Abstract
Neurophysiological studies in monkey have suggested that premotor and motor cortex may prepare for multiple movements simultaneously, sustained by cooperative and competitive interactions within and between the neural populations encoding different actions. Here, we investigate whether competition between alternative movement directions, manipulated in terms of number and spatial angle, is reflected in EEG measures of (pre)motor cortical activity in humans. EEG was recorded during performance of a centre-out pointing task in which response signals were preceded by cues providing prior information in the form of arrows pointing to one or more possible movement targets. Delay-period activity in (pre)motor cortex was modulated in the predicted manner by the number of possible movement directions and by the angle separating them. Response latencies, however, were determined not only by the amplitude of movement-preparatory activity, but also by differences in the duration of stimulus evaluation against the visuospatial memory of the cue, reflected in EEG potentials originating from posterior parietal cortex. Specifically, the spatial proximity of possible movement targets was processed differently by (pre)motor and posterior parietal cortex. Spatial proximity enhanced the amplitude of (pre)motor cortex preparatory activity during the delay period but delayed evaluation of the response signal in the posterior parietal cortex, thus producing opposite effects on response latency. The latter finding supports distributed control of movement decisions in the frontoparietal network, revealing a feature of distributed control that is of potential significance for the understanding of distracter effects in reaching and pointing.
Keywords: Electroencephalography, motor cortex, parietal cortex, movement preparation, neural competition, working memory
Introduction
Recent neurophysiological work in primates has suggested that movement-preparatory activity in premotor and motor cortex may represent multiple response options simultaneously. Cisek and Kalaska (2005) showed that neurons in the monkey dorsal premotor cortex (PMd) represented the directions of two potential reach targets during the delay period between cue and response signal. Similarly, Bastian et al. (2003) found that directional bias and firing rates of primary motor cortex (M1) neurons were shaped by the number of possible pointing targets. Simultaneous activation of multiple actions has also been inferred from visuomotor interference effects on reaching movements, elicited by distracters (Tipper et al. 2000). The coexistence of neural activity for different behavioral options is seen as being maintained through cooperative and competitive lateral interactions within and between the neural populations encoding the different actions (Erlhagen and Schöner 2002; Cisek 2006). Here, we used EEG measures of (pre)motor cortical activity to investigate whether, as a result of mutually suppressive interactions, concurrent activation of multiple potential actions can be revealed by manipulating the number of potential movement directions (Experiment 1) and the angle subtended between them (Experiment 2).
Lateral interactions between neural populations may not only serve to define different potential actions, but can also be conceived as the substrate through which decisions between alternative actions emerge, as implemented in some computational models (Cisek 2006; Erlhagen and Schöner 2002). Such models have successfully simulated the common finding of reaction time studies that response latencies increase with number of response alternatives. Computational models of that architecture (Cisek 2006; Erlhagen and Schöner 2002) also reproduce an exception to this rule, i.e., the finding that not only the number, but also the spatial layout of possible responses determines reaction times. For example, Bock and Eversheim (2000) found that reaction times in a 2-choice task were similar to those in a 5-choice task when the movement targets subtended the same spatial angle. The basis of these simulation results is that with more movement options (or the same number at wider angle) the activity associated with each option decreases due to mutually inhibitory interactions, thus taking more time to reach a decision threshold.
To examine whether the number and spatial angle of possible movement directions is reflected in delay-period preparatory activity, we adopted a movement precuing task of Bastian et al. (2003) with centre-out pointing movements, modified for EEG. Results demonstrated that preparatory activity originating in premotor and motor cortex was modulated in a manner consistent with the preparation of multiple prospective movements. Response latencies were also determined, however, by visuospatial processing effects manifested in event-related potentials (ERPs) from the posterior parietal cortex. Specifically, the spatial proximity of possible movement targets was processed differently by (pre)motor and posterior parietal cortex, mediating opposite effects on response latency. These dissociable effects, mediated by prospective movement- and retrospective visuospatial representations, indicate that spatial processing in the posterior parietal cortex can influence arm movement independent of the preparation accomplished by the premotor cortex, consistent with distributed control of reaching in frontoparietal cortex.
Materials and methods
Participants
Experiment 1 had 16 participants (9 male; age 30 ± 6 yr), fourteen of whom were right-handed (by self-report). Experiment 2 had 16 participants (11 male; age 35 ± 7 yr), all right-handed. All had normal or corrected-to-normal vision. Data of 5 further participants (2 for Experiment 1 and 3 for Experiment 2) were excluded because of excessive artifacts. All participants provided their informed consent after full explanation of the study. The study had been approved by the South Birmingham Research Ethics Committee.
Procedure and stimuli
The experimental paradigm was a cued choice-response task with stimuli presented on a computer screen and responses made by pointing movements with the index finger. In both experiments, prior information about the pointing direction was provided in three cue conditions:
Experiment 1
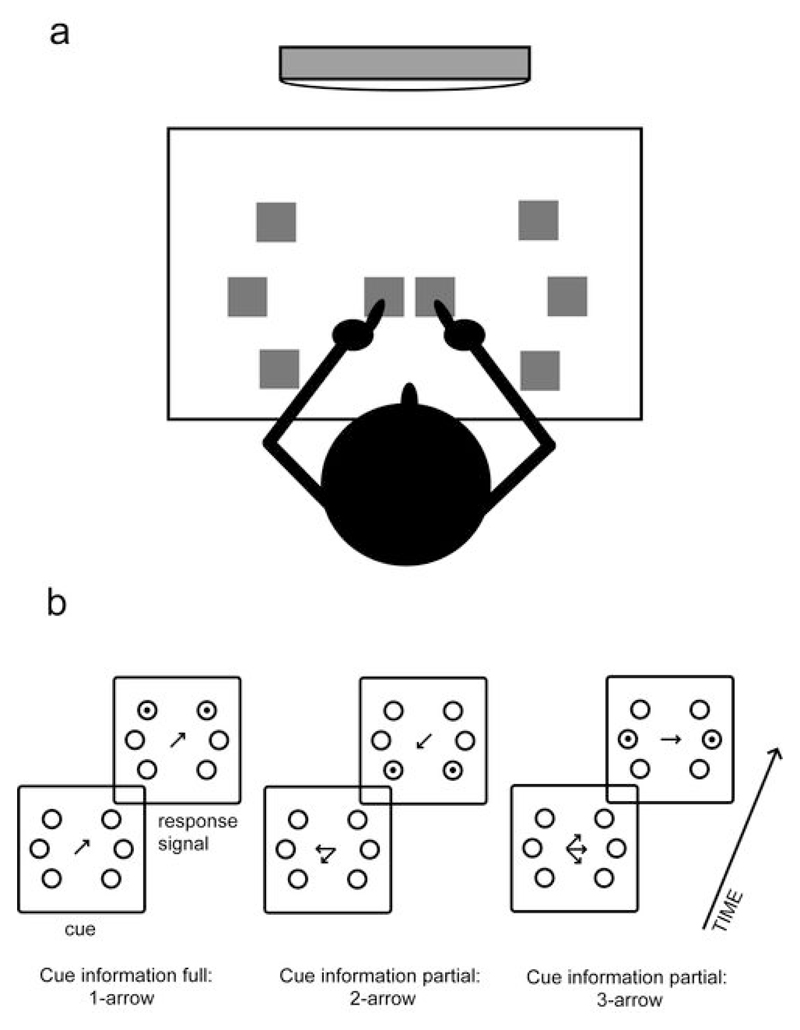
The “1-arrow” cue condition gave full information regarding the response by means of a single arrow presented at fixation, indicating the correct pointing direction. In the “2-arrow” (partial information) condition, a pair of arrows indicated two possible movement directions, always to adjacent targets. In the “3-arrow” (partial information) condition, three arrows indicated three possible movement directions to adjacent targets (see Figure 1b).
Figure 1.
(a) Schematic drawing of centre-out pointing task with central home positions (for left and right index finger) and peripheral targets. (b) Sample cue and response stimulus pairs for 1-arrow, 2-arrow, and 3-arrow conditions in Experiment 1. The delay period between cue and response signal was 1.2 second. Drawings are not accurately scaled; for accurate dimensions see Methods.
Experiment 2
Here, the three cue conditions consisted of the “3-arrow” condition and two 2-arrow conditions. In the “2-arrow-s(mall)” condition, a pair of arrows indicated two possible movement directions to adjacent targets (separation 45°), similar as in Experiment 1. In the “2-arrow-w(ide)” condition, the two possible movement directions were separated by 90°, to targets that were non-adjacent.
In both experiments, the cue was followed by a response stimulus which specified the target by means of a single arrow of the same shape and size as the cue arrows. The six possible pointing targets were arranged on a circle and were permanently displayed on the computer screen. Responses were made by sliding the index finger from a central home position to a peripheral target position on a flat surface in front of the participant. The left hand responded to targets on the left and the right hand to targets on the right. Note that in the task used by Bastian et al. (2003), which we adapted, the arrangement of targets was similar, but monkeys pointed with the same hand to all targets. The sole reason for our adaptation was to optimize the extraction of movement-related EEG activity in the form of lateralized potentials (see below).
The permanently displayed configuration of six possible pointing targets measured 3.7° by 2.7° of visual angle, with the targets lying on a circle of 1.7° of visual angle radius. The targets were indicated by circles of 0.3° of visual angle radius. The angular distance between targets (on each side of fixation) was 45°. Cue and response arrow stimuli were very small (0.6° of visual angle) and were centred on the middle of the screen (instead of having their origin at the centre), in order to minimize the asymmetry of the stimulus. The response arrow stimulus was accompanied by a white dot placed in the relevant target circle, along with a similar dot in the homologous target on the opposite side to maintain a symmetrical display. The stimuli were presented in white against a grey background. The stimulus display with six possible targets was represented in an enlarged size on the response surface, where it measured 26 by 16 cm (see Figure 1a). Here, the targets consisted of 3 x 3 cm squares of duct tape that were easily recognized by touch when subjects slid their finger from home position to target. Since pointing movements to the left and right targets were performed with the left and right index finger, respectively, home positions for the left and right index finger were immediately adjacent instead of exactly on the centre, each consisting of 3 x 3 cm squares of duct tape similar to the targets. The response surface was aligned to the participants’ body midline.
The experiments were run in a quiet, normally illuminated room and consisted of 12 blocks of ~5 minutes duration each, preceded by a practice block. Each block consisted of 72 trials with equal numbers of trials for the different cue conditions and for the left and right hand. Trial order was randomized across conditions and response hand. The interval between trials (onset to onset) was 3400 ms and the cue-response signal delay (delay period duration) was 1200 ms. Cue and response stimuli were presented for 200 ms each.
The participants were seated comfortably in an armchair, with the computer monitor at 1 m distance. Throughout each block of the experiment, the participants held their left and right index finger on the respective home positions on the response surface. They were instructed to maintain fixation on the centre of the screen, where the arrows were displayed, and not to look for the highlighted targets, which only served to better distinguish between cue and response signals. As soon as the response signal appeared, they moved their finger to the target and returned immediately to the home position. To encourage fast responding, the participants were allowed to overshoot the target instead of landing exactly on it.
Data acquisition
In Experiment 1, movements were recorded with a motion tracking system (ProReflex MCU 240, Qualisys AB, Sweden). Two retro-reflective markers were attached on the nails of the participants’ index fingers. The movement of the markers was tracked by three cameras capturing data from 500 ms before to 1500 ms after the onset of each response stimulus at a sampling rate of 200 Hz. In Experiment 2, only response latencies were measured, using force sensitive resistors under the home keys.
EEG was recorded continuously with Ag/AgCl electrodes from 130 scalp electrodes relative to CMS and DRL electrodes adjacent to the vertex electrode location Cz. The electrodes were placed according to the 10-5 extension of the International 10-20 electrode system (Oostenveld and Praamstra 2001) using a carefully positioned nylon cap. Vertical and horizontal eye movements were monitored using EOG electrodes positioned under the left and right eye and lateral to the left and right eye. EEG and EOG signals were amplified with a band-pass of 0-128 Hz by BioSemi ActiveTwo amplifiers and sampled at 512 Hz.
Data processing
Motion tracking data
For Experiment 1, reaction times were calculated on the basis of a velocity threshold of 80 mm/sec, which was taken as movement onset. Trials where participants moved their fingers during the delay period, trials where bimanual movement was recorded, and trials where a hand moved towards the incorrect side of the response surface were rejected. The criterion for erroneous movement was 2 cm deviation from the starting point. For both experiments, trials with reaction times shorter than 100 ms or longer than 800 ms were rejected.
EEG: movement- and event-related potentials
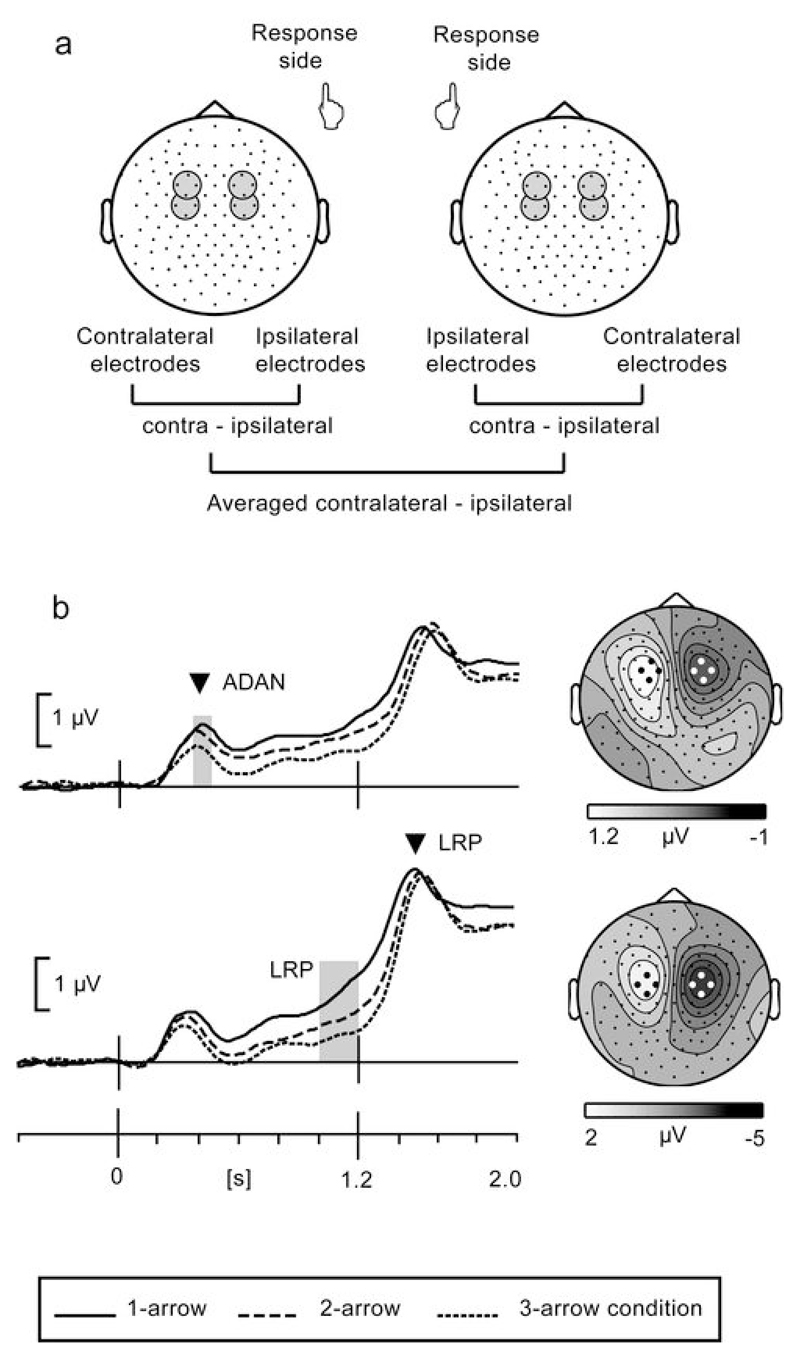
The continuous EEG data were re-referenced to an averaged mastoids reference and segmented in epochs from 500 ms before to 2000 ms after the cue stimulus. Trials containing eye-movements and other artifacts were removed before averaging using individually tailored artifact thresholds. To ensure that lateralized movement-related potentials were not contaminated by horizontal eye-movement artifacts, individual subject averages were checked, after artifact removal, to have no horizontal EOG differences > 4 μV between left and right cue conditions at any time point during the delay period (Kennett et al. 2007). The baseline was defined as the time period from 200 ms before until the onset of the cue stimulus. Averaged data were created for each participant and condition separately. Analyses focused on movement-preparatory EEG activity developing during the 1200 ms delay period between cue and response signal. To isolate movement-preparatory activity originating from primary motor cortex and lateral premotor cortex, we derived lateralized potentials by the procedure illustrated in Figure 3a (Coles et al. 1995). Activity recorded at electrodes ipsilateral to the cued response was subtracted from activity recorded at homologous electrodes at the contralateral side. Subsequently, the difference waveforms associated with left and right hand responses were averaged yielding lateralized event-related potentials (ERPs). The lateralized ERPs of primary interest were (i) the ADAN (anterior directing-attention negativity) at fronto-central locations peaking around 400 ms, and (ii) the delay period LRP (lateralized readiness potential) consisting of a slow negative shift at central electrodes overlying the motor cortex. The ADAN is labelled as an attention-directing potential (associated with control of spatial attention), but is also elicited by directional information guiding the selection of response hand or the direction of hand or eye movements (Eimer 1995; Verleger et al. 2000; Praamstra et al. 2005; Gherri et al. 2007). High-density EEG studies have localized the ADAN to the dorsal premotor cortex (Praamstra et al. 2005; Mathews et al. 2006). The ADAN is especially suitable to investigate simultaneous activation of multiple movement directions, because it is sensitive to directional information for hand movements without being affected by the probability of performing the movement (Eimer 1995; van Wijk et al. 2009; see Verleger et al. 2000 for a minor qualification). This property of the ADAN is consistent with evidence that arrow cues act like exogenous peripheral cues (Hommel et al. 2001) and supports the expectation that the ADAN is sensitive to activation of multiple responses. The LRP is associated with the preparation and execution of movements. Its main source is the primary motor cortex, with a contribution from the lateral premotor cortex for the delay period LRP (Leuthold and Jentzsch 2001; Praamstra et al. 1999). The LRP can therefore provide information on simultaneous preparation of multiple movement directions in primary and premotor cortex. The amplitude of the lateralized ERPs was quantified as the mean activity from pooled electrodes over selected time intervals, identified on the basis of grand average waveforms and scalp topographies. The ADAN was quantified from electrode pairs FC1-FC2, FC3-FC4, FCC3h-FCC4h and FFC3h-FFC4h in the time window between 375 and 425 ms (Experiment 2: 325 to 425 ms) after the directional cue. The LRP was quantified from electrode pairs C1-C2, C3-C4, FCC3h-FCC4h and CCP3h-CCP4h in the time between 200 and 0 ms before the response signal.
Figure 3.
(a) Derivation of lateralized potentials to isolate movement-preparatory activity from overlapping stimulus-related activity. The derivation involves subtraction of activity measured at electrodes ipsilateral to the side of movement from activity recorded at homologous contralateral electrode sites. (b) Movement-preparatory lateralized potentials measured from frontal electrode pool emphasizing the ADAN (top) and frontocentral electrode pool emphasizing the LRP (bottom). The displayed waveforms are averaged across all participants and represent the mean activity across four electrodes, selected on the basis of the scalp topography of the component. The grey bars indicate the latency windows for amplitude analysis. The arrow heads correspond to the latency of scalp topographies, which were produced by subtracting left and right hand movement conditions and based on data averaged across 1-, 2-, and 3-arrow conditions.
In addition to lateralized movement preparatory EEG potentials, we analyzed ERPs elicited by the response signal, i.e. the N2(00) and P3(00) components. The posterior N2 amplitude is sensitive to visual feature information (Gehring et al. 1992; Potts and Tucker 2001; Schubö et al. 2007). The P3 is an index of memory or context updating (Donchin & Coles 1988), an interpretation upheld in the motor domain as updating of an internal model of the movement environment (Krigolson et al. 2008). Its latency is selectively sensitive to manipulations affecting the duration of stimulus evaluation (Coles et al. 1995). The P3 and N2 were evaluated from an electrode group overlying the midline posterior parietal cortex, comprising electrodes Pz, CPP1h, CPP2h, PPO1h, PPO2h. Amplitudes were quantified in the window 1400-1450 ms for the N2 and at individually determined peak latencies, identified by an automatic peak detection algorithm, for the P3. P3 latency was analyzed in terms of the latency where its amplitude reached 75% of the peak amplitude.
Statistical analyses of EEG potentials and response times were performed using repeated measures MANOVA and post-hoc t-tests, with Bonferroni correction for multiple comparisons where appropriate. Since the effect of preparing for multiple movement directions, on movement preparatory activity, was predicted to consist of a reduction in amplitude, main effects of Cue information were followed up with planned comparisons. The Greenhouse-Geisser procedure was applied to all repeated measures with more than one degree of freedom. The adjusted degrees of freedom and p-values are reported. Statistical analyses of EEG data were performed on unfiltered data. Waveforms in illustrations were smoothed by a low-pass filter.
EEG: source analysis and time-frequency analysis
EEG dipole source analysis (BESA 5.1.8; MEGIS software GmbH) was applied to the N2 ERP component following the response signal, in order to evaluate whether its inferred origin in the PPC, based on interpretation of its scalp topography, was physically and neurophysiologically plausible. The analysis used a standard ellipsoid 4-shell head model. Results of source analyses are depicted in a schematic head model with dipole locations represented in terms of Talairach-Tournoux coordinates. To avoid contamination by the high amplitude contingent negative variation developing during the fore period, the N2 was analyzed relative to a baseline immediately preceding the response signal (1000-1200 ms). In addition, the data were high pass filtered (TC 0.1 s, 24 dB) to eliminate the residual contingent negative variation in the baseline epoch.
Time-frequency analyses were performed to explore task-related power changes of theta oscillatory activity, using BESA and the Matlab (MathWorks, Natick, MA) toolbox FieldTrip (http://www.ru.nl/fcdonders/fieldtrip). Following segmentation and artifact correction, each trial was transformed in the time-frequency domain using complex demodulation set to a frequency resolution of 2 Hz and temporal resolution of 25 ms in the frequency range 4-40 Hz. Time-frequency representations per channel and subject were created by averaging spectral density amplitude over trials. Inspection of the individual subject and grand average time-frequency representations enabled the identification of a time-frequency window defining the theta activity. The time-frequency data were evaluated with cluster-level randomization tests in FieldTrip, developed to handle the multiple comparison problem inherent in the statistical evaluation of high-density EEG and MEG data. The cluster-level randomization method first identifies electrodes where the difference between two conditions exceeds a chosen significance level. These electrodes are candidates for inclusion in clusters determined by a cluster-finding algorithm. The method takes the cluster with the maximum test statistic, i.e., the cluster with the maximum difference between conditions, to calculate a critical value for statistical significance under the null distribution for this test statistic. The null distribution is computed by means of a permutation method that randomly assigns replications to conditions (between-subjects design) or randomly permutates the order of paired observations (within-subjects design). This computation is performed by a Monte Carlo approximation involving a user-specified number of random draws. Since p-values for any given cluster are computed under the null distribution of the maximum cluster-level statistic, the method controls for type I errors. We used default parameters for the definition of a cluster (electrodes within 4 cm distance and a minimum of 2 neighbour channels). The number of random draws for reference distributions was set at 500.
Results
Experiment 1: Behavioral performance
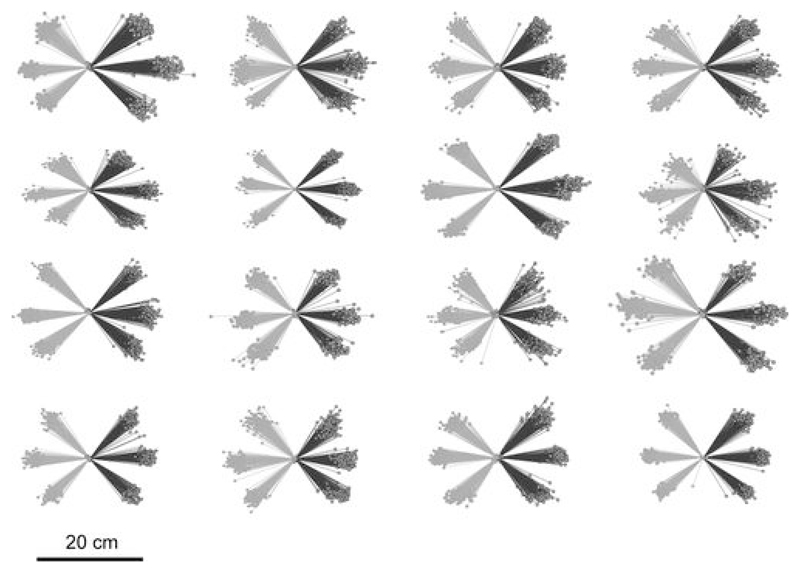
Participants performed the pointing task (see Figure 1) without visual guidance as they had to maintain their gaze and attention focused on the monitor in front of them. Nonetheless, the movement trajectories (see Figure 2) show that pointing was performed with a high degree of accuracy. Given this level of performance and in view of the fact that EEG analyses focused on preparatory activity, no further selection of trials based on pointing accuracy was performed for subsequent analyses. The number of rejected or erroneous trials was (means ± SD) 13.8 ± 19%, 3.7 ± 3.3% and 2.9 ± 2.6% for the 1-, 2- and 3-arrow conditions. The higher error rate for the 1-arrow condition was due to early responses (< 100 ms) in a few participants.
Figure 2.
Pointing trajectories of all 16 participants in Experiment 1, including all the accepted responses in the 1-arrow, 2-arrow, and 3-arrow conditions. Black and grey lines represent movements to the right and left, respectively.
Since there were no effects of response hand, we report pooled means. The average response times following one, two and three arrow cues were 288 ± 86 ms, 353 ± 81 ms and 374 ± 76 ms, respectively. Analysis confirmed a significant effect of Cue information underlying this scaling of response times (F(1.1,16.7)=87.4, p<0.001). Subsequent t-tests confirmed a significant difference between conditions, that is, t(15)=8.7, p<0.001 for the 1- vs. 2-arrow condition and t(15)=8.0, p<0.001 for the 2- vs. 3-arrow condition. These results therefore confirm not just a difference between full (1-arrow) and partial information cue conditions (2- and 3-arrow), but, more importantly, demonstrate a significant difference between the latter two. Hence, the number of possible movement directions influenced performance.
Experiment 1: Delay period activity in (pre)motor cortex
The experiments were set up in such a way that cue information, whether full or partial, always instructed for movements to the left or to the right, performed with left and right hand, respectively. This enabled participants to select the response hand and ensured that lateralized preparatory EEG potentials were elicited in premotor and motor cortex, which we expected to be modulated by the directional information of the cue. This is justified by evidence that the dorsal premotor cortex is involved in effector selection, target selection, as well as their integration (Hoshi and Tanji 2000; Beurze et al. 2006). The lateralized potentials were isolated from overlapping non-lateralized activity by a standard subtraction procedure explained in Figure 3a. The lateralized preparatory EEG potentials arising from the premotor and motor cortex are labelled ADAN and LRP. Their generation in dorsal premotor cortex and motor cortex has been established in previous work (see Materials and methods).
Based on previous findings in monkey motor and premotor cortex, and the concept of mutually suppressive interactions between potential movements, we predicted an inverse relationship between the number of possible movement directions and the amplitude of ADAN and LRP. Results (see Figure 3b) confirmed this prediction. In all conditions a significant ADAN was obtained, compared against baseline (t(15) > 7.0, p<0.001). The amplitude of the ADAN was highest in the 1-arrow and lowest in the 3-arrow condition, with intermediate values in the 2-arrow condition. This was expressed in a significant effect of Cue information (F(1.98,29.7)=12.1, p<0.001). Planned comparisons demonstrated that there was a significant difference between the 1- and the 2-arrow condition (t(15)=2.3, p=0.038), as well as between the 2- and the 3-arrow condition (t(15)= 2.6, p=0.021).
The ADAN was followed by the LRP, reflecting movement preparatory activity in the delay period and movement execution-related activity after the response signal (see Figure 3b). LRP amplitude, quantified at the end of the delay period, was significant compared to baseline in all conditions (t(15) > 4.6, p<0.001). The LRP was significantly different between conditions (F(1.3,19.9)=23.5, p<0.001), i.e. highest in the 1-arrow, lowest in the 3-arrow condition, and intermediate in the 2-arrow condition. Planned comparisons yielded a significant difference between the 1- and the 2- arrow conditions (t(15)=4.0, p=0.001), as well as between the 2- and the 3-arrow condition (t(15)=4.4, p=0.001).
Experiment 1: Preparatory effects inferred from the processing of the response signal
Movement precuing studies often focus exclusively on movement preparatory activity. However, prior information on possible movement directions will not only be encoded in a motoric representation of prospective movements, but also in a retrospective representation of the visuospatial cue information, supported by the PPC (Curtis and D’Esposito 2006). The information carried across the delay period will conceivably affect the processing of the response signal. That is, with more information provided by the cue, less information needs to be extracted from the response signal. This is most obvious for the 1-arrow cue condition, where the response stimulus is not more than a go-signal, because its spatial information does not need to be recapitulated (cf. Crammond and Kalaska 2000).
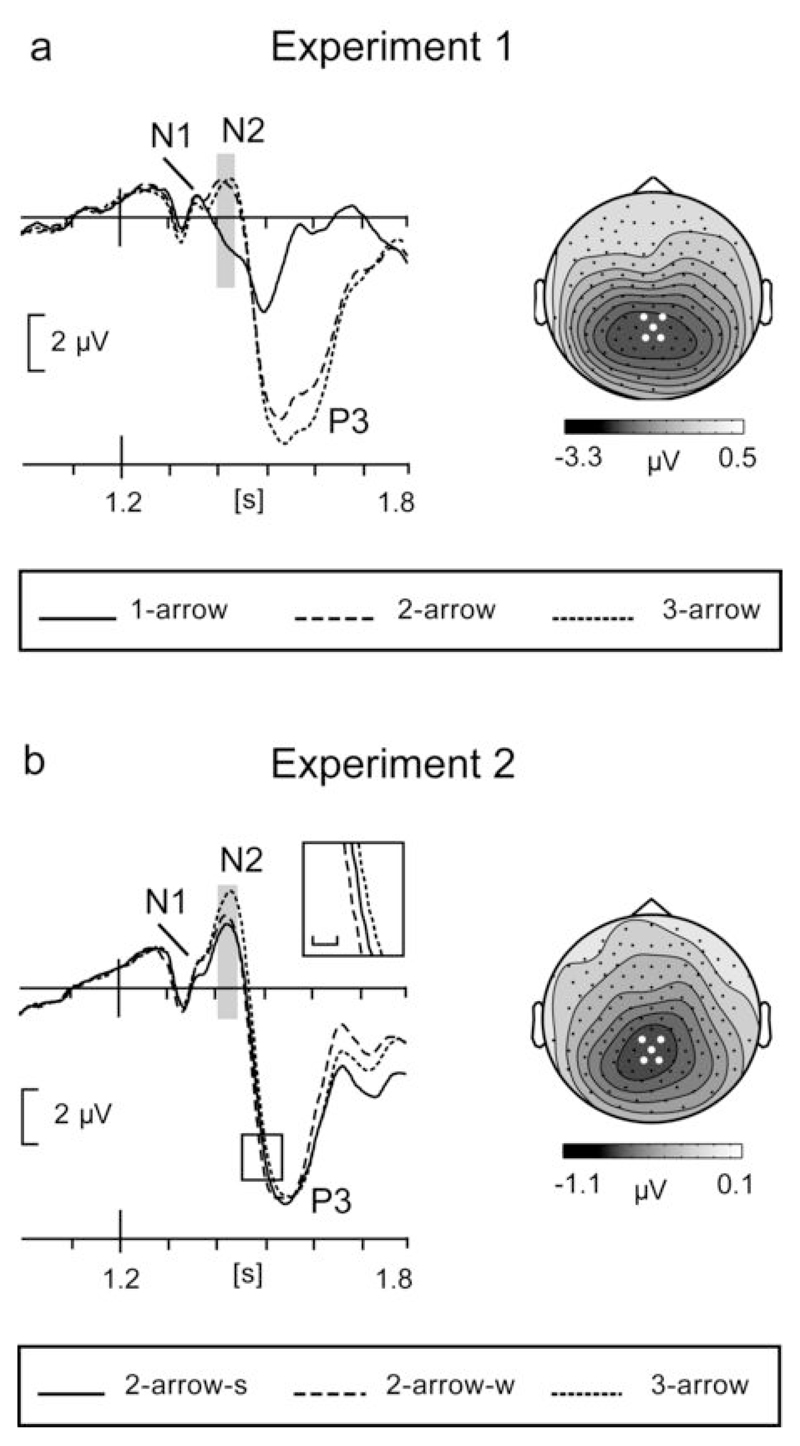
Event-related potentials following the response stimulus demonstrated a conspicuous amplitude modulation of the N2 component, associated with analysis of visual feature information. As is clear from Figure 4a, the N2 was absent in the 1-arrow condition indicating that it indeed represents processing that is redundant when prior information fully specified the movement. Analysis confirmed a significant effect of Cue information (F(1.3,19.7)=11.8, p<0.001). Subsequent t-tests confirmed a significant difference between the 1-arrow condition and each of the partial information conditions (1- vs. 2-arrow t(15)=5.0, p=0.001; 1- vs. 3-arrow t(15)=3.3, p=0.015), but no significant difference between the latter two (t(15)<1).
Figure 4.
(a) Waveforms of the N2 and P3, and scalp distribution of the N2 following the response signal in Experiment 1. The scalp distribution corresponds to the latency window indicated by the grey bar and is obtained by subtraction of the 1-arrow from the 3-arrow condition. The waveforms represent averaged data across 16 participants and the mean of 5 parietal electrodes. (b) Same for Experiment 2. The insert magnifies the P3 latency difference; scale bar = 20 ms.
The P3 latency and amplitude were quantified from the same electrode group. The amplitude scaled with the number of cued movement directions (F(1.2,17.9)=50.2, p<0.001), consistent with the notion that it is partly determined by the amount of information extracted from the eliciting stimulus (Gratton et al. 1990). Post-hoc tests on amplitude values showed a robust difference between the 1- vs. 2- arrow and 1- vs. 3-arrow conditions (t(15)=7.3, p<0.001), as well as a significant difference between 2- and 3-arrow conditions (t(15)=3.0, p=0.018). The latency of the P3 was 269 ± 50, 303 ± 27, and 304 ± 27 ms, respectively, for the 1-, 2-, and 3-arrow conditions. The latency was influenced by Cue information (F(1.1,16.1)=12.2, p<0.01), due to a difference between the full and partial cue information conditions only (1- vs. 2-arrow t(15)=3.4, p=0.012; 1- vs. 3-arrow t(15)=3.7, p=0.006; 2- vs. 3- arrow t(15)<1). We note, however, that peak latencies differed (see Figure 4a) and did yield a significant (6 ms) advantage of the 2- over the 3-arrow condition.
The N2 and P3 results provide further confirmation that participants utilized the cue information, even though the task could be performed without attending to the cues. The effects support that cue information not only induced motor activation, but was also stored in a visuospatial memory representation, against which the response signals were evaluated. Consequently, while physically identical between conditions, response signals had a different information value depending on the number of possible movement directions. This accounts for differences in stimulus evaluation time and context updating expressed in P3 latency, which might contribute, along with the different levels of movement preparatory activity, to the differences in response latency between conditions.
These results of Experiment 1, with an inverse relationship between number of response alternatives and (pre)motor cortex activation, are consistent with predictions based on the concept of mutually suppressive interactions between multiple response options. To provide further support for this explanation, we conducted Experiment 2, involving a manipulation of the angle subtended between potential movements in addition to their number.
Experiment 2: Behavioral performance
Experiment 2 included two conditions that were identical to conditions in Experiment 1, namely, a 3-arrow condition in which three left or right pointing targets were cued, and a 2-arrow-s(mall) condition in which two adjacent left or right targets were cued (see Figure 1b). In addition, there was a 2-arrow-w(ide) condition in which 2 non-adjacent left or right targets were cued. The key prediction was that the smaller separation of movement directions in the 2-arrow-s compared to the 2-arrow-w condition should produce faster responses, due to more mutually excitatory and less mutually suppressive lateral interactions between the neural populations encoding these directions. Response latencies for the 2-arrow-w condition were expected to be close to the 3-arrow condition. If the spatial layout of alternative movement directions has a prevailing influence, response latencies might turn out to be identical. If, in addition to the spatial metric, the number of response alternatives still exerts an influence, the 2-arrow-w condition might yield faster responses than the 3-arrow condition. These predictions, based on previous empirical and modelling studies (Bock and Eversheim 2000; Cisek 2006; Erlhagen and Schöner 2002; Pellizzer and Hedges 2003), were largely borne out by the results. Reaction time was fastest in the 2-arrow-s condition (397 ± 49 ms), followed by the 2-arrow-w condition (405 ± 53 ms), and the slowest 3-arrow condition (412 ± 50 ms), yielding a significant effect of Cue information (F(1.5,22.9)=22.4, p<0.001). Planned comparisons confirmed that each pair of conditions was significantly different (t(15)=3.0, p=0.01 for 2-arrow-s vs. 2-arrow-w; t(15)=3.2, p=0.006 for 2-arrow-w vs. 3-arrow condition). The key result is the non-identical reaction time for the two 2-arrow conditions, demonstrating that response speed is not only determined by the number of possible movement directions, but also by the response metric, i.e. the spatial layout of possible movements. The advantage of the 2-arrow-w over the 3-arrow condition also indicates that both these factors influence reaction time.
One might ask whether there were differences between conditions in terms of movement trajectories. For instance, the same processes that make responses faster in the 2-arrow-s compared to the 2-arrow-w condition, could cause a tendency in the former condition for movements starting off in a direction between the two targets. Whether or not such a tendency was present could not be evaluated, because no trajectories were recorded. We refer to the Supplementary materials for data and discussion of a third experiment using a joystick response device that did not find differences in cursor movement trajectories.
Experiment 2: Delay period activity in (pre)motor cortex
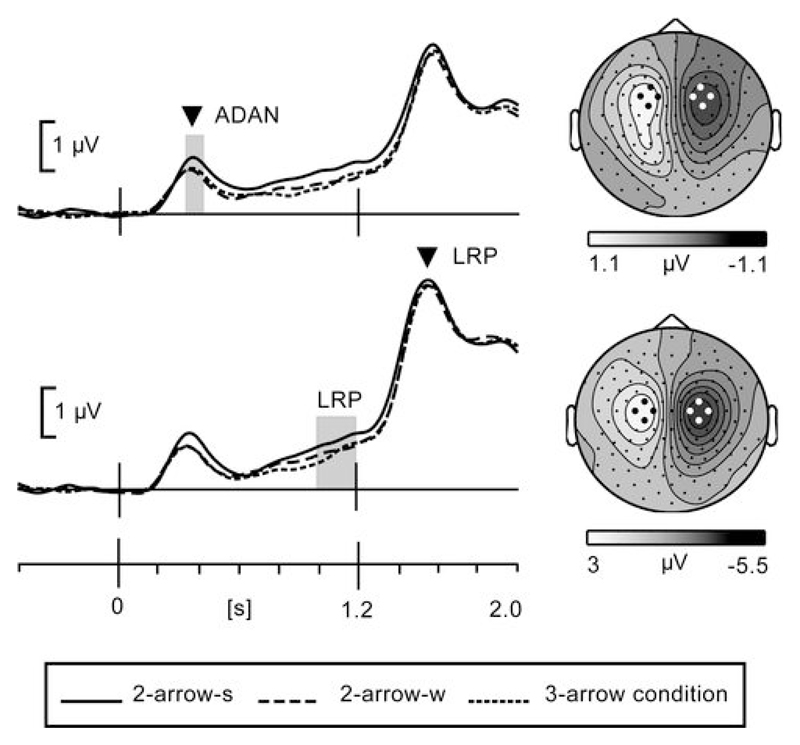
The ADAN and LRP results of Experiment 2 are illustrated in Figure 5. Both components differed significantly from baseline in all conditions (ADAN t(15) > 6.4, p<0.001; LRP t(15) > 5.1, p<0.001). Comparisons between conditions reveal an unambiguous effect of the spatial layout of possible responses. That is, the amplitude of the ADAN was significantly higher in the 2-arrow-s condition than in both the 2-arrow-w and the 3-arrow condition, yielding a significant effect of Cue information (F(1.8,26.6)=5.3, p=0.014). Planned comparisons demonstrated that there was a significant difference between the 2-arrow-s and the 2-arrow-w condition (t(15)=3.2, p=0.009) and between the 2-arrow-s and the 3-arrow condition (t(15)= 3.0, p=0.019), but not between the 2-arrow-w and 3-arrow condition (t(15)<1). The LRP was modulated in exactly the same way, with higher amplitude activity for the 2-arrow-s condition compared to the other two conditions (F(1.8,27.1)=6.3, p<0.01). Planned comparisons showed the 2-arrow-s condition to be different from the 2-arrow-w condition (t(15)=3.3, p=0.008) and from the 3-arrow condition (t(15)=2.8, p=0.018), while the latter two did not differ from each other (t(15)<1). Together, these EEG measures of premotor and motor cortex activity show the influence of spatial variables on preparatory activity, emerging already at a latency shorter than 300 ms following the cue.
Figure 5.
ADAN and LRP potentials for Experiment 2. Scalp topographies were similar and electrode selections for ADAN and LRP were identical to selections in Experiment1. The grey bars indicate the latency windows for amplitude analysis. The arrow heads correspond to the latency of scalp topographies. LRP scalp topography is plotted at peak latency to illustrate the shift to posterior relative to ADAN topography, supporting generation in primary and premotor cortex, respectively.
Experiment 2: Preparatory effects inferred from the processing of the response signal
If delay-period (pre)motoric activity alone had determined response times, it would have produced similar response latencies for the 2-arrow-w and the 3-arrow conditions. The actually faster responses in the 2-arrow-w condition may be explained by differences in the processing of the response signal. In Experiment 2, none of the cue conditions conferred full response information, thus requiring processing of the spatial information of the response signal in all conditions. Based on our interpretation of the N2 findings of Experiment 1, an N2 should therefore be present for all conditions, as is confirmed by the data illustrated in Figure 4b. Statistical evaluation of the amplitude differences between conditions revealed a significant effect of Cue information (F(1.9,27.8)=11.2, p<0.001). Post-hoc t-tests showed that this was due to a higher amplitude for the 3-arrow condition compared to both the 2-arrow-s condition (t(15)=5.1, p=0.003) and the 2-arrow-w condition (t(15)=3.5, p=0.009). There was no difference between the latter two conditions (t(15)<1). Thus, while the 2-arrow-w condition grouped with the spatially similar 3-arrow condition in terms of motoric activity, it grouped with the 2-arrow-s condition in terms of some aspect of stimulus representation or evaluation represented in the N2.
This was also borne out by analyses of the P3, which revealed no difference between conditions in amplitude (F(1.7,26.2)<1), but did show a latency effect (F(1.9,28.6)=13.5, p<0.001) indicating differences in the duration of stimulus evaluation. As illustrated in Figure 4b, the latency was longest for the 3-arrow condition (318 ± 31 ms), intermediate for the 2-arrow-s condition (311 ± 28 ms), and shortest for the 2-arrow-w condition (306 ± 32 ms). In post-hoc tests, the 2-arrow-w vs. 2-arrow-s difference approached significance (t(15)=2.3, p=0.072), while the 2-arrow-s vs. 3-arrow was significant (t(15)=3.1, p=0.016). In sum, the P3, like the N2, reveals the 2-arrow-w condition to behave more similarly to the 2-arrow-s condition than to the 3-arrow condition in terms of stimulus evaluation, contrasting with its expression of movement preparatory activity. These antagonistic effects offer a plausible explanation for response times intermediate between the 2-arrow-s (fastest) and the 3-arrow (slowest) conditions. Note, however, that an account in terms of motoric preparation being sensitive to the spatial layout of response alternatives and stimulus evaluation sensitive to their number is probably too simple. This is signaled by the borderline faster P3 latency for the 2-arrow-w compared to the 2-arrow-s condition, indicating that stimulus evaluation is sensitive to both number and spatial proximity of alternative response directions/targets.
Source reconstruction of the N2
The results presented so far raise issues beyond the question that we set out to test. In particular, results of Experiment 2 suggest that the spatial proximity of possible movement targets may be processed differently by (pre)motor and posterior parietal cortex (PPC), mediating opposite effects on response latency. Since this is of relevance to response selection and decision, we seek here and in the next section to elaborate the proposal that N2 and P3 modulations are related to the evaluation of the response stimulus against a visuospatial memory representation of the cue, a process likely supported by the PPC. This is not contentious with regard to the P3, associated with memory updating, and partly generated by the PPC (Bledowski et al. 2006). The posterior midline N2, by contrast, is not well characterized functionally or anatomically. Naranjo et al. (2007) found it modulated by the selection of reach targets and localized the N2 to the PPC/precuneus, noting its candidate role as human homologue of the parietal reach region in monkey (Astafiev et al. 2003; Connolly et al. 2003). Source reconstruction results of the present data concur with this localization.
We present spatiotemporal dipole source analysis results for the N2 in data of Experiment 2, where it had the highest amplitude, but similar results were found for Experiment 1. Figure 6 shows the N2 (3-arrow right hand condition) in a butterfly plot, emphasizing its small amplitude in comparison to the preceding visual N1 component. Given the temporal overlap of N2 with N1, the N2 could not be modeled in isolation, requiring the introduction of sources fitted to the N1. Modeling the N2 with 2 dipole sources constrained to symmetrical locations in each hemisphere, iterative fitting produced a solution with sources adopting locations close to the medial surface of the PPC, independent of the starting positions. An origin close to the midline was supported by the observation that a single source with the same Y and Z coordinates also accounted well for the data in terms of goodness-of-fit. That is, both 1- and 2-dipole solutions explained the grand average data of each condition with a goodness-of-fit >95% (window around peak latency of N2 ± 10 ms). The Talairach Tournoux coordinates of the dipole source locations for the N2 in Figure 6 are X = ±18, Y = -56, Z = 32. Locations across the three conditions, and across left and right hand responses, were almost identical, corresponding with an origin in the precuneus. These results from a dipole source reconstruction were cross-validated by applying a distributed multiple source analysis (minimum norm approach) on the same data, yielding comparable results.
Figure 6.
Results of spatiotemporal dipole source analysis of the N2 elicited by the response signal (data of Experiment 2). Top: butterfly plot with N1 and N2 components and corresponding scalp topographies. Bottom: dipole source activation time courses for N1 and N2 sources in the left hemisphere. Sources in the right hemisphere showed the same profile. Source locations and orientations are illustrated in the schematic headmodel. Note that the P3 component is attenuated, in comparison to Figure 4, due to the use of an average reference for source analysis.
The source reconstruction results distinguish the posterior midline N2 from the N2pc component associated with visuospatial attentional selection, which has a well-established origin in extrastriate cortex (Hopf et al. 2006). Neither did the small lateralization of the midline N2, when it was isolated in the same way as the N2pc, match the occipitotemporal scalp distribution of the N2pc. We note, however, that the amplitude modulation of the N2 by the number (and spatial proximity; see Supplementary material) of possible movement directions/targets resembles the modulation of the N2pc component by distracters in visual search tasks (Luck et al. 1997), suggesting mechanistic similarities.
Time-frequency analysis of the N2 and P3 time window
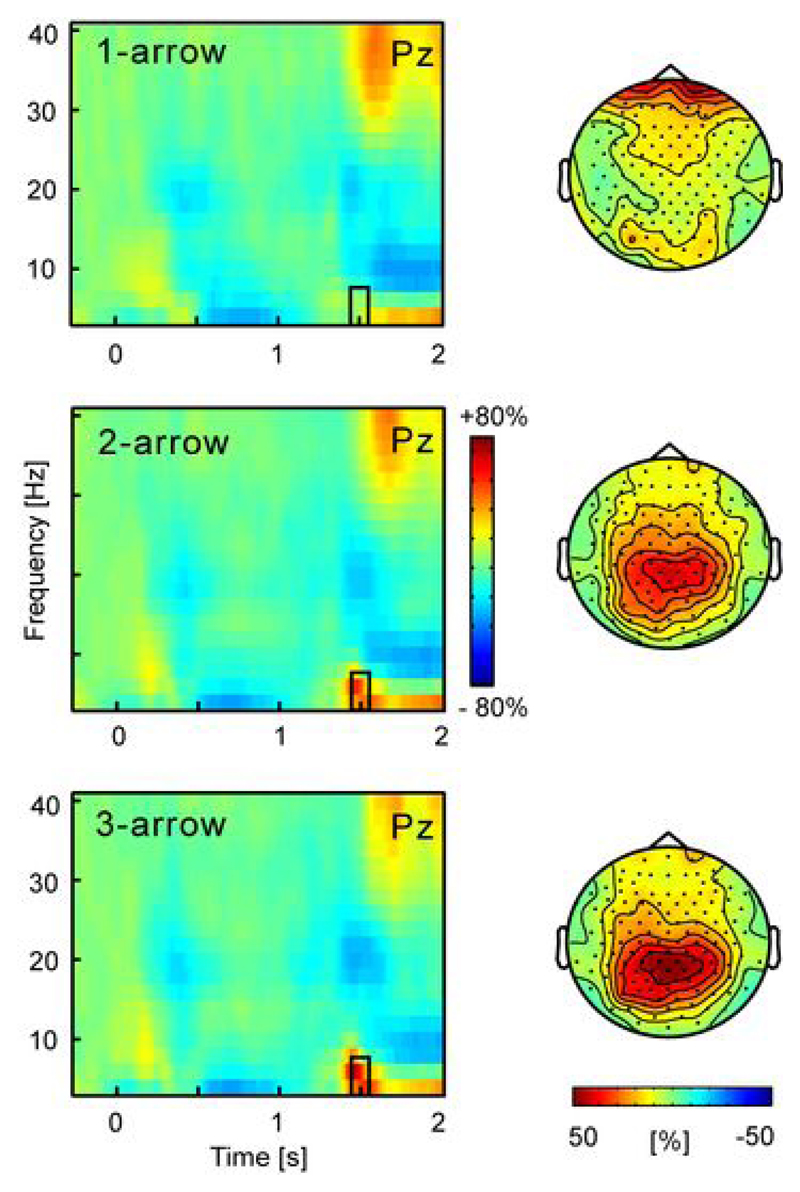
Frontal and parietal theta power and theta phase dynamics have been associated with retention of visual information and executive functions of working memory processes (Jacobs et al. 2006; Jensen and Tesche 2002; Sauseng et al. 2008). These functions comprise not only integration of current perceptual and working memory information, but also updating of motor plans on the basis of sensory input (Caplan et al. 2003). Similarly, synchronization and power increases of parietal theta have been considered as frequency-domain correlates of the memory/context updating process represented by the P3 (Klimesch et al. 1994; Makeig et al. 2004). Against this background, we explored whether the N2 and P3 effects in Experiment 1 are accompanied by a modulation of theta activity. Time-frequency spectra were derived for each condition separately and represented as change spectra relative to the baseline immediately preceding the cue, emphasizing task-related changes in power. Modulation of theta oscillatory activity was found in a small time window of ~1400-1600 ms, overlapping with the latency of the N2 and P3 ERP component (see Figure 7). For all but the 1-arrow condition theta power increased relative to baseline, assessed in a window of 1450-1550 ms. That is, for each condition cluster randomization analysis showed a significant (p<0.05) cluster of ≥30 electrodes overlying the parietal scalp region (threshold for inclusion of electrodes in cluster p<0.01). Comparisons of 3- vs. 1-arrow and 2- vs. 1-arrow conditions yielded similar significant clusters of electrodes, but a numerically higher theta burst in the 3- compared to the 2-arrow condition did not reach significance.
Figure 7.
Time-frequency representations for 1-, 2-, and 3-arrow conditions in Experiment 1. The colour scale indicates percentage power change relative to the baseline preceding the cue. A brief theta burst is seen to peak at 1500 ms (300 ms after response signal) and was quantified between 1450-1550 ms in the frequency range 4-8 Hz, as indicated by the black rectangle. Its scalp topography for each of the conditions is shown on the right, emphasizing a parietal maximum.
While a comprehensive time-frequency analysis is beyond the scope of this report, the timing and the direction of the theta modulation raise a strong possibility that the N2 at least partly arises from a phase resetting of the parietal theta activity (Makeig et al. 2002), and suggest that they reflect overlapping neural and functional processes. The absence of a theta burst in the 1-arrow condition matches the absence of an N2, and attenuated P3, and support a relation with memory updating, redundant in that condition. Finally, the scalp distribution of the theta modulation corresponds to that of the N2 and P3, consistent with the involvement of structures in the PPC in visuospatial working memory.
Discussion
Simultaneous representation of multiple potential movements
The predictions tested in this study are predicated on the view that movement parameters such as direction are encoded by populations of neurons with a range of different directional preferences. Preparation for different movement directions will shape population activity such that distinct peaks arise associated with the context-appropriate directions, maintained by mutually suppressive interactions between cell populations encoding different directions and mutually reinforcing interactions between cells with similar directional preference. Competitive interactions between a greater number of response alternatives (or same number at wider angle) will result in a narrower tuning function and weaker activity for each peak, leading to slower reaction times (Erlhagen and Schöner 2002; Cisek 2006).
Experiment 1 showed that delay period activity in (pre)motor cortex scaled inversely with the number of possible movements. This is consistent with the operation of mutually suppressive interactions between representations of concurrent movement options, but does not rule out an explanation in terms of response probability. Although response probability is traditionally regarded as acting prior to the engagement of the motor system (Sanders 1980; Goodman and Kelso 1980), evidence from primate neurophysiology shows manifestations in the form of a modulation of movement preparatory activity. For instance, Basso and Wurtz (1998) found delay period activity of buildup neurons in the superior colliculus decreased as the number of saccade targets increased. Likewise, visually responsive neurons interfacing with movement-related cells in the frontal eye field demonstrate a reduction of activity with visual target selection in the presence of more distracters (Cohen et al. 2009; Schall et al. 1995, 2004). On the one hand, these examples underline that, without direct evidence for distinct movement representations that mutually influence each other, an inverse scaling of neural activity with the number of possible movements does not necessarily reflect simultaneous activation of multiple potential movements. On the other hand, visual responses in the frontal eye field elicited by potential saccade targets can also be regarded as representing potential movements, and there is evidence for suppressive interactions between target and distracter induced activity (Schall et al. 1995, 2004) that could underlie the observed modulation by the number of distracters/potential movements (Cohen et al. 2009).
When also the results of Experiment 2 are taken into consideration, our data provide considerably stronger evidence for simultaneous activation of multiple potential movements. Here we manipulated the spatial angle between two alternative movement directions, obtaining predicted effects of spatial distance on movement preparatory activity while keeping choice uncertainty constant. These results render an explanation in terms of response probability less likely. As pointed out by Cisek (2006), effects of the spatial layout of possible responses on reaction time are difficult to account for in most computational models of decision making. They are readily simulated, however, in models where the interactions between neural populations defining different response options also mediate the decision process between them. We note that in our experiments activation of different (pre)motor cortical neural populations was helped by the use of arrow cues which specified movement options in a direct manner. Though centrally presented, arrow cues act more like exogenous peripheral cues than as endogenous symbolic cues (Hommel et al. 2001), and are therefore prone to produce relatively automatic activation of a corresponding response (Zhang et al. 1997). This feature of the experimental setup thus adds support to an explanation of the amplitude modulation of movement preparatory activity as reflecting the processing of directional information at the motor level by means of a prospective code of potential movements. NOTE.
Differential effects of spatial proximity in premotor and posterior parietal cortex
Effects of the spatial layout of possible responses on reaction time (Bock and Eversheim 2000; Favilla 1996), such as we obtained in Experiment 2, are not invariably found to prevail over the effect of the number of response alternatives (Pellizzer and Hedges 2003; Adam and Pratt 2004). The unanticipated modulation of EEG potentials elicited by the response stimulus, in this investigation, suggests as possible explanation that representations of prospective movements in the (pre)motor cortex and visuospatial representations of movement targets in the PPC make independent contributions to such effects. It has been proposed that premotor cortex and PPC partake in a distributed system for the control of action in which decision making can be biased to either structure depending on the task (Cisek and Kalaska 2005; Scherberger and Andersen 2007). We consider here the possibility that, within such a distributed system, selection operations in premotor and parietal cortex may occasionally act in such a way as to contribute opposite effects to overt behaviour.
In the present experiments, cue information modified the task-defined default representation of 6 possible movements to a representation of 1, 2, or 3 possible movements relevant in the current trial. While the (pre)motor cortex carries this information across the delay period in the form of prospective movements, the PPC carries the information in the form of a visuospatial memory representation (Curtis and D’Esposito 2006; Wolbers et al. 2008). We interpret the N2/P3 and theta modulation as indicating that the response stimulus was evaluated against this visuospatial representation and that evaluation took longer when there were more possible movements, i.e. with higher memory load. This is supported by an investigation reporting effects of memory set size on P3 latency in a task where current perceptual input was explicitly compared against a visual working memory representation (Hyun et al. 2009). We also note the relevance of recent findings with a task involving spatial selection within a memory representation, guided by directional ‘retro-cues’, expressed in BOLD signal variation on the medial aspect of the PPC, the precuneus (Lepsien et al. 2005). This research on interactions between spatial attention and working memory also suggests that activation of the PPC is not just sensitive to memory load. Another important determinant is suppression of distracters (Lepsien et al. 2005; Pollmann et al. 2003; Wojciulik and Kanwisher 1999). Distracter suppression and associated effects of a suppressive zone surrounding the locus of attention provide a potential mechanism to explain how spatial proximity of potential movement targets/directions can have differential effects in representations carried by PPC and premotor cortex.
Recall that a notable manifestation of such differential effects was found in Experiment 2, where the 2-choice-w condition grouped with the 3-choice condition in terms of delay period motoric activity, while it grouped with the 2-choice-s condition in terms of parietal activity expressed in the N2, even showing a (marginally significant) shorter P3 latency than the latter condition. The relevance of this pattern was emphasized by the results of a third experiment (see Supplementary material), where the latency advantage of the P3 in the 2-choice-w relative to the 2-choice-s condition was larger and significant (14 ms vs. 5 ms in Experiment 2). This advantage outweighed any advantage of spatial proximity on movement preparatory activity, thus producing a tendency for faster reaction times in the 2-choice-w over the 2-choice-s condition (the reverse of Experiment 2). Importantly, the longer P3 latency in the 2-choice-s condition was accompanied by a significantly higher N2 amplitude, supporting that the P3 delay was driven by the visuospatial analysis/comparison of response stimulus and memory representation. This explanation receives support from results of Bahcall and Kowler (1999) demonstrating that perceptual report from a visuospatial memory representation is worse for small compared to large target separations. Note that these results thus confirm that the parietal N2 and P3 effects are not just sensitive to the number of possible movement directions or targets, but also to their spatial relation. The feature that distinguished Experiment 3 from Experiments 1 and 2 was the use of a joystick response device, which moved a cursor in the visual stimulus display. Hence, in contrast to those experiments, the visuospatial and proprioceptive-motor representations mapped onto the same workspace, apparently giving more weight to visuospatial distracter effects (cf. Welsh et al. 1999).
The above proposed interpretation of scalp-recorded EEG signals can be linked to several aspects of PPC function, related to reaching, revealed by single unit recordings. It has to be borne in mind, however, that EEG measures post-synaptic activity as opposed to action potentials in single-unit recordings. Data from single-unit recordings in the PPC support a capacity to encode and store multiple movement goals (Baldauf et al. 2008; Scherberger and Andersen 2007). Especially interesting is the involvement of the PPC in changes of existing movement plans (Bracewell et al. 1996; Snyder et al. 1998), which might be associated with what we interpreted as comparison of the response signal against the visuopatial memory of the cue. Finally, the PPC has also been demonstrated to participate in the decision between movements to alternative goals (Scherberger and Andersen 2007), a capacity which is prerequisite for the proposition that PPC and premotor cortex may contribute opposite effects to response times. It has to be acknowledged that delay period activity, often conspicuous in recordings from the PPC (Constantinidis and Steinmetz 1996; Gnadt and Andersen 1988; Kalaska and Crammond 1995) was not evident in our lateralized EEG potentials at relevant electrode sites, although such activity might contribute to the bilaterally distributed contingent negative variation (CNV). The apparent lack of sensitivity of EEG to delay period parietal activity means that we cannot rule out that during the delay period the PPC displayed activity similar to that of the premotor cortex. We note further that some of the here cited single unit work emphasizes an interpretation of ‘sensory’ signals in the PPC in terms of movement plans towards the source of the signals. This may be seen as blurring the distinction between premotor cortex and PPC as coding prospective movement and retrospective sensory information, respectively. However, one may still argue that they differ in the degree to which they process visuospatial and visuomotor information, based both on neuroimaging (Curtis and D’Esposito 2006) and neurophysiological evidence (Kalaska and Crammond 1995).
Conclusions
The lateral interactions that shape the directional tuning of neural populations encoding different movement directions are not directly accessible (Merchant et al. 2008). However, the present data provide indirect support for the operation of such interactions by demonstrating an amplitude modulation of movement preparatory activity as a function of number and spatial layout of possible movements. Preparatory neural activity for a movement is necessarily defined over a context-dependent movement parameter space, which was redefined by the directional cues on a trial by trial basis. This makes the amplitude modulation of movement preparatory EEG signals interpretable as the result of changes in the pattern of cooperative and competitive interactions between the neural populations encoding different movement directions. The data thereby support the view that motor areas can simultaneously prepare for multiple response options, as previously proposed on the basis of recordings in monkey (pre)motor cortex (Bastian et al. 2003; Cisek and Kalaska 2005).
Whereas movement precuing studies generally focus on the modulation of movement-related activity, our data also reveal an effect of prior information on the evaluation of the response signal. We propose that this effect is due to the characteristics of a visuospatial working memory representation supported by the PPC, against which response signals are evaluated. This proposal is well-embedded in existing accounts of response precuing effects (Adam et al. 2003) and PPC involvement in visuospatial working memory (Curtis and D’Esposito 2006; Wolbers et al. 2008), and is supported by evidence on how new visual information is compared with visuospatial memories of previous input (Hyun et al. 2009; Lepsien et al. 2005). The expression of the presumed visual spatial memory effects in theta power is congruent with the role of theta activity in spatial working memory (Jensen and Tesche 2002; Sauseng et al. 2008) and provides potential means (eg. EEG coherence or hierarchical cross-frequency coupling) for examining how these effects interact with movement preparatory activity in (pre)motor cortex to produce the observed behavior.
It is recognized that competition between alternative response options is played out in different parts of the frontoparietal network at the same time, subject to different biasing influences (Cisek 2006; Kalaska et al. 2003; Scherberger and Andersen 2007). The present data show that scalp-recorded EEG measures can be used to dissociate frontal and parietally mediated effects of such competitive interactions on behaviour, creating further opportunities to address the division of labour between frontal and parietal cortex in action selection. This use of EEG is reminiscent of chronometric EEG investigations demonstrating early motor activation preceding the completion of perceptual analysis in visual search, which have been influential in rejecting strictly serial information processing views of human brain function (Smid et al. 1991; Coles et al. 1995). These methods are now also relevant to the investigation of theoretically challenging distracter effects in reaching and pointing, which have stimulated the notion of simultaneous coding of multiple actions (Tipper et al. 2000).
Acknowledgements
The authors thank Antje Meyer, Jos Adam, and Jason Braithwaite for comments on the manuscript and Nick Roach for technical support. Paul Cisek gave helpful comments on the results of Experiment 3. Experiments were realized using Cogent (http://www.vislab.ucl.ac.uk/cogent).
NOTE
If attentional selection involves covert movement preparation, as proposed in the premotor theory of attention, then the behavior of the ADAN in purely attentional variants of our tasks should resemble the behavior that we report here. On the surface, a recent publication by Seiss et al. (2009) seems to contradict this. Seiss and co-workers compared the ADAN elicited by an arrow cue indicating the left/right location of a single target with the ADAN elicited when the same target was surrounded by two distracters. Target detection was indicated by means of a verbal response. The authors found a trend (p=0.1) towards higher ADAN amplitude in the distracter condition. They found a significant difference in a later time window (550-900 ms) following the ADAN peak latency, which they labelled as “late ADAN”. These findings do not contradict our results. Firstly, in the distracter condition, attention was not cued for multiple potential locations (as movement preparation was in our tasks), because the target always occurred at the same location. Secondly, there was not a significant modulation of what is conventionally regarded as ADAN. Thirdly, the presumed ADAN effects were measured at electrode sites that were far more frontal and ventral/lateral than the electrodes overlying the (pre)motor cortex where we measured the ADAN. This distribution makes it unlikely that the effects result from dorsal frontal activity involved in the control of spatial attention or the preparation of movement.
References
- Adam JJ, Hommel B, Umiltà C. Preparing for perception and action (I): the role of grouping in the response-cuing paradigm. Cogn Psychol. 2003;46:302–58. doi: 10.1016/s0010-0285(02)00516-9. [DOI] [PubMed] [Google Scholar]
- Adam JJ, Pratt J. Dissociating visual attention and effector selection in spatial precuing tasks. J Exp Psychol Hum Percept Perform. 2004;30:1092–1106. doi: 10.1037/0096-1523.30.6.1092. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahcall DO, Kowler E. Attentional interference at small spatial separations. Vision Res. 1999;39:71–86. doi: 10.1016/s0042-6989(98)00090-x. [DOI] [PubMed] [Google Scholar]
- Baldauf D, Cui H, Andersen RA. The posterior parietal cortex encodes in parallel both goals for double-reach sequences. J Neurosci. 2008;28:10081–9. doi: 10.1523/JNEUROSCI.3423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci. 1998;18:7519–7534. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian A, Schöner G, Riehle A. Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur J Neurosci. 2003;18:2047–2058. doi: 10.1046/j.1460-9568.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- Beurze SM, de Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. J Neurophysiol. 2007;97:188–99. doi: 10.1152/jn.00456.2006. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Cohen Kadosh K, Wibral M, Rahm B, Bittner RA, Hoechstetter K, Scherg M, Maurer K, Goebel R, Linden DE. Mental chronometry of working memory retrieval: a combined functional magnetic resonance imaging and event-related potentials approach. J Neurosci. 2006;26:821–9. doi: 10.1523/JNEUROSCI.3542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock O, Eversheim U. The mechanisms of movement preparation: a precuing study. Behav Brain Res. 2000;108:85–90. doi: 10.1016/s0166-4328(99)00134-5. [DOI] [PubMed] [Google Scholar]
- Bracewell RM, Mazzoni P, Barash S, Andersen RA. Motor intention activity in the macaque's lateral intraparietal area. II. Changes of motor plan. J Neurophysiol. 1996;76:1457–64. doi: 10.1152/jn.1996.76.3.1457. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23:4726–36. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci. 2006;26:9761–9770. doi: 10.1523/JNEUROSCI.5605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Heitz RP, Woodman GF, Schall JD. Neural basis of the set-size effect in frontal eye field: timing of attention during visual search. J Neurophysiol. 2009;101:1699–704. doi: 10.1152/jn.00035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MG, Smid HGOM, Scheffers MK, Otten LJ. Mental chronometry and the study of human information processing. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind: Event-related brain potentials and cognition. Oxford: Oxford University Press; 1995. [Google Scholar]
- Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a 'parietal reach region' in the human brain. Exp Brain Res. 2003;153:140–145. doi: 10.1007/s00221-003-1587-1. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal activity in posterior parietal area 7a during the delay periods of a spatial memory task. J Neurophysiol. 1996;76:1352–5. doi: 10.1152/jn.1996.76.2.1352. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95:3923–7. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 a manifestation of context updating? Behav Brain Sci. 1988;11:357–427. [Google Scholar]
- Eimer M. Stimulus-response compatibility and automatic response activation: evidence from psychophysiological studies. J Exp Psychol Hum Percept Perform. 1995;21:837–854. doi: 10.1037//0096-1523.21.4.837. [DOI] [PubMed] [Google Scholar]
- Erlhagen W, Schöner G. Dynamic field theory of movement preparation. Psychol Rev. 2002;109:545–572. doi: 10.1037/0033-295x.109.3.545. [DOI] [PubMed] [Google Scholar]
- Favilla M. Reaching movements: programming time course is independent of choice number. Neuroreport. 1996;7:2629–2634. [PubMed] [Google Scholar]
- Gehring WJ, Gratton G, Coles MG, Donchin E. Probability effects on stimulus evaluation and response processes. J Exp Psychol Hum Percept Perform. 1992;18:198–216. doi: 10.1037/0096-1523.18.1.198. [DOI] [PubMed] [Google Scholar]
- Gherri E, van Velzen J, Eimer M. Dissociating effector and movement direction selection during the preparation of manual reaching movements: evidence from lateralized ERP components. Clin Neurophysiol. 2007;118:2031–2049. doi: 10.1016/j.clinph.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–20. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goodman D, Kelso JA. Are movements prepared in parts? Not under compatible (naturalized) conditions. J Exp Psychol Gen. 1980;109:475–495. doi: 10.1037//0096-3445.109.4.475. [DOI] [PubMed] [Google Scholar]
- Gratton G, Bosco CM, Kramer AF, Coles MG, Wickens CD, Donchin E. Event-related brain potentials as indices of information extraction and response priming. Electroencephalogr Clin Neurophysiol. 1990;75:419–32. doi: 10.1016/0013-4694(90)90087-z. [DOI] [PubMed] [Google Scholar]
- Hommel B, Pratt J, Colzato L, Godijn R. Symbolic control of visual attention. Psych Res. 2001;12:36–365. doi: 10.1111/1467-9280.00367. [DOI] [PubMed] [Google Scholar]
- Hopf J-M, Luck SJ, Boelmans K, Schoenfeld MA, Boehler CN, Rieger J, Heinze HJ. The neural site of attention matches the spatial scale of perception. J Neurosci. 2006;26:3532–3540. doi: 10.1523/JNEUROSCI.4510-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408:466–70. doi: 10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- Hyun J-S, Woodman GF, Vogel EK, Hollingworth A, Luck SJ. The comparison of visual working memory representations with perceptual inputs. J Exp Psychol Hum Percept Perform. doi: 10.1037/a0015019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: theta correlates of memory retrieval and decision making. Neuroimage. 2006;32:978–87. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–9. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex. 1995;5:410–28. doi: 10.1093/cercor/5.5.410. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Cisek P, Gosselin-Kessiby N. Mechanisms of selection and guidance of reaching movements in the parietal lobe. Adv Neurol. 2003;93:97–119. [PubMed] [Google Scholar]
- Kennett S, van Velzen J, Eimer M, Driver J. Disentangling gaze shifts from preparatory ERP effects during spatial attention. Psychophysiol. 2007;44:69–78. doi: 10.1111/j.1469-8986.2006.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–41. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Krigolson OE, Holroyd CB, Van Gyn G, Heath M. Electroencephalographic correlates of target and outcome errors. Exp Brain Res. 2008;190:401–11. doi: 10.1007/s00221-008-1482-x. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Griffin IC, Devlin JT, Nobre AC. Directing spatial attention in mental representations: Interactions between attentional orienting and working-memory load. Neuroimage. 2005;26:733–43. doi: 10.1016/j.neuroimage.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Jentzsch I. Neural correlates of advance movement preparation: a dipole source analysis approach. Brain Res Cogn Brain Res. 2001;12:207–224. doi: 10.1016/s0926-6410(01)00052-0. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Girelli M, McDermott MT, Ford MA. Bridging the gap between monkey neurophysiol-ogy and human perception: An ambiguity resolution theory of visual selective attention. Cogn Psychol. 1997;33:64–87. doi: 10.1006/cogp.1997.0660. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–4. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Makeig S, Delorme A, Westerfield M, Jung TP, Townsend J, Courchesne E, Sejnowski TJ. Electroencephalographic brain dynamics following manually responded visual targets. PLoS Biol. 2:e176. doi: 10.1371/journal.pbio.0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S, Ainsley Dean PJ, Sterr A. EEG dipole analysis of motor-priming foreperiod activity reveals separate sources for motor and spatial attention components. Clin Neurophysiol. 2006;117:2675–2683. doi: 10.1016/j.clinph.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Merchant H, Naselaris T, Georgopoulos AP. Dynamic sculpting of directional tuning in the primate motor cortex during three-dimensional reaching. J Neurosci. 2008;28:9164–72. doi: 10.1523/JNEUROSCI.1898-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Ulrich R. Locus of the effect of the number of alternative responses: Evidence from the lateralized readiness potential. J Exp Psychol Hum Percept Perf. 24:1215–1231. [Google Scholar]
- Naranjo JR, Brovelli A, Longo R, Budai R, Kristeva R, Battaglini PP. EEG dynamics of the frontoparietal network during reaching preparation in humans. Neuroimage. 2007;34:1673–1682. doi: 10.1016/j.neuroimage.2006.07.049. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol. 2001;112:713–719. doi: 10.1016/s1388-2457(00)00527-7. [DOI] [PubMed] [Google Scholar]
- Pellizzer G, Hedges JH. Motor planning: effect of directional uncertainty with discrete spatial cues. Exp Brain Res. 2003;150:276–89. doi: 10.1007/s00221-003-1453-1. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Weidner R, Humphreys GW, Olivers CN, Müller K, Lohmann G, Wiggins CJ, Watson DG. Separating distractor rejection and target detection in posterior parietal cortex--an event-related fMRI study of visual marking. Neuroimage. 2003;18:310–23. doi: 10.1016/s1053-8119(02)00036-8. [DOI] [PubMed] [Google Scholar]
- Potts GF, Tucker DM. Frontal evaluation and posterior representation in target detection. Brain Res Cogn Brain Res. 2001;11:147–56. doi: 10.1016/s0926-6410(00)00075-6. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Schmitz F, Freund HJ, Schnitzler A. Magneto-encephalographic correlates of the lateralized readiness potential. Brain Res Cogn Brain Res. 1999;8:77–85. doi: 10.1016/s0926-6410(99)00008-7. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Boutsen L, Humphreys GW. Frontoparietal control of spatial attention and motor intention in human EEG. J Neurophysiol. 2005;94:764–774. doi: 10.1152/jn.01052.2004. [DOI] [PubMed] [Google Scholar]
- Sanders AF. Stage analysis of reaction processes. In: Stelmach GR, Requin J, editors. Tutorials in Motor Behavior. Amsterdam, North Holland: pp. 331–354. [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Birbaumer N. Cross-frequency phase synchronization: a brain mechanism of memory matching and attention. Neuroimage. 2008;40:308–17. doi: 10.1016/j.neuroimage.2007.11.032. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995;15:6905–18. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Sato TR, Thompson KG, Vaughn AA, Juan CH. Effects of search efficiency on surround suppression during visual selection in frontal eye field. J Neurophysiol. 2004;91:2765–9. doi: 10.1152/jn.00780.2003. [DOI] [PubMed] [Google Scholar]
- Scherberger H, Andersen RA. Target selection signals for arm reaching in the posterior parietal cortex. J Neurosci. 2007;27:2001–2012. doi: 10.1523/JNEUROSCI.4274-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubö A, Wykowska A, Müller HJ. Detecting pop-out targets in contexts of varying homogeneity: investigating homogeneity coding with event-related brain potentials (ERPs) Brain Res. 2007;1138:136–47. doi: 10.1016/j.brainres.2006.12.059. [DOI] [PubMed] [Google Scholar]
- Seiss E, Driver J, Eimer M. Effects of attentional filtering demands on preparatory ERPs elicited in a spatial cueing task. Clin Neurophysiol. 2009;120:1087–95. doi: 10.1016/j.clinph.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Smid GH, Lamain W, Hogeboom MM, Mulder G, Mulder LJ. Psychophysiological evidence for continuous information transmission between visual search and response processes. J Exp Psychol Hum Percept Perform. 1991;17:696–714. doi: 10.1037//0096-1523.17.3.696. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Change in motor plan, without a change in the spatial locus of attention, modulates activity in posterior parietal cortex. J Neurophysiol. 1998;79:2814–9. doi: 10.1152/jn.1998.79.5.2814. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Howard LA, Houghton G. Behavioural consequences of selection from neural population codes. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and Performance XVIII. Cambridge, MA: MIT; 2000. [Google Scholar]
- Van Wijk BCM, Daffertshofer A, Roach N, Praamstra P. A role of beta oscillatory synchrony in biasing response competition? Cereb Cortex. 2009;19:1294–302. doi: 10.1093/cercor/bhn174. [DOI] [PubMed] [Google Scholar]
- Verleger R, Vollmer C, Wauschkuhn B, van der Lubbe RH, Wascher E. Dimensional overlap between arrows as cueing stimuli and responses? Evidence from contra-ipsilateral differences in EEG potentials. Brain Res Cogn Brain Res. 2000;10:99–109. doi: 10.1016/s0926-6410(00)00032-x. [DOI] [PubMed] [Google Scholar]
- Welsh TN, Elliott D, Weeks DJ. Hand deviations toward distractors. Evidence for response competition. Exp Brain Res. 1999;127:207–12. doi: 10.1007/s002210050790. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23:747–64. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Hegarty M, Büchel C, Loomis JM. Spatial updating: how the brain keeps track of changing object locations during observer motion. Nat Neurosci. 2008;11:1223–30. doi: 10.1038/nn.2189. [DOI] [PubMed] [Google Scholar]
- Zhang J, Riehle A, Requin J, Kornblum S. Dynamics of single neuron activity in monkey primary motor cortex related to sensorimotor transformation. J Neurosci. 1997;17:2227–46. doi: 10.1523/JNEUROSCI.17-06-02227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]