Abstract
Background
While patients with early stage rhabdomyosarcoma (RMS) have seen steady improvement in prognosis over the last fifty years, those with advanced stage or high grade disease continue to have a dismal prognosis. Retinoids have been shown to cause growth suppression and terminal differentiation in RMS cells, but the toxicities associated with retinoic acid (RA) limit its use. Rexinoids provide an alternative treatment approach to RA. Rexinoids primarily bind the retinoid X receptor with minimal retinoic acid receptor binding, the entity responsible for many of the toxicities of retinoid therapies. UAB30 is a novel rexinoid with limited toxicities. We hypothesized that UAB30 would lead to decreased cell survival in RMS.
Materials/Methods
Two RMS cell lines, one embryonal (RD) subtype and one alveolar (SJCRH30) subtype, were utilized. Cells were treated with UAB30 and cytotoxicity, proliferation, mobility, and apoptosis were evaluated.
Results
UAB30 significantly decreased RMS tumor cell viability and proliferation. Invasion, migration and attachment independent growth were reduced following UAB30 treatment. UAB30 also resulted in apoptosis and G1 cell cycle arrest. UAB30 affected both the alveolar and embryonal RMS cell lines in a similar fashion.
Conclusions
The results of these studies suggest a potential therapeutic role for the low toxicity synthetic retinoid X receptor selective agonist, UAB30, in RMS treatment.
Keywords: rhabdomyosarcoma, retinoid, UAB30, SJCRH30, RD
Introduction
Rhabdomyosarcoma (RMS), a malignancy of mesenchymal origin, is the most common pediatric soft tissue sarcoma of childhood [1]. Multiple prognostic factors contribute to the outcomes of RMS including age, tumor size and location, resectability, histopathologic subtype, and presence of nodal involvement or metastatic disease. The current standard of care affords excellent outcomes for patients with favorable pathology and early stage disease. However, patients with high risk disease rely on prolonged treatments with significant toxicities and an extremely poor prognosis, having less than 20% 5 year survival [1]. For clinical trial purposes, the Children’s Oncology Group (COG) defines high risk RMS as any aged patients with stage IV ARMS or patients greater than ten years old with stage IV ERMS [2]. The treatment for high risk RMS must continue to evolve to address the challenges of treating these patients. Novel therapies are needed that will minimize potential side effects while increasing long-term survival.
Previous studies by Barlow et al. demonstrated that retinoids, namely all-trans-retinoic acid (ATRA) and 9-cis-retinoic acid (CRA) caused growth suppression and differentiation in some RMS cell lines [1]. However, the use of retinoids is hampered by dose-limiting toxicities, such as hypertriglyceridemia and teratogenicity, as well as the development of chemoresistance. 9-cis-UAB30 (UAB30), a novel rexinoid compound, was developed with the goal of maintaining efficacy similar to or surpassing that of retinoic acid (RA), while achieving a more benign toxicity profile. UAB30, rather than binding to the retinoic acid receptor (RAR) which is thought to lead to a portion of the toxicities associated with RA, binds to the retinoid X receptor (RXR), and has been shown to have a limited toxicity profile. UAB30 has demonstrated anti-tumor effects in a variety of malignancies in vitro and in vivo, and is currently undergoing phase I clinical trials for adult breast cancer. Initial animal and human studies have demonstrated a benign side effect profile with virtually no issues noted in healthy volunteers [3].
Based on the anti-tumor effects of UAB30 on other solid tumor types, and the effects of ATRA and CRA on RMS noted by other investigators, we hypothesized that UAB30 would decrease proliferation and induce apoptosis in RMS. We were able to confirm this hypothesis using both alveolar (ARMS) and embryonal (ERMS) cell lines, suggesting that UAB30 warrants continued investigation and may emerge as a therapeutic intervention for RMS.
Materials and Methods
Cells and cell culture
All cell lines were maintained in standard culture conditions at 37 °C and 5% CO2. RD human ERMS cells (CCL-136™, American Type Culture Collection, ATCC®, Manassas, VA) were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 4 mM L-glutamine, 1 μM non-essential amino acids and 1 μg/mL penicillin/streptomycin. SJCRH30 human ARMS cells (CRL-2061™, ATCC®) were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum and 1 μg/mL penicillin / streptomycin. All cell lines were verified within the last year using short tandem repeat (STR) analysis by the Heflin Genomics Center, UAB, and were deemed mycoplasma free.
Antibodies and reagents
Rabbit polyclonal anti-PARP (9542S), anti-AKT (9272), anti-phospho AKT (S473, 9271), and anti-ERK1/2 (9102) were obtained from Cell Signaling Technology (Danvers, MA). Rabbit anti-phospho ERK1/2 (05-797R), mouse anti-FAK (4.47), anti-cleaved PARP (MAB3565) and mouse anti-GAPDH (MAB374, clone 6C5) were from Millipore (EMD Millipore, Billerica, MA). Mouse monoclonal anti-RXR was from Abcam (clone MOK13-17, Abcam, Cambridge, MA) and rabbit anti-phospho FAK (Y397) was from Invitrogen (Invitrogen Life Technologies, Carlsbad, CA). Mouse monoclonal anti-β-actin was from Sigma (A1978, Sigma Aldrich, St. Louis, MO).
UAB30 was synthesized as previously described [4].
Antibodies for immunofluorescence were primary anti-RXR antibody (ab2815, Abcam, 1:1000) and secondary goat anti-mouse Alexa Fluor 594 antibody (A-11045, 1:33, Thermo Fisher Scientific, Rockford, IL).
Immunoblotting
Western blots were performed as previously described [5]. Briefly, whole cell lysates were isolated using RIPA [10 mM Tris base pH 7.2, 150 mM NaCl, 1% Nadeoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS)] supplemented with protease inhibitors (Sigma), phosphatase inhibitors (Sigma) and phenylmethanesulfonylfluoride. Lysates were cleared by centrifugation at 14,000 rpm for 30 min at 4 °C. Protein concentrations were de termined using BCA Protein Assay Reagent (Pierce, Rockford, IL) and separated by electrophoresis on sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gels. Antibodies were used according to manufacturer’s recommended conditions. Molecular weight markers (Precision Plus Protein Kaleidoscope Standards, Bio-Rad, Hercules, CA) confirmed the expected size of the target proteins. Immunoblots were developed with Luminata Classico or Crescendo ECL (EMD Millipore). Blots were stripped with stripping solution (Bio-Rad) at 37 °C for 15 minutes and then re-probed with selected antibodies. Equal protein loading was confirmed by immunoblotting with antibody to β-actin or GAPDH. Densitometry was performed using Scion Image Program. Each band was normalized to background on the blot, and then normalized to their respective actin band. All bands were compared to the 0 μM treatment group, which was given the value of 1 as previously reported [6].
Immunofluorescence
Immunofluorescence staining was utilized to detect movement of RXR into the nucleus following UAB30 treatment (10 μM). Cells were plated on glass chamber slides, allowed to attach and treated with UAB30. After 48 hours, cells were fixed with 3% paraformaldehyde, permeabilized with 0.15% Triton X-100, and the primary antibody was added and incubated at room temperature (RT) for 1 hour. The Alexa Fluor 594 secondary antibody was added for 45 minutes at RT. Prolong Gold antifade reagent with DAPI (P36931, Invitrogen) was used for mounting. Imaging was performed with a Zeiss LSM 710 Confocal Scanning Microscope with Zen 2008 software (Carl Zeiss MicroImaging, LLC, Thornwood, NY) using a 63× objective with a zoom of 0.9. MetaMorph® Microscopy Image Analysis Software (Ver. 7.6, Analytical Technologies, Molecular Devices, Sunnyvale, CA) was used to analyze the images and detect overlap.
Immunohistochemistry
After institutional IRB approval (IRB number X100930009), human formalin-fixed, paraffin-embedded RMS tumor specimens were cut (6 μM sections), baked at 70 °C for 1 hour, deparaffinized, rehydrated and steamed. Sections were quenched with 3% hydrogen peroxide and blocked with blocking buffer (BSA, powdered milk, Triton X-100, PBS) for 30 minutes at 4 °C. The primary RXR antib ody (ab2815, Abcam) was added (1:200) and incubated overnight at 4 °C. After a PBS wash, secondary antibody (1:400, rabbit anti-mouse SuperPicture Polymer HRP, Invitrogen) was added for 1 hour at 22 °C. Staining reactions were developed with VECTASTAIN Elite ABC kit (PK-6100, Vector Laboratories, Burlingame, CA), TSA™ (biotin tyramide reagent, 1:400, PerkinElmer, Inc., Waltham, MA) and 3,3′-diaminobenzidine (DAB) (ImmPACT DAB, Vector Laboratories, Burlingame, CA). Slides were counterstained with hematoxylin. Negative control [mouse IgG (1 μg / mL, Invitrogen)] was included with each run. A board-certified pathologist (EMM) graded the RXR staining as either negative, weak, moderate or strongly positive.
Cell viability, proliferation, apoptosis assays
Cell viability was measured with alamarBlue® assays. Briefly, 1.5 × 104 cells per well were plated on 96-well culture plates, allowed to attach, and treated with UAB30 at increasing concentrations for 48 hours. Following treatment, 10 μL of alamarBlue® dye (Invitrogen Life Technologies, Carlsbad, CA) was added and after 4-6 hours, the absorbance at 595 nm was measured using a kinetic microplate reader (BioTek Gen5, BioTek Instruments, Winooski, VT). Viability was reported as fold change. Cell proliferation was measured with BrdU assay according to manufacturer’s instructions (Millipore BrdU Cell Proliferation Assay, Millipore Corporation, Billerica, MA). Cells (1.5 × 104 cells per well) were plated, allowed to attach and treated with UAB30 for 48 hours. Proliferation was reported as mean fold change ± SEM.
Apoptosis was detected with two methods, immunoblotting for cleavage of PARP and a colorimetric caspase 3 activation kit (KHZ0022, Invitrogen). For immunoblotting, cells were treated with UAB30 in increasing amounts and whole cell lysates utilized for SDS-PAGE. Membranes were probed with appropriate antibodies with β-actin serving as an internal control for equal protein loading. Increasing intensity of bands for cleaved products indicated apoptosis. Apoptosis was also measured by activation of caspase 3.
Cell cycle analysis
RMS cells were plated (1.0 × 106 cells) and allowed to attach overnight. Cells were then treated with UAB30 (10 μM) for 48 hours. Cells were trypsinized and washed with PBS and fixed in 100% ethanol. Ethanol was removed and cells stained with propidium iodide staining solution containing 0.3 μM propidium iodide (Invitrogen) in 0.1% Triton X and RNAse A (Qiagen, Valencia, CA) for 30 minutes at room temperature. Cells were analyzed with fluorescence-activated cell sorting (FACS) using a FACSCalibur™ Flow Cytometer (Becton Dickinson Biosciences, San Jose, CA). Data were analyzed with ModFit LT software (Verity Sofware House Inc., Topsham, ME). Negative controls were included in each flow cytometry run.
Migration assays
Migration was measured using twelve-well culture plates (TransWell®, Corning Inc., Lowell, MA) with 8 μm micropore inserts. The bottom side of the insert was coated with collagen Type I (10 mg/mL, 50 μL for 4 hours at 37 °C). RMS cells were treated with UAB30 and were placed into the insert at a concentration of 4 × 104 cells per insert. Cells were allowed to migrate through the micropore insert for 24 hours. The inserts were then fixed with 3% paraformaldehyde, stained with crystal violet, and migrated cells counted with a light microscope. Migration was reported as fold change.
Cell migration was also measured utilizing a cell monolayer wounding (scratch) assay. RMS cells (2.5 × 105 cells per well) were plated in a six-well culture dish and allowed to attach overnight. A 200 μL pipette tip was utilized to create a uniform scratch in the near-confluent cell layer and photos [Photometrics CoolSNAP HQ2 CCD camera (Tuscon, AZ)] attached to a Nikon Eclipse Ti microscope (Tokyo, Japan)] were obtained at the time the scratch was made. Cells were treated with 0, 10 or 25 μM UAB30 for 24 hours and photos were repeated. The area of the scratch was quantified by measuring the pixel count of the scratched area and comparing it to the pixel count of the same plate at the time the scratch was made and reported as fold change in scratch closure.
Invasion assay
Invasion assays were performed similar to migration. Twelve-well culture plates (TransWell®, Corning) with 8 μm micropore inserts were used. The top side of the insert was coated with Matrigel™ (BD Biosciences) (1 mg/mL, 50 μL for 4 hours at 37 °C). RD and SJCRH30 cells were treated with UAB30 and plated into the insert at a concentration of 4 × 104 cells per well. Cells were allowed to invade into the Matrigel™ (BD Biosciences) layer for 48 hours. The inserts were then fixed with 3% paraformaldehyde, stained with crystal violet, cells counted with a light microscope and invasion reported as fold change.
Attachment independent growth
Attachment-independent growth was determined using the soft agar assay. A layer of 2× culture media and 1% noble agar (BD Biosciences) in a 1:1 ratio was poured into 60 × 15 mm petri dishes and allowed to cool. A second layer containing the same ratio of culture media and agar, but also RD RMS cells (1 × 104 per dish), was added. Dishes were treated with UAB30 in fresh media twice weekly. After colonies were visible by eye, the dishes were imaged using Gel Dock Imager (Bio-Rad) and Quantity One software (Bio-Rad). The number of colonies per dish were counted using ImageJ (https://imagej.nih.gov/ij/).
Data analysis
Experiments were repeated at least in triplicate, and data reported as mean ± standard error of the mean. Student’s t-test, Fisher’s exact test, or ANOVA was used as appropriate to compare data. Statistical significance was determined at the p<0.05 level.
Results
RXR is expressed in RMS cell lines and human tumor specimens
UAB30 binds to the RXR receptor so we determined whether this receptor was present in the RMS cell lines. Immunoblotting detected RXR protein expression in both the SJCRH30 and RD RMS cell lines (Fig. 1 A). To determine potential clinical relevance, immunohistochemistry was used to stain human ARMS and ERMS tissue specimens for RXR receptor. Representative photomicrographs presented demonstrate positive staining in brown in both tumor types (Fig. 1 B). IgG negative control responded appropriately (Fig. 1 B) (insert, lower left panel). RXR receptor staining was positive in 6 of 6 ARMS specimens and 8 of 9 ERMS specimens. Binding of rexinoid or retinoid ligands to the RXRs or RARs results in translocation of the receptor to the nucleus. Immunofluorescence staining and confocal microscopy was performed to determine whether RXRs translocated into the nucleus following treatment with UAB30. Figure 1 C presents the data in graphical form, and Figures 1 D and E are representative photomicrographs of stained cells. UAB30 resulted in movement of the RXRs into the nucleus in both RD and SJCRH30 RMS cell lines, but only reached statistical significance in the RD cell line (Fig. 1 C). Baseline staining between the two cell lines was nearly the same.
Figure 1. RXR receptor is present in human RMS cells.
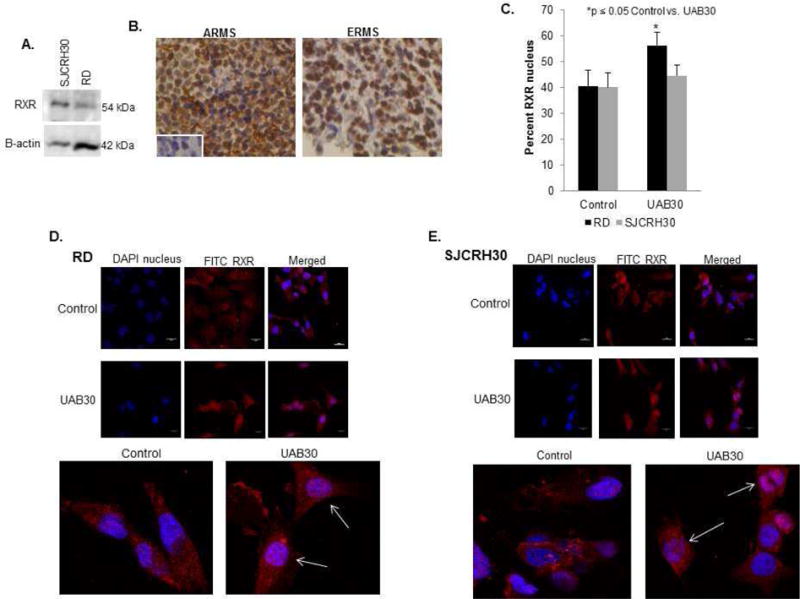
A Immunoblotting was performed to demonstrate the presence of RXR protein in SJCRH30 and RD RMS cell lines. RXR protein was expressed in both cell lines. β-actin included as protein loading control. B Immunohistochemistry was performed on formalin-fixed, paraffin-embedded human ARMS and ERMS specimens for RXR. RXR staining was seen in 6 of 6 ARMS samples and 8 of 9 ERMS samples as shown by brown stain in representative photographs. IgG negative control stained appropriately (left lower insert). C Immunofluorescence staining and confocal microscopy was used to detect RXR staining in the nucleus of SJCRH30 and RD cells with and without treatment with UAB30. The staining of RXR in the nucleus was calculated with Metamorph and reported as percent nuclear staining and represented graphically. UAB30 treatment resulted in movement of RXR into the nucleus in both RD and SJCRH30 RMS cell lines, but only reached statistical significance in the RD cell line. D. Representative photomicrographs of RD cells stained for RXR (red) and nucleus (blue) showed increased overlap after UAB30 treatment. E Representative photomicrographs of SJCRH30 cells stained for RXR (red) and nucleus (blue) show increased overlap after UAB30 treatment. Magnified views are presented below D and E.
UAB30 decreased survival and proliferation and led to apoptosis in RMS cells
Retinoids decrease cell survival and result in differentiation leading to decreased proliferation [1]. We next determined whether UAB30 treatment would decrease cell survival and proliferation in RMS cell lines. UAB30 significantly decreased survival in both the SJCRH30 and RD cell lines (Fig. 2 A). The lethal dose 50% (LD50) in the RD cell line was 26.5 μM and in the SJCRH30 cell line it was 26.1 μM. Since non-viable cells do not proliferate, we chose a concentration of UAB30 well below the LD50 to study proliferation. Proliferation of RD and SJCRH30 cells was significantly inhibited with UAB30 treatment at 10 μM (Fig. 2 B).
Figure 2. UAB30 decreased survival and proliferation and led to apoptosis in RMS cells.
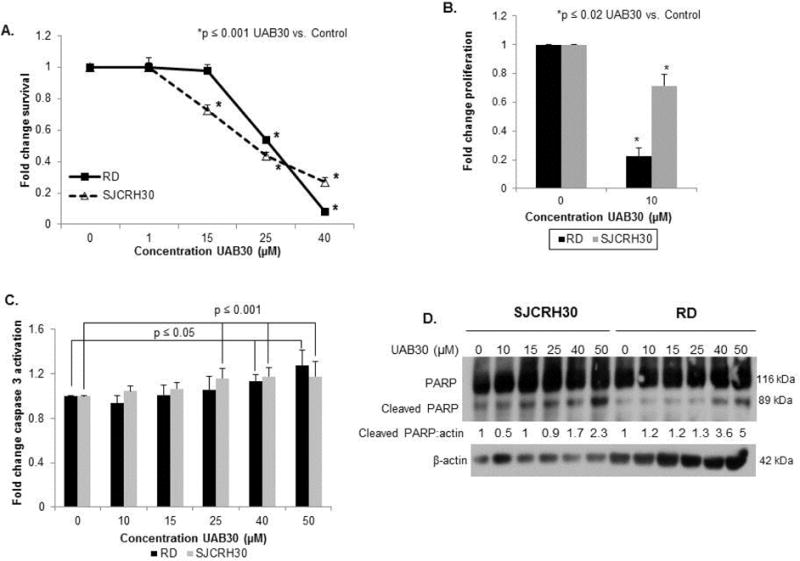
A Cell survival following treatment with UAB30 was assessed using alamarBlue®. 1.5 × 104 cells per well were plated and treated for 48 hours. Survival was reported as mean fold change ± SEM. UAB30 significantly decreased survival in both the RD and SJCRH30 cell lines. The lethal dose 50% (LD50) in the RD cell line was 26.5 μM and in the SJCRH30 cell line it was 26.1 μM. B Proliferation was assessed using BrdU Proliferation Assay. 1.5 × 104 cells per well were plated and treated for 48 hours. Proliferation was reported as mean fold change ± SEM. Proliferation of the RD and SJCRH30 cells was significantly inhibited with UAB30 treatment at 10 μM. C A caspase 3 activation assay was performed to assess effects of UAB30 on apoptosis in RMS cell lines. The results were reported as mean fold change ± SEM, and there was a statistically significant increase in caspase 3 expression following 40 μM treatment of RD cells and 25 μM treatment of SJCRH30 cells. D Immunoblotting was used to identify evidence of PARP cleavage, another marker of apoptosis. Cells were treated with increasing amounts of UAB30. Whole cell lysates were separated on SDS-PAGE and membranes probed with appropriate antibodies. β-actin served as an internal control for equal protein loading. The increase in intensity of the cleaved PARP band (89 kDa) with increasing doses of UAB30 reflects apoptosis seen in both the SJCRH30 and RD cell lines. Band densitometry further illustrated increased cleaved PARP.
UAB30 has been shown to cause apoptosis in other pediatric solid tumors as a mechanism for decreased cell survival [7]. Apoptosis was measured by two methods; caspase 3 activation and immunoblotting for PARP cleavage. RMS cells were treated with increasing concentrations of UAB30. Caspase 3 activation (Fig. 2 C) and PARP cleavage (Fig. 2 D) were both increased in the RD and SJCRH30 cell lines with UAB30, indicating apoptosis occurred.
RMS cells treated with UAB30 underwent cell cycle arrest
Retinoids [1] and UAB30 cause cell cycle arrest in the G1/S phase. RMS cells were treated with UAB30 (10 μM) and cell cycle analysis was completed using propidium iodide staining and FACS analysis. In the RD cell line, there was a significant increase in the percentage of cells in G1 and a significant decrease in the percentage of cells in S phase following treatment with UAB30. Similar results were noted in the SJCRH30 cell line. Figure 3 A shows representative histograms of the cell cycle analysis of a single experiment. Experiments were repeated in triplicate and are presented in tabular and graphic form in Figure 3 B and C, respectively. Bolded numbers in the table represent those values that were statistically significant (p ≤ 0.05).
Figure 3. RMS cells treated with UAB30 underwent cell cycle arrest.
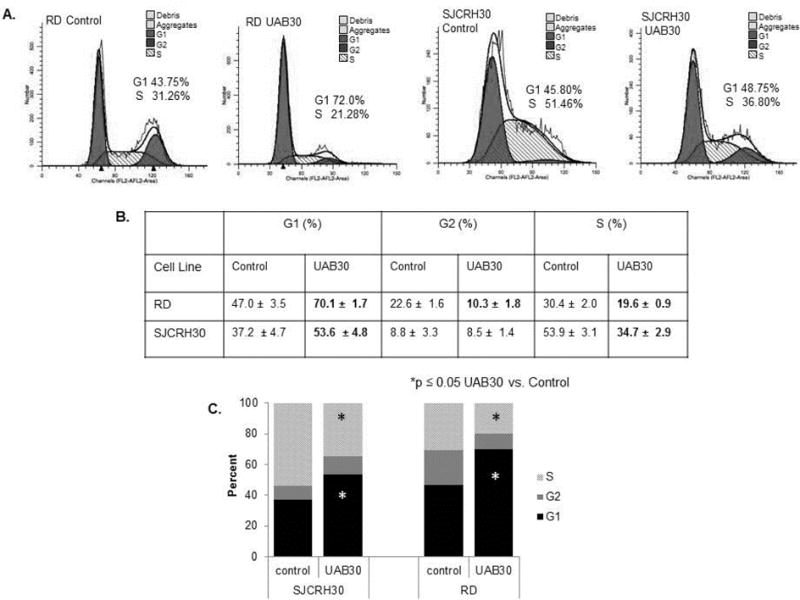
A RMS cells were plated (1.0 × 106 cells), allowed to attach, and cells were treated with UAB30 (10 μM) for 48 hours. Propidium iodine staining along with FACS analysis was used to evaluate the effects of UAB30 on the cell cycle. The histograms presented are representative of one experiment. B Cell cycle analysis was repeated in triplicate, and the table represents the results for both cell lines. In the RD and SJCRH30 cell lines, there was a significant increase in the percentage of cells in G1 and a significant decrease in the percentage of cells in S phase following treatment with UAB30. C The cell cycle results demonstrated graphically reflect the significant increase in G1 and decrease in S phase. The black bars represent percentage of cells in G1, dark grey bars represent percentage of cells in G2, and light grey bars represent the percentage of cells in S phase (*p ≤ 0.05).
UAB30 decreased migration and invasion in RMS cells
In neuroblastoma cell lines, rexinoids have been reported to decrease cell motility [7]. To determine whether UAB30 affected motility in RMS cells, migration and invasion were studied. RMS cells were treated with increasing concentrations of UAB30. Migration was measured with two methods, modified Boyden chamber and cell wounding assay. There was a significant decrease in the number of RD and SJCRH30 cells migrating across the membrane after UAB30 treatment (Fig. 4 A). Additionally, UAB30 decreased the ability of these cells to migrate across a standard scratch wound (Fig. 4 B). Invasion was measured also using a modified Boyden chamber. Following UAB30 treatment, RD and SJCRH30 cells had a significant decrease in invasion (Fig. 4 C). Finally, attachment independent growth of RD cells after UAB30 treatment was examined. UAB30 significantly diminished the number of RD cell colonies formed in soft agar (Fig. 4 D). Attachment independent growth was not assessed in the SJCRH30 cell line as these cells did not reliably form colonies on soft agar.
Figure 4.
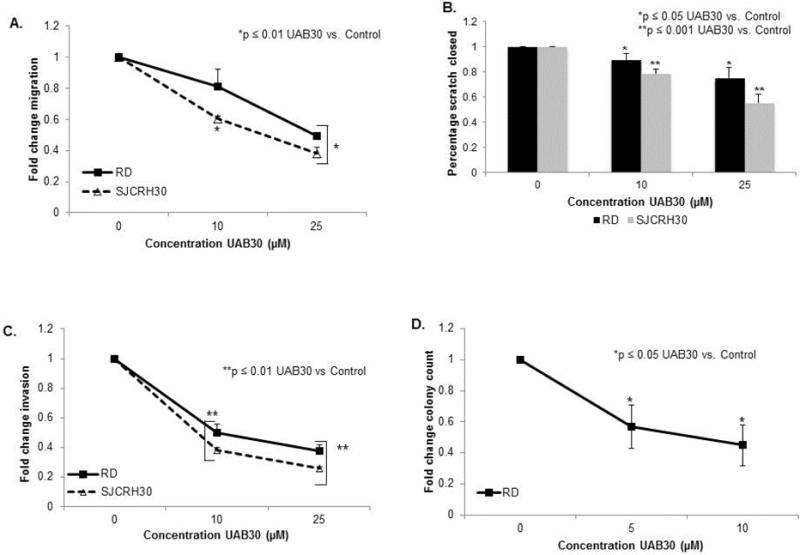
UAB30 decreased migration and invasion in RMS cells. A Cell migration following treatment with UAB30 was assessed using a modified Boyden chamber coated on the bottom with collagen Type 1. 4×104 cells were plated into each insert after being treated with UAB30 and were allowed to migrate through the 8uM micropores for 24 hours. The migrated cells were counted via light microscope and the results reported as a fold change. A significant decrease in the migration of those cells treated with UAB30 is evident in both the RD and SJCRH30 cell line. B Cell migration was also assessed using cell monolayer wounding (scratch) assay. 2.5 ×105 cells were plated, allowed to attach, and then a standard scratch was made in the plate using a 200 μL pipette tip. The cells were then treated with UAB30 and after 24hours the area of open scratch remaining was quantified. The change in scratch area was reported as a fold change ± SEM in scratch closure, and the significant decrease in scratch closure in both RD and SJCRH30 cell lines at both 10 and 25 μM is demonstrated on the bar graph. C A modified Boyden chamber, coated with Matrigel™ on top side of the insert, was utilized to assess invasion. 4 × 104 cells were plated in the inner insert. After being treated with UAB30, the cells were allowed to invade for 24 hours. A light microscope was used to count the number of invading cells and the result was reported as a fold change ± SEM. Both RD and SJCRH30 cells had decreased invasion following treatment with UAB30. D Attachment-independent growth was determined using the soft agar assay. After treatment with UAB30, the number of colonies on each soft agar plate was counted and the result was reported as a fold change ± SEM. A significant decrease in the number of colonies was seen in those plates treated with UAB30.
UAB30 treatment did not alter phosphorylation of FAK, ERK or AKT
Various pathways have been implicated as mechanisms of action of retinoids. For example, it has been shown that retinoids activated multiple kinase pathways including AKT, ERK and FAK in other tumor types [8, 9]. Therefore, the effects of UAB30 upon these kinases in RMS cell lines were assessed. SJCRH30 and RD RMS cells were treated with UAB30 at increasing concentrations for 48 hours and immunoblotting was performed on whole cell lysates to detect total and activated FAK, ERK and AKT. There was no demonstrable change in the phosphorylation of these kinases with UAB30 treatment of RMS cell lines (Fig. 5 A, B). In all instances, the changes in phosphorylation corresponded to changes in the total protein expression. These findings imply that the changes seen in differentiation and cellular survival induced by UAB30 did not likely involve these pathways and that other mechanisms are possibly involved.
Figure 5. UAB30 treatment did not alter phosphorylation of FAK, ERK or AKT.
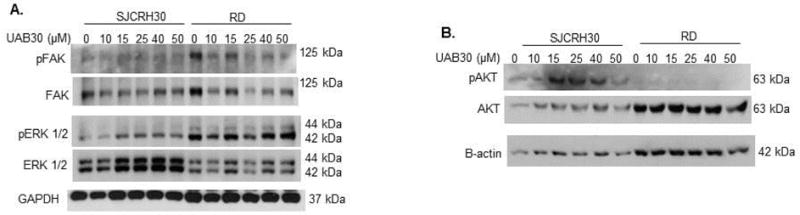
A Immunoblotting was used to assess FAK and ERK phosphorylation following treatment with UAB30. RD and SJCRH30 cells were treated with UAB30 for 48 hours. Whole cell lysates were separated on SDS-PAGE gels and appropriate antibodies were used to identify pERK and total ERK and pFAK and total FAK following treatment with UAB30. No significant difference was identified. Differences in phosphorylation band density correlated with differences in total protein. GAPDH was utilized as an internal control for equal protein loading. B Immunoblotting was utilized to detect changes in AKT phosphorylation and protein expression following 48 hour treatment with UAB30. There was no significant change in AKT phosphorylation with increasing UAB30 doses. β-actin served as an internal control for equal protein loading.
Discussion
Significant advances in the treatment of RMS have been made over the last fifty years, with cure rates increasing from 25% in the 1970s to 70% in the mid-1990s. However, patients with high risk disease continue to have survival rates less than 30% [10]. Survival rates for adolescents and young adults also lag behind those of the younger pediatric population [10].
The differentiating properties of retinoids make them a logical therapeutic for RMS which is a cancer of myogenic origin that has been arrested prior to terminal differentiation. However, the utility of retinoid compounds in the treatment of this disease has been controversial. Brodowicz et al reported decreased proliferation in the RMS cell line HTB-82 following treatment with 9-cis-RA, 13-cis-RA and ATRA, but only 9-cis-RA and ATRA induced apoptosis [11]. Crouch and Helman investigated the effects of ATRA on the ERMS cell line RD and the ARMS cell line RH30 and found that ATRA but not 13-cis-RA significantly inhibited cell growth [12]. Other authors have found RMS cell lines to be resistant to retinoid-induced apoptosis or decreased proliferation [13, 14]. In the current study we found that UAB30 not only decreased cell survival, but also resulted in decreased proliferation and cell cycle arrest.
UAB30 selectively binds to and activates RXR and demonstrates little RAR binding. Previously, Ricaud and colleagues explored the effects of RA on RMS cell lines. They found that SJCRH30 and RD cell lines were resistant to the differentiating effects of RA. They showed that these cell lines expressed little RAR and postulated that the lack of response to retinoic acid, which requires RAR to function, was explained by the paucity of RAR expression [13]. In the current study, we noted significant expression of RXR in both the SJCRH30 and RD cell lines and the human ARMS and ERMS tumor samples tested. Since UAB30 does not require RAR to function and depends upon RXR, the presence of RXR likely explains the significant responses seen following UAB30 treatment. To our knowledge, the current study is novel in its exploration and reporting of RXR protein expression in these RMS cell lines.
UAB30 caused the RXR receptor to migrate to the nucleus in both the RD and SJCRH30 cell line, but to a lesser degree in the SJCRH30 cell line. Despite this finding, the SJCRH30 cell line was as sensitive to the effects of UAB30 as the RD cell line. This observation is similar to previous studies that showed that RXR just needed to be expressed for UAB30 to function and the amount of RXR present did not correlate with phenotypic changes [7]. The decreased nuclear migration of the RXR receptor in the SJCRH30 cell line may reflect the greater treatment challenge with ARMS compared to ERMS, but further studies will be required to elucidate this relationship.
In the present study, treatment with UAB30 resulted in decreased cell proliferation, which is a hallmark of cell differentiation. Crouch and Helman [12] showed that ATRA inhibited cell growth in RD and RH30 RMS cells but did not lead to biochemical or morphologic evidence of differentiation. Barlow et al investigated the effects of 3 different retinoid compounds on 5 RMS cell lines. They found that ATRA and 9-cis-RA suppressed the growth of Rh4 and Rh28 and 9-cis-RA suppressed growth of RD, but Rh30 and Rh18 did not respond to either one. Further, cell morphology was altered in only two cell lines, Rh4, Rh28, and only by ATRA [14]. Another group studied the effects of ATRA on Rh30 and JR1 RMS cell lines and found an increased expression of myogenin indicating differentiation, but there was no cell cycle arrest or decreased proliferation [15]. Specific markers of cell differentiation were not investigated in the current study since both cell proliferation and cell cycle arrest were seen following UAB30 treatment.
We investigated previous hypotheses on the mechanism of action of RA to determine if those findings applied to UAB30 in RMS. In human solid tumors and leukemia, it has been proposed that RA-induced cell differentiation was dependent upon AKT activation [9, 16, 17], ERK activation [9, 18, 19] and activation of focal adhesion kinase (FAK) [9]. Other authors noted decreased phosphorylation of AKT in ATRA treated glioblastoma [20], decreased phosphorylation of ERK in medulloblastoma following fenretinide treatment [21] and in skin after ATRA treatment [22], and decreased FAK activation in breast cancer cells following RA treatment [23, 24, 25]. In the current investigations, phosphorylation of AKT, ERK or FAK did not change. Li and others noted similar findings in gallbladder cancer [26]. The mechanisms responsible have not been clearly defined and appear to be cell line specific. Hansen et al studied the mechanism of UAB30 in breast cancer and proposed that the compound’s effect on proliferation and survival was a result of down regulating temolerase by silencing the hTERT promoter [27]. Other investigators demonstrated that rexinoids regulate MyoD gene expression as well as histone acetylation, thereby affecting the differentiation of myoblasts, which may shed light on how rexinoids differentiate RMS cells [28]. Due to the fact that rexinoids are promiscuous ligands, these and many other potential pathways must be further explored to clarify UAB30’s mechanism of action, and this will be the focus of ongoing research.
Conclusion
In this study, we demonstrated that treatment of RMS cells with UAB30 decreased cell survival and proliferation in both embryonal and alveolar RMS cell lines. There was evidence of decreased cell motility as well as cell cycle arrest in the G1 phase and apoptosis. These results are promising for future therapeutic development using UAB30 in cases of advanced or metastatic disease alone or in combination with other therapeutic modalities.
Acknowledgments
This work was funded in part by a grant from the Mortimer A. and Josephine B. Cohen Research Acceleration and Innovation Fund (EA Beierle) and institutional grants from the National Cancer Institute including T32 CA183926 training grant in translational oncology (AP Williams) and T32 CA091078 training grant in surgical oncology (AM Waters). The content of this manuscript was solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The authors also acknowledge the UAB cores for Flow Cytometry (grants P30 AR048311 and P30 AI027767) and High Resolution Imaging (grants P30 CA013148 and P30 AR048311) with special thanks to Shawn Williams for his assistance with confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution: APW and AMW performed experiments and completed data analysis. APW wrote manuscript. JES assisted with experiments. VRA synthesized UAB30 and reviewed manuscript. EMM reviewed pathology slides and manuscript. DDM and CJG reviewed manuscript. EAB designed experiments, reviewed data and assisted in paper writing and editing.
References
- 1.Barlow JW, Wiley JC, Mous M, et al. Differentiation of rhabdomyosarcoma cell lines using retinoic acid. Pediatr Blood Cancer. 2006;47:773–784. doi: 10.1002/pbc.20650. [DOI] [PubMed] [Google Scholar]
- 2.Malempati S, Hawkins DS. Rhabdomyosarcoma: Review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer. 2012;59:5–10. doi: 10.1002/pbc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolesar JM, Hoel R, Pomplun M, et al. A Pilot, first-in-human, pharmacokinetic study of 9cUAB30 in healthy volunteers. Cancer Prev Res (Phil) 2010;3:1565–1570. doi: 10.1158/1940-6207.CAPR-10-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atigadda VR, Vines KK, Grubbs CJ, et al. Conformationally defined retinoic acid analogues. 5. Large-scale synthesis and mammary cancer chemopreventive activity for (2E,4E,6Z,8E)-8-(3′,4′-dihydro-1′(2′H)-naphthalen-1′-ylidene)-3,7-dimethyl-2,4,6-octatrienoic acid (9cUAB30) J Med Chem. 2003;46:3766–3769. doi: 10.1021/jm030095q. [DOI] [PubMed] [Google Scholar]
- 5.Beierle EA, Trujillo A, Nagaram A, et al. N-MYC regulates focal adhesion kinase expression in human neuroblastoma. J Biol Chem. 2007;282:12503–12516. doi: 10.1074/jbc.M701450200. [DOI] [PubMed] [Google Scholar]
- 6.Gillory LA, Stewart JE, Megison ML, Waters AM, Beierle EA. FAK and p53 synergistically decrease neuroblastoma cell survival. J Surg Res. 2015;196:339–349. doi: 10.1016/j.jss.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters AM, Stewart JE, Atigadda VR, et al. Preclinical evaluation of a novel RXR agonist for the treatment of neuroblastoma. Mol Cancer Ther. 2015;14:1559–1569. doi: 10.1158/1535-7163.MCT-14-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark O, Daga S, Stoker AW. Tyrosine phosphatase inhibitors combined with retinoic acid can enhance differentiation of neuroblastoma cells and trigger ERK- and AKT-dependent, p53-independent senescence. Cancer Lett. 2013;328:44–54. doi: 10.1016/j.canlet.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Qiao J, Paul P, Lee S, et al. PI3K/AKT and ERK regulate retinoic acid-induced neruoblastoma cellular differentiation. Biochem Biophys Res Commun. 2012;424:421–426. doi: 10.1016/j.bbrc.2012.06.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egas-Bejar D, Huh WW. Rhabdomyosarcoma in adolescent and young adult patients: current perspectives. Adolesc Health Med Ther. 2014;5:115–125. doi: 10.2147/AHMT.S44582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodowicz T, Wiltschke C, Kandioler-Eckersberger D, et al. Inhibition of proliferation and induction of apoptosis in soft tissue sarcoma cells by interferon-alpha and retinoids. Br J Cancer. 1999;80:1350–1358. doi: 10.1038/sj.bjc.6690528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crouch GD, Helman LJ. All-trans-retinoic acid inhibits the growth of human rhabdomyosarcoma cell lines. Cancer Res. 1991;51:4882–4887. [PubMed] [Google Scholar]
- 13.Ricaud S, Vernus B, Bonnieu A. Response of human rhabdomyosarcoma cell lines to retinoic acid: relationship with induction of differentiation and retinoic acid sensitivity. Exp Cell Res. 2005;311:192–204. doi: 10.1016/j.yexcr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Barlow JW, Wiley JC, Mous M, et al. Differentiation of rhabdomyosarcoma cell lines using retinoic acid. Pediatr Blood Cancer. 2006;47:773–784. doi: 10.1002/pbc.20650. [DOI] [PubMed] [Google Scholar]
- 15.Al-Tahan A, Sarkis O, Harajly M, et al. Retinoic acid fails to induce cell cycle arrest with myogenic differentiation in rhabdomyosarcoma. Pediatr Blood Cancer. 2012;58:877–884. doi: 10.1002/pbc.23246. [DOI] [PubMed] [Google Scholar]
- 16.García-Regalado A, Vargas M, García-Carrancá A, Aréchaga-Ocampo E, González-De la Rosa CH. Activation of Akt pathway by transcription independent mechanisms of retinoic acid promotes survival and invasion in lung cancer cells. Mol Cancer. 2013;12:44. doi: 10.1186/1476-4598-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng D, Cao Z, Li C, et al. Combination of valproic acid and ATRA restores RARβ2 expression and induces differentiation in cervical cancer through the PI3K/Akt pathway. Curr Mol Med. 2012;12:342–354. doi: 10.2174/156652412799218949. [DOI] [PubMed] [Google Scholar]
- 18.Weng XQ, Sheng Y, Ge DZ, Wu J, Shi L, Cai X. RAF-1/MEK/ERK pathway regulates ATRA-induced differentiation in acute promyelocytic leukemia cells through C/EBPβ, C/EBPε and PU.1. Leuk Res. 2016;45:68–74. doi: 10.1016/j.leukres.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Persaud SD, Park SW, Ishigami-Yuasa M, Koyano-Nakagawa N, Kagechika H, Wei LN. All trans-retinoic acid analogs promote cancer cell apoptosis through non genomic Crabp1 mediating ERK1/2 phosphorylation. Sci Rep. 2016;6:22396. doi: 10.1038/srep22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karmakar S, Banik NL, Ray SK. Combination of all-trans retinoic acid and paclitaxel-induced differentiation and apoptosis in human glioblastoma U87MG xenografts in nude mice. Cancer. 2008;112:596–607. doi: 10.1002/cncr.23223. [DOI] [PubMed] [Google Scholar]
- 21.Bassani B, Bartolini D, Pagani A, et al. Fenretinide (4-HPR) targets daspase-9, ERK 1/2 and the Wnt3a/β-Catenin pathway in medulloblastoma cells and medulloblastoma cell spheroids. PLoS One. 2016;11:e0154111. doi: 10.1371/journal.pone.0154111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheepala SB, Yin W, Syed Z, et al. Identification of the B-Raf/Mek/Erk MAP kinase pathway as a target for all-trans retinoic acid during skin cancer promotion. Mol Cancer. 2009;8:27. doi: 10.1186/14764598-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flamini MI, Gauna GV, Sottile ML, Nadin BS, Sanchez AM, Vargas-Roig LM. Retinoic acid reduces migration of human breast cancer cells: role of retinoic acid receptor beta. J Cell Mol Med. 2014;18:1113–1123. doi: 10.1111/jcmm.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YC, Chang YS, Hsieh MC, et al. All-trans retinoic acid suppresses the adhering ability of ARPE-19 cells via mitogen-activated protein kinase and focal adhesion kinase. J Pharmacol Sci. 2016;132:262–270. doi: 10.1016/j.jphs.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa K, Sogo S, Hioki K, Tokunaga R, Taketani S. Acquisition of cell adhesion and induction of focal adhesion kinase of human colon cancer Colo 201 cells by retinoic acid-induced differentiation. Differentiation. 1998;62:249–57. doi: 10.1046/j.1432-0436.1998.6250249.x. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Imai M, Hasegawa S, Yamasaki M, Takahashi N. Growth inhibition of Refractory human gallbladder cancer cells by retinol, and its mechanism of action. Biol Pharm Bull. 2017;40:495–503. doi: 10.1248/bpb.b16-00934. [DOI] [PubMed] [Google Scholar]
- 27.Hansen NJ, Wylie RC, Phipps SM, Love WK, Andrews LG, Tollefsbol TO. The low-toxicity 9-cis UAB30 novel retinoid down-regulates the DNA methyltransferases and has anti-telomerase activity in human breast cancer cells. Int J Oncol. 2007;30:641–650. [PMC free article] [PubMed] [Google Scholar]
- 28.Hamed M, Khilji S, Dixon K, et al. Insights into interplay between rexinoid signaling and myogenic regulatory factor-associated chromatin state in myogenic differentiation. Nucleic Acids Res. 2017;45:11236–11248. doi: 10.1093/nar/gkx800. [DOI] [PMC free article] [PubMed] [Google Scholar]


