(
A) The mosquito-mouse model system. Five female (TO) mice (6–8 weeks old, Harlan, UK) were treated with phenylhydrazine, and, 3 days later, were infected with 10
6
P. berghei PbPfs25DR3 (
Goodman et al., 2011). Three days later, groups of infected mice were treated with the TBV antibody. After 1 hr, the mice were anaesthetised and 500
An. stephensi mosquitoes (line SD 500, starved for 24 hr) fed randomly on the five infected mice within each group. Mosquitoes were maintained as described in (
Blagborough et al., 2013). After 10 days, a sub-sample of 50 mosquitoes were microscopically examined to measure oocyst intensity and prevalence. After 21 days post-feeding, sporozoites are present in the salivary glands and are maximally infectious to the vertebrate host (
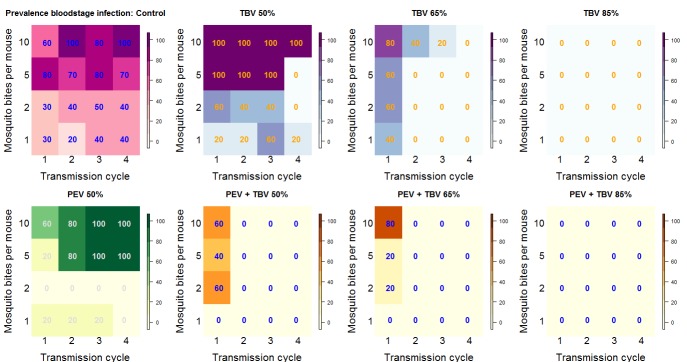
Blagborough et al., 2013). At this point, pre-defined numbers of mosquitoes (to simulate mosquito biting rates of 1, 2, 5 and 10 mosquito bites per mouse) were then randomly selected from the remaining mosquitoes and fed, for 20 min, on anesthetized mice from a naive cohort. Each group of mice (five mice per group) either received the PEV antibody or no -intervention (negative control). Engorged mosquitoes were microscopically examined immediately after feeding to determine the number of sporozoites in the salivary glands. After 10 days, blood smears from each mouse were microscopically examined to determine the percentage parasitemia. These five mice were then given either the TBV antibody at the desired dose to achieve a 50%, 65% or 85% reduction in oocyst prevalence as required, or no intervention/control (in accordance with the respective treatment arm). A new cohort of 500 naive mosquitoes was then allowed to blood feed on the mice. This mouse-to-mouse transmission cycle was repeated to a maximum of four cycles after the seeding mouse population or until no parasites had been detected in the system for two successive transmission cycles. (
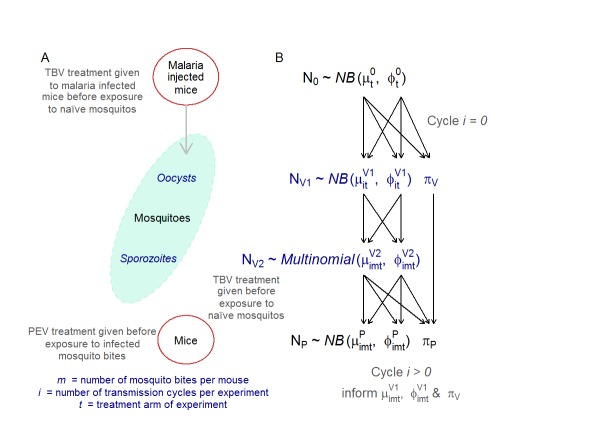
B) The statistical model mirrored the experimental set up. The initial parasite density generated by injecting mice
N0, the oocyst intensity
O’ and the parasite density in mice transmitted by mosquito bites
N, are modelled assuming zero-inflated negative binomial distributions. The sporozoite count
S data for the biting mosquitoes were censored which means that the data are modelled as a multinomial distribution. These distributions are defined by the mean (
µ,
o,
s for parasite density in mice, oocyst counts and sporozoite counts in mosquitoes) and dispersion (
φ, τ, σ for parasite density in mice, oocyst counts and sporozoite counts in mosquitoes) and zero-inflation (
πP, πV) parameters. Parameters from the previous life stage are used to inform the next (the respective parameter informing the subsequent life-stage is indicated by the arrows). The biting effect
m is modelled when sporozoites in mosquitoes propagate parasite infections in mice for each transmission cycle
i and treatment arm
t. All care and handling of animals strictly followed the Guidelines for Animal Care and Use prepared by Imperial College London and was performed under the UK Home Office Licence 70/7185. Supplementary information
Table 1—source data 1 (separate excel file). Data file of the raw data for parasite densities in mice and mosquitoes.