Abstract
The heteromeric steroid transporter organic solute transporter α/β (OSTα/β, SLC51A/B) was discovered over a decade ago, but its physiological significance in the liver remains uncertain. A major challenge has been the lack of suitable models expressing OSTα/β. Based on observations first reported here that hepatic OSTα/β is upregulated in nonalcoholic steatohepatitis, the aim of this research was to develop an in vitro model to evaluate OSTα/β function and interaction with drugs and bile acids. OSTα/β expression in human liver tissue was analyzed by quantitative RT-PCR, Western blotting, and immunofluorescence. Radiolabeled compounds were used to determine OSTα/β-mediated transport in the established in vitro model. The effect of bile acids and drugs, including those associated with cholestatic drug-induced liver injury, on OSTα/β-mediated transport was evaluated. Expression of OSTα/β was elevated in the liver of patients with nonalcoholic steatohepatitis and primary biliary cholangitis, whereas hepatocyte expression of OSTα/β was low in control liver tissue. Studies in the novel cell-based system showed rapid and linear OSTα/β-mediated transport for all tested compounds: dehydroepiandrosterone sulfate, digoxin, estrone sulfate, and taurocholate. The interaction study with 26 compounds revealed novel OSTα/β inhibitors: a biomarker for cholestasis, glycochenodeoxycholic acid; the major metabolite of troglitazone, troglitazone sulfate; and a macrocyclic antibiotic, fidaxomicin. Additionally, some drugs (e.g., digoxin) consistently stimulated taurocholate uptake in OSTα/β-overexpressing cells. Our findings demonstrate that OSTα/β is an important transporter in liver disease and imply a role for this transporter in bile acid-bile acid and drug-bile acid interactions, as well as cholestatic drug-induced liver injury.
NEW & NOTEWORTHY The organic solute transporter OSTα/β is highly expressed in hepatocytes of liver tissue obtained from patients with nonalcoholic steatohepatitis and primary biliary cholangitis. OSTα/β substrates exhibit rapid, linear, and concentration-driven transport in an OSTα/β-overexpressing cell line. Drugs associated with hepatotoxicity modulate OSTα/β-mediated taurocholate transport. These data suggest that hepatic OSTα/β plays an essential role in patients with cholestasis and may have important clinical implications for bile acid and drug disposition.
Keywords: basolateral transport, bile acid, cholestasis, drug-induced liver injury, NASH
INTRODUCTION
The basolateral membrane of hepatocytes contains several well-known proteins that transport substrates (e.g., endogenous compounds and drugs) from sinusoidal blood into and/or out of hepatocytes, e.g., organic anion-transporting polypeptides (OATPs, SLCO), sodium-taurocholate cotransporting polypeptide (NTCP, SLC10A1), and multidrug resistance-associated proteins 3 (MRP3, ABCC3) and 4 (MRP4, ABCC4). Organic solute transporter α/β (OSTα/β, SLC51A/B) is a less well known, but highly inducible, basolateral transporter. This heteromeric transport protein consisting of two subunits, OSTα (SLC51A) and OSTβ (SLC51B), was discovered over a decade ago (59), but its physiological significance remains uncertain. Notably, patients with obstructive cholestasis and primary biliary cholangitis (PBC) show significantly increased OSTα/β levels in the liver (7, 10), suggesting that OSTα/β may act as an efflux transporter and provide an alternative bile acid excretion pathway, together with MRP3 and MRP4. Unlike ATP-binding cassette (ABC) transporters, OSTα/β facilitates passive transport by moving substrates down their concentration gradient. OSTα/β transport activity and direction depend on the electrochemical gradient of the substrate (4) and, possibly, the number of transport proteins at the membrane, as demonstrated for another facilitated diffusion transporter (18).
Information regarding substrate selectivity and inhibitors of OSTα/β is sparse. In addition to bile acids, steroid hormones, such as estrone sulfate (E1S), dehydroepiandrosterone sulfate (DHEAS), pregnenolone sulfate, and digoxin (DIG), are known OSTα/β substrates (4, 20, 54). Some bile acids, steroid sulfates, and organic anions are also partial OSTα/β inhibitors (54, 59). Clearly, more information is needed to understand the potential involvement of OSTα/β in drug therapies, drug interactions, drug-induced liver injury (DILI), and diseases. The lack of suitable models has hampered investigations of OSTα/β. Rodents under normal conditions do not express OSTα and OSTβ proteins in the liver at measurable levels (4), and the in vitro models have been transient-expression systems (4, 20, 54, 59). Reproducible, higher-throughput studies require a stable system overexpressing human OSTα/β.
In the present set of experiments, the hypothesis that OSTα/β is induced in the liver of patients with nonalcoholic steatohepatitis (NASH), an advanced stage of nonalcoholic fatty liver disease (NAFLD), was tested. The recent discovery that bile acid concentrations are increased in the serum and liver of patients with NASH (22, 37) supports the finding that OSTα/β is induced in patients with NASH. In vitro data also support this hypothesis, because both hypoxia and elevated bile acid concentrations, conditions similar to patients with NASH (37, 46), induced OSTα and OSTβ expression (52). OSTα/β induction is mediated through the bile acid-activated farnesoid X receptor (FXR, NR1H4) pathway (7, 65). Because NAFLD and NASH are rapidly emerging liver diseases affecting ∼40% of the population in the United States and Europe, it is particularly important to understand the underlying molecular mechanisms of the disease (19, 49). Based on our findings described here that the expression and localization of OSTα/β were altered in the liver of NASH patients and patients with PBC, an in vitro model was developed and employed to investigate the concentration and time dependence of OSTα/β transport and to identify OSTα/β substrates and inhibitors.
MATERIALS AND METHODS
Compounds.
[3H]DHEAS (60 Ci/mmol, radiochemical purity >97%), [3H]DIG (39.8 Ci/mmol, radiochemical purity >97%), [3H]estradiol-17β-d-glucuronide (34.3 Ci/mmol, radiochemical purity >97%), [3H]E1S (45.6 Ci/mmol, radiochemical purity >97%), and [3H]taurocholic acid (TCA, 9.7–15.4 Ci/mmol, radiochemical purity >97%) were purchased from PerkinElmer Life Sciences (Boston, MA). A total of 26 compounds, which were obtained from Sigma-Aldrich (St. Louis, MO), Fisher Scientific (Fair Lawn, NJ), Toronto Research Chemicals, and Cayman Chemical (Ann Arbor, MI), were analyzed in the OSTα/β inhibition assay. Cyclosporin A was obtained from Calbiochem EMD Millipore (Darmstadt, Germany), and felodipin was purchased from Tokyo Chemical Industry (Tokyo, Japan). Compounds, except for ciprofloxacin, were dissolved in dimethyl sulfoxide (DMSO); ciprofloxacin was dissolved in 0.1 M hydrochloric acid.
Histological liver samples.
Tissues were obtained through the Liver Tissue Cell Distribution System (Minneapolis, MN, and Pittsburgh, PA). NASH and PBC samples were obtained from patients with end-stage cirrhosis undergoing liver transplant surgery. Control liver tissues that had normal histology were obtained from cancer patients with liver metastasis in the margins. All the tissue sections were fixed in formalin, embedded in paraffin, and sectioned in 4-μm slices. Clinical and demographic information about these human liver samples is shown in Table 1. Each specimen had been evaluated by the Liver Tissue Cell Distribution System medical pathologist to ensure appropriate and consistent diagnoses.
Table 1.
Demographic data for human liver donors
| Sex, M/F | Age, yr | Race | BMI, kg/m2 | Diagnosis | Medications | Ballooning of Hepatocytes | Fibrosis | |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| Subject 1 | M | 48 | White | Unknown | No pathological abnormality, colonic adenocarcinoma with liver metastasis | Not available | No | No |
| Subject 2 | M | 56 | White | 24 | No pathological abnormality, colonic adenocarcinoma with liver metastasis | Acetaminophen, lisinopril, ranitidine, ropinivole | No | No |
| Subject 3 | F | 51 | White | 29 | No pathological abnormality, colonic adenocarcinoma with liver metastasis | Amoxicillin, calcium, multivitamin | No | No |
| NASH | ||||||||
| Patient 1 | M | 53 | White | 30 | Fatty liver, NASH, cirrhosis, steatosis, diabetes | Acetylsalicylic acid, albuterol, atenolol, fluticasone, furosemide, lactulose, levofloxacin, phytonadione, potassium, rifaximin, omeprazole, spironolactone, tramadol, ursodiol, zinc | Yes | Yes |
| Patient 2 | M | 64 | White | Unknown (290 lbs) | Fatty liver, cirrhosis | Acetaminophen, benzocaine, cetirizine, ciprofloxacin, fluoxetine, furosemide, lactulose, multivitamin, ofloxacin, oxycodone, oxymetazoline, pantoprazole, pramipexole, rifaximin, simethicone, spironolactone | Yes | Yes |
| Patient 3 | F | 48 | White | 40.6 | Fatty liver, NASH, cirrhosis | Amoxicillin, esomeprazole, furosemide, potassium, propranolol, spironolactone, rifaximin, T3 liothyronine, T4 levothyroxine, zinc | Yes | Yes |
| PBC | ||||||||
| Patient 1 | M | 65 | White | 26.8 | Cirrhosis, primary biliary | Cyclobenzaprine, multivitamin, nadolol, omeprazole, simvastatin, spironolactone, trazodone, ursodiol, zinc | No | Yes |
| Patient 2 | M | 67 | White | Unknown | Cirrhosis, primary biliary | Not available | No | Yes |
| Patient 3 | F | 64 | White | 29.2 | Cirrhosis, primary biliary | Calcium, furosemide, lactulose, potassium, rifaximin, ursodiol, zinc | No | Yes |
Liver tissue from control, nonalcoholic steatohepatitis (NASH), and primary biliary cholangitis (PBC) patients was obtained from the Liver Tissue Cell Distribution System. Diagnosis was ensured by the facility pathologist. BMI, body mass index.
Human hepatocytes.
Cryopreserved human hepatocytes, induction-qualified (lot HUM4040), were purchased from Lonza (Durham, NC) and cultured for 5 days in a sandwich configuration with a few modifications to the previously described protocol (32). Briefly, sandwich-cultured human hepatocytes (SCHH) were prepared from cryopreserved hepatocytes suspended in QualGro seeding medium (Qualyst Transporter Solutions, Durham, NC) that were plated at a density of 0.5 × 106 viable cells per well onto Corning BioCoat collagen I-coated 24-well cell culture plates. After 24 h, cells were overlaid with QualGro hepatocyte overlay medium supplemented with 0.25 mg/ml Matrigel (Corning, NY). Cells were fed QualGro hepatocyte culture medium daily. After 4 days of culture, SCHH were treated with a primary bile acid, chenodeoxycholic acid (CDCA, 100 μM), in dosing solution to model the elevated bile acid levels in cholestasis. Dosing solutions were prepared fresh from DMSO stock solution (1,000× final concentration) in QualGro hepatocyte culture induction medium. Negative control cultures were treated with vehicle (0.1% DMSO). At 24 h after dosing, OSTα and OSTβ gene expression was determined.
DNA constructs.
The human SLC51A (NM_152672.5, OSTα) and human SLC51B (NM_178859.3, OSTβ) genes containing Flp-In expression vector pcDNA5.1/FRT were generated by Invitrogen GeneArt Gene Synthesis (Thermo Fisher Scientific, Waltham, MA). The OSTα and OSTβ sequences encoded the variants most common in the general population (NP_689885.4 and NP_849190.2, respectively). The Flp recombinase expression plasmid pOG44 was purchased from Invitrogen. Dh5α-competent Escherichia coli cells (Invitrogen) were propagated with 5–10 ng of pcDNA5.1/FRT/OSTα-OSTβ construct, empty pcDNA5.1/FRT vector, or pOG44 vector in superoptimal broth (S.O.C., Invitrogen) according to the manufacturer’s protocol. The Dh5α cell colonies were selected and transferred in 3 ml of Luria-Bertani medium containing 200 µg/ml ampicillin and cultured overnight in an orbital shaker (300 rpm) at 37°C. Plasmid DNA was precipitated and purified using buffers from the PureLink HiPure Plasmid Filter Midiprep Kit (Invitrogen), 3 M sodium acetate (Ambion, Thermo Fisher Scientific), and 99% ethanol. The presence of the correct construct was confirmed by DNA sequencing (Eurofins Genomics, Louisville, KY) and by restriction digestion and agarose gel electrophoresis. The clones carrying pcDNA5.1/FRT/OSTα-OSTβ, pcDNA5.1/FRT, or pOG44 (Invitrogen) were further amplified in Dh5α in 25 ml of Luria-Bertani medium and then purified with the PureLink HiPure Plasmid Filter Midiprep Kit. Prior to the transfection, the constructs were further verified by DNA sequencing.
Establishment of a stable human OSTα- and OSTβ-expressing cell line.
The human embryonic kidney 293 (HEK-293) cell line was selected because it is well characterized, easy to maintain, and shows very low endogenous transporter expression (2). The Flp-In 293 cell line, together with the Flp-In vector system (Invitrogen), enables stable and targeted gene transfection in a transcriptionally active locus and has been successful in the generation of OATP-overexpressing cell lines (33, 34, 43). The Flp-In 293 cells were grown in maintenance medium [Dulbecco’s modified Eagle’s high-glucose medium (catalog no. D11960-044, Life Technologies, Grand Island, NY)] containing 10% fetal bovine serum (Life Technologies), 2 mM l-glutamine (Life Technologies), and 100 U/ml penicillin-100 μg/ml streptomycin (Life Technologies) supplemented with 100 μg/ml Zeocin (Life Technologies). Cells were cultured at 37°C and 5% CO2 in an incubator.
To obtain Flp-In 293 cells stably expressing human OSTα and OSTβ and the control cell line, cells grown for 24 h in a 24-well plate (60% confluence, maintenance medium) were cotransfected with 80 ng of pcDNA5.1/FRT/hOSTα-hOSTβ plasmid and 720 ng of pOG44 plasmid or 80 ng of empty pcDNA5.1/FRT plasmid (Mock) and 720 ng of pOG44 plasmid using Lipofectamine 2000 transfection reagent (Invitrogen) and OptiMem (Invitrogen), as recommended by the manufacturer. Briefly, 2 µl of Lipofectamine were diluted with 48 µl of OptiMem and mixed with the DNA vectors diluted in OptiMem (50 µl). After a 20-min incubation, the Flp-In 293 cells were dosed with 100 μl of plasmid DNA-Lipofectamine mixture and incubated overnight at 37°C in 5% CO2. After 48 h of transfection, each well was subcultured with 0.25% trypsin-EDTA and transferred to a single well in a 6- or 12-well plate. Maintenance medium containing selection agent, hygromycin B (100 μg/ml; Invitrogen), was added to the cells 72 h after transfection. After 10–14 days, the single cell foci were numbered, and each was transferred into a single well in a 24- or 48-well plate with the aid of cloning rings, autoclaved silicone, and 0.25% trypsin-EDTA. At 90% confluence, the cell cultures were subcultured into T25 flasks, chamber slides, and well plates to expand the clones and analyze protein and gene expression of OSTα/β, respectively.
The established cell lines stably expressing hOSTα and hOSTβ were named OSTab, and the cells transfected with empty pcDNA5.1/FRT vector were named Mock cells. The new cell lines were cultivated in Dulbecco’s modified Eagle’s high-glucose medium (catalog no. D1190-044, Invitrogen) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 2 mM l-glutamine without selection compounds. The cells were subcultured once every week, and passages 5–20 were used in the study. Cell viability was determined by Trypan blue exclusion.
Quantitative RT-PCR analysis of OSTα and OSTβ expression.
Total RNA from OSTab, Mock, and wild-type Flp-ln293 cells, SCHH, and human liver samples was isolated using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer's instructions. RNA pellets were dissolved in nuclease-free water (GIBCO, Invitrogen) and stored at −80°C. The concentration and purity of isolated RNA were analyzed with a NanoDrop spectrophotometer (model no. ND-1000, Thermo Scientific). RNA was converted to cDNA, which was analyzed as described previously (32). Briefly, reverse transcription was performed using the High Capacity cDNA Archive Kit, following the manufacturer’s instructions (Life Technologies). SLC51A (OSTα) and SLC51B (OSTβ) cDNA were analyzed from each sample using gene-specific TaqMan assays (Hs00380895 and Hs01057182, Thermo Fisher Scientific) and a real-time PCR system (Thermo Fisher Scientific). The expression level of the studied genes was normalized against the selected reference gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the relative quantification (2ΔΔCt) was calculated against the calibrator sample: cDNA from Flp-ln-293 (wild-type) for the cell culture samples and cDNA from control livers for the human liver tissue samples. No-template control from reverse transcription was present on each PCR plate.
Western blot analysis.
Membrane proteins from human liver samples were extracted using a ProteoExtract native membrane protein extraction kit (Calbiochem, San Diego, CA). Samples for Western blots were prepared according to the manufacturer’s instructions (Invitrogen), and 25 μg were loaded onto Bis-Tris gels. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes according to standard procedures. The membranes were incubated with anti-OSTα antibody (1:250 dilution; catalog no. ab103442, Abcam, Cambridge, MA) and anti-OSTβ antibody (1:150 dilution; catalog no. HPA008533, Sigma-Aldrich) overnight in diluted 5% (wt/vol) bovine serum albumin-Tris-buffered saline with Tween 20. After they were washed, the blots were probed with horseradish peroxidase-conjugated anti-rabbit antibody for OSTα and OSTβ (1:7,000 dilution; Santa Cruz Biotechnology, Dallas, TX) and horseradish peroxidase-conjugated anti-mouse IgG secondary antibody for Na+-K+-ATPase-α (H-3) (1:10,000 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA) in 5% milk for 1 h (OSTα and Na+-K+-ATPase) and 2 h (OSTβ) at room temperature. Detection was enhanced using chemiluminescence reagents (ECL Select, GE Healthcare Bio-Sciences, Piscataway, NJ). Na+-K+-ATPase (1:5,000 dilution, 1 h, room temperature; Santa Cruz Biotechnology) was used as the loading control. The data were normalized to loading control using densitometric analysis to compare protein expression between NASH, PBC, and control liver tissue with ImageLab software version 4.1 (Bio-Rad, Hercules, CA). ImageJ was used to quantify the bands (53).
Immunocytochemistry and confocal microscopy of OSTα and OSTβ 293 cells.
Immunocytochemical staining was performed as described previously (42) with minor modifications. The cells were cultured in chamber slides (Sarstedt, Newton, NC). After fixation, permeabilization, and blocking, the cells were incubated overnight with primary antibody, anti-OSTα (Abcam), or anti-OSTβ (Sigma-Aldrich) diluted 1:50 in PBS containing 5% FBS and 0.2% bovine serum albumin. Alexa 488-labeled secondary antibody was diluted 1:200 and incubated for 1 h with the cells. After the cells were washed, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) diluted in PBS (Invitrogen). Stained cultures were mounted in ProLong Gold antifade reagent (Life Technologies) and analyzed using a Zeiss LSM 710 confocal laser scanning system with an inverted confocal laser scanning microscope and a ×40/1.30 Neo Plan oil objective. Images were acquired via three channels excited sequentially with the laser diode at 405 nm (to visualize nuclei), at 488 nm (to visualize Alexa 488), and at 594 nm (to visualize Alexa 594). Emission was collected at wavelengths of 410-495, 494-592, and 595-734 nm, respectively. Image files were processed with ImageJ (53). No deconvolution was applied.
Immunofluorescence, imaging, and digital image analysis.
Before immunofluorescence (IF) of liver tissues, the function and specificity of anti-OSTα (Abcam) and anti-OSTβ (Sigma-Aldrich) antibodies were tested with a multitissue human microarray containing kidney cortex, kidney medulla, appendix, lung, colon, pancreas, and liver (Translational Pathology Laboratory, University of North Carolina, Chapel Hill, NC). Antibody dilution and a dual-color IF protocol for anti-OSTα and anti-OSTβ were optimized first with human small bowel sections, which functioned as a positive control due to high endogenous OSTα and OSTβ expression, and then with human liver sections. Dual-color IF staining for OSTα and OSTβ was performed using the Bond-III fully automated staining system (Leica Biosystems, Norwell, MA). Tissue slides were deparaffinized in Bond dewax solution and hydrated in Bond wash solution. Epitope retrieval for both antibodies was conducted at 100°C for 20 min in Bond epitope retrieval solution 1 (pH 6.0), followed by 5 min of endogenous peroxidase blocking using Bond peroxide blocking solution; then protein blocking reagent was added for 10 min. After pretreatment, primary antibodies were applied: OSTα (1:50 dilution) for 4 h and OSTβ (1:100 dilution) for 1 h. Initially, OSTβ was detected with Bond polymer and TSA-Cy3 reagent (Perkin Elmer), while OSTα was detected with Bond polymer and TSA-Cy5 (Perkin Elmer). After completion of the first stain and before addition of the second primary antibody, the appropriate antigen retrieval protocol and peroxide blocking steps were applied. The slides were counterstained with Hoechst 33258 (Invitrogen), mounted with ProLong Gold antifade reagent, and overlaid with a coverslip. Positive and negative controls (no primary antibody) were included for IF stains. Single-stain controls were conducted for dual IF by omission of one primary antibody to ensure that cross-reactivity between the antibodies did not occur. High-resolution acquisition of IF slides in the DAPI, Cy3, and Cy5 channels was performed in the Aperio ScanScope FL (Leica) using a ×20 objective. Nuclei were visualized in the DAPI channel (blue), while OSTβ was visualized in the Cy3 (green) channel and OSTα in the Cy5 (red) channel. Image files were processed with Aperio ImageScope v12.3.1.6002.
Uptake transport studies in OSTab, Mock, and wild-type Flp-In 293 cells.
The Flp-In 293 cells were seeded on poly-d-lysine-coated Corning BioCoat 24-well plates at a density of 6 × 105 cells per well. The transport experiments were carried out after 3 days, when the cells had formed a confluent monolayer. The cells were washed with warm (37°C) buffer (HBSS-HEPES, pH 7.4; Life Technologies), extracellular fluid (ECF Na+; in mM: 122 NaCl, 25 KHCO3, 3 KCl, 0.4 KH2PO4, 10 d-glucose, 1.4 CaCl2, 1.2 MgSO4, and 10 HEPES, pH 7.4), or sodium-free ECF (ECF Na−; in mM: 122 C5H14ClNO, 25 KHCO3, 3 KCl, 0.4 KH2PO4, 10 d-glucose, 1.4 CaCl2, 1.2 MgSO4, and 10 HEPES; pH 6.4, 7.4, and 8.4) (57) and preincubated at 37°C for 10 min in the corresponding uptake buffer. The buffer was aspirated, and the uptake buffer containing a test substrate (0.003–1,000 μM unlabeled compound and 200–300 nCi/ml 3H-labeled compound) was added.
For inhibition studies, compounds at 100–1,000 μM were included in the uptake buffer. The same concentration of compounds previously tested in OSTα/β-expressing Xenopus laevis oocytes was applied (54, 59): 500 μM DIG, 200 μM E1S, 200 μM indomethacin, 1,000 μM probenecid, 200 μM spironolactone, 100 μM sulfobromophthalein, and 200 μM taurolithocholic acid sulfate (TLCAS). All other compounds were applied at 100 μM.
After 5 s–30 min of incubation at 37°C, the uptake buffer was aspirated, and the cells were washed twice with ice-cold buffer. Each cell monolayer was solubilized in 0.4 ml of lysis buffer (0.5% Triton X-100 and 0.005% antifoam A in PBS, pH 7.4), and the plates were frozen at −20°C. For determination of the radioactivity in the cells, the culture plates were thawed, the contents were vigorously mixed, and 0.3-ml aliquots of the cell lysate were added to 10 ml of Bio-Safe II counting cocktail (RPI, Mt. Prospect, IL) in scintillation vials. Radioactive counting was performed using a liquid scintillation analyzer (Tri-Carb 3100TR, PerkinElmer). Total protein in each well was determined from a 25-µl aliquot of the lysate by Pierce BCA protein assay kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. The resulting absorbance was read at 562 nm in a PowerWave XS microplate spectrophotometer (BioTek Instruments).
Compound selection.
A structurally diverse druglike data set (n = 24) was selected to investigate OSTα/β inhibitors (Table 2). This data set was chosen from compounds associated with cholestatic DILI with unknown mechanisms (n = 7) (11), as well as compounds that had been reported previously to interact with OSTα/β (n = 7) (54, 59), bile salt export pump (BSEP) (n = 4) (15, 44), and/or apical sodium-dependent bile salt transporter (ASBT) (n = 3) (64). In addition, compounds reported to interact with MRP3 (n = 8) (3, 36, 45) or MRP4 (n = 5) (36, 45) based on previously published data were included. The data set was supplemented with two bile acids: a clinically used biomarker for cholestasis, glycochenodeoxycholic acid (GCDCA), and TLCAS, which has been demonstrated to inhibit OSTα/β-mediated TCA and E1S uptake (54, 59). Eleven of the compounds are listed in the World Health Organization Model List of Essential Medicines and are in wide clinical use. Seven of the compounds are recommended by the US Food and Drug Administration (FDA) to be used as model drugs for in vitro transporter inhibitor/substrate assessment (57a). Physicochemical properties of the compounds were obtained from SciFinder Chemical Abstracts Service (American Chemical Society, Columbus, OH) and ChemSpider (Royal Society of Chemistry, Raleigh, NC) databases, and the DILI concern classification was adapted from the DILIrank reference drug list (12) and the LiverTox database (6).
Table 2.
Characteristics of 26 test compounds
| DILI Concern Score | Transporter Inhibition | Mol Wt | PSA, Å2 | LogD (pH 7.4) | WHO Essential Medicines | FDA Model Substrates or Inhibitors | Reference | |
|---|---|---|---|---|---|---|---|---|
| Exogenous compounds | ||||||||
| Amoxicillin | 2, cholestatic DILI | Not reported | 365.4 | 158 | −3.02 | X | 12 | |
| Azithromycin | 2, cholestatic DILI | Not reported | 748.98 | 180 | 1.36 | X | 12 | |
| Ciprofloxacin | 1, cholestatic DILI | Not reported | 331.34 | 72.9 | −2.23 | X | 12 | |
| Clavulanic acid | 2, cholestatic DILI (combination with amoxicillin) | Not reported | 199.16 | 87.1 | −4.72 | X | 16 | |
| Digoxin | 4 | OSTα/β inhibitor | 780.94 | 203 | 1.26 | X | x | 54, 59 |
| Dronedarone | 1 | BSEP, MRP3 inhibitor | 556.76 | 97.2 | 3.98 | x | 3, 12, 45 | |
| Fidaxomicin | 4 | MRP3 inhibitor | 1058 | 267 | 6.59 | 3 | ||
| Indomethacin | 1 | OSTα/β, ASBT, BSEP, MRP3, MRP4 inhibitor | 357.79 | 68.5 | 0.75 | 36, 45, 54, 59, 64 | ||
| Levofloxacin | 1, cholestatic DILI | Not reported | 361.37 | 73.3 | −2.08 | 6 | ||
| MK571 | 4, not in clinical use | BSEP, MRP2, MRP3, MRP4 inhibitor | 515.09 | 121 | 2.35 | 3, 45 | ||
| Paroxetine | 2 | Pgp inhibitor | 329.37 | 39.7 | 3.89 | 12 | ||
| Probenecid | 3 | OSTα/β, BSEP, MRP3, MRP4 inhibitor | 285.36 | 83.1 | 0.01 | x | 15, 36, 54, 59 | |
| Rosuvastatin | 2 | BCRP inhibitor | 481.54 | 149 | −1.77 | x | 6 | |
| Simvastatin | 2 | ASBT, BSEP inhibitor | 418.57 | 72.8 | 4.6 | X | x | 45, 64 |
| Spironolactone | 2 | OSTα/β, ASBT inhibitor | 416.57 | 85.7 | 2.78 | X | 54, 64 | |
| Sulfobromophthalein | 4 | OSTα/β inhibitor | 794 | 192.25 | −2.48 | 54 | ||
| Sulfamethoxazole | 2 (combination with trimethoprim) | Not reported | 253.28 | 107 | −0.56 | X | 6 | |
| Suramin | 4 | MRP3 inhibitor | 1297.3 | 534 | −12.23 | X | 3, 6 | |
| Toremifene | 3 | Not reported | 405.96 | 12.5 | 5.56 | 12 | ||
| Trimethoprim | 2, cholestatic DILI | MRP3, MRP4 inhibitor | 290.32 | 106 | −1.15 | X | x | 36 |
| Troglitazone | 1 | BSEP, MRP2, MRP3, MRP4 inhibitor | 441.54 | 110 | 3.26 | 15, 45 | ||
| Troglitazone sulfate* | 3 | BSEP inhibitor, MRP4 | 521.6 | 162 | −0.19 | 25, 62 | ||
| Valproic acid | 1, cholestatic DILI | Not reported | 144.21 | 37.3 | 0.1 | X | 12 | |
| Endogenous compounds | ||||||||
| Estrone sulfate | 3 | OSTα/β inhibitor | 350.4 | 89.05 | −1.05 | x | 54, 59 | |
| Glycochenodeoxycholic acid (GCDCA) | Not reported | 471.62 | 106.86 | −0.51 | 28 | |||
| Taurolithocholic acid sulfate (TLCAS) | OSTα/β inhibitor | 586.76 | 167 | −2.33 | 54, 59 | |||
Information includes score for drug-induced liver injury (DILI) concerns (1 = most, 2 = less, 3 = ambiguous, 4 = none), transporter inhibition, physicochemical properties, inclusion in the World Health Organization (WHO) model list of essential medicines (indicated by X), and listing as a US Food and Drug Administration (FDA) model substrate or inhibitor for transporters (indicated by x). ASBT, apical sodium-dependent bile salt transporter; BCRP, breast cancer resistance protein; BSEP, bile salt export pump; LogD, distribution constant; MRP, multidrug resistance-associated protein; OSTα/β, organic solute transporter α/β; Pgp, P-glycoprotein; PSA, polar surface area. Physicochemical properties, PSA, and LogD of the compounds were obtained from SciFinder, Chemical Abstracts Service (American Chemical Society, Columbus, OH), and ChemSpider (Royal Society of Chemistry, Raleigh, NC) (May 8, 2017).
Main metabolite of troglitazone.
Data analysis.
Values are means ± SD. Statistical analyses were performed using Prism 7 (GraphPad Software, La Jolla, CA). Protein expression of OSTα/β in liver tissue from NASH patients and patients with PBC was compared with that in liver tissue from controls by two-way ANOVA with Dunnett’s multiple-comparisons test. Gene expression of OSTα and OSTβ in the newly established OSTab cells was compared with that in Mock and wild-type Flp-In 293 cells, as well as with SCHH, by two-way ANOVA with Dunnett’s multiple-comparisons test. TCA uptake in OSTab cells was compared with that in wild-type Flp-ln293 cells by one-way ANOVA with Dunnett’s multiple-comparisons test. The effect of inorganic anions and pH on OSTα/β-mediated transport was identified by two-way ANOVA with Sidak’s multiple-comparisons test. In the OSTα/β inhibition screen, compounds that significantly decreased or increased OSTα/β-mediated transport were identified by two-way ANOVA with Dunnett’s multiple-comparisons test. Kinetic parameters were estimated by nonlinear and linear regression analysis.
RESULTS
OSTα/β expression is elevated in liver tissue of patients with NASH and PBC.
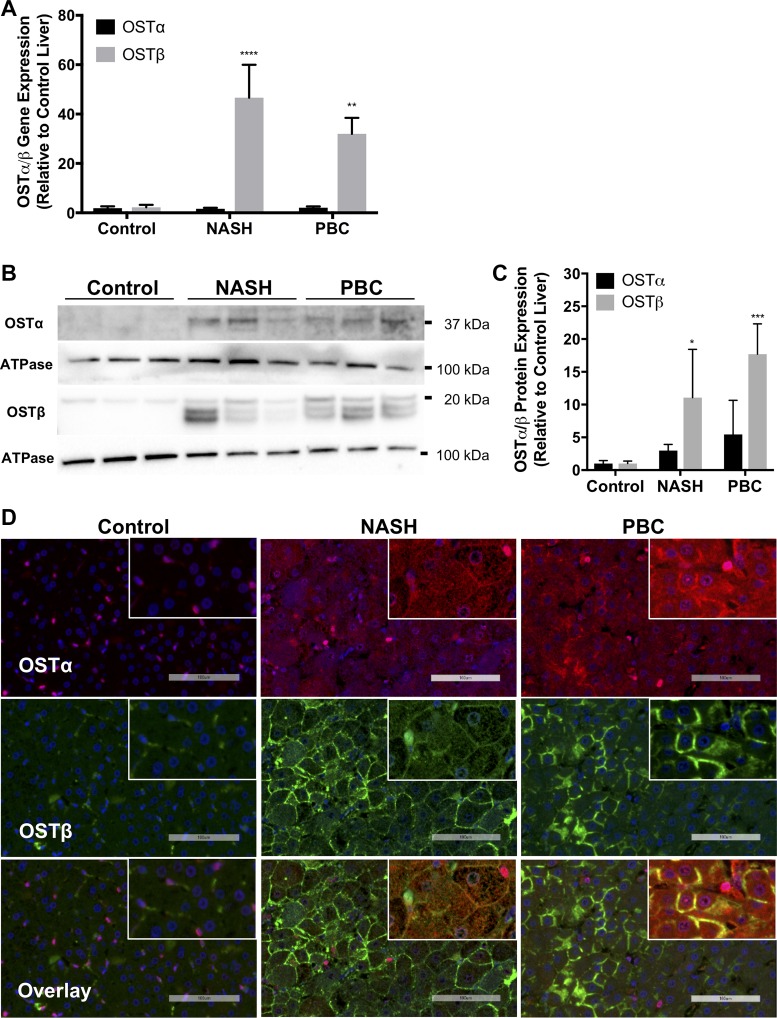
To determine whether the expression of OSTα and OSTβ was elevated in the hepatocytes of NASH and PBC patients, human liver tissue was subjected to quantitative RT-PCR, Western blotting, and double IF staining (Fig. 1). The main characteristics of the liver donors are shown in Table 1. Ballooning degeneration of hepatocytes was observed in liver tissue from all NASH patients, particularly patients 1 and 3. PBC samples exhibited extensive fibrosis, and also all NASH samples exhibited fibrosis. Control tissues did not show ballooning or fibrosis. Figure 1A demonstrates 46- and 32-fold increases in expression of OSTβ mRNA in the liver tissue of patients with NASH and PBC, respectively, whereas OSTα mRNA levels were similar in tissue from control and diseased livers. At the protein level, OSTβ expression was elevated 11-fold in NASH patients and 18-fold in PBC patients (Fig. 1, B and C). OSTα protein levels were elevated threefold in the liver of NASH patients and fivefold in liver tissue of patients with PBC compared with control liver tissue. IF revealed basolateral membrane localization of OSTα and OSTβ in the hepatocytes of NASH and PBC samples (Fig. 1D), as well as colocalization (Fig. 1D, insets). Although hepatocytes in control liver tissue expressed only weak OSTα and OSTβ staining, nonparenchymal cells expressed apparent OSTα near the nucleus and cellular OSTβ.
Fig. 1.
Expression of organic solute transporter (OST) α/β in human control livers and patients with nonalcoholic steatohepatitis (NASH) and primary biliary cholangitis (PBC). A: quantitative RT-PCR analysis of mRNA levels of OSTα and OSTβ in liver samples of NASH and PBC patients and controls. B and C: Western blots for OSTα and OSTβ proteins and corresponding densitometry. Values are means ± SD. ****P = 0.0001; ***P = 0.0007; **P < 0.005; *P = 0.0218. D: representative immunofluorescence of OSTα (red) and OSTβ (green) proteins in liver samples of NASH and PBC patients. Nuclei of cells in liver samples were stained with 4′,6-diaminido-2-phenylindole (DAPI, blue). Scale bars = 100 μm. Insets: colocalization of OSTα and OSTβ on parenchymal membrane.
Newly established OSTab cells express OSTα and OSTβ at high levels.
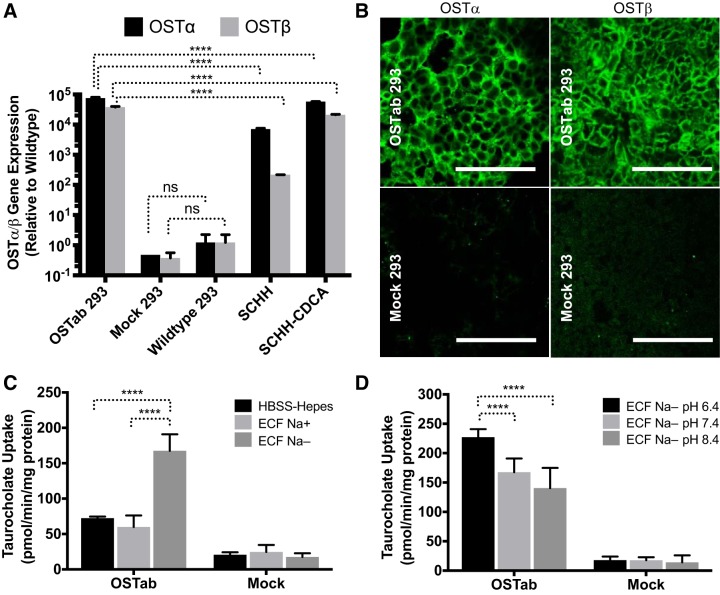
A stable human OSTα/β-overexpressing cell line (OSTab) was established to study the function, kinetics, substrates, and inhibitors of OSTα/β. The deduced amino acid sequences for the pcDNA5.1/FRT/OSTα/β construct were found to represent the major variants of human OSTα and OSTβ. Quantitative RT-PCR analysis indicated that OSTα and OSTβ mRNA was expressed at high levels in the OSTα/β-transfected (OSTab 293) cells (Fig. 2A). OSTα and OSTβ mRNA expression was >10,000-fold higher in OSTab than Mock and wild-type Flp-In 293 cells (Fig. 2A) and was even higher than the level of a constitutive gene, GAPDH (data not shown).
Fig. 2.
Expression and function of human OSTα/β in the established OSTab cell line. A: mRNA expression of human OSTα and OSTβ in Flp-In 293 cells transfected with OSTα/β vector (OSTab 293) compared with nontreated Flp-In 293 (wild-type 293) cells, Flp-in 293 cells transfected with empty vector (Mock 293), sandwich-cultured human hepatocytes (SCHH), and SCHH treated with a model bile acid, chenodeoxycholic acid (SCHH-CDCA, 100 μM, 24 h). Expression ratio of OSTα to OSTβ cannot be compared with the given normalization to wild-type. Values are means ± SD. Data were normalized to GAPDH and then compared with wild-type Flp-In 293 cells. B: immunofluorescence showing OSTα (green) and OSTβ (green) protein levels in OSTab and Mock cells. Scale bars = 100 µm. C and D: uptake of taurocholate (TCA) in OSTα/β-overexpressing Flp-In 293 cells (OSTab) and Flp-In 293 cells transfected with empty vector (Mock). C and D: Na+-free buffer and low-pH buffer increased OSTα/β-mediated uptake of TCA. OSTab and Mock cells were preincubated in HBSS-HEPES (pH 7.4) extracellular fluid (ECF) with sodium (ECF Na+, pH 7.4) or ECF in which sodium was substituted with choline (ECF Na−, pH 6.4, 7.4, and 8.4) at 37°C for 10 min. Uptake was initiated by addition of [3H]TCA or 3H-labeled estrone sulfate (300 nCi/ml, 20 µM, 2 min, 37°C) in the corresponding buffer. Values are means ± SD from 3 independent experiments. ****P = 0.0001; ns, not significant.
Expression of OSTα and OSTβ genes was >10,000-fold higher in CDCA-treated SCHH (SCHH-CDCA) than wild-type Flp-In 293 cells but was lower than in the OSTab cells, although the various OSTab clones showed some variability in gene expression (data not shown). The OSTab clone, which was selected for the functional studies, exhibited higher levels than the SCHH-CDCA (Fig. 2A). All the isolated OSTab clones exhibited higher OSTβ than OSTα mRNA when normalized to GAPDH (data not shown), resembling previously published values obtained from the bile acid-treated hepatocytes and other hepatic cells (7, 41, 52, 61).
IF showed high-intensity OSTα and OSTβ staining intracellularly and at the plasma membrane (Fig. 2B). These results demonstrate that Flp-In technology generated cells with very high OSTα and OSTβ expression, mimicking cholestatic, bile acid-treated hepatocyte cultures.
OSTab cells showed enhanced TCA uptake that was further increased in low-sodium and low-pH conditions.
OSTab cells showed enhanced uptake of TCA (Fig. 2, C and D), confirming previous results in X. laevis oocytes (54, 59). Interestingly, OSTα/β-mediated uptake of TCA was significantly higher in the sodium-free extracellular fluid buffer (ECF Na−), where sodium was isosmotically replaced by choline, than in the sodium-containing HBSS-HEPES and ECF (ECF Na+) buffers (Fig. 2C). Also, pH affected TCA uptake (Fig. 2D): lower pH (6.4 vs. 7.4 or 8.4) resulted in higher TCA uptake. Initial experiments were performed to ascertain that transfection with empty (i.e., Mock) vector did not change the nonspecific TCA uptake in Flp-In 293 (i.e., wild-type) cells (data not shown). All the uptake experiments were conducted with Mock cells as controls.
Effect of uptake time and substrate concentration on OSTα/β-mediated uptake.
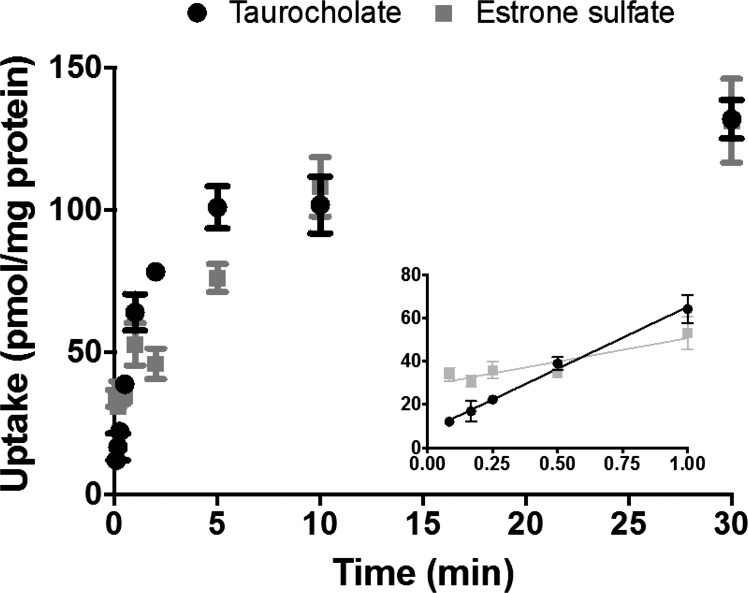
The TCA uptake data raised the question of the uptake rate in OSTab cells. Previously published OSTα/β uptake data were based on single-time-point (5, 30, or 60 min) experiments performed in X. laevis oocytes (4, 54, 59). The postulated mechanism of OSTα/β-mediated transport, facilitated diffusion, is typically a fast process. Hence, knowledge of the transport rate may be crucial for the sensitivity of the uptake assay. Thus the effect of uptake time on OSTα/β-mediated TCA and E1S transport in OSTab cells was examined (Fig. 3). Uptake in OSTab cells was rapid for both substrates, particularly within the 1st min. After 10 min, the uptake rate started to plateau, suggesting equilibrium between the uptake and efflux directions. When the amount of substrate (pmol/mg protein) taken up at 30 min was converted to concentration (μmol/l) using the published volume of HEK-293 cells (6.5 ± 0.3 μl/mg protein) (26), the concentration in OSTab cells equaled the dosing concentration (20.4 ± 2.6 μM for TCA and 20.4 ± 5.4 μM for E1S) (data not shown). These observations indicate the need to conduct OSTα/β uptake studies during the 1st min to stay in the linear range of uptake, which is shorter than the standard uptake protocols for SLCO and ABC family transporters.
Fig. 3.
Effect of uptake time (5 s–30 min) on OSTα/β-mediated TCA and estrone sulfate (E1S) transport. OSTab and Mock cells were incubated with [3H]TCA or [3H]E1S (300 nCi/ml, 20 µM) in ECF Na− buffer (pH 7.4) at 37°C for 0–30 min. Background levels derived from Mock cells were subtracted, and uptake measurements were normalized to total cell protein. Values are means ± SD from 3 independent experiments.
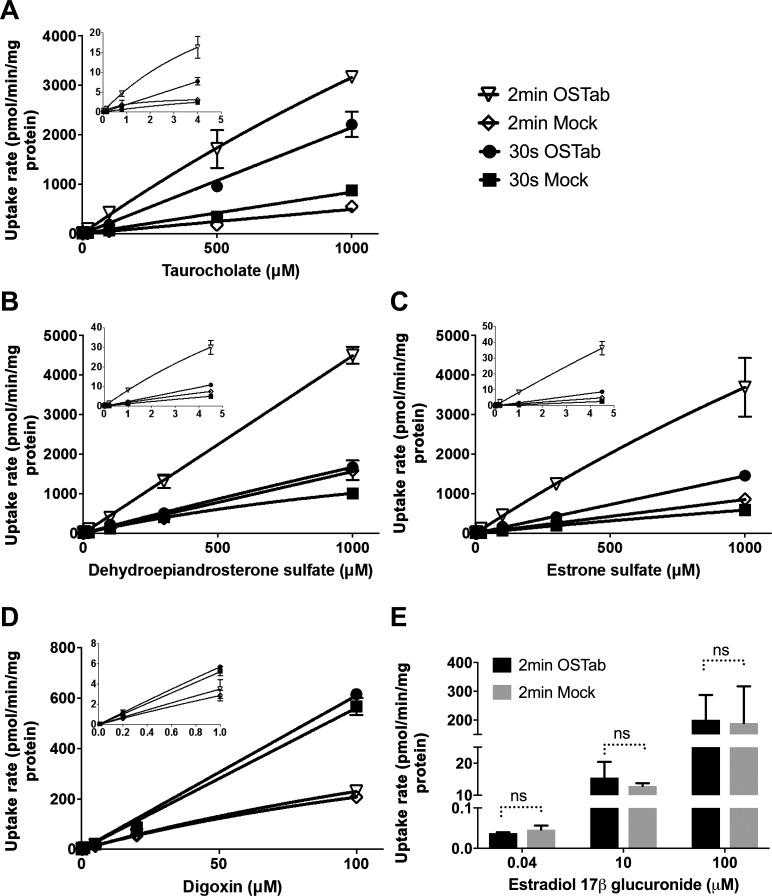
To establish the kinetics of TCA, DHEAS, E1S, and DIG transport in human OSTab cells, uptake rates of these compounds were measured at two time points (30 s and 2 min) over a concentration range of 0.003–1,000 µM for DHEAS and E1S, 0.03–1,000 µM for TCA, and 0.01–100 µM for DIG (Fig. 4). These concentrations covered the physiological and pathological plasma concentration range for TCA (0.003–2 μM) (24), DHEAS (1–5 μM) (17), and E1S (0.0004–0.004 μM) (50) but exceeded therapeutic plasma concentrations of DIG (0.001–0.002 μM) (47). Uptake of all the tested substrates, except DIG, was markedly higher in OSTab than Mock cells (Fig. 4D). Overall, DIG appeared to be a weak OSTα/β substrate. The uptake of a negative control, estradiol-17β-d-glucuronide, was equal in OSTab and Mock cells (Fig. 4E). The maximal velocity (Vmax) and Michaelis-Menten constant (Km) for substrate transport were not calculated, because uptake appeared to be linear up to substrate concentrations of 1 mM for TCA, DHEAS, and E1S and 100 µM for DIG. The lack of saturation may be due to the fact that the number of OSTα/β transporters in OSTab cells was so high that the substrate concentrations did not saturate the transporter binding site.
Fig. 4.
A–D: kinetics of TCA, dehydroepiandrosterone sulfate (DHEAS), estrone sulfate (E1S), and digoxin (DIG) uptake in cells transfected with human OSTα/β (OSTab) and empty vector (Mock). E: estradiol-17β-d-glucuronide (E217G) served as a negative control for OSTα/β-mediated uptake. OSTab and Mock cells were preincubated with ECF Na− buffer (pH 7.4, 10 min at 37°C), and uptake was initiated by addition of TCA (0.03–1,000 µM), DHEAS (0.003–1,000 µM), E1S (0.003–1,000 µM), DIG (0.01–100 µM), or E217G (0.04, 10, and 100 µM) (3H-labeled substrate concentration was ~300 nCi/ml) for 30 s or 2 min at 37°C. Uptake rate measurements were normalized to total cell protein. Values are means ± SD from 3 independent experiments. Vmax and Km were not calculated, because uptake rate appeared to be linear. ns, nonsignificant.
Effect of drugs, bile acids, and steroids on OSTα/β-mediated TCA uptake.
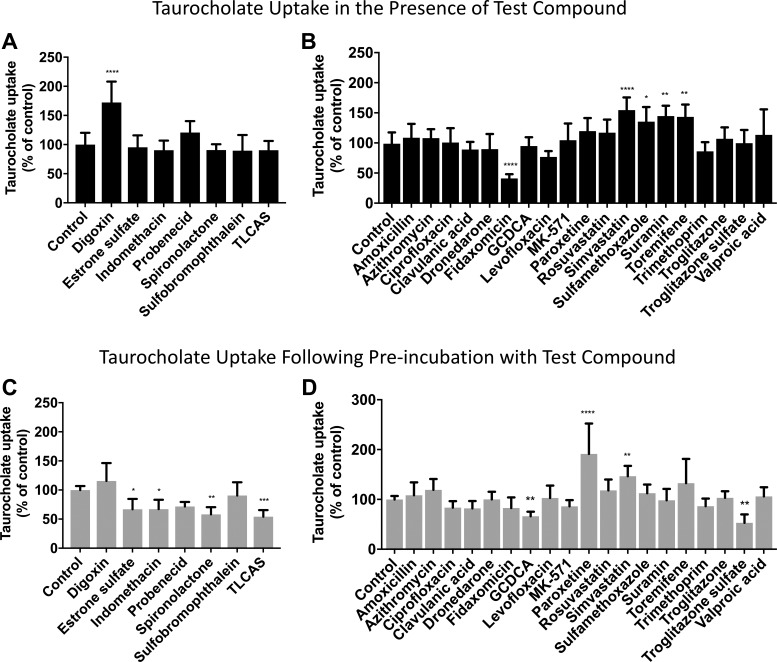
Previous studies in X. laevis oocytes revealed that some bile acids, steroids, and anionic drugs are partial OSTα/β inhibitors (20, 54, 59). The inhibitory activity of these organic compounds on OSTα/β in the newly established OSTab cells was evaluated (Fig. 5, A and C). Treatment (10 min of preincubation) with E1S, indomethacin, spironolactone, and TLCAS suppressed TCA uptake in OSTab cells (54–67% of control; Fig. 5C), in agreement with the previous reports (54, 59). Also, probenecid tended to inhibit the uptake of TCA (72% of control, P = 0.0652; Fig. 5C). These inhibitory effects were achieved only when the cells were pretreated with the compounds (Fig. 5C vs. Fig. 5A). DIG and sulfobromophthalein were not inhibitors of TCA uptake in OSTab cells; instead, DIG significantly stimulated TCA uptake when administered simultaneously with TCA without preincubation (172% of vehicle, P = 0.0001; Fig. 5A).
Fig. 5.
Effect of previously published OSTα/β inhibitors (A and C) and compounds that inhibit other bile acid transporters or compounds known to be associated with cholestatic liver injury (B and D) on OSTα/β-mediated TCA uptake. OSTab and Mock cells were preincubated with ECF Na− buffer (10 min at 37°C, pH 7.4), and uptake (30 s at 37°C) was initiated by addition of [3H]TCA (300 nCi/ml, 4 µM). Compounds were added only in the uptake phase (A and B) or only in the preincubation (10 min) phase (C and D). In A and C, concentration of known OSTα/β inhibitors was kept the same as previously published [200 μM: E1S, indomethacin, spironolactone, sulfobromophthalein, taurolithocholic acid sulfate (TLCAS); 500 μM: DIG; 1,000 μM: probenecid]. B and D: the other compounds were tested at 100 μM. Background levels derived from Mock cells were subtracted, and uptake measurements were normalized to total cell protein. Values (means ± SD from 3 independent experiments) are expressed as percentage of vehicle control. GCDCA, glycochenodeoxycholic acid. ****P = 0.0001; ***P < 0.0005; **P < 0.005; *P < 0.05.
To elucidate the potential OSTα/β inhibitory effect of compounds associated with cholestatic liver injury and compounds known to inhibit other bile acid transporters (Table 2), OSTab cells were either administered together with TCA during the uptake phase (Fig. 5B) or preincubated with the compounds (Fig. 5D). Most of these compounds did not show a significant effect on OSTα/β-mediated TCA uptake at 30 s in sodium-free conditions at pH 7.4. Fidaxomicin, a macrocyclic antibiotic, consistently inhibited OSTα/β (41% of vehicle, P = 0.0001). The percent inhibition was similar to that reported for OSTα/β-mediated E1S transport by androsterone sulfate and pregnenolone sulfate (20). Interestingly, troglitazone sulfate, a metabolite of troglitazone and a known BSEP inhibitor, decreased TCA uptake. The inhibition was observed only when troglitazone sulfate was preincubated with the cells, similar to E1S, indomethacin, spironolactone, and TLCAS. GCDCA, a biomarker for cholestasis, reduced TCA uptake to the same extent as E1S and indomethacin, but not as much as spironolactone and TLCAS. In contrast, paroxetine, simvastatin, sulfamethoxalole, suramin, and toremifene increased OSTα/β-mediated TCA uptake, suggesting that either they are substrates for OSTα/β or they inhibit OSTα/β-mediated TCA efflux.
The tested compounds exhibited a variety of physiochemical properties (Table 2). No correlation between OSTα/β inhibition and physiochemical properties [molecular weight, polar surface area, distribution constant (LogD), and pKa] was identified (data not shown). However, all the OSTα/β inhibitors, except spironolactone and fidaxomicin, were anions, with pKa <6.6. Another similarity among the inhibitors was a low LogD value (−2.33 to 0.75), except for fidaxomicin and spironolactone; which have LogD values >2.7. The compounds that stimulated TCA uptake did not share any particular physicochemical properties (both anions and cations with a range of molecular weight, polar surface area, and LogD values).
DISCUSSION
OSTα/β (SLC51A/B) has not been evaluated extensively as a transporter, although it is expressed in several human tissues, with the highest levels in liver, intestine, and kidneys (4, 54). The importance of OSTα/β in the intestine and kidneys was recognized more than a decade ago (5, 23, 65), but there is skepticism whether OSTα/β has a significant role in the liver. Data from the present study reveal for the first time that OSTα/β expression is markedly elevated in the liver of patients with NASH, similar to PBC. This finding suggests an essential role of OSTα/β in these liver diseases. The high level of OSTα/β expression in NASH is especially interesting and emphasizes the need to understand OSTα/β transport mechanisms and interactions with drugs. A major challenge in this field has been the lack of a stable in vitro model expressing OSTα/β. In this study a novel human-based in vitro model for OSTα/β was constructed. Using this stably transfected cell line, we clarified the transport mechanism of OSTα/β and evaluated the effect of DILI compounds, as well as compounds known to inhibit other bile acid transporters, on OSTα/β function.
Few studies have evaluated the impact of liver disease on transporter proteins [see review by Thakkar et al. (56)]. However, very little is known about OSTα/β expression in liver disease. As shown here, OSTα and OSTβ proteins are remarkably induced in the parenchymal cells of some NASH and PBC patients. This finding is consistent with previous observations in liver tissue from patients with NASH and PBC; bile acid uptake transporters are downregulated, whereas bile acid efflux transporters are increased (56). Interestingly, OSTα mRNA was not significantly altered in liver samples from NASH or PBC patients, in agreement with a previous study of OSTα/β expression in obstructive cholestasis (10). Two other studies reported a modest one- to-fivefold increase in OSTα mRNA in extrahepatic cholestasis and PBC (7, 51). The constant OSTα mRNA, but increased OSTα protein, can be explained by the fact that OSTα protein is unstable without OSTβ protein (14, 40, 60). In liver tissue from NASH patients and patients with PBC, OSTβ mRNA and protein are remarkably induced, which enables OSTα-OSTβ complex formation. Despite the substantial induction of OSTβ mRNA in NASH and PBC, OSTα mRNA expression is consistently higher than OSTβ mRNA expression in human liver (data not shown) (54). Elevated expression of OSTα/β in the liver of PBC patients has been reported previously (7), although only expression in whole liver samples was generated, and the localization of OSTα and OSTβ was not investigated. The present data clearly demonstrate that the increased expression of OSTα and OSTβ is localized on the basolateral membranes of hepatocytes. Control livers showed low expression in the hepatocytes, but a distinct signal for both OSTα and OSTβ was observed in nonparenchymal liver cells.
This study, as well as the previous studies, investigated transporter expression only in end-stage cirrhotic NASH, not in noncirrhotic NASH or NAFLD (1, 8, 13, 31). Therefore, we cannot conclude that OSTα/β expression is upregulated in all stages of NASH. Furthermore, the impact of severe fibrosis or inflammation cannot be excluded. The mechanism behind OSTα/β upregulation in liver tissue from patients with PBC or NASH may be elevated intrahepatic bile acid concentrations (37). Bile acids are FXR agonists, and both OSTα and OSTβ are regulated through FXR (7, 65). Particularly TCA and GCDCA are elevated in NASH liver tissue (~2- to 3-fold vs. control) (37). In the present study TCA was used as a model OSTα/β substrate, and the OSTα/β inhibitory effect of GCDCA was tested. Furthermore, OSTα and OSTβ are regulated by other transcription factors, the expression of which is altered in liver disease, including NAFLD/NASH (9). It has been reported that liver X receptor-α, hepatocyte nuclear factor 4α, and hypoxia-inducible factor-1α upregulate OSTα (48), whereas constitutive androstane receptor, retinoid X receptor, liver X receptor-α, vitamin D receptor, and the glucocorticoid receptor interact with OSTβ (35, 48, 61).
Previous investigations of OSTα/β substrates and inhibitors utilized X. laevis oocytes. The oocytes are significantly larger than human cells and present large unstirred layers and intracellular barriers that may yield misleading results regarding transporter function. Therefore, in the present study a stable, overexpressing human OSTα/β cell line, OSTab, was established to study the function, substrates, and inhibitors of OSTα/β in a reproducible manner. Functional studies in this stable cell line showed that TCA uptake was significantly (~3-fold) higher in OSTab cells than in cells transfected with the empty vector (Mock). This is consistent with previous OSTα/β studies, where a similar fold change for TCA, DHEAS, DIG, and prostaglandin E2 was reported (4, 20, 59). Low sodium and low pH further increased TCA accumulation in the OSTab cells, yielding a difference of up to 10-fold between OSTab and Mock cells. Transmembrane electrolyte or pH gradients did not affect OSTα/β transport in X. laevis oocytes when E1S was used as the substrate (4, 54), likely due to the above-mentioned differences between the oocytes and HEK-293 cells and differences in the uptake time (2 min in the present study vs. 5, 30, or 60 min in oocyte studies).
The effect of uptake time on OSTα/β-mediated TCA and E1S transport was studied. As expected, the uptake profile for OSTα/β was different from that for unidirectional ABC and SLC transporters. OSTα/β-mediated uptake was linear only during early time points. After 1 min, TCA and E1S uptake slowed and became nonlinear due to loss of the transport driving force, the concentration gradient between the extracellular and intracellular compartments.
The uptake rate profiles of OSTα/β substrates (DHEAS, E1S, TCA, and DIG) were linear over a wide concentration range, demonstrating the facilitated diffusion nature of OSTα/β. The rate-limiting factor for facilitated diffusion is the number of transporters at the plasma membrane. Expression of OSTα/β in the established OSTab cell line is high. Therefore, even at the highest substrate concentration (1 mM), transport capacity was not saturated. The uptake experiments performed at 2 min showed some nonlinearity but still did not follow Michaelis-Menten kinetics. The linear transport kinetics indicate that OSTα/β-mediated TCA, DHEAS, and E1S transport are not saturable at physiological plasma concentrations, at least when the expression is high. In contrast, studies performed in X. laevis oocytes described nonlinear uptake (20, 54). These uptake experiments were performed at 5 min, when OSTα/β transport is probably not in the linear range. A similar discrepancy between X. laevis oocytes and mammalian cells was reported with the OATP2B1 transporter: two Km values were reported based on data generated in oocytes (55), but only one uptake phase was observed in mammalian cell systems [human embryonic kidney (HEK-293), Chinese hamster ovary (CHO), and Madin-Darby canine kidney (MDCKII) cells] (29, 30).
Before the effects of DILI drugs and compounds known to inhibit other bile acid transporters were studied, the inhibitory activity of previously published OSTα/β inhibitors was investigated in the newly established OSTab cells (54, 59). The concentrations of the compounds were the same as those in the previously published studies (200–1,000 μM), and inhibitory effects were reproduced for most of the compounds. Interestingly, DIG increased TCA uptake, instead of inhibiting OSTα/β, which indicated that either DIG is an OSTα/β substrate or it inhibits OSTα/β-mediated TCA efflux. DIG appears to be an OSTα/β substrate, as previously reported (54, 59) and shown here. Because OSTα/β uptake was measured at 30 s, the inhibitory effect was tested by simultaneous dosing with the substrate and also by pretreatment, which extended the reaction time of compounds with the OSTab cells. The inhibitory effect of the known OSTα/β inhibitors was achieved only when the cells were pretreated with the compounds. This suggests that E1S, indomethacin, spironolactone, and TLCAS need to bind to OSTα/β before TCA. Another explanation for these findings may be that these compounds need to enter the cells to inhibit the OSTα/β-mediated transport, suggesting that they are also OSTα/β substrates. E1S is actually the most commonly used model substrate for OSTα/β (4, 54, 59).
The inhibitory effect of the known OSTα/β inhibitors was not strong. Additionally, the concentrations employed in inhibition studies were high. The DILI compounds and the compounds inhibiting other bile acid transporters were tested at 100 μM. Overall, the compounds with the highest DILI concern (ciprofloxacin, dronedarone, indomethacin, levofloxacin, and troglitazone valproic acid) did not alter the OSTα/β-mediated TCA transport, except for indomethacin when it was preincubated with the cells. However, the major metabolite of troglitazone, troglitazone sulfate, inhibited TCA transport when the OSTab cells were preincubated with troglitazone sulfate. Troglitazone sulfate also inhibits other bile acid transporters, including BSEP and MRP4 (25, 62), and is postulated to play a role in troglitazone-mediated hepatotoxicity, because hepatocyte concentrations of troglitazone are minimal, whereas troglitazone sulfate accumulates extensively in hepatocytes (38). Interestingly, GCDCA, a bile acid remarkably elevated in the serum and liver of NASH patients (22, 37), inhibited OSTα/β-mediated TCA transport. The inhibition was achieved only when the cells were pretreated with GCDCA, similar to E1S, indomethacin, spironolactone, and TLCAS. GCDCA is also an OSTα/β substrate (4). The effect of a macrocyclic antibiotic, fidaxomicin, was examined because studies in our laboratory indicated that it is a strong inhibitor of MRP3 (3). Surprisingly, fidaxomicin was more potent than other previously reported OSTα/β inhibitors when it was simultaneously administered with the substrate, which makes sense, because fidaxomicin is a large compound (1,058 g/mol) with minimal absorption in vivo (63). Fidaxomicin is used to treat Clostridium difficile infections in the colon, which makes this novel inhibition clinically interesting. OSTα/β is highly expressed in human small intestine and colon (4). Moreover, a side effect of fidaxomicin is diarrhea (27), which could be due to OSTα/β inhibition and impaired intestinal bile acid absorption. Another interesting finding was that simvastatin increased TCA uptake (both with and without pretreatment), suggesting that either it is an OSTα/β substrate or it inhibits OSTα/β-mediated TCA efflux, similar to DIG. Studies performed in Caco-2 cells suggest that another statin, rosuvastatin, is an OSTα/β substrate (39, 58). Although no apparent interaction was observed between rosuvastatin and OSTα/β in the present studies, it is well established that Caco-2 cells express multiple transporters with overlapping substrate specificities.
Overall, this study confirms the role of OSTα/β as a bile acid and drug transporter in the liver. The elevated hepatic expression of OSTα/β in NASH patients and patients with PBC is in agreement with the previous findings of elevated bile acid concentrations (22, 37), increased expression of efflux transporters (MRP1, MRP2, MRP3, MRP4, and breast cancer resistance protein), and decreased expression of bile acid uptake transporters (NTCP and OATP1B3) (56) in NASH and PBC liver. Moreover, the interaction of OSTα/β with multiple drugs suggests a potential role for hepatic OSTα/β in drug disposition, efficacy, and safety in patients with NASH and PBC.
In summary, this study describes the increased expression and parenchymal localization of OSTα/β in liver tissue from NASH patients as well as patients with PBC, suggesting a significant role for OSTα/β in human liver. The established OSTα/β-overexpressing cell line provides a powerful approach for investigating OSTα/β. The linear OSTα/β transport kinetics and modulation by cholestatic DILI-associated drugs further support the role of OSTα/β as a “safety valve” for bile acid efflux in cholestasis. Our findings provide new insight into the molecular mechanisms of NASH, cholestasis, and bile acid- and drug-mediated hepatotoxicity.
GRANTS
This research was supported by National Institute of General Medical Sciences (NIGMS) Grants R01-GM-041935 and R35-GM-122576 (K. L. R. Brouwer). I. Ali was supported by NIGMS Clinical Pharmacology Training Grant T32-GM-086330. M. M. Malinen was supported, in part, by the Finnish Cultural Foundation and Orion Research Foundation. The Liver Tissue Cell Distribution System (Minneapolis, MN, and Pittsburgh, PA) was funded by National Institutes of Health Contract HHSN276201200017C.
DISCLOSURES
K. L. R. Brouwer is a coinventor of the sandwich-cultured hepatocyte technology for quantification of biliary excretion (B-CLEAR®) and related technologies, which have been licensed exclusively to Qualyst Transporter Solutions, LLC.
AUTHOR CONTRIBUTIONS
M.M.M., I.A., and K.L.R.B. conceived and designed research; M.M.M., J.B., and J.J.B. performed experiments; M.M.M. and J.B. analyzed data; M.M.M., I.A., J.B., and K.L.R.B. interpreted results of experiments; M.M.M. and J.B. prepared figures; M.M.M. drafted manuscript; M.M.M., I.A., J.B., J.J.B., and K.L.R.B. edited and revised manuscript; M.M.M., I.A., J.B., J.J.B., and K.L.R.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jenni Küblbeck (University of Eastern Finland) for help with cloning methodologies; Dr. Katsuaki Ito (Teijin Pharma) for advice on the uptake study design; Kimberly Freeman and Eric Hall (Qualyst Transporter Solutions, Durham, NC) for assistance with quantitative RT-PCR analyses; Dr. Kathleen Köck for assistance with the histological samples; and Melanie Stewart, Lilly Wong, and Antti Kauttonen for assistance with the uptake assays.
REFERENCES
- 1.Aguilar-Olivos NE, Carrillo-Córdova D, Oria-Hernández J, Sánchez-Valle V, Ponciano-Rodríguez G, Ramírez-Jaramillo M, Chablé-Montero F, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. The nuclear receptor FXR, but not LXR, up-regulates bile acid transporter expression in non-alcoholic fatty liver disease. Ann Hepatol 14: 487–493, 2015. [PubMed] [Google Scholar]
- 2.Ahlin G, Hilgendorf C, Karlsson J, Szigyarto CA, Uhlén M, Artursson P. Endogenous gene and protein expression of drug-transporting proteins in cell lines routinely used in drug discovery programs. Drug Metab Dispos 37: 2275–2283, 2009. doi: 10.1124/dmd.109.028654. [DOI] [PubMed] [Google Scholar]
- 3.Ali I, Welch MA, Lu Y, Swaan PW, Brouwer KLR. Identification of novel MRP3 inhibitors based on computational models and validation using an in vitro membrane vesicle assay. Eur J Pharm Sci 103: 52–59, 2017. doi: 10.1016/j.ejps.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTα-OSTβ: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42: 1270–1279, 2005. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- 5.Ballatori N, Fang F, Christian WV, Li N, Hammond CL. Ostα-Ostβ is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol 295: G179–G186, 2008. doi: 10.1152/ajpgi.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology 63: 590–603, 2016. doi: 10.1002/hep.28323. [DOI] [PubMed] [Google Scholar]
- 7.Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTα-OSTβ in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol 290: G1124–G1130, 2006. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- 8.Canet MJ, Merrell MD, Hardwick RN, Bataille AM, Campion SN, Ferreira DW, Xanthakos SA, Manautou JE, A-Kader HH, Erickson RP, Cherrington NJ. Altered regulation of hepatic efflux transporters disrupts acetaminophen disposition in pediatric nonalcoholic steatohepatitis. Drug Metab Dispos 43: 829–835, 2015. doi: 10.1124/dmd.114.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ, Sidey J, Shi H, Aqel BA, McClain CJ, Prough RA. Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta 1859: 1083–1099, 2016. doi: 10.1016/j.bbagrm.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai J, Feng X, Zhang L, Chen S, Cheng Y, He X, Yang Y, He Y, Wang H, Wang R, Chen W. Hepatic expression of detoxification enzymes is decreased in human obstructive cholestasis due to gallstone biliary obstruction. PLoS One 10: e0120055, 2015. doi: 10.1371/journal.pone.0120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H, Gu J, Serrano J, US Drug Induced Liver Injury Network . Features and outcomes of 899 patients with drug-induced liver injury: the DILIN Prospective Study. Gastroenterology 148: 1340–1352 e1347, 2015. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Suzuki A, Thakkar S, Yu K, Hu C, Tong W. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 21: 648–653, 2016. doi: 10.1016/j.drudis.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Clarke JD, Hardwick RN, Lake AD, Lickteig AJ, Goedken MJ, Klaassen CD, Cherrington NJ. Synergistic interaction between genetics and disease on pravastatin disposition. J Hepatol 61: 139–147, 2014. doi: 10.1016/j.jhep.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter-α-β, Ostα-Ostβ, is an ileal basolateral bile acid transporter. J Biol Chem 280: 6960–6968, 2005. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson S, Stahl S, Paul N, Barber J, Kenna JG. In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos 40: 130–138, 2012. doi: 10.1124/dmd.111.040758. [DOI] [PubMed] [Google Scholar]
- 16.deLemos AS, Ghabril M, Rockey DC, Gu J, Barnhart HX, Fontana RJ, Kleiner DE, Bonkovsky HL, Drug-Induced Liver Injury Network (DILIN) . Amoxicillin-clavulanate-induced liver injury. Dig Dis Sci 61: 2406–2416, 2016. doi: 10.1007/s10620-016-4121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dharia S, Parker CR Jr. Adrenal androgens and aging. Semin Reprod Med 22: 361–368, 2004. doi: 10.1055/s-2004-861552. [DOI] [PubMed] [Google Scholar]
- 18.Dimitrakoudis D, Ramlal T, Rastogi S, Vranic M, Klip A. Glycaemia regulates the glucose transporter number in the plasma membrane of rat skeletal muscle. Biochem J 284: 341–348, 1992. doi: 10.1042/bj2840341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver (EASL)European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64: 1388–1402, 2016. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Fang F, Christian WV, Gorman SG, Cui M, Huang J, Tieu K, Ballatori N. Neurosteroid transport by the organic solute transporter OSTα-OSTβ. J Neurochem 115: 220–233, 2010. doi: 10.1111/j.1471-4159.2010.06920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer KL, Barritt AS 4th. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci 60: 3318–3328, 2015. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. Regulation of the mouse organic solute transporter-α/β, Ostα-Ostβ, by bile acids. Am J Physiol Gastrointest Liver Physiol 290: G912–G922, 2006. doi: 10.1152/ajpgi.00479.2005. [DOI] [PubMed] [Google Scholar]
- 24.Frommherz L, Bub A, Hummel E, Rist MJ, Roth A, Watzl B, Kulling SE. Age-related changes of plasma bile acid concentrations in healthy adults—results from the cross-sectional KarMeN Study. PLoS One 11: e0153959, 2016. doi: 10.1371/journal.pone.0153959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funk C, Pantze M, Jehle L, Ponelle C, Scheuermann G, Lazendic M, Gasser R. Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology 167: 83–98, 2001. doi: 10.1016/S0300-483X(01)00460-7. [DOI] [PubMed] [Google Scholar]
- 26.Gillen CM, Forbush B 3rd. Functional interaction of the K-Cl cotransporter (KCC1) with the Na-K-Cl cotransporter in HEK-293 cells. Am J Physiol Cell Physiol 276: C328–C336, 1999. doi: 10.1152/ajpcell.1999.276.2.C328. [DOI] [PubMed] [Google Scholar]
- 27.Golan Y, Epstein L. Safety and efficacy of fidaxomicin in the treatment of Clostridium difficile-associated diarrhea. Therap Adv Gastroenterol 5: 395–402, 2012. doi: 10.1177/1756283X12461294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greim H, Trülzsch D, Czygan P, Rudick J, Hutterer F, Schaffner F, Popper H. Mechanism of cholestasis. 6. Bile acids in human livers with or without biliary obstruction. Gastroenterology 63: 846–850, 1972. [PubMed] [Google Scholar]
- 29.Grube M, Köck K, Karner S, Reuther S, Ritter CA, Jedlitschky G, Kroemer HK. Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol 70: 1735–1741, 2006. doi: 10.1124/mol.106.026450. [DOI] [PubMed] [Google Scholar]
- 30.Hänggi E, Grundschober AF, Leuthold S, Meier PJ, St-Pierre MV. Functional analysis of the extracellular cysteine residues in the human organic anion transporting polypeptide, OATP2B1. Mol Pharmacol 70: 806–817, 2006. doi: 10.1124/mol.105.019547. [DOI] [PubMed] [Google Scholar]
- 31.Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos 39: 2395–2402, 2011. doi: 10.1124/dmd.111.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson JP, Freeman K, Brouwer KR. Basolateral efflux transporters: a potentially important pathway for the prevention of cholestatic hepatotoxicity. Appl In Vitro Toxicol 2: 207–216, 2016. doi: 10.1089/aivt.2016.0023. [DOI] [Google Scholar]
- 33.Karlgren M, Ahlin G, Bergström CA, Svensson R, Palm J, Artursson P. In vitro and in silico strategies to identify OATP1B1 inhibitors and predict clinical drug-drug interactions. Pharm Res 29: 411–426, 2012. doi: 10.1007/s11095-011-0564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, Haglund U, Artursson P. Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J Med Chem 55: 4740–4763, 2012. doi: 10.1021/jm300212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan AA, Chow EC, Porte RJ, Pang KS, Groothuis GM. Expression and regulation of the bile acid transporter, OSTα-OSTβ in rat and human intestine and liver. Biopharm Drug Dispos 30: 241–258, 2009. doi: 10.1002/bdd.663. [DOI] [PubMed] [Google Scholar]
- 36.Köck K, Ferslew BC, Netterberg I, Yang K, Urban TJ, Swaan PW, Stewart PW, Brouwer KL. Risk factors for development of cholestatic drug-induced liver injury: inhibition of hepatic basolateral bile acid transporters multidrug resistance-associated proteins 3 and 4. Drug Metab Dispos 42: 665–674, 2014. doi: 10.1124/dmd.113.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lake AD, Novak P, Shipkova P, Aranibar N, Robertson D, Reily MD, Lu Z, Lehman-McKeeman LD, Cherrington NJ. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol Appl Pharmacol 268: 132–140, 2013. doi: 10.1016/j.taap.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JK, Marion TL, Abe K, Lim C, Pollock GM, Brouwer KL. Hepatobiliary disposition of troglitazone and metabolites in rat and human sandwich-cultured hepatocytes: use of Monte Carlo simulations to assess the impact of changes in biliary excretion on troglitazone sulfate accumulation. J Pharmacol Exp Ther 332: 26–34, 2010. doi: 10.1124/jpet.109.156653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Wang Y, Zhang W, Huang Y, Hein K, Hidalgo IJ. The role of a basolateral transporter in rosuvastatin transport and its interplay with apical breast cancer resistance protein in polarized cell monolayer systems. Drug Metab Dispos 40: 2102–2108, 2012. doi: 10.1124/dmd.112.045666. [DOI] [PubMed] [Google Scholar]
- 40.Li N, Cui Z, Fang F, Lee JY, Ballatori N. Heterodimerization, trafficking and membrane topology of the two proteins, Ostα and Ostβ, that constitute the organic solute and steroid transporter. Biochem J 407: 363–372, 2007. doi: 10.1042/BJ20070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Lu H, Lu YF, Lei X, Cui JY, Ellis E, Strom SC, Klaassen CD. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol Sci 141: 538–546, 2014. doi: 10.1093/toxsci/kfu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malinen MM, Kanninen LK, Corlu A, Isoniemi HM, Lou YR, Yliperttula ML, Urtti AO. Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. Biomaterials 35: 5110–5121, 2014. doi: 10.1016/j.biomaterials.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Mateus A, Matsson P, Artursson P. Rapid measurement of intracellular unbound drug concentrations. Mol Pharm 10: 2467–2478, 2013. doi: 10.1021/mp4000822. [DOI] [PubMed] [Google Scholar]
- 44.Morgan RE, Trauner M, van Staden CJ, Lee PH, Ramachandran B, Eschenberg M, Afshari CA, Qualls CW Jr., Lightfoot-Dunn R, Hamadeh HK. Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci 118: 485–500, 2010. doi: 10.1093/toxsci/kfq269. [DOI] [PubMed] [Google Scholar]
- 45.Morgan RE, van Staden CJ, Chen Y, Kalyanaraman N, Kalanzi J, Dunn RT 2nd, Afshari CA, Hamadeh HK. A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci 136: 216–241, 2013. doi: 10.1093/toxsci/kft176. [DOI] [PubMed] [Google Scholar]
- 46.Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology 55: 622–633, 2012. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada RD, Hager WD, Graves PE, Mayersohn M, Perrier DG, Marcus FI. Relationship between plasma concentration and dose of digoxin in patients with and without renal impairment. Circulation 58: 1196–1203, 1978. doi: 10.1161/01.CIR.58.6.1196. [DOI] [PubMed] [Google Scholar]
- 48.Okuwaki M, Takada T, Iwayanagi Y, Koh S, Kariya Y, Fujii H, Suzuki H. LXRα transactivates mouse organic solute transporter α and β via IR-1 elements shared with FXR. Pharm Res 24: 390–398, 2007. doi: 10.1007/s11095-006-9163-6. [DOI] [PubMed] [Google Scholar]
- 49.Pais R, Barritt AS IV, Calmus Y, Scatton O, Runge T, Lebray P, Poynard T, Ratziu V, Conti F. NAFLD and liver transplantation: current burden and expected challenges. J Hepatol 65: 1245–1257, 2016. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J Clin Endocrinol Metab 81: 1460–1464, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 49: 1228–1235, 2009. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 52.Schaffner CA, Mwinyi J, Gai Z, Thasler WE, Eloranta JJ, Kullak-Ublick GA. The organic solute transporters-α and β are induced by hypoxia in human hepatocytes. Liver Int 35: 1152–1161, 2015. doi: 10.1111/liv.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seward DJ, Koh AS, Boyer JL, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTα-OSTβ. J Biol Chem 278: 27473–27482, 2003. doi: 10.1074/jbc.M301106200. [DOI] [PubMed] [Google Scholar]
- 55.Shirasaka Y, Mori T, Shichiri M, Nakanishi T, Tamai I. Functional pleiotropy of organic anion transporting polypeptide OATP2B1 due to multiple binding sites. Drug Metab Pharmacokinet 27: 360–364, 2012. doi: 10.2133/dmpk.DMPK-11-SH-080. [DOI] [PubMed] [Google Scholar]
- 56.Thakkar N, Slizgi JR, Brouwer KLR. Effect of liver disease on hepatic transporter expression and function. J Pharm Sci 106: 2282–2294, 2017. doi: 10.1016/j.xphs.2017.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchida Y, Ito K, Ohtsuki S, Kubo Y, Suzuki T, Terasaki T. Major involvement of Na+-dependent multivitamin transporter (SLC5A6/SMVT) in uptake of biotin and pantothenic acid by human brain capillary endothelial cells. J Neurochem 134: 97–112, 2015. doi: 10.1111/jnc.13092. [DOI] [PubMed] [Google Scholar]
- 57a.US Food and Drug Administration Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. Development & Approval Process (Drugs). Washington, DC: Dept. of Health and Human Services, 2016. [Google Scholar]
- 58.Wang Q, Zheng M, Leil T. Investigating transporter-mediated drug-drug interactions using a physiologically based pharmacokinetic model of rosuvastatin. CPT Pharmacometrics Syst Pharmacol 6: 228–238, 2017. doi: 10.1002/psp4.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W, Seward DJ, Li L, Boyer JL, Ballatori N. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci USA 98: 9431–9436, 2001. doi: 10.1073/pnas.161099898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu S, Soroka CJ, Sun AQ, Backos DS, Mennone A, Suchy FJ, Boyer JL. A novel di-leucine motif at the N-terminus of human organic solute transporter β is essential for protein association and membrane localization. PLoS One 11: e0158269, 2016. doi: 10.1371/journal.pone.0158269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu S, Sun AQ, Suchy FJ. A novel RARα/CAR-mediated mechanism for regulation of human organic solute transporter-β gene expression. Am J Physiol Gastrointest Liver Physiol 306: G154–G162, 2014. doi: 10.1152/ajpgi.00138.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang K, Pfeifer ND, Köck K, Brouwer KL. Species differences in hepatobiliary disposition of taurocholic acid in human and rat sandwich-cultured hepatocytes: implications for drug-induced liver injury. J Pharmacol Exp Ther 353: 415–423, 2015. doi: 10.1124/jpet.114.221564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhanel GG, Walkty AJ, Karlowsky JA. Fidaxomicin: a novel agent for the treatment of Clostridium difficile infection. Can J Infect Dis Med Microbiol 26: 305–312, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng X, Ekins S, Raufman JP, Polli JE. Computational models for drug inhibition of the human apical sodium-dependent bile acid transporter. Mol Pharm 6: 1591–1603, 2009. doi: 10.1021/mp900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-α/β in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol 290: G923–G932, 2006. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]