Abstract
Blood flow through intrapulmonary arteriovenous anastomoses (QIPAVA) occurs in healthy humans at rest and during exercise when breathing hypoxic gas mixtures at sea level and may be a source of right-to-left shunt. However, at high altitudes, QIPAVA is reduced compared with sea level, as detected using transthoracic saline contrast echocardiography (TTSCE). It remains unknown whether the reduction in QIPAVA (i.e., lower bubble scores) at high altitude is due to a reduction in bubble stability resulting from the lower barometric pressure (PB) or represents an actual reduction in QIPAVA. To this end, QIPAVA, pulmonary artery systolic pressure (PASP), cardiac output (QT), and the alveolar-to-arterial oxygen difference (AaDO2) were assessed at rest and during exercise (70–190 W) in the field (5,260 m) and in the laboratory (1,668 m) during four conditions: normobaric normoxia (NN; = 121 mmHg, PB = 625 mmHg; n = 8), normobaric hypoxia (NH; = 76 mmHg, PB = 625 mmHg; n = 7), hypobaric normoxia (HN; = 121 mmHg, PB = 410 mmHg; n = 8), and hypobaric hypoxia (HH; = 75 mmHg, PB = 410 mmHg; n = 7). We hypothesized QIPAVA would be reduced during exercise in isooxic hypobaria compared with normobaria and that the AaDO2 would be reduced in isooxic hypobaria compared with normobaria. Bubble scores were greater in normobaric conditions, but the AaDO2 was similar in both isooxic hypobaria and normobaria. Total pulmonary resistance (PASP/QT) was elevated in HN and HH. Using mathematical modeling, we found no effect of hypobaria on bubble dissolution time within the pulmonary transit times under consideration (<5 s). Consequently, our data suggest an effect of hypobaria alone on pulmonary blood flow.
NEW & NOTEWORTHY Blood flow through intrapulmonary arteriovenous anastomoses, detected by transthoracic saline contrast echocardiography, was reduced during exercise in acute hypobaria compared with normobaria, independent of oxygen tension, whereas pulmonary gas exchange efficiency was unaffected. Modeling the effect(s) of reduced air density on contrast bubble lifetime did not result in a significantly reduced contrast stability. Interestingly, total pulmonary resistance was increased by hypobaria, independent of oxygen tension, suggesting that pulmonary blood flow may be changed by hypobaria.
Keywords: echocardiography, high altitude, hypobaria, intrapulmonary arteriovenous anastomoses, microbubble dynamics
INTRODUCTION
Transthoracic saline contrast echocardiography (TTSCE) is a noninvasive method for the detection of blood flow through intrapulmonary arteriovenous anastomoses (IPAVA). Blood flow through IPAVA (QIPAVA) is determined by the number and spatial distribution of contrast bubbles appearing in the left heart after a peripheral venous injection of agitated saline contrast (45). It has been demonstrated that breathing hypoxic gas mixtures acutely at sea level increases QIPAVA in healthy humans at rest, as detected by both TTSCE (12, 20, 40) and Technetium 99m (99mTc) radiolabeled macroaggregates of albumin (1, 9), a finding that can be attributed to a reduction in arterial Po2 rather than a reduction in arterial O2 content (8). It has also been demonstrated that QIPAVA increases in healthy humans during exercise (10, 12, 14, 46, 68) and during the intravenous infusion of catecholamines at rest breathing room air (13, 39).
In a high-altitude field study, Foster et al. (20) found a reduction in QIPAVA using TTSCE in sea level inhabitants after 3 wk of acclimatization to 5,050 m (i.e., hypobaric hypoxia). However, QIPAVA increased in all of the same subjects (n = 7) when the partial pressure of end-tidal oxygen was acutely reduced to 45 mmHg at sea level (i.e., normobaric hypoxia). The inconsistencies in TTSCE determined that QIPAVA (bubble scores) between hypobaric and normobaric hypoxia could be attributed to factors such as the effect of acclimatization on cardiac output (QT), pulmonary vascular remodeling, and/or changes in blood viscosity (3). Specifically, a decrease in QT may result in a decrease in QIPAVA (13), or changes in regional pulmonary perfusion may direct blood flow away from IPAVA (44). Alternatively, there may be a previously unrecognized effect of hypobaria on the physical properties of saline contrast microbubbles created from room air that may decrease their in vivo life span. Because the density of air decreases with increasing altitude, creating saline contrast microbubbles from reduced-density air may further decrease the stability of contrast microbubbles in vivo. Moreover, reduced atmospheric pressure may result in further undersaturation of dissolved gases in the blood, which may affect in vivo bubble dynamics (67). A reduction of the persistence of air microbubbles would result in a more rapid dissolution of bubbles, thereby impairing ultrasonic detection of QIPAVA using TTSCE.
It is well established that contrast microbubbles composed of air dissolve rapidly in blood due to the outward diffusion of gas and corresponding reduction in bubble radius (49, 58, 64). For example, the time to dissolution of an air microbubble with a radius of 2.5 µm is ∼0.18 s (51, 61, 64). Factors that may affect the stability of microbubbles in blood include the surface tension at the bubble/blood interfacial boundary, molecular weight of the gas, diffusivity of the gas, gas density, blood viscosity, and solubility (in blood) of the gas from which the bubble is composed (49, 58). In the current study, we also applied a three-region mathematical model of bubble dissolution (67), which considers some of the factors affecting bubble stability (mentioned above) as part of the analysis and interpretation of our results.
The physiological significance of QIPAVA remains controversial (44). Potential roles include pulmonary pressure regulation and pulmonary gas exchange efficiency. It has been suggested that QIPAVA may be a source of right-to-left shunt (13, 44). A study conducted by Elliott et al. (13) in which subjects breathed 40% O2, which presumably eliminated any ventilation-perfusion inequality and prevented any diffusion limitation, demonstrated a shunt of ∼2% of the cardiac output when QIPAVA was detected during the intravenous infusion of epinephrine. If QIPAVA is a source of shunt and hypobaria reduces QIPAVA, then under hypobaric normoxia, pulmonary gas exchange efficiency, determined by the alveolar-to-arterial Po2 difference (AaDO2), would be expected to improve compared with normobaric normoxia (when QIPAVA is increased). This would be true under normoxic conditions when a relatively small shunt fraction (i.e., 1–3%) has a large impact on the AaDO2. However, this would not be the case in hypoxic conditions when a relatively small shunt fraction (i.e., 1–3%) has a minimal impact on the AaDO2 (73, 74).
Accordingly, the goals of this study were to determine whether 1) QIPAVA is decreased during exercise with acute ascent to high altitude (field study), 2) QIPAVA during normoxic and hypoxic exercise is decreased in acute hypobaria (chamber study), 3) the AaDO2 is less in hypobaric normoxia compared with normobaric normoxia (provided that QIPAVA is reduced in hypobaria), and 4) the reduction in barometric pressure (PB) alters the in vivo stability of contrast microbubbles via mathematical modeling or alters cardiopulmonary hemodynamics to reduce QIPAVA. We hypothesized that QIPAVA would be decreased in hypobaria compared with normobaria, and consequently, pulmonary gas exchange efficiency would improve as indicated by a decreased AaDO2.
METHODS
The field study received ethics approval from the University of Oregon, University of Colorado-Denver, and the Department of Defense. The chamber study received approval from the University of Oregon and the University of Colorado Anschutz Medical Campus. All subjects provided verbal and written, informed consent before participation, and all studies were conducted in accordance with the Declaration of Helsinki. Some data from the field study, including AaDO2 have previously been published elsewhere (15).
In the field study (5,260 m; Mt. Chacaltaya, Bolivia), six healthy subjects (1 female) without patent foramen ovale (PFO) exercised at sea level (normobaric normoxia) and after acute ascent (3-h drive with subjects breathing oxygen) from Coroico, Bolivia (1,525 m), to 5,260 m (hypobaric hypoxia) as part of the AltitudeOmics study in 2012 (15, 69, 70). Subjects exercised ∼48 h after arrival in Bolivia and within ∼2 h of arrival to 5,260 m. Bubble score was assessed using TTSCE at rest and during exercise. In the chamber study (1,668 m; Aurora, CO), eight healthy subjects (3 female) without PFO exercised inside a hypobaric chamber in four conditions: normoxia site elevation (NN; = 120.6 mmHg, PB = 625 mmHg; n = 8), normobaric hypoxia (NH; = 76.3 mmHg, PB = 625 mmHg; n = 7), hypobaric normoxia (HN; = 120.6 mmHg, PB = 410 mmHg; n = 8), and hypobaric hypoxia (HH; = 75.4 mmHg, PB = 410 mmHg; n = 7). We measured pulmonary gas exchange efficiency (AaDO2) and bubble score using TTSCE at rest and during exercise in NN, NH, HN, and HH. We also applied mathematical modeling to compare time with bubble dissolution at sea level, 1,668 m, and 5,260 m.
Subject Recruitment
Field study.
A complete description of the inclusion/exclusion criteria for the field study can be found in the overview paper of the AltitudeOmics series (70). Subjects were recruited from the University of Oregon and local area (Eugene, OR; 130 m, PB = 749 mmHg), and data from n = 6 (1 female) are presented in our previous publication (15). Data at sea level were collected in the Cardiopulmonary and Respiratory Physiology Laboratory at the University of Oregon. Data after acute ascent to high altitude were collected at the Mt. Chacaltaya Research Station in Bolivia.
Chamber study.
Subjects(n = 8; 3 females) were recruited from the University of Colorado-Denver community and local area (Aurora, CO; 1,668 m, PB = 625 mmHg). Data were collected at the Altitude Research Center using a hypobaric chamber at the University of Colorado Anschutz Medical Campus.
Spirometry, lung volumes, and diffusion capacity
Field study.
As previously described (15), pulmonary function was determined using computerized spirometry (Ultima CardiO2; MedGraphics, St. Paul, MN) according to American Thoracic Society/European Respiratory Society (ATS/ERS) standards (52). Lung volumes and capacities were determined using whole body plethysmography (Elite Plethysmograph; MedGraphics) according to ATS/ERS standards (76). Lung diffusion capacity for carbon monoxide (DLCO) was determined by the single-breath breath-hold method according to ATS/ERS standards (36, 48), using the Jones and Meade (29) method for timing.
Chambers Study.
Pulmonary function and lung volumes and capacities were determined as described in the field study according to ATS/ERS standards. DLCO was determined using a real-time diffusion rapid response multigas analyzer (Ultima PFX; MCG Diagnostics, St. Paul, MN). As with the field study, all subjects with <90% predicted for all parameters of pulmonary function and DLCO were excluded from participation.
Echocardiographic Screening
Field study.
As previously described (15), all subjects underwent a comprehensive echocardiographic screening process (Philips Sonos 5500, Eindhoven, The Netherlands) by a registered diagnostic cardiac sonographer with >25 yr of experience (R. D. Goodman) to ensure that subjects were free of cardiac abnormalities or signs of heart disease. TTSCE was used to identify the presence of a PFO (45). Saline contrast was created by mixing 1 ml of ambient air with 3 ml of normal saline (0.9% NaCl; Hospira, Lake Forest, IL) by agitation between two 10-ml syringes via a three-way stopcock. The appearance of ≥1 microbubble(s) in the left heart in any frame during the 20 cardiac cycles following right heart opacification identified subjects as either having a PFO or the transpulmonary passage of saline contrast (21, 50, 51, 61). The distinction between these two sources of left heart contrast results from the timing of microbubble appearance in the left heart following right heart opacification. Specifically, a microbubble appearing within up to three cardiac cycles is consistent with PFO, whereas a microbubble appearing after more than three cardiac cycles is consistent with transpulmonary passage (5, 26, 38, 50, 51, 61, 72). Saline contrast injections were performed during normal breathing as well as after Valsalva maneuver release, which was intended to transiently elevate right atrial pressure and create conditions optimal for detection of PFO. Effective Valsalva maneuvers, following a 15-s strain phase, were confirmed by a transient leftward deviation of the interatrial septum upon release, and multiple injections were performed as necessary when results were equivocal. PFO was defined by the appearance of at least one microbubble in the left heart within up to three cardiac cycles post-right-heart opacification (5, 38). Individuals with PFO were excluded from this study.
Chamber study.
The same comprehensive echocardiographic and PFO screening process (GE Vivid e; Amersham, UK) was performed on all subjects by a registered diagnostic cardiac sonographer with >10 yr of experience (J. E. Futral). As with the field study, all subjects with cardiac abnormalities, signs of heart disease, or a PFO were excluded from participation.
Subject Instrumentation and Exercise Protocol
Field study.
As previously described (15), subjects (n = 6) were instrumented with a 20-G radial artery catheter (Arrow International, Reading, PA) under local anesthesia (2% lidocaine), and an 18- to 22-G intravenous catheter (ProtectIV Safety IV Catheter; Smiths Medical, Minneapolis, MN) was placed in the right or left antecubital vein. Core temperature was assessed via a core temperature pill (CorTemp HQ, Palmetto, FL) ingested ∼5 h before the start of exercise. Subjects rested on an upright stationary cycle ergometer (Velotron Elite, Seattle, WA) for 10 min before performing standardized workloads of 70, 100, 130, and 160 W for 3 min each, followed by 15 W/min increments until cadence could no longer be maintained at >50 rpm.
Chamber study.
Subjects were instrumented with a radial artery catheter and intravenously, as described in the field study. Core temperature was measured by esophageal temperature probe (Mon-a-therm; Mallinckrodt, St. Louis, MO). In hypoxic conditions, subjects (n = 7) rested on an upright stationary cycle ergometer (Velotron Elite) for 10 min before performing standardized workloads of 70, 100, 130, and 160 W for 3 min each or until cadence of >50 rpm could no longer be maintained. In normoxic conditions, subjects (n = 8) rested on an upright stationary cycle ergometer (Velotron Elite) for 10 min before performing standardized workloads of 100, 130, 160, and 190 W for 3 min each or until cadence of >50 rpm could no longer be maintained. In both hypoxic and normoxic conditions, only three subjects were able to complete the exercise bout at 160 and 190 W, respectively.
Hypobaric Chamber
For HH and HN, the hypobaric chamber was decompressed to 410 mmHg (equivalent to 5,260 m) at a rate of 457 m/min and maintained at this pressure throughout the HH and HN exercise bouts. The order of HH and HN was randomized for each subject. Exercise in HH was accomplished by subjects breathing ambient air at 410 mmHg (i.e., = 75.4 mmHg), and exercise in HN was accomplished by subjects breathing 33.6% O2 at 410 mmHg (i.e., = 120.6 mmHg). After completion of both hypobaric exercise bouts, the hypobaric chamber was recompressed to site elevation (1,609 m; 625 mmHg), where subjects were given a 1-h break before normobaric exercise. Exercise in the NH condition was accomplished by subjects breathing 13.2% O2 (i.e., = 76.3 mmHg). Hypobaric conditions were always first because of safety concerns with intravascular air bubbles, and therefore, hypobaria and normobaria were not randomized. Thus, any intravascular bubbles introduced under hypobaric conditions would be smaller upon returning to ambient pressure.
Bubble Scoring
Field and chamber studies.
Using a previously published scoring system (46), a score of 0–5 was assigned after each injection based on the greatest density and spatial distribution of bubbles in the left ventricle of a single frame (recorded at >30 frames/s) during the subsequent 20 cardiac cycles. Scores were assigned as follows; 0 = zero bubbles; 1 = 1–3 bubbles; 2 = 4 –12 bubbles; 3 = more than 12 bubbles appearing in a bolus; 4 = more than 12 bubbles heterogeneously distributed in the left ventricle; and 5 = more than 12 bubbles homogenously distributed in the left ventricle. Every injection was scored by a registered diagnostic cardiac sonographer with >25 (R. D. Goodman; field study) or >10 (J. E. Futral; chamber study) yr of experience. The reproducibility of this scoring system has been validated previously in our laboratory in three separate studies (16, 40, 54), showing 92–100% agreement between independent and blinded observers, including a cardiologist. Both the field and chamber study include female subjects; however, previous work has shown that QIPAVA during exercise does not differ between women and men (34).
Arterial Blood Gases, Body Temperature, and Blood Lactate
Chamber study.
At rest and at the end of each 3-min submaximal workload, a 3-ml radial artery blood sample was drawn anaerobically over 10–15 s into a heparinized syringe and rapidly analyzed in triplicate for arterial Po2 (), arterial Pco2 (), and arterial pH with a blood-gas analyzer calibrated daily with tonometered whole human blood (RAPIDLab 248; Siemens, Erlangen, Germany). Arterial blood gases were corrected for body temperature (7, 33, 65) based on readings from the esophageal temperature probe. Arterial O2 saturation () and hemoglobin (Hb) were measured with CO-oximetry (Radiometer OSM3, Copenhagen, Denmark). Hematocrit was analyzed in duplicate at rest and in single measurements for each workload using the microcapillary tube centrifugation method (M24 Centrifuge; LW Scientific, Lawrenceville, GA). Blood lactate was analyzed in duplicate using the Lactate Plus handheld meter and lactate test strips (Nova Biomedical, Waltham, MA).
Pulmonary Artery Systolic Pressure and Cardiac Output
Chamber study.
Pulmonary artery systolic pressure (PASP) was assessed from the peak velocity of the tricuspid regurgitation using saline contrast-enhanced Doppler ultrasound (GE Vivid e). This was applied to the modified Bernoulli equation (4v2 + PRA), where v is the tricuspid regurgitation velocity envelope and PRA is right atrial pressure (based on the collapsibility of the inferior vena cava), according to the guidelines of the American Society for Echocardiography (35, 63, 80). A small volume (<0.5 ml) of air agitated with 3 ml of sterile saline was injected and used to help delineate the tricuspid regurgitation velocity envelope. Cardiac output (QT) was calculated using echocardiography (GE Vivid e) by multiplying the left ventricular outflow tract velocity time integral (LVOT VTI) by the LVOT cross-sectional area (determined during the echocardiographic screening process) and heart rate. Total pulmonary resistance (TPR) was calculated as PASP/QT. TPR was corrected for hematocrit by using the method described by Hoffman (27).
Mathematical Modeling
The Epstein and Plesset (17) model and some of the other models discussed below are known as two-region models. In these models, the entire medium around the bubble is taken to be “unstirred” so that pure diffusion (without convection) is the sole means of dissolved solute transport in the medium. The medium was taken here to represent uniformly perfused tissue that allows absorption or release of gas by the blood at every point in the tissue (67a, 67b). More recently, a three-region model that can be conveniently used to estimate bubble lifetimes has been proposed by Solano-Altamirano and Goldman (67) and is described by Eq. 1:
| 1 |
where T(diss) is time to dissolution, R is the radius of the bubble, Ro is the initial radius of the bubble, D is the diffusion constant, Pe is the external pressure, f is the undersaturation factor determined by the blood gas tensions with respect to atmospheric pressure, γ is the surface tension of the blood, and d = BT/KH (Henry’s solubility constant for an ideal gas), where B is the universal gas constant, T is temperature, and KH is Henry’s constant, that is, ,where is the gas solubility in liquid phase (67). This model consists of an interfacial boundary, a diffusive region next to the bubble where diffusion is the sole solute transport mechanism, and a well-stirred region beyond the diffusion region, where mixing is assumed perfectly efficient, so by definition, there are no solute concentration gradients in this well-mixed region (67). Convection as a mechanism of transport is introduced indirectly and qualitatively by application of a three-region model (67) and the parameter λ. As λ approaches 1, diffusion shell thickness approaches zero, which results in convection becoming the dominant mechanism of transport, whereas when λ approaches infinity, diffusion becomes the only mechanism of transport (a 2-region model). Using Eq. 1, we calculated the time to bubble dissolution for sea level pressure (Eugene, OR), a hypobaric chamber site elevation at 1,668 m, and at 5,260 m, all for a range of initial bubble radii (5–25 µm). This calculation was performed to determine the effect of atmospheric pressure on the time to bubble dissolution.
Equation 1 can be simplified to Eq. 2 (see below) as follows. Take the limit in Eq. 1, neglect the surface tension (by setting ), set , recall that (where is the gas solubility in liquid phase), use the ideal gas equation of state, and set . We then recover the dissolving time of a bubble, as given by the original Epstein and Plesset (17) model (see Eq. 2 below and Ref. 41). However, for the sake of simplicity, we will use Eq. 2 to discuss several aspects of the gas bubble stability on the basis that the same conclusions may be drawn from the complete three-region model described by Eq. 1.
Statistics
All statistical calculations were made using GraphPad Prism statistical software (version 7.0), and significance was set to P < 0.05. Measured and calculated physiological variables and total pulmonary resistance were compared across equivalent conditions (e.g., NN vs. HN) using a two-way ANOVA with Sidak’s post hoc test for comparisons between exercise intensity. Mathematical model predictions were compared by two-way ANOVA with Tukey’s post hoc test for multiple comparisons. Bubble scores were compared across equivalent conditions using a Mann-Whitney t-test.
RESULTS
Field Study
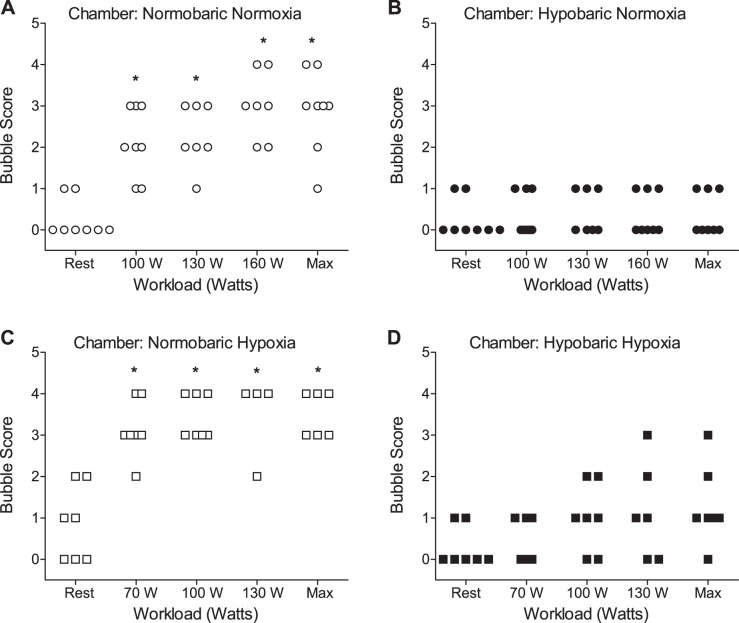
Anthropometric and pulmonary function data for field study subjects (n = 6; 1 female) are presented in Table 1. Field study bubble plots for rest and exercise in normobaric (sea level) and hypobaric (5,260 m) conditions are presented in Fig. 1, A and B. Bubble score achieved at each subject’s individual maximum workload during exercise (Max; Fig. 1, A and B) was significantly higher at sea level compared with the equivalent workload at 5,260 m.
Table 1.
Field study subject characteristics
| Characteristic | Value |
|---|---|
| Age, yr | 21.3 ± 1.5 |
| Height, cm | 180.8 ± 3.7 |
| Weight, kg | 76.1 ± 5.6 |
| FVC, liters | 5.91 ± 0.66 (104.3 ± 11.3) |
| SVC, liters | 6.13 ± 0.83 (108.5 ± 12.5) |
| FEV1, liters | 4.69 ± 0.45 (99.3 ± 4.5) |
| FEV1/FVC | 79.67 ± 5.99 (94.2 ± 7.3) |
| DLCO, ml·min−1·mmHg−1 | 44.61 ± 9.33 (129.3 ± 22.8) |
| DLCO/VA | 5.92 ± 0.86 (120.8 ± 20.7) |
Data are presented as means ± SD; n = 6 (1 female). FVC, forced vital capacity; SVC, slow vital capacity; FEV1, forced expiratory volume in 1 s; DLCO, diffusion capacity of carbon monoxide; DLCO/VA, DLCO/alveolar volume; (%predicted values).
Fig. 1.
Field study bubble scores as detected by transthoracic saline contrast echocardiography (TTSCE) in subjects performing cycle ergometer exercise at sea level (A) and within 48 h of arrival at 5,260 m (B). *P < 0.05 compared with 5,260 m. Max, highest workload [watts (W)] achieved within subject and corresponding bubble score. ○, Normobaric; ●, hypobaric.
Chamber Study
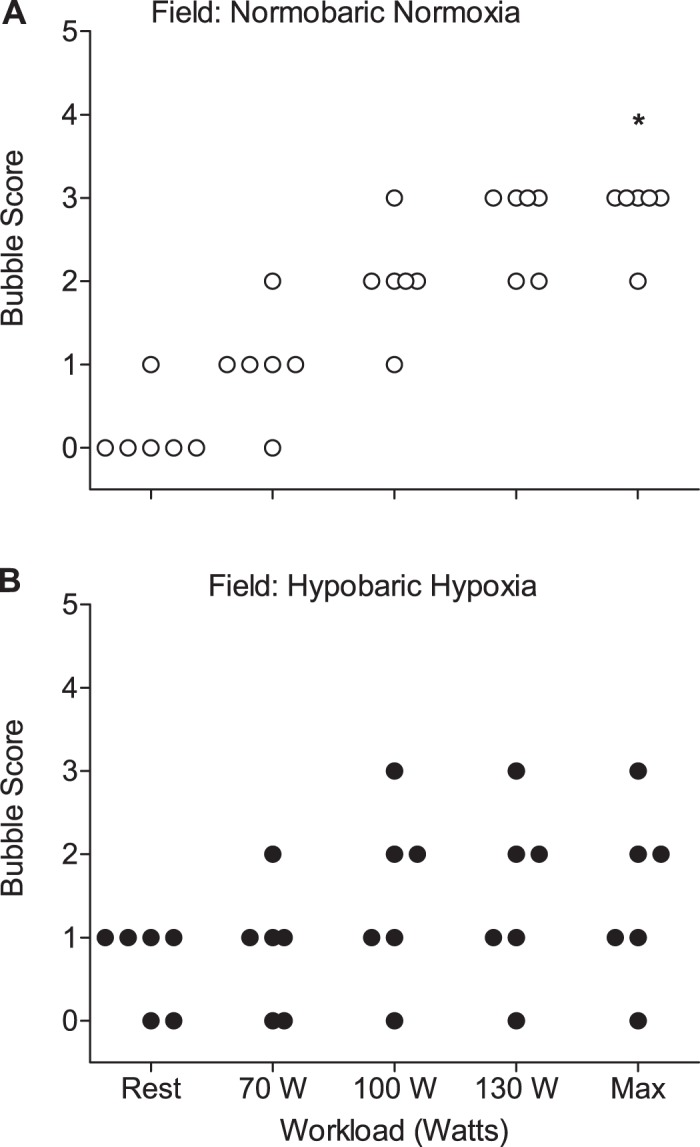
For the chamber study, baseline subject (n = 8; 3 females) anthropometric and pulmonary function data are presented in Table 2. Bubble scores are presented in Fig. 2, A–D. Bubble scores determined by TTSCE were significantly higher in NN and NH (Fig. 2, A and C) compared with HN and HH (Fig. 2, B and D), suggesting greater QIPAVA in normobaria. Cardiopulmonary and hematological data under normoxic and hypoxic conditions are presented in Tables 3 and 4, respectively. There were no significant differences in AaDO2 under comparable conditions, specifically NH vs. HH and NN vs. HN. Thus, contrast bubble detection was minimal in the HN condition, yet there was no significant difference in AaDO2 between HN and NN. A significant main effect of atmospheric pressure, i.e., normobaria vs. hypobaria (P < 0.05), was noted for total pulmonary resistance (TPR) in HN compared with NN, and this remained significant when correcting TPR for HCT. Alhough two-way ANOVA revealed no significant main effect of atmospheric pressure on ventilation (V̇e), metabolic carbon dioxide production (V̇co2), dead space to tidal volume (VD/VT), and cardiac output (QT), there were significant differences found in these parameters with respect to increases in exercise workload. Specifically, V̇e and V̇co2 were significantly higher (P < 0.05) in NH at 130 W compared with HH. This may have been caused by a difference in the number of subjects exercising at 130 W in NH (n = 5) compared with HH (n = 6). VD/VT was significantly higher (P < 0.05) in NN compared with HN at 100, 130, and 160 W. QT was significantly higher (P < 0.05) at rest and at 100 W in NN compared with HN and at 100 W in NH compared with HH.
Table 2.
Chamber study subject characteristics
| Characteristic | Value |
|---|---|
| Age, yr | 28.4 ± 9.2 |
| Height, cm | 171.3 ± 11.3 |
| Weight, kg | 68.4 ± 13.3 |
| FVC, liters | 4.56 ± 1.28 (4.73 ± 1.05) |
| SVC, liters | 4.78 ± 1.23 (4.71 ± 1.06) |
| FEV1, liters | 3.61 ± 0.80 (3.91 ± 0.78) |
| FEV1/FVC | 80.3 ± 7.3 (83.2 ± 3.4) |
| DLCO, ml·min−1·mmHg−1 | 33.3 ± 9.04 (28.75 ± 6.56) |
| DLCO/VA | 5.93 ± 0.68 (4.73 ± 0.22) |
Values are presented as means ± SD; n = 8 (3 females).
Fig. 2.
Chamber study bubble scores as detected by TTSCE in subjects performing cycle ergometer exercise in normobaric normoxia (NN; A), hypobaric normoxia (HN; B), normobaric hypoxia (NH; C), and hypobaric hypoxia (HH; D). Normoxic workloads are 100, 130, and 160 W. Hypoxic workloads are 70, 100, and 130 W. Max, highest workload achieved within subject and corresponding bubble score. *P < 0.05 for NN compared with HN and NH compared with HH. Circles are normoxia, squares are hypoxia, open symbols are normobaric, filled symbols are hypobaric.
Table 3.
Metabolic and hematologic data for normobaric normoxia and hypobaric normoxia
| Normobaric Normoxia |
Hypobaric Normoxia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Rest | 100 W | 130 W | 160 W | Rest | 100 W | 130 W | 160 W | |
| AaDO2, mmHg | 0.62 ± 2.16 | 11.4 ± 4.15 | 14.6 ± 5.65 | 17.8 ± 7.79 | 4.64 ± 5.13 | 13.4 ± 5.92 | 17.4 ± 8.80 | 19.2 ± 8.77 |
| V̇e, l/min | 15.6 ± 5.7 | 53.7 ± 6.6 | 68.9 ± 9.1 | 90.4 ± 19.9 | 11.5 ± 2.1 | 49.2 ± 8.8 | 65.3 ± 10.9 | 84.3 ± 8.9 |
| VA, l/min | 8.75 ± 4.9 | 39.2 ± 8.1 | 51.4 ± 11.3 | 66.4 ± 19.3 | 7.63 ± 2.1 | 42.1 ± 7.2 | 57.8 ± 8.6 | 75.8 ± 12.4 |
| VD/VT | 0.49 ± 0.10 | 0.31 ± 0.10† | 0.29 ± 0.11† | 0.30 ± 0.13† | 0.49 ± 0.05 | 0.22 ± 0.04 | 0.19 ± 0.05 | 0.17 ± 0.09 |
| V̇o2, l/min | 0.37 ± 0.11 | 1.70 ± 0.42 | 2.01 ± 0.45 | 2.33 ± 0.55 | 0.36 ± 0.08 | 1.70 ± 0.20 | 2.11 ± 0.17 | 2.47 ± 0.25 |
| V̇co2, l/min | 0.30 ± 0.10 | 1.46 ± 0.30 | 1.85 ± 0.40 | 2.22 ± 0.50 | 0.28 ± 0.08 | 1.53 ± 0.20 | 2.02 ± 0.21 | 2.40 ± 0.30 |
| RER | 0.80 ± 0.09 | 0.87 ± 0.11 | 0.93 ± 0.06 | 0.95 ± 0.09 | 0.77 ± 0.07 | 0.90 ± 0.06 | 0.96 ± 0.03 | 0.97 ± 0.03 |
| PAO2 | 81.2 ± 8.4 | 83.4 ± 4.8 | 86.3 ± 3.1 | 88.3 ± 5.8 | 79.7 ± 4.3 | 85.8 ± 5.0 | 87.6 ± 6.4 | 92.3 ± 6.0 |
| 80.6 ± 7.7 | 71.9 ± 6.5 | 71.7 ± 5.8 | 70.5 ± 7.5 | 75.0 ± 6.7 | 72.4 ± 6.7 | 70.2 ± 8.2 | 73.1 ± 5.8 | |
| 31.6 ± 5.1 | 32.2 ± 1.9 | 31.2 ± 2.5 | 29.7 ± 4.9 | 31.5 ± 2.6 | 31.8 ± 3.0 | 30.6 ± 4.0 | 27.9 ± 5.1 | |
| HR, beats/min | 89 ± 19 | 136 ± 24 | 149 ± 25 | 158 ± 21 | 69 ± 12 | 117 ± 17 | 143 ± 21 | 159 ± 16 |
| SV, ml | 67 ± 17 | 82 ± 11 | 90 ± 18 | 81 ± 16 | 65 ± 15 | 78 ± 13 | 88 ± 14 | 85 ± 17 |
| QT, l/min | 6.0 ± 1.8 | 11.1 ± 2.2† | 13.3 ± 2.9 | 12.8 ± 2.4 | 4.5 ± 1.0 | 9.1 ± 1.8 | 12.4 ± 2.0 | 13.2 ± 2.6 |
| PASP, mmHg | 26.0 ± 6.2 | 38.3 ± 5.8 | 44.0 ± 7.4 | 43.8 ± 6.6 | 29.3 ± 3.9 | 37.2 ± 5.0 | 41.9 ± 6.1 | 44.0 ± 7.3 |
| TPR, mmHg·l−1·min−1 | 4.76* | 3.55 | 3.42 | 3.47 | 6.73 | 4.21 | 3.44 | 3.39 |
| TPR (HCT), mmHg·l−1·min−1 | 5.60* | 3.73 | 3.80 | 3.20 | 7.08 | 4.21 | 3.40 | 3.18 |
| 95.7 ± 1.0 | 94.3 ± 1.5 | 94.3 ± 1.2 | 93.9 ± 1.7 | 94.9 ± 1.4 | 94.4 ± 1.5 | 93.7 ± 2.1 | 94.6 ± 2.3 | |
| , ml O2/dl | 18.1 ± 2.2 | 18.2 ± 2.2 | 18.5 ± 1.9 | 18.5 ± 2.3 | 18.0 ± 2.6 | 18.3 ± 2.3 | 17.8 ± 2.6 | 18.9 ± 2.3 |
| pH | 7.46 ± 0.06 | 7.43 ± 0.03 | 7.41 ± 0.04 | 7.39 ± 0.06 | 7.45 ± 0.02 | 7.41 ± 0.02 | 7.40 ± 0.02 | 7.36 ± 0.06 |
| HCT, % | 39.6 ± 6.7 | 43.1 ± 4.2 | 40.9 ± 7.1 | 42.8 ± 4.0 | 43.8 ± 3.6 | 44.9 ± 2.9 | 45.5 ± 3.2 | 46.0 ± 3.9 |
| tHb, g/dl | 13.4 ± 1.6 | 13.7 ± 1.5 | 14.1 ± 1.2 | 14.1 ± 1.7 | 13.4 ± 1.9 | 13.8 ± 1.7 | 13.9 ± 1.8 | 14.4 ± 1.7 |
| Core temp, °C | 37.1 ± 0.3 | 37.0 ± 0.6 | 37.1 ± 0.6 | 37.3 ± 0.7 | 37.0 ± 0.4 | 37.0 ± 0.4 | 37.1 ± 0.6 | 37.3 ± 0.6 |
| Lactate, mmol/l | 1.49 ± 0.62 | 3.16 ± 1.55 | 4.24 ± 2.37 | 6.76 ± 3.98 | 1.38 ± 0.82 | 3.53 ± 1.55 | 5.22 ± 2.25 | 7.18 ± 2.80 |
| n | 8 | 8 | 8 | 7 | 8 | 8 | 8 | 8 |
Values are means ± SD. VE, minute ventilation; VA, alveolar ventilation; VD/DT, dead space to tidal volume ratio; V̇o2, oxygen consumption; V̇co2, carbon dioxide production; RER, respiratory exchange ratio; PAO2, partial pressure of alveolar oxygen; , partial pressure of arterial oxygen; , arterial Pco2; HR, heart rate; SV, stroke volume; QT, cardiac output; PASP, pulmonary artery systolic pressure; TPR, total pulmonary resistance (calculated); TPR (HCT), total pulmonary resistance corrected for hematocrit; , oxygen saturation of arterial blood; , arterial oxygen content; pH, arterial pH; Hct, arterial hematocrit; tHb, total hemoglobin; Core temp, core body temperature; lactate, arterial lactate concentration.
P < 0.05 compared with hypobaric normoxia;
P < 0.05, rest vs. exercise.
Table 4.
Metabolic and hematological data for normobaric hypoxia vs. hypobaric hypoxia
| Normobaric Hypoxia |
Hypobaric Hypoxia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Rest | 70 W | 100 W | 130 W | Rest | 70 W | 100 W | 130 W | |
| AaDO2, mmHg | 2.28 ± 1.99 | 14.6 ± 4.03 | 16.9 ± 4.26 | 20.9 ± 4.44 | 1.75 ± 2.37 | 11.1 ± 3.42 | 15.0 ± 4.83 | 17.0 ± 5.48 |
| VE, l/min | 17.8 ± 3.8 | 51.5 ± 5.0 | 69.6 ± 6.4 | 101.3 ± 20.3† | 15.0 ± 3.5 | 46.3 ± 12.1 | 65.9 ± 21.3 | 82.1 ± 28.1 |
| VA, l/min | 11.5 ± 3.7 | 41.7 ± 3.4 | 57.7 ± 5.1 | 87.9 ± 17.7 | 9.64 ± 2.6 | 37.1 ± 9.5 | 55.0 ± 17.6 | 71.0 ± 24.0 |
| VD/VT | 0.41 ± 0.09 | 0.24 ± 0.07 | 0.23 ± 0.07 | 0.15 ± 0.05 | 0.38 ± 0.09 | 0.25 ± 0.06 | 0.23 ± 0.07 | 0.23 ± 0.08 |
| V̇o2, l/min | 0.38 ± 0.08 | 1.30 ± 0.17 | 1.62 ± 0.21 | 2.06 ± 0.36 | 0.37 ± 0.09 | 1.19 ± 0.26 | 1.53 ± 0.36 | 1.77 ± 0.44 |
| V̇co2, l/min | 0.36 ± 0.08 | 1.26 ± 0.12 | 1.66 ± 0.15 | 2.24 ± 0.27† | 0.30 ± 0.07 | 1.11 ± 0.26 | 1.51 ± 0.40 | 1.77 ± 0.49 |
| RER | 0.94 ± 0.06 | 0.98 ± 0.05 | 1.03 ± 0.08 | 1.10 ± 0.08 | 0.83 ± 0.08 | 0.93 ± 0.08 | 0.98 ± 0.10 | 0.99 ± 0.07 |
| PAO2 | 45.7 ± 4.7 | 49.6 ± 3.5 | 52.2 ± 3.3 | 55.8 ± 5.4 | 43.4 ± 2.4 | 47.8 ± 4.2 | 50.5 ± 5.6 | 52.7 ± 6.5 |
| 43.5 ± 5.9 | 35.0 ± 4.1 | 35.3 ± 3.8 | 35.0 ± 5.5 | 41.6 ± 1.6 | 36.6 ± 3.3 | 35.4 ± 3.5 | 35.7 ± 2.6 | |
| 27.4 ± 3.4 | 26.2 ± 2.7 | 25.0 ± 3.3 | 22.7 ± 5.1 | 27.5 ± 2.2 | 26.3 ± 2.8 | 24.6 ± 4.1 | 22.9 ± 5.5 | |
| HR, beats/min | 102 ± 23 | 143 ± 18 | 157 ± 18 | 160 ± 22 | 82 ± 21 | 128 ± 24 | 144 ± 26 | 156 ± 21 |
| SV, ml | 64 ± 11 | 82 ± 20 | 96 ± 17 | 86 ± 15 | 65 ± 11 | 88 ± 20 | 85 ± 17 | 92 ± 15 |
| QT, l/min | 6.4 ± 1.4 | 11.7 ± 2.0 | 14.9 ± 2.1† | 13.5 ± 2.0 | 5.2 ± 1.1 | 11.3 ± 3.3 | 12.3 ± 3.2 | 13.6 ± 2.5 |
| PASP, mmHg | 31.7 ± 7.2 | 44.2 ± 10.7 | 49.8 ± 9.0 | 50.0 ± 11.5 | 31.2 ± 6.7 | 39.6 ± 7.6 | 44.5 ± 7.1 | 42.8 ± 8.5 |
| TPR, mmHg·l−1·min−1 | 5.08 | 3.90 | 3.35 | 3.71 | 6.22 | 3.77 | 3.88 | 3.19 |
| TPR (HCT), mmHg·l−1·min−1 | 5.64 | 4.34 | 3.53 | 3.26 | 6.91 | 4.19 | 4.08 | 3.36 |
| 77.7 ± 7.2 | 65.7 ± 7.5 | 66.3 ± 6.9 | 65.6 ± 8.2 | 76.3 ± 2.1 | 68.6 ± 5.4 | 66.6 ± 5.8 | 67.3 ± 3.9 | |
| , ml O2/dl | 15.0 ± 2.1 | 13.3 ± 1.1 | 13.5 ± 1.4 | 13.6 ± 1.4 | 14.9 ± 2.5 | 13.5 ± 1.6 | 12.5 ± 1.0 | 13.1 ± 1.1 |
| pH | 7.50 ± 0.05 | 7.49 ± 0.03 | 7.47 ± 0.04 | 7.43 ± 0.06 | 7.50 ± 0.03 | 7.48 ± 0.03 | 7.46 ± 0.04 | 7.42 ± 0.07 |
| HCT, % | 41.1 ± 4.8 | 40.6 ± 5.3 | 42.1 ± 3.9 | 44.4 ± 2.9 | 42.3 ± 4.2 | 42.1 ± 6.2 | 44.3 ± 4.6 | 43.3 ± 6.2 |
| tHb, g/dl | 13.2 ± 1.5 | 13.4 ± 1.6 | 13.9 ± 1.5 | 14.3 ± 1.1 | 13.2 ± 2.0 | 13.3 ± 1.5 | 12.9 ± 1.6 | 13.6 ± 1.6 |
| Core temp, °C | 37.1 ± 0.4 | 36.9 ± 0.8 | 36.9 ± 0.9 | 36.7 ± 1.1 | 37.2 ± 0.3 | 36.9 ± 0.6 | 36.9 ± 0.7 | 36.9 ± 0.8 |
| Lactate, mmol/l | 1.84 ± 0.69 | 3.34 ± 0.78 | 5.23 ± 1.26 | 7.88 ± 2.26 | 1.74 ± 0.88 | 3.59 ± 1.53 | 6.11 ± 3.08 | 8.48 ± 4.81 |
| n | 7 | 7 | 7 | 5 | 7 | 7 | 7 | 6 |
Values are means ± SD.
P < 0.05 rest vs. exercise.
Mathematical Modeling
The results of the bubble model calculations for the bubble dissolution time at sea level, Aurora, CO (hypobaric chamber elevation, 1,668 m), and at 5,260 m are presented in Fig. 3 and Table 5). Although there is a significant difference (P < 0.05) on the bubble lifetimes predicted by the model for sea level vs 5,260 m, this was not the case for 1,668 m compared with 5,260 m (P > 0.05). The effect of PB is small for bubbles with an initial radius of less than ∼10 µm. The model also indicates relatively small changes in radius per unit of time compared with pulmonary transit time, suggesting that the effect of PB on bubble lifetimes cannot explain the decrease or absence of contrast appearing in the left heart at any altitude within the pulmonary transit time.
Fig. 3.
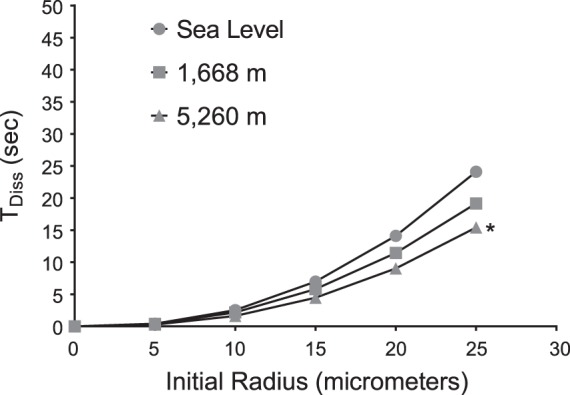
Mathematical modeling of microbubble dissolution time [time to dissolution (TDiss) vs. initial bubble radius] for the following atmospheric pressures: sea level (Eugene, OR), 1,668 m (Aurora, CO), and 5,260 m (Mt. Chacaltaya, Bolivia). Calculated using the Solano-Altamirano and Goldman (67) equation (Eq. 1). Initial radii (Ro) range from 5 to 25 µm; diffusion constant, D = 2,900 (μm2/s); undersaturation factor (f) = 0.917, 0.879, and 0.873 for sea level, 1,668 m, and 5,260 m, respectively; surface tension for venous blood (γ) = 0.5 atm μm. *P < 0.05 for 5,260 m compared with sea level.
Table 5.
Table of pressures used in mathematical modeling calculations (in mmHg)
| Altitude | PB | PASP | Pe | PvN2 | PvH2O | Pven | f | λ | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sea Level | 750 | 20 | 770 | 573* | 40* | 46* | 47* | 706 | 0.917 | 2 |
| 1,668 m | 625 | 26 | 651 | 447 | 31 | 37 | 47* | 572 | 0.879 | 2 |
| 5,260 m | 410 | 32 | 442 | 278 | 27** | 34** | 47* | 386 | 0.873 | 2 |
PB, barometric pressure; PASP, pulmonary artery systolic pressure; Pe, external pressure surrounding bubbles; PvN2, mixed venous partial pressure of nitrogen; , mixed venous partial pressure of oxygen; , mixed venous partial pressure of carbon dioxide; PvH2O, mixed venous partial pressure of water vapor; PVen, total pressure of dissolved mixed venous gases; f, undersaturation ratio, calculated as the ratio of the sum of mixed venous partial pressures gases to barometric pressure. Above values reflect resting conditions. Surface tension of venous blood: 0.5 atm µm.
George et al. (21a).
Wagner et al. (73).
DISCUSSION
The purpose of this study was to investigate the possible reason for the reduction in QIPAVA in hypobaria that was observed previously (2, 20). In the field study, we attempted to explore the effect of acclimatization on QIPAVA, but to our surprise, bubble scores were lower on acute ascent to high altitude compared with sea level. This raised the possibility that hypobaria influenced in vivo bubble dynamics such that TTSCE was less sensitive at high altitude. Consequently, we focused on carefully controlling PB and the partial pressure of inspired oxygen () in the chamber study to determine whether hypobaria per se impacts QIPAVA detection using TTSCE by determining whether QIPAVA decreases at rest and during exercise in hypobaria. This was done to ascertain whether the observed decrease seen in our other studies was, in fact, a limitation of TTSCE at high altitude. In both studies, we observed a decrease in bubble score in hypobaria regardless of exercise workload or the . However, the AaDO2 was not different in normobaric normoxia compared with hypobaric normoxia or in normobaric hypoxia compared with hypobaric hypoxia. We also found that hypobaria, independent of oxygen tension, resulted in an increase in TPR. Although there appears to be a destabilizing effect of decreasing PB on bubble stability, this effect does not appear to explain the decrease in bubble score we observed within the pulmonary transit times of interest.
Bubble Score and Pulmonary Gas Exchange Efficiency
Field study.
In the field study, bubble score (presumably indicative of QIPAVA) at the highest exercise workload achieved at sea level within a given subject was significantly higher compared with bubble scores at the same workload at 5,260 m. The decrease in bubble score, observed at 5,260 m, supports data from Foster et al. (20) and Bates et al. (2). However in both the field and chamber studies, the reduction in bubble score occurred acutely, whereas the bubble scores obtained by Foster et al. (20) and Bates et al. (2) were recorded after 3 wk of acclimatization to 5,050 m. This suggests an unknown effect of hypobaria on left heart bubble contrast, which seems to occur with or without acclimatization to extreme altitude.
Chamber study.
In the chamber study, bubble scores were significantly higher in normobaric normoxia compared with hypobaric normoxia at all workloads. Similarly, bubble scores in normobaric hypoxia were significantly higher than in hypobaric hypoxia at all workloads (Fig. 2, C and D). The highest bubble score achieved at the highest workload attained for an individual subject is represented as Max on the horizontal axis of Fig. 2. Although bubble scores were higher in normobaria compared with hypobaria, the AaDO2 was not significantly different in normobaric normoxia compared with hypobaric normoxia or in normobaric hypoxia compared with hypobaric hypoxia.
One interpretation for the observation of a similar AaDO2 in equivalent conditions is that there was no significant difference in QIPAVA in comparable conditions despite the bubble score data suggesting otherwise. This would suggest that the absolute volume of QIPAVA corresponding to a given bubble score in normobaria is not equivalent to the same bubble score in hypobaria. Thus, the hypobaria-related reduction in bubble score may be a consequence of the reduced PB and does not correspond to a reduction in QIPAVA.
Alternatively, despite a similar AaDO2 in equivalent conditions and reduced bubble scores in hypobaria, it could be that QIPAVA does not contribute significantly to AaDO2 at high altitude, as has been suggested by previous studies using the multiple inert gas elimination technique (MIGET) (73, 74). It should be noted, however, that no MIGET studies have been performed under hypobaric normoxic conditions, only normobaric hyperoxic conditions in subjects breathing 100% O2. With respect to the issue of oxygenation, the degree of hyperoxia is an important consideration when measuring QIPAVA. We have previously shown that breathing 100% O2 reduces QIPAVA, as detected by TTSCE (44), and this is supported by animal studies using conditions that enhance QIPAVA-detected microspheres (2, 53). In the current study, we used only mild hyperoxia to keep alveolar Po2 values in hypobaric conditions (17,200 ft) similar to those at Denver altitude (5,280 ft). Accordingly, the Wagner et al. (73, 74) study used 100% O2, which may have reduced QIPAVA under both SL and altitude conditions, although we did not expect to see any differences in AaDO2 in the altitude condition because the effect of shunt is reduced at altitude.
Nevertheless, previous work by our group, albeit in different experimental conditions (subjects at rest, breathing room air at sea level during the intravenous infusion of epinephrine), showed that total venous admixture was ∼2% of the QT with a correspondingly significant increase in bubble scores (13). Furthermore, when hyperoxic gas is breathed, thereby eliminating ventilation-perfusion inequality and diffusion limitation, venous admixture was ∼1% of the QT, which can only be from right-to-left shunt via bronchial and Thebesian blood flow, and QIPAVA. Importantly, this 1–2% right-to-left shunt (from all sources) is sufficient to explain the entire AaDO2 that normally occurs in healthy humans during exercise (7). Although work by Tedjasaputra et al. (71) suggests that QIPAVA may not contribute to the AaDO2 during exercise based on their TTSCE findings, bubbles scores, which constitute nonparametric data, were reported as a mean value and analyzed using parametric statistics. Furthermore, in that study the authors concluded that the differences in AaDO2 were due to ventilation-perfusion inequality, but without having their subjects breathe hyperoxic gas, this cannot be definitively concluded.
A final possibility exists. We detected a significant increase in total pulmonary resistance with hypobaric conditions (detailed in a subsequent section). These data suggest significant differences in cardiopulmonary hemodynamics, which would have likely impacted ventilation-perfusion heterogeneity and/or diffusion limitation. Thus, if hypobaria alone results in a reduction of QIPAVA but there is a concomitant increase in ventilation-perfusion and diffusion limitation, then the net effect of reducing shunt but increasing ventilation-perfusion heterogeneity and diffusion limitation would be an AaDO2 that would not change. More work in this area is required using appropriate nonparametric statistical tests and experimental designs that eliminate contributions from ventilation-perfusion inequality and diffusion limitation.
Bubble Persistence
Based on bubble persistence calculations with respect to the time from injection in the antecubital vein to detection in the right heart, contrast bubbles are presumed to have a diameter of >10 µm (28, 30). The diameter of pulmonary capillaries ranges from 7 to 10 µm (22, 62), and therefore, bubbles with diameters >10 µm will become trapped in the pulmonary microcirculation and will not be detected in the left heart (4, 51). The smaller the initial bubble radius, the shorter the expected lifetime of the bubble and the weaker the contrast effect becomes due to decrease in ultrasound scattering ability (49). Filtering of contrast bubbles by the lung can be modeled by removing 10% of bubbles with radii between 3 and 4 µm, 50% of bubbles with radii between 6 and 7 µm, and 90% with radii >10 µm (41). We detected contrast bubbles in the right heart within 1–3 s of injection under all conditions, followed by detection of contrast in the left heart with increasing cardiac output under both normobaric and hypobaric conditions. However, we detected significantly less left heart contrast in hypobaria compared with normobaria. In both cases, this supports the idea that contrast bubbles are passing through large diameter IPAVA, which are known to exist in healthy human lungs (44).
Our hypothesis for the lower bubble scores in hypobaria was based on the fact that, at altitude, air is less dense than it is at lower altitudes. Consequently, contrast bubbles created by the mixing of ambient air in saline may dissolve more rapidly at high altitude (due to the lower gas density) than those created at a higher atmospheric pressure. Equation 2 qualitatively illustrates that the stability of a bubble is augmented by gases of higher density, larger initial bubble radius, and low diffusivity (41):
| 2 |
where TD is time for complete dissolution of the bubble, Ro is initial bubble radius, ρ is gas density, Cs is solubility in the liquid phase, and D is diffusivity (41). Examination of Eq. 2 indicates that a reduction in either Ro or ρ accelerates the rate of bubble dissolution. It was not possible to control or estimate Ro of the microbubbles created by agitation of saline; however, in accordance with Eq. 2, the smaller the microbubble, the more rapidly it would be expected to dissolve. Furthermore, the smaller the Ro, the greater the Laplace pressure and the more rapidly dissolution occurs. According to Eq. 2, reduction in ρ will also reduce the time to dissolution of the bubble. The air density at our simulated altitude of 5,260 m was considerably less than the air density in Aurora, CO (1,668 m). Ambient air densities were calculated using Eq. 3 by rearrangement of the ideal gas law and solving for gas density:
| ) | 3 |
where ρ is gas density, P is pressure, MW is molecular weight, T is temperature, and B is the universal gas constant. We calculated a local air density of 9.9 × 10−1 g/l based on an atmospheric pressure of 625 mmHg (Aurora, CO) and a temperature of 20°C (room temperature at the study location) as compared with a density of 6.44 × 10−1 g/l based on an atmospheric pressure of 410 mmHg and temperature of 18°C, which was the temperature inside the hypobaric chamber. Thus, Eq. 2 predicts a shorter bubble lifetime based on the reduced air density at 410 mmHg. Although our mathematical modeling (Fig. 3) predicts a decrease in bubble stability (shorter time to dissolution) of air bubbles in blood with decreasing PB, the model does not explain the disappearance of contrast bubbles within the time interval under consideration (<5 s). Based on the model prediction, contrast bubbles of an initial radius of >10 µm would persist long enough at all three atmospheric pressures to be detected by TTSCE. Presumably, contrast bubbles with an initial radii of >10 µm would not pass through pulmonary capillaries but would either be filtered by the lungs or pass through IPAVA and be detected in the left heart (4, 26). However, we consistently observed lower bubble scores in hypobaria compared with equivalent normobaric conditions. The saline contrast was created by agitation of 3 ml of normal saline with 1 ml of air under all conditions of this study. Given the reduction in air density at high altitude, the same 1 ml of air drawn into the syringe would contain proportionately fewer moles of air molecules than the same volume of air at site elevation. As a result, fewer molecules of air were present in the saline contrast microbubbles at high altitude, potentially reducing microbubble lifetime. For the same reason (fewer gas molecules within the microbubbles at reduced air density), it may be possible that Ro may be smaller for microbubbles created at high altitude and, in accord with Eq. 2, would cause them to dissolve faster than at site elevation. This possibility is currently a subject of investigation in our laboratory. For further discussion of the physical properties of bubbles in vivo, the reader is referred to the appendix.
Mathematical Modeling
An early mathematical model that provides the rate of dissolution (or growth) of gas bubbles in pure water was derived by Epstein and Plesset (17). In this model, dissolved solute diffusion along a concentration gradient in the medium surrounding the bubble is the only mechanism for solute transport to/from the bubble. This model can be used to predict the rate of dissolution of a spherical air bubble in an unstirred liquid (19, 55). Strictly speaking, the Epstein- Plesset equation is appropriate only for microbubbles comprised of a single gas (31, 32). Furthermore, it ignores the motion of the air bubble relative to the liquid medium in which it is suspended. Therefore, when considering air microbubbles, the Epstein-Plesset equation cannot be strictly applied (to predict bubble dissolution times), since nitrogen and oxygen have different partition coefficients and diffusivities (31, 32). Several other mathematical models for predicting bubble dissolution have been developed. For example, Kabalnov and colleagues (31, 32) suggested an extension to the Epstein-Plesset model for multicomponent gas bubbles. The Rayleigh-Plesset equation and its extension modeling the non-Newtonian behavior of blood can be used to describe spherically symmetric bubbles in blood flow subjected to ultrasound oscillations (19, 47). In modeling conditions involving rapid changes in blood pressure, the Rayleigh-Plesset equation (56) describing the pressure field around the bubble can be applied to the Epstein-Plesset solution (59). As previously mentioned, the models discussed above are two-region bubble models that were defined in methods. In the current study, we applied the three-region mathematical model of bubble dissolution proposed by Solano-Altamirano and Goldman (67).
The results of our mathematical modeling indicate a small effect of atmospheric pressure on bubble lifetimes for bubbles with initial radii less than ∼10 µm. According to the mathematical model, for bubbles of this size, the dissolution time is less than ∼5 s. For bubbles of larger initial radii, at 1,668 m for example, a bubble with an initial radius of 25 µm would decrease to a radius of ∼22 µm in 5 s, whereas that same bubble would decrease to ∼20 µm in 5 s at 5,260 m (Fig. 3). Hence, in the span of time expected for pulmonary transit to occur (∼5 s), there would be a very small change in the size of a bubble whose initial radius is ∼25 µm. The effect of atmospheric pressure becomes even less significant for bubbles of smaller initial radii, where progressively higher Laplace pressure (as bubble radius decreases) becomes the dominant driving force in bubble dissolution. Therefore, we conclude that the effect of atmospheric pressure on in vivo bubble lifetimes is insignificant for the purposes of explaining the results of this study.
Factors Affecting Contrast Bubbles in Hypobaria In Vivo
Field study.
Previous work by Foster et al. (20) examining QIPAVA at sea level and at 5,050 m after 3 wk of acclimatization demonstrated a reduction in hypoxia-induced bubble score at high altitude, as assessed by TTSCE. In that study, the reduction in bubble score could have been attributed to the effects of acclimatization to high altitude and the associated decrease in AaDO2 that comes with acclimatization. Additionally, there may also have been changes to the pulmonary vasculature of the study subjects that could have occurred over the 3-wk period. However, in our field study, the subjects exercised within a few hours of arrival at 5,260 m, so the lower bubble scores were not caused by physiological changes associated with acclimatization.
Chamber study.
In the chamber study, we simulated the conditions of the field study (same exercise workloads and PB); however we added the hypobaric normoxic and normobaric hypoxic conditions to isolate the effect of hypobaria on QIPAVA. We used the same subjects in a repeated-measures design. All subjects were acutely exposed to all four conditions (normobaric normoxia, hypobaric normoxia, normobaric hypoxia, and hypobaric hypoxia), so there was no chance of any changes associated with acclimatization to altitude, i.e., increased hematocrit, pulmonary vascular remodeling, or improved pulmonary gas exchange efficiency. Importantly, there was complete right heart opacification following each bubble injection performed at 410 mmHg, which suggests that enough bubbles were initially present in the right heart. Still, we observed lower bubble scores in hypobaric conditions despite a similar AaDO2 in equivalent conditions, i.e., normobaric hypoxia vs. hypobaric hypoxia and normobaric normoxia vs. hypobaric normoxia. We designed the study such that the level of hypoxia was equivalent (normobaric hypoxia and hypobaric hypoxia), and the normobaric hypoxic condition was equivalent to breathing ambient air at the study site altitude (Aurora, CO). The only difference in the normobaric and hypobaric condition was the decreased air density. All experimental conditions were matched for . Therefore, if QIPAVA occurs in normobaric normoxia, it could be expected to occur in hypobaric normoxia (and similarly in hypobaric hypoxia compared with normobaric hypoxia) as well, unless hypobaria alone causes a reduction in QIPAVA or contrast bubble lifetime is decreased in hypobaria. The mathematical model predicting in vivo bubble dissolution times in hypobaria does not support the latter.
Blood viscosity has also been suggested to play a role in the stability of saline contrast microbubbles (2). The viscosity of blood depends on both the plasma viscosity and hematocrit (77). An increase in hematocrit implies an increase in blood viscosity; however, the relationship between blood viscosity and hematocrit is complex, and several mathematical equations for this relationship exist (77). Hematocrit is not the only factor affecting viscosity of whole blood, but it is a major contributor (27). Other factors may affect whole blood viscosity, such as blood vessel size and shear rate. For example, although blood is a non-Newtonian shear-thinning fluid, it may approximate Newtonian behavior in medium and large arteries at high shear rates, causing viscosity to become essentially constant (77). Examination of Eq. 2 indicates that bubble persistence also depends on the diffusivity, D, of a gas. The diffusivity of a gas in a liquid depends on the viscosity of the liquid (57); that is, increasing the viscosity of a liquid increases bubble stability (2, 46). However, in our study, there were no significant differences in hematocrit in our subjects between conditions. This is to be expected since all exposures to altitude were acute, limiting any chance of hematological acclimatization.
Given that the decrease in left heart contrast observed in hypobaric conditions cannot adequately be explained by a hypobaric effect on in vivo bubble lifetime (as predicted by the mathematical model), we suggest that hypobaria alone may cause a redistribution of pulmonary blood flow such that blood flow is directed away from areas of the lung with IPAVA, and therefore, QIPAVA decreases in hypobaria. It is believed that the maximal QIPAVA ranges from 2 to 5% of the cardiac output (44). Although it is unlikely that redistribution of 2–5% of the cardiac output would have a significant impact on pulmonary arterial pressure, there is an association of QIPAVA with pulmonary pressure during exercise in young and older adults (54). Specifically, high QIPAVA is associated with low pulmonary pressure, whereas low QIPAVA is associated with higher pulmonary artery pressures. Thus, the reduction in QIPAVA detected with TTSCE may be an epiphenomenon associated with redirection of blood flow away from areas of the lung that contain IPAVA. Accordingly, a reduction in QIPAVA in hypobaric conditions may suggest a redistribution of blood flow away from areas of the lung that contain IPAVA and toward areas of the lung that may contain either fewer pulmonary vessel or smaller vessels. If true, this would result in a higher pulmonary pressure for a given pulmonary blood flow. Support for this idea of a higher pulmonary pressure under hypobaric conditions under resting conditions comes from several studies. For example, it has been demonstrated previously that acute hyperoxia (100% O2) did not result in a lower pulmonary vascular resistance either at rest or during exercise after several days of acclimatization to high altitude in the Operation Everest II study (23). The authors concluded that there may have potentially been some pulmonary vascular remodeling that occurred over many days at altitude. However, the idea that acute reductions in PB may play a role in the regulation of pulmonary vascular response and fluid balance is also supported by previous studies in sheep (25, 42) in which hypobaric hypoxia, but not equivalent normobaric hypoxia, caused a significant increase in pulmonary fluid filtration and lymph flow. Moreover, Levine et al. (42) found a significant rise in pulmonary artery pressure in hypobaric normoxia (at a simulated altitude of 6,600 m) accompanied by a rise in pulmonary vascular resistance and a decrease in QT, suggesting a hypobaria-induced vasoconstriction. Similarly, in humans using direct measures of pulmonary pressures at rest and during exercise in normobaric and hypobaric normoxia (33% O2 at 484 mmHg), Eldridge et al. (11) reported a greater PVR under resting conditions in hypobaric normoxia, ∼1.4 vs. ∼2.2 Wood units, in their control subjects. Additionally, they report that during the highest exercise intensity, PVR under normobaric normoxia decreased to ∼0.63 Wood units and to ∼0.94 wood units in hypobaric normoxia. These data suggest that despite having greater PVR under resting conditions in hypobaric normoxia, PVR decreased to levels comparable with that under normobaric normoxia during exercise. However, PVR was slightly greater during exercise under hypobaric normoxia compared with normobaric normoxia. Similar to this, work by Groves et al. (23) found that the PVR during exercise also decreased but remained higher during hyperoxic hypobaric exercise compared with SL exercise, ∼1.5 vs. ∼0.5 Wood units. One possible explanation for the discrepancy between these two studies with direct measures of pulmonary pressures would be the use of 100 (23) vs. 33% O2 (11). Our work is similar to that by Eldridge et al. (11) when using similar O2 levels but different workloads and subjects known to be free of PFO. The reason for the reduction in PVR during exercise in hypobaric normoxic conditions may be explained by the work of Koizumi et al. (37) demonstrating in sheep that pulmonary pressure at rest is influenced by basal NO levels, but during exercise, PVR will decrease even when NO is blocked, suggesting that increased pressure and flow during exercise is responsible for recruitment and distention of the pulmonary circulation resulting in the reduction in PVR. In the current study, the total pulmonary resistance (PASP/QT) was significantly higher (P < 0.05) in our subjects at rest in hypobaric normoxia (6.73 mmHg·l−1·min−1) compared with normobaric normoxia (4.76 mmHg·l−1·min−1), and this remained significant when correcting TPR for HCT, suggesting an effect of hypobaria per se on the pulmonary vasculature at rest. However, TPR during exercise was similar between conditions in our study, which may be a result of our subject selection (i.e., no PFO subjects) and/or our indirect measures of pulmonary pressure.
One possible explanation for increased total pulmonary resistance (TPR) in hypobaric normoxia could be a lung volume increase associated with high altitude. Previous work has shown that hypobaric decompression can cause increases in total lung capacity, functional residual capacity, closing capacity, and residual volume (6). Coates et al. (6) attributed these increases to greater lung distensibility due to acute hypoxia. However, in accordance with Boyle’s Law (pressure and volume are inversely proportional at constant temperature), a volume of air residing (trapped) within the alveoli would be expected to increase at lower atmospheric pressure. Perhaps a plausible explanation for the increases in lung volumes seen by Coates et al. (6) is an intrinsic effect of hypobaria and not of acute hypoxia. Whatever the reason may be, increases or decreases in lung volume are known to affect pulmonary vascular resistance (24, 66) by compression of alveolar capillaries or extra-alveolar vessels at high or low lung volumes, respectively.
Another potential reason for the increase in TPR may be a hypobaria-induced reduction in nitric oxide (NO) availability. NO is an inorganic gaseous free radical involved in vasodilation as well as many other physiological processes. NO has been shown to cause pulmonary vasodilation, thus maintaining low pulmonary arterial pressure (75). A reduction in NO bioavailability could then be expected to result in pulmonary vasoconstriction and elevated pulmonary artery pressure. One previous study conducted in rats demonstrated greater reductions in serum levels of NO storage molecules with acute exposure to normobaric hypoxia and hypobaric normoxia compared with acute hypobaric hypoxia, suggesting a per se effect of hypobaria on bioavailability of NO (43). Other research groups have demonstrated altitude-dependent differences in NO availability. These differences, for example, include exaggerated oxidative stress and impaired NO bioavailability in hypobaric hypoxia compared with normobaric hypoxia and greater generation of reactive oxygen species in hypobaric hypoxia compared with normobaric hypoxia (presumably affecting NO bioavailability) (18, 60).
Given that we found no significant differences in AaDO2 in equivalent conditions, if QIPAVA indeed decreases in hypobaria, some of the AaDO2 may be attributed to hypobaria-induced changes in ventilation-perfusion inequality and/or diffusion limitation, as mentioned above. The reasons detailed above may explain the reasons for increased ventilation-perfusion heterogeneity and diffusion limitation, as well as reduced air density with hypobaria, which may alter ventilation distribution.
Summary
Despite the lower bubble scores observed in subjects exercising in hypobaric hypoxia and hypobaric normoxia compared with normobaric hypoxia and normobaric normoxia, we have shown that there was no significant difference in gas exchange efficiency in normoxic conditions, as measured by the AaDO2. This could be a potential explanation for a reduction in QIPAVA concomitant with an increase in ventilation-perfusion heterogeneity and diffusion limitation caused by the significant changes in cardiopulmonary hemodynamics under hypobaric conditions. There are two possibilities to explain the reduction in the number of contrast bubbles under hypobaric conditions: 1) hypobaria affects pulmonary blood flow in such a way as to direct blood flow away from IPAVA or areas of the lung that contain IPAVA, thereby reducing the number of contrast bubbles reaching the left heart; and 2) there was no significantly different effect on QIPAVA in hypobaria compared with normobaria, but contrast bubbles did not persist long enough to be detected in the left heart by TTSCE. Our mathematical modeling of bubble lifetime does not support the second possibility. Furthermore, hypobaria is known to impact lung volumes as well as affect NO bioavailability, and therefore, data in the present study suggest that the first possibility may warrant further investigation. Future studies should also focus on determination of initial radius of saline contrast microbubbles created at sea level and at high altitude. Additionally, performing studies in animals using microspheres to detect QIPAVA under normobaric and hypobaric conditions will provide important information to help reconcile our apparently discrepant findings.
GRANTS
This study was supported by in part by Department of Defense Grants W81XWH-11-2-0040 TATRC to R. C. Roach and W81XWH-10-2-0114 to A. T. Lovering) and by the Altitude Research Center and the Charles S. Houston Endowed Professorship, Department of Emergency Medicine, School of Medicine, University of Colorado-Denver. Further support came from the University of Oregon Office of Research, Innovation and Graduate Education (FRA 2014), the MERIC foundation, and the University of Colorado, Altitude Research Center Foundation. The project was also supported in part by the NIH/National Center for Advancing Translational Sciences Colorado CTSI Grant No. UL1-TR-000154.
DISCLAIMERS
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.A.P. and A.T.L. conceived and designed research; F.A.P., J.T.D., K.M.B., O.E., R.D.G., J.E.F., and A.T.L. performed experiments; F.A.P. and J.T.D. analyzed data; F.A.P., J.E.E., J.M.S.-A., S.G., and A.T.L. interpreted results of experiments; F.A.P. and J.E.E. prepared figures; F.A.P. drafted manuscript; F.A.P., J.T.D., K.M.B., J.E.E., A.W.S., J.M.S.-A., S.G., R.C.R., and A.T.L. edited and revised manuscript; F.A.P., J.T.D., K.M.B., O.E., J.E.E., R.D.G., J.E.F., A.W.S., J.M.S.-A., S.G., R.C.R., and A.T.L. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was part of the AltitudeOmics project that explore the basic mechanisms controlling human acclimatization to hypoxia. Many people and organizations have invested enormous amounts of time and resources to make AltitudeOmics a success. Foremost, the study was made possible by the tireless support, generosity, and tenacity of our research subjects. AltitudeOmics principal investigators were A. T. Lovering, A. W. Subudhi, and R. C. Roach. A complete list of other investigators on this multinational, collaborative effort involved in development, subject management and data collection, supporting industry partners, and people and organizations in Bolivia that made AltitudeOmics possible is available in the first paper in this series (69). We also thank Darren Drumsta and MGC Diagnostics for providing the pulmonary function, diffusing capacity, and metabolic equipment, Dr. Glen Foster for critical feedback, and Diana Dragnea for assistance with data organization and analysis.
APPENDIX
Physical Properties of Bubbles In Vivo
In the chamber and field studies, agitated saline contrast was created from room air and saline. When infused intravenously, air microbubbles dissolve rapidly, and therefore, they may not persist long enough to reach the left heart. Gas pressure inside the contrast bubble is the sum of the Laplace pressure, the equilibrium pressure, and the blood pressure. The combination of these pressures exceeds the gas pressures in the blood (64) and, consequently, results in the outward diffusion of gas and rapid dissolution of microbubbles. Metabolic oxygen consumption also reduces the partial pressure of oxygen within the venous blood. Oxygen may escape from the bubble and react with hemoglobin in the boundary layer between the blood and bubble surface, further reducing the bubble radius (64, 78, 79). Upon intravenous infusion, microbubbles can also be destroyed by ultrasound energy (49). These factors may contribute to the acceleration of contrast microbubble dissolution. Thus, there are several physical reasons for the rapid disappearance of contrast bubbles in flowing blood, and each one of these factors is discussed in more detail below.
Factor 1: Laplace pressure.
The Laplace pressure plays a significant role in the dissolution of air microbubbles within the blood. The Laplace pressure is the difference in pressure between the inside and outside of a curved surface, in this case, the bubble surface boundary, and is given by Eq. 4: ΔP = 2γ/R, where γ is surface tension and R is bubble radius. It can be seen from this equation that the Laplace pressure increases as R decreases, that is, as air diffuses out of the bubble. Increasing the Laplace pressure accelerates the rate at which the bubble dissolves. The smaller the initial R, the faster it dissolves. With decreasing R, the surface tension drives the rapid dissolution of the bubble (30).
Factor 2: gas equilibrium and oxygen metabolism.
Oxygen metabolism and diffusion from the contrast bubbles also contributes to the reduction of bubble radius. The contrast bubbles used in the field and chamber studies were composed of ambient air. Because the partial pressure of oxygen (Po2) of ambient air is higher than the Po2 of venous blood, the oxygen within the bubble diffuses into the blood, forming a thin boundary layer around the bubble. At the boundary layer, oxygen reacts with hemoglobin, initially causing a reduction in the size of the bubble (78). Once the reaction of oxygen and hemoglobin comes to equilibrium, any further reduction in bubble size with respect to oxygen is due to diffusion. Additionally, when bubbles are moving, as in flowing blood, oxygen diffuses more rapidly with increasing flow velocity (79).
Factor 3: ultrasonic destruction.
Ultrasound energy also contributes to bubble destruction because of rapid bubble contraction and expansion (49, 64). Mechanisms that contribute to ultrasonic destruction of contrast bubbles include diffusion of gas at low acoustic power and dispersion of the microbubble into smaller bubbles (49). Given that we used the same ultrasound energy under all conditions, it is not likely that ultrasound energy contributed to bubble destruction to any greater extent in hypobaria than in normobaria.
REFERENCES
- 1.Bates ML, Farrell ET, Drezdon A, Jacobson JE, Perlman SB, Eldridge MW. Hypoxia and exercise increase the transpulmonary passage of 99mTc-labeled albumin particles in humans. PLoS One 9: e101146, 2014. doi: 10.1371/journal.pone.0101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates ML, Jacobson JE, Eldridge MW. Beta adrenergic regulation of intrapulmonary arteriovenous anastomoses in intact rat and isolated rat lungs. Front Physiol 8: 218, 2017. doi: 10.3389/fphys.2017.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulet LM, Lovering AT, Tymko MM, Day TA, Stembridge M, Nguyen TA, Ainslie PN, Foster GE. Reduced blood flow through intrapulmonary arteriovenous anastomoses during exercise in lowlanders acclimatizing to high altitude. Exp Physiol 102: 670–683, 2017. doi: 10.1113/EP086182. [DOI] [PubMed] [Google Scholar]
- 4.Butler BD, Hills BA. The lung as a filter for microbubbles. J Appl Physiol Respir Environ Exerc Physiol 47: 537–543, 1979. doi: 10.1152/jappl.1979.47.3.537. [DOI] [PubMed] [Google Scholar]
- 5.Cabanes L, Coste J, Derumeaux G, Jeanrenaud X, Lamy C, Zuber M, Mas JL; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group . Interobserver and intraobserver variability in detection of patent foramen ovale and atrial septal aneurysm with transesophageal echocardiography. J Am Soc Echocardiogr 15: 441–446, 2002. doi: 10.1067/mje.2002.116718. [DOI] [PubMed] [Google Scholar]
- 6.Coates G, Gray G, Mansell A, Nahmias C, Powles A, Sutton J, Webber C. Changes in lung volume, lung density, and distribution of ventilation during hypobaric decompression. J Appl Physiol Respir Environ Exerc Physiol 46: 752–755, 1979. doi: 10.1152/jappl.1979.46.4.752. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol (1985) 87: 1997–2006, 1999. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- 8.Duke JW, Davis JT, Ryan BJ, Elliott JE, Beasley KM, Hawn JA, Byrnes WC, Lovering AT. Decreased arterial PO2, not O2 content, increases blood flow through intrapulmonary arteriovenous anastomoses at rest. J Physiol 594: 4981–4996, 2016. doi: 10.1113/JP272211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duke JW, Elliott JE, Laurie SS, Voelkel T, Gladstone IM, Fish MB, Lovering AT. Bubble and macroaggregate method differ in detection of blood flow through intrapulmonary arteriovenous anastomoses in upright and supine hypoxia in humans. J Appl Physiol (1985) 123: 1592–1598, 2017. doi: 10.1152/japplphysiol.00673.2017. [DOI] [PubMed] [Google Scholar]
- 10.Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol (1985) 97: 797–805, 2004. doi: 10.1152/japplphysiol.00137.2004. [DOI] [PubMed] [Google Scholar]
- 11.Eldridge MW, Podolsky A, Richardson RS, Johnson DH, Knight DR, Johnson EC, Hopkins SR, Michimata H, Grassi B, Feiner J, Kurdak SS, Bickler PE, Wagner PD, Severinghaus JW. Pulmonary hemodynamic response to exercise in subjects with prior high-altitude pulmonary edema J Appl Physiol (1985) 81: 911–921, 1996. doi: 10.1152/jappl.1996.81.2.911. [DOI] [PubMed] [Google Scholar]
- 12.Elliott JE, Choi Y, Laurie SS, Yang X, Gladstone IM, Lovering AT. Effect of initial gas bubble composition on detection of inducible intrapulmonary arteriovenous shunt during exercise in normoxia, hypoxia, or hyperoxia. J Appl Physiol (1985) 110: 35–45, 2011. doi: 10.1152/japplphysiol.00145.2010. [DOI] [PubMed] [Google Scholar]
- 13.Elliott JE, Duke JW, Hawn JA, Halliwill JR, Lovering AT. Increased cardiac output, not pulmonary artery systolic pressure, increases intrapulmonary shunt in healthy humans breathing room air and 40% O2. J Physiol 592: 4537–4553, 2014. doi: 10.1113/jphysiol.2014.274829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott JE, Friedman JM, Futral JE, Goodman RD, Lovering AT. Sildenafil, nifedipine and acetazolamide do not allow for blood flow through intrapulmonary arteriovenous anastomoses during exercise while breathing 100% oxygen. Exp Physiol 99: 1636–1647, 2014. doi: 10.1113/expphysiol.2014.081562. [DOI] [PubMed] [Google Scholar]
- 15.Elliott JE, Laurie SS, Kern JP, Beasley KM, Goodman RD, Kayser B, Subudhi AW, Roach RC, Lovering AT. AltitudeOmics: impaired pulmonary gas exchange efficiency and blunted ventilatory acclimatization in humans with patent foramen ovale after 16 days at 5,260 m. J Appl Physiol (1985) 118: 1100–1112, 2015. doi: 10.1152/japplphysiol.00879.2014. [DOI] [PubMed] [Google Scholar]
- 16.Elliott JE, Nigam SM, Laurie SS, Beasley KM, Goodman RD, Hawn JA, Gladstone IM, Chesnutt MS, Lovering AT. Prevalence of left heart contrast in healthy, young, asymptomatic humans at rest breathing room air. Respir Physiol Neurobiol 188: 71–78, 2013. doi: 10.1016/j.resp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Epstein PS, Plesset MS. On the stability of gas bubbles in liquid-gas solutions. J Chem Phys 18: 1505–1509, 1950. doi: 10.1063/1.1747520. [DOI] [Google Scholar]
- 18.Faiss R, Pialoux V, Sartori C, Faes C, Dériaz O, Millet GP. Ventilation, oxidative stress, and nitric oxide in hypobaric versus normobaric hypoxia. Med Sci Sports Exerc 45: 253–260, 2013. doi: 10.1249/MSS.0b013e31826d5aa2. [DOI] [PubMed] [Google Scholar]
- 19.Fischer M, Zinovik I, Poulikakos D. Diffusion and reaction controlled dissolution of oxygen microbubbles in blood. Int J Heat Mass Transfer 52: 5013–5019, 2009. doi: 10.1016/j.ijheatmasstransfer.2009.05.013. [DOI] [Google Scholar]
- 20.Foster GE, Ainslie PN, Stembridge M, Day TA, Bakker A, Lucas SJ, Lewis NC, MacLeod DB, Lovering AT. Resting pulmonary haemodynamics and shunting: a comparison of sea-level inhabitants to high altitude Sherpas. J Physiol 592: 1397–1409, 2014. doi: 10.1113/jphysiol.2013.266593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman JA, Woods TD. Use of saline contrast echo timing to distinguish intracardiac and extracardiac shunts: failure of the 3- to 5-beat rule. Echocardiography 25: 1127–1130, 2008. doi: 10.1111/j.1540-8175.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 21a.George R, Light R, Matthay M, Matthay R. Alveolar ventilation, gas exchange, oxygen delivery, and acid-base physiology. In: Chest Medicine: Essentials of Pulmonary and Critical Care Medicine. Philadelphia, PA: Lippincott Williams & Wilkins, 1983, p. 39–54. [Google Scholar]
- 22.Glazier JB, Hughes JM, Maloney JE, West JB. Measurements blood volume of capillary dimensions in rapidly frozen lungs. J Appl Physiol 26: 65–76, 1969. doi: 10.1152/jappl.1969.26.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Groves BM, Reeves JT, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS. Operation Everest II: elevated high-altitude pulmonary resistance unresponsive to oxygen. J Appl Physiol (1985) 63: 521–530, 1987. doi: 10.1152/jappl.1987.63.2.521. [DOI] [PubMed] [Google Scholar]
- 24.Hakim TS, Michel RP, Chang HK. Effect of lung inflation on pulmonary vascular resistance by arterial and venous occlusion. J Appl Physiol Respir Environ Exerc Physiol 53: 1110–1115, 1982. doi: 10.1152/jappl.1982.53.5.1110. [DOI] [PubMed] [Google Scholar]
- 25.Hirai K, Kobayashi T, Kubo K, Shibamoto T. Effects of hypobaria on lung fluid balance in awake sheep. J Appl Physiol (1985) 64: 243–248, 1988. doi: 10.1152/jappl.1988.64.1.243. [DOI] [PubMed] [Google Scholar]
- 26.Hlastala MP, Van Liew HD. Absorption of in vivo inert gas bubbles. Respir Physiol 24: 147–158, 1975. doi: 10.1016/0034-5687(75)90109-7. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman JI. Pulmonary vascular resistance and viscosity: the forgotten factor. Pediatr Cardiol 32: 557–561, 2011. doi: 10.1007/s00246-011-9954-3. [DOI] [PubMed] [Google Scholar]
- 28.Jeon DS, Luo H, Iwami T, Miyamoto T, Brasch AV, Mirocha J, Naqvi TZ, Siegel RJ. The usefulness of a 10% air-10% blood-80% saline mixture for contrast echocardiography: Doppler measurement of pulmonary artery systolic pressure. J Am Coll Cardiol 39: 124–129, 2002. doi: 10.1016/S0735-1097(01)01698-9. [DOI] [PubMed] [Google Scholar]
- 29.Jones RS, Meade F. A theoretical and experimental analysis of anomalies in the estimation of pulmonary diffusing capacity by the single breath method. Q J Exp Physiol Cogn Med Sci 46: 131–143, 1961. [DOI] [PubMed] [Google Scholar]
- 30.de Jong N, Ten Cate FJ, Lancée CT, Roelandt JRTC, Bom N. Principles and recent developments in ultrasound contrast agents. Ultrasonics 29: 324–330, 1991. doi: 10.1016/0041-624X(91)90030-C. [DOI] [PubMed] [Google Scholar]
- 31.Kabalnov A, Bradley J, Flaim S, Klein D, Pelura T, Peters B, Otto S, Reynolds J, Schutt E, Weers J. Dissolution of multicomponent microbubbles in the bloodstream: 2. Experiment. Ultrasound Med Biol 24: 751–760, 1998. doi: 10.1016/S0301-5629(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 32.Kabalnov A, Klein D, Pelura T, Schutt E, Weers J. Dissolution of multicomponent microbubbles in the bloodstream: 1. Theory. Ultrasound Med Biol 24: 739–749, 1998. doi: 10.1016/S0301-5629(98)00034-9. [DOI] [PubMed] [Google Scholar]
- 33.Kelman GR, Nunn JF. Nomograms for correction of blood Po2, Pco2, pH, and base excess for time and temperature. J Appl Physiol 21: 1484–1490, 1966. doi: 10.1152/jappl.1966.21.5.1484. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy JM, Foster GE, Koehle MS, Potts JE, Sandor GG, Potts MT, Houghton KM, Henderson WR, Sheel AW. Exercise-induced intrapulmonary arteriovenous shunt in healthy women. Respir Physiol Neurobiol 181: 8–13, 2012. doi: 10.1016/j.resp.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol 66: 493–496, 1990. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 36.Knudson RJ, Kaltenborn WT, Knudson DE, Burrows B. The single-breath carbon monoxide diffusing capacity. Reference equations derived from a healthy nonsmoking population and effects of hematocrit. Am Rev Respir Dis 135: 805–811, 1987. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- 37.Koizumi T, Gupta R, Banerjee M, Newman JH. Changes in pulmonary vascular tone during exercise. Effects of nitric oxide (NO) synthase inhibition, L-arginine infusion, and NO inhalation. J Clin Invest 94: 2275–2282, 1994. doi: 10.1172/JCI117590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, Trystram D, Coste J, Mas JL. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. Stroke 33: 706–711, 2002. doi: 10.1161/hs0302.104543. [DOI] [PubMed] [Google Scholar]
- 39.Laurie SS, Elliott JE, Goodman RD, Lovering AT. Catecholamine-induced opening of intrapulmonary arteriovenous anastomoses in healthy humans at rest. J Appl Physiol (1985) 113: 1213–1222, 2012. doi: 10.1152/japplphysiol.00565.2012. [DOI] [PubMed] [Google Scholar]
- 40.Laurie SS, Yang X, Elliott JE, Beasley KM, Lovering AT. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol (1985) 109: 1072–1079, 2010. doi: 10.1152/japplphysiol.00150.2010. [DOI] [PubMed] [Google Scholar]
- 41.Lemoigne Y, Caner A, Rahal G. Physics for Medical Imaging Applications. Dordrecht, The Netherlands: Springer, 2007. doi: 10.1007/978-1-4020-5653-6. [DOI] [Google Scholar]
- 42.Levine BD, Kubo K, Kobayashi T, Fukushima M, Shibamoto T, Ueda G. Role of barometric pressure in pulmonary fluid balance and oxygen transport. J Appl Physiol (1985) 64: 419–428, 1988. doi: 10.1152/jappl.1988.64.1.419. [DOI] [PubMed] [Google Scholar]
- 43.López-Ramos JC, Martínez-Romero R, Molina F, Cañuelo A, Martínez-Lara E, Siles E, Peinado MA. Evidence of a decrease in nitric oxide-storage molecules following acute hypoxia and/or hypobaria, by means of chemiluminescence analysis. Nitric Oxide 13: 62–67, 2005. doi: 10.1016/j.niox.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Lovering AT, Duke JW, Elliott JE. Intrapulmonary arteriovenous anastomoses in humans–response to exercise and the environment. J Physiol 593: 507–520, 2015. doi: 10.1113/jphysiol.2014.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovering AT, Goodman RD. Detection of intracardiac and intrapulmonary shunts at rest and during exercise using saline contrast echocardiography. In: Applied Aspects of Ultrasonography in Humans, edited by Ainslie PN. Rijeka, Croatia: InTech, 2012, p. 159–174. [Google Scholar]
- 46.Lovering AT, Stickland MK, Amann M, Murphy JC, O’Brien MJ, Hokanson JS, Eldridge MW. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol 586: 4559–4565, 2008. doi: 10.1113/jphysiol.2008.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacDonald CA, Sboros V, Gomatam J, Pye SD, Moran CM, Norman McDicken W. A numerical investigation of the resonance of gas-filled microbubbles: resonance dependence on acoustic pressure amplitude. Ultrasonics 43: 113–122, 2004. doi: 10.1016/j.ultras.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 48.MacIntyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CPM, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 26: 720–735, 2005. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 49.Mayer S, Grayburn PA. Myocardial contrast agents: recent advances and future directions. Prog Cardiovasc Dis 44: 33–44, 2001. doi: 10.1053/pcad.2001.26438. [DOI] [PubMed] [Google Scholar]
- 50.Meerbaum S. Principles of echo contrast. In: In Advances in Echo Imaging Using Contrast Enhancement (edited by Nanda N and Schlief R). Dordrecht, The Netherlands: Kluwer Academic, 1993, p. 9–42. doi: 10.1007/978-94-015-8126-4_2. [DOI] [Google Scholar]
- 51.Meltzer RS, Sartorius OE, Lancée CT, Serruys PW, Verdouw PD, Essed CE, Roelandt J. Transmission of ultrasonic contrast through the lungs. Ultrasound Med Biol 7: 377–384, 1981. doi: 10.1016/0301-5629(81)90048-X. [DOI] [PubMed] [Google Scholar]
- 52.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 53.Niden AH, Aviado DM Jr. Effects of pulmonary embolism on the pulmonary circulation with special reference to arteriovenous shunts in the lung. Circ Res 4: 67–73, 1956. doi: 10.1161/01.RES.4.1.67. [DOI] [PubMed] [Google Scholar]
- 54.Norris HC, Mangum TS, Duke JW, Straley TB, Hawn JA, Goodman RD, Lovering AT. Exercise- and hypoxia-induced blood flow through intrapulmonary arteriovenous anastomoses is reduced in older adults. J Appl Physiol (1985) 116: 1324–1333, 2014. doi: 10.1152/japplphysiol.01125.2013. [DOI] [PubMed] [Google Scholar]
- 55.Papadopoulou V, Tang MX, Balestra C, Eckersley RJ, Karapantsios TD. Circulatory bubble dynamics: from physical to biological aspects. Adv Colloid Interface Sci 206: 239–249, 2014. doi: 10.1016/j.cis.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Plesset MS, Prosperetti A. Bubble dynamics and cavitation. Annu Rev Fluid Mech 9: 145–185, 1977. doi: 10.1146/annurev.fl.09.010177.001045. [DOI] [Google Scholar]
- 57.Quay SC. Persistent gaseous bubbles as ultrasound contrast media. 13, 1996. [Google Scholar]
- 58.Raisinghani A, DeMaria AN. Physical principles of microbubble ultrasound contrast agents. Am J Cardiol 90, Suppl 1: 3–7, 2002. doi: 10.1016/S0002-9149(02)02858-8. [DOI] [PubMed] [Google Scholar]
- 59.Rambod E, Beizaie M, Shusser M, Milo S, Gharib M. A physical model describing the mechanism for formation of gas microbubbles in patients with mitral mechanical heart valves. Ann Biomed Eng 27: 774–792, 1999. doi: 10.1114/1.231. [DOI] [PubMed] [Google Scholar]
- 60.Ribon A, Pialoux V, Saugy JJ, Rupp T, Faiss R, Debevec T, Millet GP. Exposure to hypobaric hypoxia results in higher oxidative stress compared to normobaric hypoxia. Respir Physiol Neurobiol 223: 23–27, 2016. doi: 10.1016/j.resp.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Roelandt J. Contrast echocardiography. Ultrasound Med Biol 8: 471–492, 1982. doi: 10.1016/0301-5629(82)90079-5. [DOI] [PubMed] [Google Scholar]
- 62.Rosenzweig DY, Hughes JM, Glazier JB. Effects of transpulmonary and vascular pressures on pulmonary blood volume in isolated lung. J Appl Physiol 28: 553–560, 1970. doi: 10.1152/jappl.1970.28.5.553. [DOI] [PubMed] [Google Scholar]
- 63.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23: 685–713, 2010. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Schutt EG, Klein DH, Mattrey RM, Riess JG. Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: the key role of perfluorochemicals. Angew Chem Int Ed Engl 42: 3218–3235, 2003. doi: 10.1002/anie.200200550. [DOI] [PubMed] [Google Scholar]
- 65.Severinghaus JW. Blood gas calculator. J Appl Physiol 21: 1108–1116, 1966. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]
- 66.Simmons DH, Linde LM, Miller JH, O’Reilly RJ. Relation between lung volume and pulmonary vascular resistance. Circ Res 9: 465–471, 1961. doi: 10.1161/01.RES.9.2.465. [DOI] [Google Scholar]
- 67.Solano-Altamirano JM, Goldman S. The lifetimes of small arterial gas emboli, and their possible connection to inner ear decompression sickness. Math Biosci 252: 27–35, 2014. doi: 10.1016/j.mbs.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 67a.Srinivasan RS, Gerth WA, Powell MR. A mathematical model of diffusion-limited gas bubble dynamics in tissue with varying diffusion region thickness. Respir Physiol 123: 153–164, 2000. [DOI] [PubMed] [Google Scholar]
- 67b.Srinivasan SR, Gerth WA, Powell MR. Mathematical models of diffusion-limited gas bubble dynamics in tissue. J Appl Physiol (1985) 86: 732–741, 1999. doi: 10.1152/jappl.1999.86.2.732. [DOI] [PubMed] [Google Scholar]
- 68.Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M, Jones RL. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol 561: 321–329, 2004. doi: 10.1113/jphysiol.2004.069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Subudhi AW, Bourdillon N, Bucher J, Davis C, Elliott JE, Eutermoster M, Evero O, Fan JL, Jameson-Van Houten S, Julian CG, Kark J, Kark S, Kayser B, Kern JP, Kim SE, Lathan C, Laurie SS, Lovering AT, Paterson R, Polaner DM, Ryan BJ, Spira JL, Tsao JW, Wachsmuth NB, Roach RC. AltitudeOmics: the integrative physiology of human acclimatization to hypobaric hypoxia and its retention upon reascent. PLoS One 9: e92191, 2014. doi: 10.1371/journal.pone.0092191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subudhi AW, Fan J-L, Evero O, Bourdillon N, Kayser B, Julian CG, Lovering AT, Roach RC. AltitudeOmics: effect of ascent and acclimatization to 5260 m on regional cerebral oxygen delivery. Exp Physiol 99: 772–781, 2014. doi: 10.1113/expphysiol.2013.075184. [DOI] [PubMed] [Google Scholar]
- 71.Tedjasaputra V, Bryan TL, van Diepen S, Moore LE, Bouwsema MM, Welsh RC, Petersen SR, Stickland MK. Dopamine receptor blockade improves pulmonary gas exchange but decreases exercise performance in healthy humans. J Physiol 593: 3147–3157, 2015. doi: 10.1113/JP270238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med 117: 461–465, 1992. doi: 10.7326/0003-4819-117-6-461. [DOI] [PubMed] [Google Scholar]
- 73.Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol (1985) 61: 260–270, 1986. doi: 10.1152/jappl.1986.61.1.260. http://www.ncbi.nlm.nih.gov/pubmed/3710978. [DOI] [PubMed] [Google Scholar]
- 74.Wagner PD, Sutton JR, Reeves JT, Cymerman A, Groves BM, Malconian MK. Operation Everest II: pulmonary gas exchange during a simulated ascent of Mt. Everest. J Appl Physiol (1985) 63: 2348–2359, 1987. doi: 10.1152/jappl.1987.63.6.2348. [DOI] [PubMed] [Google Scholar]
- 75.Wang T, El Kebir D, Blaise G.. Inhaled nitric oxide in 2003: a review of its mechanisms of action. Can J Anesth 50: 839–846, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522, 2005. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 77.Westerhof N, Stergiopulos N, Noble MI. Snapshots of Hemodynamics: An Aid for Clinical Research and Graduate Education. New York: Springer Science & Business Media, 2010. doi: 10.1007/978-1-4419-6363-5 [DOI] [Google Scholar]
- 78.Yang WJ, Echigo R, Wotton DR, Hwang JB. Experimental studies of the dissolution of gas bubbles in whole blood and plasma. I. Stationary bubbles. J Biomech 4: 275–281, 1971. doi: 10.1016/0021-9290(71)90033-9. [DOI] [PubMed] [Google Scholar]
- 79.Yang WJ, Echigo R, Wotton DR, Hwang JB. Experimental studies of the dissolution of gas bubbles in whole blood and plasma. II. Moving bubbles or liquids. J Biomech 4: 283–288, 1971. doi: 10.1016/0021-9290(71)90034-0. [DOI] [PubMed] [Google Scholar]
- 80.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 70: 657–662, 1984. doi: 10.1161/01.CIR.70.4.657. [DOI] [PubMed] [Google Scholar]