Abstract
Objectives
This study sought to determine how often patients with primary prevention implantable cardioverter-defibrillators (ICDs) meet guideline-derived indications at the time of generator replacement.
Background
Professional societies have developed guideline criteria for the appropriate implantation of an ICD for the primary prevention of sudden cardiac death. It is unknown whether patients continue to meet criteria when their devices need replacement for battery depletion.
Methods
We performed a retrospective chart review of patients undergoing replacement of primary prevention ICDs at 2 tertiary Veterans Affairs Medical Centers. Indications for continued ICD therapy at the time of generator replacement included a left ventricular ejection fraction (LVEF) ≤35% or receipt of appropriate device therapy.
Results
In our cohort of 231 patients, 59 (26%) no longer met guideline-driven indications for an ICD at the time of generator replacement. An additional 79 patients (34%) had not received any appropriate ICD therapies and had not undergone reassessment of their LVEF. Patients with an initial LVEF of 30% to 35% were less likely to meet indications for ICD therapy at the time of replacement (odds ratio: 0.52; 95% confidence interval: 0.30 to 0.88; p = 0.01). Patients without ICD indications subsequently received appropriate ICD therapies at a significantly lower rate than patients with indications (2.8% vs. 10.7% annually, p < 0.001). If ICD generator explantations were performed instead of replacements in the patients without ICD indications, the cost savings would be $1.6 million.
Conclusions
Approximately 25% of patients who receive primary prevention ICDs may no longer meet guideline indications for ICD use at the time of generator replacement, and these patients receive subsequent ICD therapies at a significantly lower rate.
Keywords: ICD generator replacement, implantable cardioverter, defibrillator, sudden cardiac death
Implantable cardioverter-defibrillators (ICDs) reduce mortality in patients with reduced left ventricular function in the absence of previous sustained ventricular arrhythmias (1–3), a treatment strategy referred to as primary prevention. On the basis of the data from several randomized clinical trials, the American College of Cardiology/American Heart Association/Heart Rhythm Society as well as the Centers for Medicare & Medicaid Services have developed specific guideline criteria that patients are required to fulfill to receive an ICD for the primary prevention of sudden cardiac death (SCD) (4). These guideline criteria do not distinguish between patients receiving initial devices and those undergoing generator replacement for battery depletion.
However, after the initial ICD implantation, the clinical characteristics of patients may change. In particular, many patients who receive primary prevention ICDs may experience improvement or recovery of the left ventricular ejection fraction (LVEF) (5,6), and therefore no longer meet indications for a primary prevention ICD at the time of generator replacement.
It is possible that patients who experience improvement or recovery of LVEF may have no benefit from continued ICD therapy. Furthermore, multiple studies have shown that device replacement is associated with significant morbidity and even mortality (7–9). Patients with ICDs may also experience inappropriate therapies that have been shown to have detrimental effects including progression of heart failure, impaired psychological well-being, and impaired survival (10,11). Because ~30,000 replacement procedures are performed in the United States annually (12), ICD replacement also has a significant healthcare cost (13,14). For all of these reasons, research examining the appropriateness of ICD replacement is long overdue.
In this study, we sought to determine how often guideline-derived indications for primary prevention ICD therapy are still present when patients undergo elective ICD generator replacement. Additionally, we examined how often patients who no longer have an indication for primary prevention ICD at the time of generator replacement receive ICD therapies compared with patients who meet these indications. Finally, we sought to estimate the differential costs of replacement versus potentially withholding replacement in patients who no longer meet indications for primary prevention ICD at the time of elective generator replacement.
Methods
Study population
We performed a retrospective chart review of all patients who underwent ICD replacement at the Philadelphia Veterans Affairs (VA) Medical Center and the VA Pittsburgh Healthcare System over a period of 7 years (March 2006 through March 2013) to identify patients who had an ICD initially implanted for primary prevention of SCD on the basis of a low LVEF (≤35%). Within this subgroup, we further identified patients who underwent ICD replacement for battery depletion manifest by achievement of the device elective replacement indicator or end-of-life measure. These patients constituted our study cohort. Patients with any other indication for generator change such as lead malfunction, recall, and upgrade to a dual-chamber or cardiac resynchronization therapy (CRT) device before battery replacement indication were excluded. Patients undergoing their second or more generator change and those who were pacemaker dependent were also excluded. We also excluded patients who received the original device on the basis of MUSTT (Multicenter Unsustained Tachycardia Trial) criteria (i.e., LVEF ≤40% and inducible ventricular tachycardia or fibrillation at electrophysiological study). Clinical records of all veteran patients are maintained in the national VA-wide Computerized Patient Records System (CPRS), and we were able to review the medical records comprehensively for all study patients. The study was approved by the Philadelphia VA Medical Center and VA Pittsburgh Healthcare System Institutional Review Boards.
Data collection and definitions
Data collection included patient characteristics such as age and race, the initial indication for ICD implantation, the type of device implanted (CRT with defibrillator [CRT-D], dual-chamber ICD, or single-chamber ICD), the most recent LVEF, and the presence or absence of comorbid conditions at baseline and at the time of ICD replacement. Comorbid conditions included chronic kidney disease (stage III or greater), dialysis dependence, cognitive impairment, neoplastic disease, atrial fibrillation, hypertension, diabetes, and history of stroke. Pertinent medication use (beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and antiarrhythmic drugs) at baseline and at the time of ICD replacement was reviewed. Data were also collected from device interrogation records, which included delivery of appropriate therapies (shock or antitachycardia pacing for ventricular arrhythmia) and inappropriate therapies (shock or antitachycardia pacing for nonventricular arrhythmia events). Conventional criteria validated in previous ICD trials (3) were used to categorize patients as having ischemic cardiomyopathy (ICM) or non-ICM (NICM).
At the time of the generator replacement, patients were classified into 1 of 3 groups: 1) ICD therapy was considered to be indicated for any patient whose LVEF was ≤35% on the basis of assessment within 1 year of undergoing generator replacement or if the patient had received appropriate therapy (shock or antitachycardia pacing) from their ICD after initial implantation regardless of the LVEF; 2) ICD therapy was considered not indicated in patients who demonstrated an improvement in their LVEF to ≥40% and had not received any appropriate therapies over the lifetime of the original device; and 3) ICD therapy was considered unclear in patients who had not received any appropriate therapies over the lifetime of the original device and had also not had a reassessment of their LVEF within 1 year of undergoing ICD generator replacement. LVEF assessment was on the basis of echocardiographic or nuclear imaging studies.
Cost analysis
Three models were considered for the cost analysis: 1) replace all ICD generators regardless of LVEF; 2) explant generators in the group of patients for whom ICD therapy was considered not indicated; and 3) obtain echo-cardiograms in the group of patients with unclear indications for ICD, assume that the percent of patients for whom ICD therapy was not indicated would be the same in this group as in our overall cohort, and additionally explant generators in those patients whose LVEFs had improved (≥40%). Costs were estimated using Medicare physician and facility payment rates for procedures and Current Procedural Technology (CPT) codes. The total cost of ICD generator replacement (CPT code 33240) was estimated at $22,891 (physician cost was $379.02 and outpatient facility cost was $22,512). Total cost of ICD generator explantation (CPT code 33241) was estimated at $1,907.55 (physician cost was $224.55 and outpatient facility cost was $1,683). Total cost of an echocardiogram (CPT code 93306) was estimated at $580 (physician cost was $189.51 and facility cost was $390.49) (13,14).
Statistical analysis
The characteristics of patients at the time of initial ICD implantation and ICD replacement were compared with McNemar tests for categorical variables and paired t tests for continuous variables. Characteristics of patients who met or did not meet criteria for an ICD at the time of replacement were compared using chi-square tests for categorical variables and t tests for continuous variables. We also performed a multivariable logistic regression analysis with selected variables with known or presumed effects on cardiac remodeling and/or risk of ICD therapy, including the presence of CRT, the etiology of cardiomyopathy, comorbid conditions, medication use, and LVEF at initial implantation to determine whether these could predict whether patients would meet primary prevention ICD indications at the time of generator change. Patients were divided into tertiles of initial LVEF (<15%, 16% to 29%, and 30% to 35%) to facilitate comparisons between groups. Continuous variables are presented as mean SD. A p value <0.05 was considered to be statistically significant.
Results
Baseline characteristics
Our study cohort comprised 231 patients. The mean time between the initial implantation of an ICD and generator replacement was 61 11 months. Characteristics and comorbidities of patients at the time of the initial ICD implantation and at the time of ICD replacement are compared in Table 1. Among the comorbidities, the prevalence of chronic kidney disease (51 of 231 [22%] vs. 68 of 231 [29%]; p < 0.01), atrial fibrillation (37 of 231 [16%] vs. 56 of 231 [24%]; p < 0.01), hypertension (170 of 231 [74%] vs. 189 of 231 [82%]; p < 0.01), diabetes (99 of 231 [43%] vs. 107 of 231 [46%]; p < 0.01), and neoplastic disease (6 of 231 [3%] vs. 33 of 231 [14%]; p < 0.001) was significantly greater at the time of ICD generator replacement. Among the medications, only the rate of beta-blocker use was significantly greater at the time of ICD replacement (177 of 231 [77%] vs. 200 of 231 [87%]; p < 0.01).
Table 1.
Characteristics of Patients at Initial ICD Implantation and at the Time of ICD Replacement
| Initial Implantation (N = 231) | Generator Replacement (N = 231) | p Value | |
|---|---|---|---|
| Age, yrs | 65 ± 10 (66) | 70 ± 9 (70) | <0.01 |
|
| |||
| White race | 184 (80) | – | – |
|
| |||
| ICM | 159 (69) | – | – |
| NICM | 72 (31) | – | – |
|
| |||
| LVEF, % | 23 ± 6 (25) | 33 ± 14 (30) | <0.01 |
|
| |||
| CRT-D | 86 (37) | – | – |
|
| |||
| Comorbidities | |||
| Chronic kidney disease (stage III or greater) | 51 (22) | 68 (29) | <0.01 |
| Hypertension | 170 (74) | 189 (82) | <0.01 |
| Diabetes | 99 (43) | 107 (46) | <0.01 |
| Atrial fibrillation | 37 (16) | 56 (24) | <0.01 |
| History of stroke | 33 (14) | 37 (16) | 0.13 |
| Dialysis dependent | 1 (<1) | 2 (1) | 0.50 |
| Neoplastic disease | 6 (3) | 33 (14) | <0.01 |
| Cognitive impairment | 5 (2) | 9 (4) | 0.13 |
| Nursing facility resident | 1 (<1) | 2 (1) | 0.50 |
|
| |||
| Medication use | |||
| ACE inhibitor or ARB | 198 (86) | 194 (84) | 0.39 |
| Beta-blocker | 177 (77) | 200 (87) | <0.01 |
| Antiarrhythmic drug | 29 (13) | 37 (16) | 0.10 |
Values are mean ± SD (median) or n (%).
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CRT-D = cardiac resynchronization therapy with a defibrillator; ICD = implantable cardioverter-defibrillator; ICM = ischemic cardiomyopathy; LVEF = left ventricular ejection fraction; NICM = nonischemic cardiomyopathy.
Indications and predictors of continued ICD use at generator replacement
Of the 231 patients undergoing generator replacement, primary prevention ICD therapy was considered indicated in 93 patients (40%), not indicated in 59 patients (26%), and unclear in 79 patients (34%) (Fig. 1). Of the 93 patients who fulfilled guideline criteria for an ICD at the time of generator replacement, 50 patients (54%) continued to meet primary prevention indications, 35 patients (38%) had received appropriate ICD therapy in the intervening years and continued to have an LVEF of ≤35%, and 8 patients (8%) received appropriate ICD therapy but demonstrated improvement in LVEF to ≥40% at the time of generator replacement.
Figure 1. ICD Indications at Elective Generator Replacement.
In our cohort of 231 patients, an implantable cardioverter-defibrillator (ICD) was indicated in 93 patients (40%), not indicated in 59 patients (26%), and indications were unclear in 79 patients (34%).
Characteristics of patients who continued to meet criteria for an ICD at the time of replacement versus those who no longer met criteria are compared in Table 2. Except for a significantly higher LVEF (25 ± 11% vs. 49 ± 9%; p < 0.001), there was no other statistically significant difference between the 2 groups. Using a multivariable logistic regression analysis (Table 3), a baseline LVEF of 30% to 35% (compared with LVEF of <30%) was the only significant characteristic associated with a lower likelihood of meeting primary prevention ICD criteria at the time of generator replacement (odds ratio: 0.52; 95% confidence interval: 0.3 to 0.88; p = 0.01). Patients with ICM tended to be more likely than patients with NICM to meet criteria for ICD at the time of generator replacement (odds ratio: 1.89; 95% confidence interval: 0.90 to 3.95; p 0.09), but this did not reach statistical significance.
Table 2.
Characteristics of Patients Who Met or Did Not Meet Criteria for Primary Prevention ICD at the Time of Generator Replacement
| Met Guideline Criteria for ICD (N = 93) | Did Not Meet Guideline Criteria for ICD (N = 59) | p Value | |
|---|---|---|---|
| Age, yrs | 67 ± 9 (65) | 69 ± 9 (67) | 0.88 |
|
| |||
| White race | 68 (73) | 46 (78) | 0.63 |
|
| |||
| Single- or dual-chamber ICD | 61 (66) | 41 (69) | 0.75 |
|
| |||
| CRT-D | 32 (34) | 18 (31) | 0.75 |
|
| |||
| LVEF, % | 25 ± 11 (25) | 49 ± 9 (45) | <0.001 |
|
| |||
| ICM | 54 (58) | 35 (59) | 0.88 |
|
| |||
| Comorbidities | |||
| Chronic kidney disease (stage III or greater) | 28 (30) | 20 (34) | 0.76 |
| Hypertension | 65 (70) | 42 (71) | 0.86 |
| Diabetes | 48 (52) | 27 (46) | 0.59 |
| Atrial fibrillation | 30 (32) | 13 (22) | 0.24 |
| History of stroke or transient ischemic attack | 17 (18) | 9 (15) | 0.79 |
| Dialysis dependent | 1 (1) | 1 (2) | 0.74 |
| Neoplastic disease | 7 (8) | 8 (14) | 0.35 |
| Cognitive impairment | 6 (6) | 0 (0) | 0.12 |
| Nursing facility resident | 3 (3) | 1 (2) | 0.96 |
|
| |||
| Medication use | |||
| ACE inhibitor or ARB | 81 (87) | 46 (78) | 0.21 |
| Beta-blocker | 80 (86) | 52 (88) | 0.90 |
| Antiarrhythmic drug | 19 (20) | 8 (14) | 0.39 |
Values are mean ± SD (median) or n (%).
Abbreviations as in Table 1.
Table 3.
Selected Predictors of Meeting Indications for an ICD at Time of Generator Replacement
| OR (95% CI) | p Value | |
|---|---|---|
| Age, per 10 yrs | 0.98 (0.94–1.02) | 0.30 |
| White race vs. other | 0.87 (0.39–1.90) | 0.73 |
| Initial LVEF 30%–35% vs. <30% | 0.52 (0.30–0.88) | 0.01 |
| ICM vs. NICM | 1.89 (0.90–3.95) | 0.09 |
| CRT-D | 0.95 (0.45–2.03) | 0.90 |
| Chronic kidney disease (stage III or greater) | 0.90 (0.37–2.22) | 0.82 |
| Hypertension | 1.00 (0.47–2.16) | 0.99 |
| Atrial fibrillation | 0.58 (0.26–1.27) | 0.17 |
| ACE-I or ARB prescribed | 0.47 (0.16–1.41) | 0.18 |
| Beta-blocker prescribed | 1.34 (0.49–3.89) | 0.54 |
CI = confidence interval; OR = odds ratio; other abbreviations as in Table 1.
Subsequent ICD therapies
The 59 patients who no longer met indications for primary prevention ICD therapy at the time of generator replacement (but still underwent the replacement) were followed for a mean of 3.5 ± 2.0 years (median, 3.1 years; total of 177 person-years) after generator replacement. During this time, 5 patients (8%) received appropriate ICD therapies (4 received shocks for ventricular tachycardia or ventricular fibrillation and 1 received anti-tachycardia pacing for ventricular tachycardia). Thus, the rate of subsequent appropriate ICD therapy in these patients who no longer met primary prevention ICD indications at the time of generator replacement was 2.8% per person-year. In comparison, patients who continued to meet primary prevention ICD indications at the time of generator replacement had a significantly higher appropriate ICD therapy rate of 10.7% per person-year (log-rank, p < 0.001). Kaplan-Meier survival curves are shown in Figure 2.
Figure 2. Subsequent ICD Therapies After Elective Generator Replacement.
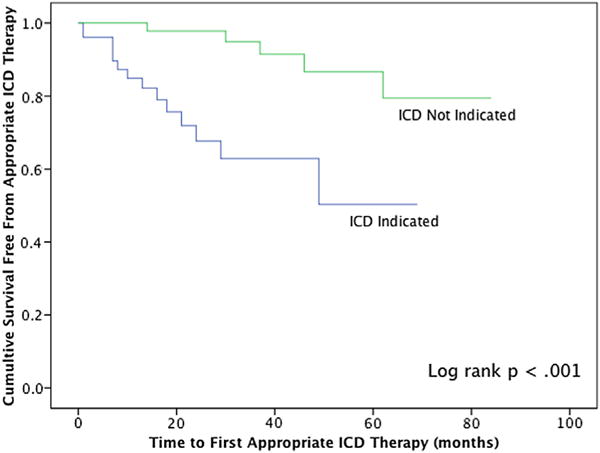
Patients with no ICD indication at the time of generator replacement subsequently receive significantly fewer ICD therapies compared with patients with an ICD indication (2.8% vs. 10.7% per person-year, p < 0.001). ICD = implantable cardioverter-defibrillator.
Cost analysis
Using the first model described in the Methods section, the total cost of replacing all ICD generators regardless of the LVEF was estimated at $5,287,821. Using the second model, the total cost of replacing ICD generators in all patients except those for whom ICD therapy was considered not indicated (and explanting generators in this group) was estimated at $4,049,797.45. Using the third model, in which echocardiograms would be obtained in the group of patients with unclear indications for ICD, and assuming that 26% (range 13% to 39%) of these would be recategorized to the group for whom ICD therapy was considered not indicated and these generators would then be explanted, the total cost was estimated at $3,654,964.55 (range $3,839,962.95 to $3,399,310.50), translating to a cost savings of $1,632,856.45 in the latter group.
Discussion
The salient findings of our study are the following: 1) 26% patients receiving initial ICD implants for primary prevention in the setting of a low LVEF no longer meet guideline-driven indications at the time of elective generator replacement; and 2) these patients subsequently receive appropriate ICD therapies at a significantly lower rate than patients who continued to meet primary prevention ICD indications. These observations, to the best of our knowledge, have never been previously reported.
Our study shows that a significant proportion of patients who receive their initial ICD for primary prevention on the basis of a low LVEF undergo generator replacement despite experiencing recovery of the LVEF to ≥40% and not experiencing any ICD interventions in the intervening years. Although the risk of SCD in patients who experience recovery of the LVEF is unknown and may still be higher than in the general population, the current guidelines for primary prevention ICD therapy are the same for patients undergoing initial implant or generator replacement. Similarly, the Centers for Medicare & Medicaid Services National Coverage Determination does not distinguish between first and subsequent implantations (15). Two recent studies concluded that ICD pulse generators should be replaced even if there is improvement in the LVEF after initial ICD implantation (16,17). However, both are limited by relatively small sample sizes and retrospective designs and base their analyses on the delivery of ICD shocks, which may not be an adequate surrogate for SCD (18,19). Furthermore, the lack of an appropriate control group precludes any possible conclusion of a mortality benefit from these studies. Our study showed that in patients who no longer fulfill primary prevention ICD therapy indications at the time of generator change, the subsequent rate of appropriate ICD therapies is significantly lower in patients who still meet these indications (2.8% vs. 10.7% per person-year; log-rank, p < 0.001). This finding would suggest that generator replacement may not always need to be performed in this population and that the lack of distinction between initial implantation and generator replacement in existing guideline criteria for appropriate use of primary prevention ICDs may be reasonable.
We also found that one-third of patients undergoing elective replacement of devices that were originally implanted for primary prevention had not had a recent assessment of their left ventricular function despite never having received appropriate therapy from their ICD over the lifetime of the device. Possible explanations for this may include a lack of awareness on the part of healthcare providers that guideline criteria for primary prevention ICD need revalidation at the time of generator replacement or the perception that once implanted, ICD is a lifelong therapy. However, in light of the findings of our study that shows significant improvement of left ventricular function in >25% of patients undergoing initial ICD implantation for primary prevention and that these patients subsequently receive ICD therapies at a significantly lower rate, an echocardiogram at the time when the original device reaches elective replacement indications may be beneficial. This is particularly true for patients undergoing prophylactic ICD implantation in the setting of an initial LVEF of 30% to 35%. Reassessment of the LVEF before ICD generator replacement may provide patients with more appropriate counseling regarding the risk-benefit profile. Recent studies have shown that patients undergoing ICD replacement may have double the risk of pocket-related infections and/or require twice as many surgical interventions for hematomas compared with those undergoing initial ICD implantation (7,8). Furthermore, patients who undergo ICD replacement despite recovery of LVEF will continue to be at risk of inappropriate shocks, which have been shown to have detrimental effects on mortality, progression of heart failure, and psychological well-being (10,11,20).
An important implication of our study pertains to the healthcare costs of generator replacement in patients who may no longer meet indications for primary prevention ICD therapy. More than 100,000 ICDs are implanted in the United States annually; of these procedures, ~30,000 are generator replacements (12). In our cohort alone, the cost savings of not replacing ICD generators in patients who did not meet criteria for ICD was >$1.5 million. In contrast, the cost of obtaining echocardiograms, which are relatively simple, noninvasive outpatient tests, to determine the LVEF in patients about to undergo ICD replacement who never received appropriate ICD therapies was <$50,000. These cost calculations would favor an approach by which every patient who receives an ICD for primary prevention and who has not received appropriate ICD therapy over the course of the original device life should undergo echocardiography when the battery reaches its elective replacement or end-of-life indicator. It is also worth mentioning that although initial ICD implantations for primary prevention have been shown to be cost-effective for the numbers of lives saved (21,22), the same may not be true after generator replacement, especially among patients with improved LVEF and/or those undergoing multiple (≥2) generator replacements.
Although using the LVEF alone as a predictor of arrhythmic death is flawed, population studies have shown quite clearly that patients with an LVEF of <30% to 35% have a much higher mortality, attributable in large part to SCD, than patients with an LVEF >40% (23,24). Although these studies examined the risk of mortality on the basis of the initial LVEF, our observations further add to this and suggest that LVEF improvement may impart a similar decrease in the risk of SCD. Although the annual rate of appropriate defibrillator discharge in patients with primary prevention ICDs in major trials is 5.1% (3), CRT responders who experience LVEF improvement to >45% have an estimated 2-year risk of <3% for appropriate ICD therapy, and CRT responders who experience complete recovery of LVEF have a risk of SCD that is comparable to the general population (25,26). These studies, as well as our observations, make a case for performing ICD explantation instead of generator replacement in patients who experience no appropriate therapies and show significant improvement of the LVEF when their devices reach elective replacement indications. In the patients in whom improvement of LVEF has occurred with the original device being CRT-D, a CRT without a defibrillator (CRT-P) device could be used instead of CRT-D for replacement.
Our study found that patients with an LVEF of 30% to 35% at the time of the initial ICD implantation were significantly more likely to not meet guideline-driven indications for primary prevention ICD therapy at the time of replacement compared with patients with an LVEF of ≤15%. Interestingly, although patients with ICM were more likely to meet guideline indications at the time of generator replacement compared with patients with NICM (22% vs. 33%), this difference did not reach statistical significance. Similarly, the type of the original device (CRT-D vs. single- or dual-chamber ICD) was also not a predictor of whether patients fulfilled guideline indications at the time of generator change. The lack of a significant difference in some of these comparisons may be due to the small sample size of our study.
Study limitations
This was a retrospective study of male veterans that examined practice patterns at 2 medical centers, and these results may not be able to be extrapolated to the general population. Although the CPRS system comprehensively captures any care that the patients receive within the VA system and non-VA health care records can also be scanned into this system, it is possible that some veterans may have received care outside of the VA system that was not documented in the CPRS system, and these data may have been missed. Because of the retrospective nature of the study, we were unable to provide accurate data regarding the specifics of ICD programming, which may have affected the rate of appropriate ICD therapies. Our cost calculations were relatively simple and did not take into account the potential cost implications of pursuing incidental findings that may be unmasked in patients undergoing echocardiography before generator change. Furthermore, even though this was a study of veteran patients, we used Medicare physician and facility payment rates for procedures. We used this method because VA healthcare does not typically generate administrative claims indicating the cost of medical care. Finally, although all patients included in this analysis met guideline-derived criteria for primary prevention ICD therapy, the retrospective nature of the study prevented us from being able to determine accurately the time frame between the diagnosis of cardiomyopathy and implantation of the original device or validate optimization of medical therapy before to initial ICD implantation.
Conclusions
Approximately 25% of patients who receive ICDs for primary prevention may no longer meet guideline-driven indications for continued ICD use when their original batteries reach elective replacement or end-of-life indicators. These patients may subsequently receive fewer ICD therapies than those who continue to meet indications. These findings have important implications on healthcare costs. Large-scale studies and/or prospective, randomized trials are needed to determine the mortality benefit and cost-effectiveness of ICD replacement among patients who demonstrate improvement in the LVEF after the initial implantation.
Acknowledgments
Dr. Epstein has received honoraria from Boston Scientific, Medtronic, and St. Jude Medical, Pittsburgh. Research grants from Biotronik, Boston Scientific, Medtronic, and St. Jude Medical. Dr. Dixit has received a research grant from Medtronic. Dr. Deo has received support from grant number K23DK089118 from the National Institutes of Health. Drs. Epstein, Bala, Riley, Deo, and Dixit have received fellowship support from Boston Scientific, Medtronic, and St. Jude Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- CPRS
Computerized Patient Records System
- CPT
Current Procedural Technology
- CRT
cardiac resynchronization therapy
- CRT-D
cardiac resynchronization therapy with a defibrillator
- CRT-P
cardiac resynchronization therapy without a defibrillator
- ICD
implantable cardioverter-defibrillator
- ICM
ischemic cardiomyopathy
- LVEF
left ventricular ejection fraction
- NICM
nonischemic cardiomyopathy
- SCD
sudden cardiac death
- VA
Veterans Affairs
References
- 1.Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease (MUSTT) N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction (MADIT-II) N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure (SCD-HeFT) N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Sharpe DN, Murphy J, Coxon R, Hannan SF. Enalapril in patients with chronic heart failure: a placebo-controlled, randomized, double-blind study. Circulation. 1984;70:271–8. doi: 10.1161/01.cir.70.2.271. [DOI] [PubMed] [Google Scholar]
- 6.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–43. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Krahn AD, Lee DS, Birnie D, et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD database. Circ Arrhythm Electrophysiol. 2011;4:136–42. doi: 10.1161/CIRCEP.110.959791. [DOI] [PubMed] [Google Scholar]
- 8.Poole JE, Gleva MJ, Mela T, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–61. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 9.Kramer DB, Kennedy KF, Noseworthy PA, et al. Characteristics and outcomes of patients receiving new and replacement implantable cardioverter-defibrillators: results from the NCDR. Circ Cardiovasc Qual Outcomes. 2013;6:488–97. doi: 10.1161/CIRCOUTCOMES.111.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52:1111–21. doi: 10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Hammill SC, Kremers MS, Stevenson LW, et al. Review of the registry’s fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7:1340–5. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. Hospital outpatient prospective payment – final rule with comment period and CY2013 payment rates. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices-Items/CMS-1589-FC.html?Page=1&DLSort=2&DLSortDir=descending. Accessed August 9, 2013.
- 14.Centers for Medicare and Medicaid Services. Medicare physician fee schedule. Available at: http://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed August 9, 2013.
- 15.Centers for Medicare and Medicaid Services. National Coverage Determination (NCD) for Implantable Automatic Defibrillators (20.4) Available at: http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=110&ncdver=3&IsPopup=y&NCAId=102&NcaName=Implantable+Defibrillators++Clinical+Trials&bc=AAAAAAAAIAAA&. Accessed October 21, 2013. [PubMed]
- 16.Schliamser JE, Kadish AH, Subacius H, et al. Significance of follow-up left ventricular ejection fraction measurements in the Defibrillators in Non-ischemic Cardiomyopathy Treatment Evaluation Trial. Heart Rhythm. 2013;10:838–46. doi: 10.1016/j.hrthm.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Naksuk N, Saab A, Li J, et al. Incidence of appropriate shock in implantable cardioverter-defibrillator patients with improved ejection fraction. J Cardiac Fail. 2013;19:426–30. doi: 10.1016/j.cardfail.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Ellenbogen KA, Levine JH, Berger RD, et al. Are implantable cardioverter-defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776–82. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- 19.Kim SG, Fogoros RN, Furman S, Connolly SJ, Kuck KH, Moss AJ. Standardized reporting of ICD patient outcome: the report of a North American Society of Pacing and Electrophysiology Policy Conference, February 9-10, 1993. Pacing and Clinical Electro-physiology. 1993;16:1358–62. doi: 10.1111/j.1540-8159.1993.tb01728.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Rees JB, Borleffs CJ, de Bie MK, et al. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. 2011;57:556–62. doi: 10.1016/j.jacc.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 21.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–80. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 22.Yao G, Freemantle N, Calvert MJ, et al. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J. 2007;28:42–51. doi: 10.1093/eurheartj/ehl382. [DOI] [PubMed] [Google Scholar]
- 23.The Multicenter Postinfarction Research Group. Risk stratification and survival after myocardial infarction. N Engl J Med. 1983;309:331–6. doi: 10.1056/NEJM198308113090602. [DOI] [PubMed] [Google Scholar]
- 24.Rouleau JL, Talajic M, Sussex B, et al. Myocardial infarction patients in the 1990sdtheir risk factors, stratification and survival in Canada: the Canadian Assessment of Myocardial Infarction (CAMI) Study. J Am Coll Cardiol. 1996;27:1119–27. doi: 10.1016/0735-1097(95)00599-4. [DOI] [PubMed] [Google Scholar]
- 25.Manfredi JA, Al-Khatib SM, Shaw LK, et al. Association between left ventricular ejection fraction post-cardiac resynchronization treatment and subsequent implantable cardioverter defibrillator therapy for sustained ventricular tachyarrhythmias. Circ Arrhythm Electrophysiol. 2013;6:257–64. doi: 10.1161/CIRCEP.112.000214. [DOI] [PubMed] [Google Scholar]
- 26.Manne M, Rickard J, Varma N, Chung MK, Tchou P. Normalization of left ventricular ejection fraction after cardiac resynchronization therapy also normalizes survival. Pacing Clin Electrophysiol. 2013;36:970–7. doi: 10.1111/pace.12174. [DOI] [PubMed] [Google Scholar]


