Autoimmune polyendocrine syndromes comprise a diverse group of clinical conditions characterized by functional impairment of multiple endocrine glands due to loss of immune tolerance. These syndromes also frequently include conditions such as alopecia, vitiligo, celiac disease, and autoimmune gastritis with vitamin B12 deficiency that affect non-endocrine organs. Failure of multiple glands in an individual patient was first described by Schmidt,1 who reported the combination of hypothyroidism and adrenal insufficiency with lymphocytic infiltration of both the thyroid and adrenal glands in 1926. We have now come to appreciate that these syndromes can be broadly categorized as rare monogenic forms such as autoimmune polyendocrine syndrome type 1 (APS-1) and a more common polygenic variety, autoimmune polyendocrine syndrome type 2 (APS-2).
Autoimmune polyendocrine syndromes are characterized by an insidious presentation, circulating autoantibodies and lymphocytic infiltration of the affected tissues or organs, eventually leading to organ failure. Presentation can occur from early infancy to old age, and new components of a given syndrome can appear throughout life. There is marked variation in the frequencies and patterns of autoimmunity in affected patients and their families, and the risk of developing various organ-specific autoimmune diseases is likely due to a combination of genetic susceptibility and environmental factors.
Monogenic autoimmune polyendocrine syndromes have provided an opportunity to learn more about specific factors that are critical for maintaining immune tolerance. In parallel, major advances in characterizing autoimmunity in patients, such as the identification of new autoantibody targets associated with distinct diseases and their manifestations have occurred. This article reviews some of these important developments and discusses approaches for the appropriate diagnosis and longitudinal follow-up of affected patients.
AUTOIMMUNE POLYENDOCRINE SYNDROME TYPE 1
Autoimmune polyendocrine syndrome type 1 (APS-1), also named autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED, OMIM 240300), is a rare autosomal recessive disease caused by mutations in the autoimmune regulator gene (AIRE).2,3 The estimated prevalence is roughly 1:80000 in most countries, with a higher prevalence in some countries such as Finland (1:25000) and Sardinia (1:14000) and among Persian Jews living in Israel (1:9000).4
CLINICAL FEATURES
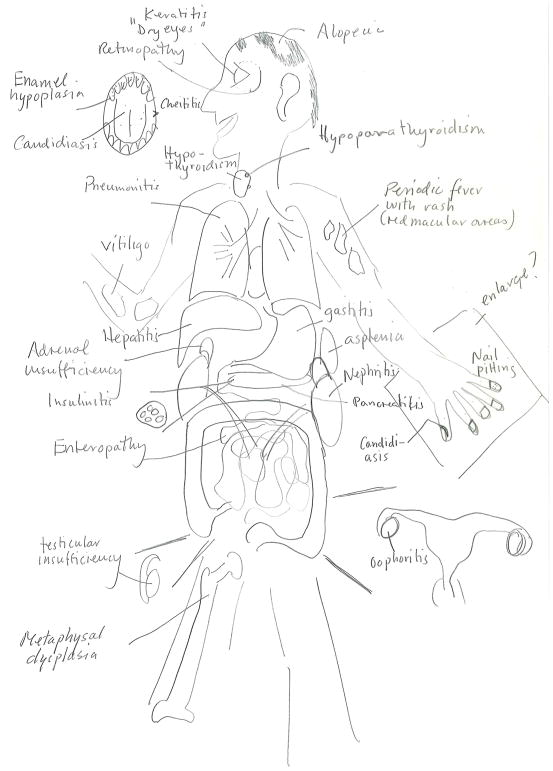
APS-1 is characterized by the development of at least two of three cardinal components during childhood -- chronic mucocutaneous candidiasis, hypoparathyroidism, and primary adrenal insufficiency (Addison’s disease).4 Other typical components include enamel hypoplasia, enteropathy with chronic diarrhea and-or constipation. Premature ovarian insufficiency, affecting about 60% of women with APS-1 before they reach 30 years of age (Fig. 1) is common. Other classic components are less frequent but may include bilateral keratitis, often accompanied by severe photophobia, and periodic fever with rash, as well as autoimmune-induction of hepatitis, pneumonitis, nephritis, exocrine pancreatitis, and functional asplenia.5–9 Such findings should prompt clinicians to consider the diagnosis of APS-1, especially in young persons. Rarely, retinitis, metaphyseal dysplasia, pure red cell aplasia8 and polyarthritis10 have been associated with APS-1 (Fig. 1).
Figure 1. Organ-specific Manifestations of Autoimmune Polyendocrine Syndromes.
The main manifestations of autoimmune polyendocrine syndrome type 1 (APS-1, red box), autoimmune polyendocrine syndrome type 2 (APS-2, blue box) and X-linked immunodysregulation polyendocrinopathy enteropathy (IPEX, green box). Primary adrenal insufficiency and type 1 diabetes are shared between APS-1 and −2, respectively (red-blue box); Type 1 diabetes is shared between Aps-2 and IPEX (blue-green box).
Several recent case series indicate that the phenotypic variation and age of symptom onset vary greatly, even within the same family,6–8,11 implying that other genes such as major histocompatibility complex genes,12 or environmental exposures, influence the phenotype and natural course. For example, a recent Norwegian survey reported that only 40 % of affected patients developed all three main components of APS-1.6 Some affected persons develop a single minor component during childhood, and the first main manifestation later, as adults. This wide variation in presentation and symptomatology makes the diagnosis of APS-1 challenging.
However, in most patients, disease manifestations develop earlier and are usually more severe than in APS-2. Typically, a given APS-1 patient develops an average of 4–5 manifestations of the syndrome, but may have as few as one or as many as 20. Due to chronic mucocutaneous candidiasis, patients are also susceptible to squamous carcinoma of the oral mucosa and esophagus over time. In general, patients with APS-1 have an increased risk of mortality,13 due to cancer, adrenal and hypocalcemic crises, and certain conditions induced by aberrant autoimmune responses, particularly hepatitis, nephritis and pneumonitis.
GENETICS AND DISEASE MECHANISMS OF APS-1
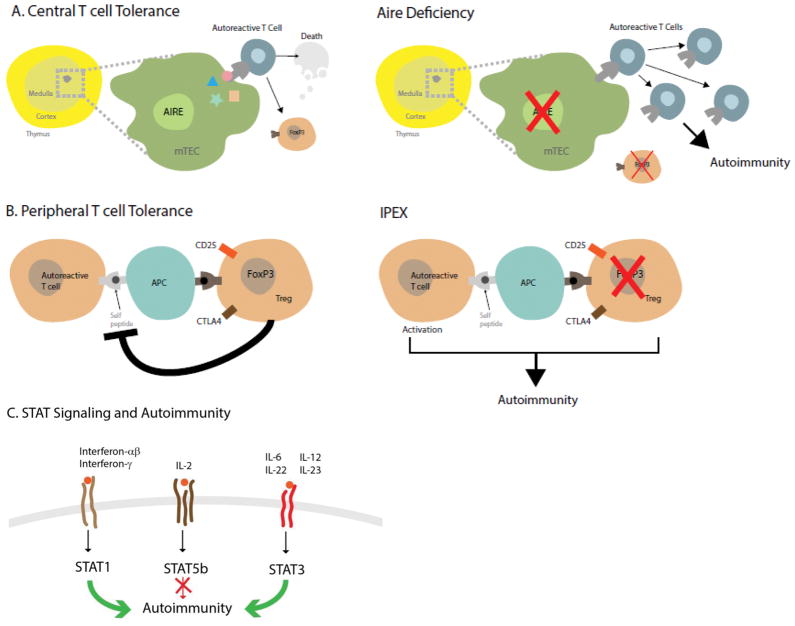
The basis for the pleomorphic spectrum of autoimmune manifestations of APS-1 has become clearer from studies of the defective gene in patients (AIRE) and mouse models (Aire). Aire which is expressed in thymic medullary epithelial cells14 and in a rare population of peripheral dendritic cells,15 mediates the ectopic expression of thousands of otherwise tissue-restricted proteins, enabling their peptides to be displayed to developing T cells (Fig. 2A). Such unique antigen presentation helps to promote the negative selection of autoreactive thymocytes, as well as self tolerance. Thus, if Aire is nonfunctional or absent, many autoreactive T cells with specificity for given antigens can escape deletion and may later be able to initiate autoimmune disease (Fig. 2A). Recent findings indicate that Aire controls immune tolerance by an additional mechanism—the induction of a unique population of FOXP3-positive T regulatory cells (Tregs) in the thymus that have the ability to suppress autoreactive cells.16,17 Thus, not only do more autoreactive cells escape deletion, but those Tregs normally in place to limit their activities are either not developed or are dysfunctional (Fig. 2A).
Figure 2. Key Immunoregulatory Pathways Involved in the Pathogenesis of Autoimmune Polyendocrine Syndromes.
Panel A, left illustrates normal central immune tolerance. The autoimmune regulator (AIRE) expressed in medullary thymic epithelial cells (mTEC) promotes expression of tissue-specific antigens (colored star, rectangle, triangle, and circle) which are expressed on the surface. Autoreactive T cells with affinity for self-proteins either die by apoptosis or become forkhead box P3 (FoxP3) expressing T regulatory cells (Tregs). When AIRE is lacking (right panel), autoreactive T cells escape to the general circulation and peripheral lymphoid organs where they can cause autoimmune reactions and the disease APS-1. Lack of Tregs also contribute to autoimmunity. Panel B, left depicts how FoxP3 positive Tregs harness autoreactive T cells by interacting with antigen presenting cells (APCs). FoxP3 mutations (right panel) or mutations in other genes key to the function of Tregs (cytotoxic T lymphocyte antigen 4 (CTLA4), cluster of differentiation 25 (CD25)) remove the inhibition of autoreactive T cells which then cause autoimmunity and immune polyendocrinopathy X-linked (IPEX) and IPEX-like syndromes. Panel C, Signal transducers and activators of transcription (STATs) are transducers of cell surface cytokine signaling (family members depicted at the cell surface). Mutations that lead to a constitutively active form of STAT1 or STAT3 promote autoimmunity (green arrows); loss of function mutations in STAT5b also lead to autoimmunity (red arrow with block). The exact mechanisms need to be further dissected but loss of STAT5b could be due to the improper expression of FOXP3, a known target of STAT5b.
Various disease-causing mutations are distributed throughout AIRE; to date, over 100 different mutations have been reported (Fig. S1 in The Supplementary Appendix). The most common is the so-called Finnish major mutation, p.R257X located in the SAND-domain (named after a range of proteins in the protein family: Sp100, AIRE-1, NucP41/75, DEAF-1). The Finnish major mutation is especially prevalent in people in Finland, Russia, and Eastern Europe.8,18 Another common mutation is the so-called 13 base pair deletion (p.C322del13) in the histone protein reading region called plant homeodomain 1 (PHD1), prevalent in persons in Norway, the British Isles, France, and North-America.6,7,19 Additionally, patients with unique dominant negative mutations in AIRE with autosomal dominant inheritance have recently been identified. These dominant negative mutations are associated with milder disease, often with accompanying pernicious anemia, vitiligo, autoimmune thyroid disease, and type 1 diabetes,20–22 and can be confused with the much more common condition, APS-2, which has a complex inheritance. The dominant gene variants are located both in the PHD1 and SAND domains (Fig. S1 in The Supplementary Appendix). Since AIRE is active as a multimer, it seems that changes in critical amino acids in mutated AIRE inhibit wild-type AIRE, thus creating the dominant negative effect. Data from the Exome Aggregation Consortium (Exac) database reveal that these variants are present in populations at frequencies of at least 0.1 % (http://exac.broadinstitute.org).21 It is likely that many families with “non-classical” dominant APS-1 remain undiagnosed.
AUTOANTIBODIES IN PATIENTS WITH APS-1
As an early marker of this T cell mediated loss of immune tolerance, disease-associated organ-specific autoantibodies may appear, often targeting intracellular proteins that have key functions in affected organs (Table 1 and Table S1 in the Supplementary Appendix). Many are fairly specific to APS-1, for example NLRP5 (also termed NALP5, an autoantibody expressed in the parathyroid and to some extent in the ovaries),23 BPI Fold Containing Family B Member 1 (BPIFB1)24, the potassium channel regulator KCNRG,25 expressed in the lung, and transglutaminase-4, expressed solely in the prostate gland.26 Other autoantibodies observed in APS-1 also appear in more common autoimmune diseases, for example, those targeting glutamic acid decarboxylase-65 in type 1 diabetes,27 21-hydroxylase in Addison’s disease,28 and side-chain cleavage enzyme in autoimmune premature ovarian insufficiency29 pointing to possible commonality in the pathogenesis of these various entities.
Table 1.
Classification and Characteristics of Autoimmune Polyendocrine Syndromes
| APS-1 | APS-2 | IPEX | |
|---|---|---|---|
| Main manifestations | Addison’s disease Hypoparathyroidism Chronic mucocutaneous candidiasis |
Addison’s disease Autoimmune thyroid disease Type 1 diabetes |
Autoimmune enteropathy Neonatal type 1 diabetes mellitus Eczema |
| Other associated manifestations | Oophoritis, autoimmune thyroid disease, type 1 diabetes Gastritis, enteritis with malabsorption, hepatitis, pancreatitis, pneumonitis, nephritis Vitiligo, alopecia, nail dystrophy, enamel hypoplasia, keratitis, retinitis |
Autoimmune gastritis, alopecia, vitiligo, celiac disease, oophoritis | Autoimmune thyroid disease, hemolytic anemia, thrombocytopenia |
| Typical age of onset | Childhood, adolescent | Adolescent to adult | Infancy |
| Frequency | 1:100 000 | 1:100 | 1:1 000 000 |
| Treatment | Replacement of hormones, anti-fungal therapy, immunosuppressive therapy for hepatitis, malabsorption, nephritis, pneumonitis, and keratitis | Replacement of hormones | Replacement of hormones, bone marrow transplantation |
| Complications, Including death | Adrenal and hypocalcemic crises, cancer in mouth and esophagus | Adrenal crisis, diabetic complications | Infections |
| Genes and mode of inheritance | AIRE, autosomal recessive and dominant | Polygenic, MHC and others | FOXP3, X-linked |
| Immune phenotype | Autoantibodies against interferon omega and alpha (>95%), and organ-specific intracellular proteins | Autoantibodies against 21-hydroxylase, GAD-65, IA2, TSH-receptor, and TPO | Autoantibodies against GAD-65, lymphocytosis, eosinophilia, overproduction of cytokines, hyper IgE |
Abbreviations: AIRE, autoimmune regulator; FOXP3, forkhead box P3; GAD, glutamic acid decarboxylase-65; IA2, Islet cell antigen; MHC, major histocompatibility complex; TPO, thyroperoxidase; TSH, thyroid stimulation hormone.
In contrast to the autoantibodies mentioned above, systemic autoantibodies to certain cytokines are highly prevalent in many, if not most, APS-1 patients. Autoantibodies to Type 1 interferons, namely interferon omega and alpha subtypes, are the most prevalent type of autoantibody in APS-1 and are present in almost all patients30,31 (except those with dominant negative mutations21). Apart from APS-1, interferon antibodies are also consistently seen in myasthenia gravis, thymomas32,33 and in patients with so-called “mild” recombination activating (RAG) gene mutations.34 Autoantibodies to the interleukin (IL) 17 family of cytokines, especially IL2235,36 are also prevalent in APS-1, reaching > 90% in some series.35
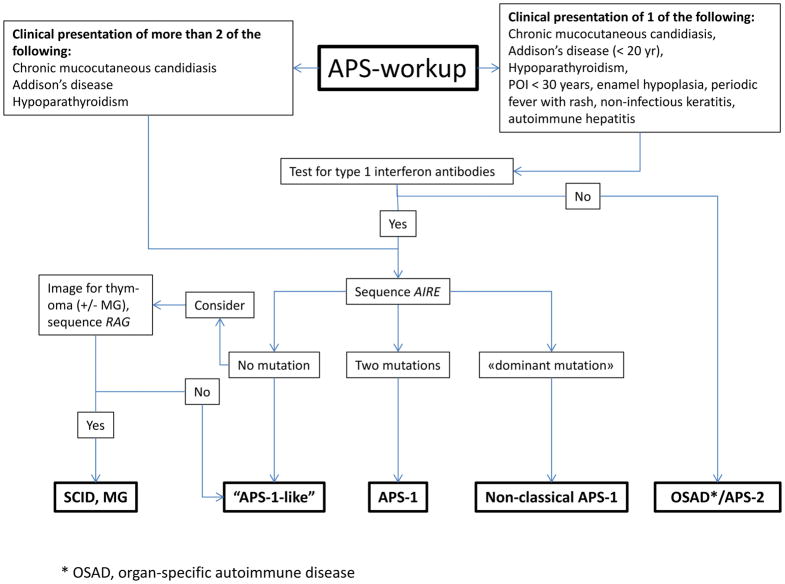
It is our experience that the diagnosis of APS-1 is often delayed and sometimes only made after death when a sibling is diagnosed. 37 Availability of AIRE sequencing and specific autoantibody tests have uncovered more atypical and milder cases in persons without two of the three main components.38 In such patients, the presence of minor components can be very helpful diagnostic hints. Some minor components of APS-1 develop early in life (keratitis, periodic fever with rash, autoimmune hepatitis),7 while others occur later (primary ovarian insufficiency under 30 years of age, enamel hypoplasia).6 Since over 95% of patients with APS-1 have autoantibodies to type 1 interferons,6,8 broad testing for such antibodies in suspected cases may be useful. In Fig. 3 we summarize current knowledge in a diagnostic workup scheme. A widely available test to detect autoantibodies quickly would provide a cost-effective tool for first-line screening prior to genetic testing.
Figure 3. Diagnostic Evaluation for APS.
Patients with a clinical diagnosis of APS-1 (upper left box) should have the AIRE gene sequenced for autoimmune regulator (AIRE) mutations. Patients with a clinical phenotype suggestive for APS-1 (upper right box) should be screened for interferon autoantibodies before AIRE sequencing. Since interferon autoantibody screening currently is available only in research laboratories, consider to go directly to sequencing of AIRE. Combined immune deficiency (CID) due to hypomorphic recombination-activation gene (RAG) mutations including Omenn syndrome, and granulomatous disease and/or autoimmunity. Other abbreviations: POI, premature ovarian insufficiency; OSAD organ-specific autoimmunity; MG, myasthenia gravis.
X-LINKED IMMUNODYSREGULATION, POLYENDOCRINOPATHY, AND ENTEROPATHY (IPEX)
X-linked immunodysregulation, polyendocrinopathy and enteropathy (OMIM 304790)-- or IPEX-- is an extremely rare inherited syndrome characterized by early onset type 1 diabetes,39,40 autoimmune enteropathy with intractable diarrhea and malabsorption, and dermatitis that may be eczematiform, ichthyosiform or psoriasiform. Eosinophilia and elevated IgE-levels are frequently present in IPEX. Some patients develop kidney disease, most often membranous glomerulonephritis or interstitial nephritis. Later manifestations may include autoimmune thyroiditis, alopecia, various autoimmune cytopenias, hepatitis and exocrine pancreatitis.41 There are many features that overlap with APS-1, but they usually develop much earlier in life. The disorder is frequently fatal in the first few years of life, unless patients are promptly treated with immunosuppressive agents or, if possible, receive an allogenic bone marrow transplant, which can cure the disease.41
There is a mouse model of a spontaneously occurring X-linked disease similar to IPEX called Scurfy. Using genetic mapping studies, the defective gene was mapped to mutations in the Foxp3 (mouse) or FOXP3 (human) genes in Scurfy and IPEX, respectively. 42–44 To date, about 70 different mutations have been reported in patients. FOXP3 is currently recognized as a master transcription factor that is highly expressed in Tregs45 in association with other key Treg elements such as CD4, cytotoxic T Lymphocyte-associated protein 4 (CTLA4), and CD25, the high affinity IL-2 receptor (Fig. 2B). The importance of CD25 in this entity has been underscored by the case of a woman presenting with IPEX-like features who had mutations in the CD25 gene, which is not on the X chromosome.46, and underscores the importance of IL-2 in promoting Treg survival and function.
Patients with IPEX, like those with APS-1, develop circulating autoantibodies that can be helpful in making the diagnosis. The majority of IPEX patients harbor autoantibodies against harmonin and villin,47 proteins involved in anchoring intestinal villi. These proteins are also expressed in the renal proximal tubule, which may be associated with the high prevalence of enteropathy and nephritis in these patients. Some patients with IPEX have autoantibodies seen in type 1 diabetes, including glutamic acid decarboxylase-65 and islet cell autoantibodies at a very early age, even a few weeks after birth.
Despite the rarity of IPEX, studies of affected patients have revealed a key pathway for self-tolerance (Fig. 2B) that has aided in the understanding of Tregs and has led to research aimed at the development of methods to enhance Treg function in transplantation and as a treatment for autoimmune disorders.48,49
OTHER INHERITED FORMS OF AUTOIMMUNE POLYENDOCRINE SYNDROMES
Using high throughput DNA-sequencing other unique monogenic syndromes with endocrine components have been characterized. Common to most is that Treg function is aberrant, giving rise to IPEX-like phenotypes. Loss-of-function mutations in STAT5b, ITCH, BACH2, and gain of function mutations in STAT1, and STAT3 are examples (see Table S2 in the Supplementary Appendix for details). Recently, an autosomal dominant syndrome characterized by hemolytic anemia, pneumonitis, lymphadenopathy, and hypogammaglobulinemia was mapped to rare variants in the CTLA4 gene60,61 that appear to destabilize Treg function and activity. However, the clinical presentation in affected patients is much milder than in patients with IPEX. A kindred with a similar clinical presentation was described in which the affected patients had mutations in the lipopolysaccharide-responsive, beige anchor (LRBA) gene.50 The mutated LRBA protein alters proper cellular trafficking of the CTLA4. Interestingly, treatment with the CTLA4-Fcreceptor fusion protein (abatacept), which binds to the ligands of CTLA4, appears to be effective in controlling symptoms in the affected patients. Several pedigrees have been identified with activating mutations in the gene STAT3, which encodes an important signaling molecule that helps to polarize T helper cell 17 (Th17) responses (Fig. 2C).51,52 The affected patients frequently develop autoimmunity that includes type 1 diabetes, autoimmune thyroid disease, hemolytic anemia, and autoimmune thrombocytopenia.
AUTOIMMUNE POLYENDOCRINE SYNDROME TYPE 2 (APS-2)
APS-2 is far more common than the syndromes already discussed. Patients with APS-2 have courses characterized by at least two of the following three endocrinopathies -- type 1 diabetes, autoimmune thyroiditis, and Addison’s disease.53 Some authors propose splitting this syndrome into further subtypes, but there is little evidence for distinct etiologies in such subcategories, so the broader term APS-2 for all of these patients seems appropriate. Women predominate among APS-2 patients. Many affected patients develop other autoimmune conditions, including celiac disease, alopecia, vitiligo, premature ovarian insufficiency, and pernicious anemia (Table 1). Additional manifestations are more frequent among APS-2 patients who have Addison’s disease.31,54
The picture emerging from genetic studies on APS-2 is that the same genes and single nucleotide polymorphisms are associated with several organ-specific autoimmune diseases. Thus, there are more similarities than specific differences when it comes to genetic associations.53 In general, associations are mostly to genes coding for key regulatory proteins in the adaptive and innate immune system, particularly in the major histocompatibility complex. For example, those patients with APS-2 at risk for celiac disease generally have variants in DR3-DQ2 and DR4-DQ8,55 and these same haplotypes confer risk to type 1 diabetes,56 autoimmune thyroid disease,57 and Addison’s disease,54 explaining why the same patients may develop all three diseases. Other well established risk genes include those that encode CTLA458, protein tyrosine phosphatase non-receptor type 22 (PTPN22),59 the transcription regulator protein,60,61 and CD25.62 Intriguingly, missense and nonsense mutations in the coding region of several of the genes noted here cause monogenic syndromes (see Table S2 in the Supplementary Appendix), pointing to their key role in immunoregulation.
Despite the major advancement in identification of disease genes, the heritability of APS-2 is complex. Erichsen found that about 10 percent of patients with APS-2 and Addison’s disease had a relative with adrenal insufficiency.54 About 10 percent of APS-2 patients with type 1 diabetes have a sibling with the same disease and even more have a sibling with autoimmune thyroid disease.63
The onset of APS-2 typically appears later than APS-1, in young adulthood. Currently, there are no unique tests to detect patients with APS-2, but testing for autoantibodies may be helpful in assessing disease risk, since the relevant autoantibodies are frequently detectable years before disease onset. Examples are antibodies to thyroid peroxidase in autoimmune thyroid disease,64 glutamic acid decarboxylase-65 in type 1 diabetes,27 and 21-hydroxylase in autoimmune Addison’s disease28 (see Table S1 in the Supplementary Appendix).
IMMUNE CHECKPOINT BLOCKADE AS A NEW TRIGGER FOR APS
Recently, there has been rapid development in the use of therapeutic antibodies to activate the immune system to treat malignancies, for example, to target the key peripheral immune tolerance regulators CTLA4 and programmed cell death protein 1 (PD-1). The wider use of monoclonal antibodies in cancer has revealed that some patients develop autoimmune side-effects.65 For example, colitis is common, and autoimmune thyroiditis has been frequently seen in patients treated with both CTLA4 and PD-1 blockade with an incidence >10%.66 Another remarkable side effect is autoimmune hypophysitis, otherwise a very rare disease, in patients treated with anti-CTLA4, especially ipilimumab.67 In addition, there are now reports of patients developing type 1 diabetes,68 and Addison’s disease69 after treatment with PD-1 blockade. These recent developments underscore how important key immune regulators are in actively suppressing autoimmune reactions.
TREATMENT AND FOLLOW-UP OF AUTOIMMUNE POLYENDOCRINE SYNDROMES
In general, management of autoimmune polyendocrine syndromes includes hormonal replacement therapy as needed, and treatment of complications. Patients with APS-1 are best followed by a multi-disciplinary team led by a pediatric or adult endocrinologist at a tertiary center. Patients should have a minimum of two follow-up visits per year due to the complexity of the entity, and asymptomatic mutations carriers should be followed at least annually. It is mandatory to check all sibling of APS-1 patients even if they are adult and seemingly well. Screening for 21-hydroxylase and NALP5 antibodies is useful in assessing the risk of development of adrenal insufficiency and hypoparathyroidism, respectively.
Oral chronic mucocutaneous candidiasis is generally best managed with oral preparations of mycostatin and amphotericin B to avoid the problem of drug resistance often encountered with continuous use of azole preparations.4 Azole drugs inhibit steroidogenesis with the risk of precipitation of adrenal insufficiency, especially if an unrecognized Addison’s disease is present. Hypoparathyroidism is managed with oral vitamin D derivatives combined with calcium and magnesium supplementation, but is sometimes difficult to control due to concomitant malabsorption.70 Some azole compounds may also inhibit the activation of alpha-calcidol, an analogue of vitamin D used for supplementation. PTH administration either by multiple injections or pump can be used, but is not recommended because of potential risk of osteosarcoma, lack of studies verifying efficacy, and cost.71 However, it can be useful in difficult cases of refractory hypocalcemia due to malabsorption.
Other symptoms, such as keratitis, pneumonitis, hepatitis, or enteritis, may need immunosuppressive treatment (see Table S3 in the Supplementary Appendix). Topical steroids and cyclosporine A may be helpful for treatment of keratitis, but many patients on such therapy develop irreversible corneal scarring.72 A new cyclosporine A prodrug, which may be used topically, improves bioavailability. Rituximab has been reported to have beneficial effects on pneumonitis and malabsorption,73 while cyclosporine A has improved pancreatic insufficiency.74 Autoimmune hepatitis in APS-1 can be aggressive and lead to hepatic failure and death if not promptly treated with high-dose steroids and azathioprine.11 More studies are needed to optimize immunosuppressive treatment. Since APS-1 patients can develop asplenia insidiously, we recommend vaccination against pneumococcus (both 13-valent and 23-valent vaccines), meningococcus, Haemophilus influenza type b, and annual influenza vaccine (Table S4 in The Supplementary Appendix).
Treatment of APS-2 should focus on replacement of missing hormones according to current guidelines for treating the main components of APS-2. Physicians should be particularly aware that a patient with APS-2 has an increased risk of developing another organ-specific autoimmune disease (Table S4 in The Supplementary Appendix). Massive family accumulation of autoimmune diseases, especially with early debut could indicate a monogenic disease, possibly a “non-classical” APS-1 especially if vitiligo and pernicious anemia is prevalent.21
NEW DIRECTIONS
The last decade has unraveled new monogenic forms of APS and better diagnostic tools, both genetic tests and autoantibody analyses. Research in the next decades should focus on prevention and targeted treatment of autoimmune diseases. More knowledge on genetic mechanisms and environmental triggers may permit subclassifying APS into distinct entities that have relevance for treatment and prognosis. Combining early and refined diagnostics with personalized genomics could enable physicians to apply early immunomodulatory therapy to stop the autoimmune process before irreversible organ damage occurs. Looking further into the future, work is currently underway to generate thymic epithelial tissue from stem cells.75 This approach could eventually be utilized to correct the expression of AIRE in APS-1 subjects and help reverse the immunopathology leading to multi-organ autoimmunity.
Supplementary Material
Acknowledgments
Studies reported herein were supported by the K.G. Jebsen-senter for autoimmune sykdommer, Novo Nordisk Foundation, The Swedish and Norwegian Research Councils, the Regional Health Authorities of Western Norway, the Torsten and Ragnar Söderberg Foundations, The National Institutes of Health, The Helmsley Charitable Trust, The Larry L. Hillblom Foundation, and the APS Type 1 Foundation. We are grateful to Dr. Cindy Wong, Dr. Mickie Cheng, and Dr. Marianne Øksnes for critically reading the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Schmidt MB. Eine biglandulare Erkrankung (Nebennieren und Schilddrüse) bei Morbus Adisonii. Verh Dtsch Ges Pathol. 1926;21:212–21. [Google Scholar]
- 2.An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. The Finnish-German APECED Consortium. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 3.Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 4.Husebye ES, Perheentupa J, Rautemaa R, Kampe O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J Intern Med. 2009;265:514–29. doi: 10.1111/j.1365-2796.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–36. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 6.Bruserud O, Oftedal BE, Landegren N, et al. A longitudinal follow-up of Autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2016;101:2975–83. doi: 10.1210/jc.2016-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferre EM, Rose SR, Rosenzweig SD, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI insight. 2016:1. doi: 10.1172/jci.insight.88782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlova EM, Sozaeva LS, Kareva MA, et al. Expanding the Phenotypic and Genotypic Landscape of Autoimmune Polyendocrine Syndrome Type 1. J Clin Endocrinol Metab. 2017;102:3546–56. doi: 10.1210/jc.2017-00139. [DOI] [PubMed] [Google Scholar]
- 9.Pollak U, Bar-Sever Z, Hoffer V, Marcus N, Scheuerman O, Garty BZ. Asplenia and functional hyposplenism in autoimmune polyglandular syndrome type 1. Eur J Pediatr. 2009;168:233–5. doi: 10.1007/s00431-008-0735-9. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez MJ, Gilson J, Zacharias J, Ishmael F, Bingham CA. Childhood Polyarthritis As Early Manifestation of Autoimmune Polyendocrinopathy with Candidiasis and Ectodermal Dystrophy Syndrome. Frontiers in immunology. 2017;8:377. doi: 10.3389/fimmu.2017.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91:2843–50. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 12.Halonen M, Eskelin P, Myhre AG, et al. AIRE mutations and human leukocyte antigen genotypes as determinants of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy phenotype. J Clin Endocrinol Metab. 2002;87:2568–74. doi: 10.1210/jcem.87.6.8564. [DOI] [PubMed] [Google Scholar]
- 13.Bensing S, Brandt L, Tabaroj F, et al. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol (Oxf) 2008;69:697–704. doi: 10.1111/j.1365-2265.2008.03340.x. [DOI] [PubMed] [Google Scholar]
- 14.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 15.Gardner JM, Devoss JJ, Friedman RS, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–7. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malchow S, Leventhal DS, Nishi S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–24. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard JD, Gilmore DC, Dileepan T, et al. Identification of Natural Regulatory T Cell Epitopes Reveals Convergence on a Dominant Autoantigen. Immunity. 2017;47:107–17. e8. doi: 10.1016/j.immuni.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruserud O, Oftedal BE, Wolff AB, Husebye ES. AIRE-mutations and autoimmune disease. Curr Opin Immunol. 2016;43:8–15. doi: 10.1016/j.coi.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Proust-Lemoine E, Saugier-Veber P, Lefranc D, et al. Autoimmune polyendocrine syndrome type 1 in north-western France: AIRE gene mutation specificities and severe forms needing immunosuppressive therapies. Hormone research in paediatrics. 2010;74:275–84. doi: 10.1159/000297714. [DOI] [PubMed] [Google Scholar]
- 20.Cetani F, Barbesino G, Borsari S, et al. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab. 2001;86:4747–52. doi: 10.1210/jcem.86.10.7884. [DOI] [PubMed] [Google Scholar]
- 21.Oftedal BE, Hellesen A, Erichsen MM, et al. Dominant Mutations in the Autoimmune Regulator AIRE Are Associated with Common Organ-Specific Autoimmune Diseases. Immunity. 2015;42:1185–96. doi: 10.1016/j.immuni.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Abbott JK, Huoh YS, Reynolds PR, et al. Dominant-negative loss of function arises from a second, more frequent variant within the SAND domain of autoimmune regulator (AIRE) J Autoimmun. 2017 doi: 10.1016/j.jaut.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alimohammadi M, Bjorklund P, Hallgren A, et al. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med. 2008;358:1018–28. doi: 10.1056/NEJMoa0706487. [DOI] [PubMed] [Google Scholar]
- 24.Shum AK, Alimohammadi M, Tan CL, et al. BPIFB1 is a lung-specific autoantigen associated with interstitial lung disease. Science translational medicine. 2013;5:206ra139. doi: 10.1126/scitranslmed.3006998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alimohammadi M, Dubois N, Skoldberg F, et al. Pulmonary autoimmunity as a feature of autoimmune polyendocrine syndrome type 1 and identification of KCNRG as a bronchial autoantigen. Proc Natl Acad Sci U S A. 2009;106:4396–401. doi: 10.1073/pnas.0809986106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landegren N, Sharon D, Shum AK, et al. Transglutaminase 4 as a prostate autoantigen in male subfertility. Science translational medicine. 2015;7:292ra101. doi: 10.1126/scitranslmed.aaa9186. [DOI] [PubMed] [Google Scholar]
- 27.Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–6. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 28.Winqvist O, Karlsson FA, Kampe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison’s disease. Lancet. 1992;339:1559–62. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
- 29.Soderbergh A, Myhre AG, Ekwall O, et al. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 2004;89:557–62. doi: 10.1210/jc.2003-030279. [DOI] [PubMed] [Google Scholar]
- 30.Meager A, Visvalingam K, Peterson P, et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalin F, Nordling Eriksson G, Dahlqvist P, et al. Clinical and immunological characteristics of Autoimmune Addison’s disease: a nationwide Swedish multicenter study. J Clin Endocrinol Metab. 2016;102:379–89. doi: 10.1210/jc.2016-2522. [DOI] [PubMed] [Google Scholar]
- 32.Cheng MH, Fan U, Grewal N, et al. Acquired autoimmune polyglandular syndrome, thymoma, and an AIRE defect. N Engl J Med. 2010;362:764–6. doi: 10.1056/NEJMc0909510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolff AS, Karner J, Owe JF, et al. Clinical and serologic parallels to APS-I in patients with thymomas and autoantigen transcripts in their tumors. J Immunol. 2014;193:3880–90. doi: 10.4049/jimmunol.1401068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter JE, Rosen LB, Csomos K, et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J Clin Invest. 2015;125:4135–48. doi: 10.1172/JCI80477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kisand K, Boe Wolff AS, Podkrajsek KT, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puel A, Doffinger R, Natividad A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–7. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolff AS, Erichsen MM, Meager A, et al. Autoimmune polyendocrine syndrome type 1 in Norway: phenotypic variation, autoantibodies, and novel mutations in the autoimmune regulator gene. J Clin Endocrinol Metab. 2007;92:595–603. doi: 10.1210/jc.2006-1873. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Streeten EA, Chan A, et al. Exome Sequencing Reveals Mutations in AIRE as a Cause of Isolated Hypoparathyroidism. J Clin Endocrinol Metab. 2017;102:1726–33. doi: 10.1210/jc.2016-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell BR, Buist NR, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr. 1982;100:731–7. doi: 10.1016/s0022-3476(82)80573-8. [DOI] [PubMed] [Google Scholar]
- 40.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–45. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barzaghi F, Amaya Hernandez LC, Neven B, et al. Long-term follow up of IPEX syndrome patients after different therapeutic strategies: an international multicenter retrospective study. The Journal of allergy and clinical immunology. 2017 doi: 10.1016/j.jaci.2017.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 43.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 44.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. The Journal of allergy and clinical immunology. 2007;119:482–7. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Lampasona V, Passerini L, Barzaghi F, et al. Autoantibodies to harmonin and villin are diagnostic markers in children with IPEX syndrome. PloS one. 2013;8:e78664. doi: 10.1371/journal.pone.0078664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Science translational medicine. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hippen KL, Merkel SC, Schirm DK, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1148–57. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo B, Zhang K, Lu W, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436–40. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 51.Milner JD, Vogel TP, Forbes L, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125:591–9. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flanagan SE, Haapaniemi E, Russell MA, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46:812–4. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med. 2004;350:2068–79. doi: 10.1056/NEJMra030158. [DOI] [PubMed] [Google Scholar]
- 54.Erichsen MM, Lovas K, Skinningsrud B, et al. Clinical, Immunological, and Genetic Features of Autoimmune Primary Adrenal Insufficiency: Observations from a Norwegian Registry. J Clin Endocrinol Metab. 2009;94:4882–90. doi: 10.1210/jc.2009-1368. [DOI] [PubMed] [Google Scholar]
- 55.Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989;169:345–50. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet. 1996;59:1134–48. [PMC free article] [PubMed] [Google Scholar]
- 57.Simmonds MJ, Gough SC. Unravelling the genetic complexity of autoimmune thyroid disease: HLA, CTLA-4 and beyond. Clin Exp Immunol. 2004;136:1–10. doi: 10.1111/j.1365-2249.2004.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 59.Bottini N, Musumeci L, Alonso A, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–8. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 60.Grant SF, Qu HQ, Bradfield JP, et al. Follow-up analysis of genome-wide association data identifies novel loci for type 1 diabetes. Diabetes. 2009;58:290–5. doi: 10.2337/db08-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eriksson D, Bianchi M, Landegren N, et al. Extended exome sequencing identifies BACH2 as a novel major risk locus for Addison’s disease. J Intern Med. 2016;280:595–608. doi: 10.1111/joim.12569. [DOI] [PubMed] [Google Scholar]
- 62.Lowe CE, Cooper JD, Brusko T, et al. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet. 2007;39:1074–82. doi: 10.1038/ng2102. [DOI] [PubMed] [Google Scholar]
- 63.Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183, e1–9. doi: 10.1016/j.amjmed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 64.Czarnocka B, Ruf J, Ferrand M, Carayon P, Lissitzky S. Purification of the human thyroid peroxidase and its identification as the microsomal antigen involved in autoimmune thyroid diseases. FEBS Lett. 1985;190:147–52. doi: 10.1016/0014-5793(85)80446-4. [DOI] [PubMed] [Google Scholar]
- 65.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. New England Journal of Medicine. 2018;378:1–10. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 66.Torino F, Corsello SM, Salvatori R. Endocrinological side-effects of immune checkpoint inhibitors. Current opinion in oncology. 2016;28:278–87. doi: 10.1097/CCO.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 67.Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. Journal of immunotherapy. 2005;28:593–8. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38:e55–7. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trainer H, Hulse P, Higham CE, Trainer P, Lorigan P. Hyponatraemia secondary to nivolumab-induced primary adrenal failure. Endocrinology, diabetes & metabolism case reports. 2016;2016 doi: 10.1530/EDM-16-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ekwall O, Hedstrand H, Grimelius L, et al. Identification of tryptophan hydroxylase as an intestinal autoantigen. Lancet. 1998;352:279–83. doi: 10.1016/S0140-6736(97)11050-9. [DOI] [PubMed] [Google Scholar]
- 71.Bollerslev J, Rejnmark L, Marcocci C, et al. European Society of Endocrinology Clinical Guideline: Treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173:G1–20. doi: 10.1530/EJE-15-0628. [DOI] [PubMed] [Google Scholar]
- 72.Chang B, Brosnahan D, McCreery K, Dominguez M, Costigan C. Ocular complications of autoimmune polyendocrinopathy syndrome type 1. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2006;10:515–20. doi: 10.1016/j.jaapos.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 73.Popler J, Alimohammadi M, Kampe O, et al. Autoimmune polyendocrine syndrome type 1: Utility of KCNRG autoantibodies as a marker of active pulmonary disease and successful treatment with rituximab. Pediatric pulmonology. 2012;47:84–7. doi: 10.1002/ppul.21520. [DOI] [PubMed] [Google Scholar]
- 74.Ward L, Paquette J, Seidman E, et al. Severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy in an adolescent girl with a novel AIRE mutation: response to immunosuppressive therapy. J Clin Endocrinol Metab. 1999;84:844–52. doi: 10.1210/jcem.84.3.5580. [DOI] [PubMed] [Google Scholar]
- 75.Parent AV, Russ HA, Khan IS, et al. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell stem cell. 2013;13:219–29. doi: 10.1016/j.stem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.