Abstract
Adult barn owl hearing is acute, but development of this sense is not well understood. We therefore measured auditory brainstem responses (ABR) in barn owls from before the onset of hearing (post hatch day 2, or P2) to adulthood (P69). The first consistent responses were detected at P4 for 1 and 2 kHz, followed by responses to 0.5 kHz and 4 kHz at P9, and 5 kHz at P13. Sensitivity to higher frequencies increased with age, with responses to 12 kHz appearing about 2 months after hatching, once the facial ruff was mature. Therefore, these altricial birds achieve their sensitivity to sound during a prolonged period of development, which coincides with maturation of the skull and facial ruff (Haresign and Moiseff 1988).
Keywords: Auditory, development, ABR, barn owl
Introduction
Barn owls (Tyto spp.) are nocturnal predators with well-developed sound localization abilities (Payne 1971; Konishi 1973; Hausmann et al. 2008), and large hindbrain auditory nuclei (Kubke et al. 2004). Generally, physiological studies of development in these owls have focused on the role of adaptive plasticity and instructed learning during development of sound localization circuits (Knudsen et al. 1984; Gold and Knudsen 2000; Miller and Knudsen 2001; Miller and Knudsen 2003; see reviews in Pena and DeBello 2010 and Knudsen 2002). Only a handful of studies have analyzed the development of their auditory sensitivity (Köppl and Nickel 2007). We therefore used ABRs to assess auditory development in young barn owls.
ABRs are relatively similar across vertebrates (Corwin et al. 1982), and have been used to study auditory sensitivity in many birds, including chickens, budgerigars, canaries, zebra finches, woodpeckers and ducks (Saunders et al. 1973; Katayama 1985; Brittan-Powell and Dooling 2004; Noirot et al. 2011; Lohr et al. 2013; Crowell et al. 2015). They have also been used to track auditory development in chickens and budgerigars (Saunders et al. 1973; Katayama 1985; Brittan-Powell and Dooling 2004). ABRs are a useful measure of hearing sensitivity, since they reflect activity in the auditory nerve and brainstem (Hood 1998; Ramos et al. 2013), with the first peak of the ABR correlated with auditory nerve activity in budgerigars (Brittan-Powell et al. 2002), cats (Melcher and Kiang 1996; Ngan and May 2001), and other vertebrates (Corwin et al. 1982).
Despite the barn owl’s prominence as an auditory model system, only three studies have measured ABRs in any species of owl. A conference proceeding showed representative ABR traces in developing barn owls (Carr et al. 1997), and Palanca-Castan et al. (2016) measured the binaural interaction components of the ABR to chirps and to 1 and 4 kHz tones in adult barn owls. ABRs were thoroughly evaluated in adult screech owls (Megascops asio) (Brittan-Powell et al. 2005). Both barn owls and screech owls have a wider hearing range than most passerines (Konishi 1973; Dyson et al. 1998; Okanoya and Dooling 1987; Dooling et al., 2002; Noirot et al. 2011). Screech owls are more vocal than barn owls (Tyto spp.), but both show similar auditory sensitivity (Brittan-Powell et al. 2005). Barn owls are noted for hunting in low-light environments, rather than for communication (Dyson et al. 1998; Brittan-Powell et al. 2005). Barn owls have a relatively small repertoire of vocalizations (Bunn et al. 2010), and show the greatest sensitivity to high sound frequencies of any bird (Fay 1988). Behavioral audiograms reveal barn owls’ lowest auditory thresholds between 4–6.3 kHz, with an average highest frequency heard of 13.4 kHz (Dyson et al. 1998, Konishi 1973).
Barn owls have a characteristic facial ruff, which improves sound localization (Keller et al. 1998, Hausmann et al. 2009). The ruff undergoes massive growth during the 60 days following hatching (Haresign and Moiseff 1988, Köppl et al. 2005). When they hatch, barn-owl chicks are small, altricial, and scantily feathered (Köppl et al. 2005, Haresign and Moiseff 1988). Growth of the head and ruff feathers occur at separate times during early maturation. Between 11 and 30 days after hatching, the diameter of the head approximately doubles. Between 35 and 60 days, facial ruff feathers emerge and grow to nearly adult length. Thus, during the first 60 days of life, young barn owls are subject to changing auditory cues (Haresign and Moiseff 1988). Köppl and Nickel (2007) measured the development of auditory sensitivity in closely related European barn owls, Tyto alba guttata and Tyto alba pratincola, during this time of development, using both cochlear microphonics and compound action potentials (CAPs). CAPs are recorded from an electrode placed at the round window of the inner ear and represent auditory nerve activity. Cochlear microphonics are the summed signals from hair cell activity in the cochlea. Köppl and Nickel (2007) used a closed sound system, so that development of the facial ruff did not affect the auditory thresholds. They found mature cochlear microphonic responses by P35, while CAP responses did not develop as quickly.
In order to incorporate the effects of facial ruff development, and to compare ABRs with CAP development, we measured ABRs in young barn owls, ages P2 to P74, and in adults. ABR measurements allowed us to sample the same barn owl over multiple time points, and provide baseline measures of auditory sensitivity during development.
Methods
Recordings from owls were performed in concordance with the NIH Guidelines for Animal Research and were approved by the Animal Care and Use Committee of the University of Maryland. ABRs were measured in 3 adult and 19 juvenile North American barn owls, Tyto furcata pratincola. Six of the juvenile owls were studied past age P65 to adulthood (P69 – P74). All owls were bred in captivity.
Apparatus
ABRs were measured in two chambers. The first part of the study was carried out in a walk-in sound-isolation booth, Model AS-114 (Industrial Acoustic Company), with a speaker (Orb Audio Mod1x, 80 Hz – 20 kHz, Orb Audio LLC, New York, NY) on a table level to the barn owl’s ear. Later ABRs were measured in a sound-attenuating chamber (24″ X 24″ X 30.5″), with the same speaker mounted to the wall, 6 inches above the bottom of the chamber. Both parts of the study used the same stimuli, speaker, and sound equipment. The software “SIGGEN” (Tucker-Davis Technologies, Gainesville, Florida) was used to create tone-bursts stimuli, which were fed through an RP2.1 (TDT, Gainesville, Florida). The PA5 programmable attenuator received input from the RP2.1 and drove the speaker directly. The TDT software “BIOSIG” was used to play stimuli and record from the three platinum subdermal needle electrodes (Grass F-E2; West Warwick, RI). The electrode signal was processed in the low-impedance Medusa Digital Biological Amplifier System (RA4L Headstage and RA16PA PreAmp; RA16BA Medusa Base station), which added 10X gain. After data collection, the signals were notch filtered at 60 Hz, low-pass filtered by 3000 Hz, and high-pass filtered by 30 Hz in the “BIOSIG” software.
Subjects
We made 2 –14 different ABR recordings per juvenile owl during their development. Age was determined using estimated hatch date, determined by daily observation, a two-day interval between hatchings, weight, and head width. Different clutches were analyzed during similar months (November, January, April, June, July) over two consecutive years. Male and female auditory sensitivity was not compared because karyotyping is needed for accurate sex determination. In total, we performed 126 ABR experiments (Table 1). Since chicks were sedated for recordings, ABR experiments were not performed over consecutive days for any individual owl.
Table 1.
One-Way ANOVA across age groups for each frequency. The age group listed has thresholds that do not differ significantly from adult thresholds. This analysis does not include inconsistent data (these data are included in Fig. 5). Groups that had no response to specific frequencies (5 kHz to 12 kHz) were excluded from the analysis at these frequencies, which explains the smaller sample size with increasing frequency.
| Frequency (Hz) | P Value | F Value | First group not significantly different from adult | Number of age groups at each frequency |
|---|---|---|---|---|
| 500 | 1.059e-6 | 4.26 | P14–P16 | 20 |
| 1000 | 1.41e-20 | 13.93 | P20–P22 | 20 |
| 2000 | 5.08e-18 | 11.53 | P35–P37 | 20 |
| 4000 | 1.12e-27 | 22.63 | P35–P37 | 20 |
| 5000 | 2.62e-16 | 9.31 | P26–P28 | 18 (starts at P11–P13) |
| 6300 | 4.25e-18 | 15.15 | P23–P25 | 17 (starts at P14–P16) |
| 8000 | 4.71e-13 | 11.1 | P32–P34 | 16 (starts at P17–P19) |
| 10000 | 2.66e-14 | 18.86 | P50–P53 | 12 (starts at P29–P31) |
| 12000 | 0.0175 | 2.43 | P29–P31 | 12 (starts at P29–P31) |
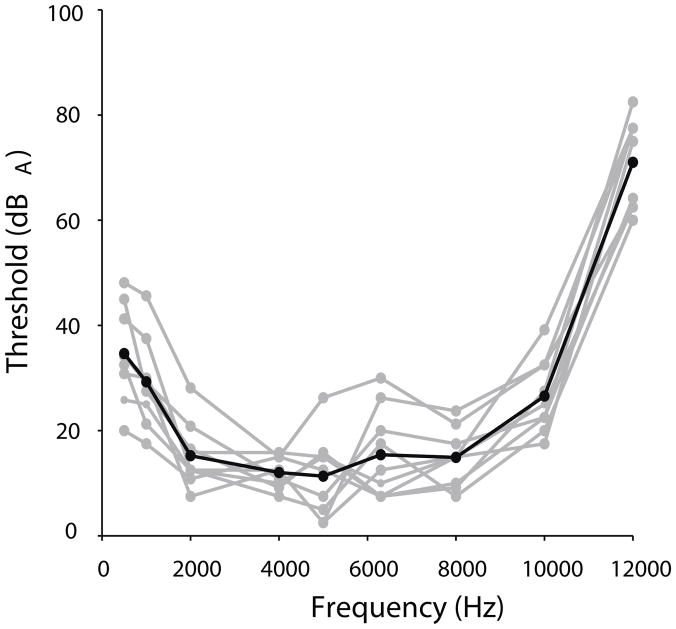
Owl chicks were grouped by age for purpose of analysis, and data were divided into 20 age groups spanning P4–P65. Typically, an age group spanned 3 consecutive days and included at least 2 birds. The number of birds in each age group varied from 3 – 11, with a mean of 6.3 per group (Online Resource 1). Two age groups spanned 4 days (P50–53 and P62–65) and one group spanned 5 days (P57–61) because of the relative maturity of the responses. Since no responses could be recorded from P2 or P3 chicks (n=2), ages P4 to P7 (n = 7) were grouped. Responses were defined as adult from 69 days posthatch and older, when all auditory thresholds were similar to those of the adults measured (Fig. 7, Table 1). Three of the nine adults measured were from our colony, and not part of the longitudinal study.
Fig. 7.
Adult thresholds for 9 individual birds in grey, with the average in black, with respect to stimulus frequency. Thresholds were considered adult by P69 because independent t-tests comparing P69-P74 owl thresholds (n = 5) to owls older than 5 months (n = 4) resulted in no significant difference in threshold for all frequencies tested (p > 0.05), thus thresholds were considered adult by P69.
Anesthesia and Data Collection
Prior to 45 days of age, barn owls were anesthetized by subcutaneous injection, and after 45 days of age by intramuscular injection of diazepam and ketamine. Doses were calculated using the weight formula (0.0004*weight (kg) = X mL diazepam, 0.00025*weight (kg) = X mL ketamine). Owls were wrapped in a heating blanket (38° C) in sternal recumbency with a temperature probe beneath the blanket. Once the barn owl was sedated, lidocaine was applied to the skin prior to inserting the electrodes subcutaneously. The negative electrode was placed behind the ear facing the speaker, the ground electrode behind the opposite ear, and the active electrode directly on the midline vertex of the barn owl’s head. The barn owl’s left ear was positioned 30 centimeters away from the speaker, directly facing the speaker. The skull was not vented; anesthesia was light, and animals were measured repeatedly during development.
Stimuli and Calibration
During each session, two trials were run for each of nine frequencies (in kHz: 0.5, 1, 2, 4, 5, 6.3, 8, 10, 12). Each trial contained an average of 300 repetitions. Stimuli were presented with multiple intensity stimulus trains that varied in frequency and intensity (Brittan-Powell et al. 2002). Each train consisted of nine frequency tone bursts that increased in intensity and were presented at a rate of 4/s. Each individual tone burst was 5 ms in duration with 1-ms rise/fall COS2 and a 20-ms ISI. Frequencies were played in a pseudorandom order. The maximum decibel levels played were 85 dB SPL for one clutch (n=4) and 100 dB SPL for the others (n=15), but note that only one threshold above 80 dB SPL was found in this study. For one clutch of four birds, the 500 Hz stimulus was not presented above 80 dB SPL due to the maximum volume of the speaker and the size of the sound-attenuating chamber.
Tone bursts and clicks were both used as stimuli. For each experiment, tone burst stimulus intensities were calibrated in the free field by placing the 0.5″ microphone of a sound level meter (System 824; Larson Davis, Inc. Provo, UT) at the approximate position of the bird’s left ear in the sound-attenuating chamber. Long duration tone bursts (1000 ms) were generated and played using the TDT SIGGEN program. Frequencies were measured using the fast-weighting A scale on the sound level meter (dB SPL).
Peak Analysis
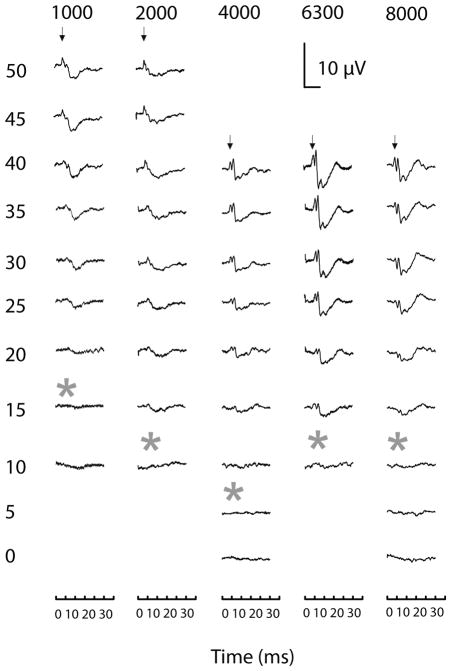
Visual peak detection was used to analyze the threshold level for each frequency because visual detection has been highly correlated with the results from algorithmic software that detects the first peak of an ABR (Caras et al. 2010, see Fig. 3 for examples of threshold). Stimuli increased in either 5 or 10 dB SPL steps. Thresholds were defined as the decibel level between the trace with a detectable peak and the trace without a detectable peak, so were either 5 dB or 2.5 dB SPL below the detectable peak. At least two thresholds were determined for each frequency per trial, and then averaged for each bird and time period. If only one threshold was measured at a particular frequency, that value was removed during analysis.
Fig. 3.
Average ABR audiograms for age groups up to P31. Thresholds decreased gradually, with lower frequency thresholds becoming adult-like first, followed by middle and higher frequencies. Plus error bars represent standard deviation in dB SPL. The adult audiogram is shown for comparison (see Fig. 7).
Threshold Analyses
A One-Way unbalanced ANOVA (MATLAB, Mathworks, Natick MA) was run between age groups for each of the nine frequencies to determine the age group at which the auditory threshold was not significantly different from the thresholds of adult barn owls. Multiple comparisons, using a Bonferroni correction (MATLAB), were used to compare threshold differences between age group for each frequency (0.5 kHz – 12 kHz).
Consistent Response Analyses
To account for variation in the responses of individual owls, we defined a consistent response for each frequency as one that was present in at least 2 owls on the day sampled, and then present in owls sampled over the next 2 days. The youngest age that met this criterion was interpreted as the consistent response. An exception was allowed for 6.3 kHz because all owls age P16 and P17 (n = 7) showed a response to 6.3 kHz stimuli, and one out of two owls tested showed no response at age P18.
Results
ABRs were used to assess the development of auditory sensitivity in barn owls from posthatch day 2 (P2) to P74 and older.
Response onsets around P4
Since the ABR is noninvasive, we were able to follow individual owls over time. Exemplar ABR waveforms in response to 1, 2, and 4 kHz tone pips are shown for an individual owl chick from 4–69 days of age (Fig. 1). ABR waveforms from the youngest birds possessed at least one long-duration positive wave, which was low in amplitude (10 μV) for an 85 dB 1 kHz stimulus. This first positive-going deflection was assumed to correspond to wave 1 of the adult barn owl ABR waveform (see Fig. 6). With increasing age, wave latencies decreased and wave amplitudes increased (Fig. 1).
Fig. 1.
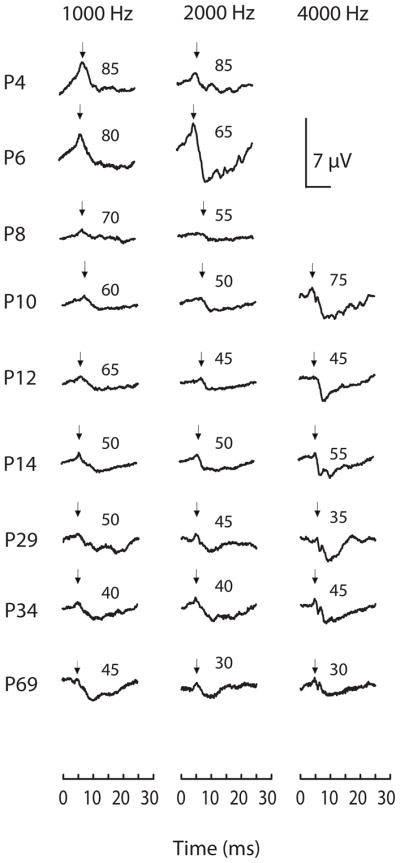
Development of auditory sensitivity across age in a single owl. Above threshold raw ABR waveforms (300 averages) for one trial for 1, 2, and 4 kHz tone-burst stimuli. The recording started at the onset of stimulus. Each waveforms is 17.5 dB SPL above threshold for each frequency and age. The left y-axis lists age, and inserts above each waveform represent dBA levels for that trace. Arrows indicate peak I.
Fig. 6.
Raw ABR traces, averaged with 300 repetitions, from adult owls from increasing to decreasing intensity. The recording started at the onset of stimulus. All of the traces were from owls at least a year old (n = 2), except for the 8 kHz tone (P70). Asterisks represent threshold, which was 2.5 dB SPL below the last waveform that showed a peak. Stimulus levels are shown on the y-axis (dBA). Arrows indicate peak I.
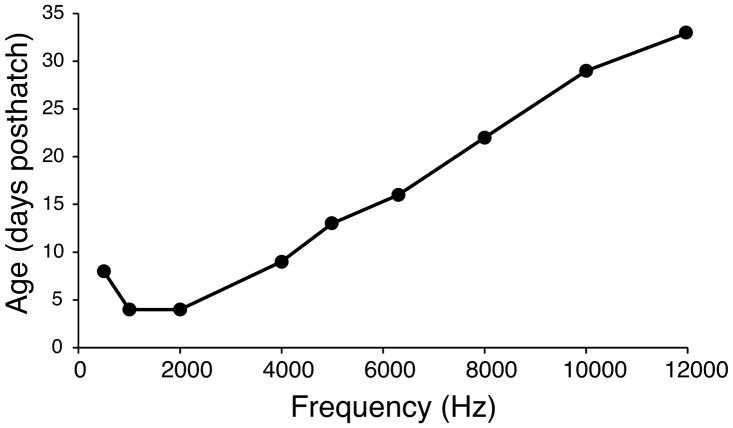
The first consistent ABR responses were detected at P4, to 1 and 2 kHz stimuli, followed by responses at P9 to 0.5 kHz and 4 kHz, and to 5 kHz at P13 (see below for definition of consistent). ABR signals changed with both stimulus frequency and intensity. Their latency increased and amplitude decreased with decreasing stimulus intensity (Hood 1998). ABRs recorded from very young owl chicks were small in amplitude, and only evoked by intense stimuli (Fig. 1, Table 1). Thresholds decreased with time (Figs. 1, 3). Responses could be elicited at all test frequencies by the third week posthatch.
Development of consistent auditory thresholds
Eight of the 9 frequencies tested showed significant differences in auditory thresholds with age (Table 1). A One-Way ANOVA was performed for each frequency across age groups. The only auditory threshold that did not change with age was 12 kHz, where thresholds remained between 65 – 80 dB SPL (Table 1, Fig. 4). The earliest responses were found for 500 Hz, 1 kHz, and 2 kHz at P4, although the 500 Hz responses were not classified as consistent because not all P6 owls displayed a response to this frequency (Fig. 1, Fig. 2). Because no responses were found in P2–3 chicks, the first consistent responses in chicks were found at P4 to 1 kHz and 2 kHz. Some P6 owls responded to 4 kHz, but responses to 4 kHz were not consistently found until P9. For 500 Hz, a consistent response was not shown until P8 (Fig. 2). One response to 5 kHz was found at P10 but consistent responses were not shown until P13 (Fig. 2). One P16 owl showed a response to 8 kHz, but no other owl showed a response to 8 kHz until P19, and a consistent response was not detected until P22. Both P24 and P27 time points included one owl that responded to 10 kHz, but only P29 owls showed consistent responses at 10 kHz. Responses were recorded to 12 kHz at P24 and P29, but again, consistent responses were not recorded until P33.
Fig. 4.
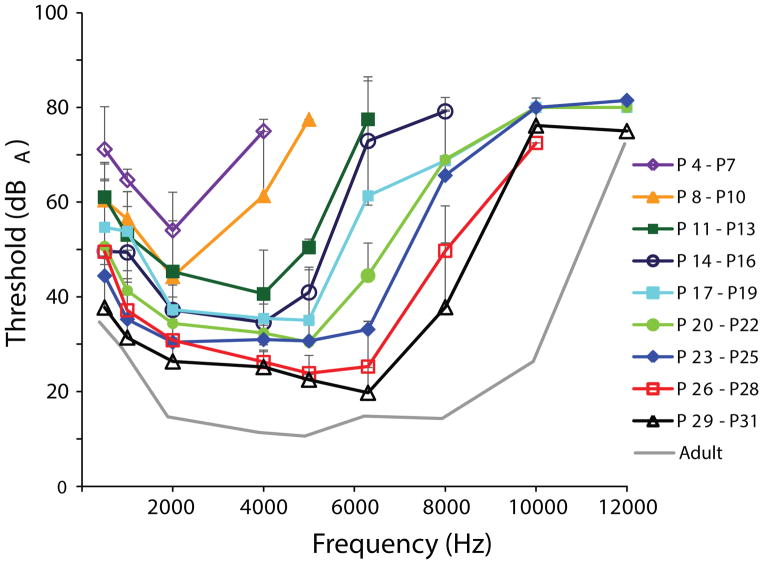
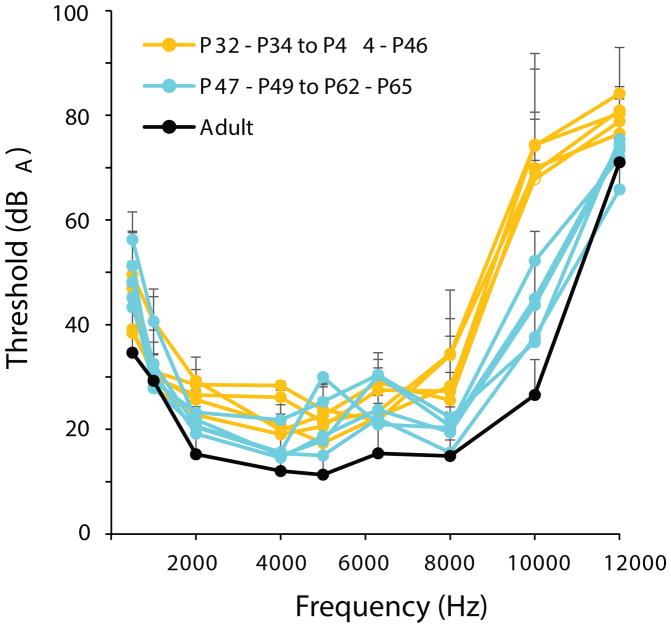
Average audiograms for age groups from P32 to adult. Orange lines represent age groups between P32 and P46, while cyan lines represent age groups between P47 and P65. The black line shows adult thresholds. Plus error bars represent standard deviation in dB SPL.
Fig. 2.
Age at which barn owls showed a consistent response to each stimulus frequency. The y-axis is age in days, and the x-axis represents frequency.
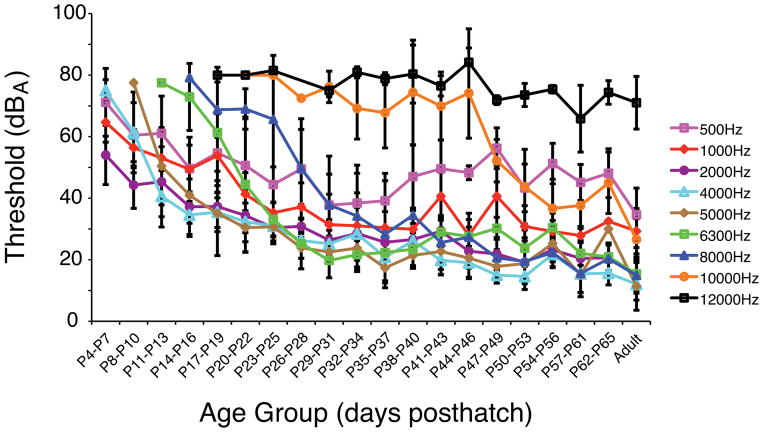
The youngest owls had a fairly flat audiogram, which increased in depth from P4–P31 (Fig. 3). Similar or lower auditory thresholds were found with increasing age, and responses to higher frequencies appeared with increasing age. The shapes of the audiograms from P32 to adult showed a similar trend of increasing sensitivity, except for the highest frequency responses (Fig. 4). The high frequency responses from the P44–46 age group and younger were different from the P47–49 and older age groups, most likely due to the lowering of the 10 kHz threshold (Fig. 4).
An unbalanced one-way ANOVA was used at each frequency to find age groups whose thresholds were not significantly different to the adult group thresholds (Table 1). Adult-like sensitivity was found in owls aged P14–P16 for 500 Hz, P20–P22 for 1 kHz, and P35–P37 for 2 kHz (Table 1, Fig. 5). In general, lower frequency responses matured earlier than higher frequency responses, with the exception of the range between 5–6.3 kHz. Interestingly, responses to both 5 kHz and 6.3 kHz matured earlier than those to 2 or 4 kHz stimuli (Fig. 5, see yellow traces in Fig. 4). Responses to 5 kHz reached adult levels sooner (P26–P28) than 2 or 4 kHz (P35–P37). Responses to 6.3 kHz reached adult thresholds even earlier, around P23–P25. For the 8 kHz stimuli, responses reached adult thresholds at P32–P34, however, thresholds showed a non-significant decrease of about 13 dB SPL between P34 and P69. The oldest age group to exhibit adult thresholds was for responses to the 10 kHz stimuli, where thresholds showed a 40 dB drop between the P47–P49 age group and the P54–P56 age group. Because the 12 kHz tone thresholds did not change very much during development, the P17–P19 age group had similarly high thresholds to adults. Only one owl in the P17–19 (n = 9) and P20–22 (n = 6) age groups showed a response to 12 kHz at 80 dBA.
Fig. 5.
Threshold versus age group for each frequency tested (0.5, 1, 2, 4, 5, 6.3, 8, 10, and 12 kHz tone-bursts). Twenty age groups, including 3 to 11 individual owls, are shown on the x-axis with increasing age. Error bars represent standard error in dB SPL.
Development of ABR waveforms
ABR responses most commonly showed multiple peaks; young barn owls typically showed two prominent positive peaks within the first 5 ms after sound reaches the bird’s external ear canal (Figs. 1, 6). At some frequencies, these peaks merged into a single peak at low stimulus intensities. 500 Hz stimuli generally produced a single peak (not shown), while 2 kHz stimuli typically produced two peaks across owls of the same age.
In adults, 2 or 3 prominent peaks were found within the first 5 ms after sound reaches the bird’s external ear canal, similar to other birds (Fig. 6) (Brittan-Powell and Dooling 2004; Brittan-Powell et al. 2005, Palanca-Castan et al. 2016). Note that the origins of ABR components are currently under study (Palanca-Castan et al. 2016). Adult ABRs showed average thresholds between 11–15 dB SPL for 2 – 8 kHz tones (* in Fig. 6, Fig. 7). Average thresholds for 0.5, 1, and 10 kHz were between 26 and 35 dB SPL, while the mean 12 kHz hearing threshold was 71 dB SPL, much higher than any other frequency tested (Fig. 7). These thresholds are comparable to, but greater than the 5–10 dB thresholds recorded for binaural chirp stimuli by Palanca-Castan et al. (2016).
Discussion
Barn owl chicks are insensitive to sound when they hatch, and first show auditory responses to 1–2 kHz around P4. Sensitivity to lower and higher frequencies increase with age, with responses to 12 kHz appearing as late as 2 months after hatching. Thus, these altricial birds achieve their sensitivity to sound during a prolonged period of posthatch development that coincides with the maturation of the skull and facial ruff (Haresign and Moiseff 1988).
Frequency Thresholds and Comparisons with CAP recordings
The development of the CAPs measured by Köppl and Nickel (2007) in European barn owls showed a very similar timeline to the ABR development up to 8 kHz, although most CAP responses appeared slightly later (2–4 days) than ABR threshold detection. There are several potential explanations for these differences. First, CAP recordings require deeper anesthesia because they are more invasive, while anesthesia for ABR recording was designed to be light to facilitate rapid recovery and return to the parent birds. Second, there could be small differences between owl subspecies; the present study was carried out on the slightly larger North American barn owl, Tyto furcata pratincola, while Köppl and Nickel (2007) studied the European barn owl, Tyto alba guttata. Third, our owls were staged on the basis of hatching date, rather than skull width or other morphometric features (Köppl et al. 2005). Fourth, the differences could reflect different techniques, since the ABR is a far field recording, and CAPs are measured close to the round window. This is the least likely, since CAP measures are generally more sensitive than ABRs, but see below for additional discussion of closed field vs. free field effects.
Side by side comparisons of recordings from Köppl and Nickel (2007) with our data showed ABR responses to 1 and 2 kHz tone pips at P4, compared to P6 for CAPs. For the 4 kHz stimuli, ABRs first revealed a response at P9, while the first CAP response was recorded at P13. For 5 kHz, the first ABR and CAP responses occurred at P13. CAPs for 6 kHz showed a response starting at P20, but the first ABRs to 6.3 kHz were found at P16. The earliest consistent ABR response at 8 kHz (P22) developed at the same time as the CAP responses. However, there was an 18-day difference between CAP and ABR responses to 10 kHz (P47 and P29, respectively; Köppl and Nickel 2007). Thus there was a wide gap in detection at 10 kHz between CAPs and ABRs, while the lower frequency response ages were similar.
Development of the outer ear
Facial ruff effects may contribute to the differences between CAP and ABR responses at high frequencies. The 10 kHz ABR response (P29) may have appeared much sooner than the CAP response (P47) because the reflector feathers of the barn owl ruff amplify sound by 6 dB, and increase directional responses (Campenhausen and Wagner 2006). Facial ruff development begins around P35, and most facial ruff feathers plateau in maximum length and width at P60 (Haresign and Moiseff 1988). Reflector feathers may specifically amplify higher frequencies before all auditory structures have fully matured. The ABR response for 10 kHz did not reach adult levels until P50–P53, and thresholds at 10 kHz did not start to drop below 80 dB until P47–P49 (Table 1), which suggests that the facial ruff may amplify responses to the 10 kHz tone pips and thus decrease auditory thresholds. Virtual ruff removal also has been shown to decrease sound localization accuracy in barn owls (Hausmann et al. 2009). The development of the skull and facial ruff may also underlie the small decrease in sensitivity to 500 Hz stimuli beginning around P35, since ruff feathers emerge around P35 (Haresign and Moiseff 1988).
The Development of Auditory Sensitivity in Altricial and Precocial Species
The developmental period for acquisition of mature auditory responses after hatching varies considerably between different species. Precocial chicks are relatively mature and mobile from the moment of hatching, while those of altricial species may be poorly coordinated, blind and deaf for extended periods after hatching (Ricklefs and Starck 1998). The two categories do, however, form a continuum with different degrees of altriciality/precociality. Chickens are precocial species, whereas the barn owls are altricial (Saunders et al. 1973; Köppl et al. 2005; Rich and Carr 1999).
Precocial chicks show mature low frequency responses as early as a day before hatching (embryonic day (E) 21), when they begin to breathe air (Saunders et al. 1973). Functional synaptic connections form much earlier; chicken embryo isolated brainstem preparations show evoked optical signals from electrical stimulation of the auditory nerve around E6–7 (Momose-Sato et al. 2006), while auditory stimuli evoke activity in the E11 brainstem (Katayama 1985). Although connections are functional, very young chicken embryos are not sensitive; E12–E13 chicks display a fairly flat audiogram, with thresholds of about 80–90 dB for the most sensitive frequencies (Saunders et al. 1973). Chicken embryos increase in auditory sensitivity and frequency range with age. Responses mature with about a 30 dB decrease in threshold between E12 and E18 (Saunders et al. 1973), leading to a more U-shaped audiogram. Chickens show only a small increase in sensitivity between E20 and 3 weeks posthatch (Saunders et al. 1973).
Both budgerigars and barn owls are altricial, and lack ABRs at hatching. In the first week posthatch, both exhibit a fairly flat, insensitive, audiogram with thresholds above 80 dB, after which thresholds decrease over the next few weeks (Brittan-Powell and Dooling 2004). Barn owls acquire mature thresholds for middle frequencies (5 kHz and 6.3 kHz) almost as quickly as for low frequencies, while budgerigars show only low frequency (0.5 to 2.0 kHz) responses by the second week posthatch (Brittan-Powell and Dooling 2004). These differences may be a reflection of the auditory range in both species, rather than the absolute frequency, since budgerigars hear up to 5.7 kHz, and barn owls up to 12 kHz. After the second week posthatch, both barn owls and budgerigars show a large drop in threshold for most frequencies (Brittan-Powell and Dooling 2004). By P16, barn owls exhibit close to adult thresholds up to 8 kHz, while they are still insensitive to 8, 10, and 12 kHz sounds. By P21, budgerigars have mature ABR thresholds up to 4.8 kHz, while they are still insensitive to their highest frequency (5.7 kHz), which took a month posthatch to reach adult thresholds (Brittan-Powell and Dooling 2004). Overall, even though barn owls have a broader range of hearing than the budgerigar, the development of auditory sensitivity in budgerigars is very similar to the timeline and development of barn owl sensitivity.
Mammals also vary greatly in their auditory capabilities at birth. In all mammals tested, however, thresholds decrease with age (for reviews, see Romand 1997, Rubel 1978, Mann and Kelley 2011). Precocial mammals, such as guinea pigs and primates, have ABR responses at birth, and for many, ABR thresholds at birth are similar to those in adults, although latencies gradually decrease with age (Dum 1984; Doyle et al. 1983). Guinea pigs demonstrate mature auditory thresholds at postnatal day 1 with ABRs for all frequencies tested; their P1 latencies are only 0.5 ms longer than adult latencies, and decrease to adult latencies within 4 weeks (Dum 1984). ABR thresholds are mature at birth in rhesus monkeys, while latencies decreased to adult levels after 12 months (Doyle et al. 1983). In humans, the auditory system becomes functional at 25 weeks’ gestation (for review, see Graven and Browne 2008), and lower frequency thresholds (500 and 2000 Hz) mature by 4 to 6 months of age (Marcoux 2011).
Altricial mammals, such as gerbils, cats, rats, and some mice, demonstrate reliable ABRs by their first (cats) or second (gerbils, rats, mice) postnatal week (Song et al. 2006; Smith and Kraus 1987; Rubel et al. 1998). Studies in gerbils provide insight into rapid increases in sensitivity during development; rapid shifts in the frequency map and decreases in threshold during cochlear maturation are consistent with tectorial membrane growth (Echteler et al. 1989; Müller 1996). Similar developmental events may underlie the 18 dB decrease in thresholds in mice for an 11.3 kHz stimulus at P12 (Song et al. 2006). Mice also show increases in sensitivity to higher frequency stimuli with development. At P12, mice showed a single ABR peak in response to 125 dB SPL tone bursts from 2.8 to 8 kHz, but did not respond to frequencies higher than 16 kHz (Song et al. 2006). By P15, mice responded to all frequencies tested, albeit at levels of 117 dB SPL. Because mice demonstrate a summating potential at postnatal day 12 at the first peak of the ABR (Song et al., 2006), we cannot rule out a contribution from similar potentials in the young barn owl, especially given the changes in peak number over time for middle to high frequencies (Fig. 1).
Cats show a similar pattern of auditory threshold development to barn owls, and respond to sound within the first postnatal week, dependent to some extent upon anesthesia. In un-anaesthetized cats, click ABR responses were detected at 135 dB SPL, and tone responses for 1 kHz at 120 dB SPL by P7 (Walsh et al. 1986a). Most kittens showed responses to tone bursts at 120 dB SPL for 0.5, 1, 2, 4, 8, and 16 kHz by P9 (Walsh et al. 1986a). Kittens anesthetized with ketamine showed ABRs at 93 dB HL at P4–6 for clicks (Shipley et al. 1980). While auditory nerve projections increase their tonotopic resolution until 6 days postnatal (Snyder and Leake 1997), a more recent study found that the tonotopy of auditory nerve projections in neonatal cats is very broad compared to adults, and neonatal cats develop an adult resolution between P0 to P3 (Leake et al. 2002). Thresholds dropped significantly between P10 and P20 (Walsh et al. 1986a; Walsh et al. 1992), and ABR wave amplitudes increased for most tones until P40 or later (Walsh et al. 1986b).
Comparison of ABR results with circuit development
Development of hearing sensitivity in young barn owls parallels development of the auditory brainstem. Interestingly, connections in the owl’s auditory brainstem form before hatching (Carr and Boudreau 1996), although ABR signals were not detected prior to P4. Thus it is likely that peripheral changes underlie the appearance of sensitivity to increasing frequencies observed in the development of Wave 1 (Köppl and Nickel 2007). The eighth nerve innervates the cochlear nucleus magnocellularis by E17, and the magnocellular axons arrive at their target in the nucleus laminaris between E17– 21 (Carr and Boudreau 1996, Kubke et al. 2002). Around E30, or two days before hatching, auditory nerve terminals in nucleus magnocellularis begin to transform into the specialized endbulb synapses, which are mature by about P12 (Carr and Boudreau 1996), when the barn owl can hear frequencies almost up to 5 kHz (detected at P13). Magnocellular and laminaris neurons and dendrites grow in the first month posthatch, and are not yet mature at the time the head reaches its adult size (Kubke and Carr 2000).
Well-myelinated tracts are required for ABRs (Hall 2007). The 8th nerve is myelinated before time of hatching, but myelination of NM and NL axons was only complete about a month after hatching (Cheng and Carr 2007), suggesting that later components of ABR waveforms may depend on both peripheral and central development. Later ABR peaks were not analyzed here, but contributions from the hindbrain auditory nuclei may be represented in peaks 2 and later of the ABR signal in budgerigars (Brittan-Powell et al. 2002) and barn owls (Palanca-Castan et al. 2016). Myelination and growth in the auditory brainstem is not uniform, but follows the tonotopic axes of all structures (Carr and Boudreau 1996). Rostro-lateral regions of nucleus magnocellularis and laminaris, or regions that develop into high best frequency regions, develop first and lead the development of the most caudal low best frequency portions of each nucleus by 2–4 days (Carr and Boudreau 1996; Kubke et al. 2002). Despite this rostral to caudal gradient of neural development, peak 1 analyses show that low frequency responses appear before high frequency ones.
Similar observations have been made in developing chickens (Lippe and Rubel 1985; Lippe 1987). Responses to low frequency stimuli appear before high frequency responses. Lippe and Rubel (1985) mapped the tonotopic map of NM and NL starting at E17 in developing chicks, and found that the antero-medial regions responded to higher frequencies, while the caudo-lateral regions responded to lower frequencies. As the chicks aged, antero-medial neurons progressively responded to higher frequencies (Lippe and Rubel 1985). More research needs to be done on the development of tonotopy, in order to relate changes in the development of ABR sensitivity to changes in brainstem circuits.
In this study, we have focused on peak I, while the sources of the peaks in the avian ABR are still unconfirmed. Wave I is assumed to originate from the auditory nerve, since avian ABR studies (budgerigar: Brittan-Powell et al., 2002; screech owl: Brittan-Powell et al., 2005) show similar Wave I latencies to avian and mammalian auditory nerve (Burkard et al., 1996; Köppl 1997). Brittan-Powell et al., (2005) suggested that activity in the nucleus laminaris might underlie wave III in screech owls and wave II in budgerigars (Brittan-Powell et al., 2004). Throughout development, most barn owls displayed two peaks in the ABR waveform for most frequencies, but older barn owls showed three peaks for high frequencies, such as 6.3, 8, and 10 kHz tone-bursts. The sources of the second and third peaks of the ABR waveform have yet to be determined.
In conclusion, barn owls hear only low frequencies (1 – 4 kHz) after hatching, and develop mature ABR thresholds for most frequencies by P35–37 (Fig. 5). With increasing age, threshold decreases and hearing range increases for high frequencies. Further, 5 and 6.3 kHz thresholds mature earlier than expected, while facial ruff development (Haresign & Moiseff 1988) may explain the large decrease in threshold at 10 kHz at the P50–P53 age group.
Acknowledgments
We acknowledge Dr. E. Brittan-Powell for help with calibration and ABR recording, and Dr. E. Smith for help with equipment, speaker setup, and calibration. This research was sponsored by the National Institute on Deafness and Other Communications Disorders (NIDCD) grant DC-000436 (CEC).
References
- Brittan-Powell EF, Dooling RJ. Development of auditory sensitivity in budgerigars (Melopsittacus undulatus) J Acoust Soc Am. 2004;115:3092–3102. doi: 10.1121/1.1739479. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ, Gleich O. Auditory brainstem responses in adult budgerigars. (Melopsittacus undulatus) J Acoust Soc Am. 2002;112:999–1008. doi: 10.1121/1.1494807. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Lohr B, Hahn DC, Dooling RJ. Auditory brainstem responses in the Eastern Screech Owl: an estimate of auditory thresholds. J Acoust Soc Am. 2005;118:314–321. doi: 10.1121/1.1928767. [DOI] [PubMed] [Google Scholar]
- Bunn DS, Warburton AB, Wilson R. The Barn Owl. London: AandC Black Publishers Ltd; 2010. [Google Scholar]
- Burkard R, McGee J, Walsh EJ. Effects of stimulus rate on the feline brain-stem auditory evoked response during development. I. Peak latencies. J Acoust Soc Am. 1996;100:978–990. doi: 10.1121/1.416209. [DOI] [PubMed] [Google Scholar]
- Campenhausen M, Wagner H. Influence of the facial ruff on the sound-receiving characteristics of the barn owl’s ears. J Comp Physiol A. 2006;192:1073–1082. doi: 10.1007/s00359-006-0139-0. [DOI] [PubMed] [Google Scholar]
- Caras ML, Brenowitz E, Rubel EW. Peripheral auditory processing changes seasonally in Gambel’s white-crowned sparrow. J Comp Physiol A. 2010;196:581–599. doi: 10.1007/s00359-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Boudreau RE. Development of the time coding pathways in the auditory brainstem of the barn owl. J Comp Neurol. 1996;373:467–483. doi: 10.1002/(SICI)1096-9861(19960930)373:4<467::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Carr CE, Kubke MF, Massoglia D, Rigby L, Moiseff A. Development of temporal coding circuits in the barn owl. Psychophysical and Physiological Advances in Hearing Edit Rees, AR Palmer and R Meddis. 1997:344–351. [Google Scholar]
- Cheng SM, Carr CE. Functional delay of myelination of auditory delay lines in the nucleus laminaris of the barn owl. Dev Neurobiol. 2007;67:1957–1974. doi: 10.1002/dneu.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Bullock TH, Schweitzer J. The auditory brain stem response in five vertebrate classes. Electroencephalogr Clin Neurophysiol. 1982;54:629–641. doi: 10.1016/0013-4694(82)90117-1. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Berlin A, Carr CE, Olsen GH, Therrien RE, Yannuzzi SE, Ketten DR. A comparison of auditory brainstem responses across diving bird species. J Comp Physiol A. 2015;201:803–815. doi: 10.1007/s00359-015-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling RJ, Lohr B, Dent ML. Comparative hearing: Birds and reptiles. Springer; New York: 2000. Hearing in birds and reptiles; pp. 308–359. [Google Scholar]
- Doyle WJ, Saad MM, Fria TJ. Maturation of the auditory brain stem response in rhesus monkeys (Macaca mulatta) Electroencephalogr Clin Neurophysiol. 1983;56:210–223. doi: 10.1016/0013-4694(83)90075-5. [DOI] [PubMed] [Google Scholar]
- Dum N. Postnatal development of the auditory evoked brainstem potentials in the guinea pig. Acta Oto-Laryngologica. 1984;97:63–68. doi: 10.3109/00016488409130965. [DOI] [PubMed] [Google Scholar]
- Dyson ML, Klump GM, Gauger B. Absolute hearing thresholds and critical masking ratios in the European barn owl: a comparison with other owls. J Comp Physiol A. 1998;182:695–702. [Google Scholar]
- Echteler SM, Arjmand E, Dallos P. Developmental alterations in the frequency map of the mammalian cochlea. Nature. 1989;341:147–149. doi: 10.1038/341147a0. [DOI] [PubMed] [Google Scholar]
- Fay RR. Hearing in Vertebrates: A Psychophysics Databook. Winnetka: Hill-Fay Associates; 1988. [Google Scholar]
- Gold JI, Knudsen EI. Abnormal auditory experience induces frequency-specific adjustments in unit tuning for binaural localization cues in the optic tectum of juvenile owls. J Neurosci. 2000;20:862–877. doi: 10.1523/JNEUROSCI.20-02-00862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven SN, Browne JV. Auditory Development in the Fetus and Infant. Newborn Infant Nurs Rev. 2008;8:187–193. [Google Scholar]
- Hall JW. New handbook of auditory evoked responses. Pearson; Boston: 2007. [Google Scholar]
- Haresign T, Moiseff A. Early Growth and Development of the Common Barn-Owl’s Facial Ruff. The Auk. 1988;105:699–705. [Google Scholar]
- Hausmann L, von Campenhausen M, Endler F, Singheiser M, Wagner H. Improvements of sound localization abilities by the facial ruff of the barn owl (Tyto alba) as demonstrated by virtual ruff removal. PLoS ONE. 2009;4:e7721. doi: 10.1371/journal.pone.0007721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann L, Plachta DTT, Singheiser M, Brill S, Wagner H. In-flight corrections in free-flying barn owls (Tyto alba) during sound localization tasks. J Exp Biol. 2008;211:2976–2988. doi: 10.1242/jeb.020057. [DOI] [PubMed] [Google Scholar]
- Hood LJ. Clinical Applications of the Auditory Brainstem Response (Evoked Potentials) 1. Clifton Park NY: Delmar Cengage Learning; 1998. [Google Scholar]
- Katayama A. Postnatal development of auditory function in the chicken revealed by auditory brainstem responses (ABRs) Electroencephalogr Clin Neurophysiol. 1985;62:388–398. doi: 10.1016/0168-5597(85)90048-6. [DOI] [PubMed] [Google Scholar]
- Keller CH, Hartung K, Takahashi TT. Head-related transfer functions of the barn owl: measurement and neural responses. Hear Res. 1998;118:13–34. doi: 10.1016/s0378-5955(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Instructed learning in the auditory localization pathway of the barn owl. Nature. 2002;417:322–328. doi: 10.1038/417322a. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF, Esterly SD. A critical period for the recovery of sound localization accuracy following monaural occlusion in the barn owl. J Neurosci. 1984;4:1012–1020. doi: 10.1523/JNEUROSCI.04-04-01012.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M. How the owl tracks its prey. American Scientist. 1973;61:414–424. [Google Scholar]
- Köppl C. Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J Neurosci. 1997;17:3312–3321. doi: 10.1523/JNEUROSCI.17-09-03312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C, Futterer E, Nieder B, Sistermann R, Wagner H. Embryonic and posthatching development of the barn owl (Tyto alba): Reference data for age determination. Dev Dyn. 2005;23:1248–1260. doi: 10.1002/dvdy.20394. [DOI] [PubMed] [Google Scholar]
- Köppl C, Nickel R. Prolonged maturation of cochlear function in the barn owl after hatching. J Comp Physiol A. 2007;193:613–624. doi: 10.1007/s00359-007-0216-z. [DOI] [PubMed] [Google Scholar]
- Kubke M, Carr CE. Development of the auditory brainstem of birds: Comparison between barn owls and chickens. Hear Res. 2000;147:1–20. doi: 10.1016/s0378-5955(00)00116-7. [DOI] [PubMed] [Google Scholar]
- Kubke M, Massoglia D, Carr CE. Bigger brains or bigger nuclei? Regulating the size of auditory structures in birds. Brain Behav Evol. 2004;2004:169–180. doi: 10.1159/000076242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubke M, Massoglia D, Carr CE. Developmental changes underlying the formation of the specialized time coding circuits in barn owls (Tyto alba) J Neuro. 2002;22:7671–7679. doi: 10.1523/JNEUROSCI.22-17-07671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe WR. Shift of tonotopic organization in brain stem auditory nuclei of the chicken during late embryonic development. Hear Res. 1987;25:205–208. doi: 10.1016/0378-5955(87)90092-x. [DOI] [PubMed] [Google Scholar]
- Lippe W, Rubel EW. Ontogeny of tonotopic organization of brain stem auditory nuclei in the chicken: implications for development of the place principle. J Comp Neuro. 1985;237:273–89. doi: 10.1002/cne.902370211. [DOI] [PubMed] [Google Scholar]
- Lohr B, Brittan-Powell EF, Dooling RJ. Auditory brainstem responses and auditory thresholds in woodpeckers. J Acoust Soc Am. 2013;133:337–42. doi: 10.1121/1.4770255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann ZF, Kelley MW. Development of tonotopy in the auditory periphery. Hear Res. 2011;276:2–15. doi: 10.1016/j.heares.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Marcoux AM. Maturation of auditory function related to hearing threshold estimations using the auditory brainstem response during infancy. Int J Pediatr Otorhinolaryngol. 2011;75:163–170. doi: 10.1016/j.ijporl.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Kiang NYS. Generators of the brainstem auditory evoked potential in cat III: Identified cell populations. Hear Res. 1996;93:52–71. doi: 10.1016/0378-5955(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Miller GL, Knudsen EI. Early auditory experience induces frequency-specific adaptive plasticity in the forebrain gaze fields of the barn owl. J Neurophysiol. 2001;85:2184–2194. doi: 10.1152/jn.2001.85.5.2184. [DOI] [PubMed] [Google Scholar]
- Miller GL, Knudsen EI. Adaptive plasticity in the auditory thalamus of juvenile barn owls. J Neurosci. 2003;23:1059–1065. doi: 10.1523/JNEUROSCI.23-03-01059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose-Sato Y, Glover J, Sato K. Development of functional synaptic connections in the auditory system visualized with optical recording: afferent-evoked activity is present from early stages. J Neurophysiol. 2006;96:1949–1962. doi: 10.1152/jn.00319.2006. [DOI] [PubMed] [Google Scholar]
- Müller M. The cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear Res. 1996;94:148–156. doi: 10.1016/0378-5955(95)00230-8. [DOI] [PubMed] [Google Scholar]
- Ngan EM, May BJ. Relationship between the auditory brainstem response and auditory nerve thresholds in cats with hearing loss. Hear Res. 2001;156:44–52. doi: 10.1016/s0378-5955(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Noirot IC, Brittan-Powell EF, Dooling RJ. Masked auditory thresholds in three species of birds as measured by the auditory brainstem response (L) J Acoust Soc Am. 2011;129:3445–3448. doi: 10.1121/1.3578452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ. Hearing in passerine and psittacine birds: a comparative study of absolute and masked auditory thresholds. J Comp Psychol. 1987;101:7–15. [PubMed] [Google Scholar]
- Palanca-Castan N, Laumen G, Reed D, Köppl C. The Binaural Interaction Component in Barn Owl (Tyto alba) Presents few Differences to Mammalian Data. JARO. 2016;17:577–589. doi: 10.1007/s10162-016-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RS. Acoustic location of prey by barn owls (Tyto alba) J Exp Biol. 1971;54:535–573. doi: 10.1242/jeb.54.3.535. [DOI] [PubMed] [Google Scholar]
- Pena JL, DeBello WM. Auditory processing, plasticity, and learning in the barn owl. ILAR J. 2010;51:338–52. doi: 10.1093/ilar.51.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos N, Almeida MG, Lewis DR. Correlation between frequency-specific auditory brainstem responses and behavioral hearing assessment in children with hearing loss. Rev CEFAC. 2013;15:796–802. [Google Scholar]
- Rich V, Carr C. Husbandry and captive rearing of barn owls. Poultry and Avian Biology Reviews. 1999;10:91–95. [Google Scholar]
- Ricklefs RE, Starck JM. Embryonic growth and development. In: Ricklefs RE, Starck JM, editors. Avian growth and development: evolution within the altricial-precocial spectrum. Oxford, New York: Oxford University Press; 1998. pp. 31–58. [Google Scholar]
- Romand R. Modification of tonotopic representation in the auditory system during development. Prog Neurobiol. 1997;51:1–17. doi: 10.1016/s0301-0082(96)00043-3. [DOI] [PubMed] [Google Scholar]
- Rubel EW. Ontogeny of structure and function in the vertebrate auditory system. In: Jacobson M, editor. Handbook of sensory physiology. Berlin: Springer; 1978. pp. 135–237. [Google Scholar]
- Rubel EW, Popper AN, Fay RR. Springer Handbook of Auditory Research. New York: Springer-Verlag; 1998. Development of the Auditory System. [Google Scholar]
- Saunders JC, Coles RB, Gates GR. The development of auditory evoked responses in the cochlea and cochlear nuclei of the chick. Brain Res. 1973;63:59–74. doi: 10.1016/0006-8993(73)90076-0. [DOI] [PubMed] [Google Scholar]
- Shipley C, Buchwald JS, Norman R, Guthrie D. Brain stem auditory evoked response development in the kitten. Brain Res. 1980;182:313–326. doi: 10.1016/0006-8993(80)91191-9. [DOI] [PubMed] [Google Scholar]
- Smith DI, Kraus N. Postnatal development of the auditory brainstem response (ABR) in the unanesthetized gerbil. Hear Res. 1987;27:157–164. doi: 10.1016/0378-5955(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Leake PA. Topography of spiral ganglion projections to cochlear nucleus during postnatal development in cats. J Comp Neurol. 1997;384:293–311. doi: 10.1002/(sici)1096-9861(19970728)384:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Song L, McGee J, Walsh EJ. Frequency- and level-dependent changes in auditory brainstem responses (ABRs) in developing mice. J Acoust Soc Am. 2006;119:2242–2257. doi: 10.1121/1.2180533. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, Gorga M, McGee J. Comparisons of the development of auditory brainstem response latencies between cats and humans. Hear Res. 1992;60:53–63. doi: 10.1016/0378-5955(92)90058-u. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, Javel E. Development of auditory evoked potentials in the cat. I. Onset of response and development of sensitivity. J Acoust Soc Am. 1986a;79:712–724. doi: 10.1121/1.393461. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, Javel E. Development of auditory-evoked potentials in the cat. III. Wave amplitudes. J Acoust Soc Am. 1986b;79:745–54. doi: 10.1121/1.393463. [DOI] [PubMed] [Google Scholar]