Abstract
Introduction.
Mixed epithelial and stromal tumour of the kidney (MEST) is a rare and distinctive neoplasm accounting for 0.2% of all renal cancers. Most of these tumours behave in a benign fashion but 13 cases with malignant transformation have already been reported. We present the first case of an extremely aggressive MEST with rapid recurrence after radical treatment, demonstrating objective response to chemotherapy.
Case presentation.
A 31-year-old female presented to the hospital complaining of gross hematuria. Computed tomography (CT) revealed an intraparenchymal mass in the left kidney forming a tumour thrombus in the inferior vena cava (IVC). Metastatic disease was ruled out and, under the clinical diagnosis of renal cell carcinoma, left radical nephrectomy with IVC thrombectomy was performed. The histopathological examination confirmed malignant MEST of the kidney. At the follow-up 12 months after surgery, a recurrent tumour in the left paravertebral area and a tumour thrombus in the IVC were detected. A second surgery was recommended and the mass from the paravertebral area was removed, so resection of the IVC with prosthetic replacement was performed. The histopathologic examination confirmed a recurrent malignant MEST. At the follow-up three months after the second surgery disease progression was diagnosed, so chemotherapy with ifosfamide and doxorubicin was initiated. The CT scan performed 14 months after the chemotherapy confirmed a stable process of the disease with no signs of progression.
Conclusions.
A literature review and our case report confirm the existence of extremely aggressive malignant MEST that shows response to chemotherapy. However, more reports are needed to improve our understanding about the biology of the MEST to develop any recommendations on personalized therapy.
Keywords: Kidney, mixed epithelial and stromal tumour, malignant, surgery, chemotherapy
Abstract
PIKTYBINIS MIŠRUS EPITELINIS IR STROMOS INKSTŲ NAVIKAS: KLINIKINIS ATVEJIS IR LITERATŪROS APŽVALGA
Santrauka
Įžanga. Mišrus epitelinis ir stromos inkstų navikas (MEST) – retas onkologinis susirgimas (sudaro 0,2 % visų inkstų vėžio susirgimų). Dažniausiai pastarieji priskiriami prie gerybinių inkstų navikų, tačiau literatūroje aprašyta 13 piktybinių MEST atvejų. Šiame straipsnyje pateikiame pirmą klinikinį atvejį, kai diagnozuotas ypač agresyvios elgsenos piktybinis MEST, po radikalaus gydymo parodęs ankstyvą ligos atkrytį ir progresavimą bei jautrumą sisteminiam gydymui.
Atvejo aprašymas. 31 metų moteris dėl intensyvios hematurijos kreipėsi į gydymo įstaigą. Diagnozei patikslinti buvo atlikta kompiuterinė tomografija, kuri parodė kairio inksto masyvų naviką su navikiniu trombu apatinėje tuščiojoje venoje. Nesant sisteminio ligos išplitimo duomenų, buvo pasirinktas radikalus operacinis gydymas – kairės pusės nefrektomija, kartu pašalinant trombą iš apatinės tuščiosios venos. Histopatologinis tyrimas nustatė piktybinį mišrų epitelinį ir stromos inksto naviką. Atliekant kontrolinius radiologinius tyrimus po operacinio gydymo praėjus 12 mėn., buvo nustatytas ligos recidyvas: navikinės masės kairėje paravertebralinėje srityje ir apatinėje tuščioje venoje. Nesant sisteminio ligos išplitimo duomenų buvo pasirinktas pakartotinas operacinis gydymas ir pašalintas navikinis židinys iš paravertebralinės srities bei dalis apatinės tuščiosios venos, kuri rekonstruota dirbtiniu protezu. Histopatologinis atsakymas patvirtino atsinaujinusį piktybinį MEST. Praėjus 3 mėn. po pakartotino operacinio gydymo buvo nustatyta tolimesnė ligos progresija, tad pradėtas sisteminis gydymas izofosfamidu ir doksorubicinu. Po sisteminio gydymo pabaigos praėjus 14 mėn. atlikta KT parodė stabilią ligą be naujų progresijos požymių.
Išvados. Literatūros apžvalga ir mūsų klinikinis atvejis patvirtina, kad egzistuoja ypač agresyvios eigos piktybiniai MEST, kurie galimai jautrūs chemoterapiniams vaistams. Be turimų žinių, būtina atskleisti daugiau klinikinių duomenų apie MEST. Ši informacija padėtų geriau suprasti naviko biologinę elgseną ir leistų formuoti individualias gydymo rekomendacijas.
Raktažodžiai: inkstai, piktybinis mišrus epitelinis ir stromos navikas, operacija, chemoterapija
INTRODUCTION
Mixed epithelial and stromal tumour (MEST) is a rare and distinctive kidney neoplasm that develops from Müllerian-like stromal cells and accounts for 0.2% of all renal cancers (1). The tumour was identified by Michal and Syrucek in 1998 and included in the WHO renal tumour classification as a separate entity in 2004 (2, 3). MEST is predominantly found in middle-aged perimenopausal women, with male to female ratio 1:10. Many of these patients have a history of long-term estrogen replacement therapy (4).
A comprehensive systematic search for material published before 1 September 2017 was conducted via PubMed. Mixed epithelial and stromal tumour, cystic hamartoma, mesoblastic nephroma, nephroblastic tumour, and cystic nephroblastoma were used as keywords. The search was limited to articles published in English. A little more than 100 cases of MEST were identified. Most of these tumours behaved in a benign fashion and only 13 cases with malignant transformation were reported: 11 cases in women and two cases in men (6–15). Current evidence suggests that the most common type of malignant MEST is undifferentiated sarcoma (7). In this paper, we present detailed clinical-pathological and radiological findings with short-term oncological outcomes of an extremely aggressive malignant MEST treated with surgery and palliative chemotherapy.
CASE PRESENTATION
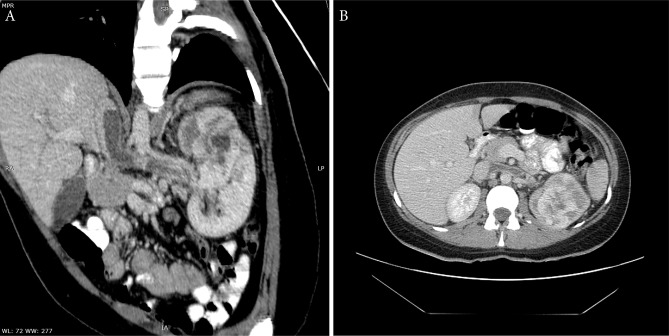
A 31-year-old female presented to the hospital complaining of gross hematuria. She had no significant medical history or family history of any malignancy, so no history of hormone therapy. Computed tomography (CT) revealed an intraparenchymal mass measuring 61 × 51 mm with focal strong inhomogenous enhancement and small cystic components in the upper part of the left kidney. The tumour was invading into the collecting system and forming a 55 × 13 mm tumour thrombus in the left renal vein and in the inferior vena cava (IVC) (Fig. 1). A metastatic disease was ruled out by a full-body CT scan and, under the clinical diagnosis of renal cell carcinoma (RCC), left radical nephrectomy with vena cava thrombectomy was performed.
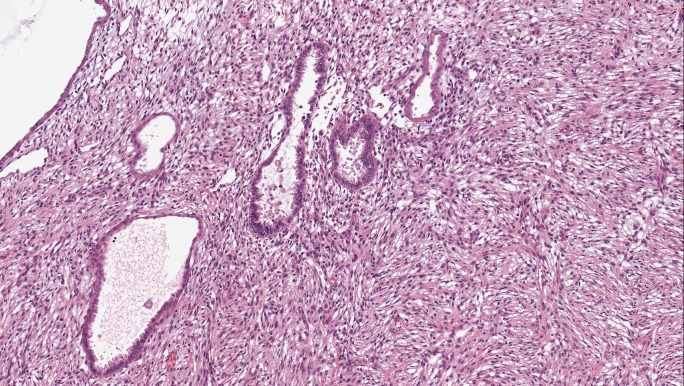
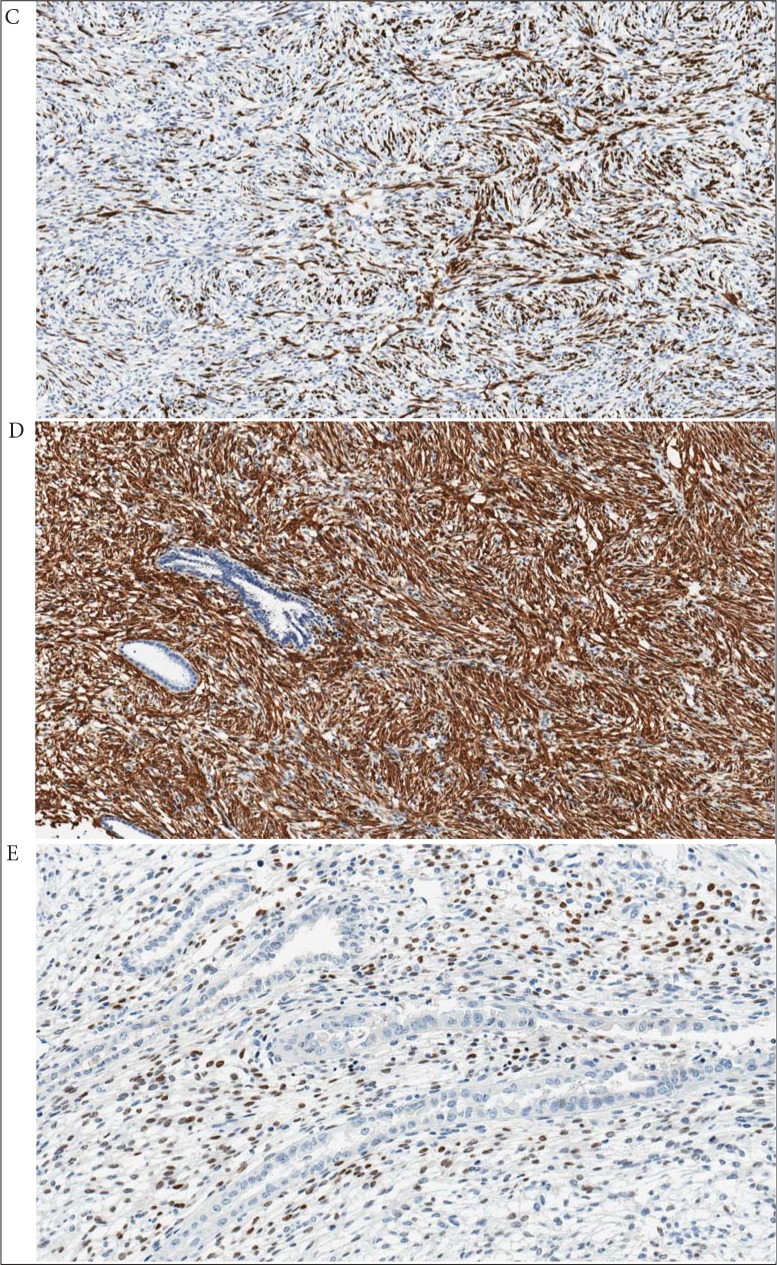
On histopathological examination, there was a 70 × 40 × 40 mm grey-brown mass within the left kidney with invasion into the left renal vein and forming a 40 × 25 × 20 mm tumour thrombus in the IVC. The tumour extended into the collecting system and renal hill adipose tissue. There were necrotic zones in the tumour which contained about 2% of the tumour volume. The tumour predominantly was composed of a solid mass and some small cystic components in the periphery (Fig. 2). Microscopically, the tumour had no capsule and was widely infiltrating renal parenchyma. The solid component was formed by spindle cells. Most of the spindle cells displayed eosinophilic cytoplasm with oval and minimally polymorphic nuclei. The mitotic activity was very low and reached a rate of 1–2 mitoses per 10 high-power fields. The cysts were lined by a single-cell layer of cuboidal ciliated cells. The epithelial cells were relatively uniform and showed no cytological atypia or mitosis. The renal parenchyma outside the tumour was unremarkable, the surgical margins of the specimen were clear. On immunohistochemistry, the stromal component showed strong and diffuse positivity for smooth muscle actin (100%), moderate to strong focal positivity for desmin (30%), H-caldesmon (15%), and progesterone receptors (10%), but was negative for melanin A, HMB45, keratins (PanCK, CAM5.2), p504S, RCC, TFE3, CD34, S-100, calretinin, inhibin B, GFAP, and oestrogen receptors (Fig. 3). The epithelial component was negative for CD10. Both histological and immunohistochemical features of the thrombus were similar to a primary tumour.
Fig. 1.
Oblique (A) and axial (B) CT views: a tumour with solid and cystic components in the left kidney. Filling defect in the left renal vein and vena cava inferior
Fig. 2.
Biological material from the first surgery. Histopathological examination: a tumour composed of stromal (spindle-shaped cells) and epithelial (medium-sized cystic and tubular structures) components (H&E, ×20)
The postoperative course was uneventful and at the first follow-up with a CT scan performed six months after surgery the patient was free of recurrence or metastases. The second follow-up with MRI was performed 12 months after surgery and revealed a huge 65 × 31 mm recurrent mass in the left paravertebral area with intraspinal masses epidurally at the level of L2-L3 and a recurrent 60 × 11 mm tumour thrombus in the IVC. Taking into account that there were no systemic metastases, a second surgery was recommended and the recurrent mass from the left paravertebral area was removed, so a resection of the IVC with a prosthetic replacement was performed. The histopathological findings confirmed recurrent MEST consisting only of malignant spindle cells with no epithelial component in the tumour. The immunohistochemistry of the recurrent stromal component showed features similar to a primary MEST. Although the second postoperative course was uneventful, the follow-up with CT three months after the second surgery revealed the progression of the disease: 14 mm and 23 mm recurrent masses in the left L2-L3 paravertebral area with multiple interaortocaval and left para-aortic lymphadenopathy up to 15 mm. To the best of our knowledge, there are no specific recommendations for systemic treatment of malignant MEST. According to the limited data in medical literature, chemotherapy with ifosfamide and doxorubicin was recommended (10). Six cycles were released of 1500 mg/m2 ifosfamide on days 1–4 and 30 mg/m2 doxorubicin on days 1–3 once every 21 days, with mesna cover. Fourteen months after systemic treatment, a follow-up with CT was performed, which confirmed a stable disease with no signs of progression.
Fig. 3.
Biological material from the first surgery. Immunohistochemical analysis: positive immunohistochemical reactions for desmin (C: ×10), smooth muscle actin (D: ×10), and progesterone receptors (E: ×20) in the stromal component
DISCUSSION
In a classic case, a MEST is biphasic and consists of mesenchymal and epithelial components. The mesenchyme element can vary from hypocellular sclerotic fibrous tissue to hypercellular proliferations of spindle cells with variable degrees of smooth muscle, collagen-associated fibroblastic or myofibroblastic differentiation. The epithelial component is usually scattered throughout the stroma and varies from regular tubules to more complex tubulopappilary structures with or without cystic dilatation (16, 17). In benign MEST, the mesenchymal component has a high expression of estrogen and progesterone receptors and resembles that of an ovarian stroma. A recent report by Turbiner et al. revealed 62% of estrogen receptor and 85% of progesterone receptor expression in the stromal component of benign MEST (18). Hormone receptors in combination with female predominance and long-term estrogen replacement therapy suggested a hormonal contribution to the pathogenesis of benign MEST. Although the expression of focal progesterone receptors has been described in malignant MEST, all cases of malignant MEST were negative for estrogen receptors, which supports the idea that estrogen hormonal milieu has no impact on the pathogenesis of malignancy (16). The hypothesis was confirmed by our immunohistochemistry data, also taking into account that the patient had no history of hormone therapy. Rhabdoid, rhabdomyosarcomatous, and chondrosarcomatous components may be seen in malignant MEST as well (6, 8, 15). Despite the hypothesis that stromal component could be the one to cause the malignant expression – which is strongly supported by our case – there are some controversial publications claiming that malignancy can be observed in either epithelial or mesenchymal parts (4, 13, 19). The latter idea was supported also by Nakagawa et al., who reported malignant MEST with no atypia in the epithelial element but with tubular structures in the extrarenally invading and recurrent tumour. It confirms the opinion that the epithelium component might be integral in the neoplastic process (8).
Expression of estrogen and progesterone receptors as such is not a diagnostic tool of MEST, and characteristic morphologic features should take precedence. To facilitate the diagnosis of malignant MEST, Jung et al. proposed diagnostic criteria to be fulfilled: (a) the epicentre of the tumour should be in the kidney; (b) clear-cut evidence of benign epithelial and stromal components with tubules or cysts lined by bland epithelial cells and a spindle-cell stroma resembling that of an ovarian-type stroma; (c) morphologically malignant components should be intimately associated with benign counterparts; and (d) primary renal sarcoma or metastases should be ruled out (6). Imaging studies in diagnosing MEST are not specific and a lack of typical radiological features makes it difficult to establish a precise diagnosis. On a CT scan, it typically manifests as a solid or solid-cystic renal mass with contrast material enhancement. The cystic component usually predominates and is associated with solid mural nodules, whereas MEST may mimic multilocular cystic RCC (16). Diagnosis of MEST by percutaneous biopsy may be particularly difficult because of sampling limitations. Malignant MEST should be considered in the case of characteristic epithelial elements with associated stromal cuffing and exclusion of the more common entities of renal neoplasms (12). The major differential diagnosis includes cystic nephroma, leiomyosarcoma, synovial sarcoma, and sarcomatoid renal cell carcinoma (4, 21).
Regardless of some papers reporting local and distant recurrences after initial treatment, the ability of malignant MEST to metastasize is not clear (8, 10, 13–15). According to our case, the rapid recurrence in the left paravertebral area supports intravascular dissemination through ascending lumbar veins as the most important pathway.
There are no established guidelines on the treatment of malignant MEST. Although nephron-sparing surgery is feasible for benign MEST, all cases with malignant transformations revealed a particularly aggressive course of disease and radical nephrectomy with extended lymphadenectomy had to be performed when malignancy was suspected (5, 17, 19, 20). There is just limited data about systemic treatment but some malignant MESTs seemed to be chemosensitive and responded to doxorubicin and ifosfamide (10). It was also confirmed by our case, which showed no progression of the disease for up to 14 months after the chemotherapy. According to some case reports, tumour spillage during primary surgery or positive surgical margins can be considered as risk factors for local recurrence and adjuvant chemotherapy or radiotherapy might be considered in selected cases (10).
CONCLUSIONS
This report demonstrates the existence of a rare malignant MEST of the kidney with an extremely aggressive behaviour. Despite the fact that two macroscopically radical resections were performed, the recurrence and progression of the disease within a very short time was observed. According to literature findings, some patients may survive recurrence-free for up to 36 months, whereas in the case of others the disease progresses rapidly with fatal outcomes. Currently, there are neither established treatment guidelines nor prognostic factors of this disease. Some limited data and our case report suggest malignant MEST to be chemosensitive, and palliative chemotherapy with doxorubicin and ifosfamide may be offered to the patients with a metastatic disease. However, it is still premature to give any recommendation and more reports are needed to improve our understanding of the biology of malignant MEST to develop any recommendations on personalized therapy.
Arnas Bakavičius, Marija Barisienė, Marius Snicorius, Dileta Valančienė, Darius Dasevičius, Algirdas Žalimas, Robertas Kvaščevičius, Henrikas Ramonas, Vitalijus Sokolovas, Feliksas Jankevičius
References
- Terao H Makiyama K Yanagisawa M Miyake M Sano F Kita K et al. . Mixed epithelial and stromal tumor of kidney: a case report. Hinyokika Kiyo. 2009; 55: 495–8. [PubMed] [Google Scholar]
- Michal M, Syrucek M. Benign mixed epithelial and stromal tumor of the kidney. Pathol Res Pract. 199; 8194: 445–8. [DOI] [PubMed] [Google Scholar]
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- Mohanty SK, Parwani AV. Mixed Epithelial and Stromal Tumors of the Kidney: An Overview. Arch Pathol Lab Med. 2009; 133: 1483–6. [DOI] [PubMed] [Google Scholar]
- Wang C, Lin Y, Xiang H, Fang D, Jiang P, Shen B. Mixed epithelial and stromal tumour of the kidney: report of eight cases and literature review. World J Surg Oncol. 2013; 11: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SJ Shen SS Tran T Jun SY Truong L Ayala AG et al. . Mixed epithelial and stromal tumor of kidney with malignant transformation: report of two cases and review of literature. Hum Pathol. 2008; 39: 463–8. [DOI] [PubMed] [Google Scholar]
- Suzuki T Hiragata S Hosaka K Oyama T Kuroda N Hes O Suzuki T Hiragata S Hosaka K et al. . Malignant mixed epithelial and stromal tumor of the kidney: report of the first male case. Int J Urol. 2013; 20: 448–50. [DOI] [PubMed] [Google Scholar]
- Nakagawa T Kanai Y Fujimoto H Kitamura H Furukawa H Maeda S et al. . Malignant mixed epithelial and stromal tumours of the kidney: a report of the first two cases with a fatal clinical outcome. Histopathology. 2004; 44: 302-4. [DOI] [PubMed] [Google Scholar]
- Svec A, Hes O, Michal M, Zachoval R. Malignant mixed epithelial and stromal tumor of the kidney. Virchows Arch. 2001; 439: 700–2. [DOI] [PubMed] [Google Scholar]
- Yap YS, Coleman M, Olver I. Aggressive mixed epithelial-stromal tumor of the kidney treated with chemotherapy and radiotherapy. Lancet Oncol. 2004; 5: 747–9. [DOI] [PubMed] [Google Scholar]
- Bisceglia M, Bacchi CE. Mixed epithelial-stromal tumor of the kidney in adults: two cases from the Arkadi M. Rywlin slide seminars. Adv Anat Pathol. 2003; 10: 223–33. [DOI] [PubMed] [Google Scholar]
- Sukov WR, Cheville JC, Lager DJ, Lewin JR, Sebo TJ, Lewin M. Malignant mixed epithelial and stromal tumor of the kidney with rhabdoid features: report of a case including immunohistochemical, molecular genetic studies and comparison to morphologically similar renal tumors. Hum Pathol. 2007; 38: 1432–7. [DOI] [PubMed] [Google Scholar]
- Kuroda N Sakaida N Kinoshita H Matsuda T Hes O Michal M et al. . Carcinosarcoma arising in mixed epithelial and stromal tumor of the kidney. APMIS. 2008; 116: 1013–5. [DOI] [PubMed] [Google Scholar]
- Zou L, Zhanq X, Xiang H. Malignant mixed epithelial and stromal tumor of the kidney: the second male case and review of literature. Int J Clin Exp Pathol. 2014; 7: 2658–63. [PMC free article] [PubMed] [Google Scholar]
- Menéndez CL Rodríguez VD Fernández-Pello S Venta Menéndez V Poch Arenas M Corrales B et al. . A new case of malignant mixed epithelial and stromal tumor of the kidney with rhabdomyosarcomatous transformation. Anal Quant Cytopathol Histpathol. 2012; 34: 331–4. [PubMed] [Google Scholar]
- Pierson CR Schober MS Wallis T Sarkar FH Sorensen PH Eble JN et al. . Mixed epithelial and stromal tumor of the kidney lacks the genetic alterations of cellular congenital mesoblastic nephroma. Hum Pathol. 2001; 32: 513–20. [DOI] [PubMed] [Google Scholar]
- Kamel MH, Davis R, Cox RM, Cole A, Eltahawy E. Enucleation/partial nephrectomy for large mixed epithelial stromal tumor and herniating into the pelvicalyceal system. Urol Ann. 2014; 6: 377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbiner J Amin MB Humphrey PA Srigley JR De Leval L Radhakrishnan A et al. . Cystic nephroma and mixed epithelial and stromal tumor of kidney: a detailed clinicopathologic analysis of 34 cases and proposal for renal epithelial and stromal tumor (REST) as a unifying term. Am J Surg Pathol. 2007; 31: 489–500. [DOI] [PubMed] [Google Scholar]
- Kalra S, Manikanda R, Dorairajan LN. Giant renal mixed epithelial and stromal tumour in a young female: a rare presentation. J Clin Diagn Res. 2015; 9: XD01–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg B Albiges L Bensalah K Bex A Giles RH Hora M et al. . European Association of Urology; Guidelines on Renal Cell Carcinoma 2018. [Google Scholar]
- Adsay NV, Eble JN, Srigley JR, Jones EC, Grignon DJ. Mixed epithelial and stromal tumor of the kidney. Am J Surg Pathol. 2000; 24: 958–70. [DOI] [PubMed] [Google Scholar]