Abstract
Purpose
Women with atypical hyperplasia (AH) on breast biopsy have an aggregate increased risk of breast cancer (BC), but existing risk prediction models do not provide accurate individualized estimates of risk in this subset of high-risk women. Here, we used the Mayo benign breast disease cohort to develop and validate a model of BC risk prediction that is specifically for women with AH, which we have designated as AH-BC.
Patients and Methods
Retrospective cohorts of women age 18 to 85 years with pathologically confirmed benign AH from Rochester, MN, and Nashville, TN, were used for model development and external validation, respectively. Clinical risk factors and histologic features of the tissue biopsy were selected using L1-penalized Cox proportional hazards regression. Identified features were included in a Fine and Gray regression model to estimate BC risk, with death as a competing risk. Model discrimination and calibration were assessed in the model-building set and an external validation set.
Results
The model-building set consisted of 699 women with AH, 142 of whom developed BC (median follow-up, 8.1 years), and the external validation set consisted of 461 women with 114 later BC events (median follow-up, 11.4 years). The final AH-BC model included three covariates: age at biopsy, age at biopsy squared, and number of foci of AH. At 10 years, the AH-BC model demonstrated good discrimination (0.63 [95% CI, 0.57 to 0.70]) and calibration (0.87 [95% CI, 0.66 to 1.24]). In the external validation set, the model showed acceptable discrimination (0.59 [95% CI, 0.51 to 0.67]) and calibration (0.91 [95% CI, 0.65 to 1.42]).
Conclusion
We have created a new model with which to refine BC risk prediction for women with AH. The AH-BC model demonstrates good discrimination and calibration, and it validates in an external data set.
INTRODUCTION
Approximately 10% of all benign breast biopsies demonstrate atypical hyperplasia (AH).1 With approximately 1 million benign biopsies per year in the United States, there are approximately 100,000 women who are newly diagnosed with AH annually. AH has been recognized for years as a phenotype of increased risk of breast cancer (BC) among women with benign breast disease (BBD).2-5 As a group, women with AH have an approximate four-fold increase in risk compared with the general population, which translates to an absolute risk of 1% to 2% per year.3,6 Because clinical management decisions, such as screening and prevention therapies, are made for individual women, a tool that provides accurate individualized risk prediction in the AH setting is needed.
Two BC risk prediction models that are commonly used for women after a benign breast biopsy—the Gail (Breast Cancer Risk Assessment Tool) and Tyrer-Cuzick International Breast Cancer Intervention Study models7,8—incorporate AH into their predictions; however, they do not provide accurate individualized estimates of risk within this subset of high-risk women.9,10 We recently developed the BBD-BC model for BC risk prediction in women with a benign breast biopsy.11 Here, we present a model for individualized risk prediction that is specifically for women with AH.
PATIENTS AND METHODS
Study Populations
Model-building sample.
The Mayo BBD cohort has been described previously12,13 and includes women age 18 to 85 years who had a benign breast biopsy between 1967 and 2001 at the Mayo Clinic (Rochester, MN). Clinical and demographic information was ascertained from medical records and questionnaires. The study breast pathologist (D.W.V.) reviewed archived hematoxylin and eosin (H&E) slides from benign biopsies and recorded histologic features. The 699 women who were found to have AH formed the model-building sample. We identified BC events, including both ductal carcinoma in situ (DCIS) and invasive, from study questionnaires, the Mayo Clinic tumor registry, and our review of medical records. This study was approved by the Mayo Clinic Institutional Review Board in keeping with the Mayo Clinic Federal-wide Assurance.12 To reduce the possibility of including undetected BC, the first 12 months of postbiopsy follow-up for each woman was removed from the analysis.
Model validation sample.
Model validation was performed in an independent external sample of women with AH from the Nashville Breast Cohort.13 This cohort similarly represents a retrospective collection of women with benign breast biopsies that were reviewed for histologic features and in whom follow-up BC events were ascertained. Within the Nashville Breast Cohort of patients who underwent breast biopsy between 1952 and 1989, 461 women had AH and no BC within 12 months of benign biopsy. H&E slides from these women were reviewed by the study pathologist (D.W.V.) to confirm AH and record the number of foci of AH using the same criteria as that of the model-building sample.
Statistical Approach
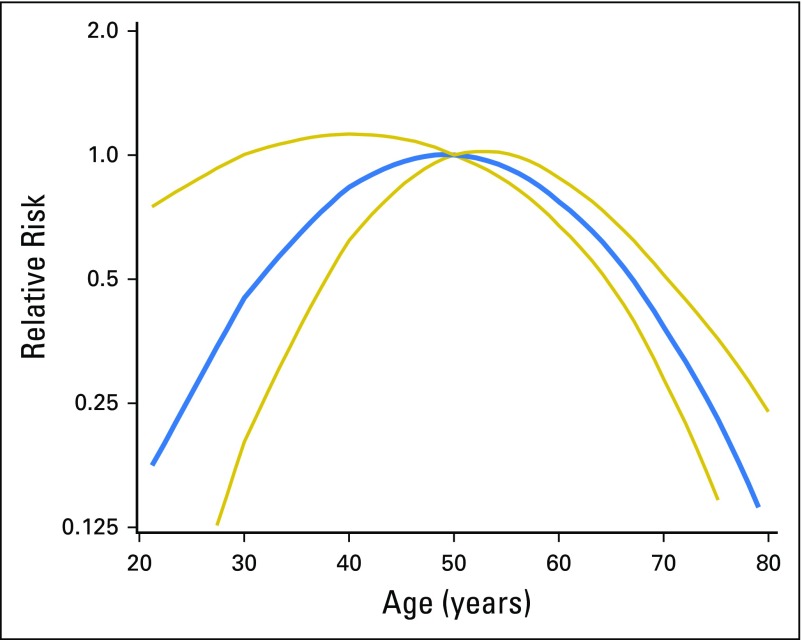
Full analysis details can be found in the Appendix (online only). In brief, follow-up was calculated as the number of days from 12 months after the initial diagnosis of AH to BC, death, prophylactic mastectomy, reduction mammoplasty, lobular carcinoma in situ, or last follow-up. Restricted B-splines14 suggested a quadratic age effect on BC risk (Fig 1); therefore, age was modeled in all analyses using both linear and quadratic terms.
Fig 1.
Predicted relative risk and 95% CI of breast cancer suggesting a quadratic association of age and breast cancer using Fine and Gray regression with age and age squared with referent at age 50 years.
Least absolute shrinkage and selection operator–based Cox proportional hazards regression was used to select risk-associated variables in the model-building sample. Age, age-squared, and 17 other clinical, demographic, and histologic variables were considered—year of biopsy, age at menarche, age at first live birth combined with number of children, breastfeeding history, body mass index, family history of BC, indication for biopsy, number of atypical foci, type of atypia, lobular involution, and the presence of a radial scar, fibroadenoma, calcifications, sclerosing adenosis, columnar alterations, cysts, or fibrosis. All analyses after this variable selection procedure were carried out using Fine and Gray regression models to account for death as a competing risk.15 Individual predicted probabilities were generated by applying the model coefficients to the observed follow-up times and were grouped into three categories to represent lower, intermediate, and higher predicted BC risk. Model discrimination was assessed using C-statistics, and internal calibration was assessed by comparing observed BC counts with predicted BC counts. Performance of the AH model was compared with our BBD-BC model, calculating absolute BC risks using both models in 5-year follow-up intervals and comparing model discrimination and calibration.
The final model was validated using the Nashville Breast Cohort. Estimated absolute risk from the model development set was applied to the external validation set for each covariate combination using parameter estimates that were derived from the Mayo cohort, and women were classified into three risk tertiles as described above.
Analyses were carried out using SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC) and R (version 3.1.1; The R Foundation, Vienna, Austria). A nomogram was created in the software package R using the nomogram function from the rms library.
RESULTS
Patient Characteristics
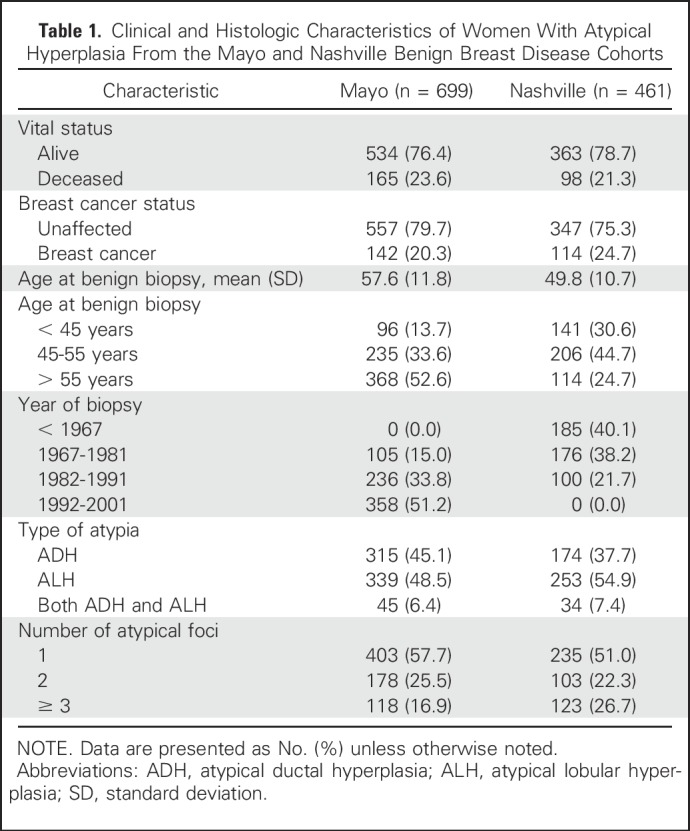
The Mayo model-building sample consisted of 699 women with 142 BC events (median follow-up, 8.1 years), and the external set from the Nashville Breast Cohort consisted of 461 women with 114 BC events (median follow-up, 11.4 years; Table 1). The two cohorts had similar characteristics, although women in the Mayo model-building set were slightly older (mean age, 57.6 years) compared with the Nashville validation set (mean age, 49.8 years). In addition, the model-building set had fewer women with 3+ atypical foci (16.9% v 26.7%).
Table 1.
Clinical and Histologic Characteristics of Women With Atypical Hyperplasia From the Mayo and Nashville Benign Breast Disease Cohorts
Model Building
Variable selection.
Frequency distributions of the features in the model-building set are shown in Appendix Table A1 (online only). Parameter tuning via cross-validation in the penalized Cox proportional hazards regression analysis indicated that the prediction model would include age (and age squared), number of atypical foci, and extent of lobular involution. However, the model C-statistic and Akaike Information Criterion were not substantially improved with involution added; therefore, for simplicity, this variable was not included in the final model.
Final AH model.
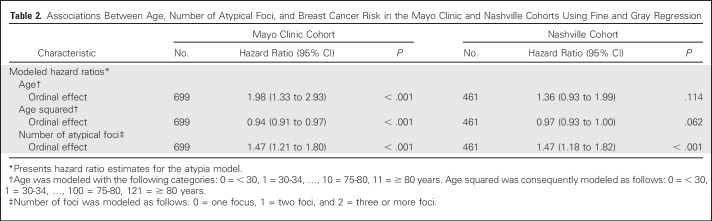
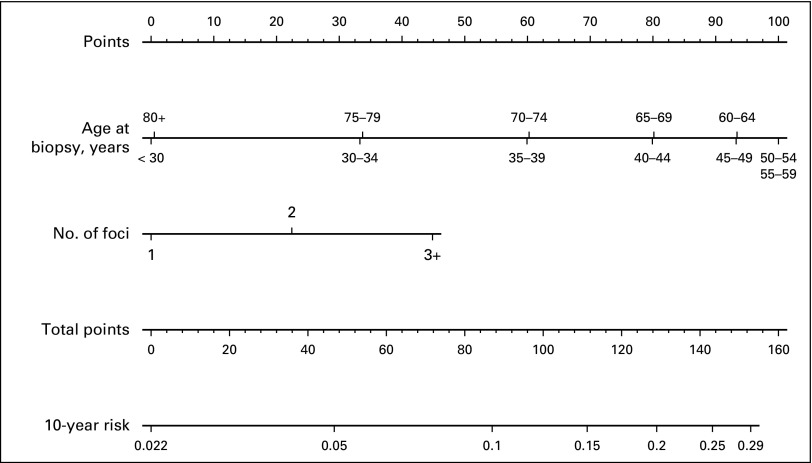
Relative risk estimates from the final model of age, age squared, and number of atypical foci for the model-building set are presented in Table 2. Younger women (age < 40 years), older women (age ≥ 75 years), and women with one focus of AH had lower risk than those who were diagnosed with AH at age 45 to 70 years and those with two or more atypical foci. Absolute risks and 95% CIs in 5-year follow-up intervals for each combination of age and number of atypical foci can be obtained either with a table (Fig 2), a nomogram (Appendix Fig A1, online only), or with an online calculator.16 The online calculator generates risk predictions after entering age and the number of atypical foci in a user-friendly format.
Table 2.
Associations Between Age, Number of Atypical Foci, and Breast Cancer Risk in the Mayo Clinic and Nashville Cohorts Using Fine and Gray Regression
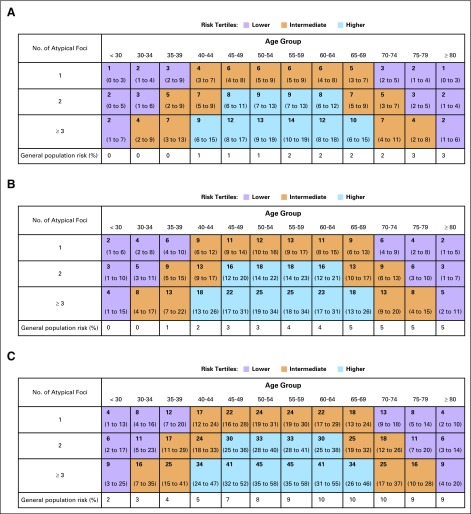
Fig 2.
Predicted absolute risk, 95% confidence interval, and population-based expected absolute risk by duration of follow-up. (A) Five-year risk; (B) 10-year risk; (C) 20-year risk. Absolute risk is expressed as percentages and displayed as predicted (95% CI) and expected. Expected risk calculated using breast cancer incidence rates from Iowa SEER. Shading represents assignments to the three risk tertiles of lower (purple), intermediate (orange) and higher (blue) risk, respectively.
Model Performance Assessment
Distribution of case-control counts among risk groups within the model-building and validation sets.
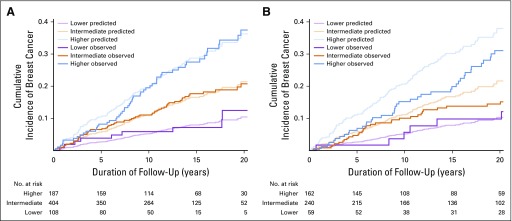
All possible combinations of covariates in the model were enumerated, and 5-year risks were calculated for each of the 36 possible combinations of age categories and number of atypical foci. The 12 combinations with the lowest 5-year risks (1% to 3%) defined the lower-risk group, the middle 14 risks (4% to 7%) defined the intermediate-risk group, and the top 10 risks (8% to 14%) defined the higher-risk group. Comparison of risk group distributions in the Mayo and Nashville sets (15.5% and 12.8% lower risk, 53.1% and 48.6% intermediate risk, and 31.5% and 38.6% higher risk, respectively) indicated that the Nashville cohort had slightly more high-risk women. Individuals in these groups demonstrated differences in the cumulative incidence of BC for both data sets (Figs 3A and 3B).
Fig 3.
(A) Predicted and observed cumulative incidences of breast cancer in women with atypical hyperplasia (grouped by tertiles of risk) in the Mayo model-building set. (B) Predicted and observed cumulative incidences of breast cancer in women with atypical hyperplasia (grouped by tertiles of risk) in the Nashville external validation set.
Model performance in the Mayo data set.
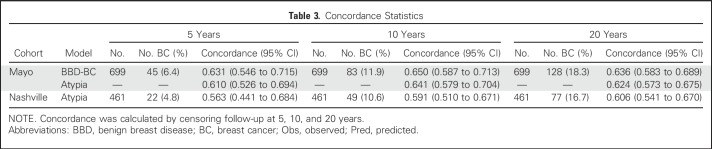
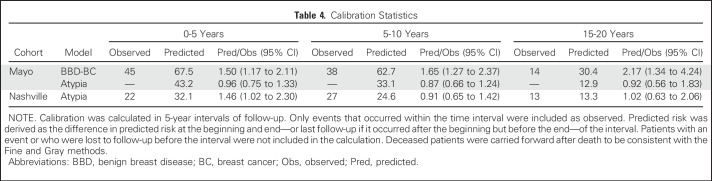
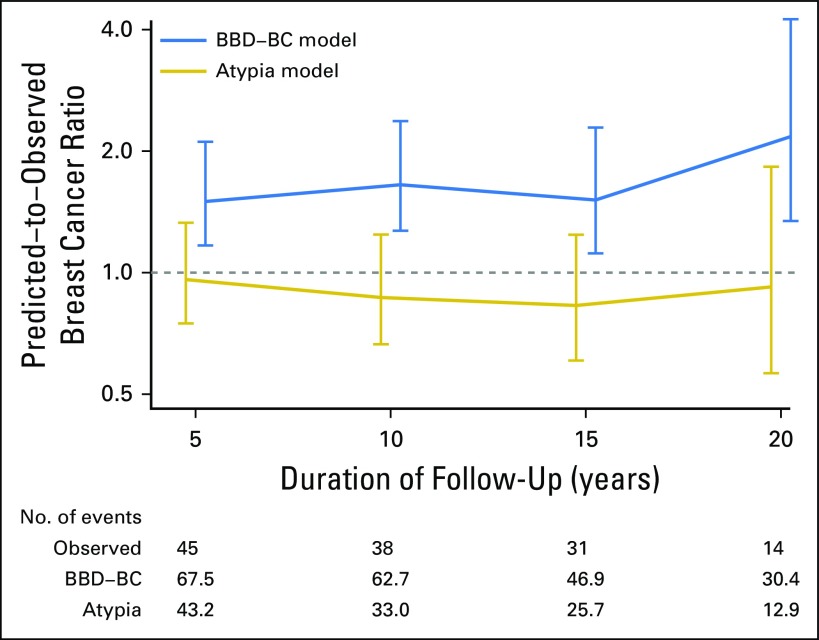
Discrimination of the AH model was good and comparable to that of the BBD-BC model that was applied to this AH subcohort, with C-statistics of 0.650 and 0.641 at 10 years, and 0.636 and 0.624 at 20 years, respectively (Table 3 and Fig 3A). The AH model displayed superior calibration compared with the BBD-BC model, as the BBD-BC model overpredicted the number of cases irrespective of the follow-up interval, whereas the new AH model was well calibrated across all time points that were assessed (ratios of predicted-to-observed cancers were 0.96, 0.87, and 0.92 at 5, 10, and 20 years, respectively; Table 4 and Appendix Fig A2, online only).
Table 3.
Concordance Statistics
Model performance in the external validation set.
Discrimination was also good in the Nashville data set, with C-statistics of 0.591 (95% CI, 0.510 to 0.671) at 10 years, and 0.606 (95% CI, 0.541 to 0.670) at 20 years. The model overpredicted the number of cancers in the Nashville cohort in the first 5 years (1.46 predicted-to-observed ratio), but was well-calibrated at 10 years (0.91 predicted-to-observed ratio) and 20 years (1.02 predicted-to-observed ratio; Tables 3 and 4). In the Nashville cohort, the model overestimated the risk in higher-risk women, but performed better in women with intermediate or lower predicted risk.
Table 4.
Calibration Statistics
DISCUSSION
We developed a parsimonious model for risk prediction in women with AH and validated this in an external cohort. In the past, we assessed the Gail and Tyrer-Cuzick models for predicting BC risk in women with AH and found that neither model performs better than chance alone in this group of high-risk women.9,10 We reported previously that the number of foci of AH stratifies future BC risk,3,13,17 and we now have demonstrated that the addition of age at biopsy further improves individualized risk estimation for women with AH. For women with AH, this model provides similar discrimination, but better calibration, than our prior BBD-BC model. Of importance, we confirmed the performance of our model—derived from the Mayo BBD cohort—in an independent external validation data set. Risk estimates that were derived from these efforts are provided in table format and have been merged into our BBD-BC Web-based platform to create one unified Web-based risk prediction tool, dubbed the BBDAH-BC model.
Accurate risk prediction for women with AH is important because clinical management can be refined on the basis of risk level. Data from independent cohorts show the incidence of BC in this group of women is approximately 1% to 2% per year in the absence of prevention therapy.6,18 Although the majority of women with AH do not develop BC, risk is substantially increased for many. Furthermore, we have reported that the number of foci of AH is a strong factor stratifying risk,18 and this finding has also been validated externally.13 In the majority with a single focus of AH, 20-year risk is approximately 17%, but among the 23% with two foci of AH, the 20-year incidence is 28%, and in the 17% of women who were found to have three foci of AH, the 20-year incidence of cancer is 40%.18 Here, we have further refined risk stratification, modeling risk with both age and the number of foci of AH. The ability to determine which individuals are at higher and lower risk makes it possible to better tailor clinical management decisions to the individual patient, whether it is to recommend aggressive surveillance or prevention strategies for women with AH who are at increased risk or to reassure women with AH who have a relatively lower risk.
The prediction algorithm that we have developed and validated can be used to better inform individual women and decisions about managing their risk. For instance, 6.7% of women in the combined Mayo and Nashville cohorts had a calculated 20-year risk of less than 12%, of which only 6.4% developed BC, which put them at or below the average population risk and ruled out the need for increased surveillance. Conversely, 30.0% of women had a predicted 20-year risk that exceeded 25%— among whom 32.7% developed BC—for whom intensive surveillance and risk reduction programs should be considered. In the American Cancer Society magnetic resonance imaging (MRI) screening guidelines from 2007,19 AH is enumerated as a condition with insufficient data to justify MRI screening. In light of growing recent literature from various sources that corroborates an absolute risk of 1% to 2% per year in women with AH,6,18 in combination with results of this study, we believe that a 20-year risk prediction of 25% from our AH model justifies MRI screening and that a lifetime risk prediction of > 25% by this model should be a qualifying addition to current MRI screening guidelines. Of more importance, women with AH who are at intermediate or higher risk should be counseled to use prevention therapy, which has high efficacy— 70% risk reduction—in women with AH.6,20-23
Early findings from our cohort suggested a cumulative risk of 1% per year in women with AH.17 Since then, we have demonstrated a similar degree and long duration of increased risk within the Nashville Breast Cohort.13 Recently, these findings were challenged by a report of lower absolute risk in women with AH who were diagnosed by core needle biopsy in the Breast Cancer Surveillance Consortium.24 In that study, 10-year BC risk was 6% (an average of 0.6% per year); however, BC risk was possibly underestimated because DCIS events were excluded, which lowers incidence by approximately 20%. Furthermore, that study depended on existing registry databases, which resulted in a higher likelihood of under ascertaining BC events. In contrast, another recent study of a cohort of women with high-risk biopsy findings (1987 to 2010) demonstrated an average 10-year cumulative absolute risk of 17% for atypical ductal hyperplasia and 24% for atypical lobular hyperplasia—even greater absolute risks than those observed in the Mayo and Nashville cohorts.6 Thus, the preponderance of data indicates that women with AH as a group have an absolute risk of at least 1% per year, including DCIS events. Furthermore, in the Mayo cohort, 82% of BCs were invasive, of which 22% were node positive at diagnosis, which indicates the potential lethality of BC subsequent to a diagnosis of AH. Our new model further stratifies BC risk for women with AH into low-, medium-, and high-risk categories, which will facilitate individualized treatment strategies.
An interesting finding was the relationship of age and risk (Figs 1 and 2). We observed a quadratic effect of age with BC risk in the Mayo cohort—and to a slightly lesser degree in the Nashville Cohort. In a prior publication, we reported that the relative risk (RR) of BC in women with AH is significantly higher for young women (RR, 6.8 for age < 45 years) compared with their peers versus an RR of 2.9 for women age > 55 years compared with their peers.17 For that analysis, RR was calculated in comparison with women of similar age in the Iowa SEER population, most of whom would not have AH. In contrast to these RR findings, the current analysis demonstrates that absolute risk in women with AH is highest in middle age years and lower at the extremes of age. Mazzola et al25 have recently reported a similar finding—that is, that absolute risk in women with AH does not follow the expected pattern of increasing risk at older age. The reason for the lower risk at younger ages is presumably a result of the lower age-specific BC incidence for younger women, whereas the lower risk for older women is a result of competing mortality from other causes—these women, on average, are not living long enough to develop BC. Regarding risk in younger women with AH, the perimenopausal years comprise the timeframe when the breast gland undergoes tissue remodeling with age-related involution, which likely presents a permissive microenvironment for premalignant epithelium to progress to cancer. A parallel may be found around the time of pregnancy—also a time of increased BC risk—during which an extensive inflammatory response has been linked to BC progression.26 Regardless of the biologic basis for our finding on age and BC risk in women with AH, from a risk-modeling perspective, this finding underscores the importance of examining the nonlinear relationships between risk factors and absolute cancer risk. We look forward to the validation of the seemingly parabolic relationship of age and BC risk for women with AH in other cohorts.
Our study has several strengths. The large group of women with AH with long-term follow-up and data on multiple clinical and pathologic features allowed for the consideration of many variables in model building. We have validated this model in an external data set and found that it works well in women with AH who are at intermediate risk and very well for women with AH who are at lower risk (Fig 3B). Furthermore, our final AH model is easy to use—either online or in table format—and its simplicity will aid the feasibility of use. Although we were not able to evaluate mammographic density as a risk factor in our model because of a lack of data in the majority of women, in the subset of women with AH in whom mammographic density is available, we recently reported no association of mammographic density with BC risk for women with AH, so this is unlikely to have affected the model.27 Our model may not apply well in the minority of women who have AH in multiple biopsies over time. Among the 699 women in our cohort with AH, only 65 underwent repeat biopsies, 23 with AH at later biopsy (only 3% of women with AH). We recently evaluated this issue and found that women with two AH biopsies over time were 75% more likely to develop BC compared with those without AH at second biopsy (RR, 1.75; 95% CI, 0.30 to 10.0), but the difference was not statistically significant (P = .53).28 Finally, although this model offers improved performance in women with AH compared with the Gail, Tyrer-Cuzick, and BBD-BC models, there is room for additional improvement in discrimination. All of our reported C-statistics cluster around 0.6 or slightly higher, which indicates that for any random case-control pair of individuals with a history of AH, our model would correctly classify the one to develop BC only 60% of the time. Development of prediction models for breast and other cancers remains challenging and lags behind that of other phenotypes, such as cardiovascular disease and diabetes, which have reported C-statistics that exceed 0.8.29,30
Improvement of prediction models through the addition of genetic variants seemed to be challenging initially, as reported by Wacholder et al31; however, continued advances in germline variant assessment32 and breast tissue–based gene expression33 hold promise for achieving clinical risk prediction with C-statistics of 0.70 and higher. Adding genomic risk information to clinical risk models can improve risk prediction34 and is a strategy that we and other research teams are actively pursuing.
In conclusion, we created the AH-BC model to predict BC risk in women with AH on the basis of age at biopsy and the number of foci of atypia. This model provides good discrimination and improved calibration in women with AH compared with the original BBD-BC model, and we have evaluated its performance in an external cohort. This model is an important step in improving risk prediction for women with AH.
ACKNOWLEDGMENT
We thank Teresa Allers and Joanne Johnson for data collection and Marilyn Churchward for assistance in manuscript preparation.
Appendix
Statistical Approach
Modeling age and risk.
To reduce the possibility of including undetected breast cancer (BC), the first 12 months of postbiopsy follow-up for each woman was removed from the analysis; thus, the duration of follow-up was calculated as the number of days since 12 months after the initial diagnosis of atypical hyperplasia (AH) to BC, death, prophylactic mastectomy, reduction mammoplasty, lobular carcinoma in situ, or last follow-up. Similar to the approach used in the Breast Cancer Risk Assessment Tool, we mapped ages into an ordinal variable that reflected 5-year age intervals (0 ≤ 30, 1 = 30 to 34, …, 10 = 75 to 79, 11 = ≥ 80) and used this ordinal variable to model stepwise changes in BC risk as a function of 5-year age categories. A restricted B-spline that was estimated in a Cox proportional hazards regression model suggested that the log hazard ratio for the association between age and BC risk in the Mayo benign breast disease (BBD) cohort was consistent with a parabolic shape, and we therefore added a squared version of the ordinal age term to capture this curvature.14 This quadratic effect persisted when modeled with the Fine and Gray approach, which accounted for death as a competing risk15 (Fig 1). Therefore, in all subsequent analyses, we modeled this relationship as a linear plus quadratic function of the ordinal age variable.
Model building.
In addition to age (and age squared) at biopsy, the model-building effort considered 17 additional variables, including clinical/demographic—year of biopsy, age at menarche, a combined categorization of age at first live birth and number of children, breastfeeding history, categorized body mass index, family history of BC, and indication for biopsy; and histologic—number of atypical foci, type of atypia (atypical ductal hyperplasia, atypical lobular hyperplasia, or both), lobular involution (none, partial, or complete; defined previously; Milanese TR, et al: J Natl Cancer Inst 98:1600-1607, 2006), and the presence of a radial scar, fibroadenoma, calcifications, sclerosing adenosis, columnar alterations, cysts, or fibrosis.
Associations of these previously identified risk factors with BC risk in the model development set were examined using penalized Cox proportional hazards regression with a least absolute shrinkage and selection operator–based (L1) penalty using the glmnet R package (Friedman J, et al: Biostatistics 9:432-441, 2008; Simon N, et al: Stat Sin 22:983-1001, 2012). Because it is a well-established risk factor for BC, age (and age squared) was forced into the least absolute shrinkage and selection operator model without penalty. Tuning parameters were selected with cross validation. All analyses after this variable selection procedure were carried out using Fine and Gray regression models to account for death as a competing risk.15 The absolute risk of BC at 5-year follow-up intervals was derived for each covariate combination by applying the model coefficients to the observed follow-up times using a product limit estimator approach (Gooley TA, et al: Stat Med 18:695-706, 1999; So Y, et al: Proc SAS Global Forum, 2015). Risk estimates were subsequently grouped into three categories—approximate tertiles—in an effort to represent lower, intermediate, and higher predicted BC risk. Predicted curves for each of the tertiles were derived by calculating weighted means of the covariate combination–specific risk estimates defined above with weights defined on the basis of their prevalence in the tertile.
Assessing model performance.
Model discrimination was assessed overall and at 5-year follow-up intervals with C-statistics that were calculated by truncating follow-up and BC status at each 5-year interval and using the method described by Ruan and Gray (Ruan PK, et al: Stat Med 27:5709-5724, 2008). Of note, although the same final model coefficients were used to calculate discrimination at each 5-year interval, differences in follow-up times as a result of competing mortal risks across women will cause predicted absolute risks to shift from interval to interval, which will result in differing C-statistics over time.
Calibration was defined as the ratio of expected-to-observed number of BC events. As with absolute risks described above, calibration was assessed at each 5-year time interval. The number of observed events was calculated as the number of BCs that occurred between the beginning and end of the interval of interest. For instance, for 5-year risk estimates, the observed number of BCs was simply the number that occurred less than 5 years after the diagnosis of AH. The expected number of events for a given time interval was estimated using methods described by Crowson et al (Crowson CS, et al: Stat Methods Med Res 25:1692-1706, 2016). The predicted BC risk of each woman was first transformed into an individual-specific expected number of events using the formula:
The total number of expected events was then calculated by simply summing the individual expected numbers. For women who were still alive at last follow-up and who were observed past the end of the interval of interest, predicted risk was calculated as the difference in risk at the beginning and end of the time period. For nondeceased women whose last follow-up occurred within the interval of interest, the difference between risk at last contact and the beginning of the interval was used. Women who died without experiencing progression to BC were used in all calculations—that is, carried forward past their death date—to be consistent with the Fine and Gray approach of accounting for competing risks. Their predicted risk was calculated as the difference in risk at the beginning of the interval and that at the end of the interval, regardless of death date.
Comparison with the BBD-BC Model.
We compared the performance of this AH model with that of our BBD-BC model in women with AH. Absolute risks of BC were calculated using both models in 5-year follow-up intervals, and model discrimination and calibration were compared.
External validation.
The final model was validated using the Nashville Breast Cohort. For comparison with the final model estimates from the model development set, hazard ratios were estimated using the Fine and Gray regression approach. Estimated absolute risk from the model development set was directly applied to the validation set for each covariate combination using parameter estimates that were derived from the Mayo cohort. Women were classified as lower-, intermediate-, and higher-risk categories using the combinations that had been identified previously in the model development set. Analyses were carried out using SAS (version 9.4) and R (version 3.1.1).
Fig A1.
Nomogram representing proposed atypia model.
Fig A2.
Calibration of the benign breast disease–breast cancer (BBD-BC) model and atypia model for Mayo participants, represented as the ratio of predicted-to-observed breast cancers at 5-year intervals.
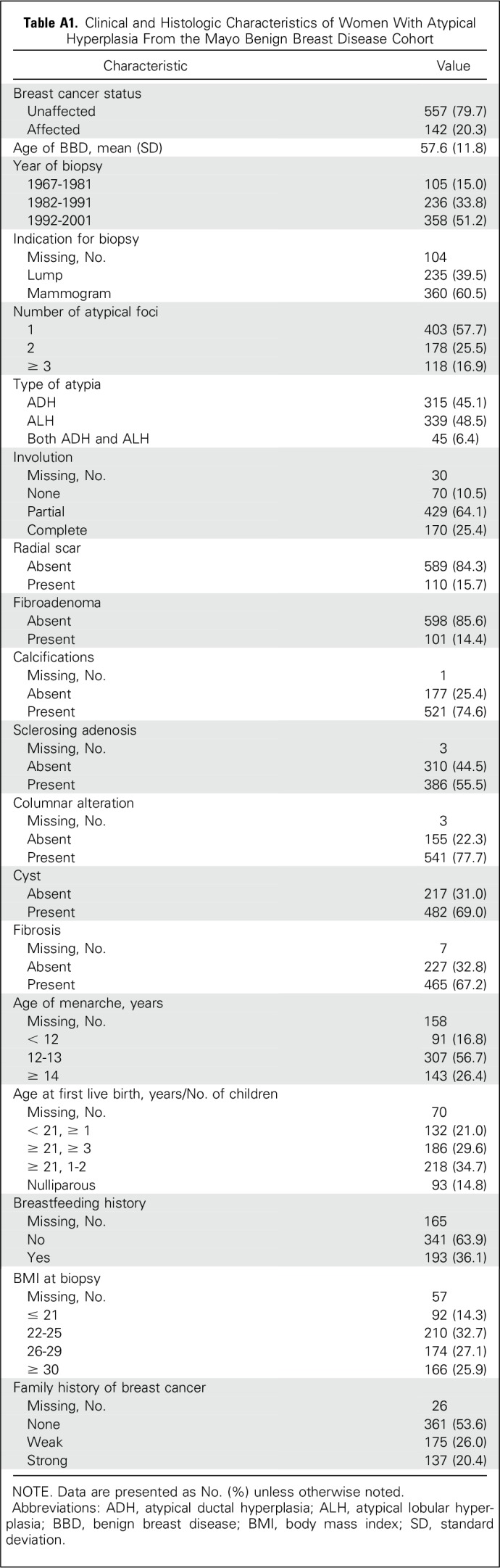
Table A1.
Clinical and Histologic Characteristics of Women With Atypical Hyperplasia From the Mayo Benign Breast Disease Cohort
Footnotes
Supported by National Cancer Institute Grant No. CA187112, the Mayo Clinic Breast Cancer—Specialized Program of Research Excellence (SPORE CA116201), and Susan B. Komen Grant No. KG110542.
Presented at the 2015 American Society of Clinical Oncology Breast Cancer Symposium, San Francisco, CA, September 25, 2015.
AUTHOR CONTRIBUTIONS
Conception and design: Amy C. Degnim, V. Shane Pankratz, William D. Dupont, Robert A. Vierkant, Marlene H. Frost, Celine M. Vachon, Karthik Ghosh, Lynn C. Hartmann, Daniel W. Visscher, Derek C. Radisky
Collection and assembly of data: Amy C. Degnim, V. Shane Pankratz, William D. Dupont, Robert A. Vierkant, Marlene H. Frost, Jodi M. Carter, Lori A. Denison, Daniel W. Visscher, Derek C. Radisky
Data analysis and interpretation: Amy C. Degnim, Stacey J. Winham, Ryan D. Frank, V. Shane Pankratz, Tanya L. Hoskin, Celine M. Vachon, Tina J. Hieken, Brendan Broderick
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Model for Predicting Breast Cancer Risk in Women With Atypical Hyperplasia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Amy C. Degnim
No relationship to disclose
Stacey J. Winham
No relationship to disclose
Ryan D. Frank
No relationship to disclose
V. Shane Pankratz
No relationship to disclose
William D. Dupont
No relationship to disclose
Robert A. Vierkant
No relationship to disclose
Marlene H. Frost
No relationship to disclose
Tanya L. Hoskin
No relationship to disclose
Celine M. Vachon
Employment: Hil-Rom (I)
Leadership: Grail
Consulting or Advisory Role: Grail
Research Funding: Grail
Patents, Royalties, Other Intellectual Property: Breast density automated measure
Travel, Accommodations, Expenses: Grail
Karthik Ghosh
No relationship to disclose
Tina J. Hieken
No relationship to disclose
Jodi M. Carter
No relationship to disclose
Lori A. Denison
No relationship to disclose
Brendan Broderick
No relationship to disclose
Lynn C. Hartmann
No relationship to disclose
Daniel W. Visscher
No relationship to disclose
Derek C. Radisky
No relationship to disclose
REFERENCES
- 1.Pearlman MD, Griffin JL: Benign breast disease. Obstet Gynecol 116:747-758, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Dupont WD, Page DL: Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 312:146-151, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Hartmann LC, Radisky DC, Frost MH, et al. : Understanding the premalignant potential of atypical hyperplasia through its natural history: A longitudinal cohort study. Cancer Prev Res (Phila) 7:211-217, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter CL, Corle DK, Micozzi MS, et al. : A prospective study of the development of breast cancer in 16,692 women with benign breast disease. Am J Epidemiol 128:467-477, 1988 [DOI] [PubMed] [Google Scholar]
- 5.London SJ, Connolly JL, Schnitt SJ, et al. : A prospective study of benign breast disease and the risk of breast cancer. JAMA 267:941-944, 1992 [PubMed] [Google Scholar]
- 6.Coopey SB, Mazzola E, Buckley JM, et al. : The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat 136:627-633, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Gail MH, Brinton LA, Byar DP, et al. : Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81:1879-1886, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Tyrer J, Duffy SW, Cuzick J: A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23:1111-1130, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Boughey JC, Hartmann LC, Anderson SS, et al. : Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol 28:3591-3596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pankratz VS, Hartmann LC, Degnim AC, et al. : Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol 26:5374-5379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pankratz VS, Degnim AC, Frank RD, et al. : Model for individualized prediction of breast cancer risk after a benign breast biopsy. J Clin Oncol 33:923-929, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann LC, Sellers TA, Frost MH, et al. : Benign breast disease and the risk of breast cancer. N Engl J Med 353:229-237, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Degnim AC, Dupont WD, Radisky DC, et al. : Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 122:2971-2978, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eilers PH, Marx BD: Flexible smoothing with B-splines and penalties. Stat Sci 11:89-121, 1996 [Google Scholar]
- 15.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assn 94:496-509, 1999 [Google Scholar]
- 16.Mayo Clinic : Breast cancer risk prediction. http://www.mayoclinic.org/medical-professionals/cancer-prediction-tools/breast-cancer
- 17.Degnim AC, Visscher DW, Berman HK, et al. : Stratification of breast cancer risk in women with atypia: A Mayo cohort study. J Clin Oncol 25:2671-2677, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hartmann LC, Degnim AC, Santen RJ, et al. : Atypical hyperplasia of the breast: Risk assessment and management options. N Engl J Med 372:78-89, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saslow D, Boetes C, Burke W, et al. : American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57:75-89, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Costantino JP, Wickerham DL, et al. : Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371-1388, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Fisher B, Costantino JP, Wickerham DL, et al. : Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 97:1652-1662, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Cuzick J, Warwick J, Pinney E, et al. : Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: A nested case-control study. J Natl Cancer Inst 103:744-752, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Goss PE, Ingle JN, Alés-Martínez JE, et al. : Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364:2381-2391, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Menes TS, Kerlikowske K, Lange J, et al. : Subsequent breast cancer risk following diagnosis of atypical ductal hyperplasia on needle biopsy. JAMA Oncol 3:36-41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzola E, Coopey SB, Griffin M, et al. : Reassessing risk models for atypical hyperplasia: Age may not matter. Breast Cancer Res Treat 165:285-291, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schedin P, O’Brien J, Rudolph M, et al. : Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia 12:71-82, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Vierkant RA, Degnim AC, Radisky DC, et al. : Mammographic breast density and risk of breast cancer in women with atypical hyperplasia: An observational cohort study from the Mayo Clinic Benign Breast Disease (BBD) cohort. BMC Cancer 17:84, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visscher DW, Frank RD, Carter JM, et al. : Breast cancer risk and progressive histology in serial benign biopsies. J Natl Cancer Inst 109:djx035, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB, Sr, Larson MG, et al. : Predicting the 30-year risk of cardiovascular disease: The Framingham Heart Study. Circulation 119:3078-3084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson PW, Meigs JB, Sullivan L, et al. : Prediction of incident diabetes mellitus in middle-aged adults: The Framingham Offspring Study. Arch Intern Med 167:1068-1074, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Wacholder S, Hartge P, Prentice R, et al. : Performance of common genetic variants in breast-cancer risk models. N Engl J Med 362:986-993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michailidou K, Lindström S, Dennis J, et al. : Association analysis identifies 65 new breast cancer risk loci. Nature 551:92-94, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degnim AC, Nassar A, Stallings-Mann M, et al. : Gene signature model for breast cancer risk prediction for women with sclerosing adenosis. Breast Cancer Res Treat 152:687-694, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shieh Y, Hu D, Ma L, et al. : Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat 159:513-525, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]