Abstract
Recent studies have shed new light on the role of the fibrinolytic system in the pathogenesis of pleural organization, including the mechanisms by which the system regulates mesenchymal transition of mesothelial cells and how that process affects outcomes of pleural injury. The key contribution of plasminogen activator inhibitor-1 to the outcomes of pleural injury is now better understood as is its role in the regulation of intrapleural fibrinolytic therapy. In addition, the mechanisms by which fibrinolysins are processed after intrapleural administration have now been elucidated, informing new candidate diagnostics and therapeutics for pleural loculation and failed drainage. The emergence of new potential interventional targets offers the potential for the development of new and more effective therapeutic candidates.
Keywords: fibrinolysis, loculation, pleural disease
INTRODUCTION TO ABERRANT FIBRIN TURNOVER IN THE PATHOGENESIS OF PLEURAL ORGANIZATION
Pleural injury proceeds through a phase of acute inflammation followed by organization, a process that involves the deposition of intrapleural fibrin and its remodeling (26, 91). Resident cells such as pleural mesothelial cells participate in the process as do lung and pleural fibroblasts, which elaborate a wide range of mediators that promote local inflammation and its repair. Among the mediators, transforming growth factor-β (TGF-β) is elaborated and plays a particularly important role in pleural organization as described in the sections that follow. Myeloid cells likewise participate in pleural organization, although the interplay between aberrant pathways of fibrin turnover and the evolution of the inflammatory component during pleural organization remains to be better understood.
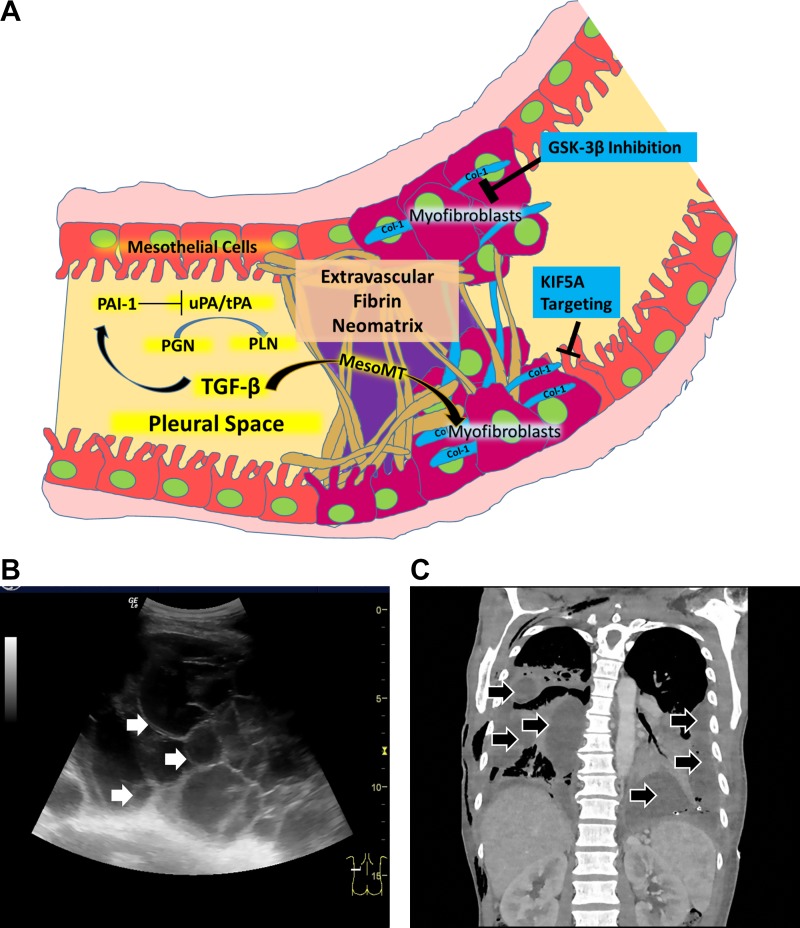
As is generally the case in tissue inflammation, injury within the pleural compartment evolves with early formation of a fibrinous transitional neomatrix and then resolves with restoration of normal architecture. Alternatively, the neomatrix undergoes organization with eventual scarification or fibrosis (3, 4, 15, 26, 91). While the pleural space is a potential compartment in normalcy, it may be occupied by pleural effusions, which are generally exudative after inflammatory insults including infections (55). The organization of inflammatory pleural fluids may occur with early formation of transitional extravascular fibrinous deposits that compose pleural adhesions, loculations, which are structurally highly organized fibrinous collections often associated with formation of a pleural rind (26) (Fig. 1A). In the aggregate, these structural derangements contribute to restrictive lung dysfunction and clinical morbidity that may ensue after pleural injury. In the setting of empyema or complicated parapneumonic pleural effusions (CPE), loculations can occur early after infection, which may impair pleural drainage and sequester the infectious agents (Fig. 1, B and C). These areas of loculation can ultimately become a source of ongoing pleural sepsis. Bacteria can contribute to the process of organization through formation of biofilms, which are complex structures whose constituents include both DNA and fibrin and which can be degraded by fibrinolysins (38, 50, 98).
Fig. 1.
Pleural organization from bench (A) to bedside (B and C). A: key events involved in the pathogenesis of pleural organization with formation of a fibrinous neomatrix, the increased presence of myofibroblasts and fibrotic remodeling within the pleural space. Arrows: facilitating interactions. Bars denote inhibition. Col 1, collagen 1; MesoMT, mesomesenchymal transition; PAI-1, plasminogen activator inhibitor-1; GSK-3β, glycogen synthase kinase-3β; KIF5A, kinesin 1 family member; PGN, plasminogen; PLN, plasmin generated by the cleavage of plasminogen buy plasminogen activators, here indicated by urokinase (uPA) or or tissue plasminogen activator (tPA), which are commonly used in clinical practice. B: pleural ultrasonography demonstrating loculation in a patient with pleural infection. Multiple adhesions have coalesced with septation of echogenic fluid into many locules that are seen throughout the imaged field. White arrows indicates the septations within the loculated pleural fluid. The cursor at the bottom right illustrates the plane of the imaged field. C; chest computed tomography imaging appearance of loculated pleural infection, which in this case illustrates extensive bilateral involvement. A representative coronal image that shows the loculated pleural process in this patient is shown here. The loculated pleural fluids are indicated within the right and left hemithoraces by black arrows.
A wide range of disease processes may cause pleural injury and organization and even fibrothoraces, including exposure to particulates such as asbestos, collagen vascular diseases, or retained hemothoraces. However, pleural organization associated with empyema or CPE is more commonly encountered in clinical practice, affecting ~80,000 patients annually in the United States and United Kingdom with a mortality rate of ~20% (9). Pleural organization assumes clinical importance if excessive scarification ensues with symptomatic lung restriction or if loculation impairs pleural drainage in patients with empyema or CPE. Almost 70 years ago, investigators first noted the fibrinous nature of loculated pleural collections and sought their resolution using relatively crude preparations of streptokinase or streptodornase (88, 89). Since that time, intrapleural fibrinolytic therapy (IPFT) has been refined, using a variety of agents including streptokinase, urokinase (uPA in the 2-chain form), or tissue plasminogen activator (tPA). The role of the fibrinolytic system in pleural remodeling has likewise undergone comprehensive investigation and is now better understood, as recently reviewed (91). These lines of investigation have yielded new candidate interventions to prevent fibrinous pleural organization and, thereby, improve patient outcomes (Fig. 1A).
The early events associated with pleural inflammation are associated with consistent local derangements of the fibrinolytic system, which set the stage for loculation and pleural remodeling (Fig. 1A). With the onset of pleural injury, local inflammation is rapidly accompanied by vascular extravasation of plasma components including coagulation substrates and procoagulants that initiate coagulation within pleural fluids and within the pleural and subpleural tissues. The procoagulant response occurs mainly via the activation of the extrinsic coagulation pathway and overexpression of tissue factor (31, 91). Local coagulation exceeds inhibition by a tissue factor pathway inhibitor, with resulting intrapleural fibrin deposition (26, 31, 35). Intrapleural fibrin deposition is accompanied by infiltration by mesenchymal and myeloid cells, which secrete proteases including plasminogen activators that are capable of remodeling the transitional neomatrix. In particular, pleural mesothelial cells (PMCs) and lung myofibroblasts also express increased amounts of plasminogen activator inhibitor-1 (PAI-1) in response to stimulation by local proinflammatory mediators (36, 37). These include TGF-β, which has been strongly implicated in the pathogenesis of pleural organization, proliferation of subpleural myofibroblasts, or PMCs undergoing mesomesenchymal transition (MesoMT) and pleural rind formation (12). Loculation is promoted and then potentiated by inhibition of local fibrinolysis, in large part by elaboration of elevated levels of PAI-1 (26). Pleural transduction experiments have provided clear evidence that excessive levels of PAI-1 increase pleural organization and can likewise block the activity of intrapleural fibrinolysins (40). Thus, as in the acute respiratory distress syndrome or interstitial lung diseases (32), fibrinous organization characterizes the neomatrix after acute, fibrosis-prone injury and is potentiated by concurrent initiation and potentiation of coagulation and impairment of local fibrinolysis (26). In a related vein, a similar linkage between inflammation and alterations of the brain fibrinolytic system has been proposed to contribute to major depressive disorder, which can occur as a complication of lung or pleural diseases (25). This postulate requires additional investigation and confirmation.
The role of the mesothelium in the pathogenesis of pleural organization and injury bears special mention. Like the lung epithelium, it elaborates components of the fibrinolytic system that in turn can regulate intrapleural organization via signal transduction, cellular phenotypic change, or alterations in cellular viability (26, 79, 91). Urokinase and its receptor, uPAR, as well as PAI-1 are all regulated by PMCs and a range of other resident lung or pleural mesothelioma cells at the transcriptional and posttranscriptional levels. The posttranscriptional interactions involve a range of mRNA binding proteins with uPA, uPAR, or PAI-1 mRNA that influence the stability of these molecules (78, 80). For example, the posttranscriptional regulation of PAI-1 in human (H) PMCs involves destabilization of PAI-1 mRNA by the interaction of a PAI-1 mRNA binding protein, identified as 6-phospho-d-gluconate-NADP oxidoreductase, which interacts with an endogenous 33-nt binding sequence of PAI-1 mRNA (80). Overexpression of 6-phospho-d-gluconate-NADP oxidoreductase in MeT-5A HPMCs and primary PMCs suppressed basal and TGF-β–induced PAI-1 expression, representing a new mode of the regulation of this inhibitor. At present, further investigation is needed to determine to what extent this pathway contributes to PAI-1 expression in vivo or whether the interaction is suppressed in the context of organizing pleural injury.
INTRAPLEURAL FIBRINOLYTIC THERAPY FOR ORGANIZING PLEURAL INFECTION: CURRENT RECOMMENDATIONS AND INTEGRATION WITH OTHER THERAPEUTICS FOR CPE AND EMPYEMA
The clinical implications of disordered fibrinolysis and organization have aggressively been pursued in the area of pleural infection over the course of many years. Pleural infection is common and has been known to be a life-threatening condition since the time of Hippocrates (c. 460–377 BC) who wrote extensively on the subject. He established the importance of evacuation of the infected fluid and stated: “if an empyema does not rupture, death will follow.” This cornerstone of pleural infection management has stood the test of time of nearly 25 centuries. Despite medical advances, reports from around the world have shown rising incidences of pleural infection (23, 56, 100). A significant resurgence of empyema mortality, the highest since the introduction of antibiotics, has also been reported (1).
The principles of treatment of pleural infection centers on antibiotics and drainage of the effusion. The latter is a clinical challenge and has been the focus of much research effort. It is hypothesized that loculation is an effective body response to contain the infected collection (54); nonetheless, its presence often prohibits effective therapeutic fluid clearance. Bacteria, especially Streptococcus pneumoniae (71), can proliferate rapidly in human pleural fluid, and loculations impair penetration of antibiotics to the contained fluid. Pleural ultrasonography has allowed appreciation of the extent of fibrinous adhesions and loculations (often noncommunicating (59)) that commonly exist within an infected pleural effusion, Fig. 1. Some investigators have reported that presence of loculations predicted adverse outcome in pleural infection (5, 6).
At least 20–30% of patients fail antibiotics and chest tube drainage (58). Traditionally, surgery (usually video-assisted thoracoscopic surgery) was the only effective option to clear the residual pleural collections. Unfortunately patients with pleural infection are often elderly, have comorbidity, and are unsuitable for surgery, which has established risks of morbidity and mortality (16). Intrapleural instillation of fibrinolytic agents to break pleural adhesions/loculations remains an attractive concept and has been a subject of many investigations. Two randomized clinical trials (RCTs) showed no significant benefits from streptokinase over placebo in adults (13, 58). Another RCT did not show convincing benefits in patients treated with single agent tPA but found that addition of deoxyribonuclease (DNase) to tPA regime produced significant synergy (73). This combination intrapleural regime has revolutionized management and has been increasingly adopted around the world. In the Multicenter Intrapleural Sepsis Trial (MIST)-2, tPA/DNase treatment significantly reduced pleural opacity on chest radiographs and shortened hospital length of stay compared with placebo. Less than 5% of tPA/DNase-treated patients required rescue surgery (73). Subsequent open-label studies (57, 60, 68, 70) have confirmed its efficacy and safety, including in patient cohorts that failed antibiotics and chest tube drainage (68). The dosing regime and delivery options have been discussed elsewhere (69). Side effects are infrequent and generally mild. Pain occurs in ~20% of patients (60, 68), especially during the first treatment dose. In 344 published cases from five series, significant pleural bleeding (defined as requiring blood transfusion) was reported only in 11 (3.2%) cases; all were managed conservatively and none was fatal. Systemic bleeding from intrapleural tPA/DNase treatment is exceedingly rare, likely because of low systemic absorption and the short half-life of tPA. The ongoing Alteplase Dose Assessment for Pleural infection Therapy (ADAPT) project (70) is a dose de-escalation approach to establish the lowest effective dose of intrapleural tPA or tPA/DNase therapy and may help to further minimize the costs and complications of treatment.
The mechanisms of action of tPA and DNase in pleural injury are not fully understood but believed to include lysis of adhesions (tPA) and reduction of viscosity of purulent fluids (DNase). Other additional benefits have also been hypothesized, including the degradation of biofilms by DNase. Intrapleural tPA, as well as other fibrinolytics, induces significant fluid formation and may offer a lavage effect to reduce infected pleural material. This class effect of fibrinolytic-induced pleural fluid formation is mediated by monocyte chemotactic protein (MCP)-1 (52), which also plays a significant part in effusion formation in other pleural pathologies (51, 83).
It should be noted that pleural infection in pediatric patients have different bacteriology and clinical course, as well as a much more favorable prognosis, when compared with adult patients. Several RCTs (81, 82) in pediatric setting have found that single agent intrapleural fibrinolytic therapy provided similar benefits when compared with video-assisted thoracoscopic surgery. Whether DNase offer additional benefits in pediatric setting requires investigations.
Active multiprong research efforts are underway to improve the management of pleural infection. The ideal approach is to stop parapneumonic fluid formation in the first place. Targeting specific candidates, such as MCP-1, or using a general approach to dampen pleural inflammatory responses is being explored. In a large study (n = 3,602) of pneumonia patients, those taking inhaled steroids were much less likely to develop parapneumonic effusions (odds ratio: 0.42) (77). A recent RCT of 60 children with parapneumonic effusion showed that high-dose intravenous dexamethasone significantly improved recovery time (85). Systemic corticosteroid therapy has also been shown to improve fluid resolution in tuberculous pleuritis (99). Approaches preventing pleural adhesion formation are intriguing. For example, animal studies of anti-TGF-β therapy significantly reduced adhesions in empyema (49).
Antibiotics are a key aspect of care that has been neglected in pleural infection research. New approaches such as measuring antibiotics penetration to pleural fluid (to allow individualized dosing) and intrapleural antibiotics administration are needed. In the meantime, studies are underway to try optimize tPA/DNase regime and patient selection and compare the ability of different fibrinolytic agents to optimally achieve relief of pleural organization and failed drainage of loculated infectious pleural effusions. It is likely the findings of such studies will contribute to better clinical care.
CURRENT INTERVENTIONAL APPROACHES FOR ORGANIZING MALIGNANT PLEURAL EFFUSIONS
Malignant pleural effusion (MPE) is a common and increasing problem, with an estimated 150,000 new cases per year in the United States (19). Recent data suggest that there were 126,825 hospital admissions in the United States for MPE in 2012 alone (86). The majority of cases of MPE are related to metastatic disease from common primary cancers including lung, breast, ovary, and colorectal (75), with the minority (~10% depending on locale) caused by primary pleural mesothelioma (75). The median survival of MPE is poor, in the order of only 3–12 mo (8, 75). There have been significant improvements in phenotyping and assessment of MPE specifically concerning prognosis, with a now published validated prognostic score in increasing clinical use (8).
The treatment intent in MPE is symptomatic (75). Large pleural collections seen in cases of MPE are associated with significant symptoms (11, 72), the most important of which is dyspnea, and the available methods to measure dyspnea are improving with the publication of validated and practical tools to achieve this specific to pleural disease (61, 72). Treatment options and evidence to support optimal outcomes in MPE are rapidly expanding. Historically, repeated large volume thoracentesis or chemically or mechanically induced pleurodesis (the adherence of the parietal to the visceral pleura to prevent further fluid accumulation) was the only available treatment. However, there is an increasing body of literature supporting the use of indwelling pleural catheters (IPCs) as effective symptomatic treatments in MPE (90, 95), accepting that the only direct randomized trial comparison of sufficient power showed no symptom advantage of IPC over standard talc pleurodesis (11). There is now further interest in combining novel ambulatory drainage strategies with talc pleurodesis (74), and this is likely to become the major treatment paradigm in the future.
FIBRIN ACCUMULATION, CLINICAL SEQUELAE, AND DERANGED FIBRINOLYSIS IN MPE
All currently available options rely on effective drainage of pleural fluid, permitting lung reexpansion and normalization of respiratory mechanics. Thus situations in which either the lung is unable to reexpand [referred to here as nonexpandable lung (NEL) but previously referred to in the literature in a number of ways, including trapped and entrapped lung] or where fluid cannot be adequately drained may result in frustrated attempts to ameliorate symptoms via available drainage methods. The most common cause of failed drainage in malignant effusion is the presence of septated, loculated pleural fluid, which is resistant to drainage via a single intercostal tube. Interestingly, the presence of septated effusion has now been validated as an independent predictor of lack of response to pleural drainage (72). This is an important signal that requires further investigation.
The entities of NEL and septated effusion are likely to have, at least in some cases, similar biological origins, in that fibrinous collections within the pleural space may create both situations. NEL is seen in a significant number of cases of MPE, with ~25% of cases in total associated with radiological evidence of NEL in a large randomized trial (14). Although several mechanisms may result in NEL, one common cause is the formation of fibrinous strands over the visceral pleura, which results in an inability to expand in the presence of negative intrapleural pressure as fluid is drained.
The precise incidence of septated MPE is unknown. Data from our own unit (Oxford, unpublished observations) suggest that up to 15% of patients with MPE have clinically significant septations/loculations. In IPC-treated patients with MPE, up to 14% develop symptomatic loculations (20, 90). A large study from Taiwan reported 125/345 (36%) of patients with MPE requiring drainage exhibited evidence of either NEL or septated effusion making drainage challenging (24).
The cause of septation within MPE is poorly understood but likely involves a progression of events similar to those that contribute to pleural organization in the context of infection as described in the Introduction. While there may be low-grade and undetected pleural infection as the driver to fibrin formation, depressed fibrinolytic activity has been demonstrated in a wide range of pleural exudates including those with MPE (31), and MPEs have been demonstrated to exhibit high levels of prothrombin factors and D-dimer, conferring potentially increased survival and invasiveness of metastatic cells within the pleura (21). While elevated D-dimer levels generally reflect fibrinolytic activity, the elevated levels in this scenario may reflect increased fibrin deposition/burden within the pleura rather than augmented endogenous fibrinolysis. The inability of local fibrinolysis to clear these deposits could be attributable to relatively greater increments in local coagulation, relatively impaired fibrinolysis, or both processes. The exact nature of the derangements in local fibrinolysis could differ based on the specific neoplasm and extent of pleural involvement.
TREATMENT OF SEPTATED/LOCULATED MPE AND THE ROLE OF INTRAPLEURAL FIBRINOLYTIC THERAPY
The difficulties associated with treatment of septated MPEs have led to the investigation of intrapleural fibrinolytic therapy in a number of studies. While a number of case series suggest “good outcomes” from intrapleural treatment, including in those with IPC-related septated effusions (87), such studies are associated with the customary selection and reporting bias associated with all case series. Furthermore, it is now established that intrapleural treatment with some forms of IPFT are associated with increased pleural fluid production via an MCP-1 dependent pathway (51). Thus surrogate outcomes of fluid output in case series of intrapleural treatment are likely to be universally flawed.
Before 2017, there were only two small randomized controlled trials in the literature addressing the use of intrapleural fibrinolytics in MPE. The first randomized a total of 47 patients with MPE (not necessarily septated) to receive either intrapleural streptokinase (250,000 IU, 3 doses) or usual drainage (no placebo) before talc pleurodesis in those with expanded lungs post drainage (64). A higher proportion of the streptokinase group achieved lung expansion sufficient to warrant pleurodesis (96 vs. 74%), and there was an increased fluid output in the streptokinase group, but no significant difference in pleurodesis success (64). The second study randomized 40 patients with septated MPEs to 4 doses of intrapleural streptokinase (250,000 IU) or to matched placebo (saline) in a double-blind design (76). This study demonstrated significantly increased fluid drainage and reduced the need for oxygen therapy in the streptokinase group and improved radiological outcomes as measured by chest computed tomography using streptokinase (85 vs. 35% with a significant improvement in computed tomography pleural shadowing, defined as more than 40% total improvement). There was a nonsignificant improvement in pleurodesis success using streptokinase (recurrent fluid in 11 vs. 45%, P = 0.07) (76).
These studies led a number of the authors of this paper to conduct the TIME3 study (62), a randomized placebo-controlled trial of urokinase in patients with nondraining MPE, with clinically meaningful outcomes defined as pleurodesis success at 1 mo and dyspnea improvement on the validated 100-mm visual analogue scale for breathlessness. TIME3 recruited a total of 71 patients in a double-blind, placebo-controlled multicenter design. The patients with nondraining MPE, defined radiologically and with ultrasound, were randomly allocated to groups receiving either three doses of 100,000 IU of intrapleural (2 chain) urokinase given 12 hourly, or matched placebo, followed by administration of intrapleural talc. No significant difference was demonstrated between the interventional and placebo groups in terms of the primary outcome measures of breathlessness (difference in visual analogue scale breathlessness 3.8 mm, P = 0.36) or pleurodesis (failure rate urokinase 37%, placebo 32%, P = 0.65) (62). These outcomes could have been influenced by the contribution of tumor deposits on the visceral pleural surfaces, which commonly occur and may impair lung expansion. Although negative for the primary outcome measure, intrapleural urokinase was associated with fascinating improvement of effusion size on chest radiograph (P < 0.001) as well as reduced hospital stay of 1 day (P = 0.049) and improved survival (median survival 69 vs. 48 days, P = 0.026). No significant excess adverse events were noted in the urokinase group. It should be noted that the recruited trial population of hospitalized patients with MPE with nondraining effusions had a very poor prognosis as noted above, and thus the trial outcomes relating to pleurodesis success and dyspnea may not be applicable to all real-life clinical populations.
Where does the accumulated evidence leave us in respect of how intrapleural fibrinolytics should be used in MPE? Three randomized trials suggest that intrapleural fibrinolytic treatment results in improved lung expansion and/or radiological outcomes (62, 64, 76), but curiously, these effects are not associated with significant improvements in either breathlessness or success of pleurodesis. On this basis, there is no clear rationale for the current use of intrapleural fibrinolytics in MPE, where the treatment intent is palliative. It should be reenforced that the small numbers of patients in the earlier trials, and the poor prognosis in the TIME3 trial, make interpretation of the study results challenging for every day clinical practice. The positive signals within the studies, with all trials suggesting improved radiology and the TIME3 trial demonstrating potential improved survival and reduced hospital stay, suggest biological activity in the pleural space and now require further assessment. It may be that fibrinolysins accelerate lymphatic clearance in addition to expediting pleural drainage, but the underlying mechanisms remain to be elucidated through additional investigation. We suggest that a follow up clinical trial should be conducted in a different population including outpatients with septated MPE. The significant number of patients now treated with IPCs associated with septated effusion is a potentially important further area of research, with only case series data currently published in this area (87). Apart from these considerations, IPFT may be considered in oncology patients with loculated, nondraining MPE/CPE who do not have contraindications, such as bleeding diathesis, severe renal failure, or intracranial metastases, and who are not surgical candidates.
PROCESSING OF INTRAPLEURALLY ADMINISTERED FIBRINOLYSINS AND OUTCOMES OF THERAPY
Endogenous PAI-1 in Pleural Fluids of Patients with Empyema and CPE: Impact on the Activity and Efficacy of IPFT
As introduced in the preceding sections, inhibition of endogenous fibrinolysis is a key feature of the pathogenesis of pleural fluid organization with loculation in empyema and CPE. PAI-1 is overexpressed in the pleural fluids of patients with pleural effusions prone to loculation or within which organization with deposition of fibrinous loculations has taken place, as generally occurs in empyema or CPE (7, 31). That this overexpression worsens pleural organization provides strong proof of concept that PAI-1 plays a critical role in the pathogenesis of pleural injury. The potential for PAI-1 to contribute to outcomes of pleural injury therefore mandates special mention.
PAI-1 limits the activity of a range of fibrinolysins (40), which can unfavorably affect clinical outcomes in patients receiving IPFT. Recently, we demonstrated that levels of both PAI-1 antigen and activity in pleural fluids from MIST2 trial subjects varied by approximately two orders of magnitude (45). In patients with higher levels of PAI-1 expression, the tPA dosing used may have been insufficient to overcome endogenous levels of PAI-1 and other inhibitors, including antiplasmins. Consequently, overall trial outcomes could have been impacted, since PAI-1 levels were not interrogated during the interventional trials of which we are aware, including MIST 2 (73). The same can be said of virtually all prior trials in which tPA or uPA were used, as both are exquisitely sensitive to inhibition by PAI-1 (27, 91).
An increase in the level of PAI-1, up to three orders of magnitude in empyema fluids, blocks plasminogen activation and its consumption. This results in accumulation of plasminogen. Notably, intrapleural levels of endogenous plasminogen in empyema vary over a relatively wide range (17). Thus the ability of pleural fluid to generate endogenous fibrinolytic activity after exogenous fibrinolytic is administered likewise varies among empyema patients up to 100-fold (17). This profound level of variation could derive from a number of factors. These include genetics and epigenetics, individual variations in the degree of the inflammatory response to a particular noxious insult, and the species and/or virulence of infectious organisms within the pleural compartment. While it is not yet possible to predict patient outcomes based on the levels of PAI-1 in pleural fluids, a new test is in early stage to address the possibility that ex vivo analyses of freshly collected pleural fluids could be used to assess outcomes or guide IPFT dosing, called the fibrinolytic potential assay (FPA) (17, 30). The FPA is an assay to measure the net fibrinolytic activity within a sample of pleural fluid that is supplemented with plasminogen activator to generate fibrinolytic activity and mimic IPFT ex vivo. The FPA is envisioned as a bedside diagnostic, which could inform the probable outcome of IPFT for a given patient. Its measurement could additionally provide the healthcare team with guidance to adjust the dose of the fibrinolysin in IPFT or the dosing frequency (30). While this assay is in the early stages of development, comparable values of the fibrinolytic potential are detectable in the empyema fluids of patients and rabbits (30), providing an excellent model animal for preclinical testing. Rabbit models of chemical (18, 40, 45–47) pleural injury have been developed to elucidate the mechanisms of intrapleural fibrinolysis during IPFT, validate the FPA, and test whether PAI-1-targeting increases the efficacy of IPFT.
A rabbit model of tetracycline (TCN)-induced pleural injury was initially used to find effective doses of single chain (sc) tPA and scuPA, doses that were well-tolerated and that cleared fibrinous, organizing intrapleural neomatrices resembling loculations (29, 34). This model was also used to determine mechanisms of intrapleural processing of the fibrinolysins (40, 47) and determine the relative rates of intrapleural fibrinolysis that derive from their local administration (17, 45). While the FPA may be useful for estimating individualized success of IPFT, the success of any form of IPFT hinges on its ability to at least transiently activate plasminogen with resultant induction of fibrinolysis within pleural fluids. Detectable levels of intrapleural plasminogen activator activity are necessary to activate newly synthesized or endogenous pleural fluid plasminogen due to the relatively slow (4–6 h) rate of intrapleural fibrinolysis that occurs with administration of IPFT (17, 30, 40, 45). If levels of PAI-1 activity exceed those of intrapleurally administered plasminogen activators, intrapleural fibrinolysis cannot occur. This fundamental concept impacts the IPFT literature as it stands to date, as dosing of intrapleural fibrinolysins has traditionally been arbitrary rather than evidence based, both in terms of actual dosing or by dosing intervals. In the absence of clear evidence of the ability of these doses of IPFT to reliable generate intrapleural fibrinolysis in the patients that were reported, it is unclear that the patient cohorts actually received sufficient amounts of the fibrinolysins that were given. Whether dosing and dosing intervals can be better informed by premarketing dose escalation testing or by the use of new diagnostics in development such as the FPA remains to be determined and represents a potentially important avenue for future studies.
The concept that PAI-1 is critical to the outcome of pleural injury now rests upon a solid foundation of recently developed evidence. An increase in the levels of intrapleural PAI-1 results in worsening both pleural fibrosis and outcomes of IPFT in a rabbit model of TCN-induced pleural injury (18, 40). On the other hand, neutralization of intrapleural PAI-1 via selective targeting of the intramolecular mechanisms of PAI-1 processing, to activation but not latency or cleavage of the inhibitor (43, 44, 48, 96), results in an increase in the efficacy of IPFT and an up to eightfold decrease in the dose of fibrinolysin (17, 18). A decrease in the effective fibrinolysin dose observed during PAI-1-targeted IPFT (17, 18) seems to reflect the amount of a plasminogen activator that circumvents inhibition by overexpressed endogenous PAI-1 that characterizes inflammatory pleural fluids. Thus targeting PAI-1 via mechanisms that neutralized its activity was successful in decreasing of the dose of fibrinolysin needed for successful IPFT (17, 18). The efficacy of IPFT can therefore be enhanced by PAI-1-targeted adjuncts, monoclonal antibodies that neutralize PAI-1, in preclinical testing. This approach could even further mitigate the relatively low risk of intrapleural bleeding associated with IPFT, although this possibility remains to be tested in the clinical trial arena. In contrast, ligands that form stable complexes with PAI-1, thereby stabilizing its active conformation, were either ineffective for IPFT (S195A-tcuPA) or worsened outcomes (18).
It is now additionally clear that the durability of plasminogen activator activity within pleural fluids substantively impact pleural injury outcomes. To be successful in a model of chemically induced pleural injury in rabbits, IPFT should support positive plasminogen activator activity for 4–6 h (18, 40, 46). While one major advantage of tPA is its high affinity to fibrin, intrapleural single chain (sc) uPA processing includes a unique mechanism for generation of durable, low-level intrapleural plasminogen activator activity via formation of relatively stable intrapleural “molecular cage” type complexes with α-macroglobulin (40, 47).
Recent studies also provide a basis for comparison of the efficacy of tPA vs. uPA, scuPA-based IPFT and processing over the same periods of time, at the same stages of disease and in the same models. The minimal effective dose (MED) of sctPA was found to be almost 3.5 times lower than that of scuPA (0.145 and 0.5 mg/kg, respectively) in a rabbit model of TCN-induced pleural injury (28, 29, 40). Specific activities of the fibrinolysins are similar, but it is important to remember that scuPA is a proenzyme that exerts very low intrinsic activity until it is activated by plasmin. Notably, the MEDs for both fibrinolysins were higher (2 mg/kg) and comparable in a rabbit model of empyema (45), reflecting differences between chemical and infectious pleural injury. The rabbit S. pneumoniae empyema model demonstrates areas of loculation, underscoring the importance of using valid animal models that recapitulate key physiological features of human disease. Nevertheless, the major mechanistic features of intrapleural fibrinolysis and the processing of fibrinolysins share considerable commonality in rabbit models of chemically induced pleural injury empyema. These models enable comparisons of the processing of fibrinolysins in pleural fluids. For example, the MED for scuPA in the rabbit Streptococcal empyema model was four times higher when compared with that identified in the tetracycline model of organizing pleural injury. For sctPA, the dose was increased by 13.8 times in infectious vs. chemically induced pleural injury. Thus scuPA, which forms “molecular cage” type complexes, could be a better alternative for treatment of empyema in humans. Testing of that postulate in humans has been initiated in an ongoing phase I safety trial, described in the next section.
Development of Single Chain Urokinase Plasminogen Activator for Treatment of Patients with Loculated Empyema/CPE
scuPA was first envisioned as a candidate for use in IPFT when it was discovered that this proenzyme was very effective and well tolerated in removing intrapleural fibrinous adhesions in TCN-induced pleural injury in rabbits, a model that is associated with prominent intrapleural fibrin deposition (34). In initial studies to examine the processing of intrapleurally administered this agent in these animals, it was determined that scuPA was effective when delivered before adhesion formation and cleared adhesions after their formation (28). Active urokinase was detectable within pleural fluids 24 h after scuPA administration, which indicated that the efficacy was associated with durable intrapleural generation of plasminogen activator activity. Subsequently, it was found that administration of intrapleural scuPA more effectively cleared intrapleural fibrin deposits and adhesions in rabbits with TCN-induced pleural injury than two chain urokinase, which was used in clinically prorated dosing (29). There was a trend toward superiority of the effectiveness of scuPA vs. clinically applied dosing of tPA prorated to the weight of the rabbits, but both tPA and scuPA effectively cleared fibrinous deposits in the model and overcame inhibition by PAI-1. We also found that scuPA generated durable plasminogen activator activity within pleural fluids of rabbits with Pasteurella-induced empyema (33). We subsequently found that scuPA was processed, in part, to a PAI-1-resistant form of uPA complexed to a2-macroglobulin, which was mainly responsible for the durable bioactivity of scuPA within inflammatory pleural fluids (47), and additional studies to more comprehensively understand the intrapleural processing of scuPA were then conducted as described above. In extensive, preclinical studies conducted over more than a decade, pleural bleeding was not encountered apart from very rare events due to trauma from pleural cannulation.
Shortly after patent protection for the use of scuPA as a treatment for loculated pleural injury was awarded, a University of Texas biotechnology initiative was obtained to initiate commercialization of scuPA through the creation of a start-up company called Lung Therapeutics through the National Heart, Lung, and Blood Institute (NHLBI) Science Moving towArds Research Translation and Therapy (SMARTT) Program (Contract No. HHSN268201100014C). Additional funding was obtained to manufacture Good Manufacturing Practices-grade scuPA in anticipation of clinical trial testing. Funding was also supplied for Good Laboratory Practice toxicology studies and regulatory support. In brief, Good Manufacturing Practices manufacturing was accomplished and the bulk drug substance obtained via the SMARTT Program, was subsequently lyophilized, and vialed for stability and release testing, which demonstrated that the material was acceptable for human administration. Good Laboratory Practice toxicology studies done under the SMARTT contract by an NHLBI subcontractor included studies done using either intravenous and intrapleural administration of scuPA, which, in brief, did not uncover bleeding events or evidence of systemic fibrinogenolysis. Subsequently and based on this trial enabling work, a phase 1a/1b dose escalation-safety trial was initiated in patients with empyema/CPE with failed drainage (ANZCT Registry Trial ID: ACTRN12616001442493). The trial is now recruiting and will close within the coming year. The objective of this trial is to determine patient safety including bleeding potential of intrapleurally administered scuPA. Efficacy signals including expedited drainage, clinical improvement, need for surgical referral, and duration of hospitalization are also being assessed. These parameters can be used to help guide dosing for a follow up phase 2 efficacy trial, which is anticipated to begin within the next 12–18 mo using a new drug supply for which manufacturing efforts have commenced. The current phase 1a/1b trial now being done represents the first dose-escalation trial for any form of IPFT of which we are aware. As such, it represents an initial step that may help overcome the empiricism now surrounding the dosing of currently available forms of IPFT.
PLEURAL REMODELING, MESOMESENCHYMAL TRANSITION OF PMCS, AND FIBROTIC REPAIR
Pleural injury, when severe, can result in scarring of the pleural surface leading to pleural fibrosis. Numerous studies have shown that the inflammatory environment of the injured pleural space can cause the relatively quiescent pleural mesothelial cells to acquire an activated cellular phenotype. Specifically, through a process termed mesomesenchymal transition (MesoMT), mesothelial cells become matrix producing myofibroblasts (Fig. 1A). While not found in normalcy, myofibroblasts are present in many types of fibrosis and are characterized by the increased expression of α-smooth muscle actin (α-SMA), collagen (Col)-1, and fibronectin. Cells undergoing mesenchymal transition also lose their epithelial and tight junction markers, including E-cadherin and zona-occludins (63, 92), allowing them to become hyperproliferative and motile. Tissue sections from patients with nonspecific pleuritis show the increased presence of α-SMA-expressing myofibroblasts at or near the pleural surface, which likely contribute to the thickening pleura (92). Collagen deposition is likewise increased in this pleural rind. Although there are multiple, potential sources of these myofibroblasts, the proximity to the site of injury and the coexpression of mesothelial cells markers, like calretinin, suggest that activated mesothelial cells play a major role in development of pleural fibrosis or, in advanced cases, fibrothorax. Interestingly, pleural mesothelial cells undergoing MesoMT can also migrate into the lung parenchyma and thereby contribute to pulmonary fibrosis via a process that can be abridged by carbon monoxide or heme oxygenase-1 (102). This important work suggests that MesoMT can affect the progression of pulmonary as well as pleural repair and fibrosis.
Fibrinolytic proteases have been reported to directly induce markers of MesoMT in vitro (92). Similar to TGF-β, uPA and plasmin induce profibrotic changes in human pleural mesothelial cell morphology and increase the expression of α-SMA and Col-1, markers of MesoMT. Neither mesothelial cell phenotype nor expression of MesoMT markers are affected by treatment with tPA. The low-density lipoprotein receptor-related protein 1 (LRP-1) binds and internalize members of the fibrinolytic pathway, including uPA and uPAR (94). Inflammatory mediators present in pleural effusions, like TNF-α and IL-1β, reduce LRP-1 expression (94). Downregulation of LRP-1 expression or activity stabilizes uPA and uPAR expression at the mesothelial cell surface, thus prolonging their activity. Consequently, uPA-mediated collagen induction is increased in LRP-1 downregulated cells (94).
The expression of components of the fibrinolytic system, in particular uPA, plasmin, and PAI-1, can also importantly influence the outcomes of pleural injury. In a carbon black/bleomycin model of pleural fibrosis, D-dimers levels, markers of plasmin-mediated fibrin dissolution, were increased in pleural fluids (92). PAI-1-deficient carbon black/bleomycin-injured mice, which demonstrate increased uPA and plasmin activity in pleural fluids, showed worse pleural injury. Myofibroblast accumulation, pleural thickening, and collagen deposition were all increased in PAI-1-deficient mice, likely reflecting locally increased plasmin activity. Paradoxically, PAI-1 deficiency also increased TF expression and thrombin anti-thrombin complexes, suggesting concurrently increased intrapleural coagulation in this model of pleural injury. These findings support the concept that cross talk between fibrinolytic and coagulation pathways exists in this form of pleural injury. Similar results were found in an infectious disease model of pleural injury. S. pneumoniae-mediated empyema in mice caused significant pleural thickening and collagen deposition. The MesoMT marker α-SMA was likewise increased in the pleural and subpleural region. While S. pneumoniae mediated infection increased fibrinolytic activity in the injured pleural space, PAI-1 deficiency worsened disease severity (93). S. pneumoniae infection in PAI-1-deficient mice showed reduced fibrin deposition and increased plasmin activity. Lethality was also increased in PAI-1-deficient mice, further suggesting that unrestricted endogenous fibrinolysis can be deleterious in the setting of infectious pleural injury.
NEW CANDIDATE THERAPEUTIC TARGETS FOR PLEURAL INJURY
Glycogen Synthase Kinase-3β
Plasmin and uPA also induce phosphatidylinositol 3-kinase/Akt and NF-κB phosphorylation in HPMCs. These pathways had previously been shown to be critical for the induction of mesenchymal transition in other cell types (53, 67, 84). In HPMCs, the phosphatidylinositol 3-kinase/Akt and NF-κB signaling pathways are linked. Blockade of either pathway significantly attenuates the induction of MesoMT (66, 92). A recent study showed that glycogen synthase kinase-3β (GSK-3β) is a critical determinant in the progression of pleural fibrosis (2). In nonspecific human pleuritis, GSK-3β expression was increased in the pleura compared with normal lung tissues. HPMCs treated with uPA or plasmin show increased GSK-3β nuclear localization and tyrosine 216 phosphorylation, indicators of increased GSK-3β activity. GSK-3β knockdown attenuated induction of MesoMT. Furthermore, the novel GSK-3β inhibitor 9ING41 blocked and reversed TGF-β, plasmin, and uPA-mediated MesoMT. GSK-3β inhibition with 9ING41 reduced tyrosine 216 phosphorylation and consequently inhibited NF-κB signaling in HPMCs (2). GSK-3β inhibition likewise improved lung function and volume in S. pneumoniae-injured mice. These mice also demonstrated less pleural thickening and fewer α-SMA-expressing myofibroblasts, suggesting that MesoMT was attenuated in vivo. This study strongly supports the therapeutic targeting of GSK-3β for the treatment of pleural injury, which is an ongoing area of active investigation.
KIF5A
During MesoMT, PMCs increase the expression and secretion of extracellular matrix (ECM) proteins such as collagen 1 (Col-1) (2, 39, 93, 94), yet the mechanism underlying this process has been poorly understood. A key gap in current knowledge is how ECM proteins are transported from perinuclear domains to the surface membrane, where they are secreted. Quite recently, Kamata et al. (39) reported that KIF5A, a kinesin 1 family member, transports Col-1-containing vesicles in HPMCs. This is a significant observation, as little is known about the mechanism of transportation and secretion of ECM proteins by PMCs. KIF5A was found to be responsible for the transportation of Col-1-containing vesicles from the perinuclear domain to the surface membrane in HPMCs based on the following findings: first, among the KIFs, KIF5A expression is notably upregulated during TGF-β-induced activation of Col-1 secretion during MesoMT. Second, Col-1 and KIF5A show notable colocalization on microtubules in HPMCs. Third, KIF5A gene silencing attenuated TGF-β-induced Col-1 localization at cell peripheries and reduced Col-1 secretion. Fourth, live cell imaging revealed KIF5A- and Col-1-containing vesicles show continuous directional comovement toward cell peripheries of HPMCs. These ex vivo results suggest that KIF5A is responsible for Col-1 transportation and facilitates Col-1 secretion from HPMCs. In mice with carbon black/bleomycin-induced pleural fibrosis, KIF5A expression is markedly increased in the thickened pleura along with increase expression of α-SMA. This coincided with marked increase in surrounding deposition of Col-1. These in vivo results suggest that upregulated KIF5A expression promotes collagen secretion in carbon black/bleomycin-induced pleural fibrosis, representing yet another potential interventional target for fibrosing pleural injury.
Reactive Oxygen and Nitrogen Radicals
As oxidant stress is strongly implicated in the pathogenesis of acute parenchymal lung injury (41, 42, 97, 101), its role in the pathogenesis of pleural injury and remodeling bears mention. Although the broad area is understudied to date, invasion of the injured pleural space by neutrophils and inflammatory cytokines is strongly implicated in the pathogenesis of pleural remodeling (26, 34). These events are linked to excessive oxidative load, but the extent of oxidative load in fibrosing pleural injury and its impact in the mesothelial remodeling remain unknown. A massive phagocytic response is likely to cause oxidative burst with overwhelming production of superoxide anion (O2·−) and hydrogen peroxide (H2O2). H2O2 could further undergo the Fenton reaction with the presence of iron producing the most damaging hydroxyl radicals (·OH) in the injured pleura. That is likely to damage the lipid bilayer of the PMC resulting in PMC dysfunction and potentially increased exudation of plasma within the pleural space. Furthermre, PMCs have been shown to produce significant nitric oxide in response to inflammatory cytokines such as TNF-α and IL-1β (65). Therefore, high levels of peroxynitrite (OONO−) are likely to be produced in the pleura due to interaction of O2·− and nitric oxide in response to an infection. OONO− is very reactive nitrogen radical that is expected to cause significant oxidative damage to all cellular components. The existence of cross talk between NADPH oxidase-mediated O2·− release triggering excessive O2·− in the mitochondria is well documented (10). Therefore, high levels of reactive oxygen/nitrogen radicals are expected to be released in the pleura, which is likely to induce accentuated oxidative stress condition due to mitochondrial reactive oxygen species release. The heightened release pleural oxidants could modulate various signaling cascades in the mesothelial cells resulting in the initiation or exacerbation of fibrogenic signaling cascades. Consistent with this hypothesis, high levels of ascorbic acid or dehydroascorbic acid have been shown to reduce pleural permeability (22). Thus better understanding pleural oxidative stress and its impact on permeability and pleural organization or fibrosis could expedite the identification of new ways to mitigate or block these responses during pleural infection or other noxious conditions.
SUMMARY
Over the past few years, the regulation of fibrin turnover has been shown to encompass derangements including the overexpression of PAI-1 that impact the organization of extravascular fluids and remodel the pleural space. It is now clear that these derangements can often be addressed by the administration of fibrinolytic therapy, with expedited pleural drainage and improved outcomes (Table1). Research gaps that remain to be addressed include identification as to how sex, age, and genetic variation influence the response to intrapleural fibrinolytic therapy. Translational areas requiring additional investigation include the development of diagnostics that reliably predict outcomes or optimize dosing of currently available intrapleural fibrinolytic therapy and predict its outcomes. The recognition that individual patients exhibit variable responses to pleural injury has led to the development of novel candidate diagnostics like the fibrinolytic potential and testing of PAI-1-resistant interventions including scuPA. A clinical area that mandates further investigation is the continuation of efforts to identify optimally tolerated, clinical, and cost-effective pharmacologic strategies to expedite pleural drainage in patients with empyema or CPE. New interventional avenues including the development of GSK-3β or PAI-1 inhibitors for pleural organization may in future expand the therapeutic options for the benefit of patents with loculation, failed pleural drainage, and lung restriction due to organizing pleural injury and fibrotic remodeling.
Table 1.
Aberrant fibrin turnover, fibrinolysis, and pleural organization: overviews of discovery, translational, and clinical literature
| Contribution | |
|---|---|
| Dvorak (15) | Seminal overview of foundational work describing the role of aberrant fibrin turnover in the pathogenesis of tissue organization after injury and scarification in the context of tissue injury and neoplasia. |
| Lee et al. (54) | Recent review of basic and translational progress in the field of infectious pleural injury, including IPFT. |
| Rahman et al. (73) | Key study that showed that a fibrinolysin alone (tissue plasminogen activator) was ineffective in improving clinical outcomes of patients with pleural infection, while tPA in combination with DNase was effective and provides a good overview of the use of combination IPFT. The key MIST1 trial (58) likewise showed that streptokinase alone was ineffective. |
| Komissarov et al. (45) | A translational study using larger animal models that contains an in depth overview of the processing of intrapleurally administered fibrinolysins in the setting of empyema. |
| Tucker and Idell (91) | Provides an overview of the role of the fibrinolytic system in the pathogenesis of both lung and pleural disease and an overview of clinical studies, some reporting the efficacy of fibrinolysins alone in IPFT, all as of 4 yr ago. |
IPFT, intrapleural fibrinolytic therapy; MIST-1, Multicenter Intrapleural Sepsis Trial-1.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-HL-118401-01 [to S. Idell, principal investigator (PI), contact, multiple principal investigator (MPI)], R01-HL-130402-01A1 (to S. Idell, MPI), 1U54-ES-027698-01 (SI site PI, subcontract), R01-HL-130133-01A1 (TAT (PI), SI, coinvestigator), R01-HL-133067-01 (SS (PI), SI, coinvestigator), and R21-ES-025815-01A1 (SS (PI), SI, coinvestigator); TLL Temple Endowed Chair in Idiopathic Pulmonary Fibrosis, Texas Lung Injury Institute Grant UO-1-HL-121841-01A1 (to S. Idell, contact PI, MPI); and NIH Science Moving towArds Research Translation and Therapy (SMARTT) Contract No. HHSN268201100014C (to S. Idell, PI). Y. C. Lee has received funding support from the National Health and Medical Research Council, Sir Charles Gairdner Research Advisory Committee, Cancer Council of Western Australia, and the Dust Disease Board of New South Wales, Australia.
DISCLOSURES
S. Idell serves as an unpaid member of the Board of Directors, Founder, and Chief Scientific Officer of Lung Therapeutics, Inc. and has an equity position in the company, as does the University of Texas Horizon Fund and The University of Texas Health Science Center at Tyler. He has a conflict of interest plan acknowledging and managing these declared conflicts of interest through The University of Texas Health Science Center at Tyler (UTHSCT). A. A. Komissarov, G. Florova, T. Tucker, R. Idell, and S. Shetty all have similar conflicts of interest plans at the same institution. All human and animal studies described in this article in publications from UTHSCT were approved by the UTHSCT Human Subjects Institutional Review Board and Institutional Animal Care and Utilization Committees, respectively. Y. C. Lee is a coinvestigator for a phase I trial (ANZCT Registry Trial ID: ACTRN12616001442493) of single chain urokinase plasminogen activator (LTI-01) sponsored by Lung Therapeutics.
AUTHOR CONTRIBUTIONS
T.A.T. and S.I. prepared figures; A.A.K., N.M.R., Y.G.L., G.F., S.S., R.I., M.I., K.D., T.A.T., and S.I. drafted manuscript; A.A.K., N.M.R., Y.G.L., G.F., S.S., R.I., M.I., K.D., T.A.T., and S.I. edited and revised manuscript; A.A.K., N.M.R., Y.G.L., G.F., S.S., R.I., M.I., K.D., T.A.T., and S.I. approved final version of manuscript.
REFERENCES
- 1.Bender JM, Ampofo K, Sheng X, Pavia AT, Cannon-Albright L, Byington CL. Parapneumonic empyema deaths during past century, Utah. Emerg Infect Dis 15: 44–48, 2009. doi: 10.3201/eid1501.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boren J, Shryock G, Fergis A, Jeffers A, Owens S, Qin W, Koenig KB, Tsukasaki Y, Komatsu S, Ikebe M, Idell S, Tucker TA. Inhibition of glycogen synthase kinase 3β blocks mesomesenchymal transition and attenuates streptococcus pneumonia-mediated pleural injury in mice. Am J Pathol 187: 2461–2472, 2017. doi: 10.1016/j.ajpath.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown LF, Dvorak AM, Dvorak HF. Leaky vessels, fibrin deposition, and fibrosis: a sequence of events common to solid tumors and to many other types of disease. Am Rev Respir Dis 140: 1104–1107, 1989. doi: 10.1164/ajrccm/140.4.1104. [DOI] [PubMed] [Google Scholar]
- 4.Chapman HA. Disorders of lung matrix remodeling. J Clin Invest 113: 148–157, 2004. doi: 10.1172/JCI20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CH, Chen W, Chen HJ, Yu YH, Lin YC, Tu CY, Hsu WH. Transthoracic ultrasonography in predicting the outcome of small-bore catheter drainage in empyemas or complicated parapneumonic effusions. Ultrasound Med Biol 35: 1468–1474, 2009. doi: 10.1016/j.ultrasmedbio.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Chen KY, Liaw YS, Wang HC, Luh KT, Yang PC. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med 19: 837–843, 2000. doi: 10.7863/jum.2000.19.12.837. [DOI] [PubMed] [Google Scholar]
- 7.Chung CL, Chen CH, Sheu JR, Chen YC, Chang SC. Proinflammatory cytokines, transforming growth factor-beta1, and fibrinolytic enzymes in loculated and free-flowing pleural exudates. Chest 128: 690–697, 2005. doi: 10.1016/S0012-3692(15)50413-3. [DOI] [PubMed] [Google Scholar]
- 8.Clive AO, Kahan BC, Hooper CE, Bhatnagar R, Morley AJ, Zahan-Evans N, Bintcliffe OJ, Boshuizen RC, Fysh ET, Tobin CL, Medford AR, Harvey JE, van den Heuvel MM, Lee YC, Maskell NA. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 69: 1098–1104, 2014. doi: 10.1136/thoraxjnl-2014-205285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corcoran JP, Hallifax R, Rahman NM. New therapeutic approaches to pleural infection. Curr Opin Infect Dis 26: 196–202, 2013. doi: 10.1097/QCO.0b013e32835d0b71. [DOI] [PubMed] [Google Scholar]
- 10.Daiber A, Di Lisa F, Oelze M, Kröller-Schön S, Steven S, Schulz E, Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol 174: 1670–1689, 2017. doi: 10.1111/bph.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, Davies CW, Grayez J, Harrison R, Prasad A, Crosthwaite N, Lee YC, Davies RJ, Miller RF, Rahman NM. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 307: 2383–2389, 2012. doi: 10.1001/jama.2012.5535. [DOI] [PubMed] [Google Scholar]
- 12.Decologne N, Kolb M, Margetts PJ, Menetrier F, Artur Y, Garrido C, Gauldie J, Camus P, Bonniaud P. TGF-beta1 induces progressive pleural scarring and subpleural fibrosis. J Immunol 179: 6043–6051, 2007. doi: 10.4049/jimmunol.179.9.6043. [DOI] [PubMed] [Google Scholar]
- 13.Diacon AH, Theron J, Schuurmans MM, Van de Wal BW, Bolliger CT. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. Am J Respir Crit Care Med 170: 49–53, 2004. doi: 10.1164/rccm.200312-1740OC. [DOI] [PubMed] [Google Scholar]
- 14.Dresler CM, Olak J, Herndon JE II, Richards WG, Scalzetti E, Fleishman SB, Kernstine KH, Demmy T, Jablons DM, Kohman L, Daniel TM, Haasler GB, Sugarbaker DJ; Cooperative Groups Cancer and Leukemia Group B; Eastern Cooperative Oncology Group; North Central Cooperative Oncology Group; Radiation Therapy Oncology Group . Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 127: 909–915, 2005. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing N Engl J Med 315: 1650–1659, 1986. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 16.Farjah F, Symons RG, Krishnadasan B, Wood DE, Flum DR. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg 133: 346–351, 2007. doi: 10.1016/j.jtcvs.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 17.Florova G, Azghani A, Karandashova S, Schaefer C, Koenig K, Stewart-Evans K, Declerck PJ, Idell S, Komissarov AA. Targeting of plasminogen activator inhibitor 1 improves fibrinolytic therapy for tetracycline-induced pleural injury in rabbits. Am J Respir Cell Mol Biol 52: 429–437, 2015. doi: 10.1165/rcmb.2014-0168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florova G, Azghani AO, Karandashova S, Schaefer C, Yarovoi SV, Declerck PJ, Cines DB, Idell S, Komissarov AA. Targeting plasminogen activator inhibitor 1 in tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol 314: L54–L68, 2018. doi: 10.1152/ajplung.00579.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 61: 975–979, 2006. doi: 10.1136/thx.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fysh ET, Waterer GW, Kendall PA, Bremner PR, Dina S, Geelhoed E, McCarney K, Morey S, Millward M, Musk AW, Lee YC. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 142: 394–400, 2012. doi: 10.1378/chest.11-2657. [DOI] [PubMed] [Google Scholar]
- 21.Gieseler F, Lühr I, Kunze T, Mundhenke C, Maass N, Erhart T, Denker M, Beckmann D, Tiemann M, Schulte C, Dohrmann P, Cavaillé F, Godeau F, Gespach C. Activated coagulation factors in human malignant effusions and their contribution to cancer cell metastasis and therapy. Thromb Haemost 97: 1023–1030, 2007. [PubMed] [Google Scholar]
- 22.Gogou E, Hatzoglou C, Chamos V, Zarogiannis S, Gourgoulianis KI, Molyvdas PA. The contribution of ascorbic acid and dehydroascorbic acid to the protective role of pleura during inflammatory reactions. Med Hypotheses 68: 860–863, 2007. doi: 10.1016/j.mehy.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 23.Grijalva CG, Zhu Y, Nuorti JP, Griffin MR. Emergence of parapneumonic empyema in the USA. Thorax 66: 663–668, 2011. doi: 10.1136/thx.2010.156406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol 1: 460–467, 2006. doi: 10.1016/S1556-0864(15)31612-9. [DOI] [PubMed] [Google Scholar]
- 25.Idell RD, Florova G, Komissarov AA, Shetty S, Girard RB, Idell S. The fibrinolytic system: A new target for treatment of depression with psychedelics. Med Hypotheses 100: 46–53, 2017. doi: 10.1016/j.mehy.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Idell S. The pathogenesis of pleural space loculation and fibrosis. Curr Opin Pulm Med 14: 310–315, 2008. doi: 10.1097/MCP.0b013e3282fd0d9b. [DOI] [PubMed] [Google Scholar]
- 27.Idell S. Update on the use of fibrinolysins in pleural disease. Clin Pulm Med 12: 184–190, 2005. doi: 10.1097/01.cpm.0000163392.76738.84. [DOI] [Google Scholar]
- 28.Idell S, Allen T, Chen S, Koenig K, Mazar A, Azghani A. Intrapleural activation, processing, efficacy, and duration of protection of single-chain urokinase in evolving tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol 292: L25–L32, 2007. doi: 10.1152/ajplung.00118.2006. [DOI] [PubMed] [Google Scholar]
- 29.Idell S, Azghani A, Chen S, Koenig K, Mazar A, Kodandapani L, Bdeir K, Cines D, Kulikovskaya I, Allen T. Intrapleural low-molecular-weight urokinase or tissue plasminogen activator versus single-chain urokinase in tetracycline-induced pleural loculation in rabbits. Exp Lung Res 33: 419–440, 2007. doi: 10.1080/01902140701703333. [DOI] [PubMed] [Google Scholar]
- 30.Idell S, Florova G, Shetty S, Tucker T, Idell R, Koenig K, Azghani A, Rahman NM, Komissarov A. Precision-guided, personalized intrapleural fibrinolytic therapy for empyema and complicated parapneumonic pleural effusions: The case for the fibrinolytic potential. Clin Pulm Med 24: 163–169, 2017. doi: 10.1097/CPM.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idell S, Girard W, Koenig KB, McLarty J, Fair DS. Abnormalities of pathways of fibrin turnover in the human pleural space. Am Rev Respir Dis 144: 187–194, 1991. doi: 10.1164/ajrccm/144.1.187. [DOI] [PubMed] [Google Scholar]
- 32.Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, Martin TR, McLarty J, Fair DS. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest 84: 695–705, 1989. doi: 10.1172/JCI114217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idell S, Jun Na M, Liao H, Gazar AE, Drake W, Lane KB, Koenig K, Komissarov A, Tucker T, Light RW. Single-chain urokinase in empyema induced by Pasturella multocida. Exp Lung Res 35: 665–681, 2009. doi: 10.3109/01902140902833277. [DOI] [PubMed] [Google Scholar]
- 34.Idell S, Mazar A, Cines D, Kuo A, Parry G, Gawlak S, Juarez J, Koenig K, Azghani A, Hadden W, McLarty J, Miller E. Single-chain urokinase alone or complexed to its receptor in tetracycline-induced pleuritis in rabbits. Am J Respir Crit Care Med 166: 920–926, 2002. doi: 10.1164/rccm.200204-313OC. [DOI] [PubMed] [Google Scholar]
- 35.Idell S, Pendurthi U, Pueblitz S, Koenig K, Williams T, Rao LV. Tissue factor pathway inhibitor in tetracycline-induced pleuritis in rabbits. Thromb Haemost 79: 649–655, 1998. [PubMed] [Google Scholar]
- 36.Idell S, Zwieb C, Boggaram J, Holiday D, Johnson AR, Raghu G. Mechanisms of fibrin formation and lysis by human lung fibroblasts: influence of TGF-beta and TNF-alpha. Am J Physiol 263: L487–L494, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Idell S, Zwieb C, Kumar A, Koenig KB, Johnson AR. Pathways of fibrin turnover of human pleural mesothelial cells in vitro. Am J Respir Cell Mol Biol 7: 414–426, 1992. doi: 10.1165/ajrcmb/7.4.414. [DOI] [PubMed] [Google Scholar]
- 38.Jørgensen NP, Zobek N, Dreier C, Haaber J, Ingmer H, Larsen OH, Meyer RL. Streptokinase treatment reverses biofilm-associated antibiotic resistance in Staphylococcus aureus. Microorganisms 4: 436, 2016. doi: 10.3390/microorganisms4030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamata H, Tsukasaki Y, Sakai T, Ikebe R, Wang J, Jeffers A, Boren J, Owens S, Suzuki T, Higashihara M, Idell S, Tucker TA, Ikebe M. KIF5A transports collagen vesicles of myofibroblasts during pleural fibrosis. Sci Rep 7: 4556, 2017. doi: 10.1038/s41598-017-04437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karandashova S, Florova G, Azghani AO, Komissarov AA, Koenig K, Tucker TA, Allen TC, Stewart K, Tvinnereim A, Idell S. Intrapleural adenoviral delivery of human plasminogen activator inhibitor-1 exacerbates tetracycline-induced pleural injury in rabbits. Am J Respir Cell Mol Biol 48: 44–52, 2013. doi: 10.1165/rcmb.2012-0183OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinnula VL. Production and degradation of oxygen metabolites during inflammatory states in the human lung. Curr Drug Targets Inflamm Allergy 4: 465–470, 2005. doi: 10.2174/1568010054526368. [DOI] [PubMed] [Google Scholar]
- 42.Kisseleva T, Brenner DA. Fibrogenesis of parenchymal organs. Proc Am Thorac Soc 5: 338–342, 2008. doi: 10.1513/pats.200711-168DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komissarov AA, Andreasen PA, Bødker JS, Declerck PJ, Anagli JY, Shore JD. Additivity in effects of vitronectin and monoclonal antibodies against alpha-helix F of plasminogen activator inhibitor-1 on its reactions with target proteinases. J Biol Chem 280: 1482–1489, 2005. doi: 10.1074/jbc.M408608200. [DOI] [PubMed] [Google Scholar]
- 44.Komissarov AA, Declerck PJ, Shore JD. Mechanisms of conversion of plasminogen activator inhibitor 1 from a suicide inhibitor to a substrate by monoclonal antibodies. J Biol Chem 277: 43858–43865, 2002. doi: 10.1074/jbc.M204110200. [DOI] [PubMed] [Google Scholar]
- 45.Komissarov AA, Florova G, Azghani AO, Buchanan A, Boren J, Allen T, Rahman NM, Koenig K, Chamiso M, Karandashova S, Henry J, Idell S. Dose dependency of outcomes of intrapleural fibrinolytic therapy in new rabbit empyema models. Am J Physiol Lung Cell Mol Physiol 311: L389–L399, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komissarov AA, Florova G, Azghani AO, Buchanan A, Bradley WM, Schaefer C, Koenig K, Idell S. The time course of resolution of adhesions during fibrinolytic therapy in tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol 309: L562–L572, 2015. doi: 10.1152/ajplung.00136.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komissarov AA, Mazar AP, Koenig K, Kurdowska AK, Idell S. Regulation of intrapleural fibrinolysis by urokinase-α-macroglobulin complexes in tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol 297: L568–L577, 2009. doi: 10.1152/ajplung.00066.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komissarov AA, Zhou A, Declerck PJ. Modulation of serpin reaction through stabilization of transient intermediate by ligands bound to alpha-helix F. J Biol Chem 282: 26306–26315, 2007. doi: 10.1074/jbc.M702089200. [DOI] [PubMed] [Google Scholar]
- 49.Kunz CR, Jadus MR, Kukes GD, Kramer F, Nguyen VN, Sasse SA. Intrapleural injection of transforming growth factor-beta antibody inhibits pleural fibrosis in empyema. Chest 126: 1636–1644, 2004. doi: 10.1378/chest.126.5.1636. [DOI] [PubMed] [Google Scholar]
- 50.Kwiecinski J, Na M, Jarneborn A, Jacobsson G, Peetermans M, Verhamme P, Jin T. Tissue plasminogen activator coating on implant surfaces reduces staphylococcus aureus biofilm formation. Appl Environ Microbiol 82: 394–401, 2015. doi: 10.1128/AEM.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lansley SM, Cheah HM, Lee YC. Role of MCP-1 in pleural effusion development in a carrageenan-induced murine model of pleurisy. Respirology 22: 758–763, 2017. doi: 10.1111/resp.12951. [DOI] [PubMed] [Google Scholar]
- 52.Lansley SM, Cheah HM, Varano Della Vergiliana JF, Chakera A, Lee YC. Tissue plasminogen activator potently stimulates pleural effusion via a monocyte chemotactic protein-1-dependent mechanism. Am J Respir Cell Mol Biol 53: 105–112, 2015. doi: 10.1165/rcmb.2014-0017OC. [DOI] [PubMed] [Google Scholar]
- 53.Le Cras TD, Korfhagen TR, Davidson C, Schmidt S, Fenchel M, Ikegami M, Whitsett JA, Hardie WD. Inhibition of PI3K by PX-866 prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Pathol 176: 679–686, 2010. doi: 10.2353/ajpath.2010.090123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee YC, Idell S, Stathopoulos GT. Translational research in pleural infection and beyond. Chest 150: 1361–1370, 2016. doi: 10.1016/j.chest.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 55.Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 77: 507–513, 1972. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 56.Lisboa T, Waterer GW, Lee YC. Pleural infection: changing bacteriology and its implications. Respirology 16: 598–603, 2011. doi: 10.1111/j.1440-1843.2011.01964.x. [DOI] [PubMed] [Google Scholar]
- 57.Majid A, Kheir F, Folch A, Fernandez-Bussy S, Chatterji S, Maskey A, Fashjian M, Cheng G, Ochoa S, Alape D, Folch E. Concurrent intrapleural instillation of tissue plasminogen activator and DNase for pleural infection. a single-center experience. Ann Am Thorac Soc 13: 1512–1518, 2016. doi: 10.1513/AnnalsATS.201602-127OC. [DOI] [PubMed] [Google Scholar]
- 58.Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, Gabe R, Rees GL, Peto TE, Woodhead MA, Lane DJ, Darbyshire JH, Davies RJUK; First Multicenter Intrapleural Sepsis Trial (MIST1) Group . U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 352: 865–874, 2005. doi: 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 59.Maskell NA, Gleeson FV, Darby M, Davies RJ. Diagnostically significant variations in pleural fluid pH in loculated parapneumonic effusions. Chest 126: 2022–2024, 2004. doi: 10.1378/chest.126.6.2022. [DOI] [PubMed] [Google Scholar]
- 60.Mehta HJ, Biswas A, Penley AM, Cope J, Barnes M, Jantz MA. Management of intrapleural sepsis with once daily use of tissue plasminogen activator and deoxyribonuclease. Respiration 91: 101–106, 2016. doi: 10.1159/000443334. [DOI] [PubMed] [Google Scholar]
- 61.Mishra EK, Corcoran JP, Hallifax RJ, Stradling J, Maskell NA, Rahman NM. Defining the minimal important difference for the visual analogue scale assessing dyspnea in patients with malignant pleural effusions. PLoS One 10: e0123798, 2015. doi: 10.1371/journal.pone.0123798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mishra EK, Rahman N. TIME3–A Randomised Controlled Trial To Evaluate Whether Use Of Intrapleural Urokinase Aids The Drainage Of Multi-Septated Pleural Effusion Compared To Placebo (Online). http://www.isrctn.com/ISRCTN12852177. [2017].
- 63.Nasreen N, Mohammed KA, Mubarak KK, Baz MA, Akindipe OA, Fernandez-Bussy S, Antony VB. Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to TGF-β1 in vitro. Am J Physiol Lung Cell Mol Physiol 297: L115–L124, 2009. doi: 10.1152/ajplung.90587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okur E, Baysungur V, Tezel C, Ergene G, Okur HK, Halezeroglu S. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann 19: 238–243, 2011. doi: 10.1177/0218492311410874. [DOI] [PubMed] [Google Scholar]
- 65.Owens MW, Grisham MB. Nitric oxide synthesis by rat pleural mesothelial cells: induction by cytokines and lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol 265: L110–L116, 1993. [DOI] [PubMed] [Google Scholar]
- 66.Owens S, Jeffers A, Boren J, Tsukasaki Y, Koenig K, Ikebe M, Idell S, Tucker TA. Mesomesenchymal transition of pleural mesothelial cells is PI3K and NF-κB dependent. Am J Physiol Lung Cell Mol Physiol 308: L1265–L1273, 2015. doi: 10.1152/ajplung.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel P, Sekiguchi Y, Oh KH, Patterson SE, Kolb MR, Margetts PJ. Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int 77: 319–328, 2010. doi: 10.1038/ki.2009.436. [DOI] [PubMed] [Google Scholar]
- 68.Piccolo F, Pitman N, Bhatnagar R, Popowicz N, Smith NA, Brockway B, Nickels R, Burke AJ, Wong CA, McCartney R, Choo-Kang B, Blyth KG, Maskell NA, Lee YC. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 11: 1419–1425, 2014. doi: 10.1513/AnnalsATS.201407-329OC. [DOI] [PubMed] [Google Scholar]
- 69.Piccolo F, Popowicz N, Wong D, Lee YC. Intrapleural tissue plasminogen activator and deoxyribonuclease therapy for pleural infection. J Thorac Dis 7: 999–1008, 2015 10.3978/j.issn.2072-1439.2015.01.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Popowicz N, Bintcliffe O, De Fonseka D, Blyth KG, Smith NA, Piccolo F, Martin G, Wong D, Edey A, Maskell N, Lee YC. Dose de-escalation of intrapleural tissue plasminogen activator therapy for pleural infection. the alteplase dose assessment for pleural infection therapy project. Ann Am Thorac Soc 14: 929–936, 2017. doi: 10.1513/AnnalsATS.201609-673OC. [DOI] [PubMed] [Google Scholar]
- 71.Popowicz N, Carson C, Chakera A, Kay I, Waterer G, Lee Y. Human pleural fluid is a potent growth medium for bacteria especially streptococcus pneumoniae. Respirology 20, Suppl 2: 126, 2015. [Google Scholar]
- 72.Psallidas I, Yousuf A, Talwar A, Hallifax RJ, Mishra EK, Corcoran JP, Ali N, Rahman NM. Assessment of patient-reported outcome measures in pleural interventions. BMJ Open Respir Res 4: e000171, 2017. doi: 10.1136/bmjresp-2016-000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, Peckham D, Davies CWH, Ali N, Kinnear W, Bentley A, Kahan BC, Wrightson JM, Davies HE, Hooper CE, Lee YCG, Hedley EL, Crosthwaite N, Choo L, Helm EJ, Gleeson FV, Nunn AJ, Davies RJO. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 365: 518–526, 2011. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]
- 74.Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 139: 1419–1423, 2011. doi: 10.1378/chest.10-1868. [DOI] [PubMed] [Google Scholar]
- 75.Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group . Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 65, Suppl 2: ii32–ii40, 2010. doi: 10.1136/thx.2010.136994. [DOI] [PubMed] [Google Scholar]
- 76.Saydam O, Karapinar K, Gokce M, Kilic L, Metin M, Oz II, Tanriverdi O. The palliative treatment with intrapleural streptokinase in patients with multiloculated malignant pleural effusion: a double-blind, placebo-controlled, randomized study. Med Oncol 32: 612, 2015. doi: 10.1007/s12032-015-0612-0. [DOI] [PubMed] [Google Scholar]
- 77.Sellares J, López-Giraldo A, Lucena C, Cilloniz C, Amaro R, Polverino E, Ferrer M, Menéndez R, Mensa J, Torres A. Influence of previous use of inhaled corticoids on the development of pleural effusion in community-acquired pneumonia. Am J Respir Crit Care Med 187: 1241–1248, 2013. doi: 10.1164/rccm.201209-1732OC. [DOI] [PubMed] [Google Scholar]
- 78.Shetty S, Idell S. New perspectives on the regulation of fibrinolysis in the lung. In: Recent Research Developments in Biological Chemistry. Trivandrum, Kerala, India: Research Signpost, 2002, p. 223–230. [Google Scholar]
- 79.Shetty S, Padijnayayveetil J, Tucker T, Stankowska D, Idell S. The fibrinolytic system and the regulation of lung epithelial cell proteolysis, signaling, and cellular viability. Am J Physiol Lung Cell Mol Physiol 295: L967–L975, 2008. doi: 10.1152/ajplung.90349.2008. [DOI] [PubMed] [Google Scholar]
- 80.Shetty S, Velusamy T, Shetty RS, Marudamuthu AS, Shetty SK, Florova G, Tucker T, Koenig K, Shetty P, Bhandary YP, Idell S. Post-transcriptional regulation of plasminogen activator inhibitor type-1 expression in human pleural mesothelial cells. Am J Respir Cell Mol Biol 43: 358–367, 2010. doi: 10.1165/rcmb.2009-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sonnappa S, Cohen G, Owens CM, van Doorn C, Cairns J, Stanojevic S, Elliott MJ, Jaffé A. Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med 174: 221–227, 2006. doi: 10.1164/rccm.200601-027OC. [DOI] [PubMed] [Google Scholar]
- 82.St Peter SD, Tsao K, Spilde TL, Keckler SJ, Harrison C, Jackson MA, Sharp SW, Andrews WS, Rivard DC, Morello FP, Holcomb GW 3rd, Ostlie DJ. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg 44: 106–111, 2009. doi: 10.1016/j.jpedsurg.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stathopoulos GT, Psallidas I, Moustaki A, Moschos C, Kollintza A, Karabela S, Porfyridis I, Vassiliou S, Karatza M, Zhou Z, Joo M, Blackwell TS, Roussos C, Graf D, Kalomenidis I. A central role for tumor-derived monocyte chemoattractant protein-1 in malignant pleural effusion. J Natl Cancer Inst 100: 1464–1476, 2008. doi: 10.1093/jnci/djn325. [DOI] [PubMed] [Google Scholar]
- 84.Sunami Y, Leithäuser F, Gul S, Fiedler K, Güldiken N, Espenlaub S, Holzmann KH, Hipp N, Sindrilaru A, Luedde T, Baumann B, Wissel S, Kreppel F, Schneider M, Scharffetter-Kochanek K, Kochanek S, Strnad P, Wirth T. Hepatic activation of IKK/NFκB signaling induces liver fibrosis via macrophage-mediated chronic inflammation. Hepatology 56: 1117–1128, 2012. doi: 10.1002/hep.25711. [DOI] [PubMed] [Google Scholar]
- 85.Tagarro A, Otheo E, Baquero-Artigao F, Navarro ML, Velasco R, Ruiz M, Penín M, Moreno D, Rojo P, Madero R, Pérez L, Herreros ML, Yebra J, Rizo J, Barrios A, Cañete A, Arguinzoniz L, Gaya F, Vázquez C, Ots C, Santos M, Saavedra J, Guillén S, Prieto L, Ramos JT, Vela C, Berghezan A, Conejo A, Paredes P, Bermejo I, Guizar M, Gutierrez D, Codesal C, Ramos F, Izquierdo C, Gomez-Herruz P, González-Tomé MI, Pérez-Caballero C, Álvarez E, Vázquez JL, Verdú C, Gómez-Zamora A, Menéndez J-J, Schuffelmann C, Borrego R, Llorente J, Fernández A, Albillos JC, Steiner M, Sanz D, Thuissard I; CORTEEC Study Group . Dexamethasone for parapneumonic pleural effusion: a randomized, double-blind, clinical trial. J Pediatr 185: 117–123.e6, 2017. doi: 10.1016/j.jpeds.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 86.Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 national inpatient sample. Chest 151: 845–854, 2017. doi: 10.1016/j.chest.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Thomas R, Piccolo F, Miller D, MacEachern PR, Chee AC, Huseini T, Yarmus L, Bhatnagar R, Lee HJ, Feller-Kopman D, Maskell NA, Tremblay A, Lee YC. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest 148: 746–751, 2015. doi: 10.1378/chest.14-2401. [DOI] [PubMed] [Google Scholar]
- 88.Tillett WS, Sherry S. The effect in patients of streptococcal fibrinolysin (Streptokinase) and streptococcal desoxyribonuclease on fibrinous, purulent, and sanguinous pleural exudations. J Clin Invest 28: 173–190, 1949. doi: 10.1172/JCI102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tillett WS, Sherry S, Read CT. The use of streptokinase-streptodornase in the treatment of postneumonic empyema. J Thorac Surg 21: 275–297, 1951. [PubMed] [Google Scholar]
- 90.Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 129: 362–368, 2006. doi: 10.1378/chest.129.2.362. [DOI] [PubMed] [Google Scholar]
- 91.Tucker T, Idell S. Plasminogen-plasmin system in the pathogenesis and treatment of lung and pleural injury. Semin Thromb Hemost 39: 373–381, 2013. doi: 10.1055/s-0033-1334486. [DOI] [PubMed] [Google Scholar]
- 92.Tucker TA, Jeffers A, Alvarez A, Owens S, Koenig K, Quaid B, Komissarov AA, Florova G, Kothari H, Pendurthi U, Mohan Rao LV, Idell S. Plasminogen activator inhibitor-1 deficiency augments visceral mesothelial organization, intrapleural coagulation, and lung restriction in mice with carbon black/bleomycin-induced pleural injury. Am J Respir Cell Mol Biol 50: 316–327, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tucker TA, Jeffers A, Boren J, Quaid B, Owens S, Koenig KB, Tsukasaki Y, Florova G, Komissarov AA, Ikebe M, Idell S. Organizing empyema induced in mice by Streptococcus pneumoniae: effects of plasminogen activator inhibitor-1 deficiency. Clin Transl Med 5: 17, 2016. doi: 10.1186/s40169-016-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tucker TA, Williams L, Koenig K, Kothari H, Komissarov AA, Florova G, Mazar AP, Allen TC, Bdeir K, Mohan Rao LV, Idell S. Lipoprotein receptor-related protein 1 regulates collagen 1 expression, proteolysis, and migration in human pleural mesothelial cells. Am J Respir Cell Mol Biol 46: 196–206, 2012. doi: 10.1165/rcmb.2011-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 26: 70–76, 2011. doi: 10.1007/s11606-010-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verhamme I, Kvassman JO, Day D, Debrock S, Vleugels N, Declerck PJ, Shore JD. Accelerated conversion of human plasminogen activator inhibitor-1 to its latent form by antibody binding. J Biol Chem 274: 17511–17517, 1999. doi: 10.1074/jbc.274.25.17511. [DOI] [PubMed] [Google Scholar]
- 97.Ward PA. Role of toxic oxygen products from phagocytic cells in tissue injury. Adv Shock Res 10: 27–34, 1983. [PubMed] [Google Scholar]
- 98.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science 295: 1487, 2002. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 99.Wyser C, Walzl G, Smedema JP, Swart F, van Schalkwyk EM, van de Wal BW. Corticosteroids in the treatment of tuberculous pleurisy. A double-blind, placebo-controlled, randomized study. Chest 110: 333–338, 1996. doi: 10.1378/chest.110.2.333. [DOI] [PubMed] [Google Scholar]
- 100.Yu D, Buchvald F, Brandt B, Nielsen KG. Seventeen-year study shows rise in parapneumonic effusion and empyema with higher treatment failure after chest tube drainage. Acta Paediatr 103: 93–99, 2014. doi: 10.1111/apa.12426. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y, Zhang X, Rabbani ZN, Jackson IL, Vujaskovic Z. Oxidative stress mediates radiation lung injury by inducing apoptosis. Int J Radiat Oncol Biol Phys 83: 740–748, 2012. doi: 10.1016/j.ijrobp.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zolak JS, Jagirdar R, Surolia R, Karki S, Oliva O, Hock T, Guroji P, Ding Q, Liu RM, Bolisetty S, Agarwal A, Thannickal VJ, Antony VB. Pleural mesothelial cell differentiation and invasion in fibrogenic lung injury. Am J Pathol 182: 1239–1247, 2013. doi: 10.1016/j.ajpath.2012.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]