Abstract
We have shown that acupuncture, including manual and electroacupuncture (MA and EA), at the P5–6 acupoints stimulates afferent fibers in the median nerve (MN) to modulate sympathoexcitatory cardiovascular reflexes through central regulation of autonomic function. However, the mechanisms underlying acupuncture activation of these sensory afferent nerves and their cell bodies in the dorsal root ganglia (DRG) are unclear. Transient receptor potential vanilloid type 1 (TRPV1) is present in sensory nerve fibers distributed in the general region of acupoints like ST36 and BL 40 located in the hindlimb. However, the contribution of TRPV1 to activation of sensory nerves by acupuncture, leading to modulation of pressor responses, has not been studied. We hypothesized that TRPV1 participates in acupuncture’s activation of sensory afferents and their associated cell bodies in the DRG to modulate pressor reflexes. Local injection of iodoresiniferatoxin (Iodo-RTX; a selective TRPV1 antagonist), but not 5% DMSO (vehicle), into the P6 acupoint on the forelimb reversed the MA’s inhibition of pressor reflexes induced by gastric distension (GD). Conversely, inhibition of GD-induced sympathoexcitatory responses by EA at P5–6 was unchanged after administration of Iodo-RTX into P5–6. Single-unit activity of Group III or IV bimodal afferents sensitive to both mechanical and capsaicin stimuli responded to MA stimulation at P6. MA-evoked activity was attenuated significantly (P < 0.05) by local administration of Iodo-RTX (n = 12) but not by 5% DMSO (n = 12) into the region of the P6 acupoint in rats. Administration of Iodo-RTX into P5–6 did not reduce bimodal afferent activity evoked by EA stimulation (n = 8). Finally, MA at P6 and EA at P5–6 induced phosphorylation of extracellular signal-regulated kinases (ERK; an intracellular signaling messenger involved in cellular excitation) in DRG neurons located at C7–8 spinal levels receiving MN inputs. After TRPV1 was knocked down in the DRG at these spinal levels with intrathecal injection of TRPV1-siRNA, expression of phosphorylated ERK in the DRG neuron was reduced in MA-treated, but not EA-treated animals. These data suggest that TRPV1 in Group III and IV bimodal sensory afferent nerves contributes to acupuncture inhibition of reflex increases in blood pressure and specifically plays an important role during MA but not EA.
Keywords: blood pressure, dorsal root ganglia, sensory receptors, somatic afferent
INTRODUCTION
Acupuncture, including both manual and electroacupuncture (MA and EA), increasingly is accepted as alternative therapies for a number of diseases, including hypertension (26, 32, 43). However, there is insufficient mechanistic evidence of its actions, particularly its actions on sensory nerves. Acupuncture applied in point-specific regions is used empirically to treat a number of diseases. In this regard, P5 (Jianshi) and P6 (Neiguan) acupoints overlying the median nerve (MN) are commonly employed to manage cardiovascular disorders (26, 40). For example, we have shown that MA and EA at P5–6 attenuate sympathoexcitatory reflex pressor responses (44) and sustained hypertension following prolonged exposure to cold in rats (25) and moderate hypertension in patients (30). The mechanisms underlying the peripheral action of acupuncture at the acupoint to lower elevated blood pressure (BP) are largely unknown.
Many acupoints are located in areas rich in sensory innervation (1, 14). Application of acupuncture at select acupoints activates underlying somatic afferent nerves including Group I to IV afferents (22, 44). In particular, our previous studies have shown that MA and EA at P5–6 stimulate both thinly myelinated Group III and unmyelinated Group IV fibers in the MN to influence central regulation of autonomic function and, hence, modulate sympathoexcitatory cardiovascular reflexes (26, 27, 39, 44, 45). Group I to IV somatic afferents can be categorized as predominately mechanosensitive, chemosensitive, thermosensitive, and polymodal (37, 38). Little is known about which functional types of somatic afferents are activated during acupuncture, more specifically during MA and EA stimulation. Furthermore, the underlying mechanisms by which acupuncture activates these sensory nerves and their cell bodies in the dorsal root ganglia (DRG) also remain unclear.
Transient receptor potential vanilloid type 1 (TRPV1) is present in both neuronal and nonneuronal cells in the general region of acupoints in the hindlimb such as ST36 [(Zusanli, beneath the knee (42)] and BL40 [(Weizhong, in the middle of the popliteal fossa (1);]. TRPV1 is a ligand-gated and nonselective cation channel that serves as a molecular target for capsaicin (ingredient in chili peppers). TRPV1 is activated by natural stimuli, including lipid derivatives, acidic protons (pH below 5.9), noxious heat (above 42°C), and mechanical stimulation (3, 5, 34, 35, 41). Stimulation of TRPV1 activates both sensory nerves and nonneuronal cells (36). Capsaicin administered into the ST36 acupoint can replicate the analgesic effect of MA in mice (42). Although these studies imply a potential association of TRPV1 at the acupoints during acupuncture, they do not prove a role for TRPV1 at the acupoints with respect to activation of sensory afferent nerves during acupuncture stimulation nor during acupuncture modulation of cardiovascular responses.
Primary sensory neuronal cell bodies in the DRG transmit sensory information from the periphery to central nervous system (CNS). Extracellular signal-regulated kinase (ERK), one of the five mitogen-activated protein kinases (MAPK) cascades identified in the intracellular signaling pathways, is activated during membrane depolarization and is involved in the excitation of DRG neurons (6, 9). Expression of phosphorylated ERK (pERK) is increased in DRG following activation of sensory afferent endings by mechanical and electrical stimuli (9). Stimulation of TRPV1 in sensory afferent nerves leads to pERK expression in DRG neurons (6, 9).
We therefore investigated the role of TRPV1 in somatic sensory afferent stimulation during MA and EA and in acupuncture modulation of cardiovascular responses. We hypothesized that TRPV1 is involved in MA and EA activation of sensory afferent nerves and cervical DRG neurons that express pERK and thus serves as an important peripheral chemical signal during acupuncture modulation of sympathoexcitatory BP reflex responses.
METHODS
Anesthesia and Surgical Preparations
All experimental preparations and protocols were reviewed and approved by the Animal Care and Use Committee of the University of California at Irvine, CA. The study conformed to the American Physiological Society's Guiding Principles for Research Involving Animals. Studies were performed on adult Sprague-Dawley male rats (350–550 g).
Anesthesia was induced with ketamine (100 mg/kg im) and maintained with α-chloralose (50–60 mg/kg iv) in terminal experiments, including cardiovascular reflexes induced by gastric distension (GD) and recordings of afferent activity. Additional doses of α-chloralose (25–30 mg/kg iv) were given as necessary to maintain an adequate depth of anesthesia by observing the absence of conjunctival reflex response. A femoral artery and vein were cannulated for measuring blood pressure (BP) and administrating drugs, respectively. The femoral artery was cannulated and attached to a pressure transducer (Statham P23 ID, Gould) to monitor systemic BP. Heart rate (HR) was derived from the pulsatile BP signal. The trachea was intubated, and respiration was maintained with a ventilator (model 661; Harvard Apparatus, Holliston, MA). Arterial blood gases and pH were measured periodically with a blood-gas analyzer (model ABL5; Radiometer, Copenhagen, Denmark) and were kept within normal physiological limits (pH 7.35–7.45, Po2 >100 mmHg and Pco2 30–40 mmHg) by adjusting the ventilation rate or volume, enriching the inspired oxygen supply and infusion of a solution of 8% sodium bicarbonate. Body temperature was kept between 36 and 38°C with a heating pad and lamp.
Induction of Pressor Reflexes
As we described in detail previously (28, 39, 44), consistent reflex increases in BP were induced by GD. In brief, a 3-cm (unstressed dimension) latex balloon was attached to a polyurethane tube (3-mm diameter) that was inserted into the stomach through the mouth and esophagus. A syringe was attached to the cannula to inflate and deflate the balloon with air. A latex balloon was inflated with 3–5 ml of air, a volume that induced a distension pressure of ∼25 mmHg. Distension pressures were selected to fall within the range that a rat normally experiences during ingestion of food and fluids in a single large meal (28). To induce increases in BP, the balloon was inflated inside the stomach. Increases in BP were observed within 30 s of inflation. The balloon was deflated within 30 s after reaching the maximal increase in BP.
Acupuncture Application
P5 and P6 acupoints are located on forelimbs correspondingly 2.5 and 4.0 mm above the flexor crease in the paw (Fig. 1), between the tendons of the palmaris longus and flexor carpi radialis muscles overlying the MN (17). Stimulation of these acupoints has been shown to evoke MN discharge and attenuate reflex cardiovascular responses (28, 39, 44). A stainless steel acupuncture needle (32-gauge; Suzhou Medical Appliance, Suzhou, China) was applied at P6 during MA, while two needles were used at P5–6 during EA. MA was performed by gentle manual rotation of the acupuncture needle twice per second guided by an audible stimulator set at 2 Hz to help maintain the frequency at that level. The needles used for EA (2 Hz, 0.3–0.5 mA, 0.5 ms) were connected to a constant-current stimulator with a stimulus isolation unit (model no. S88; Grass, West Warwick, RI). MA and EA were applied at 2 Hz since both forms of acupuncture at this frequency significantly modulate reflex-induced elevations in BP (44). During sham acupuncture, needles were inserted into the same acupoint(s) without subsequent mechanical or electrical stimulation (27, 44). Correct placement of the needle at P5 and P6 was confirmed by observing slight repetitive paw twitches with electrical stimulation of P5 and P6 (27, 44). The flexor twitches were important observations to confirm stimulation of motor fibers in the MN. We typically lowered the current during electrical stimulation to a level just below motor threshold. Of note, MN motor fiber stimulation does not participate in EA cardiovascular response, since we have shown that EA inhibition of reflex cardiovascular responses does not change following muscle paralysis (31). Gallamine triethiodide (4 mg/kg) was administered intravenously before application of MA or EA to avoid muscle movement during MN stimulation (31).
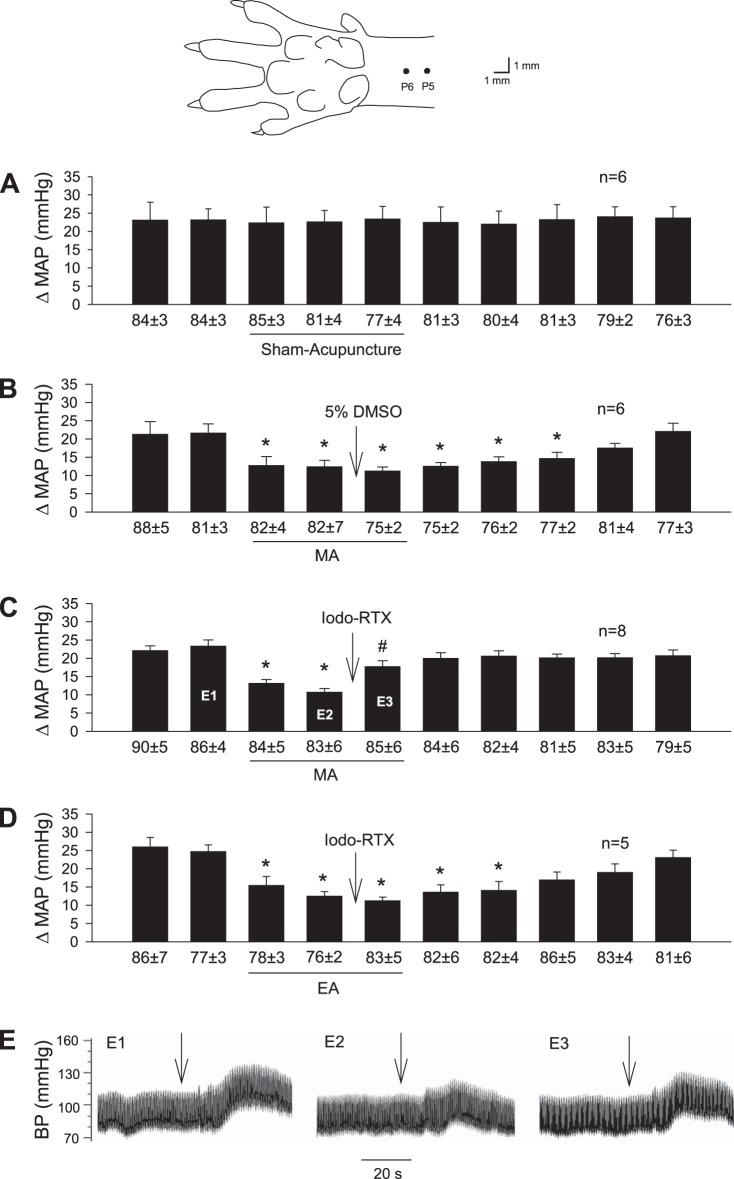
Fig. 1.
Influence of blockade of transient receptor potential vanilloid type 1 (TRPV1) in region of acupoints on manual acupuncture (MA) and electroacupuncture (EA) modulation of pressor reflexes induced by gastric distension (GD). Bars represent increases in mean arterial blood pressure (MAP) following GD. Values below each bar indicate baseline MAP values (means ± SE) before GD. A: sham acupuncture was conducted by inserting acupuncture needles into the P6 or P5–6 acupoints without manual or electrical stimulation for 30 min. The diagram above this panel displays the sites of the P5 and P6 acupoints (17). B: 5% DMSO was injected into P6 during MA. C: injection of iodoresiniferatoxin (Iodo-RTX; 0.1 mM) into P6 with MA treatment. D: injection of Iodo-RTX into P5–6 with EA treatment. *P < 0.05, after vs. before MA or EA; #P < 0.05, after vs. before microinjection into the acupoint. Labels E1–3 within bar histogram displayed in C represent original BP tracings of a rat shown in E, E1–3.
Single-Unit Somatic Afferent Recordings
Single-unit activity of afferent fibers in the MN was recorded as we described previously (39, 44). In brief, the MN was isolated in the upper forelimb near the humerus and covered with warm mineral oil. The MN then was split into fine nerve filaments under a surgical microscope (model OPMI 1-FC, Zeiss, Germany). The peripheral end of a filament was draped over one pole of a bipolar recording electrode attached to a high-impedance probe. The other pole of the electrode was grounded with a saline-saturated cotton thread to the surrounding tissue. Action potentials of each afferent fiber were amplified (50,000×) and bandpass filtered (100–3,000 Hz) through an AC amplifier (model P511 preamplifier, Grass Instruments) and then processed through an audio amplifier (model AM8B, Grass Instruments) and displayed on a storage oscilloscope (model 2201, Tektronix, UK) to allow discrimination. The activity of afferents was recorded on a computer using data acquisition and analysis software (Spike 2; Cambridge Electronic Design, Cambridge, UK) that sampled signals at 10,000 Hz through an analog-to-digital converter (micro 1401 mkII, Cambridge Electronic Design) for on- and off-line quantitative analysis. The discharge frequency was quantified using the Spike 2 software window discriminator. A histogram was created for each afferent.
The receptive field of each afferent was determined carefully by observing its responses to probing the region of the P6 acupoint with a blunt glass probe (<1 mm in diameter). The location of the afferent nerve ending was confirmed through electrical stimulation to evoke an action potential by using a stimulating electrode placed in the region of the receptive field (13, 38). Conduction distance was measured with a thread placed between the receptive field and the recording electrode along the course of the MN. Conduction time was determined by measuring the latency from the signal of electrical stimulation to the corresponding action potential in Group III and IV afferents. Conduction velocity of each afferent was calculated by dividing the conduction distance by conduction time. Classification of fibers followed established categories from previous studies on rats (39, 44). Fibers with conduction velocities <2 m/s were classified as unmyelinated Group IV (C-)fibers, whereas those with velocities between 2.0 and 20 m/s were considered to be Group III (Aδ-)fibers.
Intrathecal Injection of siRNA to Knockdown TRPV1
Rats were anesthetized with a ketamine-xylazine mixture (100/10 mg/kg ip). After applicaton of xylocaine gel in the ear canals, rats were placed on a sterile stereotaxic apparatus. The surgical area was shaved, cleaned, and disinfected with a povidone iodine-based disinfectant. Through an incision over the occipital bone, a catheter (PE10) was inserted through the atlantooccipital membrane to reach spinal level C7–8. Correct placement of the catheter was confirmed before harvesting the DRGs at the end of experiment by visualizing the tip of the catheter located at the C7–8 spinal level. The indwelling catheter was secured in place with sutures and protected with a cotton jacket placed around the neck and thorax. The wound was closed with a nonabsorbable nylon suture. The external portion of the catheter was capped. Once the rat awakened fully, buprenorphine (0.01–0.05 mg/kg im) was administered every 8–12 h for a total of 4 days to relieve pain and penicillin G procaine (7,500 U/kg im) daily to prevent infection. The animal was allowed to recover under close supervision. The siRNA (small interfering RNA) for TRPV1 was mixed with i-Fect (in vivo transfection reagent) and injected intrathecally once daily for 3–4 consecutive days under sedation with ketamine (50 mg/kg ip) to silence TRPV1 in the DRG (7, 12, 33). In an identical manner, as the control, scrambled siRNAs mixed with i-Fect were injected intrathecally. Stealth TRPV1-siRNA (RSS331240) and scrambled siRNAs were purchased from ThermoFisher Scientific (Rockford, IL). i-Fect was purchased from Neuromics (NI35150; Edina, MN). i-Fect is a novel cationic lipid-based reagent with high biocompatibility and low cytotoxicity. It efficiently delivers siRNA to DRG neurons and silences genes in vivo (33). On each day of injection, 5.4 µg of TRPV1-siRNA mixed with 12 µl of i-Fect was infused and followed by a 10-µl normal saline flush. During and after intrathecal injections, animals did not exhibit abnormal behavioral signs, abnormal stances, or posture, reclusive behavior, increased inspiratory rate, or abnormal breathing patterns. Twenty-four hours after completion of the last intrathecal injection of TRPV1-siRNA or scrambled siRNAs, animals were reanesthetized with ketamine-xylazine mixture and treated with MA or EA for 30 min (see Experimental Protocols for details). After euthanasia, DRGs (C7–8) of rats were harvested to examine expression of TRPV1 and pERK, using Western blot and immunohistochemical staining, as described in the next section. Excitation of DRG neurons was evaluated by assessing pERK expression.
Western Blots
DRG tissue was lysed with cell lysis buffer (Cell Signaling Technology, Danvers, MA), which included 137 mM NaCl, 20 mM Tris·HCl (pH 7.5), 10% glycerol, 1% Triton X-100, 0.5% Nonidet P-40, 2 mM EDTA (pH 8.0), 3 μg/ml aprotinin, 3 μg/ml leupeptin, 2 mM phenylmethylsulfonyl fluoride, 20 mM NaF, 10 mM sodium pyrophosphate, and 2 mM Na3VO4. Equal amounts of proteins were separated by SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA) and incubated with a blocking buffer (5% nonfat milk in 20 mM Tris·HCl with pH 7.5, 137 mM NaCl, and 0.1% Tween 20) for 1 h at room temperature. The membranes were incubated overnight with primary antibodies, including mouse monoclonal anti-TRPV1 (BS397, Abcam, Cambridge, MA), goat anti-β-actin (sc-1616), rabbit polyclonal anti-pERK1/2 (Thr202/Tyr204, sc-16982; both from Santa Cruz Biotechnology, Santa Cruz, CA), and mouse monoclonal anti-ERK1/2 (3A7, p44/42 MAPK; Cell Signaling Technology, Trask Lane, MA) at 4°C. The membranes then were washed three times using a solution containing 20 mM Tris·HCl (pH 7.5), 137 mM NaCl, and 0.1% Tween 20) and incubated with secondary antibodies (1:5,000 to 1:10,000 dilution) for 1 h at room temperature. The secondary antibodies were fluorescent conjugated antibodies, including donkey anti-goat (red, IRDye 680RD, 926-68074), goat anti-rabbit (red, IRDye 680RD, 926-68071), and anti-mouse (green, IRDye 800CW, 926-32210; all from LI-COR Biotechnology, Lincoln, NE). After being washed three times, the membranes were detected with Odyssey Imaging System (LI-COR Biotechnology). All comparisons were made with samples run on the same gel and examined on the same film. For final analysis, the intensity of signals was normalized to the control sample on the same gel. Data were analyzed with ImageJ (NIH). TRPV1 and pERK in the DRG were detected, with β-actin and ERK as loading controls, respectively.
Immunohistochemical Staining
Transcardial perfusion was performed using 500 ml of 0.9% saline solution followed by 500 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) following deep anesthesia with ketamine-xylazine (0.5–0.7 ml im). DRGs at C7–8 were harvested and sliced into 10-μm sections using a cryostat microtome (Leica CM1850; Heidelberger Strasse, Nussloch, Germany). DRG sections were collected serially and used for immunohistochemical labeling as described below.
After being washed for 30 min (10 min × 3 times) with phosphate-buffered saline containing 0.3% Triton X-100 (PBST, pH 7.4), DRG sections were treated for 1 h with 1% normal donkey serum (Jackson Immunoresearch Laboratories, West Grove, PA). The sections were incubated with a primary polyclonal guinea pig anti-TRPV1 antibody (PA1-29770, 1:400 dilution, ThermoFisher Scientific) or rabbit anti-pERK antibody (137F5, p44/42 MAPK, 1:400 dilution; Cell Signaling Technology) at 4°C for 48 h. Staining pERK in the DRG section was used to identify activation of DRG neurons during MA and EA (6, 9). The tissues subsequently were rinsed three times (10 min for each rinse) in PBST and incubated with a fluorescein-conjugated donkey anti-guinea pig or a rhodamine-conjugated donkey anti-rabbit (1:200 dilution, Jackson Immunoresearch Laboratories) for 24 h at 4°C. DRG sections on the slide were air-dried. The slides were coverslipped using mounting medium (Vector Laboratories, Burlingame, CA). In immunohistochemical control studies, all TRPV1 or pERK staining was abolished when 1 ml of the diluted primary antibody was preincubated with control protein of TRPV1 (10 µg; sc-12498 P, Santa Cruz Biotechnology) or pERK (10 µl, Cell Signaling Technology), respectively. In addition, no labeling was detected when the primary or secondary antibody was omitted.
Image Data Analysis
DRG sections were scanned and examined with a standard fluorescent microscope (Nikon, E400, Melville, NY). Two epifluorescence filters (B-2A, or G-2A) equipped in a fluorescent microscope were used to identify single stains appearing as green (fluorescein) or red (rhodamine) in DRG sections. Fluorescent images were captured with a Spot digital camera (RT color v.3.0; Spot Diagnostic Instruments, Sterling Heights, MI). TRPV1- and pERK-immunoreactive (IR) neurons appeared as bright green and red, respectively (Figs. 5 and 7). The numbers of TRPV1-IR and pERK-IR neurons per section were counted in each animal. The positive neurons were expressed as a percentage of total counted DRG neurons, which were determined by dividing the numbers of TRPV1-IR and pERK-IR neurons with distinctive labeling by the total number of DRG neurons in the same section (6, 9). In each rat, six sections of C7–8 DRGs were selected randomly.
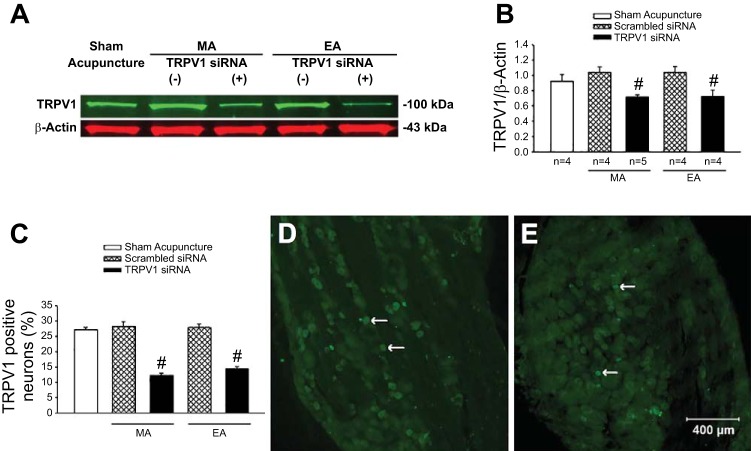
Fig. 5.
Expression of transient receptor potential vanilloid type 1 (TRPV1) in dorsal root ganglia (DRG; C7–8) of rats. Rats were treated with sham acupuncture, manual acupuncture (MA), or electroacupuncture (EA) after intrathecal injection of TRPV1 siRNA (+) or scrambled siRNA (−). A: examples of original Western blotting bands from one rat in each group. B: relative values of TRPV1/β-actin are shown in the bar graphs. C: percentage of TRPV1-labeled neurons in DRG of each group. Group data include: n = 24 DRG sections in four rats treated with TRPV1 siRNA + MA, and n = 18 DRG sections in three rats in each of four other groups, including sham acupuncture, scrambled siRNA + MA, scrambled siRNA + EA, and TRPV1 siRNA + EA. #P < 0.01, TRPV1 siRNA vs. scrambled siRNA. D and E: examples of original fluorescent images of TRPV1 labeling from a rat treated with MA after intrathecal injection of scrambled siRNA (D) or TRPV1 siRNA (E). Arrows in D and E indicate examples of single-labeled TRPV1 neurons (bright green). Scale bar in E represents 400 µm and is applied for D and E.
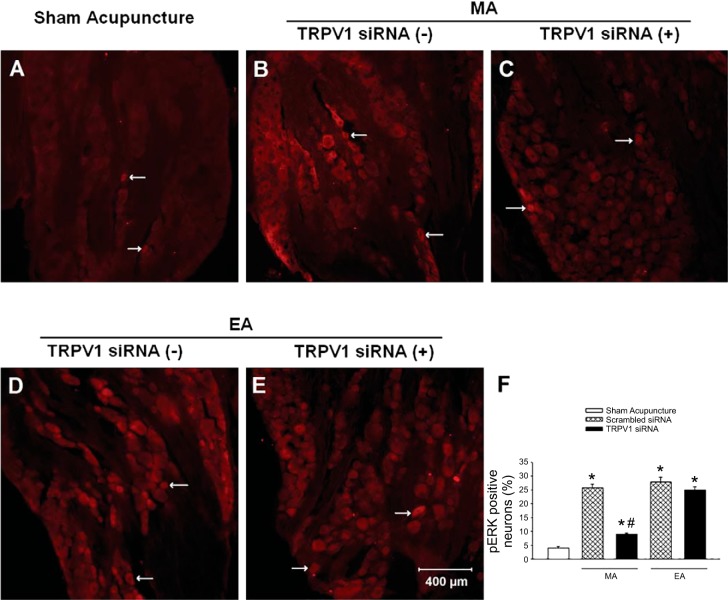
Fig. 7.
Neurons labeled with pERK1/2 in dorsal root ganglia (DRG) (C7–8) of rats. Rats were treated with sham acupuncture, manual acupuncture (MA), or electroacupuncture (EA) after intrathecal injection of transient receptor potential vanilloid type 1 (TRPV1) siRNA (+) or scrambled siRNA (−). A–E: examples of original fluorescent images of phosphorylated extracellular-signal related kinases (pERK) labeling from one rat in each group. Arrows in A–E indicate examples of single-labeled pERK neurons (bright red). Scale bar in E represents 400 µm and applies to A–E. F: bars represent percentage of pERK-labeled neurons in the DRG of each group. Group data include: n = 24 DRG sections in four rats treated with TRPV1 siRNA + MA, and n = 18 DRG sections in three rats in each of the four other groups, including sham acupuncture, scrambled siRNA + MA, scrambled siRNA + EA, and TRPV1 siRNA + EA. *P < 0.01 vs. sham acupuncture; #P < 0.01, TRPV1 siRNA vs. scrambled siRNA.
Drugs
The TRPV1 antagonist iodoresiniferatoxin (Iodo-RTX, 0.1 mM) was used to block TRPV1 (35). The vehicle for this drug is 5% dimethyl sulfoxide (DMSO). Microinjection of the vehicle into the acupoint served as a chemical control.
Experimental Protocols
Effects of TRPV1 blockade in the region of acupoints during MA and EA modulation of pressor reflexes.
pressor reflex responses induced by gastric distension.
A 30-min stabilization period was allowed after the surgical procedure. The balloon was inflated every 10 min by injecting 3–5 ml of air for 30 s. The volume of air used for distension was consistent throughout each experiment. Ten-minute intervals between inflations prevented tachyphylaxis of the cardiovascular responses (28, 44). Rats were subjected to 10 repeated GDs while BP reflex responses were monitored and recorded. After completion of each experiment, rats were euthanized with intravenous injection of potassium chloride (2 meq/ml) under deep anesthesia, and the stomach was then exposed to verify the location of the balloon. Only animals in which the balloon was observed to be within the stomach were used for data analysis.
sham acupuncture.
After two reproducible control responses to GD were recorded, acupuncture needles were inserted bilaterally into the P6 or P5–6 acupoints without manual or electrical stimulation for 30 min in rats, termed sham acupuncture. Eight additional GD-induced reflex responses were recorded during and after sham acupuncture.
blockade of trpv1 in the region of acupoints.
After two consistent BP responses to GD were established, needles were inserted bilaterally into the P6 acupoint and rotated manually at a frequency of ∼2 Hz for 5 min every 10 min during a 30-min period, while GD was repeated every 10 min eight more times. Iodo-RTX or the vehicle (5% DMSO) in 10-µl volume was injected into the region of the needle placed at P6 10 min before the 30 min of MA stimulation was terminated. A needle (28G1/2, attached to 0.5 ml of U-100 insulin syringe) used for the injection was inserted near the acupuncture needle at the same depth (3–4 mm) as the placement of the acupuncture needle (14, 42, 43). Ten microliters of blue dye injected at P6 were observed to diffuse over a region of ~3 mm3.
In separate rats, needles were inserted bilaterally at P5–6 and stimulated electrically at a frequency of 2 Hz (0.3–0.5 mA, 0.5 ms duration). Each set of electrodes was stimulated separately as a positive and negative pole, so that current did not flow from one location to the contralateral forelimb. Iodo-RTX was injected into the regions of P5 and P6.
TRPV1-mediated activation of somatic afferent nerves during MA and EA stimulation.
After identification of the receptive field of a single-unit MN afferent fiber in the region of the P6 acupoint, Von Frey filaments ranging from 0.009 to 316 g were used to examine the afferent response to gentle and strong mechanical stimulation to determine mechanical threshold. Next, the response of this afferent to capsaicin (5–10 µg in 10 µl) injected into the receptive field was tested. If the afferent was sensitive to both mechanical stimulation and capsaicin (classified as a bimodal afferent), the afferent response to MA stimulation at P6 or EA stimulation at P5–6 for 30 s before and after injection of Iodo-RTX (0.1 mM, 10 µl) into P6 or P5 and P6 accordingly was evaluated, as described above in the reflex protocols. In most instances (n = 22), the neuronal responses to MA or EA were examined in different bimodal afferents. In some cases (n = 5), the responses of the same bimodal fiber to both MA and EA were examined in random order to identify the role of TRPV1 in activation of the same population of afferent nerves by MA and EA. The response of the afferent to acupuncture stimulation before and after injection of 5% DMSO (10 µl) into the acupoint area served as control. If a fiber did not respond to both mechanical and capsaicin stimuli, the fiber was not studied further.
Activation of DRG neurons by acupuncture following knock-down of TRPV1.
The role of TRPV1 in activation of DRG neurons during acupuncture was evaluated in rats following intrathecal injection of siRNA. Twenty-four hours after the last intrathecal injection, MA (5 min of needle rotation at ~2 Hz every 10 min) at P6 or EA at 2 Hz at P5–6 was applied bilaterally for 30 min. In the sham group, acupuncture needles were inserted bilaterally into P6 or P5–6 but not manually or electrically stimulated for a 30-min period. Each rat subjected to sham acupuncture was treated identically, with the exception that normal saline was injected intrathecally in place of TRPV1-siRNA or scramble siRNA. DRGs (C7–8) then were harvested bilaterally to examine expression of TRPV1 and pERK by Western blot analysis and immunohistochemical staining.
Statistical Analyses
Data are expressed as means ± SE. Afferent activity and mean arterial pressure (MAP) were compared over time using one-way repeated-measures ANOVA followed by Tukey’s test in each group. If the data were not normally distributed, as determined by the Shapiro-Wilk test, they were compared with the Friedman repeated-measures ANOVA on Ranks followed by the Student-Newman-Keuls procedure. Expression of TRPV1 or pERK in DRGs between two groups was compared using Student's t-test. Statistical calculations were performed with SigmaStat software (Jandel Scientific Software, San Rafael, CA). Values were considered to be significantly different when P < 0.05.
RESULTS
Role of TRPV1 at the Acupoint in MA and EA Modulation of Pressor Responses
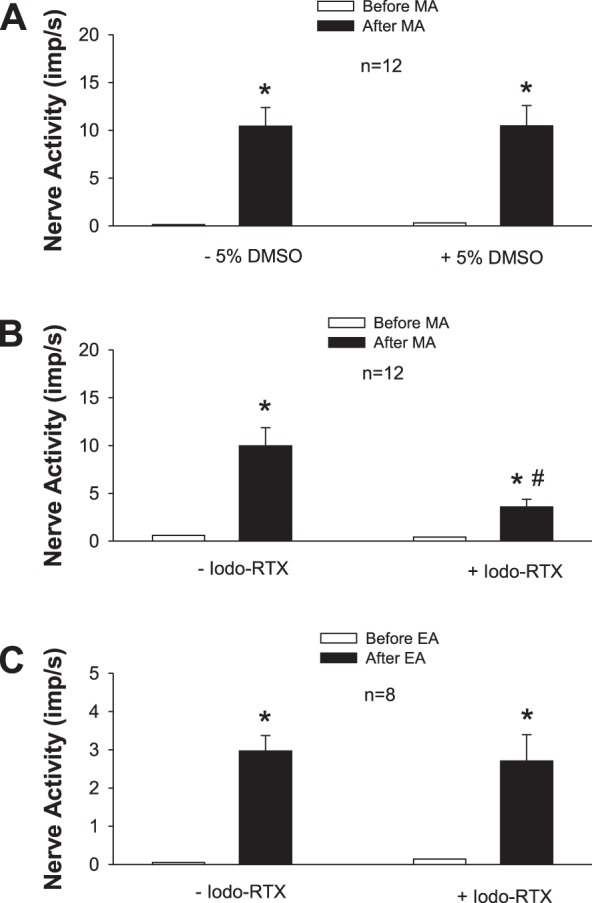
BP responses to GD in a group of rats subjected to sham acupuncture at P6 or P5–6 were consistent during 10 distensions (Fig. 1A; n = 6). HR was not affected by GD. Vehicle (5% DMSO) administered into the P6 area did not influence the response to MA (Fig. 1B; n = 6). Local injection of Iodo-RTX into the P6 region reversed the inhibitory effect of MA (Fig. 1C; n = 8) on the pressor responses (P < 0.05). Conversely, EA modulation of GD-induced pressor reflex responses was unchanged following administration of Iodo-RTX (Fig. 1D).
Effect of TRPV1 Blockade on Afferent Activity During MA and EA Stimulation:
Profile of afferents.
Twenty-seven mechanosensitive and capsaicin-sensitive bimodal afferents with receptive fields located in the region of the P6 acupoint were identified and selected for the present study. None of the afferents responded to gentle mechanical stimuli (i.e., tested with calibrated hairs of the Von Frey type < 1 g force). Their thresholds to mechanical stimulation with Von Frey hairs ranged between 5 and 114 g (average 40 g), indicating they were high-threshold mechanosensitive afferents. The conduction velocities (CVs) of the bimodal afferents ranged between 0.48 and 10.5 m/s. Fifty-nine percent (16 of 27 fibers) of the afferents were classified as Group IV fibers (C-fibers; CV = 1.27 ± 0.12 m/s) and responded to Von Frey hairs with a threshold of 53 ± 11 g. The remaining units (11 afferents) were Group III fibers (Aδ-fibers; CV = 3.43 ± 0.74 m/s) and were activated by Von Frey hairs with a threshold of 30 ± 9 g. Each identified bimodal afferent was studied with one or two experimental interventions. Table 1 indicates characteristics of afferent fibers in each experimental group, including their CVs and thresholds to Von Frey hair stimulation.
Table 1.
Characteristics of afferent fibers in experimental groups
| Group III Fibers |
Group IV Fibers |
|||||
|---|---|---|---|---|---|---|
| Groups | n | CV, m/s | Von Frey hairs threshold, g | n | CV, m/s | Von Frey hairs threshold, g |
| MA vehicle | 6 | 3.76 ± 1.35 | 21.6 ± 5.5 | 6 | 1.33 ± 0.18 | 52.0 ± 21.0 |
| MA Iodo-RTX | 5 | 3.04 ± 0.41 | 35.1 ± 20.0 | 7 | 1.08 ± 0.57 | 65.7 ± 30.3 |
| EA Iodo-RTX | 2 | 3.09 ± 0.84 | 39.1 ± 33.9 | 6 | 1.65 ± 0.06 | 46.4 ± 16.0 |
Values are means ± SE. Note: five fibers were examined for manual acupuncture (MA) and electroacupuncture (EA) with iodoresiniferatoxin (Iodo-RTX). CV, conduction velocity.
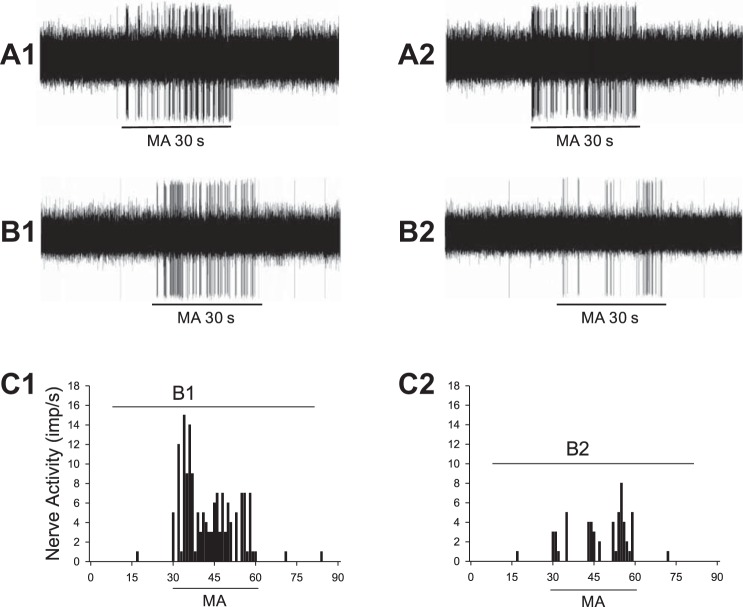
MA applied at P6 significantly increased the discharge of MN afferents (P < 0.05; Fig. 2, A and B) after a very short onset latency of 1 to 3 s (Fig. 3). Discharge frequencies during MA increased from 0.36 ± 0.20 to 9.41 ± 1.09 imp/s (n = 24). There was no difference between Group III and IV fibers with respect to resting and MA-evoked discharge (Group III fibers, from 0.06 ± 0.04 to 10.45 ± 2.28 imp/s; n = 11; Group IV fibers, from 0.62 ± 0.36 to 8.87 ± 1.00 imp/s; n = 13). MA-evoked activity of the afferents was reduced by 64% after local blockade of TRPV1 with Iodo-RTX in P6 (P < 0.05, n = 12; Figs. 2–4). In contrast, the increased activity of the afferent during MA was not influenced by the vehicle (Fig. 2A).
Fig. 2.
Activity of afferents in median nerve (MN) in response to manual acupuncture (MA) or electroacupuncture (EA) before and after local blockade of transient receptor potential vanilloid type 1 (TRPV1) in the region of the acupoint. A and B: MA at P6 before and after injection of 5% DMSO (vehicle; A) or iodoresiniferatoxin (Iodo-RTX; 0.1 mM; B) into P6. C: injection of Iodo-RTX into P5 and 6 with EA treatment. *P < 0.05, after vs. before MA or EA; #P < 0.05, after vs. before microinjection into the acupoint.
Fig. 3.
Neurograms show changes in activity of afferents in median nerve (MN) evoked by manual acupuncture (MA) before and after blockade of transient receptor potential vanilloid type 1 (TRPV1) in region of P6 acupoint. A: consistent responses of a Group IV afferent [conduction velocities (CV) = 0.48 m/s] to repeated MA stimulation before (A1) and after (A2) local injection of 5% DMSO (vehicle) at P6. B: activity of another Group IV afferent (CV = 0.63 m/s) in response to MA stimulation at P6 before (B1) and after (B2) local injection of iodoresiniferatoxin (Iodo-RTX) at the acupoint. C: histograms showing MA-evoked activity of Group IV afferent as shown in B before (C1) and after (C2) local injection of Iodo-RTX. B1 and B2 in C represent time periods shown in the neurograms in B.
Fig. 4.
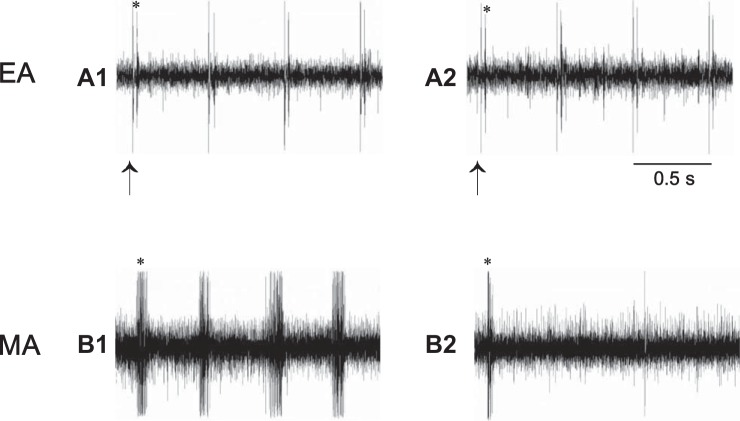
Neurograms show activity of Group IV afferent (CV = 1.69 m/s) in the median nerve (MN) in response to manual acupuncture (MA) or electroacupuncture (EA). A1 and A2: discharges of afferent evoked by EA stimulation (2 Hz) at P5–6 before and after injection of iodoresiniferatoxin (Iodo-RTX; 0.1 mM) into region of acupoints. B1 and B2: activity of afferent in response to MA stimulation (~2 Hz) at P6 before and after local administration of Iodo-RTX. Arrow, stimulus artifact; *action potential of afferent evoked by EA or MA stimulation. Note: blockade of TRPV1 reduced responses of this afferent to MA (B2) but not to EA stimulation (A2).
EA (2 Hz) applied at P5–6 increased discharge activity of eight MN afferents from 0.05 ± 0.05 to 2.97 ± 0.40 imp/s (P < 0.05; Fig. 2C). Resting and EA-evoked discharge was similar in Group III and IV fibers (Group III, from 0.20 to 3.03 imp/s, n = 2; Group IV, from 0 to 2.95 ± 0.49 imp/s, n = 6). EA-evoked activity of the afferents was unchanged after local blockade of TRPV1 with Iodo-RTX into the regions of P5 and P6 (Figs. 2 and 4). Group data (Fig. 2) showed that, both before and after TRPV1 blockade, EA-evoked activity was lower than MA-evoked activity. However, in the MA group, evoked activity of one fiber before and after blockade of TRPV1 (respectively increasing from 0.25 to 3.15 and from 0.30 to 1.11 imp/s) was observed to be lower than the overall mean activity evoked by EA, suggesting that the extent of increase in activity did not determine the response to blockade.
In five of the eight afferent fibers used in the EA group, we examined their responses to both MA and EA before and after blockade of TRPV1 in random order. The discharge frequencies induced by sequential MA and EA simulation were significantly increased (both P < 0.01). Before and after blockade of TRPV1, the responses of these five afferents to EA stimulation (from 0.08 ± 0.08 to 2.92 ± 0.51 imp/s vs. from 0.23 ± 0.23 to 3.02 ± 1.08 imp/s, before vs. after TRPV1) were similar to those of three other afferents in the EA group (from 0.00 ± 0.00 to 3.04 ± 0.80 imp/s vs. from 0.00 ± 0.00 to 2.19 ± 0.52 imp/s, before vs. after TRPV1). Also, the responses of the five afferents in response to MA stimulation (from 0.04 ± 0.03 to 10.37 ± 1.48 imp/s vs. from 0.03 ± 0.02 to 5.07 ± 1.50 imp/s, before vs. after TRPV1) were similar to those of seven other afferents included in the overall group studied during MA stimulation (from 1.02 ± 0.64 to 6.96 ± 1.03 imp/s vs. from 0.07 ± 0.56 to 2.49 ± 0.71 imp/s, before vs. after TRPV1).
Stimulation of TRPV1 in Activation of DRG Neurons by MA and EA
TRPV1 protein expression in DRGs (C7–8) was similar in rats subjected to MA or EA after intrathecal injection of scrambled siRNA and sham acupuncture. TRPV1 in the DRG was reduced significantly (P < 0.05) after administration of TRPV1 siRNA in animals treated either MA or EA (Fig. 5, A and B). Accordingly, in rats subjected to MA or EA, the number of DRG neurons labeled with TRPV1 were reduced significantly (P < 0.01) by TRPV1 siRNA, whereas the number of neurons in animals treated with scrambled siRNA TRPV1 was not reduced, similar to sham acupuncture (Fig. 5, C–E). These data indicate that this receptor was successfully knocked down in the DRG by intrathecal injection of TRPV1 siRNA.
Importantly, we also observed that both MA and EA significantly (P < 0.05) increased pERK protein expression in DRGs (C7–8) in rats treated with scrambled siRNA compared with sham acupuncture controls (Fig. 6). In contrast, intrathecal administration of TRPV1 siRNA significantly (P < 0.05) reduced pERK protein expression in the DRG of rats treated with MA but not with EA (Fig. 6). Similarly, more DRG neurons labeled with pERK were found in rats following either MA or EA than those in animals treated with sham acupuncture (P < 0.01; Fig. 7). And fewer DRG neurons labeled with pERK were noted after MA but not after EA in TRPV1 knocked-down rats (Fig. 7).
Fig. 6.
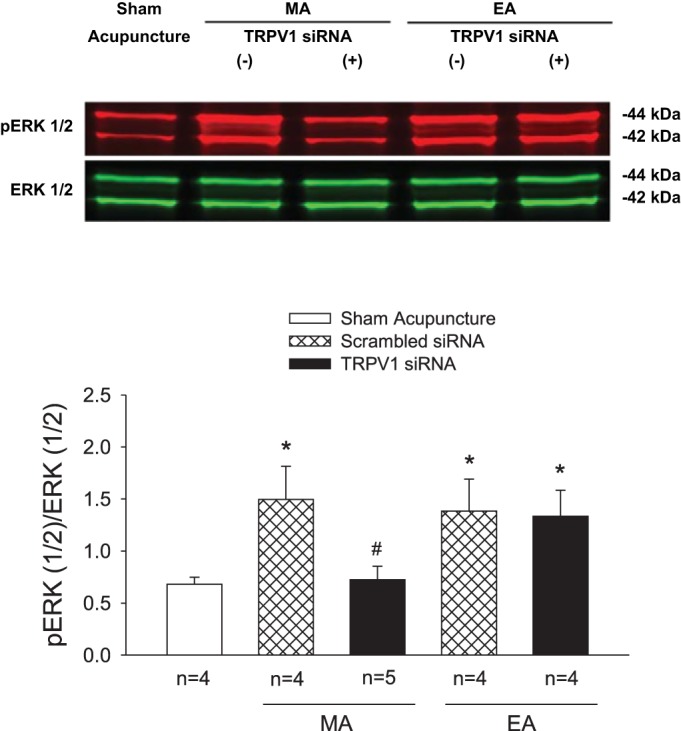
Western blot analysis of phosphorylated extracellular-signal related kinases 1 and 2 (pERK1/2) in dorsal root ganglia (DRG) (C7–8) of rats. Rats were treated with sham acupuncture, manual acupuncture (MA), or electroacupuncture (EA) after intrathecal injection of transient receptor potential vanilloid type 1 (TRPV1) siRNA (+) or scrambled siRNA (−). A: examples of original Western blotting bands from one rat in each group. B: relative values of pERK1/2 and ERK1/2. Bands indicated by 44 kDa and 42 kDa correspond to pERK1 and ERK1 and pERK2 and ERK2, respectively. *P < 0.05 vs. sham acupuncture; #P < 0.05, TRPV1 siRNA vs. scrambled siRNA.
DISCUSSION
Using a combination of physiological, anatomic, and molecular approaches, we examined peripheral neural mechanisms by which acupuncture inhibits cardiovascular reflex responses. We observed that local blockade of TRPV1 within the region of the acupoint(s) reverses inhibition of visceral sympathoexcitatory reflexes by MA but not by EA. Both MA and EA excite Group III and IV sensory neurons that are capsaicin sensitive as well as (high-threshold) mechanosensitive somatic afferents and, hence, are bimodal in function. Local inhibition of TRPV1 in the region of P5–6 attenuates the discharge response induced by MA but not by EA stimulation. Although both MA and EA increase DRG neuronal activity, as noted by changes in pERK expression, decreasing TRPV1 activity in the DRG with intrathecal TRPV1-siRNA reduces pERK in the DRG neurons of MA- but not EA-treated animals. These data support our working hypothesis that TRPV1 is responsible for acupuncture activation of high-threshold mechanosensitive Group III and IV somatic afferents and associated DRG neurons but refines our conclusion by showing that stimulation of TRPV1 in these bimodal afferents is important during MA but not EA modulation of reflex elevations in BP.
TRPV1 is widely distributed and abundantly expressed particularly in peripheral sensory neurons and in various regions in the CNS (36). TRPV1 is also expressed in nonneural tissues like keratinocytes in the epidermis, smooth muscle, glial, and mast cells as well as in macrophages (36). TRPV1 is located in both neural and nonneural tissues in the regions of acupoints like ST36 and BL40 (1, 42). Administration of capsaicin into the region of ST36 on the hindleg mimics MA analgesia in mice (42), suggesting that TRPV1 participates locally in acupuncture modulation of pain. However, this information does not prove that TRPV1 is involved in acupuncture-related analgesia, since no studies have evaluated the response of pain following inhibition of this receptor system. Furthermore, despite the association of TRPV1 with neural elements in the region of acupoints, such information does not prove a peripheral role for TRPV1 in acupuncture. MA and EA at ST36–37 and at P5–6 acupoints are known to modulate visceral reflex cardiovascular pressor responses (28, 44). However, the importance of peripheral TRPV1 in acupuncture modulation of cardiovascular responses has not been studied. The present studies provide a stepwise approach to assess this possibility. Our data show that local blockade of TRPV1 in the region of the acupoint(s) reverses acupuncture inhibition of visceral sympathoexcitatory reflex excitation during MA at P6 but not during EA at P5–6.
Although TRPV1 is present in both sensory nerves and nonneural tissues in the acupoint area, our data suggest that TRPV1 in sensory nerves likely plays an important role in acupuncture modulation of cardiovascular responses. In this regard, our current and past data show that MA and EA stimulation at P5–6 activates Group III and IV somatic afferent fibers (39, 44). Blockade of these afferents with regional anesthesia eliminates acupuncture's actions (29). Second, blockade of TRPV1 in the region of P6 attenuates increased activity of these afferent fibers, which respond immediately (within 1–3 s) to MA. Last, TRPV1 is expressed in Group III and IV primary sensory nerve fibers (4, 16) and, as we have shown here, in cell bodies of afferents responsive to acupuncture stimulation. Therefore, acupuncture and specifically MA modulates excitatory reflex responses, likely through activation of TRPV1 in Group III and IV afferents.
Regions underlying acupoints are innervated abundantly by somatic afferents located in cutaneous and subcutaneous tissues including tendon and muscle, among others (1, 43). Both Group III and IV somatic afferents are activated by MA and EA in rats (22, 44). These somatic afferents can be categorized into chemosensitive, mechanosensitive, thermosensitive, and polymodal afferents based on their physiological function (37, 38). The functional types of Group III and IV somatic afferents involved in MA and/or EA action on sensory afferents have not been studied previously. Using the single-unit afferent recording approach in the present study, we found that Group III and IV somatic afferents responsive to MA and EA were at least bimodal in rats, since they responded to mechanical stimulation with high thresholds as well as to capsaicin. The ratio of Group III and IV bimodal afferents activated by MA and/or EA was ~40:60, indicating that more Group IV than Group III bimodal afferents were activated by acupuncture. The ratio of finely myelinated to unmyelinated afferents in rats is different from the ratio of 70:30 previously reported in cats (27). The observed differences may be due to inclusion of all functional types of afferents in the previous cat study vs. only bimodal afferents in the present study and/or a species difference. Previous studies have suggested that a large portion of somatic afferents innervating subcutaneous tissues are high-threshold mechanosensitive fibers (37, 38) whereas the majority of cutaneous afferents respond to gentle mechanical stimuli, with Von Frey hairs ranged from 0.045 to 0.1 g and, hence, are low-threshold mechanosensitive (2, 19); such gentle stimuli did not stimulate any of the 27 bimodal fibers studied in the present study. Thus, our data imply that the bimodal somatic afferents with high mechanosensitive threshold responding to acupuncture at P5–6 likely were located subcutaneously.
Interestingly, blockade of TRPV1 in the acupoint area attenuated increased activity of Group III and IV afferents in response to MA, but not to EA, suggesting that the two forms of stimulation likely employ different mechanisms to activate these afferents. When an acupuncture needle is rotated repetitively, the mechanical stimulation generates action potentials, at least in part, through a TRPV1 mechanism in the afferent nerves. Conversely, low current applied to the needle directly depolarizes afferents in the region of the acupoint. Thus, TRPV1 does not contribute to EA-evoked activity of the bimodal sensory fibers, while MA requires stimulation of TRPV1 to excite both the myelinated and unmyelinated afferents.
We observed that blockade of TRPV1 reduces but does not eliminate MA-evoked activity of MN afferents or MA-modulated inhibition of pressor responses, suggesting that MA may also stimulate afferent nerves through activation of other receptors/channels in addition to TRPV1 to modulate cardiovascular responses. In this respect, MA stimulation induces local release of ATP and adenosine in the ST36 acupoint region (14). Extracellular ATP activates sensory afferents through P2 receptors and may interact with TRPV1 receptors in sensory neurons (15, 41). Adenosine contributes to MA-mediated analgesia through adenosine A1 receptors at ST36 (14), although past investigation has not defined the location of action of adenosine. However, MA could activate sensory afferent nerves through activation of P2, adenosine A1 and TRPV1 receptors as well as other yet-unstudied mediators and receptor mechanisms.
The single-unit nerve recording approach examined only a sample of sensory afferents’ responding to MA and EA stimulation. To confirm these observations and to evaluate a greater number of peripheral sensory nerves in response to MA and EA, we assessed the response of cell bodies of primary sensory afferent nerves in the C7–8 DRG that transduce and transmit sensory information from the periphery to the CNS (9). To identify activation of DRG neurons induced by MA and EA, we examined pERK expression in the DRG. pERK expression is a molecular biomarker indicating neuronal activation through MAPK/ERK intracellular transduction, an important intracellular signaling pathway (8, 9). We observed increases in pERK expression in DRG neurons (~25%) at C7–8 spinal levels following MA and EA, showing that both modalities activate sensory DRG neurons. Knocking down TRPV1 in the DRG reduced expression of pERK in the DRG neurons induced by MA, but not by EA, consistent with the aforementioned reflex and afferent data. These data further support the notion that TRPV1 at a point-specific region like P6 located over the MN on the distal forelimbs is critical during MA activation of sensory afferent nerves to modulate the pressor responses.
We noted that local blockade of TRPV1 differently affected the action of MA and EA inhibition of GD-induced pressor responses. TRPV1 pharmacological blockade reduced the action of MA but not EA on the excitatory reflex responses. Several possibilities might explain this discrepancy. First, MA applied discretely at the P6 acupoint involves mechanical stimulation that could excite local nerve endings through a TRPV1 mechanism, since TRPV1 is mechanosensitive (3, 10, 11, 21). Conversely, low-intensity EA (0.3–0.5 mA) applied through the two needles inserted into the larger P5–6 area likely stimulates both local nerve endings and axons of small fibers in the MN trunk with endings located distal to needle placement. Second, MA stimulation induces local release of ATP and possibly other chemical mediators (14). Extracellular ATP is known to excite sensory endings through interaction with TRPV1 (15, 24, 41) and, hence, may contribute to TRPV1-related MA activation of afferent endings. Importantly, the current findings, showing participation of TRPV1 in activation of sensory afferent during MA but not EA, support our other observation that TRPV1 blockade reverses inhibition of GD-induced pressor responses by MA but not by EA.
It is important to acknowledge that in the present study we did not fully explore the contributions of some subgroups of somatic afferents to acupuncture modulation of cardiovascular responses, since we selected bimodal afferents to determine the role of TRPV1 in acupuncture’s action. For instance, mechanosensitive but capsaicin-insensitive somatic afferents also could be involved in acupuncture modulation of cardiovascular responses, but likely their involvement would be through mechanisms other than the transient receptor potential system. Capsaicin-sensitive but mechanoinsensitive afferents might also participate in EA’s action in modulation of reflex elevation in BP, since EA could stimulate this subgroup of afferents by directly depolarizing nerve fibers. Future studies are warranted to determine participation of these subgroups of somatic afferents in MA and EA modulation of cardiovascular responses.
The group data showed that, both before and after TRPV1 blockade, MA-evoked activity was higher than EA-evoked activity. This raises the possibility that the action of TRPV1 blockade is effective only when there is a high level of activity as that induced during MA. We did observe, however, that activity of one fiber in the MA group was from 0.25 to 3.15 imp/s during MA stimulation. After TRPV1 blockade, the MA-induced activity increased from 0.30 to 1.11 imp/s, a level below the overall mean activity evoked by EA, suggesting that effectiveness of TRPV1 blockade likely is independent of the extent of afferent response to MA or EA.
An interesting observation was that the same extent of modulation of reflex elevations in BP occurs during EA and MA despite quite different increases in afferent discharge activity during each modality. It is possible that, although EA-evoked afferent activity is less than the MA-evoked activity, increased afferent activity evoked by EA has reached a “ceiling” of modulation of sympathoexcitatory output. Future studies are warranted to elucidate this observation.
Perspectives and Significance
Sympathoexcitatory reflexes elevate BP and have the potential to increase morbidity and mortality of patients with cardiovascular diseases (18, 23). Our past studies of the actions of acupuncture in lowering reflex-induced elevations in BP provide important clues to direct clinical management of acute pressor responses and importantly have guided our treatment of patients with essential hypertension (26, 32). Although acupuncture has been practiced for many years, its biological basis has not been well established. Our new findings indicate that both MA and EA activate bimodal sensory afferents at the P6 acupoint to modulate cardiovascular responses. Thus, stimulation of afferent nerves during either acupuncture modality is the first necessary step in effective modulation of sympathoexcitatory reflexes. Our results also show that MA and EA employ different neural mechanisms to activate the afferents and ultimately influence cardiovascular function. MA activates afferent nerves, at least in part, through a receptor-specific mechanism whereas EA either directly depolarizes nerves electrically or utilizes a mechanism other than the TRPV1 system. Since capsaicin cream is used for pain treatment in clinics, our results suggest that when this alternative approach is applied it may mimic and/or enhance acupuncture’s action (20). Future studies are required to examine these possibilities and the underlying mechanisms, but clinical use of acupuncture could be beneficial in treating cardiovascular and possibly other diseases.
In conclusion, the results from the present study demonstrate that TRPV1 plays a role in activation of Group III and IV bimodal somatic afferent nerves and the associated DRG neurons induced by MA, but not by EA, to modulate excitatory cardiovascular reflex responses. These new findings extend our knowledge of peripheral sensory receptor mechanisms underlying acupuncture’s influence on cardiovascular function.
GRANTS
This work was supported by National Center for Complementary & Integrative Health Grant AT009347 (Z.-L. Guo and J.C. Longhurst) and National Heart, Lung, and Blood Institute Grant HL-072125 (S.C. Tjen-A-Looi and J.C. Longhurst). Z.-L. Guo obtained the Department of Medicine Chair’s Research Award, University of California, Irvine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.-L.G., L.-W.F., and J.C.L. conceived and designed research; Z.-L.G., L.-W.F., and H.-F.S. performed experiments; Z.-L.G., L.-W.F., and H.-F.S. analyzed data; Z.-L.G., L.-W.F., H.-F.S., S.C.T.-A-L., and J.C.L. interpreted results of experiments; Z.-L.G., L.-W.F., and H.-F.S. prepared figures; Z.-L.G. drafted manuscript; Z.-L.G., L.-W.F., H.-F.S., S.C.T.-A-L., and J.C.L. edited and revised manuscript; Z.-L.G., L.-W.F., H.-F.S., S.C.T.-A-L., and J.C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Yash Patel.
REFERENCES
- 1.Abraham TS, Chen ML, Ma SX. TRPV1 expression in acupuncture points: response to electroacupuncture stimulation. J Chem Neuroanat 41: 129–136, 2011. doi: 10.1016/j.jchemneu.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol 32: 1025–1043, 1969. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 3.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 4.Bridges D, Rice AS, Egertová M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience 119: 803–812, 2003. doi: 10.1016/S0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517, 2001. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Geis C, Sommer C. Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci 28: 5836–5845, 2008. doi: 10.1523/JNEUROSCI.4170-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christoph T, Grünweller A, Mika J, Schäfer MK, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, Kurreck J. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun 350: 238–243, 2006. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Cruz CD, Cruz F. The ERK 1 and 2 pathway in the nervous system: from basic aspects to possible clinical applications in pain and visceral dysfunction. Curr Neuropharmacol 5: 244–252, 2007. doi: 10.2174/157015907782793630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci 22: 7737–7745, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol 583: 663–674, 2007. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci 12: 139–153, 2011. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 12.Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, Martin P, Bevan S, Fox A, Ganju P, Wishart W, Hall J. siRNA relieves chronic neuropathic pain. Nucleic Acids Res 32: e49, 2004. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu L-W, Guo ZL, Longhurst JC. Endogenous endothelin stimulates cardiac sympathetic afferents during ischaemia. J Physiol 588: 2473–2486, 2010. doi: 10.1113/jphysiol.2010.188730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci 13: 883–888, 2010. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11: 946–958, 1999. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 16.Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett 250: 177–180, 1998. doi: 10.1016/S0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- 17.Hua XB. Acupuncture manual for small animals. In: Experimental Acupuncture, edited by Hua XB. Shanghai, China: Shanghai Science and Technology Publisher, 1994, p. 269–290. [Google Scholar]
- 18.Huang H-S, Stahl GL, Longhurst JC. Cardiac-cardiovascular reflexes induced by hydrogen peroxide in cats. Am J Physiol Heart Circ Physiol 268: H2114–H2124, 1995. doi: 10.1152/ajpheart.1995.268.5.H2114. [DOI] [PubMed] [Google Scholar]
- 19.Jänig W, Grossmann L, Gorodetskaya N. Mechano- and thermosensitivity of regenerating cutaneous afferent nerve fibers. Exp Brain Res 196: 101–114, 2009. doi: 10.1007/s00221-008-1673-5. [DOI] [PubMed] [Google Scholar]
- 20.Jara-Oseguera A, Simon SA, Rosenbaum T. TRPV1: on the road to pain relief. Curr Mol Pharmacol 1: 255–269, 2008. doi: 10.2174/1874467210801030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones RC III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kagitani F, Uchida S, Hotta H, Aikawa Y. Manual acupuncture needle stimulation of the rat hindlimb activates groups I, II, III and IV single afferent nerve fibers in the dorsal spinal roots. Jpn J Physiol 55: 149–155, 2005. doi: 10.2170/jjphysiol.R2120. [DOI] [PubMed] [Google Scholar]
- 23.Kliks BR, Burgess MJ, Abildskov JA. Influence of sympathetic tone on ventricular fibrillation threshold during experimental coronary occlusion. Am J Cardiol 36: 45–49, 1975. doi: 10.1016/0002-9149(75)90866-8. [DOI] [PubMed] [Google Scholar]
- 24.Kwon SG, Roh DH, Yoon SY, Moon JY, Choi SR, Choi HS, Kang SY, Han HJ, Beitz AJ, Lee JH. Blockade of peripheral P2Y1 receptors prevents the induction of thermal hyperalgesia via modulation of TRPV1 expression in carrageenan-induced inflammatory pain rats: involvement of p38 MAPK phosphorylation in DRGs. Neuropharmacology 79: 368–379, 2014. doi: 10.1016/j.neuropharm.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Tjen-A-Looi SC, Guo ZL, Longhurst JC. Repetitive electroacupuncture attenuates cold-induced hypertension through enkephalin in the rostral ventral lateral medulla. Sci Rep 6: 35791, 2016. doi: 10.1038/srep35791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Longhurst JC. Neural mechanism of electroacupuncture’s hypotensive effects. Auton Neurosci 157: 24–30, 2010. doi: 10.1016/j.autneu.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P, Pitsillides KF, Rendig SV, Pan H-L, Longhurst JC. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation 97: 1186–1194, 1998. doi: 10.1161/01.CIR.97.12.1186. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Rowshan K, Crisostomo M, Tjen-A-Looi SC, Longhurst JC. Effect of electroacupuncture on pressor reflex during gastric distension. Am J Physiol Regul Integr Comp Physiol 283: R1335–R1345, 2002. doi: 10.1152/ajpregu.00192.2002. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Tjen-A-Looi SC, Longhurst JC.. Acupuncture's role in cardiovascular homeostasis. In: Current Research in Acupuncture, edited by Xia Y, Dong G, and Wu GC. New York: Springer Science & Business Media, 2013, p. 457–486. [Google Scholar]
- 30.Li P, Tjen-A-Looi SC, Cheng L, Liu D, Painovich J, Vinjamury S, Longhurst JC. Long-lasting reduction of blood pressure by electroacupuncture in patients with hypertension: randomized controlled trial. Med Acupunct 27: 253–266, 2015. doi: 10.1089/acu.2015.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Tjen-A-Looi SC, Longhurst JC. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am J Physiol Heart Circ Physiol 290: H2535–H2542, 2006. doi: 10.1152/ajpheart.00972.2005. [DOI] [PubMed] [Google Scholar]
- 32.Longhurst JC, Tjen-A-Looi S. Acupuncture regulation of blood pressure: two decades of research. Int Rev Neurobiol 111: 257–271, 2013. doi: 10.1016/B978-0-12-411545-3.00013-4. [DOI] [PubMed] [Google Scholar]
- 33.Luo MC, Zhang DQ, Ma SW, Huang YY, Shuster SJ, Porreca F, Lai J. An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Mol Pain 1: 29, 2005. doi: 10.1186/1744-8069-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa H, Hiura A. Capsaicin, transient receptor potential (TRP) protein subfamilies and the particular relationship between capsaicin receptors and small primary sensory neurons. Anat Sci Int 81: 135–155, 2006. doi: 10.1111/j.1447-073X.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 35.Pan HL, Chen SR. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation 110: 1826–1831, 2004. doi: 10.1161/01.CIR.0000142618.20278.7A. [DOI] [PubMed] [Google Scholar]
- 36.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol 179: 155–171, 2007. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto N, Yamashita T, Takebayashi T, Sekine M, Ishii S. An electrophysiologic study of mechanoreceptors in the sacroiliac joint and adjacent tissues. Spine 26: E468–E471, 2001. doi: 10.1097/00007632-200110150-00008. [DOI] [PubMed] [Google Scholar]
- 38.Schaible HG, Schmidt RF. Activation of groups III and IV sensory units in medial articular nerve by local mechanical stimulation of knee joint. J Neurophysiol 49: 35–44, 1983. doi: 10.1152/jn.1983.49.1.35. [DOI] [PubMed] [Google Scholar]
- 39.Tjen-A-Looi SC, Fu L-W, Zhou W, Syuu Z, Longhurst JC. Role of unmyelinated fibers in electroacupuncture cardiovascular responses. Auton Neurosci 118: 43–50, 2005. doi: 10.1016/j.autneu.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Tjen-A-Looi SC, Li P, Longhurst JC. Medullary substrate and differential cardiovascular responses during stimulation of specific acupoints. Am J Physiol Regul Integr Comp Physiol 287: R852–R862, 2004. doi: 10.1152/ajpregu.00262.2004. [DOI] [PubMed] [Google Scholar]
- 41.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA 98: 6951–6956, 2001. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu SY, Chen WH, Hsieh CL, Lin YW. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: mechanosensitive TRPV1 as an “acupuncture-responding channel”. BMC Complement Altern Med 14: 96, 2014. doi: 10.1186/1472-6882-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol 85: 355–375, 2008. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhou W, Fu L-W, Tjen-A-Looi SC, Li P, Longhurst JC. Afferent mechanisms underlying stimulation modality-related modulation of acupuncture-related cardiovascular responses. J Appl Physiol (1985) 98: 872–880, 2005. doi: 10.1152/japplphysiol.01079.2004. [DOI] [PubMed] [Google Scholar]
- 45.Zhou WY, Tjen-A-Looi SC, Longhurst JC. Brain stem mechanisms underlying acupuncture modality-related modulation of cardiovascular responses in rats. J Appl Physiol (1985) 99: 851–860, 2005. doi: 10.1152/japplphysiol.01365.2004. [DOI] [PubMed] [Google Scholar]