Abstract
Transition metals are required cofactors for many proteins that are critical for life, and their concentration within cells is carefully maintained to avoid both deficiency and toxicity. To defend against bacterial pathogens, vertebrate immune proteins sequester metals, in particular zinc, iron, and manganese, as a strategy to limit bacterial acquisition of these necessary nutrients in a process termed “nutritional immunity.” In response, bacteria have evolved elegant strategies to access metals and counteract this host defense. In mammals, metal abundance can drastically shift due to changes in dietary intake or absorption from the intestinal tract, disrupting the balance between host and pathogen in the fight for metals and altering susceptibility to disease. This review describes the current understanding of how dietary metals modulate host-microbe interactions and the subsequent impact on the outcome of disease.
Introduction
Transition metals are necessary for all forms of life and serve as critical enzymatic cofactors and protein structural components. Nearly 60% of known enzymes contain at least one metal cofactor, with zinc being the most common enzyme-associated transition metal ion followed by iron and manganese (Andreini et al., 2008). Despite their importance to numerous cellular processes, transition metals are toxic at high concentrations. Thus, transition metal levels inside the cell must be tightly controlled to maintain homeostasis.
Vertebrates face difficulties in maintaining balance between metal acquisition and utilization while preventing metal toxicity in part due to the presence of commensal bacteria, or the microbiota. Bacteria, like their mammalian hosts, require nutrient metals for essential biological processes. Given that dietary metals are rapidly complexed to host-derived molecules (Figure 1), bacteria must compete with their hosts to obtain the metals required to survive. The mechanisms detailing this multi-species competition for metal are unknown and represent a fertile area of future research.
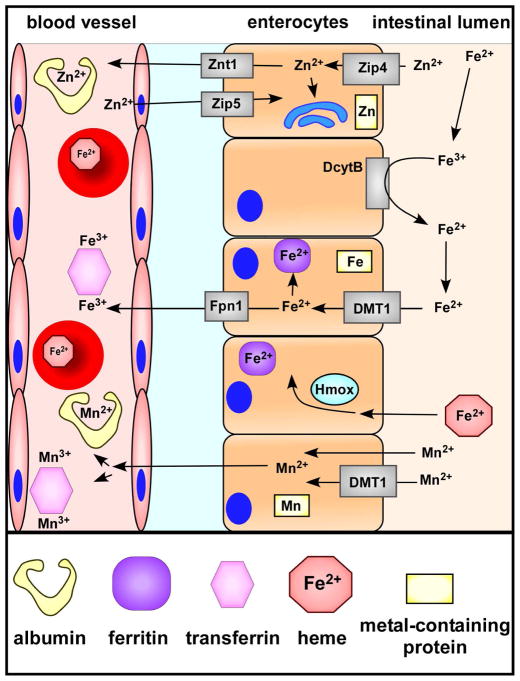
Figure 1. Zinc, iron, and manganese absorption in the intestines.
Zinc is absorbed primarily through Zip4 on the apical surface of enterocytes. Cytosolic zinc is shuttled to organelles, metal-binding proteins, or exported to systemic sites by Znt1. Excess systemic zinc is removed from the blood by enterocytes via Zip5. Non-heme ferric iron (Fe3+) is reduced to ferrous iron (Fe2+) by duodenal cytochrome b (DcytB) on the apical cell surface before being imported by a divalent metal transporter (DMT1). Cytosolic iron is stored bound to ferritin or exported via Fpn1. The extracellular labile pool of iron is often bound by circulating transferrin. Heme oxygenases (Hmox) within cells degrade heme to release iron for further transport or storage. Manganese diffuses through the enterocyte apical membrane or is transported by DMT1. In part dependent on oxidation state, circulating manganese can bind multiple circulating proteins including albumin and transferrin.
To limit bacterial growth, hosts have evolved mechanisms to sequester metals in a process termed “nutritional immunity” (Figure 2). As an overview, during an inflammatory episode interleukin-6 (IL-6) signals to the liver to release the hormone hepcidin, which promotes iron accumulation in phagocytic cells by inducing degradation of the iron exporter ferroportin (Fpn1) (Nemeth et al., 2004). Infiltrating neutrophils and macrophages block bacterial iron acquisition in part by releasing the protein lipocalin-2 (Xiao et al., 2017). Lipocalin-2 sequesters the high affinity metal binding siderophore molecules released by pathogenic bacteria to prevent uptake (Xiao et al., 2017). Neutrophils further limit nutrient metal availability by releasing lactoferrin to sequester iron (Legrand et al., 2004) and by releasing the antimicrobial protein calprotectin either as part of granules or as a component of neutrophil extracellular traps (Zackular et al., 2015). Calprotectin primarily binds zinc and manganese during infection, though recent reports revealed that calprotectin also binds ferrous iron, nickel, and copper in vitro (Besold et al., 2017; Nakashige et al., 2015; Nakashige et al., 2017). Host control of metal availability underscores how the redistribution of metals away from bacteria represents a key contributor to host-pathogen interactions.
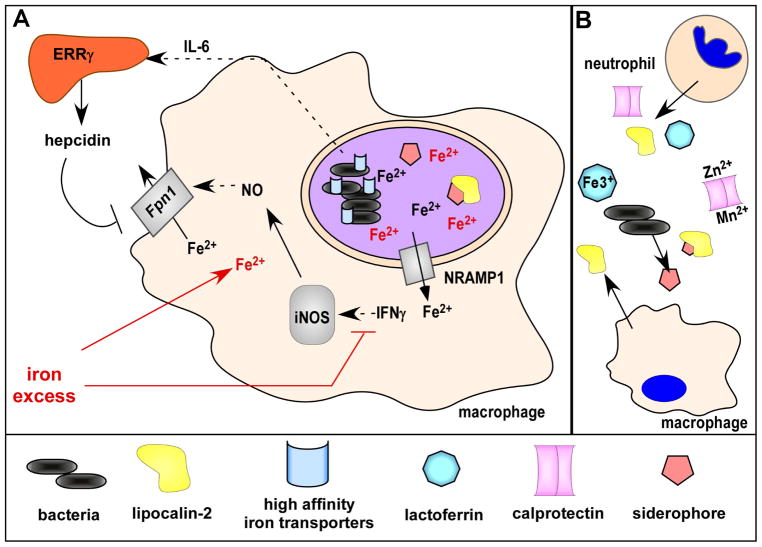
Figure 2. Nutritional immunity.
(A) During infection by intracellular pathogens, macrophages express NRAMP1 to export divalent metals, including iron, from bacteria-containing endosomes. Lipocalin-2 expression is upregulated to bind to bacterial siderophores and Fpn1 is produced to export cytosolic iron, overall limiting pathogen access to iron. Iron homeostasis is linked to other arms of the macrophage defense, as Fpn1 expression is further enhanced by interferon gamma (IFNγ) signaling to increase nitric oxide (NO) production via inducible nitric oxide synthase (iNOS). To compete for iron, intracellular bacteria express high affinity metal transporters and siderophores. Extraintestinal invasion by the bacterium S. Typhimurium additionally induces macrophage IL-6 production, resulting in hepcidin release from the liver through ERRγ signaling. Hepcidin increases Fpn1 degradation and limits iron export. Iron excess (shown in red), either through iron overload or over-supplementation, may subvert host defenses by interfering with IFN signaling and by increasing bacterial access to iron. (B) To block access to metals to extracellular bacteria, neutrophils release lipocalin-2, lactoferrin, and calprotectin while macrophages release lipocalin-2. Calprotectin binds and sequesters multiple metals including zinc and manganese. Lactoferrin binds primarily iron.
The microbiota and dietary metals in the intestines
Nutrient limitation in microbiota interactions
The intestines are a complex ecosystem in which hundreds of species interact and fill distinct niches. As with any ecosystem, resource availability is a strong force dictating population structure, with limiting resources driving competition between species and even individuals (Hornung et al., 2018). For example, the availability of oxygen during inflammation drives a disruption, or dysbiosis, of the microbiota by favoring Enterobacteriaceae expansion through respiration (Byndloss et al., 2018). Dietary metals, as a critical nutrient, also have the potential to shift the distribution and function of the microbiota. The following section describes how metal availability shapes microbe-microbe and microbe-host interactions in the intestines.
Zinc and altered susceptibility to enteric infection
Dietary changes that alter metal intake or absorption likely shape the distribution of microbial populations by affecting metal availability. For instance, zinc deficiency is a risk factor for childhood diarrhea (Walker et al., 2013) and is associated with dysbiosis of the gut microbiota (Figure 3). Specifically, zinc deficiency is correlated with low relative abundances of Clostridiales and Verrucomicrobia populations and concomitant increases in Enterobacteriaceae and Enterococcus (Reed et al., 2015; Starke et al., 2014). High levels of Akkermansia muciniphila, a member of the Verrucomicrobia, is associated with low inflammation and normal lipid and carbohydrate metabolism (Dao et al., 2016; Schneeberger et al., 2015) while high abundance of the Enterobacteriaceae are associated with intestinal dysbiosis. Thus, low intestinal zinc may push microbiota homeostasis away from beneficial bacteria such as A. muciniphila and a low inflammatory environment to favor the expansion of pathogenic bacteria.
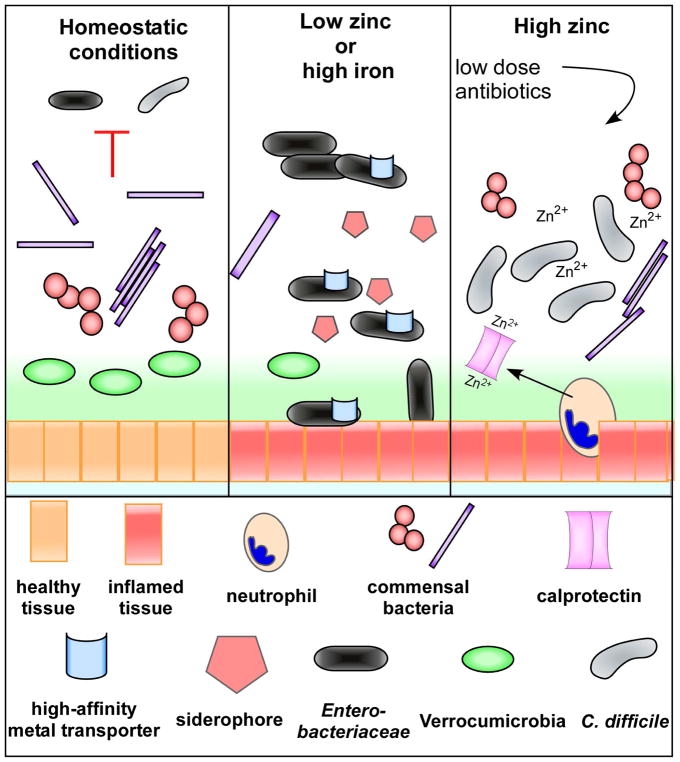
Figure 3. Changes in dietary metal availability alter the microbiota and susceptibility to pathogen colonization.
Under homeostatic conditions, a diverse commensal microbiota typically provides colonization resistance against multiple bacterial pathogens. Low zinc or high iron in the diet reduces overall commensal bacterial diversity along with key members of a healthy gut such as the Verrucomicrobia. This allows for an increase in abundance of members of the Enterobacteriaceae, many of which are pathogenic and encode high affinity metal transporters and siderophores to obtain nutrient metals. High zinc also disrupts the microbiota, reducing the amount of antibiotics needed to allow for C. difficile colonization and disease. Neutrophils recruited during C. difficile infection release calprotectin to sequester extracellular zinc, but the immune protein is overwhelmed with excess dietary zinc and is not effective at limiting zinc access to C. difficile.
Pathogenic Escherichia coli, a member of the Enterobacteriaceae, is commonly found in patients experiencing zinc deficiency and diarrhea. Enteroaggregative E. coli (EAEC), the main causative agent of traveler’s diarrhea, encodes virulence factors that allow the pathogen to attach to and aggregate on the intestinal epithelium (Okhuysen and Dupont, 2010). Zinc deficiency serves as a stressor for EAEC and leads to increased expression of virulence factors, including toxins that worsen diarrheal episodes and exaggerate weight loss (Bolick et al., 2014). Consistently, dietary zinc supplementation reverses disease progression, inhibits EAEC adherence and biofilm formation, and reduces epithelial production of pro-inflammatory cytokines (Medeiros et al., 2013). Another virulent E. coli, enteropathogenic E. coli (EPEC), experiences cell envelope stress and reduced expression of the main virulence factor, the locus of enterocyte effacement (LEE) pathogenicity island, upon exposure to high dietary zinc (Xue et al., 2015). In practice, patients receiving zinc supplementation to treat diarrhea have lessened diarrheal morbidity (Bhandari et al., 2002) and a reduced risk of prolonged diarrheal episodes (Sazawal et al., 1995). While it is tempting to speculate that zinc supplementation supports gut homeostasis by directly inhibiting pathogen virulence or by selecting for growth of beneficial microbes, in most cases the mechanisms explaining improved gut health via zinc supplementation are undetermined.
While zinc supplementation is essential for zinc-deficient individuals, excess zinc intake could disrupt the balance of gut microbial populations and predispose individuals to infection. The spore-forming Gram-positive bacterium Clostridium difficile typically does not cause disease in otherwise healthy individuals, as the microbiota effectively block C. difficile colonization (Theriot and Young, 2015)(Figure 3). However, a variety of risk factors including exposure to antibiotics and chemotherapeutic agents disrupt microbiota-mediated colonization resistance and allow C. difficile to take hold in the intestines (Abou Chakra et al., 2014). In mice, high dietary zinc generates a dysbiosis that favors expansion of Enterococcus, Porphorymonadaceae, Lachnospiraceae, and Clostridia XI while limiting the population of Turicibacter (Zackular et al., 2016). Without further insult, this dysbiotic community retains colonization resistance against C. difficile. Yet, the new community structure becomes more susceptible to antibiotics, thus lower doses are needed to overcome colonization resistance and allow C. difficile to colonize (Zackular et al., 2016). How increased zinc availability leads to perturbations of the microbiota and the mechanisms governing the altered susceptibility to C. difficile infection remain an interesting area for future work.
The battle for intestinal iron
Dietary iron has less than 20% absorption efficiency leaving the bulk of iron intake within the intestinal lumen to the microbiota (Jaeggi et al., 2015). In cases where patients naturally have low enteric pathogen burdens, the effects of iron supplementation on the gut ranges from marginally impactful (Dostal et al., 2014a) to beneficial through increasing fecal concentrations of anti-inflammatory short chain fatty acids (Dostal et al., 2014b) and lowering inflammation (Kortman et al., 2015). Yet, studies examining the effects of dietary iron suggest iron drives changes to the microbiota that may result in increased susceptibility to infection ((Constante et al., 2017; Jaeggi et al., 2015); Figure 3). For instance, heme (an iron-containing porphyrin molecule) supplementation reduces the relative abundance of Firmicutes (Constante et al., 2017), which contain members from the beneficial butyrate-producing class Clostridia. Butyrate production is associated with increased differentiation of regulatory T-cells and lowered inflammation (Sun et al., 2017). Furthermore, members of the Enterobacteriaceae are found in greater abundance following dietary heme treatment (Constante et al., 2017). In rats, oral iron administration leads to increased representation of Ruminococcaceae, Defluvitalaceae, and Coprococcus, as well as decreased representation of Lachnospiraceae (Fang et al., 2018). It is not clear if shifts in the microbiota from iron supplementation result from direct microbe interactions or indirectly through altered host inflammation as is seen in patients with irritable bowel syndrome (Bonovas et al., 2016).
The non-typhoidal Salmonella (NTS) serovar Salmonella Typhimurium initially colonizes the intestines before invading through Peyer’s patches to reach deeper tissues (Santos et al., 2009). During Salmonella-induced inflammation, the host releases lactoferrin to sequester iron and lipocalin-2 to bind bacterial siderophores and undermine bacterial iron acquisition (Raffatellu et al., 2009). To subvert host nutritional immunity, S. Typhimurium produces the stealth siderophore salmochelin that includes an additional glycosylation moiety (Fischbach et al., 2006) that prevents lipocalin-2 binding. The ability of S. Typhimurium to avoid lipocalin-2 mediated defenses provides a selective advantage during inflammation (Raffatellu et al., 2009). S. Typhimurium also increases expression of iron transport systems required for full virulence (Boyer et al., 2002; Janakiraman and Slauch, 2000) in part by providing iron to iron-sulfur (FeS) clusters necessary for respiration (Byndloss et al., 2018).
S. Typhimurium is not the only member of the Enterobacteriaceae that can take advantage of the low iron environment of the gut during inflammation. The probiotic bacterium E. coli Nissle 1917 (EcN) encodes mechanisms to uptake exogenous iron and heme as well as the siderophores yersiniabactin, aerobactin, and salmochelin (Deriu et al., 2013). EcN is able to outcompete and suppress S. Typhimurium colonization in an iron-dependent manner and only in the presence of lipocalin-2 (Deriu et al., 2013), revealing how microbiota and host-mediated iron acquisition mechanisms synergize to resist pathogen colonization.
Other enteric pathogens subvert nutritional immunity through diverse mechanisms. Yersinia enterocolitica and Y. pseudotuberculosis encode uptake receptors for yersiniabactin and other siderophores to support their iron needs (Rakin et al., 2012). Campylobacter jejuni encodes genes necessary for the uptake of heme and iron that are transcribed under iron-limited stress (Crofts et al., 2018). C. jejuni can also bind and uptake enterobactin; however, it does not encode the machinery to produce siderophores (Xu et al., 2010). This suggests a type of bacterial commensalism where C. jejuni benefits from the energy expended by other bacteria to produce siderophores. Even in the absence of other microbes, C. jejuni can steal iron directly from host lactoferrin and transferrin in a process termed iron piracy (Miller et al., 2008).
Overall, iron represents a precious resource in the gut for both the microbiota and pathogenic bacteria. A significant part of the host immune response relies on the sequestration of iron to slow pathogen growth enabling other branches of the immune system to capture and kill invading microbes. It is then not surprising that iron or heme supplementation increases susceptibility to Enterobacteriaceae and other enteric pathogens. Efforts to treat the detrimental effects of iron deficiency must take into account strategies to mitigate gut dysbiosis and bolster colonization resistance.
Manganese and nutritional immunity in the gut
While several studies have examined the impact of iron and zinc on the microbiota and bacterial pathogenesis, fewer studies have explored the role for manganese during infection. Manganese is a required coenzyme for some superoxide dismutases in the detoxification of bactericidal superoxide radicals (Damo et al., 2012) generated during inflammation and as such is important for pathogens after stimulating an immune response. In the intestinal lumen, calprotectin competes with pathogens for manganese and represses the bacterial response to superoxide radicals. S. Typhimurium bypasses calprotectin-dependent manganese sequestration by the action of the cation transporters MntH, SitABCD, and ZupT (Diaz-Ochoa et al., 2016). Acquisition of manganese then allows catalysis by manganese-dependent superoxide dismutase and catalase to collectively detoxify superoxide to water.
Subsequent to S. Typhimurium translocation through the epithelial border, macrophages phagocytose and quarantine the bacterium within Salmonella-containing vacuoles (SCVs). The cation transporter NRAMP1 localized within the SCV is responsible for pumping nutrient metals, including manganese, out of the SCV to starve S. Typhimurium and limit growth (Loomis et al., 2014). In response, S. Typhimurium increases expression of the manganese and iron transporter SitABCD, which is required for full virulence (Boyer et al., 2002; Janakiraman and Slauch, 2000).
Manganese transport and its roles in colonization and virulence in other enteric pathogens are less well known but presumably play similar critical roles as seen in S. Typhimurium (Juttukonda and Skaar, 2015). It is unknown how dietary manganese deficiency or excess in humans affects the gut population but as with other necessary nutrients it is likely that altering manganese availability within the intestines drives bacterial population shifts that may compromise microbiota function and host health.
Dietary metals at the host-pathogen interface of extraintestinal sites
Dietary metal in the intestines following ingestion of foodstuffs represents an obvious arena where metal availability can directly influence pathogen and microbiota physiology. Yet, changes to metal absorption and transport can have a significant impact on disease beyond the intestines through alterations in immune cell physiology, metal localization, and bacterial access to metals. The following section describes current understanding of the associations between changes in dietary metal and bacterial pathogenesis at extraintestinal sites.
Zinc deficiency and the host response to infection in the respiratory tract
Zinc deficiency is correlated with an increased risk of lung infection and arises from both low zinc intake and consumption of foods high in zinc-sequestering phytates and fiber to affect nearly a third of the world’s population (Wessells and Brown, 2012). In some studies, zinc deficiency in elderly individuals is associated with an increased risk of pneumonia caused by Staphylococcus aureus, Streptococcus pneumoniae, Klebsiella pneumoniae, Legionella pneumophila, and Haemophilus influenzae (Barnett et al., 2010). In children, zinc supplementation to correct zinc deficiency is reported to lower the median time to recovery from severe pneumonia, though it is important to note that some studies found no benefit to zinc supplementation (Basnet et al., 2015). The effects of low dietary zinc are hypothesized to be due to depression of the adaptive immune response to infection, where low zinc is associated with lowered antibody titers (Girodon et al., 1999), reduced lymphocyte proliferation, and reduced levels of circulating T lymphocytes, CD4+ memory T cells, and naïve T cells (Barnett et al., 2010; Fraker and King, 2004). While zinc supplementation can improve host resistance to infection, in some cases it fails to correct impairments in the immune response (Yuan et al., 2016). These data suggest that the timing of zinc supplementation is important, where basal zinc deficiencies must be restored prior to, rather than during, infection to improve disease outcome. The impact of low zinc on the innate response to infection is also not clear. In an OVA-induced allergic airway inflammation model, rats on low zinc diets experience higher eosinophil, neutrophil, and monocyte migration to the lungs following an inflammatory insult (Lu et al., 2012). These effects are reversed upon zinc supplementation and suggest that zinc may have anti-inflammatory properties and limit collateral damage during inflammation (Liu et al., 2013). However, zinc deficiency may prevent adequate pathogen clearance, as ethanol-induced zinc-deficiency in macrophages leads to impaired phagocytosis (Konomi et al., 2015). While zinc deficiency may enhance innate immune cell recruitment to combat pathogen invasion, cell dysfunction could inhibit effective bacterial killing.
Dietary modulation of zinc homeostasis can be further complicated by dysregulation of zinc-sequestering calprotectin. Calprotectin is released in response to infection to withhold zinc from bacteria, however hypercalprotectinaemia, in which patients have excessive serum calprotectin, is associated with increased incidence of recurrent infections (Sampson et al., 2002). Future work is warranted to decipher how dietary zinc differentially alters the adaptive and innate responses to bacterial infection and how those immune responses feedback to influence zinc homeostasis.
The bacterial response to zinc limitation in the respiratory tract
Adequate zinc intake and absorption is important to maintain proper immune cell function, which includes sequestering the metal to limit bacterial access. Yet, even in zinc-deplete environments pathogens have evolved mechanisms to acquire this necessary nutrient. For instance, the nosocomial Gram-negative bacterium Acinetobacter baumannii encodes several mechanisms to ensure zinc bioavailability (Mortensen and Skaar, 2013). A. baumannii infection triggers the release of calprotectin from infiltrating neutrophils to limit metal availability to extracellular bacteria while host metal transporters including NRAMP1 (Nevo and Nelson, 2006) export multiple divalent metals out of bacteria-containing endosomes. These responses collectively slow intracellular bacterial replication. In response to zinc limitation, the A. baumannii transcriptional zinc uptake regulator (Zur) de-represses the energy-dependent zinc transporter ZnuABC that is necessary for survival in murine models of pneumonia (Mortensen et al., 2014), in part by contributing to anti-oxidative stress defenses via a zinc-copper superoxide dismutase (Hassan et al., 2017). Also included in the A. baumannii Zur regulon is a histidine ammonia lysase HutH that liberates zinc from histidine and forms a labile zinc pool under zinc-deplete conditions (Nairn et al., 2016).
Pseudomonas aeruginosa, a soil bacterium that commonly colonizes the respiratory tract of cystic fibrosis patients, also expresses a ZnuABC zinc transporter under zinc-deplete conditions (Pederick et al., 2015). Zinc acquisition by P. aeruginosa transporters is complemented by the release of the siderophore pyochelin, which can bind multiple divalent cations (Brandel et al., 2012). P. aeruginosa zinc acquisition has consequences not only for bacterial replication, but also in the expression of virulence factors. Zinc triggers increased resistance to antibiotics and promotes biofilm formation within cystic fibrosis sputum (Marguerettaz et al., 2014). Pseudomonas biofilms are negatively charged owing to extracellular DNA and chelate positively charged divalent metal ions, including zinc, to promote bacterial virulence and survival (Mulcahy et al., 2008). The redundant mechanisms used by P. aeruginosa to acquire zinc illustrates how critical the metal is to survival of the pathogen within a host environment. The ability to overcome host-mediated zinc sequestration is a shared feature of respiratory pathogens including A. baumannii, P. aeruginosa, K. pneumoniae, and S. pneumoniae (Achouiti et al., 2012; Dintilhac et al., 1997; Nairn et al., 2016; Pederick et al., 2015) but there remains much to be discovered about the diverse strategies performed by these bacteria.
Systemic zinc status affects multiple aspects of immunity. As a result, zinc deficiency may drive increased susceptibility to infection because of immune dysregulation. However, an inadequate supply of zinc available to invading pathogens should also negatively influence bacterial survival. This suggests that bacterial virulence factors that compete with the host for zinc contributes significantly to pathogenesis. Teasing apart the effects of dietary zinc on immune dysfunction and bacterial virulence will be important area of future research.
Iron overload in the lung
Excess metal may also result in disruption of immune processes and modulate bacterial virulence. Dietary African iron loading is a condition characterized by high concentrations of intracellular macrophage iron due to leaching from drinking containers that is enhanced in individuals with a genetic predisposition based on a Fpn1 mutation (Gordeuk et al., 2003). This iron loading is positively correlated with an increased risk of death from the causative agent of tuberculosis, Mycobacterium tuberculosis (Cronje and Bornman, 2005). M. tuberculosis is a worldwide threat to human health and even with available treatment it remains a significant cause of mortality. After inhalation of M. tuberculosis, alveolar macrophages phagocytose the bacilli and initiate granuloma formation. Within alveolar macrophages, M. tuberculosis experiences iron starvation and, in response, upregulates genes involved in metal import (Dubnau et al., 2005) and releases the siderophore mycobactin (De Voss et al., 2000). Even with the expression of M. tuberculosis iron-acquisition factors, macrophage iron withholding effectively aids in limiting bacterial growth (Schaible et al., 2002).
However, iron-loading bypasses metal restriction by providing M. tuberculosis an iron-replete intracellular environment. Additionally, high dietary iron weakens other arms of the host defense. Iron inhibits macrophage interferon-γ signaling, a pro-inflammatory cytokine important for producing bactericidal nitric oxide gas (Weiss et al., 1992), decreases the recruitment of neutrophils (a primary innate defense against M. tuberculosis infection), and reduces pro-inflammatory cytokine expression (Agoro et al., 2017). It should be noted that moderate iron supplementation also results in increased T-cell recruitment to granulomas and enhanced reactive oxygen species production that limits M. tuberculosis replication (Agoro et al., 2017). Yet, the clinical associations between high dietary iron and the risk of death from M. tuberculosis suggests an increase in T-cell recruitment alone is not sufficient to overcome iron-mediated disruption to other aspects of the immune response.
Iron deficiency and supplementation in bacteremia
Iron deficiency affects an estimated 2 billion people worldwide (Dye et al., 2013). It is the most common type of nutrient deficiency and the only deficiency that affects a sizeable population in industrialized nations. Iron deficiency anemia, a condition characterized by a lack of iron within hemoglobin, is particularly prevalent in young children, pregnant women, and post-partum women (Miller, 2013). Immunologically, iron deficiency in animal models inhibits T cell proliferation in the liver (Bonaccorsi-Riani et al., 2015), spleen (Kuvibidila et al., 1983), and thymus (Brekelmans et al., 1994) and reduces activation of T cells and natural killer T cells (Bonaccorsi-Riani et al., 2015). B lymphocytes also show reduced proliferation (Kuvibidila et al., 1983) and long-term iron deficiency reduces production of IgG and IgM antibodies (Kochanowski and Sherman, 1985). The effects of iron deficiency on macrophage function and bactericidal radical formation is complex, as low iron reduces Fenton-mediated hydroxyl formation but also increases radical generation through defects in the respiratory chain and a reduced capacity to handle oxidative stress (Koskenkorva-Frank et al., 2013).
To correct iron deficiency, a simple strategy entails providing iron supplements. A study in Kenyan girls found that 5 months of dietary iron supplementation could restore hemoglobin levels of iron-deficient girls to levels similar to iron-replete girls. However, iron-replete girls were at an increased risk of infection with the malarial parasite Plasmodium (Leenstra et al., 2009), which acquires iron from heme largely through lysis of red blood cells (Akinosoglou et al., 2012). As such, there are concerns regarding strategies to supplement iron to correct anemia due to fears of supplying iron to the parasite (reviewed in (Spottiswoode et al., 2014)). As an example, one study found that children provided iron supplements were at an increased risk of death compared to controls, prompting early termination of the study (Sazawal et al., 2006). While not all studies find significant associations between iron supplementation and increased malaria risk (Spottiswoode et al., 2014), it is clear that caution must be taken when manipulating the balance of iron to avoid collateral alterations in infection risk.
Aside from malaria, iron supplementation in an effort to correct iron deficiency could increase the risk of co-morbidities. The incidence of NTS bacteremia is high throughout Sub-Saharan Africa and overlaps with populations suffering from malaria and iron deficiencies (van Santen et al., 2013). Plasmodium infection induces anemia and increased expression of the anti-inflammatory cytokine IL-10, which synergize to suppress phagocytic cell control of NTS and contributes to co-infection (Lokken et al., 2014). Once infected, NTS within phagocytic cells acquires ferrous and ferric iron by upregulating expression of the iron importers FeoB or FepB, respectively, (Nagy et al., 2014) and the siderophores enterochelin and salmochelin (Nagy et al., 2013). NTS also enhances iron availability through triggering expression of estrogen-related receptor gamma (ERRγ), which leads to increased hepcidin expression and accumulation of iron stores within macrophages (Kim et al., 2014). To counteract the iron scavenging of the pathogen, infected macrophages respond by increasing Fpn1 and lipocalin 2 expression, which effectively limits NTS replication (Nairz et al., 2007). Iron supplementation to treat Plasmodium-induced anemia may subvert the host response by providing NTS access to higher intracellular iron, allowing the bacteria to decrease energy expenditure on iron acquisition and devote resources to replication. To compound the effect, excess iron interferes with nitric oxide production, which feeds back to limit Fpn1 expression and increases intracellular iron stores (Nairz et al., 2013; Nairz et al., 2007)(Figure 2). Together, these studies suggest excess iron supplementation could upset efforts to correct malaria-induced anemia by increasing risks for NTS bacteremia, as others have suggested (van Santen et al., 2013), but more work is needed to clarify these relationships.
Hemoglobin holds a significant portion of the body’s iron and restoring hemoglobin levels via dietary or parenteral nutrition in iron-deficiency anemia patients is a common practice (Camaschella, 2015). However, increasing iron availability in the form of hemoglobin may also promote bacteria overgrowth at systemic sites. S. aureus is a Gram-positive bacterium that is a significant health concern and a cause of serious bloodstream infections (Johnson et al., 2014). S. aureus is well equipped to acquire iron through the toxin-dependent lysis of red blood cells followed by the uptake of heme through the iron-regulated surface determinant (Isd) system (Grigg et al., 2010). The Isd system contains high affinity receptors to remove heme from hemoglobin for transport across the cell wall (Grigg et al., 2010) and once internalized, cytosolic heme oxygenases degrade heme to release iron (Wilks and Heinzl, 2014). However heme presents its own challenge for S. aureus as electron shuttling through heme and quinone molecules in the bacterial cell membrane result in the generation of damaging superoxide molecules (Wakeman et al., 2012). To protect against heme toxicity, S. aureus senses heme through the two-component regulatory system HssRS, which under high heme conditions activates expression of the efflux pump HrtAB and limits intracellular heme availability (Stauff and Skaar, 2009). In addition, bacterial nitric oxide synthase (bNOS) protects S. aureus from heme-induced oxidative stress (Surdel et al., 2016). The ability of S. aureus to use heme as an iron source is then delicately balanced against its potential toxicity.
S. aureus heme utilization and detoxification mechanisms provide an example of why dietary interventions to restore hemoglobin levels in iron deficiency anemia should be cautiously approached to avoid promoting bacterial growth. However, other Gram-positive as well as Gram-negative pathogens have evolved diverse mechanisms to use heme as an iron source (Anzaldi and Skaar, 2010) and could also complicate efforts to correct iron deficiency by hemoglobin restoration. But, it is equally important to consider immune restoration through iron supplementation. As mentioned previously, iron deficiency suppresses multiple arms of the immune response. As an example, in S. aureus infection neutrophils are critical innate defenders that exhibit impaired killing capacities under iron limitation (Moore and Humbert, 1984). Therefore, iron supplementation to improve neutrophil bactericidal activities may balance the potential for enhanced S. aureus growth.
Limited dietary iron and extracellular bacteria
While iron deficiency impairs the immune response and prompts iron supplementation, a strategy to reduce dietary iron could reduce the burden of extracellular pathogens, particularly in genetically susceptible individuals. The Gram-negative bacterium Vibrio vulnificus is highly virulent and contributes to upwards of 50% of the mortality of blood stream infections in patients suffering from hereditary hemochromatosis (Horseman and Surani, 2011). Hereditary hemochromatosis is caused by a mutation in the HFE gene (chromosome 6p21.3) that leads to the excess absorption of iron through disruption of hepcidin and Fpn1 interactions (Adams and Barton, 2007) and increases susceptibility to multiple bacterial pathogens (Khan et al., 2007), including V. vulnificus, Y. pseudotuberculosis, Y. enterocolitica, and L. monocytogenes. Increased iron in the blood of hereditary hemochromatosis patients (Adams and Barton, 2007) may then serve as a pool of iron available for pathogens such as V. vulnificus, which increase expression of high affinity iron transporters or iron-binding siderophore molecules during iron starvation (Pajuelo et al., 2016). In accordance with increased iron availability in the blood improving bacterial survival, an iron-limited diet in hereditary hemochromatosis patients reduces the overall V. vulnificus bacterial burden (Arezes et al., 2015). However, hereditary hemochromatosis may benefit the host response to intracellular bacteria by limiting intracellular iron availability. Treatment options targeting iron homeostasis are thus highly dependent on host genetic factors and the pathogenic lifestyle of the bacteria.
Iron deficiency and the stomach
Iron deficiency is associated with an increased risk of gastric cancer (Cover and Peek, 2013). While there are likely multiple mechanisms linking iron deficiency to cancer development, infection with the Gram-negative bacterium Helicobacter pylori exacerbates carcinogenesis by increasing pathogen virulence (Noto et al., 2013). H. pylori infects nearly one-half of the world’s population and is one of the greatest risk factors for the development of distal gastric carcinomas (Cover and Peek, 2013). H. pylori infection itself is associated with iron deficiency, possibly through reducing gastric acid production and iron absorption (Dale et al., 1998) or by inducing blood loss through gastric ulcers (Beckett et al., 2016). However, there is controversy over whether H. pylori is the driver in causing iron deficiency (Banerjee and Bishop, 2005). Whereas some researchers found that Helicobacter infection does not influence absorption from iron supplementation (Herter-Aeberli et al., 2017), others discovered a positive link between H. pylori and reduced iron absorption(Beckett et al., 2016). In either case, iron deficiency increases H. pylori expression of the Cag pathogenicity island that encodes for a type 4 secretion system used to deliver CagA into host cells (Merrell et al., 2003). Interestingly, along with the secreted toxin VacA, CagA causes the mislocalization of transferrin receptors from the basolateral membrane to the apical surface where the pathogen is located (Tan et al., 2011). H. pylori subsequently acquires iron from transferrin and reveals how H. pylori manipulates host iron metabolism under iron-deplete conditions to promote survival in the stomach. Though there are well-established associations between H. pylori, gastric cancer, and iron deficiency, there are several outstanding questions regarding the mechanisms that link them together.
Dietary manganese and nutritional immunity in the heart
The role of dietary manganese and susceptibility to infectious disease is not well described, though a role for manganese in immune function has been implicated. For instance, manganese excess increases expression of pro-inflammatory cytokines from neuron-associated glial cells (Dodd and Filipov, 2011) and macrophages (Mokgobu et al., 2015) as well as enhances natural killer cell (Rogers et al., 1983) and macrophage activity (Smialowicz et al., 1985). However, high manganese also inhibits lymphocyte proliferation (Hart, 1978), exemplifying how balancing metal levels is necessary for optimum immune function. It remains unclear how the altered inflammatory environment mediated by elevated manganese from the diet affects susceptibility to bacterial colonization, but evidence points to a link between dietary manganese and nutritional immunity. In a mouse model of systemic S. aureus infection, high dietary manganese leads to increased bacterial burdens in the heart and enhanced bacterial virulence (Juttukonda et al., 2017). S. aureus invasion into organ tissue typically results in neutrophil migration to the bacterial abscesses where they release calprotectin to bind manganese. While S. aureus induces the release of calprotectin around the heart abscess, calprotectin fails to limit manganese availability when mice are provided a high manganese diet (Juttukonda et al., 2017). In this manner, high dietary manganese subverts nutritional immunity and potentiates pathogen virulence.
Dietary copper, nickel, cobalt, and molybdenum in host-pathogen responses
Zinc, iron, and manganese are the most common transition metals serving critical cofactor or structural roles in mammalian and bacterial proteins (Andreini et al., 2008). However, other metals, though not as abundant, are also critical for life. Copper, nickel, cobalt, and molybdenum are diet-derived and important for the proper functioning of several cellular processes. In humans, the causes of copper deficiency, if known, include prior gastric surgery, excessive zinc consumption, and malabsorption (Kumar, 2006). Copper deficiencies are most likely to affect host-pathogen interactions, as it is widely accepted that localized copper abundance increases at sites of bacterial invasion to cause bactericidal toxicity (Besold et al., 2016) that in E. coli is mediated in part through binding to FeS cluster assembly protein IscA and inhibiting FeS assembly (Tan et al., 2014). In animal models copper deficiency limits macrophage-mediated bactericidal killing against S. pneumoniae, M. tuberculosis, P. haemolytica, and S. Typhimurium (Johnson et al., 2015; Jones and Suttle, 1983; Newberne et al., 1968; Wolschendorf et al., 2011). In response, bacteria have evolved mechanisms to relieve host-mediated copper stress including copper exporters (Johnson et al., 2015). Additionally, copper is a component of ferroxidases, such as ceruloplasm and hephaestin, that oxidize iron(II) to iron(III) (Vashchenko and MacGillivray, 2013). Thus, copper availability both directly affects host-pathogen interactions as well as impacts iron homeostasis.
Nickel, cobalt, and molybdenum are important in many bacterial enzymes. Nickel is a cofactor in bacterial urease and NiFe hydrogenases that contribute to pathogen virulence (de Reuse et al., 2013; Remy et al., 2013). Cobalt is incorporated into coenzyme B12 and is necessary for multiple physiological processes including ethanolamine utilization that contributes to S. Typhimurium growth in the intestines (Thiennimitr et al., 2011). Molybdenum, when complexed with a pterin molecule to form a molybdenum cofactor, is necessary for the catalytic activity of reductases and dehydrogenases in bacterial respiration that are critical during infection by the Enterobacteriaceae (Lopez et al., 2016). An exciting open area of research will be the discovery of how diet-mediated changes to nickel, cobalt, and molybdenum availability affect host-pathogen interactions.
Conclusions
There is undoubtedly a strong connection between mammalian nutrition and susceptibility to infectious disease. Striking a balance between metal deficiency and toxicity requires coordinated efforts for both mammals and microbes, with disturbance of this balance causing repercussions for the survival of both parties. While several studies have elucidated associations between metal deficiencies or overloads with susceptibility to bacterial infection, often the mechanisms leading to increased susceptibility are not as clear. Complicating matters, efforts to correct the developmental or neurological abnormalities of metal imbalances through changing dietary intake quite often have adverse effects by subverting nutritional immunity and are dependent on immune status and genetic factors. Furthermore, during infection multiple metal pools are in a state of flux, with the availability of one metal often influencing the distribution of other metal cations. How nutrient metal abundances in the body can be therapeutically regulated while simultaneously maintaining resistance to bacterial colonization will be a necessary field of investigation. To accomplish this task, we need a better understanding of how the host shuttles metals under inflammatory and non-inflammatory conditions, how this contributes to nutritional immunity, and how pathogens have evolved to counter this defense mechanism. Additionally, how luminal concentrations of dietary metals shifts microbial populations in the intestines is far from understood. Metal availability could directly impact bacterial physiology to allow for certain bacterial groups to increase in abundance while decreasing the abundance of other groups. This in turn may alter colonization resistance, either through microbe-microbe interactions or by signaling changes through host immune or metabolic pathways. Equally valid, the nutrient metal status of the host could adjust immune responses to the commensal microbiota to cause dysbiosis and change susceptibility to pathogen colonization. Only by examining how nutrient metal abundance contributes to the multiple facets of host and microbe interactions will we elucidate how disturbances in that abundance drives changes to disease susceptibility and bacterial colonization.
Acknowledgments
We thank members of the Skaar laboratory for critical reading of the manuscript. The writing of this manuscript was supported by grants for C.A.L. from the Simons Foundation through the Jane Coffin Childs Memorial Fund for Medical Research, the Burroughs Wellcome Fund Postdoctoral Enrichment Program, and the National Institutes of Health (NIH)(T32AI095202) and research grants to E.P.S. from the NIH (R01 AI101171, R01 AI069233, R01 AI073843), the Vanderbilt Digestive Disease Research Center (P30DK058404), and the Ernest W. Goodpasture professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9:e98400. doi: 10.1371/journal.pone.0098400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achouiti A, Vogl T, Urban CF, Rohm M, Hommes TJ, van Zoelen MA, Florquin S, Roth J, van ‘t Veer C, de Vos AF, et al. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog. 2012;8:e1002987. doi: 10.1371/journal.ppat.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PC, Barton JC. Haemochromatosis. Lancet. 2007;370:1855–1860. doi: 10.1016/S0140-6736(07)61782-6. [DOI] [PubMed] [Google Scholar]
- Agoro R, Benmerzoug S, Rose S, Bouyer M, Gozzelino R, Garcia I, Ryffel B, Quesniaux VFJ, Mura C. An Iron-Rich Diet Decreases the Mycobacterial Burden and Correlates With Hepcidin Upregulation, Lower Levels of Proinflammatory Mediators, and Increased T-Cell Recruitment in a Model of Mycobacterium bovis Bacille Calmette-Guerin Infection. J Infect Dis. 2017;216:907–918. doi: 10.1093/infdis/jix366. [DOI] [PubMed] [Google Scholar]
- Akinosoglou KS, Solomou EE, Gogos CA. Malaria: a haematological disease. Hematology. 2012;17:106–114. doi: 10.1179/102453312X13221316477336. [DOI] [PubMed] [Google Scholar]
- Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008;13:1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- Anzaldi LL, Skaar EP. Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect Immun. 2010;78:4977–4989. doi: 10.1128/IAI.00613-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arezes J, Jung G, Gabayan V, Valore E, Ruchala P, Gulig PA, Ganz T, Nemeth E, Bulut Y. Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus. Cell Host Microbe. 2015;17:47–57. doi: 10.1016/j.chom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Bishop W. Effect of Helicobacter pylori infection on gastric acid secretion and iron absorption: can we iron out the issue? J Pediatr Gastroenterol Nutr. 2005;40:102–103. doi: 10.1097/00005176-200501000-00025. [DOI] [PubMed] [Google Scholar]
- Barnett JB, Hamer DH, Meydani SN. Low zinc status: a new risk factor for pneumonia in the elderly? Nutr Rev. 2010;68:30–37. doi: 10.1111/j.1753-4887.2009.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basnet S, Mathisen M, Strand TA. Oral zinc and common childhood infections--An update. J Trace Elem Med Biol. 2015;31:163–166. doi: 10.1016/j.jtemb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Beckett AC, Piazuelo MB, Noto JM, Peek RM, Jr, Washington MK, Algood HM, Cover TL. Dietary Composition Influences Incidence of Helicobacter pylori-Induced Iron Deficiency Anemia and Gastric Ulceration. Infect Immun. 2016;84:3338–3349. doi: 10.1128/IAI.00479-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besold AN, Culbertson EM, Culotta VC. The Yin and Yang of copper during infection. J Biol Inorg Chem. 2016;21:137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC. The role of calprotectin in withholding zinc and copper from Candida albicans. Infect Immun. 2017 doi: 10.1128/IAI.00779-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari N, Bahl R, Taneja S, Strand T, Molbak K, Ulvik RJ, Sommerfelt H, Bhan MK. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics. 2002;109:e86. doi: 10.1542/peds.109.6.e86. [DOI] [PubMed] [Google Scholar]
- Bolick DT, Kolling GL, Moore JH, 2nd, de Oliveira LA, Tung K, Philipson C, Viladomiu M, Hontecillas R, Bassaganya-Riera J, Guerrant RL. Zinc deficiency alters host response and pathogen virulence in a mouse model of enteroaggregative Escherichia coli-induced diarrhea. Gut Microbes. 2014;5:618–627. doi: 10.4161/19490976.2014.969642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorsi-Riani E, Danger R, Lozano JJ, Martinez-Picola M, Kodela E, Mas-Malavila R, Bruguera M, Collins HL, Hider RC, Martinez-Llordella M, et al. Iron Deficiency Impairs Intra-Hepatic Lymphocyte Mediated Immune Response. PLoS One. 2015;10:e0136106. doi: 10.1371/journal.pone.0136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonovas S, Fiorino G, Allocca M, Lytras T, Tsantes A, Peyrin-Biroulet L, Danese S. Intravenous Versus Oral Iron for the Treatment of Anemia in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 2016;95:e2308. doi: 10.1097/MD.0000000000002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70:6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandel J, Humbert N, Elhabiri M, Schalk IJ, Mislin GL, Albrecht-Gary AM. Pyochelin, a siderophore of Pseudomonas aeruginosa: physicochemical characterization of the iron(III), copper(II) and zinc(II) complexes. Dalton Trans. 2012;41:2820–2834. doi: 10.1039/c1dt11804h. [DOI] [PubMed] [Google Scholar]
- Brekelmans P, van Soest P, Leenen PJ, van Ewijk W. Inhibition of proliferation and differentiation during early T cell development by anti-transferrin receptor antibody. Eur J Immunol. 1994;24:2896–2902. doi: 10.1002/eji.1830241147. [DOI] [PubMed] [Google Scholar]
- Byndloss MX, Pernitzsch SR, Baumler AJ. Healthy hosts rule within: ecological forces shaping the gut microbiota. Mucosal Immunol. 2018 doi: 10.1038/s41385-018-0010-y. [DOI] [PubMed] [Google Scholar]
- Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- Constante M, Fragoso G, Calve A, Samba-Mondonga M, Santos MM. Dietary Heme Induces Gut Dysbiosis, Aggravates Colitis, and Potentiates the Development of Adenomas in Mice. Front Microbiol. 2017;8:1809. doi: 10.3389/fmicb.2017.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover TL, Peek RM., Jr Diet, microbial virulence, and Helicobacter pylori-induced gastric cancer. Gut Microbes. 2013;4:482–493. doi: 10.4161/gmic.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts AA, Poly FM, Ewing CP, Kuroiwa JM, Rimmer JE, Harro C, Sack D, Talaat KR, Porter CK, Gutierrez RL, et al. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat Microbiol. 2018;3:494–502. doi: 10.1038/s41564-018-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronje L, Bornman L. Iron overload and tuberculosis: a case for iron chelation therapy. Int J Tuberc Lung Dis. 2005;9:2–9. [PubMed] [Google Scholar]
- Dale A, Thomas JE, Darboe MK, Coward WA, Harding M, Weaver LT. Helicobacter pylori infection, gastric acid secretion, and infant growth. J Pediatr Gastroenterol Nutr. 1998;26:393–397. doi: 10.1097/00005176-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Damo S, Chazin WJ, Skaar EP, Kehl-Fie TE. Inhibition of bacterial superoxide defense: a new front in the struggle between host and pathogen. Virulence. 2012;3:325–328. doi: 10.4161/viru.19635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- de Reuse H, Vinella D, Cavazza C. Common themes and unique proteins for the uptake and trafficking of nickel, a metal essential for the virulence of Helicobacter pylori. Front Cell Infect Microbiol. 2013;3:94. doi: 10.3389/fcimb.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE., 3rd The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc Natl Acad Sci U S A. 2000;97:1252–1257. doi: 10.1073/pnas.97.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, Raffatellu M. Probiotic bacteria reduce salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 2013;14:26–37. doi: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ochoa VE, Lam D, Lee CS, Klaus S, Behnsen J, Liu JZ, Chim N, Nuccio SP, Rathi SG, Mastroianni JR, et al. Salmonella Mitigates Oxidative Stress and Thrives in the Inflamed Gut by Evading Calprotectin-Mediated Manganese Sequestration. Cell Host Microbe. 2016;19:814–825. doi: 10.1016/j.chom.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintilhac A, Alloing G, Granadel C, Claverys JP. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- Dodd CA, Filipov NM. Manganese potentiates LPS-induced heme-oxygenase 1 in microglia but not dopaminergic cells: role in controlling microglial hydrogen peroxide and inflammatory cytokine output. Neurotoxicology. 2011;32:683–692. doi: 10.1016/j.neuro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal A, Baumgartner J, Riesen N, Chassard C, Smuts CM, Zimmermann MB, Lacroix C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: a randomised, placebo-controlled intervention trial in South African children. Br J Nutr. 2014a;112:547–556. doi: 10.1017/S0007114514001160. [DOI] [PubMed] [Google Scholar]
- Dostal A, Lacroix C, Pham VT, Zimmermann MB, Del’homme C, Bernalier-Donadille A, Chassard C. Iron supplementation promotes gut microbiota metabolic activity but not colitis markers in human gut microbiota-associated rats. Br J Nutr. 2014b;111:2135–2145. doi: 10.1017/S000711451400021X. [DOI] [PubMed] [Google Scholar]
- Dubnau E, Chan J, Mohan VP, Smith I. responses of mycobacterium tuberculosis to growth in the mouse lung. Infect Immun. 2005;73:3754–3757. doi: 10.1128/IAI.73.6.3754-3757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C, Boerma T, Evans D, Harries A, Lienhardt C, McManus J, Pang T, Terry R, Zachariah R. The World Health Report: Research for Universal Health Coverage. Geneva: World Health Organization; 2013. [Google Scholar]
- Fang S, Zhuo Z, Yu X, Wang H, Feng J. Oral administration of liquid iron preparation containing excess iron induces intestine and liver injury, impairs intestinal barrier function and alters the gut microbiota in rats. J Trace Elem Med Biol. 2018;47:12–20. doi: 10.1016/j.jtemb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci U S A. 2006;103:16502–16507. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- Girodon F, Galan P, Monget AL, Boutron-Ruault MC, Brunet-Lecomte P, Preziosi P, Arnaud J, Manuguerra JC, Herchberg S. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN. VIT. AOX. geriatric network. Arch Intern Med. 1999;159:748–754. doi: 10.1001/archinte.159.7.748. [DOI] [PubMed] [Google Scholar]
- Gordeuk VR, Caleffi A, Corradini E, Ferrara F, Jones RA, Castro O, Onyekwere O, Kittles R, Pignatti E, Montosi G, et al. Iron overload in Africans and African-Americans and a common mutation in the SCL40A1 (ferroportin 1) gene. Blood Cells Mol Dis. 2003;31:299–304. doi: 10.1016/s1079-9796(03)00164-5. [DOI] [PubMed] [Google Scholar]
- Grigg JC, Ukpabi G, Gaudin CF, Murphy ME. Structural biology of heme binding in the Staphylococcus aureus Isd system. J Inorg Biochem. 2010;104:341–348. doi: 10.1016/j.jinorgbio.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Hart DA. Evidence that manganese inhibits an early event during stimulation of lymphocytes by mitogens. Exp Cell Res. 1978;113:139–150. doi: 10.1016/0014-4827(78)90094-0. [DOI] [PubMed] [Google Scholar]
- Hassan KA, Pederick VG, Elbourne LD, Paulsen IT, Paton JC, McDevitt CA, Eijkelkamp BA. Zinc stress induces copper depletion in Acinetobacter baumannii. BMC Microbiol. 2017;17:59. doi: 10.1186/s12866-017-0965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter-Aeberli I, Eliancy K, Rathon Y, Loechl CU, Marhone Pierre J, Zimmermann MB. In Haitian women and preschool children, iron absorption from wheat flour-based meals fortified with sodium iron EDTA is higher than that from meals fortified with ferrous fumarate, and is not affected by Helicobacter pylori infection in children. Br J Nutr. 2017;118:273–279. doi: 10.1017/S0007114517002045. [DOI] [PubMed] [Google Scholar]
- Hornung B, Martins Dos Santos VAP, Smidt H, Schaap PJ. Studying microbial functionality within the gut ecosystem by systems biology. Genes Nutr. 2018;13:5. doi: 10.1186/s12263-018-0594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:e157–166. doi: 10.1016/j.ijid.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64:731–742. doi: 10.1136/gutjnl-2014-307720. [DOI] [PubMed] [Google Scholar]
- Janakiraman A, Slauch JM. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Kehl-Fie TE, Klein R, Kelly J, Burnham C, Mann B, Rosch JW. Role of copper efflux in pneumococcal pathogenesis and resistance to macrophage-mediated immune clearance. Infect Immun. 2015;83:1684–1694. doi: 10.1128/IAI.03015-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NB, Hayes LD, Hoo EC, Ethier KA. CDC National Health Report: Leading causes of morbidity and mortality and associated risk and protective factors - United States, 2005–2013. MMWR Supplements. 2014:3–27. [PubMed] [Google Scholar]
- Jones DG, Suttle NF. The effect of copper deficiency on the resistance of mice to infection with Pasteurella haemolytica. J Comp Pathol. 1983;93:143–149. doi: 10.1016/0021-9975(83)90052-x. [DOI] [PubMed] [Google Scholar]
- Juttukonda LJ, Berends ETM, Zackular JP, Moore JL, Stier MT, Zhang Y, Schmitz JE, Beavers WN, Wijers CD, Gilston BA, et al. Dietary Manganese Promotes Staphylococcal Infection of the Heart. Cell Host Microbe. 2017;22:531–542 e538. doi: 10.1016/j.chom.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juttukonda LJ, Skaar EP. Manganese homeostasis and utilization in pathogenic bacteria. Mol Microbiol. 2015;97:216–228. doi: 10.1111/mmi.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan FA, Fisher MA, Khakoo RA. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis. 2007;11:482–487. doi: 10.1016/j.ijid.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Kim DK, Jeong JH, Lee JM, Kim KS, Park SH, Kim YD, Koh M, Shin M, Jung YS, Kim HS, et al. Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat Med. 2014;20:419–424. doi: 10.1038/nm.3483. [DOI] [PubMed] [Google Scholar]
- Kochanowski BA, Sherman AR. Decreased antibody formation in iron-deficient rat pups--effect of iron repletion. Am J Clin Nutr. 1985;41:278–284. doi: 10.1093/ajcn/41.2.278. [DOI] [PubMed] [Google Scholar]
- Konomi JV, Harris FL, Ping XD, Gauthier TW, Brown LA. Zinc insufficiency mediates ethanol-induced alveolar macrophage dysfunction in the pregnant female mouse. Alcohol Alcohol. 2015;50:30–38. doi: 10.1093/alcalc/agu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortman GA, Mulder ML, Richters TJ, Shanmugam NK, Trebicka E, Boekhorst J, Timmerman HM, Roelofs R, Wiegerinck ET, Laarakkers CM, et al. Low dietary iron intake restrains the intestinal inflammatory response and pathology of enteric infection by food-borne bacterial pathogens. Eur J Immunol. 2015;45:2553–2567. doi: 10.1002/eji.201545642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174–1194. doi: 10.1016/j.freeradbiomed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Kumar N. Copper deficiency myelopathy (human swayback) Mayo Clin Proc. 2006;81:1371–1384. doi: 10.4065/81.10.1371. [DOI] [PubMed] [Google Scholar]
- Kuvibidila SR, Nauss KM, Baliga SB, Suskind RM. Impairment of blastogenic response of splenic lymphocytes from iron-deficient mice. In vitro repletion by hemin, transferrin, and ferric chloride. Am J Clin Nutr. 1983;37:557–565. doi: 10.1093/ajcn/37.4.557. [DOI] [PubMed] [Google Scholar]
- Leenstra T, Kariuki SK, Kurtis JD, Oloo AJ, Kager PA, ter Kuile FO. The effect of weekly iron and vitamin A supplementation on hemoglobin levels and iron status in adolescent schoolgirls in western Kenya. Eur J Clin Nutr. 2009;63:173–182. doi: 10.1038/sj.ejcn.1602919. [DOI] [PubMed] [Google Scholar]
- Legrand D, Elass E, Pierce A, Mazurier J. Lactoferrin and host defence: an overview of its immuno-modulating and anti-inflammatory properties. Biometals. 2004;17:225–229. doi: 10.1023/b:biom.0000027696.48707.42. [DOI] [PubMed] [Google Scholar]
- Liu MJ, Bao S, Galvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep. 2013;3:386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokken KL, Mooney JP, Butler BP, Xavier MN, Chau JY, Schaltenberg N, Begum RH, Muller W, Luckhart S, Tsolis RM. Malaria parasite infection compromises control of concurrent systemic non-typhoidal Salmonella infection via IL-10-mediated alteration of myeloid cell function. PLoS Pathog. 2014;10:e1004049. doi: 10.1371/journal.ppat.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WP, Johnson ML, Brasfield A, Blanc MP, Yi J, Miller SI, Cookson BT, Hajjar AM. Temporal and anatomical host resistance to chronic Salmonella infection is quantitatively dictated by Nramp1 and influenced by host genetic background. PLoS One. 2014;9:e111763. doi: 10.1371/journal.pone.0111763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CA, Miller BM, Rivera-Chavez F, Velazquez EM, Byndloss MX, Chavez-Arroyo A, Lokken KL, Tsolis RM, Winter SE, Baumler AJ. Virulence factors enhance Citrobacter rodentium expansion through aerobic respiration. Science. 2016;353:1249–1253. doi: 10.1126/science.aag3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xin Y, Tang Y, Shao G. Zinc suppressed the airway inflammation in asthmatic rats: effects of zinc on generation of eotaxin, MCP-1, IL-8, IL-4, and IFN-gamma. Biol Trace Elem Res. 2012;150:314–321. doi: 10.1007/s12011-012-9493-7. [DOI] [PubMed] [Google Scholar]
- Marguerettaz M, Dieppois G, Que YA, Ducret V, Zuchuat S, Perron K. Sputum containing zinc enhances carbapenem resistance, biofilm formation and virulence of Pseudomonas aeruginosa. Microb Pathog. 2014;77:36–41. doi: 10.1016/j.micpath.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Medeiros P, Bolick DT, Roche JK, Noronha F, Pinheiro C, Kolling GL, Lima A, Guerrant RL. The micronutrient zinc inhibits EAEC strain 042 adherence, biofilm formation, virulence gene expression, and epithelial cytokine responses benefiting the infected host. Virulence. 2013;4:624–633. doi: 10.4161/viru.26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell DS, Thompson LJ, Kim CC, Mitchell H, Tompkins LS, Lee A, Falkow S. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect Immun. 2003;71:6510–6525. doi: 10.1128/IAI.71.11.6510-6525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CE, Rock JD, Ridley KA, Williams PH, Ketley JM. Utilization of lactoferrin-bound and transferrin-bound iron by Campylobacter jejuni. J Bacteriol. 2008;190:1900–1911. doi: 10.1128/JB.01761-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JL. Iron deficiency anemia: a common and curable disease. Cold Spring Harb Perspect Med. 2013:3. doi: 10.1101/cshperspect.a011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokgobu MI, Cholo MC, Anderson R, Steel HC, Motheo MP, Hlatshwayo TN, Tintinger GR, Theron AJ. Oxidative induction of pro-inflammatory cytokine formation by human monocyte-derived macrophages following exposure to manganese in vitro. J Immunotoxicol. 2015;12:98–103. doi: 10.3109/1547691X.2014.902877. [DOI] [PubMed] [Google Scholar]
- Moore LL, Humbert JR. Neutrophil bactericidal dysfunction towards oxidant radical-sensitive microorganisms during experimental iron deficiency. Pediatr Res. 1984;18:789–794. doi: 10.1203/00006450-198408000-00027. [DOI] [PubMed] [Google Scholar]
- Mortensen BL, Rathi S, Chazin WJ, Skaar EP. Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J Bacteriol. 2014;196:2616–2626. doi: 10.1128/JB.01650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen BL, Skaar EP. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front Cell Infect Microbiol. 2013;3:95. doi: 10.3389/fcimb.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy H, Charron-Mazenod L, Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy TA, Moreland SM, Andrews-Polymenis H, Detweiler CS. The ferric enterobactin transporter Fep is required for persistent Salmonella enterica serovar typhimurium infection. Infect Immun. 2013;81:4063–4070. doi: 10.1128/IAI.00412-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy TA, Moreland SM, Detweiler CS. Salmonella acquires ferrous iron from haemophagocytic macrophages. Mol Microbiol. 2014;93:1314–1326. doi: 10.1111/mmi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn BL, Lonergan ZR, Wang J, Braymer JJ, Zhang Y, Calcutt MW, Lisher JP, Gilston BA, Chazin WJ, de Crecy-Lagard V, et al. The Response of Acinetobacter baumannii to Zinc Starvation. Cell Host Microbe. 2016;19:826–836. doi: 10.1016/j.chom.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, Talasz H, Brandacher G, Moser PL, Muckenthaler MU, et al. Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med. 2013;210:855–873. doi: 10.1084/jem.20121946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9:2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol. 2015;11:765–771. doi: 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashige TG, Zygiel EM, Drennan CL, Nolan EM. Nickel Sequestration by the Host-Defense Protein Human Calprotectin. J Am Chem Soc. 2017;139:8828–8836. doi: 10.1021/jacs.7b01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Y, Nelson N. The NRAMP family of metal-ion transporters. Biochim Biophys Acta. 2006;1763:609–620. doi: 10.1016/j.bbamcr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Newberne PM, Hunt CE, Young VR. The role of diet and the reticuloendothelial system in the response of rats to Salmonella typhilmurium infection. Br J Exp Pathol. 1968;49:448–457. [PMC free article] [PubMed] [Google Scholar]
- Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479–492. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhuysen PC, Dupont HL. Enteroaggregative Escherichia coli (EAEC): a cause of acute and persistent diarrhea of worldwide importance. J Infect Dis. 2010;202:503–505. doi: 10.1086/654895. [DOI] [PubMed] [Google Scholar]
- Pajuelo D, Hernandez-Cabanyero C, Sanjuan E, Lee CT, Silva-Hernandez FX, Hor LI, MacKenzie S, Amaro C. Iron and Fur in the life cycle of the zoonotic pathogen Vibrio vulnificus. Environ Microbiol. 2016;18:4005–4022. doi: 10.1111/1462-2920.13424. [DOI] [PubMed] [Google Scholar]
- Pederick VG, Eijkelkamp BA, Begg SL, Ween MP, McAllister LJ, Paton JC, McDevitt CA. ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Sci Rep. 2015;5:13139. doi: 10.1038/srep13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakin A, Schneider L, Podladchikova O. Hunger for iron: the alternative siderophore iron scavenging systems in highly virulent Yersinia. Front Cell Infect Microbiol. 2012;2:151. doi: 10.3389/fcimb.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S, Neuman H, Moscovich S, Glahn RP, Koren O, Tako E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients. 2015;7:9768–9784. doi: 10.3390/nu7125497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy L, Carriere M, Derre-Bobillot A, Martini C, Sanguinetti M, Borezee-Durant E. The Staphylococcus aureus Opp1 ABC transporter imports nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol Microbiol. 2013;87:730–743. doi: 10.1111/mmi.12126. [DOI] [PubMed] [Google Scholar]
- Rogers RR, Garner RJ, Riddle MM, Luebke RW, Smialowicz RJ. Augmentation of murine natural killer cell activity by manganese chloride. Toxicol Appl Pharmacol. 1983;70:7–17. doi: 10.1016/0041-008x(83)90174-6. [DOI] [PubMed] [Google Scholar]
- Sampson B, Fagerhol MK, Sunderkotter C, Golden BE, Richmond P, Klein N, Kovar IZ, Beattie JH, Wolska-Kusnierz B, Saito Y, et al. Hyperzincaemia and hypercalprotectinaemia: a new disorder of zinc metabolism. Lancet. 2002;360:1742–1745. doi: 10.1016/S0140-6736(02)11683-7. [DOI] [PubMed] [Google Scholar]
- Santos RL, Raffatellu M, Bevins CL, Adams LG, Tukel C, Tsolis RM, Baumler AJ. Life in the inflamed intestine, Salmonella style. Trends Microbiol. 2009;17:498–506. doi: 10.1016/j.tim.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Bhan MK, Bhandari N, Sinha A, Jalla S. Zinc supplementation in young children with acute diarrhea in India. N Engl J Med. 1995;333:839–844. doi: 10.1056/NEJM199509283331304. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Collins HL, Priem F, Kaufmann SH. Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J Exp Med. 2002;196:1507–1513. doi: 10.1084/jem.20020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Everard A, Gomez-Valades AG, Matamoros S, Ramirez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowicz RJ, Luebke RW, Rogers RR, Riddle MM, Rowe DG. Manganese chloride enhances natural cell-mediated immune effector cell function: effects on macrophages. Immunopharmacology. 1985;9:1–11. doi: 10.1016/0162-3109(85)90040-2. [DOI] [PubMed] [Google Scholar]
- Spottiswoode N, Duffy PE, Drakesmith H. Iron, anemia and hepcidin in malaria. Front Pharmacol. 2014;5:125. doi: 10.3389/fphar.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke IC, Pieper R, Neumann K, Zentek J, Vahjen W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol Ecol. 2014;87:416–427. doi: 10.1111/1574-6941.12233. [DOI] [PubMed] [Google Scholar]
- Stauff DL, Skaar EP. The heme sensor system of Staphylococcus aureus. Contrib Microbiol. 2009;16:120–135. doi: 10.1159/000219376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surdel MC, Dutter BF, Sulikowski GA, Skaar EP. Bacterial Nitric Oxide Synthase Is Required for the Staphylococcus aureus Response to Heme Stress. ACS Infect Dis. 2016;2:572–578. doi: 10.1021/acsinfecdis.6b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G, Cheng Z, Pang Y, Landry AP, Li J, Lu J, Ding H. Copper binding in IscA inhibits iron-sulphur cluster assembly in Escherichia coli. Mol Microbiol. 2014;93:629–644. doi: 10.1111/mmi.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Noto JM, Romero-Gallo J, Peek RM, Jr, Amieva MR. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog. 2011;7:e1002050. doi: 10.1371/journal.ppat.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot CM, Young VB. Interactions Between the Gastrointestinal Microbiome and Clostridium difficile. Annu Rev Microbiol. 2015;69:445–461. doi: 10.1146/annurev-micro-091014-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A. 2011;108:17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen S, de Mast Q, Swinkels DW, van der Ven AJ. The iron link between malaria and invasive non-typhoid Salmonella infections. Trends Parasitol. 2013;29:220–227. doi: 10.1016/j.pt.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashchenko G, MacGillivray RT. Multi-copper oxidases and human iron metabolism. Nutrients. 2013;5:2289–2313. doi: 10.3390/nu5072289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman CA, Hammer ND, Stauff DL, Attia AS, Anzaldi LL, Dikalov SI, Calcutt MW, Skaar EP. Menaquinone biosynthesis potentiates haem toxicity in Staphylococcus aureus. Mol Microbiol. 2012;86:1376–1392. doi: 10.1111/mmi.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Fuchs D, Hausen A, Reibnegger G, Werner ER, Werner-Felmayer G, Wachter H. Iron modulates interferon-gamma effects in the human myelomonocytic cell line THP-1. Exp Hematol. 1992;20:605–610. [PubMed] [Google Scholar]
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7:e50568. doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A, Heinzl G. Heme oxygenation and the widening paradigm of heme degradation. Arch Biochem Biophys. 2014;544:87–95. doi: 10.1016/j.abb.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, Lamichhane G, Wang Y, Bossmann SH, Basaraba RJ, Niederweis M. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2011;108:1621–1626. doi: 10.1073/pnas.1009261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Yeoh BS, Vijay-Kumar M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu Rev Nutr. 2017;37:103–130. doi: 10.1146/annurev-nutr-071816-064559. [DOI] [PubMed] [Google Scholar]
- Xu F, Zeng X, Haigh RD, Ketley JM, Lin J. Identification and characterization of a new ferric enterobactin receptor, CfrB, in Campylobacter. J Bacteriol. 2010;192:4425–4435. doi: 10.1128/JB.00478-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Osborn J, Panchal A, Mellies JL. The RpoE Stress Response Pathway Mediates Reduction of the Virulence of Enteropathogenic Escherichia coli by Zinc. Appl Environ Microbiol. 2015;81:3766–3774. doi: 10.1128/AEM.00507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Qian SY, Li Z, Zhang ZZ. Effect of zinc supplementation on infants with severe pneumonia. World J Pediatr. 2016;12:166–169. doi: 10.1007/s12519-015-0072-9. [DOI] [PubMed] [Google Scholar]
- Zackular JP, Chazin WJ, Skaar EP. Nutritional Immunity: S100 Proteins at the Host-Pathogen Interface. J Biol Chem. 2015;290:18991–18998. doi: 10.1074/jbc.R115.645085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackular JP, Moore JL, Jordan AT, Juttukonda LJ, Noto MJ, Nicholson MR, Crews JD, Semler MW, Zhang Y, Ware LB, et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat Med. 2016;22:1330–1334. doi: 10.1038/nm.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]