Abstract
We investigated whether vasoactive intestinal peptide (VIP) and/or prostaglandins contribute to peripheral corticotropin-releasing factor (CRF)-induced CRF1 receptor-mediated stimulation of colonic motor function and diarrhea in rats. The VIP antagonist, [4Cl-D-Phe6, Leu17]VIP injected intraperitoneally completely prevented CRF (10 µg/kg ip)-induced fecal output and diarrhea occurring within the first hour after injection, whereas pretreatment with the prostaglandins synthesis inhibitor, indomethacin, had no effect. In submucosal plexus neurons, CRF induced significant c-Fos expression most prominently in the terminal ileum compared with duodenum and jejunum, whereas no c-Fos was observed in the proximal colon. c-Fos expression in ileal submucosa was colocalized in 93.4% of VIP-positive neurons and 31.1% of non-VIP-labeled neurons. CRF1 receptor immunoreactivity was found on the VIP neurons. In myenteric neurons, CRF induced only a few c-Fos-positive neurons in the ileum and a robust expression in the proximal colon (17.5 ± 2.4 vs. 0.4 ± 0.3 cells/ganglion in vehicle). The VIP antagonist prevented intraperitoneal CRF-induced c-Fos induction in the ileal submucosal plexus and proximal colon myenteric plexus. At 60 min after injection, CRF decreased VIP levels in the terminal ileum compared with saline (0.8 ± 0.3 vs. 2.5 ± 0.7 ng/g), whereas VIP mRNA level detected by qPCR was not changed. These data indicate that intraperitoneal CRF activates intestinal submucosal VIP neurons most prominently in the ileum and myenteric neurons in the colon. It also implicates VIP signaling as part of underlying mechanisms driving the acute colonic secretomotor response to a peripheral injection of CRF, whereas prostaglandins do not play a role.
NEW & NOTEWORTHY Corticotropin-releasing factor (CRF) in the gut plays a physiological role in the stimulation of lower gut secretomotor function induced by stress. We showed that vasoactive intestinal peptide (VIP)-immunoreactive neurons in the ileal submucosal plexus expressed CRF1 receptor and were prominently activated by CRF, unlike colonic submucosal neurons. VIP antagonist abrogated CRF-induced ileal submucosal and colonic myenteric activation along with functional responses (defecation and diarrhea). These data point to VIP signaling in ileum and colon as downstream effectors of CRF.
Keywords: c-Fos, corticotropin-releasing factor, enteric neurons, diarrhea, vasoactive intestinal peptide antagonist
INTRODUCTION
The corticotropin-releasing factor (CRF) signaling systems encompass CRF and the related peptides, urocortin 1, that interact with both CRF receptor subtypes, CRF1 and CRF2, and urocortin 2 and 3 that are specific CRF2 receptor ligands (18). The role of CRF-CRF1 receptor pathway in stress manifestations was delineated initially in the brain as a result of the widely held consensus that CRF in the central nervous system plays an important role in coordinating the stress response (1, 66). However, the CRF signaling systems localized in the gut have now emerged as important local components of acute stress-related alterations of gastrointestinal function (32, 39, 65). In particular, in the distal gut, CRF or urocortin 1 injected peripherally increases clustered spike-burst propagative activity in the cecum and proximal and distal colon, accelerates colonic transit, increases intestinal permeability, induces defecation, and spurs the onset of watery diarrhea in rodents and guinea pigs (19, 40, 41, 48, 51, 65). In vitro studies performed on isolated rat colonic preparations or muscle strips further ascertained a local site of action for CRF or urocortin 1 to stimulate the amplitude of basal myoelectric peristaltic activity and phasic contractions via mechanisms that involve the enteric nervous system (29, 40, 48).
Pharmacological studies using peripheral administration of peptide agonists selective for CRF1 receptor (cortagine and stressin1-A) (57, 67) or CRF2 receptor (urocortin 2) (55), as well as CRF antagonists (astressin) (9), established that CRF1 receptor signaling mediates the stimulatory actions of CRF and urocortin 1 on colonic motility in vivo and in vitro and the induction of diarrhea in rodents (33, 40, 41, 48, 74). By contrast, the activation of CRF2 receptor has opposite effects and counteracts the stimulation of the colon induced by peripheral CRF1 receptor agonists (15, 48).
Vasoactive intestinal peptide (VIP) is one of the most abundant neuropeptides innervating the gastrointestinal tract in several mammalian species (12). The peptide is expressed in many enteric neuronal subtypes most prominently in submucosal secretomotor neurons of the intestine (25). VIP is well known to regulate physiological processes, including intestinal secretion and motility (11, 16, 62). VIP actions are largely mediated through VCAP1 receptor expressed on apical membranes of the mucosal, cholinergic excitatory motor neurons innervating longitudinal muscles, cholinergic secretomotor neurons, and mucosal mast cells (11, 21, 27).
Recent studies indicate that VIP plays a role in acute water avoidance stress-induced alterations of ileal barrier function known to also involve CRF receptor-dependent mechanisms (27, 28). Other evidence indicates that CRF induces prostaglandin E2 (PGE2) release from rat colonic explants (3). Both VIP and PGE2 display diarrheogenic properties (5, 23, 58). However, whether VIP and/or PGs play a role in peripheral CRF-induced watery diarrhea is still to be investigated.
In the present study, we tested the influence of the VPAC1 and VPAC2 receptor antagonist [4Cl-D-Phe6,Leu17]VIP (53) and indomethacin, the nonselective inhibitor of cyclooxygenase (COX)-1 and COX-2 participating in PGs synthesis, on the stimulation of defecation and occurrence of diarrhea induced by intraperitoneal CRF. We then examined whether the VIP antagonist modulates intraperitoneal CRF-induced enteric neuronal activation (44) monitored by c-Fos induction (31) and whether CRF alters VIP levels in the ileum. Lastly, we assessed the neuroanatomical basis for CRF-VIP interactions by examining the localization of CRF1 receptor on VIP neurons in the ileal submucosal plexus.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (Harlan, San Diego, CA) weighing 225–250 g were housed under controlled conditions of temperature (22 ± 2°C) and lighting (from 6:00 AM to 6:00 PM). Animals had ad libitum access to a standard rodent diet (Prolab RMH 2500 LabDiet; PMI Nutritional, Brentwood, MO) and tap water. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All experiments were approved by the Animal Research Committee at the Veterans Affairs Greater Los Angeles Healthcare System (protocol number 9906-820).
Peptides and compounds.
Rat/human CRF and [4Cl-D-Phe6,Leu17]VIP (VIP antagonist) (53) were obtained from Dr. J. Rivier (Sentia Medical Sciences, La Jolla, CA). The peptides were stored in powder form at −80°C and then weighed and dissolved in saline immediately before administration. Indomethacin (Sigma-Aldrich, St. Louis, MO) was dissolved in 1% sodium bicarbonate. The volume of all intraperitoneal injections was 1 ml/kg.
Monitoring of defecation and diarrhea.
Each rat was placed in a new individual cage without food and water, and the pellet output and diarrhea were monitored every 15 min for 1 h posttreatment. Diarrhea was scored 0 to 3 based on the evaluation of pellets as in our previous study (70): 0 = normal, 1 = soft with color change with shape, 2 = lack of shape, and 3 = watery feces. The incidence was calculated as the number of rats with score >0 per total number of rats per group.
Whole mount preparation of submucosal and myenteric plexus.
This was performed as we described previously (73, 74). Rats were euthanized by CO2 followed by decapitation. The duodenum, jejunum, terminal ileum, and proximal and distal colon were collected. The tissues were cut along the mesenteric border, pinned flat on a Sylgard-coated Petri dish (Sylgard 184; Dow Corning, Midland, MI), and fixed by immersion with 4% paraformaldehyde and 14% saturated picric acid in 0.1 M phosphate buffer (PB, pH 7.4) overnight at 4°C. Thereafter, tissues were washed several times with 0.01 M PBS (pH 7.4). Samples were dissected under a surgical microscope to obtain the longitudinal muscle/myenteric plexus (LMMP) or submucosal whole mount preparations.
c-Fos immunohistochemistry.
Free-floating LMM and submucosal whole mount preparations were processed for c-Fos immunohistochemistry as previously described in rats (70, 73, 74). Preparations were incubated for 2 days at 4°C with rabbit polyclonal anti-Fos antibody (1:10,000; Ab-5, PC38; EMD Millipore, Billerica, MA) (42) in 0.01M PBS (pH 7.4) containing 0.3% Triton X-100. Whole mounts were then incubated with biotinylated secondary goat anti-rabbit IgG (1:1,000; Jackson ImmunoResearch, West Grove, PA) followed with avidin-biotin-peroxidase complex (1:200; Vector, Burlingame, CA), each lasting 1 h at room temperature. The chromogen was 3,3′-diaminobenzidine tetrachloride (DAB) (0.025%, Sigma-Aldrich) with hydrogen peroxide (0.01%, Sigma-Aldrich). After the staining, the whole mounts were mounted onto microscopic glass slides, air dried, dehydrated in ethanol, cleared in xylenes, and coverslipped.
Double immunostaining.
This was performed as in our previous studies (73, 76). Submucosa and LMMP whole mount preparations of terminal ileum were immunostained for c-Fos/VIP and for VIP/CRF1 receptor double labeling. For c-Fos and VIP, the whole mounts were processed first for c-Fos using the same procedures as above except for two modifications. First, the biotinylated secondary goat anti-rabbit IgG was a Fab fragment (1:1,000; Jackson ImmunoResearch). Second, the DAB solution contained 2.5% nickel ammonium sulfate and 0.001% hydrogen peroxide in 0.1 M acetate buffer (pH 6.5). After a thorough rinse, the whole mounts were incubated with rabbit anti-VIP (1:2,000, rabbit polyclonal antiserum, CURE antibody core no. 7913; UCLA, Los Angeles, CA) (7, 13) for 2 days at 4°C. The remaining processing was the same as for c-Fos. For double labeling of CRF1 receptor and VIP, the submucosal plexus preparations of ileum were incubated with a mix of goat anti-CRF1 receptor (1:200, C-20; Santa Cruz Biotechnology, Santa Cruz, CA) (74) and rabbit anti-VIP (1:1,000, CURE antibody no. 7913) (7, 13) for 2 days at 4°C. The secondary antibody mix was donkey anti-goat-Red X and donkey anti-rabbit-FITC (both at 1:500; Jackson ImmunoResearch Laboratories) and incubated for 2 h at room temperature.
Cell counting.
The counting of immunoreactive cell number was performed blindly under a light microscopy (Zeiss Axioskop II; Carl Zeiss International, Jena, Germany) in 20 ganglia randomly selected from an identical area of intestinal or colonic preparation containing submucosal or myenteric plexus. Immunostained cells were averaged and expressed as the mean number per ganglia for each intestinal or colonic segment in each rat.
VIP determinations in plasma.
One milliliter of blood was collected from the portal vein with a syringe containing 20 µl of 7.5% ethylenediaminetetraacetic acid (Sigma-Aldrich) and then put into tubes on ice containing 20 µl of aprotinin (0.6 TIU/ml; MP Biomedicals, Solon, OH) and 40 µl of 25 mg/ml of Pefabloc SC (Sigma-Aldrich). Samples were centrifuged for 5 min (4°C, 1,600 g), and plasma was stored −80°C until measurement. Terminal ileum (~1 g) was homogenized in 20 ml of 10% trifluoroacetate buffer using a Polytron and centrifuged for 10 min at 3,000 g and 4°C. The extraction procedures were similar as previously described (26). SepPak was equilibrated with 5 ml of 100% acetonitrile and 5 ml of 0.1% trifluoroacetate before extraction. The supernatant was then loaded on 1 g SepPak (Waters, Milford, MA), which was rinsed with 5 ml of 0.1% trifluoroacetate and then eluted with 5 ml of 20% acetonitrile containing 0.1% trifluoroacetic acid (TFA) and 70% acetonitrile containing 0.1% TFA. The eluates were lyophilized with 70% acetonitrile SepPak fractions by vacuum centrifugation (Speed-Vac; Thermo Quest; Marietta, OH).
VIP plasma levels and ileal concentrations were determined with VIP ELISA kit (EK-064-16; Phoenix Pharmaceuticals, Burlingame, CA), following the manufacturer’s protocol. All samples were assayed at one time. The intra-assay variation was <10%.
VIP expression by quantitative PCR.
Total RNA of terminal ileum was processed for isolation according to the manufacturer’s recommendation using TRIzol reagent (Invitrogen, Carlsbad, CA). Isolated RNA was reverse transcribed to complementary DNA (cDNA) using high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Quantitative PCR was performed using TaqMan Gene Expression Master Mix (Applied Biosystems) and StepOne Plus Real-Time PCR system (Applied Biosystems). Forward and reverse primers and probes designed for each gene were obtained from Applied Biosystems. The assay ID for housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was Rn01775763_g1 and for VIP Rn01430567_m1.
EXPERIMENTAL PROTOCOLS
All experiments were performed in freely fed rats that were pair-housed with enriched environment. In the afternoon before the experimental day, animals were placed individually in new cages to avoid the stress of novelty. On the next day, after the intraperitoneal injections, rats were put back individually in cage with clean bedding. All treatments were performed between 8:00 AM and 10:00 AM.
Effect of VIP antagonist on intraperitoneal CRF-induced stimulation of pellet output and diarrhea.
The intraperitoneal injection of VIP antagonist, [4Cl-D-Phe6,Leu17]VIP (250 µg/kg), or saline was performed immediately before that of CRF (10 µg/kg) or vehicle (saline). Fecal pellet output and diarrhea were monitored every 15 min for 1 h after the second intraperitoneal injection. The dose of CRF was selected based on our previous dose-response studies showing the maximal defecation and diarrhea in rats (61). The VIP antagonist dose was based on pilot studies assessing the influence of [4Cl-D-Phe6,Leu17]VIP injected intraperitoneally at 1.50, 0.50, 0.25, or 0.10 mg/kg on defecation and choosing the maximal dose devoid of intrinsic effect. Rats were randomly assigned to different treatment groups. Experiments were performed with Latin square crossover design with 5–7 days of washout period with rats reused one or two times.
Effect of indomethacin on intraperitoneal CRF-induced stimulation of pellet output and diarrhea.
Indomethacin (5 mg/kg) or vehicle (1% sodium bicarbonate) was injected intraperitoneally 30 min before that of CRF (10 µg/kg) or vehicle (saline). The regimen of indomethacin administration was based on our previous studies showing the complete suppression of intravenous injection of interleukin-1β or adrenomedullin-induced gastric stasis in rats (43, 64). The fecal pellet output and diarrhea were monitored every 15 min for 1 h after CRF or vehicle intraperitoneal injection.
Effect of intraperitoneal CRF on c-Fos expression in intestinal and colonic myenteric and submucosal neurons.
Two groups of naïve rats received two consecutive intraperitoneal injections, saline/saline or saline/CRF (10 µg/kg), and 1 h later rats were euthanized with CO2 followed by decapitation. The duodenum, jejunum, terminal ileum, and colon were harvested and processed for the whole mount preparations of submucosal and myenteric plexus followed by c-Fos immunostaining. The terminal ileum was also processed for double immunostaining of c-Fos and VIP and double labeling of CRF1 receptor and VIP using the immunofluorescent method.
Effect of intraperitoneal VIP antagonist on intraperitoneal CRF-induced c-Fos expression in ileal submucosal and colonic myenteric neurons.
Three groups of naïve rats received two consecutive intraperitoneal injections, saline/saline, saline/CRF (10 µg/kg), or [4Cl-D-Phe6,Leu17]VIP (0.25 mg/kg)/CRF (10 µg/kg), and 1 h later rats were euthanized with CO2 followed by decapitation. The terminal ileum and proximal colon were harvested and processed for submucosal and myenteric plexus whole mount followed by c-Fos immunostaining.
Effect of intraperitoneal CRF on VIP levels in portal plasma and ileum and ileal VIP gene expression.
Rats were anesthetized by an overdose of isoflurane 15 min or 60 min after the intraperitoneal injection of CRF (10 µg/kg) or vehicle, and 1 ml of blood was withdrawn from the portal vein. Rats were then decapitated, and ~1.5 cm of terminal ileum was collected, rinsed with cold saline, and frozen on dry ice immediately. The plasma and ileum samples were stored in −80°C until processing for VIP by ELISA and ileum for VIP mRNA determination by qPCR.
Statistical analysis.
Statistical analysis was performed using SigmaPlot 12.5 (Systat Software, San Jose, CA). Data are presented as means ± SE. Comparisons between two groups were performed with the Student’s t-test. Comparisons between multiple groups were made by one-way or two-way ANOVA followed by Tukey’s post hoc multiple comparisons. Correlations were performed by lineal regression. Differences with P < 0.05 were considered significant.
RESULTS
VIP antagonist blocks intraperitoneal CRF-stimulated fecal pellet output and diarrhea, whereas indomethacin had no effect.
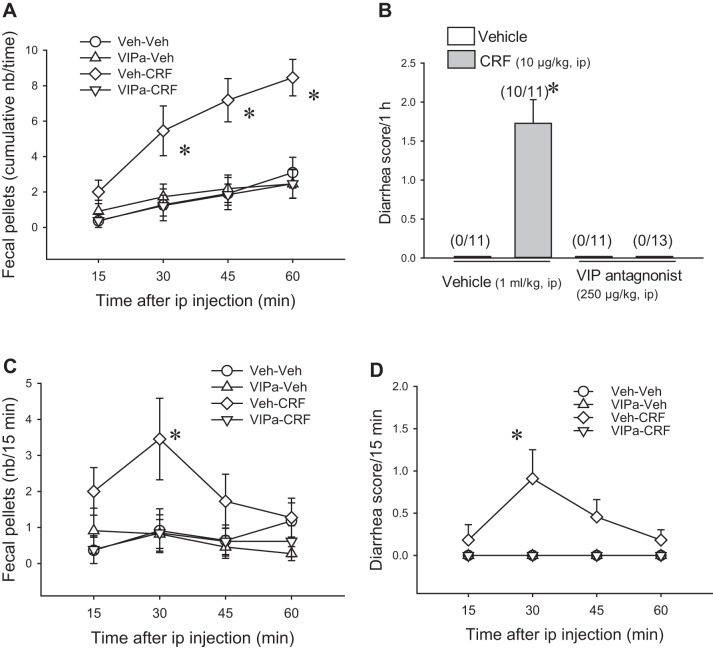
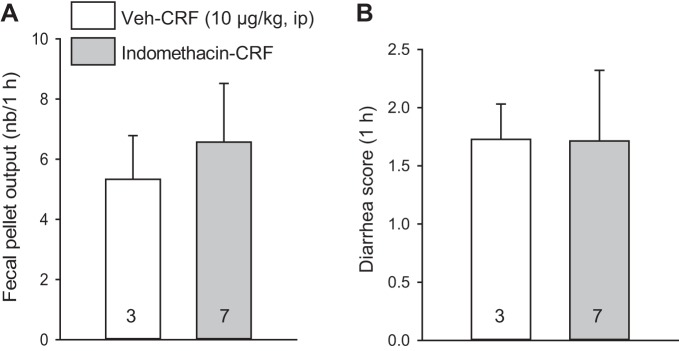
Saline/CRF (10 µg/kg ip) stimulated fecal pellet output compared with intraperitoneal vehicle (8.5 ± 1.0 pellets/h vs. 3.1 ± 0.9, n = 11/group; P < 0.01, Fig. 1A), and diarrhea occurred with a score/h of 1.7 ± 0.3 and incidence of 10/11 rats compared with none in the vehicle (saline/saline) group (P < 0.01, Fig. 1B). The peak response appeared within the 15- to 30-min period after injection (Fig. 1, C and D). The VIP antagonist (250 µg/kg ip)/saline did not influence fecal pellet output (2.5 ± 0.8, pellets/h, n = 11) and did not cause diarrhea (0 ± 0 score/h, n = 11), while completely preventing intraperitoneal CRF-induced stimulation of fecal output (2.5 ± 0.8, pellets/h, n = 13, P < 0.01) and the occurrence of diarrhea (0 ± 0 diarrhea score/h, n = 13; P < 0.01; Fig. 1). By contrast, vehicle/CRF (10 µg/kg ip) induced a similar fecal output and diarrhea score in indomethacin (5 mg/kg ip)/CRF rats (6.6 ± 2.0 vs. 5.3 ± 1.5 pellets/h; 1.7 ± 0.9 vs. 1.7 ± 0.6 diarrhea score/h; Fig. 2).
Fig. 1.
The vasoactive intestinal peptide (VIP) antagonist blocks intraperitoneal corticotropin-releasing factor (CRF)-induced stimulation of defecation and diarrhea in rats. [4Cl-D-Phe6,Leu17]VIP (250 µg/kg) was injected intraperitoneally immediately before CRF (10 µg/kg ip) in normally fed rats. Fecal output and diarrhea were monitored every 15 min for 1 h. A: cumulative fecal output. B: 1-h diarrhea score. Numbers in parentheses represent the incidence. C and D: time course of fecal output and diarrhea score. Data are means ± SE, n = 11–13 rats/group, *P < 0.001 vs. other groups (1-way ANOVA with Tukey’s post hoc multiple comparisons). 2-way ANOVA of CRF and VIP antagonist (VIPa) influence in 1-h fecal pellets and diarrhea: CRF, P < 0.01; VIPa, P < 0.001; CRF × VIPa, P < 0.01. Veh: vehicle (saline/saline).
Fig. 2.
Indomethacin does not influence intraperitoneal corticotropin-releasing factor (CRF)-induced stimulation of fecal pellet output and diarrhea score in rats. Indomethacin was injected intraperitoneally at 5 mg/kg 30 min before intraperitoneal CRF (10 µg/kg) in normally fed rats. Fecal output and diarrhea were monitored for 1 h. A: fecal output. B: diarrhea score. Data are means ± SE, n = 3–7 rats/group (P > 0.05, Student’s t-test).
Two-way ANOVA analyses showed a significant influence of CRF, VIP antagonist, and interaction between CRF and VIP antagonist on fecal pellet output (CRF: F1,42 = 9.2; VIP antagonist: F1,42 = 9.2; CRF × VIP antagonist: F1,42 = 14.1, all P < 0.01) and diarrhea scores (CRF, VIP antagonist, and CRF × VIP antagonist: all F1,42 = 35.2, P < 0.001).
Selective induction of c-Fos expression in the submucosal plexus of small intestine and myenteric plexus of colon induced by intraperitoneal CRF are prevented by VIP antagonist.
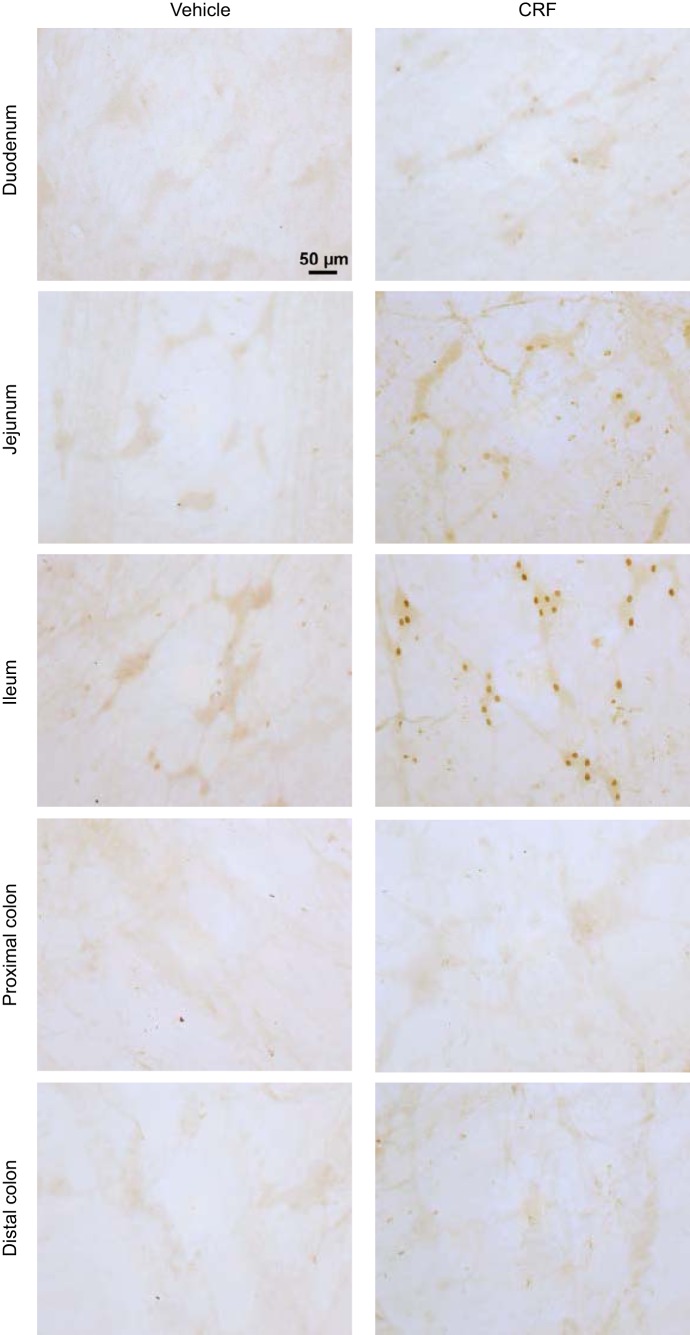
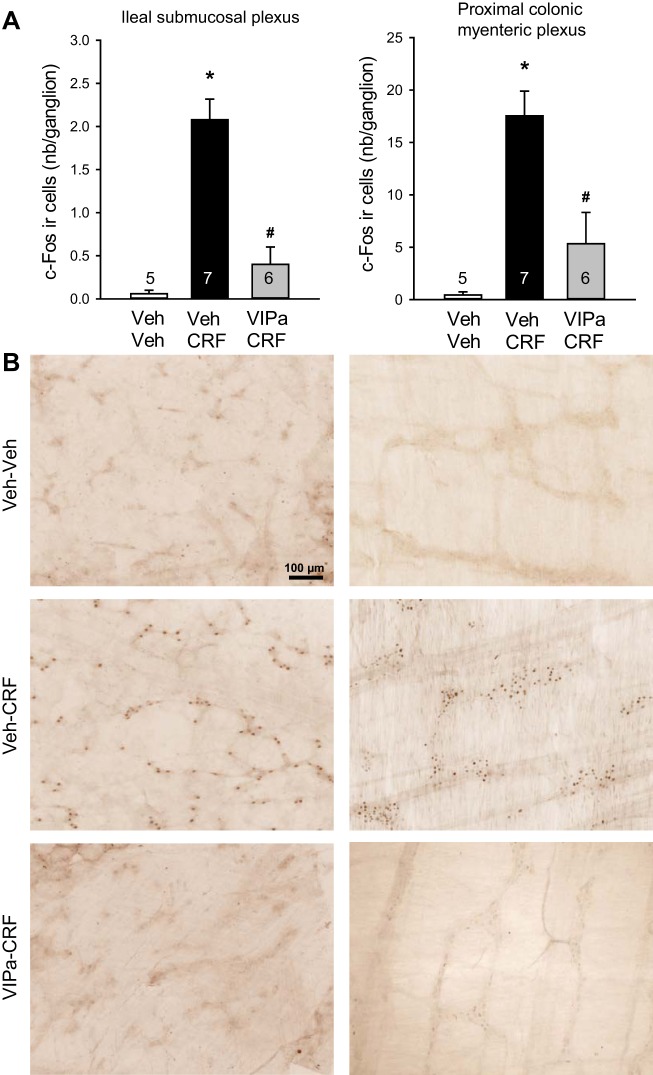
In intraperitoneal saline/saline-injected rats, there was no or a few c-Fos-immunoreactive cells in the submucosal plexus of small intestine (duodenum, jejunum, and terminal ileum) and proximal and distal colon (Fig. 3). Compared with intraperitoneal saline/saline, saline/CRF significantly increased c-Fos expression in the submucosal plexus of duodenum (0.9 ± 0.1 vs. 0.1 ± 0.1 cells/ganglion, n = 4–5, P < 0.01), jejunum (0.3 ± 0.1 vs. 0.0 ± 0.0 cells/ganglion, n = 4–5, P < 0.05), and more prominently in the terminal ileum (2.1 ± 0.4 vs. 0.1 ± 0.0 cells/ganglion, n = 5–7, P < 0.01). There was no c-Fos expression in the submucosal plexus of the colon proximal (0.0 ± 0.0 vs. 0.0 ± 0.0 cells/ganglion, n = 7–9) and distal (Fig. 3). By contrast, intraperitoneal saline/CRF compared with intraperitoneal saline/saline induced c-Fos-positive cells more prominently in the myenteric plexus of the proximal (18.0 ± 1.9 vs. 0.4 ± 0.2 cells/ganglion; P < 0.001) than the distal colon (3.0 ± 0.5 vs. 1.0 ± 0.3 cells/ganglion (P < 0.05). Few c-Fos-immunoreactive neuron was observed in the ileal myenteric plexus (0.3 ± 0.2 vs. 0.2 ± 0.2 cells/ganglion; Fig. 4). The VIP antagonist injected intraperitoneally immediately before CRF significantly inhibited c-Fos immunoreactivity both in the submucosal plexus of the ileum (0.4 ± 0.2 cells/ganglion, n = 6, P < 0.01) and in the myenteric plexus of the proximal colon (5.3 ± 3.0 cells/ganglion, n = 6, P < 0.01) by 80% and 71%, respectively, compared with saline/CRF, and values were no longer significantly different from those in saline/saline-treated rats (Fig. 5).
Fig. 3.
Representative photomicrographs of c-Fos immunoreactivity induced by intraperitoneal corticotropin-releasing factor (CRF) in the submucosal plexus of duodenum, jejunum, terminal ileum, and proximal and distal colon of rats. CRF (10 µg/kg) was injected intraperitoneally, and rats were euthanized 1 h later for c-Fos immunohistochemistry. Scale bar = 50 μm
Fig. 4.
Representative photomicrographs of c-Fos immunoreactivity induced by intraperitoneal corticotropin-releasing factor (CRF) in the myenteric plexus of terminal ileum and proximal and distal colon of rats. CRF (10 µg/kg) was injected intraperitoneally, and rats were euthanized 1 h later for c-Fos immunohistochemistry. Scale bar = 50 µm.
Fig. 5.
Vasoactive intestinal peptide (VIP) antagonist reduces intraperitoneal corticotropin-releasing factor (CRF)-induced increase in c-Fos immunoreactivity in the submucosal plexus of terminal ileum and myenteric plexus of proximal colon. Rats were injected intraperitoneally with vehicle (Veh: saline) or VIP antagonist (VIPa, 250 µg/kg) immediately before intraperitoneal CRF (10 µg/kg) or Veh (saline) and euthanized 1 h later for c-Fos immunohistochemistry. A: bar graphs are means ± SE of c-Fos-immunoreactive cell count per ganglion (average of 20/rat, and rat number is indicated at the bottom of each column). *P < 0.001 vs. Veh/Veh and #P < 0.005 vs. Veh/CRF (1-way ANOVA with Tukey’s post hoc multiple comparisons). B: representative photomicrographs of c-Fos-immunoreactive cells in the ileal submucosal plexus (left) and proximal colonic myenteric plexus (right). Scale bar = 100 µm.
In the ileal submucosal plexus, the numbers of c-Fos-positive cells were highly correlated with diarrhea (r2 = 0.90, F1,16 = 146.3, P < 0.001) and pellets as well (r2 = 0.77; F1,16 = 52.0, P < 0.001), and, in the colonic myenteric plexus, the c-Fos cells were also correlated with diarrhea and pellets [r2 = 0.74 (F1,16 = 44.8, P < 0.001) and r2 = 0.62 (F1,16 = 26.1, P < 0.001), respectively].
Intraperitoneal CRF activates VIP ileal submucosal neurons, and CRF1 receptor is colocalized on VIP-positive submucosal plexus of ileum.
CRF injected intraperitoneally induced c-Fos expression in 2.3 ± 0.1 cells/ganglia in the ileum submucosal plexus, of which 1.6 ± 0.1 cells/ganglia were also VIP positive, as shown by double labeling. On the basis of the total number of VIP-immunoreactive neurons (1.7 ± 0.2 cells/ganglia), intraperitoneal CRF activated 93.4% of VIP-immunoreactive neurons as well as 31.1% of non-VIP-immunoreactive neurons (Fig. 6). CRF1 receptor labeling was colocalized with VIP-immunoreactive neurons in the submucosal plexus of ileum that is illustrated in the representative photomicrographs in Fig. 7.
Fig. 6.
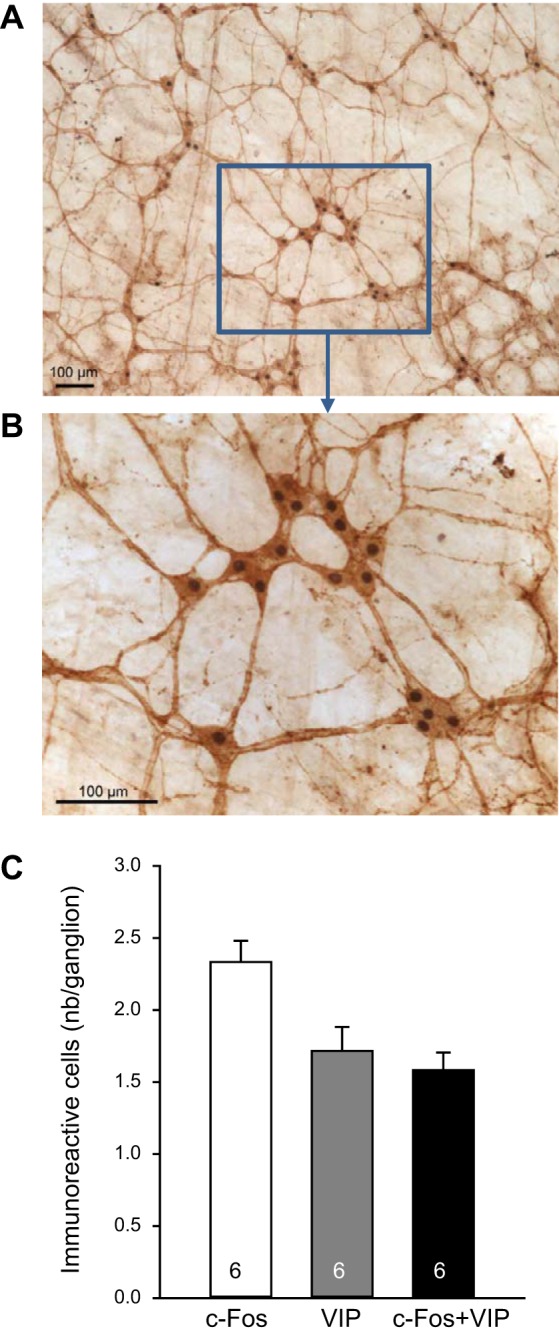
Corticotropin-releasing factor (CRF) injected intraperitoneally induces c-Fos in vasoactive intestinal peptide (VIP)-immunoreactive neurons of the submucosal terminal ileum. Rats injected with CRF (10 µg/kg ip) were euthanized 1 h later for immunohistochemistry of c-Fos and VIP. A: representative photomicrographs of double-labeled cells for c-Fos (nucleus) and VIP (cytoplasm). B: higher magnification (A and B scale bar = 100 µm). C: bar graphs, means ± SE of c-Fos, VIP, or c-Fos/VIP-immunoreactive cell count per ganglion (average of 20/rat, and rat number is indicated at the bottom of each column).
Fig. 7.
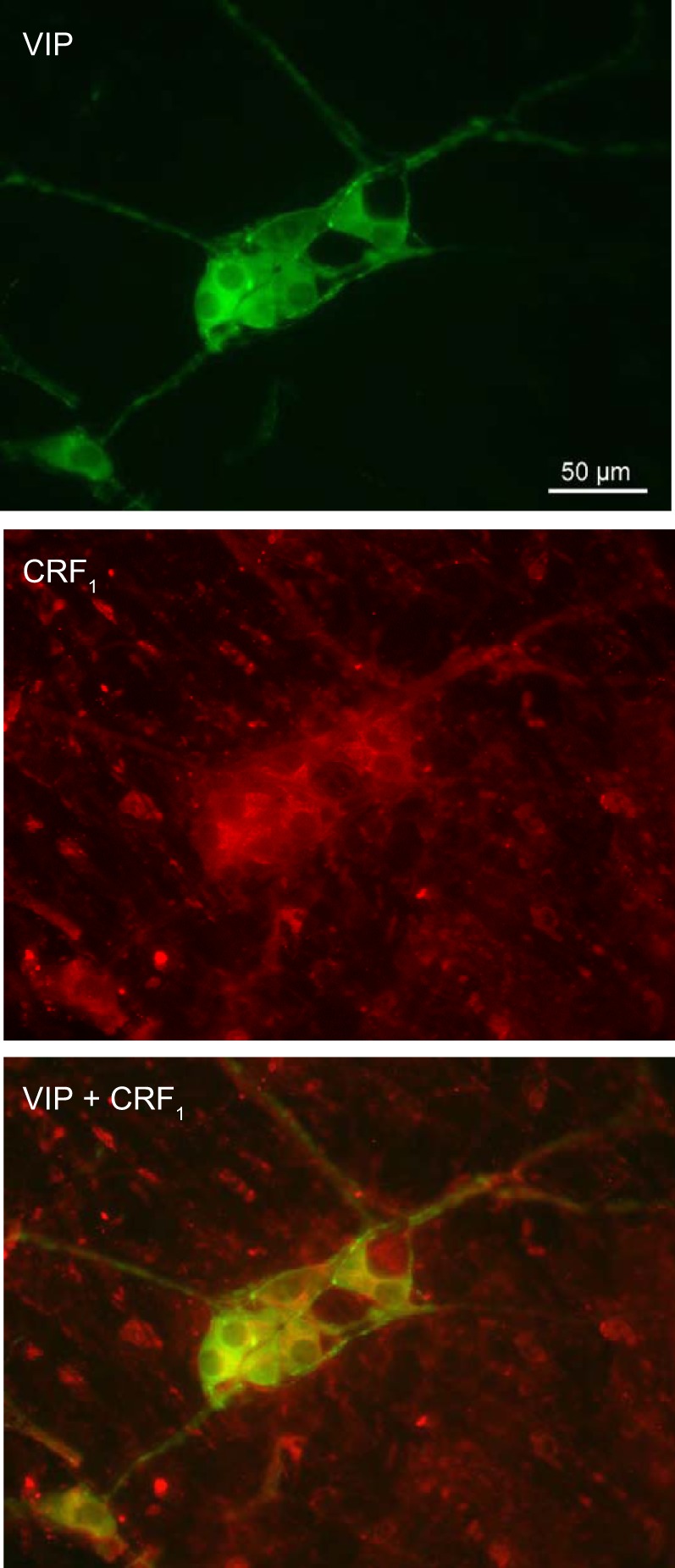
Representative photomicrographs of immunofluorescence of coexistence of vasoactive intestinal peptide (VIP) and corticotropin-releasing factor receptor 1 (CRF1) in the submucosal neurons of rat terminal ileum. Scale bar = 50 µm.
Intraperitoneal CRF decreases VIP levels in the ileum but not in the portal vein.
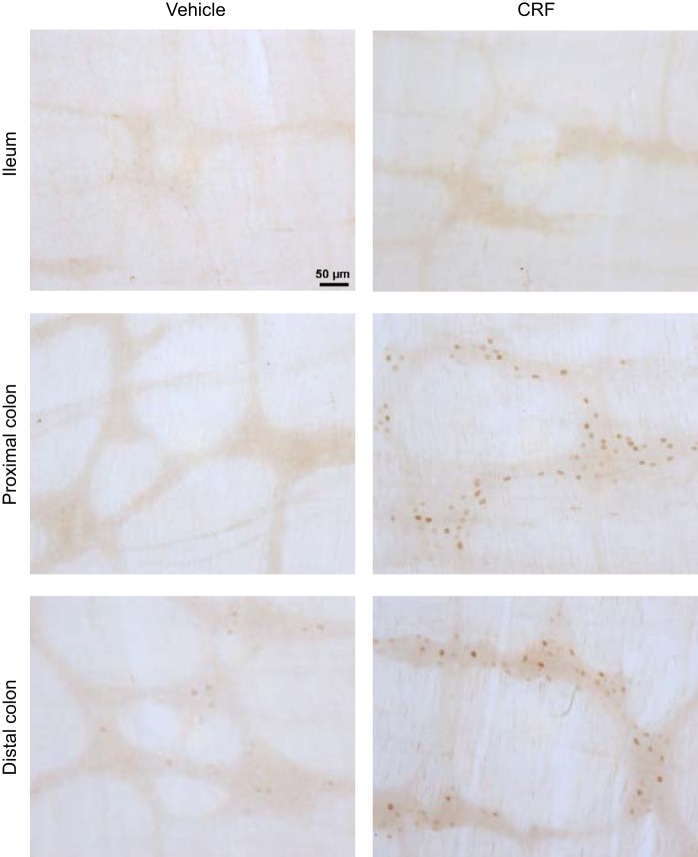
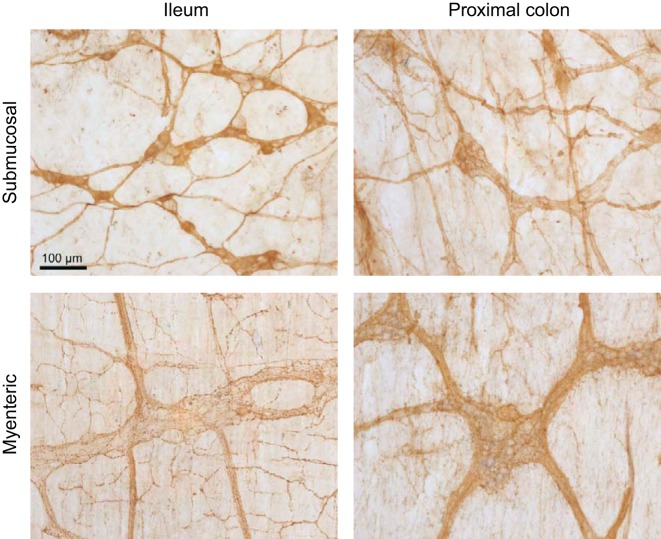
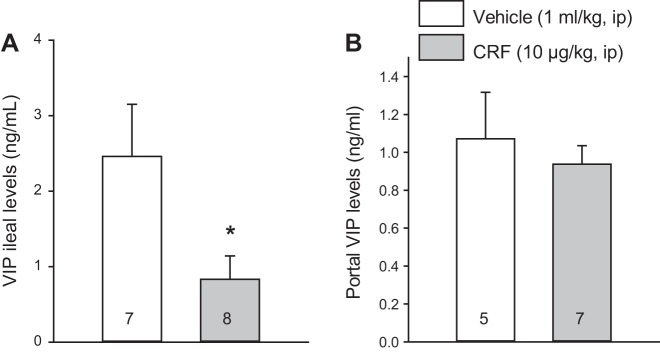
The assessment of VIP-immunoreactive neurons in the ileal and proximal colonic preparations of intraperitoneal vehicle-treated rats indicated that neurons with VIP immunoreactivity in cell bodies were prominent in the submucosal plexus of the ileum. Only few neurons with positive cell body were observed in the ileal myenteric plexus and proximal colon submucosal and myenteric plexus, where VIP immunoreactivity was mainly localized in nerve fibers (Fig. 8). Therefore, we selected the ileum where cell bodies showed localization of VIP to assess changes in VIP expression. The intraperitoneal injection of CRF induced a significant decrease in VIP levels in the terminal ileum compared with intraperitoneal saline at 60 min after injection (0.83 ± 0.31 vs. 2.46 ± 0.69 ng/g, n = 7–8, P < 0.05; Fig. 9A), which was not present at 15 min (2.11 ± 0.39 vs. 1.64 ± 0.15 ng/g, n = 5–7). In the portal vein, VIP plasma levels were not significantly different compared with vehicle at 15 min (1.20 ± 0.02 vs. 1.22 ± 0.04 ng/ml, n = 5–7) as well as at 60 min after injection (1.07 ± 0.25 vs. 0.94 ± 0.10 ng/ml, n = 7–9; Fig. 9B). There was no significant difference in VIP mRNA expression in the terminal ileum among vehicle-vehicle, vehicle-CRF, and VIP antagonist-CRF groups (1.00 ± 0.08 vs. 1.10 ± 0.14 vs. 1.08 ± 0.15, respectively, n = 5) at 60 min after the injection.
Fig. 8.
Representative photomicrographs of vasoactive intestinal peptide (VIP)-immunoreactive neurons localized primarily in the submucosal plexus of the rat ileum, whereas the proximal colon submucosal plexus, the myenteric plexus of the ileum, and proximal colon displayed VIP-immunoreactive fibers only. Scale bar = 100 µm.
Fig. 9.
Corticotropin-releasing factor (CRF) injected intraperitoneally decreases vasoactive intestinal peptide (VIP) concentrations in the terminal ileum (A) and does not change VIP levels in the portal vein (B). Rats were injected with vehicle or CRF (10 µg/kg ip) and euthanized 1 h later. Blood was withdrawn from the portal vein, and the terminal ileum was collected. Data are means ± SE, and rats/group are indicated in each bar. *P < 0.05 vs. vehicle (Student’s t-test).
DISCUSSION
We demonstrated that VIP signaling is involved in mediating peripheral injection of CRF-induced activation of ileal submucosal and colonic myenteric neurons and contributes to the rapid induction of diarrhea and stimulation of propulsive colonic motor function in rats.
The activation of CRF-CRF1 receptor signaling in the distal gut is implicated in the colonic response to acute stress (35, 39). CRF receptors and ligands are localized in various cell types, including enteric neurons (15, 30, 75, 76), enteroendocrine cells (24, 76), and mast cells (28, 69) in the colon of experimental animals and humans. Moreover, in vitro studies in colonic tissues indicate that the activation of peripheral CRF1 receptor mimicked stress-related stimulation of colonic motility and secretion and the alterations of intestinal barrier functions through a direct action on enteric cholinergic neurons, enterochromaffin, and/or mast cells (3, 34, 51, 65, 74). In further support of a role of endogenous colonic CRF signaling system, the knockdown of CRF selectively within the rat colon prevented the fecal pellet output induced by acute partial restraint stress in rats (39). There is also evidence that stress exposure modulates the expression of CRF peptides and CRF1 receptor within the distal gut (39, 49, 50, 59, 76). Lastly, pharmacological studies using peripherally restricted CRF antagonists α-helical CRF9–41, astressin, and astressin-B injected intraperitoneally or intravenously abolished or dampened the stimulation of colonic propulsive motor function and the release of mucin, PGE2, and mast cell protease II evoked by acute exposure to restraint, water avoidance, and novel environment in rats or mice (3, 14, 28, 40, 45, 46, 71).
In the present study, intraperitoneal CRF, used as a tool involved in acute stress to acutely activate the peripheral CRF receptors, results in a rapid onset of defecation associated with diarrhea in rats as previously reported (61). The return to basal defecation at 1 h may reflect the emptying of colonic feces content as observed during acute stress exposure (3, 39). At the cellular level, intraperitoneal injection of CRF induces c-Fos expression in neurons of small intestine submucosal ganglia most prominently in the terminal ileum compared with duodenum or jejunum and in colonic myenteric ganglia. By contrast, there was no c-Fos observed in ileal myenteric neurons and colonic submucosal ganglia. We previously reported that peripherally injected CRF induced c-Fos in colonic myenteric neurons (33, 44, 74), unlike in myenteric neurons of the duodenum, jejunum, and ileum (74). Collectively, these data demonstrate that peripheral CRF induces neuronal activation in the enteric nervous system with a distinct pattern between submucosal vs. myenteric neurons and regional (intestine vs. colon.) differences. The selective activation of myenteric neurons in the distal gut is correlated with the stimulation of colonic motor function (r2 = 0.74). Likewise, the lack of c-Fos expression across the small intestinal myenteric neurons is consistent with the unchanged or inhibition of small intestinal transit reported in rats after intraperitoneal or intravenous CRF injection (38, 71). In addition, the strong correlation between the numbers of c-Fos-positive cells in the submucosal plexus of terminal ileum and diarrhea (r2 = 0.90) and defecation (r2 = 0.77) points to the activation of terminal ileal submucosal neurons as an additional contributor to the secretomotor response to intraperitoneal CRF.
VIP is expressed in the majority of submucosal rat ileal neurons (77) and influences secretomotor function. In particular, the peptide stimulates water and electrolyte secretion from the intestine, leading to diarrhea in experimental animals and humans (6, 11, 37). VIP also plays a role in the peristatic reflex and exerts a prokinetic action (11, 16, 37). The present findings provide convergent neuroanatomical and pharmacological evidence that VIP signaling is involved in intraperitoneal CRF-induced CRF1 receptor-mediated diarrhea and defecation. First, we found that CRF injected intraperitoneally activates the majority (93.4%) of VIP-positive neurons located in the ileum submucosal plexus, as shown by double labeling of c-Fos and VIP immunoreactivity. Such activation may result from a direct CRF1 receptor signaling on VIP neurons of ileal submucosa plexus, as we found that VIP neurons were also immunoreactive for CRF1 receptor. This is consistent with a previous report demonstrating the presence of CRF1 receptor immunoreactivity in submucosal neurons of rat ileum although the phenotype of CRF1 receptor-expressing neurons was not identified (54). Further evidence for a role of CRF1 receptor in the submucosal neuronal activation was the demonstration that, similar to CRF, the selective CRF1 agonist, stressin1-A, injected intraperitoneally also induces c-Fos in ileal submucosal neurons (74). However, the present study cannot allow us to establish whether c-Fos expressed in VIP neurons exclusively reflects a CRF-CRF1 signaling occurring directly on these neurons or, additionally, whether it encompasses other indirect mechanisms. In particular, CRF is known to induce CRF1 receptor-mediated release of 5-HT (72), and 5-HT receptors are expressed on secretomotor neurons in the ileal mucosa (22). Second, intraperitoneal CRF results in a significant decrease of VIP levels in the terminal ileum at 60 min after injection. The decrease in ileal content may reflect an increased release of the peptide most likely from ileal neurons (36) (present study), as well as ileal mast cells known to contain VIP and expressing CRF1 receptor (27, 69). The absence of VIP increase in the portal blood further points to a local action within the gut wall.
Importantly, pharmacological approach showed that the VIP receptor antagonist, [4Cl-D-Phe6,Leu17]VIP, injected intraperitoneally completely blocked intraperitoneal CRF and induced the occurrence of defecation and diarrhea at a dose of VIP antagonist that alone had no effect on these parameters. In addition, [4Cl-D-Phe6,Leu17]VIP also prevented neuronal activation induced by intraperitoneal CRF in ileal submucosal and colonic myenteric plexi, as shown by the low numbers of c-Fos-positive neurons that were no longer significantly different from those of vehicle + vehicle-injected group. It is worth noting that c-Fos expression in these enteric plexi was not performed after intraperitoneal injection of [4Cl-D-Phe6,Leu17]VIP alone. However, we can speculate that the VIP antagonist itself does not induce c-Fos expression based on its lack of effect on colonic secretomotor function when injected alone and its inhibitory effect on both the c-Fos induction and the stimulation of defecation and diarrhea induced by CRF. VIP binds to VIP receptors VPAC1 and VPAC2; however, VPAC1 is the main form expressed at the gene and protein levels in rodents and human small intestine and colon (21) and likely to be the receptor subtype involved. We also found that intraperitoneal CRF induces c-Fos expression in 31.1% of non-VIP-positive cells in the ileal submucosal ganglia that need to be further characterized. However, the normalization of both the colonic secretomotor function linked and ileal submucosal and colonic myenteric activation by VIP antagonist in intraperitoneal CRF-treated rats indicates the primary involvement of VIP signaling.
The present study also showed that PGE2, well established to have diarrheogenic property (58) and to be released from rat colonic explants after peripheral injection of CRF (3), is not implicated. This is supported by the unaltered stimulatory actions of intraperitoneal CRF on colonic motor function and diarrhea in rats pretreated with the PGs synthesis inhibitor, indomethacin. We previously reported that the same regimen of indomethacin administration (5 mg/kg ip, −30 min) completely abolished intravenous injection of interleukin-1β and adrenomedullin-induced gastric stasis in rats (43, 64). Likewise, other reports in rats showed that indomethacin (4 mg/kg ip) pretreatment abolished HCl-induced duodenal bicarbonate secretion (20) or that indomethacin (2 mg/kg iv) blocked the inhibitory effect of oleic acid on pentagastrin-stimulated acid secretion in rats (56). Moreover, an intravenous low dose of 0.4 mg/kg inhibited PGs generation by 96% in rat stomach at 60 min after injection (60). Under these conditions of adequate eicosanoid-COX blockade, the lack of PGs involvement in intraperitoneal CRF actions is consistent with the demonstration that restraint stress-induced stimulation of fecal output is not modified by indomethacin while blocked by a peripherally acting CRF antagonist (3).
The intraperitoneal injection of CRF has been established as a model of chemically induced stress-related irritable bowel syndrome (IBS)-diarrhea (33, 35), and the involvement of VIP signaling downstream of CRF to mediate the secretomotor response may have translational application. Stress is known as an important risk factor for the development or exacerbation of IBS (4). In humans, CRF ligands are localized in colonic mucosa (47), and CRF1 mRNA expression is found in the ileum (the highest level) and the colon with CRF1 immunoreactivity showing the strongest intensity in the submucosal and myenteric neurons (75). Peripherally injected CRF in healthy human subjects largely reproduced the biological actions observed in experimental animals, namely the increased colonic motility, intestinal permeability, and mast cell activations (10, 68, 69). There are several reports showing high VIP levels in the circulation and colonic/sigmoid biopsies of patients with diarrhea-predominant IBS compared with control subjects (2, 8, 17, 52, 63, 78). Recent studies demonstrated that VIP induces mast cell-dependent increases in permeability of human ileum through activation of VIP receptors localized on the surface of mast cells (27). Likewise, ex vivo studies in rats using ileal mucosa preparation showed that VIP mimicked CRF and stress-induced increased permeability to bacteria through VPAC receptors on mast cells (27, 28). Whether ileal/colonic CRF receptor signaling activates downstream VIP release and accounts for the manifestation of sudden diarrhea in patients with IBS under stress warrants further investigations.
In summary, our results showed that intraperitoneal CRF induced selective activation of submucosal neurons in the small intestine most prominently in the ileum, along with selective activation of myenteric neurons in the colon in rats. CRF activation of ileal submucosal ganglia is taking place in the majority of VIP-containing neurons that also are immunoreactive for CRF1 receptor and is associated with the reduction of VIP levels in the ileum, indicative of local release. Additionally, peripheral injection of VIP receptor antagonist blocked the functional secretomotor response (diarrhea and defecation) along with the activation in the submucosal and colonic myenteric neurons induced by intraperitoneal CRF. These novel findings highlight the important role of VIP neuronal signaling as a downstream pathway of peripheral CRF1 receptor activation in the ileum and colon.
GRANTS
This work was supported by P30 NIDDK-41303 (Animal Core, Y. Taché, L. Wang), NIH R01 DK-57238 (Y. Taché), Veteran Administration Reab Merit Award RX001685 (L. Wang), and Veterans Administration Senior Research Career Scientist Award (Y. Taché).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.Y., L.W., K.K., K.Y., and Y.T. conceived and designed research; S.Y., L.W., H.K., and P.-Q.Y. performed experiments; S.Y., L.W., H.K., and P.-Q.Y. analyzed data; S.Y., L.W., H.K., and P.-Q.Y. interpreted results of experiments; S.Y., L.W., and Y.T. drafted manuscript; S.Y., L.W., H.K., K.Y., and Y.T. edited and revised manuscript; S.Y., L.W., H.K., P.-Q.Y., K.K., and K.Y. approved final version of manuscript; L.W. prepared figures.
ACKNOWLEDGMENTS
We are grateful to Mrs. Honghui Liang for excellent technical support and thank Dr. Jean Rivier (Sentia Medical Sciences, La Jolla, CA) for the generous supply of CRF and [4Cl-D-Phe6,Leu17]VIP antagonist.
Present addresses of S. Yakabi: Dept. of Gastroenterology, Graduate School of Medicine, The University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo, Japan, 113-0033.
Presnt address of H. Karasawa: End-Organ Disease Laboratories, Daiichi Sankyo Co., Ltd. 1-2-58, Hiromachi, Shinagawa-ku, Tokyo 140-8710, Japan.
REFERENCES
- 1.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44: 525–557, 2004. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 2.Bednarska O, Walter SA, Casado-Bedmar M, Ström M, Salvo-Romero E, Vicario M, Mayer EA, Keita AV. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology 153: 948–960.e3, 2017. doi: 10.1053/j.gastro.2017.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol Gastrointest Liver Physiol 271: G884–G892, 1996. doi: 10.1152/ajpgi.1996.271.5.G884. [DOI] [PubMed] [Google Scholar]
- 4.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology 140: 761–765, 2011. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YF, Liu TH, Chen SP, Pan GZ, Lu XH, Lu GJ, Zhong SX, Cai LX, Cui QC, Ran QY, Hou X, Tseng HC, Chen MC. Watery diarrhea syndrome caused by multihormonal malignant pancreatic islet cell tumor secreting somatostatin, vasoactive intestinal peptide, serotonin, and prostaglandin E–a clinicopathological, biochemical, immunohistochemical, and ultrastructural study. Pancreas 1: 80–89, 1986. doi: 10.1097/00006676-198601000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann NY Acad Sci 915: 77–80, 2000. doi: 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- 7.De Giorgio R, Sternini C, Anderson K, Brecha NC, Go VL. Tissue distribution and innervation pattern of peptide immunoreactivities in the rat pancreas. Peptides 13: 91–98, 1992. doi: 10.1016/0196-9781(92)90145-S. [DOI] [PubMed] [Google Scholar]
- 8.Del Valle-Pinero AY, Sherwin LB, Anderson EM, Caudle RM, Henderson WA. Altered vasoactive intestinal peptides expression in irritable bowel syndrome patients and rats with trinitrobenzene sulfonic acid-induced colitis. World J Gastroenterol 21: 155–163, 2015. doi: 10.3748/wjg.v21.i1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erchegyi J, Wang L, Gulyas J, Samant M, Perrin MH, Lewis K, Miller C, Vaughan J, Donaldson C, Fischer W, Low W, Yakabi S, Karasawa H, Taché Y, Rivier C, Rivier J. Characterization of multisubstituted corticotropin releasing factor (CRF) peptide antagonists (astressins). J Med Chem 59: 854–866, 2016. doi: 10.1021/acs.jmedchem.5b00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut 42: 845–849, 1998. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung C, Unterweger P, Parry LJ, Bornstein JC, Foong JP. VPAC1 receptors regulate intestinal secretion and muscle contractility by activating cholinergic neurons in guinea pig jejunum. Am J Physiol Gastrointest Liver Physiol 306: G748–G758, 2014. doi: 10.1152/ajpgi.00416.2013. [DOI] [PubMed] [Google Scholar]
- 12.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst 81: 87–96, 2000. doi: 10.1016/S0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 13.Furness JB, Costa M, Walsh JH. Evidence for and significance of the projection of VIP neurons from the myenteric plexus to the taenia coli in the guinea pig. Gastroenterology 80: 1557–1561, 1981. [PubMed] [Google Scholar]
- 14.Gourcerol G, Wang L, Adelson DW, Larauche M, Taché Y, Million M. Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am J Physiol Gastrointest Liver Physiol 296: G992–G1002, 2009. doi: 10.1152/ajpgi.90436.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gourcerol G, Wu SV, Yuan PQ, Pham H, Miampamba M, Larauche M, Sanders P, Amano T, Mulak A, Im E, Pothoulakis C, Rivier J, Taché Y, Million M. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology 140: 1586–96.e6, 2011. doi: 10.1053/j.gastro.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grider JR, Makhlouf GM. Colonic peristaltic reflex: identification of vasoactive intestinal peptide as mediator of descending relaxation. Am J Physiol Gastrointest Liver Physiol 251: G40–G45, 1986. doi: 10.1152/ajpgi.1986.251.1.G40. [DOI] [PubMed] [Google Scholar]
- 17.Han B. Correlation between gastrointestinal hormones and anxiety-depressive states in irritable bowel syndrome. Exp Ther Med 6: 715–720, 2013. doi: 10.3892/etm.2013.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev 55: 21–26, 2003. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 19.Hussain Z, Kim HW, Huh CW, Lee YJ, Park H. The effect of peripheral CRF peptide and water avoidance stress on colonic and gastric transit in guinea pigs. Yonsei Med J 58: 872–877, 2017. doi: 10.3349/ymj.2017.58.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isenberg JI, Smedfors B, Johansson C. Effect of graded doses of intraluminal H+, prostaglandin E2, and inhibition of endogenous prostaglandin synthesis on proximal duodenal bicarbonate secretion in unanesthetized rat. Gastroenterology 88: 303–307, 1985. doi: 10.1016/S0016-5085(85)80184-0. [DOI] [PubMed] [Google Scholar]
- 21.Jayawardena D, Guzman G, Gill RK, Alrefai WA, Onyuksel H, Dudeja PK. Expression and localization of VPAC1, the major receptor of vasoactive intestinal peptide along the length of the intestine. Am J Physiol Gastrointest Liver Physiol 313: G16–G25, 2017. doi: 10.1152/ajpgi.00081.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson PJ, Bornstein JC, Furness JB, Woollard DJ, Orrman-Rossiter SL. Characterization of 5-hydroxytryptamine receptors mediating mucosal secretion in guinea-pig ileum. Br J Pharmacol 111: 1240–1244, 1994. doi: 10.1111/j.1476-5381.1994.tb14878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane MG, O’Dorisio TM, Krejs GJ. Production of secretory diarrhea by intravenous infusion of vasoactive intestinal polypeptide. N Engl J Med 309: 1482–1485, 1983. doi: 10.1056/NEJM198312153092403. [DOI] [PubMed] [Google Scholar]
- 24.Kawahito Y, Sano H, Kawata M, Yuri K, Mukai S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology 106: 859–865, 1994. doi: 10.1016/0016-5085(94)90743-9. [DOI] [PubMed] [Google Scholar]
- 25.Keast JR, Furness JB, Costa M. Distribution of certain peptide-containing nerve fibres and endocrine cells in the gastrointestinal mucosa in five mammalian species. J Comp Neurol 236: 403–422, 1985. doi: 10.1002/cne.902360308. [DOI] [PubMed] [Google Scholar]
- 26.Keire DA, Whitelegge JP, Souda P, Faull KF, Bassilian S, Reidelberger RD, Haver AC, Reeve JR Jr. PYY(1-36) is the major form of PYY in rat distal small intestine: quantification using high-resolution mass spectrometry. Regul Pept 165: 151–157, 2010. doi: 10.1016/j.regpep.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keita AV, Carlsson AH, Cigéhn M, Ericson AC, McKay DM, Söderholm JD. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterol Motil 25: e406–e417, 2013. doi: 10.1111/nmo.12127. [DOI] [PubMed] [Google Scholar]
- 28.Keita AV, Söderholm JD, Ericson AC. Stress-induced barrier disruption of rat follicle-associated epithelium involves corticotropin-releasing hormone, acetylcholine, substance P, and mast cells. Neurogastroenterol Motil 22: 770–778, 2010. doi: 10.1111/j.1365-2982.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim KJ, Kim KB, Yoon SM, Han JH, Chae HB, Park SM, Youn SJ. Corticotropin-releasing factor stimulates colonic motility via muscarinic receptors in the rat. World J Gastroenterol 23: 3825–3831, 2017. doi: 10.3748/wjg.v23.i21.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura T, Amano T, Uehara H, Ariga H, Ishida T, Torii A, Tajiri H, Matsueda K, Yamato S. Urocortin I is present in the enteric nervous system and exerts an excitatory effect via cholinergic and serotonergic pathways in the rat colon. Am J Physiol Gastrointest Liver Physiol 293: G903–G910, 2007. doi: 10.1152/ajpgi.00066.2007. [DOI] [PubMed] [Google Scholar]
- 31.Krukoff TL. Expression of c-Fos in studies of central autonomic and sensory systems. Mol Neurobiol 7: 247–263, 1993. doi: 10.1007/BF02769178. [DOI] [PubMed] [Google Scholar]
- 32.Larauche M. Novel insights in the role of peripheral corticotropin-releasing factor and mast cells in stress-induced visceral hypersensitivity. Neurogastroenterol Motil 24: 201–205, 2012. doi: 10.1111/j.1365-2982.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- 33.Larauche M, Gourcerol G, Wang L, Pambukchian K, Brunnhuber S, Adelson DW, Rivier J, Million M, Taché Y. Cortagine, a CRF1 agonist, induces stress-like alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol 297: G215–G227, 2009. doi: 10.1152/ajpgi.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larauche M, Kiank C, Taché Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol 60, Suppl 7: 33–46, 2009. [PMC free article] [PubMed] [Google Scholar]
- 35.Larauche M, Mulak A, Taché Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol 233: 49–67, 2012. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson LI, Fahrenkrug J, Schaffalitzky De Muckadell O, Sundler F, Håkanson R, Rehfeld JR. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci USA 73: 3197–3200, 1976. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lelievre V, Favrais G, Abad C, Adle-Biassette H, Lu Y, Germano PM, Cheung-Lau G, Pisegna JR, Gressens P, Lawson G, Waschek JA. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung’s disease. Peptides 28: 1688–1699, 2007. doi: 10.1016/j.peptides.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology 94: 598–602, 1988. doi: 10.1016/0016-5085(88)90229-6. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Chang J, Long N, Beckwith K, Talhouarne G, Brooks JJ, Qu MH, Ren W, Wood JD, Cooper S, Bhargava A. Endogenous CRF in rat large intestine mediates motor and secretory responses to stress. Neurogastroenterol Motil 28: 281–291, 2016. doi: 10.1111/nmo.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology 119: 1569–1579, 2000. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]
- 41.Martínez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther 301: 611–617, 2002. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 42.Martínez V, Wang L, Taché Y. Central TRH receptor 1 antisense blocks cold-induced gastric emptying but not brain c-Fos induction. Peptides 22: 81–90, 2001. doi: 10.1016/S0196-9781(00)00359-4. [DOI] [PubMed] [Google Scholar]
- 43.Martinez V, Wang L, Taché Y. Peripheral adrenomedullin inhibits gastric emptying through CGRP8-37 -sensitive receptors and prostaglandins pathways in rats. Peptides 27: 1376–1382, 2006. doi: 10.1016/j.peptides.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Miampamba M, Maillot C, Million M, Taché Y. Peripheral CRF activates myenteric neurons in the proximal colon through CRF(1) receptor in conscious rats. Am J Physiol Gastrointest Liver Physiol 282: G857–G865, 2002. doi: 10.1152/ajpgi.00434.2001. [DOI] [PubMed] [Google Scholar]
- 45.Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JA, Zhou H, Saunders PR, Maillot C, Mayer EA, Taché Y. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res 985: 32–42, 2003. doi: 10.1016/S0006-8993(03)03027-0. [DOI] [PubMed] [Google Scholar]
- 46.Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. Am J Physiol Gastrointest Liver Physiol 274: G827–G831, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides 21: 1799–1809, 2000. doi: 10.1016/S0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 48.Nozu T, Takakusaki K, Okumura T. A balance theory of peripheral corticotropin-releasing factor receptor type 1 and type 2 signaling to induce colonic contractions and visceral hyperalgesia in rats. Endocrinology 155: 4655–4664, 2014. doi: 10.1210/en.2014-1421. [DOI] [PubMed] [Google Scholar]
- 49.O’Malley D, Dinan TG, Cryan JF. Alterations in colonic corticotropin-releasing factor receptors in the maternally separated rat model of irritable bowel syndrome: differential effects of acute psychological and physical stressors. Peptides 31: 662–670, 2010. doi: 10.1016/j.peptides.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 50.O’Malley D, Julio-Pieper M, Gibney SM, Gosselin RD, Dinan TG, Cryan JF. Differential stress-induced alterations of colonic corticotropin-releasing factor receptors in the Wistar Kyoto rat. Neurogastroenterol Motil 22: 301–311, 2010. doi: 10.1111/j.1365-2982.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 51.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One 7: e39935, 2012. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palsson OS, Morteau O, Bozymski EM, Woosley JT, Sartor RB, Davies MJ, Johnson DA, Turner MJ, Whitehead WE. Elevated vasoactive intestinal peptide concentrations in patients with irritable bowel syndrome. Dig Dis Sci 49: 1236–1243, 2004. doi: 10.1023/B:DDAS.0000037818.64577.ef. [DOI] [PubMed] [Google Scholar]
- 53.Pandol SJ, Dharmsathaphorn K, Schoeffield MS, Vale W, Rivier J. Vasoactive intestinal peptide receptor antagonist [4Cl-D-Phe6, Leu17] VIP. Am J Physiol Gastrointest Liver Physiol 250: G553–G557, 1986. doi: 10.1152/ajpgi.1986.250.4.G553. [DOI] [PubMed] [Google Scholar]
- 54.Porcher C, Juhem A, Peinnequin A, Sinniger V, Bonaz B. Expression and effects of metabotropic CRF1 and CRF2 receptors in rat small intestine. Am J Physiol Gastrointest Liver Physiol 288: G1091–G1103, 2005. doi: 10.1152/ajpgi.00302.2004. [DOI] [PubMed] [Google Scholar]
- 55.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE, Urocortin II. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA 98: 2843–2848, 2001. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhee JC, Chang TM, Lee KY, Jo YH, Chey WY. Mechanism of oleic acid-induced inhibition on gastric acid secretion in rats. Am J Physiol Gastrointest Liver Physiol 260: G564–G570, 1991. doi: 10.1152/ajpgi.1991.260.4.G564. [DOI] [PubMed] [Google Scholar]
- 57.Rivier J, Gulyas J, Kunitake K, DiGruccio M, Cantle JP, Perrin MH, Donaldson C, Vaughan J, Million M, Gourcerol G, Adelson DW, Rivier C, Taché Y, Vale W. Stressin1-A, a potent corticotropin releasing factor receptor 1 (CRF1)-selective peptide agonist. J Med Chem 50: 1668–1674, 2007. doi: 10.1021/jm0613875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robert A, Nezamis JE, Lancaster C, Hanchar AJ, Klepper MS. Enteropooling assay: a test for diarrhea produced by prostaglandins. Prostaglandins 11: 809–828, 1976. doi: 10.1016/0090-6980(76)90189-1. [DOI] [PubMed] [Google Scholar]
- 59.Sand E, Themner-Persson A, Ekblad E. Corticotropin releasing factor-distribution in rat intestine and role in neuroprotection. Regul Pept 166: 68–75, 2011. doi: 10.1016/j.regpep.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Saperas E, Kauffman G, Taché Y. Role of central prostaglandin E2 in the regulation of gastric acid secretion in the rat. Eur J Pharmacol 209: 1–7, 1991. doi: 10.1016/0014-2999(91)90002-8. [DOI] [PubMed] [Google Scholar]
- 61.Saunders PR, Maillot C, Million M, Taché Y. Peripheral corticotropin-releasing factor induces diarrhea in rats: role of CRF1 receptor in fecal watery excretion. Eur J Pharmacol 435: 231–235, 2002. doi: 10.1016/S0014-2999(01)01574-6. [DOI] [PubMed] [Google Scholar]
- 62.Shi XZ, Sarna SK. Gene therapy of Cav1.2 channel with VIP and VIP receptor agonists and antagonists: a novel approach to designing promotility and antimotility agents. Am J Physiol Gastrointest Liver Physiol 295: G187–G196, 2008. doi: 10.1152/ajpgi.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Sim J, Jang KS. Mast cell number, substance P and vasoactive intestinal peptide in irritable bowel syndrome with diarrhea. Scand J Gastroenterol 49: 43–51, 2014. doi: 10.3109/00365521.2013.857712. [DOI] [PubMed] [Google Scholar]
- 64.Sütö G, Király A, Plourde V, Taché Y. Intravenous interleukin-1-beta-induced inhibition of gastric emptying: involvement of central corticotrophin-releasing factor and prostaglandin pathways in rats. Digestion 57: 135–140, 1996. doi: 10.1159/000201326. [DOI] [PubMed] [Google Scholar]
- 65.Taché Y, Larauche M, Yuan PQ, Million M. Brain and gut CRF signaling: biological actions and role in the gastrointestinal tract. Curr Mol Pharmacol 11: 51-71, 2018. doi: 10.2174/1874467210666170224095741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol 280: G173–G177, 2001. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- 67.Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci USA 101: 9468–9473, 2004. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tóth J, Holvoet L, Farré R, Van Oudenhove L, Boeckxstaens G, Verbeke K, Tack J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 63: 1293–1299, 2014. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 69.Wallon C, Söderholm JD. Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann NY Acad Sci 1165: 206–210, 2009. doi: 10.1111/j.1749-6632.2009.04030.x. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Gourcerol G, Yuan PQ, Wu SV, Million M, Larauche M, Taché Y. Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice. Am J Physiol Gastrointest Liver Physiol 298: G45–G56, 2010. doi: 10.1152/ajpgi.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol Gastrointest Liver Physiol 253: G582–G586, 1987. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- 72.Wu SV, Yuan PQ, Lai J, Wong K, Chen MC, Ohning GV, Taché Y. Activation of type 1 CRH receptor isoforms induces serotonin release from human carcinoid BON-1N cells: an enterochromaffin cell model. Endocrinology 152: 126–137, 2011. doi: 10.1210/en.2010-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan PQ, Kimura H, Million M, Bellier JP, Wang L, Ohning GV, Taché Y. Central vagal stimulation activates enteric cholinergic neurons in the stomach and VIP neurons in the duodenum in conscious rats. Peptides 26: 653–664, 2005. doi: 10.1016/j.peptides.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan PQ, Million M, Wu SV, Rivier J, Taché Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol Motil 19: 923–936, 2007. doi: 10.1111/j.1365-2982.2007.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan PQ, Wu SV, Elliott J, Anton PA, Chatzaki E, Million M, Taché Y. Expression of corticotropin releasing factor receptor type 1 (CRF1) in the human gastrointestinal tract and upregulation in the colonic mucosa in patients with ulcerative colitis. Peptides 38: 62–69, 2012. doi: 10.1016/j.peptides.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan PQ, Wu SV, Wang L, Taché Y. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides 31: 322–331, 2010. doi: 10.1016/j.peptides.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zanoni JN, Freitas P. Effects of ascorbic acid on the vasoactive intestinal peptide synthesis in the ileum submucous plexus of normal rats. Arq Gastroenterol 42: 186–190, 2005. doi: 10.1590/S0004-28032005000300012. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H, Yan Y, Shi R, Lin Z, Wang M, Lin L. Correlation of gut hormones with irritable bowel syndrome. Digestion 78: 72–76, 2008. doi: 10.1159/000165352. [DOI] [PubMed] [Google Scholar]