Abstract
Besides its well-known action to stimulate thyroid hormone release, thyrotropin mRNA is expressed within the brain, and thyrotropin and its receptor have been shown to be present in brain areas that control feeding and gastrointestinal function. Here, the hypothesis that thyrotropin acts on receptors in the hindbrain to alter food intake and/or gastric function was tested. Fourth ventricular injections of thyrotropin (0.06, 0.60, and 6.00 µg) were given to rats with chronic intracerebroventricular cannulas aimed at the fourth ventricle. Thyrotropin produced an acute reduction of sucrose intake (30 min). The highest dose of thyrotropin caused inhibition of overnight solid food intake (22 h). In contrast, subcutaneous administration of corresponding thyrotropin doses had no effect on nutrient intake. The highest effective dose of fourth ventricular thyrotropin (6 µg) did not produce a conditioned flavor avoidance in a standardized two-bottle test, nor did it affect water intake or gastric emptying of glucose. Thyrotropin injected in the fourth ventricle produced a small but significant increase in rectal temperature and lowered plasma levels of tri-iodothyronin but did not affect plasma levels of thyroxine. In addition, there was a tendency toward a reduction in blood glucose 2 h after fourth ventricular thyrotropin injection (P = 0.056). In conclusion, fourth ventricular thyrotropin specifically inhibits food intake, increases core temperature, and lowers plasma levels of tri-iodothyronin but does not affect gastromotor function.
Keywords: conditioned flavor avoidance, ingestive behavior, thyrotropin, thyroid-stimulating hormone, water intake, tri-iodothyronine
INTRODUCTION
Thyrotropin, also known as thyroid-stimulating hormone (TSH), is a protein well known for its stimulatory role in the brain-hypophysis-thyroid axis by its action on the thyroid gland, leading to release of thyroid hormone into the circulation. It has historically been viewed as a peripheral hormone because it is known to be produced in and released from the adenohypophysis. In recent years, however, presence of TSH has been demonstrated in the brain, including areas of the brain stem and hypothalamus that are known to be involved in food intake controls (7, 9). Moreover, after peripheral injection, labeled TSH does not appear in the brain (1), suggesting that central TSH is locally translated, representing a pool separate from peripheral TSH. The possible roles for TSH and the TSH receptor in the adult brain are not fully known, although injection of TSH into the lateral brain ventricle of rats has been shown to reduce food intake (13).
In an earlier study (3), we detected the presence of immunoreactivity for the TSH receptor in nuclei of the brain stem and hypothalamus, which are known to be involved in the control of feeding, energy homeostasis, and neuroendocrine functions. Subsequent Western blot and PCR analyses confirmed these findings. Included among the sites where TSH receptor-like immunoreactivity was detected were the nucleus of the solitary tract (NTS) and the dorsal motor nucleus of the vagus (DMX), located in the caudal brain stem. Local nanoinfusion of TSH into the NTS of the caudal brain stem, where TSH receptor staining was dense, led to acute inhibition of food intake in the rat, further supporting functionality of a TSH holoreceptor protein in the circuits for food intake regulation. Together, this suggests that TSH receptor protein is present in areas of the brain that are of importance for the control of food intake and gastromotor function and further highlights a possible role for the dorsal hindbrain in TSH receptor-mediated action on feeding mechanisms.
Given the recent report of possible TSH receptor involvement in brain stem-elicited inhibitory effects on acute feeding and that thyroid hormone levels are altered in disease states characterized by inflammation and appetite loss (2, 4, 6, 17, 18, 22), we sought to further explore the possible role of a caudal brain stem TSH receptor pool with regard to food intake, food avoidance, and metabolic signaling. Here, we tested the hypothesis that a dorsal hindbrain effect of TSH on food intake is due to a true action in the brain and not elicited via a peripheral target mechanism. We did so first by comparing the dose-effect relationships of TSH injected into the fourth ventricle (fourth intracerebroventricular) and subcutaneously on intake of a liquid nutrient as well as of solid food. Furthermore, we aimed to investigate whether such a TSH-induced food intake inhibitory caudal brain stem effect is specific and not due to an adverse effect that could interfere with ingestive behavior and cause hypophagia. For example, rather than satiety, the drug could theoretically cause nausea resulting in secondary hypophagia because of avoidance. Thus we explore whether TSH induces conditioned flavor avoidance after fourth intracerebroventricular delivery. Given the evidence of functional TSH receptors in the DMX (3), the hypothesis that fourth intracerebroventricular TSH may inhibit food intake by affecting gastric emptying was also tested. We finally aimed to examine whether fourth intracerebroventricularly delivered TSH may affect metabolism through changes in body temperature and whether it may influence thyroid function or blood glucose.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing ~300 g at the time of surgery were used. The animals were housed singly in hanging cages with free access to standard chow (Prolab RMH 1000) and tap water under conditions of controlled temperature (20 ± 1°C) and humidity, on a 12-h:12-h light/dark cycle (lights on 7 AM to 7 PM). Different groups of rats were used for each experiment. Before the start of this study, the Animal Care and Use Committee at the Johns Hopkins University School of Medicine had approved the experimental protocols.
Surgery
Before surgery, the rats were anesthetized with a mixture of xylazine (8.6 mg/kg) and ketamine (57.0 mg/kg), which was injected intramuscularly in the hind leg (1.0 ml/kg body wt). For gastric emptying experiments, chronic gastric fistulas were implanted as previously described (20). The rats were food deprived overnight and anesthetized. A laparotomy was made, and a purse-string suture was sewn in the ventral forestomach. The fistula was inserted in the stomach via a central opening in the purse, and the purse-string suture was closed. After exteriorizing the distal end of the fistula through a para-midline puncture in the abdominal wall and skin, it was secured with another purse-string suture, and the incision was closed. Before the implantation of central guide cannulas, the animals were given 9 days of recovery. To allow for central administrations, some rats were implanted with chronic guide cannulas aimed at the fourth brain ventricle as previously described (20). The animal was placed in a stereotaxic frame, and a local anesthetic (lidocaine-epinephrine, 10 mg/ml + 5 µg/ml; Astra Zeneca, Uppsala, Sweden) was injected in the skin and subcutaneous scalp tissue to provide postoperative pain relief. The skull was then exposed, and a chronic guide cannula (10.0 mm × 27 gauge) aimed at the fourth ventricle was implanted 2.6 mm anterior to the occipital crest in the midsagittal line via a drilled opening and secured with screws and dental acrylic, as previously described (20). The rats were weighed and gently handled daily during another postoperative recovery period of 10 days before experimental testing sessions began. Correct fourth intracerebroventricular cannula placements were verified by a functional test performed 1 wk after the stereotaxic surgery and 7 days before the first testing session. Fourth intracerebroventricular injections of 210 µg 5-thio-d-glucose (5-TG) were given, and a doubling in blood glucose from baseline 1 h after 5-TG treatment was regarded as a correct placement (8). Animals that did not respond to 5-TG were excluded from further participation. For 7 days following cannula placement testing, the animals were weighed and handled as part of the daily routine but did not undergo any other procedures.
Drugs
TSH (human, rat; Sigma-Aldrich, St. Louis, MO) and lithium chloride (LiCl; Sigma-Aldrich) were dissolved in saline. Saline was used as the vehicle. The dissolved TSH was aliquoted in smaller vials and frozen (−20°C); a fresh aliquot was defrosted and used on each experimental day, and any excess was discarded. Saccharin (Sigma-Aldrich) was dissolved in tap water (0.15 mol/l). Lithium chloride and saccharin solutions were freshly prepared on the testing day.
Drug Administrations
For fourth intracerebroventricular injections, a 32-gauge injection needle was inserted via the guide and into the fourth ventricle. During this procedure, the animals were gently held by hand. The injection needle was attached to a Gilmont microinjector via PE 20 tubing, and 3 µl of vehicle or doses of TSH were injected into the fourth ventricle over 1 min. To minimize any risk of back flush, the injector was left in place for 30 s. After this, it was removed and replaced with an obturator. In experiments 2 and 4, 0.4 ml of vehicle, or respective dose of TSH, was injected subcutaneously in the neck.
Measurement of Body Temperature and Analysis of Blood Samples
Rectal temperature was measured using a digital thermometer (Omron, Kyoto, Japan), which provides a temperature reading in 10 s or less. The accuracy (±0.1°C) is guaranteed by the vendor.
Tail blood samples were collected for analysis of blood glucose and hormones as outlined below. The blood samples were immediately centrifuged (2,500 revolutions/min, at +4°C), and plasma was aspirated, aliquoted, and frozen (−80°C) for later analyses of thyroid hormones and glucose concentrations.
Plasma glucose concentrations were analyzed using a Glucometer DEX (Bayer, Leverkusen, Germany). Total serum thyroxine (T4) was analyzed using radioimmunoassay. A commercial kit, T4 MAb (ICN Pharmaceuticals, Orangeburg, NY) containing a monoclonal primary antibody (sp:mouse) that specifically recognizes l-thyroxine was used. Serum tri-iodothyronine was analyzed using radioimmunoassay T3 solid-phase component system with an antibody (sp:rabbit) that specifically recognizes l-tri-iodothyronine (100%) with negligible cross reactivity to l-thyroxine (<0.18%) and to di-iodinated thyronine/thyrosine structures (<0.01%).
Experimental Design
Food intake tests (experiments 1 and 2) and gastric emptying test (experiment 4).
To avoid risks of carry-over effects by the drug, the intracerebroventricular experiments were carried out every third day, and the subcutaneous experiments were performed with a 5-day wash-out period between the two testing sessions. In experiments 1 and 2, the animals had free access to water at all times and free access to food until 1 h before lights off, when drug injections were delivered and preweighed food was placed in each respective food hopper. All testing was performed in the animal’s home cages, except for in experiment 4, which is described below.
Experiment 1 was designed to examine whether fourth intracerebroventricular administration of TSH affects consumption of a nutritive fluid. Fourth intracerebroventricular injections of TSH (0.6, 1.5, or 6.0 µg) or vehicle were given 30 min before testing and food presentation in a randomized, counterbalanced change-over design. The dose range was chosen with regard to a previous report of lateral intracerebroventricular administration (13). The rats were first trained to ingest sucrose (0.3 mol/l in water) from a drinking spout through a series of daily habituation training sessions and displayed a steady baseline sucrose intake volume for at least 3 consecutive days before the first testing day. Sucrose was given at lights off; after 30 min of access, the sucrose bottle was removed, the volume ingested was recorded, and the regular maintenance chow was returned to each of the cages.
To control for whether the effect of fourth intracerebroventricular TSH occurred at a central target and not attributable to a peripheral action, for example, via intracerebroventricular TSH crossing the brain-blood barrier and entering the blood, the effects of corresponding TSH doses given peripherally at similar time points were examined. A separate, naive group of rats was used and habituated for 3 days before the start of the experiment by receiving 0.4 ml saline subcutaneously in the neck before lights off and access to food. On the subsequent testing days, 0.6 and 6.0 µg TSH or vehicle was given subcutaneously (0.4 ml) in the neck as described 30 min before testing and sucrose access. The rats underwent each condition once, in a randomized, counterbalanced, change-over design.
Experiment 2 aimed at examining whether fourth intracerebroventricular TSH inhibits solid food intake in a dose-dependent manner and exploring the dose-time profile versus vehicle. TSH (0.06, 0.60, and 6.00 µg) or vehicle was injected into the fourth ventricle 30 min before food access. Each animal received each dose once in a random, balanced, change-over design. The preweighed food hoppers were returned to the cage at lights off. Food was weighed 2, 5, and 22 h after lights off and access to food. Any food spillage was collected on preweighed aluminum trays placed under each cage and changed at the same time as the food was weighed (2, 5, and 22 h). The remaining amounts of solid food in the food hoppers as well as the dried spillages were weighed for each time point, and food consumption was calculated with correction for spill.
To exclude the possibility that the hypophagia that attended fourth intracerebroventricular-injected TSH on solid food ingestion was due to a peripheral rather than a central action, a control experiment was performed. A naive group of animals was used. For 3 days preceding the start of experimental testing sessions, the animals were habituated by receiving an subcutaneous injection of saline (0.4 ml) in the neck 30 min before lights off and food access. On the testing day, 0.4 ml of 0.6 μg TSH or saline as vehicle was given in a balanced, crossover design 30 min before meal access and lights off. Recordings of solid food intake were taken at 2, 5, and 22 h after lights out, and any spillage was collected at the same time points. There was a 5-day period between the testing days during which no experimental treatments were given to allow for any carry-over drug effects to be washed out.
Experiment 3 was designed to investigate whether fourth intracerebroventricular TSH reduces nutrient intake by inducing illness and flavor avoidance conditioning rather than causing satiety. A standardized two-bottle intake test was used, and LiCl was used as the conditioning control (21). Three groups of rats (n = 8 per group) that had passed a 5-TG cannula placement test but that were otherwise naive to drug treatments were used. The animals were pretrained to consume all of their daily water during a scheduled 2-h access period beginning at 1:00 PM Water intakes during the first 30 min were measured, and daily training continued until the animals displayed a stable baseline water intake for 4 consecutive days. On the day of conditioning, the animals were given access to 0.15% sodium saccharin dissolved in drinking water (wt/vol) and allowed to drink for 30 min. After this, the following combinations of drugs (3 µl, fourth intracerebroventricular + 20 ml/kg body wt ip) were immediately administered to the different groups of animals: group A: 6 µg TSH + saline; group B: saline + saline; and group C: saline + LiCl (0.15 mol/l), respectively. For the remaining 90 min, the animals had access to drinking water before the bottles were removed from the respective cage. The next day, flavor avoidance conditioning was assessed by presenting one bottle of 0.15% sodium saccharin and one bottle of water, for a period of 30 min. The volumes of solutions consumed were measured, followed by another 90-min period of water access.
In experiment 4, the hypothesis that TSH injected into the fourth ventricle in close proximity to the previously proposed TSH receptor substrate in the DMX (3) affects gastric emptying was tested. The during-gastric-fill nutrient gastric emptying method was used (12) to sensibly detect any small changes in gastric emptying under conditions that simulate gastric emptying dynamics during ongoing ingestion of a meal (10–12, 19). Experimental procedures were carried out every third day between 2:00 PM and 4:30 PM. Each rat was tested at approximately the same individual time point on each test day. The animals were habituated to the testing procedure by a series of four daily consecutive training sessions, during which they underwent the entire testing protocol but were not given fourth intracerebroventricular injections. The gastric fistulas were opened 1 h before testing began, and any stomach contents were gently rinsed out with water per lavage. After this, the rat was placed in a Plexiglas testing box equipped with a wire mesh floor. Vehicle or TSH (0.6 or 6.0 µg) was administered fourth intracerebroventricular in a random, balanced, crossover design 30 min before the start of intragastric glucose delivery. Immediately before testing, the fistulas were again opened, and glucose (12.5%) was infused intragastrically via the fistula at 1 ml/min for 12 min using a syringe pump infusion system (Harvard Pump 22; Harvard Instruments, South Natick, MA). After this, the remaining intragastric content was promptly evacuated, and the volume of the aspirate was recorded before the stomach was rinsed with 5 ml distilled water to retrieve any solute remaining in the stomach after the initial evacuation. The fistulas were closed, and the animal was returned to its home cage thereafter. The glucose contents for each sample and the rinse returns were determined using spectrophotometry with a glucose oxidase kit (Trinder; Sigma-Aldrich). The absorbance of each respective sample was determined (λ = 505 nm) on a Bausch & Lomb Spectrophotometer (Rochester, NY). The volumes recovered, the gastric glucose concentration of the infusate, the primary sample, and the rinse return were used to calculate amount solute emptied, volume retained, and gastric secretion volume.
In experiment 5, our aim was to explore whether caudal brain stem TSH receptor activation could impact some markers for metabolic homeostasis. For this, we recorded rectal temperature, plasma levels of thyroid hormones, and/or plasma glucose after fourth intracerebroventricular TSH injections in the preprandial but not starved state. One reason for this was to avoid differences in feeding state, which may affect plasma levels of thyroid hormones per se. Thus the drug or vehicle was administered in the final 5 h of the light period. At this time, the food hoppers were removed, and rectal temperatures were measured. Immediately after this (time = 0), fourth intracerebroventricular injections (3 µl) of TSH (6 µg) or vehicle were given as described above, in a crossover design. At 2 and 5 h after drug administration, the rectal temperatures were measured again, and tail blood samples were quickly drawn (150 µl). After this, the lights were switched off, and the rats were given free access to chow. A third measurement of rectal temperature and a final blood sample were taken at 22 h. The second testing session was performed similarly but 5 days later to allow for any carry-over effects of the drug to wash out.
Data Evaluation
STATISTICA software was used for data analysis. The data for experiments 1 and 2 were analyzed with repeated-measures ANOVA for each time point, followed by Dunnett’s test where appropriate. Data for experiment 3 were analyzed by repeated-measures ANOVA followed by Tukey’s test. In experiment 4, the data were analyzed with repeated-measures ANOVA. In experiment 5, paired two-tailed t-tests were used for the evaluation of rectal temperature, hormone levels, and plasma glucose for each time point. Throughout the study, P < 0.05 was considered significant.
RESULTS
Experiment 1
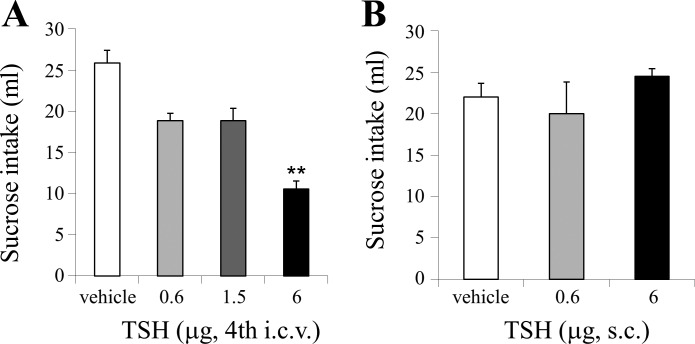
One-way repeated-measures ANOVA showed a significant main effect of fourth intracerebroventricular TSH treatment (F3,24 = 7.228, P < 0.01) on ingestion of sucrose during the 30-min test. Post hoc Dunnett’s test showed that there was a significant effect of 6 µg TSH versus vehicle but not of lower TSH doses, indicating that injection of TSH fourth intracerebroventricular produced an acute suppression of sucrose (0.3 mol/l) intake (P < 0.05; Fig. 1A). There was no main effect of subcutaneous TSH treatment (F2,10 = 0.9502, ns) on sucrose (0.3 mol/l) intake, indicating that the highest fourth intracerebroventricular TSH dose did not affect sucrose intake when provided via the peripheral route (Fig. 1B).
Fig. 1.
Dose effect of thyroid-stimulating hormone (TSH) injected in the fourth ventricle (intracerebroventricular, i.c.v.) (A) and subcutaneously (s.c.) (B), respectively, on 30-min sucrose (0.3 mol/l) intake in the rat (**P < 0.01).
Experiment 2
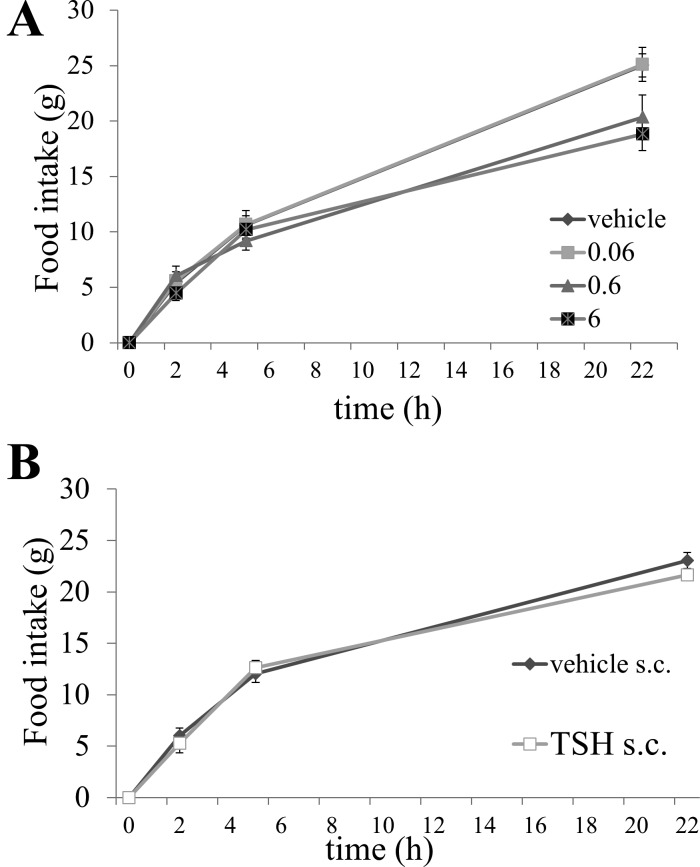
One-way repeated-measures ANOVA for each time point revealed a significant effect of TSH dose on solid food intake (F3,24 = 5.3006, P < 0.001) after 22 h but not at the 2-h (F3,24 = 0.9148, ns) or 5-h time points (F3,24 = 0.7480, ns). Post hoc Dunnett’s test showed a significant effect of 6 µg TSH compared with vehicle on solid food intake at 22 h (P < 0.01) (Fig. 2A). This indicates that, in contrast to results in experiment 1 (Fig. 1A), fourth intracerebroventricular TSH did not inhibit solid food intake in the early dark phase (0–5 h) but significantly reduced food intake at 22 h. There was no significant main effect of treatment on solid food intake after ssubcutaneous injection of TSH versus vehicle (F2,10 = 1.399, ns) (Fig. 2B).
Fig. 2.
A: dose effect of thyroid-stimulating hormone (TSH) injected in the fourth ventricle (intracerebroventricular, i.c.v.) on solid food intake in rats; 6 µg TSH vs. vehicle significantly suppressed food intake overnight (22 h) (P < 0.01). B: subcutaneous (s.c.) injection of corresponding TSH doses did not change food intake in any direction.
Experiment 3
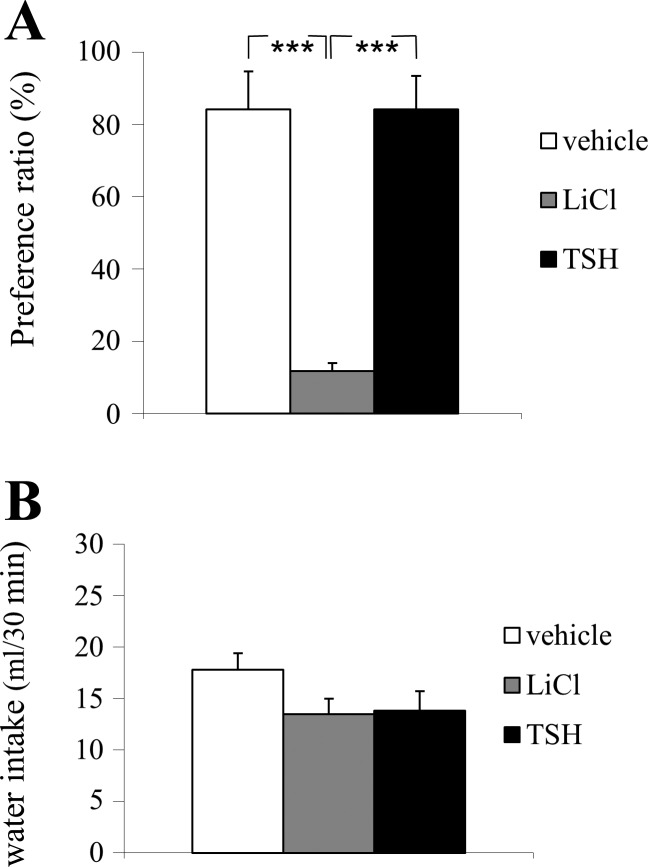
One-way ANOVA showed a main effect of treatment (F2,17 = 13.79, P < 0.0001). Post hoc Tukey’s test showed a significant effect of LiCl treatment versus vehicle on saccharin intake (P < 0.001), showing that LiCl treatment effectively induced flavor avoidance conditioning. LiCl suppressed saccharin intake significantly compared with TSH treatment (P < 0.01), but there was no effect by TSH treatment on saccharin drinking compared with vehicle (P > 0.05). One-way ANOVA showed no difference in total fluid intakes between groups (F2,17 = 3.962; P > 0.05; Fig. 3A), indicating that the observed effects by TSH or LiCl, respectively, were not due to differences in drinking per se.
Fig. 3.
A: injection of 6 µg thyroid-stimulating hormone (TSH) in the fourth ventricle (intracerebroventricular, i.c.v.) did not induce a conditioned flavor avoidance, whereas the positive control, treatment with LiCl did. B: TSH (6 µg, fourth i.c.v.) did not affect total water intake in experiment 4 (***P < 0.001).
Experiment 4
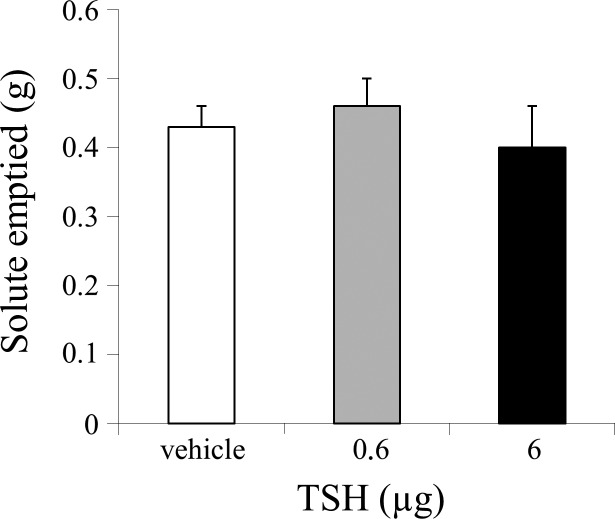
Repeated-measures ANOVA showed that there was no overall effect of treatment on either gastric emptying during gastric fill (F2,10 = 1,272, ns) (Fig. 4) or on gastric secretion volume (F2,10 = 0,998, ns), indicating that fourth intracerebroventricular application of TSH (6 µg) did not affect any of these paradigms compared with vehicle.
Fig. 4.
Injection of 6 µg thyroid-stimulating hormone (TSH) in the fourth ventricle (intracerebroventricular, i.c.v.) failed to affect gastric emptying of glucose during gastric fill.
Experiment 5
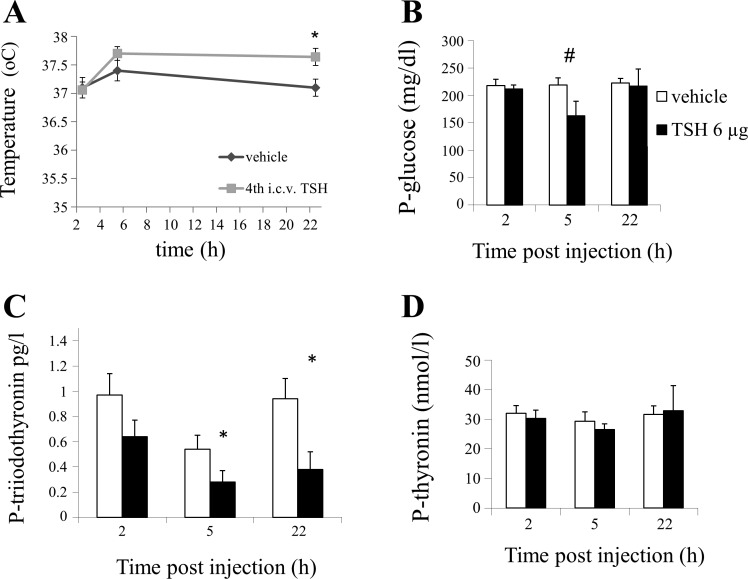
Comparisons were done between groups receiving fourth intracerebroventricular injections of TSH versus vehicle for each time point. At 22 h after TSH administration, the rectal temperature was increased in response to fourth intracerebroventricular TSH from 37.1°C to 37.6°C (P < 0.05, paired 2-tailed t-test) compared with vehicle (Fig. 5A). Plasma glucose concentrations were unchanged at 2 h and 22 h after fourth intracerebroventricular TSH. Five hours after fourth intracerebroventricular TSH administration, plasma glucose was on average lowered by 34% in the TSH-treated group from 219 to 163 mg/dl; this effect approached, but did not fully reach, statistical significance (P = 0.056; Fig. 5B). Plasma levels of thyroxine were unaffected by fourth intracerebroventricular TSH administration at all time points (P > 0.05, paired 2-tailed t-test), whereas plasma levels of tri-iodothyronin were decreased 5 and 22 h after TSH injection (P < 0.05).
Fig. 5.
A: injection of 6 µg thyroid-stimulating hormone (TSH) in the fourth ventricle (intracerebroventricular, i.c.v.) increased rectal temperature at 22 h. B: injection of 6 µg TSH in the fourth ventricle did not significantly change plasma glucose concentrations although a close to significant effect (#P = 0.056) was seen at 2 h after injection. C: after injection of 6 µg TSH into the fourth ventricle, plasma levels of tri-iodothyronine were significantly suppressed. D: plasma levels of thyroxine remained unaffected (*P < 0.05).
DISCUSSION
In this study, we explored potential caudal brain stem actions of TSH on food intake and metabolic measures in the rat. Our rationale was derived from our previous findings of the presence of functional TSH receptor protein in nuclei of the caudal brain stem (1), which are of key importance in autonomic function, including in the controls of food intake and gastric emptying.
A series of experiments were undertaken to delineate whether fourth intracerebroventricular administration of TSH inhibits food intake and whether such an effect is centrally mediated. Given the known role of peripheral TSH in governing thyroid function, we further explored the potential impact of caudal brain stem TSH receptor activation on some key markers for metabolism, including body temperature, plasma glucose levels, and thyroid hormone levels. Here, we demonstrate that administration of fourth intracerebroventricular TSH in the rat reduced intake of both a fluid nutrient (Fig. 1) and solid food (Fig. 2). As reflected by the lack of a TSH-induced conditioned flavor avoidance response (Fig. 3), the hypophagic effect of TSH appears to be by specific induction of satiety rather than by initiation of visceral illness.
We show here that, after fourth intracerebroventricular injection, TSH caused a reduction in sucrose intake, which was rapid in onset (Fig. 1A), as well as a reduction in solid food intake (Fig. 2A), which seemed more delayed. The fourth intracerebroventricular threshold dose was similar to what was previously reported after lateral intracerebroventricular injection (17), whereas there was no effect on food intake after peripheral administration of a corresponding dose (Figs. 1B and 2B, respectively). Considering that a dose subthreshold (0.6 µg) to the effective fourth intracerebroventricular dose given here was shown to cause a similar degree of hypophagia when nano-infused into the NTS (1), this suggests that the hypophagic response observed in our present feeding experiments (Figs. 1 and 2) was centrally elicited, likely involving a dorsal hindbrain receptor substrate target.
Reductions in nutritional intake can occur because of nausea or illness, rather than satiety. We therefore investigated whether TSH causes a conditioned flavor avoidance by a standardized, two-bottle test using lithium chloride as a positive control condition. Our results show clearly that TSH did not cause conditioned flavor avoidance and moreover did not affect total water intake (Fig. 3). The rapid onset in sucrose drinking inhibition seen after fourth intracerebroventricular TSH (Fig. 1) was thus likely not due to an increase in thirst. On the basis of these collective findings, we draw the conclusion that TSH at a dorsal hindbrain level inhibits food intake by inducing satiety and not by causing avoidance. To further explore underlying regulatory mechanisms for the caudal brain stem TSH hypophagia effect, we tested whether fourth intracerebroventricular TSH caused inhibition of gastric emptying. The rationale for this was first the detection of functional TSH receptors in the dorsal motor nucleus of the vagus (3), providing an anatomical basis for this possibility. Second, some compounds including cholecystokinin can act to reduce food intake by delayed gastric emptying (16). However, we found, as shown in Fig. 4, that fourth intracerebroventricularly administered TSH failed to affect gastric emptying. This suggests that the TSH-induced satiety occurs at a dorsal hindbrain level and independent of gastric cues. The lack of a gastric emptying effect does not, of course, exclude the possibility that TSH could still act in the DMX and, for example, impact gastric acid secretion or perhaps influence the release of gastrointestinal hormones.
In this study, the exogenous administration of TSH at a caudal brain stem level in close proximity to previously proposed TSH receptor subpopulations suppressed food intake. It is possible that the hypophagia that was shown to follow TSH administration reflects a mechanism that occurs as part of normal feeding control. Another and perhaps more plausible possibility is that this could be a mechanism of relevance in the response of the organism response to physical stress as seen in many states of illness. It has long been known that illnesses marked by physical stress and/or inflammatory onset often affect the thyroid axis, lowering plasma tri-iodothyronine, resulting in a state of secondary hypothyroidism (2–4, 13). The precise mechanisms underlying this syndrome, often referred to as nonthyroidal illness syndrome (NTIS) are not fully known, but central mechanisms are believed to be involved (15). As part of this study, we explored the effects of fourth intracerebroventricular delivery of TSH on plasma levels of thyroid hormones, tri-iodothyronine and thyroxine, because peripherally administered TSH is well known to stimulate release of thyroid hormones from the thyroid gland. Here, when injected into the fourth ventricle, in close proximity to the vagal complex TSH receptor substrate (3), TSH instead caused a phenomenon opposite to what would have been expected had the TSH been delivered by a peripheral route; it induced a decrease in plasma tri-iodothyronine (Fig. 5C). Plasma tri-iodothyronine levels are decreased in the fasted state (5, 14) in rodents and in humans. Here, however, the lowered plasma tri-iodothyronine levels were observed at the 5-h time point, just before food access, indicating that this occurred independent of any group differences in feeding state. Taken together, the decrease in tri-iodothyronine, in concert with increased body temperature and hypophagia induced by fourth intracerebroventricular TSH, much resembles the NTIS otherwise seen in a variety of severe illnesses and conditions featuring physical stress, such as inflammatory systemic disease and progressive cancer.
After fourth intracerebroventricular TSH delivery, body temperature was found to be slightly but significantly increased. One candidate caudal brain stem target site for TSH in initiating such a change in temperature could be the Raphe nuclei. The Raphe nuclei control temperature downstream of the anterior hypothalamus and are located in close proximity to the fourth ventricle such that they may be reached after fourth intracerebroventricular delivery. The temperature increase after fourth intracerebroventricular TSH injection found here contrasts to the decrease reported to occur after lateral intracerebroventricular administration (13). This difference may well be explained by different target sites, that the hyperthermia effect found here is most likely elicited at a dorsal hindbrain, rather than forebrain, level. It is not clear whether the lowered plasma tri-iodothyronine and the increase in temperature are linked or are parallel, independent phenomena. Although the absolute increase in temperature may be viewed as small, even such a small increase in heat production will result in an increase in energy demand. If such a combined hypophagic effect (Figs. 1A and 2A) and temperature increase (Fig. 5A), which occurred in response to fourth intracerebroventricular TSH, is prolonged, it may over time result in significant weight loss for an individual. Together, the possibility remains that TSH receptors at a dorsal hindbrain level play a role in the physiological changes in NTIS and/or cancer-associated anorexia-cachexia syndrome (17, 22).
Perspectives and Significance
In conclusion, caudal brain stem TSH receptor activation causes satiety independent of gastric cues, produces a moderate increase in body temperature, and lowers plasma levels of tri-iodothyronine. Whether this mechanism may be part of normal food intake control or perhaps is part of a central nervous system-triggered response to illness and physical stress remains to be established.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (19302), The Wenner-Gren Foundation, and the Assar Gabrielsson Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
U.S. and T.H.M. conceived and designed research; U.S. and K.A.S. performed experiments; U.S. and K.A.S. analyzed data; U.S. and T.H.M. interpreted results of experiments; U.S. prepared figures; U.S. drafted manuscript; U.S., K.A.S., and T.H.M. edited and revised manuscript; U.S., K.A.S., and T.H.M. approved final version of manuscript.
REFERENCES
- 1.Banks WA, Kastin AJ. Aluminium increases permeability of the blood-brain barrier to labelled DSIP and β-endorphin: possible implications for senile and dialysis dementia. Lancet 2: 1227–1229, 1983. doi: 10.1016/S0140-6736(83)91273-4. [DOI] [PubMed] [Google Scholar]
- 2.Bello G, Ceaichisciuc I, Silva S, Antonelli M. The role of thyroid dysfunction in the critically ill: a review of the literature. Minerva Anestesiol 76: 919–928, 2010. [PubMed] [Google Scholar]
- 3.Burgos JR, Iresjö BM, Wärnåker S, Smedh U. Presence of TSH receptors in discrete areas of the hypothalamus and caudal brainstem with relevance for feeding controls-Support for functional significance. Brain Res 1642: 278–286, 2016. doi: 10.1016/j.brainres.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Castro I, Quisenberry L, Calvo RM, Obregon MJ, Lado-Abeal J. Septic shock non-thyroidal illness syndrome causes hypothyroidism and conditions for reduced sensitivity to thyroid hormone. J Mol Endocrinol 50: 255–266, 2013. doi: 10.1530/JME-12-0188. [DOI] [PubMed] [Google Scholar]
- 5.Chopra IJ, Carlson HE, Solomon DH. Comparison of inhibitory effects of 3,5,3′-triiodothyronine (T3), thyroxine (T4), 3,3,',5′-triiodothyronine (rT3), and 3,3′-diiodothyronine (T2) on thyrotropin-releasing hormone-induced release of thyrotropin in the rat in vitro. Endocrinology 103: 393–402, 1978. doi: 10.1210/endo-103-2-393. [DOI] [PubMed] [Google Scholar]
- 6.Chopra IJ, Chopra U, Smith SR, Reza M, Solomon DH. Reciprocal changes in serum concentrations of 3,3′,5′-triiodothyronine (reverse T3) and 3,3′5-triiodothyronine (T3) in systemic illnesses. J Clin Endocrinol Metab 41: 1043–1049, 1975. doi: 10.1210/jcem-41-6-1043. [DOI] [PubMed] [Google Scholar]
- 7.DeVito WJ, Spearman TN, Connors JM, Hedge GA. Subcellular localization of immunoreactive thyroid-stimulating hormone in the rat hypothalamus. Neuroendocrinology 42: 459–466, 1986. doi: 10.1159/000124488. [DOI] [PubMed] [Google Scholar]
- 8.Flynn FW, Grill HJ. Fourth ventricular phlorizin dissociates feeding from hyperglycemia in rats. Brain Res 341: 331–336, 1985. doi: 10.1016/0006-8993(85)91072-8. [DOI] [PubMed] [Google Scholar]
- 9.Hojvat S, Anderson J, Nishimura N, Baker G, Kirsteins L, Lawrence AM. Immunoreactive thyroid stimulating hormone (TSH)(: association with synaptosomally-rich fractions in the rat hypothalamus. Brain Res 265: 259–263, 1983. doi: 10.1016/0006-8993(83)90340-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan JM, Siemers W, Grill HJ. Effect of oral versus gastric delivery on gastric emptying of corn oil emulsions. Am J Physiol Regul Integr Comp Physiol 273: R1263–R1270, 1997. doi: 10.1152/ajpregu.1997.273.4.R1263. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan JM, Siemers W, Grill HJ. Ingestion, gastric fill, and gastric emptying before and after withdrawal of gastric contents. Am J Physiol Regul Integr Comp Physiol 267: R1257–R1265, 1994. doi: 10.1152/ajpregu.1994.267.5.R1257. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan JM, Spector AC, Grill HJ. Dynamics of gastric emptying during and after stomach fill. Am J Physiol Regul Integr Comp Physiol 263: R813–R819, 1992. doi: 10.1152/ajpregu.1992.263.4.R813. [DOI] [PubMed] [Google Scholar]
- 13.Lin MT, Chu PC, Leu SY. Effects of TSH, TRH, LH and LHRH on thermoregulation and food and water intake in the rat. Neuroendocrinology 37: 206–211, 1983. doi: 10.1159/000123544. [DOI] [PubMed] [Google Scholar]
- 14.Merimee TJ, Fineberg ES. Starvation-induced alterations of circulating thyroid hormone concentrations in man. Metabolism 25: 79–83, 1976. doi: 10.1016/0026-0495(76)90162-1. [DOI] [PubMed] [Google Scholar]
- 15.Mönig H, Arendt T, Meyer M, Kloehn S, Bewig B. Activation of the hypothalamo-pituitary-adrenal axis in response to septic or non-septic diseases–implications for the euthyroid sick syndrome. Intensive Care Med 25: 1402–1406, 1999. doi: 10.1007/s001340051088. [DOI] [PubMed] [Google Scholar]
- 16.Moran TH, McHugh PR. Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am J Physiol Regul Integr Comp Physiol 242: R491–R497, 1982. doi: 10.1152/ajpregu.1982.242.5.R491. [DOI] [PubMed] [Google Scholar]
- 17.Persson H, Bennegård K, Lundberg PA, Svaninger G, Lundholm K. Thyroid hormones in conditions of chronic malnutrition. A study with special reference to cancer cachexia. Ann Surg 201: 45–52, 1985. [PMC free article] [PubMed] [Google Scholar]
- 18.Rubenfeld S. Euthyroid sick syndrome. N Engl J Med 299: 1414, 1978. doi: 10.1056/NEJM197812212992514. [DOI] [PubMed] [Google Scholar]
- 19.Smedh U, Moran TH. The dorsal vagal complex as a site for cocaine- and amphetamine-regulated transcript peptide to suppress gastric emptying. Am J Physiol Regul Integr Comp Physiol 291: R124–R130, 2006. doi: 10.1152/ajpregu.00234.2004. [DOI] [PubMed] [Google Scholar]
- 20.Smedh U, Uvnäs-Moberg K, Grill HJ, Kaplan JM. Fourth ventricle injection of corticotropin-releasing factor and gastric emptying of glucose during gastric fill. Am J Physiol Gastrointest Liver Physiol 269: G1000–G1003, 1995. doi: 10.1152/ajpgi.1995.269.6.G1000. [DOI] [PubMed] [Google Scholar]
- 21.Smith DF, Balagura S. Role of oropharyngeal factors in LiCl aversion. J Comp Physiol Psychol 69: 308–310, 1969. doi: 10.1037/h0028228. [DOI] [PubMed] [Google Scholar]
- 22.Svaninger G, Lundberg PA, Lundholm K. Thyroid hormones and experimental cancer cachexia. J Natl Cancer Inst 77: 555–561, 1986. [PubMed] [Google Scholar]