Abstract
Background
Sensory processing alterations are highly prevalent in autism spectrum disorder (ASD). Neurobiologically-based theories of ASD propose that abnormalities in the processing of temporal aspects of sensory input could underlie core symptoms of ASD. For example, rapid auditory temporal processing is critical for speech perception, and language difficulties are central to the social communication deficits defining the disorder. This study assessed visual and auditory temporal processing abilities and tested their relation to core ASD symptoms.
Methods
53 children (26 ASD, 27 TD) completed visual and auditory psychophysical gap detection tasks to measure gap detection thresholds (i.e., the minimum interval between sequential stimuli needed for individuals to perceive an interruption between the stimuli) in each domain. Children were also administered standardized language assessments such that the relation between individual differences in auditory gap detection thresholds and degree of language and communication difficulties among children with ASD could be assessed.
Results
Children with ASD had substantially higher auditory gap detection thresholds compared to children with TD, and auditory gap detection thresholds were correlated significantly with several measures of language processing in this population. No group differences were observed in the visual temporal processing.
Conclusions
Results indicate a domain-specific impairment in rapid auditory temporal processing in ASD that is associated with greater difficulties in language processing. Findings provide qualified support for temporal processing theories of ASD and highlight the need for future research testing the nature, extent, and universality of auditory temporal processing deficits in this population.
Lay Summary
Sensory symptoms are common in ASD. Temporal processing alterations are often implicated, but understudied. The ability to process rapid sensory information, particularly auditory input, is critical for language functioning. This study tested auditory and visual temporal processing in ASD and controls. Findings suggest that rapid auditory (but not visual) processing is impaired in ASD and related to language functioning. These results could provide mechanistic clues to understanding core symptoms and lead to novel intervention targets.
Keywords: ASD, audition, vision, temporal processing, language, low level perception
Autism spectrum disorder (ASD) is characterized by a dyad of symptom clusters including pervasive deficits in social reciprocity and communication, and behavioral rigidity (American Psychiatric Association, 2013). Sensory abnormalities span multiple modalities, including vision, audition, and touch (Dawson & Watling, 2000; Kanner, 1943; O’Neill & Jones, 1997; Sigman & Capps, 1997). Given their prevalence and impact, these sensory disturbances are now included in the diagnostic criteria for ASD (Worley & Matson, 2012). In reviewing the literature on sensory dysfunction in ASD, Rogers and Ozonoff (2005) concluded that, to elucidate the nature of sensory abnormalities, future research should use controlled laboratory studies, exploring across multiple sensory modalities in carefully diagnosed clinical groups and well-matched controls, and combining experimental methods with psychometrically-valid behavioral measures. The current study addresses this charge by assessing psychophysical responses to both auditory and visual stimuli in children with and without ASD and relating them to core clinical symptoms.
Whereas many studies have demonstrated intact or enhanced visual processing when utilizing low-level stimuli (Simmons et al., 2009), substantial experimental evidence suggests atypical auditory processing in ASD (Kellerman, Fan, & Gorman, 2005). Impairments have been found in language processing (Tager-Flusberg & Caronna, 2007), with deficits extending to syntactic, semantic, and pragmatic aspects of language (Rapin & Dunn, 2003a). In addition, atypical neural responses to speech sounds are reported consistently (Jeste and Nelson, 2009; Kujala et al., 2013) and have been related to the extent of language impairments in children with ASD (Oram Cardy, Flagg, Roberts, & Roberts, 2008; Roberts et al., 2011).
Many studies have reported auditory processing deficits extending to non-speech sounds (see Foss-Feig, Stone, & Wallace, 2012 for a review of these findings), including changes in the encoding and perception of timing-related aspects of auditory stimuli such as their duration and the interval between them (Lepisto et al., 2006; Oram Cardy, Flagg, Roberts, Brian, & Roberts, 2005; Orekhova et al., 2009). Perceiving rapid (i.e., on the order of milliseconds) changes in the acoustic signal is fundamental to accurately distinguishing phonemes and parsing meaningful speech from a stream of complex auditory information (Poldrack et al., 2001). Likewise, perception of temporal aspects of the speech signal allows detection of articulatory cues, discrimination of vowels versus consonants, differentiation of specific consonants, segmentation of syllables and words, and detection of prosodic cues (Rosen, 1992). Thus, rapid auditory temporal processing is critical for speech perception.
Studies of other developmental disorders characterized by language impairments, such as dyslexia (Tallal, 1980) and specific language impairment (SLI; Tallal & Piercy, 1973; Wright et al., 1997) report auditory temporal processing abnormalities. They go on to hypothesize that these abnormalities underlie core language-related difficulties, including phonetic awareness and verbal comprehension (Farmer & Klein, 1995). A similar role for auditory temporal processing abnormalities can be envisioned in ASD, as communication deficits are central to the diagnosis. Along these lines, neurobiologically-inspired models of ASD suggest abnormalities in the “temporal binding” of inputs within and across sensory modalities (Brock, Brown, Boucher, & Rippon, 2002), and it has been proposed that broad abnormalities in temporal processing could underlie many core ASD symptoms (Allman, 2011). Given that we live in a dynamic world, impairment in the capacity to resolve and respond to rapidly presented information could have broad implications not only for sensory functioning, but also for higher order communication, social, and perceptual functions. Despite these factors, the capacity to accurately process temporal aspects of sensory information has received relatively little attention compared to other aspects of sensory and social processing in ASD.
Those studies that have assessed temporal aspects of auditory processing have identified deficits both within individual stimuli and between sequential stimuli presented in rapid succession, using behavioral (Bhatara, Babikian, Laugeson, Tachdjian, & Sininger, 2013b; Kwakye, Foss-Feig, Cascio, Stone, & Wallace, 2011; Lepisto et al., 2006; Szelag, Kowalska, Galkowski, & Poppel, 2004) and neuroimaging (Oram Cardy, Flagg, Roberts, & Roberts, 2005) methods. In contrast, studies of visual temporal processing suggest intact abilities in ASD (Falter, Elliott, & Bailey, 2012; Kwakye et al., 2011). Together, these findings converge in suggesting modality-specific deficits in rapid auditory temporal processing in children with ASD. However, much work is needed to clarify the nature and extent of these deficits and the degree to which are specific to the auditory modality and related to clinical symptomatology.
The present study followed up on previous findings demonstrating increased temporal order judgment thresholds for auditory, but not visual, stimuli in children with ASD (Kwakye et al., 2011). Unlike the earlier study, the present study used a classic gap detection paradigm, measuring the minimum interval between sequential stimuli needed for individuals to perceive an interruption between the stimuli (i.e., identify that they are discontinuous, rather than a single, continuous sound). This paradigm has the advantage of being perceptually simple and placing few demands on perceptual judgment, language comprehension, or prolonged sustained attention. In addition, gap detection thresholds are generally on the order of milliseconds, providing a view into auditory temporal acuity with a resolution more than an order of magnitude better than temporal order tasks. Finally, gap detection has been utilized in other clinical populations with language processing deficits and is a robust task both for detecting differences and for relating low-level processing deficits to clinical impairments. Using psychophysical procedures, we measured gap detection thresholds in children with and without ASD, hypothesizing that, relative to children with typical development (TD), children with ASD would have larger gap detection thresholds for auditory, but not visual, stimuli. To test for associations between low-level sensory processing deficits and higher-order symptom profiles, we also examined the relation between individual differences in auditory temporal processing and degree of language and communication difficulties among children with ASD. Here, we predicted greater deficits in rapid temporal processing, indexed by gap detection thresholds, would relate to greater impairments in receptive language and phonological processing, indexed by direct clinical assessment.
Method
Participants
This study included 26 children with ASD and 27 children with TD, between 10 and 13 years of age. Of the 26 children with ASD, 20 completed both the visual and auditory tasks, 2 completed only the visual task, and 4 completed only the auditory task. Of the 27 children with TD, 24 completed both the visual and auditory tasks, while 3 children completed the auditory task only. An additional four participants with ASD were unable to complete psychophysical tasks successfully; their data were excluded. Final samples were ASD n=22, TD n=24 for the visual task and ASD n=24, TD n=27 for the auditory task. All participants had: (a) normal or corrected-to-normal vision (by parent report) and hearing (confirmed in the study with pure tone audiometric hearing screenings at 20 dB); (b) intact cognitive skills (IQ > 70); and (c) absence of genetic or neurological disorders, history of seizures, or past head injury. Children with ASD had received previous diagnosis of ASD, which was confirmed with the Autism Diagnostic Observation Schedule (Lord et al., 2000) and Autism Diagnostic Interview-Revised (Lord, Rutter, & Le Couteur, 1994); all participants in the ASD group met diagnostic criteria on both measures. Children with TD had no family history of ASD in first- or second-degree relatives and no current or past diagnosis of learning or psychological disorder. All scored below the at-risk cutoff on the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003), a screening questionnaire for ASD risk. Groups did not differ on age, gender, handedness, and IQ score, as measured by the Wechsler Abbreviated Scales of Intelligence (Weschsler, 1999) (Table 1). Parents of participants gave informed consent, and children gave assent. Procedures were approved by the Institutional Review Board.
Table 1.
Participant Demographics
| Auditory Task | Visual Task | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Group Means | Statistic | Group Means | Statistic | ||
| ASD (n=24) | TD (n=27) | ASD (n=22) | TD (n=24) | |||
| Age (years) | 11.94 ± 1.3 | 11.93 ± 1.3 | t = 0.02 | 11.96 ± 1.3 | 12.00 ± 1.3 | t = 0.09 |
| Full Scale IQ | 115.96 ± 17.4 | 114.50 ± 12.9 | t = −0.32 | 118.00 ± 16.6 | 114.70 ± 13.1 | t = −0.71 |
| Handednessa | 18 R; 5 L | 21 R; 2 L | χ2 = 1.52 | 15 R; 7 L | 19 R; 2 L | χ2 = 3.23 |
| Gender | 21 M; 3 F | 23 M; 4 F | χ2 = 0.06 | 20 M; 2 F | 20 M; 4 F | χ2 = 0.58 |
Handedness information missing for 1 child with ASD and 4 children with TD Within each task, groups were matched (i.e., there were no statistically significant differences) on all demographic variables.
Procedure
Experimental procedures included children’s completion of psychophysical tasks assessing visual and auditory gap detection thresholds and a clinical assessment battery evaluating language functioning.
Psychophysical task
Participants sat in a light- and sound-attenuated room in front of a computer monitor centered between two speakers. For both auditory and visual experiments, participants completed four practice trials that provided auditory feedback regarding accuracy, followed by the full task, which provided no performance feedback. Additional repetition of instructions and practice trials were provided as needed. Participants were monitored via closed-circuit video and, when necessary, a researcher remained in the room to cue on-task behaviors. The order of completion of auditory and visual tasks was counterbalanced across participants.
Stimuli
For the auditory task, stimuli were presented via external speakers and children sat centered between the two speakers to ensure auditory stimuli were of equal binaural amplitude. Visual fixation and cues regarding task progress were presented in white text against black background on a PC monitor (NEC Multisync FE992, 22 inch screen, 150 Hz refresh rate). These cues did not occur concurrent with task stimuli, but rather were used to focus children’s attention forward, ensuring they were positioned centrally between the speakers. Auditory stimuli were white noise bursts (20 Hz-20 kHz) created in Adobe Audition (Adobe Systems, Mountain View, CA, USA) at a sampling rate of 44100 Hz. White noise stimuli were selected because they are associated with the lowest gap detection thresholds (Shailer & Moore, 1987) (i.e., between 2 and 3 milliseconds; (Formby & Muir, 1989) and fewer irrelevant auditory cues (e.g., ramping, background masking noise). Volume of auditory stimuli was 80 dB. Stimuli were presented using PsychToolbox (www.psychtoolbox.org) within Matlab (MathWorks, Natick MA). For the visual task, stimuli were both generated and presented using PsychToolbox/Matlab. Visual stimuli consisted of a flash of light from a 5mm red LED (wavelength = 620nm; luminous intensity = 150mcd), which was centrally mounted above the computer monitor. In line with previous studies (Van Ingelghem et al., 2001), an LED stimulus was selected (i.e., as opposed to a stimulus presented on a computer screen) because monitor refresh rates prohibit presentation of the smallest gap sizes. For both modalities, stimuli were 1000ms in total duration. Within stimuli containing gaps, gap intervals ranged from 0.5ms to 100ms (in increments of 0.5ms), and were centered relative to the overall stimulus duration.
Psychophysical procedure
Psychophysical procedures were used to determine gap detection thresholds in both auditory and visual modalities. Specifically, the QUEST Bayesian adaptive psychophysical procedure (Watson & Pelli, 1983) was implemented, varying the duration of the gap within sequential trials and ultimately converging on a final performance level (85% accuracy) representing an individual’s psychophysical threshold. For each task, two blocks of two interleaved staircases, each containing 26 trials, were administered. The starting gap size for both staircases within the first block was 4.5ms for both auditory and visual tasks.
A two-interval, forced choice (2IFC) gap detection procedure was used. This method was selected because it is relatively unbiased compared to yes/no procedures (i.e., where participants indicate whether or not they perceived a gap in a single auditory stimulus per trial) (Green & Swets, 1966). Specifically, the use of the 2IFC procedure reduced the potential effect of response bias, which could theoretically differ by group (Macmillan & Creelman, 2005). For each trial, participants were presented with a pair of stimuli, one continuous and one containing a gap. For the auditory task, each trial began with a 1000ms visual fixation to cue the subject to face forward, ensuring his/her head was centrally located between bilateral speakers. Fixation was followed by onset of the pair of auditory stimuli, then by presentation of a visual response cue (i.e., question mark on the computer screen) immediately after offset of the second of the two paired stimuli. The response cue remained on screen for the entirety of a 3000ms response window, unless terminated by a button press response. If participants did not respond within 3000ms, a trial repeated itself with the same gap size and interval location. Within trials, paired stimuli were separated by 1000ms. Inter-trial interval varied randomly between 800 and 1300ms, beginning after the response to the preceding trial. The visual task was also a 2IFC procedure in which each trial consisted of a pair of stimuli (LED flashes), one containing a gap and one not, followed by a 3000ms response window. For the visual task, the PC monitor was off such that no visual fixation cued trial or response window onset. This was done to ensure total darkness, increase visibility of the LED, and minimize the degree to which participants were asked to shift visual focus from the LED to the computer screen, which could inadvertently have reduced their looking toward the stimuli themselves.
For each trial, participants made a forced choice decision regarding which interval contained the gap (i.e., first or second) using a hand-held button box. Gap presentation occurred in the first interval on 50% of trials overall, but was randomized across trials. Buttons (i.e., left or right) corresponding to the first and second interval were counter-balanced across participants.
Clinical Measures
Language assessments
Children completed all subtests from the Comprehensive Test of Phonological Processing (CTOPP; Wagner, Torgesen, & Rashotte, 1999) and select subtests from the Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4; Semel, Wiig, & Secord, 2003) to assess phonological processing abilities and language comprehension (which demands not only phonological processing, but also understanding and making meaning of heard speech stimuli), respectively. Both measures are standardized, norm-referenced assessments that were administered by a trained examiner.
The CTOPP assesses phonological awareness, phonological memory, and rapid naming. Here, we focus on the Phonological Awareness and Phonological Memory composites, which are each derived from two related subtests and yield standard scores (Mean = 100, SD = 15). To reduce the number of comparisons, we did not test relations with the Rapid Naming composite because quickly naming numbers and letters is less conceptually related to basic auditory temporal processing.
The CELF-4 subtests administered included Recalling Sentences, Concepts and Following Directions, and Word Classes – Expressive and Receptive. Because our interest related to language functioning, analyses focused on the Receptive Language Index (RLI), which is derived from the Word Classes - Receptive and Concepts and Following Directions subtest and yields a standard score (Mean = 100, SD = 15). Though subtests were administered to all children, RLI scores were only available for those ages 12 and under, as norms are unavailable for the Concepts and Following Directions subtest for the oldest children in our sample.
Data analysis
Psychophysical data preparation
For both auditory and visual experiments, psychophysical procedures yielded four threshold estimates (i.e., one from each of the two interleaved staircases, in both the first and second blocks), reported in milliseconds. Because QUEST procedures estimate thresholds in log space, the log of each threshold was computed and these four values were averaged. For auditory and visual tasks separately, this average was then transformed back to milliseconds to yield a single value representing the gap detection threshold for each participant.
Analytic plan
To test for group differences in gap detection thresholds, independent-sample t-tests were computed separately for the auditory and visual modality. To evaluate whether auditory gap detection abilities related to clinical measures of language functioning, and to test whether diagnosis moderated these relations, we conducted step-wise linear regressions using centered variables. Main effects of group and auditory gap detection threshold were entered in to Step 1, and the interaction between the two was entered into Step 2. To limit the number of analyses conducted, we ran regressions using only the relevant composite scores from the CTOPP (Phonological Awareness, Phonological Memory) and CELF-4 (RLI). Where there was a main effect of auditory gap detection but no group by auditory gap detection threshold, we followed up with bivariate correlations between auditory gap detection and the language measure with data collapsed across ASD and TD groups. Where the interaction between group and auditory gap detection threshold predicted a language measure (i.e., significant p-value and/or moderate effect size, as index by the partial correlation), follow-up bivariate correlations were conducted separately for ASD and TD. We did not correct for multiple comparisons given our modest sample size and desire to protect against missing potentially clinically meaningful relations; however, we did examine effect sizes and also conducted additional regressions with visual gap detection thresholds, group, and language assessment scores where no relations were conceptually expected in order to test for specificity of any observed effects.
Results
Tests of normality
Gap detection threshold data were tested for normality separately for the ASD and TD groups using the Kolmogorov-Smirnov Test. Data were found to be normally distributed for the auditory task (Kolmogorov-Smirnov statistic: ASD=.13, TD=.09). For the visual task, data were non-normally distributed (Kolmogorov-Smirnov statistic: ASD=.27, TD=.26); however, since transformations did not significantly improve the normality of the visual task data and two-sample t-tests are quite robust to non-normality (Lumley, Diehr, Emerson, & Chen, 2002), parametric tests with raw threshold variables were used. All between-group (auditory, visual tasks) and regression (auditory task only) results described below also held with outliers removed.
Psychophysical thresholds
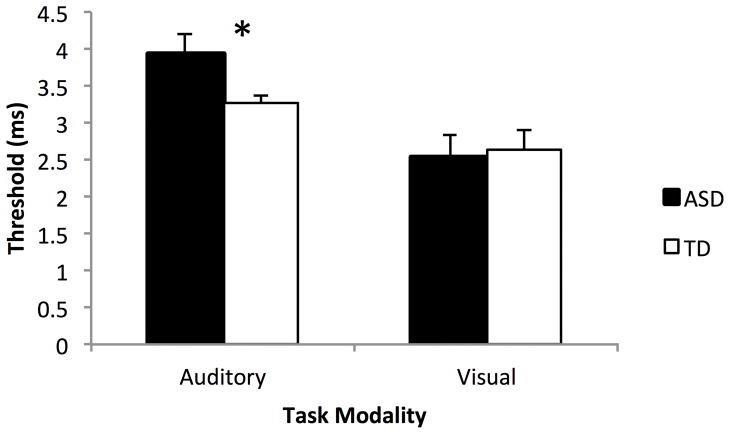
The auditory gap detection threshold was 3.92ms ± 1.36 for children with ASD and 3.25ms ± 0.66 for children with TD (Figure 1). This difference is statistically significant, t(49) = 2.28, p < 0.03, Cohen’s d = 0.66, indicating children with ASD have substantially higher auditory gap detection thresholds (i.e., require significantly longer gaps in noise to reliably detect gap presence).
Figure 1. Gap Detection Thresholds in Children with ASD and TD.
Children with ASD have significantly higher gap detection thresholds in the auditory domain (p = 0.027). In the visual domain, gap detection thresholds do not differ between groups (p = 0.80).
In the visual modality, the gap detection threshold was 2.54ms ± 0.31 for children with ASD and 2.64ms ± 0.27 in children with TD (Figure 1). This difference was not statistically significant, t(44) = 0.26, p = 0.80, Cohen’s d = 0.34. Thus, whereas children with ASD show deficits in auditory gap detection, their visual gap detection abilities remain intact.
In the subset of individuals who completed both auditory and visual tasks, thresholds across modalities were not significantly correlated with one another in either group, ASD: r(20) = .21, p=.37, TD: r(24) = .22, p=.30.
Relations with clinical variables
Regressions were conducted with composite scores from the CTOPP and CELF-4. CTOPP data were missing for one TD participant. The CELF-4 RLI could only be computed for participants under 13 years of age. Therefore, analyses were computed with a subset of 16 children with ASD and 16 with TD. Means, standard deviations, and ranges for clinical variables are presented in Table 2, and zero-order correlations are presented both within and across groups in Table 3.
Table 2.
Descriptive Information for Clinical Variables among ASD Participants
| Clinical Measure | n | Mean | SD | Study Range |
|---|---|---|---|---|
| CELF-4 Receptive Language Index | ||||
| ASD | 16 | 101.81 | 20.20 | 70–137 |
| TD | 17 | 111.24 | 11.62 | 90–131 |
| CTOPP Phonological Awareness Composite | ||||
| ASD | 24 | 108.38 | 11.22 | 79–124 |
| TD | 26 | 107.62 | 10.67 | 79–124 |
| CTOPP Phonological Memory Composite | ||||
| ASD | 24 | 100.13 | 15.51 | 64–127 |
| TD | 25 | 103.84 | 10.34 | 82–121 |
Table 3.
Zero-Order Correlations for Clinical Variables and Gap Detection Threshold
| Group | Variables | n | 1+ | 2+ | 3+ | 4+ |
|---|---|---|---|---|---|---|
| Combined | 1. Auditory Gap Detection Threshold | 51 | -- | |||
| 2. CELF-4 RLI | 33 | −.410* | -- | |||
| 3. CTOPP Phonological Awareness | 50 | −.257 | .555** | -- | ||
| 4. CTOPP Phonological Memory | 49 | −.358* | .556** | .348* | -- | |
| ASD | 1. Auditory Gap Detection Threshold | 24 | -- | |||
| 2. CELF-4 RLI | 16 | −.495* | -- | |||
| 3. CTOPP Phonological Awareness | 24 | −.303 | .565* | -- | ||
| 4. CTOPP Phonological Memory | 24 | −.397* | .599* | .542** | -- | |
| TD | 1. Auditory Gap Detection Threshold | 27 | -- | |||
| 2. CELF-4 RLI | 17 | .212 | -- | |||
| 3. CTOPP Phonological Awareness | 26 | −.289 | .603* | -- | ||
| 4. CTOPP Phonological Memory | 25 | −.155 | .317 | .097 | -- |
p ≤ .05;
p ≤ .01;
Number labels for columns refer to the four variables tested in zero-order correlations, as in the “Variables” column at left: 1. Auditory Gap Detection Threshold, 2. CELF-4 RLI, 3. CTOPP Phonological Awareness, 4. CTOPP Phonological Memory.
For the CTOPP Phonological Awareness composite, regression analyses revealed a significant main effect of auditory gap detection threshold on phonological awareness (β = −2.95; pr = −.28; p < .05). The interaction between group and auditory gap detection threshold was not a significant predictor of phonological awareness (β = −1.10; pr = −.09; p = .55). Across groups, bivariate correlations revealed a trend toward a negative relationship between gap detection threshold and phonological awareness (r = −0.26, p = .07), indicating those with better auditory temporal resolution tended to have better phonological awareness. However, this relation was of a small effect size.
For the CTOPP Phonological Memory composite, regression analyses revealed a significant main effect of auditory gap detection threshold on phonological memory (β = −4.11; pr = −.33; p = .02). The effect size of this effect was medium. The interaction between group and auditory gap detection threshold was not a significant predictor of phonological awareness (β = −1.06; pr = −.07; p = .63). Across groups, bivariate correlations revealed a significant negative relationship of medium effect size between gap detection threshold and phonological awareness (r = −0.36, p = .01), indicating those with better auditory temporal resolution had better phonological memory.
For the CELF-4 RLI, regression analyses revealed a trend for a main effect of auditory gap detection threshold on receptive language functioning (β = −4.72; pr = −.33; p < .07), where the magnitude of the effect was medium despite the lack of statistical significance at the 0.05 level. Analyses also revealed a trend for an interaction between group and auditory gap detection threshold in predicting receptive language functioning (β = −5.12; pr = −.31; p < .10). Because the interaction was of a medium effect size (despite trend-level statistical significance), bivariate correlations were conducted separately by group to explore the nature of the interaction effect. In ASD, there was a trend toward a negative relationship between gap detection threshold and receptive language functioning (r = −0.50, p = .05), with a large effect size. This finding indicates that those children with ASD and better auditory temporal resolution had better receptive language abilities. In TD, however, there was no relationship between gap detection threshold and receptive language functioning (r = 0.21, p = .41). Thus, whereas auditory temporal resolution appears to predict low-level phonological language functions across groups, the relation between auditory temporal resolution and more complex language comprehension appears to be specific to ASD.
When parallel analyses were conducted for visual gap detection thresholds, no main effect of visual gap detection threshold or interaction between group and visual gap detection threshold in predicting language function on any CELF-4 or CTOPP composite were identified (Main Effects: ps > .23; Interactions: ps > .67). These findings provide additional evidence for the specificity of relations between auditory temporal processing and language functioning.
Discussion
This study revealed increased auditory, but not visual, gap detection thresholds in high-functioning children with ASD, relative to those with TD, indicating domain-specific impairment in rapid auditory temporal processing. Importantly, worse auditory gap detection abilities were associated with lower phonological processing scores across both ASD and TD children, consistent with their role in supporting speech sound processing. Among children with ASD, worse auditory gap detection abilities were also associated with weaker receptive language skills, suggesting a potential role for low level perceptual abilities contributing to more complex communication difficulties. Relative deficits in auditory gap detection are consistent with previous findings from our group, wherein children with ASD displayed increased auditory, but not visual, temporal order judgment thresholds relative to their TD peers (Kwakye et al., 2011). The present finding extends the previous one by revealing similar auditory temporal processing deficits in a simpler task requiring less complex perceptual judgments and clarifying how auditory temporal processing may relate to core clinical features of ASD. More broadly, our findings provide new evidence in support of a domain-specific temporal processing deficit for auditory information in ASD, consistent with a growing literature reporting abnormalities in the response of individuals with ASD to rapid temporal changes, both within and between auditory stimuli (Foss-Feig et al., 2012).
Our findings are generally consistent with other studies assessing auditory temporal processing in ASD. Specifically, for high frequency (4000Hz) stimuli, Bhatara, Babikian, Laugeson, Tachdjian, and Sininger (2013a) reported increased gap detection thresholds in high-functioning adolescents with ASD and demonstrated a significant relationship between auditory gap detection threshold and speech-in-noise perception. However, the magnitude of difference in gap detection threshold between groups was quite a bit smaller in the present study than in the Bhatara et al. study (i.e., 20% higher vs. 100% higher in the two studies, respectively), even when examining results only for white noise stimuli in the latter study. Methodological differences between our study and that of Bhatara and colleagues may have affected the magnitude of results. For example, whereas in the current study we used a 2IFC procedure, Bhatara and colleagues used a three-alternative forced choice (3AFC) procedure. While psychometrically, 3AFC procedures may be advantageous in adult populations (Ennis, 1993) and perhaps in typically developing children (Sutcliffe & Bishop, 2005), they are also more susceptible to bias (Harvey, 1986) and the number of choices that must be attended to within each trial (three versus two) before a response can be provided necessarily places higher demands on working memory and attention. In addition, the paradigm utilized by Bhatara and colleagues relied on pairing auditory stimuli with visual prompts to cue responding, provided feedback after each trial, and used large initial gap size (50ms), whereas our study did not utilize cross-modal pairing, provided feedback only during practice trials, and used an initial gap size closer to threshold (i.e., 4.5ms). Thus, our study may reflect gap detection threshold values in ASD that are closer to “true” values, as we used a simpler task that more directly assessed perception while avoiding extra task parameters that might tap extraneous cognitive processes (e.g., cross-modal response cueing, responsiveness to feedback, working memory) and could differentially affect children with ASD. Nonetheless, the results of our study and that of Bhatara and colleagues are consistent in their directionality, and concur in revealing impaired auditory gap detection abilities in ASD.
The correlational finding from Bhatara and colleagues parallels those from our regression analyses, wherein impaired auditory gap detection was associated with both phonological processing and language comprehension difficulties in ASD, with some relations reaching statistical significance thresholds and all having at least medium effect sizes. Together, these studies provide evidence that rapid auditory temporal processing deficits in ASD could underlie speech perception difficulties and, as a result, communication difficulties more broadly. Additionally, converging evidence suggests particular impairments in ASD for detecting rapid (or brief) timing events within and between auditory stimuli (Kwakye et al., 2011), consistent with our findings. Finally, based on our findings of a trend-level effect of medium effect size for auditory gap detection predicting CELF-4 RLI scores across groups, we build upon Bhatara et al.’s results in showing that auditory temporal resolution is important not only for basic speech detection and perception, but also for higher level language perception and comprehension.
Our results suggest temporal processing abnormalities in ASD may be specific to audition, at least for shorter stimulus durations. Rapid temporal processing in the visual system, on the other hand, appears to be spared (as reflected in our results), or even enhanced (as suggested by other studies). Previous research revealed intact visual temporal order judgment abilities in children with ASD (Kwakye et al., 2011) and enhanced visual simultaneity judgment thresholds in adolescents and adults with ASD (Falter, Noreika, Wearden, & Bailey, 2012). Intact or enhanced temporal resolution and accumulation of information over short time intervals have been reported using other visual tasks as well (Caron, Mottron, Berthiaume, & Dawson, 2006; Foss-Feig, Tadin, Schauder, & Cascio, 2013; Scheuffgen, Happe, Anderson, & Frith, 2000; Wallace, Anderson, & Happe, 2009). Contrasted with the literature on rapid auditory temporal processing, these findings converge with ours in suggesting a modality-specific dissociation in the integrity of rapid processing of sensory information in ASD, wherein impairments are audition-specific.
The present findings confirmed our hypotheses that impaired auditory gap detection in ASD would be associated with lower performance on measures of language functioning. The capacity to perceive rapid temporal cues is fundamental for the ability to distinguish speech sounds and accurately parse the speech stream. Indeed, temporal resolution on the order of milliseconds is necessary for speech perception (Tallal, Miller, & Fitch, 1993), and our results show that more rapid auditory temporal processing is associated with better phonological processing skills across both children with ASD and TD. Deficits in processing of speech sounds and higher-level impairments in language comprehension are among the most commonly described audition-related findings in ASD (Kellerman et al., 2005). The present findings revealed a strong (large effect size) correlation between auditory (but not visual) gap detection performance and clinical assessment of receptive language functioning that was restricted to our ASD sample and not present in TD. These findings suggest a possible mechanism by which rapid auditory temporal processing deficits observed in this study may contribute to language processing impairments salient in the ASD population. Indeed, language comprehension tends to be more consistently impacted and impaired in ASD than phonological processing (Rapin & Dunn, 2003b; Tager-Flusberg, 2006; Williams, Botting, & Boucher, 2008), and the downstream effects of poorer rapid auditory temporal processing on higher-order receptive language skills were specific to our ASD group. Future studies should examine the impact of auditory temporal processing on more distal and complex aspects of language functioning, such as pragmatic language abilities or figurative language comprehension.
Impaired auditory gap detection abilities have also been reported in individuals with dyslexia (e.g., Tallal, 1980) and SLI (e.g., Tallal & Piercy, 1973). Interestingly, however, individuals with dyslexia and SLI have temporal processing deficits that span sensory modalities, affecting vision and touch, in addition to audition (Farmer & Klein, 1995; Tallal et al., 1993). If rapid temporal processing difficulties in ASD are indeed isolated to the auditory modality, this feature may differentiate ASD from other disorders where both language and temporal processing are affected. Specifically, whereas temporal processing deficits restricted to the auditory domain may be characteristic of ASD, multimodal temporal processing deficits may contribute instead to more pervasive language processing deficits, such as the speech and reading deficits seen in SLI and dyslexia, respectively. This differentiation, in turn, may indicate that the neural mechanism of temporal processing deficits in ASD may lie within auditory system-specific circuitry, whereas the brain basis of temporal processing deficits in pure language and reading disorders may lie elsewhere.
A number of theoretical models speculating about core deficits that might explain the full spectrum of ASD symptoms emphasize the possibility that diffuse temporal processing and temporal binding abnormalities may be at the heart of ASD (Allman, 2011; Belmonte, 2004; Brock, Brown, Boucher, & Rippon, 2002; Just, Cherkassky, Keller, & Minshew, 2004; Rippon, Brock, Brown, & Boucher, 2007; Wimpory, Nicholas, & Nash, 2002). Our findings support the notion of timing-related deficits in ASD, albeit not in the domain general way implied by these theoretical models. Specifically, we found deficits in the detection of silent gaps within auditory stimuli, a classic index of rapid temporal processing deficits, consistent with models implicating aberrant perception of timing-related information. However, we also saw intact visual gap detection abilities, consistent with other findings in the visual modality but inconsistent with the notion of generalized temporal processing deficits in ASD. Thus, our findings suggest temporal processing deficits in the auditory domain are likely not simply a consequence of abnormalities in brain regions responsible for timing in general (though it cannot be ruled out that audition-specific deficits result from impaired connectivity between brain regions subserving general temporal processing and auditory brain regions). It is clear that the specificity and applicability of models positing a central role for temporal processing abnormalities in ASD remain to be honed, and additional studies to test directly the degree to which theories fit experimental evidence are critical.
This study has a number of strengths, including a stringently characterized clinical sample, a well-matched TD control group, and analogous measures of auditory and visual temporal processing used in parallel within a single participant sample. Additionally, we used a simple perceptual discrimination paradigm that has shown sensitivity to deficits in clinical populations characterized by language-related difficulties and required little in terms of sustained attention, judgment, and higher-order decision-making. Finally, we also included standardized assessments of language functioning, enabling exploration of relations between markers of low-level auditory temporal processing and indices of more clinically relevant functioning. These factors contribute to the confidence with which we conclude that ASD is characterized by impaired ability to detect minute gaps in auditory, but not visual, stimuli, and that this low-level sensory processing deficit relates to clinically-relevant language vulnerabilities. One limitation of the study is that all participants were high functioning, with the mean IQ score in the High Average range and intact language scores, on average. Thus, the presence (and extent) of rapid temporal processing deficits in lower functioning individuals with ASD was not addressed. However, given the relations we observed between gap detection thresholds and language processing abilities, one might expect that auditory temporal processing deficits would hold—or be more pronounced—in lower functioning children with ASD. A second limitation is our modest sample size, which may have contributed to several of our detected effects associating gap detection thresholds with clinical functioning not meeting strict statistical significance threshold despite being of medium effect size. Though the consistency in direction and presence of detected effects and the lack of association between visual gap detection thresholds and clinical measures of language functioning provide support for the likely veracity of our findings, additional studies are needed to replicate the results we report in new and larger cohorts in order to lend confidence to the conclusions we propose. Finally, though previous research has identified delays in more general encoding auditory information in ASD (Edgar et al., 2015; Foss-Feig, et al., 2012; Roberts et al., 2010), the neural basis of impaired auditory gap detection abilities in ASD remains to be determined. Research in this area might help differentiate ASD from other disorders with language-related deficits where more diffuse, multi-modal temporal processing impairments are reported. It also may offer important clues as to specific brain regions and cognitive processes impacted in ASD.
Conclusion
Results of this study shed light on low-level differences in sensory processing that: 1) appear to be modality specific, and 2) are associated with clinically-observable differences in phonological processing and receptive language skills. The significant relation between auditory gap detection thresholds and standardized language assessment scores supports the possibility that temporal processing impairments may underlie core features of ASD in the communication domain. A better understanding of the nature, extent, and universality of auditory temporal processing deficits in ASD may increase our ability to distinguish deficits that are unique to ASD (or even a subset of individuals with ASD), which could improve clinicians’ ability to differentiate among developmental disorders, thereby increasing diagnostic accuracy. This, in turn, might lead to earlier detection of ASD as audition comes on-line in utero (Busnel, Granier-Deferre, & Lecanuet, 1992) and deficits in auditory processing may be detectable long before social communication deficits are clearly observable. In addition, if rapid temporal processing impairments indeed contribute to language processing difficulties in ASD, translational implications emerge, including interventions directly targeting auditory temporal processing, as well as modification of how auditory information is presented to children with ASD to accommodate temporal processing weaknesses. Finally, capitalizing on aspects of intact visual processing could have important translational value for psychologists and educators. In sum, increasing our understanding of auditory temporal processing impairments in ASD may have significant clinical impact; for this reason, further research clarifying the nature, extent, and specificity of auditory temporal processing deficits in ASD is warranted.
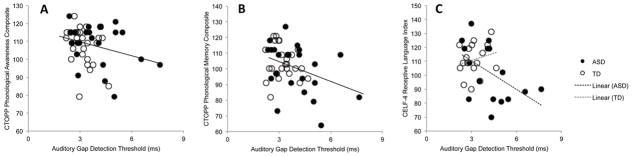
Figure 2. Auditory gap detection threshold and language score correlations.
In a combined sample of ASD and TD participants, higher auditory gap detection thresholds are associated with weaker performance on tests of Phonological Awareness (2A; p = 0.07; trend-level association; small effect size) and Phonological Memory (2B; p = 0.01; statistically significant association; moderate effect size) from the CTOPP. In the ASD group, higher auditory gap detection thresholds were also associated with weaker Receptive Language (2C; p = 0.05; statistically significant association; large effect size), as indexed by the CELF-4; however, this association was not present in the TD group (2C; p = 0.42; non-significant association).
Acknowledgments
This study was supported by a Dennis Weatherstone Predoctoral Fellowship from Autism Speaks to JHF and a Marino Autism Research Institute grant to APK and JHF. Jennifer Foss-Feig’s effort was also funded in part by a Brain and Behavior Research Foundation NARSAD Young Investigator Award to JHF, an Autism Speaks Accelerator grant to JHF, and the Seaver Foundation. The authors would like to acknowledge Rebecca Johnston, Caroline Oates, and Holly Black for their assistance with recruitment and data collection. They also would like to thank the children and families who participated in this study.
References
- Allman MJ. Deficits in temporal processing associated with autistic disorder. Front Integr Neurosci. 2011;5:2. doi: 10.3389/fnint.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A. P. DSM 5. American Psychiatric Association; 2013. [Google Scholar]
- Belmonte MK. Autism and Abnormal Development of Brain Connectivity. J Neurosci. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatara A, Babikian T, Laugeson E, Tachdjian R, Sininger YS. Impaired Timing and Frequency Discrimination in High-functioning Autism Spectrum Disorders. J Autism Dev Disord. 2013a;43(10):2312–2328. doi: 10.1007/s10803-013-1778-y. [DOI] [PubMed] [Google Scholar]
- Bhatara A, Babikian T, Laugeson E, Tachdjian R, Sininger YS. Impaired timing and frequency discrimination in high-functioning autism spectrum disorders. J Autism Dev Disord. 2013b;43(10):2312–2328. doi: 10.1007/s10803-013-1778-y. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown C, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev Psychopathol. 2002;14(2):209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev Psychopathol. 2002;14(2):209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Busnel MC, Granier-Deferre C, Lecanuet JP. Fetal audition. Dev Psychobiol. 1992;662:118–134. doi: 10.1111/j.1749-6632.1992.tb22857.x. [DOI] [PubMed] [Google Scholar]
- Caron M, Mottron L, Berthiaume C, Dawson M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 2006;129(Pt 7):1789–1802. doi: 10.1093/brain/awl072. awl072 [pii] [DOI] [PubMed] [Google Scholar]
- Dawson GG, Watling RR. Interventions to facilitate auditory, visual, and motor integration in autism: a review of the evidence. J Autism Dev Disord. 2000;30(5):415–421. doi: 10.1023/a:1005547422749. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Fisk CL, IV, Berman JI, Chudnovskaya D, Liu S, Pandey J, … Roberts TP. Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Mol Autism. 2015;6(1):69. doi: 10.1186/s13229-015-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNIS DM. The power of sensory discrimination methods. Journal of sensory studies. 1993;8(4):353–370. [Google Scholar]
- Falter C, Noreika V, Wearden J, Bailey A. More consistent, yet less sensitive: interval timing in autism spectrum disorders. Q J Exp Psychol. 2012;65(11):2093–2107. doi: 10.1080/17470218.2012.690770. [DOI] [PubMed] [Google Scholar]
- Falter CM, Elliott MA, Bailey AJ. Enhanced Visual Temporal Resolution in Autism Spectrum Disorders. PLOS One. 2012;7(3):e32774. doi: 10.1371/journal.pone.0032774.t002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer ME, Klein RM. The evidence for a temporal processing deficit linked to dyslexia: A review. Psychonomic Bulletin & Review. 1995;2(4):460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- Formby CC, Muir K. Effects of randomizing signal level and duration on temporal gap detection. Audiology. 1989;28(5):250–257. doi: 10.3109/00206098909081630. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Stone WL, Wallace MT. Processing of Non-Speech Auditory Stimuli in Individuals with Autism Spectrum Disorders: The Impact of Stimulus Characteristics. International Review of Research in Developmental Disabilities. 2012;43:87–145. [Google Scholar]
- Foss-Feig JH, Stone WL, Wallace MT. International Review of Research in Developmental Disabilities. Vol. 43. Elsevier; 2012. Processing of Non-Speech Auditory Stimuli in Individuals with Autism Spectrum Disorders: The Impact of Stimulus Characteristics; pp. 87–145. [Google Scholar]
- Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J Neurosci. 2013;33(19):8243–8249. doi: 10.1523/JNEUROSCI.1608-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Swets J. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds. Behavior research methods. 1986;18(6):623–632. [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kellerman G, Fan J, Gorman J. Auditory abnormalities in autism: toward functional distinctions among findings. CNS Spectr. 2005;10(9):748–756. doi: 10.1017/s1092852900019738. [DOI] [PubMed] [Google Scholar]
- Kwakye LD, Foss-Feig JH, Cascio CJ, Stone WL, Wallace MT. Altered auditory and multisensory temporal processing in autism spectrum disorders. Front Integr Neurosci. 2011;4:129. doi: 10.3389/fnint.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepisto T, Silokallio S, Nieminen-von Wendt T, Alku P, Naatanen R, Kujala T. Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clin Neurophysiol. 2006;117(10):2161–2171. doi: 10.1016/j.clinph.2006.06.709. S1388-2457(06)00973-4 [pii] [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, … Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- O’Neill M, Jones RS. Sensory-perceptual abnormalities in autism: a case for more research? J Autism Dev Disord. 1997;27(3):283–293. doi: 10.1023/a:1025850431170. [DOI] [PubMed] [Google Scholar]
- Oram Cardy J, Flagg E, Roberts W, Roberts T. Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 2005;16(5):521–525. doi: 10.1097/00001756-200504040-00021. 00001756-200504040-00021. [DOI] [PubMed] [Google Scholar]
- Oram Cardy J, Flagg EJ, Roberts W, Brian J, Roberts TP. Magnetoencephalography identifies rapid temporal processing deficit in autism and language impairment. Neuroreport. 2005;16(4):329–332. doi: 10.1097/00001756-200503150-00005. 00001756-200503150-00005. [DOI] [PubMed] [Google Scholar]
- Oram Cardy J, Flagg EJ, Roberts W, Roberts TPL. Auditory evoked fields predict language ability and impairment in children. Int J Psychophysiol. 2008;68(2):170–175. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofiev AO, Nygren G, Gillberg C, Elam M. The right hemisphere fails to respond to temporal novelty in autism: evidence from an ERP study. Clin Neurophysiol. 2009;120(3):520–529. doi: 10.1016/j.clinph.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JD. Relations between the neural bases of dynamic auditory processing and phonological processing: evidence from fMRI. J Cogn Neurosci. 2001;13(5):687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Rapin I, Dunn M. Update on the language disorders of individuals on the autistic spectrum. Brain Dev. 2003a;25(3):166–172. doi: 10.1016/S0387-7604(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Rapin I, Dunn M. Update on the language disorders of individuals on the autistic spectrum. Brain and development. 2003b;25(3):166–172. doi: 10.1016/s0387-7604(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: Challenges for the ‘new psychophysiology’. Int J Psychophysiol. 2007;63(2):164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, … Schmidt GL. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Research. 2010;3(1):8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TPL, Cannon KM, Tavabi K, Blaskey L, Khan SY, Monroe JF, … Edgar JC. Auditory Magnetic Mismatch Field Latency: A Biomarker for Language Impairment in Autism. Biol Psychiat. 2011;70(3):263–269. doi: 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. 2005;46(12):1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. JCPP1431 [pii] [DOI] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond B Biol Sci. 1992;336(1278):367–373. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. SCQ: Social Communication Quetionnaire. 2003. [Google Scholar]
- Scheuffgen K, Happe F, Anderson M, Frith U. High “intelligence,” low “IQ”? Speed of processing and measured IQ in children with autism. Dev Psychopathol. 2000;12(1):83–90. doi: 10.1017/s095457940000105x. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of lanauge fundementals. 4. Toronto, Canada: The Psychological Corporation; 2003. (CELF-4) [Google Scholar]
- Shailer MJ, Moore B. Gap Detection and the Auditory Filter - Phase Effects Using Sinusoidal Stimuli. J Acoust Soc Am. 1987;81(4):1110–1117. doi: 10.1121/1.394631. [DOI] [PubMed] [Google Scholar]
- Sigman M, Capps L. Children with autism: A developmental perspective. Cambridge: Harvard University; 1997. [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49(22):2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Sutcliffe P, Bishop D. Psychophysical design influences frequency discrimination performance in young children. J Exp Child Psychol. 2005;91(3):249–270. doi: 10.1016/j.jecp.2005.03.004. https://doi.org/10.1016/j.jecp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Szelag E, Kowalska J, Galkowski T, Poppel E. Temporal processing deficits in high-functioning children with autism. Br J Psychol. 2004;95(Pt 3):269–282. doi: 10.1348/0007126041528167. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Defining language phenotypes in autism. Clinical Neuroscience Research. 2006;6(3):219–224. [Google Scholar]
- Tager-Flusberg H, Caronna E. Language disorders: autism and other pervasive developmental disorders. Pediatr Clin North Am. 2007;54(3):469–481. vi. doi: 10.1016/j.pcl.2007.02.011. S0031-3955(07)00040-5 [pii] [DOI] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980;9(2):182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: a case for the preeminence of temporal processing. Ann N Y Acad Sci. 1993;682:27–47. doi: 10.1111/j.1749-6632.1993.tb22957.x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: impaired rate of non-verbal processing as a function of sensory modality. Neuropsychologia. 1973;11(4):389–398. doi: 10.1016/0028-3932(73)90025-0. [DOI] [PubMed] [Google Scholar]
- Van Ingelghem M, Van Wieringen A, Wouters J, Vandenbussche E, Onghena P, Ghesquière P. Psychophysical evidence for a general temporal processing deficit in children with dyslexia. Neuroreport. 2001;12(16):3603–3607. doi: 10.1097/00001756-200111160-00046. [DOI] [PubMed] [Google Scholar]
- Wagner R, Torgesen J, Rashotte C. Comprehensive test of phonological processing. 1999. [Google Scholar]
- Wallace GL, Anderson M, Happe F. Brief report: information processing speed is intact in autism but not correlated with measured intelligence. J Autism Dev Disord. 2009;39(5):809–814. doi: 10.1007/s10803-008-0684-1. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Weschsler D. WASI: Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment, Inc; 1999. [Google Scholar]
- Williams D, Botting N, Boucher J. Language in autism and specific language impairment: Where are the links? Psychological bulletin. 2008;134(6):944. doi: 10.1037/a0013743. [DOI] [PubMed] [Google Scholar]
- Wimpory D, Nicholas B, Nash S. Social timing, clock genes and autism: a new hypothesis. J Intellect Disabil Res. 2002;46(Pt 4):352–358. doi: 10.1046/j.1365-2788.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- Worley JA, Matson JL. Comparing symptoms of autism spectrum disorders using the current DSM-IV-TR diagnostic criteria and the proposed DSM-V diagnostic criteria. Res Autism Spectr Disord. 2012;6:965–970. [Google Scholar]
- Wright BA, Lombardino LJ, King WM, Puranik CS, Leonard CM, Merzenich MM. Deficits in auditory temporal and spectral resolution in language-impaired children. Nature. 1997;387(6629):176–178. doi: 10.1038/387176a0. [DOI] [PubMed] [Google Scholar]