Abstract
Objectives
The aim of this study was to examine sex disparity in metabolic syndrome prevalence and its risk factors among Chinese adults.
Methods
Using the 2010–2012 China National Nutrition and Health Survey (CNNHS), a nationally representative cross-sectional study on nutrition and non-communicable chronic diseases, a total of 98,042 participants aged 18 years and older were included in the analysis. Dietary information was collected with a food frequency questionnaire (FFQ). Metabolic syndrome was defined according to the updated NCEP ATP III criteria. A multivariable logistic regression model was performed to examine the associations between sociodemographic and dietary factors with metabolic syndrome prevalence, and the results are presented using odd ratios (ORs) and 95% confidence intervals (CIs).
Results
The overall standardized prevalence of metabolic syndrome was 24.2% (24.6% in men and 23.8% in women). The metabolic syndrome prevalence was positively associated with age in men and women. The prevalence of metabolic syndrome was negatively associated with the physical activity level among men and inversely associated with the education level among women (P for trend < 0.01). Frequent consumption of fungi and algae was an underlying risk factor for metabolic syndrome in men, whereas frequent consumption of nuts and pork was associated with a decreased prevalence of metabolic syndrome in women.
Conclusions
The prevalence of metabolic syndrome in men was not different from that in women. There are sex-specific associations between multiple risk factors and metabolic syndrome.
Introduction
In 2015, approximately 290 million people had cardiovascular disease (CVD). CVD is the number one cause of mortality in China and accounts for over 40% of total deaths [1]. Metabolic syndrome is characterized by a clustering of CVD risk factors, including abdominal obesity; increased blood pressure, fasting plasma glucose, and triglyceride (TG); and decreased high-density lipoprotein cholesterol (HDL-C) [2]. Exploring the cause of metabolic syndrome prevalence may provide important public health implications for the prevention and management of CVD. The prevalence of metabolic syndrome has increased dramatically worldwide [3–5]. According to the International Collaborative Study of Cardiovascular Disease in ASIA (InterASIA), the age-standardized prevalence of metabolic syndrome was 13.7% among adults aged 35–74 years in China between 2000 and 2001, using the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria [6]. Based on 2010 China Noncommunicable Disease Surveillance data assessed by NCEP ATP III criteria, the prevalence of metabolic syndrome among participants aged 18 years and older was 33.9% [7].
In China, dietary intake has changed substantially, which may be causing the rapidly rising prevalence of metabolic syndrome. Grain intake has decreased significantly, whereas fat intake has increased dramatically. The daily intake of salt is much higher, and the daily intake of vegetables and fruits is lower than recommended [8]. A cross-sectional study explored metabolic syndrome prevalence and associated dietary factors in a sample of urban Chinese adults; however, the dietary intake factors were not fully explored [9]. A prospective study based on the amount of dietary intake showed that the consumption of meat, fried food, and diet soda were adversely associated with incident metabolic syndrome risk, whereas dairy consumption was beneficial [10].
Although growing evidence has suggested that multitudinous factors are associated with metabolic syndrome prevalence [11–13], few studies have investigated the sex disparity associations between risk factors and metabolic syndrome prevalence. Therefore, we aimed to examine sex disparity in metabolic syndrome prevalence and its risk factors among the Chinese population.
Methods
Study population
The 2010–2012 China National Nutrition and Health Survey (CNNHS) is a nationally representative cross-sectional study on nutrition and non-communicable chronic diseases. This survey selected 150 survey sites (districts or counties) of 31 provinces, autonomous regions, and municipalities directly under the Chinese central government (excluding Taiwan, Hong Kong, and Macao). The country was divided into four strata according to socioeconomic characteristics: large cities, small-to-medium cities, general rural areas and poor rural areas. The first stage of sampling involved the random selection of 150 survey sites, including 34 survey sites from large cities, 41 survey sites from small-to-medium cities, 45 survey sites from general rural areas, and 30 survey sites from poor rural areas. The second stage involved the random selection of six residential committees (urban) or villages (rural). In the third sampling stage, according to the geographical location of the household, a total of 25 households is considered as a sampling unit. Three sampling units (75 households) were randomly selected from each of the residential committees or villages. In addition, participants from the first sampling unit (25 households) completed an interview with a structured food frequency questionnaire (FFQ).
Individuals with missing data on metabolic syndrome components including waist circumference, TG, HDL-C, blood pressure, and fasting plasma glucose were excluded. Participants with incomplete information on education level, household income, smoking status, drinking status, and physical activity were further excluded. A total of 98,042 participants aged 18 years and older were included for the association between sociodemographic factors and metabolic syndrome prevalence. Among them, 32,300 participants (13,741 men and 18,559 women) completed the FFQ for the association between dietary intake and metabolic syndrome prevalence. The characteristics of the inclusion and exclusion subjects are shown in S1 Table. There exists significant difference in area, smoking status, physical activity level, and blood pressure between the inclusion and exclusion subjects.
This survey was approved by the Ethical Committee of the National Institute for Nutrition and Food Safety, Chinese Center for Disease Control and Prevention. All participants provided written informed consent.
Data collection
Height, weight, and waist circumference were measured in the morning before breakfast. Height and waist circumference were accurate to 0.1 cm and weight was accurate to 0.1 kg. BMI was calculated as weight in kilograms divided by height in meters squared. Overweight and obesity were defined according to classifications for Asian populations; thus, a BMI between 24.0 and 28.0 kg/m2 is considered overweight, and a BMI ≥ 28.0 kg/m2 is considered obesity [14]. Blood pressure levels were measured three times after 5 minutes of rest in a seated position, and the set interval between measurements was 1 minute. The mean of the three measurements was used for analysis. Hypertension was defined as any of the following: systolic pressure ≥ 140 mmHg; diastolic pressure ≥ 90 mmHg; use of antihypertensive medications; or self-reported hypertension [15, 16]. Fasting plasma glucose, TG, and HDL-C were measured by the hexokinase G-6- PDH method, the GPO-HMMPS glycerol blanking method, and the direct determination method, respectively. All measurements were conducted with the Hitachi 7600 automated biochemical analyzer and all reagents were produced by Wako Pure Chemical, Ltd. Diabetes was diagnosed according to the American Diabetes Association criteria as any of the following [17]: self-report of a physician’s diagnosis of diabetes; fasting plasma glucose ≥ 7.0 mmol/L; oral glucose tolerance test (OGTT) 2-h plasma glucose ≥ 11.1 mmol/L; hemoglobin A1c ≥ 6.5%; or use of anti-diabetic medications.
Definition of metabolic syndrome
The diagnosis of metabolic syndrome was based on the updated NCEP ATPIII criteria [2] and included three or more of the following: (1) abdominal obesity (defined according to guidelines for Chinese populations as waist circumference ≥ 90 cm in men or ≥ 85 cm in women [18]); (2) TG ≥ 1.69 mmol/L; (3) HDL-C cholesterol < 1.03 mmol/L in men or < 1.29 mmol/L in women; (4) systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or use of antihypertensive medications; and (5) fasting plasma glucose ≥ 5.6 mmol/L or use of anti-diabetic medications.
Assessment of dietary intake
In the present study, dietary intake over the past year was assessed with a validated semiquantitative FFQ [19]. The FFQ includes 100 food items. Participants were asked the frequency and amount of each food consumed. Using China Food Composition data [20], we collapsed the 100 food items into 14 predefined food groups (S2 Table). According to the frequency of food intake, each food group was classified into tertiles (low, moderate, and high).
Assessment of covariates
Information including education level, household income, smoking status, drinking status, and physical activity was obtained by trained investigators from face-to-face interviews. We classified education level into uneducated, primary school, junior school, high school, and college or above. Household income was divided into < 10,000, 10,000–30,000, and ≥ 30,000 yuan. Smoking status was classified into never, ever, and current smokers. Drinking status was categorized as non-drinkers, moderate alcohol consumption (with an alcohol intake of less than 175 g by men and 105 g by women per week), and excessive alcohol consumption (with an alcohol intake of more than 175 g by men and 105 g by women per week). Physical activity level (PAL) was calculated according to the recommendation of the Institute of Medicine (IOM) [21] and was divided into quartiles.
Statistical analysis
Continuous variables with normal distribution were presented as means ± standard deviation (SD) and compared between groups using z test. Skewed distribution variables were presented as medians (interquartile ranges) and compared between groups using non-parametric statistical hypothesis test including Wilcoxon rank test and Kruskal Wallis test. Categorical variables were expressed as number (percentages) and compared by the chi-square test. The 2010–2012 CNNHS adopted a complex, multistage, probability sampling design. The standardized prevalence of metabolic syndrome was calculated using the weight coefficients to represent the overall Chinese adult population aged 18 years or older. Weight coefficients accommodated sampling weight and post stratification weight. Sampling weight was computed based on the study design. Post stratification weight was stratified by area, age, and sex, and harmonized the sample structure of the survey with that of the 2010 Chinese population census. PROC SURVEYMEANS and PROC SURVEYFREQ were used for the calculation of means and prevalence. PROC SURVEYLOGISTIC was used to calculate the odd ratios (ORs) and 95% confidence intervals (CIs) of metabolic syndrome prevalence. A 2-sided P value < 0.05 was used to determine statistical significance. Data cleaning and statistical analyses were performed using SAS version 9.2 (SAS Institute).
Results
The characteristics of the participants by sex are shown in Table 1. There was a significant difference between the two groups in terms of education level, smoking status, drinking status, and physical activity level. Men were more likely than women to be older, with higher levels of waist circumference, TG, blood pressure, and fasting plasma glucose and lower levels of BMI and HDL-C. Men had a higher prevalence of hypertension (24.9%) than women (21.5%).
Table 1. Characteristics of participants according to sex (n = 98042).
| Men | Women | P-value | |
|---|---|---|---|
| N (%) | 42036 (50.11) | 56006 (49.89) | |
| Age (years) | 52.92 ± 14.56 | 51.33 ± 14.25 | < 0.001 |
| Area, n (%) | 0.28 | ||
| Urban | 20146 (49.59) | 28504 (50.64) | |
| Rural | 21890 (50.41) | 27502 (49.36) | |
| Education, n (%) | < 0.001 | ||
| Uneducated | 2969 (4.11) | 9716 (12.87) | |
| Primary school | 11954 (21.93) | 17399 (26.43) | |
| Junior school | 16431 (46.11) | 17735 (38.59) | |
| High school | 7253 (19.12) | 7675 (14.68) | |
| College and above | 3429 (8.74) | 3481 (7.42) | |
| Income, n (%) | 0.005 | ||
| < 10000 | 21937 (52.31) | 29090 (54.28) | |
| 10000–30000 | 17115 (40.81) | 22982 (39.29) | |
| > 30000 | 2984 (6.88) | 3934 (6.43) | |
| Smoking, n (%) | < 0.001 | ||
| Current smoker | 22698 (54.68) | 1823 (2.22) | |
| Former smoker | 3201 (4.88) | 706 (1.05) | |
| Never smoker | 16137 (40.44) | 53477 (96.73) | |
| Drinking, n (%) | < 0.001 | ||
| Never drinker | 19200 (43.02) | 48394 (87.27) | |
| Moderate alcohol drinker | 15315 (39.58) | 6709 (11.53) | |
| Excessive alcohol drinker | 7521 (17.41) | 903 (1.20) | |
| Physical activity, n (%) | < 0.001 | ||
| Low | 12222 (30.43) | 12245 (25.33) | |
| Moderate | 7628 (16.12) | 16436 (28.71) | |
| High | 11230 (30.47) | 13203 (21.90) | |
| Very high | 10956 (22.99) | 14122 (24.07) | |
| BMI, n (%) | 0.24 | ||
| Normal | 23189 (57.36) | 29930 (58.46) | |
| Overweight | 14067 (30.36) | 18649 (29.74) | |
| Obesity | 4780 (12.28) | 7427 (11.80) | |
| BMI (kg/m2) | 23.54 (21.31, 25.96) | 23.69 (21.38, 26.22) | < 0.001 |
| Waist circumference (cm) | 83.61 ± 10.40 | 80.17 ± 9.95 | < 0.001 |
| TG (mmol/L) | 1.17 (0.80, 1.80) | 1.12 (0.78, 1.67) | < 0.001 |
| HDL-C (mmol/L) | 1.15 ± 0.34 | 1.22 ± 0.32 | < 0.001 |
| Systolic blood pressure (mmHg) | 126.98 ± 19.77 | 124.35 ± 21.53 | < 0.001 |
| Diastolic blood pressure (mmHg) | 80.06 ± 11.69 | 77.70 ± 11.72 | < 0.001 |
| Fasting plasma glucose (mmol/L) | 5.21 (4.74, 5.75) | 5.17 (4.72, 5.68) | < 0.001 |
| Abdominal obesity, n (%) | 12223 (27.06) | 17799 (26.62) | 0.53 |
| Diabetes, n (%) | 4561 (6.79) | 5599 (6.62) | 0.49 |
| Hypertension, n (%) | 14769 (24.93) | 17517 (21.52) | < 0.001 |
Data are mean ± standard deviation for normally distributed or medians (interquartile ranges) for skewed parameters, or number (%).
Abbreviations: BMI, body mass index; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol.
The characteristics of the participants according to metabolic syndrome status are shown in Table 2. The individuals with metabolic syndrome were more likely than the normal participants to be older, with higher levels of BMI, waist circumference, TG, blood pressure, and fasting plasma glucose, and lower levels of HDL-C. The metabolic syndrome prevalence was higher among North and East residents, but lower among North-West, South-West and North-East residents than among the general populations. Participants with metabolic syndrome were more likely to have obesity (33.6%), abdominal obesity (72.0%), diabetes (18.7%), and hypertension (52.2%).
Table 2. Characteristics of participants according to metabolic syndrome status (n = 98042).
| Variables | Normal | Metabolic syndrome | P-value |
|---|---|---|---|
| N (%) | 67451 (75.80) | 30591 (24.20) | |
| Men, n (%) | 29665 (49.82) | 12371 (50.99) | 0.15 |
| Age (years) | 49.99 ± 14.76 | 56.45 ± 12.50 | < 0.001 |
| Area, n (%) | < 0.001 | ||
| Urban | 31307 (48.30) | 17343 (55.80) | |
| Rural | 36144 (51.70) | 13248 (44.20) | |
| Smoking, n (%) | < 0.001 | ||
| Current smoker | 17820 (28.85) | 6701 (27.45) | |
| Former smoker | 2346 (2.67) | 1561 (3.90) | |
| Never smoker | 47285 (68.48) | 22329 (68.65) | |
| Drinking, n (%) | < 0.001 | ||
| Never drinker | 45696 (64.88) | 21898 (65.80) | |
| Moderate alcohol drinker | 15784 (26.15) | 6240 (23.80) | |
| Excessive alcohol drinker | 5971 (8.97) | 2453 (10.40) | |
| Physical activity level, n (%) | < 0.001 | ||
| Low | 15956 (27.54) | 8511 (28.96) | |
| Moderate | 15572 (21.69) | 8492 (24.64) | |
| High | 16518 (26.04) | 7915 (26.67) | |
| Very high | 19405 (24.74) | 5673 (19.73) | |
| Region | < 0.001 | ||
| North | 9638 (67.10) | 6310 (32.90) | |
| East | 5581 (71.09) | 3200 (28.91) | |
| South-Central | 18432 (75.53) | 8264 (24.47) | |
| North-West | 15434 (76.93) | 6774 (23.07) | |
| South-West | 10605 (82.47) | 3043 (17.53) | |
| North-East | 7761 (81.93) | 3000 (18.07) | |
| BMI (kg/m2) | 22.55 (20.66, 24.57) | 26.30 (24.24, 30.59) | < 0.001 |
| Waist circumference (cm) | 77.99 ± 8.62 | 89.70 ± 9.01 | < 0.001 |
| TG (mmol/L) | 0.96 (0.70, 1.31) | 1.89 (1.31, 2.62) | < 0.001 |
| HDL-C (mmol/L) | 1.28 ± 0.32 | 0.99 ± 0.25 | < 0.001 |
| Systolic blood pressure (mmHg) | 120.60 ± 19.15 | 136.22 ± 20.37 | < 0.001 |
| Diastolic blood pressure (mmHg) | 76.26 ± 10.93 | 84.13 ± 11.72 | < 0.001 |
| Fasting plasma glucose (mmol/L) | 5.03 (4.62, 5.43) | 5.70 (5.12, 6.33) | < 0.001 |
| Obesity, n (%) | 3241 (5.15) | 8966 (33.61) | < 0.001 |
| Abdominal obesity, n (%) | 8631 (12.41) | 21391 (72.01) | < 0.001 |
| Diabetes, n (%) | 2914 (2.88) | 7246 (18.69) | < 0.001 |
| Hypertension, n (%) | 13894 (13.99) | 18392 (52.16) | < 0.001 |
Data are mean ± standard deviation for normally distributed or medians (interquartile ranges) for skewed parameters, or number (%).
Abbreviations: BMI, body mass index; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol.
The overall metabolic syndrome prevalence and its risk factors are shown in Table 3. Participants living in urban areas (27.0%) had a higher metabolic syndrome prevalence than rural residents (21.5%). The prevalence of metabolic syndrome was positively associated with age and household income, but negatively associated with physical activity level (P for trend < 0.05). Individuals with overweight (OR: 6.40; 95% CI: 5.90–6.94) or obesity (OR: 25.04; 95% CI: 22.27–28.15) had a higher metabolic syndrome prevalence than normal individuals. The prevalence of metabolic syndrome was relatively lower among participants living in the South-Central, South-West, and North-East regions (OR = 0.73, 0.61, and 0.77, respectively) than among North residents. Significantly interactions were found for sex and age, area, education, income, drinking status, physical activity level, and BMI with metabolic syndrome prevalence (all P-interaction < 0.01).
Table 3. The metabolic syndrome prevalence and its risk factors in total sample (n = 98042).
| N | Prevalence | OR (95% CI) | P-value | P-interaction a | |
|---|---|---|---|---|---|
| Age, years | < 0.001 | ||||
| 18–44 | 30887 | 16.03 | 1.00 (ref) | ||
| 45–54 | 23264 | 32.12 | 2.27 (2.08–2.47) | < 0.001 | |
| 55–64 | 25046 | 36.97 | 3.16 (2.88–3.48) | < 0.001 | |
| ≥ 65 | 18845 | 37.81 | 4.02 (3.60–4.48) | < 0.001 | |
| P-trend | < 0.001 | ||||
| Area | < 0.001 | ||||
| Urban | 48650 | 26.95 | 1.00 (ref) | ||
| Rural | 49392 | 21.45 | 0.92 (0.82–1.04) | 0.19 | |
| Education | < 0.001 | ||||
| Uneducated | 12685 | 31.42 | 1.00 (ref) | ||
| Primary school | 29353 | 26.27 | 0.91 (0.83–1.01) | 0.07 | |
| Junior school | 34166 | 23.00 | 0.89 (0.79–0.99) | 0.03 | |
| High school | 14928 | 22.55 | 0.76 (0.68–0.86) | < 0.001 | |
| College and above | 6910 | 20.23 | 0.78 (0.64–0.94) | 0.01 | |
| P-trend | 0.07 | ||||
| Income, n (%) | < 0.001 | ||||
| < 10000 | 51027 | 23.31 | 1.00 (ref) | ||
| 10000–30000 | 40097 | 25.17 | 1.08 (0.99–1.18) | 0.07 | |
| > 30000 | 6918 | 25.53 | 1.19 (1.05–1.35) | 0.007 | |
| P-trend | 0.005 | ||||
| Smoking, n (%) | 0.05 | ||||
| Never smoker | 24521 | 24.25 | 1.00 (ref) | ||
| Current smoker | 3907 | 23.30 | 1.02 (0.95–1.10) | 0.59 | |
| Former smoker | 69614 | 31.82 | 1.04 (0.92–1.18) | 0.49 | |
| Drinking, n (%) | 0.01 | ||||
| Never drinker | 67594 | 24.46 | 1.00 (ref) | ||
| Moderate alcohol drinker | 22024 | 22.52 | 0.96 (0.89–1.04) | 0.29 | |
| Excessive alcohol drinker | 8424 | 27.01 | 0.99 (0.90–1.10) | 0.85 | |
| Physical activity, n (%) | < 0.001 | ||||
| Low | 24467 | 25.14 | 1.00 (ref) | ||
| Moderate | 24064 | 26.62 | 1.01 (0.92–1.10) | 0.89 | |
| High | 24433 | 24.65 | 0.90 (0.83–0.98) | 0.02 | |
| Very high | 25078 | 20.30 | 0.79 (0.72–0.86) | < 0.001 | |
| P-trend | < 0.001 | ||||
| BMI, kg/m2 | < 0.001 | ||||
| Normal | 53119 | 8.31 | 1.00 (ref) | ||
| Overweight | 32716 | 37.45 | 6.40 (5.90–6.94) | < 0.001 | |
| Obesity | 12207 | 67.57 | 25.04 (22.27–28.15) | < 0.001 | |
| P-trend | < 0.001 | ||||
| Region | 0.24 | ||||
| North | 15948 | 32.90 | 1.00 (ref) | ||
| East | 8781 | 28.91 | 0.76 (0.55–1.06) | 0.11 | |
| South-Central | 26696 | 24.47 | 0.73 (0.61–0.87) | < 0.001 | |
| North-West | 22208 | 23.07 | 0.89 (0.74–1.08) | 0.23 | |
| South-West | 13648 | 17.53 | 0.61 (0.51–0.72) | < 0.001 | |
| North-East | 10761 | 18.07 | 0.77 (0.64–0.92) | 0.005 |
Abbreviations: BMI, body mass index; OR, odd ratio; CI, confidence interval.
a: Interactions between sex and stratified factors. Adjusted for gender, age, area, education level, Household income, smoking status, drinking status, physical activity level, BMI, and region.
Table 4 shows the metabolic syndrome prevalence and its risk factors according to sex. The prevalence of metabolic syndrome was 24.6% in men, and 23.8% in women. The prevalence of metabolic syndrome was positively associated with age in men and women. Among men, metabolic syndrome prevalence was negatively associated with the physical activity level (P for trend < 0.001). Metabolic syndrome prevalence was inversely associated with the education level among women (P for trend < 0.001). Individuals with obesity had a higher metabolic syndrome prevalence among men and women (OR = 28.24, 20.40, respectively) than normal participants.
Table 4. The metabolic syndrome prevalence and its risk factors according to sex (n = 98042).
| Men (n = 42036) | Women (n = 56006) | |||||
|---|---|---|---|---|---|---|
| N | Prevalence | OR (95% CI) a | N | Prevalence | OR (95% CI) a | |
| Age, years | ||||||
| 18–44 | 12419 | 20.56 | 1.00 (ref) | 18468 | 11.41 | 1.00 (ref) |
| 45–54 | 9573 | 31.41 | 1.83 (1.62–2.06) *** | 13691 | 32.83 | 3.02 (2.74–3.32) *** |
| 55–64 | 10951 | 29.68 | 1.87 (1.62–2.15) *** | 14095 | 44.34 | 5.60 (4.90–6.39) *** |
| ≥ 65 | 9093 | 28.48 | 2.23 (1.94–2.56) *** | 9752 | 46.36 | 7.65 (6.61–8.85) *** |
| P-trend | < 0.001 | < 0.001 | ||||
| Area | ||||||
| Urban | 20146 | 28.63 | 1.00 (ref) | 28504 | 25.29 | 1.00 (ref) |
| Rural | 21890 | 20.70 | 0.92 (0.79–1.05) | 27502 | 22.22 | 0.94 (0.83–1.07) |
| Education | ||||||
| Uneducated | 2969 | 18.92 | 1.00 (ref) | 9716 | 35.44 | 1.00 (ref) |
| Primary school | 11954 | 21.56 | 1.13 (0.98–1.31) | 17399 | 30.20 | 1.11 (1.00–1.23) * |
| Junior school | 16431 | 24.58 | 1.23 (1.04–1.45) * | 17735 | 21.10 | 1.03 (0.91–1.17) |
| High school | 7253 | 27.86 | 1.21 (1.00–1.47) * | 7675 | 15.60 | 0.67 (0.56–0.80) *** |
| College and above | 3429 | 28.25 | 1.16 (0.86–1.56) | 3481 | 10.76 | 0.70 (0.55–0.90) ** |
| P-trend | 0.09 | 0.006 | ||||
| Income, n (%) | ||||||
| < 10000 | 21937 | 22.02 | 1.00 (ref) | 29090 | 24.57 | 1.00 (ref) |
| 10000–30000 | 17115 | 27.07 | 1.11 (1.00–1.23) | 22982 | 23.18 | 1.01 (0.91–1.12) |
| > 30000 | 2984 | 30.03 | 1.19 (0.99–1.42) | 3934 | 20.70 | 1.10 (0.92–1.31) |
| P-trend | 0.03 | 0.39 | ||||
| Smoking, n (%) | ||||||
| Never smoker | 16137 | 25.89 | 1.00 (ref) | 53477 | 23.56 | 1.00 (ref) |
| Current smoker | 22698 | 22.98 | 1.03 (0.94–1.13) | 1823 | 31.18 | 1.12 (0.93–1.35) |
| Former smoker | 3201 | 32.72 | 1.19 (1.02–1.39) * | 706 | 27.61 | 0.98 (0.73–1.32) |
| Drinking, n (%) | ||||||
| Never drinker | 19200 | 24.81 | 1.00 (ref) | 48394 | 24.29 | 1.00 (ref) |
| Moderate alcohol drinker | 15315 | 23.32 | 0.90 (0.82–0.99) * | 6709 | 19.76 | 0.95 (0.85–1.06) |
| Excessive alcohol drinker | 7521 | 27.18 | 1.00 (0.89–1.11) | 903 | 24.51 | 0.73 (0.52–1.02) |
| Physical activity, n (%) | ||||||
| Low | 12222 | 27.49 | 1.00 (ref) | 12245 | 22.31 | 1.00 (ref) |
| Moderate | 7628 | 31.43 | 1.08 (0.96–1.22) | 16436 | 23.91 | 1.02 (0.91–1.14) |
| High | 11230 | 23.65 | 0.80 (0.72–0.88) *** | 13203 | 26.04 | 1.02 (0.88–1.17) |
| Very high | 10956 | 17.39 | 0.70 (0.62–0.79) *** | 14122 | 23.09 | 0.93 (0.83–1.06) |
| P-trend | < 0.001 | 0.32 | ||||
| BMI, kg/m2 | ||||||
| Normal | 23189 | 7.52 | 1.00 (ref) | 29930 | 9.10 | 1.00 (ref) |
| Overweight | 14067 | 38.90 | 7.40 (6.72–8.14) *** | 18649 | 35.96 | 5.26 (4.71–5.86) *** |
| Obesity | 4780 | 69.32 | 28.24 (24.03–33.19) *** | 7427 | 65.75 | 20.40 (17.98–23.16) *** |
| P-trend | < 0.001 | < 0.001 | ||||
| Region | ||||||
| North | 6967 | 34.20 | 1.00 (ref) | 8981 | 31.54 | 1.00 (ref) |
| East | 3719 | 29.52 | 0.79 (0.56–1.11) | 5062 | 28.30 | 0.76 (0.54–1.06) |
| South-Central | 11699 | 25.48 | 0.79 (0.66–0.95) * | 14997 | 23.43 | 0.68 (0.55–0.83) ** |
| North-West | 9364 | 23.22 | 0.88 (0.73–1.06) | 12844 | 22.92 | 0.89 (0.72–1.10) |
| South-West | 5740 | 15.63 | 0.62 (0.51–0.76) *** | 7908 | 19.36 | 0.59 (0.48–0.72) *** |
| North-East | 4547 | 18.86 | 0.88 (0.72–1.08) | 6214 | 17.31 | 0.68 (0.56–0.84) *** |
Abbreviations: BMI, body mass index; OR, odd ratio; CI, confidence interval.
a: Adjusted for age, area, education level, Household income, smoking status, drinking status, physical activity level, BMI, and region. Except the variable of interest.
*: P < 0.05;
**: P < 0.01;
***: P < 0.001
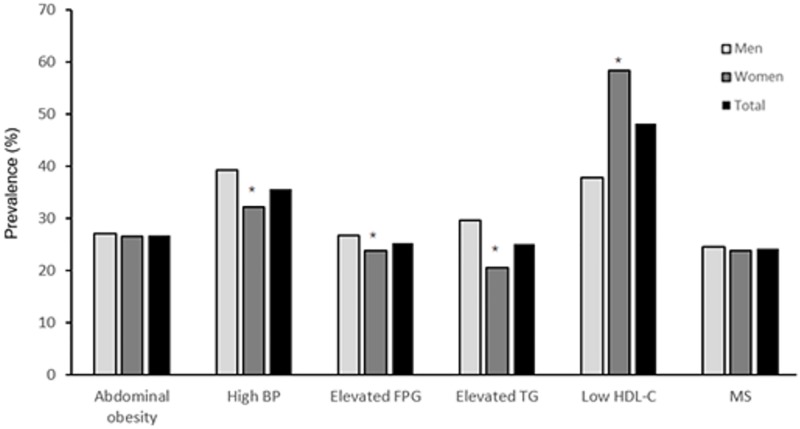
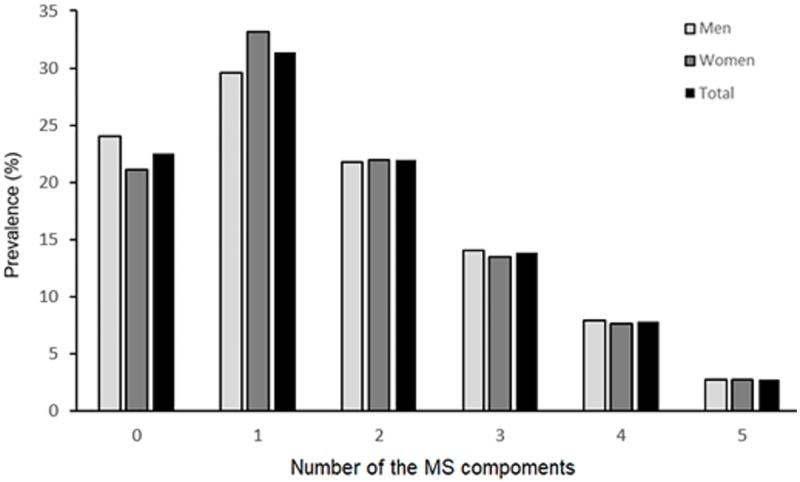
The prevalence of metabolic components is shown in Fig 1. Women had a higher prevalence of lower HDL-C (58.3%) than men (37.8%), whereas men had a higher prevalence of high blood pressure (39.2%), elevated fasting plasma glucose (26.7%), and elevated TG (29.6%) than women (32.1%, 23.8%, and 20.5%, respectively). The prevalence of abdominal obesity and metabolic syndrome was not significantly different between men and women (P > 0.05). Fig 2 shows the prevalence of one or more metabolic components. Overall, 31.4% (29.6% in men and 33.2% in women) of the participants had one metabolic component, and 24.2% of the participants (24.6% of men, and 23.8% of women) had three or more metabolic components, which is the definition of metabolic syndrome.
Fig 1. Sex disparity prevalence of metabolic components among adults in China.
BP, blood pressure; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol. *: P < 0.01 for men: women difference in prevalence.
Fig 2. Sex disparity prevalence of metabolic syndrome and number of metabolic components among adults in China.
MS, metabolic syndrome.
Table 5 shows the frequency of food intake of the participants. Men consumed more pork and organ meats and had a lower intake of fungi and algae, fruit, and dairy products than women.
Table 5. Frequency of foods intake of participants according to sex.
| Food (times/week) | Total (n = 32300) |
Men (n = 13741) |
Women (n = 18559) |
|---|---|---|---|
| Rice and rice products | 14.00 (5.00, 14.00) | 14.00 (5.00, 16.00) | 14.00 (5.00, 14.00) |
| Wheat and products | 7.00 (2.00, 10.00) | 7.00 (2.00, 10.08) | 7.00 (2.00, 10.00) |
| Starchy tubers | 1.00 (0.50, 3.00) | 1.00 (0.50, 3.00) | 1.00 (0.50, 3.00) |
| Soybean products | 2.75 (1.12, 5.50) | 2.75 (1.10, 5.31) | 2.75 (1.12, 5.50) |
| Vegetables | 15.00 (9.50, 22.00) | 15.00 (9.50, 22.00) | 15.00 (9.50, 22.00) |
| Fungi and algae | 1.12 (0.50, 2.50) | 1.08 (0.50, 2.50) * | 1.15 (0.50, 2.50) |
| Fruit | 4.00 (2.00, 7.25) | 3.75 (1.85, 7.00) * | 4.13 (2.00, 7.62) |
| Dairy products | 2.00 (0.75, 7.00) | 2.00 (0.58, 7.00) * | 2.29 (0.75, 7.00) |
| Pork | 3.00 (1.08, 7.00) | 3.02 (1.25, 7.00) * | 3.00 (1.03, 7.00) |
| Poultry | 0.50 (0.25, 1.06) | 0.50 (0.25, 1.06) | 0.50 (0.25, 1.08) |
| Organ meats | 0.25 (0.10, 0.58) | 0.26 (0.10, 0.62) * | 0.25 (0.10, 0.54) |
| Fish | 1.12 (0.42, 2.75) | 1.12 (0.44, 2.75) | 1.12 (0.40, 2.79) |
| Eggs | 3.00 (1.50, 7.00) | 3.00 (1.44, 7.00) | 3.00 (1.50, 7.00) |
| Nuts | 0.83 (0.31, 2.00) | 0.83 (0.29, 2.00) | 0.83 (0.31, 2.00) |
Data are medians (interquartile ranges).
*: Compared with women, P < 0.05
Associations between the frequency of foods intake and metabolic syndrome prevalence among men are shown in Table 6. A high frequency of fungi and algae intake was associated with an increased metabolic syndrome prevalence after adjustment for confounding factors. Associations between food intake and metabolic syndrome prevalence among women are shown in Table 7. In contrase to the first tertile of pork and nuts, the ORs (95% CIs) of metabolic syndrome prevalence for the highest tertile were 0.87 (0.78–0.95), and 0.88 (0.78–0.98), respectively.
Table 6. Associations between the frequency of foods intake and metabolic syndrome prevalence in men (n = 13741).
| Times/week | P-trend a | |||
|---|---|---|---|---|
| Low | Moderate | High | ||
| Food | ||||
| Rice and rice products | 1.00 (ref) | 0.98 (0.85–1.13) | 0.96 (0.86–1.06) | 0.77 |
| Wheat and products | 1.00 (ref) | 1.03 (0.92–1.16) | 1.01 (0.90–1.14) | 0.38 |
| Starchy tubers | 1.00 (ref) | 0.94 (0.82–1.07) | 0.96 (0.86–1.08) | 0.57 |
| Soybean products | 1.00 (ref) | 1.08 (0.97–1.21) | 0.98 (0.87–1.11) | 0.86 |
| Vegetables | 1.00 (ref) | 1.09 (0.98–1.22) | 1.08 (0.97–1.21) | 0.16 |
| Fungi and algae | 1.00 (ref) | 1.04 (0.92–1.17) | 1.24 (1.09–1.40) | < 0.001 |
| Fruits | 1.00 (ref) | 0.97 (0.87–1.09) | 0.93 (0.83–1.05) | 0.34 |
| Dairy products | 1.00 (ref) | 0.90 (0.76–1.06) | 0.99 (0.83–1.17) | 0.95 |
| Pork | 1.00 (ref) | 0.93 (0.83–1.04) | 0.89 (0.79–1.00) | 0.05 |
| Poultry | 1.00 (ref) | 0.99 (0.87–1.12) | 1.07 (0.95–1.21) | 0.19 |
| Organ meats | 1.00 (ref) | 0.99 (0.83–1.17) | 1.05 (0.90–1.24) | 0.54 |
| Fish | 1.00 (ref) | 0.99 (0.88–1.12) | 1.06 (0.94–1.20) | 0.24 |
| Eggs | 1.00 (ref) | 1.00 (0.89–1.13) | 0.99 (0.87–1.11) | 0.99 |
| Nuts | 1.00 (ref) | 0.97 (0.85–1.10) | 1.02 (0.89–1.16) | 0.74 |
BMI, body mass index.
a: Adjusted for the age, area, education, income, smoking status, drinking status, physical activity level, and BMI.
Table 7. Associations between the frequency of foods intake and metabolic syndrome prevalence in women (n = 18559).
| Times/week | P-trend a | |||
|---|---|---|---|---|
| Low | Moderate | High | ||
| Rice and rice products | 1.00 (ref) | 0.96 (0.86–1.08) | 0.93 (0.85–1.02) | 0.82 |
| Wheat and products | 1.00 (ref) | 1.01 (0.92–1.11) | 1.02 (0.93–1.13) | 0.23 |
| Starchy tubers | 1.00 (ref) | 0.94 (0.84–1.04) | 0.94 (0.85–1.04) | 0.18 |
| Soybean products | 1.00 (ref) | 0.94 (0.85–1.03) | 1.02 (0.92–1.12) | 0.61 |
| Vegetables | 1.00 (ref) | 1.03 (0.94–1.12) | 1.06 (0.96–1.16) | 0.22 |
| Fungi and algae | 1.00 (ref) | 1.04 (0.94–1.15) | 1.01 (0.90–1.12) | 0.85 |
| Fruits | 1.00 (ref) | 0.94 (0.86–1.03) | 0.91 (0.82–1.00) | 0.07 |
| Dairy products | 1.00 (ref) | 0.89 (0.77–1.02) | 0.91 (0.79–1.06) | 0.19 |
| Pork | 1.00 (ref) | 0.94 (0.86–1.03) | 0.87 (0.78–0.95) | 0.007 |
| Poultry | 1.00 (ref) | 0.95 (0.85–1.06) | 1.09 (0.98–1.22) | 0.09 |
| Organ meats | 1.00 (ref) | 1.09 (0.94–1.26) | 1.03 (0.89–1.19) | 0.71 |
| Fish | 1.00 (ref) | 0.94 (0.85–1.04) | 1.04 (0.94–1.15) | 0.39 |
| Eggs | 1.00 (ref) | 0.98 (0.89–1.08) | 0.93 (0.84–1.03) | 0.14 |
| Nuts | 1.00 (ref) | 0.92 (0.83–1.03) | 0.88 (0.78–0.98) | 0.02 |
BMI, body mass index.
a: Adjusted for the age, area, education, income, smoking status, drinking status, physical activity level, and BMI.
Discussion
In the present study, we investigated different associations by sex between metabolic syndrome prevalence and its associated factors among a Chinese population. Based on the NCEP ATP III-modified criteria, our results showed that the overall prevalence of metabolic syndrome was 24.2% (24.6% in men and 23.8% in women) among Chinese adults. Our results also suggested that the metabolic syndrome prevalence was positively associated with age. The metabolic syndrome prevalence was negatively associated with the physical activity level in men but inversely associated with the education level in women. The frequent consumption of fungi and algae was an underlying risk factor for metabolic syndrome in men, whereas the frequent consumption of nuts and pork was associated with a decreased metabolic syndrome prevalence in women.
Over the past decades, the metabolic syndrome prevalence has increased markedly worldwide [3, 5, 22], which may be explained by urbanization, an aging population, lifestyle change, and nutritional transition. Data from the National Health and Nutrition Examination Survey reported that the prevalence of metabolic syndrome among American populations aged 20 years and older increased from 32.9% in 2003–2004 to 34.7% in 2011–2012 [3]. A study conducted in urban Eastern India among adults aged 20–80 years demonstrated that the aged-standardized prevalence of metabolic syndrome was 33.5% overall: 24.9% in men and 42.3% in women [5]. The Dongfeng-Tongji Cohort study conducted in Wuhan reported that the overall metabolic syndrome prevalence was 33.2% among middle-aged and elderly Chinese populations [23]. The findings in the present study and previous surveys indicate that metabolic syndrome has become a serious public health problem and highlights the urgent need to prevent and treat metabolic syndrome in China and other populations.
The standardized prevalence of metabolic syndrome in the present study was lower than that reported in some previous studies [7, 9, 11]. The difference in prevalence is mainly explained by the definition of metabolic syndrome and the selection of study participants. The 2010 China Noncommunicable Disease Surveillance, which involvd 97,098 participants aged 18 years and older, reported that the prevalence of metabolic syndrome was 33.9% (31.0% in men and 36.8% in women) using NCEP ATP III criteria [7]. In 2012, a cross-sectional study conducted among 11,496 Chinese participants aged 35 years and older reported that metabolic syndrome prevalence was 39.0% overall and 31.4% in men and 45.6% in women, all by NCEP ATP III criteria [11]. For these studies, the cutoff for abdominal obesity was waist circumference ≥ 90 cm in men or ≥ 80 cm in women, according to the ethnic criteria for Asians [2]. The National Diabetes and Metabolic Disorders Survey conducted in 2007–2008 among 45,172 Chinese adults aged 20 years and older suggested that the prevalence of metabolic syndrome was 21.9% (25.8% in men and 18.0% in women) using the cutoffs recommended by the Chinese Joint Committee for Developing Chinese Guidelines (JCDCG) [24]; these findings were consistent with our results. According to the JCDCG criteria, abdominal obesity was defined as waist circumference ≥ 90 cm in men or ≥ 85 cm in women.
Previous studies showed that the prevalence of metabolic syndrome was higher in women than in men [3, 7]. However, our results found that the prevalence of metabolic syndrome in men (24.6%) was not significantly different from that in women (23.8%), which was consistent with other studies [9, 25, 26]. The following reasons may contribute to the sex differences in the distribution of metabolic syndrome. First, postmenopausal status is associated with an increased risk of central obesity and insulin resistance [27], which might account for the different metabolic syndrome prevalence between men and women. Second, our findings demonstrated that obesity was significantly associated with an increased risk of metabolic syndrome. A previous study indicated that pregnant women had a higher prevalence of obesity and that the more times a woman becomes pregnant, the more likely the woman is to be obesity, thus, increasing the risk of having metabolic syndrome [28].
Our results also suggested that there exist different associations of risk factors with metabolic syndrome prevalence among men and women. As shown in Table 4, with a higher level of education, the prevalence of metabolic syndrome was higher in men, but lower in women. A possible explanation for this result is that men with a higher education level and household income may spend more time sitting in the office, may have little time to exercise, may frequently consume high-fat foods, and may suffer from work-related mental health problems. Further studies are needed to investigate the potential mechanisms of these results.
The present study also suggested that the frequent consumption of pork was associated with a decreased metabolic syndrome prevalence. The possible explanation for this association is that participants with metabolic syndrome may change their lifestyles and dietary patterns, which may lead to this result. In addition, participants who frequently consumed pork also had a higher intake of vegetables, fruits, dairy products, and fish. Several prospective studies demonstrated that food groups including vegetables, fruits, dairy products, and fish were inversely associated with metabolic syndrome prevalence [29–32]. Further longitudinal surveys are needed to interpret the observed associations in terms of the cause and effect.
This study was based on data from the CHNNS, which was conducted among participants from all 31 provinces, autonomous regions, and municipalities using a complex, multistage, probability sampling design, therefore providing this study with good representativeness; its findings may be seen as convincing. However, several limitations should be considered. First, the cross-sectional design limits the ability to address causal relationships between risk factors and metabolic syndrome. Second, the prevalence of metabolic syndrome was based on a single assessment of blood samples, which may lead to minor inaccuracies. Third, because the sociodemographic characteristics and dietary information were obtained through a questionnaire, this may lead to recall bias. Fourth, the characteristics of subjects included in dietary food intake assessment differed from those of excluded subjects, which may bias our results.
Conclusion
In conclusion, the present study shows that the prevalence of metabolic syndrome in men was not significantly different from that in women. Our results also suggest sex-specific associations between multiple risk factors including sociodemographic characteristics, lifestyle, and nutrition intake, with metabolic syndrome. Our findings indicate that metabolic syndrome has become a serious public health problem, and thus, a need exists to develop strategies aimed at the prevention and treatment of metabolic syndrome in China.
Supporting information
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
The authors would like to thank all the staffs and participants working for the China National Nutrition and Health Survey 2010–2012 (CHNNS2010–2012).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Special Fund for Health-scientific Research in the Public Interest (No. 20120212) from the National Health and Family Planning Commission of the People’s Republic of China.
References
- 1.Chen W, Gao R, Liu L, Zhu M, Wang W, Wang Y, et al. Chinese cardiovascular disease report 2016: a summary. Chin Circul J. 2017: 521–530. [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112: 2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 3.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015; 313: 1973–1974. doi: 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Kim Y, Cho Y, Jun B, Oh KW. Trends in the prevalence of major cardiovascular disease risk factors among Korean adults: results from the Korea National Health and Nutrition Examination Survey, 1998–2012. Int J Cardiol. 2014; 174: 64–72. doi: 10.1016/j.ijcard.2014.03.163 [DOI] [PubMed] [Google Scholar]
- 5.Prasad DS, Kabir Z, Dash AK, Das BC. Prevalence and risk factors for metabolic syndrome in Asian Indians: A community study from urban Eastern India. J Cardiovasc Dis Res. 2012; 3: 204–211. doi: 10.4103/0975-3583.98895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu D, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005; 365: 1398–1405. doi: 10.1016/S0140-6736(05)66375-1 [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Wang L, Li M, Xu Y, Jiang Y, Wang W, et al. Metabolic Syndrome Among Adults in China: The 2010 China Noncommunicable Disease Surveillance. J Clin Endocrinol Metab. 2017; 102: 507–515. doi: 10.1210/jc.2016-2477 [DOI] [PubMed] [Google Scholar]
- 8.Hu SS, Kong LZ, Gao RL, Zhu ML, Wang W, Wang YJ, et al. Outline of the report on cardiovascular disease in China, 2010. Biomed Environ Sci. 2012; 25: 251–256. doi: 10.3967/0895-3988.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 9.Song QB, Zhao Y, Liu YQ, Zhang J, Xin SJ, Dong GH. Sex difference in the prevalence of metabolic syndrome and cardiovascular-related risk factors in urban adults from 33 communities of China: The CHPSNE study. Diab Vasc Dis Res. 2015; 12: 189–198. doi: 10.1177/1479164114562410 [DOI] [PubMed] [Google Scholar]
- 10.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008; 117: 754–761. doi: 10.1161/CIRCULATIONAHA.107.716159 [DOI] [PubMed] [Google Scholar]
- 11.Yu S, Guo X, Yang H, Zheng L, Sun Y. An update on the prevalence of metabolic syndrome and its associated factors in rural northeast China. BMC Public Health. 2014; 14: 877 doi: 10.1186/1471-2458-14-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev Med. 2013; 57: 867–871. doi: 10.1016/j.ypmed.2013.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S, Ham JO, Lee BK. Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition. 2015; 31: 111–118. doi: 10.1016/j.nut.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 14.Reynolds K, Gu D, Whelton PK, Wu X, Duan X, Mo J, et al. Prevalence and risk factors of overweight and obesity in China. Obesity. 2007; 15: 10–18. doi: 10.1038/oby.2007.527 [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003; 289: 2560–2572. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 16.Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003; 21: 1983–1992. doi: 10.1097/01.hjh.0000084751.37215.d2 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes A. Standards of medical care in diabetes—2012. Diabetes Care. 2012; 35 Suppl 1: S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinese guidelines on prevention and treatment of dyslipidemia in adults. Chin J Cardiol. 2007; 35: 390–419. [PubMed] [Google Scholar]
- 19.Li Y, Zhai F, Yang X, Hu X, Zhao W, MA Guansheng. Comparison of assessment of food intakes by using 3 dietary survey methods. Chin Prev Med. 2006: 273–280. [PubMed] [Google Scholar]
- 20.Yang Y, Wang G, Pan X. China Food Composition Tables 2002. Peking University Medical Press; 2002. [Google Scholar]
- 21.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002; 102: 1621–1630. [DOI] [PubMed] [Google Scholar]
- 22.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011; 34: 1323–1328. doi: 10.2337/dc10-2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M, He M, Min X, Pan A, Zhang X, Yao P, et al. Different physical activity subtypes and risk of metabolic syndrome in middle-aged and older Chinese people. PLoS One. 2013; 8: e53258 doi: 10.1371/journal.pone.0053258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou X, Lu J, Weng J, Ji L, Shan Z, Liu J, et al. Impact of waist circumference and body mass index on risk of cardiometabolic disorder and cardiovascular disease in Chinese adults: a national diabetes and metabolic disorders survey. PLoS One. 2013; 8: e57319 doi: 10.1371/journal.pone.0057319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshmukh PR, Kamble P, Goswami K, Garg N. Metabolic syndrome in the rural population of wardha, central India: an exploratory factor analysis. Indian J Community Med. 2013; 38: 33–38. doi: 10.4103/0970-0218.106625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Li W, Lun Z, Zhang H, Sun Z, Kanu JS, et al. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. 2016; 16: 296 doi: 10.1186/s12889-016-2870-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujimoto WY, Bergstrom RW, Boyko EJ, Chen K, Kahn SE, Leonetti DL, et al. Type 2 diabetes and the metabolic syndrome in Japanese Americans. Diabetes Res Clin Pract. 2000; 50 Suppl 2: S73–76. [DOI] [PubMed] [Google Scholar]
- 28.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med. 2013; 56: 372–378. doi: 10.1016/j.ypmed.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Zhang D, Jiang X, Jiang W. Fruit and vegetable consumption and risk of type 2 diabetes mellitus: a dose-response meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2015; 25: 140–147. doi: 10.1016/j.numecd.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 30.Wang PY, Fang JC, Gao ZH, Zhang C, Xie SY. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J Diabetes Investig. 2016; 7: 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y, Je Y. Dairy consumption and risk of metabolic syndrome: a meta-analysis. Diabet Med. 2016; 33: 428–440. doi: 10.1111/dme.12970 [DOI] [PubMed] [Google Scholar]
- 32.Kim YS, Xun P, He K. Fish consumption, long-chain omega-3 polyunsaturated fatty acid intake and risk of metabolic syndrome: a meta-analysis. Nutrients. 2015; 7: 2085–2100. doi: 10.3390/nu7042085 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.