Abstract
µ-Opioid G protein-coupled receptors (MOR) interact with ion channels to decrease neuronal excitability. In humans, intrathecal administration of the MOR agonist fentanyl inhibits the exercise pressor reflex, an effect that can be attributed to either the opening of inward rectifying potassium channels (GIRK) or the closing of N-type calcium channels. The purpose of this study was to determine if the highly selective MOR agonist [d-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO) attenuates the exercise pressor reflex and which of these two channels are responsible for this effect. In decerebrate rats, we determined the effect of intrathecal injection of either tertiapin-LQ, which blocks the GIRK channel or ω-conotoxin-GVIA, which blocks the N-type calcium channel on the exercise pressor reflex, which was evoked by contracting the triceps surae muscles. Initially, we established that intrathecal injection of DAMGO inhibited the exercise pressor reflex relative to no intrathecal injection or intrathecal saline injection (P < 0.001, n = 5). We then found that intrathecal injection of two doses of tertiapin-LQ (1 and 10 µg) had no effect on the exercise pressor reflex (n = 6 and n = 7, respectively; P > 0.05). Importantly, neither dose of tertiapin-LQ prevented the DAMGO-induced inhibition of the exercise pressor reflex. Last, we found that intrathecal injection of ω-conotoxin-GVIA markedly attenuated the exercise pressor reflex (P < 0.001, n = 7). The cardioaccelerator response to contraction did not appear to be effected in any of the experiments. We conclude that N-type voltage-gated calcium channel inhibition appears to be the mechanism by which MOR activation inhibits the exercise pressor reflex in decerebrate rats.
Keywords: arterial pressure, autonomic function, circulatory control, GIRK channels, heart rate, sympathetic nervous system
INTRODUCTION
The cardiovascular responses to exercise, regardless of whether it is dynamic or static, are for the most part caused by increases in sympathetic nervous activity and decreases in parasympathetic nervous activity. The neural mechanisms initiating these autonomically mediated events have been the subject of considerable controversy and investigation. Two mechanisms have been suggested, namely central command (8, 10) and the exercise pressor reflex (6, 19, 21). Central command postulates that these responses arise from the parallel activation of somatomotor and autonomic neural circuits in the brain. Central command is a feed forward mechanism that does not require any feedback (i.e., sensory input) from contracting muscles. The exercise pressor reflex postulates that metabolic and mechanical stimuli arising in contracting muscle increase the discharge of thin fiber (group III and IV) sensory nerves, whose synaptic input onto interneurons in laminae I, II, and IV of the dorsal horn of the spinal cord and subsequently the brainstem (15) alters via a reflex mechanism the output of the autonomic circuits controlling cardiovascular function.
In humans, distinguishing between the contributions of the exercise pressor reflex from those of central command to the autonomic responses to exercise has evoked great interest and controversy. One technique used to make this distinction is the intrathecal injection of opioids onto the lumbar and sacral portions of the spinal cord. The rationale underlying this technique is that stimulating opioid receptors on the terminal endings of thin fiber muscle afferents synapsing in the dorsal horn of the spinal cord as well as stimulating opioid receptors on the interneurons receiving synaptic input from these afferents attenuates or abolishes the exercise pressor reflex while simultaneously having no effect on central command, which arises from supraspinal structures. In humans, μ-oipoid receptor agonists, injected intrathecally, have proven to be particularly effective in investigating the roles played by the two mechanisms in causing the autonomic responses to exercise (3, 4). Specifically, intrathecal injections of fentanyl, a µ-opioid agonist, have revealed that the exercise pressor reflex plays an important role in generating these autonomic effects, which in turn are manifested as increases in arterial pressure, cardiac output, and heart rate. Moreover, intrathecal injections of fentanyl have revealed that in healthy subjects the exercise pressor reflex functions to increase arterial blood flow to exercising muscles during single leg extension (5).
When stimulated, µ-opioid receptors inhibit neuronal discharge by either closing the N-type calcium channel or by opening an inward rectifying potassium channel (GIRK), both of which are coupled to G proteins via interaction with βγ-subunits (2, 12). Nevertheless, nothing is known about which channel is functional when agonists to µ-opioid receptors are injected intrathecally or epidurally to attenuate the exercise pressor reflex. In the experiments to be described, we have used a pharmacological approach in an animal model to determine which of the two channels affected by µ-opioid agonists play a role in inhibiting the exercise pressor reflex.
METHODS
Adult male Sprague-Dawley rats (330–650 g) were acclimated in an ambient temperature-controlled facility (24 ± 1°C) and fed standard rat chow and tap water ad libitum on a 12:12-h light-dark cycle. The procedures and protocols used in this investigation were approved by the Institutional Animal Care and Use Committee of the Penn State College of Medicine. Rat core temperatures were maintained at ~37–38°C with a heat lamp throughout all surgical procedures. At the end of each experiment the rats, which were decerebrated (see below), were euthanized with an intravenous injection of saturated potassium chloride and the chest was opened.
Surgical procedures.
Rats were prepared similarly as described before (7). The rats were anesthetized with isoflurane (3–4%), the trachea was cannulated, and the lungs were mechanically ventilated (Harvard Apparatus) with the vaporized anesthetic (2%, oxygen balanced) until completion of decerebration, after which the lungs were mechanically ventilated with room air. The right and left carotid arteries were cannulated retrogradely (PE-50 catheter; Becton Dickinson) to measure arterial blood pressures (P23 XL; Statham) and to collect blood for periodic analysis of arterial gases and pH (ABL 80 FLEX, radiometer). Blood gas parameters and pH were maintained within normal limits (: 35–45 mmHg; : >90 mmHg, pH 7.35–7.45) by adjusting ventilation and or intravenous supplementation with sodium bicarbonate (8.5%).
A laminectomy (L2–L3) was performed to expose the spinal cord, after which a catheter (PE-8) was inserted under the dura. The saline-filled catheter was guided caudally until the tip was parallel to the L5 vertebrae and was then secured with Kwik Seal. A 2-mm catheter sleeve (PE-20) was fixed around the end of the catheter tip exposed to air, and a Hamilton syringe needle (25 µl capacity) was used to introduce dissolved drugs or saline into the spinal fluid. The dead space of the catheter was 8 µl total.
The triceps surae muscles and the sciatic nerve were exposed by blunt dissection. The calcaneal tendon was severed and linked with braided fishing line to a force transducer (FT10; Grass Instruments), which in turn was attached to a rack and pinion. The peroneal nerve was cut, and then the tibial nerve was very carefully isolated, after which a shielded stimulating electrode was placed under it.
Before decerebration rats were placed in a customized Kopf stereotaxic and spinal frame. Dexamethasone (Fresenius Kabi) was given (0.2 mg iv) just before beginning the precollicular decerebration to limit brainstem edema. The brain stem was sectioned with a blunt spatula 1 mm anterior to the superior colliculi, and all neural tissue rostral to the section was removed by suction. Kwik Seal was applied over and at the base of the colliculi to protect them from drying and irritation, as well as to occlude bleeding vessels, and the cranial cavity was packed with gauze and cotton. The preparation was allowed to stabilize for 60 min before beginning experimental protocols. The stereotaxic apparatus was tilted head-up 15° throughout the duration of experimental protocols to minimize migration of the injectate toward the medulla.
Experimental procedures.
The triceps surae muscles were contracted for 30 s by electrically stimulating (40 Hz; 0.01-ms pulse duration) the tibial nerve with currents that were less than two times motor threshold. For all experiments, both the functionality and stability of the preparation were confirmed by employing two contractions that evoked the pressor reflex. Twenty microliters of saline were injected into the intrathecal catheter before the second contraction. All contractions were performed on decerebrated and unanesthetized rats because previous work indicated that anesthetics inhibited the exercise pressor reflex (32). A schematic depicting the experiments performed is shown in Fig. 1.
Fig. 1.
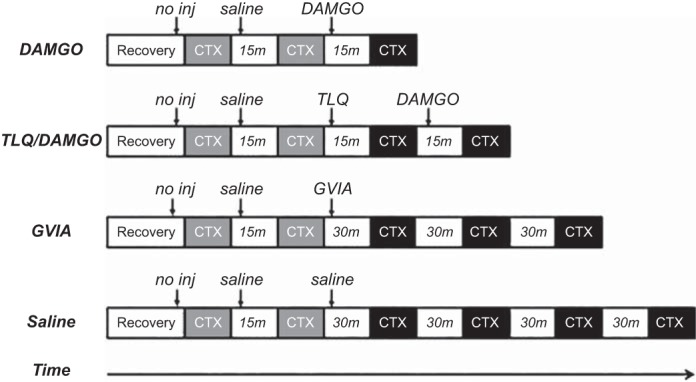
Schematic representation of protocols. Intrathecal injections (arrows) of saline or drugs dissolved in saline are indicated by arrows. After surgical procedures, rats were allowed to recover from anesthesia for 60 min before beginning experimental protocols. The first 2 contractions (CTX; gray boxes) were used to verify the viability of the preparation to express the exercise pressor reflex upon hindlimb muscle contraction. Subsequent contractions (black boxes) were used to examine the effect of the drug on the exercise pressor reflex. Series of contractions were separated by intervals of time (white boxes). DAMGO, [d-Ala2,N-MePhe4,Gly-ol]-enkephalin; GVIA, ω-conotoxin-GVIA; TLQ, tertiapin-LQ; no inj, no injection.
The first set of experiments was conducted to determine whether intrathecal injection of [d-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO) (Tocris Cookson, Bristol, UK), a µ-opioid agonist, attenuated the exercise pressor reflex elicited by contraction of the triceps surae muscles. DAMGO is ~500 times more selective for µ-opioid receptors than it is for either δ- or κ-opioid receptors. In contrast, fentanyl is only 100 times more selective for µ-opioid receptors than it is for either δ- or κ-opioid receptors (9). After verifying the integrity of the preparation, we injected 2 µg of DAMGO (n = 5) into the intrathecal catheter (in 10 µl carrier saline, followed by a 20-µl saline flush) 5 min before we initiated contraction. Ketamine (VetaKet; Akorn, Lake Forest, IL) was injected intravenously (0.5 mg) as needed to prevent movement. When given, ketamine was injected before the first contraction and was given before every subsequent contraction. The interval between ketamine injection and contraction was always 3 min.
The second set of experiments determined if intrathecal blockade of GIRK1/2 channels prevented the DAMGO-induced attenuation of the exercise pressor reflex. In one group of rats (n = 6), we injected 1 µg of tertiapin-LQ (TLQ) toxin (Alomone, Jerusalem, Israel), a GIRK1/2-channel antagonist, into the intrathecal catheter 10 min before contraction. Five minutes after the previous contraction, we injected 2 µg of DAMGO into the intrathecal catheter 5 min before the subsequent contraction. In another group of rats (n = 7), we repeated this protocol but the dose of TLQ was 10 µg instead of 1 µg. Both DAMGO and TLQ were dissolved in saline and were injected in a volume of 10 µl that was followed by a 20-µl saline flush. Tertiapin-LQ does not inhibit voltage-gated potassium channel currents (28). Tertiapin-LQ was predicted to blunt the DAMGO-induced inhibition (18) of the exercise pressor reflex, but by itself was predicted to have no effect. A large dose of TLQ (50 µg) was injected intrathecally in three experiments; this dose abruptly raised baseline blood pressure ~30–50 mmHg within 6 min of administration and caused body shaking before and during contractions. These effects induced by 50 µg of TLQ prevented its use in our experiments.
The third set of experiments determined if blockade of the N-type calcium channel CaV2.2 influenced the exercise pressor reflex. In seven rats, we intrathecally injected the specific CaV2.2 inhibitor ω-conotoxin-GVIA (1 µg; Alomone) followed by a 20-µl saline flush 30 min before contracting the triceps surae muscles. A larger dose of ω-conotoxin-GVIA was not used due to the side effects we noted and that others have reported (33).
Control experiments.
To verify that the experimental preparation remained functional over the period of time required to complete the protocols, we injected saline intrathecally (30 µl) in four rats and then contracted the triceps surae muscles at 30-min intervals over a 2-h period. The volume of saline injected was identical to that of the ω-conotoxin-GVIA injections.
At the end of each experiment, we injected pancuronium bromide (0.5 mg/kg iv) to paralyze the rats and then electrically stimulated the tibial nerve with the same frequency, current, and pulse widths that were used to contract the triceps surae muscles. We did this to confirm that the pressor responses evoked by static contraction were not caused by electrical stimulation of afferent fibers. To assess the spread of the intrathecal injections, we injected blue dye into the intrathecal catheter using a volume equal to that of the injectate volume. Visual inspection showed that the dye stained the spinal cord up to the L2 vertebrae but did not stain the cervical spinal cord and medulla.
Data analysis.
All measurements were analyzed using Sigma Plot for Windows v. 11.0 (Systat Software, San Jose, CA). Statistics for blood pressure and heart rate changes evoked by hindlimb static contraction were performed on the difference between precontraction baseline and contraction peak measures. The data are expressed as means ± SE and were analyzed by repeated-measures ANOVA. When a significant difference was detected, a Holms-Sidak post hoc test was applied to specify differences between individual means. The criterion for statistical significance was P < 0.05.
RESULTS
Intrathecal DAMGO.
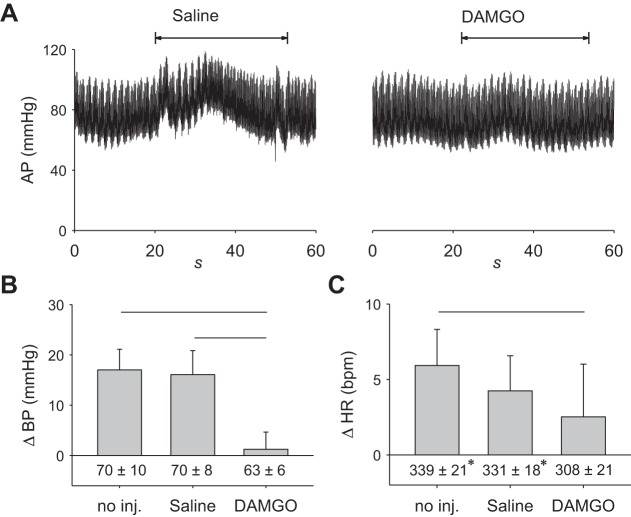
The magnitude of the exercise pressor reflex after intrathecal injection of saline was not different from the magnitude of the reflex when no injection was made (P = 0.70). In contrast, intrathecal injection of 2 µg of DAMGO (Fig. 2, A and B) decreased the magnitude of the reflex compared with that evoked after either intrathecal saline injection or no injection (both P < 0.001). Neither DAMGO nor saline injection had any effect on baseline blood pressure (P = 0.21). The modest cardioacceleration (Fig. 2C) induced reflexly by contraction was not different between control and saline injection (P = 0.12). There was a small decrease in cardioacceleration following DAMGO injection relative to control (P < 0.05) but not after saline injection (P = 0.29). In almost parallel fashion, there was a decrease in baseline heart rate following DAMGO injection relative to both control (P < 0.01) and saline (P = 0.02); however, it did not differ between control and saline (P = 0.29).
Fig. 2.
DAMGO attenuates the exercise pressor reflex (n = 5). A: 60 s of arterial pressure (AP) tracings including baseline and the onset of 30-s isometric contraction, indicated by the double arrow heads. Lines over bars represent significant differences between groups (P < 0.001). B: intrathecal injection of DAMGO (2 µg) attenuated the exercise pressor reflex. C: cardioaccelerator response to contraction following DAMGO was not only significantly different between the no injection and DAMGO conditions. Baseline heart rate (HR) was lower in the DAMGO condition relative to the no injection and saline conditions. Lines over bars represent significant differences between groups (P < 0.05). Baseline blood pressure (BP) and heart rate are given below vertical bars. DAMGO, [d-Ala2,N-MePhe4,Gly-ol]-enkephalin; no inj, no injection. *P < 0.05, significant difference relative to DAMGO only.
Intrathecal tertiapin-LQ and DAMGO.
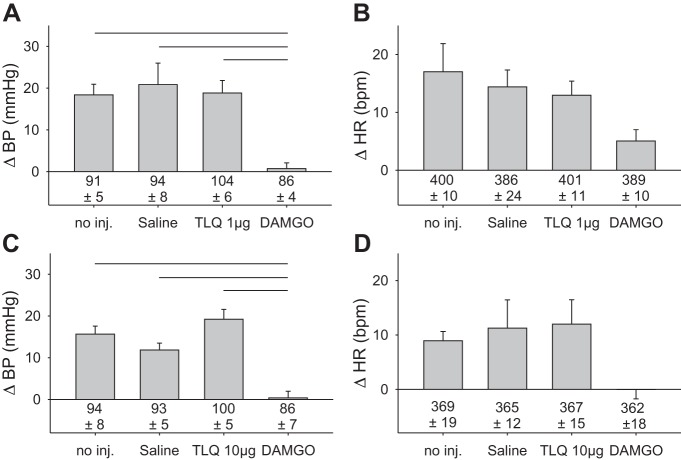
Alone, the GIRK channel blocker TLQ, injected intrathecally, had no effect on the reflex pressor response to contraction at either the 1- or 10-µg doses (Fig. 3, A and C). Most importantly, TLQ did not prevent the DAMGO-induced attenuation of the exercise pressor reflex (P < 0.001, relative to 1 and 10 µg TLQ). Baseline blood pressures were stable throughout the experiment and did not differ under any condition in the group receiving 1 µg TLQ (P = 0.10), or in the group receiving 10 µg TLQ (P = 0.53). Neither the 1-µg nor the 10-µg dose of TLQ had any effect on baseline heart rate (P = 0.84 and P = 0.90, respectively). In addition, neither dose injected alone nor after DAMGO had any effect on the modest cardioaccelerator component of the exercise pressor reflex (Fig. 3, B and D).
Fig. 3.
Intrathecal injection of tertiapin-LQ had no effect on the exercise pressor reflex. A and C: neither 1 nor 10 micrograms of tertiapin-LQ alone had any effect alone on the exercise pressor reflex; subsequent intrathecal injection of DAMGO (2 µg) still attenuated the reflex. Horizontal lines over bars represent significant differences between groups in A at P < 0.001 and in C at P < 0.01. B and D: cardioaccelerator response to contraction did not differ significantly between conditions. Baseline blood pressures and heart rates are given below vertical bars. In A and B, n = 6; in C and D, n = 7. TLQ, tertiapin-LQ; DAMGO, [d-Ala2,N-MePhe4,Gly-ol]-enkephalin; no inj, no injection.
Intrathecal ω-conotoxin-GVIA.
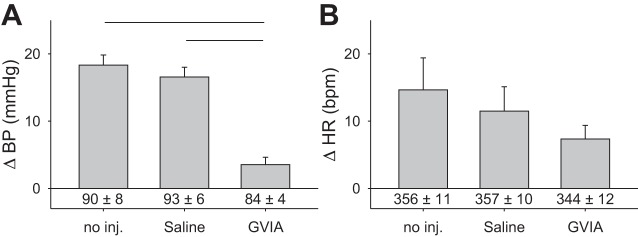
Intrathecal injection of the Cav2.2-specific antagonist (Fig. 4A) ω-conotoxin-GVIA (1 µg) blocked the exercise pressor reflex (P < 0.001). We contracted the triceps surae muscles every 30 min after intrathecal injection until the pressor response was attenuated, which in four rats occurred after 30 min, in one rat occurred after 60 min, and in two rats after 90 min. The data at these time points were combined for analysis. There were no differences in baseline blood pressures (P = 0.61), a finding that is consistent with previous observations that Cav2.2 blockade does not change baseline values when injected intrathecally in rabbits (35). Baseline heart rates were not changed by intrathecal injection of ω-conotoxin-GVIA at 1 μg (P = 0.09) at the observed time of maximal inhibition of the pressor responses to contraction. Additionally, injection of ω-conotoxin-GVIA did not change (P = 0.09) the cardioaccelerator component of the exercise pressor reflex (Fig. 4B).
Fig. 4.
Intrathecal injection of ω-conotoxin-GVIA (1 µg) attenuates the exercise pressor reflex (n = 7). A: intrathecal injection ω-conotoxin-GVIA attenuated the exercise pressor reflex. Lines over bars represent significant differences between groups (P < 0.001). B: cardioaccelerator response to contraction following ω-conotoxin-GVIA did not differ between groups. Baseline blood pressure and heart rate measurements are given below vertical bars. GVIA, ω-conotoxin-GVIA; no inj, no injection.
Intrathecal saline.
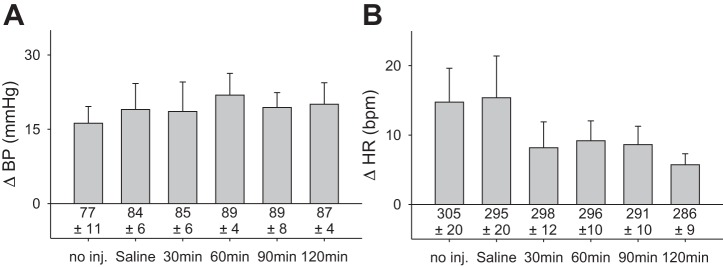
We measured the exercise pressor reflex after we injected saline intrathecally (20 µl) every 30 min for 2 h (Fig. 5). We performed this experiment to confirm that the attenuation of the exercise pressor reflex by ω-conotoxin-GVIA was not attributable to deterioration of our preparation over time. The peak blood pressure response to contraction did not decrease, and the response remained robust over the 120 min period (Fig. 5A). There were no differences in blood pressure responses between groups (P = 0.97), and baseline blood pressures remained stable (P = 0.82). Baseline heart rates did not change (P = 0.71); likewise, the cardioaccelerator component of the reflex (Fig. 5B) did not change (P = 0.14) over 120-min time period.
Fig. 5.
The exercise pressor reflex and resting arterial pressure are preserved for an extended period of time (n = 4). A: exercise pressor reflex was preserved for 120 min following intrathecal injection of saline. B: cardioaccelerator response to contraction was not significantly changed by intrathecal saline over the 120-min test period. Baseline blood pressure and heart rate are given below vertical bars; no inj, no injection.
Time-tension index during contractions.
Within experimental protocols the tension time indexes did not vary significantly between contractions (Table 1).
Table 1.
Comparison of time-tension indexes
| Groups/Contractions | TTI, g/s | SE, g/s |
|---|---|---|
| Protocol 1 (P = 0.245, n = 5) | ||
| Control | 25,598 | 2,949 |
| Saline | 32,092 | 3,280 |
| DAMGO, 2 μg | 29,735 | 1,187 |
| Protocol 2 (P = 0.489, n = 6) | ||
| Control | 22,009 | 4,450 |
| Saline | 26,005 | 3,347 |
| TLQ, 1 μg | 23,980 | 3,798 |
| TLQ + DAMGO | 25,445 | 4,825 |
| Protocol 3 (P = 0.893, n = 7) | ||
| Control | 19,444 | 2,781 |
| Saline | 19,494 | 1,919 |
| TLQ, 10 μg | 21,528 | 2,726 |
| TLQ + DAMGO | 20,667 | 2,342 |
| Protocol 4 (P = 0.114, n = 7) | ||
| Control | 33,128 | 5,239 |
| Saline | 30,824 | 3,725 |
| GVIA,* 1 μg | 25,628 | 3,193 |
| Protocol 5 (P = 0.07, n = 4) | ||
| Control | 22,924 | 1,951 |
| Saline | 32,283 | 2,750 |
| 30 min | 24,933 | 3,019 |
| 60 min | 32,878 | 3,047 |
| 90 min | 31,173 | 3,853 |
| 120 min | 29,275 | 2,593 |
Comparison of tension-time indexes within protocols. A one-way repeated-measures ANOVA revealed no differences between treatments. DAMGO, [d-Ala2,N-MePhe4,Gly-ol]-enkephalin; GVIA, ω-conotoxin-GVIA; TLQ, tertiapin-LQ; TTI, tension-time index.
Measurement recorded at time of maximal inhibition and combined for analysis.
DISCUSSION
Intrathecal injection of µ-opioid agonists, such as DAMGO and fentanyl, is firmly established to attenuate the exercise pressor reflex in both animals (13) and humans (5). The attenuation of the reflex by a µ-opioid agonist functions at the level of the neuronal membrane by either closing the N-type calcium channel or by opening an inwardly rectifying potassium channel, both of which are G protein coupled. Closure of the N-type calcium channel decreases the release of neurotransmitter by a neuron, whereas opening of the potassium channel, termed GIRK, hyperpolarizes the neuron.
In our experiments, we found that blocking the N-type calcium channel mimicked the attenuating effect of DAMGO, a µ-opioid agonist, on the exercise pressor reflex, whereas blocking the GIRK channel had no effect on the DAMGO-induced attenuation of the exercise pressor reflex. N-type calcium channels are found both on the terminal endings of thin fiber primary afferents synapsing in the dorsal horn as well as on the laminae I and II interneurons receiving this input (34). In contrast, GIRK channels are sparse on the endings of thin fiber primary afferents but are plentiful on laminae I and II interneurons of the dorsal horn. In particular, lamina II, also known as the substantia gelatinosa, is a plentiful site of interneurons containing GIRK channels (17).
We used two doses of TLQ to block GIRK channels in our experiments and found that neither had any effect on the DAMGO-induced attenuation of the exercise pressor reflex. This finding suggests that GIRK channels are not involved in the DAMGO-induced attenuation of the exercise pressor reflex. This lack of effect raises the possibility that the spinal concentration of TLQ created was not sufficient to block GIRK channels. To address this possibility, we calculated the concentration of TLQ bathing the spinal cord in our experiments. This calculation was based on an intrathecal injection of 10 µg of TLQ diluted by the injection volume of 22 µl plus 90 µl of cerebrospinal fluid (27). The concentration was calculated to be ~37 µM, which is a value that is over 30-fold greater than that (i.e., 1 µM) needed to prevent the DAMGO-induced depression of breathing when this µ-opioid agonist was applied to the pre-Botzinger complex of rats (22). Previously, Nakamura et al. (23) found that TLQ took effect within 10 min, a period of time that is very similar to that used in our experiments. Consequently, the doses of TLQ used in our experiments were likely to reveal a role played by spinal GIRK channels in the attenuation of the exercise pressor reflex by DAMGO if such a role existed.
Intrathecal injection or topical application of ω-conotoxin-GVIA to the surface of the spinal cord has been previously shown to attenuate various responses to noxious and nonnoxious stimuli. For example, in a behavioral experiment in rats, Malmberg and Yaksh (16) reported that intrathecal injection of a ω-conotoxin-GVIA increased the latency to respond to a noxious thermal stimulus. In an electrophysiological experiment in anesthetized rats, Neugebauer et al. (24) found that topical administration of ω-conotoxin-GVIA decreased the responses of wide dynamic range dorsal horn neurons to both innocuous and noxious mechanical stimulation of the knee joint. The latter report is consistent with the notion that N-type calcium channels play a role in attenuating input from thin fiber afferents responding to nonnoxious input, including that arising from group III mechanoreceptors.
Any interpretation of our findings must consider two limitations. First, our finding that intrathecal injection of ω-conotoxin-GVIA that attenuated the exercise pressor reflex cannot distinguish between pre- and postsynaptic mechanisms. Specifically, when injected intrathecally, the conotoxin blocked N-type calcium channels on both the terminal endings of thin fiber afferents as well as on dorsal horn interneurons, many of which are located in the substansia gelatinosa (34). Second, any interpretation of our finding that GIRK channels played no role in the DAMGO-induced attenuation of the exercise pressor reflex must consider the nature of the stimulus used in our experiments. We evoked the exercise pressor reflex by contracting the triceps surae muscles while they were freely perfused, a stimulus that most would consider to be relatively mild when compared with contracting these muscles while they were ischemic or while the muscles were subjected to damaging or intensely painful stimuli. We cannot exclude the possibility that GIRK channels play a role in μ-opioid-induced analgesia when these severe stimuli were present in muscle, such as those found in rats with ligated femoral arteries. Indeed, evidence for a role played by GIRK channels in μ-opioid receptor-induced analgesia under intense nociceptive stimuli, but not under milder nociceptive stimuli, has been previously reported (18).
Although intrathecal injection of ω-conotoxin-GVIA attenuated the exercise pressor reflex in our experiments, the onset of this effect was relatively long and variable. We can offer no explanation as to why this occurred, but we are not the first to report this finding. For example, Sanger et al. (29) reported that ω-conotoxin-GVIA required 50–60 min to maximally attenuate an electrically induced contraction of human vas deferens smooth muscle. In addition, Scott et al. (30) found that the latency to peak attenuation of tactile allodynia caused by intrathecal injection of ω-conotoxin-GVIA in rats was 120 min. In addition, Hannon and Atchison (11) demonstrated the slow association kinetics of ω-conotoxin-GVIA in rat spinal cord slices.
The variability in latency of effect for ω-conotoxin-GVIA found in our experiments in adult rats was unlikely to be explained by age or weight. This variability has been reported in vitro for cells taken from rats homogeneous for age. If age or weight was a factor, then either should have played a role in the increasing variability of the latency of effect in our experiments in which we injected either ω-conotoxin or DAMGO intrathecally. This did not appear to be the case.
Activation of N-type voltage-gated calcium channels on the presynaptic terminals of group III and IV fibers is necessary to release neurotransmitters and neuromodulators, such as glutamate and substance P, onto postsynaptic neurons in the dorsal horn of the spinal cord. Antagonists to N-type calcium channels have been shown to reduce substance P release in the spinal cord (20, 33). Findings such as this prompt us to speculate that in our experiments that intrathecal injection of ω-conotoxin-GVIA reduced the release of both glutamate and substance P by the contraction-induced activation of thin fiber muscle afferents synapsing in the dorsal horn of the spinal cord. This speculation is supported by previous reports from our laboratory that intrathecal injection of either a neurokinin 1 receptor antagonist (14) or an NMDA and AMPA receptor antagonist (1) attenuated the exercise pressor reflex.
In conclusion, we have demonstrated that attenuation of the exercise pressor reflex via blockade of Cav2.2 channels with ω-conotoxin-GVIA mimics the attenuation of the exercise pressor reflex by the μ-opioid receptor agonist DAMGO. A previous report has demonstrated that coapplication of very-low-dose morphine and ω-conotoxin-GVIA acts synergistically to alleviate pain (26). Together these findings suggest that μ-opioid receptor activation inhibits afferent excitability, thereby attenuating the exercise pressor reflex, by blocking the N-type Cav2.2 channel. In the freely perfused rat at least, GIRK channels within contraction sensitive afferent pathways are not opened by μ-opioid receptor activation.
Perspectives and Significance
To our knowledge this is the first demonstration of a significant role for the Cav2.2 channel in the exercise pressor reflex. However, we assume here that Cav2.2 channels are primarily clustered presynaptically within primary sensory afferent fibers. We do not know if additional channels are also found presynaptically on intermediate neurons within the dorsal horn. Mice lacking Cav2.2 have a reduced capacity to respond to inflammatory and neuropathic pain, and sympathetic deficits for heart rate and blood pressure control, which have been reviewed by others (31). Thus, pharmacologically blocking Cav2.2 channels within the spinal cord is potentially undesirable. On the other hand, blockade of peripheral Cav2.2 channels may be therapeutically beneficial in the setting of peripheral artery disease and hypertension (25). These observations prompt further investigations on the effects of blocking peripheral calcium channels in various pathological cardiovascular conditions.
GRANTS
This work was supported by National Institutes of Health Grants R01-AR-059397 and P01-HL-134609.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.E. and M.P.K. conceived and designed research; J.A.E. performed experiments; J.A.E. analyzed data; J.A.E. interpreted results of experiments; J.A.E. prepared figures; J.A.E. and M.P.K. drafted manuscript; J.A.E. and M.P.K. edited and revised manuscript; J.A.E. and M.P.K. approved final version of manuscript.
REFERENCES
- 1.Adreani CM, Hill JM, Kaufman MP. Intrathecal blockade of both NMDA and non-NMDA receptors attenuates the exercise pressor reflex in cats. J Appl Physiol (1985) 80: 315–322, 1996. doi: 10.1152/jappl.1996.80.1.315. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115: 1363–1381, 2011. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589: 5299–5309, 2011. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copp SW, Stone AJ, Yamauchi K, Kaufman MP. Effects of peripheral and spinal κ-opioid receptor stimulation on the exercise pressor reflex in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 307: R281–R289, 2014. doi: 10.1152/ajpregu.00156.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211: 844–846, 1981. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- 9.Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther 271: 1630–1637, 1994. [PubMed] [Google Scholar]
- 10.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannon HE, Atchison WD. Omega-conotoxins as experimental tools and therapeutics in pain management. Mar Drugs 11: 680–699, 2013. doi: 10.3390/md11030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan B, Kim JS, Farrag M, Kaufman MP, Ruiz-Velasco V. Alteration of the mu opioid receptor: Ca2+ channel signaling pathway in a subset of rat sensory neurons following chronic femoral artery occlusion. J Neurophysiol 112: 3104–3115, 2014. doi: 10.1152/jn.00630.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol (1985) 68: 2466–2472, 1990. doi: 10.1152/jappl.1990.68.6.2466. [DOI] [PubMed] [Google Scholar]
- 14.Hill JM, Pickar JG, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static contraction by an NK-1 receptor antagonist. J Appl Physiol (1985) 73: 1389–1395, 1992. doi: 10.1152/jappl.1992.73.4.1389. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto GA, Waldrop TG, Kaufman MP, Botterman BR, Rybicki KJ, Mitchell JH. Pressor reflex evoked by muscular contraction: contributions by neuraxis levels. J Appl Physiol (1985) 59: 459–467, 1985. doi: 10.1152/jappl.1985.59.2.459. [DOI] [PubMed] [Google Scholar]
- 16.Malmberg AB, Yaksh TL. Effect of continuous intrathecal infusion of omega-conopeptides, N-type calcium-channel blockers, on behavior and antinociception in the formalin and hot-plate tests in rats. Pain 60: 83–90, 1995. doi: 10.1016/0304-3959(94)00094-U. [DOI] [PubMed] [Google Scholar]
- 17.Marker CL, Luján R, Colón J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci 26: 12251–12259, 2006. doi: 10.1523/JNEUROSCI.3693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marker CL, Luján R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids. J Neurosci 25: 3551–3559, 2005. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meintjes AF, Nóbrega AC, Fuchs IE, Ally A, Wilson LB. Attenuation of the exercise pressor reflex. Effect of opioid agonist on substance P release in L-7 dorsal horn of cats. Circ Res 77: 326–334, 1995. doi: 10.1161/01.RES.77.2.326. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 22.Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, Horner RL. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology 124: 641–650, 2016. doi: 10.1097/ALN.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura A, Fujita M, Ono H, Hongo Y, Kanbara T, Ogawa K, Morioka Y, Nishiyori A, Shibasaki M, Mori T, Suzuki T, Sakaguchi G, Kato A, Hasegawa M. G protein-gated inwardly rectifying potassium (KIR3) channels play a primary role in the antinociceptive effect of oxycodone, but not morphine, at supraspinal sites. Br J Pharmacol 171: 253–264, 2014. doi: 10.1111/bph.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neugebauer V, Vanegas H, Nebe J, Rümenapp P, Schaible HG. Effects of N- and L-type calcium channel antagonists on the responses of nociceptive spinal cord neurons to mechanical stimulation of the normal and the inflamed knee joint. J Neurophysiol 76: 3740–3749, 1996. doi: 10.1152/jn.1996.76.6.3740. [DOI] [PubMed] [Google Scholar]
- 25.Ohno S, Yokoi H, Mori K, Kasahara M, Kuwahara K, Fujikura J, Naito M, Kuwabara T, Imamaki H, Ishii A, Saleem MA, Numata T, Mori Y, Nakao K, Yanagita M, Mukoyama M. Ablation of the N-type calcium channel ameliorates diabetic nephropathy with improved glycemic control and reduced blood pressure. Sci Rep 6: 27192, 2016. doi: 10.1038/srep27192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omote K, Kawamata M, Satoh O, Iwasaki H, Namiki A. Spinal antinociceptive action of an N-type voltage-dependent calcium channel blocker and the synergistic interaction with morphine. Anesthesiology 84: 636–643, 1996. doi: 10.1097/00000542-199603000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Pardridge WM. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS 8: 7, 2011. doi: 10.1186/2045-8118-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramu Y, Xu Y, Lu Z. Engineered specific and high-affinity inhibitor for a subtype of inward-rectifier K+ channels. Proc Natl Acad Sci USA 105: 10774–10778, 2008. doi: 10.1073/pnas.0802850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanger GJ, Ellis ES, Harries MH, Tilford NS, Wardle KA, Benham CD. Rank-order inhibition by omega-conotoxins in human and animal autonomic nerve preparations. Eur J Pharmacol 388: 89–95, 2000. doi: 10.1016/S0014-2999(99)00830-4. [DOI] [PubMed] [Google Scholar]
- 30.Scott DA, Wright CE, Angus JA. Actions of intrathecal omega-conotoxins CVID, GVIA, MVIIA, and morphine in acute and neuropathic pain in the rat. Eur J Pharmacol 451: 279–286, 2002. doi: 10.1016/S0014-2999(02)02247-1. [DOI] [PubMed] [Google Scholar]
- 31.Simms BA, Zamponi GW. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82: 24–45, 2014. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. doi: 10.1113/jphysiol.2001.012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takasusuki T, Yaksh TL. Regulation of spinal substance p release by intrathecal calcium channel blockade. Anesthesiology 115: 153–164, 2011. doi: 10.1097/ALN.0b013e31821950c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci 18: 6319–6330, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright CE, Robertson AD, Whorlow SL, Angus JA. Cardiovascular and autonomic effects of omega-conotoxins MVIIA and CVID in conscious rabbits and isolated tissue assays. Br J Pharmacol 131: 1325–1336, 2000. doi: 10.1038/sj.bjp.0703701. [DOI] [PMC free article] [PubMed] [Google Scholar]