Abstract
The gut microbiome plays a critical role in the onset and progression of obesity and the metabolic syndrome. However, it is not well documented whether the cecal vs. the fecal microbiome is more relevant when assessing their contributions to these diseases. Here, we amplified the V4 region of the 16S rRNA gene from cecal and fecal samples of female Ossabaw swine fed a low-fat control diet (10.5% fat, n = 4) or Western diet (43.0% fat, 17.8% high fructose corn syrup, 2% cholesterol; n = 3) for 36 wk. Obesity significantly lowered alpha-diversity (P < 0.05), and there was clear separation in beta-diversity between lean and obese pigs, as well as between cecal and fecal samples (P < 0.05). Obesity dramatically increased (P < 0.05) the Firmicutes:Bacteroidetes ratio in fecal samples, and Actinobacteria was higher (P < 0.05) in fecal vs. cecal samples in obese pigs. Cyanobacteria, Proteobacteria, and Fusobacteria were increased (P < 0.05), while Spirochaetes, Tenericutes, and Verrucomicrobia were decreased (P < 0.05) in obese vs. lean pigs. Prevotellaceae was reduced (P < 0.05) in obese fecal vs. cecal samples. Moreover, cecal samples in obese had greater (P < 0.05) predicted metabolic capacity for glycan biosynthesis and metabolism and LPS biosynthesis compared with fecal. Obese pigs also had greater (P < 0.05) capacity for carbohydrate metabolism, which was driven by obese fecal rather than cecal samples and was opposite in lean pigs (P < 0.05). The observed differences in pro-inflammatory microbiota and their metabolic capacity in cecal vs. fecal samples of obese pigs provide new insight into evaluating the microbiome in the pathogenesis of obesity and metabolic disease.
Keywords: alpha diversity, beta diversity, metabolic syndrome, microbiome, Ossabaw swine
INTRODUCTION
The gut is enumerated with trillions of microorganisms that together along with their genes are known as the microbiome. The healthy fecal human microbiome composition at the phylum taxonomic level is characterized by a predominance of Firmicutes and Bacteroidetes, with lower abundances of Actinobacteria and Proteobacteria (23). Changes in the relative abundances of these phyla and their constituent genera can be influenced by a number of factors including diet and lifestyle that can significantly impact host metabolism (33, 44). Furthermore, more recent evidence has linked increases in gram-negative Proteobacteria in response to diet-induced obesity to inflammation and progression of metabolic syndrome (7, 39).
The majority of preclinical obesity and microbiome studies have been in rodent models, which despite their advantages, have been questioned as to their suitability for human obesity and metabolic diseases. The pig has been proposed as a more suitable model for human research due to its more similar gastrointestinal (GI) tract and cardiovascular system (40). As in human obesity and metabolic syndrome, previous evidence in the female Ossabaw swine model have shown Western diet-induced obesity as well as signs of cardiovascular disease, glucose intolerance, insulin resistance, dyslipidemia, and abdominal adiposity (5, 10, 22, 29, 45). Furthermore, Pederson et al. (36) characterized the ileal and colonic microbiome composition of lean vs. metabolic syndrome/obese Ossabaw pigs and found 1) similarities in obesity-induced microbiome changes to humans and 2) distinct differences between the proximal vs. distal GI tract. Indeed, some evidence in rodents shows compositional differences between cecal and fecal microbiomes (34); however, it is not fully understood whether the cecal or fecal microbiome is more relevant to understanding its connection to obesity onset. Here, our objectives were to 1) characterize the cecal vs. the fecal microbiome in both lean and Western diet-fed obese Ossabaw swine and 2) determine differences in predicted metagenomic function in both cecal and fecal microbiomes as it relates to obesity. Overall, our findings suggest significant differences in both composition and predicted metagenomic function between cecal and fecal samples of obese pigs and, thus, may have significant implications in assessing the microbiome’s role in obesity.
METHODS
Prior to the initiation of the study, approval was received from the Animal Care and Use Committee at the University of Missouri. Female Ossabaw pigs were generously provided by Michael Sturek, Ph.D., in the Ossabaw Swine Resource, Comparative Medicine Program at Purdue University and Indiana University School of Medicine. Previous evidence in this model has shown that female pigs develop morbid obesity, nonalcoholic fatty liver disease, and the metabolic syndrome (10, 22, 29, 36, 45), and we therefore sought to replicate this phenotype. Indeed, human gut microbiome differences exist between obese females and males (15); however, whether these differences explain the heterogeneity in the susceptibility to obesity is still largely unknown.
Five-week-old Ossabaw pigs were randomly divided into two experimental groups and fed either chow diet (5L80, Lab Diet; 3.03 kcal/g: 10.5, 71, and 18.5% by energy for fat, carbohydrate, and protein, respectively; no high fructose corn syrup and cholesterol < 0.1%) or high-fat/high-fructose corn syrup/high-cholesterol (5B4L, Lab Diet; 4.14 kcal/g: 43, 40.8, and 16.2% by energy for fat, carbohydrate, and protein, respectively; 17.8% high-fructose corn syrup, and 2% cholesterol) diets for 36 wk (n = 4 for chow/lean pigs and n = 3 for Western diet/obese pigs). All pigs were individually housed in a core animal care facility at the University of Missouri under temperature-controlled conditions (20–23°C) with a 12 h:12 h light-dark cycle. Animals were provided food daily. A portion of the animal characteristics for this study has been reported previously (45).
Cecal content DNA extraction and sequencing.
Fecal and cecal contents were collected from all pigs at study completion, snap-frozen in liquid nitrogen, and stored at −80°C until DNA extraction. Bacterial DNA was isolated from cecal contents with a QIAamp Fast DNA stool mini kit (Qiagen, Valencia, CA), including a bead-beating step. Amplification of the V4 variable region of 16S rRNA gene, dual indexing, sequencing, processing, and quality filtering were performed as previously described by our group (32). A major limitation to our study is amplifying 16S rRNA gene limits analysis to predicted microbial metabolic function rather than direct measurement by shotgun/whole genome sequencing.
Filtered reads were demultiplexed within Quantitative Insights into Microbial Ecology (QIIME), and samples with fewer than 5,000 reads were excluded from further analysis. UCLUST was used to cluster sequences into operational taxonomical units (OTU) based on 97% identity (11). OTU picking was performed by an open-reference method that encompasses clustering of reads against a reference sequence collection and also performs de novo OTU picking on the reads that fail to align to any known reference sequence in the database The resulting OTU tables were checked for mislabeling sequences (18). Representative sequences were further aligned with PyNAST with the Greengenes core-set alignment template (28). Construction of the phylogenetic tree, alpha rarefaction, and beta diversity estimation were conducted and analyzed in QIIME as previously described by our group (32).
Functional metagenomic annotations on the basis of 16S rRNA data were predicted by phylogenetic investigation of communities by reconstruction of unobserved states (PICRUST) (21). Briefly, a closed-reference OTU table was generated from the original sequence files in QIIME (8). The closed-reference OTU table was normalized to 16S rDNA copy, and function was categorized in reference to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in PICRUST. The resulting biom file was then analyzed with STAMP (35).
Statistical analysis.
All mean OTU, alpha-diversity, and predicted metagenomics data were analyzed as a two-way ANOVA using the MIXED procedure of SAS (SAS Institute, Cary, NC), and significance was set at P < 0.05. Significant interactions were followed up with a Tukey’s post hoc adjustment. Differences in beta-diversity were measured by two-sample Monte-Carlo t-test. A permutational multivariate analysis of variance (PERMNOVA) with 500 permutations of log-shifted Bray-Curtis dissimilarities was used to assess differences between groups in beta-diversity using R packages. All heat-map data are normalized raw relative abundance OTU data to supplement mean relative abundances.
RESULTS
Animal characteristics.
Animal characteristics of Ossabaw pigs have been reported previously (45). In brief, pigs provided Western diet for 36 wk developed severe obesity through 2.5 times greater weight gain, dyslipidemia, hypertension, and insulin resistance compared with lean pigs. We utilized these data to provide post hoc correlations with microbiome composition.
Microbiome analyses.
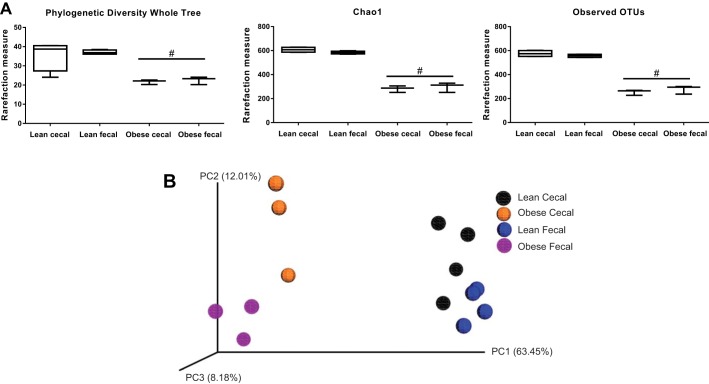
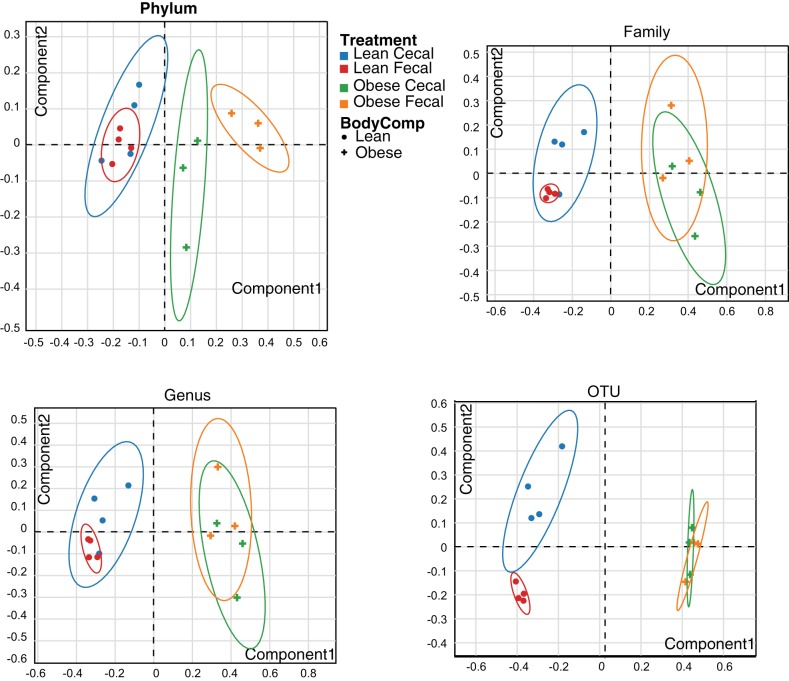
All indexes of alpha diversity (Chao1, phylogenetic diversity whole tree, and observed OTUs) were greater (P < 0.05) in lean vs. obese animals (Fig. 1A). No significant differences in species richness were noted between fecal and cecal samples. Figure 1B displays the weighted Unifrac distances as a Principal Coordinates Analysis (PCoA) plot. Obese pigs were more similar to each other than lean pigs (P < 0.05). Within obese pigs, cecal samples were more similar to each other than fecal samples (P < 0.05); this was not as evident between cecal and fecal samples in lean pigs. Likewise, PERMANOVA results of Bray-Curtis dissimilarities (beta diversity) also showed highly significant differences between lean and obese pigs (P < 0.01 at every taxonomic level), and differences in cecal vs. fecal samples only within lean pigs (P < 0.05, at family, genus, and OTU). No differences in overall beta diversity were observed via PERMANOVA between cecal and fecal sample types in obese pigs at any taxonomic level (PCoA plots represented in Fig. 2).
Fig. 1.
Indexes of cecal and fecal alpha-diversity including phylogenetic diversity of whole tree, Chao1, and observed operational taxonomic units (A) and beta diversity of weighted unifrac distances represented as a principal coordinates analysis (PCoA) plot (B) in low fat diet-fed (lean; n = 4 per group) and Western diet-fed (obese; n = 3 per group) pigs for 36 wk. All alpha-diversity/rarefaction box plots are means ± SE #Represents significant (P < 0.05) difference between lean and obese.
Fig. 2.
Principal Coordinate Analysis (PCA) ordination of the OTU dissimilarity-based index (Bray-Curtis) of β-diversity in cecal and fecal DNA of rats from Lean and Obese groups at Phylum (A), Family (B), Genus (C), and operational taxonomic unit (OTU) level (D). Statistical significance was determined by using PERMANOVA by comparing the true F statistic to randomly permuted F statistics defaulted to 500 permutations.
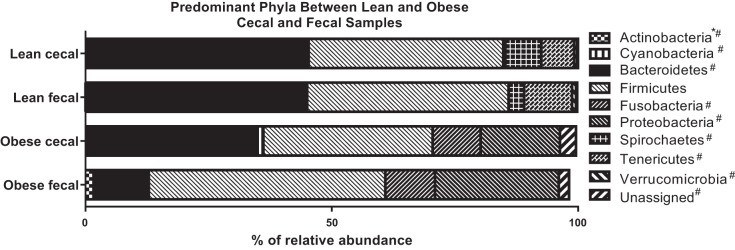
Mean relative abundances at the phylum, family, and genus levels between cecal and fecal microbiomes in lean and obese pigs are reported in Table 1. The predominant phyla in lean cecal and fecal samples consisted of Bacterioidetes, Firmicutes, Spirochaetes, and Tenericutes, while obese cecal and fecal samples consisted of Bacteroidetes, Firmicutes, Proteobacteria, and Fusobacteria (Fig. 3). Lean pigs had low relative abundances of Unassigned phyla, Actinobacteria, Cyanobacteria, Proteobacteria, and Verrucomicrobia, while obese pigs had low Unassigned phyla, Actinobacteria, and Cyanobacteria. Regardless of sample type, obese pigs had greater (P < 0.05) relative abundances of Unassigned phyla, Bacteroidetes, Cyanobacteria, Fusobacteria, and Proteobacteria and less (P < 0.05) relative abundance of Spirochaetes, Tenericutes, and Verrucomicrobia compared with lean pigs. Fecal Actinobacteria was greater (P < 0.05) in obese fecal vs. obese cecal samples and both lean cecal and fecal samples.
Table 1.
Relative abundances of bacterial phyla, families, and genera in cecal and fecal samples of female Ossabaw swine fed low-fat diet (lean) or Western diet (Obese) for 36 wk
| Lean (n = 4 each) |
Obese (n = 3 each) |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Cecal | Fecal | Cecal | Fecal | Sample Type | Body Composition | Interaction | |
| Unassigned* | 0.13 ± 0.05 | 0.23 ± 0.13 | 3.27 ± 1.47 | 2.10 ± 1.44 | 0.5512 | 0.0158 | 0.4808 |
| Methanobrevibacter | 0.05 ± 0.03 | 0.08 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.5250 | 0.0067 | 0.5250 |
| Methanosphaera | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.20 ± 0.10 | 1.80 ± 0.71 | 0.1087 | 0.0441 | 0.1087 |
| Actinobacteria | 0.18 ± 0.06 | 0.00 ± 0.00 | 0.13 ± 0.09 | 1.63 ± 0.61 | 0.0296 | 0.0123 | 0.0094 |
| Coriobacteriaceae† | 0.10 ± 0.04 | 0.00 ± 0.00 | 0.13 ± 0.09 | 1.60 ± 0.61 | 0.0245 | 0.0101 | 0.0126 |
| Unassigned | 0.10 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.20 ± 0.10 | 0.3250 | 0.3250 | 0.0111 |
| Collinsella‡ | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.13 ± 0.09 | 1.33 ± 0.54 | 0.0265 | 0.0096 | 0.0265 |
| Bacteroidetes | 45.1 ± 3.32 | 45.0 ± 1.59 | 34.8 ± 3.23 | 10.9 ± 1.85 | 0.0011 | <.0001 | 0.4348 |
| Unassigned | 15.7 ± 3.37 | 7.55 ± 1.94 | 0.10 ± 0.06 | 0.00 ± 0.00 | 0.1035 | 0.0005 | 0.1111 |
| Bacteroidaceae | 0.28 ± 0.05 | 0.15 ± 0.06 | 1.57 ± 1.03 | 0.37 ± 0.12 | 0.1588 | 0.1137 | 0.2449 |
| Porphyromonadaceae | 0.18 ± 0.09 | 1.18 ± 0.33 | 0.07 ± 0.03 | 0.10 ± 0.00 | 0.0277 | 0.0146 | 0.0368 |
| Parabacteroides | 0.18 ± 0.09 | 1.15 ± 0.33 | 0.07 ± 0.03 | 0.10 ± 0.00 | 0.0359 | 0.0193 | 0.0471 |
| Prevotellaceae | 9.18 ± 2.73 | 10.2 ± 0.60 | 26.6 ± 4.17 | 9.57 ± 1.72 | 0.0093 | 0.0072 | 0.0049 |
| RF16 | 0.08 ± 0.03 | 0.15 ± 0.06 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.3814 | 0.0206 | 0.3814 |
| Rikenellaceae | 0.25 ± 0.22 | 0.30 ± 0.15 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.8755 | 0.1076 | 0.8755 |
| S24-7 | 1.78 ± 0.38 | 2.95 ± 0.70 | 2.90 ± 1.96 | 0.70 ± 0.06 | 0.5999 | 0.5654 | 0.1048 |
| Paraprevotellaceae | 16.5 ± 1.56 | 12.2 ± 2.09 | 3.53 ± 1.19 | 0.17 ± 0.03 | 0.0404 | <.0001 | 0.7854 |
| Unassigned | 0.93 ± 0.50 | 0.43 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.4492 | 0.0594 | 0.4492 |
| CF231 | 4.53 ± 1.41 | 1.48 ± 0.19 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.1001 | 0.0051 | 0.1001 |
| YRC22 | 5.85 ± 0.94 | 5.90 ± 0.66 | 0.03 ± 0.03 | 0.00 ± 0.00 | 0.9952 | <.0001 | 0.9666 |
| Prevotella | 5.13 ± 1.29 | 4.38 ± 1.97 | 3.47 ± 1.19 | 0.17 ± 0.03 | 0.2012 | 0.0727 | 0.4187 |
| p-2534-18B | 0.95 ± 0.49 | 10.3 ± 2.92 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.0238 | 0.0094 | 0.0238 |
| Cyanobacteria | 0.03 ± 0.03 | 0.00 ± 0.00 | 1.03 ± 0.55 | 0.33 ± 0.12 | 0.1547 | 0.0173 | 0.1823 |
| Firmicutes | 39.5 ± 3.46 | 40.9 ± 1.37 | 34.5 ± 8.75 | 48.0 ± 1.94 | 0.1188 | 0.8192 | 0.1947 |
| Lactobacillaceae | 5.10 ± 2.57 | 0.05 ± 0.03 | 8.23 ± 3.83 | 6.47 ± 2.67 | 0.1987 | 0.0826 | 0.5223 |
| Streptococcaceae | 2.10 ± 1.35 | 0.13 ± 0.03 | 1.90 ± 1.14 | 5.97 ± 2.88 | 0.5077 | 0.0936 | 0.0753 |
| Turicibacteraceae | 0.53 ± 0.17 | 0.48 ± 0.09 | 0.00 ± 0.00 | 0.03 ± 0.03 | 0.9429 | 0.0017 | 0.7212 |
| Clostridiales (other) | 0.43 ± 0.13 | 0.48 ± 0.10 | 0.17 ± 0.07 | 0.13 ± 0.03 | 0.9375 | 0.0160 | 0.6961 |
| Unassigned | 6.75 ± 0.89 | 6.65 ± 0.68 | 0.93 ± 0.83 | 0.93 ± 0.39 | 0.9124 | <.0001 | 0.9124 |
| Christensenellaceae | 0.10 ± 0.07 | 0.43 ± 0.10 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.0527 | 0.0053 | 0.0527 |
| Clostridiaceae | 1.33 ± 0.41 | 2.58 ± 0.37 | 0.17 ± 0.12 | 0.63 ± 0.22 | 0.0319 | 0.0011 | 0.2820 |
| Unassigned | 0.53 ± 0.26 | 2.00 ± 0.46 | 0.10 ± 0.10 | 0.53 ± 0.24 | 0.0130 | 0.0094 | 0.1593 |
| Clostridium | 0.75 ± 0.21 | 0.55 ± 0.15 | 0.07 ± 0.03 | 0.07 ± 0.07 | 0.5308 | 0.0036 | 0.5308 |
| Lachnospiraceae | 11.1 ± 2.49 | 9.05 ± 1.45 | 3.83 ± 1.30 | 9.00 ± 0.67 | 0.4030 | 0.0729 | 0.0763 |
| Other | 0.68 ± 0.17 | 0.25 ± 0.09 | 0.03 ± 0.03 | 0.20 ± 0.06 | 0.2829 | 0.0125 | 0.0265 |
| Unassigned | 3.65 ± 0.84 | 5.20 ± 0.89 | 1.87 ± 0.43 | 4.47 ± 0.52 | 0.0236 | 0.1368 | 0.5150 |
| Blautia | 0.18 ± 0.09 | 0.00 ± 0.00 | 0.40 ± 0.12 | 1.43 ± 0.47 | 0.0653 | 0.0025 | 0.0155 |
| Coprococcus | 0.65 ± 0.13 | 0.28 ± 0.06 | 0.40 ± 0.26 | 1.47 ± 0.88 | 0.4025 | 0.2614 | 0.0984 |
| Dorea | 5.20 ± 2.37 | 2.83 ± 0.87 | 0.70 ± 0.50 | 0.60 ± 0.12 | 0.4316 | 0.0501 | 0.4687 |
| Lachnospira | 0.35 ± 0.09 | 0.18 ± 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.2664 | 0.0022 | 0.1141 |
| Roseburia | 0.25 ± 0.12 | 0.13 ± 0.09 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.5128 | 0.1251 | 0.5128 |
| Ruminococcus | 0.10 ± 0.00 | 0.23 ± 0.10 | 0.40 ± 0.20 | 0.67 ± 0.23 | 0.0005 | <.0001 | 0.0001 |
| Peptostreptococcaceae | 0.10 ± 0.04 | 0.00 ± 0.00 | 0.10 ± 0.10 | 1.50 ± 0.80 | 0.0842 | 0.0513 | 0.0513 |
| Ruminococcaceae | 9.85 ± 1.46 | 19.4 ± 1.39 | 1.30 ± 0.55 | 4.80 ± 2.07 | 0.0014 | <.0001 | 0.0691 |
| Other | 0.03 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.4178 | 0.4178 | 0.4178 |
| Unassigned | 7.08 ± 1.44 | 18.2 ± 1.45 | 1.10 ± 0.45 | 4.37 ± 1.94 | 0.0006 | <.0001 | 0.0238 |
| Ruminococcus | 1.65 ± 0.18 | 0.35 ± 0.03 | 0.03 ± 0.03 | 0.13 ± 0.09 | 0.0005 | <.0001 | 0.0001 |
| Veillonellaceae | 1.40 ± 0.40 | 0.53 ± 0.05 | 17.6 ± 1.39 | 17.2 ± 3.61 | 0.7121 | <.0001 | 0.8796 |
| Unassigned | 0.08 ± 0.05 | 0.03 ± 0.03 | 1.00 ± 0.30 | 0.93 ± 0.58 | 0.8372 | 0.0078 | 0.9766 |
| Acidaminococcus | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.10 ± 0.75 | 0.43 ± 0.12 | 0.3192 | 0.0366 | 0.3192 |
| Anaerovibrio | 0.43 ± 0.20 | 0.00 ± 0.00 | 1.80 ± 0.57 | 0.23 ± 0.07 | 0.0040 | 0.0132 | 0.0587 |
| Megamonas | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.50 ± 1.85 | 5.23 ± 3.43 | 0.6070 | 0.0233 | 0.6070 |
| Megasphaera | 0.03 ± 0.03 | 0.00 ± 0.00 | 4.93 ± 1.87 | 6.73 ± 0.71 | 0.3130 | <.0001 | 0.3003 |
| Mitsuokella | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.07 ± 0.03 | 0.13 ± 0.03 | 0.1218 | 0.0005 | 0.1218 |
| Phascolarctobacterium | 0.70 ± 0.19 | 0.45 ± 0.06 | 5.20 ± 1.79 | 3.57 ± 0.85 | 0.2866 | 0.0011 | 0.4277 |
| Veillonella | 0.13 ± 0.08 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.1893 | 0.1893 | 0.1893 |
| Eubacterium | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.06 ± 0.06 | 0.16 ± 0.03 | 0.1399 | 0.0038 | 0.1399 |
| p-75-a5 | 0.68 ± 0.17 | 0.93 ± 0.34 | 0.00 ± 0.00 | 0.06 ± 0.03 | 0.5008 | 0.0070 | 0.6944 |
| Fusobacteria | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.73 ± 2.29 | 10.1 ± 3.83 | 0.9167 | 0.0003 | 0.9167 |
| Proteobacteria | 0.43 ± 0.17 | 0.00 ± 0.00 | 16.1 ± 3.55 | 25.1 ± 7.45 | 0.2428 | 0.0002 | 0.2024 |
| Desulfovibrionaceae | 0.03 ± 0.03 | 0.00 ± 0.00 | 1.73 ± 0.34 | 0.60 ± 0.26 | 0.0093 | <.0001 | 0.0118 |
| Unassigned | 0.03 ± 0.03 | 0.00 ± 0.00 | 1.30 ± 0.20 | 0.07 ± 0.03 | <.0001 | <.0001 | <.0001 |
| Bilophila | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.06 ± 0.03 | 0.10 ± 0.06 | 0.5634 | 0.0136 | 0.5634 |
| Desulfovibrio | 0.03 ± 0.03 | 0.00 ± 0.00 | 0.33 ± 0.15 | 0.43 ± 0.20 | 0.7294 | 0.0056 | 0.5663 |
| Campylobacteraceae | 0.25 ± 0.12 | 0.00 ± 0.00 | 0.20 ± 0.20 | 0.00 ± 0.00 | 0.1063 | 0.1063 | 0.1063 |
| Succinivibrionaceae | 0.00 ± 0.00 | 0.00 ± 0.00 | 13.3 ± 4.16 | 15.2 ± 6.21 | 0.7715 | 0.0010 | 0.7715 |
| Succinivibrio | 0.00 ± 0.00 | 0.00 ± 0.00 | 13.3 ± 4.12 | 15.2 ± 6.21 | 0.7670 | 0.0010 | 0.7670 |
| Enterobacteriaceae | 0.03 ± 0.03 | 0.00 ± 0.00 | 1.03 ± 0.46 | 9.27 ± 5.74 | 0.1191 | 0.0587 | 0.1172 |
| Spirochaetes | 7.28 ± 2.05 | 3.15 ± 0.92 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.1519 | 0.0029 | 0.1519 |
| Tenericutes | 6.70 ± 1.65 | 9.70 ± 2.18 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.3749 | 0.0005 | 0.3749 |
| Verrucomicrobia | 0.60 ± 0.32 | 0.83 ± 0.27 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.6567 | 0.0158 | 0.6567 |
| RFP12 | 0.25 ± 0.12 | 0.73 ± 0.23 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.1284 | 0.0078 | 0.1284 |
All values are means ± SE. Boldface P value denotes a significant difference. Relative abundances of
bacterial phyla,
families,
and genera.
Fig. 3.
Depiction of representative cecal and fecal microbial phyla in low fat diet-fed (lean; n = 4 per group) and Western diet-fed (obese; n = 3 per group) pigs for 36 wk. All values represented are means, and statistical differences between groups were determined using SE *Significant (P < 0.05) difference between obese cecal and obese fecal samples. #Significant (P < 0.05) difference between obese and lean.
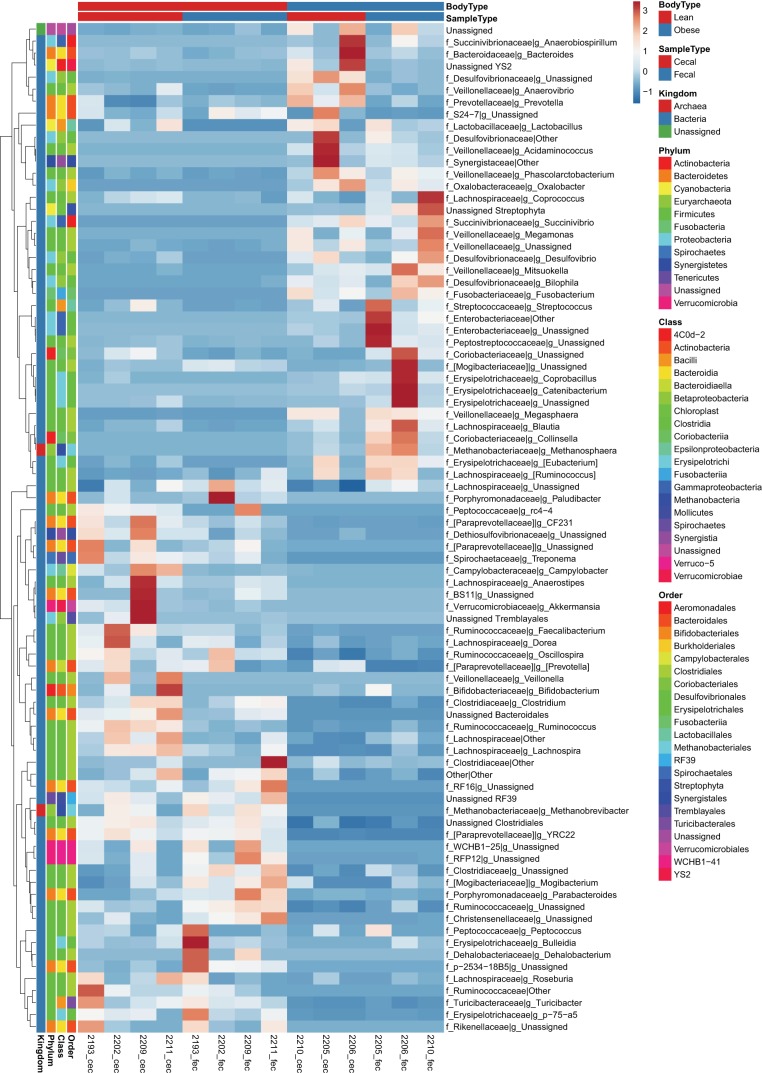
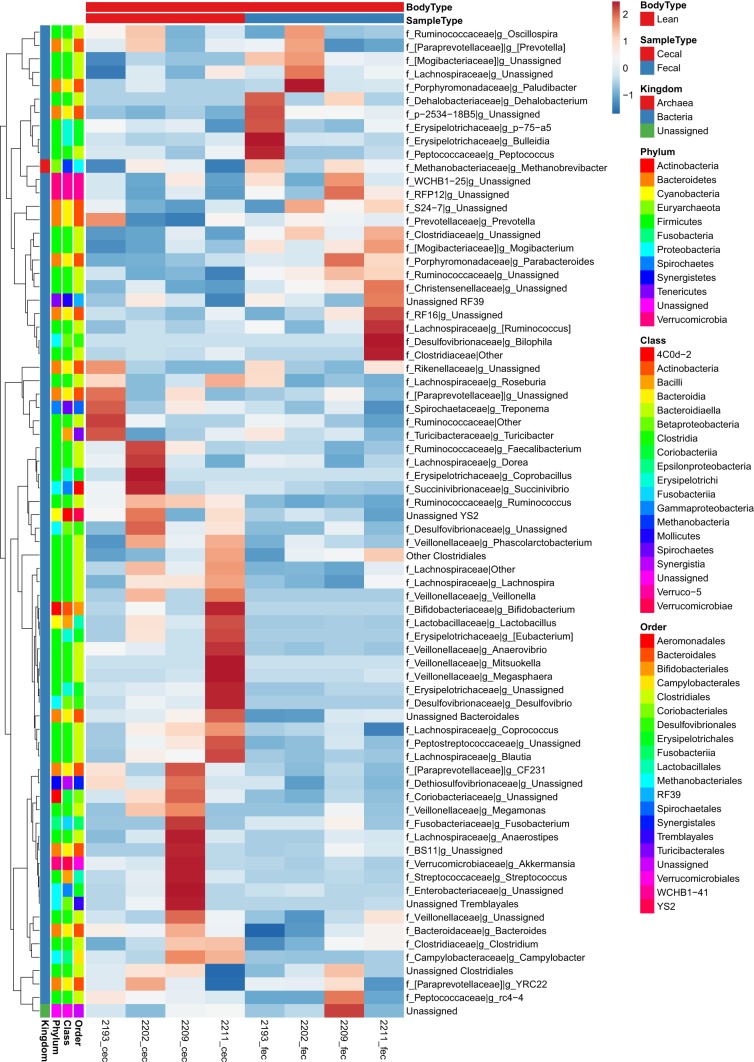
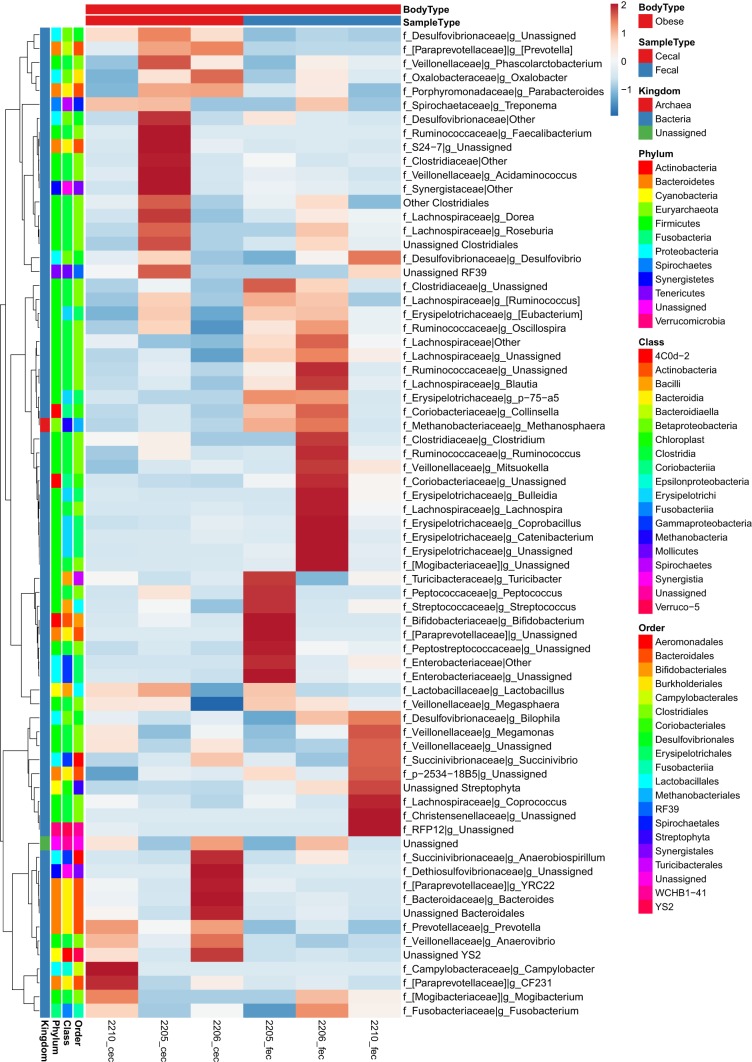
To provide a depiction of individual variability in OTUs and the magnitude of differences between samples we created heat maps to supplement the data shown in Table 1. At the family and genus taxonomic levels, fold change for all individual samples and between sample types within lean and obese are depicted in Figs. 4, 5, and 6 as heat maps. Within Actinobacteria, Unassigned Coriobacteriaceae and Collinsella were greater (P < 0.05) in obese fecal compared with obese cecal samples. Within Bacteroidetes, Porphyromonadaceae and Parabacteroides were reduced (P < 0.05) in obese vs. lean pigs and in cecal vs. fecal samples (P < 0.05), while Prevotellaceae was greatest (P < 0.05) in obese cecal samples compared with lean animals and obese cecal samples. Obese pigs had reduced (P < 0.05) Paraprevotellaceae, CF231, and YRC22 in both cecal and fecal samples compared with lean samples. Both obese cecal and fecal samples exhibited no change in Bacteroidetes family p-2534-18B; however, lean fecal samples had greater (P < 0.05) relative abundances compared with lean cecal and obese cecal/fecal samples.
Fig. 4.
Heat map of individual normalized counts of cecal and fecal microbial families and genera from most abundant (top) to least abundant (bottom) in low fat diet-fed (lean; n = 4 each) and Western diet-fed (obese; n = 3 each) pigs for 36 wk. All values are based off raw operational taxonomic unit (OTU)/relative abundance values. Dark red represents highest abundance, and dark blue is lowest relative abundance.
Fig. 5.
Heat map of individual normalized counts of cecal and fecal microbial families and genera from most abundant (top) to least abundant (bottom) in low fat diet-fed (lean; n = 4 each) pigs for 36 wk. All values are based off raw operational taxonomic unit (OTU)/relative abundance values. Dark red represents highest abundance, and dark blue is lowest relative abundance.
Fig. 6.
Heat map of individual normalized counts of cecal and fecal microbial families and genera from most abundant (top) to least abundant (bottom) in Western diet-fed (obese; n = 3 each) pigs for 36 wk. All values are based off raw operational taxonomic unit (OTU)/relative abundance values. Dark red represents highest abundance, and dark blue is lowest relative abundance.
Within Firmicutes, Turcibacteriaceae, other Clostridiales, and Unassigned genera, were reduced (P < 0.05) in obese compared with lean pigs. Cecal and fecal Christensenellaceae was lower in obese pigs, while lean pig fecal samples had greater (P < 0.05) relative abundance compared with lean cecal samples. Obese cecal and fecal Clostridiaceae, Unassigned Clostridiaceae, and Clostridium relative abundances were reduced (P < 0.05) compared with lean samples. Obese cecal Blautia was reduced (P < 0.05) compared with obese fecal samples; whereas, lean cecal samples had greater (P < 0.05) relative abundances compared with lean fecal samples. Obese pigs in general had lower (P < 0.05) Dorea and Lachnospira and greater (P < 0.05) Ruminococcus compared with lean pigs. Fecal Peptostreptococcaceae was greater (P < 0.05) in obese pigs compared with obese cecal samples and lean pigs; whereas Ruminococcaceae was reduced (P < 0.05) in obese vs. lean samples. Unassigned Ruminococcaceae was reduced (P < 0.05) in obese vs. lean pigs, and was greater (P < 0.05) in fecal vs. cecal samples in both lean and obese animals. Obese pigs also had robust increases (P < 0.05) in cecal and fecal Veillonellaceae, including unassigned genera within, Acidaminococcus, Anaerovibrio, Megamonas, Megasphaera, Mitsuokella, Phascolarctobacterium, and Eubacterium, as well as decreased (P < 0.05) p-75-a5 cecal and fecal relative abundances.
Within Proteobacteria, cecal Desulfovibrionaceae and unassigned Desulfovibrionaceae were greatest (P < 0.05) in obese cecal vs. obese fecal samples, as well as compared with lean samples. Obese pigs also exhibited greater (P < 0.05) Bilophila, Desulfovibrio, Succinivibrionaceae (Succinivibrio), and a tendency to have greater (P = 0.06) Enterobacteriaceae compared with lean pigs. Within Verrucomicrobia, genus RFP12 was greater (P < 0.05) in lean vs. obese pigs.
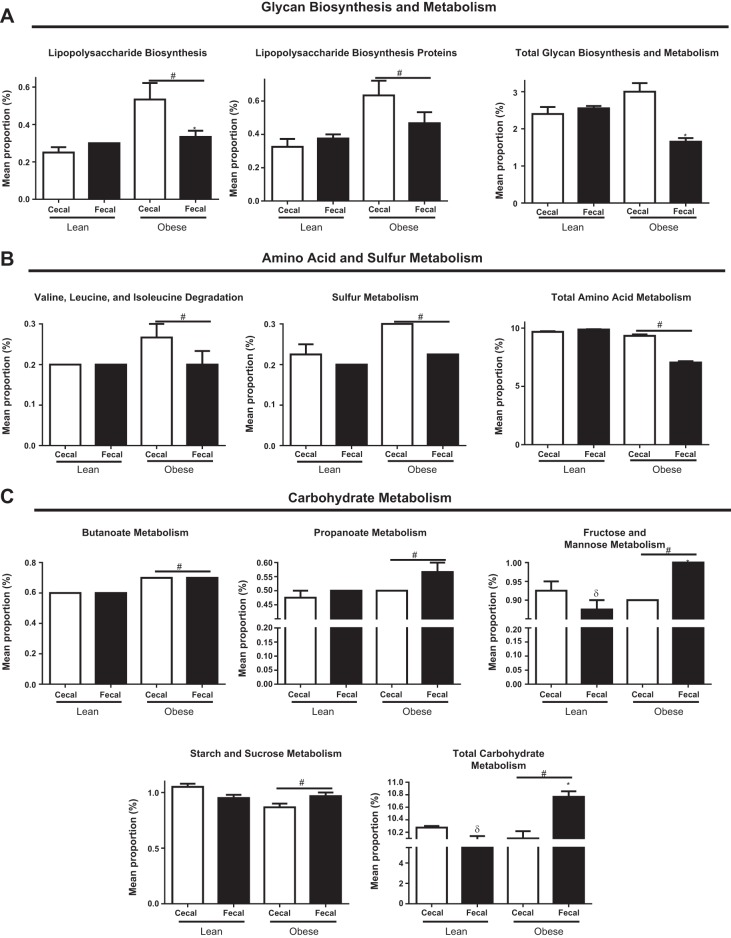
We assessed predicted metabolic function of the microbiome between obese and lean cecal and fecal samples (Fig. 7, A–C). Obese cecal samples exhibited a greater (P < 0.05) metabolic capacity for lipopolysaccharide (LPS) biosynthesis compared with obese fecal samples, as well as lean samples. Obese cecal and fecal microbiomes exhibited a greater (P < 0.05) predicted metabolic capacity for LPS binding proteins, which tended (P = 0.08) to be greater in obese cecal vs. obese fecal samples. Predicted metabolic function of total glycan biosynthesis was also greater (P < 0.05) in obese cecal vs. obese fecal samples; no differences were noted between lean cecal and fecal samples. Obese pigs exhibited greater (P < 0.05) microbial predicted metabolic function for valine, leucine, and isoleucine degradation and sulfur metabolism, while predicted metabolic function for total amino acid metabolism was greater (P < 0.05) in lean vs. obese pigs. Predicted metabolic function for both butanoate and propanoate metabolism was greater (P < 0.05) in obese vs. lean pigs. Overall, obese pigs exhibited greater (P < 0.05) microbial metabolic capacity for fructose and mannose metabolism and total carbohydrate metabolism compared with lean pigs. However, obese cecal vs. fecal samples had significantly reduced (P < 0.05) predicted metabolic function for fructose and mannose metabolism as well as total carbohydrate metabolism, while this was increased (P < 0.05) in lean cecal vs. lean fecal samples. Furthermore, obese pigs exhibited lower (P < 0.05) predicted metabolic function for starch and sucrose metabolism compared with lean pigs.
Fig. 7.
Cecal- and fecal-predicted metagenomic differences as measured by phylogenetic investigation of communities by reconstruction of unobserved states (PICRUST) for glycan biosynthesis and metabolism (A), amino acid and sulfur metabolism (B), and carbohydrate metabolism (C) in low fat diet-fed (lean; n = 4 per group) and Western diet-fed (obese; n = 3 per group) for 36 wk. All values are means ± SE *Significant (P < 0.05) difference between obese cecal and obese fecal samples. #Significant (P < 0.05) difference between obese and lean. δSignificant (P < 0.05) difference between lean cecal and lean fecal samples.
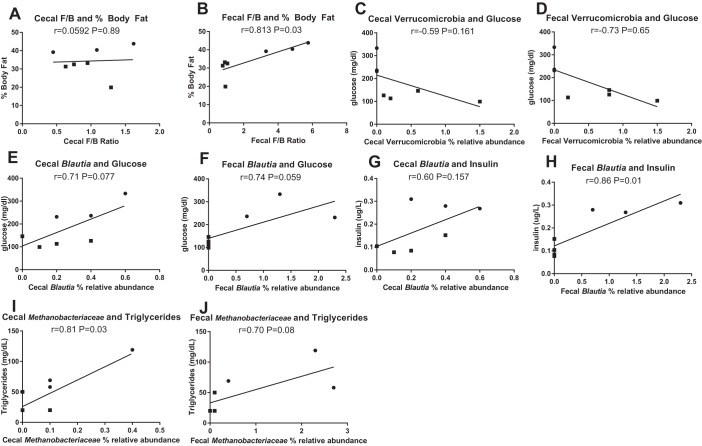
We assessed whether differences in microbiota composition between cecal and fecal samples within lean and obese pigs are associated with markers of obesity and metabolic syndrome through post hoc Pearson’s correlations (Fig. 8, A–J). Interestingly, the cecal Firmicutes-to-Bacteroidetes ratio did not correlate with body fat percent (Fig. 8A, r = 0.0592; P = 0.89); whereas this displayed a strong positive association in fecal samples (Fig. 8B, r = 0.81; P = 0.01). Cecal and fecal Verrucomicrobia showed a trend toward a positive correlation with serum glucose (Fig. 8, C and D; r = −0.59, P = 0.16 and r = −0.73, P = 0.06, respectively). Cecal and fecal Blautia relative abundances also exhibited a tendency to be positively correlated with serum glucose concentrations (Fig. 8, E and F; r = 0.71, P = 0.08 and r = 0.74, P = 0.06, respectively). However, fecal Blautia exhibited a strong positive correlation with insulin (Fig. 8H, r = 0.87; P = 0.01), whereas no association was observed in cecal samples (Fig. 8G; r = 0.60, P = 0.16). Cecal Methanobacteriaceae relative abundances were positively associated with serum triglyceride (Fig. 8I, r = 0.81; P = 0.03), while this association was not as strong in fecal samples (Fig. 8J, r = 0.70; P = 0.08).
Fig. 8.
Pearson’s correlations of cecal and fecal Firmicutes to Bacteroidetes ratio and percent body fat (A, B), cecal and fecal Verrucomicrobia and serum glucose (C, D), cecal and fecal Blautia and serum glucose (E, F) and insulin (G, H), cecal and cecal and fecal Methanobacteriaceae and serum triglycerides (I, J) in low fat diet-fed (lean, n = 4 per group, ■) and Western diet-fed (obese, n = 3 per group, ●) pigs for 36 wk.
DISCUSSION
It is well established that the gut microbiome is a critical factor in determining the onset and progression of obesity and the metabolic syndrome (2, 3, 43). In human obesity, the vast majority of studies analyze fecal rather than cecal microbiome because of the ease of collection and lack of invasiveness. Rodent models are often utilized as preclinical models in studying gut microbiome-host interactions where the cecal microbiome harbors the greatest density and diversity of microbes. The Ossabaw pig model is being recognized as a suitable animal model for human obesity and metabolic syndrome because of its diet-induced obesity causing dyslipidemia, insulin resistance, abdominal adiposity, and elevated glucose concentrations (10, 22, 29, 45). Furthermore, swine have similarities in physiology, immunology, and gastrointestinal anatomy to what is observed in humans (24). More recently, a study analyzed differences in the ileal vs. colonic microbiome in Ossabaw pigs and discovered similar diet-induced obesity to humans including increased Firmicutes-to-Bacteroidetes ratio (36). Indeed, compositional differences in the cecal vs. the fecal microbiome have been reported in mice (34); however, whether these differences are an indication of the susceptibility to diet-induced obesity is still largely unknown. Here, we characterize the cecal and fecal microbiome compositions and predicted metabolic function of 36 wk Western diet fed-induced obese and low fat diet/chow-fed lean Ossabaw swine to examine differences in diet-driven obesity and the microbiome.
We found obese pigs have markedly lower species richness/alpha-diversity compared with lean pigs independent of sampling location. However, beta-diversity revealed unique microbiome signatures depending upon obesity status and sampling location. This separation between cecal and fecal microbiomes was not as apparent in the obese animals. Furthermore, the impact of obesity on remodeling the global microbiome was far greater that differences observed between cecal and fecal samples. Seminal work in studying the microbiome and obesity show decreases in microbial diversity with obesity (42); however, a recent meta-analysis revealed that obese individuals only show modest decreases in species richness, evenness, and diversity (41). Firmicutes-to-Bacteroidetes ratio was much more pronounced in obese fecal vs. obese cecal samples in the current study, which is often interpreted as increased energy harvest from the diet and a signature for the obese microbiome and is consistent with previous reports in obese Ossabaw swine (36, 42). Besides analyzing this ratio, we also found that fecal samples from obese pigs had the greatest relative abundance of Actinobacteria compared with obese cecal and lean samples, which is consistent in human obesity (42), but not with what has been previously reported in the Ossabaw pig (36). Many reasons may also contribute to differences in taxonomic changes due to Western diet relative to a previous report (36), including diet composition (lack of high cholesterol), housing conditions, DNA extraction method, 16S rRNA gene region amplified to infer microbial ecology (V1–3, V4–5 vs. V4), as well as bioinformatics methods for taxonomic classification. Western-diet induced obesity also caused a robust increase in Fusobacteria and Proteobacteria. A recent study in an obese Japanese population found increased fecal Fusobacteria, which is a phylum that may be linked to proinflammatory stimulus to host epithelial cells and has been associated with colorectal carcinoma (1, 19); however, the role of this phylum in obesity is still largely unknown. More recent evidence has shown a strong link between increased proinflammatory gram-negative Proteobacteria families such as Enterobacteriaceae and Desulfovibrionaceae in human obesity and in high fat diet-fed mice, which may be a source of LPS to cause systemic inflammation (6, 48). Overall, changes at the phylum taxonomic level from both sampling locations exhibited some consistency between Ossabaw pig and what has been reported in the human obesity literature.
Changes between cecal and fecal microbiomes within obesity were evident at the family and genus taxonomic levels. Collinsella, a genus previously linked to serum insulin concentrations in obese women (14), was greater in obese fecal vs. obese cecal as well as compared with the lean fecal/cecal samples. Interestingly, Prevotellaceae was robustly increased in cecal vs. fecal samples in obese pigs. Elevated H2-producing Prevotellaceae have been shown in obese humans (49), which has been interpreted as H2-producing bacterium transferring hydrogen gas to H2-using methanogens that can increase host energy extraction by breaking down indigestible polysaccharides (37, 49). Furthermore, Nod-like receptor-6 inflammasome knockout mice exhibited robust increases in Prevotellaceae and elevated risk for colitis, suggesting a role in enteric mucosal integrity (13). Similarly, changes in Proteobacteria families have been linked to proinflammation in metabolic diseases including obesity and nonalcoholic fatty liver disease and have been considered a marker of an unstable microbial community (i.e., dysbiosis) (39). Several proinflammatory Proteobacteria families and genera including Desulfovibrionaceae, Bilophila, and Enterobacteriaceae (P = 0.06) were also increased with Western diet-induced obesity, all of which have been linked to gut inflammation and a source of LPS to promote inflammation (4, 25, 46). Only Desulfovibrionaceae, a sulfate-reducing bacteria that is often enriched after high-fat diet and contains LPS associated with microbiota inflammatory properties (47), was significantly greater in obese cecal vs. fecal samples. These outcomes would suggest that the cecal vs. fecal microbiome might be more revealing of Western diet-induced proinflammatory stimuli (i.e., LPS, bacterial DNA, and other pathogen-associated molecular patterns).
Predicted microbial metabolic function revealed differences between lean and obese and between cecal and fecal samples within obese that may be pertinent to the disease. One major outcome, glycan biosynthesis (i.e., LPS biosynthesis and LPS binding protein), was markedly increased in obese cecal vs. fecal samples. Indeed, obesity and metabolic syndrome are associated with systemic low-grade inflammation potentially due to gut-derived LPS (9). Specifically, high fat diet-induced obesity has been shown to promote an altered microbiome that results in increased gut permeability and metabolic endotoxemia (6, 7). Our data support that evaluation of the proximal large bowel (cecal) rather than distal (fecal) microbiome and may indicate that the cecal sampling is more applicable in evaluating Western-diet induced inflammation. Indexes of predicted microbial carbohydrate metabolism were greater in obese pigs. Indeed, fecal short chain fatty acids (SCFA) are fermentative end products of fiber/carbohydrate that are increased in the feces of obese humans (38), highlighting increased “energy harvest” from the diet. Surprisingly, total carbohydrate and fructose and mannose metabolism was increased in obese fecal vs. obese cecal samples, while lean pigs exhibited an opposite effect. This may be attributed to obese pigs exhibiting greater food intake and Western diet altering gut transit rate, thereby influencing carbohydrate metabolism in the large bowel. Together these data suggest that Western diet-induced changes of the cecal microbiome may be more relevant toward host inflammation, while fecal samples may be more relevant in assessing differences in in energy harvesting potential.
Another novel finding from our study is that obese cecal and fecal samples had greater predicted metagenomic function for branch-chain amino acids (BCAA) degradation and synthesis compared with lean pigs. Microbial fermentation of the BCAA valine, isoleucine, and leucine yield branched-chain fatty acids (BCFA) isobutyrate, valerate, and isovalerate. Proteolytic fermentation is normally more prevalent in the distal colon due to lower carbon substrates for bacterial fermentation, causing digestion pH to increase (16). However, Western diets are very low in complex carbohydrates and fiber and high in saturated fat and simple sugars. Some evidence has linked isobutyrate and isovalerate to insulin resistance and metabolic syndrome (30); however, the connection between BCFA and obesity and metabolic syndrome is largely unexplored. Furthermore, amino acids can also serve as precursors to SCFA, which have been implicated as sources of energy derived from the gut microbiota and contributing to the disease.
Lastly, we wanted to assess post hoc whether microbial composition differences in the cecal contents vs. feces had any implications on anthropometric and serum markers of obesity and metabolic syndrome. We have previously shown that increases in cecal Proteobacteria and families within are positively associated with hepatic toll-like receptor 4 and ileal LPS-binding protein expression and negatively associated with ileal tight junction proteins mRNA expression (31). Here, we found that the ratio of Firmicutes to Bacteroidetes was positively associated with percent body fat in fecal samples, but not in cecal samples. This supports our predicted metagenomic function data supporting greater “energy harvest” potential in obese fecal vs. cecal samples. Interestingly, our current study found fecal relative abundances of Verrucomicrobia tended to be negatively associated and Blautia was positively associated with increased serum glucose, insulin, and cholesterol concentrations, respectively, whereas these associations were not as strong in cecal contents. These phylum and genus observations have been observed in fecal samples of patients with glucose intolerance and patients with atherosclerosis (12, 17, 20, 50). However, Methanobacteriaceae exhibited a stronger positive correlation to serum triglycerides in cecal contents than in fecal samples. This family consists of methanogen species, including Methanobrevibacter smithii, that have been linked to host energy metabolism potentially through increased SCFA production/increased energy harvest and slowing gut motility in both rodents and humans (26, 27). Furthermore, abundance of this family of microbiota has been shown to be increased in the small vs. the large bowel (26), suggesting the impact of this bacteria may have different implications on host metabolism in the proximal vs. distal GI tract. Specifically, fecal microbiota composition may be more indicative of energy harvest, and potentially impact glucose, insulin, and cholesterol metabolism, whereas cecal microbiota samples may be more indicative of methanogen and potentially inflammation and hepatic health (31). These data suggest that differences in microbiome composition in proximal vs. distal colon may reveal different implications on host metabolism and warrant further investigation.
Conclusions
Overall, our data suggest significant differences between lean and obese Ossabaw pig microbiome compositions and predicted metagenomic function. Furthermore, we found differences between the cecal and fecal microbiomes primarily at the family and genus taxonomic levels that may have different implications for obesity and metabolic syndrome. More specifically, some families within Bacteroidetes and Proteobacteria that suggest increased methanogens and LPS-containing bacteria are greater in cecal contents than in fecal samples, thereby potentially promoting more energy harvesting potential from the diet and inflammation and gut permeability. Furthermore, evaluation of energy extraction from the diet may be more revealing in the fecal microbiome. In conclusion, our data suggest that Western diet causes severe dysbiosis differentially between the proximal and distal GI tracts in the Ossabaw pig model.
Future Directions
Although difficult to obtain, future evaluation of the proximal vs. the distal colon microbiome as it relates to obesity and metabolic syndrome in humans could prove to be very important in determining the impact of the microbiome on host metabolism and metabolic disease risk. To this end, understanding the compositional and microbial metagenomics of other GI sites (i.e., jejunum, ileum, and proximal colon) as they relate to host metabolism in this animal model would help supplement this work. Observed differences in cecal and fecal predicted metagenomic capacities in this study prompt microbiome transfer studies that investigate the host metabolic consequences of using cecal vs. fecal samples. A limitation to this study is the amplification of 16S rDNA at the V4 region provides a limited microbial composition compared with shotgun metagenomic sequencing. Future studies analyzing whole genome sequencing between cecal and fecal microbiomes to directly measure composition and metabolic capacity as they relate to obesity would also be invaluable to the field.
GRANTS
This work was supported by grants from Mizzou Advantage, The Allen Foundation, USDA-Agricultural Research Service Project 6026-51000-010-05S (K. Shankar) and was partially supported by VA-Merit Grant I01BX003271-01 (R. S. Rector). We acknowledge National Institutes of Health Grants RR-013223 and HL-062552 to M. Sturek and the Comparative Medicine Program of Indiana University School of Medicine and Purdue University for the female Ossabaw swine. This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.R.P., U.D.W., S.V.C., K.S., and R.S.R. performed experiments; M.R.P., U.D.W., S.V.C., K.S., and R.S.R. analyzed data; M.R.P., U.D.W., S.V.C., K.S., and R.S.R. interpreted results of experiments; M.R.P. and R.S.R. prepared figures; M.R.P. and R.S.R. drafted manuscript; M.R.P., U.D.W., S.V.C., K.S., and R.S.R. edited and revised manuscript; M.R.P., U.D.W., S.V.C., K.S., and R.S.R. approved final version of manuscript; R.S.R. conceived and designed research.
REFERENCES
- 1.Andoh A, Nishida A, Takahashi K, Inatomi O, Imaeda H, Bamba S, Kito K, Sugimoto M, Kobayashi T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J Clin Biochem Nutr 59: 65–70, 2016. doi: 10.3164/jcbn.15-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäckhed F. Changes in intestinal microflora in obesity: cause or consequence? J Pediatr Gastroenterol Nutr 48, Suppl 2: S56–S57, 2009. doi: 10.1097/MPG.0b013e3181a11851. [DOI] [PubMed] [Google Scholar]
- 3.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101: 15718–15723, 2004. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beerens H, Romond C. Sulfate-reducing anaerobic bacteria in human feces. Am J Clin Nutr 30: 1770–1776, 1977. doi: 10.1093/ajcn/30.11.1770. [DOI] [PubMed] [Google Scholar]
- 5.Bellinger DA, Merricks EP, Nichols TC. Swine models of type 2 diabetes mellitus: insulin resistance, glucose tolerance, and cardiovascular complications. ILAR J 47: 243–258, 2006. doi: 10.1093/ilar.47.3.243. [DOI] [PubMed] [Google Scholar]
- 6.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 8.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336, 2010. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O’Toole PW, Cotter PD. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes 3: 186–202, 2012. doi: 10.4161/gmic.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56: 35–45, 2006. [PubMed] [Google Scholar]
- 11.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461, 2010. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 12.Egshatyan L, Kashtanova D, Popenko A, Tkacheva O, Tyakht A, Alexeev D, Karamnova N, Kostryukova E, Babenko V, Vakhitova M, Boytsov S. Gut microbiota and diet in patients with different glucose tolerance. Endocr Connect 5: 1–9, 2016. doi: 10.1530/EC-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145: 745–757, 2011. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M; SPRING Trial Group . Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes 65: 2214–2223, 2016. doi: 10.2337/db16-0278. [DOI] [PubMed] [Google Scholar]
- 15.Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, Quintana-Navarro GM, Landa BB, Navas-Cortés JA, Tena-Sempere M, Clemente JC, López-Miranda J, Pérez-Jiménez F, Camargo A. Intestinal microbiota is influenced by gender and body mass index. PLoS One 11: e0154090, 2016. doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes R, Magee EA, Bingham S. Protein degradation in the large intestine: relevance to colorectal cancer. Curr Issues Intest Microbiol 1: 51–58, 2000. [PubMed] [Google Scholar]
- 17.Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3: 1245, 2012. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knights D, Kuczynski J, Charlson ES, Zaneveld J, Mozer MC, Collman RG, Bushman FD, Knight R, Kelley ST. Bayesian community-wide culture-independent microbial source tracking. Nat Methods 8: 761–763, 2011. doi: 10.1038/nmeth.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J, Liu C, Shivdasani RA, Ogino S, Birren BW, Huttenhower C, Garrett WS, Meyerson M. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22: 292–298, 2012. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahti L, Salonen A, Kekkonen RA, Salojärvi J, Jalanka-Tuovinen J, Palva A, Orešič M, de Vos WM. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. PeerJ 1: e32, 2013. doi: 10.7717/peerj.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31: 814–821, 2013. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee L, Alloosh M, Saxena R, Van Alstine W, Watkins BA, Klaunig JE, Sturek M, Chalasani N. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology 50: 56–67, 2009. doi: 10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075, 2005. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Litten-Brown JC, Corson AM, Clarke L. Porcine models for the metabolic syndrome, digestive and bone disorders: a general overview. Animal 4: 899–920, 2010. doi: 10.1017/S1751731110000200. [DOI] [PubMed] [Google Scholar]
- 25.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2: 204, 2007. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Mathur R, Kim G, Morales W, Sung J, Rooks E, Pokkunuri V, Weitsman S, Barlow GM, Chang C, Pimentel M. Intestinal Methanobrevibacter smithii but not total bacteria is related to diet-induced weight gain in rats. Obesity (Silver Spring) 21: 748–754, 2013. doi: 10.1002/oby.20277. [DOI] [PubMed] [Google Scholar]
- 27.Mbakwa CA, Penders J, Savelkoul PH, Thijs C, Dagnelie PC, Mommers M, Arts IC. Gut colonization with methanobrevibacter smithii is associated with childhood weight development. Obesity (Silver Spring) 23: 2508–2516, 2015. doi: 10.1002/oby.21266. [DOI] [PubMed] [Google Scholar]
- 28.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6: 610–618, 2012. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neeb ZP, Edwards JM, Alloosh M, Long X, Mokelke EA, Sturek M. Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comp Med 60: 300–315, 2010. [PMC free article] [PubMed] [Google Scholar]
- 30.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15: 606–614, 2012. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panasevich MR, Meers GM, Linden MA, Booth FW, Perfield JW 2nd, Fritsche KL, Wankhade UD, Chintapalli SV, Shankar K, Ibdah JA, Rector RS. High-fat, high fructose, high-cholesterol feeding causes severe NASH and cecal microbiota dysbiosis in juvenile Ossabaw swine. Am J Physiol Endocrinol Metab 314: E78–E92, 2018. doi: 10.1152/ajpendo.00015.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panasevich MR, Morris EM, Chintapalli SV, Wankhade UD, Shankar K, Britton SL, Koch LG, Thyfault JP, Rector RS. Gut microbiota are linked to increased susceptibility to hepatic steatosis in low-aerobic-capacity rats fed an acute high-fat diet. Am J Physiol Gastrointest Liver Physiol 311: G166–G179, 2016. doi: 10.1152/ajpgi.00065.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panasevich MR, Peppler WT, Oerther DB, Wright DC, Rector RS. Microbiome and NAFLD: potential influence of aerobic fitness and lifestyle modification. Physiol Genomics 49: 385–399, 2017. doi: 10.1152/physiolgenomics.00012.2017. [DOI] [PubMed] [Google Scholar]
- 34.Pang W, Vogensen FK, Nielsen DS, Hansen AK. Faecal and caecal microbiota profiles of mice do not cluster in the same way. Lab Anim 46: 231–236, 2012. doi: 10.1258/la.2012.011128. [DOI] [PubMed] [Google Scholar]
- 35.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30: 3123–3124, 2014. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen R, Ingerslev HC, Sturek M, Alloosh M, Cirera S, Christoffersen BO, Moesgaard SG, Larsen N, Boye M. Characterisation of gut microbiota in Ossabaw and Göttingen minipigs as models of obesity and metabolic syndrome. PLoS One 8: e56612, 2013. doi: 10.1371/journal.pone.0056612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci USA 103: 10011–10016, 2006. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18: 190–195, 2010. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 39.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33: 496–503, 2015. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr 138: 397–402, 2008. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 41.Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. MBio 7: 7e01018-16, 2016. doi: 10.1128/mBio.01018-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature 457: 480–484, 2009. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 44.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1: 6ra14, 2009. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vieira-Potter VJ, Lee S, Bayless DS, Scroggins RJ, Welly RJ, Fleming NJ, Smith TN, Meers GM, Hill MA, Rector RS, Padilla J. Disconnect between adipose tissue inflammation and cardiometabolic dysfunction in Ossabaw pigs. Obesity (Silver Spring) 23: 2421–2429, 2015. doi: 10.1002/oby.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol 10: 18–26, 2017. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang-Sun W, Augusto LA, Zhao L, Caroff M. Desulfovibrio desulfuricans isolates from the gut of a single individual: structural and biological lipid A characterization. FEBS Lett 589: 165–171, 2015. doi: 10.1016/j.febslet.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C, Zhao G, Chen Y, Zhao L. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J 4: 232–241, 2010. [Erratum in ISME J 4: 312–313, 2010.] doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA 106: 2365–2370, 2009. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One 8: e71108, 2013. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]