Abstract
Intermittent spinal serotonin receptor activation elicits phrenic motor facilitation (pMF), a form of spinal respiratory motor plasticity. Episodic activation of either serotonin type 2 (5-HT2) or type 7 (5-HT7) receptors elicits pMF, although they do so via distinct cellular mechanisms known as the Q (5-HT2) and S (5-HT7) pathways to pMF. When coactivated, these pathways interact via mutual cross-talk inhibition. Although we have a rudimentary understanding of mechanisms mediating cross-talk interactions between spinal 5-HT2 subtype A (5-HT2A) and 5-HT7 receptor activation, we do not know if similar interactions exist between 5-HT2 subtype B (5-HT2B) and 5-HT7 receptors. We confirmed that either spinal 5-HT2B or 5-HT7 receptor activation alone elicits pMF and tested the hypotheses that 1) concurrent activation of both receptors suppresses pMF due to cross-talk inhibition; 2) 5-HT7 receptor inhibition of 5-HT2B receptor-induced pMF requires protein kinase A (PKA) activity; and 3) 5-HT2B receptor inhibition of 5-HT7 receptor-induced pMF requires NADPH oxidase (NOX) activity. Selective 5-HT2B and 5-HT7 receptor agonists were administered intrathecally at C4 (3 injections, 5-min intervals) to anesthetized, paralyzed, and ventilated rats. Whereas integrated phrenic nerve burst amplitude increased after selective spinal 5-HT2B or 5-HT7 receptor activation alone (i.e., pMF), pMF was no longer observed with concurrent 5-HT2B and 5-HT7 receptor agonist administration. With concurrent receptor activation, pMF was rescued by inhibiting either NOX or PKA activity, demonstrating their roles in cross-talk inhibition between these pathways to pMF. This report demonstrates cross-talk inhibition between 5-HT2B- and 5-HT7 receptor-induced pMF and that NOX and PKA activity are necessary for that cross-talk inhibition.
Keywords: intermittent hypoxia, long-term facilitation, NADPH oxidase, protein kinase A, respiratory plasticity
INTRODUCTION
Plasticity is a hallmark of the neural system controlling breathing. One well-studied model of respiratory motor plasticity is phrenic long-term facilitation (pLTF), a prolonged increase in phrenic nerve activity lasting hours after brief exposure to moderate acute intermittent hypoxia (AIH) (2, 26, 28). Episodic spinal serotonin 2 receptor (5-HT2) activation is necessary and sufficient for phrenic motor facilitation (pMF, a more general term that includes pLTF); since 5-HT2 receptors are coupled to Gq proteins, and multiple Gq protein-coupled receptors elicit similar pMF, this mechanism is referred to as the “Q pathway to pMF” (5). Episodic spinal serotonin 7 receptor (5-HT7) activation also elicits pMF but through a distinct cellular cascade; since 5-HT7 receptors are Gs protein coupled, and multiple Gs protein-coupled receptors elicit similar pMF, this mechanism is referred to as the “S pathway” (5).
When the Q and S pathways are activated concurrently, pMF is attenuated or even abolished (9, 10, 12, 18, 20). Thus, the Q and S pathways interact via mutual cross-talk inhibition. Although we have learned a great deal concerning cellular mechanisms giving rise to the Q and S pathways (7–9, 14), little is known concerning the mechanisms of mutual cross-talk inhibition.
Initial insights concerning Q and S pathway interactions came from studies of the bell-shaped pMF dose-response curve to episodic spinal serotonin administration (23); low serotonin doses elicit robust pMF, whereas pMF is no longer observed at high serotonin doses unless spinal 5-HT7 receptors are blocked (23). Similarly, spinal 5-HT7 receptor inhibition enhances 5-HT2 receptor-dependent pLTF following moderate AIH (20). Protein kinase A (PKA) activity is necessary and sufficient for 5-HT7 receptor inhibition of 5-HT2 receptor-dependent pMF, since spinal PKA inhibition enhances, and PKA activation suppresses, 5-HT2 receptor-dependent pLTF following moderate AIH (20) and spinal 5-HT2A receptor agonist-induced pMF (12).
There is little evidence concerning sources of inhibition from 5-HT2 receptors to the 5-HT7 receptor-induced S pathway. NOX inhibition blocks 5-HT2B, but not 5-HT2A, receptor-induced pMF (25); this unique sensitivity of 5-HT2B-induced pMF to NOX activity suggests that it may operate via some Q pathway variant with differential sensitivity to reactive oxygen species (ROS). NOX activity generates ROS, particularly superoxide anions that are rapidly converted to hydrogen peroxide (29). Hydrogen peroxide depresses adenyl cyclase and cAMP signaling in heart tissue (16, 17); since cAMP signaling is essential for 5-HT7 receptor-induced pMF, NOX activity could inhibit the S pathway (14). Since exchange protein activated by cAMP (EPAC) activity is necessary and sufficient for 5-HT7 receptor-induced pMF (14), we hypothesize that 5-HT2B receptor activation inhibits the S pathway to pMF and that it does so via NOX-dependent mechanisms.
The fundamental goals of this study were to test the hypotheses that 1) concurrent spinal 5-HT2B and 5-HT7 receptor activation cancels pMF due to cross-talk inhibition; 2) 5-HT2B receptors inhibit 5-HT7 receptor-induced pMF by a mechanism that requires spinal NOX activity; and 3) spinal PKA activity is necessary for 5-HT7 receptor inhibition of 5-HT2B receptor-induced pMF. We confirm that 5-HT2B and 5-HT7 receptors both elicit pMF, but concurrent 5-HT2B and 5-HT7 receptor activation undermines pMF. Furthermore, cross-talk interactions between 5-HT2B and 5-HT7 receptors require NOX and PKA, reflecting 5-HT2B to 5-HT7 and 5-HT7 to 5-HT2B interactions, respectively. The functional significance of these complex cross-talk interactions is not yet fully understood, but they may be critical for emergent properties of phrenic motor plasticity, such as pattern sensitivity and metaplasticity (13). Greater understanding of these complex interactions will accelerate progress in our attempts to harness “low-dose” intermittent hypoxia as a treatment for devastating clinical disorders that compromise breathing and nonrespiratory movements (6, 13, 30).
METHODS
Animals.
Experiments were conducted on adult (300–400 g) male Sprague-Dawley rats (208A Colony, Envigo, IN) maintained on a 12:12-h light-dark cycle with access to food and water ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Florida (protocol no. 201408657).
Neurophysiological experiments.
Rats were prepared as described previously (14, 20). Rats were anesthetized with 3% isoflurane in a closed chamber and transferred to a heated surgical table where anesthesia was maintained (3% in 60% O2, balanced with N2) through a nose cone. A rectal thermometer (Fisher Scientific) was used to measure body temperature, maintained within 37.5 ± 1°C. Rats were tracheotomized, mechanically ventilated (~70 breaths/min, tidal volume ~2.5 ml; Rodent Ventilator, model 683, Harvard Apparatus), and bilaterally vagotomized through a ventral midline incision to prevent entrainment of respiratory neural activity with the ventilator. Rats were then slowly converted to urethane anesthesia (2.1 g/kg iv) while being weaned from isoflurane (over 20 min). Adequacy of anesthesia was assessed as a lack of pressor or respiratory neural responses to a toe pinch with a hemostat. Once rats were converted to urethane, the neuromuscular paralytic pancuronium bromide was delivered over 3 min (3 mg/kg iv; Sigma-Aldrich, St. Louis, MO) before we determined the CO2 apneic/recruitment thresholds. An intravenous 4:1 mixture of lactated Ringer solution and 8.4% sodium bicarbonate was infused (1–4 ml/h) though a tail vein catheter to maintain the standard base excess (sBE) within ± 3 meq/l. After conversion to urethane anesthesia, at least 1 h was allowed for stabilization before experiments commenced.
A polyethylene catheter (PE50, ID: 0.58 mm, OD: 0.965 mm, Intramedic) was inserted into the right femoral artery to monitor blood pressure (baseline mean arterial pressure: 90–150 mmHg, ≤30 mmHg change at the end of the experiment; Argon Pressure Transducer, DTXPlus) and blood gases (ABL 90 Flex, Radiometer). End-tidal CO2 was monitored throughout experiments using a flow-through capnogard with sufficient response time to detect end-tidal CO2 levels in rats (Novametrix).
Muscles overlying the cervical spinal cord were separated to give access to C1–C2 cervical vertebrae. The spinal cord was exposed via dorsal C2 laminectomy and durotomy to enable intrathecal drug administration. Then a silicone catheter (OD 0.6 mm; Access Technologies; primed with drug or vehicle) was inserted through a small hole in the dura and advanced caudally, resting just over the spinal C3 region. This catheter was not placed until right before starting the experiments so as to minimize unintended drug diffusion from the catheter.
The left phrenic and hypoglossal nerves were dissected via a dorsal approach, cut distally, desheathed, and covered with a saline-soaked cotton ball to prevent desiccation. Nerve activities were recorded using bipolar glass suction electrodes held in micromanipulators (Narishige, Tokyo, Japan). Signals were amplified (10,000×), band-pass filtered (0.3–5 kHz) and acquired with an A/D converter (CED 1401; Cambridge Electronic Design, Cambridge, UK) to a computer using Spike 2 software (version 8.08). Nerve activities were recorded in absolute units (V) and analyses performed offline in rectified and smoothed signals.
The apneic threshold was determined by progressively lowering inspired CO2 until respiratory nerve activity ceased for 60 s. The recruitment threshold was then determined by slowly raising inspired CO2 until rhythmic nerve activity resumed. During baseline, end-tidal CO2 was maintained ~2 mmHg above recruitment threshold as before (1). Arterial blood samples were drawn using heparinized capillaries (70 μl) to monitor blood gases at baseline and 30, 60, and 90 min postintrathecal drug administration. Arterial O2 pressure () was maintained above 150 mmHg; arterial CO2 pressure () was maintained within 1.5 mmHg of baseline values by adjusting the inspired CO2 fraction. , , sBE, and mean arterial pressure were maintained within specified ranges during the experiments (Tables 1, 2, and 3). At the end of the experiments, rats were euthanized with an intravenous urethane bolus.
Table 1.
Physiological variables at baseline and 30-, 60-, and 90-min postintrathecal drug injections of vehicle, 5-HT2B, 5-HT7, or 5-HT2B and 5-HT7 receptor agonists
| Time, min | Vehicle | 5-HT2B | 5-HT7 | 5-HT2B + 5-HT7 | |
|---|---|---|---|---|---|
| , mmHg | Baseline | 45.0 ± 0.5 | 48.2 ± 1.5 | 45.7 ± 1.5 | 45.3 ± 0.9 |
| 30 | 45.3 ± 0.8 | 48.6 ± 1.4 | 46.5 ± 1.7 | 45.9 ± 1.2 | |
| 60 | 45.5 ± 0.5 | 49.1 ± 1.6 | 46.2 ± 1.6 | 45.5 ± 1.1 | |
| 90 | 45.0 ± 0.7 | 48.3 ± 1.6 | 45.9 ± 1.5 | 45.3 ± 1 | |
| , mmHg | Baseline | 303 ± 13 | 284 ± 20 | 262 ± 18 | 319 ± 15 |
| 30 | 291 ± 12 | 277 ± 25 | 264 ± 18 | 306 ± 20 | |
| 60 | 292 ± 9 | 273 ± 25 | 258 ± 22 | 297 ± 21 | |
| 90 | 288 ± 9 | 262 ± 19 | 263 ± 17 | 293 ± 27 | |
| sBE, mmol/l | Baseline | 0.4 ± 0.5 | 0.8 ± 0.7 | −0.4 ± 0.6 | −0.7 ± 0.6 |
| 30 | −0.2 ± 0.5 | 0.7 ± 0.5 | −0.2 ± 0.6 | −0.3 ± 0.7 | |
| 60 | 0.7 ± 0.5 | 0.2 ± 0.6 | −0.1 ± 0.7 | −0.5 ± 0.4 | |
| 90 | −0.6 ± 0.4 | −0.2 ± 0.4 | 0.4 ± 0.8 | −0.6 ± 0.5 | |
| MAP, mmHg | Baseline | 131 ± 6 | 128 ± 7 | 136 ± 7 | 114 ± 5 |
| 30 | 130 ± 7 | 123 ± 7 | 138 ± 7 | 104 ± 3 | |
| 60 | 126 ± 7 | 123 ± 8 | 137 ± 6 | 98 ± 3 | |
| 90 | 124 ± 5 | 118 ± 8 | 132 ± 7 | 94 ± 3 |
Values are means ± SE. , arterial O2 pressure; , arterial CO2 pressure; 5-HT2B, serotonin 2 receptor; 5-HT7, serotonin 7 receptor; sBE, standard excess base; MAP, mean arterial pressure.
Table 2.
Physiological variables at baseline and 30-, 60-, and 90-min postintrathecal drug injections of vehicle, 5-HT2B, 5-HT7, or 5-HT2B and 5-HT7 receptor agonists in rats pretreated with apocynin
| Apocynin |
|||||
|---|---|---|---|---|---|
| Time, min | Vehicle | 5-HT2B | 5-HT7 | 5-HT2B + 5-HT7 | |
| , mmHg | Baseline | 45.7 ± 1.1 | 45.8 ± 1.6 | 43.3 ± 0.9 | 45.3 ± 1.3 |
| 30 | 45.0 ± 1.0 | 46.2 ± 1.4 | 43.2 ± 1.2 | 45.9 ± 1.4 | |
| 60 | 45.6 ± 1.2 | 45.4 ± 1.6 | 43.4 ± 1.3 | 45.4 ± 1.8 | |
| 90 | 45.6 ± 0.9 | 45.6 ± 1.6 | 43.4 ± 1.4 | 44.9 ± 1.2 | |
| , mmHg | Baseline | 326 ± 11 | 314 ± 16 | 307 ± 7 | 285 ± 21 |
| 30 | 338 ± 6 | 299 ± 10 | 305 ± 13 | 306 ± 15 | |
| 60 | 336 ± 8 | 287 ± 6 | 291 ± 15 | 302 ± 11 | |
| 90 | 336 ± 11 | 283 ± 10 | 295 ± 18 | 294 ± 19 | |
| sBE, mmol/l | Baseline | 0.6 ± 0.4 | 1.9 ± 0.4 | 0.7 ± 0.1 | 0.5 ± 0.6 |
| 30 | 1.1 ± 0.6 | 1.3 ± 0.3 | 1.4 ± 0.5 | 0.8 ± 0.6 | |
| 60 | 1.1 ± 0.5 | 0.4 ± 0.3 | 1.0 ± 0.7 | 0.4 ± 0.5 | |
| 90 | 0.9 ± 0.6 | 0.5 ± 0.6 | 1.4 ± 0.5 | 0.2 ± 0.4 | |
| MAP,mmHg | Baseline | 125 ± 7 | 136 ± 6 | 103 ± 11 | 116 ± 6 |
| 30 | 117 ± 6 | 138 ± 7 | 105 ± 11 | 116 ± 7 | |
| 60 | 117 ± 6 | 134 ± 7 | 107 ± 9 | 111 ± 7 | |
| 90 | 115 ± 6 | 131 ± 7 | 110 ± 6 | 109 ± 8 | |
Values are means ± SE. , arterial O2 pressure; , arterial CO2 pressure; 5-HT2B, serotonin 2 receptor; 5-HT7, serotonin 7 receptor; sBE, standard excess base; MAP, mean arterial pressure.
Table 3.
Physiological variables at baseline and 30-, 60-, and 90 -min postintrathecal drug injections of vehicle, 5-HT2B, 5-HT7, or 5-HT2B and 5-HT7 receptor agonists in rats pretreated with PKAi
| PKAi |
|||||
|---|---|---|---|---|---|
| Time, min | Vehicle | 5-HT2B | 5-HT7 | 5-HT2B + 5-HT7 | |
| , mmHg | Baseline | 43.8 ± 0.8 | 44.3 ± 1.2 | 42.2 ± 1.3 | 44.9 ± 0.9 |
| 30 | 44.2 ± 0.6 | 43.6 ± 0.9 | 42.2 ± 1.2 | 45.0 ± 1.1 | |
| 60 | 44.2 ± 0.7 | 44.2 ± 0.6 | 42.7 ± 1.0 | 45.4 ± 0.9 | |
| 90 | 44.0 ± 0.8 | 43.9 ± 0.5 | 42.4 ± 1.0 | 44.7 ± 1.0 | |
| , mmHg | Baseline | 331 ± 6 | 312 ± 26 | 323 ± 11 | 320 ± 7 |
| 30 | 314 ± 21 | 297 ± 23 | 321 ± 7 | 320 ± 8 | |
| 60 | 323 ± 12 | 281 ± 21 | 309 ± 9 | 319 ± 6 | |
| 90 | 309 ± 11 | 264 ± 31 | 303 ± 10 | 314 ± 6 | |
| sBE, mmol/l | Baseline | 0.7 ± 0.5 | 1.4 ± 0.6 | 1.0 ± 0.5 | 0.5 ± 0.6 |
| 30 | 1.2 ± 0.6 | −0.2 ± 1.3 | 1.9 ± 0.7 | 0.2 ± 0.6 | |
| 60 | 0.8 ± 0.5 | 0.1 ± 1.0 | 1.7 ± 0.6 | 0.8 ± 0.7 | |
| 90 | 0.9 ± 0.5 | 0.5 ± 1.0 | 1.8 ± 0.5 | 0.4 ± 0.6 | |
| MAP, mmHg | Baseline | 127 ± 6 | 134 ± 11 | 129 ± 7 | 124 ± 4 |
| 30 | 121 ± 7 | 131 ± 19 | 118 ± 13 | 116 ± 5 | |
| 60 | 121 ± 7 | 120 ± 15 | 119 ± 17 | 115 ± 5 | |
| 90 | 113 ± 4 | 119 ± 14 | 117 ± 19 | 119 ± 6 | |
Values are means ± SE. PKAi, protein kinase A inhibitor , arterial O2 pressure; , arterial CO2 pressure; 5-HT2B, serotonin 2 receptor; 5-HT7, serotonin 7 receptor; sBE, standard excess base; MAP, mean arterial pressure.
Drug administration.
BW 723C86 hydrochloride (5-HT2B receptor agonist) and AS-19 (5-HT7 receptor agonist) were obtained from Tocris Biosciences; Apocynin (NOX inhibitor) was obtained from Sigma-Aldrich, and Rp-8-Br-cAMPS (PKA inhibitor) was obtained from Santa Cruz Biotechnology. All drugs were initially dissolved in 100% dimethyl sulfoxide (DMSO) and the aliquots of stock solution stored frozen at −20°C. On experimental days, stock solutions were thawed and diluted in sterile saline to their final concentration with a DMSO concentration between 0 and 10%. Intrathecal drug doses were chosen based on prior studies from our laboratory, using the minimal dose required to induce consistent pMF (20, 25).
Selectivity of 5-HT2B (3 × 6 µl,1 mM) and 5-HT7 receptor agonists (3 × 6 µl,10 µM) was well documented in vivo. 5-HT2B-induced pMF is not affected by the 5-HT-2A/2C receptor antagonist ketanserin (1 × 15 µl, 500 µM) but is blocked by a selective 5-HT2B receptor antagonist (1 × 15 µl, 300 µM). On the other hand, 5-HT7-induced pMF remains in the absence of new brain-derived neurotrophic factor (BDNF) synthesis using siRNAs targeting BDNF mRNA, and with MEK/ERK inhibition (19); however, it is blocked by a selective 5-HT7 receptor antagonist (SB269970; 1 × 7 µl, 5 mM) (19). 5-HT2B and 5-HT7 receptors are expressed in the hypoglossal motor nucleus, and their activation increases hypoglossal nerve activity (15, 34). Thus, we assessed hypoglossal nerve activity and burst frequency as internal controls for unintended brain stem drug distribution following intrathecal cervical spinal injections, as done previously (3).
Protocols.
To evaluate the effect of simultaneous 5-HT2B and 5-HT7 receptor activation on pMF, rats were treated with three episodic intrathecal injections (6 μl each, 5-min intervals) of the selective 5-HT2B (1 mM) and 5-HT7 (10 μM) receptor agonists. In separate rat groups, 5-HT2B or 5-HT7 receptors were activated alone to confirm that the drugs and doses used induce pMF.
To evaluate the mechanisms of cross-talk inhibition, rats were pretreated with either the intrathecal NOX (12 μl apocynin, 600 μM) or PKA inhibitors (10 μl PKAi, 1 mM). Twenty minutes after drug injections, rats were treated with both 5-HT2B and 5-HT7 receptor agonists through a second intrathecal catheter. Vehicle control rats were treated similarly, but with intrathecal injections of sterile saline with the same DMSO concentration used to dilute the drugs.
Data analyses.
The assumption of normally distributed data was confirmed by visual inspection of histograms and normal probability plots. A mixed, two-way ANOVA with a repeated-measures design for time was used to test the null hypothesis that the dependent variables (phrenic and hypoglossal nerve activity and respiratory frequency) did not differ between and within groups. When appropriate, individual comparisons were made using Tukey's post hoc tests. Data were expressed as means ± SE; differences were considered significant at P < 0.05. Statistical analyses were performed using Sigma Plot (version 13).
RESULTS
5-HT2B and 5-HT7 receptor activation each elicits pMF, but concurrent activation cancels pMF.
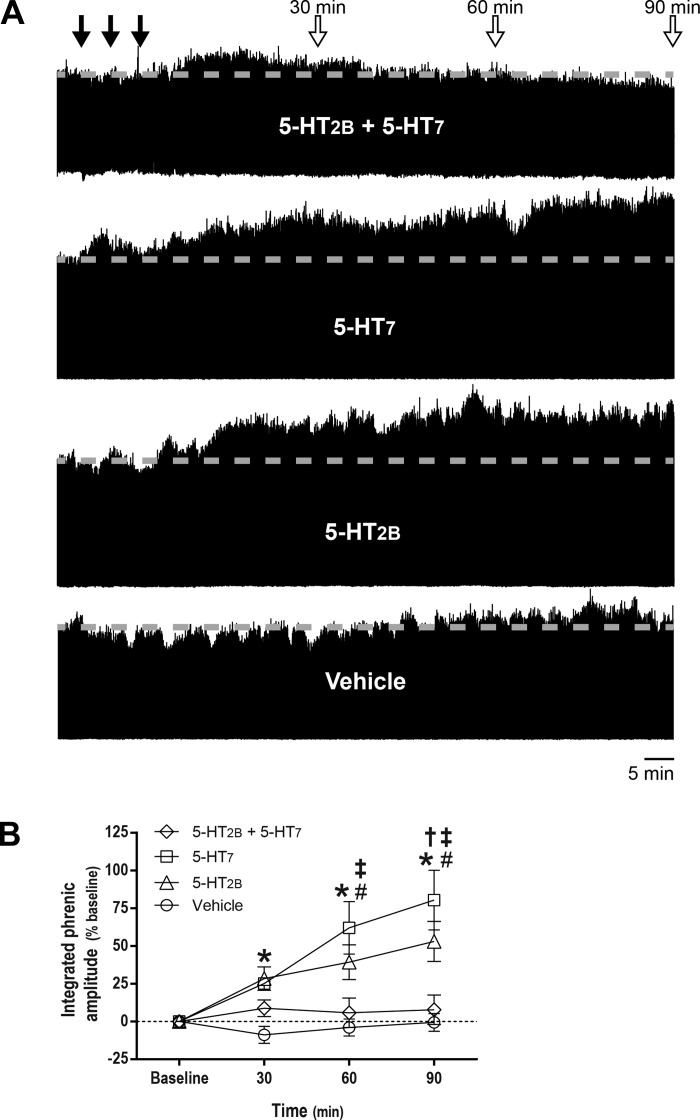
Figure 1A shows time-compressed neurograms illustrating integrated phrenic nerve burst amplitude at baseline and following intrathecal serotonin receptor agonist injections. Integrated phrenic nerve burst amplitude increased significantly from baseline at 30 (28 ± 8%, P = 0.032), 60 (39 ± 12%, P = 0.001), and 90 min (55 ± 13%, P < 0.001) after episodic 5-HT2B receptor activation; similar pMF was observed at 60 (62 ± 18%, P < 0.001) and 90 min (80 ± 20%, P < 0.001) after episodic 5-HT7 receptor activation. However, when 5-HT2B and 5-HT7 receptors were activated concurrently, there was no longer a postinjection increase in integrated phrenic nerve burst amplitude (P > 0.05; Fig. 1B). Furthermore, there were no time-dependent changes in phrenic nerve burst amplitude in vehicle-treated rats (P > 0.05).
Fig. 1.
Although serotonin 2B receptor (5-HT2B) and serotonin 7 (5-HT7) receptor activation elicit phrenic motor facilitation (pMF), concurrent activation blocks pMF. A: representative traces of compressed, integrated phrenic nerve burst amplitude at baseline and during and after intrathecal episodic drug injections. Gray dashed line in each trace marks baseline amplitude of phrenic nerve activity. Top closed arrows depict episodic intrathecal drug injections; top open arrows indicate 30-, 60-, and 90-min blood samples. B: average (± SE) values from baseline to 90 min after episodic intrathecal drug injections (expressed as %baseline). Lines are from rats treated with 5-HT2B receptor agonist (BW 723C86 hydrochloride, 1 mM, 3 × 6 μl, n = 8), 5-HT7 receptor agonist (AS-19; 10 μM; 3 × 6 μl; n = 8), 5-HT2B and 5-HT7 receptor agonists (3 × 6 μl; n = 8), and vehicle (3 × 6 μl; n = 7). Integrated phrenic nerve burst amplitude was significantly different from baseline at 30, 60, and 90 min (*) post-5-HT2B receptor activation and at 60 and 90 min post-5-HT7 receptor activation (#). At 90 min post-5-HT2B receptor activation (†), and at 60 and 90 min post-5-HT7 receptor activation (‡), integrated phrenic nerve burst amplitude was significantly higher than in rats treated with combined 5-HT2B and 5-HT7 receptor agonist. Mixed two-way ANOVA with repeated measures for time was used to detect overall differences between and within groups (interaction between time and groups was included in the model; F, 5.610; P < 0.001; df, 9), followed by Tukey post hoc tests.
Intrathecal drug injections are restricted to the spinal cord.
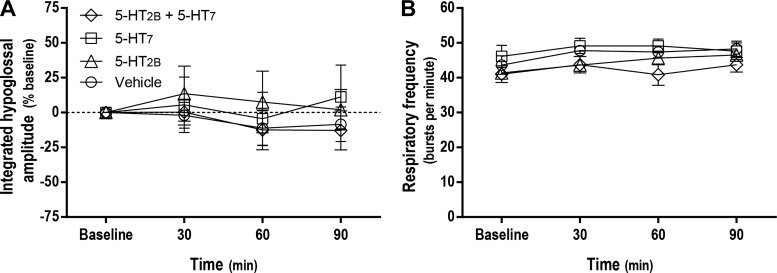
Episodic intrathecal injections of 5-HT2B, 5-HT7, or combined 5-HT2B and 5-HT7 receptor agonists had no effects on 1) hypoglossal nerve activity at any time postinjection (P > 0.05; Fig. 2A), or 2) respiratory frequency during any condition (P > 0.05; Fig. 2B). Thus, drugs did not reach the brain stem at effective concentrations; their effects appeared restricted to the spinal cord as in previous studies (3).
Fig. 2.
Intrathecal drug injections failed to elicit changes in hypoglossal nerve activity or phrenic burst frequency, demonstrating that effective drug concentrations were restricted to the spinal cord. Average (± SE) values from baseline to 90 min postepisodic intrathecal drug injections are shown (expressed as %baseline). Lines are from rats treated with serotonin 2B receptor (5-HT2B) agonist (BW 723C86 hydrochloride; 1 mM; 3 × 6 μl), serotonin 7 receptor (5-HT7) agonist (AS-19; 10 μM; 3 × 6 μl), 5-HT2B and 5-HT7 receptor agonists (3 × 6 μl), and vehicle (3 × 6 μl). There were no significant changes from baseline in integrated hypoglossal nerve burst amplitude (A) or respiratory burst frequency (B) at any time evaluated. Mixed two-way ANOVA with repeated measures for time was used to detect overall differences between and within groups (interaction between time and groups was included in the model). For hypoglossal nerve activity as the dependent variable: F, 0.336; P = 0.959; df, 9). For respiratory frequency as the dependent variable: F, 0.885; P > 0.05; df, 9).
NOX or PKA inhibitors restore pMF with joint receptor activation.
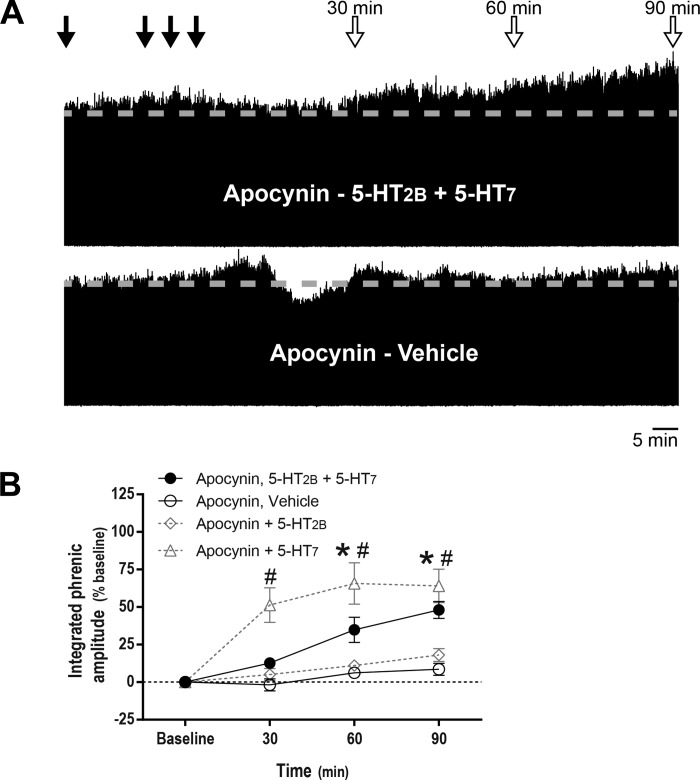
Figures 3A and 4A show time-compressed neurograms in rats primed with apocynin or PKA inhibitor, illustrating pMF despite joint intrathecal receptor agonist injections. In rats pretreated with apocynin, integrated phrenic nerve burst amplitude was increased significantly from baseline at 60 (35 ± 8%, P < 0.001) and 90 min (48 ± 6%, P < 0.001) after combined episodic spinal 5-HT2B and 5-HT7 receptor activation. With apocynin pretreatment, 5-HT7 receptor activation alone still elicited pMF; on the other hand, 5-HT2B-dependent pMF was blunted in apocynin-treated rats. There were no time-dependent changes in phrenic burst amplitude in vehicle-treated rats primed with apocynin (P > 0.05; Fig. 3B).
Fig. 3.
NADPH oxidase inhibitor apocynin restores phrenic motor facilitation (pMF) after concurrent serotonin 2B receptor (5-HT2B) and serotonin 7 (5-HT7) receptor activation. A: representative traces of compressed, integrated phrenic nerve burst amplitude at baseline and during and after intrathecal drug injections. Gray dashed line in each trace marks baseline amplitude of phrenic nerve activity. Top closed arrows depict intrathecal apocynin (600 μM, 12 μl) injections followed by episodic intrathecal drug injections; top open arrows depict 30-, 60-, and 90-min blood samples. B: average (± SE) values from baseline to 90 min after episodic intrathecal drug injections (expressed as %baseline). Lines are from rats pretreated with intrathecal apocynin injections followed by either 5-HT2B (BW 723C86 hydrochloride; 1 mM) plus 5-HT7 receptor agonists (AS-19; 10 μM, 3 × 6 μl, n = 8), or vehicle (3 × 6 μl, n = 6). In apocynin-pretreated rats, integrated phrenic nerve burst amplitude was significantly higher than baseline at 60 and 90 min post-5-HT2B and 5-HT7 receptor activation (*), and integrated phrenic nerve burst amplitude was also significantly higher than in vehicle-pretreated rats at 30, 60 and 90 min post-5-HT2B and 5-HT7 receptor activation (#). Although 5-HT7-dependent pMF was not affected by apocynin pretreatment (3 × 6 μl, n = 3), 5-HT2B-dependent pMF was blunted (3 × 6 μl; n = 5). Differences were considered significant at P < 0.05. Mixed, two-way ANOVA with repeated measures for time was used to detect overall differences between and within groups (interaction between time and groups was included in the model; F, 7.751; P < 0.001; df, 9), followed by Tukey's post hoc test.
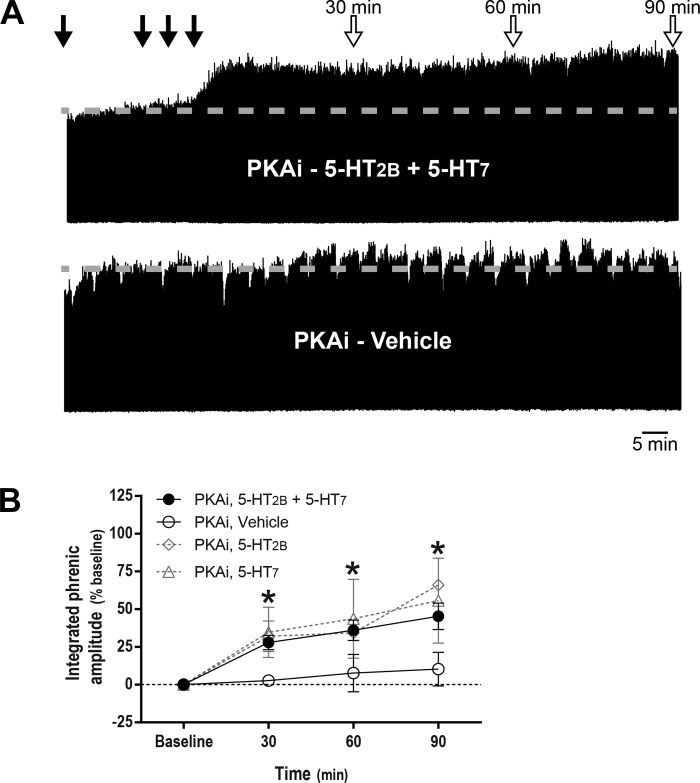
Fig. 4.
Protein kinase A inhibitor (PKAi) restores phrenic motor facilitation (pMF) after concurrent serotonin 2B receptor (5-HT2B) and serotonin 7 (5-HT7) receptor activation. A: representative traces of compressed, integrated phrenic neurograms at baseline, during and after intrathecal drug injections. Gray dashed line in each trace marks baseline amplitude of phrenic nerve activity. Top closed arrows depict intrathecal PKAi (1 mM, 10 μl) injections followed by episodic intrathecal drug injections; top open arrows on depict 30-, 60-, and 90-min blood samples. B: average (± SE) values from baseline to 90 min after episodic intrathecal drug injections (expressed as %baseline). Lines are from rats pretreated with intrathecal PKAi injections followed by either 5-HT2B (BW 723C86 hydrochloride; 1 mM) plus 5-HT7 receptor agonists (AS-19, 10 μM, 3 × 6 μl, n = 8) or vehicle (3 × 6 μl, n = 6). *Integrated phrenic nerve burst amplitude was significantly different from baseline and vehicle-treated rats at 30, 60, and 90 min post-5-HT2B and post-5-HT7 receptor activation. Magnitude of pMF at 90 min after either 5-HT2B (3 × 6 μl, n = 3) or 5-HT7 receptor activation (3 × 6 μl, n = 3) was unaffected by pretreatment with PKAi. Differences considered significant at P < 0.05. Mixed two-way ANOVA with repeated measures for time was used to detect overall differences between and within groups (interaction between time and groups was included in the model; F, 2.074; P < 0.05; df, 9), followed by Tukey's post hoc test.
In rats pretreated with PKA inhibitor, integrated phrenic nerve burst amplitude was increased above baseline at 30 (28 ± 5%, P = 0.004), 60 (36 ± 7%, P < 0.001), and 90 min (45 ± 9%, P < 0.001) following combined episodic spinal 5-HT2B and 5-HT7 receptor activation. The magnitude of pMF 90 min following 5-HT2B or 5-HT7 receptor activation alone was unaffected by PKA inhibition. There were no time-dependent changes in phrenic nerve burst amplitude in vehicle-treated rats with PKA inhibition (P > 0.05; Fig. 4B).
DISCUSSION
Spinal 5-HT2B and 5-HT7 receptor-induced pMF involve distinct intracellular signaling cascades (19, 25). Whereas pMF elicited by Gq protein-coupled 5-HT2B receptors requires spinal NOX activity (25), pMF elicited by Gs protein-coupled 5-HT7 receptors requires signaling via EPAC and mammalian target of rapamycin (14). We now show that concurrent 5-HT2B and 5-HT7 receptor activation undermines pMF, demonstrating powerful cross-talk inhibitory interactions between these competing pathways.
Apocynin pretreatment restores pMF during concurrent 5-HT2B and 5-HT7 receptor activation, demonstrating that NOX underlies Q to S pathway inhibition. PKA inhibition also restores pMF, confirming previous reports that PKA underlies S to Q pathway inhibition (12, 20). The present findings extend these earlier reports to a new serotonin receptor subtype (5-HT2B). Collectively, these results advance our understanding of competing mechanisms of spinal serotonin-dependent phrenic motor plasticity and how these interactions could give rise to emergent properties, such as pattern sensitivity (10) or metaplasticity (13).
Although both 5-HT2B and 5-HT7 receptor subtypes are expressed in the phrenic motor nucleus (14, 25), and activation of either receptor is sufficient to elicit pMF when acting alone, they do so via distinct cellular mechanisms. Few studies targeting mechanisms of 5-HT2B receptor-induced phrenic motor plasticity have been performed to date, but these Gq protein-coupled receptors likely elicit pMF via the Q pathway (5), implicating, ERK MAP kinase, new BDNF protein synthesis, tyrosine receptor kinase B (TrkB) receptor activation, and protein kinase Cθ activity (2, 5, 7, 8, 21). However, these findings pertain to moderate AIH-induced 5-HT2 receptor-dependent pLTF versus 5-HT2B or 5-HT2A receptor-induced pMF per se. The lone study demonstrating that 5-HT2B receptors elicit pMF revealed a unique feature that differentes 5-HT2B from 5-HT2A receptor-induced pMF; specifically, cervical spinal 5-HT2B receptor-induced pMF requires NOX-dependent ROS formation, whereas 5-HT2A receptor-induced pMF does not (25).
Spinal NOX activity and ROS formation are also necessary for moderate AIH-induced pLTF (24) and spinal serotonin-induced pMF (23). Indeed, our results confirm that 5-HT2B receptor-induced (but not 5-HT7 receptor-induced) pMF is abolished in apocynin-pretreated rats (25), suggesting that apocynin rescues 5-HT7 receptor-induced pMF with concurrent 5-HT2B and 5-HT7 receptor activation by preventing inhibitory cross-talk from the Q to S pathways. NOX generated ROS may constrain Gs protein-coupled receptor effects (16, 17) via inhibition of adenyl cyclase activity and cAMP formation (17).
Cervical spinal 5-HT7 receptor activation elicits pMF by a mechanism that requires cAMP-dependent EPAC activation, activity of Akt (not ERK) and mammalian target of rapamycin (mTORC1), and new TrkB protein synthesis (14, 19). Interactions between spinal 5-HT7 receptors and 5-HT2 receptor-dependent pMF were initially revealed by the demonstration that spinal 5-HT7 receptor inhibition enhances moderate AIH-induced pLTF, which typically arises from the Q pathway (20). A similar response occurs with spinal inhibition of another Gs protein-coupled receptor, adenosine 2A receptors (18). Collectively, these studies established the concept of cross-talk inhibition between competing mechanisms of phrenic motor plasticity (5, 9, 12). Interactions between other serotonin receptors have been reported in other models of neuroplasticity (33), including 5-HT1 and 5-HT7 receptor interactions in memory formation (34). In our model, PKA activity is the source of cross-talk inhibition from 5-HT7 receptors to 1) 5-HT2-dependent, moderate AIH-induced pLTF (20) and 2) 5-HT2A receptor-induced pMF (12).
Although PKA is not necessary for 5-HT7 receptor-induced pMF, we now demonstrate that 5-HT7 receptor-dependent PKA activity constrains 5-HT2B receptor-induced pMF through divergent cAMP signaling (12). PKA phosphorylates cytosolic p47phox subunits necessary for NOX complex activation (4, 22, 31), potentially diminishing the ROS formation necessary for 5-HT2B receptor-induced pMF (25). Thus, PKA could constrain the Q pathway to pMF, representing a key molecule in orchestrating complex interactions between competing mechanisms of pMF. This explanation cannot explain PKA inhibition of 5-HT2A receptor-induced pMF, which does not require NOX activity (12). Since PKA requires nanomolar cAMP concentrations (11), whereas EPAC activation requires persistent and higher levels of cAMP (32), 5-HT7 receptors may still constrain 5-HT2B receptor-induced pMF through cAMP-dependent PKA activity despite NOX/ROS inhibition of cAMP-dependent EPAC activity. Thus, attenuation of adenyl cyclase activity by NOX may compromise EPAC-dependent pMF without blocking the cross-talk constraint from cAMP-dependent PKA activity.
Perspectives and Significance
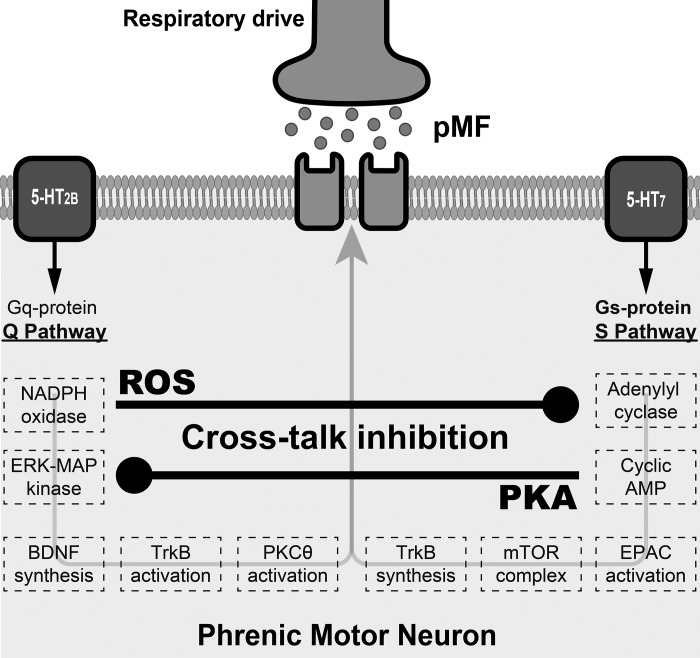
Our results inspire a novel model of cross-talk interactions between competing mechanisms of phrenic motor plasticity elicited by specific serotonin receptor subtypes. Using a selective pharmacological approach, we identified relevant molecules giving rise to mutual cross-talk inhibition that undermines phrenic motor plasticity (Fig. 5). A better understanding of the molecular interactions giving rise to respiratory plasticity suggests new targets for therapeutic intervention with drugs or genetic manipulation as we attempt to harness respiratory motor plasticity for therapeutic advantage in the treatment of life-threatening neurorespiratory disorders such as spinal injury or motor neuron disease (27).
Fig. 5.
Schematic representation of multiple intracellular pathways independently leading to phrenic motor plasticity. Simultaneous pharmacological activation of the Q (5-HT2B receptors) and S (5-HT7 receptors) pathways abolishes phrenic motor plasticity via Q pathway-dependent NADPH oxidase activation and S pathway-dependent protein kinase A (PKA) activation. ROS, reactive oxygen species; BDNF, brain-derived neurotrophic factor; TrkB, tyrosine receptor kinase B; PKC, protein kinase C; EPAC, exchange protein activated by cAMP.
GRANTS
Support was provided by National Heart, Lung, and Blood Institute Grants HL-69064 and HL-111598 and the McKnight Brain Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.R.P., D.P.F., and G.S.M. conceived and designed research; R.R.P. and D.P.F. performed experiments; R.R.P. analyzed data; R.R.P., D.P.F., and G.S.M. interpreted results of experiments; R.R.P. prepared figures; R.R.P., D.P.F., and G.S.M. drafted manuscript; R.R.P., D.P.F., and G.S.M. edited and revised manuscript; R.R.P., D.P.F., and G.S.M. approved final version of manuscript.
REFERENCES
- 1.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 2.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 3.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bengisgarber C, Gruener N. Protein kinase A downregulates the phosphorylation of p47 phox in human neutrophils: a possible pathway for inhibition of the respiratory burst. Cell Signal 8: 291–296, 1996. doi: 10.1016/0898-6568(96)00052-6. [DOI] [PubMed] [Google Scholar]
- 5.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29: 39–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale EA, Fields DP, Devinney MJ, Mitchell GS. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Exp Neurol 287: 130–136, 2017. doi: 10.1016/j.expneurol.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS. Phrenic long-term facilitation requires PKCθ activity within phrenic motor neurons. J Neurosci 35: 8107–8117, 2015. doi: 10.1523/JNEUROSCI.5086-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devinney MJ, Nichols NL, Mitchell GS. Sustained hypoxia elicits competing spinal mechanisms of phrenic motor facilitation. J Neurosci 36: 7877–7885, 2016. doi: 10.1523/JNEUROSCI.4122-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dostmann WR, Taylor SS. Identifying the molecular switches that determine whether (Rp)-cAMPS functions as an antagonist or an agonist in the activation of cAMP-dependent protein kinase I. Biochemistry 30: 8710–8716, 1991. doi: 10.1021/bi00099a032. [DOI] [PubMed] [Google Scholar]
- 12.Fields DP, Mitchell GS. Divergent cAMP signaling differentially regulates serotonin-induced spinal motor plasticity. Neuropharmacology 113: 82–88, 2017. doi: 10.1016/j.neuropharm.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields DP, Mitchell GS. Spinal metaplasticity in respiratory motor control. Front Neural Circuits 9: 2, 2015. doi: 10.3389/fncir.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields DP, Springborn SR, Mitchell GS. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol 114: 2015–2022, 2015. doi: 10.1152/jn.00374.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Günther S, Maroteaux L, Schwarzacher SW. Endogenous 5-HT2B receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol 66: 949–961, 2006. doi: 10.1002/neu.20253. [DOI] [PubMed] [Google Scholar]
- 16.Haenen GR, Plug HJ, Vermeulen NP, Timmerman H, Bast A. Contribution of 4-hydroxy-2,3-trans-nonenal to the reduction of beta-adrenoceptor function in the heart by oxidative stress. Life Sci 45: 71–76, 1989. doi: 10.1016/0024-3205(89)90437-2. [DOI] [PubMed] [Google Scholar]
- 17.Haenen GR, Veerman M, Bast A. Reduction of beta-adrenoceptor function by oxidative stress in the heart. Free Radic Biol Med 9: 279–288, 1990. doi: 10.1016/0891-5849(90)90002-Z. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol 588: 255–266, 2010. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 589: 1397–1407, 2011. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience 250: 632–643, 2013. doi: 10.1016/j.neuroscience.2013.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol (1985) 113: 1184–1193, 2012. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JS, Diebold BA, Babior BM, Knaus UG, Bokoch GM. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J Biol Chem 282: 34787–34800, 2007. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- 23.MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 587: 5469–5481, 2009. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol 587: 1931–1942, 2009. doi: 10.1113/jphysiol.2008.165597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41: 87–103, 1980. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders? In: Genetic Basis for Respiratory Control Disorders, edited by Gaultier C. New York: Springer, 2007, p. 291–311. [Google Scholar]
- 28.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. Invited review. Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90: 2466–2475, 2001. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 29.Nauseef WM. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim Biophys Acta 1840: 757–767, 2014. doi: 10.1016/j.bbagen.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307: R1181–R1197, 2014. doi: 10.1152/ajpregu.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nogueira-Machado JA, Lima e Silva FC, Medina LO, Costa DC, Chaves MM. Modulation of the reactive oxygen species (ROS) generation mediated by cyclic AMP-elevating agents or Interleukin 10 in granulocytes from type 2 diabetic patients (NIDDM): a PKA-independent phenomenon. Diabetes Metab 29: 533–537, 2003. doi: 10.1016/S1262-3636(07)70068-X. [DOI] [PubMed] [Google Scholar]
- 32.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep 5: 1176–1180, 2004. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson-White A, Stratakis CA. Protein kinase A signaling: “cross-talk” with other pathways in endocrine cells. Ann NY Acad Sci 968: 256–270, 2002. doi: 10.1111/j.1749-6632.2002.tb04340.x. [DOI] [PubMed] [Google Scholar]
- 34.Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA 90: 8547–8551, 1993. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]