Abstract
Social isolation is a major source of stress and can lead to activation of the hypothalamic-pituitary-adrenal (HPA) axis. The presence of a close social partner can reduce the magnitude of the HPA-axis response during a stressor, a phenomenon known as social buffering. The oxytocin (OXT) system has been identified as one candidate for mediating social buffering due to its role in the facilitation of social bonding and the expression of prosocial behavior. The goal of the present study was to determine whether the OXT system contributes to social buffering of HPA-axis activity in response to stressor exposure in marmoset monkeys (Callithrix jacchus). Male and female marmosets experienced a standardized psychogenic stressor with and without their long-term mate under OXT-treatments (Pro8-OXT, Leu8-OXT, OXT antagonist, and saline); we assessed HPA-axis activity by measuring urinary cortisol across the stressor. We found that blocking, but not augmenting, the OXT system altered patterns of cortisol and proximity behavior in response to a stressor. We demonstrated that (1) the presence of a mate during a stressor significantly attenuated HPA-axis activity in female, but not male, marmosets; (2) male, but not female, marmosets treated with an OXT antagonist had significantly higher HPA-axis activity across the stressor than when they were treated with saline, suggesting that the OXT system may reduce the stressor-induced rise in cortisol levels; (3) male and female marmosets treated with an OXT antagonist spent significantly less time in close proximity to their mate during the first 30 min of the stressor than when they were treated with saline, suggesting that the OXT system may be important for the expression of partner-seeking behavior during a stressor. Thus, the OXT system and social context differentially influenced how the HPA-axis responded to a stressor in male and female marmosets, and may modulate HPA-axis activity by promoting the expression of proximity behavior with a close social partner.
Keywords: Stress, Cortisol, HPA-axis, Social buffering, Pro8-OXT, Proximity
1. Introduction
Social disruption, isolation, and neglect are major sources of stress and can negatively impact health and well-being (McEwen, 2008), as well as contribute to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Smith and Wang, 2012). Long-lasting social relationships and positive social interactions can serve as resilience factors against stressors. These relationships, including the bonds between parents and offspring, peer friendships, and adult male–female bonds provide critical social resources that operate as protective mechanisms against environmental challenges, including predation, inter- and intra-group aggression, disease, as well as psychogenic stressors (Ditzen and Heinrichs, 2014). The social support originating from these close social partnerships can help mitigate the deleterious consequences of social stress through HPA-axis attenuation (Ditzen et al., 2008; Smith et al., 1998), a phenomenon known as social buffering (Cohen and Willis, 1985). Thus, the formation and preservation of long-lasting, social relationships may be an adaptive behavioral strategy for maintaining physiological and psychological well-being and reducing the detrimental health consequences of psychogenic stress.
While there are a number of biological pathways that contribute to the stress-buffering effect of social support (Hostinar et al., 2014), the oxytocin (OXT) system has been identified as a leading candidate for the regulation of social buffering due to its role in the facilitation of social bonding and the expression of prosocial behavior (Johnson and Young, 2015). The HPA-axis activity reducing, and anxiolytic properties of OXT have been identified in a variety of model species, including rodents, humans, and non-human primates (Neumann and Landgraf, 2012). For instance, OXT-deficient mice displayed more anxiety-like behavior and had higher corticosterone levels than wild-type mice following a psychogenic stressor (Amico et al., 2004). In socially monogamous New World primates, social isolation increased HPA-axis activity and anxiety-like behaviors (Fernandez-Duque et al., 1997; Rukstalis and French, 2005; Smith et al., 1998), and it appears that OXT treatment can reduce the magnitude of the physiological stress response. In particular, chronic intranasal OXT administration reduced circulating adrenocorticotropic hormone (ACTH) following 90 min of social isolation (Parker et al., 2005). In humans, central and peripheral OXT release during social support from a long-term partner following a stressor facilitated the attenuation of the HPA-axis and anxiety-like behavior (Grewen et al., 2005; Heinrichs et al., 2003). When OXT was administered intranasally during conflict in marital couples, positive communication between partners increased and circulating cortisol levels decreased (Ditzen et al., 2009), relative to individuals that received a placebo. Furthermore, positive couple interactions have a stress buffering effect on total daily cortisol secretion (Ditzen et al., 2008). Thus, the salubrious effects of social support occur in large part as a result of the positive social interactions among close social partners, and it appears that OXT maybe be a key anxiolytic agent and HPA-axis modulator during stressors.
Marmosets (Callithrix jacchus) are a highly social, cooperatively-breeding New World primate that readily form and maintain long-term, male–female relationships (Digby, 1995; Schaffner et al., 1995). Marmosets employ several long-term mating behaviors, including expressing high levels of sociality with a pair-mate (Schaffner et al., 1995), a pronounced stress response to social disruption (Rukstalis and French, 2005), aversion to an opposite-sex stranger in the presence of a pair-mate (Inglett et al., 1990), and aggressive responses to potential same-sex rivals (Ross et al., 2004). In marmosets, a single nucleotide substitution in the coding region of the OXT gene leads to a unique OXT ligand (Pro8-OXT), with distinct structural and physicochemical properties from consensus mammalian OXT (Leu8-OXT; Lee et al., 2011; Ren et al., 2015; Vargas-Pinilla et al., 2015). Furthermore, this ligand variation is associated with significant changes in the marmoset oxytocin receptor (OXTR; Ren et al., 2015) and social phenotype (Cavanaugh et al., 2015, 2014; Mustoe et al., 2015; Taylor and French, 2015).
Previous experimental work in marmosets has shown that the OXT system is involved in the expression of social behavior between pair-mates. Blocking endogenous OXT activity by administering an oral OXT antagonist (OXTA) significantly diminished sociality in newly-formed pairs (Smith et al., 2010). Additionally, administration of Pro8-OXT, but not Leu8-OXT, facilitated partner fidelity by reducing the time spent in close proximity with, and sociosexual behavior toward, an opposite-sex stranger (Cavanaugh et al., 2014), and reduced prosocial responses toward an opposite-sex stranger during an altruistic food-sharing task (Mustoe et al., 2015). However, we have also demonstrated that both the Pro8 and Leu8 variants of OXT influence social phenotype. Administration of Pro8-OXT or Leu8-OXT altered marmosets’ stimulus properties in such a way as to enhance the social attractiveness of an OXT-treated individual during social interactions with their pair-mate (Cavanaugh et al., 2015). In a similar vein, intranasal administration of Leu8-OXT subtly augmented sociality in newly formed marmoset pairs (Smith et al., 2010), and intracerebroventricular (icv) administration of Leu8-OXT enhanced paternal tolerance for food sharing with offspring (Saito and Nakamura, 2011). Thus, the threshold for alteration of social behavior via OXT ligand administration may differ across measures of social behavior (i.e., offspring care, food sharing, partner preference, proximity, grooming). Furthermore, Pro8-OXT and Leu8-OXT likely produce differential binding affinity with the OXTR (Ren et al., 2015), and neural circuits underlying marmoset sociality may be differentially sensitive to the two forms of OXT. Thus, we sought to examine the influence of both Pro8-OXT and Leu8-OXT on social buffering in marmosets.
The current study examined the potential that the OXT system mediates the attenuation of the stress response from social support by measuring changes in HPA-axis activity, as well as affiliative and anxiety-like behavior, during stressor exposure in marmosets. If social relationships are an important mediator of social buffering, then excreted levels of cortisol and the expression of anxiety-like behavior should be reduced when an individual’s pair-mate is present, relative to when an individual’s pair-mate is absent, during a novel housing stressor. If the OXT system regulates these social buffering effects, then stressor-exposed marmosets treated with an OXTA should fail to display reductions in cortisol and anxiety-like behavior when their pair-mate is present, relative to when their pair-mate is absent. Treatment with an OXTA is also expected to reduce, while treatment with an OXT agonist is expected to enhance, measures of social behavior during a stressor when an individual’s pair-mate is present. Furthermore, stressor-exposed marmosets treated with an OXT agonist should display reductions in cortisol and anxiety-like behavior regardless of whether their pair-mate is absent or present. If structural changes in the OXT ligand are biologically relevant, then marmosets treated with Pro8-OXT, but not Leu8-OXT, should display enhanced social behavior when their pair-mate is present and decreased cortisol secretion regardless of whether their pair-mate is absent or present.
2. Method
2.1. Subjects
Five adult male and five adult female white-tufted ear marmosets (C. jacchus), housed at the Callitrichid Research Center (CRC) at the University of Nebraska at Omaha, experienced a standardized novel housing stressor known to reliably elevate cortisol in adult marmosets (Rukstalis and French, 2005; Smith et al., 1998). Animals were 4.3 ± 0.2 (mean ± SEM) years of age at the start of the study, and had cohabitated with the same partner for 15 months in large indoor wire-mesh enclosures (1.0 × 2.5 × 2.0 m), equipped with a sleeping hammock, natural branches for climbing and various enrichment materials. Visual access was restricted between enclosures, but auditory and olfactory cues were not. Colony rooms at the CRC were maintained on a 12 h:12 h light:dark cycle and at a temperature range between 19 °C and 22 °C. For all dietary and husbandry protocols please refer to (Schaffner et al., 1995). The University of Nebraska at Omaha/University of Nebraska Medical Center Institutional Animal Care and Use Committee evaluated and approved all procedures: 12-099-12-FC.
2.2. Behavioral paradigm
Marmosets were exposed to a standardized novel housing stressor both with and without their long-term mate during four counterbalanced OXT-treatment conditions, over a series of eight treatment periods (2 contexts × 4 OXT-treatments). On the day of the stressor, the subject was removed from its home enclosure at 0830 h, administered an OXT-treatment, and transferred to a transport enclosure (0.3 × 0.3 × 0.3 m) located in a room some distance from the colony room that contained the pair’s home enclosure. The marmoset was then transferred to a larger enclosure (0.6 × 0.6 × 0.6 m) at 0900 h and remained in this environment until 1600 h. Each member of a pair was treated separately during each treatment period, and treatments were administered in a counterbalanced order. Sequence of treatments within each individual was also counterbalanced. There was a 14–28 day washout period between drug treatments.
For each drug treatment condition, marmosets experienced the novel housing stressor once with their long-term mate present and once with their long-term mate absent. The treated marmoset had visual, acoustic, and olfactory, but not physical, access to their partner during the partner-present condition. The untreated partner remained in the home-enclosure during the partner-absent condition. We measured the latency, rate, and duration of behavioral responses (Table 1) during two 30-min videotaped focal observations at 0900 h and 1300 h during the stressor. Social interactions between pair-mates were also observed the day prior to, the day of, and the day after the stressor at 1600 h during 20-min focal observations in the pair’s home enclosure. All observers were trained to achieve a level of reliability (κ > 0.90) on scoring marmoset behavior. All focal animal observations were recorded using Stopwatch + software (Emory University).
Table 1.
Ethogram.
| Behavior | Operational definition |
|---|---|
| Affiliative behavior | |
| Solicit grooma | Orientation of body or head to present to mate for grooming |
| Allogrooma | Manipulation of pelage of mate (or self) by parting the fur with hands and removing particles with hands or teeth |
| Approacha | Moving to a distance of ≤10 cm from mate |
| Leavea | Moving to a distance of >10 cm from mate |
| Proximitya | Duration that focal animal is ≤10 cm from mate |
| Proximityb | Duration that focal animal is ≤30 cm of adjacent enclosure |
| Huddlinga | The resident pair is in physical contact |
| Sexual behavior | |
| Open-mouth displaya | A rhythmic opening and closing of the mouth (i.e., lip smacking, tongue flicking) directed toward a potential mate |
| Mountinga | Male grasps female’s back and thrusts pelvis with an erect phallus |
| Copulationa | Adult male licks erect phallus after successfully mounting |
| Anxiety-like behavior | |
| Scratchingb | Self-directed rubbing, itching, or scraping of pelage |
| Locomotionb | Marmoset moves from one location to another |
| Agonistic behavior | |
| Phee callb | Vocalization that is used as a contact call, long in duration and high in pitch |
| Scent marka,b | Rub anogenital region across branches or other surfaces, often preceded by gnawing on surface |
| Genital displaya | Exposing genital area by lifting the tail |
| Enclosure manipulationb | Marmoset grasps or bites the wire of the enclosure |
Behaviors observed during home-enclosure focal.
Behaviors observed during psychogenic stress paradigm.
2.3. Drug treatments
2.3.1. Intranasal administration of OXT agonists
Marmosets received an intranasal treatment of saline or one of two different OXT agonists (25 IU; 50 µg/100 µL saline solution) that yielded a dose of 150 µg/kg. The dose was determined based on previous primate literature (Cavanaugh et al., 2014; Heinrichs et al., 2003; Mustoe et al., 2015; Parker et al., 2005; Smith et al., 2010). Marmosets were administered Pro8-OXT (i.e., the marmoset variant of OXT; synthesized by Anaspec Corp., California), Leu8-OXT (i.e., the consensus mammalian variant of OXT; synthesized by Maurice Manning, University of Toledo), or saline 30 min prior to stressor exposure, via intranasal administration during a brief (~3 min.) manual restraint. Intranasal administration was accomplished using a 100-µL Eppendorf pipette to administer 50 µL of solution to each nostril drop-wise (30 s between each nostril), and is a relatively well-tolerated, non-invasive method of administration.
Peptides administered intranasally are quickly absorbed into the bloodstream via the nasal passage (Pires et al., 2009), and some fraction of the peptides appear to bypass the blood-brain barrier (BBB) to access the central nervous system (CNS) via the olfactory bulb and the maxillary branch of the trigeminal nerve (MacDonald and Feifel, 2013). Intranasal neuropeptides are transported to the CNS and accumulate in the cerebrospinal fluid (CSF) in humans (Striepens et al., 2013) and macaques (Dal Monte et al., 2014). In rats and mice, OXT levels were increased in microdialysates from the hippocampus and amygdala, and in plasma 30–60 min after intranasal administration (Neumann et al., 2013). Elevated circulating levels of OXT after intranasal treatment persist for up to, but no more than 7-h in humans (van IJzendoorn et al., 2012). These results suggest that intranasal administration rapidly increases OXT levels in the brain and plasma during the timeframe of our behavioral testing, and that OXT clears the system several hours after testing.
2.3.2. Oral administration of OXT antagonist
Marmosets were treated with 20 mg/kg of an OXTA, or saline, 90 min prior to stressor exposure, via oral administration in a preferred food treat. Marmosets received a saline-treated food treat 90 min prior to stressor exposure during Pro8-OXT, Leu8-OXT, and saline intranasal treatment conditions. Marmosets also received intranasal saline 30 min prior to stressor exposure during the OXTA treatment condition. The OXTA (L368,899®; provided by Dr. Peter Williams, Merck & Co., Inc.) is readily absorbed by the bloodstream after passage through the digestive system (Thompson et al., 1997), penetrates the CNS after peripheral administration, accumulates in areas of the limbic system (Boccia et al., 2007), and reduces affiliative social behavior in newly-paired marmosets (Smith et al., 2010).
2.4. Hormone analysis
Urine samples were collected across six days to measure excreted cortisol prior to, during, and following the stressor. First-void urine samples were collected using non-invasive techniques described by French et al. (1996) between 0600 and 0800 h on days 1–6 (two days prior to the stressor, the morning of the stressor, and three days following the stressor; stressor occurred on Day 3). Urine samples were also collected once per hour between 0900 h and 1600 h during the stressor, and at 1700 h and 1800 h post pair-mate reunion in their home enclosure (Fig. 1). The clearance rate from cortisol secretion to cortisol excretion in urine occurs within 2.5 h in marmosets (Bahr et al., 2000). After collection, urine samples were centrifuged at 2000 rpm for five minutes to separate sediment from the sample. The supernatant was then transferred to a clean vial and stored at −20 °C pending assay.
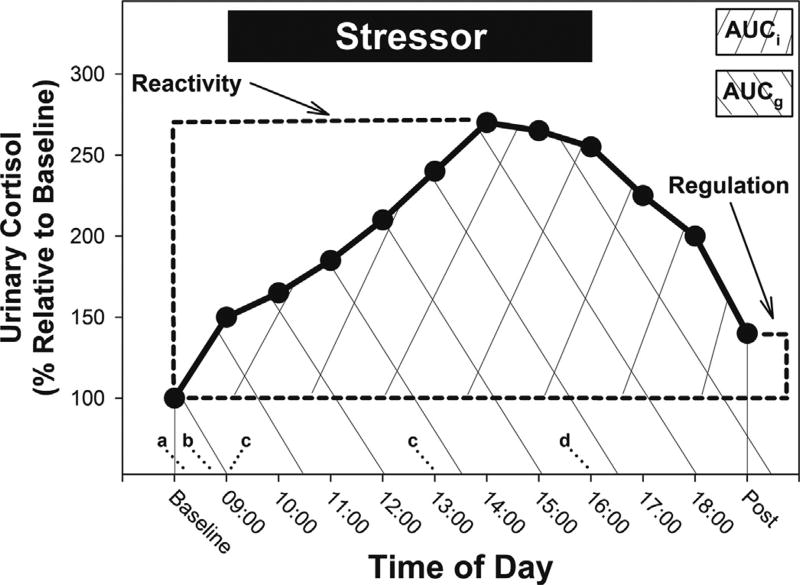
Fig. 1.
Schematic of basic and derived measures of HPA-axis activity to a standardized psychosocial stressor in marmosets (adapted with permission; French et al., 2012). Baseline = mean first-void cortisol concentration from Days 1–3 (stressor occurred on Day 3). Post = mean first-void cortisol concentration from Days 4–6. Reactivity = maximum cortisol concentration during stressor—Baseline. Regulation = Post—Baseline. Area Under Curve-ground (AUCg) = areaunder the cortisol response curve with respect to a cortisol concentration of 0 µg/mg creatinine. AUCi = area under the cortisol response curve with respect to Baseline. aAdministration of oral OXTA or saline; bAdministration of intranasal Pro8-OXT, Leu8-OXT, or saline; c30-min videotaped observation; dReunion with pair-mate in home enclosure.
Excreted cortisol concentrations were measured via enzyme immunoassay, which has been validated for use in marmosets (Smith et al., 1998). Briefly, urine samples were diluted in distilled water (1:3,200 or 1:6,400) to fall within the standard curve. Microtitre plates were coated with cortisol antibody (3.6.07), diluted to 1:25,000 in bicarbonate coating buffer, and incubated for 12 h. Cortisol standards ranged from 1000 to 7.8 pg/well. Labeled cortisol horseradish peroxidase (HRP; R4866) was diluted 1:30,000 in PBS. After the 12-h incubation, 50 µL of PBS was added to each well, followed by 50 µL of the extracted urine samples or cortisol standards. After 50 µL of HRP was added, the plates were set to incubate for two hours. Free and bound hormones were separated, after which an EIA substrate (ABTS, H2O2) was added. Absorbance at 405-nm was measured in a microplate reader. The mass of cortisol is expressed in µg per mg of creatinine (Cr) to control for variation in the solute concentration of the urine samples. Creatinine was measured using a standard Jaffé reaction colorimetric assay (French et al., 1996). Intra-assay coefficients of variation (CV) for high and low concentration pools were 5.39% and 4.02%, respectively. Inter-assay CVs for the same high and low concentration pools were 14.24% and 12.02%, respectively.
2.5. Data analysis
To assess whether OXT treatment altered HPA-axis activity (measured by cortisol excretion) we calculated several derived variables, Reactivity, AUCg, AUCi, and Regulation. Baseline cortisol values were calculated by averaging first-void cortisol concentrations from days 1–3. The post-stressor cortisol value was calculated by averaging first-void cortisol concentrations from days 4–6. Hourly samples (0900–1800 h) during collection were collapsed into 2-h blocks (extrapolation for missing samples was not necessary; there was never two consecutive hours of missing samples). First, Reactivity was defined as the difference between the maximum cortisol concentration during the stressor and the baseline value (Reactivity was also calculated as a percentage relative to baseline: Reactivity%). Second, we calculated the area under the curve (AUC) of the cortisol response across the stressor in two ways. AUCg was calculated using the trapezoidal method to measure the area under the cortisol response curve with respect to a cortisol value of 0 µg/mg creatinine. AUCi was calculated by measuring the area under the cortisol response curve with respect to baseline. Each measure provides different information about how the HPA-axis responds to the stressor (AUCg is an estimate of total cortisol exposure, AUCi is an estimate of cortisol increase over baseline; (French et al., 2012; Pruessner et al., 2003). Lastly, Regulation was defined as the difference between the post-stressor value and the baseline value). We evaluated the effect of drug treatment and social context on cortisol across the stressor using several-mixed model ANOVAs, with time of day, OXT-treatment, and partner presence or absence as within-subject factors, and sex as a between-subject factor (7 × 4 × 2 × 2). The effects of drug treatment and social context on derived measures of cortisol and behavior exhibited during the stressor were also evaluated (4 × 2 × 2). If main effects or interactions were significant, post-hoc comparisons were made using Bonferroni’s correction. All alpha levels were set at p < 0.05.
3. Results
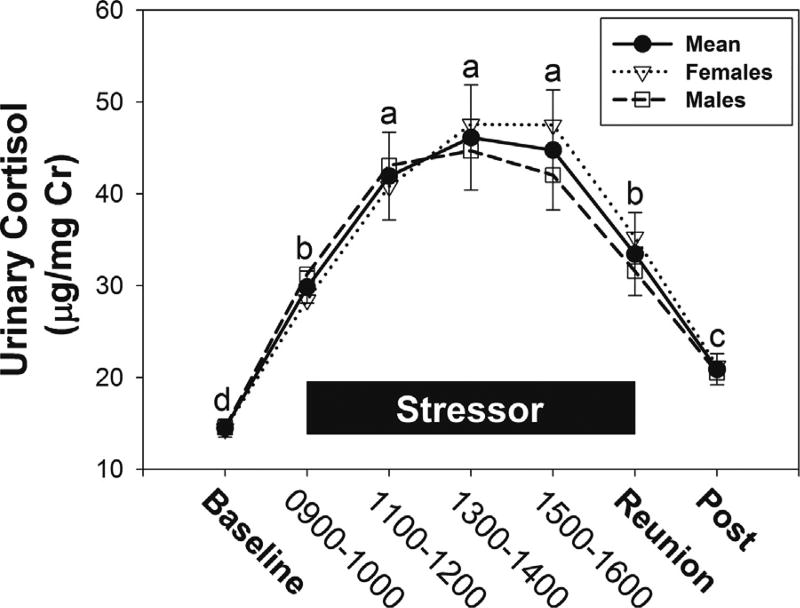
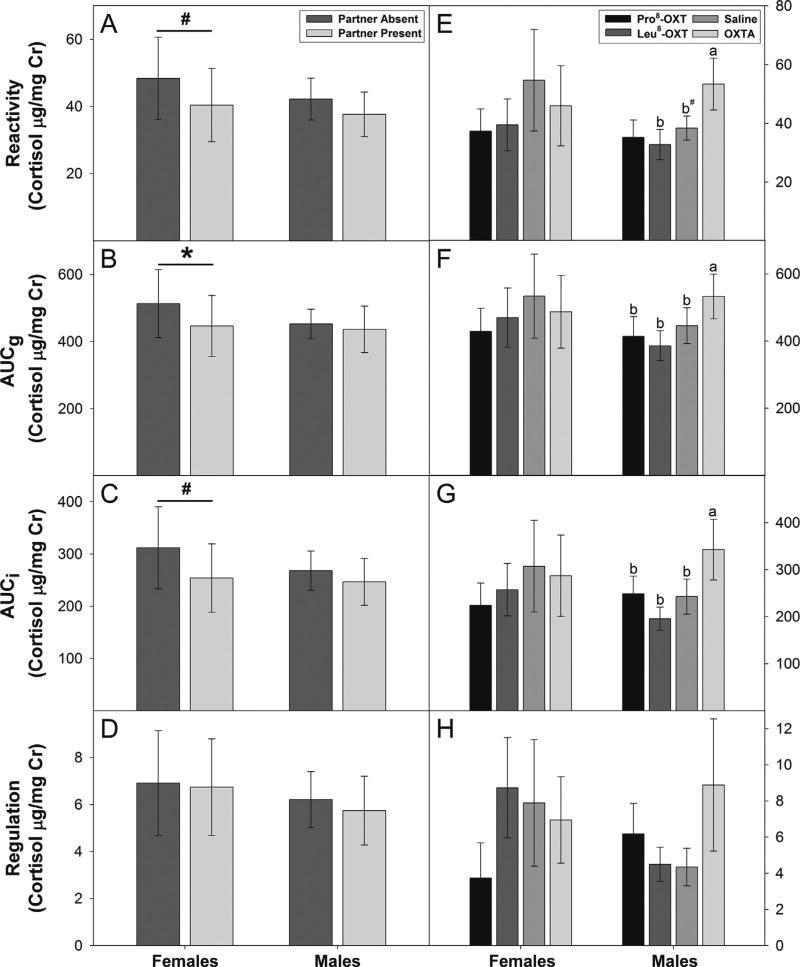
Exposure to a novel housing stressor had pronounced effects on HPA-axis activity, indicated by a three-fold increase in excreted cortisol levels, in male and female marmosets [F(6,48) = 20.47, p < 0.001, η2 = 0.72] (Fig. 2). Female, but not male, marmosets that were exposed to a stressor with their partner present had lower derived measures of HPA-axis activity than females exposed to a stressor alone (Fig. 3A–C). Female marmosets exposed to a stressor with their partner present had significantly lower AUCg [F(1,4) = 30.72, p = 0.005, η2 = 0.89], and tended to have lower cortisol reactivity [F(1,4) = 7.07, p = 0.056, η2 = 0.64] and AUCi [F(1,4) = 6.76, p = 0.06, η2 = 0.63] than females exposed to a stressor with their partner absent. The presence or absence of their partner did not alter any derived measures of HPA activity in male marmosets [F’s < 0.55, n.s.] (Fig. 3A–C). The presence or absence of their partner did not influence regulation of excreted cortisol levels in male and female marmosets after cessation of the novel housing stressor [F(1,8) = 0.10, n.s.] (Fig. 3D).
Fig. 2.
Mean (±SEM) cortisol levels across the stressor period in male and female marmosets (n = 10), collapsed across OXT-treatment. Reunion = Mean cortisol 1–2 h post-reunion. a > b > c > d at p < 0.05.
Fig. 3.
Mean values (±SEM) for components of the stress response in female and male marmosets (n = 10): (A) Reactivity, (B) AUCg, (C) AUCi, and (D) Regulation for marmosets that experienced a stressor with and without their partner. *p < 0.05, #p < 0.06. (E) Reactivity, (F) AUCg, (G) AUCi, and (H) Regulation for marmosets that received an OXT-treatment: Marmoset OXT agonist (Pro8-OXT); Consensus mammalian OXT agonist (Leu8-OXT); saline; OXT antagonist (OXTA: L368,899®). a > b at p < 0.05, #p < 0.10.
OXT-treatment significantly influenced derived measures of HPA-axis activity in male, but not female, marmosets. OXT-treatment significantly altered cortisol reactivity [F(3,12) = 6.88, p = 0.006, η2 = 0.63], AUCg [F(3,12) = 14.45, p < 0.001, η2 = 0.78], and AUCi [F(3,12) = 7.15, p = 0.005, η2 = 0.64] in male marmosets, but not in females [F’s < 2.10, n.s.]. Males treated with an OXTA had significantly higher cortisol reactivity (Fig. 3E), AUCg (Fig. 3F), and AUCi (Fig. 3G) than when they were treated with Pro8-OXT, Leu8-OXT or saline. Regulation of excreted cortisol levels after cessation of the novel housing stressor was unaffected by OXT-treatment in males and females [F(3,24) = 0.69, n.s.] (Fig. 3H). OXT-treatment did not significantly alter derived measures of HPA-axis activity differentially between conditions when an individual’s partner was present versus absent in males and females [F’s < 0.59, n.s.].
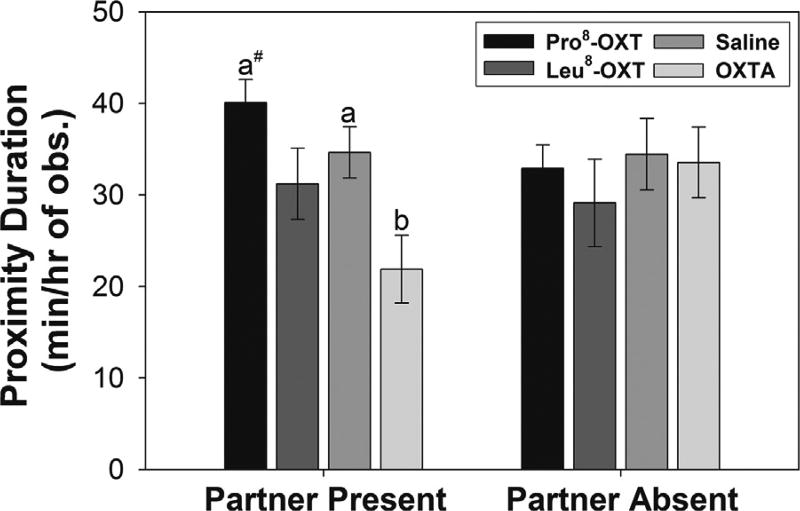
The expression of proximity behavior during the novel housing stressor was modulated by OXT-treatment in male and female marmosets when their partner was present, but not when their partner was absent. Male and female marmosets administered an OXTA spent significantly less time in close proximity to the adjacent enclosure during the first 30 min of the stressor when their partner was present, regardless of the location of their untreated partner in the adjacent enclosure, relative to when they were treated with Pro8-OXT or saline [F(3,24) = 4.80, p = 0.009, η2 = 0.38] (Fig. 4). OXT-treatment did not significantly alter proximity to the adjacent enclosure during the first 30 min of the stressor when their partner was absent [F < 0.40, n.s.]. Furthermore, neither OXT-treatment nor the presence or absence of their partner modulated the expression of anxiety-like behavior or agonistic behavior during the stressor [F’s < 1.77, n.s.] (Table 2). Although, males and females tended to display more contact vocalizations (i.e., phee calls) when their partner was absent than when their partner was present [F(1,8) = 4.80, p = 0.06, η2 = 0.38].
Fig. 4.
Mean (±SEM) time spent in proximity to the adjacent enclosure during the first 30 min of the stressor for marmosets (n = 10) that that received an OXT-treatment, and experienced a stressor with and without their partner. a > b at p < 0.05, #p < 0.10.
Table 2.
Effects of OXT treatment and partner presence or absence on anxiety-like behavior and agonistic behavior.
| Behavior | Pro8-OXT | Leu8-OXT | Saline | OXTA | F value | p value | Partner absent | Partner present | F value | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| Anxiety-like behavior | ||||||||||
| Scratchinga | 6.0 (1.8) | 5.0 (1.0) | 6.0 (2.4) | 4.0 (1.2) | 0.57 | 0.64 | 4.4 (1.0) | 6.2 (1.6) | 1.59 | 0.24 |
| Locomotionb | 2.5 (0.5) | 2.4 (0.5) | 3.1 (0.7) | 2.6 (0.7) | 0.84 | 0.49 | 2.7 (0.7) | 2.6 (0.5) | 0.02 | 0.90 |
| Agonistic behavior | ||||||||||
| Phee calla | 8.4 (4.0) | 10.8 (3.2) | 9.2 (4.0) | 12.4 (5.2) | 0.35 | 0.80 | 16.0 (5.6) | 4.2 (2.0) | 4.80 | 0.06 |
| Scent markinga | 5.4 (2.4) | 2.8 (1.2) | 1.8 (1.0) | 2.8 (1.6) | 1.06 | 0.39 | 2.0 (1.2) | 4.4 (1.6) | 1.47 | 0.26 |
| Enclosure manipulationa | 1.4 (0.8) | 1.4 (1.0) | 3.0 (1.8) | 1.2 (0.4) | 0.68 | 0.57 | 2.4 (1.2) | 1.0 (0.4) | 1.14 | 0.32 |
Data are expressed as a function of OXT treatment and partner presence or absence during the stressor. N = 10.
The mean (±SEM) frequency (per hour of observation).
The mean (±SEM) duration (minutes per hour of observation) of each behavior.
4. Discussion
Exposure to positive social interactions and social support from a close social partner during a stressful event has a substantial impact on short-term well-being. Several neurobiological pathways have been proposed as regulators of social buffering, including the sympathetic nervous system, HPA-axis, and the OXT system (Ditzen and Heinrichs, 2014). Here we examined how the HPA-axis responded to a psychogenic stressor in marmoset monkeys, and whether partner-presence and OXT-treatment altered the stressor-induced rise in cortisol levels. We demonstrated that the presence of a mate during a stressor significantly attenuated HPA-axis activity in female, but not male, marmosets, relative to experiencing a stressor when their partner was absent. This suggests that the mere presence of a close social partner during a stressor, even devoid of physical contact, buffers HPA-axis activity in female marmosets. The OXT system appears to be important in attenuating the physiological stress response. Male marmosets treated with an OXTA had significantly higher HPA-axis activity across the stressor than when they were treated with saline, suggesting that the OXT system may reduce the stressor-induced rise in cortisol levels in male, but not female, marmosets. However, intranasal administration of an OXT agonist did not alter measures of HPA-axis activity in male and female marmosets, regardless of partner presence or absence. Additionally, male and female marmosets treated with an OXTA spent significantly less time in close proximity to their pair-mate during the first 30 min of the stressor, relative to when they were treated with saline, suggesting that the OXT system may be important for the expression of partner-seeking behavior during a stressor.
Male and female marmoset’s HPA-axis differentially responded to the presence or absence of a long-term mate during a stressor. Females had reduced cortisol levels across the stressor when their partner was present, relative to when their partner was absent, while males appeared to be unaffected by the presence or absence of a partner, suggesting that females benefited more from the presence of their partner than males. This finding is in line with the “tend-and-befriend” behavioral response to stressors put forth by Taylor et al. (2000). They proposed that some species appear to utilize a different suite of behavioral responses to a threat/challenge than the traditional “fight or flight” describes, namely by nurturing offspring (i.e., tending pattern) and enhancing affiliation with group members (i.e., befriending pattern) as a means to ameliorate the deleterious consequences of stress (Geary and Flinn, 2002; Taylor, 2006). Both male and female marmosets respond positively to vocal contact, as isolated marmosets exposed to only a partner’s contact vocalization (i.e., phee call) had significantly lower cortisol levels than marmosets exposed to a stranger’s vocalization or no vocalization (Rukstalis and French, 2005). In the current study, male and female marmosets displayed moderately more contact calls when their partner was absent than when their partner was present, suggesting that isolated marmosets are motivated to reestablish contact with their pair-mate. Male marmosets may have been less responsive to the presence of a pair-mate during a stressor in the current study because their female partner may not have provided them with the requisite social support, males may not have sufficiently perceived the social support, or males may have required physical contact to buffer against psychogenic stress.
Cohen and Willis (1985) proposed two models of social support: (1) embedding within a social network generates positive outcomes by providing stability, predictability, and an improved ability to cope with stressors; (2) benefits of social support occur directly at the time of the stressor, and could be produced by either the presence of a social partner or by providing social support via active intervention (e.g., vocal reassurance, physical contact). In either case, an individual’s social partner may provide social support by modulating the psychological (i.e., perceived intensity of the stressful stimuli) and/or physiological (i.e., autonomic nervous system activity) response (Levine, 1993; Smith et al., 1998). In the current study, it is clear that the presence of a long-term mate buffered HPA-axis activity in female marmosets. However, it is not clear if females’ stress responses were buffered as a result of relationship stability or active social interaction during stressor exposure. Female marmosets stress response was likely attenuated from simply having a long-term mate (Model 1) and from the presence of their long-term mate during a stressor (Model 2).
There is burgeoning evidence that the OXT system has a neuromodulatory role in the attenuation of the stress response. In the current study, male, but not female, marmosets administered an OXTA had significantly higher HPA-axis activity across the stressor, relative to when they were treated with saline. This suggests that the OXT system may attenuate the stressor-induced rise in cortisol levels in male marmosets. The OXT system likely modulates the physiological stress response via mechanisms within the paraventricular nucleus of the hypothalamus (PVN; Neumann et al., 2000; van den Burg et al., 2015), as well as the interaction between the PVN and the bed nucleus of the stria terminalis (BNST; Dabrowska et al., 2011). While a reciprocal neuroanatomical connection between the PVN and BNST may explain how OXT influence stress buffering generally (MacDonald and Feifel, 2014), it does not explain why only male marmosets were influenced by an OXTA intervention. Perhaps female marmosets had sufficient endogenous OXT activity to overcome the OXTA intervention. However, there are currently no data showing that female marmosets have differential OXTR distribution across neural regions, greater expression of OXTR within the PVN or BNST, or higher circulating levels of OXT, than males. It is also possible that functional differences, rather than neuroanatomical ones, in the OXT system of males and females may underlie this difference (de Vries, 2008).
While OXTR blockade by OXTA appears to increase HPA-axis activity, intranasal administration of OXT agonists did not attenuate HPA-axis activity or alter behavioral responses to a stressor in marmosets, regardless of partner presence or absence. Intranasal administration of OXT has been shown to buffer cortisol responses in humans (Cardoso et al., 2013a), and in combination with social support, OXT enhanced cortisol reduction, relative to social support alone (Heinrichs et al., 2003). Administration of intranasal OXT (Leu8-OXT: the consensus mammalian variant or Pro8-OXT: the marmoset variant) in marmosets did not significantly ameliorate stressor-induced increases in cortisol. Previously, we demonstrated that intranasal Pro8-OXT exerted more potent effects than intranasal Leu8-OXT on fidelity-threatening behaviors in well-established marmoset pairs, by decreasing social motivation to interact with an opposite-sex stranger (Cavanaugh et al., 2014; Mustoe et al., 2015). Administration of intranasal Leu8-OXT has also been shown to modulate social behavior in marmoset pairs (Cavanaugh et al., 2015; Smith et al., 2010), as well as father-offspring interactions (Saito and Nakamura, 2011). Thus, it appears that the threshold for alteration of social behavior by OXTR activation may differ across measures of social behavior. However, it is currently unknown if Pro8-OXT and Leu8-OXT have differential binding affinities for the OXTR, if there are differential intracellular signaling cascades associated with each OXT variant, or if neural circuits that mediate different aspects of the social phenotype are differentially sensitive to the two variants of OXT.
These data also raise the intriguing possibility that ligand variation in OXT may produce differential social phenotypes in part as a consequence of interactions with vasopressin receptors, particularly V1aR. We know that pharmacologically (Gimpl and Fahrenholz, 2001), physiologically (Meyer-Lindenberg et al., 2011), and behaviorally (Schorscher-Petcu et al., 2010; Song et al., 2014) there is considerable crosstalk between OXT and V1aR, lending considerable credence to this possibility. However, the binding affinity of Pro8-OXT and Leu8-OXT for marmoset arginine-vasopressin (AVP) receptors is currently unknown. While OXT appears to have HPA-axis activity reducing properties (Neumann and Landgraf, 2012), activation of AVP receptors has been shown to increase HPA-axis activity (Ebstein et al., 2009; Shalev et al., 2011). OXT and AVP appear to have opposing effects on the HPA-axis response to stressors, with AVP augmenting, and OXT attenuating HPA-axis activity (Legros, 2001). Thus, this cross talk could potentially diminish the OXT agonist specific effects by activating both AVP and OXT receptors, rather than specifically targeting the OXT system.
While there is no evidence that male and female marmosets have dissimilar distributions of OXT immunoreactive neurons in the PVN, supraoptic nucleus of the hypothalamus (SON), BNST, or the medial amygdala (Wang et al., 1997), the BNST (an important relay site within the HPA-axis; Choi et al., 2007) is sexually dimorphic in AVP distribution, with male marmosets expressing more AVP+ neurons than females (Wang et al., 1997). Thus, the sex difference observed in marmosets’ HPA-axis response to a psychogenic stressor might be mediated by activation of both OXT and AVP systems. Additionally, the interaction between the hypothalamic-pituitary-gonadal (HPG) axis (particularly via estrogen receptors α and β) and the OXT system (Choleris et al., 2003; Gabor et al., 2012; Tribollet et al., 1990) may explain some of the observed sex differences. Estrogens appear to regulate OXT production in the PVN through ER-β, and regulate OXTR transcription in the amygdala through ER-α (Choleris et al., 2003). Thus, there is a distinct likelihood that differential estrogen receptor activity in male and female marmosets may have impacted OXTR responsiveness to OXT treatment. Further examination of the interaction between the OXT system and the HPG-axis would provide valuable insight into sex-specific responses to psychogenic stressors.
Marmosets characteristically utilize proximity and grooming behavior to facilitate the maintenance of their bond (Cavanaugh et al., 2015; Schaffner et al., 1995). Thus, one potential explanation that intranasal OXT did not buffer physiological and behavioral responses to a stressor is that marmosets were not receiving the compulsory social support from their pair-mate. Since both members of the pair experienced the stressor, and physical access was restricted, the untreated partner may not have been able to give and receive physical social support. Moreover, it is possible that vicarious anxiety (i.e., seeing one’s partner experiencing a stressor) counteracted the beneficial stress-buffering properties of their partner’s presence. One emerging hypothesis is that OXT enhances the sensation of socio-emotional features of the environment. Thus, in conditions when an individual does not receive social support and has increased levels of OXT they may be more sensitive to the social stress (Eckstein et al., 2014). This adverse effect of OXT may be due to its ability to enhance self-referential processing and awareness of one’s own current state (Hurlemann and Scheele, 2015), consequently increasing the magnitude of the stress response. Through enhancing the salience of social cues OXT treatment may make individuals more sensitive to social cues, both positive and negative in nature, resulting in enhanced sensitivity to emotional stimuli (McQuaid et al., 2014).
While OXT agonists did not significantly influence behavioral responses, treatment with an OXTA did alter marmosets’ expression of affiliative behavior during a psychogenic stressor. Male and female marmosets treated with an OXTA spent significantly less time in close proximity to their partner’s enclosure during a stressor, relative to when they were treated with saline. Thus, one effect of OXTR blockade may be reduced motivation to engage in affiliative behavior with a partner during a stressor, which subsequently limits the amount of social support an individual can receive. This result provides further support for the “tend-and-befriend” hypothesis. The OXT system has been proposed as a regulator of the “tend-and-befriend” behavioral response to stressors as a motivator to seek social support during threat/challenge (Cardoso et al., 2013b; Taylor, 2006; Taylor et al., 2000). In the context of the current study, marmosets treated with an OXTA spent less time in proximity with their long-term mate during a psychogenic stressor, suggesting that the OXT system plays an important role in promoting contact with a social partner during stressors.
In a recent report, non cooperatively-breeding capuchin monkeys treated with an intranasal OXT agonist spent less time in proximity with a social partner in a food-sharing task (Brosnan et al., 2015). This finding is in stark contrast to the effects of OXT in the cooperatively-breeding marmoset, where administration of an intranasal OXT agonist enhanced affiliation with social partner (Cavanaugh et al., 2014; Mustoe et al., 2015; Smith et al., 2010). Brosnan et al. (2015) suggested that treatment with OXT elicited an anxiolytic response, thus diminished the motivation, and potentially the need, for social contact (which can be anxiolytic itself; Ditzen and Heinrichs, 2014). In the current study, the greater distance between pair-mates during the stressor as a result of OXTA administration may have limited the positive effects of the partner’s presence. Therefore, as opposed to endogenous OXT acting directly on the HPA-axis and reducing the physiological stress response, another explanation is that the endogenous OXT system acts to promote affiliative behavior between social partners, and this increase in positive social behavior subsequently regulates HPA-axis activity. We demonstrated that administration of an OXTA reduced proximity to long-term mate, which may have had the consequence of limiting the amount of social support the subject was able to receive. These findings suggest that the OXT system may be an important regulator of partner-seeking behavior during stressors.
Surprisingly, neither OXT-treatment nor the presence or absence of a partner modulated the expression of anxiety-like behaviors during a psychogenic stressor in marmosets. OXT has been shown to influence anxiety-like behavior in several gregarious (e.g., rats and mice) and socially monogamous (e.g., prairie voles) rodent species. Diminished endogenous OXT activity increased the expression of anxiety-like behavior (Amico et al., 2004), while administering exogenous OXT reduced anxiety-like behavior (Grippo et al., 2009) during social isolation. Though OXT treatment has been shown to reduce anxiety-like behavior in rodents, OXT may not influence the sufficiently more complex anxiety-like behavior in non-human primates, which can manifest differently among individuals as a result of variation in genetics, development, experience with different kinds of stressors, as well as differs across species and social systems (Coleman and Pierre, 2014). Research in humans has demonstrated that OXT-treatment reduces anxiety-like behavior in some contexts and increases anxiety-like behavior in other contexts, which has brought into question OXT’s standing as an anxiolytic compound (MacDonald and Feifel, 2014), and suggests that OXT treatment may not necessarily be a useful long-term treatment for human anxiety disorders. The results of the current study suggest that the OXT system may be involved in the termination of the physiological stress response, potentially via enhancing partner-seeking behavior, but may not necessarily influence the expression of anxiety-like behavior in marmosets.
5. Concluding remarks
Intranasal OXT has gained support as a therapeutic agent due to its potential for treating anxiety-like psychological disorders (Meyer-Lindenberg et al., 2011), as well as its relatively noninvasive method of administration. The results of the current study suggest that the OXT system may serve an important mechanistic role in the attenuation of the stress response. OXT-treatment and social context differentially influenced how the HPA-axis responded to stressors in male and female marmosets. The OXT system may modulate HPA-axis activity by promoting the expression of proximity behavior with a close social partner. While the results of the current study do not intimately link the OXT system with social buffering, we demonstrated that treatment with an OXTA increased the magnitude of the stress response, regardless of the presence of absence of a long-term mate, and that treatment with an OXTA reduced proximity to a pair-mate during a stressor. Thus, the OXT system may be an important regulator of partner-seeking behavior during stressful life events, as a means to buffer against social stress.
Acknowledgments
We would like to thank April Harnisch and Kelsey Kane, who assisted in the data collection process, and Aaryn Mustoe and Jack Taylor for providing valuable comments on earlier drafts of the manuscript. We also give thanks to Heather Jensen and Liz Gunkelman for providing excellent care of the marmoset colony. We would also like to thank Maurice Manning (University of Toledo) and Peter Williams (Merck & Co., Inc.) for their professional courtesies offering material support.
Funding
This work was supported in part by funds from the National Institutes of Health (HD 042882) awarded to Jeffrey A. French, and by funds from University of Nebraska—Omaha’s Graduate Research and Creative Activity Committee awarded to Jon Cavanaugh.
Footnotes
Conflict of interest
In submitting this manuscript, all authors declare no conflict of interest, monetary or otherwise.
Contributors
J. Cavanaugh designed the study, wrote the protocol, collected the data, conducted the statistical analysis, and wrote the first draft of the manuscript. S. Carp assisted in data collection and provided comments/edits on previous drafts of the manuscript. C. Rock assisted in data collection. J. French provided comments/edits on previous drafts of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J. Neuroendocrinol. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Bahr NI, Palme R, Möhle U, Hodges JK, Heistermann M. Comparative aspects of the metabolism and excretion of cortisol in three individual nonhuman primates. Gen. Comp. Endocrinol. 2000;117:427–438. doi: 10.1006/gcen.1999.7431. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Goursaud A-PS, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899®, accumulates in limbic brain areas: a new pharmacological tool for the study of social motivation in non-human primates. Horm. Behav. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. http://dx.doi.org/10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, Talbot CF, Essler JL, Leverett K, Flemming T, Dougall P, Heyler C, Zak PJ. Oxytocin reduces food sharing in capuchin monkeys by modulating social distance. Behaviour. 2015;152:941–961. http://dx.doi.org/10.1163/1568539x-00003268. [Google Scholar]
- Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology. 2013a;38:399–407. doi: 10.1016/j.psyneuen.2012.07.013. http://dx.doi.org/10.1016/j.psyneuen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Ellenbogen MA, Serravalle L, Linnen A-M. Stress-induced negative mood moderates the relation between oxytocin administration and trust: evidence for the tend-and-befriend response to stress? Psychoneuroendocrinology. 2013b;38:2800–2804. doi: 10.1016/j.psyneuen.2013.05.006. http://dx.doi.org/10.1016/j.psyneuen.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Huffman MC, Harnisch AM, French JA. Marmosets treated with oxytocin are more socially attractive to their long-term mate. Front. Behav. Neurosci. 2015;9:251. doi: 10.3389/fnbeh.2015.00251. http://dx.doi.org/10.3389/fnbeh.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, French JA. Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology. 2014;49:1–10. doi: 10.1016/j.psyneuen.2014.06.020. http://dx.doi.org/10.1016/j.psyneuen.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J. Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Gustafsson J-A, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-α and -β knockout mice. Proc. Natl. Acad. Sci. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Willis TA. Stress, social support, and the buffering hypothesis. Psychol. Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- Coleman K, Pierre PJ. Assessing anxiety in nonhuman primates. ILAR J. 2014;55:333–346. doi: 10.1093/ilar/ilu019. http://dx.doi.org/10.1093/ilar/ilu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo J-D, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. http://dx.doi.org/10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS One. 2014;9:e103677. doi: 10.1371/journal.pone.0103677. http://dx.doi.org/10.1371/journal.pone.0103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ. Progress in Brain Research. Elsevier; 2008. Sex differences in vasopressin and oxytocin innervation of the brain; pp. 17–27. [DOI] [PubMed] [Google Scholar]
- Digby LJ. Social organization in a wild population of Callithrix jacchus: II. Intragroup social behavior. Primates. 1995;36:361–375. [Google Scholar]
- Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor. Neurol. Neurosci. 2014;32:149–162. doi: 10.3233/RNN-139008. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Hoppmann C, Klumb P. Positive couple interactions and daily cortisol: on the stress-protecting role of intimacy. Psychosom. Med. 2008;70:883–889. doi: 10.1097/PSY.0b013e318185c4fc. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, Gritsenko I, Riebold M, Salomon S, Yirmiya N. Arginine vasopressin and oxytocin modulate human social behavior. Ann. N.Y. Acad. Sci. 2009;1167:87–102. doi: 10.1111/j.1749-6632.2009.04541.x. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Scheele D, Weber K, Stoffel-Wagner B, Maier W, Hurlemann R. Oxytocin facilitates the sensation of social stress: oxytocin and social stress. Hum. Brain Mapp. 2014;35:4741–4750. doi: 10.1002/hbm.22508. http://dx.doi.org/10.1002/hbm.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque E, Mason WA, Mendoza SP. Effects of duration of separation on responses to mates and strangers in the monogamous titi monkey (Callicebus moloch) Am. J. Primatol. 1997;43:225–237. doi: 10.1002/(SICI)1098-2345(1997)43:3<225::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- French JA, Brewer JK, Schaffner CM, Schalley J, Hightower-Merritt D, Smith T, Bell SM. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied’s black tufted-ear marmosets (Callithrix kuhlii) Am. J. Primatol. 1996;40:231–245. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- French JA, Smith AS, Gleason AM, Birnie AK, Mustoe A, Korgan A. Stress reactivity in young marmosets (Callithrix geoffroyi): ontogeny, stability, and lack of concordance among co-twins. Horm. Behav. 2012;61:196–203. doi: 10.1016/j.yhbeh.2011.12.006. http://dx.doi.org/10.1016/j.yhbeh.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci. 2012;126:97–109. doi: 10.1037/a0026464. http://dx.doi.org/10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- Geary DC, Flinn MV. Sex differences in behavioral and hormonal response to social threat: commentary on Taylor et al. (2000) Psychol. Rev. 2002;109:745–750. doi: 10.1037/0033-295x.109.4.745. http://dx.doi.org/10.1037//0033-295x.109.4.745. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico JA, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom. Med. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. http://dx.doi.org/10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. http://dx.doi.org/10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. http://dx.doi.org/10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol. Bull. 2014;140:256–282. doi: 10.1037/a0032671. http://dx.doi.org/10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Scheele D. Dissecting the role of oxytocin in the formation and loss of social relationships. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.013. http://dx.doi.org/10.1016/j.biopsych.2015.05.013. [DOI] [PubMed]
- Inglett French BJJA, Dethlefs TM. Patterns of social preference across different contexts in golden lion tamarins (Leontopithecus rosalia) J. Comp. Psychol. 1990;104:131–139. doi: 10.1037/0735-7036.104.2.131. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Curr. Opin. Behav. Sci. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. http://dx.doi.org/10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Cool DR, Grunwald WC, Neal DE, Buckmaster CL, Cheng MY, Hyde SA, Lyons DM, Parker KJ. A novel form of oxytocin in New World monkeys. Biol. Lett. 2011;7:584–587. doi: 10.1098/rsbl.2011.0107. http://dx.doi.org/10.1098/rsbl.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros JJ. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- Levine S. The influence of social factors on the response to stress. Psychother. Psychosom. 1993;60:33–38. doi: 10.1159/000288677. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Oxytocin’s role in anxiety: a critical appraisal. Brain Res. 2014;1580:22–56. doi: 10.1016/j.brainres.2014.01.025. http://dx.doi.org/10.1016/j.brainres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front. Neurosci. 2013;7 doi: 10.3389/fnins.2013.00035. http://dx.doi.org/10.3389/fnins.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Abizaid A, Anisman H. Making room for oxytocin in understanding depression. Neurosci. Biobehav. Rev. 2014;45:305–322. doi: 10.1016/j.neubiorev.2014.07.005. http://dx.doi.org/10.1016/j.neubiorev.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. http://dx.doi.org/10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mustoe AC, Cavanaugh J, Harnisch AM, Thompson BE, French JA. Do marmosets care to share? Oxytocin treatment reduces prosocial behavior toward strangers. Horm. Behav. 2015;71:83–90. doi: 10.1016/j.yhbeh.2015.04.015. http://dx.doi.org/10.1016/j.yhbeh.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. http://dx.doi.org/10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J. Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. http://dx.doi.org/10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Pires A, Fortuna A, Alves G, Falcão A. Intranasal drug delivery: how, why and what for? J. Pharm. Pharm. Sci. 2009;12:288–311. doi: 10.18433/j3nc79. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. http://dx.doi.org/10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ren D, Lu G, Moriyama H, Mustoe AC, Harrison EB, French JA. Genetic diversity in oxytocin ligands and receptors in New World monkeys. PLoS One. 2015;10:e0125775. doi: 10.1371/journal.pone.0125775. http://dx.doi.org/10.1371/journal.pone.0125775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C, French J, Patera K. Intensity of aggressive interactions modulates testosterone in male marmosets. Physiol. Behav. 2004;83:437–445. doi: 10.1016/j.physbeh.2004.08.036. http://dx.doi.org/10.1016/j.physbeh.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, French JA. Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Horm. Behav. 2005;47:1–7. doi: 10.1016/j.yhbeh.2004.09.004. http://dx.doi.org/10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Nakamura K. Oxytocin changes primate paternal tolerance to offspring in food transfer. J. Comp. Physiol. A. 2011;197:329–337. doi: 10.1007/s00359-010-0617-2. http://dx.doi.org/10.1007/s00359-010-0617-2. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in wied’s black tufted-ear marmosets (Callithrix kuhli) Am. J. Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu S-B, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J. Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. http://dx.doi.org/10.1523/jneurosci.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Israel S, Uzefovsky F, Gritsenko I, Kaitz M, Ebstein RP. Vasopressin needs an audience: neuropeptide elicited stress responses are contingent upon perceived social evaluative threats. Horm. Behav. 2011;60:121–127. doi: 10.1016/j.yhbeh.2011.04.005. http://dx.doi.org/10.1016/j.yhbeh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. http://dx.doi.org/10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm. Behav. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. http://dx.doi.org/10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhli) Horm. Behav. 1998;34:211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeil JK, IV, Larkin TE, II, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 2013;3 doi: 10.1038/srep03440. http://dx.doi.org/10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, French JA. Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Horm. Behav. 2015;75:154–159. doi: 10.1016/j.yhbeh.2015.10.002. http://dx.doi.org/10.1016/j.yhbeh.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Tend and befriend biobehavioral bases of affiliation under stress. Curr. Dir. Psychol. Sci. 2006;15:273–277. [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Thompson KL, Vincent SH, Miller RR, Colletti AE, Alvaro RF, Wallace MA, Feeney WP, Chiu SHL. Pharmacokinetics and disposition of the oxytocin receptor antagonist L-368,899 in rats and dogs. Drug Metab. Dispos. 1997;25:1113–1118. [PubMed] [Google Scholar]
- Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ. Gonadal steroids regulate oxytocin receptors but no vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Res. 1990;511:129–140. doi: 10.1016/0006-8993(90)90232-z. [DOI] [PubMed] [Google Scholar]
- van den Burg EH, Stindl J, Grund T, Neumann ID, Strauss O. Oxytocin stimulates extracellular Ca2+ influx through TRPV2 channels in hypothalamic neurons to exert its anxiolytic effects. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.147. http://dx.doi.org/10.1038/npp.2015.147. [DOI] [PMC free article] [PubMed]
- van IJzendoorn MH, Bhandari R, van der Veen R, Grewen KM, Bakermans-Kranenburg MJ. Elevated salivary levels of oxytocin persist more than 7 h after intranasal administration. Front. Neurosci. 2012;6 doi: 10.3389/fnins.2012.00174. http://dx.doi.org/10.3389/fnins.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Pinilla P, Paixão-Côrtes VR, Paré P, Tovo-Rodrigues L, Vieira CM, de Xavier AGA, Comas D, Pissinatti A, Sinigaglia M, Rigo MM, Vieira GF, Lucion AB, Salzano FM, Bortolini MC. Evolutionary pattern in the OXT-OXTR system in primates: coevolution and positive selection footprints. Proc. Natl. Acad. Sci. 2015;112:88–93. doi: 10.1073/pnas.1419399112. http://dx.doi.org/10.1073/pnas.1419399112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, Insel TR. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus) Synapse. 1997;27:14–25. doi: 10.1002/(SICI)1098-2396(199709)27:1<14::AID-SYN2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]