Abstract
Physical activity is critically important for Type 2 diabetes management, yet adherence levels are poor. This might be partly due to disproportionate exercise intolerance. Submaximal exercise tolerance is highly sensitive to muscle oxygenation; impairments in exercising muscle oxygen delivery may contribute to exercise intolerance in Type 2 diabetes since there is considerable evidence for the existence of both cardiac and peripheral vascular dysfunction. While uncompromised cardiac output during submaximal exercise is consistently observed in Type 2 diabetes, it remains to be determined whether an elevated cardiac sympathetic afferent reflex could sympathetically restrain exercising muscle blood flow. Furthermore, while deficits in endothelial function are common in Type 2 diabetes and are often cited as impairing exercising muscle oxygen delivery, no direct evidence in exercise exists, and there are several other vasoregulatory mechanisms whose dysfunction could contribute. Finally, while there are findings of impaired oxygen delivery, conflicting evidence also exists. A definitive conclusion that Type 2 diabetes compromises exercising muscle oxygen delivery remains premature. We review these potentially dysfunctional mechanisms in terms of how they could impair oxygen delivery in exercise, evaluate the current literature on whether an oxygen delivery deficit is actually manifest, and correspondingly identify key directions for future research.
Keywords: cardiac dysfunction, exercise habits, microvascular flow, muscle blood flow, vasodilation
INTRODUCTION
The criteria for diagnosis of Type 2 diabetes as outlined by the American Diabetes Association are the consistent manifestation of any one of the following: a hemoglobin A1c (HbA1c) level of 6.5% or higher, fasting plasma glucose of 126 mg/dl (7 mmol/l) or higher, a 2-h plasma glucose level of 200 mg/dl (11.1 mmol/l) or higher during a 75-g oral glucose tolerance test, or a random plasma glucose of 200 mg/dl or higher in patients with classic symptoms of hyperglycemia (1). In Type 2 diabetes this compromise to blood glucose regulation is a result of a progressive decline in β-cell insulin secretion under a background of insulin resistance.
Exercise is a cornerstone of diabetes management, benefiting glycemia and insulin sensitivity and having a protective influence on cardiovascular health (20, 101). In addition, cardiovascular fitness is a strong independent predictor of mortality in persons with Type 2 diabetes (19). Unfortunately, adherence to physical activity in persons with this disease is quite poor (70, 110, 123). Studies investigating exercise responses in persons with Type 2 diabetes consistently document a reduced peak aerobic capacity and slower rates of increase in oxygen consumption (V̇o2) with the onset of exercise compared with control subjects (9, 44, 50, 72, 73, 88, 89). There is also some preliminary evidence for increased ratings of perceived exertion (RPE) during submaximal exercise even when controlling for differences in relative exercise intensity at the same relative exercise intensity (38) in women, although more recent work failed to replicate this finding in men across a broad range of relative exercise intensities (46). These indices of aerobic response to exercise are associated with reduced tolerance for a given absolute exercise intensity. As such they have been interpreted to represent disproportionate exercise intolerance in persons with Type 2 diabetes (9, 15, 38, 88–90, 117).
Such disproportionate exercise intolerance may be an important contributor to poor exercise adherence. Therefore, understanding and combating the causes of exercise intolerance will be critical and may help address problems with exercise adherence and have important implications for improving Type 2 diabetes management. In this regard, it is important to recognize that exercise responses during submaximal exercise intensity, and not peak exercise capacity, are most relevant for assessment of exercise intolerance related to participation in physical activity (32). It is also important to consider whether the exercise intolerance is disease mediated or a consequence of excessive chronic physical inactivity.
Compromised oxygen delivery to exercising muscle (mO2del) as a consequence of reduced blood flow and/or reduced arterial oxygen content results in faster development of muscle fatigue during submaximal exercise in healthy persons (4, 42), thereby impairing exercise tolerance. Initial investigations into whether impairment in mO2del is inherent in persons with Type 2 diabetes took place in the early 1990s (64, 121). In a recent review of the literature, Reusch et al. (90) concluded that “As such, nutritive blood flow appears to be an important contributor to the impaired response of the skeletal muscle to exercise in people with T2D [Type 2 diabetes].” However, closer examination of the literature reveals that although there is evidence for impaired exercising mO2del, evidence against impairment is also considerable (see Table 1). Furthermore, the degree to which a compromised mO2del, if it exists, might explain disproportionate exercise intolerance in Type 2 diabetes is currently unknown.
Table 1.
Summary of studies that examined exercising muscle oxygen delivery in humans with Type 2 diabetes
| Studies identified by medication status and by oxygen delivery findings | ||||||
|---|---|---|---|---|---|---|
| Medication Status Not Reported | Medications Withdrawn During Testing | Medications Continued During Testing | Adjustment of Muscle Blood Flow Impaired | Adjustment of Muscle Blood Flow Preserved | Submaximal Steady-State Muscle Blood Flow Impaired | Submaximal Steady-State Muscle Blood Flow Preserved |
| 1, 5, 8, 9, 17 | 4, 7, 16 | 2, 3, 6, 10, 11, 12, 13, 14, 15 | 1, 3, 6, 10, 11, 13, 14 | 3, 6, 11, 13, 14 | 2, 4, 5, 8, 9, 10, 16, 17 | 2, 3, 6, 7, 9, 10, 11, 12, 14, 15, 16 |
| Specific study details | ||||||
|---|---|---|---|---|---|---|
| Table Reference/Number Reference | Study Participants: n, age, BMI | Duration of Diagnosis and HbA1c in T2D | Medication(s) and Status During Testing (SDT) | Study Protocol | Evidence for and against impairment | Measurement Method and Potential Limitations |
| 1. Bauer et al., 2007 (9) | T2D: n = 11 (6 ♀, 5 ♂), ~47 ± 4 yr 31 ± 4 kg/m2C: n = 11 (5 ♀, 6 ♂), ~47 ± 6 yr 28 ± 3 kg/m2 | not reported6.8 ± 1.2% | T2D: not reportedC: noneSDT: not reportedExclusions: insulin, thiazolidinediones, α-glucosidase inhibitors, β-blockers, calcium channel blockers | Exercise transitions from unloaded to moderate cycling exercise (~85% of each subject’s lactate threshold) | Evidence for adjustment impairment:1. Phase 2: slower microvascular blood flow adjustment | Capillary blood flow adjustment estimated via Fick principle using gas exchange measures of V̇o2 combined with NIRS measures of deoxyhemoglobinValidity of NIRS to reflect arteriovenous oxygen difference is questionable (71) |
| 2. Joshi et al., 2010 (40) | T2D:Group 1: HbA1c<8, n = 31, 50 ± 7 yr; 26 ± 3 kg/m2Group 2: HbA1c≥8, n = 38, 50 ± 5 yr 27 ± 4 kg/m2C: n = 32, 49 ± 5 yr 24 ± 3 kg/m2 (♂/♀ not indicated) | Group 1: ~4 yr <8%Group 2: ~5 yr ≥8% | T2D (Group 1, Group 2): secretagogues (71, 74%), sensitizers (74, 76%), β-blockers (32, 5%), antihypertensives (52, 34%), diuretics (6, 3%), statins (10, 0%)C: noneSDT: on medicationsExclusions: insulin | 10-min cycling at 50 W | Evidence for steady-state impairment: | Gastrocnemius microvascular perfusion via laser Doppler |
| In persons with HbA1c ≥ 81. ↓ % increase in laser Doppler measured gastrocnemius red blood cell velocity2. ↑ Hb deoxygenationEvidence against steady-state impairment:In persons with HbA1c < 81. No compromise to % increase in laser Doppler measured gastrocnemius red blood cell velocity2. No compromise to hemoglobin deoxygenation | Gastrocnemius Hb deoxygenation via white light spectroscopyMeasurement technique may not be valid for muscle perfusion estimates (10) | |||||
| 3. Kiely et al., 2014 (44) | T2D: n = 30 ♂, 57 ± 7 yr, 30 ± 3.4 kg/m2 n = 14 ♀, 57 ± 5 yr, 31.9 ± 4.9 kg/m2C: n = 17♂, 56 ± 10 yr, 29.3 ± 3 kg/m2 n = 18 ♀, 52 ± 9 yr, 29.6 ± 3.9 kg/m2 | ♂ = 4.1 ± 2.5 yr, 6.58 ± 0.97%♀ = 4.8 ± 3.0 yr, 6.54 ± 0.82% | T2D: diet treated (32%), metformin (50%), metformin + sulphonylurea (18%), ACE inhibitors (9%), AIIRA (9%), ACE inhibitors + AIIRA (9%)C: noneSDT: on medicationsExclusions: β-blockers, evidence of peripheral artery disease, HbA1c > 9%, uncontrolled hypertension, kidney dysfunction, liver dysfunction, insulin or thiazolidinedione treatment | Calf plantar flexion exercise (6-s duty cycle, 2-s contraction:4-s relaxation):6 min at 30% MVC and 70% MVCIncremental exercise test to failure (♂ 200 N increments every 2 min; ♀ 150 N increments every 2 min) | Evidence for adjustment impairment:1. Phase 1: ↓ amplitude of CVC at 70% MVC exercise intensity within each of ♀♂2. Phase 2: slower increase in CVC at 70% MVC exercise intensity within each of ♀♂Evidence against adjustment impairment:1. Phase 1: no compromise to amplitude of CVC at 30% MVC exercise intensity within each of ♀♂2. Phase 2: no compromise to rate of increase in CVC at 30% MVC exercise intensity within each of ♀♂Evidence against steady-state impairment:1. No compromise to CBF or CVC at any submaximal exercise intensities within each of ♀♂ | CBF between contractions with venous occlusion strain gauge plethysmography |
| 4. Kingwell et al., 2003 (48) | T2D: n = 9 ♂, 48 ± 1 yr 28 ± 1 kg/m2 | not reported6.2 ± 0.4% | T2D: metformin (22%), gliclazide (11%) | 25-min cycling at 60% V̇o2peak | Evidence for steady-state impairment: | LBF via thermodilution |
| C: n = 9 ♂, 46 ± 2 yr 26 ± 1 kg/m2 | C: noneSDT: held medications for 24 h before testing | (also assessed leg blood flow (LBF) responses to intrafemoral arterial infusion of Ach and SNP) | 1. ↓ steady-state LBF | |||
| 5. Lalande et al., 2008 (50) | T2D: n = 8 ♂, 53 ± 1 yr 29 ± 1 kg/m2 | 5 ± 3 yr6.6 ± 0.3% | Not reportedExclusions: insulin, any cardiovascular medications (including β-blockers, ACE inhibitors) | Low-intensity knee extensor exercise (1.5-kg ankle weights, 60 ± 5 rpm, ~15 min) | Evidence for steady-state impairment: | LBF via magnetic resonance imaging Arterial spin labeling |
| C: n = 11 ♂, 49 ± 2 yr 28 ± 1 kg/m2 | 1. ↓ steady-state LBF indexed to thigh lean mass | |||||
| 6. MacAnaney et al., 2011 (56) | T2D: n = 9 ♀ 49 ± 6 yr 33 kg/m2C: n = 10 ♀ (heavy), 43 ± 13 yr 29 kg/m2n = 8 ♀ (lean) 44 ± 8 yr 23 kg/m2BMI values are medians | Range: 1–4 yr6.8 ± 1.0% | T2D: metformin (56%), avandmen (11%), statins (33%), ACE inhibitor (11%)C: noneSDT: on medicationsExclusions: insulin, β-blockers, calcium channel blockers, any other anti-hypertensive drug | 6 min of intermittent calf contractions (2 s static contraction: 4 s relaxation at 70% MVC) | Evidence for adjustment impairment:1. Phase 2: Slower increase in CVCEvidence against adjustment impairment:1. Phase 1: no blunting of CVC amplitudeEvidence against steady-state impairment:1. No compromise to steady-state CBF or CVC | CBF between contractions via venous occlusion Strain gauge plethysmography |
| 7. Martin et al., 1995 (60) | T2D: n = 8 ♂, 51 ± 2 yr 24 ± 0.3 kg/m2C: n = 7 ♂, 53 ± 1 yr24 ± 1 kg/m2 | 4.7 ± 1.3 yr7.1 ± 0.6% | T2D: oral hypoglycemic agents (38%)C: noneSDT: held medications for 48 h before testing | 40-min cycling exercise at 60% V̇o2max | Evidence against steady-state impairment:1. No compromise to steady-state LBF | LBF via indocyanine green dye infusion in femoral arterystudy cohort was considerably more aerobically fit than typical persons with Type 2 diabetes |
| 8. – Menon et al., 1992 (64) | T1D: n = 10 ♂, 23–40 yrT2D: n = 11 ♂, 35–50 yrC: n = 15 ♂, (age and BMI not reported) | T1D: 1–10 yrT2D: 2–10 yrHbA1c in both T1D and T2D:9.5 ± 1.2% | T1D and C: not reportedT2D: oral hypoglycemic agentsSDT: not reported | Plantar and dorsiflexion exercise (80 repetitions in 150 s) | Evidence for steady-state impairment:1. ↓ early postexercise CBF | CBF via 133Xe clearance“Early” postexercise blood flow may not be early enough to reflect exercise specific flow but rather postexercise perfusion mechanisms |
| 9. Mohler et al., 2006 (66) | T2D: n = 17 (3 ♀, 14 ♂) 58 ± 6 yr 29 ± 3 kg/m2C: n = 25 (15 ♀, 10 ♂) 66 ± 10 yr 26 ± 5 kg/m2 | not reported | not reported | 30-plantar flexion over 1 min, and a progressive treadmill test | Evidence for steady-state impairment:1. ↓ calf BV expansion (interpreted to represent improved capillary perfusion) from rest to steady state in treadmill walking exerciseEvidence against steady-state impairment:1. No compromise to muscle oxygenation change from rest to steady-state plantar flexion or treadmill walking exercise | Calf microvascular BV and deoxygenation via NIRSBV expansion is not a valid surrogate for blood flow (peripheral arterial disease group also studied had enhanced blood volume change vs. control, but this was not reported as improved perfusion) |
| 10. Pak et al., 2010 (77) | T2D: n = 4 ♂, 54 ± 4 yr 34 ± 2 kg/m2C: n = 6 ♂, 44 ± 3 yr 27 ± 1 kg/m2 | 5.8 ± 2.6 yr7.4 ± 0.9% | T2D: metformin (50%), statin (75%), anti-hypertensive (50%), aspirin (50%)C: noneSDT: on medications | Two-leg knee extension/flexion exercise: 1) incremental test (1 min at each level) to exhaustion, and 2) step increases to low and moderate-intensity work rates | Evidence for adjustment impairment:1. Phase 1: ↓ amplitude of LBF increase in low-intensity exercise | LBF via Doppler ultrasoundSmall subject pool results in low power to detect differences between groups |
| Evidence for steady-state impairment:1. ↓ LBF at moderate-to-heavy work rates during incremental exerciseEvidence against steady-state impairment:1. No compromise to LBF in low-intensity exercise during incremental or low-intensity step increase | • Possible that LBF did not have time to reach “steady-state” levels at times of measurement in the incremental protocol | |||||
| 11. Poitras et al. 2015a (81) | T2D: n = 13 ♂, 63.2 ± 9.5 yr 32.1 ± 4.9 kg/m2 | 5.9 ± 5.4 yr | T2D: COX inhibitor (62%), ACE inhibitor (100%), HMG-CoA Reductase inhibitor (“statin”) (100%), Thienopyridine (or derivative; “anti-platelet”) (31%), Cholesterol absorption inhibitor (23%), Biguanide (69%), Insulin/analog (8%), Other (54%) | Single-leg isometric knee extension exercise: rest to 6-kg and 6-kg to 12-kg resistance transitions | Evidence for adjustment impairment:1. Phase 1: ↓ amplitude of LBF increase in both rest to 6-kg and 6-kg to 12-kg transitions | LBF via Doppler ultrasound |
| C: n = 11 ♂, 62.7 ± 11.2 yr 30.4 ± 5.0 kg/m2 | 7.3 ± 1.4% | C: COX inhibitor (82%), ACE inhibitor (64%), HMG-CoA reductase inhibitor (“statin”) (91%), Thienopyridine (or derivative; “anti-platelet”),Cholesterol absorption inhibitor (0%), Biguanide (0%), Insulin/analog (0%), Other (82%)SDT: on medicationsExclusions: Stage 3 or greater renal disease, current smoker or smoking within last 12 mo, β-blockers (unless they could be safely withdrawn), nitroglycerine or other nitric oxide donors | Evidence against adjustment impairment: 1. No impairment in rate of increase of LBF in either Phase 1 or Phase 2Evidence against steady-state impairment:1. No compromise to steady-state LBF in either 6-kg or 12-kg work rates | |||
| 12. Poitras et al. 2015b (82) | T2D: n = 8 ♂, 61.8 ± 8.9 yr, 31.8 ± 4.4 kg/m2C: n = 8 ♂, 62.6 ± 10.7 yr, 29.5 ± 4.5 kg/m2 | 6.9 ± 6.2 yr7.3 ± 1.6% | T2D: COX Inhibitor (Aspirin) (88%), ACE inhibitor (100%), Angiotensin II receptor blocker (0%), HMG-CoA reductase inhibitor (100%), Thienopyridine (or derivative; “anti-platelet”) (38%), Cholesterol absorption inhibitor (13%), Biguanide (88%), Insulin/analog (13%), Other (50%) | Forearm critical impulse test: 10 min of maximal effort rhythmic isometric forearm handgrip contractions (1-s contraction/2-s relaxation duty cycle) | Evidence against impairment:1. No compromise to FBF or FVC at critical impulse exercise intensity | FBF via Doppler ultrasound |
| C: COX Inhibitor (Aspirin) (75%), ACE inhibitor (75%), Angiotensin II receptor blocker (25%), HMG-CoA reductase inhibitor (88%), Thienopyridine (or derivative; “anti-platelet”) (38%), Cholesterol absorption inhibitor (0%), Biguanide (0%), Insulin/analog (0%), Other (88%) | ||||||
| SDT: on medications | ||||||
| Exclusions: Stage 3 or greater renal disease, current smoker or smoking within last 12 mo, β-blockers (unless they could be safely withdrawn), nitroglycerine or other nitric oxide donors | ||||||
| 13. Sanchez et al., 2011 (95) | T2D: n = 8 (3 ♀, 5♂) 37 ± 2 yr 34 ± 2 kg/m2C: n = 8, BMI-matched (3 ♀, 5♂) 37 ± 2 yr 33 ± 2 kg/m2n = 8 lean (3 ♀, 5♂) 38 ± 1 yr 23 ± 0.6 kg/m2 | 4.0 ± 2.9 yr7.1 ± 0.4% | T2D: blood glucose control medications (89%; ~1.6 ± 1.0 per subject), insulin (25%)C: not reportedSDT: on medications | 10-s isometric dorsiflexion contractions at 50 and 100% MVC | Evidence for adjustment impairment:1. ↓ EDL muscle blood volume expansion following 100% MVC contraction2. ↑ EDL muscle deoxygenation following 100% MVC contractionEvidence against adjustment impairment:• No compromise to muscle blood volume expansion following 50% MVC in EDL and 50 and 100% MVC in TA• No compromise to muscle oxygenation change following 50% MVC in EDL and 50 and 100% MVC in TA | MRI sequences used to measure variations in blood volume and % HbO2 saturation immediately after contractions in TA; predominantly type I fibers) and EDL (mostly type II fibers)• Whether MRI blood volume measures reflect blood flow has not been validated: peak blood volume signal occurs 20–30 s postcontraction which does not reflect pattern of post- contraction blood flow |
| 14. Slade et al., 2011 (102) | T2D: n = 16 (11 ♀, 5♂) 47 ± 2 yr 36 ± 2 kg/m2C: n = 16 (11 ♀, 5♂) 45 ± 2 yr 36 ± 2 kg/m2 | median duration:2.4 yr7.6 ± 0.3% | T2D: glucose control medications [biguanides (75%), thiazolidinediones (50%)], ACE inhibitor (31%), angiotensin II receptor blocker (13%), β-blocker (13%), hydrochlorothiazide (13%)C: average of 1.1 medications per day (type not reported)SDT: on medications | Rapid initial response: 1-s maximal isometric ankle dorsiflexionSteady-state response: 2 min of dynamic ankle dorsiflexion exercise at 40% MVC (1-s contraction: 1-s relaxation) | Evidence for adjustment impairment:1. Longer time for dorsiflexor microvascular perfusion to reach peak following brief contractionEvidence against adjustment impairment:1. No compromise to dorsiflexor microvascular perfusion peak response following brief contractionEvidence against steady-state impairment:1. No compromise to steady-state micro- or macrovascular perfusion | Microvascular perfusion of dorsiflexor muscles via MRI BOLD signal. Macrovascular perfusion via phase contrast MRI of popliteal and tibialis arteries.Extrapolation of post exercise blood flow to represent exercise blood flow assumes exponential post exercise decay |
| 15. Thaning et al., 2011 (109) | T2D: n = 10 (4♀, 6♂) 55 ± 2 yr 29 ± 1 kg/m2C: n = 10 (4♀, 6♂) 55 ± 2 yr 27 ± 1 kg/m2 | median duration: 6 yr (range 2–13)7.6 ± 1.2% | T2D: insulin (40%); all participants (100%): lipid-lowering medication, oral hypoglycemic agents, anti-hypertensive treatmentC: noneSDT: all medications continued during testing except insulin (stopped on the day of the study) | 2 min of knee extensor exercise at 15 W with or without tyramine | Evidence against steady-state impairment:1. No compromise to steady-state LBF | LBF via Doppler ultrasound |
| 16. Womack et al., 2009 (119) | T2D: n = 22 (19 ♀, 3♂) 53 yr (median) 34 ± 6 kg/m2T2D + microvascular complications (MC): n = 8 (5♀, 3♂) 54 yr 35 ± 5 kg/m2C: n = 20 (11 ♀, 9 ♂) 47 yr 23 ± 3 kg/m2 | T2D: 2.5 ± 4 yr 6.9 ± 2.2%T2D+MC: 7.0 ± 5.0 yr 8.5 ± 2.2% | T2D and T2D+MC: aspirin or clopidogrel (41, 63%), β-blocker (0, 25%), ACE inhibitor or angiotensin receptor blocker (41, 50%), statin (55, 75%)C: noneSDT: held medications 3 days before testing | Low- or high-intensity handgrip exercise (25% and 80% MVC); duty cycle: 1-s contraction: 4-s relaxation for 2 min; then 1-s contraction: 19-s relaxation for 3 min | Evidence for steady-state impairment:1. ↓ forearm microvascular perfusion by 60–70% in Type 2 diabetes group with MC for both work ratesEvidence against steady-state impairment:1. No compromise to steady-state forearm macrovascular perfusion in Type 2 diabetes groups with or without microvascular complications for both work rates2. No compromise to steady-state forearm microvascular perfusion in Type 2 diabetes group without MC for both work rates | Forearm macrovascular perfusion via Doppler ultrasound. Forearm microvascular perfusion via contrast-enhanced ultrasound20-s period between contractions to obtain perfusion measurements may mean nature of impairment identified is recovery perfusion, not exercise perfusion |
| 17. Young et al., 1991 (121) | T2D: n = 10 ♂ 58 ± 8 yrC: n = 7 ♂ 56 ± 5 yr (BMI not reported) | At least 10 yraverage duration and HbA1c not reported | T2D: insulin; others not reportedC: not reportedSDT: not reported | 5-min cycling exercise at 75 W | Evidence for steady-state impairment: | Microvascular LBF via 133Xe Clearance |
AIIRA, angiotensin II receptor antagonist; ACE inhibitor, angiotensin-converting enzyme inhibitor; Ach, acetylcholine (endothelium-dependent vasodilator); BMI, body mass index; BOLD, blood oxygen level dependent; BV, blood volume; C, control; CBF, calf blood flow; COX, cyclooxygenase; CVC, calf vascular conductance; EDL, extensor digitorum longus; FBF, forearm blood flow; FVC, forearm vascular conductance; [HHb], deoxygenated hemoglobin/myoglobin; %HbO2, oxygen saturation; %HbA1c, % glycosylated hemoglobin; LBF, leg blood flow; MC, microvascular complications; MRI, magnetic resonance imaging; MVC, maximal voluntary contraction; NIRS, near-infrared spectroscopy; SDT, status during testing; SNP, sodium nitroprusside (endothelium-independent vasodilator); T1D, Type 1 diabetes; T2D, Type 2 diabetes; TA, tibialis anterior; ♂ = men; ♀ = women.
Therefore the purpose of this perspectives paper is 1) to address the issue of exaggerated exercise intolerance as being disease vs. inactivity mediated, 2) to provide a framework for understanding how muscle oxygenation (PmyoO2) is established by the cardiovascular system and how PmyoO2 influences muscle metabolism and processes related to fatigue, 3) to critically assess the current understanding of oxygen delivery to exercising muscle (i.e., muscle blood flow) and oxygen diffusion into the exercising muscle fibers in Type 2 diabetes and their relationship to exercise intolerance, and 4) based on this assessment, to identify key research questions to guide future advancement in the field.
This review focuses on findings from studies in humans that have Type 2 diabetes. Obesity, insulin resistance, and metabolic syndrome often precede diagnosed Type 2 diabetes, and it is likely that the progression of vascular and cardiac dysfunction are already in progress. There has been considerable work in animal models focusing on the microvascular consequences of these conditions, but these will not be discussed or integrated in the content of the current review. The reader is referred to the excellent reviews by Lemaster et al. (51) and Chantler and Frisbee (17) for further reading on that topic.
EXAGGERATED EXERCISE INTOLERANCE: DISEASE OR INACTIVITY MEDIATED?
Adherence to physical activity is poor in persons with Type 2 diabetes (70, 110, 123). The evidence strongly supports reduced maximal aerobic capacity (typically ~15–20%) and a slowing of the increase in V̇o2 at the onset of exercise (9, 15, 38, 44, 72, 73, 89, 117). Both of these characteristics would predict reduced performance at a given absolute work rate, reflecting poorer exercise tolerance. Unfortunately, confirmation of such submaximal tolerance/performance compromise via the necessary confirmation of this in submaximal exercise challenges such as time to exhaustion at a fixed work rate or time to completion of a fixed distance have not been performed.
However, another approach to assessment of submaximal exercise tolerance/performance is to examine RPE. There is preliminary evidence for increased perceived exertion during submaximal exercise, even after adjusting for relative work rate. Huebschmann et al. (38) compared the RPE at two fixed work rates (20 and 30 W cycling) in women ~42 yr old with vs. without Type 2 diabetes. As the Type 2 diabetes group had a lower V̇o2peak, these work rates were significantly greater in Type 2 diabetes between groups in terms of relative exercise intensity compared with both overweight and normal weight controls (20 W, ~54 vs. 39 and 36%; 30 W, ~60 vs. 45 and 42%). The authors statistically accounted for these relative exercise intensity differences, which eliminated the RPE difference at 30 W but not at 20 W.
In contrast, a recent study by Kim et al. (46) in men (albeit an older cohort of ~61 yr) found no difference in RPE at the same relative exercise intensities spanning from 25 to 100% peak workload. Since the Type 2 diabetes group had a peak workload that was 20% less than controls, their RPE at a given workload was significantly higher. These investigators also measured cerebral perfusion and oxygenation and found that it was significantly reduced in Type 2 diabetes, both when comparing at absolute and at relative percentage of workloads. An intriguing hypothesis put forward by Kim et al. (46) was that this cerebral hypoperfusion may have contributed to elevated RPE. However, RPE would be expected to be greater at a given absolute workload if that represented a greater relative workload as it is scaled within subjects to their peak, and RPE was in fact not different between Type 2 diabetes and control when compared at the same relative percentage of workload. Nevertheless, the need to consider potential impact of compromised cerebral oxygenation in Type 2 diabetes remains. RPE was found to be higher in the Type 2 diabetes group, which also had a lower V̇o2peak. In summary, the findings discussed are consistent with the notion that a reduced aerobic capacity in persons with Type 2 diabetes can be interpreted to indicate reduced tolerance for a given absolute submaximal exercise workload. However, whether relative workload tolerance is reduced specific to the disease requires the assessment of RPE in persons with Type 2 diabetes who are matched for V̇o2peak with controls.
Whether this reduced exercise tolerance is a result of the disease or simply a result of exaggerated physical inactivity is an important question to ask. Researchers have typically matched activity levels between control and Type 2 diabetes groups based on physical activity questionnaires (7, 9, 15, 89), reporting no differences. Whether this approach is valid for detection of differences in physical activity in already sedentary groups may be questioned (84, 100). This is because when self-assessment is compared with objective measures of physical activity there is low to moderate correlation, even when the range of physical activity in a cohort is substantial (84). Given that range would be substantively restricted when comparing cohorts of low physical activity, this problem would be exacerbated for comparison of controls vs. persons with Type 2 diabetes.
In contrast, three recent studies used objective measures (5-day accelerometry measures) to quantify habitual physical activity (44, 72, 73). In all three studies the Type 2 diabetes groups had reduced maximal aerobic capacity, but in two of the three the Type 2 diabetes group also had significantly attenuated habitual physical activity (44, 73) as reflected by increased inactivity and decreased moderate to vigorous activity. In the third study, O’Connor et al. (72) found no difference in habitual physical activity in either a middle-aged or older cohort.
A key limitation of such habitual physical activity measures is the assumption that the few days of objective measurement reflect the activity levels that over a number of years contributed to a decline in aerobic capacity. Furthermore, the findings are at once consistent and inconsistent with the hypothesis that exaggerated physical inactivity, not Type 2 diabetes per se, is the cause of reduced exercise tolerance. Consistent with an inactivity-mediated reduction in exercise tolerance, the Type 2 diabetes groups in two of these studies did have reduced aerobic capacity together with reduced physical activity levels (44, 73). Inconsistent with this mechanism, a reduction in aerobic capacity was observed by O’Connor et al. (72) in the absence of a difference in habitual physical activity. In addition, in the male cohort habitual activity was only slightly lower in Type 2 diabetes compared with substantially lower in the female cohort, yet the compromise to aerobic capacity was much greater for men. In other words, there was a disproportionate disparity between inactivity and impaired aerobic capacity.
A final consideration is that, when measuring physical activity after persons have had a chronic disease for a number of years, it becomes very difficult to disentangle whether the disease and its effects on exercise tolerance led to greater inactivity, or if greater inactivity coincident with the disease mediated the exercise intolerance. Furthermore, it may also be the case that persons with Type 2 diabetes are more vulnerable to the effects of inactivity per se on exercise tolerance. The role of disease vs. physical inactivity in exercise intolerance therefore remains to be determined, and investigators should not assume a disease-mediated phenomenon.
CARDIOVASCULAR DETERMINATION OF SKELETAL MUSCLE FIBER OXYGENATION
A primary role of the cardiovascular system during exercise is to provide the skeletal muscle with adequate O2 to support aerobic metabolism (Fig. 1). The effectiveness of this role is ultimately reflected by the partial pressure of oxygen within skeletal muscle fibers (PmyoO2) at a given V̇o2. PmyoO2 at physiological levels is a key determinant of submaximal exercise skeletal muscle contractile and metabolic function (113). Of particular importance is the fact that PmyoO2 can vary at the same V̇o2 depending on the rate of oxygen delivery from the lungs to the skeletal muscle capillaries (i.e., mO2del) and the diffusive conductance of the muscle for oxygen (mDKO2). Diffusive conductance refers to the relative ease with which oxygen can diffuse, and is generally dependent on surface area and distance characteristics between the capillary lumen and the mitochondria in the myocyte. Recently, it has also been increasingly recognized that heterogeneity of spatial microvascular distribution of convective oxygen delivery (blood flow) has an important impact on muscle oxygenation (61, 62).
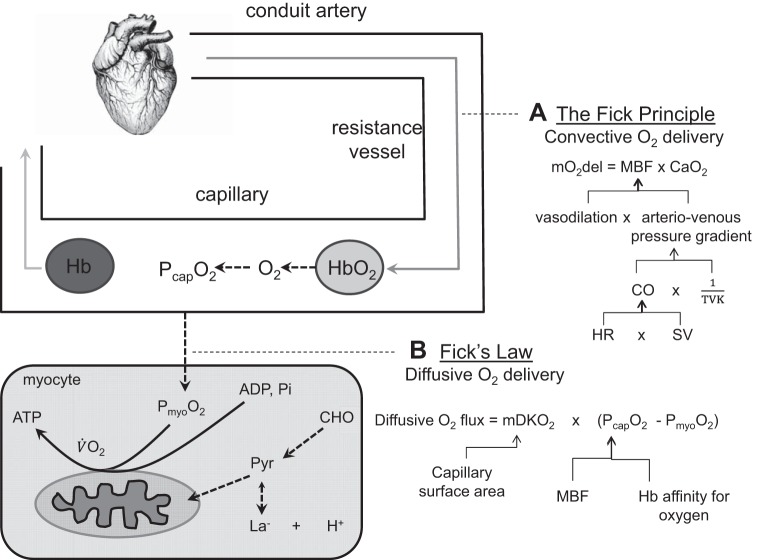
Fig. 1.
Cardiovascular pathway for oxygen delivery to the mitochondria. The “flow” of oxygen has a convective component delivering oxygen to the capillaries, where a diffusive component determines the delivery from the capillaries into the myocytes. A: the Fick principle identifies the role of vasodilation and the arteriovenous pressure gradient in increasing convective oxygen delivery (mO2del) via increasing muscle blood flow (MBF). Arterial pressure is ultimately determined by the cardiac supply of blood to the arteries (cardiac output, CO) in balance with total systemic vascular tone (total vascular conductance, TVK). Heart rate (HR) and stroke volume (SV) responses determine CO. B: Fick’s law identifies the role of diffusive conductance (mDKO2) and the capillary plasma oxygen level (PcapO2) in determining the diffusion of oxygen into the myocyte. A combination of increased MBF supplying oxygen to the capillaries and reduced hemoglobin (Hb) affinity for oxygen determine PcapO2 during exercise. If Type 2 diabetes impairs oxygen delivery to the mitochondria, it would be as a result of problems with one or a number of these determinants along the cardiovascular pathway. Note that myocellular oxygenation (PmyoO2), which plays a key role in determining muscle metabolic and contractile function, can be different at the same V̇o2; changes in ADP and inorganic phosphate (Pi) can compensate for reductions in PmyoO2 (due to impaired convective and/or diffusive oxygen flow) to maintain V̇o2. , arterial oxygen content; CHO, carbohydrate, H+, hydrogen ion associated with lactate formation; La−, lactate; Pyr, pyruvate.
The Fick principle describes the relationship between muscle V̇o2, oxygen delivery (mO2del), and oxygen extraction (arteriovenous O2 difference). Muscle oxygen delivery is the product of muscle blood flow (MBF) and arterial oxygen content (), and it establishes the partial pressure of oxygen in the capillaries (PcapO2; the driving pressure for diffusion of O2 from the capillaries to the inside of the myocytes). Therefore it interacts with mDKO2 to determine the diffusive flux of oxygen into the myocyte according to Fick’s law and thereby establishes PmyoO2 at a given V̇o2.
For example, at a fixed submaximal V̇o2, and a fixed diffusive conductance, a reduction in convective delivery of O2 to the capillary space relative to its removal via diffusion into the myocyte leads to a decrease in PcapO2. This reduces the pressure gradient for the diffusion of O2 into the myocytes such that the influx of O2 into the muscle fibers is less than its consumption by the mitochondria. As a result, PmyoO2 will decrease until the pressure gradient for diffusive flux is restored, and diffusion into the myocyte once again equals V̇o2, but at a lower PmyoO2. Thus all other things being equal, any factor compromising the convective O2 delivery [in terms of rate of increase (kinetics) and magnitude (at steady state)] or the diffusive capacity of the muscle for O2 will result in reduced PmyoO2.
Although not the focus of this review, it must also be recognized that alterations in muscle perfusion can affect muscle fatigue progression independent of oxygen delivery (8), implicating the potential for metabolite removal impacting exercise tolerance.
How Does PmyoO2 Determine Exercise Tolerance?
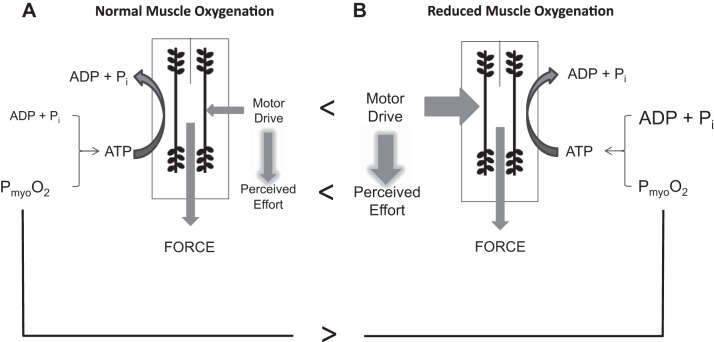
Even slight reductions in exercising muscle PmyoO2 can significantly compromise the muscle’s metabolic environment and force production capability, making exercise feel more difficult (for review see Ref. 113) (Fig. 2). For example, a decrease in PmyoO2 results in reduced force production at a given motor neuron activation level independent of a fatigue effect (120). This translates to 1) an obligatory reduction in exercise intensity, or 2) greater motor drive being required to maintain the workload, which would be experienced as increased perceived exertion (i.e., exercise feels more difficult). In addition, with reduced PmyoO2 greater changes in muscle fiber ADP and inorganic phosphate (Pi) levels are required to maintain the same aerobic supply of ATP (36). Elevated levels of Pi evoke muscle fatigue (116), while elevated levels of ADP stimulate glycolysis out of proportion to oxidative phosphorylation, leading to metabolic acidosis (106). Muscle fatigue would require further increases in motor drive to maintain force production (4) and thereby increase perceived exertion. Clearly, in the presence of reduced O2 supply one’s ability and willingness to sustain a given level of physical activity would be compromised. It is important to remember that these identified compromises are not necessarily accompanied by a reduced V̇o2 and are therefore not a case of inadequate aerobic ATP production requiring the addition of anaerobic ATP production. This is because the rate of oxygen consumption can be maintained at lower levels of PmyoO2 by increased levels of other substrates relative to products for mitochondrial respiration (118).
Fig. 2.
Effect of PmyoO2 on muscle metabolism and contractile function. A: normal PmyoO2: schematic representation of the factors influencing muscle force production (i.e., ADP + Pi, PmyoO2, motor drive). Perceived effort is a function of the degree of motor drive for a given force production. B: a reduced PmyoO2 (comparison with normal indicated by >) necessitates both an increase in ADP + Pi levels and an increase in motor drive (comparison with normal indicated by <) to maintain the given aerobic supply of ATP and exercise intensity. This would be experienced as increased perceived effort (comparison with normal indicated by <) due to increased motor drive. [Borrowed with permission from Tschakovsky and Pyke (113).]
Although the body of literature identifying oxygen delivery compromise as impacting exercise performance and tolerance is considerable, it has not addressed this phenomenon from the perspective of a “dose response.” In other words, it is not known what the minimal exercise intensity for a noticeable compromise to exercise tolerance (either increased perceived exertion or limits to duration of exercise, for example) with reduced oxygen delivery is. Nor is it known what the minimal decrease in oxygen delivery capable of evoking compromise is. Therefore it is not clear whether activities of daily living or participation in clinically relevant exercise programs are compromised by the level of reduced oxygen delivery observed in some of the studies we will address.
CARDIOVASCULAR CONTRIBUTIONS TO EXERCISE INTOLERANCE IN TYPE 2 DIABETES
Limitations to exercise tolerance that are due to the cardiovascular system are thus due to the muscle oxygenation environment (PmyoO2) that is established by the convective delivery of O2 from the lungs to the skeletal muscle capillaries, and the diffusive capacity for the movement of O2 from the capillaries to the inside of the myocytes.
Convective Muscle O2 Delivery: Potential Mechanisms of Impairment
The existence of cardiac and vascular dysfunction in Type 2 diabetes form the basis for hypothesizing compromised mO2del in exercise. Diabetic cardiomyopathy, defined as abnormal cardiac structure and function in the absence of hypertension or coronary artery disease, is common in Type 2 diabetes (16, 99), and is likely influenced by the duration and complications of the disease (99). In terms of the vasculature, a recent systematic review and meta-analysis of 27 observational reports (1,042 individuals with Type 2 diabetes and 601 control subjects) identified both macro- and microvessel endothelial (conduit artery flow mediated dilation, acetylcholine infusion) and vascular smooth muscle (oral nitroglycerine, sodium nitroprusside infusion) dysfunction as characteristic of persons with Type 2 diabetes (68). These impairments would predict compromised mO2del in exercise. It is critical to recognize, however, that dysfunction identified under these nonexercise conditions will not necessarily impact muscle oxygen delivery during exercise, in which multiple mechanisms and interaction complexities are at work (41). This becomes apparent when examining the data from actual exercise responses.
Impaired MBF: is there a central (cardiac) contribution?
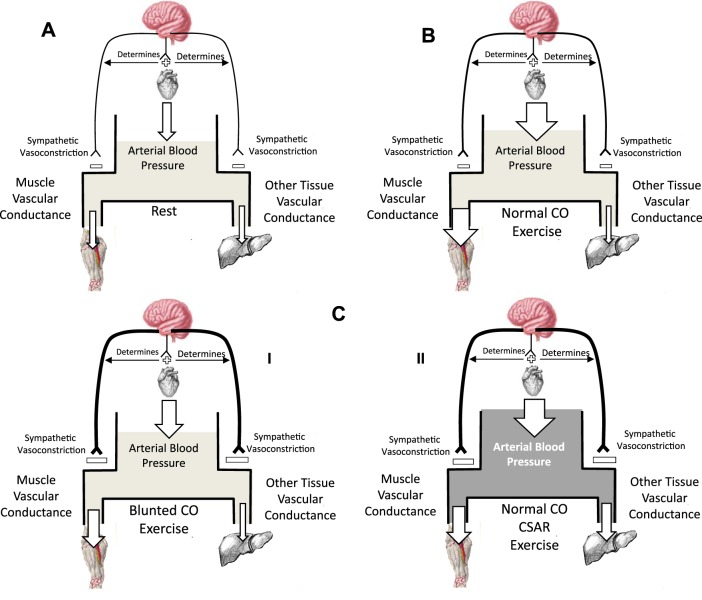
The interactions between cardiac output and regional vascular conductances in determining arterial blood pressure and the increase in MBF at a given exercise intensity are illustrated in Fig. 3. The total peripheral perfusion, which is determined by arterial blood pressure and total peripheral vascular conductance, cannot exceed the cardiac output, or arterial blood pressure will drop. Therefore, the increase in exercising MBF is ultimately constrained by the increase in cardiac output and the amount of peripheral blood flow reduction to other vascular beds via vasoconstriction.
Fig. 3.
Schematic representation of the systemic circulation with arterial blood pressure as a reservoir determined by the balance between inflow (cardiac output) and outflow (muscle and other tissue blood flow). The two outflows are determined by their respective vascular conductance and the arterial driving pressure. Arrow size represents flow magnitude. A: rest represents rest. B: normal cardiac output (CO) response in submaximal exercise in which vasodilation in the exercising muscle is matched by an increase in cardiac output CO and potentially a decrease in other tissue blood flow due to sympathetic vasoconstriction, preserving the normal increases in arterial blood pressure and increasing muscle blood flow. Sympathetic vasoconstriction (magnitude indicated by thickness of neural pathway) also normally restrains exercising muscle vasodilation to some extent. The degree to which this restraint of peripheral vasodilation is required may be dictated by the magnitude of the cardiac output CO increase. C I illustrates the way in which a blunted CO response cardiac contribution to impaired exercising muscle blood flow would manifest. C I: blunted cardiac output; blunted CO increase requires an increase in sympathetic restraint of exercising muscle vasodilation and therefore blood flow to maintain arterial blood pressure. C II: normal CO response, but cardiac sympathetic afferent reflex (CSAR) increases sympathetic vasoconstriction and mediates increased arterial blood pressure and blunted muscle blood flow.
Since persons with Type 2 diabetes are normotensive during exercise, or may even have exaggerated increases in blood pressure responses (48, 72), a cardiac contribution to compromised mO2del would have to take the form of either 1) (in a normotensive exercise response) a blunted cardiac output and subsequent blunted exercising muscle vasodilation, most likely as a result of blood pressure regulation-mediated increased sympathetic vasoconstriction, or 2) (in a hypertensive exercise response) a normal cardiac output, but blunted exercising muscle vasodilation.
Functional deficits of diabetic cardiomyopathy include left ventricular diastolic (LVDD) (16, 99) and systolic (LVSD) (34) dysfunction and might only present with an exercise challenge (34, 87). Mechanisms thought to be responsible include impaired Ca2+ handling in cardiac myocytes, increased renin angiotensin system activation leading to increased oxidative damage and interstitial fibrosis, increased reactive oxygen species (ROS) production, and reduced mitochondrial efficiency effecting greater disturbance to myocyte cellular homeostasis [for review see Boudina and Abel (13)]. Recently, Reusch et al. (90) concluded that “there are impairments in muscle substrate delivery and utilization presumed secondary to decreased cardiac function.” This conclusion was based on findings of an association between resting LVDD and reduced maximal exercise capacity (28, 80), evidence for LVDD at peak exercise (87), potentially compromised cardiac perfusion in exercise (67, 87) and the above-mentioned mechanisms of cardiomyopathy. Although the associations cited suggest the possibility of cardiac dysfunction as a contributor to compromised maximal exercising mO2del [see also Baldi et al. (6) for an excellent review on this], a closer look at the literature reveals that studies have so far been consistent in their findings of normal steady state (7, 33, 50, 72, 73) and dynamic adjustments (55, 73) of cardiac output, with one exception (46). These findings suggest that adequate cardiac output is available for distribution to exercising muscle and do not support a role for impaired cardiac pump function at submaximal exercise intensities, which would necessitate sympathetic vasoconstriction of exercising muscle to regulate blood pressure.
In contrast, the hypothesis that heightened cardiac sympathetic afferent reflex (CSAR)-mediated activation might evoke increased sympathetic vasoconstriction (52, 122) in exercising muscle represents a heretofore unconsidered mechanism. The CSAR is a positive-feedback sympathoexcitatory reflex (18) that leads to increases in blood pressure, heart rate, and myocardial contractile function (52). It is sensitive to a reduced perfusion environment and increases in endothelin-1 (18), ROS, protons, and adenosine (52), all of which could be elevated in the diabetic heart. CSAR augmentation in diabetes has recently been demonstrated in a rat model of streptozotocin-induced diabetes (122). A disproportionately large increase in blood pressure during exercise in persons with Type 2 diabetes (48, 121), together with elevated catecholamines (121), is consistent with this in humans. It remains to be determined if this reflex results in restraint of exercising skeletal muscle vasodilation in excess of the increase in arterial blood pressure, and therefore impairs mO2del.
Impaired MBF: is there a peripheral (vascular) contribution?
Virtually all studies examining vasoregulatory mechanisms in Type 2 diabetes have been done in resting skeletal muscle, which lacks validity for drawing conclusions about exercise hyperemia. Mechanisms of vasodilation in exercising muscle are numerous and interdependent, and their complex integrated function to match mO2del to demand remains poorly understood (41).
feed-forward mechanisms.
Skeletal muscle resistance vessels dilate immediately with the first increase in contraction intensity, resulting in an immediate but incomplete increase in MBF that reaches a plateau within ~5–7 s (termed “Phase 1” of the MBF response) (57). Recent work by Crecelius et al. (23) and Ross et al. (94) have identified K+, nitric oxide, vasodilating prostaglandins, and adenosine as vasodilatory mechanisms that together account for virtually all of the rapid vasodilation in healthy microcirculation. At present we do not know how these mechanisms might be compromised by Type 2 diabetes.
feedback mechanisms.
Within ~15–20 s of the increase in contraction intensity, a second slower feedback-mediated increase in MBF is initiated (termed “Phase 2”) that adjusts MBF to steady state (57). Feedback control is thought to come from vasodilatory factors in proportion to metabolic demand and from the deoxygenation state of hemoglobin (Hb), which indicates the mismatch between O2 demand and delivery. At present, nothing is known about the function of exercising muscle metabolic feedback mechanisms in persons with Type 2 diabetes; future studies are required to determine if these mechanisms are impaired. In contrast, there is evidence for impairment in the following deoxygenation-related feedback mechanisms.
rbc-o2 sensor.
Increases in skeletal muscle O2 demand results in localized red blood cell (RBC) Hb desaturation that triggers proportional ATP release from the RBC (105). This ATP binds to endothelial purinergic (P2y) receptors and evokes nitric oxide (NO) and non-NO-dependent vasodilation that ascends the arteriolar tree (conducted vasodilation) and increases MBF (25, 105). In this way, O2 supply may be spatially matched to O2 demand.
A signal transduction pathway for the release of ATP from RBCs has been proposed (for review see Ref. 27). This pathway contains the heterotrimeric G protein Gi (74), which activates adenylyl cyclase, a transmembrane protein that catalyzes the conversion of ATP to cyclic AMP (cAMP). Accumulation of cAMP leads to an increase in ATP efflux from the RBC (103). RBCs of persons with Type 2 diabetes have a diminished ATP release in response to Hb desaturation, potentially due to a reduced expression of the membrane-bound Gi protein (104). In addition, reduced RBC deformability due to increased glycosylation may also contribute since mechanical deformation of RBCs, as might occur in contracting muscle where O2 demand has increased, is also a stimulus for the release of ATP (103). Finally, recent evidence indicates that the function of endothelial purinergic receptors for ATP is attenuated in Type 2 diabetes (108). However, no studies to date have examined the functioning of the RBC-O2 sensor mechanism in vivo, and its contribution to any impairment in MBF and mO2del in persons with Type 2 diabetes has yet to be determined.
endocrine-like nitric oxide bioavailability.
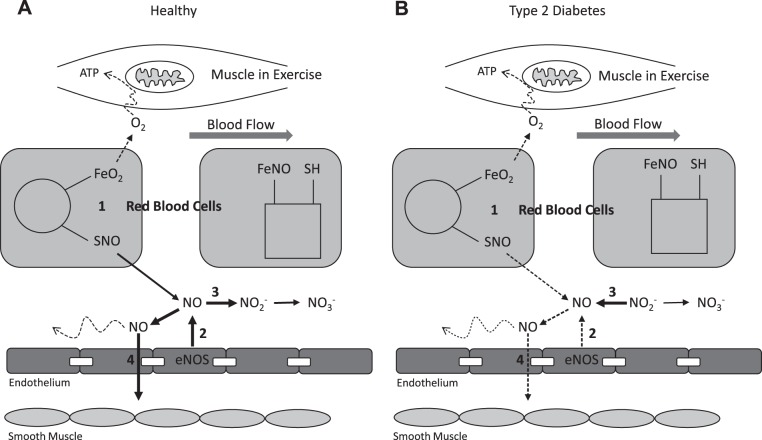
Recently a feedback mechanism of NO-mediated vasoregulation has been proposed in which NO bioavailability is determined by deoxygenation-sensitive NO-containing compounds in the blood [e.g., S-nitrosothiol (SNO) in the RBC and nitrite () in plasma] (97, 107) (Fig. 4). With deoxygenation, RBCs release SNO that, in the presence of ambient thiols, can then transfer NO to the vasculature and promote vasodilation (107). Exposure to deoxygenation also leads to increased conversion of to NO (79). A potential role for plasma in exercise tolerance is supported by whole body plasma nitrite flux (change from pre- to postexercise), being a strong predictor of peak exercise capacity across subjects ranging from cardiovascular risk-factor-but-healthy to peripheral artery disease (PAD) to PAD + Type 2 diabetes (2, 3).
Fig. 4.
Schematic of proposed dysfunction of endocrine-like nitric oxide (NO) bioavailability. A: healthy: muscle oxygen consumption in exercise leads to red blood cell (RBC) deoxygenation and S-nitrosothiol (SNO) release (1) to supplement endothelial NO synthase (eNOS) NO production (2). Excess NO converts to nitrite (), which is a biomarker of NO bioavailability (3), and eventually nitrate (). NO initiates direct and conducted vasodilation to help match oxygen delivery to demand (4). B: Type 2 diabetes: muscle oxygen consumption in exercise leads to RBC deoxygenation, but SNO release is impaired (1). eNOS NO production is also impaired (2), such that plasma nitrite is now converted to NO (3), but this is not able to maintain required NO bioavailability. Therefore direct and conducted NO-mediated vasodilation (4) is inadequate to allow matching of oxygen delivery to muscle oxygen demand in exercise. FeNO, partially nitrosylated blood; ,oxygenated blood; SH, thiol functional group.
Evidence for potential defects in RBC and plasma NO transport in persons with Type 2 diabetes is emerging. For instance, excessive glycation of Hb results in enhanced NO binding to the RBC (65), and as a result release of NO from these RBCs at sites of low oxygen concentration may be impaired (39). Plasma nitrite levels may also be reduced (2). The current evidence supporting inadequate NO bioavailability as a potentially important contributor to impaired mO2del in exercise indicates that this is a mechanism deserving closer scrutiny, especially in human in vivo conditions.
shear-mediated support of feed-forward and feedback mechanisms: endothelial function.
Endothelial cells respond to mechanical forces (e.g., shear stress) and pharmacological agents (e.g., ATP, as mentioned previously, or acetylcholine) by releasing vasodilators into the interstitium (namely NO, vasodilatory prostaglandins, and endothelium-derived hyperpolarizing factor) (24). Of these vasodilators, NO has received by far the most attention. In most studies, endothelial function is impaired in persons with Type 2 diabetes vs. healthy controls (68). However, it is critical to point out that endothelial function has only been examined in resting muscle in persons with Type 2 diabetes, and evidence to support the common contention that it is the mechanism responsible for impaired exercising mO2del (9, 48, 50) is based on correlation of resting endothelial function with exercising MBF across combined data sets of healthy controls and persons with Type 2 diabetes (e.g., 48). Such analysis must be viewed with caution as it is often the case that covariates differing between groups are not causally linked (i.e., in this case endothelial function and MBF).
no bioavailability: summary.
Evidence suggests that NO bioavailability is reduced at rest as a result of Type 2 diabetes. However, extrapolating these findings to exercise is problematic because exercising muscle vasodilatory control demonstrates considerable redundancy (41). To determine if impaired NO bioavailability compromises mO2del will require studies in which NO bioavailability is acutely improved during exercise in persons with Type 2 diabetes. These studies are currently underway in our laboratory.
sympathetic restraint in exercising muscle.
The skeletal muscle vascular bed normally experiences “sympathetic restraint” (sympathetic adrenergic vasoconstriction) of MBF during exercise as part of the integrated cardiac and peripheral resistance regulation of arterial blood pressure. Since increasing sympathetic restraint to exercising muscle has been demonstrated to reduce MBF (43), a plausible mechanism for reduced MBF in persons with Type 2 diabetes might be exaggerated sympathetic restraint in exercise.
Preliminary evidence in support of this comes from work by Hogikyan et al. (37) who observed 1) greater vasodilation in response to α1-receptor blockade (phentolamine), indicative of greater underlying adrenergic vasoconstrictor tone, and 2) greater vasoconstriction in response to norepinephrine infusion, indicating greater adrenergic vasoconstrictor responsiveness in persons with Type 2 diabetes vs. controls at rest. If this increased sympathetic vasoconstriction is present during exercise, then it may represent a mechanism for reduced exercising MBF in Type 2 diabetes. Studies which employ α-adrenergic receptor blockade in exercising muscle are needed to determine if this is indeed the case. Secondary to this would be identifying the cause of heightened sympathetic restraint, with possible contenders including the CSAR, skeletal muscle metaboreflex, and/or the baroreflex.
functional sympatholysis in exercising muscle.
Although sympathetic restraint of muscle does occur, it is well established that this restraint is normally blunted by local factors in the exercising muscle (93, 114). This “functional sympatholysis” effect increases with exercise intensity (114). ATP (93, 108) and NO (92, 114) have been identified as sympatholytic agents, although data in support of NO is not unequivocal, and its effect may depend on an interaction with prostaglandins (26). Nevertheless, if NO bioavailability is reduced in persons with Type 2 diabetes, either via reduced production or enhanced degradation as studies suggest (48, 107), then this blunting effect may also be reduced such that sympathetic vasoconstriction restrains MBF to a greater degree than in healthy individuals. However, only one study has investigated functional sympatholysis in persons with Type 2 diabetes, and no impairment was evident (109). These initial findings are limited in that the subjects with Type 2 diabetes had intact endothelial function, and functional sympatholysis was only examined during moderate-intensity exercise. Further studies are needed to replicate these findings and to examine a range of exercise intensities and patient populations to determine how these might influence functional sympatholysis and subsequently contribute to exercising MBF.
Convective Muscle O2 Delivery During Exercise in Type 2 Diabetes: Is It Really Impaired?
Given the evidence for cardiac and peripheral vascular impairments in Type 2 diabetes, it is reasonable to postulate that mO2del may be impaired in persons with Type 2 diabetes. Since does not appear to be compromised in Type 2 diabetes (88), impaired mO2del would be a function of MBF. In the following sections we examine the evidence for and against such impairment. The reader is referred to Table 1 for a comprehensive summary of the nature of the evidence, both for and against, as well as the specifics of exercise modality and protocol across studies.
Is adjustment of MBF at the onset of exercise slower in Type 2 diabetes?
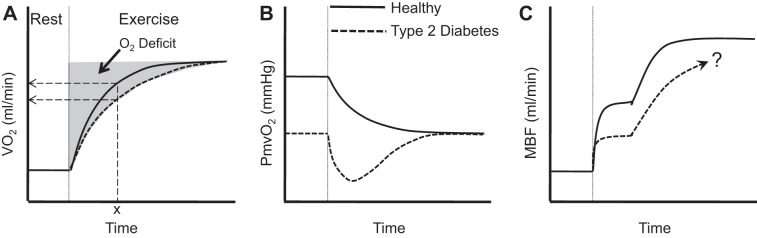
A slower adjustment of mO2del in response to exercise can reduce the rate at which aerobic ATP production increases (termed V̇o2 kinetics) to meet ATP demand (112) and result in a greater accumulation of metabolites that can contribute to fatigue (e.g., ADP, Pi) (21). A slowed V̇o2 kinetic response has been observed in persons with Type 2 diabetes at various exercise intensities (15, 89) (Fig. 5A), as has a faster PCr breakdown (98).
Fig. 5.
Schematic of proposed exercise responses in persons with Type 2 diabetes (dashed lines) vs. healthy controls (solid lines). A: slowed V̇o2 kinetics in Type 2 diabetes. The magnitude of O2 deficit is determined by V̇o2 kinetics. Dashed arrows indicate a lower V̇o2 at time “x” for the dashed vs. solid curve. This is significant because it indicates that Type 2 diabetes results in the need for a greater reliance on substrate-level phosphorylation to meet the identical ATP demand. [Borrowed with permission from Lukin and Ralston (54).] B: overshoot of microvascular deoxygenation (PcapO2, partial pressure of oxygen in the capillaries) in Type 2 diabetes is suggestive of a greater MBF/V̇o2 mismatch at the onset of exercise. Responses in A and B are consistent with observations in the literature. C: hypothesized skeletal muscle blood flow (MBF) dynamics accounting for these responses; “?” indicates that in Type 2 diabetes a slower second phase response may depend on the exercise intensity, and there is inadequate consensus on whether steady state is impaired.
Studies in animal models of Type 2 diabetes suggest slower adjustment of mO2del. In situ diabetic rat skeletal muscle has been observed to have reduced resting microvascular oxygen partial pressure (PcapO2), which declines more quickly and “undershoots” the eventual steady state in response to muscle electrical stimulation compared with control (11, 75) (Fig. 5B). While this is consistent with slower mO2del adjustment and compromised PmyoO2, PcapO2 eventually stabilizes at the same steady-state level in both normal and Type 2 diabetes animals, suggesting steady-state mO2del may not be impaired.
Evidence from human studies for and against slower mO2del adjustment in persons with Type 2 diabetes is summarized in Table 1. In total there are seven studies in humans (9, 44, 56, 77, 81, 95, 102).
initial feed-forward hyperemia.
The six studies that have reported on the initial exercise hyperemia have failed to provide consensus. Both Sanchez et al. (95) and Slade et al. (102) measured the immediate response of muscle perfusion following a single contraction. This would reflect the immediate response of “feed-forward” vasodilation. Sanchez et al. (95) observed a reduced expansion of muscle blood volume and greater deoxygenation following a maximal voluntary contraction (MVC) in extensor digitorum longus (type II fibers) but not tibialis anterior muscle (type I fibers). However, there was no compromise to blood volume expansion or muscle deoxygenation in either muscle following a 50% MVC. Slade et al. (102) found that an MVC of the dorsiflexors evoked the same peak microvascular deoxygenation (blood oxygen level dependent BOLD MRI signal); however, the time to reach peak microvascular response was slower in those with Type 2 diabetes, suggesting slower blood flow kinetics. Poitras et al. (81) measured femoral artery blood flow response to the transition from rest to rhythmic isometric quadriceps contractions, and then a further transition to a higher exercise intensity. In both transitions, the amplitude of the initial rapid increase in blood flow was blunted in Type 2 diabetes. Consistent with this were the findings of Kiely et al. (44), demonstrating blunted initial vasodilation response to 70% MVC rhythmic calf plantar flexion exercise and Pak et al.’s (77) findings of blunted initial hyperemia in low-intensity rhythmic lower limb extension/flexion exercise. However, Kiely et al. (44) found no such blunting for 30% MVC contractions, and MacAnaney et al. (56) did not observe a blunting of the initial vasodilation in 70% MVC calf plantar flexion exercise.
Reasons for this virtually equivocal evidence for and against an impairment to the initial feed-forward-evoked increase in MBF are not readily apparent. Examination of the evidence (Table 1) indicates no consistent characteristics of study cohorts in which impairment was observed vs. those where it was not.
delayed, slower feedback hyperemia.
Of the four studies reporting on this part of the exercise hyperemia response, three found slower feedback hyperemia adjustment (9, 44, 56). However, in one of these studies the impairment only occurred at a higher exercise intensity (70% but not 30% MVC) (44), and in another Bauer et al. (9) employed an indirect estimation technique with potentially limited validity (71) to quantify the rate of blood flow adaptation. Briefly, Bauer et al. (9) measured pulmonary V̇o2 and skeletal muscle hemoglobin + myoglobin deoxygenation [HHb], using near-infrared spectroscopy (NIRS), and estimated microvascular perfusion during exercise transitions from unloaded to moderate cycling exercise. This was done via rearrangement of the Fick principle, whereby the [HHb] is assumed to be a valid surrogate of arteriovenous oxygen difference. Slower V̇o2 and estimated microvascular blood flow kinetics were observed in the Type 2 diabetes group. In the fourth study no impairment was observed in either rest-to-mild or mild-to-moderate rhythmic isometric knee extension exercise intensity transitions (81). However, it must be noted that the authors investigated the effects of Type 2 diabetes beyond characteristic comorbidities and medications in this patient population. Thus differences in the control group between studies may explain the disparate findings.
In summary, it appears that the perfusion and vasodilatory adjustment to an increase in exercise intensity can be impaired in persons with Type 2 diabetes; however, a lack of impairment in some studies would indicate that this is not an obligatory characteristic of the disease. evidence is far from convincing that slower feedback-mediated adjustment in mO2del is a characteristic of persons with Type 2 diabetes, and the necessary experiments to provide conclusive evidence remain to be performed.
Is steady-state MBF reduced in Type 2 diabetes?
Menon et al. (64) demonstrated significantly reduced MBF in persons with Type 2 diabetes immediately following submaximal exercise (unloaded, 80 repetitions in 150 s). A limitation of these findings is that postexercise measurements rest on the assumption that differences in the rate of decline of perfusion following exercise does not occur independent of actual exercise hyperemia magnitude. Similarly, Young et al. (121) found that the MBF response (measured by Xe133 clearance) to 5 min of cycling at 75 W was significantly lower. Kingwell et al. (48) also observed reduced steady-state leg MBF responses (measured via thermodilution) to moderate-intensity cycling exercise (60% V̇o2peak). Lalande et al. (50) observed that steady-state femoral blood flow measured using MRI during low-intensity knee extensor exercise (1.5 kg ankle weights, 60 ± 5 rpm, ~15 min), indexed to lean thigh mass, was significantly lower in persons with Type 2 diabetes. Unfortunately, absolute values for MBF were not given, hence it is unclear whether absolute mO2del was different between groups for a given V̇o2. Pak et al. (77) also observed reduced leg MBF at moderate-to-heavy work rates during incremental two-leg knee extension/flexion exercise, but leg MBF was not compromised at low work rates during either incremental or single-step increases. Mohler et al. (66) used NIRS measurement of blood volume expansion at the probe site to infer capillary perfusion changes and observed less blood volume expansion during calf plantar flexion; however, this was not the case for treadmill walking exercise. Furthermore, no compromise in the change in muscle oxygenation from rest to exercise was evident in either plantar flexion or treadmill walking exercise. It should be pointed out that the use of NIRS blood volume expansion to infer perfusion is problematic. In fact, the peripheral arterial disease group they studied demonstrated enhanced blood volume expansion compared with control, but this was not reported as improved perfusion. Finally, Joshi et al. (40) estimated gastrocnemius perfusion during cycling at 50 W via white light spectroscopy and laser Doppler and found it to be blunted but only in the group whose HbA1c was ≥8% (64 mmol/mol). However, this measurement technique has only been validated for nonmuscle perfusion (10) and may not provide valid estimates of MBF.
In contrast, Martin et al. (60) found no difference in exercising leg blood flow between Type 2 diabetes and control subjects (matched for age, BMI, and V̇o2max) during 40 min of bicycle exercise at 60% V̇o2max. This discrepancy may be due to the recruitment of a fitter cohort [peak V̇o2 achieved by participants was on average 1 l/min greater than that of the participants in the study by Kingwell et al. (48)]. However, a recent investigation in diabetic rats in which a relative “fitness” issue would not be manifest found no impairment to exercising MBF (22). Similarly, Thaning et al. (109) found no impairment in MBF during knee extensor exercise at 15 W; however, unlike Kingwell et al. (48), their subjects did not have impaired endothelial function. MacAnaney et al. (56) found no compromise to steady-state calf MBF during intermittent calf contractions at 70% MVC, nor did Kiely et al. (44) during calf plantar flexion exercise at 30 or 70% MVC. Poitras et al. (81) found no difference in steady-state femoral artery blood flow during low- or moderate-intensity rhythmic isometric quadriceps exercise between participants with Type 2 diabetes and controls, or in forearm blood flow during 10 min of repeated maximal isometric handgrip contractions (82). Slade et al. (102) measured popliteal and anterior tibial artery blood flow with arterial spin labeling at two time points following 2 min of rhythmic dorsiflexion at 40% MVC. Exercise blood flow was extrapolated from the postexercise blood flow assuming an exponential decay. This indirect estimation of exercising MBF was not different between control and Type 2 diabetes. Finally, in a recent study Womack et al. (119) measured brachial artery blood flow to exercising forearm as well as contrast-enhanced ultrasound measures of forearm flexor capillary recruitment and found no deficits in persons with well-controlled Type 2 diabetes. However, others diagnosed with microvascular complications, based on spot urine analysis for proteinuria, demonstrated considerable capillary recruitment deficits, despite no evidence of impaired brachial artery blood flow. Since measurements were made during exercise consisting of 1-s contractions separated by 20 s of rest, it is possible that this reflects deficits in postexercise hyperemia rather than steady-state exercising MBF. Of additional interest are the data from animal models of diabetes that have measured microvascular O2 pressures that, while finding evidence for slower mO2del, also observed a recovery of muscle oxygenation in steady state equal to that in controls (11, 75), which would be inconsistent with a reduced steady-state mO2del.
In summary, a definitive conclusion that mO2del is impaired in Type 2 diabetes remains premature. A problem with interpreting the cumulative evidence to date is the small number of studies conducted and the small sample sizes within these studies, and the range of potentially confounding comorbidities (e.g., cardiovascular disease, neuropathy) and individual characteristics (e.g., age, adiposity, glycemic control) across studies that can influence outcomes of interest (see Table 1 for summary). Moreover, medication status during testing differs widely across studies (including only medication-free subjects, completing testing after having subjects abstain from taking their medications for a standardized period of time, or testing subjects while on their various medications) (Table 1). Another possibility not ordinarily considered is that some persons with Type 2 diabetes may have diabetes-evoked impairment while others may not, and therefore the inconsistent findings may represent impaired vs. unimpaired cohorts, superimposed on moderating influences of medications and comorbidities. In other words, some persons may be more susceptible to a negative impact of the disease than others, and this propensity may depend on other moderating factors. The proposed V̇o2, PcapO2, and MBF responses during exercise in healthy vs. persons with Type 2 diabetes, as based on current evidence, are depicted in Fig. 5. Future studies are required to investigate steady-state mO2del in persons with Type 2 diabetes to better define the specific exercise conditions and patient characteristics in which impairment does vs. does not manifest.
Diffusive O2 Delivery: Potential Mechanisms of Impairment
In persons with Type 2 diabetes, the diffusive flux of O2 may be hindered by 1) increased affinity of Hb for O2 (affecting the pressure gradient for diffusive flux of O2 into the myocyte), and 2) a reduced diffusive conductance due in part to capillary rarefaction.
Is Hb affinity for O2 increased in Type 2 diabetes?
“Glycosylated Hb,” or HbA1c, is a variant of Hb that is formed via the nonenzymatic binding of glucose to the Hb protein; the rate of formation of HbA1c is directly proportional to the ambient glucose concentration (31). The glycation of Hb increases its affinity for O2, causing a leftward shift of the Hb-O2 dissociation curve. This predicts a requirement of lower PcapO2 for O2 offloading from RBCs. The importance of this effect on PmyoO2 and exercise tolerance in persons with Type 2 diabetes has yet to be determined and will require studies that acutely restore normal Hb-O2 affinity and examine the effect on muscle metabolism and exercise tolerance.
Is capillary morphology altered in Type 2 diabetes?
Histological analysis of skeletal muscle in Type 2 diabetes reveals a structural (58) and functional (76, 119) (no compromise to anatomical capillary density, just compromised recruitment) capillary rarefaction that reduces the surface area, and increases the distance, for O2 diffusion. Prolonged vessel nonperfusion can progress to its structural loss (83), suggesting functional rarefaction might predict structural rarefaction.
Is disproportionate exercise intolerance in Type 2 diabetes explained by impaired muscle oxygen supply?
Direct manipulation of oxygen supply in young healthy subjects has clearly demonstrated the sensitivity of muscle metabolism, contractile function, and exercise tolerance to muscle oxygenation in this group (4, 42). Current evidence suggests the possibility that both mO2del and mDKO2 may be impaired during exercise in persons with Type 2 diabetes. Given the likely impact of these impairments on PmyoO2, it is reasonable to hypothesize that cardiovascular dysfunction contributes to the disproportionate exercise intolerance in this population. However, current support for this hypothesis is based on the assumption that impaired O2 delivery observed in some studies (9, 48, 50) explains the reduced exercise tolerance observed in those and other studies. Studies in which mO2del is acutely restored are required to identify whether and how much exercise intolerance is due to impaired mO2del in persons with Type 2 diabetes.
Other possible contributors to exercise intolerance in Type 2 diabetes.
It is important to acknowledge that although cardiovascular dysfunction may contribute to exercise intolerance in Type 2 diabetes, there are other possible factors. For instance, in Type 2 diabetes there is evidence for “mitochondrial dysfunction,” manifest as a reduction in mitochondrial content (86) with an overall reduction in oxidative phosphorylation capacity per muscle mass (14, 91). Although this dysfunction is generally thought to be composed solely of a reduced mitochondrial volume without a change in functional capacity, in one investigation the decrement in electron transport chain activity (succinate oxidase activity) was greater than could be accounted for by a reduced mitochondrial content, suggesting a functional impairment (91). In general, the mitochondrial dysfunction appears to be independent of muscle fiber type (35), present systemically in both locomotor and nonlocomotor muscles (45) and related to adiposity (35). A coordinated downregulation of genes involved in oxidative phosphorylation (69, 78) and significant reduction in the expression of PGC-1α [peroxisome proliferator-activated receptor γ coactivator-1α; the “master regulator” of mitochondrial biogenesis (78, 85)] are likely responsible. Mitochondrial dysfunction could serve to limit O2 consumption (e.g., slowed V̇o2 kinetics) or to cause a greater perturbation of the intracellular environment at a given workload, and observations of greater changes in ADP and Pi in exercising muscle in Type 2 diabetes are consistent with this (98). Although these alterations in mitochondrial morphology are pervasive in investigations in Type 2 diabetes, it is unclear whether they are inherent to the disease or are secondary to a sedentary lifestyle (86).
Similarly, it is possible that differences in muscle morphology contribute to decreased exercise tolerance in persons with Type 2 diabetes since there is generally a trend toward reduced type I and increased type II muscle fiber content in this population (30, 58) since type II fibers are known to have greater fatigability (111). Observations of twofold higher leg lactate output and greater muscle lactate content during exercise (cycling at 60% V̇o2peak) in Type 2 diabetes (60) are consistent with this potential mechanism, although others have observed no difference in exercising leg lactate release at that same work rate (47). In addition, it has been proposed that different fiber types mandate different magnitudes of contraction-induced hyperemia, such that type I fibers exhibit an augmented MBF-to-V̇o2 relationship (63). Thus it is possible that a change in muscle morphology characteristic of the population could explain the differential microvascular oxygen response described earlier (9, 11, 75). However, these differences in fiber-type composition are not always observed (35), and when they are present it is again unclear whether they result from the disease or habitual inactivity.
Lastly, psychological factors such as depression and emotional stress, which are commonly comorbid with Type 2 diabetes (5, 29), are known to increase perceived exertion and could contribute to reduced exercise tolerance in this population (115). Interestingly, there is some evidence that reduced brain-derived neurotrophic factor (BDNF) may be involved in the etiology of both depression and Type 2 diabetes, which could explain the clustering of these symptoms in epidemiological studies (49). BDNF also is thought to mediate the relationship between “energetic challenges” (i.e., exercise) and mood (59). Thus if persons with Type 2 diabetes have less BDNF, perhaps they also experience a lesser “mood elevating effect” of exercise, which could result in reduced motivation to be active. Although speculative, this possibility illustrates that there is much that remains to be determined regarding exercise intolerance in Type 2 diabetes.
SUMMARY AND FUTURE DIRECTIONS
Cardiovascular support of exercising muscle can determine submaximal muscle metabolic and contractile function and thereby directly influence the capacity for, and perceived effort of, exercise, thus impacting exercise tolerance. A key issue that remains difficult to resolve is whether the exaggerated exercise intolerance typical of persons with Type 2 diabetes is a consequence of the disease or prolonged excessive inactivity. At present, any and all findings regarding physiological contributions to this exercise intolerance require acknowledgment of this distinction.
Although evidence does exist to support the hypothesis that impaired convective and diffusive exercising muscle oxygen supply occurs as a result of Type 2 diabetes, a careful review of existing literature directly assessing cardiac and skeletal muscle vascular responses during exercise reveals that evidence contravening this hypothesis exists in equal measure. Therefore it remains premature to conclude that delivery of oxygen to exercising skeletal muscle is an important contributor to exercise intolerance in persons with Type 2 diabetes. The current body of scientific evidence on this issue is limited by small sample size, superimposition of a range of potentially confounding comorbidities (e.g., cardiovascular disease, neuropathy), and individual characteristics (e.g., age, adiposity, glycemic control) that can influence outcomes of interest, as well as differences in medication status during testing across studies that can complicate interpretation.
Nevertheless, there is good evidence for the existence of specific cardiac and vascular structural and mechanistic impairments as summarized in Fig. 6. Definitively establishing the impact of these impairments on mO2del and exercise tolerance will require studies that are able to adequately control for the above-mentioned confounds. Key research questions that need to be addressed are summarized in Table 2 and can be grouped into at least three categories. First, are mO2del and/or mDKO2 consistently compromised during exercise in Type 2 diabetes? Second, what are the cardiac and peripheral vascular mechanisms that contribute to these deficiencies? Third, does compromised mO2del and/or mDKO2 explain some or all of the disproportionate exercise intolerance?
Fig. 6.
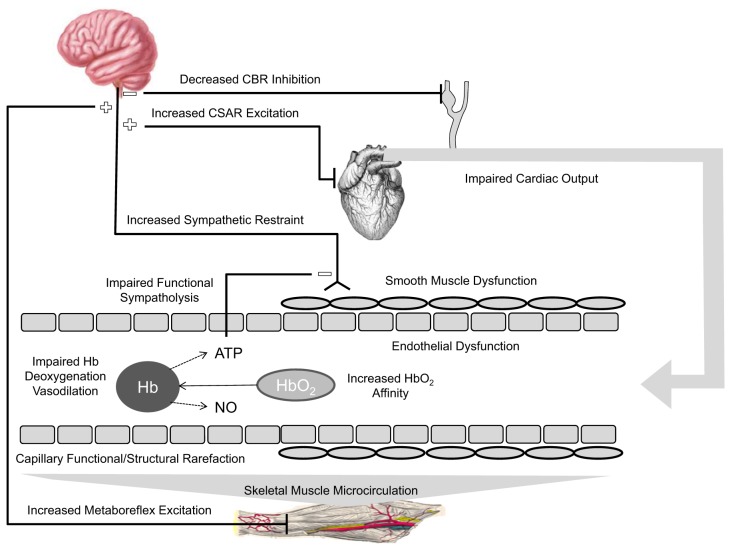
Schematic of potential central and peripheral cardiovascular impairments that could compromise steady-state convective and diffusive oxygen delivery to the contracting skeletal muscle myocyte, thereby contributing to exercise intolerance. The vessel shows capillary (left side) and upstream arteriolar (right side) levels of the vascular tree. Sympathetic restraint of exercising skeletal muscle vasodilation could be enhanced due to 1) reduced CBR inhibition of sympathetic neural outflow to compensate for inadequate CO and maintain arterial blood pressure, 2) CSAR stimulation in a diabetic heart, and/or 3) MMR activation. Impaired functional sympatholysis would reduce blunting of sympathetic restraint. The vasodilatory response to increased muscle metabolic demand for oxygen in contracting skeletal muscle could be compromised due to 1) endothelial and/or 2) smooth muscle dysfunction, and/or 3) impaired RBC release of ATP and/or nitric oxide (NO) with hemoglobin (Hb) deoxygenation. Diffusive flux of oxygen could be compromised by increased Hb affinity for O2 and/or structural and functional capillary rarefaction. CBR, carotid baroreflex; CO, cardiac output; CSAR, cardiac sympathetic afferent reflex; MMR, muscle metaboreflex.
Table 2.
Summary of important future directions
| Does Type 2 diabetes lead to compromised convective mO2del and/or diffusive O2 flux? If so: what are the cardiac and peripheral mechanisms? |
| • Convective mO2del |
| o Is the initial rapid onset vasodilatory response diminished? If so: |
| ▪ Is this due to impaired NO, prostaglandin, adenosine, and/or K+-mediated dilation? |
| o Is the rate and magnitude of increase to steady state diminished? If so: |
| ▪ Is this due to impaired RBC ATP release or NO bioavailability? |
| ▪ Is this due to elevated sympathetic restraint as a result of the CSAR, skeletal muscle metaboreflex, or baroreflex? |
| • What role does impaired cardiac function play in evoking this sympathetic restraint? |
| ▪ Is this due to diminished functional sympatholysis? |
| o Is there an exercise intensity dependence of impairment? |
| o Is there a muscle mass or exercise modality dependence of impairment? |
| • Diffusive O2 Flux |
| o Is mDKO2 impaired in Type 2 diabetes? If so: |
| ▪ Is this due to increased Hb-O2 affinity, and/or structural or functional capillary rarefaction? |
| Does dysfunctional cardiovascular support of exercising muscle in Type 2 diabetes explain some or all of the disproportionate exercise tolerance? |
| • Does acute restoration of mO2del and/or mDKO2 diminish or abolish the exercise intolerance in persons with Type 2 diabetes? |
CSAR, cardiac sympathetic afferent reflex; Hb, hemoglobin; mDKO2, muscle diffusive conductance for oxygen; mO2del, muscle oxygen delivery; NO, nitric oxide; RBC, red blood cell.
GRANTS
V. Poitras was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) doctoral student grant. M. Tschakovsky was supported by NSERC Discovery Grant RGPIN\250367-2011. M. Tschakovsky is the guarantor of this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.J.P., R.W.H., and M.E.T. conceived and designed research; V.J.P. and M.E.T. prepared figures; V.J.P., R.W.H., and M.E.T. drafted manuscript; V.J.P., R.W.H., and M.E.T. edited and revised manuscript; V.J.P. and M.E.T. approved final version of manuscript.
REFERENCES
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 31, Suppl 1: S55–S60, 2008. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 2.Allen JD, Miller EM, Schwark E, Robbins JL, Duscha BD, Annex BH. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide 20: 231–237, 2009. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, Dobrosielski DA, Annex BH. Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3 months of exercise training. Free Radic Biol Med 49: 1138–1144, 2010. doi: 10.1016/j.freeradbiomed.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Romer LM, Pegelow DF, Jacques AJ, Hess CJ, Dempsey JA. Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J Appl Physiol (1985) 101: 119–127, 2006. doi: 10.1152/japplphysiol.01596.2005. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 24: 1069–1078, 2001. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 6.Baldi JC, Wilson GA, Wilson LC, Wilkins GT, Lamberts RR. The Type 2 diabetic heart: its role in exercise intolerance and the challenge to find effective exercise interventions. Sports Med 46: 1605–1617, 2016. doi: 10.1007/s40279-016-0542-9. [DOI] [PubMed] [Google Scholar]
- 7.Baldi JC, Aoina JL, Oxenham HC, Bagg W, Doughty RN. Reduced exercise arteriovenous O2 difference in Type 2 diabetes. J Appl Physiol (1985) 94: 1033–1038, 2003. doi: 10.1152/japplphysiol.00879.2002. [DOI] [PubMed] [Google Scholar]
- 8.Barclay JK. A delivery-independent blood flow effect on skeletal muscle fatigue. J Appl Physiol (1985) 61: 1084–1090, 1986. doi: 10.1152/jappl.1986.61.3.1084. [DOI] [PubMed] [Google Scholar]
- 9.Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in Type 2 diabetes. Diabetes Care 30: 2880–2885, 2007. doi: 10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- 10.Beckert S, Witte MB, Königsrainer A, Coerper S. The impact of the micro-lightguide O2C for the quantification of tissue ischemia in diabetic foot ulcers. Diabetes Care 27: 2863–2867, 2004. doi: 10.2337/diacare.27.12.2863. [DOI] [PubMed] [Google Scholar]
- 11.Behnke BJ, Kindig CA, McDonough P, Poole DC, Sexton WL. Dynamics of microvascular oxygen pressure during rest-contraction transition in skeletal muscle of diabetic rats. Am J Physiol Heart Circ Physiol 283: H926–H932, 2002. doi: 10.1152/ajpheart.00059.2002. [DOI] [PubMed] [Google Scholar]
- 12.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 115: 3213–3223, 2007. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 14.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. Patients with Type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50: 790–796, 2007. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with Type 2 diabetes. Diabetes Care 22: 1640–1646, 1999. doi: 10.2337/diacare.22.10.1640. [DOI] [PubMed] [Google Scholar]
- 16.Brassard P, Legault S, Garneau C, Bogaty P, Dumesnil JG, Poirier P. Normalization of diastolic dysfunction in Type 2 diabetics after exercise training. Med Sci Sports Exerc 39: 1896–1901, 2007. doi: 10.1249/mss.0b013e318145b642. [DOI] [PubMed] [Google Scholar]
- 17.Chantler PD, Frisbee JC. Arterial function in cardio-metabolic diseases: from the microcirculation to the large conduits. Prog Cardiovasc Dis 57: 489–496, 2015. doi: 10.1016/j.pcad.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Chen AD, Xiong XQ, Gan XB, Zhang F, Zhou YB, Gao XY, Han Y. Endothelin-1 in paraventricular nucleus modulates cardiac sympathetic afferent reflex and sympathetic activity in rats. PLoS One 7: e40748, 2012. doi: 10.1371/journal.pone.0040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 27: 83–88, 2004. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 20.Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ; American College of Sports Medicine; American Diabetes Association . Exercise and Type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and Type 2 diabetes. Med Sci Sports Exerc 42: 2282–2303, 2010. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 21.Conley KE, Kemper WF, Crowther GJ. Limits to sustainable muscle performance: interaction between glycolysis and oxidative phosphorylation. J Exp Biol 204: 3189–3194, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Copp SW, Hageman KS, Behnke BJ, Poole DC, Musch TI. Effects of Type II diabetes on exercising skeletal muscle blood flow in the rat. J Appl Physiol (1985) 109: 1347–1353, 2010. doi: 10.1152/japplphysiol.00668.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG Jr. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol 278: H1294–H1298, 2000. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 26.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- 27.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 24: 107–116, 2009. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang ZY, Sharman J, Prins JB, Marwick TH. Determinants of exercise capacity in patients with Type 2 diabetes. Diabetes Care 28: 1643–1648, 2005. doi: 10.2337/diacare.28.7.1643. [DOI] [PubMed] [Google Scholar]
- 29.Fisher L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U, Glasgow R, Laurencin G. Clinical depression versus distress among patients with Type 2 diabetes: not just a question of semantics. Diabetes Care 30: 542–548, 2007. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- 30.Gaster M, Staehr P, Beck-Nielsen H, Schrøder HD, Handberg A. GLUT4 is reduced in slow muscle fibers of Type 2 diabetic patients: is insulin resistance in Type 2 diabetes a slow, type 1 fiber disease? Diabetes 50: 1324–1329, 2001. doi: 10.2337/diabetes.50.6.1324. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, Sacks DB. Tests of glycemia in diabetes. Diabetes Care 27: 1761–1773, 2004. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin ML, Harris JE, Hernández A, Gladden LB. Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol 1: 558–569, 2007. doi: 10.1177/193229680700100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gusso S, Hofman P, Lalande S, Cutfield W, Robinson E, Baldi JC. Impaired stroke volume and aerobic capacity in female adolescents with Type 1 and Type 2 diabetes mellitus. Diabetologia 51: 1317–1320, 2008. doi: 10.1007/s00125-008-1012-1. [DOI] [PubMed] [Google Scholar]
- 34.Ha JW, Lee HC, Kang ES, Ahn CM, Kim JM, Ahn JA, Lee SW, Choi EY, Rim SJ, Oh JK, Chung N. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart 93: 1571–1576, 2007. doi: 10.1136/hrt.2006.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in Type 2 diabetes and obesity. Diabetes 50: 817–823, 2001. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- 36.Hogan MC, Welch HG. Effect of altered arterial O2 tensions on muscle metabolism in dog skeletal muscle during fatiguing work. Am J Physiol 251: C216–C222, 1986. doi: 10.1152/ajpcell.1986.251.2.C216. [DOI] [PubMed] [Google Scholar]
- 37.Hogikyan RV, Galecki AT, Halter JB, Supiano MA. Heightened norepinephrine-mediated vasoconstriction in Type 2 diabetes. Metabolism 48: 1536–1541, 1999. doi: 10.1016/S0026-0495(99)90242-1. [DOI] [PubMed] [Google Scholar]
- 38.Huebschmann AG, Reis EN, Emsermann C, Dickinson LM, Reusch JE, Bauer TA, Regensteiner JG. Women with Type 2 diabetes perceive harder effort during exercise than nondiabetic women. Appl Physiol Nutr Metab 34: 851–857, 2009. doi: 10.1139/H09-074. [DOI] [PubMed] [Google Scholar]
- 39.James PE, Lang D, Tufnell-Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res 94: 976–983, 2004. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 40.Joshi D, Shiwalkar A, Cross MR, Sharma SK, Vachhani A, Dutt C. Continuous, non-invasive measurement of the haemodynamic response to submaximal exercise in patients with diabetes mellitus: evidence of impaired cardiac reserve and peripheral vascular response. Heart 96: 36–41, 2010. doi: 10.1136/hrt.2009.177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol 583: 855–860, 2007. doi: 10.1113/jphysiol.2007.135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katayama K, Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Effect of arterial oxygenation on quadriceps fatigability during isolated muscle exercise. Am J Physiol Regul Integr Comp Physiol 292: R1279–R1286, 2007. doi: 10.1152/ajpregu.00554.2006. [DOI] [PubMed] [Google Scholar]
- 43.Keller DM, Fadel PJ, Ogoh S, Brothers RM, Hawkins M, Olivencia-Yurvati A, Raven PB. Carotid baroreflex control of leg vasculature in exercising and non-exercising skeletal muscle in humans. J Physiol 561: 283–293, 2004. doi: 10.1113/jphysiol.2004.071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiely C, O’Connor E, O’Shea D, Green S, Egaña M. Hemodynamic responses during graded and constant-load plantar flexion exercise in middle-aged men and women with Type 2 diabetes. J Appl Physiol (1985) 117: 755–764, 2014. doi: 10.1152/japplphysiol.00555.2014. [DOI] [PubMed] [Google Scholar]
- 45.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279: E1039–E1044, 2000. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 46.Kim YS, Seifert T, Brassard P, Rasmussen P, Vaag A, Nielsen HB, Secher NH, van Lieshout JJ. Impaired cerebral blood flow and oxygenation during exercise in Type 2 diabetic patients. Physiol Rep 3: e12430, 2015. doi: 10.14814/phy2.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Nitric oxide synthase inhibition reduces glucose uptake during exercise in individuals with Type 2 diabetes more than in control subjects. Diabetes 51: 2572–2580, 2002. doi: 10.2337/diabetes.51.8.2572. [DOI] [PubMed] [Google Scholar]
- 48.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003. doi: 10.2337/diacare.26.3.899. [DOI] [PubMed] [Google Scholar]
- 49.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK. Brain-derived neurotrophic factor (BDNF) and Type 2 diabetes. Diabetologia 50: 431–438, 2007. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 50.Lalande S, Gusso S, Hofman PL, Baldi JC. Reduced leg blood flow during submaximal exercise in Type 2 diabetes. Med Sci Sports Exerc 40: 612–617, 2008. doi: 10.1249/MSS.0b013e318161aa99. [DOI] [PubMed] [Google Scholar]
- 51.Lemaster K, Jackson D, Goldman D, Frisbee JC. Insidious incrementalism: the silent failure of the microcirculation with increasing peripheral vascular disease risk. Microcirculation 24: e12332, 2017. doi: 10.1111/micc.12332. [DOI] [PubMed] [Google Scholar]
- 52.Longhurst JC, Tjen-A-Looi SC, Fu LW. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann N Y Acad Sci 940: 74–95, 2001. doi: 10.1111/j.1749-6632.2001.tb03668.x. [DOI] [PubMed] [Google Scholar]
- 54.Lukin L, Ralston HJ. Oxygen deficit and repayment in exercise. Int Z Angew Physiol 19: 183–193, 1962. [DOI] [PubMed] [Google Scholar]
- 55.Mac Ananey O, Malone J, Warmington S, O’Shea D, Green S, Egaña M. Cardiac output is not related to the slowed O2 uptake kinetics in Type 2 diabetes. Med Sci Sports Exerc 43: 935–942, 2011. doi: 10.1249/MSS.0b013e3182061cdb. [DOI] [PubMed] [Google Scholar]
- 56.MacAnaney O, Reilly H, O’Shea D, Egaña M, Green S. Effect of Type 2 diabetes on the dynamic response characteristics of leg vascular conductance during exercise. Diab Vasc Dis Res 8: 12–21, 2011. doi: 10.1177/1479164110389625. [DOI] [PubMed] [Google Scholar]
- 57.MacDonald MJ, Shoemaker JK, Tschakovsky ME, Hughson RL. Alveolar oxygen uptake and femoral artery blood flow dynamics in upright and supine leg exercise in humans. J Appl Physiol (1985) 85: 1622–1628, 1998. doi: 10.1152/jappl.1998.85.5.1622. [DOI] [PubMed] [Google Scholar]
- 58.Mårin P, Andersson B, Krotkiewski M, Björntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17: 382–386, 1994. doi: 10.2337/diacare.17.5.382. [DOI] [PubMed] [Google Scholar]
- 59.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab 25: 89–98, 2014. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin IK, Katz A, Wahren J. Splanchnic and muscle metabolism during exercise in NIDDM patients. Am J Physiol 269: E583–E590, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Mason McClatchey P, Wu F, Olfert IM, Ellis CG, Goldman D, Reusch JE, Frisbee JC. Impaired tissue oxygenation in metabolic syndrome requires increased microvascular perfusion heterogeneity. J Cardiovasc Transl Res 10: 69–81, 2017. doi: 10.1007/s12265-017-9732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McClatchey PM, Frisbee JC, Reusch JEB. A conceptual framework for predicting and addressing the consequences of disease-related microvascular dysfunction. Microcirculation 24: e12359, 2017. doi: 10.1111/micc.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563: 903–913, 2005. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menon RK, Grace AA, Burgoyne W, Fonseca VA, James IM, Dandona P. Muscle blood flow in diabetes mellitus. Evidence of abnormality after exercise. Diabetes Care 15: 693–695, 1992. doi: 10.2337/diacare.15.5.693. [DOI] [PubMed] [Google Scholar]
- 65.Milsom AB, Jones CJ, Goodfellow J, Frenneaux MP, Peters JR, James PE. Abnormal metabolic fate of nitric oxide in Type I diabetes mellitus. Diabetologia 45: 1515–1522, 2002. doi: 10.1007/s00125-002-0956-9. [DOI] [PubMed] [Google Scholar]
- 66.Mohler ER III, Lech G, Supple GE, Wang H, Chance B. Impaired exercise-induced blood volume in Type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopy. Diabetes Care 29: 1856–1859, 2006. doi: 10.2337/dc06-0182. [DOI] [PubMed] [Google Scholar]
- 67.Moir S, Hanekom L, Fang ZY, Haluska B, Wong C, Burgess M, Marwick TH. Relationship between myocardial perfusion and dysfunction in diabetic cardiomyopathy: a study of quantitative contrast echocardiography and strain rate imaging. Heart 92: 1414–1419, 2006. doi: 10.1136/hrt.2005.079350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montero D, Walther G, Pérez-Martin A, Vicente-Salar N, Roche E, Vinet A. Vascular smooth muscle function in Type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetologia 56: 2122–2133, 2013. doi: 10.1007/s00125-013-2974-1. [DOI] [PubMed] [Google Scholar]
- 69.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 70.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care 30: 203–209, 2007. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 71.Murias JM, Spencer MD, Pogliaghi S, Paterson DH. Noninvasive estimation of microvascular O2 provision during exercise on-transients in healthy young males. Am J Physiol Regul Integr Comp Physiol 303: R815–R823, 2012. doi: 10.1152/ajpregu.00306.2012. [DOI] [PubMed] [Google Scholar]
- 72.O’Connor E, Green S, Kiely C, O’Shea D, Egaña M. Differential effects of age and Type 2 diabetes on dynamic vs. peak response of pulmonary oxygen uptake during exercise. J Appl Physiol (1985) 118: 1031–1039, 2015. doi: 10.1152/japplphysiol.01040.2014. [DOI] [PubMed] [Google Scholar]
- 73.O’Connor E, Kiely C, O’Shea D, Green S, Egaña M. Similar level of impairment in exercise performance and oxygen uptake kinetics in middle-aged men and women with Type 2 diabetes. Am J Physiol Regul Integr Comp Physiol 303: R70–R76, 2012. doi: 10.1152/ajpregu.00012.2012. [DOI] [PubMed] [Google Scholar]
- 74.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol 286: H940–H945, 2004. doi: 10.1152/ajpheart.00677.2003. [DOI] [PubMed] [Google Scholar]
- 75.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of Type II diabetes on muscle microvascular oxygen pressures. Respir Physiol Neurobiol 156: 187–195, 2007. doi: 10.1016/j.resp.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of Type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol Heart Circ Physiol 291: H2439–H2444, 2006. doi: 10.1152/ajpheart.00290.2006. [DOI] [PubMed] [Google Scholar]
- 77.Pak M, Moynes J, Poitras V, Tschakovsky ME. Is oxygen consumption and oxygen delivery during leg exercise compromised in Type II diabetes? Med Sci Sports Exerc 42: 242, 2010. doi: 10.1249/01.MSS.0000384301.18771.9d. [DOI] [Google Scholar]
- 78.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pinder AG, Pittaway E, Morris K, James PE. Nitrite directly vasodilates hypoxic vasculature via nitric oxide-dependent and -independent pathways. Br J Pharmacol 157: 1523–1530, 2009. doi: 10.1111/j.1476-5381.2009.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled Type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 24: 5–10, 2001. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]
- 81.Poitras VJ, Bentley RF, Hopkins-Rosseel DH, LaHaye SA, Tschakovsky ME. Independent effect of Type 2 diabetes beyond characteristic comorbidities and medications on immediate but not continued knee extensor exercise hyperemia. J Appl Physiol (1985) 119: 202–212, 2015. doi: 10.1152/japplphysiol.00758.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poitras V, Bentley RF, Hopkins-Roseel DH, Lahaye SA, Tschakovsky ME. No independent effect of Type 2 diabetes beyond characteristic co-morbidities and medications on exercising small muscle mass blood flow and exercise tolerance. Physiol Rep 3: e12487, 2015. doi: 10.14814/phy2.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prewitt RL, Chen II, Dowell R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am J Physiol 243: H243–H251, 1982. doi: 10.1152/ajpheart.1982.243.2.H243. [DOI] [PubMed] [Google Scholar]
- 84.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 5: 56, 2008. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90, 2003. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 86.Rabøl R, Boushel R, Dela F. Mitochondrial oxidative function and Type 2 diabetes. Appl Physiol Nutr Metab 31: 675–683, 2006. doi: 10.1139/h06-071. [DOI] [PubMed] [Google Scholar]
- 87.Regensteiner JG, Bauer TA, Reusch JE, Quaife RA, Chen MY, Smith SC, Miller TM, Groves BM, Wolfel EE. Cardiac dysfunction during exercise in uncomplicated Type 2 diabetes. Med Sci Sports Exerc 41: 977–984, 2009. doi: 10.1249/MSS.0b013e3181942051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc 27: 875–881, 1995. doi: 10.1249/00005768-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 89.Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelsong AM, Smith S, Wolfel EE, Eckel RH, Hiatt WR. Abnormal oxygen uptake kinetic responses in women with Type II diabetes mellitus. J Appl Physiol (1985) 85: 310–317, 1998. doi: 10.1152/jappl.1998.85.1.310. [DOI] [PubMed] [Google Scholar]
- 90.Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord 14: 77–86, 2013. doi: 10.1007/s11154-012-9234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and Type 2 diabetes. Diabetes 54: 8–14, 2005. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 92.Rosenmeier JB, Fritzlar SJ, Dinenno FA, Joyner MJ. Exogenous NO administration and α-adrenergic vasoconstriction in human limbs. J Appl Physiol (1985) 95: 2370–2374, 2003. doi: 10.1152/japplphysiol.00634.2003. [DOI] [PubMed] [Google Scholar]
- 93.Rosenmeier JB, Yegutkin GG, González-Alonso J. Activation of ATP/UTP-selective receptors increases blood flow and blunts sympathetic vasoconstriction in human skeletal muscle. J Physiol 586: 4993–5002, 2008. doi: 10.1113/jphysiol.2008.155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ross GA, Mihok ML, Murrant CL. Extracellular adenosine initiates rapid arteriolar vasodilation induced by a single skeletal muscle contraction in hamster cremaster muscle. Acta Physiol (Oxf) 208: 74–87, 2013. doi: 10.1111/apha.12060. [DOI] [PubMed] [Google Scholar]
- 95.Sanchez OA, Copenhaver EA, Chance MA, Fowler MJ, Towse TF, Kent-Braun JA, Damon BM. Postmaximal contraction blood volume responses are blunted in obese and Type 2 diabetic subjects in a muscle-specific manner. Am J Physiol Heart Circ Physiol 301: H418–H427, 2011. doi: 10.1152/ajpheart.00060.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med 348: 1483–1485, 2003. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 98.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with Type 2 diabetes. Circulation 107: 3040–3046, 2003. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- 99.Shapiro LM, Leatherdale BA, Mackinnon J, Fletcher RF. Left ventricular function in diabetes mellitus. II: Relation between clinical features and left ventricular function. Br Heart J 45: 129–132, 1981. doi: 10.1136/hrt.45.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med 37: 197–206, 2003. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and Type 2 diabetes. Diabetes Care 27: 2518–2539, 2004. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 102.Slade JM, Towse TF, Gossain VV, Meyer RA. Peripheral microvascular response to muscle contraction is unaltered by early diabetes but decreases with age. J Appl Physiol (1985) 111: 1361–1371, 2011. doi: 10.1152/japplphysiol.00009.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281: C1158–C1164, 2001. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 104.Sprague RS, Stephenson AH, Bowles EA, Stumpf MS, Lonigro AJ. Reduced expression of G(i) in erythrocytes of humans with Type 2 diabetes is associated with impairment of both cAMP generation and ATP release. Diabetes 55: 3588–3593, 2006. doi: 10.2337/db06-0555. [DOI] [PubMed] [Google Scholar]
- 105.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab 18: 350–355, 2007. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 106.Spriet LL, Howlett RA, Heigenhauser GJ. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med Sci Sports Exerc 32: 756–763, 2000. doi: 10.1097/00005768-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 107.Stabler T, Kenjale A, Ham K, Jelesoff N, Allen J. Potential mechanisms for reduced delivery of nitric oxide to peripheral tissues in diabetes mellitus. Ann N Y Acad Sci 1203: 101–106, 2010. doi: 10.1111/j.1749-6632.2010.05599.x. [DOI] [PubMed] [Google Scholar]
- 108.Thaning P, Bune LT, Hellsten Y, Pilegaard H, Saltin B, Rosenmeier JB. Attenuated purinergic receptor function in patients with Type 2 diabetes. Diabetes 59: 182–189, 2010. doi: 10.2337/db09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thaning P, Bune LT, Zaar M, Saltin B, Rosenmeier JB. Functional sympatholysis during exercise in patients with Type 2 diabetes with intact response to acetylcholine. Diabetes Care 34: 1186–1191, 2011. doi: 10.2337/dc10-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas N, Alder E, Leese GP. Barriers to physical activity in patients with diabetes. Postgrad Med J 80: 287–291, 2004. doi: 10.1136/pgmj.2003.010553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thorstensson A, Karlsson J. Fatiguability and fibre composition of human skeletal muscle. Acta Physiol Scand 98: 318–322, 1976. doi: 10.1111/j.1748-1716.1976.tb10316.x. [DOI] [PubMed] [Google Scholar]
- 112.Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol (1985) 86: 1101–1113, 1999. doi: 10.1152/jappl.1999.86.4.1101. [DOI] [PubMed] [Google Scholar]
- 113.Tschakovsky ME, Pyke KE. Cardiovascular responses to exercise and limitations to human performance. In: Physiological Bases of Human Performance During Work and Exercise, edited by Taylor N, Groeller H. New York: Elsevier Health Sciences, 2008, p. 5–27. [Google Scholar]
- 114.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watt B, Grove R. Perceived exertion. Antecedents and applications. Sports Med 15: 225–241, 1993. doi: 10.2165/00007256-199315040-00002. [DOI] [PubMed] [Google Scholar]
- 116.Westerblad H, Allen DG. Cellular mechanisms of skeletal muscle fatigue. Adv Exp Med Biol 538: 563–571, 2003. doi: 10.1007/978-1-4419-9029-7_50. [DOI] [PubMed] [Google Scholar]
- 117.Wilkerson DP, Poole DC, Jones AM, Fulford J, Mawson DM, Ball CI, Shore AC. Older Type 2 diabetic males do not exhibit abnormal pulmonary oxygen uptake and muscle oxygen utilization dynamics during submaximal cycling exercise. Am J Physiol Regul Integr Comp Physiol 300: R685–R692, 2011. doi: 10.1152/ajpregu.00479.2010. [DOI] [PubMed] [Google Scholar]
- 118.Wilson W, Marsden CA, Maughan RJ. Factors affecting the rate and energetics of mitochondrial oxidative phosphorylation. Med Sci Sports Exerc 26: 37–43, 1994. doi: 10.1249/00005768-199405001-00213. [DOI] [PubMed] [Google Scholar]
- 119.Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with Type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol 53: 2175–2183, 2009. doi: 10.1016/j.jacc.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wright JR, McCloskey DI, Fitzpatrick RC. Effects of systemic arterial blood pressure on the contractile force of a human hand muscle. J Appl Physiol (1985) 88: 1390–1396, 2000. doi: 10.1152/jappl.2000.88.4.1390. [DOI] [PubMed] [Google Scholar]
- 121.Young JL, Pendergast DR, Steinbach J. Oxygen transport and peripheral microcirculation in long-term diabetes. Proc Soc Exp Biol Med 196: 61–68, 1991. doi: 10.3181/00379727-196-43164. [DOI] [PubMed] [Google Scholar]
- 122.Zhang L, Xiong XQ, Fan ZD, Gan XB, Gao XY, Zhu GQ. Involvement of enhanced cardiac sympathetic afferent reflex in sympathetic activation in early stage of diabetes. J Appl Physiol (1985) 113: 47–55, 2012. doi: 10.1152/japplphysiol.01228.2011. [DOI] [PubMed] [Google Scholar]
- 123.Zhao G, Ford ES, Li C, Mokdad AH. Compliance with physical activity recommendations in US adults with diabetes. Diabet Med 25: 221–227, 2008. doi: 10.1111/j.1464-5491.2007.02332.x. [DOI] [PubMed] [Google Scholar]