Abstract
Obesity is associated with dysregulation of vagal neurocircuits controlling gastric functions, including food intake and energy balance. In the short term, however, caloric intake is regulated homeostatically although the precise mechanisms responsible are unknown. The present study examined the effects of acute high-fat diet (HFD) on glutamatergic neurotransmission within central vagal neurocircuits and its effects on gastric motility. Sprague-Dawley rats were fed a control or HFD diet (14% or 60% kcal from fat, respectively) for 3–5 days. Whole cell patch-clamp recordings and brainstem application of antagonists were used to assess the effects of acute HFD on glutamatergic transmission to dorsal motor nucleus of the vagus (DMV) neurons and subsequent alterations in gastric tone and motility. After becoming hyperphagic initially, caloric balance was restored after 3 days following HFD exposure. In control rats, the non- N-methyl-d-aspartate (NMDA) receptor antagonist, 6,7-dinitroquinoxaline-2,3-dione (DNQX), but not the NMDA receptor antagonist, amino-5-phosphonopentanoate (AP5), significantly decreased excitatory synaptic currents and action potential firing rate in gastric-projecting DMV neurons. In contrast, both AP5 and DNQX decreased excitatory synaptic transmission and action potential firing in acute HFD neurons. When microinjected into the brainstem, AP5, but not DNQX, decreased gastric motility and tone in acute HFD rats only. These results suggest that acute HFD upregulates NMDA receptor-mediated currents, increasing DMV neuronal excitability and activating the vagal efferent cholinergic pathway, thus increasing gastric tone and motility. Although such neuroplasticity may be a persistent adaptation to the initial exposure to HFD, it may also be an important mechanism in homeostatic regulation of energy balance.
NEW & NOTEWORTHY Vagal neurocircuits are critical to the regulation of gastric functions, including satiation and food intake. Acute high-fat diet upregulates glutamatergic signaling within central vagal neurocircuits via activation of N-methyl-d-aspartate receptors, increasing vagal efferent drive to the stomach. Although it is possible that such neuroplasticity is a persistent adaptation to initial exposure to the high-fat diet, it may also play a role in the homeostatic control of feeding.
Keywords: brainstem, high-fat diet, glutamate, N-methyl-d-aspartate, vagus
INTRODUCTION
The recent increase in rates of obesity, as well as its comorbid disorders such as type II diabetes, hypertension, and heart disease, has underscored the importance of understanding the neural mechanisms that are involved in energy homeostasis and visceral functions such as feeding and digestion. Although obesity is a multifactorial disorder that involves genetic, epigenetic, and environmental factors (41–43, 52), a growing body of evidence suggests that diet, especially a high-fat diet (HFD), can have profound effects on synaptic transmission in the neurocircuits that are involved in regulation of gastric functions, feeding behavior, and energy homeostasis (11, 24, 26, 38, 39, 57).
Although the enteric nervous system endows the gastrointestinal (GI) tract with a significant degree of autonomy over function (31), the upper GI tract and stomach in particular receive dense extrinsic innervation, particularly from the parasympathetic nervous system via the vagus nerve (20, 53). Understanding the vagally dependent control of gastric functions, including motility, compliance, and emptying is crucial to addressing the altered meal patterns and food intake that are central to the disruption in energy homeostasis that is responsible for the development of obesity. Sensory inputs from the GI tract are transduced by the peripheral terminals of vagal afferent (sensory) nerves, the cell bodies of which lie in the nodose ganglia, and the central terminals of which enter the brainstem via the tractus solitarius, terminating upon neurons of the nucleus tractus solitarius (NTS). NTS neurons integrate this peripheral sensory information with inputs from brainstem and higher CNS centers involved in autonomic homeostasis and relay the resulting integrated signal to the adjacent dorsal motor nucleus of the vagus (DMV), which provides the parasympathetic efferent (motor) input to the GI tract (20, 53). Together with the area postrema, the NTS and DMV form the dorsal vagal complex (DVC), which functions as a crucial intersection in the integration of both ascending interoceptive as well as descending visceromotor signals related to food intake, satiation, and energy balance.
It is clear that the ingestion of food has direct effects on the control of gastric tone and motility through vagally mediated reflexes. It is well recognized that, upon introduction to an HFD, rodents become hyperphagic initially although caloric balance is restored shortly thereafter (7, 9, 60); the mechanisms responsible for this regulation have not been elucidated fully, however. After continued exposure to HFD, this homeostatic compensation is lost, and rodents increase meal size and/or meal frequency, which contribute to the onset and development of obesity (29, 56). Recent human and animal studies have shown that appropriate gastric functions are critical to the regulation of satiety and energy balance, whereas obesity is frequently associated with gastric dysmotility, altered gastric emptying, and increased compliance of the stomach, increasing the amount of food required to signal satiation and terminate feeding (22, 36, 46).
Both chronic HFD exposure and diet-induced obesity (DIO) have profound effects on vagal neurocircuits controlling gastric motility, emptying, food intake, and energy homeostasis (11, 24, 26, 38, 39, 57). Fewer studies have examined the effects of acute exposure to HFD on vagal neurocircuits before the development of obesity (11, 47, 57), however. Understanding how neurosignaling is modulated during periods of homeostatic energy balance will also be critical to elucidating the dysregulated neuroplasticity that is associated with increased meal size, meal frequency, and obesity.
The unique biophysical and membrane properties of DMV neurons mean they are spontaneously active pacemakers, firing action potentials (APs) at ~1 Hz (54, 55). The excitability of DMV neurons is modulated continuously by synaptic inputs, particularly from the adjacent NTS, which provides glutamatergic, GABAergic, and catecholaminergic inputs (25, 48, 55). Although DMV neurons, as preganglionic parasympathetic motoneurons, are cholinergic, they synapse onto postganglionic myenteric neurons that form one of two opposing pathways, either an excitatory cholinergic pathway or an inhibitory nonadrenergic, noncholinergic (NANC) pathway (20, 53). Alterations in synaptic inputs, therefore, may exert profound effects on DMV neuronal excitability and, in turn, upon vagal efferent control of gastric functions.
Inhibitory GABAergic signaling has long been considered to be the predominant neurotransmitter at the NTS-DMV synapse; indeed DMV neurons regulating gastric functions are tonically inhibited by NTS GABAergic synaptic inputs (50, 55). Although glutamatergic synaptic inputs appear more important in pancreatic function (4), their potential role in regulation of gastric functions are frequently overlooked because of their apparently minimal role in the regulation of DMV neuronal activity. The role of glutamatergic transmission to DMV neurons under pathophysiological conditions, however, has received less attention. The aims of this study were to examine the effects of acute HFD exposure on glutamatergic signaling within central vagal neurocircuits, particularly during those periods when homeostatic regulation of caloric intake has been reestablished, as well as the resulting effects on gastric motility.
MATERIALS AND METHODS
All experiments were conducted with the approval of the Penn State University College of Medicine Institutional Animal Care and Use Committee and according to National Institute of Health regulations.
Animals.
Male Sprague-Dawley (SD) rats (n = 31; Charles River, Kingston, NY) were used for in vivo gastric motility recordings. Both male and female SD rats (n = 34) were used for in vitro electrophysiological experiments and food intake studies. All rats were fed a normal chow (fat:protein:carbohydrate content 14:27:59% kcal; Purina Mills, Gray Summit, MO), unless assigned an acute HFD (fat:protein:carbohydrate content 60:20:20%; D12492; Research Diets, New Brunswick, NJ), which was provided for 3–5 days before experimentation. All rats had ad libitum access to food and water, except during in vivo gastric recordings, where rats were fasted overnight with ad libitum access to water before experimentation. All rats were 4–8 wk of age during in vitro and in vivo experiments.
Food intake measurements.
Rats were housed individually for 4 days to allow acclimation before exposure to HFD. Food intake and body mass were measured twice daily, within 1 h of lights on/off.
Identification of gastric-projecting DMV neurons.
All animals that were used for electrophysiological experiments had the retrograde tracer DiI [octadecyl (C18) indocarbocyanine; Life Technologies, Grand Island, NY] applied to discrete gastric regions as previously described (17). Briefly, rats were anesthetized with isoflurane (2.5% in 100% O2), and an abdominal laparotomy was performed. The stomach was exposed, and DiI was applied to either the corpus or antrum/pylorus. The dye was then secured in place with a fast-hardening epoxy resin. The rats then received 2 wk of rest following the surgery to allow the tracer sufficient time to reach the DMV and fluorescently label the gastric-projecting DMV neurons.
Brainstem preparation.
After the labeling of gastric-projecting DMV neurons, rats were anesthetized with isoflurane before being euthanized via administration of a bilateral pneumothorax, as described previously (17, 47). Briefly, the brainstems were quickly removed and mounted on a vibratome while submerged in cold, oxygenated Krebs solution. The brainstems were sliced into 300-μm sections and placed in a warm (30°C) oxygenated Krebs solution for 90 min before recording.
Electrophysiological recordings from brainstem slices.
A single brainstem slice was placed in a perfusion chamber (volume 500 μl) on the stage of a Nikon E600FN microscope equipped with tetramethylrhodamine isothiocyanate (TRITC) epifluorescent filters and maintained at 32°C via perfusion with warmed Krebs solution containing the following (in mM): 126.0 NaCl, 25.0 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, and 10.0 D-glucose, maintained at pH 7.4 by bubbling with 95%O2-5% CO2 at a rate of 2.0–2.5 ml/min. Once identified, gastric-projecting DMV neurons were recorded electrophysiologically under bright-field illumination. Whole cell patch-clamp recordings were made using patch pipettes of 2–4 MΩ when filled with potassium gluconate intracellular solution (in mM: 128.00 potassium gluconate, 10.00 KCl, 0.30 CaCl2, 1.00 MgCl2, 10.00 HEPES, 1.00 EGTA, 1.00 NaATP, and 0.25 NaGTP adjusted to pH 7.35) and a single-electrode voltage-clamp amplifier (Axopatch 200B; Molecular Devices, Union City, CA). Data were filtered at 2 kHz, digitized via a Digidata 1440 Interface, and stored and analyzed on a PC utilizing pClamp 10 software (Molecular Devices). Recordings with a series resistance of >20 MΩ were eliminated from the study.
To examine glutamatergic miniature excitatory postsynaptic currents (mEPSCs; i.e., AP-independent neurotransmitter release), neurons were voltage clamped at −50 mV in the presence of tetrodotoxin (TTX; 0.3 μM), and the amplitude, frequency, and charge transfer of mEPSCs in the absence and presence of N-methyl-d-aspartate (NMDA)- and non-NMDA receptor-selective antagonists, amino-5-phosphonopentanoate (AP5) (25 μM) and 6,7-dinitroquinoxaline-2,3-dione (DNQX) (30 μM) respectively, were examined using MiniAnalysis (Synaptosoft, Leonia, NJ).
To analyze evoked currents, a bipolar stimulating electrode with tip separation ~125 μm (WPI, Sarasota, FL) was placed in the adjacent NTS and used to electrically evoke EPSCs (eEPSCs). Electrical stimuli (10–500 μA, 0.05–1.00 ms) were applied every 10 s throughout the recording to evoke submaximal currents. Neurons were voltage clamped at potentials between −80 mV and +40 mV, and the amplitude of eEPSCs in the absence and presence of DNQX (30 μM) were used to examine the current-voltage (I–V) relationship of NMDA and non-NMDA currents.
Drug application and statistical analysis.
All drugs were prepared freshly and diluted in Krebs solution just before use. Drugs were used at concentrations determined previously to be effective; effects of AP5 (25 μM) and DNQX (30 μM) (25, 33, 61) on mEPSC properties such as frequency, amplitude, and charge transfer were examined. For the characterization of AP firing rate, neurons were current clamped at a holding potential that allowed an AP firing rate of ~1 Hz, before AP5 and DNQX application. Antagonists were applied for a period of time sufficient for the response to reach plateau, or at least 5 min if no response was observed. Each neuron served as its own control, i.e., the response of any neuron was assessed before and after drug application using a paired Student’s t-test. Intergroup comparisons were made using the χ2-test. Minimum variation of ≥20% in mEPSC amplitude and ≥25% change in AP firing rate was taken as indicative of a responsive neuron. Only responsive neurons were included in the statistical analyses, which were conducted using GraphPad Prism software (La Jolla, CA). Results are expressed as means ± SE, with significance defined as P < 0.05.
In vivo recordings of gastric tone and motility.
Rats were anesthetized with Inactin (thiobutabarbital; 125–150 mg/lg ip); once a deep plane of anesthesia was obtained (abolition of the foot pinch withdrawal reflex), a tracheal catheter was fitted, and an abdominal laparotomy was performed to expose the anterior stomach. Miniaturized strain gauges (AT Engineering, Hershey, PA) were attached to the ventral corpus and antrum of male rats, in alignment with the circular smooth muscle, as described previously (47). The strain gauge leads were exteriorized before the abdominal incision was closed. Rats were placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA), and temperature was maintained at 37°C. The strain gauge signal was filtered (AT Engineering; low-pass cutoff 0.5 Hz) and amplified (EXP CLSG-2; QuantaMetrics, Newton, PA) and recorded on a computer using Axotape 10 software (Molecular Devices).
The brainstem was exposed via blunt dissection, the meningeal membranes above the vagal trigone were removed, and the exposed brainstem was covered with warmed saline during a recovery period of at least 60 min. A micropipette (~20-μm tip diameter) was lowered into the left DVC at coordinates (in mm) +0.4–0.5 rostro-caudal from calamus scriptorius, +0.2–0.4 medio-lateral from midline, and −0.6–0.065 dorso-ventral from brainstem surface. Drugs were microinjected in 60-nl volumes using a picospritzer (Toohey, Fairfield, IL) over a period of ~60 s. All drugs were dissolved in PBS (in mM: 115.0 NaCl, 75.0 Na2HPO4, and 7.5 KH2PO4). The effects of drug application on corpus and antrum motility and tone were measured as described previously (47).
In five rats, the vagally dependent nature of the effects of ionotropic glutamate receptor antagonists were assessed. The effect of kynurenic acid was assessed first because it will antagonize all ionotropic glutamate receptors; the effects of the selective NMDA and non-NMDA receptor antagonists, AP5 or DNQX, respectively, were assessed subsequently only if a response to kynurenic acid was observed. Before placement of the miniaturized strain gauges, the posterior gastric branch of the vagus was isolated and sectioned at the level of the lower esophagus. A suture thread was then placed around the left cervical vagus and exteriorized for later transection. In these rats, after recovery from microinjection of kynurenic acid, the ligature was withdrawn, severing the remaining vagal innervation to the stomach. After 30–60 min of recovery, kynurenic acid was reapplied.
To assess which vagal pathway the effects of DVC application of ionotropic glutamate receptor antagonists were modulating, in another group of rats (n = 8), a jugular cannula was inserted to permit intravenous administration of drugs. To assess the contribution of the inhibitory NANC pathway, the effects of kynurenic acid microinjection on the modulation of gastric tone and motility were assessed before and after intravenous infusion with the nitric oxide inhibitor nitro-l-arginine methyl ester (l-NAME; 10 mg/kg in 500 μl). To assess the contribution of the excitatory cholinergic pathway, the effects of kynurenic acid were assessed before and after intravenous infusion with the muscarinic agonist, bethanechol (30 μg/kg in 500 μl). As shown previously, this dose of bethanechol produces a maximal activation of the gastric smooth muscle cholinergic receptors, overcoming any actions of brainstem drug microinjection to activate or inhibit the vagal efferent cholinergic pathway (14, 44).
After experimentation, rats were euthanized and perfused transcardially with saline followed by 4% paraformaldehyde in PBS (composition in mM: 115.0 NaCl, 75.0 Na2HPO4, 7.5 KH2PO). The brainstems were removed and postfixed for 3 days in 4% paraformaldehyde + 20% sucrose. The brainstems were then frozen, and 50-μm sections were cut throughout the rostro-caudal extent of the DVC using a freezing microtome to permit post hoc verification of microinjection sites using a Nikon E400 microscope.
In vivo data and statistical analysis.
Strain gauges were calibrated before use, and drug-induced effects on gastric motility and tone were extrapolated from the average calibration value. Although basal gastric tone was not preset to a fixed value, the gastric circular muscle provided a basal tension of ~500 mg. Corpus and antrum tone are reported as absolute values relative to baseline; because of individual variations in animal size, surgical placement of the strain gauges, etc., which lead to minor variations in absolute values, each rat served as its own control. Corpus and antrum motility were calculated using the following formula: motility index (%) = 100 × [(N1 × 1) + (N2 × 2) + (N3 × 4) + (N4 × 8)]/t, where Nx = number of motility peaks in a given force range and t = time period over which motility was measured. With the assumption that the absence of motility produced a 0-mV signal, the peak-to-peak motility waves reflected N1 = 25–50 mg; N2 = 51–100 mg, N3 = 101–200 mg, and N4 >201 mg.
Each rat served as its own control, i.e., gastric responses were assessed before and after DVC microinjection using a paired Student’s t-test. Intergroup comparisons were made using the χ2-test. Only responsive rats were included in the statistical analyses, which were conducted using GraphPad Prism software. Results are expressed as means ± SE, with significance defined as P < 0.05.
RESULTS
Acute HFD exposure does not alter caloric intake.
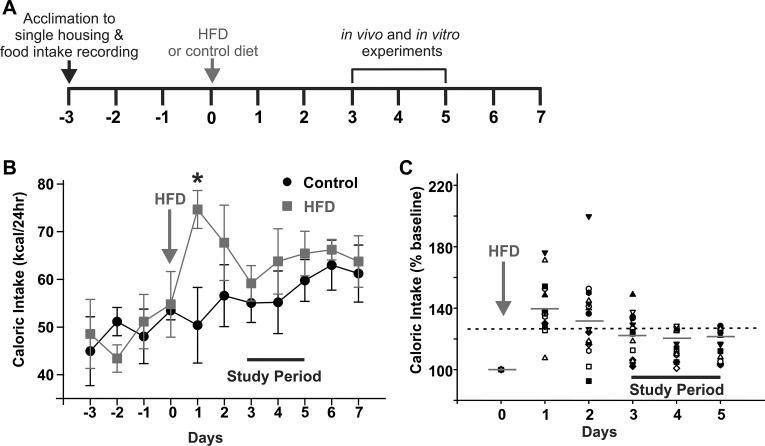
Food intake was measured in 4 control and 17 acute HFD rats. Although a significant increase in caloric intake was apparent immediately (1 day) after HFD exposure, by day 3, food intake was decreased, regulating caloric intake to that of control-fed rats (Fig. 1). Briefly, daily caloric intake increased in 16 of 17 HFD rats from 50 ± 7.9 kcal/day to 74.7 ± 4.0 kcal/day (P < 0.05) after 1 day of HFD exposure but was restored to within 25% of baseline levels by 2 days in 7/16 rats and by 3–5 days in the remaining 9/16 rats, when rats ingested 55 ± 4.1 kcal/day vs. 59 ± 3.7 kcal/day of HFD vs. control diet, respectively (P > 0.05). In addition, there was no significant difference in body weight between control and HFD rats at any time point.
Fig. 1.
Restoration of caloric balance occurs within 3 days of exposure to a high-fat diet (HFD). A: graphical illustration of the experimental timeline. B: graphical summary of caloric intake following exposure to an HFD. Single-housed rats were fed a control diet and, after acclimation, continued on a control diet (n = 4) or fed an HFD (n = 17). Rats were weighed daily, and their total food intake was calculated. Note that rats became hyperphagic and consumed a larger number of calories immediately upon exposure to the HFD (day 1). By day 3 onward, however, energy balance had been restored, and there were no differences in caloric intake between control and HFD-fed rats. *P < 0.05 (Student’s paired t-test). C: graphical summary of caloric intake following exposure to an HFD, expressed as a percentage of averaged baseline control intake. Note that caloric intake increased in 16/17 rats after 1 day of food intake but was restored to within 25% of baseline levels by 2 days in 7/16 rats, and by 3–5 days in the remaining 9/16 rats. Mean values for each day are marked by gray horizontal lines; the black dashed line indicates 125% of baseline caloric intake.
These data suggest that, following exposure to HFD, rats initially increase caloric intake, but energy balance is restored after 3 days.
Regulation of caloric intake following acute HFD exposure is associated with increased NMDA receptor activation and DMV neuronal excitability.
Neither qualitative nor quantitative differences were observed in the electrophysiological responses of corpus- and antrum/pylorus-labeled DMV neurons. Similarly, significant differences were not observed in responses between male and female rats; all results were therefore combined.
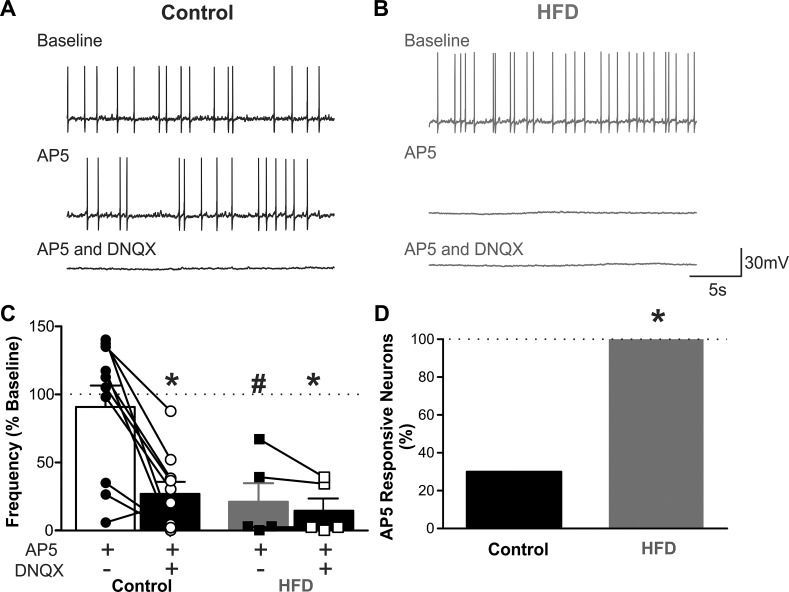
To assess the NMDA- vs. non-NMDA-dependent nature of glutamatergic inputs to gastric-projecting DMV neurons, the effects of AP5 (25 μM) and DNQX (30 μM), respectively, to modulate AP firing rates of gastric-projecting DMV neurons were assessed. Application of the AP5 decreased AP firing rate in 3/10 DMV neurons from control rats, from 25 ± 6 to 5 ± 2 AP/30 s, i.e., a 78 ± 8% decrease in firing rate (P < 0.05) with the remaining seven neurons being unresponsive (40 ± 5 vs. 48 ± 6 AP/30 s, i.e., 120 ± 6% of control; P > 0.05). In contrast, after 3–5 days of HFD exposure, AP5 decreased AP firing rate in all five DMV neurons tested (P < 0.05 vs. control), from 27 ± 5 to 5 ± 3 AP/30 s, i.e., 79 ± 14% decrease (P < 0.05). Further addition of DNQX further decreased AP firing rate in two of the three responding control DMV neurons and six of the seven nonresponding control neurons, as well as in four of the five acute HFD DMV neurons (Fig. 2).
Fig. 2.
The N-methyl-d-aspartate (NMDA) receptor-selective antagonist, amino-5-phosphonopentanoate (AP5), decreases action potential (AP) firing rate in acute high-fat diet (HFD), but not control, dorsal motor nucleus of the vagus (DMV) neurons. A: representative traces from a gastric-projecting DMV neuron from a control diet rat, current clamped at a potential that allowed AP firing of ~1 Hz. Perfusion with AP5 (25 μM) had no effect on AP firing rate in 7/10 neurons tested (3 rats). Subsequent application of 6,7-dinitroquinoxaline-2,3-dione (DNQX) (30 μM) decreased AP firing rate in 8/10 neurons tested (3 rats). B: representative traces from a gastric-projecting DMV neuron from an acute HFD rat, current clamped at a potential to allow AP firing of ~1 Hz. Perfusion with AP5 (25 μM) decreased AP firing in all 5 neurons (from 3 rats) tested. Subsequent application of DNQX (30 μM) did not affect AP firing rate further. C: graphical summary of the responses of control (left; n = 10 neurons from 3 rats) and acute HFD (right; n = 5 neurons from 3 rats) gastric-projecting DMV neurons to perfusion with AP5 and DNQX. Note that AP5 decreased the firing rate in all acute HFD neurons but in only a minority of control neurons. *P < 0.05 vs. baseline (Student’s paired t-test), #P < 0.05 vs. control (Student’s paired t-test). D: graphical summary of the proportion of gastric-projecting DMV neurons responding to AP5 with a decrease in AP firing rate.*P < 0.05 vs. control (χ2-test).
In contrast, AP5 had no effect on the excitability of DMV neurons after 1 day of HFD exposure, when rats were hyperphagic and before caloric homeostasis was attained. In five acute HFD gastric-projecting DMV neurons tested, AP5 had no effect on AP firing in any neuron (19 ± 3 vs. 19 ± 4 AP/30 s, i.e., 98 ± 9% of control firing rate; P > 0.05; data not shown).
Previous studies have demonstrated that glutamatergic synaptic inputs regulate the neuronal excitability of a subpopulation of gastric-projecting DMV neurons (4). Data from this present study suggest that the increased NMDA receptor activation resulting from acute HFD exposure alters DMV neuronal excitability, increasing AP firing rates. Furthermore, this increased NMDA receptor activation occurs after 3 days of HFD exposure and correlates with the period after which food intake is regulated to achieve a similar caloric intake to control-fed rats.
Acute HFD increases activation of synaptic NMDA receptors.
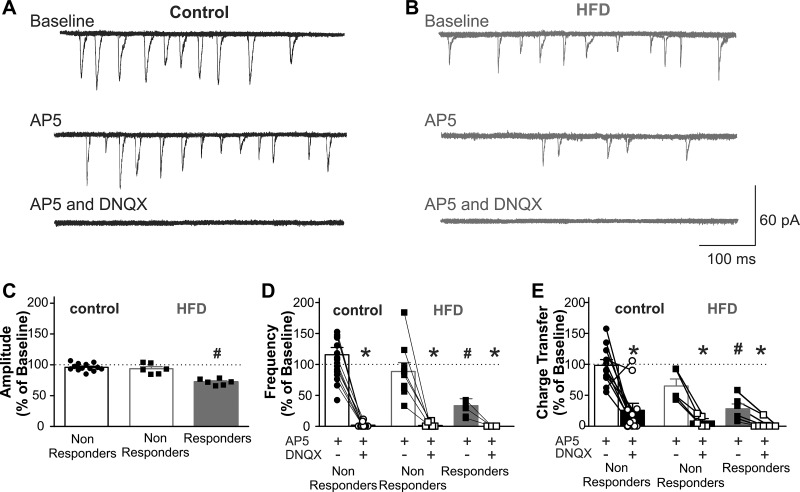
To determine whether acute HFD exposure altered the activation of synaptic NMDA vs. non-NMDA receptors in gastric-projecting DMV neurons, the effects of the antagonists AP5 (25 μM) and DNQX (30 μM), respectively, to modulate mEPSCs in gastric-projecting DMV neurons voltage clamped at −50 mV were examined. In control diet rats, only 1 out of 18 DMV neurons was responsive to AP5 with the remaining 17 neurons being unaffected (19 ± 1.3 pA vs. 18 ± 1.3 pA, P > 0.05). In contrast, 9 out of 15 DMV neurons from acute HFD rats responded to AP5 with a decrease in mEPSC amplitude, frequency, and charge transfer (amplitude = 25 ± 2.9 pA vs. 18 ± 1.7 pA, i.e., 28 ± 2.0% reduction; frequency = 1.4 ± 0.3 events/s vs. 0.5 ± 0.1 events/s, i.e., 65 ± 9.4% reduction; charge transfer = 156 ± 61 pA·ms vs. 37 ± 13 pA·ms, i.e., 72 ± 8% reduction; P < 0.05 for each vs. control; Fig. 3). Further application of DNQX (30 μM) abolished the remaining mEPSCs in control and HFD neurons (Fig. 3).
Fig. 3.
The N-methyl-d-aspartate receptor (NMDA-R)-selective antagonist, amino-5-phosphonopentanoate (AP5), decreases excitatory synaptic transmission to acute high-fat diet (HFD), but not control, dorsal motor nucleus of the vagus (DMV) neurons. A: 6 overlapping consecutive traces from a control gastric-projecting DMV neuron voltage clamped at −50 mV illustrating miniature excitatory postsynaptic currents (mEPSCs). Perfusion with AP5 (25 μM), had no effect on mEPSC frequency or amplitude in 17/18 neurons. Subsequent application of 6,7-dinitroquinoxaline-2,3-dione (DNQX) (30 μM) abolished all mEPSCs, confirming their glutamatergic nature. B: sample trace of mEPSCs in an acute HFD gastric-projecting DMV neuron, voltage clamped at −50 mV (6 overlapping consecutive traces). Perfusion with AP5 decreased mEPSC amplitude and frequency in 6/15 neurons; subsequent application of DNQX abolished all mEPSCs, confirming the role of both NMDA-R and non-NMDA-R (presumably α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, AMPA-R) in synaptic glutamatergic currents. C: graphical summary of the effects of AP5 on mEPSC amplitude in control (left; n = 17, 8 rats) and acute HFD (right; n = 6 responding neurons and 9 nonresponding neurons, 8 rats) gastric-projecting DMV neurons. Neurons were classified as responsive based on whether there was a significant change in amplitude >25% from baseline. D: graphical summary of the effects of AP5 and DNQX on mEPSC frequency in control (left; n = 17 neurons, 8 rats) and acute HFD (right; n = 6 responding neurons and 9 nonresponding neurons, 8 rats) gastric-projecting DMV neurons. Neurons were classified as responsive based upon the ability of AP5 to decrease mEPSC amplitude. ● and ■ indicated response to AP5; ○ and □ indicate response to AP5 and DNQX. All mEPSCs were abolished by DNQX (control) or a combination of AP5 and DNQX (acute HFD). E: graphical summary of the effects of AP5 and DNQX on mEPSC charge transfer in control (left; n = 17 neurons, 8 rats) and acute HFD (right; n = 6 responding neurons and 9 nonresponding neurons, 8 rats) gastric-projecting DMV neurons. Neurons were classified as responsive based on the ability of AP5 to decrease mEPSC amplitude. ● and ■ indicate response to AP5; ○ and □ indicate response to AP5 and DNQX. *P < 0.05 vs. baseline (Student's paired t-test). #P < 0.05 vs. control (Student's paired t-test).
Additional experiments were conducted in four control and seven acute HFD DMV neurons in which the effects of DNQX on mEPSC properties were assessed first, before subsequent application of AP5. In control neurons, DNQX decreased the frequency of mEPSCs in 4/4 control neurons from 1.7 ± 0.2 events/s to 0.3 ± 0.1 events/s (i.e., to 20.1 ± 6.8% of control), whereas DNQX decreased firing rate in 4/7 acute HFD neurons from 1.38 ± 0.35 events/s to 0.71 ± 0.26 events/s (i.e., to 48 ± 17% of baseline). In both groups, subsequent application of AP5 further reduced mEPSC frequency in control and HFD neurons to 0.19 ± 0.01 events/s and 0.15 ± 0.10 events/s, respectively. These data suggest that acute HFD increases DMV neuronal excitability via actions to increase activation of synaptic NMDA receptors.
Acute HFD alters the AMPA:NMDA ratio in DMV neurons.
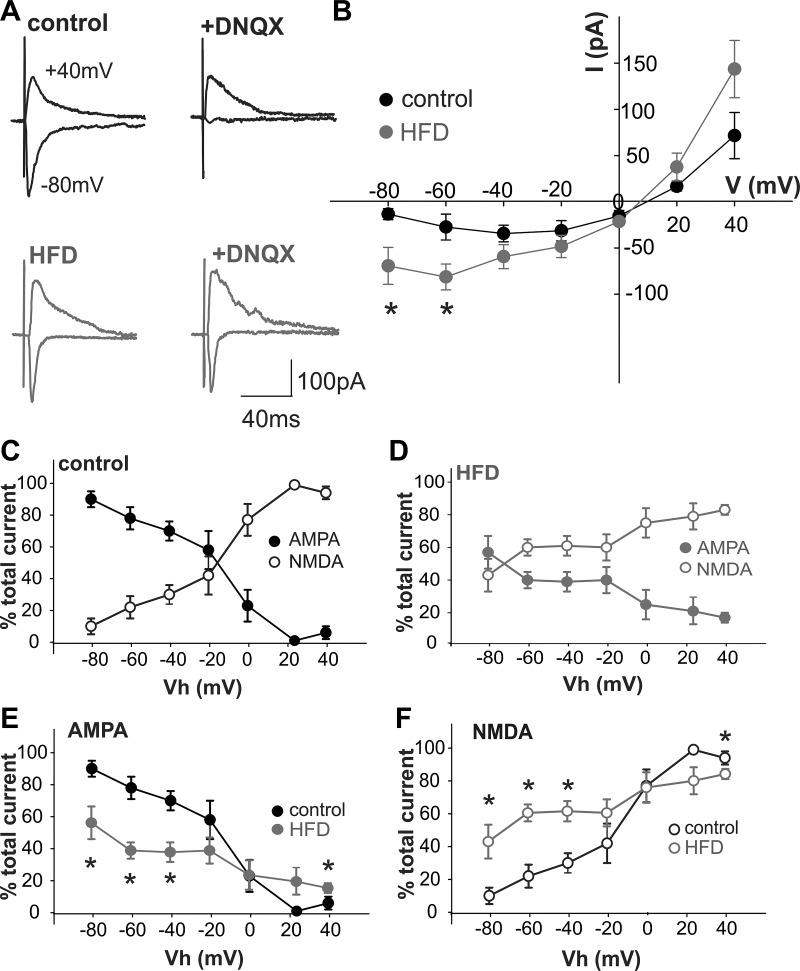
To assess whether acute HFD exposure altered the voltage dependency of NMDA receptor-mediated currents, the current-voltage (I–V) relationships for NMDA- and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated currents were compared in control and acute HFD DMV neurons. As shown previously in central vagal neurocircuits (55), in control DMV neurons, eEPSCs were composed primarily of AMPA receptor-mediated currents at membrane potentials negative to −20 mV, with NMDA receptor-mediated currents becoming more apparent at potentials positive to −20 mV (Fig. 4). In contrast, following acute HFD exposure, eEPSCs were composed primarily of NMDA receptor-mediated currents at potentials positive to −60 mV (Fig. 4). Indeed, the NMDA receptor-mediated current in acute HFD DMV neurons was ~150% larger than in control DMV neurons at resting membrane potential (~−50 mV; Fig. 4B).
Fig. 4.
Acute high-fat diet (HFD) exposure alters the N-methyl-d-aspartate (NMDA): α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) ratio in dorsal motor nucleus of the vagus (DMV) neurons. Glutamate currents were evoked electrically via stimulation of the adjacent nucleus tractus solitarius (NTS) with a bipolar electrode. Gastric-projecting DMV neurons were voltage clamped at potentials between −80 mV and +40 mV in the absence and presence of the non-NMDA receptor (R)-selective antagonist, 6,7-dinitroquinoxaline-2,3-dione (DNQX) (30 μM) to reveal the proportion of NMDA-R-mediated currents at each potential. A: representative evoked excitatory postsynaptic currents (eEPSCs) in control (top, black) and acute HFD (bottom, gray) gastric-projecting DMV neurons at −80 mV and +40 mV, in the absence (left) and presence (right) of DNQX. Note that, in control DMV neurons, DNQX almost abolished the eEPSC at −80 mV, suggesting that NMDA-R activation contributed little, if at all, to the glutamatergic eEPSC at this potential, whereas AMPA-R activation contributed little to the eEPSC at +40 mV. In contrast, the effects of DNQX to inhibit the eEPSC at −80 mV were attenuated significantly in acute HFD DMV neurons, suggesting that NMDA-R activation contributed significantly to the glutamatergic current at this potential. B: magnitude of the NMDA-R-mediated current in control and acute HFD DMV neurons was calculated at holding potentials between −80 mV and +40 mV to create a current-voltage relationship. Note that, in acute HFD DMV neurons, the NMDA-R-mediated current is increased significantly at potentials negative to −40 mV, suggesting a significant contribution to glutamatergic-mediated synaptic currents at rest (n = 3–8 neurons from 3 to 4 rats, respectively), *P < 0.05 vs. control (Student’s t-test). C: graphical representation of NMDA-R-mediated (○) and AMPA-R-mediated (●) currents as a percentage of total eEPSC at potentials between −80 mV and +40 mV in control gastric-projecting DMV neurons (n = 3–8 neurons, 3 rats). D: graphical representation of NMDA-R-mediated (○) and AMPA-R-mediated (●) currents as a percentage of total eEPSC at potentials between −80 mV and +40 mV in acute HFD gastric-projecting DMV neurons (n = 3–8 neurons, 4 rats). E: graphical representation of AMPA-R-mediated currents as a percentage of total eEPSC at potentials between −80 mV and +40 mV in control (black) and acute HFD (gray) gastric-projecting DMV neurons (n = 3–8 neurons for each, 3–4 rats). *P < 0.05 vs. control (Student’s t-test). F: graphical representation of NMDA-R-mediated currents as a percentage of total eEPSC at potentials between −80 mV and +40 mV in control (black) and acute HFD (gray) gastric-projecting DMV neurons (n = 3–8 neurons for each, 3–4 rats; *P < 0.05 vs. control (Student’s t-test).
These data suggest that acute HFD exposure altered the voltage dependency of NMDA receptor activation, and evoked glutamatergic currents were mediated principally by NMDA dependent currents at resting membrane potentials.
The acute HFD-induced alteration in glutamatergic signaling in the DMV increases gastric tone and motility in a vagally dependent manner.
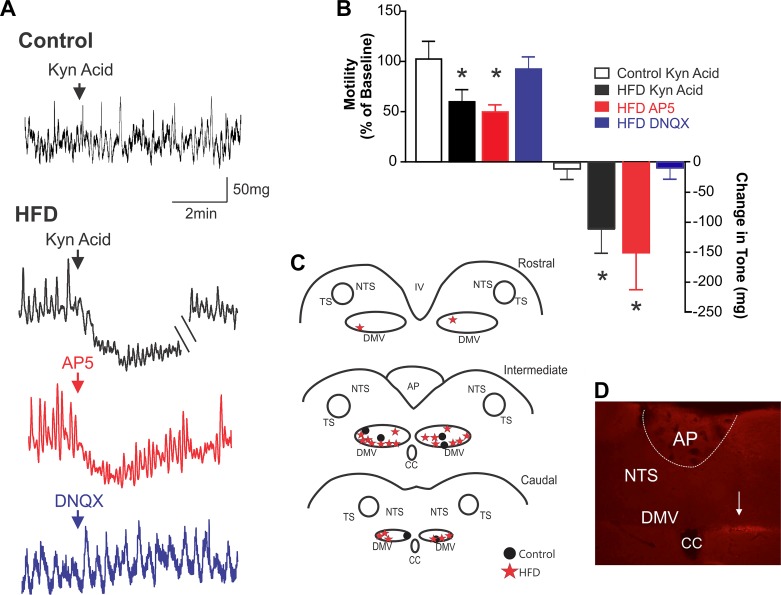
As shown previously (50), DVC microinjection of kynurenic acid (100 pmol/60 nl) in control rats has no effect on either corpus or antrum tone and motility (Fig. 5). In contrast, following acute HFD exposure, DVC microinjection of kynurenic acid induced a significant inhibition in tone and motility in both the corpus and antrum (n = 7/10 rats; P < 0.05 for each; note, for the sake of clarity, only antrum motility and tone are shown graphically in Figs. 5 and 6, and a summary of results can be found in Table 1). When repeated after 45-min recovery, reapplication of kynurenic acid in four acute HFD rats induced a similar decrease in antrum (63.0 ± 13.1% vs. 64.0 ± 10.7% and −82.0 ± 24.7 mg vs. −79.0 ± 9.4 mg) and corpus (30.0 ± 22.8% vs. 42.0 ± 29.8% and −95.0 ± 83.2 mg vs. −76.0 ± 1.5 mg; n = 4; P > 0.05 for each, data not shown) motility and tone, respectively. To determine whether the actions of kynurenic acid were mediated via antagonism of NMDA receptor vs. non-NMDA receptor, in four acute HFD rats in which kynurenic acid induced an inhibition in gastric tone and motility, subsequent microinjection of AP5 (500 pmol/60 nl) but not DNQX (10 pmol/60 nl) decreased corpus and antrum tone and motility (Fig. 5; Table 1), thus indicating a relevant NMDA-mediated component in the synaptic activity of acute HFD rats.
Fig. 5.
Brainstem microinjection of ionotropic glutamate receptor antagonists inhibits gastric tone and motility in acute high-fat diet (HFD) rats. A: representative recording demonstrating that dorsal vagal complex (DVC) microinjection of the nonselective glutamate receptor antagonist, kynurenic acid (100 pmol/60 nl) had no effect on antrum tone or motility in control diet rats (top). In contrast, DVC microinjection of kynurenic acid (100 pmol/60 nl; black trace), amino-5-phosphonopentanoate (AP5) (500 pmol/60 nl; red), but not 6,7-dinitroquinoxaline-2,3-dione (DNQX) (10 pmol/60 nl; blue), significantly inhibits antrum tone and motility in acute HFD rats. B: graphical representation of effects of brainstem microinjection of glutamate receptor antagonists on antrum motility (left) and tone (right) (n = 3–8 per data point; *P < 0.05 vs. baseline; paired Student’s t-test). C: map illustrating all brainstem microinjection sites, divided into rostral (top), intermediate (middle), and caudal (bottom) areas. Note that, for the sake of clarity, injection sites are marked bilaterally although all experiments were conducted using microinjections into the left DVC because recordings of motility and tone were made from the ventral stomach. D: photomicrograph illustrating a brainstem microinjection (arrow) at the level of the intermediate DVC. AP, area postrema; NTS, nucleus tractus solitarius; DMV, dorsal motor nucleus of the vagus; CC, central canal.
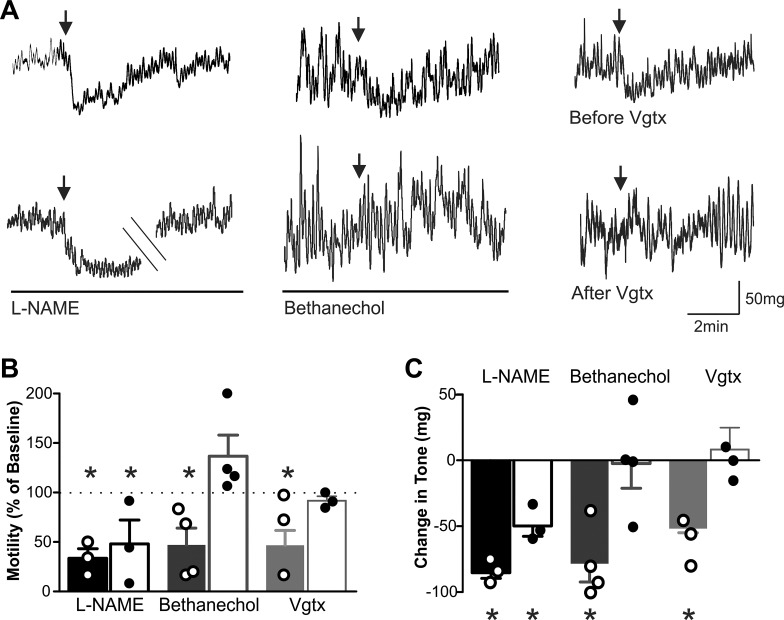
Fig. 6.
Brainstem microinjection of N-methyl-d-aspartate (NMDA) receptor antagonists inhibits gastric motility and tone in a vagally dependent manner involving the efferent cholinergic pathway. A: representative recordings demonstrating that the effects of dorsal vagal complex (DVC) microinjection of kynurenic acid (100 pmol/60 nl; top) are blocked by complete subdiaphragmatic vagotomy (Vgtx; right) and infusion of bethanechol (30 μg·kg−1·0.5 ml−1; middle) but not by infusion of nitro-l-arginine methyl ester (l-NAME) (10 mg·kg−1·0.5 ml−1; left). B: graphical summary of the effects of DVC microinjection of kynurenic acid on antrum motility before and after perfusion of l-NAME, perfusion of bethanecol, and complete subdiaphragmatic Vgtx, perfusion of l-NAME, and perfusion of bethanechol (n = 3–5 per data point; *P < 0.05 vs. baseline paired Student’s t-test). C: graphical summary of the effects of DVC microinjection of kynurenic acid on antrum tone, before and after complete subdiaphragmatic Vgtx, perfusion of l-NAME, and perfusion with bethanechol (n = 3–5 per data point; *P < 0.05 vs. baseline paired Student’s t-test).
Table 1.
Effects of brainstem microinjection of glutamatergic antagonists on gastric motility and tone
| Antrum |
Corpus |
||||
|---|---|---|---|---|---|
| Condition | Treatment | Motility | Tone | Motility | Tone |
| Control | Kynurenic acid | 102.5 ± 17.6% | −14.3 ± 19.3 mg | 109.4 ± 4.8% | −0.01 ± 0.24 mg |
| HFD | Kynurenic acid | 63.8 ± 12.6%* | −117.0 ± 42.8 mg* | 55.1 ± 13.4%* | −73.7 ± 22.8 mg* |
| AP5 | 49.7 ± 6.5%* | −150.9 ± 59.3 mg* | 57.2 ± 8.9%* | −40.4 ± 57.2 mg* | |
| DNQX | 75.9 ± 10.2% | −50.4 ± 31.7 mg | 62.0 ± 19.0% | −22.7 ± 70.7 mg | |
| Kynurenic acid before vagotomy | 62.1 ± 23.9%* | −60.9 ± 10.3 mg* | 56.2 ± 8.2%* | −108.6 ± 24.1 mg* | |
| Kynurenic acid after vagotomy | 91.8 ± 4.5% | −3.7 ± 12.4 mg | 87.8 ± 4.6% | −0.6 ± 0.1 mg | |
| Kynurenic acid before l-NAME | 33.6 ± 9.6%* | −84.4 ± 5.2 mg* | 76.9 ± 12.1%* | −95.8 ± 9.9 mg* | |
| Kynurenic acid after l-NAME | 48.1 ± 24.1%* | −48.9 ± 7.9 mg* | 47.8 ± 24.8% | −118.9 ± 33.5 mg* | |
| Kynurenic acid before bethanechol | 47.1 ± 16.9%* | −78.4 ± 14.0 mg* | 73.8 ± 5%* | −105.4 ± 31.7 mg* | |
| Kynurenic acid after bethanechol | 136.8 ± 21.4% | −1.4 ± 19.8 mg | 122.2 ± 22.2% | −86.9 ± 47.8 mg | |
Values are means ± SE. HFD, high-fat diet; AP5, amino-5-phosphonopentanoate; DNQX, 6,7-dinitroquinoxaline-2,3-dione; l-NAME, nitro-l-arginine methyl ester.
P < 0.05 vs. baseline.
To confirm the vagally dependent nature of these actions, the effects of kynurenic acid (100 pmol/60 nl) on gastric tone and motility were assessed before and after complete subdiaphragmatic vagotomy; following vagotomy, kynurenic acid had no effect on corpus or antrum tone or motility (Fig. 6; Table 1). To determine the vagal efferent pathway involved in the gastric inhibition induced by DVC microinjection of ionotropic glutamate receptor antagonists, the response to kynurenic acid (100 pmol/60 nl) was assessed before and after intravenous infusion of nitro-l-arginine methyl ester (10.0 mg·kg−1·0.5 ml−1) and bethanechol (30 μg·kg−1·0.5 ml−1). Briefly, in three rats in which kynurenic acid induced an inhibition in corpus and antrum tone and motility, subsequent injection of kynurenic acid during nitro-l-arginine methyl ester infusion still produced a significant inhibition in antrum and corpus tone and motility (Fig. 6; Table 1). In contrast, in four rats in which kynurenic acid induced a significant inhibition in tone and motility, subsequent reapplication of kynurenic acid during infusion of bethanechol no longer resulted in an inhibition in corpus or antrum motility and tone (Fig. 6;Table 1).
These data suggest that the acute HFD-induced increased activation of the NMDA receptor increases gastric tone and motility through activation of a vagally dependent efferent cholinergic pathway.
DISCUSSION
Data from the present study suggest that acute (3–5 days) exposure to an HFD 1) increases synaptic NMDA receptor-mediated currents in gastric-projecting DMV neurons, 2) alters the voltage dependency and magnitude of NMDA receptor signaling at potentials positive to −60 mV, 3) increases DMV neuronal excitability and AP firing rate, and 4) increases gastric motility and tone in a manner involving activation of the efferent vagal excitatory cholinergic pathway. Furthermore, these changes in glutamatergic signaling occur over a time period consistent with the homeostatic regulation of caloric intake and body mass.
Multiple studies in both humans and rodent models have highlighted the dysregulation of vago-vagal neurocircuits that occurs after chronic exposure to an HFD or following with development of DIO (15, 23, 24, 38, 39, 45). Determining whether such changes are diet induced vs. obesity induced have proven difficult, however, especially because previous studies suggest that they may exert distinct effects on vagal functions (15). Despite the prominent role of vagal neurocircuits in the homeostatic regulation of energy balance and food intake (8, 10, 27), few studies have investigated whether vagal dysfunction contributes to the development of, or occurs in response to, obesity. Even less attention has been paid to the effects of acute HFD exposure, despite previous studies noting compromised vagal functions within short periods of time (3 days) (57). Alterations within autonomic neurocircuits in response to acute HFD exposure may provide valuable insights into homeostatic regulation within these networks because several studies, including the present one, demonstrate restricted food intake to balance caloric demand within the short term (7, 9, 60). Results of the present study indicate that the observed alterations in glutamatergic signaling parallel the temporal pattern of homeostatic adaptation, suggesting that such synaptic plasticity may play a role in the acute regulation of food intake and energy balance. It is also possible, however, that, despite the lack of observable alterations in NMDA receptor-mediated neuronal excitability after 1 day of HFD exposure, the subsequent neuroplasticity may be merely a persistent response to this initial HFD exposure and, therefore, unrelated to homeostatic compensation. Altered NMDA signaling has been shown to occur rapidly within central vagal neurocircuits in a manner consistent with the ongoing control and maintenance of synaptic fidelity, however (61), suggesting a common pattern of involvement in homeostatic regulation. Nevertheless, further experiments will be required to delineate the sufficiency, necessity, and temporal patterning of such glutamatergic modulation in the autonomic regulation of food intake. There were no observed differences, either qualitative or quantitative, between male and female rats in any of the electrophysiological studies. Because it is well recognized that female rats display estrus-related fluctuations in gastric motility, only male rats were used for the in vivo experiments. The electrophysiological data, however, suggest that there are no significant differences in the responses between male and female rats following an acute HFD.
Modulation of glutamatergic signaling is a hallmark of adaptive neuronal plasticity (2, 28, 35, 49). Although several studies from this and other laboratories have demonstrated that DMV neurons receive glutamatergic synaptic inputs (5, 25, 55), and that glutamatergic synaptic transmission is modulated by a variety of neurotransmitters and neuromodulators (16, 18, 19, 32, 34, 51, 59), it is GABA that has long been considered the primary synaptic neurotransmitter regulating vagal efferent motoneuron activity (50, 55). The present study suggests that exposure to an acute HFD upregulates glutamatergic signaling to gastric-projecting DMV neurons through increased activation of NMDA receptor. This, in turn, increases DMV neuronal excitability and increases vagal efferent input to the stomach via activation of the excitatory cholinergic pathway.
The present study also examined the physiological outcomes of altered glutamatergic signaling at NTS-DMV synapses; microinjection of kynurenic acid had no effect on gastric functions in control rats but significantly decreased gastric tone and motility following acute HFD exposure. Our in vivo results further confirmed our in vitro studies, demonstrating that these effects were due to actions at NMDA receptor and additionally suggested that these effects were due to vagally dependent actions involving the efferent cholinergic pathway. Thus our results suggest that the altered glutamatergic signaling observed centrally increases the tonic vagal efferent cholinergic drive to the stomach. Given the role that gastric motility, compliance, and emptying exert on meal size and food intake (22, 36, 45), such neuroplasticity may be, at least in part, responsible for the regulation of caloric intake observed over the experimental study period.
Previous studies investigating glutamatergic signaling at central vagal neurocircuits have shown that, at higher frequencies of stimulation, a frequency-dependent facilitation of glutamatergic signaling at NTS neurons occurs via postsynaptic NMDA receptors; this maintains synaptic fidelity under conditions where AMPA receptor is desensitized, preserving the fidelity of synaptic transmission by allowing NMDA receptor to increase postsynaptic neuronal excitability (61). In contrast, the present study suggests that upregulation of glutamatergic signaling via recruitment of NMDA receptor activation in central vagal neurocircuits may also occur at spontaneous levels of neurotransmission. Although the mechanism by which acute HFD exposure-increased NMDA receptor-mediated glutamatergic signaling is unknown, it appears to occur via postsynaptic sites of action. The decrease in mEPSC amplitude in response to AP5 is to be expected if acute HFD exposure results in the activation of synaptic NMDA receptor as well as AMPA receptor. Although the significant decrease in mEPSC frequency following AP5 application may also suggest a presynaptic site of action, it is more likely to be due to a threshold effect, when the antagonist decreases mEPSC amplitude below detectable levels. The present study did not investigate whether acute HFD exposure altered the proportion of DMV neurons expressing NMDA receptors. Previous studies, however, determined that >80% of gastric-projecting DMV neurons express NMDA and/or AMPA receptors (1, 13), suggesting that an acute HFD-induced increase in receptor expression is unlikely to be responsible for the observed changes. Although the period of HFD exposure (3–5 days) is most likely sufficient to allow altered expression and/or trafficking of NMDA receptors, the tonic activation of these receptors at or close to resting membrane potential implies a significant alteration in their voltage dependency of activation, irrespective of putative changes in receptor number.
Previously, the activation of extrasynaptic NMDA receptors has been shown to induce a localized postsynaptic depolarization sufficient to remove the Mg2+ block of synaptic NMDA receptor-gated cation channels (40). Further studies will be required to determine whether a similar mechanism is responsible for the activation of synaptic NMDA receptors observed in the present study, whether this involves a similar activation of extrasynaptic NMDA receptors, and, if so, the source of the glutamate. Indeed, although altered glutamate uptake and synaptic overspill may modulate glutamatergic signaling, previous studies have demonstrated that altered glutamatergic signaling may also be due to modified astroglial function and release of gliotransmitters, including glutamate itself or d-serine, the essential cofactor for the synaptic NMDA receptor (12, 30, 37, 40). In this regard, it is interesting to note that, within the hypothalamus, acute HFD exposure activates astroglia within a similar time frame (3, 6, 21, 58). Whether acute HFD activates astroglia and/or induces gliotransmission within vagal brainstem neurocircuits remains to be elucidated.
In conclusion, the results of the present study suggest that 3–5 days of exposure to a HFD is sufficient to cause an alteration in glutamatergic signaling in the DMV. Specifically, we observed a tonic activation of NMDA receptor in DMV neurons that increased AP firing rates. Because DMV neurons are pacemakers and have a resting membrane potential within 1–2 mV of AP firing threshold (54), even small alterations in synaptic input can have profound effects on DMV neuronal excitability, as well as dramatic effects on vagally dependent gastric functions. Previous studies have suggested that the gastric dysfunctions associated with obesity are caused by excessive weight gain, metabolic dysregulation, and peripheral inflammation. The present study suggests, however, that vagal neurocircuit dysregulation occurs well in advance of the development of obesity. Although the exact mechanisms responsible for the altered glutamatergic signaling following acute HFD exposure remain to be elucidated, their potential involvement in the homeostatic regulation of energy balance remains of interest.
GRANTS
This work was supported by National Institutes of Health Grant DK-78364 and NSF IOS 114978.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.C., R.A.T., and K.N.B. conceived and designed research; C.C., R.A.T., and K.N.B. performed experiments; C.C., R.A.T., and K.N.B. analyzed data; C.C., R.A.T., and K.N.B. interpreted results of experiments; C.C. and K.N.B. prepared figures; C.C. and K.N.B. drafted manuscript; C.C., R.A.T., and K.N.B. edited and revised manuscript; C.C., R.A.T., and K.N.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank W. Nairn Browning, as well as Cesare M. and Zoraide Travagli, for support and encouragement.
REFERENCES
- 1.Ammori JB, Zhang W, Newman EA, Mulholland MW. Glutamate-induced calcium transients in rat neurons of the dorsal motor nucleus of the vagus. J Gastrointest Surg 11: 1016–1024, 2007. doi: 10.1007/s11605-007-0176-1. [DOI] [PubMed] [Google Scholar]
- 2.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol 22: 461–469, 2012. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astiz M, Pernía O, Barrios V, Garcia-Segura LM, Diz-Chaves Y. Short-term high-fat diet feeding provides hypothalamic but not hippocampal protection against acute infection in male mice. Neuroendocrinology 104: 40–50, 2017. doi: 10.1159/000444527. [DOI] [PubMed] [Google Scholar]
- 4.Babic T, Browning KN, Kawaguchi Y, Tang X, Travagli RA. Pancreatic insulin and exocrine secretion are under the modulatory control of distinct subpopulations of vagal motoneurones in the rat. J Physiol 590: 3611–3622, 2012. doi: 10.1113/jphysiol.2012.234955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol 300: G21–G32, 2011. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belegri E, Eggels L, Unmehopa UA, Mul JD, Boelen A, la Fleur SE. The effects of overnight nutrient intake on hypothalamic inflammation in a free-choice diet-induced obesity rat model. Appetite 120: 527–535, 2018. doi: 10.1016/j.appet.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Berthoud HR. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity (Silver Spring) 14, Suppl 5: 197S–200S, 2006. doi: 10.1038/oby.2006.308. [DOI] [PubMed] [Google Scholar]
- 8.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept 149: 15–25, 2008. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthoud H-R. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite 43: 315–317, 2004. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Berthoud H-R. The caudal brainstem and the control of food intake and energy balance. In: Neurobiology of Food and Fluid Intake, edited by Stricker E, Woods S. New York: Plenum, 2004. doi: 10.1007/0-306-48643-1_9. [DOI] [Google Scholar]
- 11.Bhagat R, Fortna SR, Browning KN. Exposure to a high fat diet during the perinatal period alters vagal motoneurone excitability, even in the absence of obesity. J Physiol 593: 285–303, 2015. doi: 10.1113/jphysiol.2014.282806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonansco C, Couve A, Perea G, Ferradas CA, Roncagliolo M, Fuenzalida M. Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur J Neurosci 33: 1483–1492, 2011. doi: 10.1111/j.1460-9568.2011.07631.x. [DOI] [PubMed] [Google Scholar]
- 13.Broussard DL, Li H, Altschuler SM. Colocalization of GABA(A) and NMDA receptors within the dorsal motor nucleus of the vagus nerve (DMV) of the rat. Brain Res 763: 123–126, 1997. doi: 10.1016/S0006-8993(97)00344-2. [DOI] [PubMed] [Google Scholar]
- 14.Browning KN, Babic T, Toti L, Holmes GM, Coleman FH, Travagli RA. Plasticity in the brainstem vagal circuits controlling gastric motor function triggered by corticotropin releasing factor. J Physiol 592: 4591–4605, 2014. doi: 10.1113/jphysiol.2014.278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol 591: 2357–2372, 2013. doi: 10.1113/jphysiol.2012.249268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci 22: 2998–3004, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol 517: 521–532, 1999. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Browning KN, Travagli RA. Characterization of the in vitro effects of 5-hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV). Br J Pharmacol 128: 1307–1315, 1999. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browning KN, Travagli RA. Neuropeptide Y and peptide YY inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Physiol 549: 775–785, 2003. doi: 10.1113/jphysiol.2003.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol 4: 1339–1368, 2014. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckman LB, Thompson MM, Lippert RN, Blackwell TS, Yull FE, Ellacott KL. Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Mol Metab 4: 58–63, 2014. doi: 10.1016/j.molmet.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology 148: 1219–1233, 2015. doi: 10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept 86: 83–88, 2000. doi: 10.1016/S0167-0115(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 24.Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol 589: 2857–2870, 2011. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017: 208–217, 2004. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav 105: 100–105, 2011. doi: 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dockray GJ. The versatility of the vagus. Physiol Behav 97: 531–536, 2009. doi: 10.1016/j.physbeh.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Dore K, Stein IS, Brock JA, Castillo PE, Zito K, Sjöström PJ. Unconventional NMDA receptor signaling. J Neurosci 37: 10800–10807, 2017. doi: 10.1523/JNEUROSCI.1825-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11: 845–851, 2003. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- 30.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43: 729–743, 2004. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 32.Glatzer NR, Smith BN. Modulation of synaptic transmission in the rat nucleus of the solitary tract by endopmorphin-1. J Neurophysiol 93: 2530–2540, 2005. 10.1152/jn.00429.2004. [DOI] [PubMed] [Google Scholar]
- 33.Glaum SR, Miller RJ. Activation of metabotropic glutamate receptors produces reciprocal regulation of ionotropic glutamate and GABA responses in the nucleus of the tractus solitarius of the rat. J Neurosci 13: 1636–1641, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. J Physiol 587: 4749–4759, 2009. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron 80: 704–717, 2013. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther 33: 880–894, 2011. doi: 10.1111/j.1365-2036.2011.04609.x. [DOI] [PubMed] [Google Scholar]
- 37.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10: 331–339, 2007. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 38.Kentish S, Li H, Philp LK, O’Donnell TA, Isaacs NJ, Young RL, Wittert GA, Blackshaw LA, Page AJ. Diet-induced adaptation of vagal afferent function. J Physiol 590: 209–221, 2012. doi: 10.1113/jphysiol.2011.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kentish SJ, Vincent AD, Kennaway DJ, Wittert GA, Page AJ. High-fat diet-induced obesity ablates gastric vagal afferent circadian rhythms. J Neurosci 36: 3199–3207, 2016. doi: 10.1523/JNEUROSCI.2710-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. Astrocytic control of synaptic NMDA receptors. J Physiol 581: 1057–1081, 2007. doi: 10.1113/jphysiol.2007.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levin BE. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philos Trans R Soc Lond B Biol Sci 361: 1107–1121, 2006. doi: 10.1098/rstb.2006.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levin BE. Developmental gene x environment interactions affecting systems regulating energy homeostasis and obesity. Front Neuroendocrinol 31: 270–283, 2010. doi: 10.1016/j.yfrne.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin BE. Interaction of perinatal and pre-pubertal factors with genetic predisposition in the development of neural pathways involved in the regulation of energy homeostasis. Brain Res 1350: 10–17, 2010. doi: 10.1016/j.brainres.2009.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol 543: 135–146, 2002. doi: 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity–oral and gastrointestinal sensory contributions. Physiol Behav 104: 613–620, 2011. doi: 10.1016/j.physbeh.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 46.Little TJ, Horowitz M, Feinle-Bisset C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: implications for the pathophysiology of obesity. Am J Clin Nutr 86: 531–541, 2007. doi: 10.1093/ajcn/86.3.531. [DOI] [PubMed] [Google Scholar]
- 47.McMenamin CA, Travagli RA, Browning KN. Perinatal high fat diet increases inhibition of dorsal motor nucleus of the vagus neurons regulating gastric functions. Neurogastroenterol Motil, 2018. In press. doi: 10.1111/nmo.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol 285: R479–R489, 2003. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth RH, Zhang Y, Huganir RL. Dynamic imaging of AMPA receptor trafficking in vitro and in vivo. Curr Opin Neurobiol 45: 51–58, 2017. doi: 10.1016/j.conb.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil 10: 305–313, 1998. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 51.Smith BN, Davis SF, Van Den Pol AN, Xu W. Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience 115: 707–714, 2002. doi: 10.1016/S0306-4522(02)00488-8. [DOI] [PubMed] [Google Scholar]
- 52.Tamashiro KL, Moran TH. Perinatal environment and its influences on metabolic programming of offspring. Physiol Behav 100: 560–566, 2010. doi: 10.1016/j.physbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol 13: 389–401, 2016. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Travagli RA, Gillis RA. Hyperpolarization-activated currents, IH and IKIR, in rat dorsal motor nucleus of the vagus neurons in vitro. J Neurophysiol 71: 1308–1317, 1994. doi: 10.1152/jn.1994.71.4.1308. [DOI] [PubMed] [Google Scholar]
- 55.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol 260: G531–G536, 1991. [DOI] [PubMed] [Google Scholar]
- 56.Treesukosol Y, Moran TH. Analyses of meal patterns across dietary shifts. Appetite 75: 21–29, 2014. doi: 10.1016/j.appet.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Troy AE, Browning KN. High fat diet decreases glucose-dependent modulation of 5-HT responses in gastrointestinal vagal afferent neurons. J Physiol 594: 99–114, 2016. doi: 10.1113/JP271558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waise TM, Toshinai K, Naznin F, NamKoong C, Md Moin AS, Sakoda H, Nakazato M. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun 464: 1157–1162, 2015. doi: 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- 59.Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 148: 1868-1881, 2007. doi: 10.1210/en.2006-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woods SC, Seeley RJ, Porte D Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science 280: 1378–1383, 1998. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 61.Zhao H, Peters JH, Zhu M, Page SJ, Ritter RC, Appleyard SM. Frequency-dependent facilitation of synaptic throughput via postsynaptic NMDA receptors in the nucleus of the solitary tract. J Physiol 593: 111–125, 2015. doi: 10.1113/jphysiol.2013.258103. [DOI] [PMC free article] [PubMed] [Google Scholar]