Abstract
Background.
Around 13% of patients undergoing parathyroidectomy for primary hyperparathyroidism (PHPT) postoperatively develop a condition known as the hungry bone syndrome (HBS). Although the condition is quite prevalent, the research in this field is very limited. The aim of our study was to determine possible risk factors of developing HBS after parathyroidectomy for PHPT.
Materials and methods.
In this study we enrolled patients who underwent parathyroidectomy for PHPT from January 2005 to December 2016 and performed a retrospective analysis. We used the definition of HBS as hypocalcaemia with normal or elevated PTH values. Patients were divided into two groups by the postoperative HBS prevalence: patients with postoperative HBS and those without postoperative HBS.
Results.
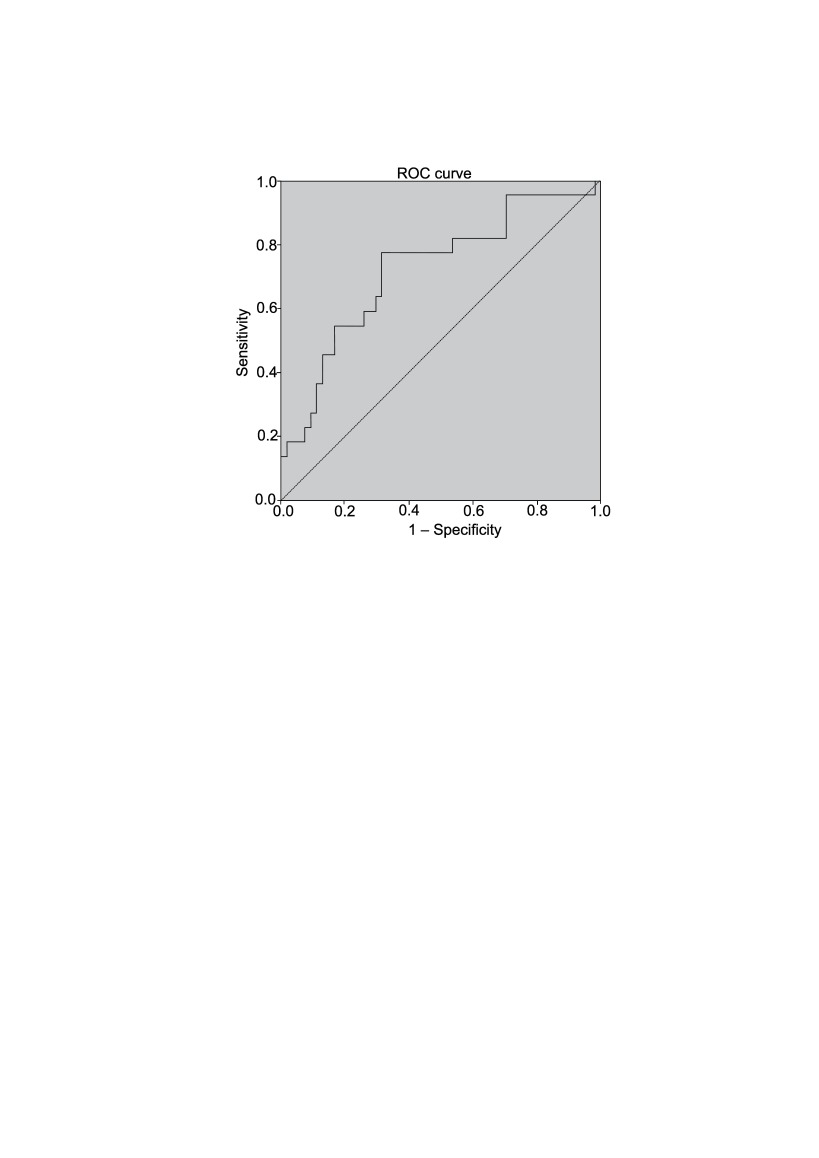
In all, 94 patients were included into the final analysis. We found that patients who developed HBS more often underwent parathyroidectomies simultaneously with a thyroid surgery, underwent longer operations (73.9 ± 41.7 vs. 102.4 ± 44.8 minutes; p = 0.001), and had heavier parathyroid glands removed (0.6 (0.3–8.0) vs. 0.8 (0.15–14.0) g; p = 0.041). Also, these patients had higher preoperative PTH values (15.3 (6.1–63.7) vs. 22.4 (9.2–47.8) pmol/l; p = 0.003). From the ROC curve of the preoperative PTH values and the development of the hungry bone syndrome (AUC = 0.721 (95% CI 0.59–0.85); p = 0.003) we found a 45 pmol/l PTH cut-off value that shows a 90% tendency to develop postoperative HBS.
Conclusions.
Patients undergoing longer parathyroidectomies and those with heavier removed parathyroid glands tend to develop HBS. A preoperative PTH value higher than 45 pmol/l determines an over 90% risk of developing HBS.
Keywords: hungry bone syndrome, primary hyperparathyroidism, parathyroidectomy, parathryroid adenoma
Abstract
ALKANŲ KAULŲ SINDROMO RIZIKOS VEIKSNIAI PO PRIESKYDINIŲ LIAUKŲ OPERACIJŲ DĖL PIRMINIO HIPERPARATIROIDIZMO
Santrauka
Įvadas. Apie 13 % pacientų po prieskydinių liaukų operacijų dėl pirminio hiperparatiroidizmo vystosi vadinamasis alkanų kaulų sindromas (AKS). Nors tai pakankamai dažnai pasitaikanti būklė, tačiau tyrimų šioje srityje atlikta nedaug. Mūsų tyrimo tikslas buvo įvertinti rizikos veiksnius, lemiančius AKS išsivystymą po prieskydinių liaukų operacijų dėl pirminio hiperparatiroidizmo.
Tyrimo metodai. Į tyrimą įtraukėme pacientus, kuriems nuo 2005 m. sausio iki 2016 m. gruodžio mėn. buvo atliktos prieskydinių liaukų operacijos dėl pirminio hiperparatiroidizmo, ir atlikome retrospektyvinę analizę. AKS apibrėžėme kaip hipokalcemiją esant normaliai arba padidėjusiai parathormono koncentracijai. Pacientai buvo suskirstyti į dvi grupes: (1) po operacijos pasireiškė AKS; (2) AKS nebuvo stebėtas.
Rezultatai. Galutiniame tyrime dalyvavo 94 pacientai. Nustatėme, kad pacientams, kuriems po operacijos vystėsi AKS, dažniau buvo atliktos simultaninės prieskydinių liaukų ir skydliaukės operacijos, taip pat jų operacijos truko ilgiau (73,9 ± 41,7 vs. 102,4 ± 44,8 minutės; p = 0,001), pašalintos prieskydinės liaukos buvo sunkesnės (0,6 (0,3–8,0) vs. 0,8 (0,15–14,0) g; p = 0,041), be to, jie turėjo didesnę parathormono koncentraciją kraujyje (15,3 (6,1–63,7) vs. 22,4 (9,2–47,8) pmol/l; p = 0,003). Atlikę ROC analizę tarp priešoperacinės PTH koncentracijos ir AKS vystymosi (AUC = 0,721 (95 % CI 0,59–0,85); p = 0,003), nustatėme ribinę 45 pmol/l reikšmę. Esant šiai reikšmei, 90 % pacientų vystosi pooperacinis AKS.
Išvados. Atliekant ilgesnes prieskydinių liaukų operacijas, taip pat esant sunkesnės būklės patologinei prieskydinei liaukai, pacientams kyla didesnė pooperacinio AKS rizika. Esant 45 pmol/l priešoperacinio parathormono koncentracijai, net 90 % pacientų gresia pooperacinis AKS.
Raktažodžiai: alkanų kaulų sindromas, pirminis hiperparatiroidizmas, prieskydinių liaukų operacija, prieskydinių liaukų adenoma
INTRODUCTION
Primary hyperparathyroidism (PHPT) is a condition when one or more of the parathyroid glands are over-active. Most of the time clinical symptoms are scarce; however, it can result in renal stone formation, bone demineralization, fatigue, and other conditions (1, 2). Laboratory tests show elevated values of parathyroid hormone (PTH) and calcium. Several surgical approaches ranging from traditional parathyroidectomy to video-assisted parathyroidectomy can be used to remove over-active glands. According to several large case series, around 13% of patients undergoing parathyroidectomy for PHPT develop a condition known as the hungry bone syndrome postoperatively (3–5). Hungry bone syndrome (HBS) is described as a prolonged hypocalcaemia after parathyroidectomy for hyperparathyroidism, followed by normal or elevated PTH levels. Pathogenesis of hypocalcaemia is explained by disturbed equilibrium between bone formation and resorption as a result of diminished PTH after removal of an over-active parathyroid gland (6–8). Although the condition is quite prevalent, research in this field is very limited: only few studies, all dating back to the 1980s, searched for the risk factors of this condition (3, 4, 9). The aim of our study was to determine possible risk factors for developing HBS after parathyroidectomy for primary hyperparathyroidism.
MATERIALS AND METHODS
Study population and outcomes
In this study we enrolled patients who underwent parathyroidectomy for PHPT at Vilnius University Hospital Santaros Klinikos during the 11-year period from January 2005 to December 2016 and performed a retrospective analysis. The final study cohort consisted of patients followed-up at the same hospital for at least 30 days and without any other PTH or calcium concentration anomalies other than those that define HBS (hypocalcaemia with normal or elevated PTH values). Basic demographic information such as sex and age was obtained. Preoperative symptoms and relevant conditions such as fatigue, osteoporosis, kidney stones, heart rate abnormalities, and neurasthenic symptoms were accounted for. Moreover, preoperative PTH and ionized calcium (iCa) values were collected. Furthermore, perioperative and postoperative data were gathered. Perioperative data included the length of surgery, type of operation, number of the parathyroid glands removed, and the change in the PTH value during the operation. Information about the removed parathyroid gland such as weight, volume, and final histology was carefully examined. The gland volume was calculated using the formula for an ellipsoid (V = 4/3 × πabc). If several parathyroid glands were operated on, only the heaviest gland was included into the analysis. Postoperative metabolic response was evaluated by testing PTH and iCa values. Laboratory values from 1.2 to 7.3 pmol/l for PTH and from 1.05 to 1.30 mmol/l for iCa were considered normal.
Statistical analysis
Patients were divided into two groups by the postoperative prevalence of HBS: patients with postoperative HBS and without postoperative HBS. Previously mentioned factors were analyzed between groups. The Mann-Whitney U test was used for continuous variables and the Fisher test for dichotomous variables. A ROC analysis was performed in order to determine the PTH cut-off value for 90% risk of developing HBS. Factors were statistically significant when the p value was less than 0.05. Statistical analysis was performed using IBM SPSS® version 21.
Results
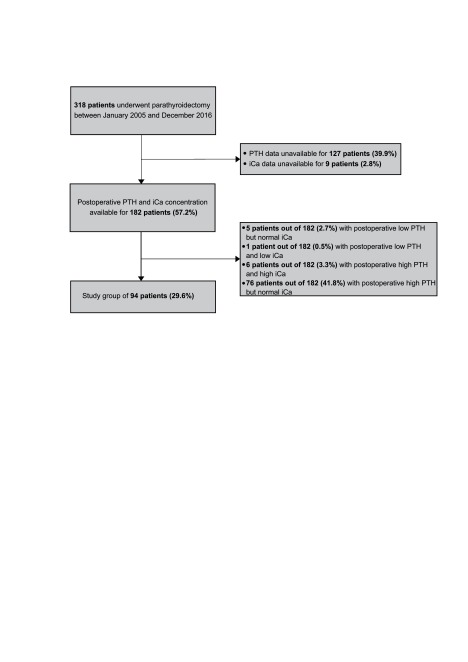
Of the 318 patients who underwent parathyroidectomy during our investigated time period, only 94 patients (29.6%) were included into the final analysis (Fig. 1). We did not have pre- or postoperative PTH or iCA data on almost half of the 318 patients. Of the 182 patients who had the necessary laboratory results, six (3.3%) had PHPT recurrence.
Table 1 contains the demographic and clinical data about the whole study cohort. In this table we also report the final histological results of the removed parathyroid gland. Twenty-seven patients (28.7%) had postoperative HBS and the overall HBS frequency between patients with available PTH and iCa values was 14.8%.
Table 1.
Demographic and clinical data
| Study cohort (N = 94) | |
|---|---|
| Patients’ age at the time of the operation (years) [mean ± SD] | 61.5 ± 12.2 |
| Sex [n (%)] | |
| Male | 14 (14.9) |
| Female | 80 (85.1) |
| Operating time (min) [mean ± SD] | 71.9 ± 40.4 |
| Hospital stay (days) [mean ± SD] | 3.6 ± 1.4 |
| Parathyroid weight (g) [median (range)] | 1 (0.15–33) |
| Parathyroid volume (cm3) [median (range)] | 4.5 (0.3–266.5) |
| Preoperative iCa value (mmol/l) [median (range)] | 1.4 (1.2-1.8) |
| Preoperative PTH value (pmol/l) [median (range)] | 18 (6.1–231.7) |
| Postoperative hungry bone syndrome [n (%)] | |
| Yes | 27 (28.7) |
| No | 67 (71.3) |
| Histology results | |
| Normal histology [n (%)] | 4 (4.3) |
| Hyperplasia [n (%)] | 25 (26.6) |
| Adenoma [n (%)] | 62 (66.0) |
| Carcinoma [n (%)] | 3 (3.1) |
In Table 2 we report possible postoperative HBS risk factors. Here we can see that younger patients tended to develop HBS more often (61.0 ± 11.5 vs. 52.9 ± 17.8 years; p = 0.045). None of the postoperative symptoms had a statistically significant difference between both groups, however, we observed a tendency that patients without HBS had osteoporosis preoperatively more often. Furthermore, the type of operation was significantly different between the two groups. Patients who underwent parathyroidectomies simultaneously with a thyroid surgery (parathyroidectomy + lobisthmectomy, or parathyroidectomy + total thyroidectomy) developed HBS more often. By analyzing other continuous variables, we discovered that patients with HBS underwent longer operations (73.9 ± 41.7 vs. 102.4 ± 44.8 minutes; p = 0.001). In addition, the removed parathyroid glands of patients with HBS were heavier (0.6 (0.3–8.0) vs. 0.8 (0.15–14.0) g; p = 0.041) and these patients had statistically significant higher preoperative PTH values (15.3 (6.1–63.7) vs. 22.4 (9.2–47.8) pmol/l; p = 0.003).
Table 2.
Risk factors of the hungry bone syndrome
| Variables | Patients without hungry bone syndrome (n = 67) | Patients with hungry bone syndrome (n = 27) | p value |
|---|---|---|---|
| Sex [n (%)] | 0.105 | ||
| Male | 7 (10.4) | 7 (25.9) | |
| Female | 60 (89.6) | 20 (74.1) | |
| Age at the time of the operation (years) [mean ± SD] | 61.0 ± 11.5 | 52.9 ± 17.8 | 0.045 |
| Preoperative symptoms and conditions [n (%)] | |||
| No symptoms | 21 (31.3) | 9 (33.3) | 0.851 |
| Fatigue | 16 (23.9) | 9 (33.3) | 0.348 |
| Osteoporosis | 21 (31.3) | 4 (14.8) | 0.101 |
| Kidney stones | 12 (17.9) | 3 (11.1) | 0.542 |
| Heart rate abnormalities | 9 (13.4) | 4 (14.8) | 1.000 |
| Neurasthenic symptoms | 9 (13.4) | 4 (14.8) | 1.000 |
| Type of operation [n (%)] | 0.040 | ||
| Traditional parathyroidectomy | 22 (32.8) | 6 (22.2) | |
| Focused parathyroidectomy | 34 (50.7) | 8 (29.6) | |
| Video-assisted parathyroidectomy | 1 (1.5) | 1 (3.7) | |
| Subtotal parathyroidectomy | 2 (3.0) | 1 (3.7) | |
| Parathyroidectomy + lobisthmectomy | 3 (4.5) | 6 (22.2) | |
| Parathyroidectomy + total thyroidectomy | 5 (7.5) | 5 (18.5) | |
| Number of removed parathyroid glands [n (%)] | 0.670 | ||
| 1 | 59 (88.0) | 24 (88.9) | |
| 2 | 3 (4.5) | 2 (7.4) | |
| 3 | 3 (4.5) | 0 | |
| 3.5 | 2 (3.0) | 1 (3.7) | |
| Operating time (min) [mean ± SD] | 73.9 ± 41.7 | 102.4 ± 44.8 | 0.001 |
| Hospital stay (days) [mean ± SD] | 3.7 ± 1.6 | 4.3 ± 1.5 | 0.039 |
| Parathyroid weight (g) [median (range)] | 0.6 (0.3–8.0) | 0.8 (0.15–14.0) | 0.041 |
| Parathyroid volume (cm3) [median (range)] | 4.4 (1.3–113.1) | 9.5 (0.3–25.9) | 0.722 |
| Preoperative iCa value (mmol/l) [median (range)] | 1.4 (1.2–1.8) | 1.4 (1.3–1.6) | 0.672 |
| Preoperative PTH value (pmol/l) [median (range)] | 15.3 (6.1–63.7) | 22.4 (9.2–47.8) | 0.003 |
| Change of intraoperative PTH values (%) [mean ± SD] | 81.2 ± 19.4 | 88.1 ± 8.8 | 0.906 |
Fig. 1.
Flow chart of the study population
Figure 2 presents the ROC curve of the preoperative PTH values and development of the hungry bone syndrome (AUC = 0.721 (95% CI 0.59–0.85); p = 0.003). From the statistical data that came with this curve, we determined that patients with a 45 pmol/l PTH cut-off value have a 90% tendency to develop postoperative HBS.
Fig. 2.
The ROC curve of the preoperative PTH values and development of the hungry bone syndrome
DISCUSSION
Postoperative HBS is one of the most common conditions following parathyroidectomy for PHPT. The prevalence of HBS in our study (14.8%) was very similar to the 13% reported in older high-volume studies (3–5). This contradicts the findings in the literature review by Witteveen et al., which claims that the prevalence has decreased during the last two decades (9). In our study, we found a correlation between younger age and HBS (61.0 ± 11.5 vs. 52.9 ± 17.8 years; p = 0.045), this only finding contradicts the results presented by Brasier et al. (4). Their study found that patients of older age had a greater risk in developing postoperative HBS. We did not find any literature nor could suggest a theory that would explain why younger patients developed HBS more often. Furthermore, the operating time had been significantly longer for patients who later developed HBS. This finding has not been described in any other article yet. In our opinion, it could be partially explained by the fact that patients with postoperative HBS underwent parathyroidectomies simultaneously with a thyroid surgery more often and these types of surgeries tend to be longer by their nature. During these simultaneous operations, the risk of traumatisation of other parathyroid gland is greater; it can lead to a partially decreased functional activity and the development of postoperative hypocalcaemia. In our study, we determined a statistically significant relation between heavier removed parathyroid glands and HBS, and these results coincided with the findings by Zamboni et al. (3) and Brasier et al. (4). Our most significant and clinically relevant finding is the difference of preoperative PTH between groups. This is important because a preoperative PTH value can help us predict the HBS development. We plotted a ROC curve and determined from the additional data that 90% of patients with a preoperative value of 45 pmol/l or more developed HBS during the postoperative period. Our study lacks data about other laboratory markers that represent bone metabolism, such as alkaline phosphatase and vitamin D. Several studies report that depleted vitamin D could be a risk factor for the HBS development (4, 10), other studies show that patients who later develop HBS have greater alkaline phosphatase levels (4, 11).
Currently there is no evidence for possible preoperative prevention options, although we could determine patients with a high risk of developing HBS preoperatively. As mentioned above, a preoperative deficiency of vitamin D has been established as a risk factor for HBS, however, the use of vitamin D supplements prior to surgery is still a topic of debate. Some guidelines advise the use of vitamin D supplements before the operation (12–14). Recently, several studies analysed the impact of vitamin D correction prior to parathyroidectomy, found that it had an insignificant impact on the outcomes, and suggested that correction of vitamin D deficiency should not delay the operation (15, 16). Moreover, theoretically the use of bisphosphonates could reduce the rate of HBS, because they inhibit osteoclasts and reduce bone demineralization. Several studies managed to use bisphosphonates preoperatively with success (1, 17, 18), but a lack of high quality randomized, controlled studies that would also support the use of bisphosphonates preoperatively remains the main drawback. The use of a calcimimetic agent cinacalcet has also been proposed. Although it is very efficient in treating patients with PHPT who cannot undergo a parathyroidectomy, no studies have been conducted with PHPT patients and the preoperative use of cinacalcet (19, 20). Wirowski et al. reports a study with patients who had secondary hyperparathyroidism and used cinacalcet preoperatively; he states that the patients’ perioperative course did not alter and the impact on postoperative hypocalcemia remains unclear (21).
CONCLUSIONS
Our study found that patients undergoing longer parathyroidectomies and patients with heavier removed parathyroid glands (>0.8 g) tend to develop HBS after the operation. Furthermore, we found that high preoperative PTH values indicate a greater chance to develop HBS postoperatively, and a value greater than 45 pmol/l determines a risk of over 90% of developing HBS. The use of preoperative HBS prevention needs further investigation.
Matas Jakubauskas, Virgilijus Beiša, Kęstutis Strupas
References
- Malabu UH, Founda MA. Primary hyperparathyroidism in Saudi Arabia: a review of 46 cases. Med J Malaysia. 2007; 62(5): 394. [PubMed] [Google Scholar]
- Bhansali A Masoodi SR Reddy KS Behera A Das Radotra B Mittal BR et al. . Primary hyperparathyroidism in north India: a description of 52 cases. Ann Saudi Med. 2005; 25(1): 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni WA, Folse R. Adenoma weight: a predictor of transient hypocalcemia after parathyroidectomy. Am J Surg. 1986. Dec; 152(6): 611–5. [DOI] [PubMed] [Google Scholar]
- Brasier AR, Nussbaum SR. Hungry bone syndrome: clinical and biochemical predictors of its occurrence after parathyroid surgery. Am J Med. 1988. April; 84(4): 654–60. [DOI] [PubMed] [Google Scholar]
- Anderberg B, Gillquist J, Larsson L, Lundström B. Complications to subtotal parathyroidectomy. Acta Chir Scand. 1981; 147(2): 109–13. [PubMed] [Google Scholar]
- Stankus N. Hungry bone syndrome. In: Lang F. editor. Encyclopedia of molecular mechanisms of disease. Springer Berlin Heidelberg; 2009. p. 865–6. [Google Scholar]
- Eriksen EF, Mosekilde L, Melsen F. Trabecular bone remodeling and balance in primary hyperparathyroidism. Bone. 1986; 7(3): 213–21. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The actions of parathyroid hormone on bone: Relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease: Part III of IV parts: PTH and osteoblasts, the relationship between bone turnover and bone loss, and the state of the bones in primary hyperparathyroidism. Metabolism. 1976; 25(9): 1033–69. [DOI] [PubMed] [Google Scholar]
- Witteveen JE, van Thiel S, Romijn JA, Hamdy NAT. Therapy of endocrine disease: Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol. 2013. February 20; 168(3): R45–53. [DOI] [PubMed] [Google Scholar]
- Graal MB, Wolffenbuttel BHR. Consequences of long-term hyperparathyroidism. Neth J Med. 1998; 53(1): 37–42. [DOI] [PubMed] [Google Scholar]
- Lee C-H, Tseng L-M, Chen J-Y, Hsiao H-Y, Yang A-H. Primary Hyperparathyroidism in Multiple Endocrine Neoplasia Type 1: Individualized Management With Low Recurrence Rates. Ann Surg Oncol. 2006. January; 13(1): 103–9. [DOI] [PubMed] [Google Scholar]
- Eastell R, Brandi ML, Costa AG, D’Amour P, Shoback DM, Thakker RV. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014. Oct; 99(10): 3570–9. [DOI] [PubMed] [Google Scholar]
- Marcocci C, Bollerslev J, Khan AA, Shoback DM. Medical management of primary hyperparathyroidism: proceedings of the Fourth International Workshop on the management of asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab. 2014. October; 99(10): 3607–18. [DOI] [PubMed] [Google Scholar]
- Bilezikian JP Brandi ML Eastell R Silverberg SJ Udelsman R Marcocci C et al. . Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014. Oct; 99(10): 3561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolighed L Rejnmark L Sikjaer T Heickendorff L Vestergaard P Mosekilde L et al. . No beneficial effects of vitamin D supplementation on muscle function or quality of life in primary hyperparathyroidism: results from a randomized controlled trial. Eur J Endocrinol. 2015. March 26; 172(5): 609–17. [DOI] [PubMed] [Google Scholar]
- Randle RW, Balentine CJ, Wendt E, Schneider DF, Chen H, Sippel RS. Should vitamin D deficiency be corrected before parathyroidectomy? J Surg Res. 2016. July; 204(1): 94–100. [DOI] [PubMed] [Google Scholar]
- Lee I-T, Sheu WH-H, Tu S-T, Kuo S-W, Pei D. Bisphosphonate pretreatment attenuates hungry bone syndrome postoperatively in subjects with primary hyperparathyroidism. J Bone Miner Metab. 2006. April 24; 24(3): 255–8. [DOI] [PubMed] [Google Scholar]
- Mayilvaganan S, Vijaya Sarathi HA, Shivaprasad C. Preoperative zoledronic acid therapy prevent hungry bone syndrome in patients with primary hyperparathyroidism. Indian J Endocrinol Metab. 2017; 21(1): 76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostoglou-Athanassiou I, Athanassiou P, Xanthakou E, Gkountouvas A, Kaldrymides P. AB0831 management of primary hyperparathyroidism with cinacalcet. Ann Rheum Dis Lond. 2014. June; 73: 1077. [Google Scholar]
- Peacock M Bolognese MA Borofsky M Scumpia S Sterling LR Cheng S et al. . Cinacalcet treatment of primary hyperparathyroidism: biochemical and bone densitometric outcomes in a five-year study. J Clin Endocrinol Metab. 2009. Dec; 94(12): 4860–7. [DOI] [PubMed] [Google Scholar]
- Wirowski D, Goretzki PE, Schwarz K, Lammers BJ. Cinacalcet effects on the perioperative course of patients with secondary hyperparathyroidism. Langenbecks Arch Surg. 2013. January; 398(1): 131–8. [DOI] [PubMed] [Google Scholar]