Abstract
Background.
Infantile haemangioma is the most common childhood vascular tumour, which causes great anxiety to parents and treating first-line physicians due to its proliferative nature. It accounts for a large percentage of a tertiary centre consultations, thus delaying consultation time for patients in need of immediate care.
Materials and methods.
Review of literature and experience of treatment and observation of infantile haemangiomas in a tertiary centre of paediatric surgery.
Results.
Based on the gathered information, we established an observation guideline of infantile haemangiomas for first-line physicians.
Conclusions.
First-line physicians must recognise the infantile haemangioma that requires immediate referral to a tertiary centre in order to prevent the appearance of associated complications. The remaining population of the patients of infantile haemangioma can be actively monitored once a month for at least a year by the treating pediatrician or family doctor. New and easy to use protocoled diagnostic tests such as thermography would greatly benefit first-line and tertiary-centre physicians in the follow-up of infantile haemangiomas.
Keywords: infantile haemangioma, observation, referral guideline, infrared thermography, conservative treatment
Abstract
ĮGIMTŲ HEMANGIOMŲ SEKIMO PROTOKOLAS PIRMINIO LYGIO LIGONINĖJE
Santrauka
Tikslas. Įgimta hemangioma (IH) yra dažniausias vaikų kraujagyslinis navikas. Dėl IH natūralios proliferacinės eigos tai sukelia didelį tėvų ir gydančių gydytojų nerimą. Viena dažniausių tretinio lygio chirurgų konsultacijų priežastis – IH. Daugumą sergančiųjų galėtų stebėti pirminio lygio specialistai, taip atsirastų daugiau galimybių pacientams su komplikuotos eigos IH patekti į IH gydymo centrą dar prieš pasireiškiant komplikacijoms.
Medžiaga ir metodai. Apžvelgti naujausi literatūros duomenys apie IH sekimą ir komplikacijas.
Rezultatai. Remiantis mūsų, tretinio lygio centro, gydymo ir IH sekimo patirtimi bei naujausios literatūros apžvalga, pristatome IH sekimo protokolą pirminio lygio specialistui.
Išvados. Pirminio lygio specialistai privalo atpažinti IH ir nustatyti, kam būtinas sekimas ar gydymas tretinio lygio ligoninėje, kad būtų laiku užkirstas kelias komplikacijoms. Likusieji IH atvejai gali būti prižiūrimi pirminio lygio specialisto. Pirmaisiais gyvenimo metais reikėtų tikrintis kartą per mėnesį. Nauji standartizuoti diagnostiniai tyrimas kaip termografija galėtų palengvinti IH sekimą tiek pirminiame, tiek tretiniame lygyje. Tačiau šio tyrimo standartizavimui ir sekimo protokolo algoritmo sukūrimui reikalingi papildomi tyrimai.
Raktažodžiai: įgimta hemangioma, stebėjimas, konsultacija
INTRODUCTION
Infantile haemangioma (IH) is the most common childhood vascular tumour. It can appear anywhere in the body but is most frequent in the head and neck region. Various methods of following and treating IHs have been reported, most selected individually based on the haemangioma and the treating doctor’s experience. Because of its natural proliferative course, IHs cause great anxiety to parents, thus constituting a large group of a tertiary surgery centre consultations. This causes long patient waiting times for a consultation, and, consequently, delays the time of the consultation for the patients whose treatment should be initiated immediately. Although complicated IHs should be treated in a tertiary hospital setting or at a haemangioma research centre, many IHs could be monitored by the treating primary centre physician. This article aims to review the course of IHs, possible complications, diagnostic tests reported in literature, and to present a short outline protocol for first-line physicians to follow, so that they could differentiate and refer the appropriate patients to tertiary centres.
MATERIALS AND METHODS
We reviewed the newest literature concerning IH observation, complications, and treatment. Based on the recommendations presented in literature and on our experience of treating IH in a tertiary centre, we established a protocol of IH observation for first-line physicians, which should help them direct only the risk-associated patients to an IH specialist before any complications arise.
LITERATURE REVIEW
IH prevalence differs between ethnic groups, but it is estimated to be around 4–5% in term neonates and 2.6–9.9% in children of up to one year of age (1–4). It is even more common among preterm babies. Its highest prevalence is among preterm babies under the weight of 2500 g (5). According to literature, girls have a slightly higher prevalence of IH, with a girl to boy ratio from 3:1 to 4:1 (2, 3, 6). In preterm babies this ratio changes to 1:1, most likely due to the fact that the majority of preterm babies are boys (7, 8).
Even though the aetiology and pathology of haemangiomas remain up for discussion, some risk factors have been identified. They are associated with Eurasian descent, prematurity, low birth rate, female sex polyhydramnion, mother’s age and pregnancy associated disorders such as preeclampsia, placenta previa, or vaginal bleeding (9).
In 1982, Mulliken and Glowacki published the first biological classification of congenital vascular tumours and malformations, in which two main groups are identified: haemangiomas and vascular malformations. This classification was later modified by the International Society for the Study of Vascular Anomalies (ISSVA), which also identified two groups, those of vascular malformations and vascular tumours. Haemangiomas are classified by their morphology, the affected surface area, and the growth phase, as summarized in Table 1. According to their morphology, haemangiomas are divided into three groups: superficial, deep, and compound. Depending on the affected surface area, they are divided into focal (67%), segmental (13%), undetermined (16.4%), and multifocal (3.6%) (3). Another classification of IHs depends on the stage of growth that the IH is in: proliferating IH, plateau, or involuting IH. This classification is most frequently used in clinical practice, on its own or in a combination with the classification based on the affected surface area, e.g., a proliferating focal IH.
Table 1.
Types and subtypes of infantile haemangioma classification based on morphology, affected surface area and growth phase, and their descriptive features
| Morphology | Features | Affected surface area | Features | Growth phase | Features |
|---|---|---|---|---|---|
| Superficial | A bulging out reddish discoloration of the skin | Focal | Solitary tumour | Proliferating | An increase in size is observed at consecutive visits |
| Deep | A soft tissue tumour, with a slight bluish discoloration of the skin | Segmental | Few segments fused and extending across an anatomical region | Plateau | Size stabilization/intensity of growth decrease observed at consecutive visits |
| Compound | Traits of both superficial and deep IH | Multifocal | Two or more separate focal haemangiomas in the same anatomical region | Involuting | Size or discoloration decrease observed at consecutive visits |
| Undetermined | Does not match any other of the group descriptions |
An IH can be differentiated from other vascular tumours and malformations by its clinical appearance and the course of disease. Congenital haemangiomas and vascular malformations are present at birth, whereas IHs usually appear within the first eight weeks of life as a discoloration of the skin or telangiectasis (10, 11).
Proliferation of the IH starts immediately after its appearance: a superficial IH increases in size, discolours the skin, bulges out. On palpation the surface is hard, non-pulsing. A deep IH presents as a soft tissue tumour, with a slight bluish discoloration of the skin. Compound IHs have traits of both superficial and deep IH. Eighty per cent of IHs reach their largest size by the third month of the patient’s life. From the fifth month they start to increase proportionally to the child (12). In rare cases, they can proliferate up to 24 months (10). At the end of their proliferative stage, 80% of IHs are 3 cm and less (3).
Proliferation is followed by a plateau phase, and at 6–12 months of age the involution of IH begins. During the involution, the IH flattens and its centre becomes paler. By the age of five years, around 50–60% of IHs will have involuted, however, this phase may take up to nine years, by which time 90% of all IHs have been reported to reach full involution (13). According to literature, in 50–70% of patients, even after the regression of the IH some skin damage is observed – teleangiectasis, fibrofatty tissue residues, pigmentation, skin texture abnormalities, or scarring (3, 10).
Every IH should be first approached by answering two main questions: whether the lesion appeared right after birth or the patient was born with it, and whether the lesion is increasing in size. If so, is it increasing proportionally to the child’s growth or is it growing faster, decreasing in size, or unchanging. A lesion presenting from birth, with proportional increase in size to the child is associated with vascular malformations, whereas the appearance of a vascular anomaly within eight weeks of birth and rapid growth are typical of IH. A haemangioma presenting from birth and decreasing in size is associated with a rapidly involuting congenital haemangioma (RICH), whereas a slight decrease of size/unchanging haemangioma is typical of a non-involuting congenital haemangioma (NICH), which requires a different therapeutical approach to RICH.
Most IHs can be diagnosed from their typical presentation and appearance, but some might require additional testing in order to confirm the diagnosis. Ultrasound or magnetic resonance are usually used in such cases. Orbital, nose region, or multiple haemangiomas also require additional specific testing due to associated complications. Thermography has been reported as another diagnostic tool used for the follow up of an IH during the course of treatment or active observation.
Even though around 80% of IHs regress spontaneously, they can cause psycho-emotional damage along with life-or function-threatening complications. According to Haggstrom et al., nearly a third of patients referred to tertiary paediatric surgery centres present with complications and require immediate treatment (14). The most frequent complications that require observation and treatment in a tertiary centre are summarized in Table 2. Ulceration of the IH is the most common complication, no matter the location of the lesion; however, it most frequently develops from anogenital, head and neck IHs. Life-threatening complications occur less often and are usually associated with the location of the IH, such as IH of the GI, airway, or the liver. Any IH of the frontal neck region should be referred to a tertiary centre, as it may be associated with an airway IH, which may cause airway obstruction during its proliferation. Periorbital, nasal, lip, or breast region IHs are associated with a loss of function (15), not to mention the associated pshycho-emotional damage. Morphology of IH can also help predict the risk of complications: segmental haemangiomas have an 11 times greater complication risk and require treatment eight times more often than focal haemangiomas (14). Five and more haemangiomas of at least two different regions require additional testing for liver IH, which may lead to cardiac failure, hepatomegaly, and even hypothyroidism (16, 17).
Table 2.
Possible complications of infantile haemangioma depending on the localization of the haemangioma
| Location | Possible complication | |
|---|---|---|
| Life-threatening conditions | Airway IH | Airway obstruction |
| Liver IH | Hepatomegaly Cardiac failure Hypothyroidism |
|
| Gastrointestinal IH | GI tract bleeding | |
| Loss of function | Periorbital region IH | Amblyopia |
| Auricular region | Hearing impairment | |
| Nasal IH | Obstruction of breathing, loss of smell, aesthetic problems | |
| Anogenital region | Loss of sensation due to ulceration | |
| Aesthetic, psychological problems | IH of the face | Ulceration, residues after involution |
| Breast IH | Loss of function, residues after involution |
Treatment of IH remains controversial and largely depends on the IH and the experience of the treating clinician. However, all clinicians agree that the best time for treatment is before any serious complications arise. In order to get the best results, treatment should be initiated early in the proliferation stage (15). Due to the anxiety of parents, many patients with low-risk IHs are referred to tertiary centres thus creating long waiting times for those patients who do need immediate treatment or close follow-up of experienced vascular anomaly surgeons. Based on the newest literature reviews, we present a protocol for referral of an IH patient to a tertiary centre in order to identify risk-associated IHs, treat them before any complications arise, and to minimize superfluous numbers of consultations.
The main goals of treatment are best described by Frieden (18):
Prevent the development of life-threatening complications;
Minimize the possibility of forming skin deformities after regression of an IH;
Reduce the stress and psychological damage of the patient and his/her parents;
Avoid aggressive treatment that can leave scarring or deformations of IH that could be treated conservatively;
Prevent the risk of ulceration of IH and ensure proper ulcerated IH treatment to reduce pain, infection, and scarring risk.
RESULTS
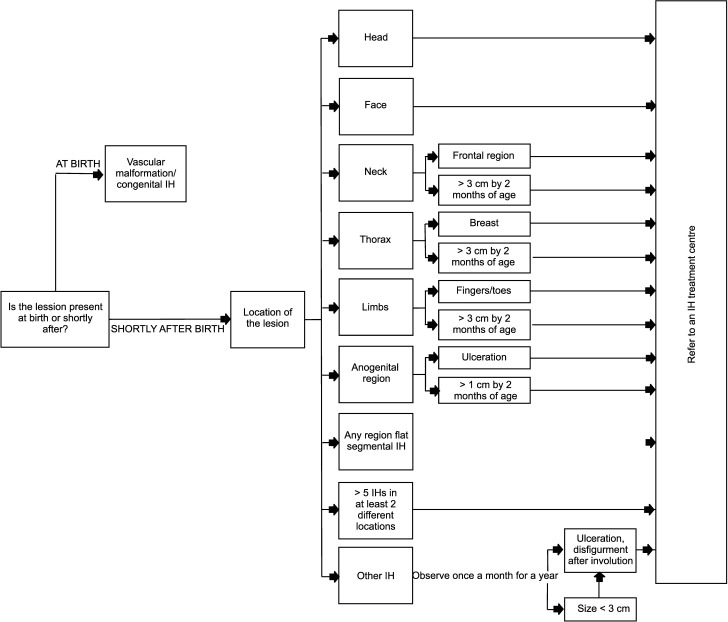
Many cases of IH do not fall into any of these risk-involved IHs and require active observation by a general practitioner or paediatrician. However, it is necessary to recognize the risk-associated IH before any complications occur. Children with risk factors of developing an IH must be identified and examined for an IH at least twice during the first eight weeks after birth. If present, active observation is crucial during the proliferative stage of an IH and a visit must be scheduled at least once per month for a year. Children with a higher risk of functional loss or ulceration should be monitored once every 1–2 weeks. It is necessary for the treating clinician to explain the risk of a developing complication to the parents very clearly as the course of a risk-associated IH can worsen very quickly during the proliferating stage. The treating clinician must teach the parents to recognize the symptoms requiring immediate care. Based on the literature review above, we present a proposal of an approach to an IH referral to a vascular anomaly treatment centre (Figure).
Figure.
Infantile haemangioma observation guidelines for first-line physicians
CONCLUSIONS
Infantile haemangioma is the most common childhood vascular tumour, which causes great anxiety to parents due to its proliferative nature. Although treatment greatly depends on the consulting surgeon, certain indications for treatment and risk factors of complication occurrence have been identified. The patients with the associated-risk factors should be identified early on and referred to appropriate centres. The remaining population of IH patients can usually be actively monitored by the treating paediatrician or family doctor once a month for at least a year. Even though here we presented an IH approach protocol, objective diagnostic methods of identifying risk-associated IH and following the course of an IH would benefit the primary centre doctor and the tertiary centre consultant. Certain methods are currently under investigation, but thermography of the IH is reported to be a safe, cheap, and useful tool for this purpose. However, protocols for establishing the use of thermography, indications for referral to a consultant, or IH treatment have not been presented yet and further research is required to establish them.
Arūnas Strumila, Rūta Vilija Dagilytė, Virgilijus Beiša
References
- Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008; 25(2): 168–73. [DOI] [PubMed] [Google Scholar]
- Hoornweg MJ, Smeulders MJ, Ubbink DT, van der Horst CM. The prevalence and risk factors of infantile haemangiomas: a case-control study in the Dutch population. Paediatr Perinat Epidemiol. 2012; 26(2): 156–62. [DOI] [PubMed] [Google Scholar]
- Darrow DH Greene AK Mancini AJ Nopper AJ Section On Dermatology SOO-H Neck S et al. . Diagnosis and Management of Infantile Hemangioma. Pediatrics. 2015; 136(4): e1060–104. [DOI] [PubMed] [Google Scholar]
- Goelz R, Poets CF. Incidence and treatment of infantile haemangioma in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2015; 100(1): F85–91. [DOI] [PubMed] [Google Scholar]
- Drolet BA, Swanson EA, Frieden IJ. Hemangioma Investigator Group. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008; 153(5): 712–5, 5 e1. [DOI] [PubMed] [Google Scholar]
- Hemangioma Investigator Group, Haggstrom AN Drolet BA Baselga E Chamlin SL Garzon MC et al. . Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr. 2007; 150(3): 291–4. [DOI] [PubMed] [Google Scholar]
- Amir J, Metzker A, Krikler R, Reisner SH. Strawberry hemangioma in preterm infants. Pediatr Dermatol. 1986; 3(4): 331–2. [DOI] [PubMed] [Google Scholar]
- Achauer BM, Chang CJ, Vander Kam VM. Management of hemangioma of infancy: review of 245 patients. Plast Reconstr Surg. 1997; 99(5): 1301–8. [DOI] [PubMed] [Google Scholar]
- Puttgen KB. Diagnosis and management of infantile hemangiomas. Pediatr Clin North Am. 2014; 61(2): 383–402. [DOI] [PubMed] [Google Scholar]
- Leaute-Labreze C, Prey S, Ezzedine K. Infantile haemangioma: part I. Pathophysiology, epidemiology, clinical features, life cycle and associated structural abnormalities. J Eur Acad Dermatol Venereol. 2011; 25(11): 1245–53. [DOI] [PubMed] [Google Scholar]
- Bauland CG, Luning TH, Smit JM, Zeebregts CJ. Spauwen PH. Untreated hemangiomas: growth pattern and residual lesions. Plast Reconstr Surg. 2011; 127(4): 1643–8. [DOI] [PubMed] [Google Scholar]
- Chang LC Haggstrom AN Drolet BA Baselga E Chamlin SL Garzon MC et al. . Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008; 122(2): 60–7. [DOI] [PubMed] [Google Scholar]
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014; 18(Suppl 1): S117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggstrom AN Drolet BA Baselga E Chamlin SL Garzon MC Horii KA et al. . Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006; 118(3): 882–7. [DOI] [PubMed] [Google Scholar]
- Leaute-Labreze C, Prey S, Ezzedine K. Infantile haemangioma: part II. Risks, complications and treatment. J Eur Acad Dermatol Venereol. 2011; 25(11): 1254–60. [DOI] [PubMed] [Google Scholar]
- Kulungowski AM, Alomari AI, Chawla A, Christison-Lagay ER, Fishman SJ. Lessons from a liver hemangioma registry: subtype classification. J Pediatr Surg. 2012; 47(1): 165–70. [DOI] [PubMed] [Google Scholar]
- Huang SA Tu HM Harney JW Venihaki M Butte AJ Kozakewich HP et al. . Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000; 343(3): 185–9. [DOI] [PubMed] [Google Scholar]
- Frieden IJ. Which hemangiomas to treat – and how? Archives of Dermatology. 1997; 133(12). [PubMed] [Google Scholar]