved by the Institutional Review Boards at both sites as well as the National Institute for Minority Health and Health Disparities. Participants were recruited from waiting rooms in primary care, prenatal, and family planning clinics. A total of five clinics were used for recruitment, one in Baltimore and four in the USVI. Data collection utilized an audio computer assisted self-interview (ACASI), which allowed for participation of women with varying levels of literacy as well as an additional level of privacy for women completing the questionnaire. Women were compensated $20 upon completion of the questionnaire. Participation was restricted to women who met the inclusion criteria of being age 18–55, reporting African American, African Caribbean, or of racial or ethnic heritage that included African descent, and reporting being in a relationship within the past 2 years. In Baltimore, inclusion was additionally limited to English-speaking participants; while in the USVI English and Spanish-speaking participants were included to more accurately reflect the site's demographic.
Case–control selection
Cases and controls were identified following a brief set of screening questions. Cases of IPV were defined as women reporting physical or sexual abuse by a current or recent (within the past 2 years) partner on the Abuse Assessment Screen (AAS),19 or current extreme controlling behaviors on the Women's Experience of Battering (WEB) tool.20 Controls were never-abused women according to their lifetime reports on the AAS who reported having an intimate partner during the past 2 years. Based on prior prevalence estimates, the number of potentially eligible controls participants was hypothesized to exceed the number of cases, thus control selection was randomized by computer programming according to the 10 to 1 ratio identified in prior prevalence estimates.21 The prevalence in the actual population was almost twice as high as predicted, resulting in a lower number of controls (n = 358) than cases (n = 543).
Measures
Variables for strangulation came from one question on the Danger Assessment (“Does he ever try to choke you?”) and one question from the Severity of Violence Against Women Scales (“In the past 12 months has your partner choked you?”).22 Head injuries were measured using six questions from the Miller Abuse Physical Symptoms and Injury Scale (MAPSAIS).23,24 Each included question from the MAPSAIS asks about past year injuries to the head or face (see Table 1 for a list of all six included injuries). These variables were dichotomized into ever or never for both injury to the head and strangulation. A combined dichotomous probable TBI variable, including participants who reported any injury to the head or strangulation event, was also created for analyses. While strangulation results in anoxic brain injury which is sometimes defined as distinct category of acquired brain injury separate from TBI, the potential neurological consequences of the two are similar.25,26 Thus, for this analysis of the similarity in mechanism (blunt force injuries and strangulation, both being external sources of traumatic injury) and sequela, we include both mechanisms in our probably TBI variable.
Table 1.
Proportion of Participants Reporting Injury(ies) to the Head or Strangulation in Cases and Controls
| Cases: reporting any lifetime IPV (n = 537) | Controls: reporting no lifetime IPV (n = 357) | p | |
|---|---|---|---|
| Head injuries with loss of consciousness | 40 (7) | 5 (1) | <0.001 |
| Broken/dislocated jaw | 20 (4) | 4 (1) | 0.02 |
| Eye injuries | 66 (12) | 17 (5) | <0.001 |
| Head injury with damage to the ear | 28 (5) | 5 (1) | 0.003 |
| Facial injuries (e.g., black eye, bloody nose) | 88 (16) | 15 (4) | <0.001 |
| Dental injuries | 70 (13) | 34 (10) | 0.11 |
| Head injurya | 168 (31) | 56 (15) | <0.001 |
| Strangulationb | 194 (36) | — | — |
Values are presented as n (%). n = 894, participants with complete MAPSAIS head injury data.
Any of the preceding six head injuries (past year reported).
Only measured in cases (lifetime reported).
IPV, intimate partner violence; MAPSAIS, Miller Abuse Physical Symptoms and Injury Scale.
CNS symptoms were measured using the MAPSAIS and included nine specific symptoms (see Table 2 for a list of all nine symptoms included in the analysis).23 For each symptom, women were asked: “How often have you had this problem in the past year for any reason?” they could respond on a Likert scale (0–4) with the choices: “never/not at all; once; a few times; many times; or every day/almost every day.” In addition to examining the proportion of women who experienced each particular symptom, we created an overall frequency score that totaled their responses to all nine symptoms, which resulted in a possible range of scores from 0 (never experienced any of the CNS symptoms in the past year) to 36 (experienced each of the CNS symptoms every day/almost every day in the past year).
Table 2.
Proportions of Participants Reporting Central Nervous System Symptoms
| Case (n = 534) | Control (n = 356) | p | Probable TBIa(n = 265) | No probable TBIa(n = 269) | p | |
|---|---|---|---|---|---|---|
| Headaches | 476 (89) | 311 (87) | 0.416 | 242 (91) | 234 (87) | 0.108 |
| Memory loss | 213 (40) | 58 (16) | <0.001 | 126 (48) | 87 (32) | <0.001 |
| Blacking out | 122 (23) | 33 (9) | <0.001 | 80 (30) | 42 (16) | <0.001 |
| Ears ringing | 224 (42) | 86 (24) | <0.001 | 132 (50) | 92 (34) | <0.001 |
| Dizzy spells | 320 (60) | 133 (37) | <0.001 | 173 (65) | 147 (55) | 0.012 |
| Seizures | 32 (6) | 6 (2) | 0.002 | 17 (6) | 15 (6) | 0.683 |
| Vision problems | 261 (49) | 116 (33) | <0.001 | 150 (57) | 111 (41) | <0.001 |
| Hearing problems | 111 (21) | 29 (8) | <0.001 | 69 (26) | 42 (16) | 0.003 |
| Difficulty concentrating | 290 (54) | 81 (23) | <0.001 | 167 (63) | 123 (46) | <0.001 |
Values are presented as n (%). n = 890, participants with complete MAPSAIS CNS data.
Among cases only.
CNS, central nervous system.
Symptoms of post-traumatic stress disorder (PTSD) and depression were measured using the 3-item Primary Care PTSD Screen (PC-PTSD)27 and the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10),28 respectively. Both screening tools have been validated in diverse, clinic-based samples.27–29 A cut-off score of 10 on the CESD-10 and 3 on the PC-PTSD were used to determine whether participants met criteria for having experienced past week symptoms of depression or lifetime symptoms of PTSD.
Analyses
Analyses were conducted using SPSS Version 22.30 Data were cleaned and reviewed for missing data, which was less than 2% for the included variables. Frequencies and descriptive statistics were used to examine all variables of interest. Chi-square analysis was used to examine differences in probable TBI and CNS symptoms between cases and controls, as well as to examine differences in CNS symptoms between cases who had experienced a probable TBI (any reported strangulation event or head/face injury—see Table 1) and those who had not. Multivariable linear regression was used to examine the effects of multiple variables on the CNS symptom frequency score. The regression model included relevant covariates available in the dataset based on prior literature.13
Results
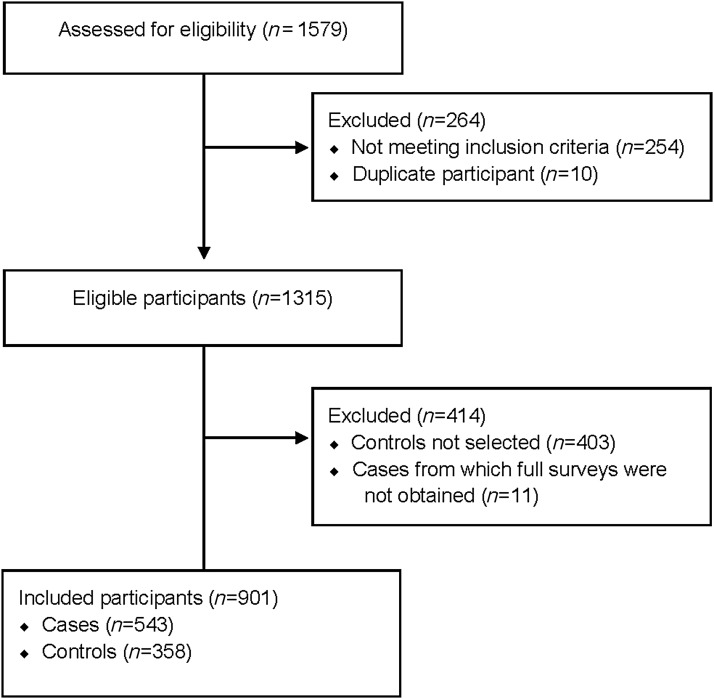
In total, 1579 women were screened for inclusion in the study, and 901 (n = 358 controls, n = 543 cases) met all eligibility criteria, were selected for participation, provided consent, and completed survey measures (Fig. 1). From the USVI, data were collected from 384 cases and 169 controls, while in Baltimore the breakdown was 159 cases and 189 controls.
FIG. 1.
Eligibility and inclusion flow diagram.
The median age of participants was 27 (IQR: 22–35, range: 18–55). There were no significant differences in age, education, monthly income, or employment between IPV cases and controls. Additional details on the demographics and site-specific differences of the sample can be found in other publications.24,31,32
Overall, 270 abused women reported at least one of the six types of head injuries inquired about in the MAPSAIS. As shown in Table 1, in all categories of head and face injuries, abused women reported more injuries relative to nonabused women. These differences were significant in all categories except dental injuries. Strangulation was only measured in women reporting IPV, and 194 women reported at least one lifetime strangulation event by an intimate partner. Reported head injuries and strangulation events were similar between the two sites. Symptoms consistent with PTSD were more commonly reported by women in the USVI than in Baltimore (16% vs. 10%, p = 0.015).
CNS symptoms were also more commonly reported among cases than controls (Table 2). Headaches were the only CNS symptom, in which a significant difference was not noted. Among cases only, each of the CNS symptoms was reported by more women who had experienced a probable TBI than those who had not. Seven out of nine symptoms reached significance, with headaches and seizures being the two that did not. CNS frequency scores in this sample ranged from 0 to 28 (median: 6; IQR: 2–10). In a linear regression model, the probable TBI variable (which included a history of lifetime strangulation and/or a past-year injury to the head) was associated with an increase in overall CNS symptom frequency score of three to four points (β: 3.76, confidence interval [95% CI]: 3.07–4.45, p < 0.001).
In a multivariable linear regression model controlling for age and site, we examined the effect of probable TBI and mental health covariates on the overall CNS symptom frequency score (Table 3), probable TBI remained significantly associated with an increase in CNS symptom frequency score of approximately two points (β: 2.24, 95% CI: 1.55–2.93, p < 0.001). In addition, lifetime IPV, PTSD, and depression were also significantly associated with increases in the CNS symptom frequency score of one to three points (β: 1.91, 95% CI: 1.20–2.62, p < 0.001; β: 1.84, 95% CI: 0.90–2.79, p < 0.001; and β: 2.82, 95% CI: 2.15–3.50, p < 0.001). There was also a statistical association between the 45 and 55-year-old (oldest) age group and an increase of CNS symptom frequency score (β: 1.12, 95% CI: −0.01 to 2.25, p = 0.05).
Table 3.
Associations of Covariates with Central Nervous System Symptom Frequency Score
| Unadjusted results | Adjusted results | ||||||
|---|---|---|---|---|---|---|---|
| n (%) | β | 95% CI | p | β | 95% CI | p | |
| TBI status | |||||||
| Probable TBI | 321 (36) | 3.76 | 3.07–4.45 | <0.001 | 2.24 | 1.55–2.93 | <0.001 |
| No reported TBI | 569 (64) | Ref. | Ref. | ||||
| IPV status | |||||||
| Any lifetime IPV exposure | 534 (60) | 3.61 | 2.93–4.29 | <0.001 | 1.91 | 1.20–2.62 | <0.001 |
| No lifetime IPV exposure | 356 (40) | Ref. | Ref. | ||||
| PC-PTSD screen | |||||||
| Positive (≥3) | 122 (14) | 4.19 | 3.21–5.18 | <0.001 | 1.84 | 0.90–2.79 | <0.001 |
| Negative (<3) | 768 (86) | Ref. | Ref. | ||||
| CES-D depression screen | |||||||
| Positive (≥10) | 342 (38) | 4.17 | 3.49–4.84 | <0.001 | 2.82 | 2.15–3.50 | <0.001 |
| Negative (<10) | 548 (62) | Ref. | Ref. | ||||
| Site | |||||||
| US Virgin Islands | 543 (61) | −0.19 | −0.92 to 0.53 | 0.60 | −0.61 | −1.26 to 0.04 | 0.07 |
| Baltimore, MD | 347 (39) | Ref. | Ref. | ||||
| Age | |||||||
| 45+ | 80 (9) | 0.83 | −0.47 to 2.14 | 0.21 | 1.12 | −0.01 to 2.25 | 0.05 |
| 35–44 | 146 (16) | 0.77 | −0.27 to 1.81 | 0.14 | 0.47 | −0.43 to 1.37 | 0.30 |
| 25–34 | 321 (36) | −0.11 | 0.93–0.71 | 0.79 | −0.50 | −1.20 to 0.21 | 0.17 |
| 18–24 | 343 (39) | Ref. | Ref. | ||||
n = 890, participants with complete MAPSAIS CNS symptom frequency data.
CESD, Center for Epidemiologic Studies-Depression; CI, confidence interval; PTSD, post-traumatic stress disorder; PC-PTSD, Primary Care-PTSD Screen; Ref, reference category; TBI, reported traumatic brain injury.
Discussion
The prevalence of reported probable TBI (from injury to the head or strangulation) in women who experienced IPV was 50% in this sample. Prior literature on the topic presents a wide range of TBI prevalence among women who have experienced IPV (40%–92%).13,33 Difficulty in determining a more specific prevalence estimate stems from challenges in measurement and sampling. Many studies of TBI in patients experiencing IPV rely on samples of victims who are seeking healthcare or advocacy services, which may not be representative of the larger population of abused women.14,34 In addition, lack of care seeking following IPV-related head injuries is common,16,17,35 limiting prospective clinical and research evaluation, thus leading to reliance on retrospective reporting in research. Similar to other studies that examined women in ED and shelter settings, having experienced a probable TBI was associated with increased reporting of CNS symptoms.16,17,35 Our findings are unique in that they were obtained from an outpatient clinic-based sample (women not currently seeking violence specific or acute care services), and that we were able to compare rates of reported CNS symptoms with abused women who had not experienced an injury to the head or strangulation event. Multivariable analysis allowed us to control for comorbid PTSD and depression symptoms, which often manifest similarly.
Implications for practice
These findings are of utmost concern to providers who (1) may not screen women for past or present IPV and (2) if they are screening for IPV, may be more likely to attribute CNS symptoms to concomitant mental health issues faced by women who have experienced IPV. This is important as TBI-related CNS symptoms require interventions that greatly differ from those of mental health symptoms. For example, neuropsychological testing, occupational and physical therapy evaluations may be sought for symptoms associated with TBI. For this reason, additional assessment and interventions are likely needed in to identify and treat women who are experiencing TBI symptoms in the context of a current or past abusive relationship. Women who have experienced IPV-related TBIs may enter the healthcare system through various avenues, including primary care, emergency departments (EDs) mental health settings, and drug and alcohol care. Incorporating brief but specific screening for TBI such as the HELPS Brain Injury Screening Tool36 may help to identify women who could benefit from referrals to neurology and brain rehabilitation specialists.37 Work in other high-risk populations has shown that TBI screening can be successfully implemented across various settings, including healthcare systems and community-based services providers.37,38 Additional emerging risk stratification techniques such as neuroimaging may help to identify women at high risk for postconcussive syndrome and may aid clinicians in decision-making.39 Partnerships with law enforcement and advocacy agencies providing services to abused women, as the first point of system contact for abused women, may further improve case-finding efforts and access to appropriate care. Concerted efforts to increase collaborating agencies' awareness about the link between IPV and mTBI may provide reciprocal long-term benefits to the justice and advocacy system in the form of better documentation of physiologic injury and improved symptomology with appropriate treatment.
While screening for TBI is an important initial step in assuring women that they receive an appropriate care, much additional work is needed to determine the short- and long-term risks and benefits of implementing screening and referral protocols. As interventions for mTBI detection and treatment continue to improve, it is important that IPV providers and mTBI experts collaborate to establish protocols that are appropriate to the needs of this population. There are a few best practice protocols that have been developed by various organizations for acute care of IPV victims of strangulation40 and a separate initiative for IPV head injury victims.41 However, these existing protocols are lacking in features to actively capture both blunt force and strangulation injuries and CNS symptoms indicative of postconcussive syndrome. It is also important that screening efforts for TBI among IPV survivors occur in nonacute settings as prior work has shown that the minority of women seek medical care following an incident of head injury or strangulation.16,17,35 While mental health disorders (PTSD and depression) were associated with increased CNS symptom reporting in this sample, the importance of mental health diagnosis, treatment, and follow-up for these patients cannot be underestimated. Attention to both physical health and physiologic mechanisms associated with their symptomology may also help to validate women's experiences with violence.
Limitations
This study relied on cross-sectional, self-reported data and not on a clinical diagnosis, neuroimaging, or validated screening tool for TBI, possibly resulting in incorrect recall by participants. Variation in reporting time frame based on measure may compound this issues, as IPV and CNS symptom measures except the strangulation variable used for this analysis were time limited to past year or past 2 years, mental health symptoms were lifetime (PTSD) or current (past 2 weeks) reports. This has the potential to lead to variation in prevalence estimates and ability to detect associations. TBI, especially, may be underestimated with the past 1-year time frame, while lifetime screening of PTSD symptoms may not be reflective of current health status. As health outcomes more broadly were the topics of the study, TBI and CNS symptoms were secondary analyses. The measures used may therefore not completely or accurately capture all potential TBI causes or CNS symptoms experienced by participants. Future research should utilize clinical records or a more formal screening and diagnostic aids to identify patients who meet the diagnostic criteria for having experienced a TBI. Previous literature suggests that this population may face multiple head injuries as well as multiple nonfatal strangulation events over time.33,42 A measure that more accurately collected information related to repeated head trauma from blunt force facial injuries (from punches, blows with weapon) and strangulation events, over time, would have been helpful in capturing any dose–response relationships between CNS symptoms and repeated TBI.
Similarly, CNS symptoms were self-reported, with no testing of neurological deficits undertaken either by physical exam or by neuropsychological testing, and the MAPSAIS has not been validated against clinical diagnosis. The implications of differences in reported symptoms using the MAPSAIS score are therefore unknown. While the MAPSAIS CNS score did not meet the normality assumption for linear regression, it was deemed the most appropriate analysis as no cut-off score has been defined in the literature. Additional covariates such as acute anxiety or stress and other neurological diagnoses would also have been ideally added to strengthen the conclusions of association of outcomes with TBI. Finally, while the sample was selected from two diverse locations in the US and USVI, it was not a population-based study, and therefore, generalizability is limited.
Strengths
This study adds to the body of evidence, suggesting that women who experience IPV are more likely to suffer from symptoms consistent with mTBI and shows an association between these symptoms and reported injuries to the head and strangulation events. While the study design and setting limit generalizability, it is unique in examining reported probable TBI occurrences and symptoms among abused women who are not currently seeking either acute medical care or specific IPV services. This study also importantly adds to the very limited literature regarding IPV experiences in Caribbean women.
Conclusions
The impact of TBI may contribute to the long-term negative outcomes of women who have experienced IPV. In this study, women reporting probable TBI and IPV had more CNS symptoms than women who had never been abused or who reported no probable TBIs within abusive relationships. Specific protocols for a variety of healthcare settings as well as for IPV service organizations for the proper identification, diagnosis, and treatment of TBIs in these women may provide opportunities to improve long-term health and functional outcomes.
Acknowledgments
Funding/Support: This work was supported by the National Institute on Minority Health and Health Disparities (P20MD002286). Dr. J.C.A received support from the National Institute of Mental Health (F31MH100995) and the National Institute of Child Health and Development (T32HD087162). The opinions, findings, conclusion, and recommendations expressed are those of the authors and do not necessarily reflect the view of the NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Menon DK, Schwab K, Wright DW, et al. Position statement: Definition of traumatic brain injury. Arch Phys Med Rehabil 2010;91:1637–1640 [DOI] [PubMed] [Google Scholar]
- 2.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and death 2002–2006. Atlanta, GA: Center for Disease Control and Prevention, 2010 [Google Scholar]
- 3.Manley GT, Maas AI. Traumatic brain injury: An international knowledge-based approach. JAMA 2013;310:473–474 [DOI] [PubMed] [Google Scholar]
- 4.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveillance Summ 2017;66:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawryluk GW, Manley GT. Classification of traumatic brain injury: Past, present, and future. Handb Clin Neurol 2015;127:15–21 [DOI] [PubMed] [Google Scholar]
- 6.Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien) 1976;34:45–55 [DOI] [PubMed] [Google Scholar]
- 7.Setnik L, Bazarian JJ. The characteristics of patients who do not seek medical treatment for traumatic brain injury. Brain Inj 2007;21:1–9 [DOI] [PubMed] [Google Scholar]
- 8.Minen MT, Boubour A, Walia H, Barr W. Post-concussive syndrome: A focus on post-traumatic headache and related cognitive, psychiatric, and sleep issues. Curr Neurol Neurosci Rep 2016;16:100. [DOI] [PubMed] [Google Scholar]
- 9.Sahler CS, Greenwald BD. Traumatic brain injury in sports: A review. Rehabil Res Pract 2012;2012:659652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136(Pt 1):43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black MC, Basile KC, Breiding MJ. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 summary report. Atlanta, GA: Center for Disease Control and Prevention, 2011 [Google Scholar]
- 12.World Health Organization. Understanding and addressing violence against women. Geneva, Switzerland: World Health Organization, 2012 [Google Scholar]
- 13.St Ivany A, Schminkey D. Intimate partner violence and traumatic brain injury: State of the science and next steps. Fam Community Health 2016;39:129–137 [DOI] [PubMed] [Google Scholar]
- 14.Kwako LE, Glass N, Campbell J, Melvin KC, Barr T, Gill JM. Traumatic brain injury in intimate partner violence: A critical review of outcomes and mechanisms. Trauma Violence Abuse 2011;12:115–126 [DOI] [PubMed] [Google Scholar]
- 15.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury—An update. Phys Med Rehabil Clin N Am 2016;27:373–393 [DOI] [PubMed] [Google Scholar]
- 16.Smith DJ, Jr., Mills T, Taliaferro EH. Frequency and relationship of reported symptomology in victims of intimate partner violence: The effect of multiple strangulation attacks. J Emerg Med 2001;21:323–329 [DOI] [PubMed] [Google Scholar]
- 17.Wilbur L, Higley M, Hatfield J, et al. Survey results of women who have been strangled while in an abusive relationship. J Emerg Med 2001;21:297–302 [DOI] [PubMed] [Google Scholar]
- 18.Valera E, Kucyi A. Brain injury in women experiencing intimate partner-violence: Neural mechanistic evidence of an “invisible” trauma. Brain Imaging Behav 2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.McFarlane J, Parker B, Soeken K, Bullock L. Assessing for abuse during pregnancy. Severity and frequency of injuries and associated entry into prenatal care. JAMA 1992;267:3176–3178 [DOI] [PubMed] [Google Scholar]
- 20.Smith PH, Earp JA, DeVellis R. Measuring battering: Development of the Women's Experience with Battering (WEB) Scale. Women's Health 1995;1:273–288 [PubMed] [Google Scholar]
- 21.Breiding MJ, Black MC, Ryan GW. Prevalence and risk factors of intimate partner violence in eighteen U.S. states/territories, 2005. Am J Prev Med 2008;34:112–118 [DOI] [PubMed] [Google Scholar]
- 22.Marshall LL. Development of the severity of violence against women scales. J Fam Violence 1992;7:103–121 [Google Scholar]
- 23.Miller CD, Campbell JC. Reliability and validity of the Miller Abuse Physical Symptom and Injury Scale (MAPSAIS). Chicago, IL: Midwest Nursing Research Society, 1993 [Google Scholar]
- 24.Anderson JC, Stockman JK, Sabri B, Campbell DW, Campbell JC. Injury outcomes in African American and African Caribbean women: The role of intimate partner violence. J Emerg Nurs 2015;41:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah MK, Carayannopoulos AG, Burke DT, Al-Adawi S. A comparison of functional outcomes in hypoxia and traumatic brain injury: A pilot study. J Neurol Sci 2007;260:95–99 [DOI] [PubMed] [Google Scholar]
- 26.Anderson CA, Arciniegas DB. Cognitive sequelae of hypoxic-ischemic brain injury: A review. NeuroRehabilitation 2010;26:47–63 [DOI] [PubMed] [Google Scholar]
- 27.Freedy JR, Steenkamp MM, Magruder KM, et al. Post-traumatic stress disorder screening test performance in civilian primary care. Fam Prac 2010;27:615–624 [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;3:385–401 [Google Scholar]
- 29.Canady RB, Stommel M, Holzman C. Measurement properties of the Centers for Epidemiological Studies Depression Scale (CES-D) in a sample of African American and non-Hispanic White pregnant women. J Nurs Meas 2009;17:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IBM SPSS Statistics for Windows [computer program]. Version 22.0. Armonk, NY: IBM Corporation, 2013 [Google Scholar]
- 31.Stockman JK, Lucea MB, Bolyard R, et al. Intimate partner violence among African American and African Caribbean women: Prevalence, risk factors, and the influence of cultural attitudes. Global Health Action 2014;7:24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabri B, Stockman JK, Bertrand DR, Campbell DW, Callwood GB, Campbell JC. Victimization experiences, substance misuse, and mental health problems in relation to risk for lethality among African American and African Caribbean women. J Interpers Violence 2013;28:3223–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson H, Philip E, Nuttal R, Diller L. Traumatic brain injury: A hidden consequence for battered women. Prof Psychol Res Pract 2002;33:39–45 [Google Scholar]
- 34.Pritchard AJ, Reckdenwald A, Nordham C. Nonfatal strangulation as part of domestic violence: A review of research. Trauma Violence Abuse 2017;18(4):407–424 [DOI] [PubMed] [Google Scholar]
- 35.Strack GB, McClane GE, Hawley D. A review of 300 attempted strangulation cases. Part I: Criminal legal issues. J Emerg Med 2001;21:303–309 [DOI] [PubMed] [Google Scholar]
- 36.Picard M, Scarisbrick D, Paluck R. HELPS brain injury screening tool. New York: International Center for the Disabled, TBI-NET, US Department of Education, Rehabilitation Services Administration, 1991 [Google Scholar]
- 37.Hux K, Schneider T, Bennett K. Screening for traumatic brain injury. Brain Inj 2009;23:8–14 [DOI] [PubMed] [Google Scholar]
- 38.Dams-O'Connor K, Cantor JB, Brown M, Dijkers MP, Spielman LA, Gordon WA. Screening for traumatic brain injury: Findings and public health implications. J Head Trauma Rehabil 2014;29:479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lui G, Ghimire P, Pang H, Wu G, Shi H. Improved sensitivity of 3.0 Tesla susceptibility-weighted imaging in detecting traumatic bleeds and its use in predicting outcomes in patients with moderate traumatic brain injury. Acta Radiol 2015;56:1256–1263 [DOI] [PubMed] [Google Scholar]
- 40.Faugno D, Waszak D, Strack GB, Brooks MA, Gwinn CG. Strangulation forensic examination: Best practice for health care providers. Adv Emerg Nurs J 2013;35:314–327 [DOI] [PubMed] [Google Scholar]
- 41.New York State Office for the Prevention of Domestic Violence. Domestic abuse and traumatic brain injury. Albany, NY.: Author. Available at: http://www.opdv.ny.gov/professionals/tbi/dvandtbiprint.pdf Accessed October24, 2017 [Google Scholar]
- 42.Valera EM, Berenbaum H. Brain injury in battered women. J Consult Clin Psychol 2003;71:797–804 [DOI] [PubMed] [Google Scholar]