Abstract
The SLC4 family Cl−/ cotransporters (NBCe1, NBCe2, NBCn1, and NBCn2) contribute to a variety of vital physiological processes including pH regulation and epithelial fluid secretion. Accordingly, their dysfunction can have devastating effects. Disorders such as epilepsy, hemolytic anemia, glaucoma, hearing loss, osteopetrosis, and renal tubular acidosis are all genetically linked to SLC4-family gene loci. This review summarizes how studies of Slc4-modified mice have enhanced our understanding of the etiology of SLC4-linked pathologies and the interpretation of genetic linkage studies. The review also surveys the novel disease signs exhibited by Slc4-modified mice which could either be considered to presage their description in humans, or to highlight interspecific differences. Finally, novel Slc4-modified mouse models are proposed, the study of which may further our understanding of the basis and treatment of SLC4-linked disorders of -transporter dysfunction.
Keywords: acid-base, disease, epithelia, knockout, pH
INTRODUCTION
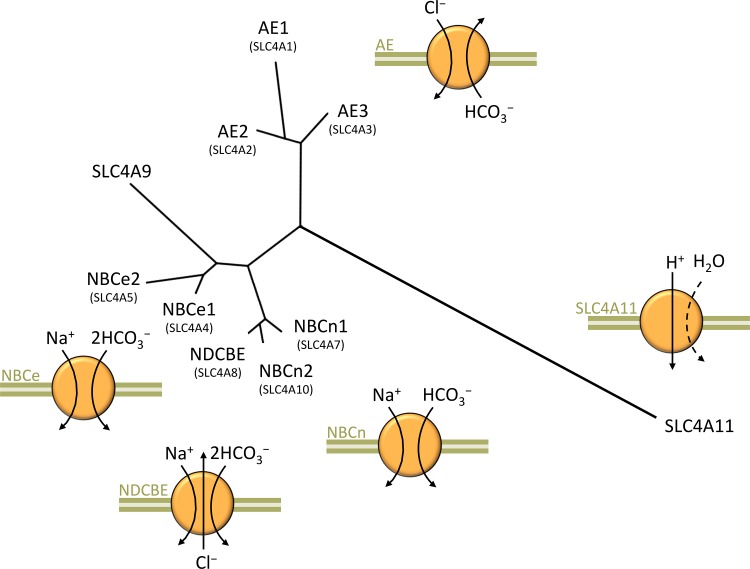
The SLC4 family of acid-base transporters (Fig. 1 and Ref. 145) includes three Na+-independent Cl−/ exchangers (the anion exchangers AE1, AE2, and AE3), two electrogenic Na+- cotransporters (NBCe1, and NBCe2), two electroneutral Na+- cotransporters (NBCn1 and NBCn2), one Na+-driven Cl−/ exchanger (NDCBE), one unusual member (SLC4A9) which has been variously described as being capable of most of the above actions (30, 102, 151), and one H2O-permeable H+/OH− conductor (SLC4A11) that does not transport (139, 204). Each SLC4-family transporter is expressed in multiple cell types such that disruption of any single gene often has devastating and complex pathological consequences (Table 1). Fortunately, most of these diseases are rare, but the paucity of clinical reports and tissue samples from affected individuals provides a challenge to understanding the basis of, and developing treatments for, these orphan diseases. The study of Slc4-modified mice has enabled investigators to probe more deeply into the consequences of Slc4 dysfunction and learn about the physiological roles of Slc4 proteins.
Fig. 1.
The SLC4 family of acid-base transporters. A dendrogram of protein sequence similarity, based on human sequences, was generated using the TreeDyn tool (37) at http://www.phylogeny.fr/index.cgi. Murine slc4 sequences have a similar relationship, being 82–98% identical to their human orthologs according to pairwise BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) results.
Table 1.
Diseases associated with SLC4-linked transporter dysfunction
| Gene | Human | Cattle | Dog |
|---|---|---|---|
| SLC4A1 | • Hereditary spherocytosis with distal renal tubular acidosis (164, 167) | • Hereditary spherocytosis with distal renal tubular acidosis (86) | |
| • Hereditary spherocytosis (without distal renal tubular acidosis) (93, 152) | |||
| • Distal renal tubular acidosis (without hereditary spherocytosis) (27) | |||
| • Southeast Asian ovalocytosis (92) | |||
| • Acanthocytosis (28) | |||
| • Cryohydrocytosis (25) | |||
| SLC4A2 | • Primary biliary cholangitis (2, 157) | • Osteopetrosis (131) | |
| SLC4A3 | • Idiopathic generalized epilepsy (171) | • Progressive retinal atrophy (54) | |
| • Short QT syndrome (199) | |||
| SLC4A4 | • Proximal renal tubular acidosis with ocular abnormalities (83) | ||
| • Band keratopathy (without proximal renal tubular acidosis) (148) | |||
| SLC4A5 | • Hypertension (29, 82, 195, 196) | ||
| SLC4A7 | • Hypertension (88, 123) | ||
| • Breast cancer susceptibility (1, 36, 121, 191) | |||
| SLC4A10 | • Autism with idiopathic generalized epilepsy (16, 71, 106, 173) |
Disorders shown in boldface have been recapitulated in Slc4-modified mice.
Mice have proven to be a very useful model for Slc4 studies; mice express orthologs of all SLC4 genes, and most SLC4 protein variants. In most cases the distribution of SLC4 proteins is the same or similar between humans and mice and, as we will see, in the majority of cases Slc4-modified mice recapitulate many of the major disease signs that are linked to SLC4 dysfunction in humans.
Although this review focuses on the modeling of human diseases, there are also several cases of naturally occurring, pathological mutations in the Slc4 genes of cattle and dogs which are considered here both as models of human disease and as economically important diseases in their own right to be modeled in mice. No human diseases have yet been associated with the SLC4A8 (NDCBE) or SLC4A9 genes; thus the phenotypes of Slc4a8-null mice [compromised renal Na+ reabsorption and increased seizure threshold (114, 179, 180)] and Slc4a9-null mice [impaired salivary fluid secretion (150)] are not considered further in this review. Disruption of SLC4A11 is associated with blindness (Congenital Hereditary Endothelial Dystrophy, Fuchs’ dystrophy) often with progressive hearing loss (Harboyan Syndrome) in humans; both phenotypes are well modeled in mice (149). However, as these are not directly related to transporter dysfunction (139, 204), discussion of SLC4A11-linked disease falls beyond the scope of the present review.
For the remaining genes, a comparison of the human versus mouse gene structure is presented followed by a summary of the signs of the associated human/bovine/canine diseases that have been modeled in mice, a listing of published mouse models and their phenotypes, and a consideration of the strengths and weaknesses of these models. Finally, each section includes suggestions of yet-to-be-generated mouse models, which may add to our knowledge base. For comprehensive reviews of SLC4-linked physiology and pathology the reader is directed to recent reviews by Parker and Boron (145), Romero et al. (165), and Alka and Casey (4). Reviews of SLC4 dysfunction in the kidney or choroid plexus as modeled in mice have been published by Eladari and Kumai (56) and Christensen et al. (39).
Slc4a1-MODIFIED MICE: MODELS OF HEREDITARY SPHEROCYTOSIS AND DISTAL RENAL TUBULAR ACIDOSIS
SLC4A1 and the Cl−/ Exchanger AE1
Human.
SLC4A1 (chromosomal location 17q21.31) encodes the Na+-independent Cl−/ exchanger AE1 (anion exchanger 1). An erythroid (eAE1) and a renal (kAE1) variant of AE1 are expressed from alternative promoters, with the result that eAE1 is 65 amino acids longer than kAE1 [the translation of kAE1 begins at the equivalent of Met66 of eAE1 (6)]. This genetic arrangement permits the selective disruption of eAE1. eAE1 is expressed in the erythrocyte membrane, whereas kAE1 is expressed in the basolateral membrane of α-intercalated (H+ secreting) epithelial cells of the renal collecting duct (8, 55). Both variants of AE1 comprise a cytosolic NH2-terminal domain (Nt) with defined structure, a transmembrane domain (TMD) composed of 12–14 transmembrane spans, and a shorter unstructured cytosolic COOH-terminal domain (118). The Nt of AE1 is dispensable for anion exchange activity (69).1
Murine.
Slc4a1 (Chr 11 66.29 cM)2 encodes orthologs of both AE1 variants. Mouse and human AE1 are 82% identical at the protein level,3 the major difference being several small insertions in the open reading frame of the AE1 Nt compared with the human gene, which add 18 additional amino acids to the murine ortholog of eAE1 [14 of which are located in the unique eAE1 Nt appendage, such that eAe1 is 79 amino acid residues longer than kAe1 in mice (24)]. The consequence of these differences is unknown.
AE1 Function and Dysfunction
Erythrocyte integrity and hemolytic anemia including hereditary spherocytosis.
In red cells, eAE1-mediated Cl−/ exchange promotes gas exchange [the chloride shift (44)], while the Nt of eAE1 forms a critical bridge between the erythrocyte membrane and the underlying cytoskeleton (26, 50). This structural role helps to define the mechanical properties of circulating erythrocytes; loss of this function is the chief cause of SLC4A1-associated blood disease. There are various dominantly inherited blood disorders linked to AE1 dysfunction: Southeast Asian ovalocytosis (SAO), hereditary spherocytosis (HS), cryohydrocytosis (CHC), and acanthocytosis. SAO and HS are hemolytic anemias in which a disturbance of the AE1-mediated linkage between the erythrocyte membrane and cytoskeleton alters the cell shape, increasing membrane rigidity and fragility (92, 93, 135). Although these dominantly inherited anemias are often mild, the rarer recessive inheritance of these mutations can result in severe dyserythropoietic anemia together with distal renal tubular acidosis and may even be lethal (155, 164). A subtype of HS (HS-LTL) is an atypical form of hereditary stomatocytosis (HSt) in which spherocytic cells also exhibit a low-temperature cation leak. The leak is associated with mutations in the latter half of the TMD of AE1 that create or enhance a pathological cation leak pathway (25, 190). The leak compromises the ability of the otherwise intact cells to regulate their volume, causing the erythrocytes to swell (25). CHC is a variant of HSt in which the cell swelling is greatly exacerbated by cooling of the cells (e.g., for storage) (25). Finally, acanthocytosis—the otherwise asymptomatic presence of crenated erythrocytes—is associated with recessive inheritance of a variant of AE1 that exhibits an enhanced Vmax for anion transport (28).
Urinary acidification and distal renal tubular acidosis.
The normal range of plasma [] is 22–28 mM. The majority of the filtered at the glomerulus is reabsorbed by proximal tubule epithelia, thus very little is normally delivered to the distal tubule. To maximize urinary acid elimination, α-intercalated cells in the distal tubule secrete H+ across their apical membrane. (Fig. 2) These H+ are titrated by non- buffers such as phosphate and excreted in urine. This apical secretion of H+ is balanced by the basolateral absorption of by AE1, maintaining intercalated cell pHi. When SLC4A1 mutations impair this supportive function, the ability of the kidney to eliminate acid is compromised and can result in a decrease in plasma []: a disease known as distal renal tubular acidosis (dRTA) (27, 134).
Fig. 2.
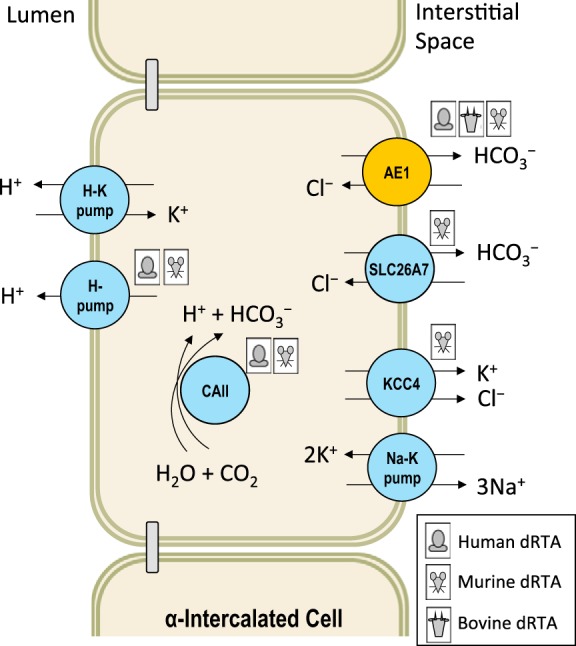
Defects in the mechanism of urinary acidification: distal renal tubular acidosis (dRTA) in humans, cattle, and mice. The consequences of AE1 loss in all three species are discussed in the text. Mutations in the two H+-pump subunits can also cause dRTA in humans (99, 182) and mice (60, 143). Mutations in carbonic anhydrase II are associated with a generalized renal tubular acidosis in humans (181) and mice (115). In addition, disruption of Slc26a7 (212) and Kcc4 (21) in mice results in subtypes of dRTA that have yet to be observed in humans.
dRTA can be inherited in dominant or recessive forms. Depending on the impact of the mutation on AE1 function, dRTA can be complete (spontaneous metabolic acidosis with [] as low as 5 mM and failure to acidify urine; e.g., Ref. 186) or incomplete (acidosis only manifested upon administration of an acid load). In some cases, a mutation that causes incomplete dRTA in heterozygous form can cause complete dRTA in homozygous form (e.g., Ref. 32), in other cases the heterozygous inheritance of a mutation can result in complete dRTA in one individual yet incomplete dRTA in another, suggesting variable expressivity (94). Acid-base imbalance is the only pathognomic feature of dRTA although individuals often exhibit short stature, defective Ca2+ handling (hypercalciuria, nephrocalcinosis, nephrolithiasis, rickets), defective K+ handling (hypokalemia, muscle weakness), and sometimes one of the aforementioned blood disorders (see below). Less frequently reported are signs such as hypocitraturia [which sensitizes the individual to renal calcium deposition (32, 62, 186)], seizures/epilepsy (164, 187), and susceptibility to infection (174, 202).
Determinants of co-occurrence of pRTA with a blood disorder.
SLC4A1 mutations can cause either a blood disorder, pRTA, or both in the same individual. Presumably the physiological importance of a structurally intact Nt for eAE1 function versus the physiological importance of Cl−/ exchange for kAE1 function, in part influences the phenotypic result of each mutation. What is known of the genotype-phenotype relationships between AE1 mutation and disease is neatly illustrated in a review by Alper (7). There are three notable situations in which the genotype-phenotype correlation is understood: 1) one AE1 mutation specifically affects the Nt of eAE1 and therefore results in a blood-specific phenotype (152); 2) at least two AE1 mutations that ought to affect both eAE1 and kAE1 cause a folding defect that is specifically corrected in erythrocytes by the erythroid-specific AE1 chaperone glycophorin A, resulting in a kidney-specific phenotype (190, 194); 3) several AE1 mutations are predicted to cause premature termination of the AE1 polypeptide, resulting in the loss of both eAE1 and kAE1 resulting in both HS and dRTA (e.g., Ref. 97).
Complete abrogation of AE1 expression results in severe HS and complete dRTA, a condition so rare that it was once considered to have been lethal (97, 164). One report describes two pregnancies in an AE1-heterozygous mother in which the fetus stopped moving near term; the first—presumed to be AE1-null—was stillborn and the second—demonstrated to be AE1-null and diagnosed with HS and dRTA—was delivered by emergency caesarian section (164). These data indicate that total AE1 loss could have a negative impact on fertility. HS with dRTA has also been described in cattle with a spontaneous nonsense mutation in Slc4a1 (86).
Slc4a1-Modified Mice
Strains.
Four strains of Slc4a1-modified mice have been characterized; the details of their genotype are provided below. Note that the names of these mice have been altered for internal consistency within this review: their original names and their official designations are listed in Table 2.
Table 2.
Nomenclature for the Slc4-modified mice used in this review
| Human Gene | Mouse Model |
||
|---|---|---|---|
| This Review | Original Report | MGI Database | |
| SLC4A1 |
Slc4a1−/−(NeoR) Slc4a1eAE1−/−(NeoR) Slc4a1Q85X/Q85X Slc4a1R607H/R607H |
AE1−/− Band 3−/− C3H/HeJwan/wan Ae1R607H/R607H |
Slc4a1tm1Llp Slc4a1tm1Ahc Slc4a1wan |
| SLC4A2 |
Slc4a2a,b−/− Slc4a2 −/−(NeoR) Slc4a2CTSK−/− Slc4a2 −/−(ind) |
Ae2−/−, Ae2a,b−/− AE2−/− cKO Slc4a2Δ/Δ |
Slc4a2tm1Jmed Slc4a2tm1Ges Slc4a2tm2Ges |
| SLC4A3 |
Slc4a3−/− Slc4a3−/−(NeoR) |
AE3-knockout Slc4a3−/− |
Slc4a3tm1Cahb Slc4a3tm1Ges |
| SLC4A4 |
Slc4a4−/−(NeoR) Slc4a4W516X/W516X |
NBC1−/− NBC1W516X/W516X |
Slc4a4tm1Ges Slc4a4tm1.1Slin |
| SLC4A5 |
Slc4a5−/−(NeoR) Slc4a5−/− |
Slc4a5−/− Slc4a5Δ7/ Δ7 |
Slc4a5Gt(OST447853)Lex Slc4a5tm1Boet |
| SLC4A7 |
Slc4a7−/−(NeoR) Slc4a7b,c,d,e−/−(NeoR) |
Slc4a7−/− Nbcn1−/− |
Slc4a7tm1Krtz Slc4a7Gt(40G1)Cmhd |
| SLC4A10 |
Slc4a10−/− Slc4a10L647P/L647P |
Slc4a10 KO Slc4a10trmb/trmb |
Slc4a10tm1.1Cahb Slc4a10mpc96H |
Precise details of the genotypes of these mice together with details of other available Slc4-modified mice, yet to be fully characterized, are available on the MGI database (www.informatics.jax.org).
Slc4a1−/−(NeoR). In this strain, exons 9–11 of Slc4a1 are disrupted by a neomycin resistance cassette (NeoR), a maneuver that should prevent the expression of both eAe1 and kAe1. Original experimental mice had a mixed 129/Sv-C57BL/6J genetic background (10, 153, 189).
Slc4a1eAE1−/−(NeoR). In this strain, exon 3 of Slc4a1 is disrupted by NeoR, a maneuver that should selectively prevent the expression of eAe1. Original experimental mice had a C57BL/6J genetic background (183).
Slc4a1Q85X/Q85X. This is not a deliberately engineered mouse, but its genome includes a nonsense mutation in the Slc4a1 gene that arose spontaneously in a colony of inbred C3H/HeJ mice (154). The mutant Ae1 is truncated early in the Nt (p.Gln85X), preventing the expression of both eAe1 and kAe1.
Slc4a1R607H/R607H. In this strain a missense mutation is introduced into exon 14 of slc4a1 to recapitulate the human mutant p.Arg589His that is associated with dominant inheritance of dRTA without coinheritance of a blood disorder4 (94). Original experimental mice had a mixed 129/Sv-C57BL/6J genetic background (138).
HS modeled in Slc4a1-modified mice.
Clearly not all blood disorders can be modeled by a single strain of Slc4a1-null mouse: SAO is associated with a specific deletion at the Nt/TMD interface of AE1, HS is associated with a variety of Ae1-disabling mutations, while HSt, CHC, and acanthocytosis are associated with specific gain-of-function missense mutations (7, 25). While HS is also associated with a variety of missense mutations in dominant and recessively inherited form, it is also the only blood disorder associated with premature termination codons in SLC4A1.
Heterozygous Slc4a1+/−(NeoR) and Slc4a1+/Q85X mice ought to recapitulate the dominantly inherited form of disease in which nonsense or frameshift mutations cause a mild HS with incomplete (167) or absent/undiagnosed dRTA (5, 91, 95); homozygous Slc4a1−/−(NeoR) and Slc4a1Q85X/Q85X mice ought to recapitulate the recessively inherited form of disease in which nonsense mutations cause severe HS with complete dRTA (97, 164). Slc4a1eAE1−/−(NeoR) mice model severe HS without accompanying dRTA caused by specific loss of eAE1 (152).
Indeed most of these strains exhibit signs of HS, although to a large extent the phenotype of these mice appears to be milder than that of their human counterparts. Regarding the heterozygotes: Slc4a1+/−(NeoR) mice exhibit a very subtle spherocytosis without dRTA (153), whereas Slc4a1+/R85X mice are described as hematologically normal (154). No blood phenotype is reported for Slc4a1eAE1+/−(NeoR) mice (183). Regarding the homozygotes: acidotic Slc4a1−/−(NeoR) mice, presumably-acidotic Slc4a1R85X/R85X mice, and (presumably nonacidotic) Slc4a1eAE1−/− mice all exhibit severe hemolytic anemia with spherocytic erythrocytes, and increased mortality [although no evidence of fetal mortality (153, 183)]. Other secondary signs of anemia such as pallor, growth retardation, splenomegaly, and cardiac hypertrophy have also been reported in these Ae1-null mice (10, 153, 183). The extent of the anemia varies among models (2–10 fold deficiency of hematocrit and hemoglobin concentration) but is consistent with the extent of anemia in AE1-null individuals (97). Also important with respect to the integrity of the model is the observation that Slc4a1+/R607H mice—a model of dRTA without HS—do not exhibit any blood phenotype, even in homozygous form (138).
dRTA modeled in Slc4a1-modified mice.
dRTA associated with dominant inheritance of SLC4A1 mutations exhibits variable expressivity in humans. For example, the R589H mutant (modeled in Slc4a1+/R607H) can result in complete or incomplete forms of dRTA in affected individuals (94). Nonetheless, as with AE1-related blood disorders, the signs of dRTA are milder in mice than humans; neither heterozygous Slc4a1+/−(NeoR) nor Slc4a1+/R607H mice exhibit the metabolic acidosis or urinary acidification defects associated with dominantly inherited dRTA (138, 189). However, homozygous Slc4a1−/−(NeoR) do exhibit the complete dRTA that is characteristic of AE1-null individuals as well as other dRTA associated signs such as hypercalciuria and hypocitraturia, but not hypokalemia (138). Slc4a1R607H/R607H mice exhibit only an incomplete dRTA but they do recapitulate several features that have been observed in biopsy samples from individuals with dRTA such as fewer α-intercalated cells, and mistargeting of the H+-ATPase (138, 205).
Novel disease signs in Slc4a1-modified mice.
Slc4a1−/−(NeoR) mice exhibit a mild urine concentrating defect similar to nephrogenic diabetes insipidus that is related to loss of apical aquaporin 2 (AQP2) expression from the inner medullary collecting duct (189), which is not reported in individuals with SLC4A1 mutations.
Overview and Outlook
Slc4a1-modified mice recapitulate all of the major features of HS and dRTA. The main disparity between the effects of SLC4A1 deletion in humans and mice lies in the severity of the phenotypes. Autosomal dominant inheritance of HS or dRTA is not recapitulated in mouse models whereas the modeled autosomal recessive inheritance is variably lethal, indicating that modifying factors ameliorate the phenotype. Studies of Slc4a1R85X/R85X mice reveal that genetic background can influence the severity of the anemia and the lethality of the phenotype. One such genetic modifier locus includes Spnb1 which encodes the erythrocyte cytoskeletal component β-spectrin (154). Regarding the expressivity of signs of dRTA in mice, it has also been noted that laboratory mice have a more alkaline diet than humans which may temper the impact of Ae1 loss (138). A particularly intriguing avenue of research is the recreation of specific missense mutations in Slc4a1 for the following reason: heterologous expression studies have long been used to assess the appropriate plasma-membrane targeting of transporter mutants and to test small molecular therapies to restore their correct trafficking. Human biopsies suggest that some predicted-to-be-mistargeted AE1 mutants are simply absent from the renal epithelia of affected individuals and reveal unanticipated renal adaptations (202, 205). The study of Slc4a1R607H/R607H mice also shows the reduced expression of the mutant, rather than its predicted mistargeting and altered expression of the H+-pump (138); thus these and similar mice will be critical for the development and testing of therapeutic strategies.
Slc4a2-MODIFIED MICE: MODELING PRIMARY BILIARY CHOLANGITIS AND OSTEOPETROSIS
SLC4A2 and the Cl−/ Exchanger AE2
Human.
SLC4A2 (chromosomal location 7q36.1) encodes the Na+-independent Cl−/ exchanger AE2 (anion exchanger 2). Alternative promoter choice and transcript processing allows the expression of four AE2 variants that each have a different NH2-terminal appendage, that influences the activity, plasma-membrane expression, and pH dependence of the transporter (e.g., Ref. 109). The longest variants are AE2a, AE2b1, and AE2b2 (collectively AE2a/b), differ by only a few amino acids at their extreme Nt, and have a broad distribution. AE2a/b expression is typical in the basolateral membranes of intestinal, renal, and dental epithelia and in the contra-lacunar membrane of osteoclasts (145). An atypical apical presence of AE2a/b has been described in cholangiocytes (liver bile-duct epithelia) (13, 125, 185).
Murine.
The murine ortholog Slc4a2 (Chr 5 11.74 cM) is capable of expressing orthologs of AE2a/b as well as two shorter variants Ae2c1 and Ae2c2. These are ~200 aa shorter than AE2a/b and it is unclear if there is a human ortholog of AE2c1. Ae2c2 is considered to be a mouse-specific variant and does not accumulate well in the plasma membrane by itself: its role is unknown (108). Mouse and human AE2a are 94% identical at the protein level.
AE2 Function and Dysfunction
Cholangiocyte protection and primary biliary cholangitis.
AE2 acts as an acid-loader, removing excess from cells in exchange for Cl−. In the apical membrane of cholangiocytes, the action of AE2 contributes to the secretion of -rich bile and also creates a protective “umbrella” at the apical surface of the cells that protects cells from bile-acid-induced injury (79). Furthermore, AE2 action limits the accumulation of intracellular [], preventing pathological activation of the HCO3−-sensing apoptosis-regulator soluble adenylyl cyclase (31). Primary biliary cholangitis (PBC), also known as primary biliary cirrhosis, is a chronic liver disease—mostly exhibited in middle-aged women—in which bile ducts are damaged and the tissue becomes fibrotic. PBC has an autoimmune component; antimitochondrial (auto)antibodies (AMAs) are found in the serum of >90% of patients (52). Signs and symptoms of PBC can include abdominal pain, jaundice, edema, internal bleeding, and osteoporosis (11).
There is no confirmed single heritable cause of PBC, although the susceptibility to and progression of PBC correlates with environmental factors and certain single nucleotide polymorphism (SNPs) (119), some of which are located within the SLC4A2 gene (2, 157). Indeed, loss of AE2 transcript and protein abundance as well as loss of AE2 function are a feature of duct cells isolated from PBC patients (127, 130, 160, 161). Some studies show that AE2 loss in PBC follows other primary disturbances such as upregulation of an miRNA or altered composition of hepatic bile acids (14, 76), suggesting that local loss of AE2, predisposed by SNPs, may be a more common etiology than the direct abrogation of AE2 caused by SLC4A2 mutation. Nonetheless, many studies including the recapitulation of signs of PBC in Slc4a2-null mice, discussed below, reveal the importance of AE2 dysfunction in cholangiocytes and T cells to the etiology of PBC.
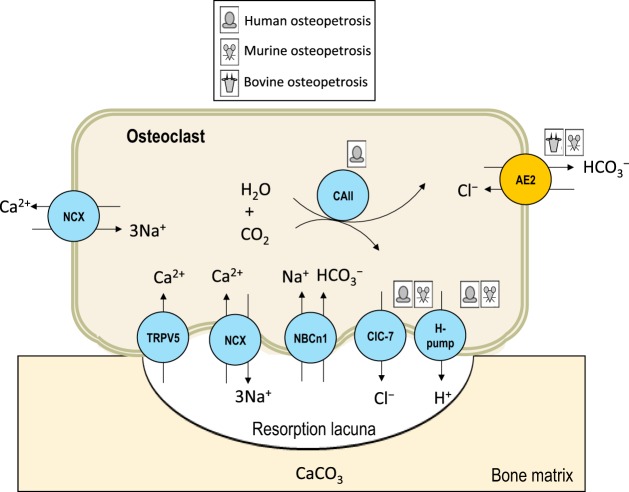
Acid secretion by osteoclasts and osteopetrosis.
AE2 in the contra-lacunar membrane of osteoclasts supports the secretion of H+ that is necessary to resorb and remodel bone (Fig. 3). Osteopetrosis is a group of diseases characterized by increased bone density caused by osteoclast dysfunction. Signs and symptoms include short stature, proneness to fracture, compressive neuropathies, and hypocalcemia (188). The genetic basis of osteopetrosis is known in ~70% of cases and involves mutations in any of 13 genes, including several that encode the acid-base handling machinery of osteoclasts such as CA2, CLCN7, and TCIRG1 (188). Each subtype of osteopetrosis has a unique set of signs that related to its unique genetic basis. Although AE2 is also considered part of the H+-secreting machinery in osteoclasts, SLC4A2 has yet to be linked to osteopetrosis in humans. However, in cattle a spontaneous deletion of the 2.8-kb region, which encompasses exons 2 and part of exon 3 of bovine Slc4a2, is linked to recessive inheritance of osteopetrosis with early mortality and neurological signs (131, 144). These cattle provide an insight into the possible features of AE2-null patients. Affected cattle are usually aborted; necropsies reveal thickened skull and bones, a reduction in osteoclast number, impacted and unerupted teeth, as well as compressed and calcified brains (131).
Fig. 3.
Defects in the mechanism of bone resorption: osteopetrosis in humans, cattle, and mice. The consequences of Ae2 loss in cattle and mice are discussed in the text. Loss of carbonic anhydrase II is associated with osteopetrosis in humans (181), but not mice (115). Loss of subunits of the ClC-7 channel and the H+-pump is associated with osteopetrosis in humans and mice (61, 104, 116).
Slc4a2-Modified Mice
Strains.
Four strains of Slc4a2-modified mice have been described.
Slc4a2a,b−/−. This strain is on a mixed C57BL/6, 129/Ola, FVB genetic background (128). Slc4a2a,b−/− mice have a 1.5-kb deletion in Slc4a2 that is predicted to prevent expression of Ae2a/b without disrupting the expression of Ae2c1 or the inactive Ae2c2. However, in practice, Ae2c1 expression is also virtually absent from these mice and so these mice are essentially Ae2-null (163).
Slc4a2−/−(NeoR). This strain was studied on a mixed 129S6/SvEv, Black Swiss genetic background and has NeoR inserted in place of four Slc4a2 exons (65). This disruption is further downstream than that introduced in Slc4a2a,b−/− mice and is predicted to abrogate expression of all Ae2 variants.
Slc4a2CTSK−/−. Slc4a2fl/fl mice (a C57BL/6 strain in which an exon common to all Ae2 variants is “floxed”: flanked by loxP sites) were crossed with a strain of cathepsin-K-Cre mice to generate an osteoclast-specific knockout of Ae2. However, Ae2 expression was only partially abrogated (70% reduction) in the osteoclasts of these mice, reflecting incomplete deletion of the floxed alleles (43).
Slc4a2−/−(ind). Slc4a2fl/fl mice were crossed with a strain of Mx1-Cre mice to produce this strain of inducible Ae2-null mouse (43). In these mice, an intraperitoneal injection with polyinosinic-polycytidinic acid (poly I:C) induced the expression of Cre recombinase causing the excision of the floxed Slc4a2 exon, preventing Ae2 expression in all cells (107).
Slc4a2a,b−/− and Slc4a2−/−(NeoR) mouse strains are both effectively Ae2-null, and both exhibit a failure to thrive with increased mortality. However, although perinatal mortality is greater in Slc4a2a,b−/− versus Slc4a2−/−(NeoR) mice (80% versus 25%), the surviving Slc4a2a,b−/− mice thrive normally, whereas no Slc4a2−/−(NeoR) mice survive beyond 40 days. Slc4a2−/−(NeoR) also exhibit a more severe skeletal defect (see below). It is possible, although unlikely, that the small difference in Ae2c1 and AE2c2 expression between the strains could underlie this difference. One other obvious difference between the two strains is the genetic background, although the milder phenotype of Slc4a2a,b−/− mice is reportedly no worse when backcrossed onto several other genetic backgrounds (90). It is also possible that the expression of the neomycin resistance gene (or the expression of partial Ae2 polypeptides) in Slc4a2−/−(NeoR) cells might exacerbate the phenotype in those mice; the exact reason for the difference has not been determined.
PBC modeled in Slc4a2-modified mice.
Individual Slc4a2a,b−/− mice exhibit many of the signs of PBC with varying degrees of severity. Signs include the presence of AMAs, splenomegaly, cholestatic liver damage, and portal inflammation surrounding bile ducts (169). Consistent with the multifactorial etiology of PBC in humans, disturbances are detected in both the cholangiocytes and the T cells of Slc4a2a,b−/− mice. In common with the cholangiocytes isolated from PBC patients, the cholangiocytes of these mice exhibit a loss of cAMP-stimulated -efflux (but no increase in resting pHi) (130, 169, 200). The cholangiocytes of Slc4a2a,b−/− mice also exhibit signs of metabolic stress and increased antigen presentation which makes them more susceptible to autoimmune injury (169). Concomitantly, Slc4a2a,b−/− mice exhibit a reduction in the number of helper CD4-positive (CD4+) T cells and an increase in the number of cytotoxic CD8-positive (CD8+) T cells, which disposes their bodies to autoimmune attack (169). This increase in CD8+ cells appears to be directly linked to Ae2 loss: a key observation from studies of mice is that CD8+ cells also express Ae2 and the loss of Ae2 from these cells causes a rise in steady-state pHi that promotes CD8+ proliferation, activation, and survival (41, 42).
Although the trigger for PBC in humans remains unknown, the recapitulation of PBC in Ae2-null mice makes it clear that AE2 loss is a critical factor in the pathogenesis of PBC and that these mice appear to faithfully reproduce many of the signs of PBC. However, the female gender-bias that is a feature of human PBC is not evident in Ae2-null mice, suggesting either a species difference or that there is an X-linked component upstream of AE2 downregulation that contributes to the etiology of PBC in humans (133). Another consideration is that, unlike PBC patients in which AE2 loss may be posttranscriptional and conditional to certain cells types, Ae2-null mice exhibit other pathologies associated with the global loss of AE2, such as osteopetrosis, that may modify the hepatic and immunological phenotype.
Osteopetrosis modeled in Slc4a2-modified mice.
Signs of osteopetrosis have been observed in four strains of Slc4a2-modified mice. Slc4a2a,b−/− and Slc4a2−/−(Neo) mice most closely recapitulate the global loss of Ae2 predicted to underlie the bovine disease. As mentioned earlier, Slc4a2−/−(NeoR) mice exhibit an inexplicably more severe phenotype than Slc4a2a,b−/− mice. Slc4a2−/−(NeoR) mice exhibit a general failure to thrive together with a failure to remodel the primary skeleton, loss of bone marrow cavities, a reduction in the number of osteoclasts, jaw malformation, and unerupted teeth (23, 65, 96, 211). Those osteoclasts that are present show increased signs of apoptosis, lacked a ruffled border, and are incapable of forming acidic resorption pits (96, 211). On the other hand, Slc4a2a,b−/− mice exhibit a far less severe phenotype; mild growth retardation with signs of osteopetrosis is most evident in long bones. The skull and jaw appear normal, but teeth are hypomineralized (23, 90, 124). Clearly, the phenotype of Slc4a2−/−(NeoR) mice better reflects the signs of the bovine disease and adds credence to the possibility that the lack of reports of AE2-linked osteopetrosis in humans may lie in the lethality of global AE2 loss.
The osteoclast-specific, but partial loss of Ae2 in Slc4a2CTSK−/− mice resulted in a mild bone pathology (increased bone volume with remnants of cartilage and decrease marrow space) and partly reduced osteoclast function (43). Osteoclast remodeling activity was also reduced in Ae2-null cells isolated from poly-I:C-injected Slc4a2−/−(ind) mice, although no gross morphological consequences were reported (43).
Novel disease signs in Slc4a2-modified mice.
Several novel phenotypes have been reported from studies of Slc4a2-modified mice that may presage their genetic linkage to AE2 dysfunction in humans.
Sterility. Slc4a2a,b−/− males are sterile due to disruption of spermiogenesis (128).
Deafness. Initial reports of Slc4a2a,b−/− and Slc4a2−/−(NeoR) mice both cite unpublished observations of hearing loss.
Achlorhydria. Despite normal parietal cell abundance, both Slc4a2a,b−/− and Slc4a2−/−(NeoR) mice exhibit defects in gastric acid secretion (65, 163). Again, the Slc4a2−/−(NeoR) mice appear to exhibit a worse phenotype and are described as achlorhydric. It is possible that differences in the expression of the parietal Ae2c1 variant, partially preserved in Slc4a2a,b−/− mice, could underlie this difference. It has been noted that AE2 is downregulated in gastric cancers, which may contribute to the achlorhydria experienced by those patients (213).
Susceptibility to infection. Although not a phenotype described in whole animals, Ae2-null macrophages isolated from poly-I:C-injected Slc4a2−/−(ind) mice exhibit a reduced ability to phagocytose the pathogenic fungus Candida albicans due to reduced expression of a key surface receptor (201).
Overview and Outlook
The variable expressivity of osteopetrosis among strains of Slc4a2-modified mice provides an opportunity to search for environmental or genetic modifiers. One of these may turn out to be the AE2c variant that has no known ortholog in humans; if so, mice might be “humanized” by identifying and inactivating the Ae2c promoter(s). At least this may reveal the role of the Ae2c variants. PBC appears to be related to a later-onset loss of AE2 expression secondary to other factors that, although well modeled in Slc4a2-null mice, may be more faithfully recreated by induction of Ae2 loss in Slc4a2−/−(ind) mice. It would be especially powerful if the AE2 loss could be conditionally induced in T cells versus cholangiocytes to tease apart the pathological importance of each site of Ae2 loss. Finally, further studies are warranted on the basis of the hearing loss reported in two strains of Slc4a2-modified mice as many studies show extensive AE2 expression throughout the inner ear of various species (81, 132, 218).
Slc4a3-MODIFIED MICE: MODELS OF IDIOPATHIC GENERALIZED EPILEPSY, RETINAL DEGENERATION, AND CARDIAC DYSFUNCTION
SLC4A3 and the Cl−/ Exchanger AE3
Human.
SLC4A3 (chromosomal location 2q35) encodes the Na+-independent Cl−/ exchanger AE3 (anion exchanger 3). Alternative promoter choice allows the expression of two versions of AE3 that each have a different NH2-terminal appendage. The longer version, known as full-length AE3 (AE3fl, also known as brain AE3: bAE3), is expressed in the brain and heart. A second variant known as cardiac AE3 (cAE3) is predominantly expressed in the heart (117).
Murine.
Slc4a3 (Chr 1, 39.16 cM) expresses orthologs of AE3fl and cAE3 (117). Mouse and human AE3fl are 96% identical at the protein level.
AE3 Function and Dysfunction
Neuronal excitability and epilepsy.
AE3 is abundantly expressed in neurons of the CA3 region of the hippocampus where its acid-loading action would tend to lower pHi and dampen neuronal excitement. Consistent with this proposed role, an SNP in SLC4A3—Ala867Asp of AE3fl (which reduces AE3 activity 2-fold)—confers a small degree of susceptibility to epilepsy (171, 203).
Retinal health and retinal degeneration.
The site of expression and the role of AE3 in the human eye are unknown and there are no descriptions of human vision defects associated with SLC4A3. However, a frameshift mutation in the Slc4a3 gene of golden retriever dogs that is predicted to disrupt the TMD is associated with the recessive inheritance of progressive retinal atrophy (PRA)—the equivalent of retinitis pigmentosa in humans—a disease caused by photoreceptor degeneration (54).
Cardiac function and short QT syndrome.
AE3 is expressed in the sarcolemma and T-tubules of ventricular cardiomyocytes (162), where it contributes to pHi and Cl− regulation (34, 113). Dominant inheritance of a missense mutation (Arg370His of AE3fl per GenBank accession NP_963868)5 in the Nt of AE3 is associated with short QT syndrome (SQTS), a shortening of the duration of the repolarization phase (“QT” interval) of the action potential that can lead to arrhythmia, cardiac arrest, and sudden death (199). In model systems, the mutation reduces the plasma membrane abundance of AE3 and results in a predictable increase in resting pHi, a perturbation that itself is sufficient to shorten the QT interval, presumably via effects on pH-sensitive ion channels. The decrease in intracellular [Cl−] that is predicted to follow AE3 loss is also capable of shortening the QT interval (199).
Slc4a3-Modified Mice
Strains.
Two strains of Slc4a3-modified mice have been characterized.
Slc4a3−/−. This strain is on a mixed 129Sv/C57BL6 genetic background. Exons 6–19, common to both Ae3 variants, have been removed by Cre recombination (74). These mice have also been studied following backcross to isogeny with C57BL6 mice (168).
Slc4a3−/−(NeoR). This strain is on a mixed 129SvJ/Black Swiss genetic background. Exons 6 and 7, common to both Ae3 variants, have been replaced by NeoR (9).
Idiopathic generalized epilepsy modeled in Slc4a3-modified mice.
Slc4a3−/− mice do not seize spontaneously, but exhibit a decreased seizure threshold and increased seizure-induced mortality (74). The recapitulation of enhanced neuronal excitability in these mice strengthens the link between the human SNP and epilepsy. Intriguingly, although AE3 is not expressed in hippocampal astrocytes, hippocampal astrocytes isolated from Slc4a3−/− mice have a reduced ability to extrude acid into the extracellular space around neurons which could itself contribute to the hyperexcitable phenotype (168).
PRA modeled in Slc4a3-modified mice.
Both Ae3 variants are expressed in the rodent retina; AE3fl in the Müller (glial) cells and cAE3 in the horizontal (neuronal) cells (103). Slc4a3−/−(NeoR) mice exhibit disorganization of astrocytic processes and vasculature within their retinas and have reduced phototransducing ability (9).
Cardiac dysfunction modeled in Slc4a3-modified mice.
Slc4a3−/−(NeoR) mice have apparently normal cardiac function and even some resistance to development of cardiac hypertrophy (184) but, in combination with other stressors, Ae3 loss can exacerbate cardiac dysfunction (3, 158, 159). Ae3-null mice have yet to be examined for electrophysiological evidence of SQTS, but QT interval length is shortened in the hearts of Ae3-knockdown zebrafish embryos (199), indicating that the phenomenon is also likely to be observed in mice.
Novel disease signs in Slc4a3-modified mice.
No other major pathologies have been reported in Ae3-null mice, but Slc4a3−/− mice exhibit a reduced respiratory rate (129).
Overview and Outlook
Slc4a3-modified mice appear to be useful models of human epilepsy and canine PRA. Successful recapitulation and therapeutic correction of SQTS in AE3-null zebrafish embryos promises that Ae3-null mice (or even Slc4a3+/R370H mice) could be valuable models in which to develop therapies for SLC4A3-linked SQTS. The current absence of descriptions of a human retinal phenotype is suggestive of interspecific differences or the ability of the human retina to compensate for AE3 dysfunction. The ability to generate variant specific knockouts of AE3fl versus cAE3 has yet to be capitalized upon: studies of these mice could elucidate the importance of horizontal cell versus Müller cell dysfunction to the pathogenesis of PRA. It might also be desirable to recreate the A867D mutation in Slc4a3 to see if that in itself is sufficient to allow expression of the epilepsy phenotype.
Slc4a4-MODIFIED MICE: MODELS OF PROXIMAL RENAL TUBULAR ACIDOSIS
SLC4A4 and the Electrogenic Na+-2 Cotransporter NBCe1
Human.
SLC4A4 (chromosomal location 4q13.3) is one of five members of the SLC4-family that encode an Na+ and -cotransporting membrane-protein (145). The SLC4A4 product is NBCe1, a Na+-2 cotransporter6 that is expressed in proximal tubule epithelia as well as in a wide variety of nonrenal epithelia and excitable cells (166). NBCe1 has a similar domain structure to the AEs, but the presence of the Nt is critical to NBCe1 action: the TMD of NBCe1 appears incapable of transport without it, and variations in the sequence of the Nt affect the rate of Na+-2 cotransport (57, 126). The NH2-terminal sequence variations are genetically encoded: alternative promoter choice determines that the renal NBCe1 gene product NBCe1-A includes a sequence within its Nt that enables constitutively rapid Na+-2 cotransport, whereas the nonrenal variants NBCe1-B and NBCe1-C (collectively NBCe1-B/C) include a different sequence in their Nts that confers a slower, but stimulatable, rate of Na+-2 cotransport (112, 126, 176). This genetic arrangement allows for the selective disruption of NBCe1-A versus NBCe1-B/C. NBCe1-B/C exhibits a broader distribution pattern than NBCe1-A; of particular relevance to the consequences of SLC4A4 disruption is the presence of NBCe1-B/C in ameloblasts of the enamel organ, corneal endothelium, pancreas, myocytes, neurons, and glia (145).
Murine.
Slc4a4 (Chr 5, 44.10 cM) expresses orthologs of all human NBCe1 variants (117). Mouse and human NBCe1-A are 97% identical at the protein level.
NBCe1 Function and Dysfunction
Plasma [] maintenance and proximal renal tubular acidosis with ocular abnormalities.
The nephron reabsorbs 99.9% of the filtered at the glomerulus, with the majority (~80%) being reabsorbed by the proximal tubule. In healthy individuals, the proximal tubule is able to generate new to replace that depleted by acid titration. Both of these processes—reabsorption and repletion—require the expression of NBCe1 function in the basolateral membrane of proximal-tubule epithelial cells (Fig. 4). Recessive inheritance7 of SLC4A4 mutations results in pRTA: a dramatic reduction of plasma [] and a rise in the amount of wasted in the urine. pRTA is also associated with ocular defects and a diverse array of other signs and sequelae (83). Typically, the heterozygous parents of these individuals exhibit no signs of pRTA There are only 15 reports of pRTA-linked SLC4A4 mutations (49, 51, 80, 83–85, 87, 101, 120, 140, 175, 192, 193) in contrast with over 120 documented cases of dRTA. Among the described cases of SLC4A4-linked pRTA, in a variety of individuals 3–50 years old, plasma [] is 3–17 mM (normal range 22–28 mM), and pH = 7.04–7.27 (normal range 7.35–7.45). The fact that the metabolic acidosis is not worse speaks to the ability of the distal nephron, faced with an unusual load of , to perform some compensatory reabsorption. However, the shortfall of renal reabsorption is revealed when plasma [] is raised to normal range by intravenous infusion of Na: fractional urinary excretion, which is trivial in unaffected individuals, can rise to ~40% of the filtered load (136).
Fig. 4.
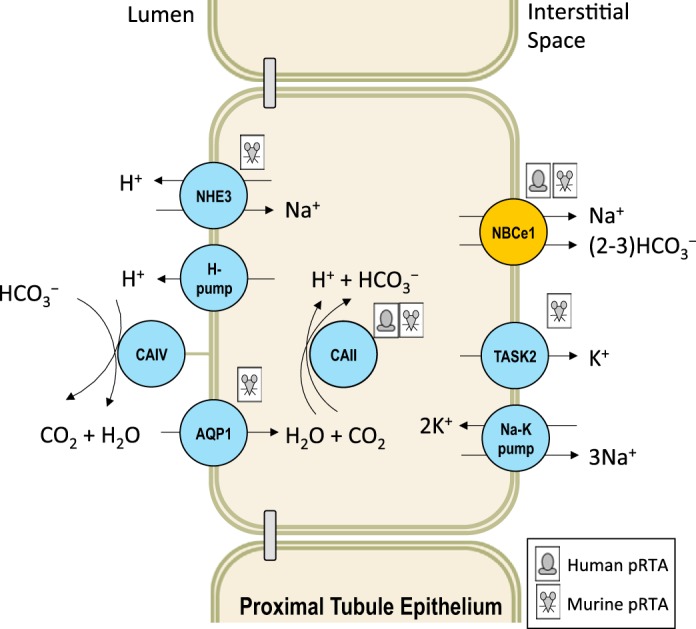
Defects in the mechanism of reabsorption: proximal renal tubular acidosis (pRTA) in humans and mice. The consequences of NBCe1 loss in humans and mice are discussed in the text. Mutations in carbonic anhydrase II are associated with a generalized renal tubular acidosis in humans (181) and mice (115). In addition, disruption of Nhe3 (172), and Task2 (208) in mice results in subtypes of pRTA yet to be observed in humans. Although Aqp1-null mice do not exhibit pRTA per se, preliminary data suggest that their isolated proximal tubules exhibit an impaired ability to reabsorb (216).
SLC4A4-linked pRTA is associated with a variety of signs and sequelae with diverse expressivity and penetrance which may be sequelae of acidemia and/or consequences of NBCe1-B/C loss from nonrenal cells. The two aspects that might be considered pathognomic are ocular abnormalities (bilateral glaucoma,8 usually accompanied by band keratopathy and cataracts), and/or short stature which have been noted in all cases to date (see pRTA references in previous paragraph). In many, but not all cases, individuals with pRTA are noted to be underweight, to have developmental delays (intellectual and motor skills), and to suffer a diverse array of neurological/neuromuscular signs such as epilepsy, migraine, paraplegia, dyskinesia, and ataxia [9 out of 15 individuals (48, 49, 87, 101, 120, 140, 175, 193)]. Computed tomography scans of several individuals with neurological/neuromuscular signs revealed bilateral calcifications of the basal ganglia [5 out of 15 individuals (80, 87, 101, 120, 140)]. It is noteworthy that individuals with hereditary bilateral calcifications of the basal ganglia unrelated to SLC4A4 mutation also exhibit migraines and movement disorders (105, 142). A less frequently reported sign is dental abnormalities of the permanent teeth: misaligned or deformed teeth with defective enamel [4 out of 15 individuals (49, 51, 87, 140)]. However, the absence of a dental phenotype is not specifically noted in other cases so its prevalence may be underreported. Other sporadically reported features are elevated serum amylase [a sign of pancreatic disease: 3 out of 15 individuals (80, 83)] and hypokalemia [3 out of 15 individuals (48, 101, 120)].
Valuable insights into the etiology of these signs are provided by the case report of one 12-year-old girl with a Q29X mutation, a conversion that is predicted to specifically abrogate expression of NBCe1-A without affecting the expression of NBCe1-B/C (84). Her signs were limited to glaucoma, short stature, and intellectual impairment. This case suggests that the other signs associated with pRTA may be primary signs of, or may be exacerbated by, NBCe1-B/C dysfunction in nonrenal cells. Additional insight comes from a report of a mutation in the 3′-untranslated region (UTR) of SLC4A4 which is predicted to destabilize all SLC4A4 transcripts, perhaps in a tissue-specific manner; the five individuals who carry this mutation exhibit progressive band keratopathy and/or glaucoma without any of the other signs of pRTA (148).
Slc4a4-Modified Mice
Strains.
Two strains have been characterized:
Slc4a4−/−(NeoR). In this strain, studied on a mixed 129S6/SvEv and Black Swiss genetic background, exon 9 of Slc4a4 is disrupted by NeoR, a maneuver that should prevent the expression of all Nbce1 variants (64).
Slc4a4W516X/W516X. In this strain, a mutant exon 11 carrying a W516X mutation replaces the wild-type exon. This strain was characterized on a 129S genetic background and homozygotes are intended to model an individual with pRTA caused by this same mutation (120).
pRTA modeled in Slc4a4-modified mice.
Neither strain of Nbce1-null mouse thrives well: both exhibit 100% mortality within 3 weeks, perhaps speaking to the scarcity of individuals with pRTA.9 Both strains of null mice exhibit the acidemia and low plasma [] that is characteristic of pRTA. Both mice also have developmental delays; they are underweight and, as formally demonstrated in the case of Slc4a4W516X/W516X mice, have reduced bone dimensions. Slc4a4−/−(NeoR) mice exhibit enamel defects from birth. Histological examination shows normal proximal tubule morphology with some flattened epithelia and tubular atrophy. Unexpectedly, heterozygous Slc4a4+/W516X mice also exhibit a reduction in plasma [] although not to the extent of Slc4a4W516X/W516X mice (120).
The mortality of Nbce1-null mice perhaps precedes the manifestation of other signs of pRTA, but in mice whose lifespans have been extended by alkali administration, corneal edema is noted which perhaps presages the characteristic ocular abnormalities of pRTA (120).
Novel disease signs in Slc4a4-modified mice.
NBCe1 is one of a number of proteins that supports the secretion of anions, and thereby fluid, across intestinal epithelia. Nbce1-null mice exhibit impacted intestines. This phenotype is considered to be due to a combination of defective fluid secretion caused by Nbce1-b/c loss and epithelial Na+ channel (ENaC)-mediated hyper absorption secondary to acidosis (64, 215). Although intestinal secretory defects are not a known feature of pRTA, the SLC4A4 locus has been linked to meconium ileus in newborns with cystic fibrosis (53).
Overview and Outlook
The greatest drawback of Slc4a4-modified mice is their enhanced mortality, which precludes the ability to perform studies of adult mice and to monitor the development of human phenotypes such as band keratopathy that have yet to be seen in young mice. The exact cause of their death is unknown, but considering the importance of Nbce1 to diverse physiological processes, the best future approach may be to develop inducible and/or tissue-specific-conditional knockouts of Nbce1. Preliminary reports of the development of variant-specific knockouts of Nbce1a and Nbce1b/c knockout mice show the value of such an approach (33, 170) and promise to reveal much about the pathophysiology of the signs and sequelae of pRTA.
Slc4a5-MODIFIED MICE: MODELS OF HYPERTENSION
SLC4A5 and the Electrogenic Na+-2 Cotransporter NBCe2
Human.
SLC4A5 (chromosomal location 4q13.3) encodes NBCe2, the second of two electrogenic Na+-2 cotransporters. Only one functional gene product, NBCe2c, is confirmed to be expressed from the human gene (145). Of particular relevance to the present review, NBCe2 is expressed in the kidney and choroid plexus.
Murine.
Slc4a5 (Chr 6, 35.94 cM) encodes an Nbce2c ortholog that is 91% identical at the protein level to human NBCe2c. Alternative promoter choice allows the expression of two major variants of NBCe2 in rodents. The second, Nbce2g is truncated in the Nt by ~115 aa (63). It is not clear whether this variant is expressed in humans, but both types are predicted to be deleted in the strains of mice discussed below.
NBCe2 Function and Dysfunction
Genetic linkage to hypertension.
Numerous genetic linkage studies have pointed to an association between polymorphisms in the SLC4A5 gene locus and elevated blood pressure traits such as elevated systolic (82, 195, 196) and diastolic blood pressure (82, 195), pulse pressure and rate (15), and salt sensitivity of hypertension (29). Those variants are intronic so their effect is not easy to predict nor to model in mice. Moreover, these phenotypes have different expressivity in different races (15) and co-vary with other factors (197). However, as we shall see, the recapitulation of hypertension in one strain of Nbce2-null mouse suggests that these SNPs tend to repress NBCe2 expression.
Slc4a5-Modified Mice
Strains.
Phenotypes have been reported in two strains of Slc4a5-null mice.
Slc4a5−/−(NeoR). This is an Slc4a5 gene-trapped mouse on a mixed 129/SvEvBrd C57BL/6J genetic background into which NeoR is integrated after exon 15 to prevent expression of NBCe2 (98).
Slc4a5−/−. In these mice, exon 7 of Slc4a5 was floxed and the exon removed by Cre recombination. The phenotype of this mouse has been reported on a mixed 129/C57BL6 genetic background (67) and on a C57BL6 isogenic background (209, 210).
Hypertension modeled in Slc4a5-modified mice.
Slc4a5−/− mice exhibit a complex, pH-dependent elevation of blood pressure (67, 210). On the mixed 129/C57BL6 genetic background, the mice exhibit a compensated metabolic acidosis with spontaneous elevation of systolic and diastolic blood pressures that can be corrected by making the mice alkalotic (67). On the other hand, Slc4a5−/− mice that are on the isogenic C57BL6 background have a normal mean arterial pressure (MAP) until an acid load is administered; the acidosis induces a rise in MAP (210). Although numerous studies indicate that NBCe2 is expressed in the distal nephron, lack of consensus regarding the polarity of its distribution—and therefore whether NBCe2 is positioned to contribute to Na+-sparing reabsorption or Na+ secretion—complicates our understanding of the basis of the disease. A hypothesis based on the apical location of NBCe2 suggests that NBCe2 contributes to Na+-sparing reabsorption and that, in the absence of Nbce2, the upregulation of other -reabsorbing proteins with a greater Na+: coupling stoichiometry such as NBCn1 causes increased Na+ retention (67). A second hypothesis, based on the basolateral expression of NBCe2, suggests that the electrogenic uptake of Na+ and from plasma into collecting-duct epithelial cells normally puts an electrochemical brake on ENaC activity, which is released with acidosis (210). The association between acidity and SLC4A5-linked hypertension has not been studied in humans.
Novel disease signs in Slc4a5-modified mice.
Aside from the unanticipated spontaneous metabolic acidosis in one strain of Slc4a5−/− mice, the other major signs observed in mice but not humans are neurological. Slc4a5−/−(NeoR) mice exhibit traits such as retinal detachment and degeneration, increased seizure threshold, reduced intracranial pressure, and reduced intracerebral volume (98). The signs are consistent with impaired cerebrospinal fluid secretion, caused by loss of NBCe2-supported anion secretion from choroid plexus epithelia. However, ventricular volume was normal in Slc4a5−/− mice, leading those authors to suggest that this follows a difference in the manner of Slc4a5 modification between the strains; Slc4a5−/−(NeoR) mice express a longer partial NBCe2 product than Slc4a5−/− mice which includes part of the NBCe2 TMD. Expression of this partial membrane protein may provide additional stress to choroid plexus epithelial cells that promotes the choroid plexus phenotype (67).
Overview and Outlook
The variable expressivity of the choroid plexus phenotype provides another opportunity to screen for pathology-modifying influences. It would also be interesting to learn whether Slc4a5−/− mice exhibit the retinal phenotype to determine the strain specificity of this novel phenotype.
Slc4a7-MODIFIED MICE: MODELS OF HYPERTENSION AND BREAST CANCER SUSCEPTIBILITY
SLC4A7 and the Electroneutral Na+- Cotransporter NBCn1
Human.
SLC4A7 (chromosomal location 3p24.1) is one of five Na+-coupled transporters and is the archetypal electroneutral Na+- cotransporter NBCn1. NBCn1 is also substantially leaky to Na+ in a -independent manner (38). Alternative promoter choice and several splice cassettes allow the expression of more than twenty NBCn1 variants, although the consequence of such diversity remains obscure (118). NBCn1 is widely expressed, but its presence in tumors, vascular endothelia, vascular smooth muscle cells, neurons, and intestinal epithelia (reviewed in Ref. 145) are most pertinent to this section.
Murine.
Murine Slc4a7 (Chr 14, 7.08 cM) is presumed capable of expressing all orthologs of all NBCn1 variants (117).
NBCn1 Function and Dysfunction
Genetic linkage to hypertension.
Several SNPs within or near the currently defined boundaries of the SLC4A7 gene locus are associated with elevated blood pressure (88, 123). The most robustly associated SNPs are located upstream of the known transcriptional start sites of NBCn1 and are associated either with 1) enhanced NBCn1 expression or with 2) no effect on NBCn1 expression but are located in a region that in isolation has intrinsic transcriptional enhancer activity (141, 207).
Genetic linkage to breast cancer susceptibility.
Numerous SNPs within the SLC4A7 are associated with breast cancer susceptibility in various populations (1, 36, 121, 191). One SNP in particular, located in the region that encodes the 3′-UTR of NBCn1 transcripts, is associated with risk for a subtype of estrogen-receptor-positive, BRCA2-mutation-carrying tumor (12, 70, 73, 137). The same SNP is also linked to mammographic density in premenopausal women (59), but is not prognostic of survival (58). The influence of the SNP on NBCn1 expression is complex (66), although SLC4A7 expression is generally considered to promote tumor survival by increasing acid extrusion (18, 111).
Slc4a7-Modified Mice
Strains.
Phenotypes have been reported in two strains of Slc4a7-null mice.
Slc4a7−/−(NeoR). In this strain, exon 5 is disrupted by NeoR. Experimental animals were of a mixed 129SvEvBrd/C57BL6 albino genetic background (22).
Slc4a7b,c,d,e−/−(NeoR). In this strain, backcrossed to isogeny with C57BL/6J, Slc4a7 is gene trapped in such a way that ought to preclude expression of most Nbcn1 variants (20) Although the consequences of this disruption on global Nbcn1 expression are hard to predict, Nbcn1 appears to be largely absent in the specific tissues that are studied in these mice (20, 110).
A third strain, Slc4a7lacZ/lacZ, has insertion of a lacZ cassette between exons 3 and 4 of Slc4a7 that creates an imperfect gene trap. The result is a ~70% reduction of Nbcn1 protein abundance. These mice are on a C57BL6/129S1/Sv genetic background and have been used for studying the location of Slc4a7 promoter activity. No phenotypes are reported for these mice (19).
Hypertension modeled in Slc4a7-modified mice.
Slc4a7b,c,d,e−/−(NeoR) mice are mildly hypertensive (20). The basis of this phenotype is considered to be pHi dysregulation in vascular endothelial cells impairing the activity of nitric oxide synthase with consequences for the tone and contractility of vascular smooth muscle cells (20, 198). Although the phenotype of these mice appears to corroborate the linkage between SLC4A7 SNPs and hypertension in humans, in humans the SNPs are not predicted to impair NBCn1 activity. Thus it remains to be seen whether these mice model the effects of the SNPs described above. On the other hand, vascular smooth muscle cells isolated from Slc4a7b,c,d,e−/−(NeoR) mice exhibit a reduced ability to proliferate and migrate, suggesting that NBCn1 is critical for arterial remodeling, implicating excessive NBCn1 activity in the pathogenesis of arterial occlusion (17).
Cancer susceptibility modeled in Slc4a7-modified mice.
In Slc4a7b,c,d,e−/−(NeoR) mice, Slc4a7 abrogation increased the latency of chemically induced tumors and resulted in their slower growth and reduced aggressiveness (110). These findings are consistent with the hypothesis that pathological activation of NBCn1 promotes tumor survival.
Novel disease signs in Slc4a7-modified mice.
Two novel phenotypes have been reported from studies of Slc4a7-modified mice that may presage their genetic linkage to NBCn1 dysfunction in humans.
Blindness and deafness. Slc4a7−/−(NeoR) mice exhibit retinal degeneration and progressive hearing loss that was at one point considered to be a candidate for a subtype of Usher Syndrome (22, 122). However, a linkage between such signs in humans and the SLC4A7 locus has yet to be described.10
Intestinal fluid secretion defects. Studies of Slc4a7b,c,d,e−/−(NeoR) mice reveal a duodenal secretion defect (35) as well as colonic mucosal thinning (177), suggesting that NBCn1 expression could influence the expressivity of intestinal diseases such as impaction or ulceration.
Overview and Outlook
The association between human disease and SLC4A7 is unique among SLC4s inasmuch as the pathology is predicted to result from greater functional expression of the gene, rather than its loss. In that sense, neither of the mouse models discussed here are faithful models of disease. However, it may be possible to mimic the enhancement of NBCn1 activity in a mouse by inactivating, via mutagenesis, the autoinhibitory domain that is located in the Nt of NBCn1 (147). These mice might be expected to exhibit a worse tumor prognosis and may exhibit hypertension due to an alternative mechanism to the one suggested for Slc4a7-null mice.
Slc4a10-MODIFIED MICE: MODELS OF AUTISM WITH IDIOPATHIC GENERALIZED EPILEPSY
SLC4A10 and the Electroneutral Na+- cotransporter NBCn2
Human.
SLC4A10 (chromosomal location 2q24.2) encodes the electroneutral Na+- cotransporter, NBCn2. The protein also transports Cl− although controversy surrounds its ability to perform net Na+-coupled Cl−/ exchange (the basis of its alternative acronym NCBE) under physiological conditions (45, 146, 206). The expression of NBCn2 is predominantly neuronal, but it is also expressed in other cells types such as choroid plexus epithelia. SLC4A10 encodes a variety of NBCn2 variants (118), but all are predicted to be disrupted in the mouse strain described in this section.
Murine.
Slc4a10 (Chr 2, 35.79 cM) is presumed capable of expressing orthologs of all NBCn2 variants.
NBCn2 Function and Dysfunction
Genetic linkage to autism with idiopathic generalized epilepsy.
Neurological signs have been reported in five girls with large genomic deletions that include all or part of SLC4A10 (16, 71, 106, 173). All of the girls exhibit autistic features. The three for whom additional data are presented exhibit impaired motor and language skills. Two of the girls exhibited seizures at age 7 and 10 (71, 106). A third girl was only 14 month old at the time of report (16). The linkage is complicated by the co-loss of other genes.
Slc4a10-Modified Mice
Strains.
Phenotypes have been reported in two strains of Slc4a10-null mice
Slc4a10−/−. In this strain, exon 12 is excised by Cre recombination, which ought to disable expression of all Nbcn2 variants (89). This mouse has been studied on a mixed 129/C57 background as well as on an isogenic C57 background (40, 46, 47, 178).
Slc4a10L647P/L647P. This strain was isolated from a screen of chemically induced mutant alleles associated with age-related diseases in mice. The mutation is located at the extracellular end of TM span 5. This strain was studied on a mixed C57BL/6J-C3H genetic background (156).
Idiopathic generalized epilepsy modeled in Slc4a10-modified mice.
Slc4a10−/− mice have a reduced brain ventricular volume (89), although demonstration of the direct importance of NBCn2 to choroid plexus fluid secretion is complicated by the disturbed expression of numerous other membrane transport proteins in the choroid plexus epithelia of these mice (40). These mice also exhibit an increased seizure threshold (89). Although that observation appears to be at odds with the human linkage to epilepsy, neuronal excitability is enhanced in these mice (178).
Novel features of Slc4a10-modified mice.
Two novel phenotypes have been reported from studies of Slc4a10-modified mice that may presage their genetic linkage to NBCn2 dysfunction in humans.
Overview and Outlook
It is promising that Slc4a10−/− mice recapitulate signs of epilepsy, and these mice should also be examined for behavioral signs associated with mouse models of autism. Although no other mutations were described in the chemically induced mouse model of hearing loss, it would be desirable to verify that phenotype in aged Slc4a10−/− mice.
CONCLUSIONS AND FUTURE PROSPECTS
Slc4-modified mice have proven to be useful models of a wide variety of SLC4-linked diseases. In considering the human versus murine-modeled pathologies a common theme emerges. Both human and murine pathologies can have variable expressivity due to modifying environmental or genetic factors and can depend on the genetic background of the mouse model being studied. Thus on occasion the phenotype has been unexpectedly lethal in mice, precluding detailed study of disease progression, and in other cases has been unexpectedly mild. Thus there may be value in backcrossing existing strains to a variety of genetic backgrounds in order to appreciate the full spectrum of disease and to begin to screen for modifiers that may have physiological and clinical relevance (72). On a similar note, some strains of inbred mice are predisposed to certain disorders such as the retinal degeneration and progressive hearing loss that have been frequently observed in Slc4-modified mice, but not yet in humans. It will be important to understand the influence of mouse-specific modifiers on the expressivity of these novel mouse phenotypes in order to understand the likelihood of observing such pathologies in humans. Another source of variable expressivity comes from the way in which the SLC4/Slc4 gene has been modified. The complete loss of gene expression will often have a different result than the disruption of a gene in such a way that a partial/misfolded protein is expressed, which could exert additional stress on the cells in which it is expressed. Thus the best way to model a disease is to recreate the exact genetic modification that underlies the human disease. So far those studies are in the minority, presumably because of the time, expense, and risk of generating such a mouse model by homologous recombination. However, the dawn of the new era of genome-editing technologies will facilitate the production of a wealth of new models that will greatly increase our understanding of the basis of SLC4-linked disorders and reconcile current disparities among existing models. Finally, beyond observing and understanding SLC4-linked pathologies as modeled in mice, we may eventually hope to use Slc4-modified mice for the development and testing of corrective therapies.
Glossary
- AE1
Anion exchanger 1
- AE3fl
“Full-length” AE3 aka bAE3
- AMA
Antimitochondrial (auto)antibody
- AQP2
Aquaporin 2
- bAE3
“Brain” variant of AE3 aka AE3fl
- BLAST
Basic local alignment search tool
- BRCA2
Breast cancer susceptibility gene 2
- CA2
Carbonic anhydrase 2 gene
- CA3
Cornis ammonis region 3 of the hippocampus
- cAE3
“Cardiac” variant of AE3
- CHC
Cryohydrocytosis
- Chr
Chromosome
- CLCN7
Voltage-dependent Cl−-channel gene aka ClC-7
- cM
Centimorgan
- dRTA
Distal renal tubular acidosis
- eAE1
Erythroid variant of AE1
- HS
Hereditary spherocytosis
- HS-LTL
Hereditary spherocytosis with low temperature ion leak
- HSt
Hereditary stomacytosis
- kAE1
Kidney variant of AE1
- MAP
Mean arterial pressure
- NBCe1
Electrogenic sodium bicarbonate cotransporter 1
- NBCn1
Electroneutral sodium bicarbonate cotransporter 1
- NDCBE
Sodium driven chloride bicarbonate exchanger
- NeoR
Neomycin resistance cassette encoding aminoglycoside phosphotransferase
- Nt
Amino-terminal domain
- PBC
Primary biliary cholangitis aka primary biliary cirrhosis
- pHi
Intracellular pH
- Poly I:C
Polyinosinic-polycytidinic acid
- PRA
Progressive retinal atrophy
- pRTA
Proximal renal tubular acidosis
- SAO
Southeast Asian ovalocytosis
- SLC4A1
Solute carrier family 4 member 1
- Spnb1
β-Spectrin gene
- TCIRG1
V-type H+-ATPase, subunit A3 gene
- TMD
Transmembrane domain
- X
Amino-acid codon replaced by termination codon (e.g., Gln29X)
GRANTS
The author acknowledges the Department of Physiology and Biophysics and the Dean of the Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo for the start-up funding that supported the writing of this manuscript. The author is also supported by National Eye Institute Grant EY028580.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.D.P. prepared figures; drafted manuscript; edited and revised manuscript; approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Aniko Marshall for assistance with gathering reference material.
Footnotes
See glossary for abbreviations.
Cytogenic locations for mouse Slc4 genes are taken from the Mouse Genome Informatics website (http://www.informatics.jax.org/).
Protein sequence alignments were performed using the blastp suite at https://blast.ncbi.nlm.nih.gov/Blast.cgi.
This location is considered a mutational hotspot with incidences of dRTA linked to inheritance and/or de novo occurrence of R589H, R598C, and R598S (100, 186).
The GenBank database curates two versions of the AE3fl sequence that differ in the inclusion of 27 amino-acid Nt cassette. The archetypal AE3fl sequence (NP_005061) lacks this cassette; thus, in archetypal AE3fl, the mutation locus is Arg343.
Controversy surrounds the precise mode of action of NBCe1 in the proximal tubule; the presumed electrochemical gradients ought not support efflux mediated by a Na+-2 cotransporter (68, 214). Thus either our understanding of local electrochemical gradients in the vicinity of NBCe1-A is incomplete or NBCe1-A operates in a more efflux-favorable Na+-3 cotransport mode in these cells. However, the ability of NBCe1 to undergo a shift in stoichiometry is not universally agreed upon, as recently reviewed in Refs. 145, 217.
In one case, one mutant allele is paternally inherited, while the second contains a de novo occurrence of the same mutation (120).
Or elevated intraocular pressure in the case of one 3-year-old patient.
There is little data regarding mortality in humans, although 4/15 patients were adult at the time of report (the oldest being 50) so this issue is either specific to mice or there is unappreciated perinatal mortality in humans.
REFERENCES
- 1.Ahmed S, Thomas G, Ghoussaini M, Healey CS, Humphreys MK, Platte R, Morrison J, Maranian M, Pooley KA, Luben R, Eccles D, Evans DG, Fletcher O, Johnson N, dos Santos Silva I, Peto J, Stratton MR, Rahman N, Jacobs K, Prentice R, Anderson GL, Rajkovic A, Curb JD, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Diver WR, Bojesen S, Nordestgaard BG, Flyger H, et al. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet 41: 585–590, 2009. doi: 10.1038/ng.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiba Y, Nakamura M, Joshita S, Inamine T, Komori A, Yoshizawa K, Umemura T, Horie H, Migita K, Yatsuhashi H, Nakamuta M, Fukushima N, Saoshiro T, Hayashi S, Kouno H, Ota H, Muro T, Watanabe Y, Nakamura Y, Komeda T, Shimada M, Masaki N, Komatsu T, Yagura M, Sugi K, Koga M, Tsukamoto K, Tanaka E, Ishibashi H; PBC Study Group in NHOSLJ . Genetic polymorphisms in CTLA4 and SLC4A2 are differentially associated with the pathogenesis of primary biliary cirrhosis in Japanese patients. J Gastroenterol 46: 1203–1212, 2011. doi: 10.1007/s00535-011-0417-7. [DOI] [PubMed] [Google Scholar]
- 3.Al Moamen NJ, Prasad V, Bodi I, Miller ML, Neiman ML, Lasko VM, Alper SL, Wieczorek DF, Lorenz JN, Shull GE. Loss of the AE3 anion exchanger in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure. J Mol Cell Cardiol 50: 137–146, 2011. doi: 10.1016/j.yjmcc.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alka K, Casey JR. Bicarbonate transport in health and disease. IUBMB Life 66: 596–615, 2014. doi: 10.1002/iub.1315. [DOI] [PubMed] [Google Scholar]
- 5.Alloisio N, Maillet P, Carré G, Texier P, Vallier A, Baklouti F, Philippe N, Delaunay J. Hereditary spherocytosis with band 3 deficiency. Association with a nonsense mutation of the band 3 gene (allele Lyon), and aggravation by a low-expression allele occurring in trans (allele Genas). Blood 88: 1062–1069, 1996. [PubMed] [Google Scholar]
- 6.Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol 91: 153–161, 2006. doi: 10.1113/expphysiol.2005.031765. [DOI] [PubMed] [Google Scholar]
- 7.Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol 212: 1672–1683, 2009. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA 86: 5429–5433, 1989. doi: 10.1073/pnas.86.14.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez BV, Gilmour GS, Mema SC, Martin BT, Shull GE, Casey JR, Sauvé Y. Blindness caused by deficiency in AE3 chloride/bicarbonate exchanger. PLoS One 2: e839, 2007. doi: 10.1371/journal.pone.0000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez BV, Kieller DM, Quon AL, Robertson M, Casey JR. Cardiac hypertrophy in anion exchanger 1-null mutant mice with severe hemolytic anemia. Am J Physiol Heart Circ Physiol 292: H1301–H1312, 2007. doi: 10.1152/ajpheart.00449.2006. [DOI] [PubMed] [Google Scholar]
- 11.American Liver Foundation Primary Biliary Cholangitis (PBC) [Online]. New York: American Liver Foundation; https://www.liverfoundation.org/for-patients/about-the-liver/diseases-of-the-liver/primary-biliary-cholangitis [2017]. [Google Scholar]
- 12.Antoniou AC, Beesley J, McGuffog L, Sinilnikova OM, Healey S, Neuhausen SL, Ding YC, Rebbeck TR, Weitzel JN, Lynch HT, Isaacs C, Ganz PA, Tomlinson G, Olopade OI, Couch FJ, Wang X, Lindor NM, Pankratz VS, Radice P, Manoukian S, Peissel B, Zaffaroni D, Barile M, Viel A, Allavena A, Dall’Olio V, Peterlongo P, Szabo CI, Zikan M, Claes K, Poppe B, Foretova L, Mai PL, Greene MH, Rennert G, et al. Common breast cancer susceptibility alleles and the risk of breast cancer for BRCA1 and BRCA2 mutation carriers: implications for risk prediction. Cancer Res 70: 9742–9754, 2010. doi: 10.1158/0008-5472.CAN-10-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aranda V, Martínez I, Melero S, Lecanda J, Banales JM, Prieto J, Medina JF. Shared apical sorting of anion exchanger isoforms AE2a, AE2b1, and AE2b2 in primary hepatocytes. Biochem Biophys Res Commun 319: 1040–1046, 2004. doi: 10.1016/j.bbrc.2004.05.080. [DOI] [PubMed] [Google Scholar]
- 14.Banales JM, Sáez E, Uriz M, Sarvide S, Urribarri AD, Splinter P, Tietz Bogert PS, Bujanda L, Prieto J, Medina JF, LaRusso NF. Upregulation of mir-506 leads to decreased AE2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology 56: 687–697, 2012. doi: 10.1002/hep.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, Turner ST, Weder AB, Boerwinkle E; Family Blood Pressure Program . Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension 43: 477–482, 2004. doi: 10.1161/01.HYP.0000111585.76299.f7. [DOI] [PubMed] [Google Scholar]
- 16.Belengeanu V, Gamage TH, Farcas S, Stoian M, Andreescu N, Belengeanu A, Frengen E, Misceo D. A de novo 2.3 Mb deletion in 2q24.2q24.3 in a 20-month-old developmentally delayed girl. Gene 539: 168–172, 2014. doi: 10.1016/j.gene.2014.01.060. [DOI] [PubMed] [Google Scholar]
- 17.Boedtkjer E, Bentzon JF, Dam VS, Aalkjaer C. Na+, HCO3−-cotransporter NBCn1 increases pHi gradients, filopodia, and migration of smooth muscle cells and promotes arterial remodelling. Cardiovasc Res 111: 227–239, 2016. doi: 10.1093/cvr/cvw079. [DOI] [PubMed] [Google Scholar]
- 18.Boedtkjer E, Moreira JMA, Mele M, Vahl P, Wielenga VT, Christiansen PM, Jensen VED, Pedersen SF, Aalkjaer C. Contribution of Na+,HCO3−-cotransport to cellular pH control in human breast cancer: a role for the breast cancer susceptibility locus NBCn1 (SLC4A7). Int J Cancer 132: 1288–1299, 2013. doi: 10.1002/ijc.27782. [DOI] [PubMed] [Google Scholar]
- 19.Boedtkjer E, Praetorius J, Füchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+- − cotransporter NBCn1 (slc4a7) in mice. Am J Physiol Cell Physiol 294: C591–C603, 2008. doi: 10.1152/ajpcell.00281.2007. [DOI] [PubMed] [Google Scholar]
- 20.Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Füchtbauer AC, Simonsen U, Füchtbauer EM, Aalkjaer C. Disruption of Na+,HCO3− cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in mice. Circulation 124: 1819–1829, 2011. doi: 10.1161/CIRCULATIONAHA.110.015974. [DOI] [PubMed] [Google Scholar]
- 21.Boettger T, Hübner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature 416: 874–878, 2002. doi: 10.1038/416874a. [DOI] [PubMed] [Google Scholar]
- 22.Bok D, Galbraith G, Lopez I, Woodruff M, Nusinowitz S, BeltrandelRio H, Huang W, Zhao S, Geske R, Montgomery C, Van Sligtenhorst I, Friddle C, Platt K, Sparks MJ, Pushkin A, Abuladze N, Ishiyama A, Dukkipati R, Liu W, Kurtz I. Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nat Genet 34: 313–319, 2003. doi: 10.1038/ng1176. [DOI] [PubMed] [Google Scholar]
- 23.Bronckers AL, Lyaruu DM, Jansen ID, Medina JF, Kellokumpu S, Hoeben KA, Gawenis LR, Oude-Elferink RP, Everts V. Localization and function of the anion exchanger Ae2 in developing teeth and orofacial bone in rodents. J Exp Zoolog B Mol Dev Evol 312B: 375–387, 2009. doi: 10.1002/jez.b.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brosius FC III, Alper SL, Garcia AM, Lodish HF. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem 264: 7784–7787, 1989. [PubMed] [Google Scholar]
- 25.Bruce LJ. Hereditary stomatocytosis and cation-leaky red cells–recent developments. Blood Cells Mol Dis 42: 216–222, 2009. doi: 10.1016/j.bcmd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, Tanner MJA. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101: 4180–4188, 2003. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 27.Bruce LJ, Cope DL, Jones GK, Schofield AE, Burley M, Povey S, Unwin RJ, Wrong O, Tanner MJ. Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest 100: 1693–1707, 1997. doi: 10.1172/JCI119694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruce LJ, Kay MM, Lawrence C, Tanner MJ. Band 3 HT, a human red-cell variant associated with acanthocytosis and increased anion transport, carries the mutation Pro-868–Leu in the membrane domain of band 3. Biochem J 293: 317–320, 1993. doi: 10.1042/bj2930317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, Sun B, Raby B, Lasky-Su J, Hopkins PN, Adler GK, Williams SM, Jose PA, Felder RA. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension 60: 1359–1366, 2012. doi: 10.1161/HYPERTENSIONAHA.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hübner CA, Eladari D. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci USA 110: 7928–7933, 2013. doi: 10.1073/pnas.1221496110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang JC, Go S, de Waart DR, Munoz-Garrido P, Beuers U, Paulusma CC, Oude Elferink R. Soluble adenylyl cyclase regulates bile salt-induced apoptosis in human cholangiocytes. Hepatology 64: 522–534, 2016. doi: 10.1002/hep.28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheidde L, Vieira TC, Lima PR, Saad ST, Heilberg IP. A novel mutation in the anion exchanger 1 gene is associated with familial distal renal tubular acidosis and nephrocalcinosis. Pediatrics 112: 1361–1367, 2003. doi: 10.1542/peds.112.6.1361. [DOI] [PubMed] [Google Scholar]
- 33.Chen AP, Holmes HL, Chang M, Romero MF. Targeted NBCe1A deletion causes proximal RTA: whole nbce1 (sh_nbce1) KO versus nbce1A KO mice (Abstract) J Am Soc Nephrol 25: 71A, 2014. [Google Scholar]
- 34.Chen HP, He M, Mei ZJ, Huang QR, Peng W, Huang M. Anion exchanger 3 is required for sasanquasaponin to inhibit ischemia/reperfusion-induced elevation of intracellular Cl− concentration and to elicit cardioprotection. J Cell Biochem 112: 2803–2812, 2011. doi: 10.1002/jcb.23195. [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Praetorius J, Zheng W, Xiao F, Riederer B, Singh AK, Stieger N, Wang J, Shull GE, Aalkjaer C, Seidler U. The electroneutral Na+:HCO3− cotransporter NBCn1 is a major pHi regulator in murine duodenum. J Physiol 590: 3317–3333, 2012. doi: 10.1113/jphysiol.2011.226506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Zhong R, Ming J, Zou L, Zhu B, Lu X, Ke J, Zhang Y, Liu L, Miao X, Huang T. The SLC4A7 variant rs4973768 is associated with breast cancer risk: evidence from a case-control study and a meta-analysis. Breast Cancer Res Treat 136: 847–857, 2012. doi: 10.1007/s10549-012-2309-9. [DOI] [PubMed] [Google Scholar]
- 37.Chevenet F, Brun C, Bañuls A-L, Jacq B, Christen R. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7: 439, 2006. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405: 571–575, 2000. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- 39.Christensen HL, Nguyen AT, Pedersen FD, Damkier HH. Na+ dependent acid-base transporters in the choroid plexus; insights from slc4 and slc9 gene deletion studies. Front Physiol 4: 304, 2013. doi: 10.3389/fphys.2013.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christensen IB, Gyldenholm T, Damkier HH, Praetorius J. Polarization of membrane associated proteins in the choroid plexus epithelium from normal and slc4a10 knockout mice. Front Physiol 4: 344, 2013. doi: 10.3389/fphys.2013.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Concepcion AR, Salas JT, Sáez E, Sarvide S, Ferrer A, Portu A, Uriarte I, Hervás-Stubbs S, Oude Elferink RP, Prieto J, Medina JF. CD8+ T cells undergo activation and programmed death-1 repression in the liver of aged Ae2a,b–/– mice favoring autoimmune cholangitis. Oncotarget 6: 28588–28606, 2015. doi: 10.18632/oncotarget.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Concepcion AR, Salas JT, Sarvide S, Sáez E, Ferrer A, López M, Portu A, Banales JM, Hervás-Stubbs S, Oude Elferink RP, Prieto J, Medina JF. Anion exchanger 2 is critical for CD8+ T cells to maintain pHi homeostasis and modulate immune responses. Eur J Immunol 44: 1341–1351, 2014. doi: 10.1002/eji.201344218. [DOI] [PubMed] [Google Scholar]
- 43.Coury F, Zenger S, Stewart AK, Stephens S, Neff L, Tsang K, Shull GE, Alper SL, Baron R, Aliprantis AO. SLC4A2-mediated Cl–/HCO3– exchange activity is essential for calpain-dependent regulation of the actin cytoskeleton in osteoclasts. Proc Natl Acad Sci USA 110: 2163–2168, 2013. doi: 10.1073/pnas.1206392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crandall ED, Bidani A. Effects of red blood cell HCO3–/Cl– exchange kinetics on lung CO2 transfer: theory. J Appl Physiol Respir Environ Exerc Physiol 50: 265–271, 1981. [DOI] [PubMed] [Google Scholar]
- 45.Damkier HH, Aalkjaer C, Praetorius J. Na+-dependent HCO3– import by the slc4a10 gene product involves Cl– export. J Biol Chem 285: 26998–27007, 2010. doi: 10.1074/jbc.M110.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damkier HH, Praetorius J. Genetic ablation of Slc4a10 alters the expression pattern of transporters involved in solute movement in the mouse choroid plexus. Am J Physiol Cell Physiol 302: C1452–C1459, 2012. doi: 10.1152/ajpcell.00285.2011. [DOI] [PubMed] [Google Scholar]
- 47.Damkier HH, Prasad V, Hübner CA, Praetorius J. Nhe1 is a luminal Na+/H+ exchanger in mouse choroid plexus and is targeted to the basolateral membrane in Ncbe/Nbcn2-null mice. Am J Physiol Cell Physiol 296: C1291–C1300, 2009. doi: 10.1152/ajpcell.00062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deda G, Ekim M, Güven A, Karagöl U, Tümer N. Hypopotassemic paralysis: a rare presentation of proximal renal tubular acidosis. J Child Neurol 16: 770–771, 2001. doi: 10.1177/088307380101601013. [DOI] [PubMed] [Google Scholar]
- 49.Demirci FYK, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: a novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1). Mol Vis 12: 324–330, 2006. [PubMed] [Google Scholar]
- 50.Ding Y, Casey JR, Kopito RR. The major kidney AE1 isoform does not bind ankyrin (ANK1) in vitro. An essential role for the 79 NH2-terminal amino acid residues of band 3. J Biol Chem 269: 32201–32208, 1994. [PubMed] [Google Scholar]
- 51.Dinour D, Chang M-H, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279: 52238–52246, 2004. doi: 10.1074/jbc.M406591200. [DOI] [PubMed] [Google Scholar]
- 52.Doniach D, Walker G. Immunopathology of liver disease. Prog Liver Dis 4: 381–402, 1972. [PubMed] [Google Scholar]
- 53.Dorfman R, Li W, Sun L, Lin F, Wang Y, Sandford A, Paré PD, McKay K, Kayserova H, Piskackova T, Macek M, Czerska K, Sands D, Tiddens H, Margarit S, Repetto G, Sontag MK, Accurso FJ, Blackman S, Cutting GR, Tsui L-C, Corey M, Durie P, Zielenski J, Strug LJ. Modifier gene study of meconium ileus in cystic fibrosis: statistical considerations and gene mapping results. Hum Genet 126: 763–778, 2009. doi: 10.1007/s00439-009-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Downs LM, Wallin-Håkansson B, Boursnell M, Marklund S, Hedhammar Å, Truvé K, Hübinette L, Lindblad-Toh K, Bergström T, Mellersh CS. A frameshift mutation in golden retriever dogs with progressive retinal atrophy endorses SLC4A3 as a candidate gene for human retinal degenerations. PLoS One 6: e21452, 2011. doi: 10.1371/journal.pone.0021452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drenckhahn D, Schlüter K, Allen DP, Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science 230: 1287–1289, 1985. doi: 10.1126/science.2933809. [DOI] [PubMed] [Google Scholar]
- 56.Eladari D, Kumai Y. Renal acid-base regulation: new insights from animal models. Pflugers Arch 467: 1623–1641, 2015. doi: 10.1007/s00424-014-1669-x. [DOI] [PubMed] [Google Scholar]
- 57.Espiritu DJ, Bernardo AA, Arruda JA. Role of NH2 and COOH termini in targeting, stability, and activity of sodium bicarbonate cotransporter 1. Am J Physiol Renal Physiol 291: F588–F596, 2006. doi: 10.1152/ajprenal.00361.2005. [DOI] [PubMed] [Google Scholar]
- 58.Fasching PA, Pharoah PD, Cox A, Nevanlinna H, Bojesen SE, Karn T, Broeks A, van Leeuwen FE, van’t Veer LJ, Udo R, Dunning AM, Greco D, Aittomäki K, Blomqvist C, Shah M, Nordestgaard BG, Flyger H, Hopper JL, Southey MC, Apicella C, Garcia-Closas M, Sherman M, Lissowska J, Seynaeve C, Huijts PE, Tollenaar RA, Ziogas A, Ekici AB, Rauh C, Mannermaa A, Kataja V, Kosma VM, Hartikainen JM, Andrulis IL, Ozcelik H, Mulligan AM, et al. The role of genetic breast cancer susceptibility variants as prognostic factors. Hum Mol Genet 21: 3926–3939, 2012. doi: 10.1093/hmg/dds159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez-Navarro P, Pita G, Santamariña C, Moreno MP, Vidal C, Miranda-García J, Ascunce N, Casanova F, Collado-García F, Herráez B, González-Neira A, Benítez J, Pollán M. Association analysis between breast cancer genetic variants and mammographic density in a large population-based study (Determinants of Density in Mammographies in Spain) identifies susceptibility loci in TOX3 gene. Eur J Cancer 49: 474–481, 2013. doi: 10.1016/j.ejca.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 60.Finberg KE, Wagner CA, Bailey MA, Paunescu TG, Breton S, Brown D, Giebisch G, Geibel JP, Lifton RP. The B1-subunit of the H+ ATPase is required for maximal urinary acidification. Proc Natl Acad Sci USA 102: 13616–13621, 2005. doi: 10.1073/pnas.0506769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 25: 343–346, 2000. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- 62.Fry AC, Su Y, Yiu V, Cuthbert AW, Trachtman H, Karet Frankl FE. Mutation conferring apical-targeting motif on AE1 exchanger causes autosomal dominant distal RTA. J Am Soc Nephrol 23: 1238–1249, 2012. doi: 10.1681/ASN.2012020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukuda H, Hirata T, Nakamura N, Kato A, Kawahara K, Wakabayashi S, Chang M-H, Romero MF, Hirose S. Identification and properties of a novel variant of NBC4 (Na+/HCO3− co-transporter 4) that is predominantly expressed in the choroid plexus. Biochem J 450: 179–187, 2013. doi: 10.1042/BJ20121515. [DOI] [PubMed] [Google Scholar]
- 64.Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/ cotransporter. J Biol Chem 282: 9042–9052, 2007. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- 65.Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Mice with a targeted disruption of the AE2 Cl−/ exchanger are achlorhydric. J Biol Chem 279: 30531–30539, 2004. doi: 10.1074/jbc.M403779200. [DOI] [PubMed] [Google Scholar]
- 66.Gorbatenko A, Olesen CW, Loebl N, Sigurdsson HH, Bianchi C, Pedraz-Cuesta E, Christiansen J, Pedersen SF. Oncogenic p95HER2 regulates Na+-HCO3− cotransporter NBCn1 mRNA stability in breast cancer cells via 3′UTR-dependent processes. Biochem J 473: 4027–4044, 2016. doi: 10.1042/BCJ20160054. [DOI] [PubMed] [Google Scholar]
- 67.Gröger N, Vitzthum H, Fröhlich H, Krüger M, Ehmke H, Braun T, Boettger T. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet 21: 1025–1036, 2012. doi: 10.1093/hmg/ddr533. [DOI] [PubMed] [Google Scholar]
- 68.Gross E, Kurtz I. Structural determinants and significance of regulation of electrogenic Na+- cotransporter stoichiometry. Am J Physiol Renal Physiol 283: F876–F887, 2002. doi: 10.1152/ajprenal.00148.2002. [DOI] [PubMed] [Google Scholar]
- 69.Groves JD, Tanner MJ. Co-expressed complementary fragments of the human red cell anion exchanger (band 3, AE1) generate stilbene disulfonate-sensitive anion transport. J Biol Chem 270: 9097–9105, 1995. doi: 10.1074/jbc.270.16.9097. [DOI] [PubMed] [Google Scholar]
- 70.Guo J, Sueta A, Nakamura K, Yoshimoto N, Baba M, Ishida N, Hagio K, Toyama T, Iwase H, Tamakoshi A, Yamashita H. Genetic and environmental factors and serum hormones, and risk of estrogen receptor-positive breast cancer in pre- and postmenopausal Japanese women. Oncotarget 8: 65759–65769, 2017. doi: 10.18632/oncotarget.20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gurnett CA, Veile R, Zempel J, Blackburn L, Lovett M, Bowcock A. Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental retardation. Arch Neurol 65: 550–553, 2008. doi: 10.1001/archneur.65.4.550. [DOI] [PubMed] [Google Scholar]
- 72.Hamilton BA, Yu BD. Modifier genes and the plasticity of genetic networks in mice. PLoS Genet 8: e1002644, 2012. doi: 10.1371/journal.pgen.1002644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han MR, Deming-Halverson S, Cai Q, Wen W, Shrubsole MJ, Shu XO, Zheng W, Long J. Evaluating 17 breast cancer susceptibility loci in the Nashville Breast Health Study. Breast Cancer 22: 544–551, 2015. doi: 10.1007/s12282-014-0518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hübner CA. Mice with a targeted disruption of the Cl−/HCO3− exchanger AE3 display a reduced seizure threshold. Mol Cell Biol 26: 182–191, 2006. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hilgen G, Huebner AK, Tanimoto N, Sothilingam V, Seide C, Garcia Garrido M, Schmidt K-F, Seeliger MW, Löwel S, Weiler R, Hübner CA, Dedek K. Lack of the sodium-driven chloride bicarbonate exchanger NCBE impairs visual function in the mouse retina. PLoS One 7: e46155, 2012. doi: 10.1371/journal.pone.0046155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hisamoto S, Shimoda S, Harada K, Iwasaka S, Onohara S, Chong Y, Nakamura M, Bekki Y, Yoshizumi T, Ikegami T, Maehara Y, He XS, Gershwin ME, Akashi K. Hydrophobic bile acids suppress expression of AE2 in biliary epithelial cells and induce bile duct inflammation in primary biliary cholangitis. J Autoimmun 75: 150–160, 2016. doi: 10.1016/j.jaut.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 77.Hmani M, Ghorbel A, Boulila-Elgaied A, Ben Zina Z, Kammoun W, Drira M, Chaabouni M, Petit C, Ayadi H. A novel locus for Usher syndrome type II, USH2B, maps to chromosome 3 at p23-24.2. Eur J Hum Genet 7: 363–367, 1999. doi: 10.1038/sj.ejhg.5200307. [DOI] [PubMed] [Google Scholar]
- 78.Hmani-Aifa M, Benzina Z, Zulfiqar F, Dhouib H, Shahzadi A, Ghorbel A, Rebaï A, Söderkvist P, Riazuddin S, Kimberling WJ, Ayadi H. Identification of two new mutations in the GPR98 and the PDE6B genes segregating in a Tunisian family. Eur J Hum Genet 17: 474–482, 2009. doi: 10.1038/ejhg.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hohenester S, Maillette de Buy Wenniger L, Paulusma CC, van Vliet SJ, Jefferson DM, Oude Elferink RP, Beuers U. A biliary HCO3− umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 55: 173–183, 2012. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 80.Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al-Gazali L, Shimadzu M, Seki G, Fujita T. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol 16: 2270–2278, 2005. doi: 10.1681/ASN.2004080667. [DOI] [PubMed] [Google Scholar]
- 81.Hosoya M, Fujioka M, Kobayashi R, Okano H, Ogawa K. Overlapping expression of anion exchangers in the cochlea of a non-human primate suggests functional compensation. Neurosci Res 110: 1–10, 2016. doi: 10.1016/j.neures.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Hunt SC, Xin Y, Wu LL, Cawthon RM, Coon H, Hasstedt SJ, Hopkins PN. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10-year follow-up blood pressures. Hypertension 47: 532–536, 2006. doi: 10.1161/01.HYP.0000196949.26088.3c. [DOI] [PubMed] [Google Scholar]
- 83.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet 23: 264–266, 1999. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- 84.Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, Takeshima Y, Takumi T, Takahashi T, Yoshikawa N, Nakamura H, Endou H. Novel nonsense mutation in the Na+/HCO3− cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol 12: 713–718, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Igarashi T, Sekine T, Inatomi J, Seki G. Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol 13: 2171–2177, 2002. doi: 10.1097/01.ASN.0000025281.70901.30. [DOI] [PubMed] [Google Scholar]
- 86.Inaba M, Yawata A, Koshino I, Sato K, Takeuchi M, Takakuwa Y, Manno S, Yawata Y, Kanzaki A, Sakai J, Ban A, Ono K, Maede Y. Defective anion transport and marked spherocytosis with membrane instability caused by hereditary total deficiency of red cell band 3 in cattle due to a nonsense mutation. J Clin Invest 97: 1804–1817, 1996. doi: 10.1172/JCI118610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, Kawahara K, Moriyama N, Kudo A, Kawakami H, Shimadzu M, Endou H, Fujita T, Seki G, Igarashi T. Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch 448: 438–444, 2004. doi: 10.1007/s00424-004-1278-1. [DOI] [PubMed] [Google Scholar]
- 88.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sõber S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109, 2011. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacobs S, Ruusuvuori E, Sipilä ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynelä J, Praetorius J, Voipio J, Hübner CA. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci USA 105: 311–316, 2008. doi: 10.1073/pnas.0705487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jansen ID, Mardones P, Lecanda F, de Vries TJ, Recalde S, Hoeben KA, Schoenmaker T, Ravesloot J-H, van Borren MM, van Eijden TM, Bronckers AL, Kellokumpu S, Medina JF, Everts V, Oude Elferink RP. Ae2a,b-deficient mice exhibit osteopetrosis of long bones but not of calvaria. FASEB J 23: 3470–3481, 2009. doi: 10.1096/fj.08-122598. [DOI] [PubMed] [Google Scholar]
- 91.Jarolim P, Murray JL, Rubin HL, Taylor WM, Prchal JT, Ballas SK, Snyder LM, Chrobak L, Melrose WD, Brabec V, Palek J. Characterization of 13 novel band 3 gene defects in hereditary spherocytosis with band 3 deficiency. Blood 88: 4366–4374, 1996. [PubMed] [Google Scholar]
- 92.Jarolim P, Palek J, Amato D, Hassan K, Sapak P, Nurse GT, Rubin HL, Zhai S, Sahr KE, Liu SC. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc Natl Acad Sci USA 88: 11022–11026, 1991. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jarolim P, Rubin HL, Brabec V, Chrobak L, Zolotarev AS, Alper SL, Brugnara C, Wichterle H, Palek J. Mutations of conserved arginines in the membrane domain of erythroid band 3 lead to a decrease in membrane-associated band 3 and to the phenotype of hereditary spherocytosis. Blood 85: 634–640, 1995. [PubMed] [Google Scholar]
- 94.Jarolim P, Shayakul C, Prabakaran D, Jiang L, Stuart-Tilley A, Rubin HL, Simova S, Zavadil J, Herrin JT, Brouillette J, Somers MJ, Seemanova E, Brugnara C, Guay-Woodford LM, Alper SL. Autosomal dominant distal renal tubular acidosis is associated in three families with heterozygosity for the R589H mutation in the AE1 (band 3) Cl−/HCO3− exchanger. J Biol Chem 273: 6380–6388, 1998. doi: 10.1074/jbc.273.11.6380. [DOI] [PubMed] [Google Scholar]
- 95.Jenkins PB, Abou-Alfa GK, Dhermy D, Bursaux E, Féo C, Scarpa AL, Lux SE, Garbarz M, Forget BG, Gallagher PG. A nonsense mutation in the erythrocyte band 3 gene associated with decreased mRNA accumulation in a kindred with dominant hereditary spherocytosis. J Clin Invest 97: 373–380, 1996. doi: 10.1172/JCI118425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Josephsen K, Praetorius J, Frische S, Gawenis LR, Kwon TH, Agre P, Nielsen S, Fejerskov O. Targeted disruption of the Cl−/HCO3− exchanger Ae2 results in osteopetrosis in mice. Proc Natl Acad Sci USA 106: 1638–1641, 2009. doi: 10.1073/pnas.0811682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kager L, Bruce LJ, Zeitlhofer P, Flatt JF, Maia TM, Ribeiro ML, Fahrner B, Fritsch G, Boztug K, Haas OA. Band 3 nullVIENNA, a novel homozygous SLC4A1 p.Ser477X variant causing severe hemolytic anemia, dyserythropoiesis and complete distal renal tubular acidosis. Pediatr Blood Cancer 64: e26227, 2017. doi: 10.1002/pbc.26227. [DOI] [PubMed] [Google Scholar]
- 98.Kao L, Kurtz LM, Shao X, Papadopoulos MC, Liu L, Bok D, Nusinowitz S, Chen B, Stella SL, Andre M, Weinreb J, Luong SS, Piri N, Kwong JMK, Newman D, Kurtz I. Severe neurologic impairment in mice with targeted disruption of the electrogenic sodium bicarbonate cotransporter NBCe2 (Slc4a5 gene). J Biol Chem 286: 32563–32574, 2011. doi: 10.1074/jbc.M111.249961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21: 84–90, 1999. doi: 10.1038/5022. [DOI] [PubMed] [Google Scholar]
- 100.Karet FE, Gainza FJ, Györy AZ, Unwin RJ, Wrong O, Tanner MJ, Nayir A, Alpay H, Santos F, Hulton SA, Bakkaloglu A, Ozen S, Cunningham MJ, di Pietro A, Walker WG, Lifton RP. Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci USA 95: 6337–6342, 1998. doi: 10.1073/pnas.95.11.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kari JA, El Desoky SM, Singh AK, Gari MA, Kleta R, Bockenhauer D. The case | Renal tubular acidosis and eye findings. Kidney Int 86: 217–218, 2014. doi: 10.1038/ki.2013.320. [DOI] [PubMed] [Google Scholar]
- 102.Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K. AE4 is a DIDS-sensitive Cl−/ exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol 283: C1206–C1218, 2002. doi: 10.1152/ajpcell.00512.2001. [DOI] [PubMed] [Google Scholar]
- 103.Kobayashi S, Morgans CW, Casey JR, Kopito RR. AE3 anion exchanger isoforms in the vertebrate retina: developmental regulation and differential expression in neurons and glia. J Neurosci 14: 6266–6279, 1994. doi: 10.1523/JNEUROSCI.14-10-06266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kornak U, Kasper D, Bösl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104: 205–215, 2001. doi: 10.1016/S0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 105.Kostić VS, Petrović IN. Brain calcification and movement disorders. Curr Neurol Neurosci Rep 17: 2, 2017. doi: 10.1007/s11910-017-0710-9. [DOI] [PubMed] [Google Scholar]
- 106.Krepischi ACV, Knijnenburg J, Bertola DR, Kim CA, Pearson PL, Bijlsma E, Szuhai K, Kok F, Vianna-Morgante AM, Rosenberg C. Two distinct regions in 2q24.2-q24.3 associated with idiopathic epilepsy. Epilepsia 51: 2457–2460, 2010. doi: 10.1111/j.1528-1167.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 107.Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science 269: 1427–1429, 1995. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 108.Kurschat CE, Shmukler BE, Jiang L, Hevi S, Kim EH, Stewart AK, Alper SL. Mouse strain-specific coding polymorphism in the Slc4a2/Ae2 gene encodes Ae2c2 variants differing in isoform-specific dominant negative activity. Exp Physiol 93: 458–467, 2008. doi: 10.1113/expphysiol.2007.040931. [DOI] [PubMed] [Google Scholar]
- 109.Kurschat CE, Shmukler BE, Jiang L, Wilhelm S, Kim EH, Chernova MN, Kinne RKH, Stewart AK, Alper SL. Alkaline-shifted pHo sensitivity of AE2c1-mediated anion exchange reveals novel regulatory determinants in the AE2 N-terminal cytoplasmic domain. J Biol Chem 281: 1885–1896, 2006. doi: 10.1074/jbc.M509734200. [DOI] [PubMed] [Google Scholar]
- 110.Lee S, Axelsen TV, Andersen AP, Vahl P, Pedersen SF, Boedtkjer E. Disrupting Na+, HCO3−-cotransporter NBCn1 (Slc4a7) delays murine breast cancer development. Oncogene 35: 2112–2122, 2016. doi: 10.1038/onc.2015.273. [DOI] [PubMed] [Google Scholar]
- 111.Lee S, Mele M, Vahl P, Christiansen PM, Jensen VED, Boedtkjer E. Na+,HCO3−-cotransport is functionally upregulated during human breast carcinogenesis and required for the inverted pH gradient across the plasma membrane. Pflugers Arch 467: 367–377, 2015. doi: 10.1007/s00424-014-1524-0. [DOI] [PubMed] [Google Scholar]
- 112.Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na-HCO3 cotransporter NBCe1-B: role of IRBIT vs. amino-terminal truncation. Am J Physiol Cell Physiol 302: C518–C526, 2012. doi: 10.1152/ajpcell.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol 517: 159–180, 1999. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lewis SE, Erickson RP, Barnett LB, Venta PJ, Tashian RE. N-ethyl-N-nitrosourea-induced null mutation at the mouse Car-2 locus: an animal model for human carbonic anhydrase II deficiency syndrome. Proc Natl Acad Sci USA 85: 1962–1966, 1988. doi: 10.1073/pnas.85.6.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li YP, Chen W, Liang Y, Li E, Stashenko P. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet 23: 447–451, 1999. doi: 10.1038/70563. [DOI] [PubMed] [Google Scholar]
- 117.Linn SC, Askew GR, Menon AG, Shull GE. Conservation of an AE3 Cl−/HCO3− exchanger cardiac-specific exon and promoter region and AE3 mRNA expression patterns in murine and human hearts. Circ Res 76: 584–591, 1995. doi: 10.1161/01.RES.76.4.584. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y, Yang J, Chen LM. Structure and function of SLC4 family transporters. Front Physiol 6: 355, 2015. doi: 10.3389/fphys.2015.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lleo A, Marzorati S, Anaya JM, Gershwin ME. Primary biliary cholangitis: a comprehensive overview. Hepatol Int 11: 485–499, 2017. doi: 10.1007/s12072-017-9830-1. [DOI] [PubMed] [Google Scholar]
- 120.Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai JD, Yu IS, Lin SW, Lin SH. Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney Int 79: 730–741, 2011. doi: 10.1038/ki.2010.523. [DOI] [PubMed] [Google Scholar]
- 121.Long J, Shu XO, Cai Q, Gao YT, Zheng Y, Li G, Li C, Gu K, Wen W, Xiang YB, Lu W, Zheng W. Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol Biomarkers Prev 19: 2357–2365, 2010. doi: 10.1158/1055-9965.EPI-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lopez IA, Acuna D, Galbraith G, Bok D, Ishiyama A, Liu W, Kurtz I. Time course of auditory impairment in mice lacking the electroneutral sodium bicarbonate cotransporter NBC3 (slc4a7). Brain Res Dev Brain Res 160: 63–77, 2005. doi: 10.1016/j.devbrainres.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 123.Lu X, Wang L, Lin X, Huang J, Charles Gu C, He M, Shen H, He J, Zhu J, Li H, Hixson JE, Wu T, Dai J, Lu L, Shen C, Chen S, He L, Mo Z, Hao Y, Mo X, Yang X, Li J, Cao J, Chen J, Fan Z, Li Y, Zhao L, Li H, Lu F, Yao C, Yu L, Xu L, Mu J, Wu X, Deng Y, et al. Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet 24: 865–874, 2015. doi: 10.1093/hmg/ddu478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lyaruu DM, Bronckers AL, Mulder L, Mardones P, Medina JF, Kellokumpu S, Oude Elferink RP, Everts V. The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol 27: 119–127, 2008. doi: 10.1016/j.matbio.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martínez-Ansó E, Castillo JE, Díez J, Medina JF, Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology 19: 1400–1406, 1994. doi: 10.1002/hep.1840190613. [DOI] [PubMed] [Google Scholar]
- 126.McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol 127: 639–658, 2006. doi: 10.1085/jgp.200609520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Medina JF, Martínez-Ansó E, Vazquez JJ, Prieto J. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology 25: 12–17, 1997. doi: 10.1002/hep.510250104. [DOI] [PubMed] [Google Scholar]
- 128.Medina JF, Recalde S, Prieto J, Lecanda J, Saez E, Funk CD, Vecino P, van Roon MA, Ottenhoff R, Bosma PJ, Bakker CT, Oude Elferink RP. Anion exchanger 2 is essential for spermiogenesis in mice. Proc Natl Acad Sci USA 100: 15847–15852, 2003. doi: 10.1073/pnas.2536127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meier S, Hübner CA, Groeben H, Peters J, Bingmann D, Wiemann M. Expression of anion exchanger 3 influences respiratory rate in awake and isoflurane anesthetized mice. J Physiol Pharmacol 58, Suppl 5: 371–378, 2007. [PubMed] [Google Scholar]
- 130.Melero S, Spirlì C, Zsembery A, Medina JF, Joplin RE, Duner E, Zuin M, Neuberger JM, Prieto J, Strazzabosco M. Defective regulation of cholangiocyte Cl−/HCO3− and Na+/H+ exchanger activities in primary biliary cirrhosis. Hepatology 35: 1513–1521, 2002. doi: 10.1053/jhep.2002.33634. [DOI] [PubMed] [Google Scholar]
- 131.Meyers SN, McDaneld TG, Swist SL, Marron BM, Steffen DJ, O’Toole D, O’Connell JR, Beever JE, Sonstegard TS, Smith TPL. A deletion mutation in bovine SLC4A2 is associated with osteopetrosis in Red Angus cattle. BMC Genomics 11: 337, 2010. doi: 10.1186/1471-2164-11-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mhatre AN, Charachon G, Alper SL, Lalwani AK. The guinea pig cochlear AE2 anion exchanger: cDNA cloning and in situ localization within the cochlea. Biochim Biophys Acta 1414: 1–15, 1998. doi: 10.1016/S0005-2736(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 133.Miozzo M, Selmi C, Gentilin B, Grati FR, Sirchia S, Oertelt S, Zuin M, Gershwin ME, Podda M, Invernizzi P. Preferential X chromosome loss but random inactivation characterize primary biliary cirrhosis. Hepatology 46: 456–462, 2007. doi: 10.1002/hep.21696. [DOI] [PubMed] [Google Scholar]
- 134.Mohebbi N, Wagner CA. Pathophysiology, diagnosis and treatment of inherited distal renal tubular acidosis. J Nephrol. In press. doi: 10.1007/s40620-017-0447-1. [DOI] [PubMed] [Google Scholar]
- 135.Moriyama R, Ideguchi H, Lombardo CR, Van Dort HM, Low PS. Structural and functional characterization of band 3 from Southeast Asian ovalocytes. J Biol Chem 267: 25792–25797, 1992. [PubMed] [Google Scholar]
- 136.Morris RC., Jr Renal tubular acidosis. Mechanisms, classification and implications. N Engl J Med 281: 1405–1413, 1969. doi: 10.1056/NEJM196912182812508. [DOI] [PubMed] [Google Scholar]
- 137.Mulligan AM, Couch FJ, Barrowdale D, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Robson M, Sherman M, Spurdle AB, Wappenschmidt B, Lee A, McGuffog L, Healey S, Sinilnikova OM, Janavicius R, Hansen T, Nielsen FC, Ejlertsen B, Osorio A, Muñoz-Repeto I, Durán M, Godino J, Pertesi M, Benítez J, Peterlongo P, Manoukian S, Peissel B, Zaffaroni D, Cattaneo E, Bonanni B, Viel A, Pasini B, Papi L, Ottini L, et al. Common breast cancer susceptibility alleles are associated with tumour subtypes in BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res 13: R110, 2011. doi: 10.1186/bcr3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mumtaz R, Trepiccione F, Hennings JC, Huebner AK, Serbin B, Picard N, Ullah AK, Păunescu TG, Capen DE, Lashhab RM, Mouro-Chanteloup I, Alper SL, Wagner CA, Cordat E, Brown D, Eladari D, Hübner CA. Intercalated cell depletion and vacuolar H+-ATPase mistargeting in an Ae1 R607H knockin model. J Am Soc Nephrol 28: 1507–1520, 2017. doi: 10.1681/ASN.2016020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Myers EJ, Marshall A, Jennings ML, Parker MD. Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH− conductance pathway that is stimulated by rises in intracellular and extracellular pH. Am J Physiol Cell Physiol 311: C945–C959, 2016. doi: 10.1152/ajpcell.00259.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Myers EJ, Yuan L, Felmlee MA, Lin YY, Jiang Y, Pei Y, Wang O, Li M, Xing XP, Marshall A, Xia WB, Parker MD. A novel mutant Na+/HCO3− cotransporter NBCe1 in a case of compound-heterozygous inheritance of proximal renal tubular acidosis. J Physiol 594: 6267–6286, 2016. doi: 10.1113/JP272252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ng FL, Boedtkjer E, Witkowska K, Ren M, Zhang R, Tucker A, Aalkjær C, Caulfield MJ, Ye S. Increased NBCn1 expression, Na+/HCO3− co-transport and intracellular pH in human vascular smooth muscle cells with a risk allele for hypertension. Hum Mol Genet 26: 989–1002, 2017. doi: 10.1093/hmg/ddx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nicolas G, Charbonnier C, de Lemos RR, Richard AC, Guillin O, Wallon D, Legati A, Geschwind D, Coppola G, Frebourg T, Campion D, de Oliveira JR, Hannequin D; the collaborators from the French IBGC study Group . Brain calcification process and phenotypes according to age and sex: lessons from SLC20A2, PDGFB, and PDGFRB mutation carriers. Am J Med Genet B Neuropsychiatr Genet 168: 586–594, 2015. doi: 10.1002/ajmg.b.32336. [DOI] [PubMed] [Google Scholar]
- 143.Norgett EE, Golder ZJ, Lorente-Cánovas B, Ingham N, Steel KP, Karet Frankl FE. Atp6v0a4 knockout mouse is a model of distal renal tubular acidosis with hearing loss, with additional extrarenal phenotype. Proc Natl Acad Sci USA 109: 13775–13780, 2012. doi: 10.1073/pnas.1204257109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O’Toole D, Swist S, Steadman L, Johnson GC. Neuropathology and craniofacial lesions of osteopetrotic Red Angus calves. Vet Pathol 49: 746–754, 2012. doi: 10.1177/0300985811412621. [DOI] [PubMed] [Google Scholar]
- 145.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013. doi: 10.1152/physrev.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl− self-exchange activity. J Biol Chem 283: 12777–12788, 2008. doi: 10.1074/jbc.M707829200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parker MD, Skelton LA, Daly CM, Boron WF. IRBIT binds to and functionally enhances the electroneutral Na+-coupled bicarbonate transporters NBCn1, NDCBE and NCBE (Abstract) FASEB J 21: A1285, 2007. [Google Scholar]
- 148.Patel N, Khan AO, Al-Saif M, Moghrabi WN, AlMaarik BM, Ibrahim N, Abdulwahab F, Hashem M, Alshidi T, Alobeid E, Alomar RA, Al-Harbi S, Abouelhoda M, Khabar KSA, Alkuraya FS. A novel mechanism for variable phenotypic expressivity in Mendelian diseases uncovered by an AU-rich element (ARE)-creating mutation. Genome Biol 18: 144, 2017. doi: 10.1186/s13059-017-1274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Patel SP, Parker MD. SLC4A11 and the pathophysiology of congenital hereditary endothelial dystrophy. BioMed Res Int 2015: 475392, 2015. doi: 10.1155/2015/475392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peña-Münzenmayer G, Catalán MA, Kondo Y, Jaramillo Y, Liu F, Shull GE, Melvin JE. Ae4 (Slc4a9) anion exchanger drives Cl− uptake-dependent fluid secretion by mouse submandibular gland acinar cells. J Biol Chem 290: 10677–10688, 2015. doi: 10.1074/jbc.M114.612895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Peña-Münzenmayer G, George AT, Shull GE, Melvin JE, Catalán MA. Ae4 (Slc4a9) is an electroneutral monovalent cation-dependent Cl−/HCO3− exchanger. J Gen Physiol 147: 423–436, 2016. doi: 10.1085/jgp.201611571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Perrotta S, Borriello A, Scaloni A, De Franceschi L, Brunati AM, Turrini F, Nigro V, del Giudice EM, Nobili B, Conte ML, Rossi F, Iolascon A, Donella-Deana A, Zappia V, Poggi V, Anong W, Low P, Mohandas N, Della Ragione F. The N-terminal 11 amino acids of human erythrocyte band 3 are critical for aldolase binding and protein phosphorylation: implications for band 3 function. Blood 106: 4359–4366, 2005. doi: 10.1182/blood-2005-07-2806. [DOI] [PubMed] [Google Scholar]
- 153.Peters LL, Shivdasani RA, Liu SC, Hanspal M, John KM, Gonzalez JM, Brugnara C, Gwynn B, Mohandas N, Alper SL, Orkin SH, Lux SE. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell 86: 917–927, 1996. doi: 10.1016/S0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 154.Peters LL, Swearingen RA, Andersen SG, Gwynn B, Lambert AJ, Li R, Lux SE, Churchill GA. Identification of quantitative trait loci that modify the severity of hereditary spherocytosis in wan, a new mouse model of band-3 deficiency. Blood 103: 3233–3240, 2004. doi: 10.1182/blood-2003-08-2813. [DOI] [PubMed] [Google Scholar]
- 155.Picard V, Proust A, Eveillard M, Flatt JF, Couec M-L, Caillaux G, Fénéant-Thibault M, Finkelstein A, Raphaël M, Delaunay J, Bruce LJ, Pissard S, Thomas C. Homozygous Southeast Asian ovalocytosis is a severe dyserythropoietic anemia associated with distal renal tubular acidosis. Blood 123: 1963–1965, 2014. doi: 10.1182/blood-2014-01-548149. [DOI] [PubMed] [Google Scholar]
- 156.Potter PK, Bowl MR, Jeyarajan P, Wisby L, Blease A, Goldsworthy ME, Simon MM, Greenaway S, Michel V, Barnard A, Aguilar C, Agnew T, Banks G, Blake A, Chessum L, Dorning J, Falcone S, Goosey L, Harris S, Haynes A, Heise I, Hillier R, Hough T, Hoslin A, Hutchison M, King R, Kumar S, Lad HV, Law G, MacLaren RE, Morse S, Nicol T, Parker A, Pickford K, Sethi S, et al. Novel gene function revealed by mouse mutagenesis screens for models of age-related disease. Nat Commun 7: 12444, 2016. doi: 10.1038/ncomms12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Poupon R, Ping C, Chrétien Y, Corpechot C, Chazouillères O, Simon T, Heath SC, Matsuda F, Poupon RE, Housset C, Barbu V. Genetic factors of susceptibility and of severity in primary biliary cirrhosis. J Hepatol 49: 1038–1045, 2008. doi: 10.1016/j.jhep.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 158.Prasad V, Bodi I, Meyer JW, Wang Y, Ashraf M, Engle SJ, Doetschman T, Sisco K, Nieman ML, Miller ML, Lorenz JN, Shull GE. Impaired cardiac contractility in mice lacking both the AE3 Cl−/HCO3− exchanger and the NKCC1 Na+-K+-2Cl− cotransporter: effects on Ca2+ handling and protein phosphatases. J Biol Chem 283: 31303–31314, 2008. doi: 10.1074/jbc.M803706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Prasad V, Lorenz JN, Lasko VM, Nieman ML, Al Moamen NJ, Shull GE. Loss of the AE3 Cl−/HCO−3 exchanger in mice affects rate-dependent inotropy and stress-related AKT signaling in heart. Front Physiol 4: 399, 2013. doi: 10.3389/fphys.2013.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Prieto J, García N, Martí-Climent JM, Peñuelas I, Richter JA, Medina JF. Assessment of biliary bicarbonate secretion in humans by positron emission tomography. Gastroenterology 117: 167–172, 1999. doi: 10.1016/S0016-5085(99)70564-0. [DOI] [PubMed] [Google Scholar]
- 161.Prieto J, Qian C, García N, Díez J, Medina JF. Abnormal expression of anion exchanger genes in primary biliary cirrhosis. Gastroenterology 105: 572–578, 1993. doi: 10.1016/0016-5085(93)90735-U. [DOI] [PubMed] [Google Scholar]
- 162.Pucéat M, Korichneva I, Cassoly R, Vassort G. Identification of band 3-like proteins and Cl−/HCO3− exchange in isolated cardiomyocytes. J Biol Chem 270: 1315–1322, 1995. doi: 10.1074/jbc.270.3.1315. [DOI] [PubMed] [Google Scholar]
- 163.Recalde S, Muruzábal F, Looije N, Kunne C, Burrell MA, Sáez E, Martínez-Ansó E, Salas JT, Mardones P, Prieto J, Medina JF, Oude Elferink RP. Inefficient chronic activation of parietal cells in Ae2a,b−/− mice. Am J Pathol 169: 165–176, 2006. doi: 10.2353/ajpath.2006.051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ribeiro ML, Alloisio N, Almeida H, Gomes C, Texier P, Lemos C, Mimoso G, Morlé L, Bey-Cabet F, Rudigoz RC, Delaunay J, Tamagnini G. Severe hereditary spherocytosis and distal renal tubular acidosis associated with the total absence of band 3. Blood 96: 1602–1604, 2000. [PubMed] [Google Scholar]
- 165.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3−) transporters. Mol Aspects Med 34: 159–182, 2013. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998. [DOI] [PubMed] [Google Scholar]
- 167.Rysavá R, Tesar V, Jirsa M Jr, Brabec V, Jarolím P. Incomplete distal renal tubular acidosis coinherited with a mutation in the band 3 (AE1) gene. Nephrol Dial Transplant 12: 1869–1873, 1997. doi: 10.1093/ndt/12.9.1869. [DOI] [PubMed] [Google Scholar]
- 168.Salameh AI, Hübner CA, Boron WF. Role of Cl−-HCO3− exchanger AE3 in intracellular pH homeostasis in cultured murine hippocampal neurons, and in crosstalk to adjacent astrocytes. J Physiol 595: 93–124, 2017. doi: 10.1113/JP272470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Salas JT, Banales JM, Sarvide S, Recalde S, Ferrer A, Uriarte I, Oude Elferink RP, Prieto J, Medina JF. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology 134: 1482–1493, 2008. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 170.Salerno EE, Patel SP, Marshall A, Mballo CS, Parker MD. NBCe1-B/C knockout mice exhibit signs associated with proximal renal tubular acidosis (Abstract) FASEB J 31 2017. [Google Scholar]
- 171.Sander T, Toliat MR, Heils A, Leschik G, Becker C, Rüschendorf F, Rohde K, Mundlos S, Nürnberg P. Association of the 867Asp variant of the human anion exchanger 3 gene with common subtypes of idiopathic generalized epilepsy. Epilepsy Res 51: 249–255, 2002. doi: 10.1016/S0920-1211(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 172.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 173.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimäki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science 316: 445–449, 2007. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shayakul C, Jarolim P, Zachlederova M, Prabakaran D, Cortez-Campeao D, Kalabova D, Stuart-Tilley AK, Ideguchi H, Haller C, Alper SL. Characterization of a highly polymorphic marker adjacent to the SLC4A1 gene and of kidney immunostaining in a family with distal renal tubular acidosis. Nephrol Dial Transplant 19: 371–379, 2004. doi: 10.1093/ndt/gfg557. [DOI] [PubMed] [Google Scholar]
- 175.Shiohara M, Igarashi T, Mori T, Komiyama A. Genetic and long-term data on a patient with permanent isolated proximal renal tubular acidosis. Eur J Pediatr 159: 892–894, 2000. doi: 10.1007/PL00008363. [DOI] [PubMed] [Google Scholar]
- 176.Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1). Proc Natl Acad Sci USA 103: 9542–9547, 2006. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Singh AK, Xia W, Riederer B, Juric M, Li J, Zheng W, Cinar A, Xiao F, Bachmann O, Song P, Praetorius J, Aalkjaer C, Seidler U. Essential role of the electroneutral Na+-HCO3− cotransporter NBCn1 in murine duodenal acid-base balance and colonic mucus layer build-up in vivo. J Physiol 591: 2189–2204, 2013. doi: 10.1113/jphysiol.2012.247874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sinning A, Liebmann L, Hübner CA. Disruption of Slc4a10 augments neuronal excitability and modulates synaptic short-term plasticity. Front Cell Neurosci 9: 223, 2015. doi: 10.3389/fncel.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sinning A, Liebmann L, Kougioumtzes A, Westermann M, Bruehl C, Hübner CA. Synaptic glutamate release is modulated by the Na+ -driven Cl−/HCO3− exchanger Slc4a8. J Neurosci 31: 7300–7311, 2011. doi: 10.1523/JNEUROSCI.0269-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sinning A, Radionov N, Trepiccione F, López-Cayuqueo KI, Jayat M, Baron S, Cornière N, Alexander RT, Hadchouel J, Eladari D, Hübner CA, Chambrey R. Double knockout of the Na+-driven Cl−/HCO3− exchanger and Na+/Cl− cotransporter induces hypokalemia and volume depletion. J Am Soc Nephrol 28: 130–139, 2017. doi: 10.1681/ASN.2015070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sly WS, Whyte MP, Sundaram V, Tashian RE, Hewett-Emmett D, Guibaud P, Vainsel M, Baluarte HJ, Gruskin A, Al-Mosawi M, Sakati N, Ohlsson A. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med 313: 139–145, 1985. doi: 10.1056/NEJM198507183130302. [DOI] [PubMed] [Google Scholar]
- 182.Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26: 71–75, 2000. doi: 10.1038/79208. [DOI] [PubMed] [Google Scholar]
- 183.Southgate CD, Chishti AH, Mitchell B, Yi SJ, Palek J. Targeted disruption of the murine erythroid band 3 gene results in spherocytosis and severe haemolytic anaemia despite a normal membrane skeleton. Nat Genet 14: 227–230, 1996. doi: 10.1038/ng1096-227. [DOI] [PubMed] [Google Scholar]
- 184.Sowah D, Brown BF, Quon A, Alvarez BV, Casey JR. Resistance to cardiomyocyte hypertrophy in ae3−/− mice, deficient in the AE3 Cl−/HCO3− exchanger. BMC Cardiovasc Disord 14: 89, 2014. doi: 10.1186/1471-2261-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Spirlì C, Granato A, Zsembery K, Anglani F, Okolicsányi L, LaRusso NF, Crepaldi G, Strazzabosco M. Functional polarity of Na+/H+ and Cl−/HCO3− exchangers in a rat cholangiocyte cell line. Am J Physiol Gastrointest Liver Physiol 275: G1236–G1245, 1998. [DOI] [PubMed] [Google Scholar]
- 186.Sritippayawan S, Kirdpon S, Vasuvattakul S, Wasanawatana S, Susaengrat W, Waiyawuth W, Nimmannit S, Malasit P, Yenchitsomanus PT. A de novo R589C mutation of anion exchanger 1 causing distal renal tubular acidosis. Pediatr Nephrol 18: 644–648, 2003. [DOI] [PubMed] [Google Scholar]
- 187.Sritippayawan S, Sumboonnanonda A, Vasuvattakul S, Keskanokwong T, Sawasdee N, Paemanee A, Thuwajit P, Wilairat P, Nimmannit S, Malasit P, Yenchitsomanus PT. Novel compound heterozygous SLC4A1 mutations in Thai patients with autosomal recessive distal renal tubular acidosis. Am J Kidney Dis 44: 64–70, 2004. doi: 10.1053/j.ajkd.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 188.Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis 4: 5, 2009. doi: 10.1186/1750-1172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl−/HCO3− exchanger (slc4a1). J Am Soc Nephrol 18: 1408–1418, 2007. doi: 10.1681/ASN.2006101072. [DOI] [PubMed] [Google Scholar]
- 190.Stewart AK, Vandorpe DH, Heneghan JF, Chebib F, Stolpe K, Akhavein A, Edelman EJ, Maksimova Y, Gallagher PG, Alper SL. The GPA-dependent, spherostomatocytosis mutant AE1 E758K induces GPA-independent, endogenous cation transport in amphibian oocytes. Am J Physiol Cell Physiol 298: C283–C297, 2010. doi: 10.1152/ajpcell.00444.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sueta A, Ito H, Kawase T, Hirose K, Hosono S, Yatabe Y, Tajima K, Tanaka H, Iwata H, Iwase H, Matsuo K. A genetic risk predictor for breast cancer using a combination of low-penetrance polymorphisms in a Japanese population. Breast Cancer Res Treat 132: 711–721, 2012. doi: 10.1007/s10549-011-1904-5. [DOI] [PubMed] [Google Scholar]
- 192.Suzuki M, Vaisbich MH, Yamada H, Horita S, Li Y, Sekine T, Moriyama N, Igarashi T, Endo Y, Cardoso TP, de Sá LC, Koch VH, Seki G, Fujita T. Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflugers Arch 455: 583–593, 2008. doi: 10.1007/s00424-007-0319-y. [DOI] [PubMed] [Google Scholar]
- 193.Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Riant F, De Jonghe P, Li Y, Sekine T, Igarashi T, Fujimoto I, Mikoshiba K, Shimadzu M, Shiohara M, Braverman N, Al-Gazali L, Fujita T, Seki G. Defective membrane expression of the Na+-HCO3− cotransporter NBCe1 is associated with familial migraine. Proc Natl Acad Sci USA 107: 15963–15968, 2010. doi: 10.1073/pnas.1008705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tanphaichitr VS, Sumboonnanonda A, Ideguchi H, Shayakul C, Brugnara C, Takao M, Veerakul G, Alper SL. Novel AE1 mutations in recessive distal renal tubular acidosis. Loss-of-function is rescued by glycophorin A. J Clin Invest 102: 2173–2179, 1998. doi: 10.1172/JCI4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Taylor JY, Maddox R, Wu CY. Genetic and environmental risks for high blood pressure among African American mothers and daughters. Biol Res Nurs 11: 53–65, 2009. doi: 10.1177/1099800409334817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Taylor JY, Sampson D, Taylor AD, Caldwell D, Sun YV. Genetic and BMI risks for predicting blood pressure in three generations of West African Dogon women. Biol Res Nurs 15: 105–111, 2013. doi: 10.1177/1099800411419026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Taylor JY, Sun YV, Barcelona de Mendoza V, Ifatunji M, Rafferty J, Fox ER, Musani SK, Sims M, Jackson JS. The combined effects of genetic risk and perceived discrimination on blood pressure among African Americans in the Jackson Heart Study. Medicine (Baltimore) 96: e8369, 2017. doi: 10.1097/MD.0000000000008369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Thomsen ABK, Kim S, Aalbaek F, Aalkjaer C, Boedtkjer E. Intracellular acidification alters myogenic responsiveness and vasomotion of mouse middle cerebral arteries. J Cereb Blood Flow Metab 34: 161–168, 2014. doi: 10.1038/jcbfm.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Thorsen K, Dam VS, Kjaer-Sorensen K, Pedersen LN, Skeberdis VA, Jurevičius J, Treinys R, Petersen IMBS, Nielsen MS, Oxvig C, Morth JP, Matchkov VV, Aalkjær C, Bundgaard H, Jensen HK. Loss-of-activity-mutation in the cardiac chloride-bicarbonate exchanger AE3 causes short QT syndrome. Nat Commun 8: 1696, 2017. doi: 10.1038/s41467-017-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Uriarte I, Banales JM, Sáez E, Arenas F, Oude Elferink RP, Prieto J, Medina JF. Bicarbonate secretion of mouse cholangiocytes involves Na+-HCO3− cotransport in addition to Na+-independent Cl−/HCO3− exchange. Hepatology 51: 891–902, 2010. doi: 10.1002/hep.23403. [DOI] [PubMed] [Google Scholar]
- 201.Urso K, Charles JF, Shull GE, Aliprantis AO, Balestrieri B. Anion exchanger 2 regulates dectin-1-dependent phagocytosis and killing of Candida albicans. PLoS One 11: e0158893, 2016. doi: 10.1371/journal.pone.0158893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vichot AA, Zsengellér ZK, Shmukler BE, Adams ND, Dahl NK, Alper SL. Loss of kAE1 expression in collecting ducts of end-stage kidneys from a family with SLC4A1 G609R-associated distal renal tubular acidosis. Clin Kidney J 10: 135–140, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vilas GL, Johnson DE, Freund P, Casey JR. Characterization of an epilepsy-associated variant of the human Cl−/HCO3(−) exchanger AE3. Am J Physiol Cell Physiol 297: C526–C536, 2009. doi: 10.1152/ajpcell.00572.2008. [DOI] [PubMed] [Google Scholar]
- 204.Vilas GL, Loganathan SK, Liu J, Riau AK, Young JD, Mehta JS, Vithana EN, Casey JR. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet 22: 4579–4590, 2013. doi: 10.1093/hmg/ddt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Walsh S, Turner CM, Toye A, Wagner C, Jaeger P, Laing C, Unwin R. Immunohistochemical comparison of a case of inherited distal renal tubular acidosis (with a unique AE1 mutation) with an acquired case secondary to autoimmune disease. Nephrol Dial Transplant 22: 807–812, 2007. doi: 10.1093/ndt/gfl662. [DOI] [PubMed] [Google Scholar]
- 206.Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl−/HCO3− exchanger. Cloning, tissue distribution, and functional characterization. J Biol Chem 275: 35486–35490, 2000. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- 207.Wang L, Li H, Yang B, Guo L, Han X, Li L, Li M, Huang J, Gu D. The hypertension risk variant Rs820430 functions as an enhancer of SLC4A7. Am J Hypertens 30: 202–208, 2017. doi: 10.1093/ajh/hpw127. [DOI] [PubMed] [Google Scholar]
- 208.Warth R, Barrière H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R, Guy N, Bendahhou S, Lesage F, Poujeol P, Barhanin J. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA 101: 8215–8220, 2004. doi: 10.1073/pnas.0400081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wen D, Yuan Y, Cornelius RJ, Li H, Warner PC, Wang B, Wang-France J, Boettger T, Sansom SC. Deficient acid handling with distal RTA in the NBCe2 knockout mouse. Am J Physiol Renal Physiol 309: F523–F530, 2015. doi: 10.1152/ajprenal.00163.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wen D, Yuan Y, Warner PC, Wang B, Cornelius RJ, Wang-France J, Li H, Boettger T, Sansom SC. Increased epithelial sodium channel activity contributes to hypertension caused by Na+-HCO3− cotransporter electrogenic 2 deficiency. Hypertension 66: 68–74, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wu J, Glimcher LH, Aliprantis AO. HCO3−/Cl− anion exchanger SLC4A2 is required for proper osteoclast differentiation and function. Proc Natl Acad Sci USA 105: 16934–16939, 2008. doi: 10.1073/pnas.0808763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xu J, Song P, Nakamura S, Miller M, Barone S, Alper SL, Riederer B, Bonhagen J, Arend LJ, Amlal H, Seidler U, Soleimani M. Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J Biol Chem 284: 29470–29479, 2009. doi: 10.1074/jbc.M109.044396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang Y, Wu PP, Wu J, Shen WW, Wu YL, Fu AF, Zheng L, Jin XL, Fu GH. Expression of anion exchanger 2 in human gastric cancer. Exp Oncol 30: 81–87, 2008. [PubMed] [Google Scholar]
- 214.Yoshitomi K, Burckhardt BC, Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch 405: 360–366, 1985. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]
- 215.Yu Q, Liu X, Liu Y, Riederer B, Li T, Tian DA, Tuo B, Shull G, Seidler U. Defective small intestinal anion secretion, dipeptide absorption, and intestinal failure in suckling NBCe1-deficient mice. Pflugers Arch 468: 1419–1432, 2016. doi: 10.1007/s00424-016-1836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhou Y, Zhao J, Bouyer P, Boron WF. Evidence from renal proximal tubules that HCO3− and solute reabsorption are acutely regulated not by pH but by basolateral HCO3− and CO2. Proc Natl Acad Sci USA 102: 3875–3880, 2005. doi: 10.1073/pnas.0500423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhu Q, Shao XM, Kao L, Azimov R, Weinstein AM, Newman D, Liu W, Kurtz I. Missense mutation T485S alters NBCe1-A electrogenicity causing proximal renal tubular acidosis. Am J Physiol Cell Physiol 305: C392–C405, 2013. doi: 10.1152/ajpcell.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zimmermann U, Köpschall I, Rohbock K, Bosman GJ, Zenner HP, Knipper M. Molecular characterization of anion exchangers in the cochlea. Mol Cell Biochem 205: 25–37, 2000. doi: 10.1023/A:1007002916772. [DOI] [PubMed] [Google Scholar]