Abstract
The circuit controlling visually guided behavior in nonmammalian vertebrates, such as Xenopus tadpoles, includes retinal projections to the contralateral optic tectum, where visual information is processed, and tectal motor outputs projecting ipsilaterally to hindbrain and spinal cord. Tadpoles have an intertectal commissure whose function is unknown, but it might transfer information between the tectal lobes. Differences in visual experience between the two eyes have profound effects on the development and function of visual circuits in animals with binocular vision, but the effects on animals with fully crossed retinal projections are not clear. We tested the effect of monocular visual experience on the visuomotor circuit in Xenopus tadpoles. We show that cutting the intertectal commissure or providing visual experience to one eye (monocular visual experience) is sufficient to disrupt tectally mediated visual avoidance behavior. Monocular visual experience induces asymmetry in tectal circuit activity across the midline. Repeated exposure to monocular visual experience drives maturation of the stimulated retinotectal synapses, seen as increased AMPA-to-NMDA ratios, induces synaptic plasticity in intertectal synaptic connections, and induces bilaterally asymmetric changes in the tectal excitation-to-inhibition ratio (E/I). We show that unilateral expression of peptides that interfere with AMPA or GABAA receptor trafficking alters E/I in the transfected tectum and is sufficient to degrade visuomotor behavior. Our study demonstrates that monocular visual experience in animals with fully crossed visual systems produces asymmetric circuit function across the midline and degrades visuomotor behavior. The data further suggest that intertectal inputs are an integral component of a bilateral visuomotor circuit critical for behavior.
NEW & NOTEWORTHY The developing optic tectum of Xenopus tadpoles represents a unique circuit in which laterally positioned eyes provide sensory input to a circuit that is transiently monocular, but which will be binocular in the animal’s adulthood. We challenge the idea that the two lobes of tadpole optic tectum function independently by testing the requirement of interhemispheric communication and demonstrate that unbalanced sensory input can induce structural and functional plasticity in the tectum sufficient to disrupt function.
Keywords: excitatory/inhibitory balance, experience-dependent plasticity, intertectal, retinotectal, STDP
INTRODUCTION
Visual detection of threats and escape responses are essential for survival. Escape behaviors are among the earliest to develop, and an immature and plastic neural circuit must be capable of producing them reliably. Generation of robust escape behaviors in response to looming stimuli is conserved across many species (Dong et al. 2009; Dunn et al. 2016; Gray et al. 2010; Khakhalin et al. 2014; Liu et al. 2011; Santer et al. 2012; Wallace et al. 2013; Wu et al. 2005; Zhao et al. 2014). The sensorimotor integration that produces escape behavior in response to visual detection of threats occurs in the optic tectum (or its mammalian counterpart, the superior colliculus) (Dong et al. 2009; King 2004). The optic tectum is the direct target of multiple sensory inputs, including the retina, and it projects to motor regions (Deeg et al. 2009; Hiramoto and Cline 2009; Pratt and Aizenman 2009). In Xenopus tadpoles, the optic tectum is required for a visual avoidance behavior in which the tadpole turns to avoid a perceived collision with a looming stimulus (Dong et al. 2009); however, the specific circuitry responsible for this behavior is unknown.
Visual experience drives visual system development and refinement, resulting in functional, efficient circuits. In animals with laterally positioned eyes, such as birds, fish, and amphibians, the visual system is fully crossed; each hemisphere of the brain receives input almost exclusively from the contralateral eye (Cowan et al. 1961; Munz et al. 2014; Sharma 1972). In contrast, animals with forward-facing eyes, such as cats and primates, have partially crossed visual systems in which the inputs from both eyes are represented in both hemispheres, resulting in binocular vision (Pietrasanta et al. 2012). In binocular systems, perturbations in the amount, quality, or correlation of visual experience between the two eyes during development result in changes to neural response properties and synaptic connectivity, and disrupt visually driven behavior (Mitchell and Duffy 2014). In humans, this leads to conditions such as amblyopia (Mitchell and Duffy 2014). Whether and how imbalanced visual experience during development affects plasticity and function of the visual circuit and visually guided behavior in animals with a fully crossed visual circuit is unclear.
In Xenopus laevis tadpoles, each eye projects axons almost exclusively to the contralateral optic tectum, the primary visual processing center (Munz et al. 2014). Furthermore, tadpoles lack binocularly responsive tectal neurons (Udin 2012), leading to the supposition that the two hemispheres operate independently and that visual experience affects only the optic tectal hemisphere that receives direct innervation from the contralateral eye. This suggests that bilateral development of the functional visuomotor circuit in animals with crossed visual projections arises from comparable visual experience to both eyes, independent of communication across the midline.
Visuomotor circuit function can be assessed in tadpoles by their successful performance of optic tectum-dependent behavior. In tadpoles, injury to one tectum interfered with visually guided behavior regardless of the direction of approach of the looming stimulus (McKeown et al. 2013), suggesting that communication between tectal lobes may be important for visuomotor behavior and that the two optic tectal hemispheres are not independent. Tadpoles have a band of intertectal connections including excitatory and inhibitory axons, which cross the midline to connect the two tectal lobes (Gambrill et al. 2016). Intertectal connections are potentially a component of the visuomotor circuit; however, visuomotor behavior has not been tested in animals in which intertectal communication has been disrupted or in animals in which tectal circuitry differs across the midline.
Experience-dependent cellular and circuit changes that result from bilateral visual stimulation provided to tadpoles are well documented (Pratt et al. 2016; Ruthazer and Aizenman 2010), including improvements in visual avoidance behavior, mentioned above (Liu and Cline 2016; Shen et al. 2014). Visual experience promotes refinement of the topographic map (Ruthazer and Cline 2004), elaboration of tectal neuron dendrites (Sin et al. 2002), retinotectal synaptogenesis (Aizenman and Cline 2007; Ruthazer et al. 2006), neuronal excitability (Aizenman et al. 2003), and development of excitatory and inhibitory circuitry underlying visual information processing (Pratt and Aizenman 2007; Pratt et al. 2008). Visual experience shapes many of these properties through spike timing-dependent plasticity (STDP) mechanisms (Pratt et al. 2008; Richards et al. 2010a), akin to the role of STDP in other developing circuits (D’amour and Froemke 2015; Feldman 2012; Vogels et al. 2013). Via STDP, inputs arriving in close temporal proximity regulate the development of tectal neuron direction selectivity (Engert et al. 2002; Mu and Poo 2006), receptive field size (Vislay-Meltzer et al. 2006), recurrent network activity (Pratt et al. 2008), and retinotopic maps (Ruthazer and Cline 2004). Inhibition plays an integral role in the development of these neuronal and circuit properties by affecting neuronal spiking and STDP (Pratt et al. 2008; Richards et al. 2010b; Shen et al. 2011; Tao and Poo 2005), consistent with studies showing that an optimal level of inhibition or the balance of excitation to inhibition (E/I) is critical during development (Feldman 2012; Fritschy 2008; Froemke 2015; Richards et al. 2010b).
Developmental disruptions in tectal E/I balance by manipulating excitation or inhibition affect tectal neuron dendrite arbor growth and plasticity (Haas et al. 2006; Shen et al. 2009), receptive field size (Shen et al. 2011; Tao and Poo 2005), spike timing precision (Richards et al. 2010b; Shen et al. 2011), the synaptic integration window (Shen et al. 2011), multisensory integration (Felch et al. 2016), and sensitivity to visual stimuli (Khakhalin et al. 2014). The proper development of these features in the tectum is necessary for visuomotor behavior in tadpoles (Dong et al. 2009; Khakhalin et al. 2014; Liu et al. 2016; Shen et al. 2011). Together these studies suggest that normal visual experience drives bilaterally symmetric maturation of the visual circuit in a manner that preserves visually evoked E/I balance (He et al. 2016); however, the importance of E/I balance across the midline is unclear.
In the current study, we tested the role of intertectal inputs in the sensorimotor circuit in Xenopus tadpoles, using surgical disruption of the intertectal commissure, asymmetric visual experience-dependent rearing protocols, and molecular genetic strategies to alter tectal circuit development and E/I balance across the midline. Transecting the intertectal commissure disrupted visual avoidance behavior, illustrating that intertectal communication is an important component governing the output of the visuomotor circuit. If visuomotor behavior arises from two independent circuits segregated by the midline, monocular visual experience-dependent maturation of the tectal circuit contralateral to the stimulated eye would not affect visual avoidance behavior in response to a visual stimulus approaching the unstimulated eye. In contrast, if the circuit is bilateral, monocular visual experience should disrupt behavior in response to a stimulus approaching either eye. We showed that monocular visual experience disrupts visually guided behavior independent of the direction of stimulus approach, suggesting that visuomotor circuit function is indeed coordinated across the midline. We then explored the cellular mechanisms by which visual experience might affect connectivity of intertectal inputs within visuomotor circuitry. We found that spike timing-dependent mechanisms underlie the incorporation of intertectal inputs into tectal circuitry, analogous to the role of STDP in other developmental plasticity events in the tectum. In addition, monocular visual experience drives intertectal structural plasticity in a manner distinct from bilateral visual experience (Gambrill et al. 2016). Furthermore, we demonstrated that monocular visual experience created hemisphere-specific changes in E/I in tectal neurons, which disrupted E/I balance between the hemispheres. Last, we showed that shifting E/I values unilaterally by interfering with GABAA or AMPA receptor trafficking was sufficient to disrupt visuomotor circuit function.
Our experiments expand a well-established body of theoretical and experimental investigation of sensory activity-induced plasticity in developing circuits. In contrast to long-held assumptions that visual information processing is highly lateralized in the tadpole visual circuit, we show that intertectal communication is important for efficient visuomotor circuit function and behavior. The tadpole tectal hemispheres do not operate independently, even in this fully crossed visual circuit. Rather, the visuomotor circuit is bilateral, and behavioral output is coordinated between the hemispheres. Furthermore, we describe a previously unrecognized function for intertectal fibers in the tadpole visual circuit. Finally, we demonstrate mechanisms of cellular and circuit plasticity which result in physiologically relevant changes to behavior. These experiments take advantage of the Xenopus tadpole’s uniquely accessible and plastic visual circuit to provide broadly applicable knowledge about how differences in sensory experience can drive asymmetric plasticity between brain hemispheres and affect sensory circuit function and behavior.
MATERIALS AND METHODS
Animals.
Albino X. laevis tadpoles of either sex were obtained by in-house breeding or purchased from Xenopus Express (Brooksville, FL). Tadpoles were reared in 0.1× Steinberg’s solution in a 12:12-h light-dark cycle at 22–23°C and used for time-lapse imaging and electrophysiology experiments beginning at stage 46 (Nieuwkoop and Faber 1956). Animals were fed beginning at stage 47 and were anesthetized in 0.02% tricaine methanesulfonate (MS-222; catalog no. A5040; Sigma) before all procedures. All animal protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Visual avoidance behavior.
Stage 47 tadpoles were screened for the optomotor response (OMR) to evaluate overall health. To determine their baseline visual avoidance response, tadpoles with normal OMR were placed in a clear tank and randomly moving dots were presented for 90 s using a microprojector positioned below the tank (McKeown et al. 2013). Tadpoles were visualized with infrared light-emitting diodes (LEDs), and videos of tadpole behavior were captured with a high-speed charge-coupled device (CCD) camera. To quantify the visual avoidance response, videos were analyzed post hoc, frame by frame. An avoidance response was scored when a tadpole displayed a sharp turn within 500 ms of a dot moving perpendicularly toward the eye. The avoidance index was quantified as the fraction of encounters between a swimming tadpole and a moving dot that resulted in an avoidance response. Animals were only included in the analysis if they swam normally and if they had 10 or more moving dot encounters within the 90-s video. To determine left vs. right bias, videos of individual animals and moving dot encounters were examined to determine if the stimulus approached the animal from the left or right side. Data were analyzed blind to experimental group.
Surgery to cut intertecal axons.
Tadpoles were anesthetized in 0.02% MS-222 and split into two groups: surgery and sham surgery control. In the surgery group, a microsurgical knife was used to cut the intertectal axons at the tectal midline along the entire rostrocaudal extent of the optic tectum. In the sham surgery control, a hole was poked through the skin along the midline above the two tectal lobes caudal to the anterior commissure. Tadpoles were placed in 0.1× Steinberg’s to recover following surgery. At 2 and 5 days after surgery, tadpoles were screened for the OMR, and animals with normal OMR were retested for their visual avoidance response. The avoidance index was calculated as described above and normalized to its value before surgery. To visualize intertectal axons before and following surgery, 1 mM FM 4-64 dye (catalog no. T-3166; ThermoFisher) was injected into the caudal portion of the ventricle. Animals were imaged within 10 min of FM 4-64 injection using a Perkin-Elmer Ultraview Vox spinning disk confocal microscope with a ×25 water-immersion lens [1.1 numerical aperture (NA)].
Electrophysiology.
Following anesthesia in 0.2% MS-222, brains were removed and cut along the ventral midline to expose the tectal cell layers while preserving the dorsal intertectal axons. The dissected brain was placed in room temperature extracellular saline (115 mM NaCl, 4 mM KCl, 3 mM CaCl2, 0.5 mM MgCl2, 5 mM HEPES, 10 mM glucose, pH 7.2, osmolality of 255 mosmol/kgH2O). When stated, 100 µM picrotoxin (PTX; Tocris) or 20 µM 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX; Tocris) and 50 µM dl-2-amino-5-phosphonovaleric acid (APV; Tocris) were added to the bath to block GABAA receptors (GABAAR) or AMPA (AMPAR) and N-methyl-d-aspartate (NMDA) receptors (NMDAR), respectively. Electrical stimulation was done using bipolar electrodes (Frederick Haer, Bowdoin, ME) placed in either the opposite tectal hemisphere or the lateral optic tract. Baseline responses to electrical stimulation were collected in voltage-clamp configuration at 0.05 Hz. Recordings containing back-propagated action potentials were discarded. Experiments in which AMPA-to-GABA ratios were assayed were performed in voltage clamp at −60 and 0 mV, with the immediate peak of current collected over a 10-ms window for each holding potential. For AMPA-to-NMDA ratios, cells were clamped at −60 and +40 mV. The amplitude of NMDAR-mediated currents were measured at +40 mV in a 20-ms window beginning 50 ms after initial peak AMPAR-mediated current. AMPAR-mediated currents were measured during a 10-ms window of the peak current at −60 mV. To confirm that current recorded at −60 mV was excitatory, postsynaptic currents (PSCs) were recorded at −60 mV, and PTX (100 µM) was then added to the bath. Following a 2-min incubation during which recording was paused, optic tract stimulation and recording were resumed. Similarly, inhibitory currents recorded at 0 mV were confirmed by addition of NBQX (20 µM) and APV (50 µM) to the bath.
Synaptic plasticity (see Figs. 3 and 4) was induced in current-clamp configuration using the following protocol: the recording was switched to current-clamp mode, and the baseline of the cell was allowed to stabilize. Current was injected to maintain the resting potential at −55 ± 5 mV. The stimulating electrode was triggered. Following a 50-ms interval, current injection sufficient to trigger an action potential or the second bipolar electrode was triggered. This pairing was repeated once after an interval of 950 ms. The cell was then allowed to rest for 29 s. This protocol was repeated 10 times. The recording was then switched back to voltage clamp, and response collection at 0.05 Hz was resumed.
Fig. 3.
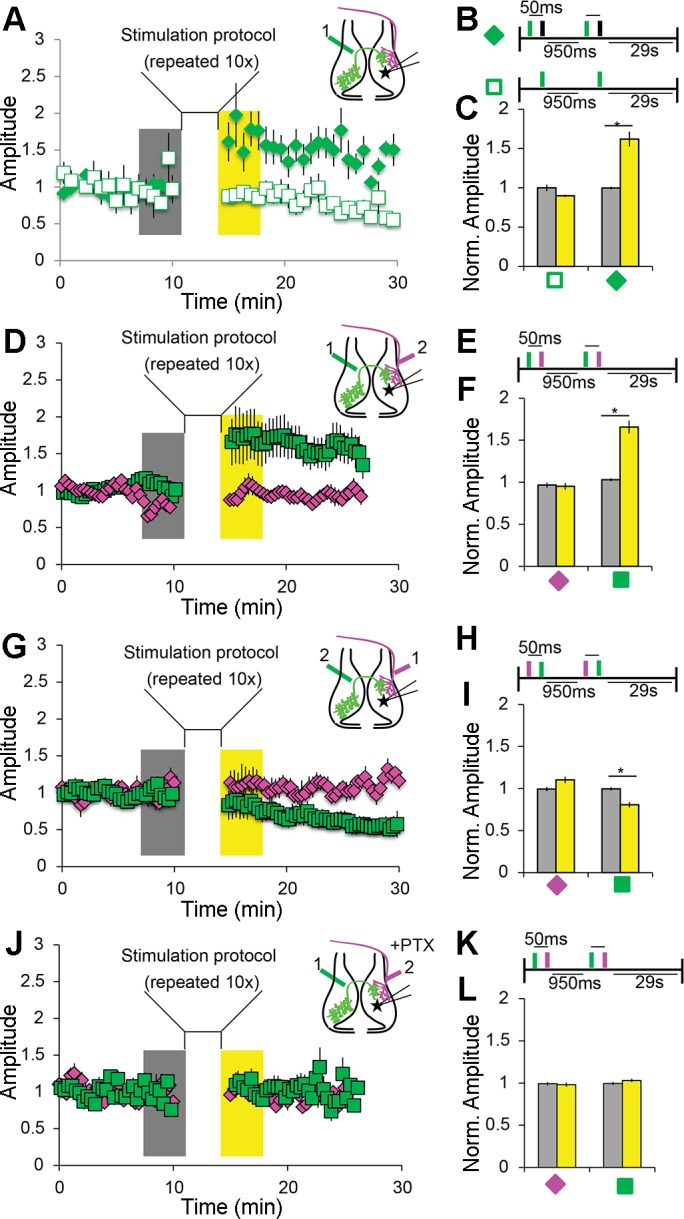
Spike timing-dependent plasticity (STDP) protocols induce intertectal synaptic plasticity. A–C: pairing IT axon stimulation with postsynaptic current injection potentiates IT responses. A: IT axon stimulation was paired with direct depolarizing current injection into tectal neurons in the contralateral tectum. Inset: diagram showing location of IT stimulation (green; 1) and recording location (star). When current injection followed IT stimulation by <50 ms, IT response amplitude was potentiated. Excitatory postsynaptic currents (EPSCs) were recorded in voltage clamp at −60 mV. Stimuli were given at 0.05 Hz. B: schematic showing protocols for pairing depolarization (black bar) and IT stimulation (green bar) and unpaired control. C: summary data showing potentiation of IT responses from cells exposed to the pairing protocol (solid green diamond; n = 16) and no change when unpaired (open square; n = 10). Amplitudes were measured during the time periods highlighted before (gray) and after pairing (yellow) as shown in A. Amplitudes were normalized to baseline before pairing. Only cells that fired action potentials after stimulation were included in the analysis. D–F: pairing IT before retinotectal (RT) stimuli potentiates IT responses. D: pairing protocol as in A, except that current injection is replaced by RT input stimulation. Inset: diagram showing location and timing of IT (1) and RT (2) stimulating electrodes and the recording location (star). E: schematic of pairing protocol with IT stimulation (green bar) preceding RT stimulation (magenta bar). F: summary data showing potentiation of IT responses (green square) and no change in RT responses (magenta diamond) when IT stimulation preceded RT stimulation (interval = 50 ms, n = 25). Data are presented as in C. G–I: pairing RT before IT stimulation depresses IT responses. G: stimulation protocol. Inset: diagram showing location and timing of stimulation electrodes and recording site. H: schematic of pairing protocol. RT stimulation (magenta bar) precedes IT stimulation (green bar). I: summary data as in F showing depression of IT responses but no change in RT responses (n = 7). Data are presented as in C. J–L: pairing-induced IT potentiation requires circuit inhibition. J: stimulation protocol. Inset: diagram showing location and timing of stimulation electrodes and recording site. Recordings were done as in D, with the addition of 100 µM picrotoxin (PTX) in the bath. K: schematic of pairing protocol. IT stimulation precedes RT. L: summary data as in F showing no pairing-induced change in the amplitude of IT or RT PSCs in PTX (n = 13). Data are means ± SE. *P < 0.05 determined using Student’s t-test for PSC amplitude vs. baseline.
Fig. 4.
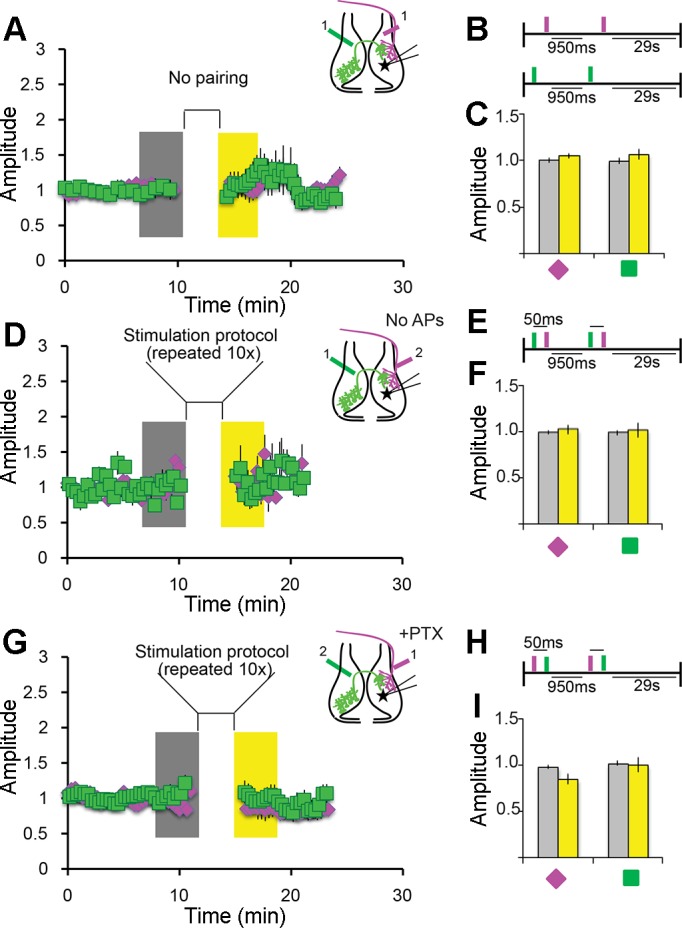
STDP depends on temporal proximity of input and postsynaptic depolarization. A–C: unpaired stimulation does not drive plasticity. A: bipolar electrode stimulation of IT (green squares) or RT (magenta diamonds) axons produced no plasticity. When stimuli were presented independently or with an interval >50 ms (data not shown), EPSC amplitudes were not significantly different from baseline. B: stimulation protocol presented as a schematic. C: summary data showing no potentiation. Data were collected as described in Fig. 3; n = 7 IT and 7 RT cells. D–F: postsynaptic depolarization is required for potentiation. D: a subset of cells recorded in the experiment represented in Fig. 3D failed to fire action potentials in response to RT or IT stimulation. No potentiation was observed in these cells. EPSCs were recorded and stimulation protocol was as described in Fig. 3, D–F. E: stimulation protocol. F: summary data showing no potentiation. Data were collected as in Fig. 3; n = 13 cells. G–I; inhibition is required for pairing-induced depression. G: RT stimulation before IT stimulation depressed IT responses (Fig. 3, G–I). PTX (100 µM) blocked the pairing-induced depression. H: pairing protocol as in Fig. 3H. I: summary data showing that the depression seen in Fig. 3, G–I is absent. Data were collected as in Fig. 3; n = 13 cells.
Recordings were made using glass micropipettes (5–15 MΩ) containing either a Cs-based (80 mM Cs-methanesulfonate, 5 mM MgCl2·6H2O, 20 mM tetraethylammonium-Cl, 10 mM EGTA, 20 mM HEPES, 2 mM ATP, 0.3 mM GTP, pH 7.2, 255 mosmol/kgH2O) (see Figs. 2, 5, and 6) or K-based internal solution (100 mM K-gluconate, 8 mM KCl, 5 mM NaCl, 1.5 mM MgCl2, 10 mM EGTA, 20 mM HEPES, 2 mM ATP, 0.3 mM GTP, pH 7.2, 255 mosmol/kgH2O) (see Figs. 3 and 4). Input and series resistance were monitored for the duration of the recording. Recordings in which these values change by >10% were discarded. Data were acquired and analyzed using MultiClamp 700A, Clampex 9.2, and Clampfit 10.2 (Molecular Devices).
Fig. 2.
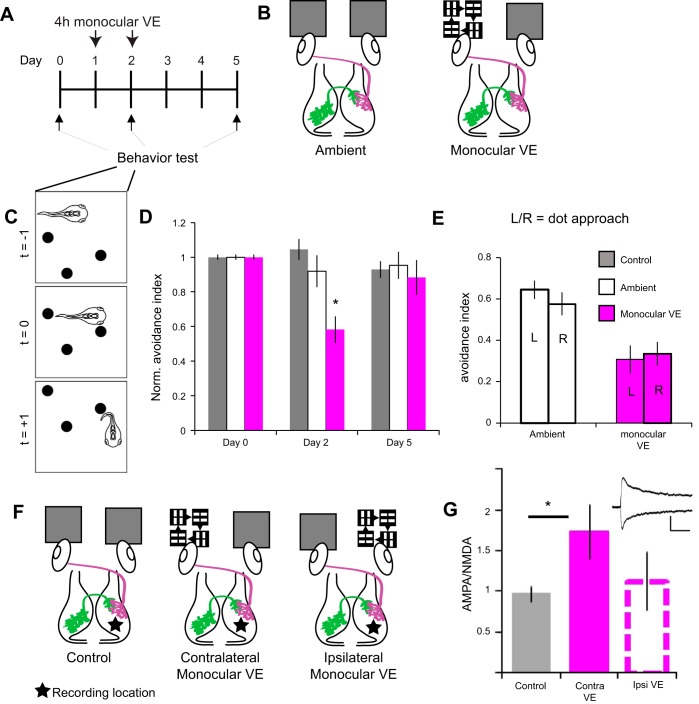
Monocular visual experience (VE) disrupts visual avoidance behavior. A and B: experimental timeline and schematic of experimental groups. A: baseline visual avoidance behavior was assayed on day 0. Animals were immobilized in agarose, and the left eye was exposed to 4 h of monocular VE or ambient light on days 1 and 2. Visual avoidance behavior was assayed immediately after monocular VE on day 2 and again on day 5. An additional control group exposed to ambient light was tested. B: schematic of control and monocular VE conditions showing retinotectal axons (magenta) and intertectal axons (green) projecting to the contralateral optic tectum. In the ambient condition, both eyes were exposed to ambient light; for monocular VE, the left eye was exposed to light bars moving in 4 random directions. C: schematic of the avoidance behavioral response to a laterally approaching visual stimulus. Freely swimming tadpoles (t = −1) turn to avoid approaching dots (t = +1) within 500 ms of the stimulus encounter (t = 0). D: monocular VE impairs visual avoidance behavior. Visual avoidance behavior assessed on day 2, right after the 2nd bout of monocular VE, was significantly worse than in tadpoles exposed to ambient light (day 2, *P = 0.006). Avoidance behavior returned to baseline 3 days after monocular VE (day 5). Data were pooled from 3 biological replicates and are normalized to baseline (day 0). Bars represent control, nonimmobilized (gray; n = 28 tadpoles), ambient (white bars, n = 15 tadpoles), and monocular VE conditions (magenta bars, n = 18 tadpoles). E: direction of stimulus approach does not affect avoidance behavior. Stimuli approached the left (L) or right (R) eye. Successful avoidance was similar regardless of the direction of approach for animals exposed to ambient light (P = 0.326) and monocular VE (P = 0.751). F: schematic of experimental groups for electrophysiology experiments. Tadpoles were exposed to ambient light in both eyes (control), monocular VE to the eye contralateral to recording location, or monocular VE to the eye ipsilateral to the recording location. Tectal cell recordings were made at −60 and +40 mV in dissected brains from the right tectal lobe (star) with stimulation electrodes placed on retinotectal (magenta) axons to evoke postsynaptic currents. G: summary data of AMPA-to-NMDA ratios (AMPA/NMDA) recorded 1–3 days after visual stimulation as described in F. Monocular VE increases the retinotectal AMPA/NMDA in the contralateral tectum compared with control (*P = 0.037). Inset: example traces of recordings in control condition at −60 and +40 mV (scale bar: 100 ms, 50 ms). AMPA amplitude was measured as the initial peak; NMDA amplitude was collected in a 20-ms window beginning 50 ms after initial peak AMPA receptor-mediated current. Data are means ± SE for control (n = 22 cells), contralateral VE (n = 20 cells), and ipsilateral VE (n = 16 cells) conditions. A 2-tailed Student’s t-test was used to determine significance.
Fig. 5.
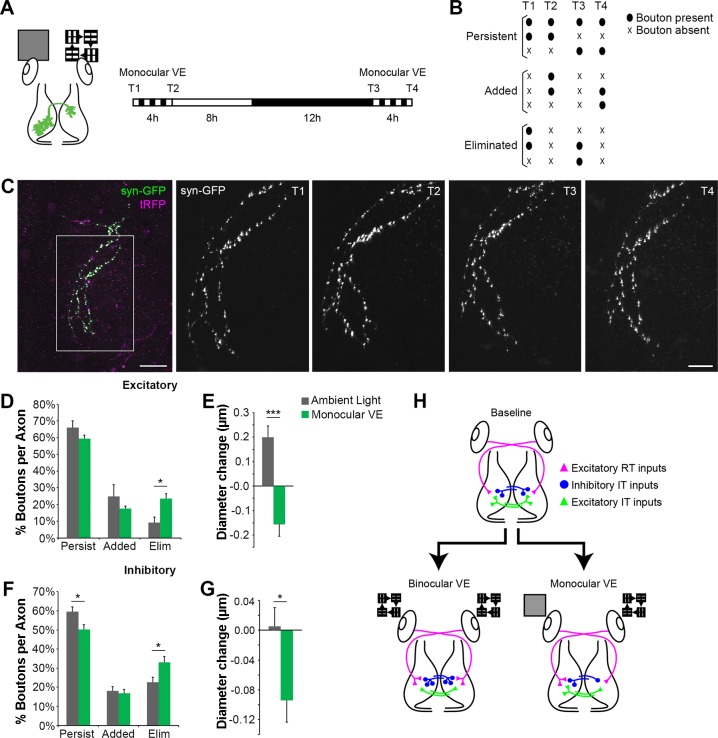
Monocular VE drives structural plasticity in intertectal axon boutons in vivo. A: experimental protocol. In vivo time-lapse 2-photon images of intertectal neuronal axons were collected before (T1) and after (T2) tadpoles were exposed to 4-h monocular VE provided to the eye contralateral to the neuronal cell bodies of the IT axons (schematized at left). Animals were returned to normal light-dark cycle. The next day, in vivo images were collected again before (T3) and after (T4) a second 4-h bout of monocular VE. This protocol tests the effect of visually driven activity on axonal outputs of IT-projecting tectal neurons. B: boutons were categorized as persistent, added, or eliminated in response to monocular VE as schematized. C: 2-photon Z projections of an IT axon expressing synaptophysin tagged with GFP (syn-GFP; green) and cytoplasmic turboRFP (tRFP; magenta). Scale bar: 50 µm. The boxed region was imaged repeatedly at higher magnification (right, T1–T4; scale bar: 25 µm). D and F: quantification of the percentage of intertectal boutons that persist, are added, or are eliminated during monocular VE. Monocular VE increases loss of excitatory (D) and inhibitory (F) boutons compared with ambient light. [excitatory (D): monocular VE, n = 6 axons; ambient light, n = 5 axons; *P = 0.014 eliminated, 2-tailed Student’s t-test; inhibitory (F): monocular VE, n = 11 axons; ambient light, n = 6 axons; *P = 0.040 persistent, *P = 0.038 eliminated, 2-tailed Student’s t-test]. E and G: quantification of the average change in diameter of persistent intertectal boutons. Persistent excitatory (E) and inhibitory (G) boutons shrink with monocular VE compared with ambient light [excitatory (E): monocular VE, n = 216 boutons; ambient light, n = 177 boutons; ***P < 0.0001, Mann-Whitney; inhibitory (G): monocular VE, n = 545 boutons; ambient light, n = 469 boutons; *P = 0.016, Mann-Whitney). Data are means ± SE. H: schematic illustrating the differences in structural plasticity of axon inputs after binocular VE and monocular VE. At baseline, each tectal hemisphere receives input from RT axons (magenta triangles), excitatory IT axons (green triangles), and inhibitory IT axons (blue circles). Binocular VE results in bilaterally symmetric addition of RT synapses and inhibitory IT synapses and elimination of excitatory IT synapses (bottom left). In contrast, monocular VE increases RT synapses in the tectum contralateral to the stimulus, and both excitatory and inhibitory IT neurons projecting axons from the stimulated tectum eliminate more synapses in the tectal hemisphere ipsilateral to the visual stimulus compared with ambient light. These data indicate that monocular VE produces bilaterally asymmetric structural plasticity. Data in binocular VE condition are from Gambrill et al. (2016).
Fig. 6.
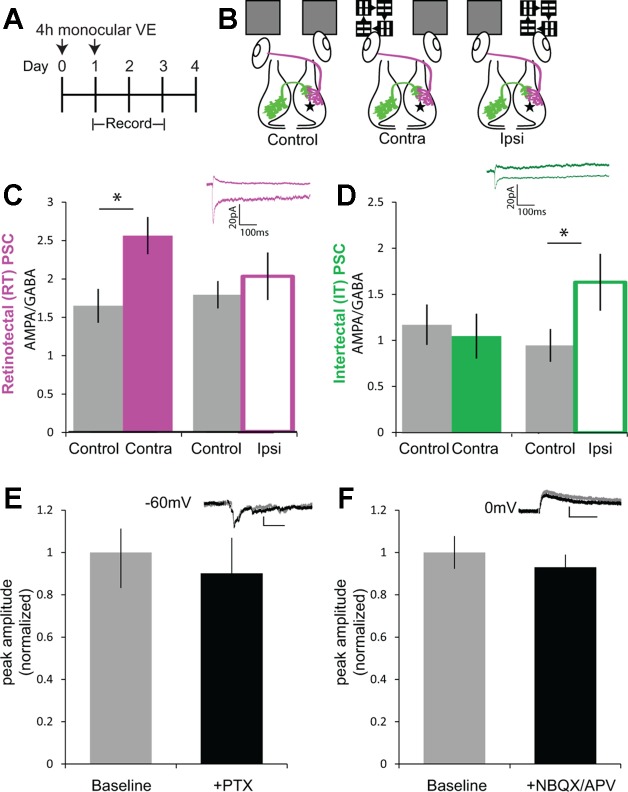
Monocular visual experience induced plasticity disrupts bilateral excitatory/inhibitory (E/I) balance. A: experimental timeline. Monocular VE was provided to the left or right eye on day 0 and day 1. Recordings were made immediately after monocular VE on day 1 and on days 2 and 3. B: schematic of experimental groups. “Control” tadpoles received bilateral ambient light to both eyes. “Contra” tadpoles received monocular VE exposure to the eye contralateral to recording location. “Ipsi” tadpoles received monocular VE to the eye ipsilateral to the recording location. Tectal cell recordings were made at −60 and 0 mV in dissected brains with electrodes placed on RT (magenta) or IT (green) axons to evoke PSCs. C: two 4-h treatments with contralateral monocular VE increased retinotectal AMPA/GABA compared with control. Summary data of ratios of PSC amplitudes at −60 to 0 mV (n = 22 control, 20 contra, and 16 ipsi cells; control vs. contra: P = 0.0043). Inset shows representative retinotectal PSC recorded from control at −60 and 0 mV (scale bar: 100 ms, 20 pA). D: two 4-h treatments with ipsilateral monocular VE increased intertectal AMPA/GABA compared with control. Summary data of ratios of PSC amplitudes at −60 to 0 mV (control vs. ipsi: P = 0.025). Inset shows representative intertectal PSC recorded from control at −60 and 0 mV (scale bar: 100 ms, 20 pA). Data are means ± SE. *P < 0.05, 2-tailed Student’s t-test. E: PSCs recorded at −60 mV are insensitive to PTX (100 µM) added to the bath. PSC amplitudes recorded in the presence of PTX are not significantly different from those recorded during the baseline period. Inset: examples of PSCs before (gray) and after (black) PTX addition (scale bar: 20 pA, 50 ms). Data are means ± SE presented as normalized current amplitude (n = 5 cells). P > 0.05, 2-tailed Student’s t-test. F: PSCs recorded at 0 mV are insensitive to 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX; 20 µM) and dl-2-amino-5-phosphonovaleric acid (APV; 50 µM). Inset: examples of PSCs before (gray) and after (black) NBQX and APV addition (scale bar: 50 ms, 20 pA). Data are means ± SE presented as normalized current amplitude (n = 6 cells). P > 0.05, 2-tailed Student’s t-test.
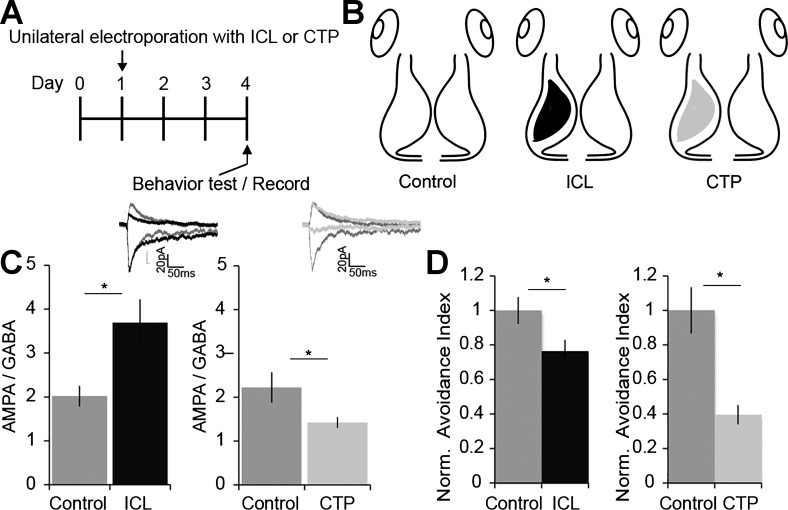
Unilateral electroporation.
Stage 47 tadpoles were prescreened for OMR and avoidance behavior. Animals that performed both behaviors were electroporated with plasmids into the left tectal lobe. For electroporation, plasmids were injected into the brain ventricle, platinum electrodes were then placed on each side of the midbrain, and voltage pulses were applied across the midbrain (Haas et al. 2002). The left tecta were electroporated with a dual cytomegalovirus (CMV) promoter plasmid expressing enhanced green fluorescent protein (eGFP) and a peptide corresponding to the intracellular loop of the gamma 2 subunit of the GABAA receptor (ICL), eGFP and a peptide corresponding to the cytoplasmic tail of the GluA2 subunit of the AMPAR (CTP), or eGFP alone at 1 μg/μl concentration (Shen et al. 2011). Three days following electroporation, animals were screened for OMR and animals with normal OMR were tested for visual avoidance behavior. Data were analyzed as described above and blind to experimental group.
Monocular visual experience protocol.
Animals were exposed to monocular visual experience before electrophysiology and behavior experiments by being presented with visual stimuli, as described previously (Hiramoto and Cline 2014). Stage 47 tadpoles were anesthetized in 0.02% MS-222 and then placed in a transparent chamber containing a layer of Sylgard (catalog no, 24236-10; Electron Microscopy Science). Each chamber contained a custom-sized well carved into the Sylgard that allowed the animal to rest on its side, with either the right or left eye facing the transparent floor of the chamber. The animal’s head was fixed in place with a small application of agarose. The tail remained free. The entire preparation was submerged in fresh 1× Steinberg’s solution, and the tadpole was allowed to recover from anesthesia. The chamber was placed 15 mm from a liquid crystal display (LCD) screen, which showed pseudorandomly moving bar stimuli modified from Hiramoto and Cline (2014). The moving bars consisted of alternating 9-mm-wide white bars and 27-mm-wide black bars moving at 78 mm/s. Animals were exposed to this visual experience for 4 h for 2 sequential days. The same eye was stimulated for each animal on subsequent days.
In vivo time-lapse imaging.
To visualize the synaptic boutons of intertectal axons, one tectal lobe of stage 46 animals was electroporated with 1 μg/μl VGAT::gal4 + 2 μg/μl UAS::synaptophysin-GFP + 1 μg/μl UAS::turboRFP to enhance fluorescent protein expression with the gal4-upstream activation sequence (UAS) system (Haas et al. 2002). Synaptophysin tagged with green fluorescent protein (syn-GFP) was used to label presynaptic boutons (Ruthazer et al. 2006), and cytosolic red fluorescent protein (TurboRFP) was used to label the entire axonal arbor. Seven days after electroporation, animals with sparse intertectal axon labeling were imaged on a custom-built two-photon microscope with a ×20 (1.05 NA) water-immersion lens. First, a 512- × 512-pixel image of the entire axon was acquired, and then a region of the axonal arbor was selected and imaged at 1024 × 1024 pixels with ×2–3 digital scan zoom (T1). Tadpoles were exposed to monocular visual experience or ambient light for 4 h, and the selected axonal subregion was imaged again immediately thereafter (T2). Animals were placed in a normal light-dark cycle until the next day (8:12-h light-dark) when the protocol was repeated: image (T3), 4-h monocular visual experience or ambient light, image (T4). To compare boutons from single axons over time, the same imaging parameters were used for all four images.
Immediately after imaging, animals were fixed for post hoc vesicular GABA transporter (VGAT) immunostaining. Tadpoles were anesthetized with 0.02% MS-222, immersed in 4% paraformaldehyde, and fixed using two bouts of microwave fixation at 150 W for 1 min followed by overnight fixation at 4°C. Brains were dissected and sectioned at 40 μm on a vibratome. Sections were blocked and permeabilized in 5% normal donkey serum and 2% Triton X-100 for 1 h at room temperature. Sections were then incubated in 1:400 rabbit anti-VGAT (catalog no. 131-003; Synaptic Systems) for 3 days at 4°C, followed by 2 h in 1:200 anti-rabbit Alexa Fluor 647 (Life Technologies) at room temperature. Sections were mounted in Gel mount (Accurate) and imaged with an Olympus FluoView500 confocal microscope. Confocal Z stacks were first acquired with a ×20 (0.8 NA) or ×40 (1.0 NA) oil-immersion lens to determine the location of the GFP+ axon from in vivo imaging. Two-channel GFP and VGAT/Alexa647 Z stacks were then acquired from axons with a ×60 (1.4 NA) oil-immersion lens at ×2 digital scan zoom to yield a pixel size of 0.10 μm. To determine colocalization between GFP and VGAT, we used the Colocalization plugin for ImageJ (Gambrill et al. 2016). The percentage of GFP+ boutons that were VGAT+ was calculated, and axons were categorized as excitatory (VGAT−) and inhibitory (VGAT+) using previously determined criteria (Gambrill et al. 2016). For presentation purposes, autofluorescing skin cells were removed from the image in Fig. 5C.
To identify and measure boutons at each time point, we used the 3D Object Counter plugin for ImageJ (Gambrill et al. 2016). The background fluorescence intensity for each axon was measured along the axonal backbone, and the threshold was set as two times background level. Each bouton with at least two neighboring pixels above threshold was identified (pixel sizes ranged from 0.23 to 0.35 μm depending on the digital scan zoom). Images were compared across time points to quantify bouton dynamics in response to monocular visual experience or ambient light. We examined each bout of monocular visual experience or ambient light (T1 to T2 and T3 to T4) and categorized boutons as persistent, added, or eliminated. Boutons included in this analysis had the following behavioral profiles (schematized in Fig. 5B): Persistent boutons were 1) persistent from T1 to T4, 2) persistent from T1 to T2 and eliminated overnight, or 3) added overnight and persistent from T3 to T4. Added boutons were 1) added from T1 to T2 and then eliminated overnight, 2) added from T1 to T2, eliminated overnight, and added again from T3 to T4, or 3) added from T3 to T4. Eliminated boutons were 1) eliminated from T1 to T2, 2) eliminated from T1 to T2, added overnight, and eliminated again from T3 to T4, or 3) added overnight and eliminated from T3 to T4. Approximately 85% of boutons were included in this analysis. The remaining ~15% were present during the entire imaging session but did not have consistent behaviors during the two bouts of visual experience. The fraction of boutons exhibiting each dynamic behavior was calculated by dividing the number of boutons that were persistent, added, or eliminated by the total number of boutons that exhibited these behaviors for each axon. These data are presented as the average percentage of boutons per axon. The diameters of boutons present at each time point were measured at each boutons widest point. For persistent boutons, we quantified the change in bouton diameter during each bout of visual experience (T2 − T1 and T4 − T3). The cumulative change in diameter was calculated by adding the change in diameter during the two bouts of visual experience: (T2 − T1) + (T4 − T3).
Statistical analysis.
All data are means ± SE or individual data points. For normally distributed data, statistical differences were determined using two-tailed Student’s t-tests. For data that were not normally distributed, statistical differences were determined using the Mann-Whitney test.
RESULTS
Cutting the intertectal commissure impairs visual avoidance behavior.
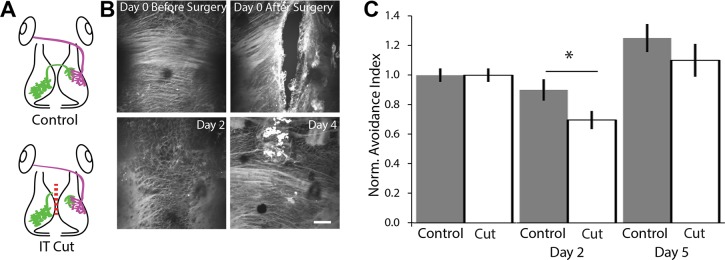
Excitatory and inhibitory tectal neurons extend axons through the dorsally located intertectal commissure. These axons elaborate widely in the contralateral optic tectum and make synapses onto retinorecipient tectal neurons (Gambrill et al. 2016), as schematized in green in Fig. 1A. To explore whether intertectal axons play a role in visual avoidance behavior, we tested animals for baseline visual avoidance behavior and divided the animals into two groups, one of which received surgery to transect the intertectal commissure and another that received sham surgery. We then evaluated visual avoidance behavior (Fig. 1, B and C). The dorsal position of the intertectal commissure allows it to be transected without disrupting other brain structures. Images of FM 4-64 staining before and after surgery showed that intertectal axons were successfully cut and began extending into the injury site 2 days after surgery. Considerable axon regrowth was apparent 4 days after surgery (Fig. 1B). Tadpoles with the commissure transected displayed a deficit in visual avoidance behavior 2 days after surgery (Fig. 1C). Behavior recovered to control values by 5 days after surgery. The ability to avoid looming objects is not lost completely when the commissure is cut. This may reflect, in part, our strict inclusion criteria, which require that animals be actively swimming and have 10 encounters with the stimulus within the first 90 s of the visual avoidance test. Fewer animals with their commissure severed met the criterion for inclusion (64%) compared with sham controls (77%). In addition, the residual behavioral capacity after commissure transection suggests that there are other pathways that can transmit information between the tectal lobes. Indeed, we previously reported that some tectal neurons extend an axon through the floor of the midbrain to the contralateral tectum (Foa et al. 2001). Nevertheless, these results indicate that intertectal axons play a role in visual avoidance behavior and that behavioral recovery after cutting intertectal axons correlates with their regeneration.
Fig. 1.
Intertectal commissure transection impairs visual avoidance behavior. A: schematic of experimental groups. Tadpoles received sham surgery (control) or surgery in which the intertectal (IT) commissure was transected (IT cut). Green axons, intertectal; magenta axons, retinotectal. B: in vivo confocal images of FM 4-64-labeled intertectal axons before and after surgery. Axons were completely cut. Axon regeneration occurs over 4 days after surgery. Scale bar: 50 µm. C: cutting the IT commissure impairs visual avoidance behavior. Data are presented as an avoidance index: the number of times over 10 encounters that a tadpole avoids an anticipated collision, normalized to baseline before surgery (day 0). Tadpoles with cut IT axons had a deficit in avoidance behavior 2 days after surgery. Tadpoles recover avoidance behavior comparable to sham controls after 5 days. Data are means ± SE on day 0 (before surgery), day 2 (n = 36 control and 28 cut axons; *P = 0.042), and day 5 (n = 18 control and 17 cut axons). A 2-tailed Student’s t-test was used to determine significance. Norm., normalized.
Monocular visual experience disrupts visual avoidance behavior.
Prior work demonstrated that bilateral visual experience induces plasticity in the tectal circuit and enhances visuomotor behavior (Pratt et al. 2016; Ruthazer and Aizenman 2010; Shen et al. 2014). We tested if we could use monocular visual experience to drive plasticity exclusively in the contralateral tectal circuit and whether this would interfere with visuomotor behavior. We provided tadpoles with 4 h of visual experience to one eye (monocular visual experience) on 2 sequential days and assayed avoidance behavior before, directly after, and several days after monocular visual experience (Fig. 2, A–E).
After a baseline visual avoidance test, tadpoles were split into three groups: a control group without manipulation, a monocular visual experience group in which tadpoles were immobilized in agarose and positioned so that the left eye was exposed to bars moving in randomized directions, and an ambient group that was immobilized and the left eye exposed to ambient light (Fig. 2B). Immobilization was well tolerated, and tadpoles began swimming normally immediately after release from the agarose. Inclusion in the avoidance behavior assay requires that all animals swim normally. The visual avoidance behavior of the ambient animals was not significantly different at any time point (Fig. 2D). Tadpoles exposed to monocular visual experience had impaired visual avoidance behavior compared with ambient light (Fig. 2D), whereas previously published work demonstrated that bilateral visual stimulation produced improvements in the same visual behavior assay (Liu and Cline 2016; Shen et al. 2014). This deficit was fully resolved 3 days after monocular visual experience treatment, during which tadpoles were exposed to normal, binocular visual experience (Fig. 2D, day 5), indicating that coordinated visual experience to both eyes restored circuit function.
If the tectal hemispheres operate independently, we predict a difference in behavior performance depending on whether the visual stimulus approaches the stimulated or unstimulated eye. To test this, we analyzed behavioral responses to stimulus encounters based on the direction of approach of the stimulus. We observed no sidedness to the deficit: regardless of direction of approach, monocular visual experience degraded behavior (Fig. 2E).
To test whether monocular visual experience affected retinotectal responses in only one hemisphere of the tectum, we measured AMPA-to-NMDA ratios of tectal neurons in response to retinotectal axon stimulation (Fig. 2, F and G). Short-term bilateral visual experience is known to produce shifts in the AMPA-to-NMDA ratio (Aizenman and Cline 2007). We recorded PSCs at −60 and +40 mV from control tadpoles, tadpoles whose left eye received monocular visual experience, and tadpoles whose right eye received monocular visual experience, in each case recording from tectal neurons located in the right tectal hemisphere (Fig. 2F). Monocular visual experience to the eye contralateral, but not ipsilateral, to the recording location significantly increased the retinotectal AMPA-to-NMDA ratio (Fig. 2G), indicating monocular stimulation is capable of selectively modifying retinotectal inputs to a single tectal hemisphere. Together, these results suggest that asymmetric visual experience induced asymmetric circuit plasticity between the tectal lobes and produced a deficit in the visuomotor circuit resulting in impaired behavior.
Spike timing-dependent plasticity protocols induce intertectal plasticity.
On the basis of the demonstrated role of STDP in the development of the tectal circuit (Mu and Poo 2006; Pratt et al. 2008) and prior work showing that monosynaptic intertectal and retinotectal inputs converge on tectal neurons (Gambrill et al. 2016), we tested whether activity-dependent synaptic plasticity mechanisms affect the integration of intertectal connections into the tectal circuit by stimulating intertectal and retinotectal axons in close temporal proximity.
First, to test whether intertectal synapses are capable of plasticity, we paired bipolar stimulation of intertectal axons with direct depolarizing current injection in tectal neurons (Fig. 3, A–C). We recorded a 10-min baseline of excitatory postsynaptic excitatory currents (EPSCs) in response to stimulating intertectal inputs. We paired intertectal axon stimulation with depolarizing current injection and measured the EPSC amplitude in the 5 min following the pairing protocol (Fig. 3B). When intertectal stimulation preceded current injection by <50 ms, intertectal EPSCs were potentiated (Fig. 3C), but no potentiation was observed when intertectal axons were stimulated without paired current injection (Fig. 4, A–C).
We next tested how interactions between intertectal and retinotectal inputs could modify the tectal circuit. We placed a second bipolar electrode in the optic tract to stimulate retinotectal axons and stimulated these two independent fiber populations in various temporally correlated patterns (Fig. 3, D–F). We recorded a 10-min baseline of intertectal and retinotectal EPSCs, introduced the pairing protocol (Fig. 3E), and assessed intertectal and retinotectal synaptic plasticity over the 5 min following pairing. Intertectal EPSCs were significantly potentiated when intertectal stimulation preceded retinotectal stimulation by <50 ms (Fig. 3F), comparable to that when intertectal stimulation preceded tectal cell depolarization by current injection. No significant plasticity in synaptic strength was observed when current injection failed to generate action potentials (Fig. 4, D–F).
Reversing the order of stimulation so that retinotectal stimulation preceded intertectal stimulation depressed intertectal EPSCs without affecting retinotectal EPSCs (Fig. 3, G–I). Finally, bath application of picrotoxin (PTX; 100 µM) blocked potentiation and depression of intertectal inputs (Fig. 3, J–L, and Fig. 4, G–I), suggesting that intact circuit inhibition is required for intertectal EPSC plasticity. These results suggest that spike timing-dependent mechanisms affect connectivity of intertectal inputs within the developing visuomotor circuit, similar to the role of STDP in the maturation of retinotectal and intratectal connections (Mu and Poo 2006; Pratt et al. 2008).
Monocular visual experience drives structural plasticity in excitatory and inhibitory intertectal axons.
The experiments described above suggest that intertectal inputs are an integral part of the tectal circuit. To probe their potential contribution to circuit dysfunction following monocular visual experience, we assessed whether and how monocular visual experience drives plasticity in intertectal synapses. First, we examined the effect of monocular visual experience on the structural plasticity of intertectal synaptic boutons. We labeled intertectal axons and presynaptic boutons by electroporating one tectal lobe with plasmids expressing cytoplasmic TurboRFP (tRFP) and synaptophysin tagged with GFP (syn-GFP). Previous electron microscopy studies indicated that 95% of syn-GFP+ boutons are bona fide synapses (Ruthazer et al. 2006). We used in vivo two-photon time-lapse imaging of sparsely electroporated intertectal axons to measure changes in syn-GFP+ bouton dynamics in response to two 4-h periods of monocular visual experience or ambient light provided to the eye contralateral to the labeled tectal neurons on sequential days (Fig. 5, A–C).
We collected a low-magnification image of intertectal axons in the optic tectum (Fig. 5C). We then selected a region of the arbor to image at higher magnification for the remainder of the experiment. After a baseline image (T1) was collected, tadpoles were exposed to 4 h of monocular visual experience or ambient light as a control and then imaged again (T2). Animals were placed in a normal light-dark cycle for the remaining 20 h of the day and then imaged (T3), exposed to 4-h monocular visual experience or ambient light, and imaged a final time (T4) the next day (Fig. 5A). Tadpoles were fixed immediately following T4, and we conducted post hoc immunolabeling for the vesicular GABA transporter (VGAT) to determine whether imaged axons were excitatory or inhibitory.
We quantified the percentage of boutons that persisted, were added, or were eliminated in response to monocular visual experience. Bouton behaviors are schematized in Fig. 5B. Excitatory and inhibitory intertectal axons eliminated relatively more boutons in response to monocular visual experience compared with ambient light (Fig. 5, D and F). In addition, excitatory and inhibitory boutons that persisted through monocular visual experience shrank compared with those in ambient light (Fig. 5, E and G). Monocular visual experience produces similar changes in excitatory and inhibitory intertectal synapses, but binocular visual experience selectively decreases excitatory intertectal synaptic inputs and increases inhibitory intertectal synaptic inputs (Gambrill et al. 2016). In response to binocular visual experience, intertectal axons undergo plasticity, which keeps tectal neurons operating within functional limits in the face of increasing retinotectal input, and the bilateral nature of this plasticity maintains a balance of excitation to inhibition between the tectal lobes. In contrast, monocular visual experience creates an intertectal imbalance in each lobe’s operating state (Fig. 5H).
Monocular visual experience-induced plasticity disrupts excitatory/inhibitory balance.
Given that monocular visual experience drives structural plasticity in both excitatory and inhibitory intertectal axons, and the important role E/I balance plays in sensory circuit formation (Froemke 2015; Miraucourt et al. 2012; Murphy et al. 2005; Sprekeler 2017; Tatti et al. 2017), we tested whether monocular visual experience could selectively shift E/I in response to different sets of inputs to tectal neurons. Binocular visual experience drives refinement and potentiation of retinotectal and intertectal synapses (Aizenman and Cline 2007; Gambrill et al. 2016; Ruthazer et al. 2006) in a manner that preserves circuit E/I balance (He et al. 2016). In contrast, monocular visual experience only potentiates the direct retinotectal connections in the tectal lobe contralateral to the stimulated eye, seen as an increase in AMPA/NMDA, but does not affect retinotectal AMPA-to-NMDA ratios in the opposite tectal hemisphere, ipsilateral to the stimulated eye (Fig. 2G). Retinotectal synaptic inputs activate recurrent excitatory and inhibitory connections in the tectum (Pratt et al. 2008). We hypothesized that monocular visual experience might drive plasticity in the excitatory and inhibitory connections in the tectum contralateral to the stimulated eye and that these changes in neuronal activity may be conveyed to the opposite tectum via tectal cells extending intertectal axons. We further postulated that we could assess this visual experience-dependent plasticity by recording AMPA-to-GABA ratios in the tecta contralateral and ipsilateral to the stimulated eye (Fig. 6B). Monocular visual experience would thus create an E/I imbalance between the hemispheres, contributing to the observed detriments in behavior (Fig. 2).
As in Fig. 2, we provided monocular visual experience for 2 sequential days to the same eye while the other eye was exposed to ambient light (Fig. 6A). In the days following monocular visual experience, we tested for asymmetric changes in E/I by recording PSCs from tectal neurons held at −60 and 0 mV to capture AMPAR- and GABA receptor-mediated responses to stimulation of retinotectal or intertectal axons. Inhibitory responses to optic tract stimulation of retinotectal axons are feed forward inhibitory responses and all recordings were made during the developmental age in which GABAergic reversal potential is stable and GABA currents are hyperpolarizing (Akerman and Cline 2006). We recorded from tectal neurons from three groups: those that received monocular visual experience to the eye contralateral to the recording location, those that received monocular visual experience to the eye ipsilateral to the recording location, and those that received only ambient light to both eyes (control; Fig. 6B).
AMPA-to-GABA ratio in response to retinotectal stimulation increased significantly in response to contralateral visual experience, but not ipsilateral visual experience (Fig. 6C). Together with recordings of the retinotectal AMPA-to-NMDA ratio (Fig. 2G), these results indicate monocular visual experience stimulates one eye in isolation of the other and that this stimulation shifts E/I in affected synapses in the contralateral tectum. In contrast, intertectal synapses were not affected by contralateral visual experience, but AMPA-to-GABA ratios were significantly increased in response to ipsilateral visual experience, a condition in which visual experience activates the somata of intertectally projecting tectal neurons (Fig. 6D). In a separate experiment, we confirmed that PSCs recorded at −60 mV were majority excitatory by applying a GABAA antagonist (PTX, 100 µM) to the bath (Fig. 6E). The response amplitude at −60 mV was not significantly affected by PTX. Likewise, majority inhibitory current at 0 mV was confirmed by the addition of AMPAR (NBQX, 20 µM) and NMDAR (APV, 50 µM) antagonists (Fig. 6F), which did not significantly affect the PSC amplitude. This indicates that our protocol of monocular visual experience selectively induced input-specific synaptic plasticity capable of inducing change in E/I. These synaptic changes, which create differences in the relative E/I ratios across the midline, may explain the observed monocular visual experience-induced deficits in visual avoidance behavior.
Unilateral E/I shifts disrupt circuit function.
To test whether unilateral shifts in E/I are sufficient to disrupt behavior, we used two molecular genetic strategies to shift E/I in a single tectal hemisphere. We prescreened animals for visual avoidance responses and unilaterally electroporated tecta with a plasmid that expresses a peptide corresponding to the intracellular loop (ICL) of the γ2-subunit of the GABAAR or with a plasmid that expresses a peptide corresponding to the cytoplasmic tail (CTP) of the GluA2 subunit of the AMPAR, together with GFP (Fig. 7, A and B). ICL expression interferes with anchoring the γ2-subunit of GABAAR at postsynaptic sites, which decreases GABAergic synaptic input to neurons within 2 days after transfection (Shen et al. 2009). CTP expression interferes with trafficking of GluA2-containing AMPARs into synapses, which decreases AMPA-mediated synaptic transmission onto transfected neurons (Haas et al. 2006; Shi et al. 2001).
Fig. 7.
Unilateral E/I shifts are sufficient to disrupt visual avoidance behavior. A and B: experimental timeline (A) and schematic of experimental groups (B). Baseline visual avoidance behavior was assayed on day 0. On day 1, tadpoles were electroporated in one tectal lobe with plasmids expressing green fluorescent protein (GFP) and a peptide corresponding to the intracellular loop of the γ2-subunit of the GABAA receptor (ICL) or a peptide corresponding to the cytoplasmic tail of the GluA2 subunit of the AMPA receptor (CTP). Control tadpoles were electroporated with a GFP expression plasmid. Visual avoidance behavior was assayed again 3 days after electroporation. C: unilateral expression of ICL or CTP disrupts E/I balance. Recordings from GFP+ neurons were made in the left tectal lobe at −60 and 0 mV in dissected brains (B). PSCs were generated by stimulating IT axons. ICL increased AMPA/GABA (black; n = 10) compared with control (n = 13; *P = 0.01). CTP decreased AMPA/GABA (light gray; n = 11) compared with control (n = 14; *P = 0.04). Insets show representative PSCs recorded from control (dark gray), ICL-expressing (black), and CTP-expressing neurons (light gray) at −60 and 0 mV (scale bar: 50 ms, 20 pA). D: unilateral ICL expression (black) or CTP expression (light gray) produces a significant deficit in visual avoidance behavior. Avoidance indexes were normalized to control. Data were collected from 2 to 3 biological replicates and are means ± SE. Left, control, 1.0 ± 0.07 (n = 14 tadpoles) vs. ICL, 0.76 ± 0.06 (n = 21 tadpoles). *P = 0.026, 2-tailed Student’s t-test. Right, control, 1.0 ± 0.13 (n = 3 batches, 20–40 animals per group) vs. CTP, 0.40 ± 0.06. *P = 0.03, 2-tailed Student’s t-test.
We tested the effect of ICL and CTP expression on E/I by recording PSCs at −60 and 0 mV from GFP-expressing neurons in the transfected tecta. ICL expression significantly increased AMPA-to-GABA ratios, compared with nontransfected controls, consistent with decreased GABAAR-mediated current, as reported previously (Shen et al. 2011) (Fig. 7C). CTP expression significantly decreased AMPA-to-GABA ratios, consistent with loss of AMPAR-mediated current, as reported previously (Haas et al. 2006) (Fig. 7C). We then assayed the behavior of tadpoles 3 days after electroporation (Fig. 7, A and D). Unilateral expression of either ICL or CTP significantly impaired visual avoidance behavior compared with controls (Fig. 7D), indicating unilateral changes in E/I are sufficient to disrupt visuomotor behavior.
DISCUSSION
In this study, we showed that intertectal inputs are a component of the functional visuomotor circuit in Xenopus tadpoles. Cutting intertectal axons impaired visual avoidance behavior, suggesting intertectal fibers carry information between the tectal hemispheres that is necessary for optimal performance. Motivated by evidence that visual experience promotes maturation of the functional tectal circuit, we found that monocular visual experience induced input-specific plasticity in the optic tectum, which was bilaterally asymmetric and which disrupted visuomotor behavior, regardless of the direction of approach of a looming stimulus. We demonstrated intertectal synapses are capable of structural and functional plasticity, consistent with the idea that visual experience modifies intertectal connectivity in the tectal circuit. Monocular visual experience induced input- and hemisphere-specific changes in E/I, leading us to hypothesize that asymmetric E/I across the tectal midline might be sufficient to disrupt circuit function. To test this, we unilaterally expressed peptides that disrupt GABAA or AMPA receptor trafficking, to disrupt symmetrical E/I, and showed that both manipulations impair visual behavior. Together, these data demonstrate that communication between the optic tectal lobes, mediated by intertectal axons, is an integral component of the bilateral tectal circuit and is important for sensorimotor behavior.
Role of intertectal inputs in sensorimotor integration.
The optic tectum/superior colliculus is crucial for sensorimotor integration, in both developing and adult animals. The importance of intercollicular communication in visuomotor behavior was first demonstrated in cats, where a unilateral lesion to visual cortex rendered animals blind in one visual hemifield, but subsequent transection of the intercollicular commissure restored visually guided behavior in response to stimuli in the affected hemifield (Sprague 1966). Since then, neuroanatomical tracing and electrophysiological experiments have demonstrated that intercollicular inputs are excitatory and inhibitory, and suggested these fibers selectively reinforce or suppress activation of collicular neurons to shape their receptive fields and output (Edwards 1977; Sprague 1966; Takahashi et al. 2007; Waleszczyk et al. 1993).
Intertectal inputs are also present in the fully crossed visual circuit of nonmammalian vertebrates such as frogs and fish (Gambrill et al. 2016; Herrero et al. 1999; Miraucourt et al. 2012), but their role in the tectal circuit and sensorimotor integration in these species is unclear. Early studies of visual circuitry in adult frogs showed that the capacity of unilateral stimuli to evoke responses in the ipsilateral tectum is acquired as a result of metamorphic rewiring of the circuit and that transection of the intertectal commissure failed to eliminate these responses (Gaze and Jacobson 1963; Keating and Gaze 1970). In studies recording from neurons in the tadpole optic tectum, the intertectal commissure is routinely cut to facilitate recording access to tectal neurons. Consequently, the potential role intertectal inputs may play in circuit development and function in tadpoles has been neglected. In our study, tadpoles in which the intertectal commissure was cut had defects in avoidance of looming visual stimuli. Tadpoles recovered the behavior after intertectal fibers regenerated. Together with previous work showing that unilateral tectal injury was sufficient to disrupt avoidance behavior regardless of the side of the approach of a looming stimulus (McKeown et al. 2013), this result demonstrates that cutting the intertectal commissure interferes with the tectal function of coordinating bilateral sensory input with motor output. This indicates that the integration of visual information from both eyes is crucial to survival, even at this early developmental stage when adult circuitry for binocular vision has not yet developed (Udin 2012).
In fish, unilateral optic tectal lesions produce deficits in behavior to stimuli only in the lesioned hemifield, not the intact hemifield (Temizer et al. 2015). This suggests that in fish, the tectal hemispheres may operate in isolation with respect to visuomotor behavior. Frogs are unique among animals with fully crossed visual systems in that they acquire binocular vision during metamorphosis when the eyes undergo dorsomedial migration (Grant and Keating 1989). Binocular visual inputs are critical for the development of binocular vision in the frog, but our data suggest the necessity for symmetric visual input begins much earlier. Direct intertectal input may mediate visuomotor behavior transiently during tadpole stages and may have a role in establishing the tecto-isthmo-tectal circuit, which mediates binocular vision later in development.
Monocular disruptions to visual input in partially and fully crossed visual systems.
A comprehensive body of work documents the development of partially crossed visual circuits. Experimental deprivation of sensory input to a single eye has played a key role in establishing how activity drives the development, plasticity, and refinement of the visual circuit (Cooke and Bear 2013; Espinosa and Stryker 2012; Hensch and Fagiolini 2005). Monocular deprivation results in changes to axonal projections (Hubel et al. 1977), the organization of ocular dominance columns (Wiesel and Hubel 1963), and the response properties of neurons in visual processing regions (Wang et al. 2010). Critical periods, or windows of heightened circuit plasticity, can also be modified by changes in sensory input. In mammalian visual cortex, visual experience drives maturation of inhibitory circuits (Jiang et al. 2005) and E/I balance establishes the timing of the critical period (Fagiolini et al. 2003; Hensch et al. 1998; Iwai et al. 2003). Furthermore, shifts in E/I facilitate open eye potentiation and deprived eye depression in response to monocular deprivation (Ma et al. 2013; Maffei et al. 2006). The observation that transient visual deprivation can induce a return to a more plastic state has led researchers to investigate how therapies involving dark exposure or retinal inactivation can help correct visual disorders such as amblyopia, in which imbalances in the strength of input to visual processing regions between the two eyes lead to visual deficits (Fong et al. 2016; Freeman and Olson 1982; He et al. 2007; Mitchell and Duffy 2014). Dark exposure may facilitate recovery from amblyopia induced by early monocular deprivation by resetting the E/I balance to a less mature, more excitatory, state (He et al. 2006). Indeed, other manipulations that decrease cortical inhibition have also been shown to permit juvenile-like plasticity in the adult visual cortex (Hübener and Bonhoeffer 2014).
In fully crossed visual circuits such as the optic tectum of Xenopus tadpoles, the effect of providing differing amounts of input to the two eyes was unknown. When visual input across the midline is grossly asymmetric, as occurs as a result of our monocular visual experience manipulation, intertectally mediated integration of normal visual activity across the midline and sensorimotor behavior are disrupted (Fig. 2). Under normal sensory conditions with binocular visual experience, retinotectal and intertectal synapses are similarly active in each tectal lobe, resulting in similar experience-dependent plasticity in the bilateral tectal circuit. However, monocular visual experience drives asymmetric plasticity between tectal lobes, and monocular visual experience shifts E/I in select intertectal inputs. Under normal visual conditions, the plasticity of intertectal input might act as a homeostatic regulator to maintain bilateral tectal circuit function within a dynamic range that gauges visual inputs from both eyes. The concept that coordinated development and function of the hemispheres is important for visual function and that proper E/I may be necessary to detect imbalances in activity between the two eyes has been proposed in partially crossed visual systems (Hensch and Fagiolini 2005; Pietrasanta et al. 2012); however, our discovery that this is also the case in a developing organism with a fully crossed visual system is novel and surprising.
Excitation-to-inhibition ratios in visual circuit function.
E/I balance changes during development and is dynamically regulated by visual experience (Froemke 2015; Jiang et al. 2005; Vogels et al. 2013). As the balance of E/I shifts over development (Akerman and Cline 2006; Liu 2004; Liu et al. 2007), the strength of excitatory and inhibitory inputs becomes more correlated, excitatory and inhibitory receptive fields align, and modifications in either excitation or inhibition result in homeostatic changes in the other (He et al. 2016; Liu 2004; Sprekeler 2017; Tao and Poo 2005). In tadpoles, short-term exposure to bilateral visual stimuli increases GABA expression in tectal interneurons (Miraucourt et al. 2012) and drives plasticity in excitatory and inhibitory tectal synapses (Aizenman et al. 2002; Gambrill et al. 2016; Liu et al. 2007). Interestingly, the polarity of experience-dependent plasticity in GABAergic synapses is dependent on the tectal E/I balance (Liu et al. 2007). These modifications are part of a circuit-wide adjustment which ensures that tectal neurons remain dynamically responsive to a wide range of stimuli. In addition, normal E/I is important for establishing the size and alignment of tectal receptive fields (Shen et al. 2011; Tao and Poo 2005), synaptic and multisensory integration (Felch et al. 2016; Shen et al. 2011), sensitivity to visual stimuli (Khakhalin et al. 2014), and visuomotor behavior (Shen et al. 2011).
Although bilateral manipulations of E/I have produced functional or developmental deficits (Khakhalin et al. 2014; Richards et al. 2010b; Shen et al. 2009, 2011; Tao and Poo 2005), it is often difficult to separate the role of changes in the E/I from the effect of bulk loss of excitatory or inhibitory current. Furthermore, with the use of bilateral manipulations, it is not possible to specifically test the importance of E/I balance between the two hemispheres. We directly tested these concepts in two ways. First, we showed that monocular visual experience drives acute structural and functional plasticity in the contralateral retinotectal circuit and the ipsilateral intertectal projections, leading to bilateral asymmetry in E/I. Interestingly, monocular and binocular visual experiences drive different types of changes in the intertectal circuit. We previously found that binocular visual experience induces addition of new inhibitory intertectal synapses, increasing GABAAR-mediated current, and refines excitatory intertectal synapses so that weak synapses are preferentially lost, leading to an increase in AMPAR-mediated EPSCs (Gambrill et al. 2016). Importantly, these modifications are symmetric across the tectal midline, so the E/I ratio of intertectal inputs is maintained with bilateral visual experience. In contrast, we found in the current study that the E/I ratio of intertectal inputs increases unilaterally in response to monocular visual experience, even though both excitatory and inhibitory intertectal synaptic boutons are eliminated. Together, these findings suggest that decreasing excitatory intertectal bouton numbers reflects loss of weak synapses, leading to a unilateral increase in AMPAR-mediated current. However, inhibitory intertectal boutons are lost with monocular visual experience instead of added, leading to a unilateral decrease in the GABAAR-mediated current and bilateral asymmetry in E/I.
Second, we demonstrated that unilateral disruption of inhibitory or excitatory neurotransmission by manipulating AMPA or GABAA receptor trafficking led to a deficit in visuomotor behavior. The parallel loss of behavior in response to manipulating either excitation or inhibition suggests that E/I itself is crucial for circuit function. This also suggests that the E/I asymmetry created by monocular visual experience might be responsible for behavioral abnormalities after monocular visual experience. Importantly, whether the E/I shifts were elicited by pathway-specific plasticity to the tectum (monocular visual experience) or postsynaptic neuronal manipulations (ICL and CTP), the affect on visuomotor behavior was the same. Our data indicate that E/I is critical for establishing the response properties of tectal neurons and that imbalanced E/I between the hemispheres may disrupt communication between the tectal hemispheres concerning properties of visual stimuli, resulting in disruption of behaviors that require motor activity in response to pertinent, moving stimuli.
There are several ways in which imbalances in E/I might affect circuit function and lead to deficits in visuomotor behavior (Tatti et al. 2017). The gating of STDP by E/I is one candidate mechanism (Froemke 2015). STDP is known to be important for the development and plasticity of visual circuits (Feldman 2012; Richards et al. 2010a; Ruthazer and Aizenman 2010). At early developmental stages, tadpoles exhibit STDP in retinotectal synapses, and STDP plays a critical role in visual circuit refinement (Mu and Poo 2006; Pratt et al. 2008; Richards et al. 2010b; Vislay-Meltzer et al. 2006; Zhang et al. 1998). Later in development, retinotectal STDP is difficult to induce (Tsui et al. 2010), but STDP at intratectal synapses (Pratt et al. 2008) and, as we have shown in this study, intertectal synapses, continues to modify tectal responsiveness. Inhibition regulates spike timing (Shen et al. 2011; Tao and Poo 2005) and is required for intertectal STDP. In addition to the function of synapses, E/I balance is critical for the experience-dependent regulation of dendritic arbor size and, by extension, synapse number (Haas et al. 2006; Shen et al. 2009). E/I regulates other circuit features such as the jitter in spike latency (Pouille and Scanziani 2001; Richards et al. 2010b; Shen et al. 2011) and temporal receptive fields (Shen et al. 2011). These properties affect the timing of responses to visual input and the window in which synaptic inputs are integrated (Tiesinga et al. 2008) and might be critical in signaling and integration across the midline. Local and intertectal inputs provide feedforward excitation and inhibition that would dynamically shape the E/I balance in the tectal circuit (Gambrill et al. 2016; Pratt et al. 2008). In response to monocular visual experience, unilateral retinotectal excitation drives plasticity in feedforward intertectal inputs in the contralateral hemisphere, creating a discrepancy between the E/I balance in the two hemispheres. Over time, this would result in differences in how each tectal lobe processes visual input. We have shown that when those processing differences arise, integration and coordination across the midline are perturbed and behavioral output suffers. In conclusion, intertectal input provides an important pathway for information to cross between the tectal hemispheres, allowing a coordination that leads to functional visuomotor behavior.
GRANTS
This work was supported by NIH grants 5F32NS084749 (to A. C. Gambrill), 5F32NS071807 (to R. L. Faulkner), and EY011261 and EY027437 (to H. T. Cline), Helen Dorris Scholars awards (to A. C. Gambrill and R. L. Faulkner), and an endowment from the Hahn Family Foundation (to H. T. Cline).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C.G., R.L.F., and H.T.C. conceived and designed research; A.C.G. and R.L.F. performed experiments; A.C.G. and R.L.F. analyzed data; A.C.G., R.L.F., and H.T.C. interpreted results of experiments; A.C.G. and R.L.F. prepared figures; A.C.G., R.L.F., and H.T.C. drafted manuscript; A.C.G., R.L.F., and H.T.C. edited and revised manuscript; A.C.G., R.L.F., and H.T.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of the Cline laboratory for helpful discussions and Drs. Carlos Aizenman and Ed Ruthazer for comments on the manuscript.
REFERENCES
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39: 831–842, 2003. doi: 10.1016/S0896-6273(03)00527-0. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Cline HT. Enhanced visual activity in vivo forms nascent synapses in the developing retinotectal projection. J Neurophysiol 97: 2949–2957, 2007. doi: 10.1152/jn.00452.2006. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Muñoz-Elías G, Cline HT. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron 34: 623–634, 2002. doi: 10.1016/S0896-6273(02)00674-8. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci 26: 5117–5130, 2006. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bear MF. How the mechanisms of long-term synaptic potentiation and depression serve experience-dependent plasticity in primary visual cortex. Philos Trans R Soc Lond B Biol Sci 369: 20130284, 2013. [Erratum in Philos Trans R Soc Lond B Biol Sci 369: 20140021, 2014.] 10.1098/rstb.2013.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan WM, Adamson L, Powell TP. An experimental study of the avian visual system. J Anat 95: 545–563, 1961. [PMC free article] [PubMed] [Google Scholar]
- D’amour JA, Froemke RC. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron 86: 514–528, 2015. doi: 10.1016/j.neuron.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg KE, Sears IB, Aizenman CD. Development of multisensory convergence in the Xenopus optic tectum. J Neurophysiol 102: 3392–3404, 2009. doi: 10.1152/jn.00632.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Lee RH, Xu H, Yang S, Pratt KG, Cao V, Song YK, Nurmikko A, Aizenman CD. Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. J Neurophysiol 101: 803–815, 2009. doi: 10.1152/jn.90848.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TW, Gebhardt C, Naumann EA, Riegler C, Ahrens MB, Engert F, Del Bene F. Neural circuits underlying visually evoked escapes in larval zebrafish. Neuron 89: 613–628, 2016. doi: 10.1016/j.neuron.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SB. The commissural projection of the superior colliculus in the cat. J Comp Neurol 173: 23–40, 1977. doi: 10.1002/cne.901730103. [DOI] [PubMed] [Google Scholar]
- Engert F, Tao HW, Zhang LI, Poo MM. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature 419: 470–475, 2002. doi: 10.1038/nature00988. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron 75: 230–249, 2012. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-d-aspartate receptor 2A signaling. Proc Natl Acad Sci USA 100: 2854–2859, 2003. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felch DL, Khakhalin AS, Aizenman CD. Multisensory integration in the developing tectum is constrained by the balance of excitation and inhibition. eLife 5: e15600, 2016. doi: 10.7554/eLife.15600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron 75: 556–571, 2012. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa L, Rajan I, Haas K, Wu GY, Brakeman P, Worley P, Cline H. The scaffold protein, Homer1b/c, regulates axon pathfinding in the central nervous system in vivo. Nat Neurosci 4: 499–506, 2001. doi: 10.1038/87447. [DOI] [PubMed] [Google Scholar]
- Fong MF, Mitchell DE, Duffy KR, Bear MF. Rapid recovery from the effects of early monocular deprivation is enabled by temporary inactivation of the retinas. Proc Natl Acad Sci USA 113: 14139–14144, 2016. doi: 10.1073/pnas.1613279113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RD, Olson C. Brief periods of monocular deprivation in kittens: effects of delay prior to physiological study. J Neurophysiol 47: 139–150, 1982. doi: 10.1152/jn.1982.47.2.139. [DOI] [PubMed] [Google Scholar]
- Fritschy JM. Epilepsy, E/I balance and GABAA receptor plasticity. Front Mol Neurosci 1: 5, 2008. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC. Plasticity of cortical excitatory-inhibitory balance. Annu Rev Neurosci 38: 195–219, 2015. doi: 10.1146/annurev-neuro-071714-034002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill AC, Faulkner RF, Cline HT. Experience-dependent plasticity of excitatory and inhibitory intertectal inputs in Xenopus tadpoles. J Neurophysiol 116: 2281–2297, 2016. doi: 10.1152/jn.00611.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze RM, Jacobson M. Proceedings of the Physiological Society, 9–10 November 1962; Mill Hill Meeting: Communications. J Physiol 165, Suppl: 60–82, 1963. doi: 10.1113/jphysiol.1963.sp007086. [DOI] [Google Scholar]
- Grant S, Keating MJ. Changing patterns of binocular visual connections in the intertectal system during development of the frog, Xenopus laevis. I. Normal maturational changes in response to changing binocular geometry. Exp Brain Res 75: 99–116, 1989. doi: 10.1007/BF00248534. [DOI] [PubMed] [Google Scholar]
- Gray JR, Blincow E, Robertson RM. A pair of motion-sensitive neurons in the locust encode approaches of a looming object. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 196: 927–938, 2010. doi: 10.1007/s00359-010-0576-7. [DOI] [PubMed] [Google Scholar]
- Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electroporation in Xenopus tadpoles in vivo–from single cells to the entire brain. Differentiation 70: 148–154, 2002. doi: 10.1046/j.1432-0436.2002.700404.x. [DOI] [PubMed] [Google Scholar]
- Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci USA 103: 12127–12131, 2006. doi: 10.1073/pnas.0602670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci 26: 2951–2955, 2006. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci 10: 1134–1136, 2007. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- He HY, Shen W, Hiramoto M, Cline HT. Experience-dependent bimodal plasticity of inhibitory neurons in early development. Neuron 90: 1203–1214, 2016. doi: 10.1016/j.neuron.2016.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res 147: 115–124, 2005. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282: 1504–1508, 1998. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero L, Pérez P, Núnez Abades P, Hardy O, Torres B. Tectotectal connectivity in goldfish. J Comp Neurol 411: 455–471, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Hiramoto M, Cline HT. Convergence of multisensory inputs in Xenopus tadpole tectum. Dev Neurobiol 69: 959–971, 2009. doi: 10.1002/dneu.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto M, Cline HT. Optic flow instructs retinotopic map formation through a spatial to temporal to spatial transformation of visual information. Proc Natl Acad Sci USA 111: E5105–E5113, 2014. doi: 10.1073/pnas.1416953111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci 278: 377–409, 1977. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Hübener M, Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell 159: 727–737, 2014. doi: 10.1016/j.cell.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci 23: 6695–6702, 2003. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev 50: 126–133, 2005. doi: 10.1016/j.brainresrev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Keating MJ, Gaze RM. The ipsilateral retinotectal pathway in the frog. Q J Exp Physiol Cogn Med Sci 55: 284–292, 1970. [DOI] [PubMed] [Google Scholar]
- Khakhalin AS, Koren D, Gu J, Xu H, Aizenman CD. Excitation and inhibition in recurrent networks mediate collision avoidance in Xenopus tadpoles. Eur J Neurosci 40: 2948–2962, 2014. doi: 10.1111/ejn.12664. [DOI] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr Biol 14: R335–R338, 2004. doi: 10.1016/j.cub.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci 7: 373–379, 2004. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- Liu HH, Cline HT. Fragile X mental retardation protein is required to maintain visual conditioning-induced behavioral plasticity by limiting local protein synthesis. J Neurosci 36: 7325–7339, 2016. doi: 10.1523/JNEUROSCI.4282-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang LI, Tao HW. Heterosynaptic scaling of developing GABAergic synapses: dependence on glutamatergic input and developmental stage. J Neurosci 27: 5301–5312, 2007. doi: 10.1523/JNEUROSCI.0376-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Wang Q, Li B. Neuronal responses to looming objects in the superior colliculus of the cat. Brain Behav Evol 77: 193–205, 2011. doi: 10.1159/000327045. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hamodi AS, Pratt KG. Early development and function of the Xenopus tadpole retinotectal circuit. Curr Opin Neurobiol 41: 17–23, 2016. doi: 10.1016/j.conb.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Ma WP, Li YT, Tao HW. Downregulation of cortical inhibition mediates ocular dominance plasticity during the critical period. J Neurosci 33: 11276–11280, 2013. doi: 10.1523/JNEUROSCI.5598-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature 443: 81–84, 2006. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- McKeown CR, Sharma P, Sharipov HE, Shen W, Cline HT. Neurogenesis is required for behavioral recovery after injury in the visual system of Xenopus laevis. J Comp Neurol 521: 2262–2278, 2013. doi: 10.1002/cne.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraucourt LS, da Silva JS, Burgos K, Li J, Abe H, Ruthazer ES, Cline HT. GABA expression and regulation by sensory experience in the developing visual system. PLoS One 7: e29086, 2012. doi: 10.1371/journal.pone.0029086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE, Duffy KR. The case from animal studies for balanced binocular treatment strategies for human amblyopia. Ophthalmic Physiol Opt 34: 129–145, 2014. doi: 10.1111/opo.12122. [DOI] [PubMed] [Google Scholar]
- Mu Y, Poo MM. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron 50: 115–125, 2006. doi: 10.1016/j.neuron.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Munz M, Gobert D, Schohl A, Poquérusse J, Podgorski K, Spratt P, Ruthazer ES. Rapid Hebbian axonal remodeling mediated by visual stimulation. Science 344: 904–909, 2014. doi: 10.1126/science.1251593. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Beston BR, Boley PM, Jones DG. Development of human visual cortex: a balance between excitatory and inhibitory plasticity mechanisms. Dev Psychobiol 46: 209–221, 2005. doi: 10.1002/dev.20053. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J, editors. Normal Table of Xenopus laevis (Daudin): a Systematical and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis. Amsterdam: North-Holland, 1956. [Google Scholar]
- Pietrasanta M, Restani L, Caleo M. The corpus callosum and the visual cortex: plasticity is a game for two. Neural Plast 2012: 838672, 2012. doi: 10.1155/2012/838672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293: 1159–1163, 2001. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci 27: 8268–8277, 2007. doi: 10.1523/JNEUROSCI.1738-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Multisensory integration in mesencephalic trigeminal neurons in Xenopus tadpoles. J Neurophysiol 102: 399–412, 2009. doi: 10.1152/jn.91317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Dong W, Aizenman CD. Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci 11: 467–475, 2008. doi: 10.1038/nn2076. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Hiramoto M, Cline HT. An evolutionarily conserved mechanism for activity-dependent visual circuit development. Front Neural Circuits 10: 79, 2016. doi: 10.3389/fncir.2016.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards BA, Aizenman CD, Akerman CJ. In vivo spike-timing-dependent plasticity in the optic tectum of Xenopus laevis. Front Synaptic Neurosci 2: 7, 2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards BA, Voss OP, Akerman CJ. GABAergic circuits control stimulus-instructed receptive field development in the optic tectum. Nat Neurosci 13: 1098–1106, 2010b. doi: 10.1038/nn.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Aizenman CD. Learning to see: patterned visual activity and the development of visual function. Trends Neurosci 33: 183–192, 2010. doi: 10.1016/j.tins.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Cline HT. Insights into activity-dependent map formation from the retinotectal system: a middle-of-the-brain perspective. J Neurobiol 59: 134–146, 2004. doi: 10.1002/neu.10344. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci 26: 3594–3603, 2006. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer RD, Rind FC, Simmons PJ. Predator versus prey: locust looming-detector neuron and behavioural responses to stimuli representing attacking bird predators. PLoS One 7: e50146, 2012. doi: 10.1371/journal.pone.0050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SC. The retinal projections in the goldfish: an experimental study. Brain Res 39: 213–223, 1972. doi: 10.1016/0006-8993(72)90796-2. [DOI] [PubMed] [Google Scholar]
- Shen W, Da Silva JS, He H, Cline HT. Type A GABA-receptor-dependent synaptic transmission sculpts dendritic arbor structure in Xenopus tadpoles in vivo. J Neurosci 29: 5032–5043, 2009. doi: 10.1523/JNEUROSCI.5331-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Liu HH, Schiapparelli L, McClatchy D, He HY, Yates JR 3rd, Cline HT. Acute synthesis of CPEB is required for plasticity of visual avoidance behavior in Xenopus. Cell Reports 6: 737–747, 2014. doi: 10.1016/j.celrep.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, McKeown CR, Demas JA, Cline HT. Inhibition to excitation ratio regulates visual system responses and behavior in vivo. J Neurophysiol 106: 2285–2302, 2011. doi: 10.1152/jn.00641.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331–343, 2001. doi: 10.1016/S0092-8674(01)00321-X. [DOI] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419: 475–480, 2002. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 153: 1544–1547, 1966. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Sprekeler H. Functional consequences of inhibitory plasticity: homeostasis, the excitation-inhibition balance and beyond. Curr Opin Neurobiol 43: 198–203, 2017. doi: 10.1016/j.conb.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sugiuchi Y, Shinoda Y. Commissural mirror-symmetric excitation and reciprocal inhibition between the two superior colliculi and their roles in vertical and horizontal eye movements. J Neurophysiol 98: 2664–2682, 2007. doi: 10.1152/jn.00696.2007. [DOI] [PubMed] [Google Scholar]
- Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron 45: 829–836, 2005. doi: 10.1016/j.neuron.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Tatti R, Haley MS, Swanson OK, Tselha T, Maffei A. Neurophysiology and Regulation of the Balance Between Excitation and Inhibition in Neocortical Circuits. Biol Psychiatry 81: 821–831, 2017. doi: 10.1016/j.biopsych.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temizer I, Donovan JC, Baier H, Semmelhack JL. A visual pathway for looming-evoked escape in larval zebrafish. Curr Biol 25: 1823–1834, 2015. doi: 10.1016/j.cub.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Tiesinga P, Fellous JM, Sejnowski TJ. Regulation of spike timing in visual cortical circuits. Nat Rev Neurosci 9: 97–107, 2008. doi: 10.1038/nrn2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui J, Schwartz N, Ruthazer ES. A developmental sensitive period for spike timing-dependent plasticity in the retinotectal projection. Front Synaptic Neurosci 2: 13, 2010. doi: 10.3389/fnsyn.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udin SB. Binocular maps in Xenopus tectum: visual experience and the development of isthmotectal topography. Dev Neurobiol 72: 564–574, 2012. doi: 10.1002/dneu.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vislay-Meltzer RL, Kampff AR, Engert F. Spatiotemporal specificity of neuronal activity directs the modification of receptive fields in the developing retinotectal system. Neuron 50: 101–114, 2006. doi: 10.1016/j.neuron.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Vogels TP, Froemke RC, Doyon N, Gilson M, Haas JS, Liu R, Maffei A, Miller P, Wierenga CJ, Woodin MA, Zenke F, Sprekeler H. Inhibitory synaptic plasticity: spike timing-dependence and putative network function. Front Neural Circuits 7: 119, 2013. doi: 10.3389/fncir.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleszczyk WJ, Dec K, Hekimian AA. Influence of the intertectal connection upon visual responses in the cat’s superior colliculus. Acta Neurobiol Exp (Wars) 53: 409–414, 1993. [PubMed] [Google Scholar]
- Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JN. Rats maintain an overhead binocular field at the expense of constant fusion. Nature 498: 65–69, 2013. doi: 10.1038/nature12153. [DOI] [PubMed] [Google Scholar]
- Wang BS, Sarnaik R, Cang J. Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 65: 246–256, 2010. doi: 10.1016/j.neuron.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol 26: 1003–1017, 1963. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wu LQ, Niu YQ, Yang J, Wang SR. Tectal neurons signal impending collision of looming objects in the pigeon. Eur J Neurosci 22: 2325–2331, 2005. doi: 10.1111/j.1460-9568.2005.04397.x. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature 395: 37–44, 1998. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- Zhao X, Liu M, Cang J. Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice. Neuron 84: 202–213, 2014. doi: 10.1016/j.neuron.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]