Abstract
In amyotrophic lateral sclerosis (ALS), loss of motoneuron function leads to weakness and, ultimately, respiratory failure and death. Regardless of the initial pathogenic factors, motoneuron loss follows a specific pattern: the largest α-motoneurons die before smaller α-motoneurons, and γ-motoneurons are spared. In this article, we examine how homeostatic responses to this orderly progression could lead to local microcircuit dysfunction that in turn propagates motoneuron dysfunction and death. We first review motoneuron diversity and the principle of α-γ coactivation and then discuss two specific spinal motoneuron microcircuits: those involving proprioceptive afferents and those involving Renshaw cells. Next, we propose that the overall homeostatic response of the nervous system is aimed at maintaining force output. Thus motoneuron degeneration would lead to an increase in inputs to motoneurons, and, because of the pattern of neuronal degeneration, would result in an imbalance in local microcircuit activity that would overwhelm initial homeostatic responses. We suggest that this activity would ultimately lead to excitotoxicity of motoneurons, which would hasten the progression of disease. Finally, we propose that should this be the case, new therapies targeted toward microcircuit dysfunction could slow the course of ALS.
Keywords: excitotoxicity, α-motoneurons, γ-motoneurons, muscle spindles, proprioceptive afferents, Renshaw cells
INTRODUCTION
Many diverse provinces of the central nervous system are involved in the production of movement, and, through their interconnections, the coordination of activity of circuits in these regions leads to organized behavior. Microcircuits within and between many regions of the cerebral cortex, basal ganglia, cerebellum, brain stem, and spinal cord each play a role in movement, whether as selection circuits, command neurons, organization circuits, or the final common path leading to muscle contraction. These circuits are remarkably adaptive: movement is well controlled in a multitude of environmental conditions. To do so, multiple modalities of sensory input are involved in the moment-to-moment adjustments of motor output to ensure appropriate coordination and function of these disparate motor circuits.
There are homeostatic processes in neurons and circuits that ensure that the output of neurons, in the form of trains of action potentials, is maintained within a specific range (Turrigiano and Nelson 2004) needed for the behavior. This homeostatic regulation is necessary to maintain activity throughout the life cycle of an organism, for example, in relation to the short timescale of protein turnover (Marder and Goaillard 2006; O’Leary et al. 2014). In the vertebrate motor system, for example, it is necessary to maintain muscle force production in the range necessary for movement (e.g., consider body weight support). Thus homeostatic processes in neurons (motoneurons) and circuits underlie homeostatic processes of the organism (movement) (see Fig. 2A).
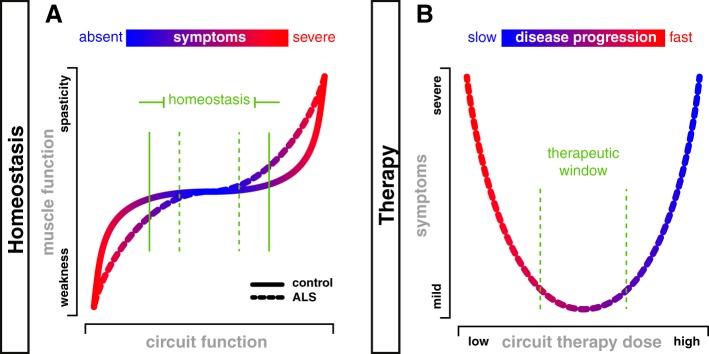
Fig. 2.
Homeostasis and microcircuit therapy: targeting microcircuits to slow progression? A: chair-shaped homeostatic curve (Nijhout et al. 2014) demonstrating a region in which normal motor function can be maintained despite increases and decreases in circuit function (solid green vertical bars). Increases beyond this range would lead to positive motor symptoms such as spasticity, whereas reductions would lead to weakness. As circuits degenerate in amyotrophic lateral sclerosis (ALS) and fewer α-motoneurons (α-MNs) are available to these circuits, the homeostatic plateau would narrow (dashed green vertical bars). B: microcircuit therapy for ALS, as defined here, would be aimed at reversing at least one of the arrows in Fig. 1. For example, a therapy to reduce γ-MN activity, or to increase Renshaw cell activity, could reverse the imbalance in these circuits, potentially slowing MN death as depicted by the color scale. We predict that this would reduce symptoms by preserving α-MNs. However, at higher “doses,” such therapy could in itself lead to weakness through reducing α-MN activity. We suggest that there would be a therapeutic window (dashed green vertical bars) in which progression could be slowed and the duration of time that people will have functional muscle contraction would increase, thus leading to improvements in quality of life.
Homeostatic mechanisms also play important roles in maintaining movement following damage to the nervous system. For example, after spinal cord injury, spinal circuits can regain activity needed for locomotor function (Bui et al. 2016; Martinez et al. 2011). Similarly, homeostasis is also seen in neurodegenerative diseases, in which symptoms do not become apparent until a significant proportion of neurons dies. For example, Parkinson’s disease is asymptomatic until an estimated 30% of nigral dopaminergic neurons die (Fearnley and Lees 1991; Greffard et al. 2006; Ma et al. 1997), and amyotrophic lateral sclerosis (ALS) remains asymptomatic until at least 30% of vulnerable motoneurons (MNs) degenerate (Lalancette-Hebert et al. 2016; Zang et al. 2005). In some pools, up to 70% of motor units may have degenerated by the time of symptom onset (Hegedus et al. 2007). Thus circuit homeostasis plays an important role in maintaining quality of life in the face of neurological disease or injury.
Conversely, there are situations in which circuit homeostasis may be maladaptive. For example, following spinal cord injury, adaptations including changes in MN serotonin receptors (Murray et al. 2010) and/or chloride homeostasis (Boulenguez et al. 2010) lead to spasticity, sometimes to a degree that can significantly impair quality of life (Holtz et al. 2016), and plasticity of autonomic motor systems can lead to autonomic dysreflexia (reviewed in Brown and Weaver 2012), which can be life-threatening. Understanding the mechanisms of maladaptive plasticity is thus important for the development of strategies to improve quality of life in people with neurological diseases.
In this review, we ask whether maladaptive plasticity of motor circuits can contribute to progression of neurodegenerative diseases. We focus on ALS, describing two fundamental spinal circuits involving MNs. We do not suggest that circuit dysfunction is causative of ALS, but rather propose that ALS-induced changes in these circuits disrupt normal homeostatic mechanisms and may thus accelerate the progression of MN degeneration. We present an hypothesis whereby maladaptive plasticity of MN circuits leads to excessive glutamate receptor activation, excitotoxicity, and hence further MN dysfunction and, ultimately, death. We therefore suggest that the development of strategies to target microcircuits involved in these maladaptive processes could slow the progression of disease.
SELECTIVE VULNERABILITY OF MOTONEURONS IN ALS
ALS was described by Charcot in the 19th century (Charcot and Joffroy 1869). ALS is a fatal adult-onset neurodegenerative condition associated with progressive loss of MNs, leading to weakness and eventually death by respiratory failure (for review, see Kiernan et al. 2011). Although ALS can affect other central nervous system functions, we focus on MN degeneration.
The underlying causes of ALS are not clear and are not explored in this review. Various cellular and molecular hypotheses have been proposed to explain MN death, e.g., aggregation of toxic proteins, defects in RNA metabolism, or disrupted axonal transport (for review, see Peters et al. 2015; Taylor et al. 2016). Importantly, the death of MNs is not considered to be a cell autonomous process (Ditsworth et al. 2017).
The clinical presentations of ALS are heterogeneous. For example, initial MN loss may be in the brain stem (bulbar) or spinal cord. The time of onset varies considerably, although it is most commonly diagnosed between the 6th and 8th decades of life, and the speed of progression is quite variable (for review, see Swinnen and Robberecht 2014). Despite these differences, there are some commonalities in the pathology. For example, some MN types are vulnerable to degenerative processes, whereas others are resistant (reviewed in Nijssen et al. 2017), and the motor symptoms and signs of ALS tend to start in a certain location and spread to adjacent regions in an orderly manner (reviewed in Ravits 2014).
Regarding selective vulnerability, the pattern of MN loss is remarkably consistent regardless of the etiology of the disease. In the spinal cord, MNs of the lateral motor column (LMC) are affected to a greater extent than those of the medial motor column (MMC). Furthermore, sacral MNs that innervate external anal and urethral sphincter muscles (Onuf’s nucleus) are spared (Iwata and Hirano 1979; Mannen et al. 1977; Schrøder and Reske-Nielsen 1984). In the brain stem, ALS affects trigeminal MNs that innervate the muscles of mastication, facial MNs that supply the superficial muscles of the face, hypoglossal MNs innervating the muscles of the tongue, and ambiguus MNs supplying the muscles of the soft palate, pharynx, and larynx. In contrast, oculomotor, trochlear, and abducens MNs (II, IV, VI nuclei) innervating the extraocular muscles are spared (Iwata and Hirano 1979; Nimchinsky et al. 2000; Valdez et al. 2012), as is the parasympathetic dorsal motor nucleus of the vagus (Iwata and Hirano 1979). The reasons why some populations are spared are not clear (Hedlund et al. 2010), and although not addressed directly by our hypothesis, the circuits that we discuss, as we point out below, are different in these populations. We emphasize that our hypothesis is related to disease progression rather than causation. Of note, there is recent evidence that the factors underlying disease onset and progression in ALS are different (Ditsworth et al. 2017).
There is also selective vulnerability within motor pools, the populations of MNs that innervate a single muscle. α-MNs, those that innervate extrafusal muscle fibers responsible for force production, are vulnerable, whereas γ-MNs, those that innervate the contractile elements of muscle spindles to regulate proprioceptive feedback, are resistant to degeneration (Kawamura et al. 1981; Lalancette-Hebert et al. 2016; Mohajeri et al. 1998; Vaughan et al. 2015). Furthermore, the largest α-MNs that innervate fast-twitch muscle fibers degenerate before the smaller α-MNs that innervate slow-twitch muscle fibers (Frey et al. 2000; Pun et al. 2006). Again, the mechanisms underlying this orderly death are not clear.
MOTONEURON TYPES
Given these differences in MN vulnerability, we next explore local microcircuits involving different types of MNs. Spinal MNs, termed the “final common path” for movement by Sherrington (1904), receive and integrate inputs from supraspinal, spinal, and sensory neurons and project axons outside the central nervous system to innervate muscles and thus effect movement. Despite this common role, they do not constitute a uniform population.
It is perhaps useful to consider MN types from an evolutionary standpoint. After the evolution of contractile muscles and their innervating α-MNs (Fig. 1A, c), muscle sensory feedback evolved, with the development of muscle spindles and associated afferent fibers to relay stretch length and velocity data back to the central nervous system (for review, see Manuel and Zytnicki 2011) (Fig. 1A, c). Spindles developed contractile elements such that their tension could be regulated during muscle shortening. Early in vertebrate evolution, spindle contractile elements were innervated by the same MNs (β-MNs) that innervated extrafusal fibers (Adal and Barker 1965; Bessou et al. 1965), but in mammalian evolution, the roles divided such that many spindles became innervated by an independent class of MNs termed γ-MNs (Burke et al. 1977; Kuffler et al. 1951) (Fig. 1A, b). About 30% of most MN pools are composed of γ-MNs, which overlap in size with small α-MNs, have simpler dendritic branching patterns (Westbury 1982), and can be distinguished by diminished expression of NeuN (Friese et al. 2009; Shneider et al. 2009). The intermixture of α- and γ-MNs in each motor pool is similar from rostral to caudal pools (Burke et al. 1977).
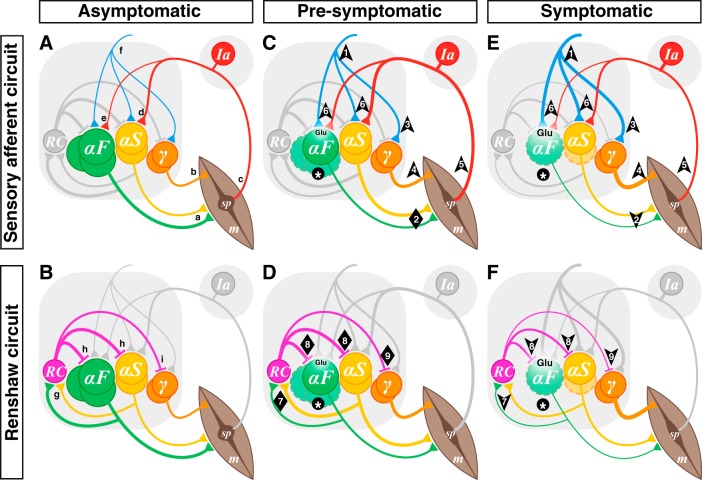
Fig. 1.
Hypothesis: α-motoneuron (α-MN) death leads to microcircuit imbalance and disease progression. Sensory afferent circuits (A, C, E) and Renshaw circuits (B, D, F) in asymptomatic (A, B), presymptomatic (C, D), and symptomatic (E, F) amyotrophic lateral sclerosis (ALS). A and B: normal spinal MN circuits. MN pools comprise multiple types: α-MNs can be defined by the extrafusal muscle fiber (m) types they innervate as either fast (αF; FF and FR types depicted together) or slow (αS) (a). γ-MNs innervate muscle spindles (sp; b), which convey length and velocity information back to α-MNs primarily via group Ia afferents (c), which form monosynaptic connexions with α-MNs (d, e). During most movement, α- and γ-MNs are coactivated by spinal and supraspinal neurons (f). α-MNs also innervate Renshaw cells (RC; g), which in turn inhibit both α- (h) and γ-MNs (i). C and D: in presymptomatic ALS, α-MNs (F-type) become dysfunctional and start to die (*), but γ-MNs are preserved. Homeostatic mechanisms include increased input to α-MNs from spinal and supraspinal circuits (1) to ensure that force production is preserved (2). Thus the input to the coactivated γ-MNs would also increase (3), leading to increased intrafusal fiber contraction (4) out of proportion to extrafusal fibers. This α-γ imbalance would result in an increase in spindle afferent input to α-MNs (6). The increasing glutamatergic (Glu) excitation from these inputs would initially maintain the homeostatic response despite a reduction of activity of fast high-force-producing muscle fibers. In addition, the loss of α-MNs (particularly type F; *) would concomitantly lead to a reduction of output from MN pools to RCs, initially compensated by increased α-MN activity (particularly type S; 7). Thus Renshaw inhibition would at first be maintained in all MN types (8, 9). Together, these processes would lead to increased glutamatergic excitation of vulnerable α-MNs and, hence, excitotoxicity. E and F: in symptomatic stages, type F α-MNs continue to die and type S α-MNs start to degenerate and ultimately die at later stages of the disease (*), but γ-MNs are completely spared. The processes that started in presymptomatic stages would continue, there would be runaway from homeostatic processes, and further excitotoxicity would lead to disease progression. It would no longer be possible to maintain muscle contraction (2), compounding the α-γ imbalance (2 and 4, 5), and the resulting loss of input to RCs (7) would reduce Renshaw inhibition of α-MNs (8) and also diminish γ-MN inhibition (9), thereby contributing to increased excitation of remaining α-MNs but a further imbalance of α-γ output. Note that the thickness of each line represents the integral of synaptic transmission over the number of synaptic contacts on the target cells. In the interest of simplicity and clarity, static and dynamic γ-MNs are represented as a single population, group II sensory afferents are not shown, and MN types are represented as distinct groups, although they are intermingled in each MN pool. Arrows indicate direction of change, and diamonds indicate no net change.
Two main classes of γ-MNs contribute to the modulation of muscle proprioceptive feedback. Static γ-MNs innervate nuclear chain and bag2 fibers and regulate the stretch sensitivity of primary and secondary endings (Ia and II sensory afferents) conveying feedback related to length. Dynamic γ-MNs innervate nuclear bag1 fibers and regulate the dynamic sensitivity of primary endings (Ia sensory afferents) conveying information on lengthening velocity (Brown and Butler 1973; Jansen and Matthews 1962; Matthews 1962; Murphy 1982). Thus, during behavior, α-MNs produce movement and γ-MNs regulate sensory feedback from muscles.
A second type of diversification of MNs is that α-MNs themselves are not homogeneous. α-MN types can be defined by the contractile properties of the muscle fibers they innervate (Bakels and Kernell 1993; Gardiner 1993; Manuel and Heckman 2012), with each MN forming synapses with muscle fibers of similar structural and functional properties (Edström and Kugelberg 1968). Thus a single α-MN innervates multiple similar muscle fibers, which together constitute a motor unit, the functional unit of the motor system (Buchthal and Schmalbruch 1980; Liddell and Sherrington 1925). Accordingly, α-MNs can be divided into three main subtypes: slow (S), fast fatigue resistant (FR), and fast fatigable (FF) (Burke et al. 1973). S-MNs form synapses with slow-twitch fatigue-resistant type I muscle fibers, forming type S motor units; FR-MNs form synapses with fast-twitch fatigue-resistant type IIa (fast oxidative) muscle fibers, constituting FR motor units; and FF-MNs form synapses with fast-twitch (fast glycolytic) fatigable type IIb muscle fibers, forming FF motor units (Burke et al. 1973; McDonagh et al. 1980). Within a motor pool innervating a muscle with a mixture of fiber types, α-MNs of various types are intermingled with each other (Burke et al. 1977) (and with γ-MNs).
The different types of MNs have different biophysical, morphological, and molecular properties (for a detailed review, see Kanning et al. 2010; Stifani 2014). In general, type S motor units are responsible for low-amplitude forces of long duration, whereas FF motor units are responsible for high-amplitude force ballistic movements. Corresponding to these forces, S-MNs have longer postspike afterhyperpolarizations (AHPs) (Eccles et al. 1957a), fire at lower frequencies than F-MNs (Kernell 1979; Zengel et al. 1985), and can produce prolonged self-sustained tonic firing (Lee and Heckman 1998), whereas FF-MNs have shorter AHPs and fire at higher frequencies and in phasic discharge patterns (Burke 1968a). These MN properties thus correspond to the muscle fiber types they innervate (Bakels and Kernell 1993; Eccles et al. 1958; Schiaffino and Reggiani 2011).
In response to uniform input to a motor pool, there is orderly recruitment of MNs from S to FR to FF (Denny-Brown and Pennybacker 1938; Henneman 1957). This results from their electrophysiological properties: type S MNs are smaller (Cullheim et al. 1987; Ulfhake and Kellerth 1982) and have higher input resistances and lower rheobase currents (Kernell and Monster 1981; Zajac and Faden 1985; Zengel et al. 1985). Thus multiple MN types are coordinated in a specific manner to produce a given movement.
INPUTS TO MOTONEURONS: α-γ COACTIVATION AND BALANCE
During muscle contraction, the activity in α- and γ-MNs is generally balanced to prevent spindle unloading and thus maintain spindle sensitivity. The initial evolution of β-MNs ensured coactivation of spindles and extrafusal muscle fibers. The emergence of γ-MNs provided the central nervous system with some control over proprioceptive feedback, potentially leading to a form of “active sensing.”
Nonetheless, in many behaviors, there is evidence in animals and humans that α- and γ-MNs are, at least to a degree, coactivated (for review, see Prochazka and Ellaway 2012). Many different inputs (spinal and supraspinal) lead to coactivation of α- and γ-MNs (Granit 1975; Grillner 1969; Sjöström and Zangger 1975, 1976; Vallbo et al. 1979) (Fig. 1A, f). Although there is some variability, in many rhythmic movements α- and γ (both static and dynamic)-MNs are coactivated (Ellaway et al. 2015; Murphy and Martin 1993). For example, during locomotion, α-γ coactivation has been shown in both extensor (Bessou et al. 1986) and flexor (Bessou et al. 1990) motor pools (Edgerton et al. 1976). This coactivation could promote a degree of servo-assisted muscle contraction (Hagbarth 1993; Watanabe and Hirayama 1976). In support of this, sectioning of γ-MN efferents in cats led to a reduction of α-MN activity evoked by supraspinal inputs (Severin 1966). In general, coactivation of α- and γ-MNs is necessary to prevent spindle unloading that would occur with extrafusal muscle fiber contraction alone (Murphy and Martin 1993).
It should be noted that it is not a rule that α- and γ-MNs are always coactivated; if that were the case, then there would have been no evolutionary pressure to move beyond β-MNs to the independent control afforded by γ-MNs. There are several examples of differential control of α- and γ-MNs. First, group Ia spindle afferents, which comprise the major direct proprioceptive inputs to MNs, form synapses with and thus activate only α-MNs, thus avoiding a possible positive feedback loop that would be created with γ-MN activation. Second, spinal circuits differentially activate static vs dynamic γ-MNs during locomotion, with each type serving a different purpose (Ellaway et al. 2015). Third, selective inputs to γ-MNs would allow the nervous system to independently adjust afferent sensitivity during different behaviors (Prochazka et al. 1985; Vallbo and Hulliger 1981). This has been nicely demonstrated during an attention task (Hospod et al. 2007). Thus, although α-γ coactivation is common during movement, the nervous system has the ability to tune the balanced relationship between contraction and spindle sensitivity by weighting inputs to either α- or γ-MNs.
SENSORY FEEDBACK CIRCUITS
Muscle spindles act as stretch-sensitive mechanoreceptors to provide information about muscle length and lengthening velocity to the nervous system (Hunt 1951). These data are transmitted by groups Ia and II proprioceptive sensory afferents.
Group Ia afferents project directly to homonymous and synergist α-MNs (Brown and Fyffe 1981; Eccles 1946; Lloyd 1943a, 1943b, 1946a, 1946b) (Fig. 1A, d and e), but not to γ-MNs (Appelberg et al. 1983; Eccles et al. 1960; Friese et al. 2009; Shneider et al. 2009). A single Ia afferent fiber contacts virtually all α-MNs of the homonymous pool, with each MN receiving afferents from almost all spindles of the muscle it innervates (Brown and Fyffe 1978, 1981; Mendell and Henneman 1968). The strength of heteronymous connections is typically weaker than that of homonymous connections (Burke and Glenn 1996; Eccles et al. 1957b; Mendelsohn et al. 2015).
The strength of monosynaptic group Ia excitation differs on different MN types. Whereas the average number of sensory collaterals and boutons is similar on all MN types (Burke and Glenn 1996), the strength of Ia excitation varies according to MN size. That is, Ia excitation is strongest on type S MNs (Fig. 1A, d) and weakest on type FF MNs (Fig. 1A, e) (Burke 1968b; Burke and Rymer 1976; Eccles et al. 1957b; Heckman and Binder 1988).
There is evidence that group II afferents also project directly to homonymous MNs, but these connections are weak (Fyffe 1979; Hongo 1992; Kirkwood and Sears 1974). Contrary to group Ia afferents, group II afferents project to only about one-half of homonymous MNs, and the resulting excitation is equal across α-MN types (Munson et al. 1982). In addition, although γ-MNs receive few primary sensory afferent inputs (Shneider et al. 2009), about one-third of γ-MNs seem to receive monosynaptic input from group II afferents converging from a variety of muscles (Gladden et al. 1998), although there is minimal anatomical evidence of this, with few proprioceptive boutons apposing γ-MNs (Shneider et al. 2009). Thus primary and secondary spindle afferents provide direct excitation to MN pools, but with different distributions.
Not all motor pools receive direct spindle afferent input. In the spinal respiratory motor columns, for example, despite the presence of a few spindles in the diaphragm (Corda et al. 1965b), there is no monosynaptic afferent input to phrenic MNs; thus phrenic MNs do not respond to muscle stretch (Corda et al. 1965a). On the other hand, intercostal muscles have many spindles (Huber 1902) and intercostal MNs (which are MMC MNs) receive monosynaptic homonymous Ia connections (Corda et al. 1965a; Kirkwood and Sears 1982). In the brain stem, facial MNs do not have spindle afferent input (there are no spindles in facial muscles), but trigeminal MNs, innervating the muscles of mastication, do. Thus innervation of MNs by primary afferents varies across the neuraxis.
Monosynaptic connectivity between proprioceptive afferents and MNs has been less well studied in ALS-resistant motor pools. The external urethral and anal sphincters have few, if any, spindles (Chennells et al. 1960; Garry and Garven 1957; Todd 1964; Walker 1959), including in humans (Lassmann 1984a, 1984b). Although MNs in Onuf’s nucleus showed weak monosynaptic responses to dorsal root stimulation (Mackel 1979), no anatomical evidence of proprioceptive afferent input directly to these MNs has been found (e.g., Lalancette-Hebert et al. 2016). The presence of proprioceptive feedback from extraocular muscles is even less clear. Extraocular muscles of some species completely lack muscle spindles, whereas in others, their number and morphology vary considerably (for review, see Büttner-Ennever et al. 2006; Donaldson 2000; Maier et al. 1974). These muscles contain palisade endings that may function as proprioceptors (Dogiel 1906; Lienbacher and Horn 2012). However, it seems clear that there are no proprioceptive afferents from extraocular muscles that form monosynaptic connexions with extraocular MNs (Keller and Robinson 1971; reviewed in Rao and Prevosto 2013). Thus ALS-resistant MN pools get little, if any, monosynaptic proprioceptive feedback directly from the muscles they innervate.
RENSHAW CELL CIRCUITS
α-MNs have targets in addition to muscle: they have axon collaterals that form synapses with inhibitory interneurons in the ventral horn, Renshaw cells (Alvarez et al. 1999; Lagerbäck et al. 1981; Lagerbäck and Ronnevi 1982; for review, see Alvarez and Fyffe 2007) (Fig. 1B, g). A single Renshaw cell is excited by axon collaterals from several α-MNs, which can be from different motor pools (Eccles et al. 1954, 1961b; Moore et al. 2015; Renshaw 1946).
Renshaw cells are differentially innervated by different types of α-MNs, with the relative contribution from larger MNs being greater: the ratio of type S, FR, and FF MNs to the excitation of Renshaw cells is ~1:2:4 (Cullheim and Kellerth 1978; Hultborn et al. 1988). In contrast to α-MNs, however, γ-MNs almost completely lack axon collaterals (Westbury 1982) and hence have minimal, if any, contribution to Renshaw inhibition (for review, see Windhorst 1990).
Renshaw cells have several targets (Hultborn et al. 1971; Ryall 1970; Wilson et al. 1964), but in this review we focus on their MN targets. Renshaw cells project back to and form inhibitory synapses (glycinergic and/or GABAergic) with MNs (Cullheim and Kellerth 1981; Eccles et al. 1954; Renshaw 1946; Schneider and Fyffe 1992), for the most part in the same or adjacent segments (Jankowska and Smith 1973; Kirkwood et al. 1981; Ryall et al. 1971; Saywell et al. 2013; van Keulen 1979) (Fig. 1B, h). In addition to projecting to the MNs that excite them (Moore et al. 2015), Renshaw cells project to homonymous and synergistic MNs (Eccles et al. 1954, 1961a; Renshaw 1946), and, as with other systems that coactivate α- and γ-MNs, Renshaw cells inhibit γ-MNs too (Ellaway 1971), although likely to a lesser extent than they inhibit α-MNs (Ellaway and Murphy 1981; Granit et al. 1957) (Fig. 1B, i).
It is not clear whether the effects of Renshaw inhibition differ on different MN types. Initially, it was thought that Renshaw inhibition was weighted toward tonically active MNs (i.e., S > FR > FF) (Friedman et al. 1981; Granit et al. 1957). On the other hand, synaptic currents produced by Renshaw cells have been shown to be similar across different MN types (Lindsay and Binder 1991). Whether there are differential functional effects of this inhibition is not known. For example, persistent firing in type S MNs (Lee and Heckman 1998) may be particularly sensitive to Renshaw inhibition (Bui et al. 2008; Hultborn et al. 2003). The specific effects of Renshaw inhibition of γ-MNs are not known.
The functional role of Renshaw inhibition is not clear, with many hypotheses having been raised (Alvarez and Fyffe 2007; Brownstone et al. 2015; Hultborn et al. 1979; Windhorst 1996). For example, Renshaw inhibition may serve to limit MN firing (Noga et al. 1987), curtail plateau potentials and persistent firing (Bui et al. 2008; Hultborn et al. 2003), or even support high firing rates of MNs (Obeidat et al. 2014). In addition, it has been proposed that they serve at the motor pool level to restructure recruitment (Hultborn et al. 1979) or at the microcircuit level to guide the tuning of MN properties (Brownstone et al. 2015).
Examination of the evolution and distribution of Renshaw cells has failed to unearth a unifying hypothesis of their function. Although about one-half of frog lumbar MNs have recurrent axon collaterals (Chmykhova and Babalian 1993), they do not lead to recurrent inhibition (Holemans and Meij 1968). There is evidence of a Renshaw-like feedback pathway in lampreys (Quinlan and Buchanan 2008), and of Renshaw-type neurons in chicks (Wenner and O’Donovan 1999), showing that they arose early in vertebrate evolution. In mammals, Renshaw cells form circuits with MNs innervating intercostal (Kirkwood et al. 1981) and other MMC MNs (Jankowska and Odutola 1980), neck MNs (Brink and Suzuki 1987), and phrenic MNs (Lipski et al. 1985). In circuits for limb MNs, Renshaw cells are involved in proximal motor pools more so than distal-innervating pools: Renshaw cells appear to be absent in motor pools innervating intrinsic hand and foot muscles in humans, as well as in distal muscles of the cat fore and hind limbs (for review, see Illert and Kümmel 1999; Pierrot-Desseilligny and Burke 2005; Piotrkiewicz and Młoźniak 2016). They are also absent from other pools, such as those innervating muscles of mastication (Shigenaga et al. 1989; Türker et al. 2007). Hence, although Renshaw inhibition evolved during vertebrate evolution, these neurons are involved more so with the control of MNs innervating proximal large muscles than in those involved in control of digits.
Whereas it is clear that Renshaw cells are not involved in circuits for all vulnerable MNs, Renshaw cell circuits have not been identified in circuits of ALS-resistant motor pools. Motor axon collaterals have been observed from neurons in Onuf’s nucleus (Sasaki 1994), but Renshaw inhibition appears to be absent (Mackel 1979). There is no evidence of Renshaw-type inhibition of oculomotor neurons (Baker and Precht 1972; Sasaki 1963).
In summary, Renshaw cells are activated by axon collaterals of α-MNs, in particular those in proximal motor pools that are vulnerable in ALS, and in turn inhibit α- and γ-MNs in homonymous and synergist MN pools.
HYPOTHESIS: MICROCIRCUIT IMBALANCE RESULTING FROM MOTONEURON DEATH LEADS TO DISEASE PROGRESSION
In ALS, the underlying neurodegenerative process, regardless of how it starts, ultimately leads to death of α-MNs. FF MNs degenerate first, followed by FR MNs, whereas S MNs are well preserved until late stages of the disease (Frey et al. 2000; Pun et al. 2006). Clearly, homeostatic mechanisms compensate for this loss given that the disease is not symptomatic until at least 30% of a MN pool degenerates (Lalancette-Hebert et al. 2016; Zang et al. 2005) (Fig. 1, C and D, and Fig. 2A, solid line). These homeostatic processes (Nijhout et al. 2014) likely involve multiple sites in the nervous system, from descending to spinal cord circuits to MNs and to neuromuscular junctions (NMJs). Below we explore an hypothesis in which the imbalance of spinal cord microcircuits caused by the initial loss of FF MNs leads to runaway circuit function and hence progression of MN dysfunction and, ultimately, death. [We direct the reader to van Zundert et al. (2012) for a complementary discussion.]
The underlying assumption of this hypothesis is that MNs are susceptible to excitotoxic cell death (for review, see van Zundert et al. 2012; King et al. 2016). We do not review mechanisms of excitotoxicity, which have been well studied and reviewed elsewhere (e.g., Dong et al. 2009; King et al. 2016; Vucic et al. 2014). Briefly, excessive activation of glutamate receptors can lead to neuronal death via the resulting high levels of intracellular calcium activity. This process has been known for decades and used experimentally to produce excitotoxic lesions via focal injections of kainic acid, a glutamate receptor agonist (e.g., Coyle and Schwarcz 1976; McGeer and McGeer 1976). Pathologically, excitotoxicity has been well studied, for example, in stroke, in which death of cells at the stroke core results in a high concentration of extracellular glutamate, which subsequently results in further neuronal death (for review, see Arundine and Tymianski 2004). Thus it is well established that excessive glutamate receptor activity can lead to neuronal death.
The hypothesis we present is also based on two key underlying principles: 1) γ-MNs are not affected in ALS (Mohajeri et al. 1998; Lalancette-Hebert et al. 2016; Vaughan et al. 2015); and 2) during most movement, α-MNs and γ-MNs are for the most part coactivated (vide supra; Granit 1975; Grillner 1969; Hagbarth 1993; Sjöström and Zangger 1975, 1976).
We will assume that the first step in the degenerative process is α-MN dysfunction (Fig. 1, asterisks), defined as a reduction in the capacity of a MN to activate its associated muscle fibers appropriately for the task at hand. α-MNs are known to be affected at presymptomatic stages (Schütz 2005), so the homeostatic processes would start very early, long before symptoms appear (Fig. 1, C and D). For a given input to a motor pool, the associated loss of NMJ activation, particularly affecting fast, high-force-producing muscle fibers, would result in a reduction in the force of muscle contraction. The homeostatic responses to this reduction would be aimed at facilitating force production such that normal movement could proceed. These responses could include the following: 1) changes at NMJs, including changes in synaptic transmission at the NMJ (Tremblay et al. 2017) and/or collateral sprouting at the NMJ following retraction of axon terminals such that slow motor axons transiently reoccupy vacated fast NMJs (Hegedus et al. 2008; Pun et al. 2006; Schaefer et al. 2005); 2) changes in MN physiology, including increased excitability or increased glutamate receptors to produce higher rates of firing in response to a given input (although it is unlikely that these intrinsic changes contribute to MN death) (Leroy et al. 2014); and/or 3) changes in circuits, including increased synaptic input to drive MNs to higher firing rates.
Given that initial dysfunction lies in the high force-producing activity of FF MNs, it is likely that homeostatic responses at the NMJ alone would be insufficient to compensate for this loss, and the homeostatic response of the nervous system would be to increase the firing rates of MNs. Although this could be accomplished in a cell autonomous process, e.g., by increasing membrane voltage-gated calcium channels (Heckman et al. 2003) or increasing available glutamate receptors, it seems likely that cell autonomous processes have evolved to maintain firing rate within set limits for any neuron type (O’Leary et al. 2014; Turrigiano and Nelson 2004). That is, in the absence of a Hebbian process, it seems unlikely that MNs would autonomously increase their firing frequency beyond their normal operating range. We thus propose that a key driving homeostatic response lies in circuit function, that is, increasing excitation of MNs by premotor circuits (Jiang et al. 2009) (Fig. 1, C and E, 1).
This increased activity in premotor circuits would lead to increased output of functional α-MNs as well as γ-MNs, with the former being the homeostatic response leading to restoration of force production. As an increasing number of MNs becomes affected, the homeostatic range would narrow, because it would become increasingly difficult for the remaining functional MNs and their associated circuits to produce the required force output (Fig. 2A, dashed line). In addition, this increased input to the motor pool would lead to increased γ-MN activity, as well (Fig. 1, C and E, 3), such that intrafusal fibers would contract out of proportion to extrafusal fibers (Fig. 1, C and E, 2 and 4). Increased attention to the task secondary to any perceived weakness may also increase γ-MN activity (Hospod et al. 2007). This in turn would lead to a relative increase in spindle sensitivity and thus increased afferent activity from the spindles, perhaps even during muscle contractions (Fig. 1C, 5). This could initially assist the homeostatic response, compensating for the reduction in motor pool output via “servo-assistance” (Hagbarth 1993; Watanabe and Hirayama 1976). Furthermore, such a compensatory response would be weighted to α-MNs, leading to some normalization of α-γ balance (although weighted to S over F). However, both the increased premotor input and the spindle afferent input will lead to increased activation of glutamate receptors (Fig. 1C, 6) and will thus contribute to excitotoxicity (Fig. 1, Glu). That is, following initial compensatory processes, there would be an escape from the homeostatic responses (Nijhout et al. 2014), ultimately resulting in MN degeneration and weakness (to the left on the curve in Fig. 2A).
Interestingly, a significant proportion of humans with ALS have abnormal sensory function, as well (Dyck et al. 1975; Hammad et al. 2007). Recent evidence from two different animal models has shown that the peripheral innervation of spindles by group Ia and II fibers is diminished in the presymptomatic stages of disease, even though the sensory neuron somata are unaffected at this time point (Dal Canto and Gurney 1995; Vaughan et al. 2015), and central synapses are affected only late in the disease process (Vaughan et al. 2015). These changes occur in parallel with α-MN degeneration in one animal model of familial ALS (SOD1G93A) but before changes in motor axons in another (TDP43A315T) (Vaughan et al. 2015). This reduction in peripheral innervation would limit the excitotoxic effects described above (Fig. 1E, thinner red line compared with Fig. 1C), whether the degeneration is a primary effect of the disease or a homeostatic response to an α-γ mismatch.
However, this peripheral circuit would not be the only dysfunctional MN microcircuit. In motor pools that have Renshaw inhibition, the reduction in α-MN activity would also lead to a loss of input to Renshaw cells (Fig. 1F, 7), which would reduce Renshaw pool activity. Although this would diminish α-MN inhibition (Fig. 1F, 8) and thus could aid in a homeostatic compensatory process leading toward normalization of muscle contraction, it would also diminish γ-MN inhibition (Fig. 1F, 9). That is, the loss of Renshaw cell activation by α-MNs would lead to a further imbalance of α-γ output (Fig. 1, C and D, 2 and 4), leading to a relative increase in spindle contraction (Fig. 1C, 5). This in turn would lead to an increase in spindle afferent activity (Fig. 1C, 6) and, as above, contribute to excitotoxic cell death (Fig. 1, Glu). In addition, given that Renshaw cells have been shown to limit plateau potentials in α-MNs (Bui et al. 2008; Hultborn et al. 2003), reduction in their activity could also lead to hyperexcitability of α-MNs and increased calcium entry, thus directly contributing to excitotoxic cell death. Although we propose that this circuit dysfunction may contribute to the degenerative process, it is clear that it is not necessary for MN degeneration, because not all vulnerable MN pools have Renshaw cell circuits.
Note that the selective vulnerability of large fast MNs/motor units to ALS may initially be due to their specific cellular profile, that is, the combination of particular molecular, physiological, and metabolic properties. However, the central and peripheral pattern of connectivity of these MNs, that is, their local microcircuits, may play a key role in the progression and propagation of MN degeneration.
Is there evidence of reduced Renshaw cell activity in ALS? The role of Renshaw cells in ALS has been reviewed elsewhere (Mazzocchio and Rossi 2010; Ramírez-Jarquín et al. 2014). There is evidence that recurrent inhibition is reduced in people with ALS (Raynor and Shefner 1994). At early asymptomatic stages in ALS animal models, Renshaw cells are spared (Knirsch et al. 2001; Morrison et al. 1996) (Fig. 1D, 8 and 9). At these presymptomatic stages, there is evidence of axonal sprouting of Renshaw cells leading to transient upregulation of glycinergic synapses on MNs (Chang and Martin 2009; Wootz et al. 2013). However, as the disease progresses, Renshaw cells receive progressively less input from MNs, with some Renshaw cells being completely denervated (Wootz et al. 2013). A proportion of Renshaw cells then dies over the course of the disease (Chang and Martin 2009). Thus there is evidence that a reduction in MN inputs to Renshaw cells leads to a reduction in recurrent inhibition but that Renshaw cells initially compensate by sprouting on remaining viable MNs (Wootz et al. 2013). These initial changes would be homeostatic, but as disease progresses, there would be an escape from this homeostatic process.
Although there is no evidence to date of α-γ imbalance in ALS, the hypothesis could be readily tested. Though studies of short-latency reflexes in reduced preparations in ALS models can highlight changes in the integrity of these pathways (the afferents, the synapses on MNs, and the motor output), they reveal neither afferent nor γ-MN activity in the intact animal (Jiang et al. 2009). Reflex studies in the awake, intact animal could examine underlying spindle tone compared with that in wild-type mice, but perhaps the ideal experiment would involve microneurography in humans at different stages of ALS, where activity in identified axons can be recorded (Dimitriou 2014; Edin and Vallbo 1990). The hypothesis presented here could thus be readily studied.
One clinical sign that would be seen with this maladaptive plasticity would be spasticity. ALS patients with supraspinal disease tend to have a greater degree of spasticity than those with predominantly spinal disease (Gordon et al. 2009). The mechanisms underlying this spasticity may therefore be similar to those in spinal cord injury, such as changes in serotonin receptors (Murray et al. 2010) or chloride reversal potentials (Boulenguez et al. 2010). However, the α-γ MN imbalance described above could also contribute to spasticity, with muscle stretches producing excessive spindle afferent activity due to the high γ-MN tone (Gladden et al. 1998). That is, if sufficient numbers of α-MNs remain functional, the relative increase in γ-MN activity would lead to an escape to the right of the homeostatic curve (Fig. 2A).
In summary, we propose that the homeostatic response to reduced neuromuscular activity following early MN dysfunction would be for the nervous system to increase inputs to α-MNs. With ongoing loss of α-MN function, these increased inputs would lead to a shift in the α-γ balance, leading to increased afferent input to α-MNs. Concomitantly, in some motor pools there would be a reduction in Renshaw cell inhibition resulting from reduced MN excitation of Renshaw cells. The changes to both Ia-MN and MN-Renshaw cell circuits would initially be homeostatic but would ultimately lead to excitotoxic death of MNs. Furthermore, we note that the increased inputs to MNs will affect more than a single motor pool [consider muscle synergies, e.g., Takei et al. (2017); respiratory-locomotor coupling, e.g., Romaniuk et al. (1994); reticulospinal effects on multiple body segments, e.g., Drew and Rossignol (1990)] and hence could contribute to symptomatic spread of the disease from one MN pool to another (Ravits 2014). That is, though not a cause of ALS, such circuit dysfunction could contribute to its progression within and beyond motor pools.
TARGETING MICROCIRCUITS TO SLOW DISEASE PROGRESSION?
If this hypothesis is correct, then therapies that target this microcircuit dysfunction should slow the progression of disease (Fig. 2B). Microcircuit therapy for ALS, as defined in this article, could thus be devised to target, for example, γ-MNs, to reduce their activity, or Renshaw cells, to increase their activity. Whereas we predict on the basis of the hypothesis presented here that either approach would slow the progression of the disease, overzealous treatment of either could also lead to a reduction in MN output and hence weakness. We suggest, however, that there would be a therapeutic window in which disease progression could be slowed and quality of life thus improved by increasing the duration of time that people will have functional muscle contraction (Fig. 2B).
There is no evidence in humans that such therapies might be effective, and we do not yet have the knowledge or methods to specifically target these microcircuits, but there is some recent evidence provided by animal models of ALS. In two models, the progression of disease was slowed concomitant with a reduction in γ-MN activity. In the first, muscle spindles were targeted, and their degeneration was associated with a loss of γ-MNs (Lalancette-Hebert et al. 2016). In the second, γ-MNs were selectively targeted genetically, reducing their population by half. In both instances, there was a slower rate of α-MN loss, signs of ALS were significantly delayed, and survival was prolonged (Lalancette-Hebert et al. 2016). These findings are consistent with the hypothesis presented here.
To target these circuits in humans, if there are sufficient data to support the concept, a gene therapy approach could be considered (e.g., https://clinicaltrials.gov/ct2/show/NCT03306277 for spinal muscular atrophy). As we learn more about gene expression profiles in mature MNs or Renshaw cells, for example, promoters for these genes could be used in viral approaches to drive expression confined to these populations. For example, introduction of potassium channels to γ-MNs or inhibitory “designer receptors exclusively activated by designer drugs” (DREADDs) could be expressed to reduce activity (in the latter case, with oral clozapine). Perhaps the advantage of a DREADD approach would be that dosage could be titrated and the therapeutic window thus defined (Fig. 2B).
In neurodegenerative diseases, much research is necessarily focused on causation, with the concept being that identification of the cause of the disease will lead to strategies to prevent or cure the disease. On the other hand, clinical treatment is focused on symptom amelioration, because that is where our state of knowledge is. We suggest that there is a third possibility for treatment that may be unrelated to the cause of the disease: treatment of dysfunctional microcircuits to slow the progression of the disease.
GRANTS
R. Brownstone is supported by Brain Research UK, and the motoneuron work in his laboratory is supported by Wellcome (grant no. 110193).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.B. conceived and designed research; C.L. prepared figures; R.M.B. and C.L. drafted manuscript; R.M.B. and C.L. edited and revised manuscript; R.M.B. and C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The concepts in this paper were first presented in two fora by R. M. Brownstone. The first was a P2ALS meeting held at Columbia University in May, 2011 and supported by Project A.L.S. The second was a Cold Spring Harbor Symposium entitled “Development and Evolution of the Human Motor System in relation to ALS & FTD,” held in April 2013 and funded by the Greater New York Chapter of the ALS Association. We are grateful to these organizations for funding these meetings, for the education about ALS, and for the associated stimuli to focus our thoughts on this hypothesis.
REFERENCES
- Adal MN, Barker D. Intramuscular branching of fusimotor fibres. J Physiol 177: 288–299, 1965. doi: 10.1113/jphysiol.1965.sp007592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Dewey DE, McMillin P, Fyffe RE. Distribution of cholinergic contacts on Renshaw cells in the rat spinal cord: a light microscopic study. J Physiol 515: 787–797, 1999. doi: 10.1111/j.1469-7793.1999.787ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Fyffe RE. The continuing case for the Renshaw cell. J Physiol 584: 31–45, 2007. doi: 10.1113/jphysiol.2007.136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B, Hulliger M, Johansson H, Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group I muscle afferent fibres in the hind limb of the cat. J Physiol 335: 237–253, 1983. doi: 10.1113/jphysiol.1983.sp014531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci 61: 657–668, 2004. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakels R, Kernell D. Matching between motoneurone and muscle unit properties in rat medial gastrocnemius. J Physiol 463: 307–324, 1993. doi: 10.1113/jphysiol.1993.sp019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R, Precht W. Electrophysiological properties of trochlear motoneurons as revealed by IVth nerve stimulation. Exp Brain Res 14: 127–157, 1972. doi: 10.1007/BF00234796. [DOI] [PubMed] [Google Scholar]
- Bessou P, Cabelguen JM, Joffroy M, Montoya R, Pagès B. Efferent and afferent activity in a gastrocnemius nerve branch during locomotion in the thalamic cat. Exp Brain Res 64: 553–568, 1986. doi: 10.1007/BF00340493. [DOI] [PubMed] [Google Scholar]
- Bessou P, Emonet-Dénand F, Laporte Y. Motor fibres innervating extrafusal and intrafusal muscle fibres in the cat. J Physiol 180: 649–672, 1965. doi: 10.1113/jphysiol.1965.sp007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P, Joffroy M, Montoya R, Pagès B. Evidence of the co-activation of alpha-motoneurones and static gamma-motoneurones of the sartorius medialis muscle during locomotion in the thalamic cat. Exp Brain Res 82: 191–198, 1990. doi: 10.1007/BF00230851. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 16: 302–307, 2010. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- Brink EE, Suzuki I. Recurrent inhibitory connexions among neck motoneurones in the cat. J Physiol 383: 301–326, 1987. doi: 10.1113/jphysiol.1987.sp016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Weaver LC. The dark side of neuroplasticity. Exp Neurol 235: 133–141, 2012. doi: 10.1016/j.expneurol.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol 274: 111–127, 1978. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat’s lumbosacral spinal cord. J Physiol 313: 121–140, 1981. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Butler RG. Studies on the site of termination of static and dynamic fusimotor fibres within muscle spindles of the tenuissimus muscle of the cat. J Physiol 233: 553–573, 1973. doi: 10.1113/jphysiol.1973.sp010323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Bui TV, Stifani N. Spinal circuits for motor learning. Curr Opin Neurobiol 33: 166–173, 2015. doi: 10.1016/j.conb.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Buchthal F, Schmalbruch H. Motor unit of mammalian muscle. Physiol Rev 60: 90–142, 1980. doi: 10.1152/physrev.1980.60.1.90. [DOI] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK. Relative location of inhibitory synapses and persistent inward currents determines the magnitude and mode of synaptic amplification in motoneurons. J Neurophysiol 99: 583–594, 2008. doi: 10.1152/jn.00718.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Stifani N, Akay T, Brownstone RM. Spinal microcircuits comprising dI3 interneurons are necessary for motor functional recovery following spinal cord transection. eLife 5: e21715, 2016. doi: 10.7554/eLife.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Firing patterns of gastrocnemius motor units in the decerebrate cat. J Physiol 196: 631–654, 1968a. doi: 10.1113/jphysiol.1968.sp008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol 196: 605–630, 1968b. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Glenn LL. Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. J Comp Neurol 372: 465–485, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE 3rd. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234: 723–748, 1973. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Rymer WZ. Relative strength of synaptic input from short-latency pathways to motor units of defined type in cat medial gastrocnemius. J Neurophysiol 39: 447–458, 1976. doi: 10.1152/jn.1976.39.3.447. [DOI] [PubMed] [Google Scholar]
- Burke RE, Strick PL, Kanda K, Kim CC, Walmsley B. Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. J Neurophysiol 40: 667–680, 1977. doi: 10.1152/jn.1977.40.3.667. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Konakci KZ, Blumer R. Sensory control of extraocular muscles. Prog Brain Res 151: 81–93, 2006. doi: 10.1016/S0079-6123(05)51003-3. [DOI] [PubMed] [Google Scholar]
- Chang Q, Martin LJ. Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a quantitative confocal analysis. Am J Pathol 174: 574–585, 2009. doi: 10.2353/ajpath.2009.080557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charcot JM, Joffroy A. Deux cas d’atrophie musculaire progressive avec lésion de la substance grise et des faisceaux antéro-latéraux de la moelle épinière. Arch Physiol 2: 744–760, 1869. [Google Scholar]
- Chennells M, Floyd W, Gould R. Muscle spindles in the external anal sphincter of the cat (Abstract). J Physiol 151, Suppl: 23P–24P, 1960. [Google Scholar]
- Chmykhova NM, Babalian AL. Structure of recurrent axon collaterals of frog lumbar motoneurons as revealed by intracellular HRP labelling. Brain Res 603: 289–295, 1993. doi: 10.1016/0006-8993(93)91250-V. [DOI] [PubMed] [Google Scholar]
- Corda M, Eklund G, Von Euler C. External intercostal and phrenic alpha-motor responses to changes in respiratory load. Acta Physiol Scand 63: 391–400, 1965a. doi: 10.1111/j.1748-1716.1965.tb04079.x. [DOI] [PubMed] [Google Scholar]
- Corda M, Voneuler C, Lennerstrand G. Proprioceptive innervation of the diaphragm. J Physiol 178: 161–177, 1965b. doi: 10.1113/jphysiol.1965.sp007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington’s chorea. Nature 263: 244–246, 1976. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Fleshman JW, Glenn LL, Burke RE. Membrane area and dendritic structure in type-identified triceps surae alpha motoneurons. J Comp Neurol 255: 68–81, 1987. doi: 10.1002/cne.902550106. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Kellerth JO. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different functional types of muscle unit. J Physiol 281: 301–313, 1978. doi: 10.1113/jphysiol.1978.sp012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S, Kellerth JO. Two kinds of recurrent inhibition of cat spinal alpha-motoneurones as differentiated pharmacologically. J Physiol 312: 209–224, 1981. doi: 10.1113/jphysiol.1981.sp013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res 676: 25–40, 1995. doi: 10.1016/0006-8993(95)00063-V. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D, Pennybacker JB. Fibrillation and fasciculation in voluntary muscle. Brain 61: 311–312, 1938. doi: 10.1093/brain/61.3.311. [DOI] [Google Scholar]
- Dimitriou M. Human muscle spindle sensitivity reflects the balance of activity between antagonistic muscles. J Neurosci 34: 13644–13655, 2014. doi: 10.1523/JNEUROSCI.2611-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditsworth D, Maldonado M, McAlonis-Downes M, Sun S, Seelman A, Drenner K, Arnold E, Ling SC, Pizzo D, Ravits J, Cleveland DW, Da Cruz S. Mutant TDP-43 within motor neurons drives disease onset but not progression in amyotrophic lateral sclerosis. Acta Neuropathol 133: 907–922, 2017. doi: 10.1007/s00401-017-1698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogiel A. Die Endigungen der sensiblen Nerven in den Augenmuskeln und deren Sehnen beim Menschen und den Säugetieren. Arch Mikrosk Anat 68: 501–526, 1906. doi: 10.1007/BF02979882. [DOI] [Google Scholar]
- Donaldson IM. The functions of the proprioceptors of the eye muscles. Philos Trans R Soc Lond B Biol Sci 355: 1685–1754, 2000. doi: 10.1098/rstb.2000.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 30: 379–387, 2009. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J Neurophysiol 64: 767–781, 1990. doi: 10.1152/jn.1990.64.3.767. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Stevens JC, Mulder DW, Espinosa RE. Frequency of nerve fiber degeneration of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. Morphometry of deep and superficial peroneal nerves. Neurology 25: 781–785, 1975. doi: 10.1212/WNL.25.8.781. [DOI] [PubMed] [Google Scholar]
- Eccles JC. Synaptic potentials of motoneurones. J Neurophysiol 9: 87–120, 1946. doi: 10.1152/jn.1946.9.2.87. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Iggo A, Ito M. Distribution of recurrent inhibition among motoneurones. J Physiol 159: 479–499, 1961a. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Iggo A, Lundberg A. Electrophysiological studies on gamma motoneurones. Acta Physiol Scand 50: 32–40, 1960. doi: 10.1111/j.1748-1716.1960.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Iggo A, Lundberg A. Electrophysiological investigations on Renshaw cells. J Physiol 159: 461–478, 1961b. doi: 10.1113/jphysiol.1961.sp006821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Durations of after-hyperpolarization of motoneurones supplying fast and slow muscles. Nature 179: 866–868, 1957a. doi: 10.1038/179866b0. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol 137: 22–50, 1957b. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol 142: 275–291, 1958. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol 126: 524–562, 1954. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Grillner S, Sjöström A, Zangger P. Central generation of locomotion in vertebrates. In: Neural Control of Locomotion. Advances in Behavioral Biology, edited by Herman RM, Grillner S, Stein PS, Stuart DG. Boston, MA: Springer, vol. 18, 1976, p. 439–464. [Google Scholar]
- Edin BB, Vallbo AB. Muscle afferent responses to isometric contractions and relaxations in humans. J Neurophysiol 63: 1307–1313, 1990. doi: 10.1152/jn.1990.63.6.1307. [DOI] [PubMed] [Google Scholar]
- Edström L, Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry 31: 424–433, 1968. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol 216: 419–439, 1971. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Murphy PR. A comparison of the recurrent inhibition of alpha- and gamma-motoneurones in the cat. J Physiol 315: 43–58, 1981. doi: 10.1113/jphysiol.1981.sp013731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Taylor A, Durbaba R. Muscle spindle and fusimotor activity in locomotion. J Anat 227: 157–166, 2015. doi: 10.1111/joa.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114: 2283–2301, 1991. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci 20: 2534–2542, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WA, Sypert GW, Munson JB, Fleshman JW. Recurrent inhibition in type-identified motoneurons. J Neurophysiol 46: 1349–1359, 1981. doi: 10.1152/jn.1981.46.6.1349. [DOI] [PubMed] [Google Scholar]
- Friese A, Kaltschmidt JA, Ladle DR, Sigrist M, Jessell TM, Arber S. Gamma and alpha motor neurons distinguished by expression of transcription factor Err3. Proc Natl Acad Sci USA 106: 13588–13593, 2009. doi: 10.1073/pnas.0906809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe RE. The morphology of group II muscle afferent fibre collaterals (Abstract). J Physiol 296 Suppl: 39P–40P, 1979. [PubMed] [Google Scholar]
- Gardiner PF. Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. J Neurophysiol 69: 1160–1170, 1993. doi: 10.1152/jn.1993.69.4.1160. [DOI] [PubMed] [Google Scholar]
- Garry RC, Garven HS. The ganglia, afferent nerve-endings and musculature of the urethra in the cat (Abstract). J Physiol 139 Suppl: 1P–2P, 1957. [PubMed] [Google Scholar]
- Gladden MH, Jankowska E, Czarkowska-Bauch J. New observations on coupling between group II muscle afferents and feline gamma-motoneurones. J Physiol 512: 507–520, 1998. doi: 10.1111/j.1469-7793.1998.507be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PH, Cheng B, Katz IB, Mitsumoto H, Rowland LP. Clinical features that distinguish PLS, upper motor neuron-dominant ALS, and typical ALS. Neurology 72: 1948–1952, 2009. doi: 10.1212/WNL.0b013e3181a8269b. [DOI] [PubMed] [Google Scholar]
- Granit R. The functional role of the muscle spindles–facts and hypotheses. Brain 98: 531–556, 1975. doi: 10.1093/brain/98.4.531. [DOI] [PubMed] [Google Scholar]
- Granit R, Pascoe JE, Steg G. The behaviour of tonic alpha and gamma motoneurones during stimulation of recurrent collaterals. J Physiol 138: 381–400, 1957. doi: 10.1113/jphysiol.1957.sp005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffard S, Verny M, Bonnet AM, Beinis JY, Gallinari C, Meaume S, Piette F, Hauw JJ, Duyckaerts C. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol 63: 584–588, 2006. doi: 10.1001/archneur.63.4.584. [DOI] [PubMed] [Google Scholar]
- Grillner S. Supraspinal and segmental control of static and dynamic gamma-motoneurones in the cat. Acta Physiol Scand Suppl 327: 1–34, 1969. [PubMed] [Google Scholar]
- Hagbarth KE. Microneurography and applications to issues of motor control: Fifth Annual Stuart Reiner Memorial Lecture. Muscle Nerve 16: 693–705, 1993. doi: 10.1002/mus.880160702. [DOI] [PubMed] [Google Scholar]
- Hammad M, Silva A, Glass J, Sladky JT, Benatar M. Clinical, electrophysiologic, and pathologic evidence for sensory abnormalities in ALS. Neurology 69: 2236–2242, 2007. doi: 10.1212/01.wnl.0000286948.99150.16. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Analysis of effective synaptic currents generated by homonymous Ia afferent fibers in motoneurons of the cat. J Neurophysiol 60: 1946–1966, 1988. doi: 10.1152/jn.1988.60.6.1946. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci 26: 688–695, 2003. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hedlund E, Karlsson M, Osborn T, Ludwig W, Isacson O. Global gene expression profiling of somatic motor neuron populations with different vulnerability identify molecules and pathways of degeneration and protection. Brain 133: 2313–2330, 2010. doi: 10.1093/brain/awq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 28: 154–164, 2007. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol 586: 3337–3351, 2008. doi: 10.1113/jphysiol.2007.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Holemans KC, Meij HS. An analysis of some inhibitory mechanisms in the spinal cord of the frog (Xenopus laevis). Pflugers Arch 303: 289–310, 1968. [PubMed] [Google Scholar]
- Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB. Prevalence and effect of problematic spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil 98: 1132–1138, 2016. doi: 10.1016/j.apmr.2016.09.124. [DOI] [PubMed] [Google Scholar]
- Hongo T. Patterns of spinal projection of muscle spindle group II fibres. In: Muscle Afferents and Spinal Control of Movement, edited by Jami L, Pierrot-Deseilligny E, Zytnicki D. Oxford: Pergamon, 1992, p. 389–394. [Google Scholar]
- Hospod V, Aimonetti J-M, Roll JP, Ribot-Ciscar E. Changes in human muscle spindle sensitivity during a proprioceptive attention task. J Neurosci 27: 5172–5178, 2007. doi: 10.1523/JNEUROSCI.0572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber GC. Neuro-muscular spindles in the intercostal muscles of the cat. Am J Anat 1: 520–521, 1902. [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol 552: 945–952, 2003. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Jankowska E, Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol 215: 591–612, 1971. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Lindström S, Wigström H. On the function of recurrent inhibition in the spinal cord. Exp Brain Res 37: 399–403, 1979. doi: 10.1007/BF00237722. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Lipski J, Mackel R, Wigström H. Distribution of recurrent inhibition within a motor nucleus. I. Contribution from slow and fast motor units to the excitation of Renshaw cells. Acta Physiol Scand 134: 347–361, 1988. doi: 10.1111/j.1748-1716.1988.tb08502.x. [DOI] [PubMed] [Google Scholar]
- Hunt CC. The reflex activity of mammalian small-nerve fibres. J Physiol 115: 456–469, 1951. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illert M, Kümmel H. Reflex pathways from large muscle spindle afferents and recurrent axon collaterals to motoneurones of wrist and digit muscles: a comparison in cats, monkeys and humans. Exp Brain Res 128: 13–19, 1999. doi: 10.1007/s002210050812. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirano A. Current problems in the pathology of amyotrophic lateral sclerosis. In: Progress in Neuropathology, edited by Zimmerman HM. New York: Raven, 1979, p. 277–298. [Google Scholar]
- Jankowska E, Odutola A. Crosses and uncrossed synaptic actions on motoneurones of back muscles in the cat. Brain Res 194: 65–78, 1980. doi: 10.1016/0006-8993(80)91319-0. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Smith DO. Antidromic activation of Renshaw cells and their axonal projections. Acta Physiol Scand 88: 198–214, 1973. doi: 10.1111/j.1748-1716.1973.tb05447.x. [DOI] [PubMed] [Google Scholar]
- Jansen JK, Matthews PB. The effects of fusimotor activity on the static responsiveness of primary and secondary endings of muscle spindles in the decerebrate cat. Acta Physiol Scand 55: 376–386, 1962. doi: 10.1111/j.1748-1716.1962.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Jiang M, Schuster JE, Fu R, Siddique T, Heckman CJ. Progressive changes in synaptic inputs to motoneurons in adult sacral spinal cord of a mouse model of amyotrophic lateral sclerosis. J Neurosci 29: 15031–15038, 2009. doi: 10.1523/JNEUROSCI.0574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci 33: 409–440, 2010. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Dyck PJ, Shimono M, Okazaki H, Tateishi J, Doi H. Morphometric comparison of the vulnerability of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 40: 667–675, 1981. doi: 10.1097/00005072-198111000-00008. [DOI] [PubMed] [Google Scholar]
- Keller EL, Robinson DA. Absence of a stretch reflex in extraocular muscles of the monkey. J Neurophysiol 34: 908–919, 1971. doi: 10.1152/jn.1971.34.5.908. [DOI] [PubMed] [Google Scholar]
- Kernell D. Rhythmic properties of motoneurones innervating muscle fibres of different speed in m. gastrocnemius medialis of the cat. Brain Res 160: 159–162, 1979. doi: 10.1016/0006-8993(79)90612-7. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Threshold current for repetitive impulse firing in motoneurones innervating muscle fibres of different fatigue sensitivity in the cat. Brain Res 229: 193–196, 1981. doi: 10.1016/0006-8993(81)90756-3. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet 377: 942–955, 2011. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- King AE, Woodhouse A, Kirkcaldie MT, Vickers JC. Excitotoxicity in ALS: Overstimulation, or overreaction? Exp Neurol 275: 162–171, 2016. doi: 10.1016/j.expneurol.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature 252: 243–244, 1974. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol 322: 287–314, 1982. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA, Westgaard RH. Recurrent inhibition of intercostal motoneurones in the cat. J Physiol 319: 111–130, 1981. doi: 10.1113/jphysiol.1981.sp013895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knirsch U, Sturm S, Reuter A, Bachus R, Gosztonyi G, Voelkel H, Ludolph AC. Calcineurin A and calbindin immunoreactivity in the spinal cord of G93A superoxide dismutase transgenic mice. Brain Res 889: 234–238, 2001. doi: 10.1016/S0006-8993(00)03048-1. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Hunt CC, Quilliam JP. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol 14: 29–54, 1951. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- Lagerbäck PA, Ronnevi LO. An ultrastructural study of serially sectioned Renshaw cells. I. Architecture of the cell body, axon hillock, initial axon segment and proximal dendrites. Brain Res 235: 1–15, 1982. doi: 10.1016/0006-8993(82)90192-5. [DOI] [PubMed] [Google Scholar]
- Lagerbäck PA, Ronnevi LO, Cullheim S, Kellerth JO. An ultrastructural study of the synaptic contacts of alpha 1-motoneuron axon collaterals. II. Contacts in lamina VII. Brain Res 222: 29–41, 1981. doi: 10.1016/0006-8993(81)90938-0. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Sharma A, Lyashchenko AK, Shneider NA. Gamma motor neurons survive and exacerbate alpha motor neuron degeneration in ALS. Proc Natl Acad Sci USA 113: E8316–E8325, 2016. doi: 10.1073/pnas.1605210113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann G. [Afferent innervation of musculus sphincter ani externus of men]. Acta Neuropathol 62: 254–256, 1984a. doi: 10.1007/BF00691860. [DOI] [PubMed] [Google Scholar]
- Lassmann G. [Muscle spindles and sensory nerve endings in the urethral sphincter]. Acta Neuropathol 63: 344–346, 1984b. doi: 10.1007/BF00687343. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Leroy F, Lamotte d’Incamps B, Imhoff-Manuel RD, Zytnicki D. Early intrinsic hyperexcitability does not contribute to motoneuron degeneration in amyotrophic lateral sclerosis. eLife 3: e04046, 2014. doi: 10.7554/eLife.04046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell EG, Sherrington CS. Recruitment and some other factors of reflex inhibition. Proc R Soc Lond B Biol Sci 97: 488–518, 1925. doi: 10.1098/rspb.1925.0016. [DOI] [Google Scholar]
- Lienbacher K, Horn AKE. Palisade endings and proprioception in extraocular muscles: a comparison with skeletal muscles. Biol Cybern 106: 643–655, 2012. doi: 10.1007/s00422-012-0519-1. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Binder MD. Distribution of effective synaptic currents underlying recurrent inhibition in cat triceps surae motoneurons. J Neurophysiol 65: 168–177, 1991. doi: 10.1152/jn.1991.65.2.168. [DOI] [PubMed] [Google Scholar]
- Lipski J, Fyffe RE, Jodkowski J. Recurrent inhibition of cat phrenic motoneurons. J Neurosci 5: 1545–1555, 1985. doi: 10.1523/JNEUROSCI.05-06-01545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DP. Conduction and synaptic transmission of the reflex response to stretch in spinal cats. J Neurophysiol 6: 317–326, 1943a. doi: 10.1152/jn.1943.6.4.317. [DOI] [Google Scholar]
- Lloyd DP. Neuron patterns controlling transmission of ipsilateral hind limb reflexes in cat. J Neurophysiol 6: 293–315, 1943b. doi: 10.1152/jn.1943.6.4.293. [DOI] [Google Scholar]
- Lloyd DP. Facilitation and inhibition of spinal motoneurons. J Neurophysiol 9: 421–438, 1946a. doi: 10.1152/jn.1946.9.6.421. [DOI] [PubMed] [Google Scholar]
- Lloyd DP. Integrative pattern of excitation and inhibition in two-neuron reflex arcs. J Neurophysiol 9: 439–444, 1946b. doi: 10.1152/jn.1946.9.6.439. [DOI] [PubMed] [Google Scholar]
- Ma SY, Röyttä M, Rinne JO, Collan Y, Rinne UK. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson’s disease using disector counts. J Neurol Sci 151: 83–87, 1997. doi: 10.1016/S0022-510X(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Mackel R. Segmental and descending control of the external urethral and anal sphincters in the cat. J Physiol 294: 105–122, 1979. doi: 10.1113/jphysiol.1979.sp012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, DeSantis M, Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J Morphol 143: 397–408, 1974. doi: 10.1002/jmor.1051430404. [DOI] [PubMed] [Google Scholar]
- Mannen T, Iwata M, Toyokura Y, Nagashima K. Preservation of a certain motoneurone group of the sacral cord in amyotrophic lateral sclerosis: its clinical significance. J Neurol Neurosurg Psychiatry 40: 464–469, 1977. doi: 10.1136/jnnp.40.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Heckman CJ. Simultaneous intracellular recording of a lumbar motoneuron and the force produced by its motor unit in the adult mouse in vivo. J Vis Exp: e4312, 2012. doi: 10.3791/4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Zytnicki D. Alpha, beta and gamma motoneurons: functional diversity in the motor system’s final pathway. J Integr Neurosci 10: 243–276, 2011. doi: 10.1142/S0219635211002786. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7: 563–574, 2006. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S. Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry. J Neurophysiol 106: 1969–1984, 2011. doi: 10.1152/jn.00368.2011. [DOI] [PubMed] [Google Scholar]
- Matthews PB. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci 47: 324–333, 1962. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Rossi A. Role of Renshaw cells in amyotrophic lateral sclerosis. Muscle Nerve 41: 441–443, 2010. doi: 10.1002/mus.21602. [DOI] [PubMed] [Google Scholar]
- McDonagh JC, Binder MD, Reinking RM, Stuart DG. Tetrapartite classification of motor units of cat tibialis posterior. J Neurophysiol 44: 696–712, 1980. doi: 10.1152/jn.1980.44.4.696. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Duplication of biochemical changes of Huntington’s chorea by intrastriatal injections of glutamic and kainic acids. Nature 263: 517–519, 1976. doi: 10.1038/263517a0. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Henneman E. Terminals of single Ia fibers: distribution within a pool of 300 homonymous motor neurons. Science 160: 96–98, 1968. doi: 10.1126/science.160.3823.96. [DOI] [PubMed] [Google Scholar]
- Mendelsohn AI, Simon CM, Abbott LF, Mentis GZ, Jessell TM. Activity regulates the incidence of heteronymous sensory-motor connections. Neuron 87: 111–123, 2015. doi: 10.1016/j.neuron.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri MH, Figlewicz DA, Bohn MC. Selective loss of alpha motoneurons innervating the medial gastrocnemius muscle in a mouse model of amyotrophic lateral sclerosis. Exp Neurol 150: 329–336, 1998. doi: 10.1006/exnr.1998.6758. [DOI] [PubMed] [Google Scholar]
- Moore NJ, Bhumbra GS, Foster JD, Beato M. Synaptic connectivity between Renshaw cells and motoneurons in the recurrent inhibitory circuit of the spinal cord. J Neurosci 35: 13673–13686, 2015. doi: 10.1523/JNEUROSCI.2541-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BM, Gordon JW, Ripps ME, Morrison JH. Quantitative immunocytochemical analysis of the spinal cord in G86R superoxide dismutase transgenic mice: neurochemical correlates of selective vulnerability. J Comp Neurol 373: 619–631, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Munson JB, Sypert GW, Zengel JE, Lofton SA, Fleshman JW. Monosynaptic projections of individual spindle group II afferents to type-identified medial gastrocnemius motoneurons in the cat. J Neurophysiol 48: 1164–1174, 1982. doi: 10.1152/jn.1982.48.5.1164. [DOI] [PubMed] [Google Scholar]
- Murphy PR. The patterns of discharge of static and dynamic gamma motoneurones in the decerebrate rabbit. J Physiol 333: 29–37, 1982. doi: 10.1113/jphysiol.1982.sp014436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, Martin HA. Fusimotor discharge patterns during rhythmic movements. Trends Neurosci 16: 273–278, 1993. doi: 10.1016/0166-2236(93)90181-K. [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D’Amico J, Harvey PJ, Li X, Harris RLW, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16: 694–700, 2010. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout HF, Best J, Reed MC. Escape from homeostasis. Math Biosci 257: 104–110, 2014. doi: 10.1016/j.mbs.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Nijssen J, Comley LH, Hedlund E. Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis. Acta Neuropathol 133: 863–885, 2017. doi: 10.1007/s00401-017-1708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Young WG, Yeung G, Shah RA, Gordon JW, Bloom FE, Morrison JH, Hof PR. Differential vulnerability of oculomotor, facial, and hypoglossal nuclei in G86R superoxide dismutase transgenic mice. J Comp Neurol 416: 112–125, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Noga BR, Shefchyk SJ, Jamal J, Jordan LM. The role of Renshaw cells in locomotion: antagonism of their excitation from motor axon collaterals with intravenous mecamylamine. Exp Brain Res 66: 99–105, 1987. doi: 10.1007/BF00236206. [DOI] [PubMed] [Google Scholar]
- O’Leary T, Williams AH, Franci A, Marder E. Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron 82: 809–821, 2014. [Erratum in Neuron 88: 1308, 2015.] doi: 10.1016/j.neuron.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeidat AZ, Nardelli P, Powers RK, Cope TC. Modulation of motoneuron firing by recurrent inhibition in the adult rat in vivo. J Neurophysiol 112: 2302–2315, 2014. doi: 10.1152/jn.00358.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters OM, Ghasemi M, Brown RH Jr. Emerging mechanisms of molecular pathology in ALS. J Clin Invest 125: 1767–1779, 2015. doi: 10.1172/JCI71601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Desseilligny E, Burke DC. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge, UK: Cambridge University Press, 2005, p. 151–196. doi: 10.1017/CBO9780511545047.005. [DOI] [Google Scholar]
- Piotrkiewicz M, Młoźniak D. Are human digit muscles devoid of recurrent inhibition? Front Cell Neurosci 9: 507, 2016. doi: 10.3389/fncel.2015.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Ellaway P. Sensory systems in the control of movement. Compr Physiol 2: 2615–2627, 2012. doi: 10.1002/cphy.c100086. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Zangger P, Appenteng K. ‘Fusimotor set’: new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res 339: 136–140, 1985. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci 9: 408–419, 2006. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Quinlan KA, Buchanan JT. Cellular and synaptic actions of acetylcholine in the lamprey spinal cord. J Neurophysiol 100: 1020–1031, 2008. doi: 10.1152/jn.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Jarquín UN, Lazo-Gómez R, Tovar-Y-Romo LB, Tapia R. Spinal inhibitory circuits and their role in motor neuron degeneration. Neuropharmacology 82: 101–107, 2014. doi: 10.1016/j.neuropharm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Rao HM, Prevosto V. Proprioceptive eye position signals are still missing a sensory receptor. J Neurosci 33: 10585–10587, 2013. doi: 10.1523/JNEUROSCI.1594-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravits J. Focality, stochasticity and neuroanatomic propagation in ALS pathogenesis. Exp Neurol 262: 121–126, 2014. doi: 10.1016/j.expneurol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Raynor EM, Shefner JM. Recurrent inhibition is decreased in patients with amyotrophic lateral sclerosis. Neurology 44: 2148–2153, 1994. doi: 10.1212/WNL.44.11.2148. [DOI] [PubMed] [Google Scholar]
- Renshaw B. Central effects of centripetal impulses in axons of spinal ventral roots. J Neurophysiol 9: 191–204, 1946. doi: 10.1152/jn.1946.9.3.191. [DOI] [PubMed] [Google Scholar]
- Romaniuk JR, Kasicki S, Kazennikov OV, Selionov VA. Respiratory responses to stimulation of spinal or medullary locomotor structures in decerebrate cats. Acta Neurobiol Exp (Wars) 54: 11–17, 1994. [PubMed] [Google Scholar]
- Ryall RW. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol 33: 257–270, 1970. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- Ryall RW, Piercey MF, Polosa C. Intersegmental and intrasegmental distribution of mutual inhibition of Renshaw cells. J Neurophysiol 34: 700–707, 1971. doi: 10.1152/jn.1971.34.4.700. [DOI] [PubMed] [Google Scholar]
- Sasaki K. Electrophysiological studies on oculomotor neurons of the cat. Jpn J Physiol 13: 287–302, 1963. doi: 10.2170/jjphysiol.13.287. [DOI] [PubMed] [Google Scholar]
- Sasaki M. Morphological analysis of external urethral and external anal sphincter motoneurones of cat. J Comp Neurol 349: 269–287, 1994. doi: 10.1002/cne.903490209. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Ford TW, Kirkwood PA. Axonal projections of Renshaw cells in the thoracic spinal cord. Physiol Rep 1: e00161, 2013. doi: 10.1002/phy2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol 490: 209–219, 2005. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Schneider SP, Fyffe RE. Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J Neurophysiol 68: 397–406, 1992. doi: 10.1152/jn.1992.68.2.397. [DOI] [PubMed] [Google Scholar]
- Schrøder HD, Reske-Nielsen E. Preservation of the nucleus X-pelvic floor motosystem in amyotrophic lateral sclerosis. Clin Neuropathol 3: 210–216, 1984. [PubMed] [Google Scholar]
- Schütz B. Imbalanced excitatory to inhibitory synaptic input precedes motor neuron degeneration in an animal model of amyotrophic lateral sclerosis. Neurobiol Dis 20: 131–140, 2005. doi: 10.1016/j.nbd.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Severin FV. [Role of the gamma-motor system in performance of movements evoked by natural stimulation of supraspinal centres]. Fiziol Zh SSSR Im I M Sechenova 52: 453–457, 1966. [PubMed] [Google Scholar]
- Sherrington CS. Correlation of reflexes and the principle of the common path. Nature 70: 460–473, 1904. [Google Scholar]
- Shigenaga Y, Doe K, Suemune S, Mitsuhiro Y, Tsuru K, Otani K, Shirana Y, Hosoi M, Yoshida A, Kagawa K. Physiological and morphological characteristics of periodontal mesencephalic trigeminal neurons in the cat–intra-axonal staining with HRP. Brain Res 505: 91–110, 1989. doi: 10.1016/0006-8993(89)90119-4. [DOI] [PubMed] [Google Scholar]
- Shneider NA, Brown MN, Smith CA, Pickel J, Alvarez FJ. Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival. Neural Dev 4: 42, 2009. doi: 10.1186/1749-8104-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström A, Zangger P. α-γ-Linkage in the spinal generator for locomotion in the cat. Acta Physiol Scand 94: 130–132, 1975. doi: 10.1111/j.1748-1716.1975.tb05869.x. [DOI] [PubMed] [Google Scholar]
- Sjöström A, Zangger P. Muscle spindle control during locomotor movements generated by the deafferented spinal cord. Acta Physiol Scand 97: 281–291, 1976. doi: 10.1111/j.1748-1716.1976.tb10265.x. [DOI] [PubMed] [Google Scholar]
- Stifani N. Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci 8: 293, 2014. doi: 10.3389/fncel.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol 10: 661–670, 2014. doi: 10.1038/nrneurol.2014.184. [DOI] [PubMed] [Google Scholar]
- Takei T, Confais J, Tomatsu S, Oya T, Seki K. Neural basis for hand muscle synergies in the primate spinal cord. Proc Natl Acad Sci USA 114: 8643–8648, 2017. doi: 10.1073/pnas.1704328114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Brown RH Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nature 539: 197–206, 2016. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JK. Afferent impulses in the pudendal nerves of the cat. Q J Exp Physiol Cogn Med Sci 49: 258–267, 1964. [DOI] [PubMed] [Google Scholar]
- Tremblay E, Martineau É, Robitaille R. Opposite synaptic alterations at the neuromuscular junction in an ALS mouse model: when motor units matter. J Neurosci 37: 8901–8918, 2017. doi: 10.1523/JNEUROSCI.3090-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türker KS, Schmied A, Rossi A, Mazzocchio R, Sowman PF, Vedel JP. Is the human masticatory system devoid of recurrent inhibition? Exp Brain Res 179: 131–144, 2007. doi: 10.1007/s00221-006-0774-2. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Kellerth JO. Does alpha-motoneurone size correlate with motor unit type in cat triceps surae? Brain Res 251: 201–209, 1982. doi: 10.1016/0006-8993(82)90738-7. [DOI] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One 7: e34640, 2012. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hulliger M. Independence of skeletomotor and fusimotor activity in man? Brain Res 223: 176–180, 1981. doi: 10.1016/0006-8993(81)90819-2. [DOI] [PubMed] [Google Scholar]
- van Keulen LC. Axon trajectories of Renshaw cells in the lumbar spinal cord of the cat, as reconstructed after intracellular staining with horseradish peroxidase. Brain Res 167: 157–162, 1979. doi: 10.1016/0006-8993(79)90270-1. [DOI] [PubMed] [Google Scholar]
- van Zundert B, Izaurieta P, Fritz E, Alvarez FJ. Early pathogenesis in the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Cell Biochem 113: 3301–3312, 2012. doi: 10.1002/jcb.24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan SK, Kemp Z, Hatzipetros T, Vieira F, Valdez G. Degeneration of proprioceptive sensory nerve endings in mice harboring amyotrophic lateral sclerosis-causing mutations. J Comp Neurol 523: 2477–2494, 2015. doi: 10.1002/cne.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Rothstein JD, Kiernan MC. Advances in treating amyotrophic lateral sclerosis: insights from pathophysiological studies. Trends Neurosci 37: 433–442, 2014. doi: 10.1016/j.tins.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Walker LB. Neuromuscular spindles in the external anal sphincter of the cat. Anat Rec 133: 347, 1959. [Google Scholar]
- Watanabe S, Hirayama K. Alpha-gamma linkage in man during varied contraction. Prog Brain Res 44: 339–351, 1976. doi: 10.1016/S0079-6123(08)60743-8. [DOI] [PubMed] [Google Scholar]
- Wenner P, O’Donovan MJ. Identification of an interneuronal population that mediates recurrent inhibition of motoneurons in the developing chick spinal cord. J Neurosci 19: 7557–7567, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbury DR. A comparison of the structures of alpha and gamma-spinal motoneurones of the cat. J Physiol 325: 79–91, 1982. doi: 10.1113/jphysiol.1982.sp014137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VJ, Talbot WH, Kato M. Inhibitory convergence upon Renshaw cells. J Neurophysiol 27: 1063–1079, 1964. doi: 10.1152/jn.1964.27.6.1063. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Activation of Renshaw cells. Prog Neurobiol 35: 135–179, 1990. doi: 10.1016/0301-0082(90)90020-H. [DOI] [PubMed] [Google Scholar]
- Windhorst U. On the role of recurrent inhibitory feedback in motor control. Prog Neurobiol 49: 517–587, 1996. doi: 10.1016/0301-0082(96)00023-8. [DOI] [PubMed] [Google Scholar]
- Wootz H, Fitzsimons-Kantamneni E, Larhammar M, Rotterman TM, Enjin A, Patra K, André E, Van Zundert B, Kullander K, Alvarez FJ. Alterations in the motor neuron-renshaw cell circuit in the Sod1G93A mouse model. J Comp Neurol 521: 1449–1469, 2013. doi: 10.1002/cne.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac FE, Faden JS. Relationship among recruitment order, axonal conduction velocity, and muscle-unit properties of type-identified motor units in cat plantaris muscle. J Neurophysiol 53: 1303–1322, 1985. doi: 10.1152/jn.1985.53.5.1303. [DOI] [PubMed] [Google Scholar]
- Zang DW, Lopes EC, Cheema SS. Loss of synaptophysin-positive boutons on lumbar motor neurons innervating the medial gastrocnemius muscle of the SOD1G93A G1H transgenic mouse model of ALS. J Neurosci Res 79: 694–699, 2005. doi: 10.1002/jnr.20379. [DOI] [PubMed] [Google Scholar]
- Zengel JE, Reid SA, Sypert GW, Munson JB. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol 53: 1323–1344, 1985. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]