Abstract
The renal aldosterone-sensitive distal tubule (ASDT) is crucial for sodium reabsorption and blood pressure regulation. The ASDT consists of the late distal convoluted tubule (DCT2), connecting tubule (CNT), and collecting duct. Due to difficulties in isolating epithelial cells from the ASDT in large quantities, few transcriptome studies have been performed on this segment. Moreover, no studies exist on isolated DCT2 and CNT cells (excluding intercalated cells), and the role of aldosterone for regulating the transcriptome of these specific cell types is largely unknown. A mouse model expressing eGFP in DCT2/CNT/initial cortical collecting duct (iCCD) principal cells was exploited to facilitate the isolation of these cells in high number and purity. Combined with deep RNA sequencing technology, a comprehensive catalog of chronic aldosterone-regulated transcripts from enriched DCT2/CNT/iCCD principal cells was generated. There were 257 significantly downregulated and 290 upregulated transcripts in response to aldosterone (P < 0.05). The RNA sequencing confirmed aldosterone regulation of well-described aldosterone targets including Sgk1 and Tsc22d3. Changes in selected transcripts such as S100a1 and Cldn4 were confirmed by RT-qPCR. The RNA sequencing showed downregulation of Nr3c2 encoding the mineralocorticoid receptor (MR), and cell line experiments showed a parallel decrease in MR protein. Furthermore, a large number of transcripts encoding transcription factors were downregulated. An extensive mRNA transcriptome reconstruction of an enriched CNT/iCCD principal cell population was also generated. The results provided a comprehensive database of aldosterone-regulated transcripts in the ASDT, allowing development of novel hypotheses for the action of aldosterone.
Keywords: aldosterone, aldosterone-sensitive distal tubule (ASDT), kidney, mineralocorticoid receptor (MR), mRNA transcripts
INTRODUCTION
Aldosterone-mediated activation of nongenomic and genomic pathways in the aldosterone-sensitive distal tubule (ASDT) tightly regulates renal sodium reabsorption and potassium secretion (13, 46, 50). The genomic effects of aldosterone are predominantly through activation of the mineralocorticoid receptor (MR), which translocates to the nucleus to stimulate mRNA transcription of target genes such as serum and glucocorticoid-regulated kinase 1 (Sgk1) (15) and the epithelial sodium channel alpha-subunit (αENaC) (1, 57). The importance of the ASDT is clear from mouse models with ENaC deletion in various parts of the ASDT (6, 36, 40, 43), which suffer from various disorders of sodium and potassium balance, including difficulties maintaining blood pressure.
Large-scale studies of the ASDT have been limited by the inability to isolate the segment in large numbers and purity. Microdissection of late distal convoluted tubules (DCT2)/connecting tubules (CNT) from mice (41, 59) and rats (25) is possible, but the technique is highly difficult and does not exclude the intercalated cells, which are mainly responsible for acid/base homeostasis (1). To navigate this problem, a transgenic mouse model with eGFP expression controlled by the transient receptor potential cation channel subfamily V member 5 (TRPV5) promoter was recently generated (14). This model has in the kidney eGFP expression exclusively in the DCT2 cells, CNT cells, and initial cortical collecting duct (iCCD) principal cells, allowing collection of these cells by fluorescence-activated cell sorting (FACS) (20). This model was recently used to study short-term regulatory effects of aldosterone on the proteome of ASDT with protein mass spectrometry (20).
Due to its high sensitivity, transcriptomic analysis using microarrays is a good alternative approach for large-scale analysis of low amounts of starting material (9, 37, 59). Using this technique, Zuber et al. (59) made a transcriptome reconstruction of microdissected mouse DCT2/CNTs (containing a heterogeneous cell population consisting of DCT2 cells, CNT cells, and intercalated cells), and Fakitsas et al. (9) identified 22 gene products in microdissected mouse distal nephrons that were increased in abundance 1 h after aldosterone injection. Recently, RNA sequencing has largely replaced the use of microarrays due to its improved sensitivity and greater dynamic range (54). RNA sequencing of various renal tubule segments microdissected from rat kidney has demonstrated the power of this technique (25). In the latter study, whole DCT2 and the CNT tubules were isolated and sequenced (25).
The present study combines the use of the TRPV5 eGFP model with FACS and RNA sequencing to 1) examine the effects of 6-day aldosterone administration on the transcriptome of enriched DCT2/CNT/iCCD principal cells, 2) generate an extensive mRNA transcriptome of enriched CNT/iCCD principal cells, and 3) generate a DCT2 cell transcriptome by comparison of the two pools of data. The results open up new avenues within the aldosterone biology field for studying regulation of signaling processes within the ASDT.
MATERIALS AND METHODS
Experimental protocols and animals.
Two animal studies were performed using TRPV5 eGFP male mice and littermates (genetic background: C57BL/6). In the DCT2/CNT/iCCD aldosterone study, TRPV5 eGFP mice (8–13 wk) (14) were kept at 27°C in metabolic cages with free access to water and powdered standard mouse chow (Altromin Spezialfutter, Lage, Germany). Mice were allowed 48 h of acclimation before baseline metabolic measurements were recorded over a 24 h period. Subsequently, mice were administered vehicle control (5% DMSO in physiological saline; n = 7) or aldosterone (100 µg aldosterone kg−1·24 h−1 in 5% DMSO in physiological saline, n = 7) for 6 days via osmotic minipumps (Alzet model 1007D; Alza, Palo Alto, CA) inserted subcutaneously while the mice were under isoflurane anesthesia. The mice were kept in metabolic cages for the duration of the study, where after the kidneys were collected with the mice under isoflurane anesthesia. Due to the application of FACS, one control and one aldosterone-administrated mouse were processed each day. In the CNT/iCCD transcriptome reconstruction study, TRPV5 eGFP mice (8–13 wk) (14) (n = 6; cells from all mice were pooled before RNA sequencing) were kept in regular cages with free access to water and pelleted standard mouse chow (Altromin Spezialfutter) until mice were euthanized and their kidneys were collected under isoflurane anesthesia. All animal experiments were performed in agreement with a license issued by the Danish Animal Experiments Inspectorate, Ministry of Food, Agriculture, and Fisheries, Danish Veterinary and Food Administration.
Collection and analyses of urine and blood.
Urine was collected in metabolic cages and cleared by centrifugation at 1000 g for 4 min. Sodium and potassium concentrations were measured with an IL943TM flame photometer (Instrumentation Laboratory, Bedford, MA) or measured commercially by MRC Harwell (Oxfordshire, UK). Blood was collected from the right ventricle and immediately centrifuged at 12,000 g for 4 min. Plasma concentrations of sodium and potassium were measured by MRC Harwell with an ion-selective electrode (AU680; Beckman Coulter, Brea, CA). Blood plasma aldosterone concentrations were analyzed with an enzyme immunoassay kit (EIA-5298; DRG International, Springfield, NJ). Osmolality of urine and plasma was measured with a freezing point depression osmometer (Advanced model 3320 Micro-Osmometer; Advanced Instruments, Norwood, MA).
Enzyme digestion of tissue.
A single-cell kidney suspension was produced by enzymatic digestion of whole kidney (WK) tissue. Mice were perfused through the left ventricle with a dissociation buffer prewarmed to 37°C [1.5 mg/l Collagenase B (Roche Diagnostics, Mannheim, Germany), 2.0 mg/ml Pronase (Roche Diagnostics), 0.05 mg/ml DNase I (Sigma-Aldrich, St. Louis, MO), 0.38 mg/ml glycine, 140 mM NaCl, 0.4 mM KH2PO4, 1.6 mM K2HPO4, 1 mM MgSO4, 10 mM Na-Acetate, 1 mM α-ketoglutarate, 1.3 mM Ca-gluconate, 10 mM glucose, pH: 7.4]. Kidneys were removed and immediately minced in 37°C tissue dissociation buffer. The kidney pieces were mixed at 850 rpm (37°C) with an Eppendorf Thermomixer Compact (Eppendorf, Hamburg, Germany). After 10, 20, and 30 min, dissociated tubules were removed, and fresh tissue dissociation buffer was added. After 40 min, all dissociated tubule fractions were combined and sedimented by low-speed centrifugation (2 min at 500 rcf). The pellet was washed and incubated in a trypsin buffer (trypsin supplemented with 0.45 mg/ml DNase I, 0.7 mM MgSO4, 9 mM glucose, 9 mM HEPES) for 15 min at 37°C to separate cells. During the incubation period, the tubule suspension was pipetted every 5 min to detach cells mechanically. Subsequently, cells were washed, filtered through a 40 µm mesh, and stored in 4°C GIBCO DMEM cell medium (Life Technologies, Carlsbad, CA; supplemented with 0.7 mg DNase I, 9 mM HEPES, and 9 mM glucose) until FACS (~30 min).
FACS.
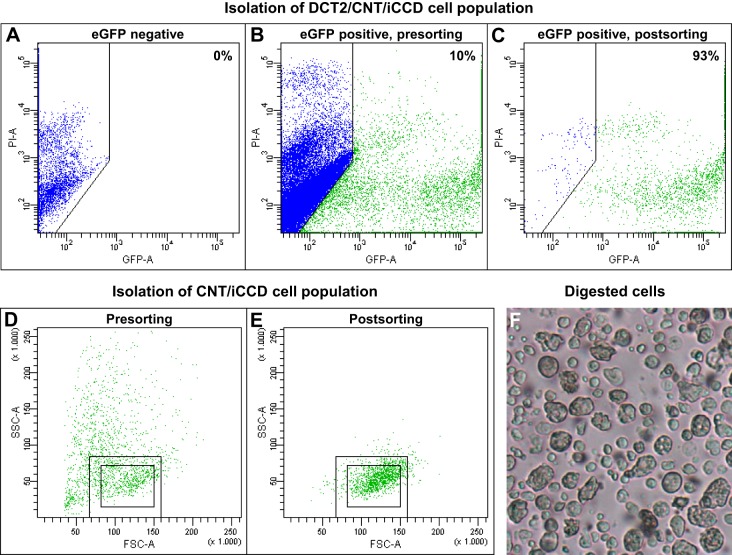
Before sorting, propidium iodide (PI) was added to the cell suspension to stain dead cells that were subsequently excluded from the final pool of eGFP-positive cells. The cell suspension was sorted at 4°C using a BD FACSAria III Cell Sorter (BD Biosciences, San Jose, CA). In the DCT2/CNT/iCCD aldosterone study, gates were set to exclude the smallest eGFP-positive events (likely to represent cellular fragments) and events that were considered to represent duplicates (i.e., cells that were attached to each other). Finally, a gate was set to collect eGFP-positive cells. After sorting, ~2,000 cells from the sorted pool were analyzed on the cell sorter to determine sorting purity (Fig. 1, A–C). The sorting purity was analyzed with BD FACSDiva Software (BD Biosciences), and background fluorescence was determined with an eGFP-negative cell suspension (Fig. 1A). To collect CNT/iCCD principal cells, gates in Fig. 1, B and C were used. However, an additional gate was set to collect eGFP-positive with low side scatter and medium forward scatter (i.e., cells inside small rectangle), and to exclude “DCT2” cells (cells outside large rectangle). These cells were expected to represent CNT/CCD principal cells because they have a lower side scatter than DCT2 cells. For RNA sequencing, cells were immediately lysed with a lysis buffer (Buffer RLT Plus; Qiagen, Venlo, Netherlands) and stored at −80°C.
Fig. 1.
Evaluation of fluorescence-activated cell sorting (FACS) sorting purity. A: an eGFP-negative cell population from whole kidney was used to determine background fluorescence. B, C: based on the background fluorescence, the mean presorting and postsorting eGFP concentrations of the late distal convoluted tubule (DCT2)/connecting tubule (CNT)/initial cortical collecting duct (iCCD) cell population were measured to be ~10 and ~93% (n = 14), respectively. D, E: in the CNT/iCCD transcriptome reconstruction study, gates in B and C were used; however, an additional gate was set to collect eGFP-positive CNT cells and CCD principal cells, which have low side scatter and medium forward scatter (i.e., cells inside small rectangle), and to exclude “DCT2” cells (cells outside large rectangle). F: in the enzymatic digested cell suspension, most cells were separated from each other confirming that the cell suspension subjected to the FACS mainly contained single cells.
Cell culture and cell experiments.
Mouse (m)CCDcl1 cells (10) were grown in DMEM/F12 (GIBCO) supplemented with insulin (10 µg/ml), human apotransferrin (5.5 µg/ml), Na-selenate (6.7 µg/l), dexamethasone (50 nM), T3 (10−9 M), penicillin-streptomycin, EGF (10 ng/µl), and FCS (2%). Cells were seeded on semipermeable filters (Transwell, 0.4 µm pore size, Corning) at a density of 8.4 × 104 cells/cm2 and grown for further 7 days reaching formation of polarized monolayers. Subsequently, cells were treated with 300 nM aldosterone for 4 days. The experiments were performed in either the medium described above (two experiments, n = 3 per group in each experiment) or in the medium described above excluding FCS and dexamethasone (one experiment, n = 3 per group).
Western blotting.
FACS-sorted cells or (m)CCDcl1 cells were sonicated at 4°C in a dissection buffer containing protease and phosphatase inhibitors and Laemmli sample buffer containing 15 mg/ml DTT. Samples were run on 12% Tris-HEPES gels or 4–15% Criterion TGX Precast Gels (Bio-Rad Laboratories, Hercules, CA). Coomassie-stained gels were used to adjust for different protein concentrations between the samples. For Western blotting, proteins were transferred to Hybond-P PVDF membranes (GE Healthcare, Little Chalfont, UK) and incubated overnight at 4°C with primary rabbit antibodies against aquaporin-2 [AQP2, 7661 (33), dilution 1:1,000], Na+-Cl− cotransporter (NCC; SPC-402D, StressMarq Biosciences, Victoria, BC, Canada; dilution 1:1,000), aquaporin-1 [AQP1 (34), dilution 1:1,000], αENaC [(48), dilution 1:1,000], MR (rMR1-18 1D5-s, Developmental Studies Hybridoma Bank, dilution 1:500). After secondary antibody incubation, each blot was visualized using an Enhanced Chemiluminescence system (GE Healthcare) or SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific, Rockford, IL).
RNA sequencing.
Purification of mRNA, library construction, and RNA sequencing were carried out by AROS Applied Biotechnology (Aarhus, Denmark) by industry standard methods. RNA was extracted with Qiagen’s RNeasy Micro kit. RNA sequencing libraries were prepared with Illumina’s TruSeq RNA Sample prep kit using 400 ng RNA as input. The steps in library preparation included capture of mRNA on oligo-dT-beads, concomitant fragmentation and primer annealing, first and second strand synthesis, 3′ enzymatic steps resulting in end repair, 3′ adenylation, adapter ligation, and library amplification (PCR). Sequencing was performed with a Hiseq 2000 (Illumina, San Diego, CA) and 100 bp paired-end reads. In the DCT2/CNT/iCCD aldosterone study, 39.8 ± 1.0, million reads (n = 14) were generated per sample, while in the CNT/iCCD mRNA transcriptome reconstruction study, 214.1 million reads were generated (n = 1, FACS-sorted cells from six mice were pooled to get sufficient amount of RNA for sequencing).
RT-qPCR.
Total RNA was isolated with the Ambion Ribopure kit (Invitrogen, Carlsbad, CA), treated with DNase I (Invitrogen), and reverse transcribed using Superscript II and random primers (Invitrogen); all steps were performed according to the manufacturer’s instructions. All primers spanned an intron and the product size was < 200 bp (for primers see Table 1). A control reaction without the reverse transcriptase enzyme was performed to exclude genomic DNA amplification. Specificity of the amplified product was evaluated with melting curve analysis software and by running products on a gel to confirm the predicted size. Amplification was performed on 5 pmol of each primer and with SYBR Green I Master Taq (Roche Applied Science). Cycling conditions were: 95°C for 5 min, followed by 40 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 30 s. RT-qPCR reactions were run on a LightCycler 480 (Roche), with fluorescence measured at the end of each elongation step to calculate Ct values. Relative quantitation of gene expression was determined by the comparative Ct method. Differences in the amount of starting cDNA were corrected for using the geometric mean of 18s, Polr2a, and GAPDH.
Table 1.
Primers used for RT-qPCR
| Gene | Forward Primer | Reverse Primer | Product Size, bp |
|---|---|---|---|
| 18s | GGATCCATTGGAGGGCAAGT | ACGAGCTTTTTAACTGCAGCAA | 91 |
| Polr2a | ATGGCTGAGGAGTTTCGGCT | AGGATGGGCAATGGCTTGGT | 90 |
| GAPDH | CCGTGTTCCTACCCCCAAT | ATGTCATCATACTTGGCAGGTTTC | 75 |
| S100a1 | GCCCTTCTGTCGAGAATCTGT | GCTCAGAGCCCATTTCAGCA | 71 |
| B2m | CTCGGTGACCCTGGTCTTTC | GGATTTCAATGTGAGGCGGG | 164 |
| Atp5e | TGAGGCTACTCTGAAGCGAC | TCTTCAGGGCATCCCTCACT | 141 |
| Atp5k | CTCATCAAGTTCGGCCGGTA | TCCTCCGCTGCTATTCTCCT | 113 |
| Cldn4 | CAACTGCATGGAGGACGAGA | GGGTTGTAGAAGTCGCGGAT | 135 |
| Car15 | GCCTGGCTGTACATTCCTCAT | GCAGGTGCTAGTTCCTTCCA | 193 |
Bioinformatics analysis.
All index and annotation files were obtained from the webpage of John Hopkins University, Center for Computational Biology, Baltimore, MD. Alignment and annotation were performed with TopHat 2.0.8. and Cufflinks 2.0.2, respectively, without options allowing for identification of novel genes, as described in Trapnell et al. (51). Transcript abundances were calculated as Fragments Per Kilobase of the transcript per Million mapped reads (FPKM) (5, 30).
For the DCT2/CNT/iCCD aldosterone study, reads were aligned to the Mus musculus genome index [mm10, University of California, Santa Cruz (UCSC)]. Data were further analyzed with the Cuffdiff software (51) to identify downregulated and upregulated transcripts (i.e., genes whose mean transcript isoform abundance was downregulated or upregulated) (51). FPKMs were required to be > 1 in either the control or the aldosterone-administrated group, while transcripts were included in a reference transcriptome if the FPKM for either the control or the aldosterone-administrated group was > 0. To estimate the number of false positively regulated transcripts identified when applying uncorrected P values, the control group was randomly divided into two groups (n = 4 and 3, respectively). The uncorrected and FDR (false discovery rate)-corrected P values revealed 74 and five significantly regulated transcripts, respectively. In addition, four control and three aldosterone-administrated mice were tested similarly, and here the uncorrected and FDR-corrected P values revealed 535 and 62 significantly regulated transcripts, respectively. Thus, we estimated that when using uncorrected P values, 14% (74 out of 535) of the transcripts were false positively regulated, while FDR correction reduced the fraction to 8% (5 out of 62). In the tables representing the controls (n = 7) vs. the aldosterone-administrated mice (n = 7) (Supplemental Tables S1 and S2), the significance levels are presented as both uncorrected (P, 547 significantly regulated transcripts) and FDR-corrected (Q, 76 significantly regulated transcripts). (The online version of this article contains supplemental material.) Transcripts found to be differentially regulated (uncorrected P < 0.05) were grouped into gene ontology (GO) terms using the generic GO term mapper developed by the Bioinformatics Group at the Lewis-Sigler Institute (http://go.princeton.edu/cgi-bin/GOTermMapper). For the CNT/iCCD transcriptome reconstruction study, reads were aligned to the M. musculus genome index, NCBIM37, [European Bioinformatics Institute, UK/the Wellcome Trust Sanger Institute, UK (Ensemble)]. To identify connections between differentially regulated transcripts involved in oxidative phosphorylation in the aldosterone study, data were analyzed with Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources 6.7 (Frederick, MD) (16, 17). Data are presented in Fig. 5 with permission from Kyoto Encyclopedia of Genes and Genomes (KEGG) (21–23). Hierarchical clustering analyses were performed on data from both the DCT2/CNT/iCCD aldosterone study and the CNT/iCCD transcriptome reconstruction study with Perseus software (version 1.5.4.1) (PMID: 27348712) and Microsoft Excel (Redmond, WA). Nearness of data was measured by using Euclidean distance, and distance between clusters was defined by average linkage. All transcripts with an FPKM value ≥ 1 were included, and the FPKM values were transformed with log2 (FPKM+1) before entering the clustering algorithm.
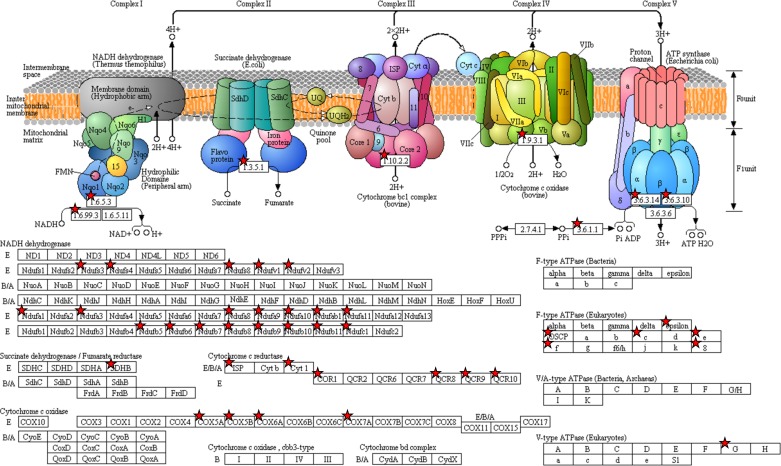
Fig. 5.
DAVID analysis shows aldosterone-induced upregulation of a high number of transcripts involved in oxidative phosphorylation (red asterisks).
Statistics.
Densitometric data meeting statistical assumptions of normality and variance homogeneity were analyzed by Student’s two-sided t-test, while data only meeting assumptions of normality were analyzed by Satterthwaite's two-sided unequal variance t-test. Data not meeting assumptions of normality were ln-transformed or square-root transformed in accordance with Sokal and Rohlf (47) and analyzed by the appropriate t-tests. If data did not fulfill assumptions of normality after transformation, untransformed data were analyzed by Mann-Whitney U-test. For GO-term analyses, significant differences between the number of downregulated/upregulated transcripts and the reference transcriptome were determined by Fisher’s exact test (47). Within each GO-term category, P values were corrected for multiple comparisons by FDR correction (31). Statistical tests were carried out with Stata 12.0 (StataCorp, College Station, TX) for Windows. All values are presented as means ± SE.
RESULTS AND DISCUSSION
Evaluation of the physiological animal model.
Plasma aldosterone levels were significantly increased in the aldosterone-administrated group (Table 2), and the mice were hypokalemic, consistent with previous studies (8, 32, 38). Aldosterone-administered mice had increased water intake, increased urine output, and decreased urine osmolality; this manifestation has been shown previously to be associated with decreased apical expression of AQP2 in the CCD (8). The physiological data presented here are consistent with our recently published study (38) where we used an identical aldosterone protocol. In that study, we furthermore showed that the administrated dose of aldosterone (100 µg aldosterone kg−1·24 h−1) resulted in an expected upregulation of NCC and αENaC proteins (38). The upregulation of these two proteins was confirmed in the present study (data not shown).
Table 2.
Physiological parameters in mice after 6 days aldosterone administration
| Control |
Aldosterone |
||||
|---|---|---|---|---|---|
| Parameter | Mean | SE | Mean | SE | P Value |
| Blood aldosterone, 6 days, pg/ml | 145 | 34 | 506 | 61 | 0.003† |
| Blood Na+, 6 days, mmol/l | 149.7 | 0.8 | 153.4 | 0.9 | 0.011* |
| Blood K+, 6 days, mmol/l | 5.7 | 0.2 | 4.5 | 0.1 | 0.001‡ |
| Blood osmolality, 6 days, mosmol/kgH2O | 317 | 1 | 322 | 2 | 0.053 |
| Urine Na+, baseline, µmol·(g BW)−1·(24 h)−1 | 6.4 | 1.2 | 6.5 | 0.6 | 0.949 |
| Urine Na+, 6 days, µmol·(g BW)−1·(24 h)−1 | 5.8 | 0.7 | 8.4 | 0.9 | 0.047* |
| Urine K+, baseline, µmol·(g BW)−1·(24 h)−1 | 24.9 | 2.8 | 25.7 | 1.5 | 0.825 |
| Urine K+, 6 days, µmol·(g BW)−1·(24 h)−1 | 22.8 | 1.3 | 25.0 | 4.0 | 0.655 |
| Body weight, baseline, g | 22.33 | 0.92 | 21.90 | 0.88 | 0.742 |
| Body weight, 6 days, g | 23.26 | 0.87 | 22.74 | 0.78 | 0.669 |
| Food intake, baseline, g·(g BW) −1·(24 h)−1 | 0.15 | 0.04 | 0.18 | 0.01 | 0.168 |
| Food intake, 6 days, g·(g BW) −1·(24 h)−1 | 0.14 | 0.02 | 0.16 | 0.01 | 0.793 |
| Water intake, baseline, g·(g BW) −1·(24 h)−1 | 0.28 | 0.02 | 0.25 | 0.02 | 0.305 |
| Water intake, 6 days, g·(g BW) −1·(24 h)−1 | 0.25 | 0.01 | 0.38 | 0.02 | 0.001† |
| Urine output, baseline, g·(g BW) −1·(24 h)−1 | 0.07 | 0.01 | 0.06 | 0.01 | 0.281 |
| Urine output, 6 days, g·(g BW) −1·(24 h)−1 | 0.07 | 0.01 | 0.14 | 0.02 | 0.005† |
| Urine osmolality, baseline, mosmol/kgH2O | 2,089 | 106 | 2,661 | 319 | 0.131 |
| Urine osmolality, 6 days, mosmol/kgH2O | 2,053 | 122 | 1,137 | 136 | <0.001‡ |
BW, body weight. n = 5–7 per group;
P < 0.05,
P < 0.01,
P < 0.001.
Evaluation of the cell type-specific sorting.
Using FACS, we collected cell populations enriched for DCT2 cell/CNT cells/iCCD principal cells and CNT cells/iCCD principal cells. When we ignored the smallest events/particles, the eGFP-positive cell concentration was ~10% before sorting (Fig. 1B). In the DCT2/CNT/iCCD aldosterone study, the eGFP-positive cell concentration was increased to 93 ± 1% (n = 14, Fig. 1C) after sorting. In the CNT/iCCD transcriptome reconstruction study, identical gates were used as in the DCT2/CNT/iCCD aldosterone study (Fig. 1, B and C) except that an additional gate was set to exclude DCT2 cells, which have high side scatter (cells outside large rectangle Fig. 1, D and E), and thereby primarily collect eGFP-positive CNT cells and CCD principal cells, which have low side scatter and medium forward scatter (i.e., cells inside small rectangle; Fig. 1, D and E). The sorting purity in the CNT/iCCD transcriptome reconstruction study was 94 ± 1% (n = 6). Figure 1F shows the cell suspension after enzymatic digestion. Most cells are separated from each other, confirming that the cell suspension subjected to the FACS contained mainly single cells.
To confirm the enrichment of cell types of interest, we investigated the protein abundances of NCC, AQP2, and AQP1 in the FACS-sorted cell populations (Fig. 2). Compared with the WK cell population, NCC abundance was reduced in the DCT2/CNT/iCCD cell population due to the exclusion of DCT1 cells in which NCC is highly abundant (26, 38). AQP2 levels were clearly higher in the DCT2/CNT/iCCD cell population compared with the WK cell population due to the enrichment of CNT cells and iCCD principal cells. In the CNT/iCCD cell population, NCC was virtually undetectable, while it was detected in the DCT2/CNT/iCCD cell population and the “DCT2” cell population. The DCT2 cell population was located outside the gate set to collect the CNT/iCCD cell population. However, it was highly contaminated with eGFP-negative cells and contained also AQP2-positive cells. Therefore, the DCT2 cell population was not analyzed further. AQP1 was highly expressed in the WK cell population, and only a very weak signal was present in the DCT2/CNT/iCCD and CNT/iCCD cell populations, confirming that these cell populations were highly enriched.
Fig. 2.
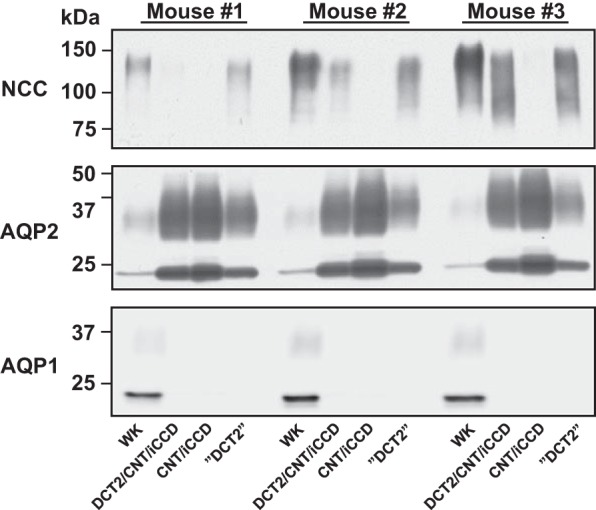
Confirmation of enriched DCT2/CNT/iCCD and CNT/iCCD cell populations. Compared with the whole kidney (WK) cell population, the NCC abundance was reduced in the DCT2/CNT/iCCD cell population (i.e., DCT2 cells, CNT cells, and iCCD principal cells) due to exclusion of DCT1 cells. Furthermore, the AQP2 abundance was clearly higher in the DCT2/CNT/iCCD cell population due to enrichment of CNT cells and iCCD principal cells. In the CNT/iCCD cell population, NCC abundance was reduced compared with the DCT2/CNT/iCCD cell population and the DCT2 cell population (cells located outside the gate collecting CNT/iCCD cell population) due to loss of DCT2 cells. By contrast, the AQP2 abundance was higher in the CNT/iCCD cell population due to enrichment of CNT cells and iCCD principal cells. Compared with the WK cell population, the AQP1 abundance was very weak in the DCT2/CNT/iCCD and CNT/iCCD cell populations confirming that contamination of AQP1-positive cells ( = eGFP negative cells) was minimal. One membrane was used for simultaneous examination of NCC, AQP2, and AQP1 abundances. AQP, aquaporin; CNT, connecting tubule; DCT2, late distal convoluted tubule; iCCD, initial cortical collecting duct; NCC, Na+-Cl− cotransporter.
Effect of long-term aldosterone administration on the DCT2/CNT/iCCD transcriptome.
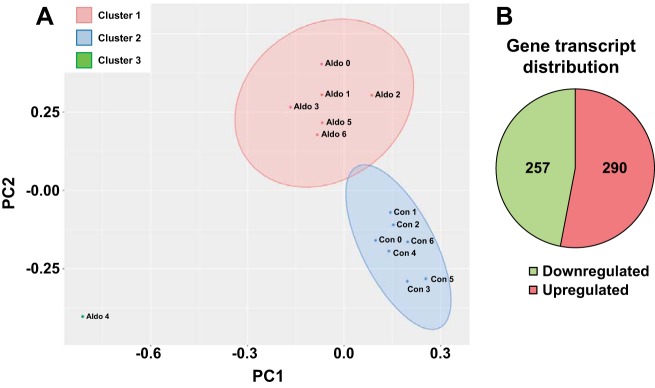
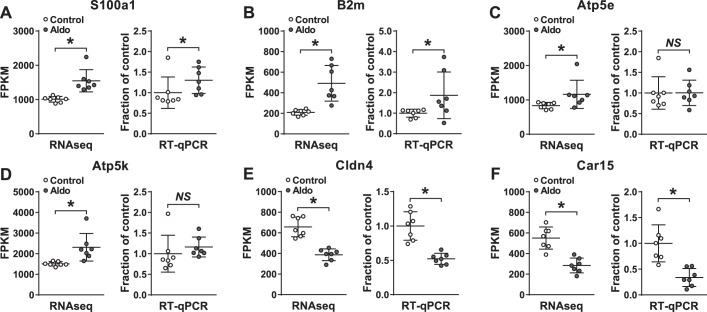
RNA sequencing identified products from 17,883 genes [FPKM > 0 in either the control or aldosterone group; only transcripts with test status = OK (see http://cole-trapnell-lab.github.io/cufflinks/cuffdiff/) were further considered for quantitative analysis]. Principal component analysis of all transcripts from the control and aldosterone-administered group (with FPKM ≥ 1 in at least 3 samples) revealed two distinct clusters (Fig. 3A), indicating that the aldosterone-administered group expressed a different transcriptome profile compared with the control group. Based on uncorrected P values, 547 transcripts (only included if FPKM status = OK and either control or aldosterone FPKM > 1) were regulated by aldosterone (downregulated, 257; upregulated, 290; Fig. 3B, Supplemental Tables S1 and S2). To confirm the RNA sequencing data, we investigated changes in mRNA levels of six randomly selected regulated transcripts by RT-qPCR using the samples that were subjected to RNA sequencing (Fig. 4). Four of the six transcripts were found to be regulated both with RNA sequencing and RT-qPCR (S100a1, B2m, Cldn4, and Car15; Fig. 4, A, B, E, and F). Thus, RNA sequencing is a robust technique for investigating aldosterone-induced transcript regulation.
Fig. 3.
Comparison of transcripts in the control and aldosterone-administered group. A: principal component analysis of all transcripts from the control and the aldosterone-administered groups revealed 2 distinct clusters. B: in the DCT2/CNT/iCCD cell population, 257 transcripts were downregulated and 290 were upregulated in response to aldosterone. CNT, connecting tubule; DCT2, late distal convoluted tubule; iCCD, initial cortical collecting duct.
Fig. 4.
Quantitative analysis of identical samples analyzed by RNA-Seq or RT-qPCR. Statistical comparisons of control and aldosterone administration were performed differently for RNA-Seq and RT-qPCR data as described in materials and methods. n = 7 per group. *P < 0.05. NS, nonsignificant.
DAVID analysis of the RNA sequencing data identified increased expression of a high number of transcripts involved in oxidative phosphorylation (Fig. 5), suggesting that mitochondrial ATP production is clearly regulated by aldosterone administration. Other interesting transcripts identified to be regulated by aldosterone included Sgk1 (FPKM: control 121.2, aldosterone 271.1; log2-ratio: 1.2; P = 0.003, Supplemental Table S2) and Tsc22d3 encoding the transcriptional factor Gilz (FPKM: control 10.8, aldosterone 17.3; log2-ratio: 0.68; P = 0.022, Supplemental Table S2). Both transcripts have been reported previously to be aldosterone-induced proteins (15, 27, 49). Some regulated transcripts were identified that encoded proteins not thought to be present in eGFP-positive cells. For example, Slc26a4, coding for the “intercalated cell-specific” protein pendrin, was increased by aldosterone (Supplemental Table S2) as previously described (53), and Foxi1, encoding the transcription factor Foxi1 that is also present in intercalated cells (Table 3) (52), was detected. However, whether these genes encode transcripts with low abundance in the eGFP-positive cells or, despite high FACS sorting purity, are due to the 6–7% eGFP-negative cell contamination from e.g., intercalated cells or cells from the connective tissue remains unknown (Fig. 1).
Table 3.
Upregulated kinases, E3 ligases, and transcriptional factors in response to 6 days aldosterone administration
| FPKM |
Log2- | ||||||
|---|---|---|---|---|---|---|---|
| Gene Symbol | Gene Name | Con | Aldo | Ratio | Ratio | P | Q |
| Kinase | |||||||
| Sgk1 | serine/threonine-protein kinase Sgk1 isoform a | 121.2 | 271.1 | 1.2 | 2.2 | 3.0e-03 | 2.8e-01 |
| E3 ligase | |||||||
| Herc6 | E3 ISG15-protein ligase Herc6 | 4.1 | 6.9 | 0.7 | 1.7 | 3.2e-03 | 2.8e-01 |
| Transcriptional factor | |||||||
| Irf7 | interferon regulatory factor 7 isoform 3 | 2.6 | 17.2 | 2.7 | 6.6 | 2.4e-06 | 7.9e-04 |
| Batf3 | basic leucine zipper transcriptional factor ATF-like 3 | 1.8 | 4.8 | 1.4 | 2.7 | 1.5e-02 | 6.7e-01 |
| Hmx2 | homeobox protein HMX2 | 6.1 | 14.0 | 1.2 | 2.3 | 1.2e-04 | 2.1e-02 |
| Rorc | nuclear receptor ROR-gamma | 6.1 | 12.7 | 1.1 | 2.1 | 1.2e-04 | 2.1e-02 |
| Zbtb16 | zinc finger and BTB domain-containing protein 16 | 1.9 | 3.9 | 1.0 | 2.0 | 1.1e-03 | 1.3e-01 |
| Stat1 | signal transducer and activator of transcription 1 isoform 2 | 6.2 | 11.3 | 0.9 | 1.8 | 1.2e-03 | 1.4e-01 |
| Tsc22d3 | TSC22 domain family protein 3 isoform 2 | 10.8 | 17.4 | 0.7 | 1.6 | 2.2e-02 | 7.8e-01 |
| Foxi1 | forkhead box protein I1 | 27.4 | 44.0 | 0.7 | 1.6 | 3.6e-03 | 3.0e-01 |
| Klf15 | Krüppel-like factor 15 | 8.4 | 13.1 | 0.6 | 1.6 | 1.5e-02 | 6.6e-01 |
| Stat2 | signal transducer and activator of transcription 2 | 3.4 | 5.3 | 0.6 | 1.5 | 2.2e-02 | 7.8e-01 |
| Arnt2 | aryl hydrocarbon receptor nuclear translocator 2 | 2.6 | 3.8 | 0.6 | 1.5 | 4.3e-02 | 1.0e+00 |
| Emx2 | homeobox protein EMX2 | 14.6 | 20.8 | 0.5 | 1.4 | 3.1e-02 | 8.8e-01 |
| Epas1 | endothelial PAS domain-containing protein 1 | 4.6 | 6.5 | 0.5 | 1.4 | 4.4e-02 | 1.0e+00 |
Aldo, aldosterone; Con, control; FPKM, Fragments Per Kilobase of the transcript per Million mapped reads. n = 7 per group.
A clustering analysis of the transcripts encoding protein kinases, E3 ubiquitin ligases, and transcription factors showed coclustering of samples within each experimental group (aldosterone-administrated vs. control, data not shown). All 17,883 genes identified in the DCT2/CNT/iCCD transcriptome (designated as the “reference transcriptome”) and the 547 regulated transcripts (divided into down- and upregulated transcripts) were annotated with the GO slim terms into three categories, i.e., cellular component, molecular function, and biological process. Based on the reference transcriptome, the 12 most frequently occurring terms within each category are listed in Fig. 6, A–C). Within the cellular component category (Fig. 6A), we found an overrepresentation of the “nucleus” term among the downregulated transcripts (P < 0.05). This result correlated with the molecular function term “DNA binding” within the downregulated transcripts (Fig. 6B). It could be speculated that aldosterone overall reduced the repression of DNA transcription by downregulating gene products responsible for inhibition of DNA transcription. Six members of the transcription factor family Kruppel-like factors (Klf) were downregulated (Supplemental Table S1), in which four of them (Klf9, -10, -11, and -13) are known to interact with SIN3 transcription regulator family member A (Sin3a was downregulated in the present study, Supplemental Table S1) to form a transcriptional repressor complex [reviewed in (28)]. Other downregulated transcription factor transcripts include Srf, encoding the serum response factor (Supplemental Table S1), which is involved in cell cycle, cell differentiation, and apoptosis (4). This finding correlates with an overrepresentation of the biological process terms “cell differentiation” and “cell death” within the downregulated transcripts (Fig. 6C).
Fig. 6.
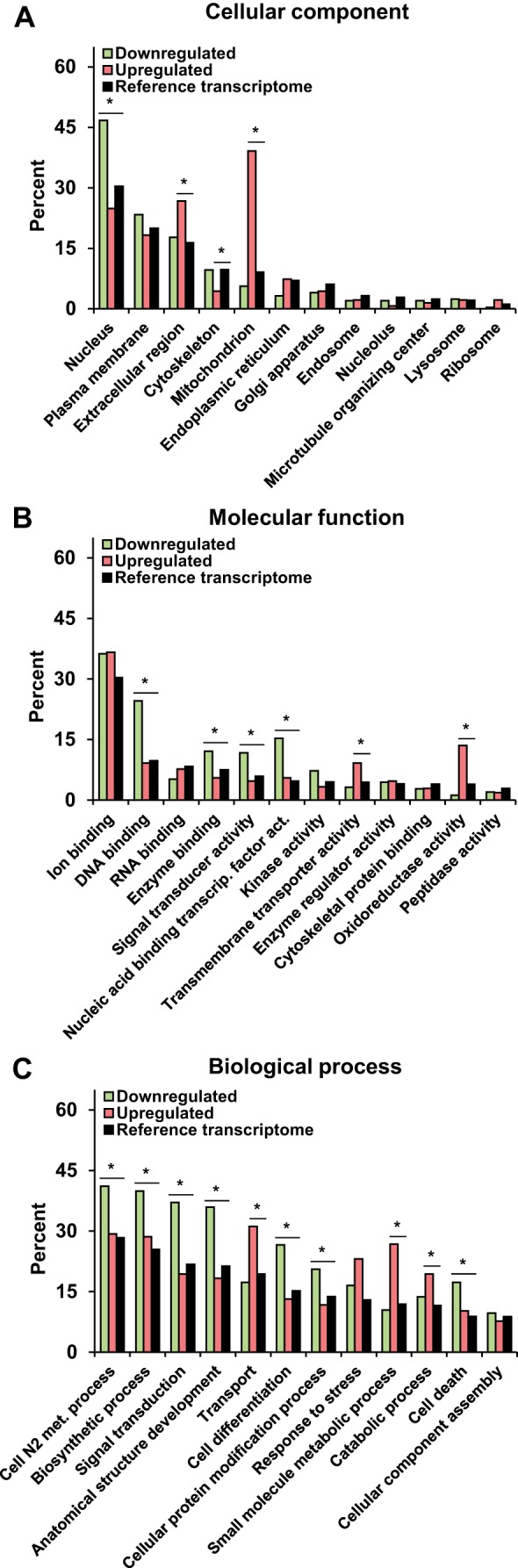
Annotation of significantly regulated genes into Gene ontology (GO) slim term categories in the DCT2/CNT/iCCD aldosterone study. Within the categories cellular component (A), molecular function (B), and biological process (C), down- and upregulated transcripts were compared with the reference transcriptome. Within each category, the 12 most abundantly expressed groups in the reference transcriptome are presented. *Significant difference between percent of down-/upregulated transcripts and the reference transcriptome (P < 0.05). CNT, connecting tubule; DCT2, late distal convoluted tubule; iCCD, initial cortical collecting duct.
Bioinformatic analysis identified several upregulated transcription factor transcripts (Table 3). These transcripts include Zbtb16, which encodes the transcription factor PLZF (promyelocytic zinc finger), which has previously been found to be highly expressed in heart (45). Upon angiotensin II stimulation, PLZF has been shown to interact with the angiotensin II type 2 receptor (AT2) at the plasma membrane followed by translocation to the nucleus and activation of phosphatidylinositol-3 kinase (PI3K-p85alpha) (45). Similarly, PLZF was found to interact with the prorenin/renin receptor, also leading to activation of PI3K-p85alpha (44).
Several E3 ubiquitin ligase transcripts were downregulated in response to aldosterone administration (Table 4), for example, Nedd4-l, encoding the E3 ubiquitin-protein ligase NEDD4-like isoform 1 (Nedd4–2), which ubiquitinates ENaC, leading to internalization and subsequent degradation of ENaC (2), and Rnf2, coding for E3 ubiquitin-protein ligase RING2, which interacts with the aldosterone sensitive transcription factor Af9 in mIMCD3 CD cells, leading to decreased α-ENaC transcription, a process reversed by aldosterone (58).
Table 4.
Downregulated kinases and E3 ligases in response to 6 days aldosterone administration
| FPKM |
Log2- | ||||||
|---|---|---|---|---|---|---|---|
| Gene Symbol | Gene Name | Con | Aldo | Ratio | Ratio | P | Q |
| Kinase | |||||||
| Tbck | TBC domain-containing protein kinase-like protein isoform 1 | 23.7 | 14.3 | −0.7 | 0.6 | 4.3e-03 | 3.4e-01 |
| Nuak1 | NUAK family SNF1-like kinase 1 | 2.8 | 1.8 | −0.7 | 0.6 | 3.5e-02 | 9.2e-01 |
| Mast4 | microtubule-associated serine/threonine-protein kinase 4 | 2.9 | 1.9 | −0.6 | 0.7 | 1.7e-02 | 7.0e-01 |
| Map3k2 | mitogen-activated protein kinase kinase kinase 2 | 10.7 | 6.5 | −0.7 | 0.6 | 2.0e-03 | 2.1e-01 |
| Map3k1 | mitogen-activated protein kinase kinase kinase 1 | 25.2 | 18.1 | −0.5 | 0.7 | 3.9e-02 | 9.5e-01 |
| Erbb3 | receptor tyrosine-protein kinase erbB-3 precursor | 13.0 | 9.4 | −0.5 | 0.7 | 4.9e-02 | 1.0e+00 |
| Epha2 | ephrin type-A receptor 2 precursor | 5.7 | 2.8 | −1.0 | 0.5 | 5.7e-04 | 7.5e-02 |
| Cdkl1 | cyclin-dependent kinase-like 1 | 201.5 | 135.9 | −0.6 | 0.7 | 4.8e-02 | 1.0e+00 |
| E3 ligase | |||||||
| Rnf43 | E3 ubiquitin-protein ligase RNF43 precursor | 8.7 | 5.9 | −0.6 | 0.7 | 2.3e-02 | 7.9e-01 |
| Rnf2 | E3 ubiquitin-protein ligase RING2 | 20.6 | 14.5 | −0.5 | 0.7 | 3.1e-02 | 8.8e-01 |
| Nedd4l | E3 ubiquitin-protein ligase NEDD4-like isoform 1 | 26.6 | 17.7 | −0.6 | 0.7 | 1.1e-02 | 5.6e-01 |
| Mylip | E3 ubiquitin-protein ligase MYLIP | 23.4 | 14.4 | −0.7 | 0.6 | 2.6e-03 | 2.5e-01 |
| Mib1 | E3 ubiquitin-protein ligase MIB1 | 9.3 | 6.3 | −0.6 | 0.7 | 3.0e-02 | 8.6e-01 |
| Mdm4 | protein Mdm4 | 11.2 | 7.9 | −0.5 | 0.7 | 4.8e-02 | 1.0e+00 |
| March7 | E3 ubiquitin-protein ligase MARCH7 | 18.5 | 12.6 | −0.5 | 0.7 | 2.2e-02 | 7.9e-01 |
| Map3k1 | mitogen-activated protein kinase kinase kinase 1 | 25.2 | 18.1 | −0.5 | 0.7 | 3.9e-02 | 9.5e-01 |
| Cbl | E3 ubiquitin-protein ligase CBL | 4.6 | 2.9 | −0.7 | 0.6 | 2.1e-02 | 7.7e-01 |
Aldo, aldosterone; Con, control; FPKM, Fragments Per Kilobase of the transcript per Million mapped reads. n = 7 per group.
There was an overrepresentation of the cellular component term “extracellular region” among the upregulated transcripts (Fig. 6A). The extracellular region contains subcomponents such as extracellular exosomes and extracellular space, which include proteins secreted by cells. This extracellular region category contained e.g., transcripts coding for the proteins NCC and FXYD domain containing ion transport regulator 2, which are present in urinary exosomes (11, 18).
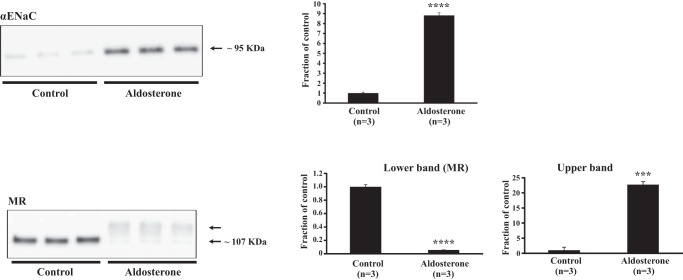
To investigate whether changes in mRNA transcripts paralleled changes in protein expression, we stimulated mCCD cells [originating from the mouse cortical CD (10)] with aldosterone. The experiment was performed both in the absence (Fig. 7) and presence (data not shown) of serum-dexamethasone in the medium. The αENaC subunit was upregulated by aldosterone (Fig. 7) when we used medium in the absence of serum/dexamethasone as previously described (10). By contrast, no significant effect was observed when we performed the experiment in the presence of serum-dexamethasone (not shown, P = 0.22, n = 6 per group). The RNA sequencing showed that the Nr3c2 mRNA transcript encoding MR was downregulated (Supplemental Table S1). A parallel downregulation of MR protein (~107 kDa) levels was found in the aldosterone-treated mCCD cells (Fig. 7), which is in accordance with data on COS-1 cells (56). This downregulation was also shown in the presence of serum-dexamethasone (data not shown, P < 0.0001, n = 6 per group). Interestingly, the MR antibody recognized a higher MW band in the aldosterone-stimulated mCCD cells (Fig. 7). Whether this band represents modified MR or an association between the MR and another protein, or whether the band is unspecific is not known. Furthermore, RNA sequencing showed a higher expression although not significant of Slc12a3 encoding NCC (FPKM: control 140.1, aldosterone 207.5; log2-ratio: 0.566 P = 0.058). This parallels our previously published data showing an upregulation of NCC protein specifically in the DCT2 (38).
Fig. 7.
Western blots showing epithelial sodium channel alpha-subunit (αENaC) and mineralocorticoid receptor (MR) protein expression in mouse (m)CCD cells treated with aldosterone for 4 days (n = 3 per group). The experiment was performed in medium without serum and dexamethasone. The αENaC subunit was highly upregulated in response to aldosterone (top), whereas MR was markedly downregulated (bottom panel, lower band). The antibody against MR also recognized a higher MW band in the aldosterone-treated samples (bottom panel, upper band). ***P < 0.001 and ****P < 0.0001.
Profiling of the CNT/iCCD transcriptome.
Profiling of the CNT/iCCD transcriptome was performed on cells isolated from untreated control littermates. We identified 69,287 transcripts (FPKM > 0; Supplemental Table S3, https://hpcwebapps.cit.nih.gov/ESBL/Database/ASDT_Transcriptome/CNT_iCCD_transcriptome.xlsx) distributed on 21,070 genes. Of the 69,287 transcripts, 25,294 were expressed at FPKM levels ≥ 1 and distributed on 11,907 genes. Among the highly expressed transcripts, a large number were involved in oxidative phosphorylation. This finding corresponds well with CNT/iCCD segments being actively transporting epithelia with high Na+-K+-ATPase activity (24). Other transcripts with high expression included Aqp2 and Aqp3 coding for water channel proteins aquaporin-2 (FPKM = 2,962) and aquaporin-3 (FPKM = 1,859), respectively, and Klk1 coding for Kallikrein (FPKM = 3,867). These proteins are highly expressed in CNT cells (7, 39, 42). In contrast, Aqp4 encoding aquaporin-4 was expressed at a lower level (FPKM = 7) compared with Aqp2; this expression pattern was also seen in the CNT transcriptome of isolated rat tubules (25).
Comparison between the DCT2/CNT/iCCD transcriptome and CNT/iCCD transcriptome.
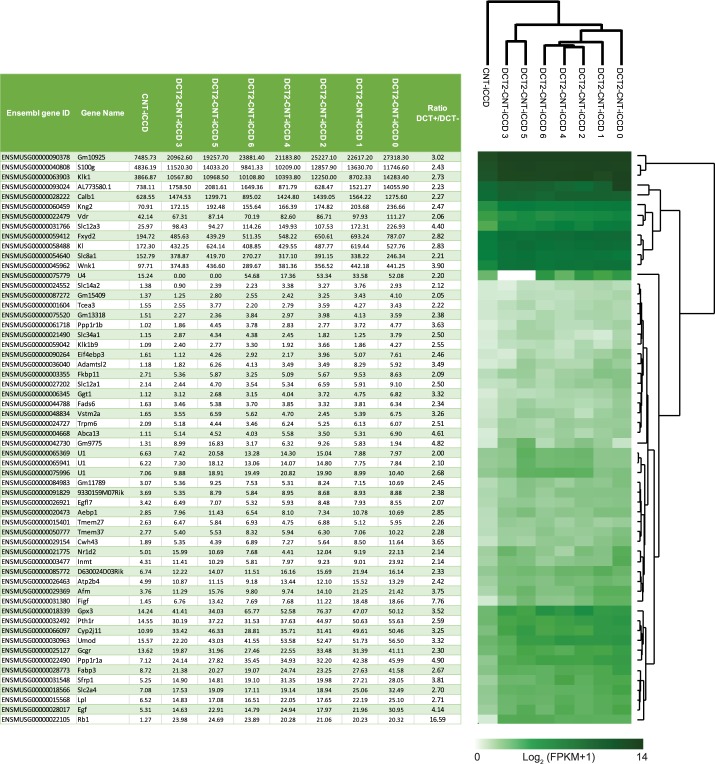
To obtain information about transcripts enriched in DCT2 cells, we made a comparison between the DCT2/CNT/iCCD transcriptome of the control mice (n = 7) from the aldosterone study and the CNT/iCCD transcriptome (n = 1) (Fig. 8). A heat-map representation of transcripts encoding expressed protein kinases, E3 ubiquitin ligases, and transcription factors showed a coclustering of transcripts within the DCT2/CNT/iCCD samples, segregating from the CNT/iCCD transcriptome (data not shown). Slc12a3 encoding NCC and Wnk1 encoding WNK lysine-deficient protein kinase 1 were enriched in the DCT2/CNT/iCCD cells compared with the CNT/iCCD cells (Fig. 8; ratios DCT+/DCT−: 4.40 and 3.90, respectively) consistent with the presence of NCC in the DCT2 (26) and the role of WNK1 as an activator of NCC through SPAK/OSR1 (12). Fxyd2, encoding the Na+-K+-ATPase regulatory protein FXYD2, was also enriched in the DCT2/CNT/iCCD samples compared with the CNT/iCCD sample (ratio DCT+/DCT−: 2.82) consistent with the Na+-K+-ATPase being abundantly expressed in the basolateral membrane of DCT cells. Calb1 encoding calbindin 28 kDa was highly expressed in both the DCT2/CNT/iCCD and the CNT/iCCD samples with a DCT+/DCT− ratio of 2.27 (Fig. 8) consistent with the known higher abundance of the calbindin protein in mouse DCT2 cells compared with CNT cells (26). Sfrp1 encoding secreted frizzled related protein 1 (SFRP1) was also more abundant in the DCT2/CNT/iCCD cells (ratio DCT+/DCT−: 3.81). Sfrp1 is a Wnt signaling antagonist that has been shown to be involved in inhibition of renal tubulointerstitial fibrosis (29). Figf (FIGF, c-Fos-induced growth factor; VEGF-D, vascular endothelial growth factor) was highly enriched in the DCT2/CNT/iCCD cells (ratio DCT+/DCT−: 7.76). This gene encodes the vascular endothelial growth factor (VEGF-D), which is important for growth and development of endothelial and vascular tissues (3). Ppp1r1a encoding the protein phosphatase 1 regulatory inhibitor subunit 1A (I-1) was also enriched in the DCT2/CNT/iCCD cells (ratio DCT+/DCT−: 4.90). This transcript has been shown previously to be enriched in DCT cells isolated by an alternative cell sorting approach, where it plays an important role in regulating NCC function (37). A limitation to the comparison in Fig. 8 was that the CNT/iCCD transcriptome equals one sample (cells from six mice were pooled to obtain enough tissue for the sequencing). Nevertheless, data provide novel information on differential transcript expression in DCT2 vs. CNT/iCCD.
Fig. 8.
Transcripts enriched in the DCT2/CNT/iCCD cell population compared with the CNT/iCCD cell population. Comparison of the DCT2/CNT/iCCD transcriptome of control mice (n = 7) and the CNT/iCCD transcriptome (n = 1). Transcripts enriched in the DCT2 (DCT+/DCT- ratio > 2) are presented in the table (left) and the corresponding heat map (right). CNT, connecting tubule; DCT2, late distal convoluted tubule; FPKM, Fragments Per Kilobase of the transcript per Million mapped reads; iCCD, initial cortical collecting duct.
Summary
The strength of the present study is the use of highly sensitive deep sequencing technology to generate an unprecedented map of gene products regulated by long-term aldosterone stimulation. Furthermore, the use of a highly enriched population of aldosterone-sensitive DCT2 cells, CNT cells, and iCCD principal cells creates a novel framework for identifying regulatory pathways belonging to these specific cells and the basis for extensive further investigations. In total 547 transcripts significantly regulated by aldosterone were identified. We cannot exclude the possibility that the changes in some of the mRNA transcripts are not directly caused by aldosterone, because primary hyperaldosteronism induces secondary physiological effects. These effects may include increased GFR (38) and hypervolemia or hyperosmolality inducing vasopressin release (35). Hypokalemia is another secondary effect, which may be associated with stimulation of e.g., pendrin and NCC (19, 55). Furthermore, we exploited the TRPV5 eGFP mice to isolate a cell population enriched for CNT cells and iCCD principal cells, and we identified more than 69,000 mRNA transcripts. Collectively, our study provides a comprehensive database of aldosterone-regulated transcripts in the ASDT, allowing development of novel hypotheses for the action of aldosterone, and a novel deep sequencing profiling of the CNT cell/iCCD principal cell transcriptome.
GRANTS
Funding for this study was provided by the Lundbeck Foundation (B. M. Christensen), the Danish Council for Independent Research (B. M. Christensen), and the Faculty of Health Sciences, Aarhus University (S. B. Poulsen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.B.P., R.A.F., and B.M.C. conceived and designed research; S.B.P. performed experiments; S.B.P., K.L., T.P., and B.M.C. analyzed data; S.B.P., K.L., T.P., and B.M.C. interpreted results of experiments; S.B.P., K.L., and B.M.C. prepared figures; S.B.P., T.P., and B.M.C. drafted manuscript; S.B.P., R.A.F., T.P., and B.M.C. edited and revised manuscript; S.B.P., K.L., R.A.F., T.P., and B.M.C. approved final version of manuscript.
ENDNOTE
At the request of the authors, readers are alerted to additional materials related to this manuscript that may be found at the institutional website of the authors, which at the time of publication they indicate is: https://hpcwebapps.cit.nih.gov/ESBL/Database/ASDT_Transcriptome/. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
Supplemental Data
ACKNOWLEDGMENTS
We thank Bernard Rossier and Edith Hummler (Department of Pharmacology and Toxicology, University of Lausanne) for providing the mCCD cell line and Johannes Loffing (Institute of Anatomy, University of Zurich) for the αENaC antibody. We thank Christine Nardini, Liu Yuanhua, and Jennifer Dent (the Partner Institute for Computational Biology, Shanghai, China) for guidance on bioinformatical analysis of RNA sequencing data. We thank Christian Westberg, Tina Drejer, and Helle Høyer for excellent technical assistance and Jeppe Praetorius for help with planning the isolation of cells from the TRPV5 eGFP mice. FACS was performed at the FACS Core Facility, Aarhus University, Aarhus, Denmark.
REFERENCES
- 1.Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, Loffing J. In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol 299: F1473–F1485, 2010. doi: 10.1152/ajprenal.00437.2010. [DOI] [PubMed] [Google Scholar]
- 2.Bobby R, Medini K, Neudecker P, Lee TV, Brimble MA, McDonald FJ, Lott JS, Dingley AJ. Structure and dynamics of human Nedd4-1 WW3 in complex with the αENaC PY motif. Biochim Biophys Acta 1834: 1632–1641, 2013. doi: 10.1016/j.bbapap.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Breen EC. VEGF in biological control. J Cell Biochem 102: 1358–1367, 2007. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- 4.Chai J, Tarnawski AS. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol 53: 147–157, 2002. [PubMed] [Google Scholar]
- 5.Chen G, Li R, Shi L, Qi J, Hu P, Luo J, Liu M, Shi T. Revealing the missing expressed genes beyond the human reference genome by RNA-Seq. BMC Genomics 12: 590, 2011. doi: 10.1186/1471-2164-12-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, Malsure S, Ronzaud C, Stehle JC, Rossier BC, Hummler E. Sodium and potassium balance depends on αENaC expression in connecting tubule. J Am Soc Nephrol 21: 1942–1951, 2010. doi: 10.1681/ASN.2009101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen BM, Wang W, Frøkiaer J, Nielsen S. Axial heterogeneity in basolateral AQP2 localization in rat kidney: effect of vasopressin. Am J Physiol Renal Physiol 284: F701–F717, 2003. doi: 10.1152/ajprenal.00234.2002. [DOI] [PubMed] [Google Scholar]
- 8.de Seigneux S, Nielsen J, Olesen ET, Dimke H, Kwon TH, Frøkiaer J, Nielsen S. Long-term aldosterone treatment induces decreased apical but increased basolateral expression of AQP2 in CCD of rat kidney. Am J Physiol Renal Physiol 293: F87–F99, 2007. doi: 10.1152/ajprenal.00431.2006. [DOI] [PubMed] [Google Scholar]
- 9.Fakitsas P, Adam G, Daidié D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol 18: 1084–1092, 2007. doi: 10.1681/ASN.2006080902. [DOI] [PubMed] [Google Scholar]
- 10.Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16: 878–891, 2005. doi: 10.1681/ASN.2004121110. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 20: 363–379, 2009. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadchouel J, Ellison DH, Gamba G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78: 367–389, 2016. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- 13.Haseroth K, Gerdes D, Berger S, Feuring M, Günther A, Herbst C, Christ M, Wehling M. Rapid nongenomic effects of aldosterone in mineralocorticoid-receptor-knockout mice. Biochem Biophys Res Commun 266: 257–261, 1999. doi: 10.1006/bbrc.1999.1771. [DOI] [PubMed] [Google Scholar]
- 14.Hofmeister MV, Fenton RA, Praetorius J. Fluorescence isolation of mouse late distal convoluted tubules and connecting tubules: effects of vasopressin and vitamin D3 on Ca2+ signaling. Am J Physiol Renal Physiol 296: F194–F203, 2009. doi: 10.1152/ajprenal.90495.2008. [DOI] [PubMed] [Google Scholar]
- 15.Hou J, Speirs HJ, Seckl JR, Brown RW. Sgk1 gene expression in kidney and its regulation by aldosterone: spatio-temporal heterogeneity and quantitative analysis. J Am Soc Nephrol 13: 1190–1198, 2002. doi: 10.1097/01.ASN.0000013702.73570.3B. [DOI] [PubMed] [Google Scholar]
- 16.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 18.Huebner AR, Cheng L, Somparn P, Knepper MA, Fenton RA, Pisitkun T. Deubiquitylation of protein cargo is not an essential step in exosome formation. Mol Cell Proteomics 15: 1556–1571, 2016. doi: 10.1074/mcp.M115.054965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizawa K, Xu N, Loffing J, Lifton RP, Fujita T, Uchida S, Shibata S. Potassium depletion stimulates Na-Cl cotransporter via phosphorylation and inactivation of the ubiquitin ligase Kelch-like 3. Biochem Biophys Res Commun 480: 745–751, 2016. doi: 10.1016/j.bbrc.2016.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen TB, Pisitkun T, Hoffert JD, Jensen UB, Fenton RA, Praetorius HA, Knepper MA, Praetorius J. Assessment of the effect of 24-hour aldosterone administration on protein abundance in fluorescence-sorted mouse distal renal tubules by mass spectrometry. Nephron, Physiol 121: p9–15, 2012. doi: 10.1159/000346832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45, D1: D353–D361, 2017. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27–30, 2000. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 44, D1: D457–D462, 2016. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz AI, Doucet A, Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol Renal Physiol 237: F114–F120, 1979. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- 25.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loffing J, Loffing-Cueni D, Valderrabano V, Kläusli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001. doi: 10.1152/ajprenal.0085.2001. [DOI] [PubMed] [Google Scholar]
- 27.Loffing-Cueni D, Flores SY, Sauter D, Daidié D, Siegrist N, Meneton P, Staub O, Loffing J. Dietary sodium intake regulates the ubiquitin-protein ligase nedd4-2 in the renal collecting system. J Am Soc Nephrol 17: 1264–1274, 2006. doi: 10.1681/ASN.2005060659. [DOI] [PubMed] [Google Scholar]
- 28.Lomberk G, Urrutia R. The family feud: turning off Sp1 by Sp1-like KLF proteins. Biochem J 392: 1–11, 2005. doi: 10.1042/BJ20051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuyama M, Nomori A, Nakakuni K, Shimono A, Fukushima M. Secreted Frizzled-related protein 1 (Sfrp1) regulates the progression of renal fibrosis in a mouse model of obstructive nephropathy. J Biol Chem 289: 31526–31533, 2014. doi: 10.1074/jbc.M114.584565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621–628, 2008. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 31.Narum SR. Beyond Bonferroni: less conservative analyses for conservation genetics. Conserv Genet 7: 783–787, 2006. doi: 10.1007/s10592-005-9056-y. [DOI] [Google Scholar]
- 32.Nielsen J, Kwon TH, Frøkiaer J, Knepper MA, Nielsen S. Maintained ENaC trafficking in aldosterone-infused rats during mineralocorticoid and glucocorticoid receptor blockade. Am J Physiol Renal Physiol 292: F382–F394, 2007. doi: 10.1152/ajprenal.00212.2005. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen J, Kwon TH, Praetorius J, Frøkiaer J, Knepper MA, Nielsen S. Aldosterone increases urine production and decreases apical AQP2 expression in rats with diabetes insipidus. Am J Physiol Renal Physiol 290: F438–F449, 2006. doi: 10.1152/ajprenal.00158.2005. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen S, Smith BL, Christensen EI, Knepper MA, Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol 120: 371–383, 1993. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nogueira-Silva L, Blanchard A, Curis E, Lorthioir A, Zhygalina V, Bergerot D, Baron S, Amar L, Bobrie G, Plouin PF, Ménard J, Azizi M. Deciphering the role of vasopressin in primary aldosteronism. J Clin Endocrinol Metab 100: 3297–3303, 2015. doi: 10.1210/JC.2015-2007. [DOI] [PubMed] [Google Scholar]
- 36.Perrier R, Boscardin E, Malsure S, Sergi C, Maillard MP, Loffing J, Loffing-Cueni D, Sørensen MV, Koesters R, Rossier BC, Frateschi S, Hummler E. Severe salt-losing syndrome and hyperkalemia induced by adult nephron-specific knockout of the epithelial sodium channel α-subunit. J Am Soc Nephrol 27: 2309–2318, 2016. doi: 10.1681/ASN.2015020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picard N, Trompf K, Yang CL, Miller RL, Carrel M, Loffing-Cueni D, Fenton RA, Ellison DH, Loffing J. Protein phosphatase 1 inhibitor-1 deficiency reduces phosphorylation of renal NaCl cotransporter and causes arterial hypotension. J Am Soc Nephrol 25: 511–522, 2014. doi: 10.1681/ASN.2012121202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poulsen SB, Christensen BM. Long-term aldosterone administration increases renal Na+-Cl− cotransporter abundance in late distal convoluted tubule. Am J Physiol Renal Physiol 313: F756–F766, 2017. doi: 10.1152/ajprenal.00352.2016. [DOI] [PubMed] [Google Scholar]
- 39.Poulsen SB, Kim YH, Frøkiær J, Nielsen S, Christensen BM. Long-term vasopressin-V2-receptor stimulation induces regulation of aquaporin 4 protein in renal inner medulla and cortex of Brattleboro rats. Nephrol Dial Transplant 28: 2058–2065, 2013. doi: 10.1093/ndt/gft088. [DOI] [PubMed] [Google Scholar]
- 40.Poulsen SB, Praetorius J, Damkier HH, Miller L, Nelson RD, Hummler E, Christensen BM. Reducing αENaC expression in the kidney connecting tubule induces pseudohypoaldosteronism type 1 symptoms during K+ loading. Am J Physiol Renal Physiol 310: F300–F310, 2016. doi: 10.1152/ajprenal.00258.2015. [DOI] [PubMed] [Google Scholar]
- 41.Pradervand S, Zuber Mercier A, Centeno G, Bonny O, Firsov D. A comprehensive analysis of gene expression profiles in distal parts of the mouse renal tubule. Pflugers Arch 460: 925–952, 2010. doi: 10.1007/s00424-010-0863-8. [DOI] [PubMed] [Google Scholar]
- 42.Proud D, Knepper MA, Pisano JJ. Distribution of immunoreactive kallikrein along the rat nephron. Am J Physiol Renal Physiol 244: F510–F515, 1983. doi: 10.1152/ajprenal.1983.244.5.F510. [DOI] [PubMed] [Google Scholar]
- 43.Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC. Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112: 554–565, 2003. doi: 10.1172/JCI16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res 99: 1355–1366, 2006. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 45.Senbonmatsu T, Saito T, Landon EJ, Watanabe O, Price E Jr, Roberts RL, Imboden H, Fitzgerald TG, Gaffney FA, Inagami T. A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. EMBO J 22: 6471–6482, 2003. doi: 10.1093/emboj/cdg637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheader EA, Wargent ET, Ashton N, Balment RJ. Rapid stimulation of cyclic AMP production by aldosterone in rat inner medullary collecting ducts. J Endocrinol 175: 343–347, 2002. doi: 10.1677/joe.0.1750343. [DOI] [PubMed] [Google Scholar]
- 47.Sokal RR, Rohlf FJ. Biometry. New York: Freeman, 1995. [Google Scholar]
- 48.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 49.Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem 280: 39970–39981, 2005. doi: 10.1074/jbc.M508658200. [DOI] [PubMed] [Google Scholar]
- 50.Thomas W, Harvey BJ. Mechanisms underlying rapid aldosterone effects in the kidney. Annu Rev Physiol 73: 335–357, 2011. doi: 10.1146/annurev-physiol-012110-142222. [DOI] [PubMed] [Google Scholar]
- 51.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578, 2012. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trepiccione F, Capasso G, Nielsen S, Christensen BM. Evaluation of cellular plasticity in the collecting duct during recovery from lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 305: F919–F929, 2013. doi: 10.1152/ajprenal.00152.2012. [DOI] [PubMed] [Google Scholar]
- 53.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10: 57–63, 2009. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, Uchida S, Shibata S. Hypokalemia and pendrin induction by aldosterone. Hypertension 69: 855–862, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08519. [DOI] [PubMed] [Google Scholar]
- 56.Yokota K, Shibata H, Kobayashi S, Suda N, Murai A, Kurihara I, Saito I, Saruta T. Proteasome-mediated mineralocorticoid receptor degradation attenuates transcriptional response to aldosterone. Endocr Res 30: 611–616, 2004. doi: 10.1081/ERC-200043783. [DOI] [PubMed] [Google Scholar]
- 57.Yu Z, Kong Q, Kone BC. Aldosterone reprograms promoter methylation to regulate αENaC transcription in the collecting duct. Am J Physiol Renal Physiol 305: F1006–F1013, 2013. doi: 10.1152/ajprenal.00407.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu ZY, Kong Q, Kone BC. Physical and functional interaction of Rnf2 with Af9 regulates basal and aldosterone-stimulated transcription of the α-ENaC gene in a renal collecting duct cell line. Biosci Rep 33: e00076, 2013. doi: 10.1042/BSR20130086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA 106: 16523–16528, 2009. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.