Abstract
Oxytocin (OT) neurons exhibit larger afterhyperpolarizations (AHPs) following spike trains during late pregnancy and lactation, times when these neurons fire in bursts and release more OT associated with labor and lactation. Calcium-dependent AHPs mediated by SK channels show this plasticity, and are reduced when the channel complex is phosphorylated by casein kinase 2 (CK2), and increased when dephosphorylated by protein phosphatase (PP)2A, by altering Ca2+ sensitivity. We compared AHP currents in supraoptic OT neurons after CK2 inhibition with 4,5,6,7-tetrabromobenzotriazole (TBB), or PP1-PP2A inhibition with okadaic acid (OA), to determine the roles of these enzymes in AHP plasticity, focusing on the peak current at 100 ms representing the SK-mediated, medium AHP (ImAHP). In slices from virgin and two groups of pregnant rats [embryonic days (E18–19, or E20–21], ImAHPs were evoked with 3-, 10-, and 17-spike trains (20 Hz). With 3-spike trains, TBB increased the ImAHP to the greatest extent in virgin compared with both groups of pregnant animals. A difference between virgins and E20–21 rats was also evident with a 10-spike train but the increases in ImAHPs were similar among groups with 17-spike trains. In contrast, OA, while consistently reducing the ImAHP in all cases, showed no differential effects among groups. In Western blots, CK2α, CK2β, PP2A-A, PP2A-B, and PP2A-C were found in supraoptic lysates, and expression of CK2α and CK2β was reduced in E20–21 rats. Coimmunoprecipitation revealed that calmodulin, CK2α, and PP2A-C were associated with SK3 protein. The results suggest that a downregulation of SK3-associated CK2α during late pregnancy may increase the sensitivity of the SK calmodulin (Ca2+) sensor for ImAHP, contributing to the enhanced ImAHP.
NEW & NOTEWORTHY The article demonstrates for the first time that enhancement in spike afterhyperpolarizations in oxytocin neurons during pregnancy may be related to a downregulation in the small-conductance Ca2+-activated potassium channels (SK)/calmodulin binding protein casein kinase 2, which phosphorylates the SK channel complex and reduces its Ca2+ sensitivity.
Keywords: casein kinase 2, oxytocin, protein phosphatase 2A, vasopressin
INTRODUCTION
During parturition and lactation, the neurohypophysial hormone oxytocin (OT) is released in large quantities to promote uterine contractions and milk ejection, respectively (Armstrong 2015). In mammals, the periodic, synchronous bursts of action potentials in OT neurons drive pulsatile OT release from axon terminals in the neurohypophysis during these periods (Poulain and Wakerley 1982). A range of studies has shown that OT neurons and associated neuroglia undergo dramatic changes during late pregnancy and lactation that could contribute to this specialized activity: changes in morphology, synaptic efficacy, and intrinsic membrane properties (Armstrong 2015; Hatton 2002; Theodosis et al. 2008).
Ca2+-dependent, K+-mediated spike afterhyperpolarizations (AHPs) in OT neurons are enhanced beginning in late pregnancy (Teruyama and Armstrong 2002; Teruyama et al. 2008) and these changes persist into lactation (Stern and Armstrong 1996; Teruyama and Armstrong 2002, 2005). The enhanced AHPs can be divided into a medium-duration (decay tau ~500 ms) variety (mAHP) that peaks ~100 ms posttrain and that is mediated by small-conductance Ca2+-activated potassium channels (SK3) that are blocked by apamin (Armstrong et al. 1994; Bourque and Brown 1987; Erickson et al. 1993; Greffrath et al. 1998; Stocker and Pedarzani 2000), and a slower Ca2+-dependent AHP (sAHP), the mechanism of which is still in question (Ghamari-Langroudi and Bourque 2004; Greffrath et al. 1998; Teruyama and Armstrong 2005; Teruyama et al. 2008).
We previously examined whole cell Ca2+ current density and somatic intracellular Ca2+ concentration ([Ca2+]i) in virgin and lactating animals and found that neither changed during lactation (Teruyama and Armstrong 2005), suggesting that changes in the AHP channels or their modulation are more likely to underlie plasticity. Recent studies have documented regulation of the Ca2+ sensitivity of SK channels by actions of the intracellular enzymes casein kinase 2 (CK2) and protein phosphatase 2A (PP2A) on the SK-calmodulin (CaM) complex. Phosphorylation of the Ca2+ sensor CaM by CK2 inhibits SK channels, an effect reversed by PP2A (Allen et al. 2007; Bildl et al. 2004; Maingret et al. 2008). While most of this work has been done in expression systems, SK2 channels in outer hair cells (Bildl et al. 2004) and in peripheral ganglion cells (Maingret et al. 2008) are modulated in this manner. These findings suggested to us that the changes in the AHP during the reproductive cycle might involve modulation by CK2 or PP2A, at least for the mAHP and underlying SK3 channels.
In the present study, we examined OT neurons from virgin and pregnant animals to determine whether inhibition of CK2 or PP2A differentially affected AHP currents associated with unclamped spike trains in voltage clamp. Our overarching hypothesis is that a change in the ratio of these two factors, favoring PP2A over CK2, contributes to enhanced AHPs during pregnancy. If so, these effects should be most pronounced with smaller AHPs associated with smaller changes in [Ca2+]i.
MATERIALS AND METHODS
Animals and slice preparation.
Adult, Sprague-Dawley albino female virgin (random cycling) and pregnant rats were used (Harlan Laboratories, Indianapolis, IN) for both recordings and Western blot analyses. The rats had free access to food and water and were housed in cages in a room under a 12:12-h light-dark cycle. We used two groups of pregnant animals for the electrophysiology studies, those from embryonic days 18–19 (E18–19) and another group from E20–21.
The rats were deeply anesthetized with pentobarbital sodium (50 mg/kg ip) and perfused transcardially with ice-cold, low-Na+ (NaCl was replaced by an equiosmolar amount of sucrose) artificial cerebrospinal fluid (aCSF), which had been oxygenated with 95% O2 and 5% CO2. Slices from the extracted brain were cut at a thickness of 250 μm into the same sucrose-aCSF slush, incubated in normal, oxygenated aCSF at 32–34°C for 1 h, then maintained at room temperature until transfer to a recording chamber. The aCSF contained (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1.0 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 0.45 ascorbic acid, and 20 d-glucose (pH = 7.4; ~290 mosmol/kgH2O). The recording chamber was perfused continuously with oxygenated aCSF at ~2 ml/min at 32–34°C. The Institutional Animal Care and Use Committee approved all protocols used in the study.
Electrophysiological recordings.
Patch pipettes (3–5 MΩ) were prepared from thin-walled borosilicate capillary glass (OD = 1.5 mm, ID = 1.17 mm, Warner Instrument) using a horizontal micropipette puller (P-80 or P-1000, Sutter Instruments, Novato, CA). Most experiments used an internal solution containing (in mM): 135 K-MeSO4, 8 NaCl, 10 HEPES, 4 Mg-ATP, 0.3 Na-GTP, 6 phosphocreatine, 0.1 leupeptin, and 0.2 EGTA. The pH of the pipette solution was adjusted to 7.3 with 1 M KOH, and osmolality was adjusted to 290–295 mosmol/kgH2O. Biocytin (0.1%, Sigma) was added to internal solutions to label neurons for immunochemical identification.
Whole-cell recordings were obtained with Multiclamp 700A or 700B (Molecular Devices, Sunnyvale, CA) amplifiers using recently published procedures (Wang and Armstrong 2012; Wang et al. 2015). Supraoptic nucleus (SON) neurons were identified on a BX50WI upright microscope (Olympus Optical, Tokyo, Japan) using an Imago Sensicam CCD camera (Till Photonics; now FEI Munich, Graefelfing, Germany). The membrane currents were recorded without series resistance compensation and filtered at 2 kHz and digitized at 20 kHz with a Digidata interface (1322A or 1440A) in conjunction with pClamp 9 or 10 software (Molecular Devices). Using periodic measures of input resistance and series resistance, cells were excluded from analysis if the series resistance exceeded 20 MΩ or changed by more than 20%. AHP currents were generated by trains of unclamped action potentials evoked by a fixed number of voltage pulses (−60 mV to +10 mV; 5 ms) in voltage clamp, holding at −60 mV. We focused measurements on the peak current at 100 ms posttrain, as this represents primarily the SK-mediated, medium AHP (ImAHP) mediated by SK3 channels (Armstrong et al. 1994; Ghamari-Langroudi and Bourque 2004; Kirchner et al. 2017; Teruyama and Armstrong 2005). Measurements of amplitude were always averaged over 10–20 sampling points around the peak.
All the experiments were conducted with dl-2-amino-5-phosphonopentanoic acid (AP5; 40 µM), 6,7-dinitroquinoxaline-2,3(1h,4h)-dione (DNQX; 10 µM) and picrotoxin (100 µM) in the aCSF to block most fast synaptic events, and 5 mM CsCl2 to block the time-overlapping depolarizing afterpotential (Ghamari-Langroudi and Bourque 1998; Teruyama and Armstrong 2005). No corrections were made for the pipette liquid junction potentials (~10 mV).
Ca2+ imaging.
Simultaneous whole cell recording and Ca2+ imaging fluorescence records were acquired according to previously published procedures (Roper et al. 2003, 2004; Teruyama and Armstrong 2005) using 100 µM fura-2 (penta K+ salt) as the Ca2+ indicator. Fura-2 was excited at 380 nm using a Polychrome V monochromater (FEI Munich). Fluorescence changes in the soma were measured at 520 ± 40 nm. The frame rate was 50 Hz with pixels binned (4 × 4) at run time. Images were corrected for photobleaching and autofluorescence. More detailed information is available in Roper et al. (2003, 2004). Since in these previous studies we found that the relative change in fura-2 fluorescence (%∆F/F) was linearly related to [Ca2+]i for the values observed here (≤50%∆F/F), %∆F/F was used to assess whether changes in [Ca2+]i accompanied the effects of OA or 4,5,6,7-tetrabromo-2-azabenzimidazole (TBB) on AHPs.
Drugs for electrophysiological experiments.
Okadaic acid (OA) was purchased from either Enzo Life Sciences (Farmingdale, NY) or Tocris-R&D Bioscience (Ellesville, MO). TBB was purchased from Tocris-R&D Bioscience. At our flow rate of ~2 ml/min, complete media exchange took 2–3 min. Drug effects typically began ~2 min after the exchange. Unless otherwise stated, all other reagents were purchased from Sigma-Aldrich Chemical (St. Louis, MO).
Immunocytochemistry.
We identified OT and vasopressin (VP) neurons using recently published protocols (Kirchner et al. 2017; Wang and Armstrong 2012; Wang et al. 2015). Briefly, fixed slices were treated for immunochemical identification of VP neurons using a polyclonal antibody provided by Alan Robinson (University of California, Los Angeles) raised in rabbit against VP-neurophysin and used at a 1:20,000 dilution. OT neurons were identified by a monoclonal antibody (PS36 or PS 38, provided by Harold Gainer (NIH) raised in mouse against OT-neurophysin and used at a 1:5,000 dilution. After incubation in primary antibodies and rinsing, slices were incubated in a cocktail of secondary antibodies and avidin-7-amino-4-methylcoumarin-3-acetic acid (avidin-AMCA, Vector Laboratories, Burlingame, CA) for 4–6 h at room temperature. The secondary antibodies were diluted 1:200 in PBS-TX and included Alexa Fluor 488-conjugated anti-rabbit and Alexa Fluor 594-conjugated goat anti-mouse IgGs (Invitrogen, Eugene, OR). Avidin-AMCA (1:100–200) was included to label the biocytin-filled neurons. Photographs were captured with a Nikon, DS-Fi1 digital camera, using Nikon NIS Elements software on a Nikon 90i Eclipse fluorescent microscope (Nikon Instruments, Melville, NY). Neurons were considered as either OT or VP types only if positive staining for one antibody was accompanied by a negative reaction for the other.
SON lysate preparation.
The rats were deeply anesthetized with sodium pentobarbital (50 mg/kg) and perfused through the heart with cold sucrose solution (in mM: 20 D-glucose, 0.45 ascorbic acid, 2.5 KCl, 1 MgSO4, 1.25 NaH2PO4·H2O, 26 NaHCO3, 210.35 sucrose, and 2 CaCl2). The brains were excised and sectioned in this cold solution at 250 μm on a vibrating microtome (VT1000, Leica, Bannockburn, IL). The SON was microdissected under a stereomicroscope, then transferred to sterile vials containing lysis buffer (pH 7.4) (in mM: 20 HEPES, 150 NaCl, 0.01% Igepal) with 0.01% protease inhibitor (Sigma-Aldrich, no. P8340) and 0.01% phosphatase inhibitor 2 (Sigma-Aldrich, no. P5726) cocktails. These SON pieces were homogenized manually by mortar and pestle and were passed 3× through a 19-gauge needle, then a 25-gauge needle followed by vortex. The homogenized lysates were then centrifuged at 12,000 rpm for 15 min and the supernatant was transferred to a fresh sterile vial, followed by protein estimation with BCA protein assay kit (Pierce, Thermo Scientific, Waltham, MA). The entire process of lysate preparation was carried out on ice.
Western blots.
For Western blots and coimmunoprecipitation (Co-IP) experiments, antibodies against the following proteins used were: CK2α (Santa Cruz Biotechnology, Dallas, TX, no. sc-12738), CK2β (Santa Cruz, no. sc-135856), PP2A-A (Cell Signaling, Danvers, MA, no. 2039S), PP2A-B (Cell Signaling, no. 4953S), PP2A-C (Cell Signaling, no. 2038S), SK3 (Santa Cruz, no. sc-28621), calmodulin (CaM; AbCam, Cambridge, MA, no. ab45689), and ERK1/2 (Cell Signaling, no. 4695S).
For standard Western blots, 100 μg of protein obtained from each SON lysate were mixed with 5–10 µl of 2× Laemmli sample buffer (or as described under CO-IP) and boiled at 95°C for 5 min and proteins were separated on 10–12% SDS-PAGE gel. Proteins were transferred to nitrocellulose membranes in electrophoresis buffer (25 mM Tris, 200 mM glycine, and 20% methanol) for 120 min at 95 V at +4°C. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing (in mM: 1,000 Tris, 50 NaCl, pH 8.0 adjusted with HCl and 0.001% Tween 20) (TBST) for 60 min at room temperature. The primary antibodies (dilution 1:500–1:1,000) were added to the blocking buffer and incubated overnight with the blot at +4°C. The blots were washed 3× with TBST buffer and incubated 60 min with goat anti-mouse/rabbit (horseradish peroxidase-conjugated) secondary antibody diluted 1:3,000 in the blocking buffer at room temperature. They were washed 3× with wash buffer and visualized with enhanced chemiluminescence reagents (Pierce-ECL Western Blotting Substrate, Thermo Scientific) using Classic BX autoradiography film (MIDSCI, Valley Park, MO). Immunoblot densities from film were scanned at 300 dpi and analyzed using ImageJ. For each gel, lysates from one animal from each group were processed in one of three lanes, first for primary antibodies. An additional lane was initially run as a positive control using lysates from human embryonic kidney (HEK) cells (Santa Cruz) or NIH 3T3 cells (Cell Signaling). Gels were then stripped by incubating for 30 min at +50°C in 30 ml stripping buffer (2% SDS, 62.5 mM Tris·HCl, pH 6.7 and 210 μl β-mercaptoethanol), then reprobed for β-tubulin. For statistical comparisons, primary antibody values were normalized against β-tubulin for each run in the normal Western blots. To control for variability in exposures across the eight to nine runs, the primary antibody/β-tubulin ratios were ranked within each of the runs, with each run including lysates from a pregnant and virgin rat.
Co-IP.
Either 500 or 600 μg of protein obtained from each SON lysate from four animals was precleared and incubated overnight with SK3 (2–4 μg) at +4°C, followed by a 120 min incubation with protein A or G beads at +4°C on an agitator. With preparations having more the 600 µg or protein, we used 600 µg, and for those having less than 600 µg, we used 500 µg. The beads were centrifuged and washed with ice-cold PBS and finally resuspended in 30 µl of 2× Laemmli sample buffer to proceed further as Western blots using various antibodies as described above. These pulldown lysates were successively probed with the six antibodies used for Western blots after stripping the previous antibody by incubating for 30 min at +50°C in 30 ml of stripping buffer (2% SDS, 62.5 mM Tris HCl, pH 6.7 and 210 µl of β-mercaptoethanol) before reprobing.
The beads were purchased from GE Healthcare (Chicago, IL; A beads, no. 17-0780-01; G beads, no. 17-0618-01). In addition to the antibodies listed above for Western blots, we also used an antibody to CaM (AbCam, Cambridge, MA, no. ab45689) as an additional positive control, since CaM is known to be the Ca2+ sensor for SK3 channels, and an antibody to ERK1/2 (Cell Signaling Technology; see Chandaka et al. 2016), which should not be associated with SK3, and finally we ran the SK3 antibody once again to verify SK3 presence in the pulldown lysates. An additional lane was run using human embryonic kidney cell lysates or an SON lysate from one of the animals.
Data analysis.
For the analysis of voltage clamp data following drug treatment, we used a Kruskal-Wallis (K-W) nonparametric ANOVA to compare virgins, E18–19, and E20–21 pregnant animals, so that each spike train was compared separately across the 3 groups. Post hoc multiple comparisons were done with the Benjamini, Krieger, and Yekutieli (BKY) false discovery method. To compare differences across spike trains in Fig. 1, Friedman’s nonparametric repeated-measures ANOVA was used, followed by Benjamini, Krieger, and Yekutieli (BKY) false discovery method, adjusted for paired data. Ca2+ imaging data were compared with the nonparametric Wilcoxon signed rank test for matched pairs, and rankings from Western blots from virgin and E20–21 pregnant rats were compared with the nonparametric Mann-Whitney test. In all cases, probability values of P ≤ 0.05 were considered statistically significant. All data are presented as means ± SE. Tests were run with JMP Pro 12.0.1 (SAS Institute, Cary, NC) or Prism 7 (GraphPad, San Diego, CA).
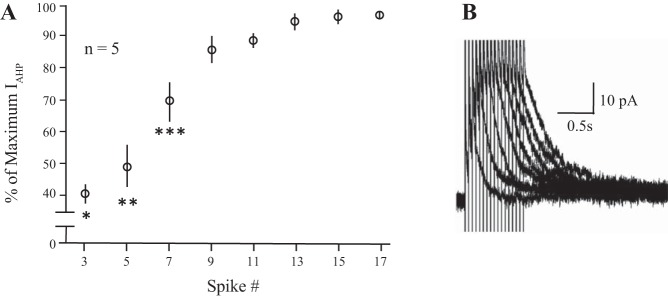
Fig. 1.
The IAHP increases with spike number. A: changes in the peak ImAHP (left y-axis) are plotted as a percentage of maximum against increasing spike numbers during 20 Hz trains in voltage clamp. Friedman’s nonparametric repeated-measures ANOVA: P ≤ 0.0002; post hoc tests: *3 spikes less than 9 (P ≤ 0.04), 11 (P ≤ 0.03), 13 (P ≤ 0.01), 15 (P ≤ 004 and 17 spikes (P ≤ 0.004); **5 spikes less than 9 (P ≤ 0.05), 11 (P ≤ 0.04); 13 (P ≤ 0.01), 15 (P ≤ 0.005) and 17 spikes (P ≤ 0.004); ***7 spikes less than 15 (P ≤ 0.05) and 17 spikes (P ≤ 0.02). Five OT neurons from virgin rats. B: example voltage clamp traces from an OT neuron given 20-Hz trains containing 3, 5, 9, 11, 13, 15, and 17 spikes. IAHP, afterhyperpolarization; ImAHP, small-conductance Ca2+-activated potassium channel-mediated, medium afterhyperpolarization; OT, oxytocin.
RESULTS
Spike dependence of afterhyperpolarization.
Magnocellular neurons exhibit Ca2+-dependent mAHPs and sAHPs following spike trains, and a faster (<50 ms), BK-mediated fast Ca2+-dependent AHP (fAHP) after single spikes (Ghamari-Langroudi and Bourque 2004; Roper et al. 2003). The apamin-blockable mAHP is first evoked by short (2–3 spikes) trains, and with longer trains, the peak AHP (~100 ms posttrain) is still dominated by this apamin-sensitive mAHP (Armstrong et al. 1994; Bourque and Brown 1987; Kirchner et al. 2017; Teruyama and Armstrong 2005). The sAHP peaks much later (~1 s) and requires longer trains than we have used here to reach its maximum (Ghamari-Langroudi and Bourque 2004). The sAHP is not considered further in the present study. Using voltage clamp in virgin animals (n = 5), the peak ImAHP in OT neurons occurred after 9–13 spikes (Fig. 1). To compare the effects of drugs in virgin and pregnant animals, each cell was given 3, 10, and 17 pulse trains (+10 mV, 5 ms pulses) at 20 Hz, from a holding potential of −60 mV. From two to four tests were given at each train, and the results were averaged. We compared the peak ImAHP of the three groups, combining the results for each spike train before both drug treatments, and found a significance difference (K-W, P ≤ 0.0001). Post hoc tests revealed differences among all three groups (in pA): virgin (36.2 ± 1.2; n = 29), E18–19 (30.3 ± 1.4; n = 22), E20–21 (40.0 ± 1.3; n = 29) as follows: virgin vs. E20–21, P ≤ 0.048; virgin vs. E18–19 P ≤ 0.002; E20–21 vs. E18–19, P < 0.0001.
TBB and OA affect afterhyperpolarization without changing [Ca2+]i.
TBB is the most specific, membrane-permeable CK2 inhibitor commercially available (Battistutta et al. 2001; Pagano et al. 2008; Sarno et al. 2001, 2002). In pilot studies, the CK2 inhibitor TBB was first tested on several cells at doses of 1, 5, and 10 µM. We found that at 10 µM, TBB cells appeared unhealthy, becoming leaky within a few minutes of exposure. These effects were also observed at the lower doses to a lesser degree, but only late in the recording (>8 min after exposure), and only well after TBB’s enhancement of the AHP current (IAHP) was first observed, beginning at ~3 min and peaking at 5 min of exposure. We chose 5 µM as our test dose since effects were greater than at 1 µM and the deleterious effects were no different than the lower dose. The PP1 and PP2A inhibitor OA was used at a previously tested dose of 25 nM (Vogalis et al. 2004), a dose that, in contrast to TBB, had little effect on cell health yet produced pronounced changes in AHPs within a few minutes of application.
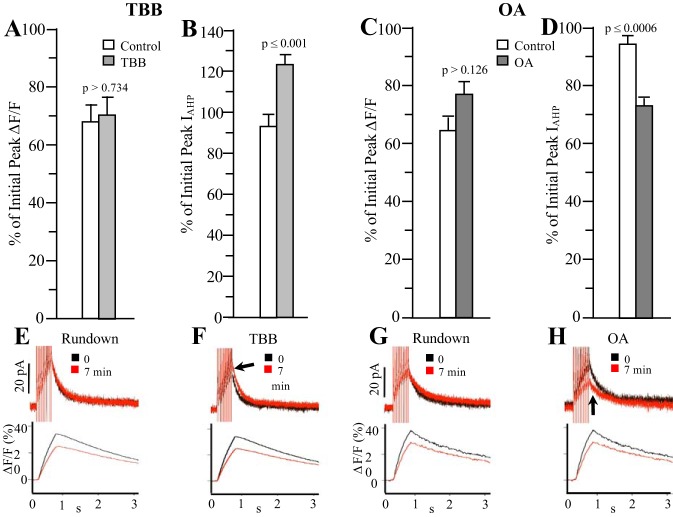
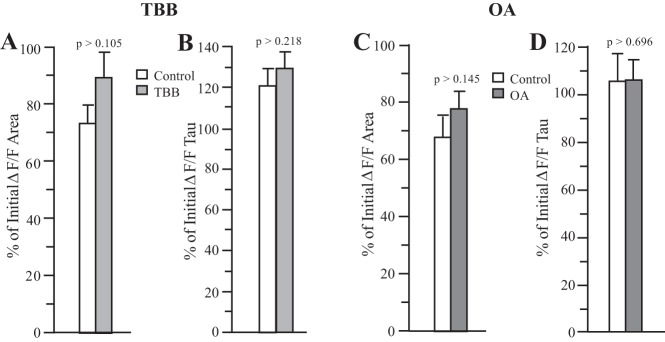
We first tested OA and TBB in virgin rats using a 10-spike train at 20 Hz, to verify their effects on IAHPs and to ensure these compounds had no effects on [Ca2+]i. As shown in Fig. 2A and B, TBB did not affect peak [Ca2+]i (estimated from %∆F/F) beyond what would be expected for normal rundown (Control), while still increasing the peak IAHP in these neurons as predicted. In addition, on average neither the area under the curve nor the decay tau (fitted with a single exponential) of the [Ca2+]i transient was affected by TBB (Fig. 3, A and B). Similarly, OA reduced the peak IAHP but did not significantly alter the peak [Ca2+]i (Fig. 2, C and D), and had no effect on the decay of the [Ca2+]i transient or the area under the curve (Fig. 3, C and D). Thus, neither the increase in IAHP in response to TBB nor its decrease in response to OA was associated with any significant changes in bulk somatic [Ca2+]i as estimated by changes in fura-2 fluorescence (%∆F/F). For both test compounds, the set of control cells was different, and control cells were recorded in the same series of experiments as the respective drug test. Measurements were made ~5 min from the initially recorded IAHP for control cells, the same time period wherein both TBB and OA had exerted their maximal effects.
Fig. 2.
Effect of 5 µM TBB or 25 nM OA on ImAHP and [Ca2+]i in virgin rats. Following a 10-spike train at 20 Hz, neither TBB (A and B) nor OA (C and D) significantly altered the peak [Ca2+]i (as estimated from %ΔF/F) while increasing or decreasing the ImAHP, respectively. Control neurons were untreated and their mean percentages are reported for 7 min after the initial stimulus to represent the normal rundown with time compared with the initial peak because both OA and TBB exerted maximal effects by 7 min with no obvious ill effects on the neurons. E–H: example current (top) and Ca2+ traces (bottom) from control cells (Rundown, E and G) and TBB- (F) or OA- (H) treated cells. The time scales have been expanded to better see changes in peak current after drug (arrow) application; n = 10 for TBB; 10 for TBB controls; 10 for OA; 8 for OA controls. [Ca2+]i, intracellular Ca2+ concentration; IAHP, afterhyperpolarization; ImAHP, small-conductance Ca2+-activated potassium channel-mediated, medium afterhyperpolarization; OA, okadaic acid; TBB, 4,5,6,7-tetrabromobenzotriazole.
Fig. 3.
Effect of 5 µM TBB or 25 nM OA on the area and decay of the [Ca2+]i. transient in virgin rats. Neither TBB (A and B) nor OA (C and D) significantly altered the area or the decay of the [Ca2+]i transient (as estimated from %ΔF/F). As in Fig. 2, control neurons were untreated and percentages represent changes normally associated with time (7 min). n = 10 for TBB; 10 for TBB controls; 10 for OA; 8 for OA controls. [Ca2+]i, intracellular Ca2+ concentration; %∆F/F, fura-2 fluorescence; OA, okadaic acid; TBB, 4,5,6,7-tetrabromobenzotriazole.
Effects of TBB.
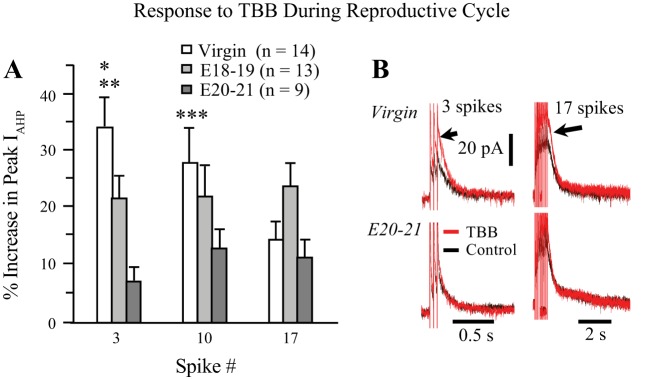
As shown in Fig. 4, TBB enhanced the peak ImAHP in all groups, but the magnitude of its effects was dependent on spike number and reproductive state. The effects on virgins and early pregnant (E18–19) animals were each greater than those on E20–21 animals, but only for the short three-spike train (Fig. 4A). Virgin animals were not significantly different from E18–19 pregnant animals in this regard. Thus, TBB had a greater effect on the smaller ImAHPs generated by three spikes in virgin and E18–19 animals and a lesser effect on the larger ImAHPs evoked with longer trains in pregnant animals, when ImAHPs are naturally enhanced (Teruyama and Armstrong 2002; Teruyama et al. 2008).
Fig. 4.
Effect of 5 µM TBB on the ImAHP during reproductive state is dependent on spike number. A: TBB increased the peak ImAHP to a much greater extent in OT neurons from virgin rats compared with both groups of pregnant animals with short, 3 spike trains. (K-W, 3 spike P ≤ 0.0001; *P ≤ 0.0001, virgin vs. E18–19; **P ≤ 0.046, virgin vs. E20–21). The effect of reproductive state was marginally significant at 10 spikes (K-W 10 spike P ≤ 055) with the significant difference lying between the virgin and E20–21 groups (***P ≤ 0.016), but was not significantly different with 17 spikes (K-W, P > 0.104). B: example traces from 3- and 17-spike trains in virgins and E20–21 pregnant rats showing the larger increase to TBB with the 3 spike trains (arrows). Note the difference in the time scales for 3 vs.17 spike trains. E18–19, embryonic days 18–19; E20–21, embryonic days 20–21; ImAHP, small-conductance Ca2+-activated potassium channel-mediated, medium afterhyperpolarization; K-W, Kruskal-Wallis; OA, okadaic acid; OT, oxytocin; TBB, 4,5,6,7-tetrabromobenzotriazole.
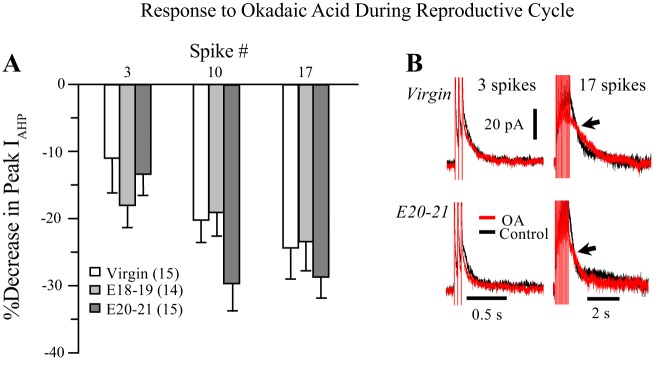
Effects of OA.
The effects of OA were opposite from those of TBB, as OA consistently reduced the peak ImAHP, especially with longer trains. However, OA’s effects were similar in virgin and pregnant rats (Fig. 5A, B). OA’s effects were more complicated than those of TBB, sometimes slowing the decay of ImAHP (not shown). This slowing was inconsistent, however, and appeared to affect an AHP component slower than the ImAHP.
Fig. 5.
Effect of 25 nM OA on the ImAHP during reproductive state is dependent on spike number. A: OA decreased the peak IAHP with all 3 trains, but with a similar effect among the 3 groups (K-W 3 spike, P > 0.178; 10 spike, P > 0.089, 17 spike, P > 0.476). B: example traces from 3- and 17-spike trains in virgins and E20–21 pregnant rats showing the larger decrease to OA with the 17 spike train (arrows). Note the difference in the time scales for 3 vs.17 spike trains. E18–19, embryonic days 18–19; E20–21, embryonic days 20–21; IAHP, afterhyperpolarization; ImAHP, small-conductance Ca2+-activated potassium channel-mediated, medium afterhyperpolarization; K-W, Kruskal-Wallis; OA, okadaic acid.
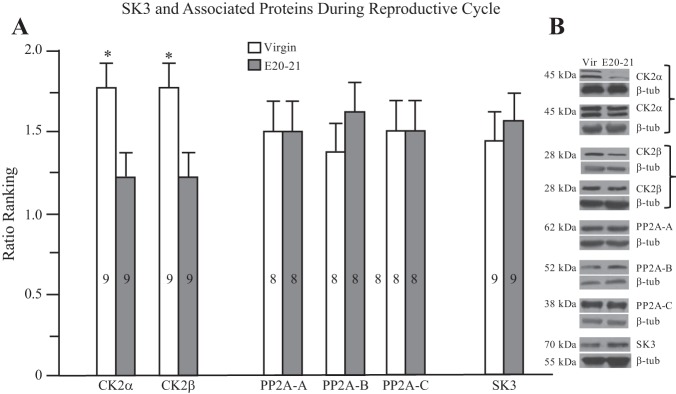
Western blots.
The majority of our results suggested that changes in the sensitivity to TBB are most pronounced in late pregnancy. This corresponds with previous data on synaptic and neuroglial plasticity in the SON (see Armstrong 2015; Hatton 2002; Theodosis et al. 2008, for reviews) as well as our recent findings of a pronounced upregulation of ERK1/2 in OT neurons at late pregnancy (Chandaka et al. 2016). We thus compared blots from SON lysates from virgin and late (E20–21) pregnant rats for three PP2A subunits (A, B and C), two CK2 subunits (CK2α and CK2β), and SK3. As shown in Fig. 6, while each CK and PP2A subunit and SK3 were present in the SON lysates, the only significant difference we found between virgin and E20–21 rats was in the decreased amount of CK2α and CK2β. CK2α consistently expressed as two contiguous bands in our lysates. This has been previously reported in the literature for this monoclonal antibody, which recognizes the two catalytic subunits CK2α and CK2α′ catalytic subunits (e.g., Rosenberger et al. 2016). Alternatively, the two bands may reflect some consistent degradation of CK2α, or some unresolved nonspecific binding. The latter seems unlikely since each band reflected a similar change in individual blots.
Fig. 6.
Relative changes in SK3 and associated proteins in virgins and E20–21 pregnant rats. A: ratio ranking from Western blots indicates a reduction in CK2α and CK2β in the SON of E20–21 compared with virgin rats, no changes in PP2A proteins or in SK3. The number of animals is shown within the bars. *P ≤ 0.025. B: examples of Western blots for each protein analyzed. Two runs each are shown for CK2α and CK2β. E20–21, embryonic days 20–21; SON, supraoptic nucleus; β-tub, β-tubulin; Vir, virgin.
Co-IP.
Since the ImAHP is underlain by SK3 channels, we used an SK3 antibody to pulldown protein, and then determined which of the CK2 and PP2A subunits might be associated with SK3 protein. As a positive control, we examined whether CaM was present in SK3 pulldown lysates, since this is the Ca2+ sensor for SK channels (Xia et al. 1998). As shown in Fig. 7, a band associated with the appropriate molecular weight for CaM (~17 kDa) was present when applying CaM antibody. CK2α and PP2A-C were also found associated with SK3, but none of the other subunits were found in these pulldowns. Some degradation of PP2A-C over time seems likely due to the appearance of two bands compared with controls, and to the previous Western blots. Since the antibody to PP2A-B labeled a band in Western blots with a molecular weight close to the heavy IgG chain band (~50 kDa) present in Co-IP gels, we couldn’t be certain of PP2A-B’s apparent inability to associate with SK3. SON lysates have abundant amounts of ERK1/2 (Chandaka et al. 2016) and we found that ERK1/2 was not associated with SK3 protein, serving as a negative control. These results were verified in four animals from each group. As a last positive control, we verified the presence of SK3 in the pulldown lysates. Due to the repeated stripping needed to probe several antibodies, we did not attempt to quantify and compare the amount of coimmunoprecipitates across these groups.
Fig. 7.
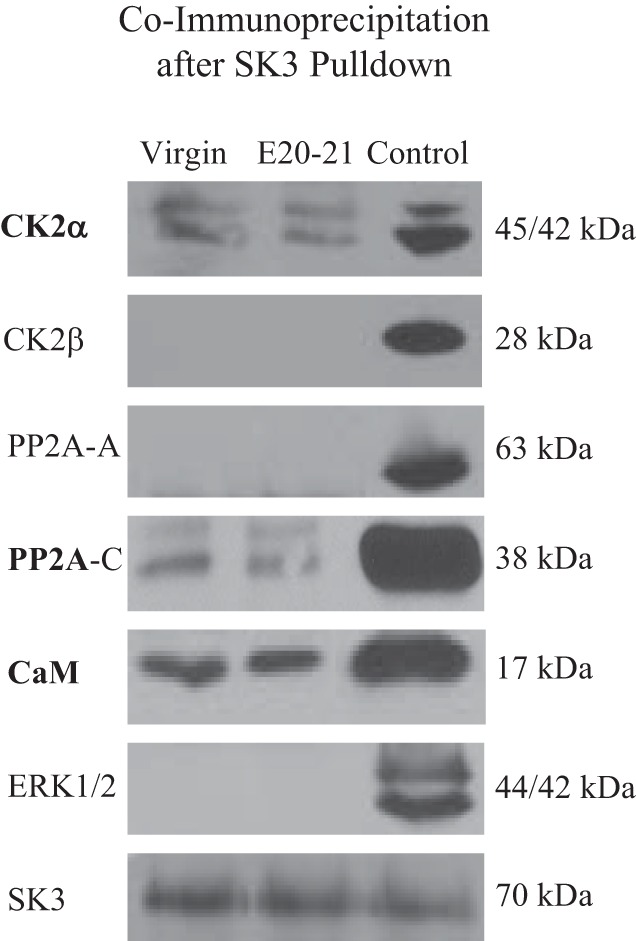
Coimmunoprecipitation of CK2 and PP2A proteins after SK3 pulldown in virgin and E20–21 rats. Western blots from SK3 pulldown lysates show that CK2α and PP2A-C were associated with SK3. As controls, the SK3 Ca2+ sensor CaM was found associated with SK3, whereas ERK1/2 was not. The presence of SK3 in lysates was verified (bottom gel). For each pulldown lysate run, control lysates from normal rats were also run with each antibody on the same gel. Similar results were found in 4 animals.
DISCUSSION
We investigated whether the upregulation of the Ca2+-dependent ImAHP we previously observed in OT neurons during late pregnancy (Teruyama and Armstrong 2002; Teruyama et al. 2008) was associated with a change in the regulation of the underlying SK3 channels by the enzymes CK2 and PP2A, enzymes known to adjust the Ca2+ sensitivity of SK channels (Adelman et al. 2012; Fakler and Adelman 2008). Since a previous study showed that AHP plasticity was not associated with changes in whole cell Ca2+ current or [Ca2+]i (Teruyama and Armstrong 2005), we hypothesized that changes in these two CaM-SK binding proteins might increase the sensitivity of AHPs to Ca2+. We used spike trains that produced different sized ImAHPs to test whether changes in CK2 and PP2A differentially alter ImAHPs in virgins compared with pregnant rats. We examined two stages of pregnancy, E18–19 and E20–21, in anticipation that changes in OT reproductive plasticity might occur most significantly later in pregnancy, 24–48 h before birth (Armstrong 2015; Hatton 2002; Theodosis et al. 2008).
Our results show for the first time 1) activity- and state-dependent effects of CK2 inhibition on the ImAHP; 2) the presence of CK2α, CK2β, and three PP2A (A–C) subunits in SON lysates; 3) a decrease in the amount of CK2α and CK2β during late pregnancy; and 4) the Co-IP of the catalytic subunits of the CaM-SK binding proteins CK2α and PP2A with SK3. The latter result is only the second report of this association in native brain tissue for SK proteins, the first being the discovery of CK2 and PP2A subunits associated with SK2 in whole mouse brain lysates (Allen et al. 2007; Bildl et al. 2004). Although the CK2α and CK2β subunits associate with both the NH2- and COOH-termini of the SK channel, the Ca2+ sensor CaM is only associated with the COOH-terminus, and it is CK2α that phosphorylates CaM to reduce SK activity by decreasing its Ca2+ sensitivity (Adelman et al. 2012; Fakler and Adelman 2008). Also, TBB inhibits CK2 by specifically binding to CK2α (Battistutta et al. 2001). In addition, CK2α is critical for neurotransmitter modulation of SK channels (Maingret et al. 2008). While we found both CK2 subunits in SON lysates and that both decrease their levels during late pregnancy, we did not find the regulatory subunit CK2β in the SK3 pulldowns. Since these two subunits bind to different parts of the CaM-SK complex, it’s possible the more stringent and prolonged procedures involved in preparing the Co-IP assays may have dissociated CK2β from CaM-SK3.
TBB produced its greatest effect in virgin animals with short spike trains that would produce smaller changes in [Ca2+]i (Roper et al. 2003, 2004), where changes in Ca2+ sensitivity would be more apparent. This finding is consistent with a downregulation of CK2 during pregnancy. Furthermore, with TBB we observed a graded effect among the three groups using short trains when examining the peak current, with the changes occurring 24–48 h before birth being the most prominent. This latest period was also critical for changes in ERK1/2 in OT neurons (Chandaka et al. 2016). While we cannot rule out that changes in SK3 density may also contribute to plasticity, we found no evidence for SK3 upregulation during late pregnancy in SON lysates. TBB did not affect [Ca2+]i when enhancing ImAHPs, suggesting that TBB targeted CK2 at the SK3 channel complex, as predicted.
Complementary control of SK channels is exerted by PP2A, a bound phosphatase that dephosphorylates CaM-SK (Adelman et al. 2012; Fakler and Adelman 2008), and the reduction of the ImAHP we observed with OA suggests a role for PP2A in SON neurons. In addition, we found the catalytic subunit PP2A-C, the regulatory β subunit (PP2A-B) and a scaffolding subunit (PP2A-A) in SON lysates, and we also found that PP2A-C coprecipitated with SK3 (As stated above for CK2β, it is possible the harsher conditions involved with preparing the SK3 pulldowns prevented coprecipitation of PP2A-A and PP2A-B; see also Bildl et al. 2004). However, none of the PP2A subunits changed with pregnancy. Furthermore, while the PP1-PP2A inhibitor OA reduced the peak ImAHP in all groups and with each spike train, we found no difference in the sensitivity to OA across the groups. Thus, while inhibition of CK2 with TBB permits dephosphorylation by PP2A, and hence, a greater sensitivity of the channel to Ca2+, our data suggest that it is primarily the reduction in CK2 that contributes to AHP plasticity. We have previously observed plasticity during lactation (Teruyama and Armstrong 2005) and late pregnancy (Teruyama et al. 2008) in the sAHP and the SK3-mediated mAHP. Although there is a clear role demonstrated for PP2A in the regulation of SK2 channels (Adelman et al. 2012), we could find no study demonstrating a specific inhibition of SK-mediated ImAHPs by OA. Reductions in sAHPs have also been previously reported in response to OA in sympathetic neurons (Vogalis et al. 2004). We did note inconsistent changes with OA later (i.e., >100 ms) in the evoked IAHP that might reflect effects on the IsAHP; however, verifying an effect on the IsAHP would require further investigation with its isolation. Finally, as was the case with TBB, OA did not alter [Ca2+]i when reducing ImAHPs, again suggesting OA affected the SK3 channel complex, and not Ca2+ entry during spike trains.
We picked two groups of pregnant animals to compare with virgins to determine whether the critical period (24–48 h before birth, or E20–21) we previously observed for ERK1/2 expression was relevant to AHP plasticity, and because of the known involvement of sex steroid changes during late pregnancy (Bridges 1984) and in plasticity (Theodosis et al. 2008). Pregnancy, and particularly this late period before parturition, also coincides with increases in glutamatergic, GABAergic, and noradrenergic synapses (Theodosis et al. 2008), an increase in norepinephrine release (Herbison et al. 1997), and increased OT receptor expression (Bealer et al. 2006) within magnocellular nuclei. It is not known whether these phenomena help induce the intrinsic plasticity we observe, or whether they simply correlate with it. While there was a graded difference between virgin rats and the two groups of pregnant animals with TBB with 3 spikes, with 10 spikes the difference only lay between virgins and E20–21 rats, and no differences were found with the 17-spike train across groups. Thus, while broadly our results support significant differences between virgin and pregnant rats, changes did not appear only in the later (E20–21) pregnant animals, a period characterized by the precipitous fall in progesterone before birth, association with maternal behavior (Bridges 1984), and permissiveness for neuronal plasticity (Theodosis et al. 2008). It should be noted that changes in OT receptor binding, while greatest in late pregnancy, commence as early as E15 (Bealer et al. 2006), and central OT receptor blockade from this time until E21 prevents AHP plasticity (Teruyama et al. 2008). Thus, it seems likely that plasticity during pregnancy is multidimensional, involving as it does functional synaptic and intrinsic properties, as well as morphological rearrangement among glia and neurons, which may occur over different time scales, but all of which are needed to support enhanced peripheral OT release needed for parturition and lactation.
Finally, it should be noted that recent evidence suggests that OT and VP neurons are glucose sensitive (Song et al. 2014). Although similar to previous papers in our laboratories, the 20 mM glucose we used would likely be saturating for the transient effects these authors observed on increased [Ca2+]i and increased hormone release in hypothalamic explants. Interestingly, OT release was not very sensitive to glucose in virgin rats in the absence of additional insulin (Song et al. 2014) but was more sensitive to during lactation (Sladek et al. 2016). While these effects were independent of spike activity, suggesting the spike AHPs were not critical to increased hormone release or [Ca2+]i in response to glucose, any effects on [Ca2+]i could potentially alter Ca2+-dependent AHPs. Thus differential glucose sensitivity of AHPs during pregnancy would be an interesting avenue to follow in future studies.
GRANTS
This research was supported by NIH grants R01-HD072056 (W. E. Armstrong) and R01-NS044163 (R. C. Foehring). L. Wang was partially supported by the Neuroscience Center of Excellence, University of Tennessee Health Science Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.W., R.C.F., and W.E.A. conceived and designed research; L.W. and G.K.C. performed experiments; L.W., G.K.C., and W.E.A. analyzed data; L.W., G.K.C., R.C.F., J.C.C., and W.E.A. interpreted results of experiments; L.W. and W.E.A. prepared figures; L.W. and W.E.A. drafted manuscript; L.W., G.K.C., R.C.F., and W.E.A. edited and revised manuscript; L.W., G.K.C., R.C.F., J.C.C., and W.E.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Matt Kirchner for reading a previous version of the manuscript.
REFERENCES
- Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 74: 245–269, 2012. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- Allen D, Fakler B, Maylie J, Adelman JP. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci 27: 2369–2376, 2007. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong WE. Central nervous system control of oxytocin secretion during lactation. In: Knobil and Neill’s Physiology of Reproduction, edited by Plant T, Zelezkni A (4th ed.). New York: Elsevier/Academic, 2015, p. 527–563. doi: 10.1016/B978-0-12-397175-3.00013-2. [DOI] [Google Scholar]
- Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. J Physiol 475: 115–128, 1994. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistutta R, De Moliner E, Sarno S, Zanotti G, Pinna LA. Structural features underlying selective inhibition of protein kinase CK2 by ATP site-directed tetrabromo-2-benzotriazole. Protein Sci 10: 2200–2206, 2001. doi: 10.1110/ps.19601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bealer SL, Lipschitz DL, Ramoz G, Crowley WR. Oxytocin receptor binding in the hypothalamus during gestation in rats. Am J Physiol Regul Integr Comp Physiol 291: R53–R58, 2006. doi: 10.1152/ajpregu.00766.2005. [DOI] [PubMed] [Google Scholar]
- Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, Fakler B. Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron 43: 847–858, 2004. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Brown DA. Apamin and d-tubocurarine block the afterhyperpolarization of rat supraoptic neurosecretory neurons. Neurosci Lett 82: 185–190, 1987. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology 114: 930–940, 1984. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Chandaka GK, Wang L, Senogles S, Armstrong WE. Late pregnancy is a critical period for changes in phosphorylated mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 in oxytocin neurones. J Neuroendocrinol 28: 12398, 2016. doi: 10.1111/jne.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KR, Ronnekleiv OK, Kelly MJ. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendocrinology 57: 789–800, 1993. doi: 10.1159/000126438. [DOI] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron 59: 873–881, 2008. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones. J Physiol 510: 165–175, 1998. doi: 10.1111/j.1469-7793.1998.165bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci 24: 7718–7726, 2004. doi: 10.1523/JNEUROSCI.1240-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffrath W, Martin E, Reuss S, Boehmer G. Components of after-hyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro. J Physiol 513: 493–506, 1998. doi: 10.1111/j.1469-7793.1998.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI. Glial-neuronal interactions in the mammalian brain. Adv Physiol Educ 26: 225–237, 2002. doi: 10.1152/advan.00038.2002. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Voisin DL, Douglas AJ, Chapman C. Profile of monoamine and excitatory amino acid release in rat supraoptic nucleus over parturition. Endocrinology 138: 33–40, 1997. doi: 10.1210/endo.138.1.4859. [DOI] [PubMed] [Google Scholar]
- Kirchner MK, Foehring RC, Wang L, Chandaka GK, Callaway JC, Armstrong WE. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulates afterhyperpolarizations in oxytocin neurons of the supraoptic nucleus. J Physiol 595: 4927–4946, 2017. doi: 10.1113/JP274219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Coste B, Hao J, Giamarchi A, Allen D, Crest M, Litchfield DW, Adelman JP, Delmas P. Neurotransmitter modulation of small-conductance Ca2+-activated K+ channels by regulation of Ca2+ gating. Neuron 59: 439–449, 2008. doi: 10.1016/j.neuron.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano MA, Bain J, Kazimierczuk Z, Sarno S, Ruzzene M, Di Maira G, Elliott M, Orzeszko A, Cozza G, Meggio F, Pinna LA. The selectivity of inhibitors of protein kinase CK2: an update. Biochem J 415: 353–365, 2008. doi: 10.1042/BJ20080309. [DOI] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience 7: 773–808, 1982. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Roper P, Callaway J, Armstrong W. Burst initiation and termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical, electrical, and calcium fluorescence study. J Neurosci 24: 4818–4831, 2004. doi: 10.1523/JNEUROSCI.4203-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper P, Callaway J, Shevchenko T, Teruyama R, Armstrong W. AHP’s, HAP’s and DAP’s: how potassium currents regulate the excitability of rat supraoptic neurones. J Comput Neurosci 15: 367–389, 2003. doi: 10.1023/A:1027424128972. [DOI] [PubMed] [Google Scholar]
- Rosenberger AF, Morrema TH, Gerritsen WH, van Haastert ES, Snkhchyan H, Hilhorst R, Rozemuller AJ, Scheltens P, van der Vies SM, Hoozemans JJ. Increased occurrence of protein kinase CK2 in astrocytes in Alzheimer’s disease pathology. J Neuroinflammation 13: 4, 2016. doi: 10.1186/s12974-015-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno S, Moro S, Meggio F, Zagotto G, Dal Ben D, Ghisellini P, Battistutta R, Zanotti G, Pinna LA. Toward the rational design of protein kinase casein kinase-2 inhibitors. Pharmacol Ther 93: 159–168, 2002. doi: 10.1016/S0163-7258(02)00185-7. [DOI] [PubMed] [Google Scholar]
- Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’). FEBS Lett 496: 44–48, 2001. doi: 10.1016/S0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- Sladek CD, Stevens W, Song Z, Johnson GC, MacLean PS. The “metabolic sensor” function of rat supraoptic oxytocin and vasopressin neurons is attenuated during lactation but not in diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 310: R337–R345, 2016. doi: 10.1152/ajpregu.00422.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Levin BE, Stevens W, Sladek CD. Supraoptic oxytocin and vasopressin neurons function as glucose and metabolic sensors. Am J Physiol Regul Integr Comp Physiol 306: R447–R456, 2014. doi: 10.1152/ajpregu.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci 16: 4861–4871, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci 15: 476–493, 2000. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Teruyama R, Armstrong WE. Changes in the active membrane properties of rat supraoptic neurones during pregnancy and lactation. J Neuroendocrinol 14: 933–944, 2002. doi: 10.1046/j.1365-2826.2002.00844.x. [DOI] [PubMed] [Google Scholar]
- Teruyama R, Armstrong WE. Enhancement of calcium-dependent afterpotentials in oxytocin neurons of the rat supraoptic nucleus during lactation. J Physiol 566: 505–518, 2005. doi: 10.1113/jphysiol.2005.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruyama R, Lipschitz DL, Wang L, Ramoz GR, Crowley WR, Bealer SL, Armstrong WE. Central blockade of oxytocin receptors during mid-late gestation reduces amplitude of slow afterhyperpolarization in supraoptic oxytocin neurons. Am J Physiol Endocrinol Metab 295: E1167–E1171, 2008. doi: 10.1152/ajpendo.90620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev 88: 983–1008, 2008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Harvey JR, Furness JB. Suppression of a slow post-spike afterhyperpolarization by calcineurin inhibitors. Eur J Neurosci 19: 2650–2658, 2004. doi: 10.1111/j.0953-816X.2004.03369.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Armstrong WE. Tonic regulation of GABAergic synaptic activity on vasopressin neurones by cannabinoids. J Neuroendocrinol 24: 664–673, 2012. doi: 10.1111/j.1365-2826.2011.02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ennis M, Szabó G, Armstrong WE. Characteristics of GABAergic and cholinergic neurons in perinuclear zone of mouse supraoptic nucleus. J Neurophysiol 113: 754–767, 2015. doi: 10.1152/jn.00561.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503–507, 1998. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]