Abstract
Purpose
To compare the toxicities and cost of proton radiation and stereotactic body radiotherapy (SBRT) with intensity-modulated radiotherapy (IMRT) for prostate cancer among men younger than 65 years of age with private insurance.
Methods
Using the MarketScan Commercial Claims and Encounters database, we identified men who received radiation for prostate cancer between 2008 and 2015. Patients undergoing proton therapy and SBRT were propensity score–matched to IMRT patients on the basis of clinical and sociodemographic factors. Proportional hazards models compared the cumulative incidence of urinary, bowel, and erectile dysfunction toxicities by treatment. Cost from a payer’s perspective was calculated from claims and adjusted to 2015 dollars.
Results
A total of 693 proton therapy patients were matched to 3,465 IMRT patients. Proton therapy patients had a lower risk of composite urinary toxicity (33% v 42% at 2 years; P < .001) and erectile dysfunction (21% v 28% at 2 years; P < .001), but a higher risk of bowel toxicity (20% v 15% at 2 years; P = .02). Mean radiation cost was $115,501 for proton therapy patients and $59,012 for IMRT patients (P < .001). A total of 310 SBRT patients were matched to 3,100 IMRT patients. There were no significant differences in composite urinary, bowel, or erectile dysfunction toxicities between SBRT and IMRT patients (P > .05), although a higher risk of urinary fistula was noted with SBRT (1% v 0.1% at 2 years; P = .009). Mean radiation cost for SBRT was $49,504 and $57,244 for IMRT (P < .001).
Conclusion
Among younger men with prostate cancer, proton radiation was associated with significant reductions in urinary toxicity but increased bowel toxicity at nearly twice the cost of IMRT. SBRT and IMRT were associated with similar toxicity profiles; SBRT was modestly less expensive than IMRT.
INTRODUCTION
From 2000 to 2010, intensity-modulated radiotherapy (IMRT) became the most common radiation treatment modality for localized prostate cancer.1,2 Although more expensive than the historical standard of three-dimensional conformal radiation therapy, IMRT allowed for improved sparing of normal tissues that reduced treatment toxicity while facilitating modest dose escalation that improved biochemical disease-free survival.3,4 Newer radiation techniques, such as proton radiation and stereotactic body radiotherapy (SBRT), seek to build on these gains. Specifically, proton therapy decreases low-dose radiation exposure to uninvolved organs, which potentially translates into lower risks of treatment toxicity and second malignancy.5-7 Alternatively, SBRT decreases the number of treatment fractions to only five or fewer, thereby improving convenience and lowering cost.8
Recent studies have shown that prostate cancer is among the most common indications for treatment with these advanced radiation modalities.9 Despite their potential benefits, high-level evidence supporting either modality as a replacement for IMRT has been difficult to gather through randomized trials, which has prompted analyses of claims data to evaluate their comparative effectiveness. The current literature suggests that proton radiation costs more than IMRT, with no definite evidence of clinical benefit,2,10 whereas SBRT provides cost savings at the expense of increased genitourinary (GU) toxicity.11,12
The available literature is derived almost exclusively from Medicare claims and limited to older men. However, patients younger than 65 years of age account for over 40% of prostate cancer diagnoses13 and are unique because of smaller prostate volumes and fewer comorbidities.14 Furthermore, reimbursement from private plans is considerably higher than Medicare. As such, it would be inappropriate to extrapolate existing comparative effectiveness and cost data to younger men. Thus, our goal was to evaluate the toxicity profile and cost of proton radiation and SBRT compared with IMRT in a cohort of younger men with incident prostate cancer using a contemporary private insurance claims database.
METHODS
Study Cohort
We used the MarketScan Commercial Claims and Encounter database (Truven Health Analytics, Ann Arbor, MI), a nationwide, employment-based convenience sample of medical claims data of employees and dependents younger than 65 years of age aggregated from over 100 payers.15 Patients were included if they received IMRT, proton therapy, or SBRT (Appendix Table A1, online only) for a primary diagnosis of prostate cancer between 2008 and 2015 and had continuous coverage from 6 months before through 6 months after starting treatment. Patients were excluded if they received brachytherapy or combined radiation modalities, or if pretreatment claims indicated metastatic disease, radical prostatectomy, or other malignancy.
Defining Treatment
All time-to-event analyses were indexed to the start of treatment, defined as the date of first radiation treatment. Radiation treatment was defined as at least three fractions of SBRT or 20 fractions of IMRT or proton radiation within 90 days of starting radiation. Any androgen deprivation therapy (ADT) in claims from 6 months before through 3 months after starting radiation was considered treatment with concurrent ADT (Appendix Table A2, online only).
Covariables
Patient-level covariables extracted from MarketScan included age, metropolitan service area (MSA), geographic region, treatment year, insurer relation (employee v spouse/dependent), and insurance type (health maintenance organization or capitated v noncapitated plans). MSA-level median household income was obtained from the US Census Bureau16 and divided into quartiles. Modified Charlson comorbidity index was determined using pretreatment claims.17
Outcomes
Treatment toxicity was defined a priori by the presence of specific diagnosis or procedure codes selected based on literature review2,10-12,18,19 and expert opinion (Appendix Table A3, online only). Toxicity was initially divided into composite categories of urinary toxicity, bowel toxicity, and erectile dysfunction (ED). Urinary and bowel toxicities were further subcategorized for additional detail (Appendix Table A3). The most common diagnosis and procedure codes are listed in Appendix Table A4 (online only). The presence of each toxicity as a preradiation comorbidity was determined using pretreatment claims.
Costs were adjusted to 2015 US dollars using the Medical Care Consumer Price Index20 and reported from a payer perspective unless otherwise noted. Radiation cost included treatment planning, treatment delivery, and patient management spanning 1 month before through 6 months after treatment. Out-of-pocket radiation costs were also computed. Complication cost was defined as the cost of all claims on days where toxicity was recorded. Total health care cost included all medical and pharmacy claims starting 1 month before treatment.
Statistical Analysis
Baseline covariables among the treatment groups of IMRT, proton radiation, and SBRT were compared using the χ2 test. Propensity score-matched cohorts were created to account for differences in baseline covariables. Propensity scores were computed using logistic models with dependent variables of IMRT versus proton and IMRT versus SBRT and independent variables of age, residence type, median household income, geographic region, treatment year, employee relation, capitated insurance plan, medical comorbidity, baseline GU/bowel comorbidity, and concurrent ADT. Patients were matched using a greedy algorithm and a maximum allowed caliper distance of 0.1.21 Covariable balance was assessed by postmatch standardized difference, with less than 10% indicating a similar distribution.22 The number of matched IMRT patients was maximized while preserving the number of included proton and SBRT patients.
Within each matched cohort, separate Cox proportional hazards models stratified by matched pair were constructed to determine the hazard ratio of proton radiation or SBRT relative to IMRT for developing each toxicity. The proportional hazards assumption was confirmed by inspection of log (−log [survival]) curves. The modeled cumulative incidence of each toxicity at 6, 12, 24, and 36 months after the start of radiation is reported by treatment modality. Sensitivity analyses included (1) dividing the toxicity profile of treatment into early (up to 12 months) versus late (after 12 months) per existing literature,2,10 (2) including only procedure codes (and excluding diagnosis codes) in toxicity assessment as a surrogate of severity, and (3) assessing toxicity as combinations of procedure and diagnosis codes previously validated for five severe toxicities (cystitis, rectal complications, urethral stricture, ureteral stricture, and urinary/rectal fistula) after pelvic radiation.19 Toxicity analysis was performed using SAS, version 9.4 (SAS Institute, Cary, NC).
Mean radiation cost within matched cohorts was compared using the Wilcoxon rank sum test. Mean values of complication and total health care cost were estimated at various time points while accounting for censored data using the general representation theorem for missing data processes and were compared as asymptotically normally distributed values.23 Cost analysis was conducted using Stata software, version 14 (STATA, College Station, TX) using the hcost module.24
RESULTS
Patient Characteristics
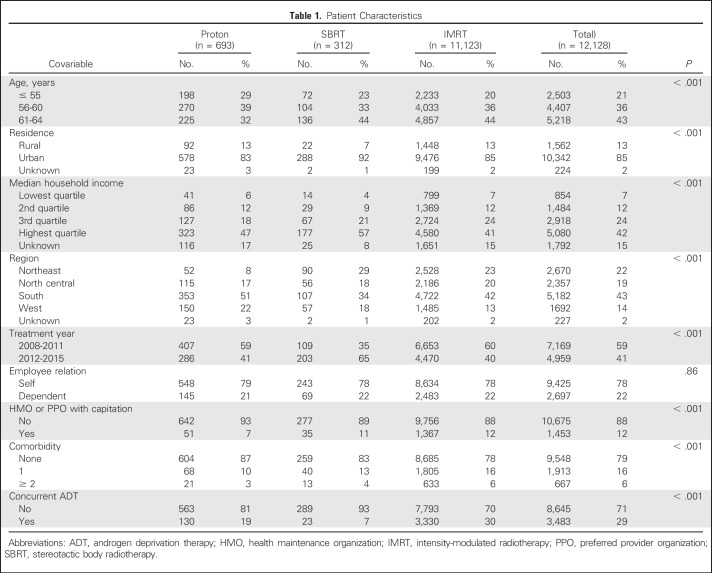
A total of 12,128 patients met the study selection criteria (Table 1), which included 11,123 IMRT patients (92%), 693 proton therapy patients (6%), and 312 SBRT patients (3%). The median number of treatment fractions was 42 for IMRT (interquartile range [IQR], 38 to 44), 39 for proton radiation (IQR, 39 to 44), and five for SBRT (IQR, 5 to 5). IMRT patients were more likely to reside in MSAs with lower median household income, have greater medical comorbidity, and receive concurrent ADT. Proton therapy patients were younger and more likely to participate in a noncapitated insurance plan. SBRT patients were more likely to be treated in the latter half of the study period and reside in urban locations. Pretreatment urinary comorbidity was lower among proton therapy patients, bowel comorbidity was similar among treatment groups, and ED was higher among IMRT patients (Appendix Table A5, online only).
Table 1.
Patient Characteristics
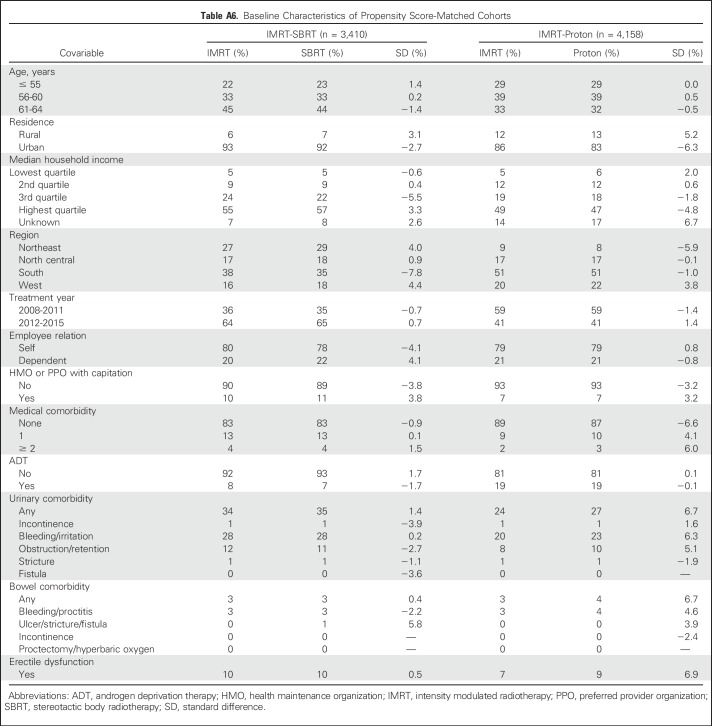
A total of 693 proton therapy patients (median follow-up, 23 months) were matched to 3,465 IMRT patients (median follow-up, 23 months), and 310 SBRT patients (median follow-up, 18 months) were matched to 3,100 IMRT patients (median follow-up, 21 months). Postmatch baseline covariables, including pretreatment urinary, bowel, and ED comorbidity, were similar between treatment groups (Appendix Table A6, online only).
Proton-IMRT Comparison
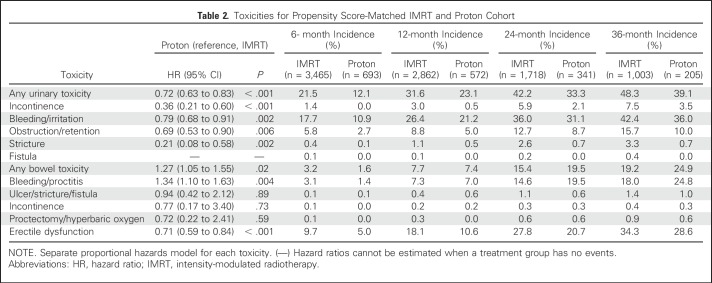
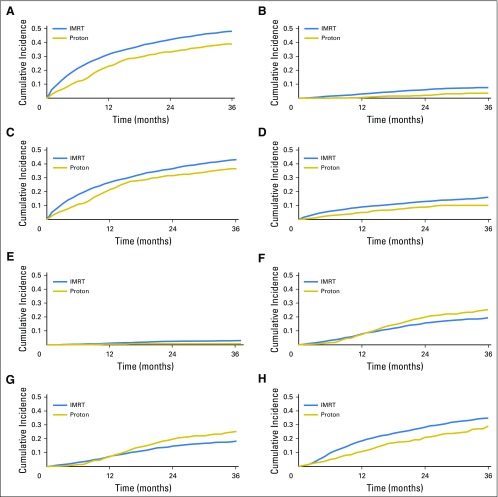
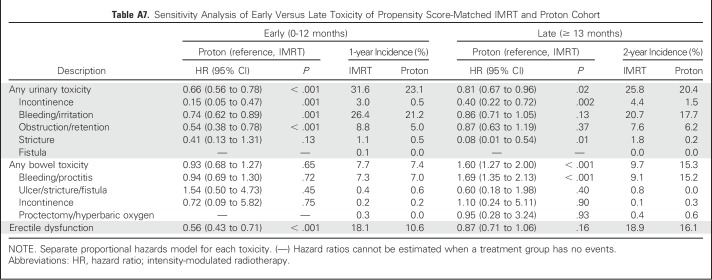
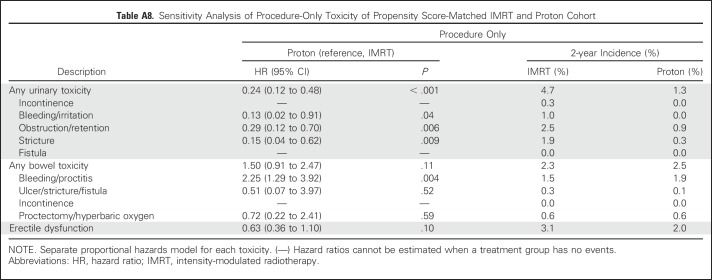
Comparative toxicities of patients receiving proton therapy and IMRT are listed in Table 2. Proton therapy patients had a lower risk of composite urinary toxicity (33% v 42% at 2 years; P < .001), which was persistent on sensitivity analysis when assessed as early, late, or procedure-only toxicity (Appendix Tables A7 and A8, online only). This urinary benefit with proton radiation was seen across multiple domains, including incontinence, bleeding/irritation, obstruction, and stricture (Figs 1A to 1E). Sensitivity analysis demonstrated reduction in urinary bleeding/irritation and obstruction/retention in the early period, stricture in the late period, and incontinence in both periods (Appendix Table A7). Additional sensitivity analysis using previously validated pelvic radiation severe toxicity criteria also demonstrated a lower risk of urethral stricture with proton radiation (0% v 1% at 2 years; P = .03; Appendix Table A9, online only). Bowel toxicity was higher among proton therapy patients (20% v 15% at 2 years; P = .02), which was principally late bleeding/proctitis (Figs 1F to 1G; Appendix Table A7) and confirmed on procedure-only sensitivity analysis (Appendix Table A8). ED was less common among proton therapy patients (21% v 28% at 2 years; P < .001; Fig 1H), but the difference did not persist when assessed as procedure-only toxicity (Appendix Table A8).
Table 2.
Toxicities for Propensity Score-Matched IMRT and Proton Cohort
Fig 1.
Cumulative incidence of genitourinary and bowel toxicities among propensity score-matched intensity-modulated radiotherapy (IMRT) and proton cohort: (A) any urinary toxicity; (B) urinary incontinence; (C) urinary bleeding/irritation; (D) urinary obstruction; (E) urinary stricture; (F) any bowel toxicity; (G) bowel bleeding/irritation; and (H) erectile dysfunction.
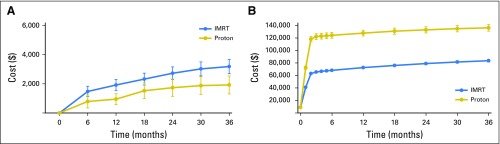
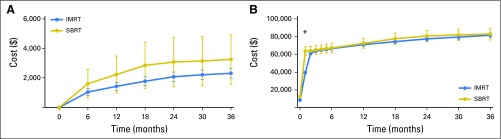
The mean radiation cost for protons and IMRT to the payer was $115,501 and $59,012 (P < .001), respectively, and to the patient was $2,269 and $1,714 (P < .001), respectively. Proton therapy patients had a lower mean complication cost ($1,737 v $2,730 at 2 years; P = .008; Fig 2A) but higher mean total health care cost ($133,220 v $79,209 at 2 years; P < .001; Fig 2B).
Fig 2.
Complication and total health care cost comparison between propensity score–matched intensity-modulated radiotherapy (IMRT) and proton cohort: (A) complication cost and (B) total cost. P < .05 at all time points.
SBRT-IMRT Comparison
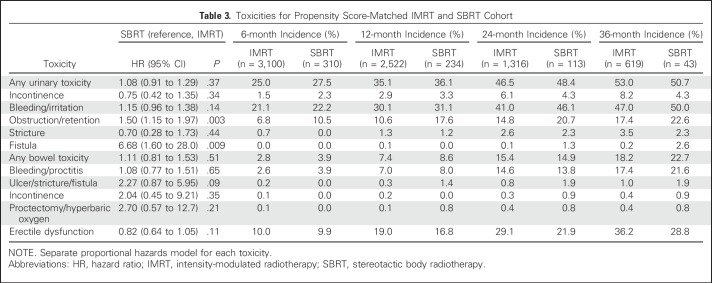
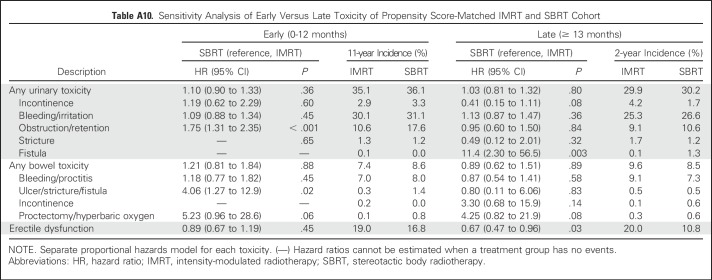
Comparative toxicities of SBRT and IMRT patients are listed in Table 3. There were no statistically significant differences between SBRT and IMRT patients in composite urinary, composite bowel, or ED toxicities. SBRT was associated with a higher risk of urinary toxicity within the specific domains of obstruction/retention (21% v 15% at 2 years; P = .003) and fistula (1% v 0.1% at 2 years; P = .009). Sensitivity analysis demonstrated that the increased risk of obstruction/retention was limited to the early period, whereas the increased risk of fistula was limited to the late period (Appendix Table A10, online only). There were no differences between SBRT and IMRT when toxicity evaluation was conducted using only procedure codes (Appendix Table A11, online only) or previously validated pelvic radiation severe toxicity criteria (Appendix Table A12, online only).
Table 3.
Toxicities for Propensity Score-Matched IMRT and SBRT Cohort
The mean radiation cost for SBRT and IMRT was $49,504 and $57,244 (P < .001) to the payer, respectively, and $1,015 and $1,560 (P < .001) to the patient, respectively. SBRT and IMRT patients had a similar mean complication cost ($3,084 v $2,079 at 2 years; P = .25; Fig 3A) and mean total health care cost ($80,786 v $77,539 at 2 years; P = .36; Fig 3B).
Fig 3.
Complication and total health care cost comparison between propensity score–matched intensity-modulated radiotherapy (IMRT) and stereotactic body radiotherapy (SBRT) cohort: (A) complication cost and (B) total cost. (*) Denotes P <. 05 at measured time point.
DISCUSSION
Given its high economic burden25 and multiple effective treatment options, localized prostate cancer is a high-priority area for comparative effectiveness research.26 As randomized trials of proton radiation accrue27 and trials of SBRT mature,28 large cohort studies provide key evidence to evaluate these modalities. To our knowledge, this study is the first to report comparative toxicities and private insurance cost of these treatment options in younger men.
The recent rapid expansion of proton centers in the United States has been driven by a combination of medical promise and competitive market pressures.29 Although proton prostate radiation offers a theoretical benefit of lower delivered dose to normal pelvic structures,6,7 controversy remains around its continued use in the absence of demonstrable clinical benefit within existing comparative effectiveness literature. A Medicare study of early toxicity found a transient reduction in GU toxicity at 6 months and no difference in bowel toxicity,10 whereas a SEER-Medicare study of late toxicity found no difference in GU toxicity and an increase in bowel toxicity with proton radiation.2
A strength of this study was further a priori refinement of toxicity criteria from prior studies to maximize the specificity of our measured result for radiation toxicity, such as inclusion of irritative bladder symptoms and exclusion of endoscopies that did not include modifiers of bleeding control. Although the prior SEER-Medicare study was conducted when only a single proton center was located within the SEER catchment area,30 this study has increased generalizability because of its inclusion of nationwide proton facilities. Within this context, our finding of increased late bowel toxicity of bleeding/proctitis among proton therapy patients within a younger, private insurance data set corroborated the prior observation among older men.
To our knowledge, this study is the first to identify possible benefit associated with proton radiation compared with IMRT for prostate cancer, with results suggesting decreased multidomain urinary toxicity. To put in perspective the effect size of proton radiation in this study, the magnitude of the decreased risk of urinary toxicity (33% v 43% at 2 years) compares favorably with population-based studies showing a reduction in bowel complications (19% v 23% at 2 years) between patients with prostate cancer treated with three-dimensional conformal radiation therapy and IMRT.18
The observed toxicity differences may be partially explained by the dose-volume characteristics of each technology. The available evidence suggests that the high dose volume to the rectum is correlated with late toxicity,31 whereas there is no clearly established bladder dose-volume relationship with GU toxicity. One dosimetric study comparing proton radiation with IMRT showed that proton radiation had increased high-dose rectal exposure, improved target/prostate dose homogeneity, and increased low-dose bladder sparing.32 These dosimetric findings could correspond to our respective observations of increased late rectal bleeding/proctitis, decreased urethral stricture, and decreased other urinary toxicity with protons. However, other dosimetric studies suggest either decreased7 or similar6 rectal high-dose exposure with protons compared with IMRT. In addition, other treatment-related factors, such as stricter prostate immobilization in proton radiation due to the increased sensitivity of proton dosimetry through varying tissue densities, may contribute to our observed toxicity differences. Given the conflicting dosimetric studies and potential nondosimetric factors affecting dose delivered to organs at risk, the empiric findings in this study provide valuable observational toxicity data.
With the current emphasis on cost-effective health care, our toxicity findings must be considered in the context of differing cost profiles. Although there was a durable statistically significant reduction in complication cost for proton therapy patients, differences in overall health care expenditure were driven by private insurance reimbursing nearly twice the amount for proton radiation compared with IMRT. It is important to note that these radiation cost differences represent national averages that can differ significantly from reimbursement rates negotiated between individual treatment centers and specific payers, and some institutions offer proton radiation as a cost-neutral option to IMRT.33 The reported toxicity risks and cost can facilitate the design of models to evaluate the likelihood that proton radiation is cost-effective across a spectrum of societal willingness to pay or of proton-IMRT cost differences.34 Ultimately, innovative delivery strategies to reduce the cost of proton radiation are likely necessary for it to be considered cost effective while balancing its potential for reduced GU toxicity with increased bowel toxicity.
Alongside the adoption of proton radiation, there has been longstanding interest in hypofractionated radiation in the treatment of prostate cancer.35 Multiple randomized trials have recently published initial results demonstrating the noninferiority of moderately hypofractionated prostate radiation regimens compared with conventional fractionation.36-38 SBRT represents an extreme form of hypofractionation that recently became possible with improved radiation technology. As such, there is less robust follow-up with SBRT, and published comparative effectiveness research is limited to population-based studies from Medicare and SEER-Medicare showing greater GU toxicity, including urethritis, obstruction, and incontinence11,12 with SBRT compared with IMRT.
Although our study did not show differences in composite urinary or bowel toxicity between SBRT and IMRT, it similarly demonstrated additional early toxicity within the specific urinary domain obstruction/retention among younger men. Given the concern for the late toxicity of fistula with SBRT, we notably also found a statistically significant higher risk of urinary fistula. However, it was a small absolute risk detected based on diagnosis codes alone without associated procedure codes and was comparable to the risk of grade 3+ urinary/bowel toxicity reported in prospective SBRT studies.39 It is reassuring that 2-year toxicity data from a randomized trial comparing SBRT with conventionally fractionated IMRT recently reported in abstract form showed no long-term difference in physician- or patient-reported bowel or GU toxicity.28 While we await final publication of these data, the currently available comparative effectiveness data suggest that it is appropriate to counsel patients on the potential of increased urinary toxicity with SBRT and to reduce the risk of post-treatment obstructive symptoms with appropriate patient selection.
In our study, private insurance reimbursement for SBRT was 14% less than IMRT, and out-of-pocket cost was 35% less. Despite this upfront cost savings, overall long-term health care expenditures between the two patient groups were similar. However, other potential cost savings from the reduced treatment time of SBRT, including less patient time away from work and improved radiotherapy resource use are unaccounted for in this comparison. The combined toxicity and cost comparison between SBRT and IMRT suggest that SBRT is a well-tolerated and good-value treatment alternative to conventionally fractionated or moderately hypofractionated radiation in appropriately selected patients.
The strengths of using claims data for comparative effectiveness research include the assessment of real-world cost and outcomes that are externally valid and representative. However, there are also limitations to this approach. Although patients with metastatic disease were excluded, other prognostic information, including Gleason score, prostate-specific antigen level, and clinical stage, and treatment information, such as radiation field and dose, were unavailable. Importantly, propensity score matching thus could not account for these and other potential unmeasured confounders that could differ between treatment groups and influence measured outcomes. In addition, median follow-up was relatively short, reflecting frequent changes in insurance coverage endemic to the private insurance market. Reassuringly, however, follow-up did not vary by type of treatment, indicating that informative censoring bias was unlikely a concern. Claims data do not distinguish between passive scatter proton radiation and more modern intensity-modulated proton therapy, which may have differing toxicity profiles.40 Determination of toxicity grade is limited by the lack of physician- and patient-reported outcomes, and there are few validated algorithms for claims-based toxicity assessment of pelvic radiation.19,41,42 Differences in the proportion of hospital-based versus freestanding treatment facility between radiation modalities cannot be assessed and may partially account for cost differences. Alternative treatment options, including surveillance, brachytherapy, and surgery, were not included in this study, but comparative toxicities have been previously reported.43-46 Finally, follow-up within a private insurance cohort is less robust than Medicare, which limits the ability to study long-term toxicity or efficacy.
Nevertheless, to our knowledge, this study is unique in its assessment of the toxicity and cost of prostate radiation treatment options in the previously understudied but significant patient population of younger men with private insurance. Our findings include sustained reductions in urinary toxicity but increased bowel toxicity with proton therapy and modestly increased domain-specific urinary toxicity with SBRT. These key findings, coupled with the real-world private insurance cost reported herein, will be useful for patients selecting the most appropriate treatment and for researchers designing cost-effectiveness models to guide treatment decisions in prostate cancer.
Appendix
Table A1.
Inclusion and Exclusion Criteria Procedure and Diagnosis Codes
Table A2.
Androgen Deprivation Therapy Procedure Codes
Table A3.
Treatment Toxicity Diagnosis and Procedure Codes
Table A4.
Most Common Toxicity Codes
Table A5.
Preradiation Genitourinary and Bowel Comorbidity in Unmatched Cohort
Table A6.
Baseline Characteristics of Propensity Score-Matched Cohorts
Table A7.
Sensitivity Analysis of Early Versus Late Toxicity of Propensity Score-Matched IMRT and Proton Cohort
Table A8.
Sensitivity Analysis of Procedure-Only Toxicity of Propensity Score-Matched IMRT and Proton Cohort
Table A9.
Sensitivity Analysis of Previously Validated Severe Toxicity Criteria After Pelvic Radiation of Propensity Score-Matched IMRT and Proton Cohort
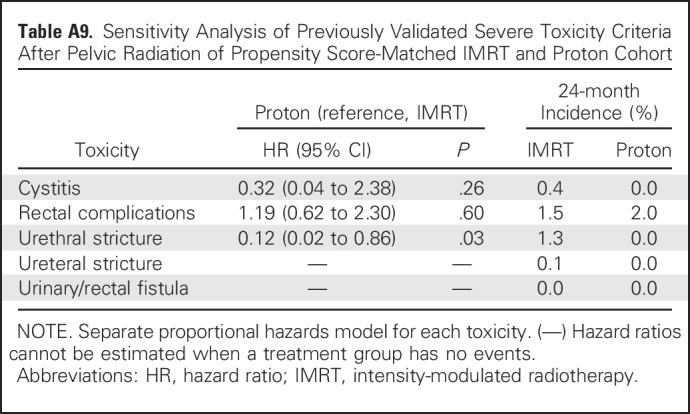
Table A10.
Sensitivity Analysis of Early Versus Late Toxicity of Propensity Score-Matched IMRT and SBRT Cohort
Table A11.
Sensitivity Analysis of Procedure-Only Toxicity of Propensity Score-Matched IMRT and SBRT Cohort
Table A12.
Sensitivity Analysis of Previously Validated Severe Toxicity Criteria After Pelvic Radiation of Propensity Score-Matched IMRT and SBRT Cohort

Footnotes
Supported in part by the Cancer Prevention and Research Institute of Texas (CPRIT; Grant No. RP160674), National Cancer Institute (Grant No. R01 CA207216), and Varian Medical Systems. B.D.S. is supported by the Andrew Sabin Family Fellowship. Support was also provided through the Biostatistics Shared Resource through the Cancer Center Support Grant No. CA16672 (PI: R. DePinho, MD Anderson Cancer Center). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
See accompanying Oncology Grand Rounds on page 1780
AUTHOR CONTRIBUTIONS
Conception and design: Hubert Y. Pan, Karen E. Hoffman, Ya-Chen Tina Shih, Benjamin D. Smith
Collection and assembly of data: Hubert Y. Pan, Jing Jiang
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Comparative Toxicities and Cost of Intensity-Modulated Radiotherapy, Proton Radiation, and Stereotactic Body Radiotherapy Among Younger Men With Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Hubert Y. Pan
Research Funding: Varian Medical Systems
Jing Jiang
No relationship to disclose
Karen E. Hoffman
No relationship to disclose
Chad Tang
Stock or Other Ownership: Corvus Pharmaceuticals
Research Funding: Varian Medical Systems
Patents, Royalties, Other Intellectual Property: Patent #9,175,079
Travel, Accommodations, Expenses: Varian Medical Systems
Seungtaek L. Choi
No relationship to disclose
Quynh-Nhu Nguyen
No relationship to disclose
Steven J. Frank
Leadership: C4 Imaging
Stock or Other Ownership: C4 Imaging
Honoraria: Varian Medical Systems
Consulting or Advisory Role: Varian Medical Systems
Research Funding: Elekta, Hitachi
Patents, Royalties, Other Intellectual Property: C4 Imaging
Travel, Accommodations, Expenses: Varian Medical Systems
Mitchell S. Anscher
Stock or Other Ownership: CivaTech Oncology
Ya-Chen Tina Shih
Research Funding: Novartis (Inst)
Benjamin D. Smith
Research Funding: Varian Medical Systems
REFERENCES
- 1.Nguyen PL, Gu X, Lipsitz SR, et al. : Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol 29:1517-1524, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheets NC, Goldin GH, Meyer AM, et al. : Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307:1611-1620, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuban DA, Tucker SL, Dong L, et al. : Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 70:67-74, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Spratt DE, Pei X, Yamada J, et al. : Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 85:686-692, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slater JD, Rossi CJ, Jr, Yonemoto LT, et al. : Proton therapy for prostate cancer: The initial Loma Linda University experience. Int J Radiat Oncol Biol Phys 59:348-352, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Trofimov A, Nguyen PL, Coen JJ, et al. : Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: A treatment planning comparison. Int J Radiat Oncol Biol Phys 69:444-453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas C, Fryer A, Mahajan C, et al. : Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 70:744-751, 2008 [DOI] [PubMed] [Google Scholar]
- 8.King CR, Freeman D, Kaplan I, et al. : Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 109:217-221, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Pan HY, Jiang J, Shih YT, et al. : Adoption of radiation technology among privately insured nonelderly patients with cancer in the United States, 2008 to 2014: A claims-based analysis. J Am Coll Radiol 14:1027-1033.e2, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Yu JB, Soulos PR, Herrin J, et al. : Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst 105:25-32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halpern JA, Sedrakyan A, Hsu WC, et al. : Use, complications, and costs of stereotactic body radiotherapy for localized prostate cancer. Cancer 122:2496-2504, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu JB, Cramer LD, Herrin J, et al. : Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: Comparison of toxicity. J Clin Oncol 32:1195-1201, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Howlader N, Noone A, Krapcho M, et al: SEER Cancer Statistics Review, 1975-2014. Bethesda, MD, National Cancer Institute, 2017. [Google Scholar]
- 14.Salinas CA, Tsodikov A, Ishak-Howard M, et al. : Prostate cancer in young men: An important clinical entity. Nat Rev Urol 11:317-323, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. IBM: Putting research data into your hands with the MarketScan Database. http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases.
- 16.United States Census Bureau : American FactFinder. https://factfinder.census.gov/
- 17.Klabunde CN, Potosky AL, Legler JM, et al. : Development of a comorbidity index using physician claims data. J Clin Epidemiol 53:1258-1267, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Bekelman JE, Mitra N, Efstathiou J, et al. : Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys 81:e325-e334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sewell JM, Rao A, Elliott SP: Validating a claims-based method for assessing severe rectal and urinary adverse effects of radiotherapy. Urology 82:335-340, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Bureau of Labor Statistics : Measuring price change for medical care in the CPI. https://www.bls.gov/cpi/factsheets/medical-care.htm
- 21.Austin PC: An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399-424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC: A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 27:2037-2049, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Tian L: On estimating medical cost and incremental cost-effectiveness ratios with censored data. Biometrics 57:1002-1008, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Rolfes J, Zhao HW: Estimation of mean health care costs and incremental cost-effectiveness ratios with possibly censored data. Stata J 15:698-711, 2015 [Google Scholar]
- 25.Mariotto AB, Yabroff KR, Shao Y, et al. : Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 103:117-128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Institute of Medicine: Initial national priorities for comparative effectiveness research. Washington, DC, National Academies Press, 2009. [Google Scholar]
- 27. US National Institute of Health: Proton therapy vs. IMRT for low of intermediate risk prostate cancer (PARTIQoL). https://clinicaltrials.gov/ct2/show/NCT01617161.
- 28.Widmark A, Gunnlaugsson A, Beckman L, et al. : Extreme hypofractionation versus conventionally fractionated radiotherapy for intermediate risk prostate cancer: Early toxicity results from the Scandinavian Randomized Phase III Trial “HYPO-RT-PC.” Int J Radiat Oncol Biol Phys 96:938-939, 2016 [Google Scholar]
- 29.Steinberg ML, Konski A: Proton beam therapy and the convoluted pathway to incorporating emerging technology into routine medical care in the United States. Cancer J 15:333-338, 2009 [DOI] [PubMed] [Google Scholar]
- 30. International Cancer Institute: About the SEER registries. https://seer.cancer.gov/registries/
- 31.Michalski JM, Gay H, Jackson A, et al. : Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys 76:S123-S129, 2010. (3, suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Dong L, Lee AK, et al. : Effect of anatomic motion on proton therapy dose distributions in prostate cancer treatment. Int J Radiat Oncol Biol Phys 67:620-629, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekelman JE, Hahn SM: Reference pricing with evidence development: A way forward for proton therapy. J Clin Oncol 32:1540-1542, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konski A, Speier W, Hanlon A, et al. : Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol 25:3603-3608, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Fowler JF: The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol 44:265-276, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Catton CN, Lukka H, Gu CS, et al. : Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 35:1884-1890, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Dearnaley D, Syndikus I, Mossop H, et al. : Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 17:1047-1060, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee WR, Dignam JJ, Amin MB, et al. : Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol 34:2325-2332, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King CR, Collins S, Fuller D, et al. : Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: Results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys 87:939-945, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Pugh TJ, Munsell MF, Choi S, et al. : Quality of life and toxicity from passively scattered and spot-scanning proton beam therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 87:946-953, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer AM, Kuo TM, Chang Y, et al. : Using procedure codes to define radiation toxicity in administrative data: The devil is in the details. Med Care 55:e36-e43, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Potosky AL, Warren JL, Riedel ER, et al. : Measuring complications of cancer treatment using the SEER-Medicare data. Med Care 40:IV62-IV68, 2002. (8, suppl) [DOI] [PubMed] [Google Scholar]
- 43.Frank SJ, Pisters LL, Davis J, et al: An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J Urol 177:2151-2156, 2007; discussion 2156 [DOI] [PubMed] [Google Scholar]
- 44.Resnick MJ, Koyama T, Fan KH, et al. : Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 368:436-445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanda MG, Dunn RL, Michalski J, et al. : Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 358:1250-1261, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Donovan JL, Hamdy FC, Lane JA, et al. : Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 375:1425-1437, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]