Abstract
Every year, enteric infections and associated diarrhea kill millions of people. The situation is compounded by increases in the number of enteric pathogens that are acquiring resistance to antibiotics, as well as (hitherto) a relative paucity of information on host molecular targets that may contribute to diarrhea. Many forms of diarrheal disease depend on the dysregulation of intestinal ion transporters, and an associated imbalance between secretory and absorptive functions of the intestinal epithelium. A number of major transporters have been implicated in the pathogenesis of diarrheal diseases and thus an understanding of their expression, localization, and regulation after infection with various bacteria, viruses, and protozoa likely will prove critical in designing new therapies. This article surveys our understanding of transporters that are modulated by specific pathogens and the mechanism(s) involved, thereby illuminating targets that might be exploited for new therapeutic approaches.
Keywords: Ion Transport, Diarrhea, Enteric Pathogen, Epithelium
Abbreviations used in this paper: ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; cAMP, adenosine 3′,5′-cyclic monophosphate; CDI, Clostridium difficile infection; CFTR, cystic fibrosis transmembrane conductance regulator; CLCA1, chloride channel accessory 1; CT, cholera toxin; CXCR2, C-X-C motif chemokine receptor 2; DRA, down-regulated in adenoma; ENaC, epithelial sodium channel; EPEC, enteropathogenic Escherichia coli; EspG, Escherichia coli secreted protein G; ETEC, enterotoxigenic Escherichia coli; GPR39, G-protein coupled receptor 39; KCC, potassium-chloride cotransporter; LPA, lysophosphatidic acid; LT, heat-labile toxin; NHE, sodium/hydrogen exchanger; NHERF2, sodium/hydrogen exchanger regulatory factor 2; NKCC, sodium-potassium-2 chloride cotransporter; ORT, oral rehydration therapy; PKC, protein kinase C; SGLT1, sodium-glucose cotransporter 1; SLC, solute carrier; ST, heat-stabile toxin; Tcd, Clostridium difficile toxin; TNF, tumor necrosis factor; ZnR, zinc sensing receptor
Summary.
Intestinal ion transporters ensure fluid and electrolyte homeostasis. Several are modulated during enteric infections, potentially contributing to diarrhea. This review surveys changes in the abundance and/or regulation of transporters that occur in these conditions, pointing to possible novel targets for therapy.
The intestinal epithelium is responsible for absorbing nutrients, such as sugars and peptides, as well as electrolytes and water.1 Most water absorption occurs in the small intestine, with residual water absorption occurring in the colon. Absorptive processes are predominant in villi whereas secretory processes are predominant in the crypts. To facilitate solute and water absorption, the intestines rely on transporters that permit the movement of solutes through the cell membrane. Water then follows passively via both paracellular and transcellular routes. The transporters that mediate solute uptake or secretion are expressed differentially throughout the intestines, and have a wide range of substrates. Under normal conditions, the various transporters work together to provide an optimum balance between absorption and secretion, with absorption predominating to reclaim the 8–9 L of fluid that are used daily during digestion and absorption of meals in human beings. However, during pathologic states, such as infections with diarrheal pathogens, this balance is disrupted, with either increased secretion, loss of absorption, or both.1 Although the gut has a substantial reserve capacity for absorption, ultimately this imbalance can cause diarrhea.
Diarrhea is an almost ubiquitous sign of enteric infection, leading to the question of what benefit it provides for the microbe or the host. For the microbe, diarrhea presumably facilitates the colonization of additional hosts, particularly in settings in which sanitation is compromised. For the host, the diarrheal response, although potentially harmful in terms of dehydration, also may represent a primitive host defense mechanism, reducing microbial colonization and perhaps restricting cellular entry by invasive species.2 Because of its risks, diarrhea often calls for treatment in serious cases and/or particularly vulnerable hosts. However, most currently available antidiarrheal agents may have side effects, target motility rather than transport processes themselves, and often are relatively ineffective, particularly in the setting of life-threatening infectious diarrhea. There is therefore a need for new therapies, for which it is important to understand the underlying mechanism(s) of diarrhea.
Overview of Epithelial Transport Function
The transport of ions across the plasma membrane is crucial for cellular homeostasis. There are 3 major mediators of ion transport: (1) transporters (both cotransporters and exchangers), (2) ion channels, and (3) pumps.
Transporters are transmembrane proteins that mediate the transport of ions and sometimes other solutes, such as glucose or amino acids. Some also may transport drugs or metabolites. Cotransporters bind to their substrates on one side of the membrane, causing a conformational change that releases the substrates on the other side of the membrane. Exchangers transfer a solute into the cell in exchange for one that is secreted out of the cell. In either case, the activity of transporters is driven by the prevailing combined electrochemical gradients for the solutes in question.
Ion channels are pore-forming transmembrane proteins that open as gates in response to a variety of cellular signals, allowing high-capacity solute passage. The direction of ion movement depends on the electrochemical gradient for that solute across the membrane.
Pumps expend cellular energy, in the form of adenosine triphosphate (ATP) hydrolysis, and allow for uphill transport of one or more of their substrates. An example is the Na+,K+ adenosine triphosphatase (ATPase), which exports 3 sodium ions for every 2 potassium ions taken up into the cell, maintaining a low intracellular sodium concentration and sustaining the negative membrane potential.
Intestinal epithelial cells control the secretion and absorption of electrolytes through various arrangements of the ion transporters described earlier, which function together to maintain fluid balance; this fluid balance is impaired during diarrhea.1 Impairments in transporter function can occur during infections and in inflammatory diseases, or may be caused by genetic mutations.
Major Transporters Implicated in Infectious Diarrhea
Although the intestines express a large array of distinct transport proteins, only a subset have been examined for their possible contributions to infectious diarrhea (Table 1). Thus, we focus on those transporters here (Figures 1 and 2). They include the following.
-
1.
Sodium/hydrogen exchangers (NHEs): NHE3 (solute carrier [SLC]9A3) (and to a lesser extent, NHE2 [SLC9A2]) are responsible for electroneutral NaCl absorption in the small intestine and colon, by functioning in partnership with a chloride/bicarbonate exchanger.3
-
2.
Sodium/glucose cotransporter (SGLT1, SLC5A1): this transporter is responsible for the absorption of both glucose and sodium ions postprandially.4
-
3.
Down-regulated in adenoma (DRA [SLC26A3]): this transporter is a Cl-/HCO3- exchanger, and is responsible for Cl- absorption (and also transports SO42-). DRA functions in concert with NHEs in the electroneutral absorption of NaCl.5
-
4.
Epithelial sodium channel (ENaC): this channel mediates electrogenic Na+ absorption and is localized to the distal colon.6
-
5.
Ca2+-activated chloride channels7: these channels mediate the efflux of chloride ions and are activated by increases in intracellular Ca2+ concentration. Their precise molecular identity in the gut still is controversial, although one candidate is chloride channel accessory 1 (CLCA1).8 Other studies have implicated transmembrane protein 16A9, 10 (anoctamin 1), although the precise relative roles, if any, for both channels is still under investigation.
-
6.
Sodium/potassium/chloride cotransporter 1 (NKCC1 [SLC12A2]): this transporter mediates the uptake of Na+, K+, and 2Cl- ions across the basolateral membrane, and thereby supplies chloride for secretion.11
-
7.
Cystic fibrosis transmembrane conductance regulator (CFTR): this is an adenosine 3′,5′-cyclic monophosphate (cAMP)- and guanosine 3′,5′-cyclic monophosphate–regulated chloride channel present primarily at the apical surfaces of epithelial cells, and mediates chloride efflux as part of the chloride secretory mechanism.1 It also can transport bicarbonate.
-
8.
Na+,K+ ATPase: This establishes and maintains a low intracellular Na+ concentration that is a driving force for several different transport mechanisms, both secretory and absorptive.12
Table 1.
Major Ion Transporters Targeted by Enteric Infections
| Transport function | Transporter | Location | Examples of regulation by enteric pathogens |
|---|---|---|---|
| Absorption | NHEs | Apical membrane of small intestinal villus and surface epithelial cells in colon | Function of NHE2 and NHE3 decreased in response to cholera toxin29, 50 EHEC toxin Stx2 shown to prevent trafficking of NHE2 to the apical membrane40 Rotavirus decreases NHE334, 61 EPEC increases NHE2 activity24, 25 C difficile TcdB decreases NHE3 activity57, 58 |
| SGLT1 | Apical membrane of small intestinal villi108 | Function decreased by EPEC infection29, 30 Rotavirus decreases SGLT134, 61 |
|
| ENaC | Apical membrane of surface cells in distal colon | Decreased by Salmonella infection in mice17 | |
| DRA | Apical membrane of small intestinal villous cells and surface cells in colon109, 110, 111 | Decreased by EPEC, C rodentium, and Salmonella infection19, 21, 29, 34, 112 | |
| Secretion | CaCC | For CLCA1 in human beings, apical membrane of small intestinal and colonic crypt epithelial cells (and goblet cells)113 | Possibly stimulated by E histolytica, G lamblia, cholera (ACE toxin), V parahaemolyticus, and rotavirus34, 53 |
| NKCC1 | Basolateral membrane of small intestinal and colonic crypt epithelial cells114 | In cell lines and tissue ex vivo, expression increased by enteroinvasive E coli and Salmonella Dublin43 | |
| CFTR | Apical membrane of small intestinal and colonic epithelial cells with expression decreasing from crypt to villus115 | Increased activity after infection with ETEC, enteroinvasive E coli, or Salmonella Dublin; the latter also may increase expression in cell lines8, 32 | |
| Redistributed into epithelial cytosol without a change in expression after Salmonella infection in mice17 | |||
| E histolytica and norovirus increase chloride secretion34, 116, 117 | |||
| Absorption and secretion | Na+, K+ ATPase | Basolateral membrane of epithelial cells throughout the small intestine and colon | Salmonella infection in mice accompanied by redistribution from basolateral to apical membrane17 |
| Activated by the magnesium transporter C (MgtC) virulence factor of Salmonella118 |
ACE, accessory cholera enterotoxin; CaCC, Ca2+-activated chloride channel; CLCA1, chloride channel accessory 1; EHEC, enterohemorrhagic E coli; Stx2, Shiga toxin 2.
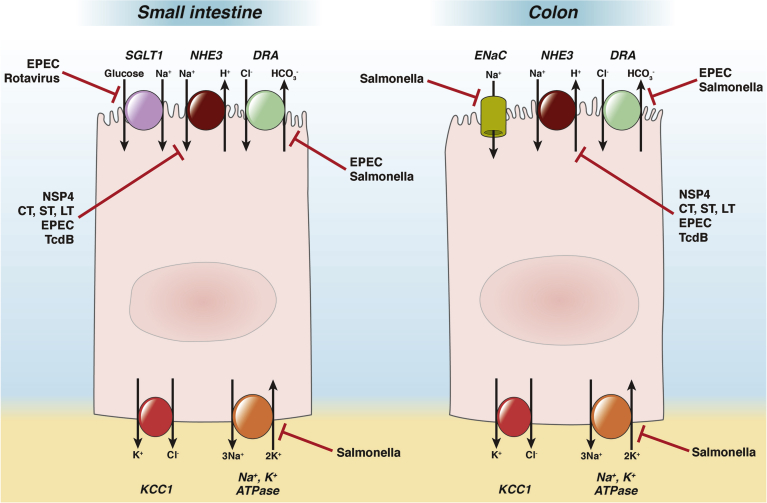
Figure 1.
Localization of absorptive ion transporters (discussed in text) in the small intestine and colon, and their regulation by pathogens or their secreted toxins. The figure is not intended to imply that the illustrated transporters are necessarily expressed in the same cells. Note particularly that ENaC is present only in the distal colon. The red bars indicate inhibitory effects. The effect shown for Salmonella on the Na+, K+ ATPase consists of mislocalization to the apical membrane that would be expected to disrupt absorptive transport; however, note that a stimulatory effect of a Salmonella effector on the ATPase also has been reported118 (not shown). CT, cholera toxin; DRA, down-regulated in adenoma; ENaC, epithelial sodium channel; EPEC, enteropathogenic E. coli; KCC1, potassium chloride cotransporter-1; NHE, sodium hydrogen exchanger; NSP4, Rotavirus non-structural protein 4; SGLT1, sodium glucose cotransporter-1; ST, heat-stable toxin of E. coli; LT, heat-labile toxin of E. coli; TcdB, C. difficile toxin B.
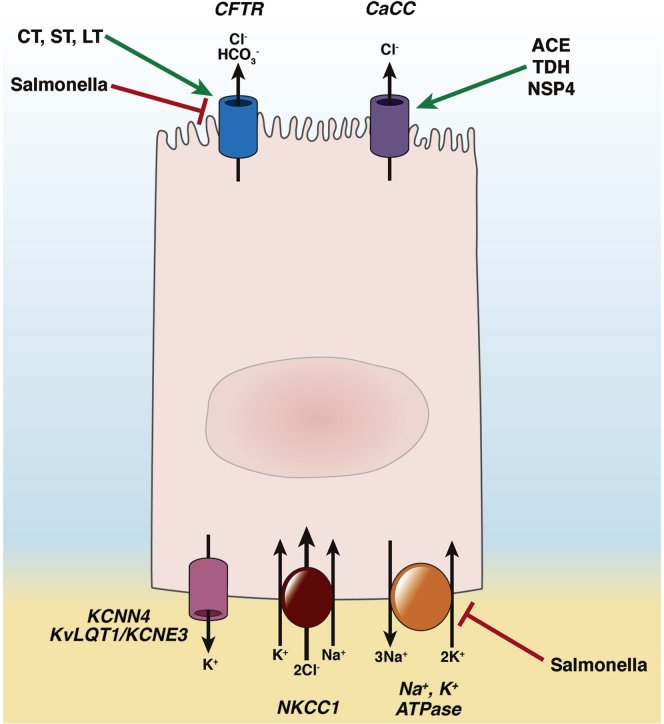
Figure 2.
Chloride secretory mechanism in the small intestine and colon, and regulation of its constituent transporters by pathogens or their secreted toxins. The green arrows and red bars represent stimulatory and inhibitory effects, respectively. CaCC, calcium-activated chloride channel; ACE, accessory cholera enterotoxin; TDH, thermostable direct hemolysin of V parahemolyticus; NSP4, rotavirus nonstructural protein-4; KCNN4, calcium-activated potassium channel; KvLQT1/KCNE3, cAMP-activated potassium channel.
Regulation of Transport
The transporters discussed earlier can be regulated in 3 main ways to effect changes in overall levels of epithelial transport.1 First, changes in transcription/translation of a given transporter will result in changes in its abundance, and associated changes in the capacity of the epithelium for transport function. Second, transport activity may be controlled by trafficking of a given transporter into or out of the plasma membrane. Finally, transporter activity may be acutely regulated by post-translational modifications, such as phosphorylation by various kinases, or may be modulated directly by intracellular second messengers such as free cytosolic calcium. Each of these mechanisms has been implicated in dysregulated transport in the setting of infection.
Epithelial Dysfunction in Diarrhea: Relative Roles of Secretion and Absorption
Intestinal epithelial cells play the key role in diarrheal pathogenesis. The epithelium is the first line of defense and host-microbe interactions are crucial in the development of infectious diarrhea. In addition to its transport functions, moreover, the epithelium also forms a barrier that may protect the host from the intrusion of microbial pathogens or toxins. Intestinal barrier dysfunction also may play a significant role in diarrheal disease (so-called leak-flux diarrhea).13, 14, 15 However, in this review, we have focused mostly on the role of ion transporters in infectious diarrhea.
A classic view of infectious diarrhea implicated direct stimulation of epithelial chloride secretion, with associated loss of fluid, as the primary driving force for diarrheal symptoms (eg, in the setting of infection with Vibrio cholerae or enterotoxigenic strains of Escherichia coli [ETEC]). However, it has become increasingly obvious that changes in electrolyte absorptive processes also are involved in disease pathogenesis. For example, an increase in epithelial cAMP not only activates chloride secretion, but also inhibits electroneutral NaCl absorption.16 Furthermore, studies with invasive pathogens such as nontyphoidal Salmonella species or enteropathogenic E coli (EPEC) have failed to uncover any evidence of active anion secretion in the setting of infection.17, 18 Rather, diarrheal disease may result from the specific suppression of absorptive transport mechanisms.17, 19
Specific Transporters Implicated in the Pathogenesis of Infectious Diarrhea
In this section, we summarize evidence for the modulation of transporters by selected bacteria, protozoa, and viruses that cause diarrheal illness. Of note, studies to date have used a variety of models, including colon cancer cell lines, tissue explants, xenografts, and whole animals (typically mice), which raises questions about the extent to which all conclusions can be extrapolated to human patients. The recent introduction of organoid models, as well as monolayers derived from these, should offer benefits in developing an enhanced understanding of diarrheal mechanisms.20
Bacterial Diarrhea
A major cause of diarrheal diseases in developing countries is infection by bacteria, such as enterotoxigenic and enteropathogenic E coli, Salmonella, Shigella, and V cholerae. Bacterial pathogens remain important causes of foodborne illness in developed countries as well.
E coli
Although many strains of E coli are harmless commensals, several are diarrheagenic, albeit with distinct mechanisms. For example, EPEC can be distinguished from other diarrheagenic E coli by its ability to form attaching/effacing lesions at its sites of attachment to the epithelium, resulting in loss of microvilli. Infection decreases the abundance of absorptive transporters in the epithelial apical membrane secondary to this disruption, but also by more rapid effects that are independent of the loss of microvilli. In particular, EPEC causes internalization of DRA from the apical surface of enterocytes and presumably causes subsequent diarrhea by inhibiting electroneutral NaCl absorption.21 EPEC uses a type III secretion system to inject bacterial effectors into host cells. The effectors E coli secreted protein G (EspG) and EspG2 cause disruption of the host microtubule network and are necessary for the internalization of DRA, although the exact mechanism is not yet known.19 EPEC also disrupts tight junctions and thereby impairs barrier function, which in turn provokes infiltration by inflammatory cells.22, 23
EPEC infection also acutely increases NHE2 activity, but decreases that of NHE3 (of note, NHE3 is the major isoform contributing to NaCl absorption in vivo in association with DRA).24, 25 Protein kinase C (PKC) is thought to be involved in EPEC-mediated up-regulation of NHE2, and the kinase also can suppress NHE3 activity, although whether this accounts for the action of EPEC needs to be investigated.26 DRA and NHE3 are thought to be coupled via NHE regulatory factor 2 (NHERF2), but the bacterial effectors required to decrease DRA after infection (EspG and EspG2) are not required for the decrease in NHE3; rather, another effector, EspF, has been implicated.27, 28 Unlike many other enteric pathogens, EPEC not only inhibits electrolyte absorptive mechanisms, but also is capable of inhibiting the activity of SGLT1.29, 30 This has been advanced as an explanation for the fact that diarrhea associated with EPEC infection is poorly responsive to oral rehydration therapy (ORT).
ETEC is a major cause of traveler’s diarrhea as well as childhood diarrhea in developing countries,31 and causes disease by elaborating toxins: heat-labile toxins (LTs), heat-stable toxins (STs), or both. LTs activate an increase in Cl- secretion mediated by CFTR via their ability to increase cAMP. STs, on the other hand, stimulate CFTR in a guanosine 3′,5′-cyclic monophosphate–dependent manner.32 The toxin also inhibits NHE3 activity by dysregulating trafficking of the transporter.33 Some studies have suggested that ST causes net secretion, whereas others have suggested that it is only anti-absorptive.34, 35 ST also may stimulate HCO3- secretion via a mechanism independent of CFTR, but with possible involvement of DRA or putative anion transporter 1.36 LT-expressing strains of ETEC, which functionally can be considered to cause diarrhea in a manner analogous to that occurring in the setting of cholera, presumably also cause a cAMP-dependent decrease in the activity and trafficking of NHEs.29
Enterohemorrhagic E coli is a highly pathogenic strain capable of causing severe bloody diarrhea as well as systemic sequelae such as endothelial injury and renal failure. Many of the effects of enterohemorrhagic E coli infection relate to its ability to elaborate Shiga toxins 1 and 2, which are known to cause epithelial apoptosis and barrier dysfunction with an associated inflammatory response, although some effects of the pathogen on tight junctions are independent of Shiga toxins.37, 38, 39 The inflammatory infiltrate is most likely to mediate the diarrheal response rather than a direct effect of infection on transporters per se, although Shiga toxin 1 also has been shown to prevent NHE2 trafficking to the apical membrane secondary to depleting intracellular galectin-3.40
Salmonella
Nontyphoidal Salmonella species, such as S enterica serovar Typhimurium, are invasive bacteria that use a type III secretion system to deliver a variety of effectors into intestinal epithelial cells. They are leading causes of foodborne diarrhea in many settings.41 They were long considered to trigger diarrhea predominantly via their ability to trigger a host inflammatory response, although they likely also exert direct effects on epithelial transport function.42 Studies in cell lines and human intestinal xenografts suggested up-regulation of both the activity and expression of NKCC1 and CFTR after infection, secondary to the ability of the bacteria to induce cyclooxygenase-2 and inducible nitric oxide synthase.17, 43 More recent studies in a murine model of Salmonella diarrhea, however, failed to show an increase in CFTR or chloride secretion.17 Rather, infection resulted in diarrhea that was associated with a reduction in DRA and ENaC expression in the proximal and distal colon, respectively, redistribution of CFTR into the epithelial cytosol, mislocalization of Na+, K+ ATPase to the apical membrane, and an expansion of the compartment expressing NKCC1.17 Furthermore, both diarrheal symptoms and down-regulation of DRA in infected mice were independent of neutrophil infiltration.44 Thus, at least initially, diarrhea in the setting of Salmonella infection may reflect a direct inhibition of electrolyte absorption in the gut, perhaps secondary to epithelial immaturity induced by effectors delivered by the type III secretion system.
Shigella
The mechanism of fluid loss after Shigella infection differs from toxigenic diarrheas caused by V cholerae and ETEC. Shigella is an invasive pathogen that also generates Shiga toxins and stimulates an inflammatory infiltrate and watery and/or bloody diarrhea. Most of the diarrheal response is caused by the toxin as well as bacterial effectors that activate inflammatory cytokines, attract polymorphonuclear cells, and activate chloride secretion.45 Infection with Shigella flexneri may generate acute dysentery, watery diarrhea, or both.46 At least in monkeys, dysentery alone was associated with diminished colonic absorption or even net colonic secretion, whereas although these were also present in the setting of diarrhea, jejunal secretion additionally was seen.47 However, the specific transporters that account for these effects have not been identified. Shiga toxin also decreased water absorption in the human colon without changing short circuit current, implying it influences an electroneutral process such as NaCl absorption,48 but the mechanism is unknown.
Vibrio
V cholerae causes diarrhea predominantly by activating net secretion of chloride ions. Its major virulence factor is cholera toxin (CT), which binds to apical GM1 receptors on host epithelial cells, thereby allowing translocation of the toxin into the cell.49 CT adenosine diphosphate-ribosylates and thereby up-regulates the activity of a guanosine triphosphatase that governs adenylate cyclase, resulting in irreversible increases in cAMP production. In turn, protein kinase A is activated and increases CFTR activity and subsequent chloride secretion.32 CT also inhibits Na+ absorption by down-regulating both NHE2 and NHE3, although through different mechanisms (post-translational vs post-transcriptional).50 cAMP is responsible for this decrease in NHE abundance, and also has an acute effect on NHE activity mediated via NHERF2.50, 51 In addition, the accessory cholera toxin stimulates CLCA.34 The overall result is an increase in both sodium and chloride ions in the lumen, leading to diarrhea. However, SGLT1 activity is unaffected, accounting for the efficacy of ORT in cholera.52 Similarly, Vibrio parahaemolyticus, frequently acquired from contaminated seafood, elaborates a thermostable direct hemolysin toxin that activates CLCA via activation of PKC and thus Ca2+-dependent Cl- secretion.53
Clostridium difficile
C difficile infection (CDI) causes often debilitating diarrhea, frequently is precipitated by the systemic use of antibiotics or acquired in health care settings, and generates health costs of $1 billion per year.54 The bacteria elaborate toxins A and B (TcdA and TcdB), as well as an additional toxin called binary toxin.55 Most of the diarrheal effects of CDI have been attributed to the ability of TcdA to trigger epithelial injury, barrier dysfunction, an inflammatory infiltrate, and activation of subepithelial elements such as nerve endings and mast cells, rather than effects on transport function.56 However, TcdB causes a pronounced inhibition of NHE3 activity in cell lines.57 Furthermore, patients with CDI have decreased NHE3 in the apical membranes of their enterocytes.58 In fact, bezlotoxumab, a monoclonal antibody that neutralizes TcdB, recently received Food and Drug Administration approval for the prevention of CDI recurrence.59
Viral Diarrhea
Important viral pathogens that cause diarrhea include rotavirus, norovirus, sapovirus, adenovirus, and astrovirus. Among these, rotavirus is the most common cause of severe diarrhea and diarrheal mortality in infants and young children worldwide.60 Rotavirus invades enterocytes of the small intestine. It produces a toxin, rotavirus nonstructural protein 4, that increases intracellular Ca2+ levels, and thereby activates Cl- secretion and decreases activity of NHE3.61 Rotavirus infection also results in malabsorption of sodium and glucose owing to decreases in SGLT1.34, 61 Interestingly, rotavirus infection also disrupts cellular Na+ and K+ homeostasis, which in theory could impair intestinal absorption, but without altering abundance of the Na+, K+ ATPase.62 However, the involvement of ion transport dysregulation in the pathogenesis of the other viral infections mentioned remains to be examined.34
Parasite-Mediated Diarrhea
Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum are common causes of water-borne diarrhea. Giardia tropozoites strongly adhere to the epithelial surface of the intestine via a ventral adhesive disc. Giardia causes a loss of the absorptive surface similar to EPEC. It decreases NaCl and glucose absorption owing to this loss of absorptive surface area.34, 63, 64 Giardia also apparently directly stimulates intestinal chloride secretion secondary to activation of PKC.34, 64, 65 Entamoeba histolytica, on the other hand, may be directly toxic to epithelial cells, resulting in barrier defects and consequent diarrhea.66 There is also evidence that the parasites can produce serotonin and prostaglandin E2, which are direct chloride secretagogues.67, 68 Finally, C parvum causes a loss of absorptive villous enterocytes in the small intestine with an associated reduction in SGLT1 abundance and function, which has been assumed to underlie diarrheal pathogenesis along with host cell prostaglandin production and diminished barrier function.69, 70, 71
Inflammatory Diarrhea
As indicated earlier, some pathogens, particularly invasive bacteria, alter the function of ion transporters by virtue of their ability to provoke a mucosal inflammatory response. In fact, there is substantial evidence that inflammation alone is sufficient to modify ion channels and other transporters.72, 73 For example, inflammatory cytokines/chemokines can influence cell proliferation and the census of ion transporters varies in less vs more differentiated epithelial cells, with a predominance of secretory transporters in the former cells. Many inflammatory cytokines also impact tight junction integrity, which indirectly alters ion transport. In ulcerative colitis, the expression of ENaC is reduced, perhaps secondary to an effect of tumor necrosis factor (TNF)-α.74 Inflammatory mediators present in Crohn’s disease also decrease ENaC expression.75 Furthermore, mice lacking the interleukin 8 receptor, C-X-C motif chemokine receptor 2 (CXCR2), are prone to severe diarrhea in the setting of infection with Citrobacter rodentium. This finding has been attributed to the role that CXCR2 plays in neutrophil recruitment; when CXCR2 signaling is absent, the numbers of luminal bacteria increase owing to impaired host defense. There is an accompanying down-regulation of CFTR and DRA, and an exacerbation of infection-mediated diarrhea.76 On the other hand, the ability of Salmonella to induce down-regulation of DRA as well as diarrhea in mice was independent of a neutrophilic infiltrate because these responses were intact in animals lacking CXCR2.44 The exact mechanisms of DRA regulation are not fully understood. Nevertheless, it is of interest that TNF-α−overexpressing (TNF+/Δ AU-rich elements (ARE)) transgenic mice with high levels of interleukin 1β and interferon-γ in both the ileum and colon showed decreased expression of DRA.77 The effect of TNF-α on DRA also has been investigated in human intestinal epithelial cells. In these studies, TNF-α activated nuclear factor-κB, which in turn reduced expression of DRA secondary to direct binding of p65 to the DRA promoter.78 This has been advanced as a contributing mechanism in inflammatory bowel disease–associated diarrhea, although the relevance of this mechanism in infectious diarrhea has yet to be studied.
Taken together, these findings therefore illustrate the complex cross-talk that may occur between infection, inflammation, and epithelial function. They likewise underscore the fact that inflammation may not always be associated with triggering a diarrheal response in the setting of infection, but rather may be protective in certain circumstances.
Implications for Treatment of Diarrhea
The discussion thus far implies that efforts to reverse infection-associated changes in transporter expression and/or function may offer new and more specific approaches to much-needed therapies for infectious diarrhea, particularly because conventional antidiarrheal agents that impair intestinal motility may be counterindicated and/or may have side effects. We discuss how this applies to various existing treatments as well as modalities that could be repurposed for therapies or are currently under development (Table 2).
Table 2.
Examples of Therapeutics Targeting Ion Transporters That Are in Use or in Development for Infectious Diarrhea
| Treatment | Transporters | Targeted pathway | Status |
|---|---|---|---|
| Natural products: cocoa-derived flavonoids Lysophosphatidic acid Red wine and green tea extracts, tannins (gelatin tannate) |
CFTR | Flavonoids inhibit chloride secretion by blocking CFTR89 | Preclinical in vitro study |
| CFTR, DRA, NHE3 | LPA inhibits intestinal chloride secretion,92 up-regulates DRA expression,104 and stimulates NHE3 activity102 | Preclinical in vitro studies as well as in mice with DSS colitis or treated with CT | |
| CaCC | Red wine and green tea extracts, resveratrol dimer, and tannic acid inhibit CaCC92, 93, 119 | Preclinical in vitro studies as well as a neonatal mouse model of rotaviral diarrhea Gelatin tannate in clinical trials |
|
| L acidophilus | NHE3 | Up-regulates NHE387, 88 | Preclinical in vitro studies |
| DRA | Up-regulates DRA86 | Preclinical in vitro studies | |
| (R)-Benzopyrimido-pyrrolo-oxazine-dione-27 | CFTR | Inhibits CFTR95 | Preclinical studies in mice treated with CT or ST, and in human enteroids |
| Thiazolidione, pyrimido-pyrrolo-quinoxalinedione/benzopyrimido-pyrrolo-oxazinedione, and glycine hydrides | CFTR | CFTR inhibitors120 | Preclinical in vitro and mouse studies |
| Crofelemer | CFTR, CaCC | Partial antagonist of CFTR, relatively strong inhibitor of CaCCs98 | Approved |
| Clotrimazole | KCNN4 | Blocks basolateral K+ channels, which prevents chloride secretion100 | Approved for antifungal use but no trials in diarrheal disease |
| Zinc | KCC1 | ZnR activation stimulates chloride absorption83 | Approved as supplement to ORS |
| Antisecretory factor, Salovum (AS-Faktor AB, Stockholm) | Undefined | Prevents intestinal fluid secretion induced by CT, ST, TcdA121 | Shown to be effective against diarrhea in small trials |
CaCC, Ca2+-activated chloride channel; DSS, dextran sulfate sodium; KCNN, calcium-activated potassium channel.
Oral Rehydration Therapy
The use of prepackaged mixtures of glucose and salt that can be dissolved and delivered orally to patients with severe, dehydrating diarrhea was one of the simplest but most impactful clinical advances of the past century, and has doubtless saved countless lives in developing countries where provisions for intravenous rehydration are absent. ORT drives water reabsorption in diseases such as cholera by taking advantage of the fact that although the electroneutral NaCl absorptive process is impaired by the disease, the function of SGLT1 is intact and can mediate sodium ion and fluid absorption if glucose is provided. This addresses acute water loss caused by diarrhea, even if it does not combat the root cause of the diarrheal episode (which is often self-limiting). Newer forms of ORT include those that incorporate starch and/or zinc.79 Starch-based ORT drives Na+ absorption by providing short-chain fatty acids in the colon,79 and has been shown to be more effective than conventional ORT. Zinc-based ORT also has been proven to be more effective than conventional ORT, but the mechanism is not fully understood. In vitro studies have suggested that zinc inhibits basolateral K+ channels,80, 81 which would prevent chloride secretion. The zinc-sensing receptor (ZnR/G-protein coupled receptor 39 (GPR39)) also may be involved in the effect of zinc and is another possible drug target.82, 83 In Caco-2 cells, activation of ZnR/GPR39 increased Cl- absorption by up-regulating K+/Cl- cotransporter (KCC1) activity.83 Conversely, knockdown of ZnR/GPR39 decreased barrier function in colonic epithelial cells.82, 84
Ion Transporters as Drug Targets
Agents currently used to combat diarrhea include antimotility agents and probiotics. Antimotility agents are useful for the traveler in combating the most distressing phase of an acute diarrheal attack, but often are contraindicated in severe infectious diarrhea because of potential adverse side effects such as paralytic ileus and ischemic colitis. Furthermore, they have no direct impact on any ion transport defects. There is, on the other hand, high-quality clinical evidence that probiotics are of value in the treatment of acute infectious diarrhea.85 There are likely several possible mechanisms underlying this beneficial effect. However, based on in vitro and animal studies, certain strains of probiotics have the potential to reduce the incidence of diarrhea via effects on ion transport, including by restoring the expression of transporters down-regulated by infection, such as DRA.86 Lactobacillus acidophilus also up-regulates intestinal NHE3 expression and function.87, 88
One strategy for antidiarrheal drug discovery is to look for active constituents of traditional remedies. Cocoa-derived flavonoids inhibit chloride secretion by blocking CFTR.89 Other examples of natural products that block enterotoxin-induced secretory diarrheas are lysophosphatidic acid90 (egg yolk, cabbage, tomato) and tannins or tannic acid91 (grape seed, oak, and tea), which inhibit smooth muscle contraction and intestinal chloride secretion.92 Similarly, 3-acyl-2-aminothiophene, tannic acid, and red wine extract also are Ca2+-activated chloride channel inhibitors derived from natural compounds that potentially could be useful in the treatment of diarrhea.93
It also remains attractive to consider that infectious diarrhea might be effectively targeted by small molecules that act specifically on transporters implicated in the disease. For example, small-molecule modulators of CFTR function could be useful not only in the treatment of cystic fibrosis, but also in secretory diarrhea.94 (R)-Benzopyrimido-pyrrolo-oxazinedione-27 inhibits CFTR and was shown to be effective in animal models of secretory diarrhea caused by cholera and E coli enterotoxins.95 Thiazolidione, pyrimido-pyrrolo-quinoxalinedione (PPQ)/benzopyrimido-pyrrolo-oxazinedione, and glycine hydrides are CFTR inhibitors that currently are being explored in this context.96, 97 Similarly, although it has not reportedly been used in infectious diarrhea in human beings, the antisecretory agent crofelemer is approved for the treatment of human immunodeficiency virus–induced diarrhea and appears to act by inhibiting chloride secretion via CFTR and CLCA.98 It also is being evaluated for the treatment of diarrhea occurring in the setting of cancer chemotherapy.99 The antifungal agent clotrimazole blocks basolateral K+ channels, which then prevents apical chloride secretion.100 NKCC1 is a potential target for antidiarrheal agents, but selective agents would be difficult to develop because of the need for the inhibitor to target NKCC1 in the intestines as opposed to NKCC2 present in the kidneys; inhibition of NKCC2 would result in a diuretic action. It also may be a drawback that NKCC1 is expressed basolaterally, and may not be readily accessible to agents delivered orally, because oral administration is attractive to avoid systemic side effects.
Finally, a novel approach would be to activate transport by NHE and/or DRA, which are targeted frequently by diarrheal pathogens, or to increase their abundance. In this regard, the probiotic L acidophilus has been shown to increase messenger RNA for DRA in colonic epithelial cell lines by transcriptional activation of its promoter.86 Similarly, lysophosphatidic acid (LPA) is a small, bioactive glycerophospholipid that regulates intestinal electrolyte transport. It stimulates NHE3 trafficking to the apical membrane and its activity,101, 102 inhibits CFTR-dependent Cl- secretion,103 and stimulates Cl-/OH- exchange, and both expression of and apical abundance of DRA in Caco-2 cells.104, 105 The actions of LPA are mediated via LPA receptors and it also increases the apical expression of the epidermal growth factor receptor.106 LPA thereby activates the mitogen-activated protein kinase (MAPK)/ERK-extracellular signal-regulated kinases (MEK-ERK) pathway, RhoA and Rho-associated kinase, and proline-rich tyrosine kinase 2. Knockdown of proline-rich tyrosine kinase 2 blocked activation of NHE3 by LPA. NHE3 activation by LPA is attributable to the LPA5 receptor and was abolished in the absence of NHERF2.102, 107 Similarly, LPA increases DRA promoter activity, which involves the LPA2 receptor and the phosphatidylinositol 3-kinase/AKT serine/threonine kinase 1 (PI3K/AKT) pathway.104 In total, these results suggest that LPA, or agents that target its receptor, might be useful as antidiarrheal agents. Indeed, LPA inhibits intestinal fluid accumulation in response to cholera toxin in mice.103
Conclusions
In conclusion, numerous enteric pathogens cause diarrhea, at least in part, by specifically targeting the expression, abundance, and/or function of ion transporters. An emerging theme in this area of research is the importance of down-regulated absorptive pathways, such as NHE3 and DRA, in diarrheal pathogenesis, in addition to the classic up-regulation of active chloride secretion that is produced by infections such as cholera. Knowledge about the molecular mechanisms of infectious diarrheal disease is already yielding possible advances in targeted therapies, as well as a rationale to repurpose existing agents. Although there may be challenges in bringing new antidiarrheal drugs to market in light of the predominant incidence of diarrheal diseases in developing countries, the societal value of such agents is clear to address the ongoing burden of diarrheal illness and its long-term sequelae.
Footnotes
Author contributions All authors participated in drafting the manuscript as well as critically reviewing the manuscript for important intellectual content.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Barrett K.E., Keely S.J. Integrative physiology and pathophysiology of intestinal electrolyte transport. In: Johnson L.R., editor. 4th ed. Vol. 1 and 2. Academic Press; San Diego: 2006. pp. 1931–1951. (Physiology of the Gastrointestinal Tract). [Google Scholar]
- 2.Tsai P.Y., Zhang B., He W.Q., Zha J.M., Odenwald M.A., Singh G., Tamura A., Shen L., Sailer A., Yeruva S., Kuo W.T., Fu Y.X., Tsukita S., Turner J.R. IL-22 upregulates epithelial claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe. 2017;21:671–681 e4. doi: 10.1016/j.chom.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato A., Romero M.F. Regulation of electroneutral NaCl absorption by the small intestine. Ann Rev Physiol. 2011;73:261–281. doi: 10.1146/annurev-physiol-012110-142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright E.M., Loo D.D., Hirayama B.A. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 5.Schweinfest C.W., Spyropoulos D.D., Henderson K.W., Kim J.H., Chapman J.M., Barone S., Worrell R.T., Wang Z., Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem. 2006;281:37962–37971. doi: 10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- 6.Kunzelmann K., Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 7.Pauli B.U., Abdel-Ghany M., Cheng H.C., Gruber A.D., Archibald H.A., Elble R.C. Molecular characteristics and functional diversity of CLCA family members. Clin Exp Pharmacol Physiol. 2000;27:901–905. doi: 10.1046/j.1440-1681.2000.03358.x. [DOI] [PubMed] [Google Scholar]
- 8.Roussa E., Wittschen P., Wolff N.A., Torchalski B., Gruber A.D., Thevenod F. Cellular distribution and subcellular localization of mCLCA1/2 in murine gastrointestinal epithelia. J Histochem Cytochem. 2010;58:653–668. doi: 10.1369/jhc.2010.955211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ousingsawat J., Mirza M., Tian Y., Roussa E., Schreiber R., Cook D.I., Kunzelman K. Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflugers Arch. 2011;461:579–589. doi: 10.1007/s00424-011-0947-0. [DOI] [PubMed] [Google Scholar]
- 10.Ousingsawat J., Martins J.R., Schreiber R., Rock J.R., Harfe B.D., Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J Biol Chem. 2009;284:28698–28703. doi: 10.1074/jbc.M109.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Andrea L., Lytle C., Matthews J.B., Hofman P., Forbush B., 3rd, Madara J.L. Na:K:2Cl cotransporter (NKCC) of intestinal epithelial cells. Surface expression in response to cAMP. J Biol Chem. 1996;271:28969–28976. doi: 10.1074/jbc.271.46.28969. [DOI] [PubMed] [Google Scholar]
- 12.Robinson J.D., Flashner M.S. The (Na+ + K+)-activated ATPase. Enzymatic and transport properties. Biochim Biophys Acta. 1979;549:145–176. doi: 10.1016/0304-4173(79)90013-2. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M., Sellin J.H., Barrett K.E. Pathophysiology, evaluation, and management of chronic watery diarrhea. Gastroenterology. 2017;152:515–532 e2. doi: 10.1053/j.gastro.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttman J.A., Finlay B.B. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 2009;1788:832–841. doi: 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Turner J.R. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke L.L., Harline M.C. CFTR is required for cAMP inhibition of intestinal Na+ absorption in a cystic fibrosis mouse model. Am J Physiol. 1996;270:G259–G267. doi: 10.1152/ajpgi.1996.270.2.G259. [DOI] [PubMed] [Google Scholar]
- 17.Marchelletta R.R., Gareau M.G., McCole D.F., Okamoto S., Roel E., Klinkenberg R., Guiney D.G., Fierer J., Barrett K.E. Altered expression and localization of ion transporters contribute to diarrhea in mice with Salmonella-induced enteritis. Gastroenterology. 2013;145:1358–1368. doi: 10.1053/j.gastro.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hecht G., Koutsouris A., Enteropathogenic E. coli attenuates secretagogue-induced net intestinal ion transport but not Cl- secretion. Am J Physiol. 1999;276:G781–G788. doi: 10.1152/ajpgi.1999.276.3.G781. [DOI] [PubMed] [Google Scholar]
- 19.Gill R.K., Borthakur A., Hodges K., Turner J.R., Clayburgh D.R., Saksena S., Zaheer A., Ramaswamy K., Hecht G.A., Dudeja P.K. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest. 2007;117:428–437. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh V., Yang J., Chen T.E., Zachos N.C., Kovbasnjuk O., Verkman A.S., Donowitz M. Translating molecular physiology of intestinal transport into pharmacologic treatment of diarrhea: stimulation of Na+ absorption. Clin Gastroenterol Hepatol. 2014;12:27–31. doi: 10.1016/j.cgh.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gujral T., Kumar A., Priyamvada S., Saksena S., Gill R.K., Hodges K., Alrefai W.A., Hecht G.A., Dudeja P.K. Mechanisms of DRA recycling in intestinal epithelial cells: effect of enteropathogenic E. coli. Am J Physiol Cell Physiol. 2015;309:C835–C846. doi: 10.1152/ajpcell.00107.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michail S.K., Halm D.R., Abernathy F. Enteropathogenic Escherichia coli: stimulating neutrophil migration across a cultured intestinal epithelium without altering transepithelial conductance. J Pediatr Gastroenterol Nutr. 2003;36:253–260. doi: 10.1097/00005176-200302000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Spitz J., Yuhan R., Koutsouris A., Blatt C., Alverdy J., Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 24.Gawenis L.R., Stien X., Shull G.E., Schultheis P.J., Woo A.L., Walker N.M., Clarke L.L. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G776–G784. doi: 10.1152/ajpgi.00297.2001. [DOI] [PubMed] [Google Scholar]
- 25.Hecht G., Hodges K., Gill R.K., Kear F., Tyagi S., Malakooti J., Ramaswamy K., Dudeja P.K. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G370–G378. doi: 10.1152/ajpgi.00432.2003. [DOI] [PubMed] [Google Scholar]
- 26.Hodges K., Gill R., Ramaswamy K., Dudeja P.K., Hecht G. Rapid activation of Na+/H+ exchange by EPEC is PKC mediated. Am J Physiol Gastrointest Liver Physiol. 2006;291:G959–G968. doi: 10.1152/ajpgi.00274.2005. [DOI] [PubMed] [Google Scholar]
- 27.Cha B., Donowitz M. The epithelial brush border Na+/H+ exchanger NHE3 associates with the actin cytoskeleton by binding to ezrin directly and via PDZ domain-containing Na+/H+ exchanger regulatory factor (NHERF) proteins. Clin Exp Pharmacol Physiol. 2008;35:863–871. doi: 10.1111/j.1440-1681.2008.04931.x. [DOI] [PubMed] [Google Scholar]
- 28.Hodges K., Alto N.M., Ramaswamy K., Dudeja P.K., Hecht G. The enteropathogenic Escherichia coli effector protein EspF decreases sodium hydrogen exchanger 3 activity. Cell Microbiol. 2008;10:1735–1745. doi: 10.1111/j.1462-5822.2008.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viswanathan V.K., Hodges K., Hecht G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat Rev Microbiol. 2009;7:110–119. doi: 10.1038/nrmicro2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dean P., Maresca M., Schuller S., Phillips A.D., Kenny B. Potent diarrheagenic mechanism mediated by the cooperative action of three enteropathogenic Escherichia coli-injected effector proteins. Proc Natl Acad Sci U S A. 2006;103:1876–1881. doi: 10.1073/pnas.0509451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotloff K.L. The burden and etiology of diarrheal illness in developing countries. Pediatr Clin North Am. 2017;64:799–814. doi: 10.1016/j.pcl.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Golin-Bisello F., Bradbury N., Ameen N. STa and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol. 2005;289:C708–C716. doi: 10.1152/ajpcell.00544.2004. [DOI] [PubMed] [Google Scholar]
- 33.Chen T., Kocinsky H.S., Cha B., Murtazina R., Yang J., Tse C.M., Singh V., Cole R., Aronson P.S., de Jonge H., Sarker R., Donowitz M. Cyclic GMP kinase II (cGKII) inhibits NHE3 by altering its trafficking and phosphorylating NHE3 at three required sites: identification of a multifunctional phosphorylation site. J Biol Chem. 2015;290:1952–1965. doi: 10.1074/jbc.M114.590174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodges K., Gill R. Infectious diarrhea: cellular and molecular mechanisms. Gut Microbes. 2010;1:4–21. doi: 10.4161/gmic.1.1.11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaandrager A.B. Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol Cell Biochem. 2002;230:73–83. [PubMed] [Google Scholar]
- 36.Sellers Z.M., Mann E., Smith A., Ko K.H., Giannella R., Cohen M.B., Barrett K.E., Dong H. Heat-stable enterotoxin of Escherichia coli (STa) can stimulate duodenal HCO3- secretion via a novel GC-C- and CFTR-independent pathway. FASEB J. 2008;22:1306–1316. doi: 10.1096/fj.06-7540com. [DOI] [PubMed] [Google Scholar]
- 37.Roxas J.L., Koutsouris A., Bellmeyer A., Tesfay S., Royan S., Falzari K., Harris A., Cheng H., Rhee K.J., Hecht G.A. Enterohemorrhagic E. coli alters murine intestinal epithelial tight junction protein expression and barrier function in a Shiga toxin independent manner. Lab Invest. 2010;90:1152–1168. doi: 10.1038/labinvest.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philpott D.J., McKay D.M., Mak W., Perdue M.H., Sherman P.M. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect Immun. 1998;66:1680–1687. doi: 10.1128/iai.66.4.1680-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurley B.P., Thorpe C.M., Acheson D.W. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immun. 2001;69:6148–6155. doi: 10.1128/IAI.69.10.6148-6155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laiko M., Murtazina R., Malyukova I., Zhu C., Boedeker E.C., Gutsal O., O'Malley R., Cole R.N., Tarr P.I., Murray K.F., Kane A., Donowitz M., Kovbasnjuk O. Shiga toxin 1 interaction with enterocytes causes apical protein mistargeting through the depletion of intracellular galectin-3. Exp Cell Res. 2010;316:657–666. doi: 10.1016/j.yexcr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wadamori Y., Gooneratne R., Hussain M.A. Outbreaks and factors influencing microbiological contamination of fresh produce. J Sci Food Aric. 2017;97:1396–1403. doi: 10.1002/jsfa.8125. [DOI] [PubMed] [Google Scholar]
- 42.Santos R.L. Pathobiology of salmonella, intestinal microbiota, and the host innate immune response. Front Immunol. 2014;5:252. doi: 10.3389/fimmu.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Resta-Lenert S., Barrett K.E. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: role of iNOS and COX-2. Gastroenterology. 2002;122:1070–1087. doi: 10.1053/gast.2002.32372. [DOI] [PubMed] [Google Scholar]
- 44.Marchelletta R.R., Gareau M.G., Okamoto S., Guiney D.G., Barrett K.E., Fierer J. Salmonella-induced diarrhea occurs in the absence of IL-8 receptor (CXCR2)-dependent neutrophilic inflammation. J Infect Dis. 2015;212:128–136. doi: 10.1093/infdis/jiu829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sansonetti P.J., Phalipon A., Arondel J., Thirumalai K., Banerjee S., Akira S. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 46.Kotloff K.L., Riddle M.S., Platts-Mills J.A., Pavlinac P., Zaidi A.K.M. Shigellosis. Lancet. 2018;391:801–812. doi: 10.1016/S0140-6736(17)33296-8. [DOI] [PubMed] [Google Scholar]
- 47.Rout W.R., Formal S.B., Giannella R.A., Dammin G.J. Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterology. 1975;68:270–278. [PubMed] [Google Scholar]
- 48.Creydt V.P., Miyakawa M.F., Martin F., Zotta E., Silberstein C., Ibarra C. The Shiga toxin 2 B subunit inhibits net fluid absorption in human colon and elicits fluid accumulation in rat colon loops. Braz J Med Biol Res. 2004;37:799–808. doi: 10.1590/s0100-879x2004000600004. [DOI] [PubMed] [Google Scholar]
- 49.Muanprasat C., Chatsudthipong V. Cholera: pathophysiology and emerging therapeutic targets. Future Med Chem. 2013;5:781–798. doi: 10.4155/fmc.13.42. [DOI] [PubMed] [Google Scholar]
- 50.Subramanya S.B., Rajendran V.M., Srinivasan P., Nanda Kumar N.S., Ramakrishna B.S., Binder H.J. Differential regulation of cholera toxin-inhibited Na-H exchange isoforms by butyrate in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2007;293:G857–G863. doi: 10.1152/ajpgi.00462.2006. [DOI] [PubMed] [Google Scholar]
- 51.Murtazina R., Kovbasnjuk O., Chen T.E., Zachos N.C., Chen Y., Kocinsky H.S., Hogema B.M., Seidler U., de Jonge H.R., Donowitz M. NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol. 2011;301:C126–C136. doi: 10.1152/ajpcell.00311.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alam N.H., Ashraf H. Treatment of infectious diarrhea in children. Paediatr Drugs. 2003;5:151–165. doi: 10.2165/00128072-200305030-00002. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi A., Sato Y., Shiomi Y., Cantarelli V.V., Iida T., Lee M., Honda T. Mechanisms of chloride secretion induced by thermostable direct haemolysin of Vibrio parahaemolyticus in human colonic tissue and a human intestinal epithelial cell line. J Med Microbiol. 2000;49:801–810. doi: 10.1099/0022-1317-49-9-801. [DOI] [PubMed] [Google Scholar]
- 54.Kyne L., Hamel M.B., Polavaram R., Kelly C.P. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34:346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 55.Kuehne S.A., Cartman S.T., Heap J.T., Kelly M.L., Cockayne A., Minton N.P. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 56.Pothoulakis C., Lamont J.T. Microbes and microbial toxins: paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol. 2001;280:G178–G183. doi: 10.1152/ajpgi.2001.280.2.G178. [DOI] [PubMed] [Google Scholar]
- 57.Hayashi H., Szaszi K., Coady-Osberg N., Furuya W., Bretscher A.P., Orlowski J., Grinstein S. Inhibition and redistribution of NHE3, the apical Na+/H+ exchanger, by Clostridium difficile toxin B. J Gen Physiol. 2004;123:491–504. doi: 10.1085/jgp.200308979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engevik M.A., Engevik K.A., Yacyshyn M.B., Wang J., Hassett D.J., Darien B., Yacyshyn B.R., Worrell R.T. Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. Am J Physiol Gastrointest Liver Physiol. 2015;308:G497–G509. doi: 10.1152/ajpgi.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kufel W.D., Devanathan A.S., Marx A.H., Weber D.J., Daniels L.M. Bezlotoxumab: a novel agent for the prevention of recurrent Clostridium difficile infection. Pharmacotherapy. 2017;37:1298–1308. doi: 10.1002/phar.1990. [DOI] [PubMed] [Google Scholar]
- 60.Lanata C.F., Fischer-Walker C.L., Olascoaga A.C., Torres C.X., Aryee M.J., Black R.E. Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Halaihel N., Lievin V., Ball J.M., Estes M.K., Alvarado F., Vasseur M. Direct inhibitory effect of rotavirus NSP4(114-135) peptide on the Na+-D-glucose symporter of rabbit intestinal brush border membrane. J Virol. 2000;74:9464–9470. doi: 10.1128/jvi.74.20.9464-9470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.del Castillo J.R., Ludert J.E., Sanchez A., Ruiz M.C., Michelangeli F., Liprandi F. Rotavirus infection alters Na+ and K+ homeostasis in MA-104 cells. J Gen Virol. 1991;72:541–547. doi: 10.1099/0022-1317-72-3-541. [DOI] [PubMed] [Google Scholar]
- 63.Buret A.G. Pathophysiology of enteric infections with Giardia duodenalius. Parasite. 2008;15:261–265. doi: 10.1051/parasite/2008153261. [DOI] [PubMed] [Google Scholar]
- 64.Troeger H., Epple H.J., Schneider T., Wahnschaffe U., Ullrich R., Burchard G.D., Jelinek T., Zeitz M., Fromm M., Schulzke J.D. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut. 2007;56:328–335. doi: 10.1136/gut.2006.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorowara S., Ganguly N.K., Mahajan R.C., Walia B.N. Study on the mechanism of Giardia lamblia induced diarrhoea in mice. Biochim Biophys Acta. 1992;1138:122–126. doi: 10.1016/0925-4439(92)90051-n. [DOI] [PubMed] [Google Scholar]
- 66.Leroy A., Lauwaet T., De Bruyne G., Cornelissen M., Mareel M. Entamoeba histolytica disturbs the tight junction complex in human enteric T84 cell layers. FASEB J. 2000;14:1139–1146. doi: 10.1096/fasebj.14.9.1139. [DOI] [PubMed] [Google Scholar]
- 67.McGowan K., Kane A., Asarkof N., Wicks J., Guerina V., Kellum J., Baron S., Gintzler A.R., Donowitz M. Entamoeba histolytica causes intestinal secretion: role of serotonin. Science. 1983;221:762–764. doi: 10.1126/science.6308760. [DOI] [PubMed] [Google Scholar]
- 68.Dey I., Keller K., Belley A., Chadee K. Identification and characterization of a cyclooxygenase-like enzyme from Entamoeba histolytica. Proc Natl Acad Sci U S A. 2003;100:13561–13566. doi: 10.1073/pnas.1835863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X.T., Gong A.Y., Wang Y., Chen X., Lim S.S., Dolata C.E., Chen X.M. Cryptosporidium parvum infection attenuates the ex vivo propagation of murine intestinal enteroids. Physiol Rep. 2016;4:24. doi: 10.14814/phy2.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laurent F., Kagnoff M.F., Savidge T.C., Naciri M., Eckmann L. Human intestinal epithelial cells respond to Cryptosporidium parvum infection with increased prostaglandin H synthase 2 expression and prostaglandin E2 and F2alpha production. Infect Immun. 1998;66:1787–1790. doi: 10.1128/iai.66.4.1787-1790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kapel N., Huneau J.F., Magne D., Tome D., Gobert J.G. Cryptosporidiosis-induced impairment of ion transport and Na+-glucose absorption in adult immunocompromised mice. J Infect Dis. 1997;176:834–837. doi: 10.1086/517316. [DOI] [PubMed] [Google Scholar]
- 72.Yang H., Jiang W., Furth E.E., Wen X., Katz J.P., Sellon R.K., Silberg D.G., Antalis T.M., Schweinfest C.W., Wu G.D. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol. 1998;275:G1445–G1453. doi: 10.1152/ajpgi.1998.275.6.G1445. [DOI] [PubMed] [Google Scholar]
- 73.Sugi K., Musch M.W., Field M., Chang E.B. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology. 2001;120:1393–1403. doi: 10.1053/gast.2001.24045. [DOI] [PubMed] [Google Scholar]
- 74.Greig E., Sandle G.I. Diarrhea in ulcerative colitis. The role of altered colonic sodium transport. Ann N Y Acad Sci. 2000;915:327–332. doi: 10.1111/j.1749-6632.2000.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 75.Zeissig S., Bergann T., Fromm A., Bojarski C., Heller F., Guenther U., Zeitz M., Fromm M., Schulzke J.D. Altered ENaC expression leads to impaired sodium absorption in the noninflamed intestine in Crohn's disease. Gastroenterology. 2008;134:1436–1447. doi: 10.1053/j.gastro.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 76.Spehlmann M.E., Dann S.M., Hruz P., Hanson E., McCole D.F., Eckmann L. CXCR2-dependent mucosal neutrophil influx protects against colitis-associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J Immunol. 2009;183:3332–3343. doi: 10.4049/jimmunol.0900600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao F., Juric M., Li J., Riederer B., Yeruva S., Singh A.K., Zheng L., Glage S., Kollas G., Dudeja P., Tian D.A., Zhu J., Bachmann O., Seidler U. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3- secretion in murine ileocolonic inflammation. Inflamm Bowel Dis. 2012;18:101–111. doi: 10.1002/ibd.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar A., Chatterjee I., Gujral T., Alakkam A., Coffing H., Anbazhagan A.N., Borthakur A., Saksena S., Gill R.K., Alrefai W.A., Dudeja P.K. Activation of nuclear factor-kappaB by tumor necrosis factor in intestinal epithelial cells and mouse intestinal epithelia reduces expression of the chloride transporter SLC26A3. Gastroenterology. 2017;153:1338–1350 e3. doi: 10.1053/j.gastro.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Binder H.J., Brown I., Ramakrishna B.S., Young G.P. Oral rehydration therapy in the second decade of the twenty-first century. Curr Gastroenterol Rep. 2014;16:376. doi: 10.1007/s11894-014-0376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoque K.M., Rajendran V.M., Binder H.J. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2005;288:G956–G963. doi: 10.1152/ajpgi.00441.2004. [DOI] [PubMed] [Google Scholar]
- 81.Hoque K.M., Sarker R., Guggino S.E., Tse C.M. A new insight into pathophysiological mechanisms of zinc in diarrhea. Ann N Y Acad Sci. 2009;1165:279–284. doi: 10.1111/j.1749-6632.2009.04442.x. [DOI] [PubMed] [Google Scholar]
- 82.Cohen L., Sekler I., Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014;5:e1307. doi: 10.1038/cddis.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sunuwar L., Asraf H., Donowitz M., Sekler I., Hershfinkel M. The Zn2+-sensing receptor, ZnR/GPR39, upregulates colonocytic Cl(-) absorption, via basolateral KCC1, and reduces fluid loss. Biochim Biophys Acta. 2017;1863:947–960. doi: 10.1016/j.bbadis.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sunuwar L., Gilad D., Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, in health and disease. Front Biosci (Landmark Ed) 2017;22:1469–1492. doi: 10.2741/4554. [DOI] [PubMed] [Google Scholar]
- 85.Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2011;45:S149–S153. doi: 10.1097/MCG.0b013e3182257e98. [DOI] [PubMed] [Google Scholar]
- 86.Raheja G., Singh V., Ma K., Boumendjel R., Borthakur A., Gill R.K., Saksena S., Alrefai W.A., Ramaswamy K., Dudeja P.K. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol. 2010;298:G395–G401. doi: 10.1152/ajpgi.00465.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar A., Anbazhagan A.N., Coffing H., Chatterjee I., Priyamvada S., Gujral T., Saksena S., Gill R.K., Alrefair W.A., Borthakur A., Dudeja P.K. Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. Am J Physiol Gastrointest Liver Physiol. 2016;311:G817–G826. doi: 10.1152/ajpgi.00173.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh V., Raheja G., Borthakur A., Kumar A., Gill R.K., Alakkam A., Malakooti J., Dudeja P.K. Lactobacillus acidophilus upregulates intestinal NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1393–G1401. doi: 10.1152/ajpgi.00345.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schuier M., Sies H., Illek B., Fischer H. Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia. J Nutr. 2005;135:2320–2325. doi: 10.1093/jn/135.10.2320. [DOI] [PubMed] [Google Scholar]
- 90.Tanaka T., Kassai A., Ohmoto M., Morito K., Kashiwada Y., Takaishi Y., Urikura M., Morishige J., Satouchi K., Tokamura A. Quantification of phosphatidic acid in foodstuffs using a thin-layer-chromatography-imaging technique. J Agric Food Chem. 2012;60:4156–4161. doi: 10.1021/jf300147y. [DOI] [PubMed] [Google Scholar]
- 91.Chung K.T., Wong T.Y., Wei C.I., Huang Y.W., Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr. 1998;38:421–464. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- 92.Namkung W., Thiagarajah J.R., Phuan P.W., Verkman A.S. Inhibition of Ca2+-activated Cl- channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 2010;24:4178–4186. doi: 10.1096/fj.10-160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ko E.A., Jin B.J., Namkung W., Ma T., Thiagarajah J.R., Verkman A.S. Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut. 2014;63:1120–1129. doi: 10.1136/gutjnl-2013-305663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verkman A.S., Lukacs G.L., Galietta L.J. CFTR chloride channel drug discovery–inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr Pharm Des. 2006;12:2235–2247. doi: 10.2174/138161206777585148. [DOI] [PubMed] [Google Scholar]
- 95.Cil O., Phuan P.W., Gillespie A.M., Lee S., Tradtrantip L., Yin J., Tse M., Zachos N.C., Lin R., Donowitz M., Verkman A.S. Benzopyrimido-pyrrolo-oxazine-dione CFTR inhibitor (R)-BPO-27 for antisecretory therapy of diarrheas caused by bacterial enterotoxins. FASEB J. 2017;31:751–760. doi: 10.1096/fj.201600891R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayol J.M., Arbeo-Escolar A., Alarma-Estrany P., Adame-Navarrete Y., Fernandez-Represa J.A. Progesterone inhibits chloride transport in human intestinal epithelial cells. World J Surg. 2002;26:652–656. doi: 10.1007/s00268-001-0284-0. [DOI] [PubMed] [Google Scholar]
- 97.Thiagarajah J.R., Broadbent T., Hsieh E., Verkman A.S. Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology. 2004;126:511–519. doi: 10.1053/j.gastro.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 98.Tradtrantip L., Namkung W., Verkman A.S. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol. 2010;77:69–78. doi: 10.1124/mol.109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao J.J., Tan M., Pohlmann P.R., Swain S.M. HALT-D: a phase II evaluation of crofelemer for the prevention and prophylaxis of diarrhea in patients with breast cancer on pertuzumab-based regimens. Clin Breast Cancer. 2017;17:76–78. doi: 10.1016/j.clbc.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rufo P.A., Merlin D., Riegler M., Ferguson-Maltzman M.H., Dickinson B.L., Brugnara C., Alper S.L., Lencer W.I. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. Demonstration of efficacy in intact rabbit colon and in an in vivo mouse model of cholera. J Clin Invest. 1997;100:3111–3120. doi: 10.1172/JCI119866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee-Kwon W., Kawano K., Choi J.W., Kim J.H., Donowitz M. Lysophosphatidic acid stimulates brush border Na+/H+ exchanger 3 (NHE3) activity by increasing its exocytosis by an NHE3 kinase a regulatory protein-dependent mechanism. J Biol Chem. 2003;278:16494–16501. doi: 10.1074/jbc.M300580200. [DOI] [PubMed] [Google Scholar]
- 102.Lin S., Yeruva S., He P., Singh A.K., Zhang H., Chen M., Lamprecht G., de Jonge H.R., Tse M., Donowitz M., Hogema B.M., Chun J., Seidler U., Yun C.C. Lysophosphatidic acid stimulates the intestinal brush border Na+/H+ exchanger 3 and fluid absorption via LPA(5) and NHERF2. Gastroenterology. 2010;138:649–658. doi: 10.1053/j.gastro.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li C., Dandridge K.S., Di A., Marrs K.L., Harris E.L., Roy K., Jackson J.S., Makarova N.V., Fujiwara Y., Farrar P.L., Nelson D.J., Tigyi G.J., Naren A.P. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med. 2005;202:975–986. doi: 10.1084/jem.20050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singla A., Kumar A., Priyamvada S., Tahniyath M., Saksena S., Gill R.K., Alrefai W.A., Dudeja P.K. LPA stimulates intestinal DRA gene transcription via LPA2 receptor, PI3K/AKT, and c-Fos-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2012;302:G618–G627. doi: 10.1152/ajpgi.00172.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singla A., Dwivedi A., Saksena S., Gill R.K., Alrefai W.A., Ramaswamy K., Dudeja P.K. Mechanisms of lysophosphatidic acid (LPA) mediated stimulation of intestinal apical Cl-/OH- exchange. Am J Physiol Gastrointest Liver Physiol. 2010;298:G182–G189. doi: 10.1152/ajpgi.00345.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoo B.K., He P., Lee S.J., Yun C.C. Lysophosphatidic acid 5 receptor induces activation of Na+/H+ exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol. 2011;301:C1008–C1016. doi: 10.1152/ajpcell.00231.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin R., Murtazina R., Cha B., Chakraborty M., Sarker R., Chen T.E., Lin Z., Hogema B.M., de Jong H.R., Seidler U., Turner J.R., Li X., Kovbasnjuk O., Donowitz M. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology. 2011;140:560–571. doi: 10.1053/j.gastro.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wood I.S., Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- 109.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silberg D.G., Wang W., Moseley R.H., Traber P.G. The down regulated in adenoma (dra) gene encodes an intestine-specific membrane sulfate transport protein. J Biol Chem. 1995;270:11897–11902. doi: 10.1074/jbc.270.20.11897. [DOI] [PubMed] [Google Scholar]
- 111.Jacob P., Rossmann H., Lamprecht G., Kretz A., Neff C., Lin-Wu E., Gregor M., Groneberg D.A., Kere J., Seidler U. Down-regulated in adenoma mediates apical Cl-/HCO3- exchange in rabbit, rat, and human duodenum. Gastroenterology. 2002;122:709–724. doi: 10.1053/gast.2002.31875. [DOI] [PubMed] [Google Scholar]
- 112.Petri W.A., Jr., Miller M., Binder H.J., Levine M.M., Dillingham R., Guerrant R.L. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gruber A.D., Elble R.C., Ji H.L., Schreur K.D., Fuller C.M., Pauli B.U. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl- channel proteins. Genomics. 1998;54:200–214. doi: 10.1006/geno.1998.5562. [DOI] [PubMed] [Google Scholar]
- 114.Jakab R.L., Collaco A.M., Ameen N.A. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G82–G98. doi: 10.1152/ajpgi.00245.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strong T.V., Boehm K., Collins F.S. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest. 1994;93:347–354. doi: 10.1172/JCI116966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lejeune M., Moreau F., Chadee K. Prostaglandin E2 produced by Entamoeba histolytica signals via EP4 receptor and alters claudin-4 to increase ion permeability of tight junctions. Am J Pathol. 2011;179:807–818. doi: 10.1016/j.ajpath.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Troeger H., Loddenkemper C., Schneider T., Schreier E., Epple H.J., Zeitz M., Fromm M., Schulzke J.D. Structural and functional changes of the duodenum in human norovirus infection. Gut. 2009;58:1070–1077. doi: 10.1136/gut.2008.160150. [DOI] [PubMed] [Google Scholar]
- 118.Gunzel D., Kucharski L.M., Kehres D.G., Romero M.F., Maguire M.E. The MgtC virulence factor of Salmonella enterica serovar Typhimurium activates Na+, K+-ATPase. J Bacteriol. 2006;188:5586–5594. doi: 10.1128/JB.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu B., Jiang Y., Zhang B., Yang H., Ma T. Resveratrol dimer trans-ε-viniferin prevents rotaviral diarrhea in mice by inhibition of the intestinal calcium-activated chloride channel. Pharmacol Res. 2018;129:453–461. doi: 10.1016/j.phrs.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 120.Verkman A.S., Synder D., Tradtrantip L., Thiagarajah J.R., Anderson M.O. CFTR inhibitors. Curr Pharm Design. 2013;19:3529–3541. doi: 10.2174/13816128113199990321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lange S., Lonnroth I. The antisecretory factor: synthesis, anatomical and cellular distribution, and biological action in experimental and clinical studies. Int Rev Cytol. 2001;210:39–75. doi: 10.1016/s0074-7696(01)10003-3. [DOI] [PubMed] [Google Scholar]