Abstract
Pulmonary hypertension (PH) is a progressive and often fatal illness presenting with nonspecific symptoms of dyspnea, lower extremity edema, and exercise intolerance. Pathologically, endothelial dysfunction leads to abnormal intimal and smooth muscle proliferation along with reduced apoptosis, resulting in increased pulmonary vascular resistance and elevated pulmonary pressures. PH is subdivided into five World Health Organization groups based on the disease pathology and specific cause. While there are Food and Drug Administration-approved medications for the treatment of pulmonary arterial hypertension (PAH; Group 1 PH), as well as for chronic thromboembolic PH (Group 4 PH), the morbidity and mortality remain high. Moreover, there are no approved therapies for other forms of PH (Groups 2, 3, and 5) at present. New research has identified molecular targets that mediate vasodilation, anti-inflammatory, and antifibrotic changes within the pulmonary vasculature. Given that PAH is the most commonly studied form of PH worldwide and because recent studies have led to better mechanistic understanding of this devastating disease, in this review we attempt to provide an updated overview of new therapeutic approaches under investigation for the treatment of PH, with a particular focus on PAH, as well as to offer guidelines for future investigations.
INTRODUCTION
Pulmonary hypertension (PH) is a progressive illness often presenting with nonspecific symptoms including dyspnea, dizziness, lower extremity edema, and decreased exercise tolerance (58). At the cellular level, it is characterized by endothelial cell dysfunction and increased contractility of the small pulmonary arteries, which lead to abnormal intimal and smooth muscle proliferation together with resistance to apoptosis (108, 122, 128, 129). Pulmonary vascular remodeling is a prominent feature of PH independent of the etiology (17, 58). This remodeling increases pulmonary vascular resistance (PVR), which eventually leads to failure of the right ventricle (RV) due to rising afterload. Many symptoms of PH, including lower extremity edema and dyspnea, arise from RV failure (108, 128, 129).
DEFINITION OF PH
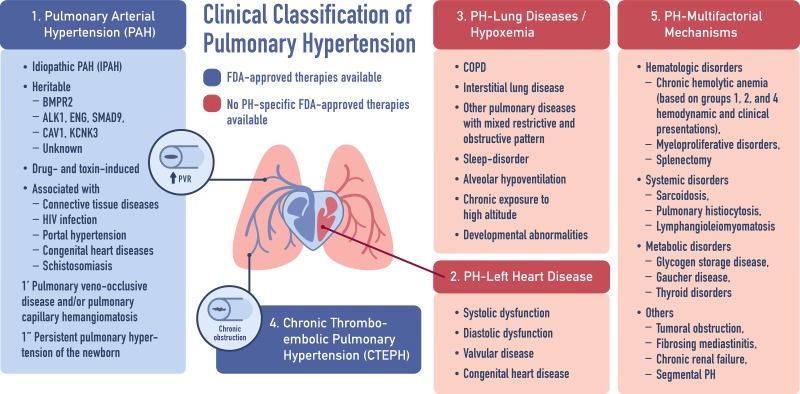
PH is defined by end-expiratory mean pulmonary artery pressure ≥25 mmHg and PVR >3 Wood units at rest (58, 124). PH is a nonspecific umbrella term, which covers elevated pulmonary artery pressure regardless of the etiology. The initial clinical classification of PH has arisen from a World Health Organization-sponsored international meeting in 1973 (53). In 1998, PH was subdivided into five World Health Organization groups based on the disease pathology and specific cause. Pulmonary arterial hypertension (PAH; Group 1 PH) specifically refers to disease processes, which result in vasoconstriction and stiffening of the small arteries in the lungs secondary to cell proliferation, fibrosis, as well as the development of in situ thrombi or plexiform lesions. This pathology both defines PAH and unifies the multiple etiologies, which may lead to the development of the disease. PAH can be idiopathic, can be heritable, and can be associated with connective tissue disease, HIV, drug use, etc. (see Fig. 1 for the updated clinical classification of PH from the 5th World Symposium held in Nice, France, in 2013) (58, 118). There are other pathologies in which PH presents as a secondary disease, including left heart disease (Group 2), chronic lung diseases and/or hypoxia (Group 3), chronic thromboembolic pulmonary hypertension (CTEPH, Group 4), and miscellaneous or multifactorial etiologies (Group 5).
Fig. 1.
Clinical classification of pulmonary hypertension (PH) from the 5th World Symposium held in Nice, France, in 2013. [Adapted from Simonneau et al. (118) with permission from the publisher, copyright 2013, Elsevier] BMPR2, bone morphogenetic protein receptor 2; PVR, pulmonary vascular resistance; FDA, Food and Drug Administration; COPD, chronic obstructive pulmonary disease.
CURRENT THERAPIES FOR PH
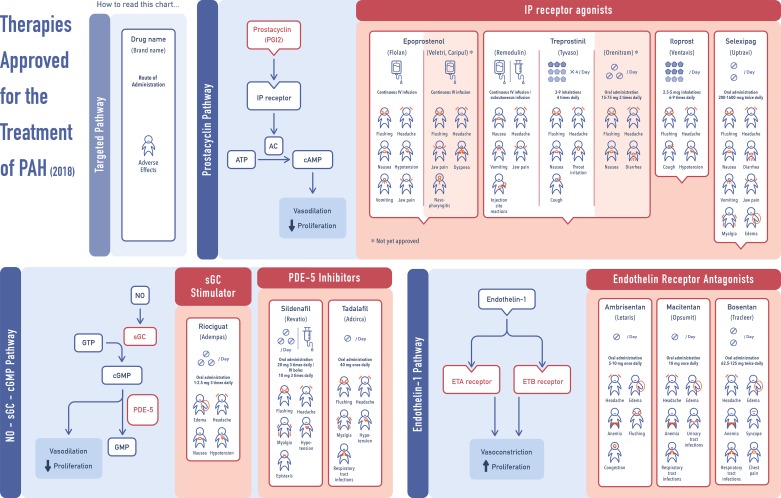
Current therapies targeting endothelial function and vasodilation/antiproliferation via three major pathways associated with prostacyclin (PGI2), nitric oxide (NO), and endothelin-1 (ET-1) have led to the rapid clinical development of >10 major Food and Drug Adminisration (FDA)-approved medications for the treatment of PAH (Fig. 2) (7, 61, 74, 106). In 2013, riociguat, a member of soluble guanylyl cyclase stimulators, has also been approved for the treatment of both PAH and CTEPH (43, 44). Although there are 14 FDA-approved drugs for the treatment of PAH available on the market, class-specific side effects (hypotension and myalgia for phosphodiesterase type-5 inhibitors; significant hypotension with no evident myalgia for soluble guanylate cyclase stimulator; anemia and edema for endothelin receptor antagonists; see Fig. 2 for more details) are commonly reported, and mortality with the current therapies remains high, with the estimated rates of 15, 30, and 45% at 1, 2, and 3 yr from diagnosis, respectively (58). At present, there are no approved therapies for other forms of PH (Groups 2, 3, and 5). Moreover, therapies approved for PAH, such as treatment with sildenafil and tadalafil, have been found to be ineffective or exhibited controversial results in patients with Groups 2 and 3 PH (22, 45, 48, 79, 110). Thus there is an important unmet need in identifying new therapeutic approaches to provide a significant positive impact on disease progression and to improve patient outcomes. Given that PAH is the most commonly studied form of PH worldwide and because recent studies have led to better mechanistic understanding of this devastating disease, the emerging targets and therapies for PAH is the main focus of this review.
Fig. 2.
Current therapies approved for treatment of pulmonary arterial hypertension (PAH) among prostacyclin, nitric oxide (NO), and endothelin (ET)-1 pathways. sGC, soluble guanylyl cyclase; PDE-5, phosphodiesterase type 5; PPARγ, peroxisome proliferator-activated receptor-γ.
EMERGING TARGETS AND THERAPIES FOR PAH
Here, we will first focus on drugs that modulate the vasodilator/vasoconstrictor balance, and then, we will review drugs that target the proliferative antiapoptotic behavior of the pulmonary endothelial and smooth muscle cells. New approaches targeting inflammation and disordered metabolism will also be discussed.
VASODILATORS
Apelin
Apelin is a ligand for the apelin receptor (also known as APJ, APLNR, and AGTRL1), a G protein-coupled receptor predominantly located in the lung, heart, endothelium, kidney, and brain (64, 68). In the lung, apelin is localized exclusively in endothelial cells of small pulmonary arteries and is regulated by hypoxia-inducible factor-1α, as well as bone morphogenetic protein receptor 2 (BMPR2) (68, 69). The apelin-APJ axis modulates vascular tone, apoptosis, NO-dependent vasodilation, and cardiac contractility (68).
There are several downstream effectors of apelin, such as microRNAs (miR) 424/503, which inhibits fibroblast growth factor 2 (2, 68, 69). Reduced expression of miR 424/503, together with the subsequent increased fibroblast growth factor 2 expression, has been shown to induce pulmonary vascular remodeling and vasoconstriction in pulmonary artery endothelial cells (PAECs) from patients with PAH and in monocrotaline (MCT) rat model of PAH (69). In fact, administration of apelin or miR 420/503 has been shown to prevent and rescue preclinical models of PAH (2, 37, 69). Nevertheless, miR 424/503 is not exclusively regulated by apelin. In mammary epithelium, this miR cluster has been shown to be transcriptionally controlled by transforming growth factor-β (TGF-β), where induction of miR 424/503 regulates cell apoptosis and cell cycle arrest through downregulation of CDC25A (86). Apelin has also been reported to regulate the ATPase activity of the endothelial enzyme CD39, which is significantly downregulated in the lungs and PAECs from patients with PAH (54). Moreover, suppression of CD39 is associated with vascular dysfunction and remodeling in lungs and cultured PAECs from patients with PAH (54).
No less important is another pathway in which APJ activates AMPK, Kruppel-like factor 2, and endothelial nitric oxide synthase (eNOS), which enhance NO production and vasodilation. Another newly discovered mediator, Elabela/Toddler, an endogenous agonist of the APJ, has been shown to be downregulated in lungs from PAH patients, as well as in both MCT and Sugen/hypoxia (SuHx) rat models of PAH (136). Administration of Elabela/Toddler attenuates RV systolic pressures, pulmonary vascular remodeling, and RV hypertrophy in rats with PAH-induced by MCT (136). Clinical trials have shown that apelin increases cardiac output and stoke volume in patients with PAH, although no changes in mean pulmonary artery pressure and systemic vascular resistance were observed (16).
Dehydroepiandrosterone
Dehydroepiandrosterone (DHEA) is a cholesterol-derived hormone made in and secreted from the adrenal cortex. It serves as a precursor for estrogens and androgens in the body. Low DHEA levels in men have been associated with PAH (130). DHEA treatment has been shown to prevent and reverse chronic hypoxic-PH (12). In addition, DHEA treatment attenuates severe PAH in SuHx rats and protects against MCT-induced PAH in pneumonectomized rats (4, 59).
At the molecular level, DHEA inhibits PI3K/Akt, which phosphorylates and inactivates glycogen synthase kinase-3β. Glycogen synthase kinase-3β decreases the mitochondrial membrane potential, promotes apoptosis, and inhibits the activation of the transcription factor nuclear factor of the activated T cells, which downregulates the voltage-gated potassium (Kv) channels like Kv1.5, leading to cell depolarization, increased calcium levels, and cell proliferation. DHEA also inhibits the signal transducers and activators of transcription-3, one of the key mediators responsible for proliferative and antiapoptotic phenotypes in PAH, which also upregulates survivin proteins (102). DHEA has additionally been shown to attenuate PAH by inhibiting NADPH or glucose-6 phosphate dehydrogenase activity in pulmonary artery smooth muscle cells (PASMCs) (4, 25, 100). Alternatively, DHEA may inhibit RhoA/Rho-kinases (ROCK) signaling, which is known to contribute pathologically in numerous models of PH (59) (see below for more details).
Although data related to the use of DHEA in PAH are limited, DHEA treatment has been recently reported to improve 6-min walk test distance (6MWD), pulmonary hemodynamics, and the diffusing capacity of the lung for carbon oxide in patients with PH associated with chronic obstructive pulmonary disease (35).
Serotonin
Multiple studies have implicated serotonin (5-HT), a monoamine neurotransmitter found in the gastrointestinal tract, blood, and central nervous system, in the development of PAH (94, 140). Serotonin is produced in PAECs by tryptophan hydroxylase (TPH1) and acts at the 5-HT (1B and 2A) receptor and the serotonin receptor transporter (SERT or 5-HTT) to mediate constriction and proliferation of vascular smooth muscle cells (84, 94).
Treatment with the most potent 5-HT1B antagonist LY393558, alone or in combination with fluoxetine and SB224289, has been shown to reduce 5-HT-induced pulmonary vasoconstriction in rats exposed to 2-wk hypoxia (94). C-122, a novel antagonist that blocks the 5-HT2B receptor, has also been shown to lower pulmonary pressures, decrease RV hypertrophy, and reduce pulmonary vascular remodeling in MCT rat model of PAH (140). A recent study also demonstrated that the antagonist of 5-HT2B receptor, SB204741, prevented heritable PAH (HPAH) (134). Additionally, RP5063, a multimodal dopamine (DA) and 5-HT modulator with high affinity for DA2/3/4 and 5-HT2A/2B/7 receptors and moderate affinity for SERT, has been recently reported to prevent MCT and SuHx models of PAH (10, 11). Moreover, supplementation with 5-HT1B antagonists and 5-HTT antagonist, alone or in combination, increases apoptosis and reduces proliferation of PASMCs through downregulation of extracellular signal-regulated kinase-1/2 and pyruvate dehydrogenase kinase (PDK) signaling pathways (84). Although a recent study by De Raaf et al. (32) demonstrated that serotonin transporter is not required for the development of severe PH in SERT knockout (KO) rats exposed to SuHx, serotonin-targeting strategies remain a promising therapeutic option for the treatment of PAH.
RhoA/Rho-Kinase
The ROCK pathway was originally discovered in 1995 as an effector of the G protein RhoA. Increased RhoA/ROCK activity is implicated in a number of pathophysiological events ranging from abnormal vasoconstriction to promotion of vascular inflammation and remodeling, all of which are associated with numerous cardiovascular diseases, including PAH (25). ROCK inhibits calcium-independent myosin light chain phosphatase activity by phosphorylating myosin phosphatase-targeting subunit 1 and a smaller subunit M20, thus inducing vascular smooth muscle cell contraction (41). Additional vasoconstriction and remodeling are mediated by inhibition of eNOS, monocyte chemoattractant protein-1, and plasminogen activator inhibitor-1 (98). Fasudil, the first generation RhoA/ROCK inhibitor, has been shown to suppress hypertension in both animals and humans (90, 116). Moreover, fasudil has been shown to lower pulmonary artery pressure, improve pulmonary vascular remodeling, and RV hypertrophy in rats with PAH induced by MCT and SuHx, as well as in mice with pulmonary fibrosis and PH induced by bleomycin (9, 41, 98). Most recently, the use of combination therapy with fasudil and SOD was reported to slow PAH progression in MCT and SuHx rats (51).
Clinical trials of fasudil on extensive background therapy have shown a significant improvement in pulmonary artery pressures and PVR in PAH patients (41). Two recent studies have also demonstrated that acute fasudil treatment reduces pulmonary pressures and PVR in 12 and 35 patients with congenital heart disease and severe PAH (81, 135). Lastly, the use of AT-877ER, an extended release formulation of fasudil, has also been recently reported to improve pulmonary hemodynamics in patients with PAH (42). However, these beneficial effects require further verification in future clinical trials.
Vasoactive Intestinal Peptide
Vasoactive intestinal peptide (VIP) is a peptide hormone that is produced throughout the body and acts on type II G coupled-protein receptors (VPAC1 and VPAC2) to exert prominent vasodilation, antiproliferation, and anti-inflammatory properties via activation of cAMP and cGMP systems (137). Recent animal studies have shown that VIP KO mice developed moderate PAH, accompanied by RV hypertrophy, pulmonary vascular remodeling, and infiltration of perivascular inflammatory cells (112). Replenishment of VIP attenuated both vascular remodeling and RV hypertrophy in VIP KO mice (112).
Low serum concentrations of VIP have also been described in patients with PAH. These patients were reported as having received a significant improvement of pulmonary hemodynamics after chronic external VIP substitution via an aerosol (105). Other work by Leuchte et al. (77) also showed that inhalation of VIP (aviptadil) improved PVR and oxygenation in patients with PAH, patients with PH associated with lung disease, and patients with CTEPH. Because VIP is known to have a short half-life due to being rapidly inactivated by neuropeptidases (NEP) located on the lung surface, recent work from the same group evaluated the combined treatment with VIP and thiorphan, an NEP 24.11 inhibitor, in isolated rabbit lungs exposed to thromboxane A2 mimetic U46619, which induces pulmonary vasoconstriction. Remarkably, thiorphan significantly augmented the hemodynamic effects of VIP on PH (78). Finally, inhalation of the cyclic VIP analog RO 25-1553 has also been shown to induce potent and sustained vasodilatory effects in ex vivo and in vivo models with hypoxia exposure, without detectable adverse effects (137). Still, the clinical effects of this type of treatment need to be further investigated.
Inhaled Nitrite
Nitrite () is reduced to form NO in blood and tissues by a variety of heme- and molybdopterrin-containing proteins (such as hemoglobin, myoglobin, neuroglobin, xanthine oxidase, sulfite oxidase, and mARC enzymes) (88). Inhaled and orally administered nitrite has been shown to reduce pulmonary pressures in sheep, mouse, and rat models of PH (20, 62, 75, 141) and has now advanced through phase 1 safety studies to phase 2A proof-of-concept studies in the catheterization laboratory (13, 14, 111). A recent study by Simon et al. (117) evaluated inhaled nitrite in 20 patients with PAH on extensive background therapy and in 10 and 6 patients with Groups 2 and 3 PH, respectively. While nitrite was safe, well tolerated, and reduced pulmonary pressures in all patients, the hemodynamic profile appeared most favorable in Groups 2 and 3 PH (117). This is based on a significant reduction in all pressures, including central venous, pulmonary artery, and pulmonary artery occlusion pressures (PAOPs). The significant drop in PAOP limited the decrease in PVR. Other investigators have recently reported that inhaled and intravenous nitrite also reduces PAOP during exercise in patients with Group 2 PH (particularly the subset of patients with heart failure with preserved ejection fraction), which is associated with improved exercise capacity (13, 14). This drug is now being evaluated by the National Heart, Lung, and Blood Institute Heart Failure Network and is under development by United Therapeutics (NCT02742129) (109).
ANTIPROLIFERATIVE/ANTI-INFLAMMATORY
Bone Morphogenic Protein Receptor Type 2
Since the discovery of mutations in the gene encoding BMPR2 in families with PAH, BMPR2 has been studied extensively and has become a central player in PAH. BMPR2 is a member of the TGF-β signaling family. Mutations in BMPR2 have been found in 80% of patients with HPAH, as well as in 40% of patients with sporadic or idiopathic PAH (3). BMPR2 signaling regulates cell growth via stimulation of Smad1/5/8, which has downstream effects on Smad4. BMPR2 is involved in maintaining balance between the smooth muscle cells and the vascular endothelium. Loss of BMPR2 in PAECs has been shown to increase susceptibility of cells to apoptosis (126). Pulmonary endothelium-specific BMPR2 KO mice have also been shown to be predisposed to PAH development (60). Moreover, a recent study reported that mice bearing a heterozygous knockin allele of a human BMPR2 mutation, R899X, developed spontaneous PAH (87).
Therapeutic benefits targeting the BMPR2 pathway have been investigated greatly and have now progressed into clinical trials. High-throughput screening of 3,765 FDA-approved drugs has identified FK506 (tacrolimus), a calcineurin inhibitor often used in organ transplantation for immune suppression, as an activator of BMPR2. In addition to acting as a calcineurin inhibitor, FK506 also binds FK-binding protein-12, which is a repressor of BMP signaling, to activate BMPR2. FK506 has been shown to prevent the development of PAH in mice with endothelial deletion of BMPR2 and reverse established PAH in MCT and SuHx rat models (121). The use of low-dose FK506 has led to positive clinical results in patients with PAH in an ongoing phase 2A clinical trial, but these beneficial effects need to be further evaluated in the near future (120).
Enhancement of endothelial BMPR2 signaling with selective ligand BMP9 has also been recently reported to reverse established PAH in mice with heterozygous R899X mutation, as well as in MCT and SuHx rat models of PAH (87). In addition, ataluren, a drug that promotes ribosomal read-through of premature stop codons, was found to increase BMP-mediated miRNA processing in six out of eight patients with HPAH with nonsense mutations in BMPR2 and Smad9 (34). Other work has demonstrated that the immunoglobulin-Fc fusion protein of TGF-β (TGFBRII-Fc), which serves as a selective TGF-β 1/3 ligand trap, improves pulmonary hemodynamics, vascular remodeling, and survival in MCT rat, as well as in SuHx-exposed mice and rats (138). Restoration of BMPR2 signaling via activation of the transcription factor Forkhead box O1 with the chemotherapeutic agent paclitaxel has also been shown to reverse pulmonary vascular remodeling and RV hypertrophy in animal models of PAH (113). Furthermore, the elastase inhibitor elafin was recently reported to reverse severe PAH in SuHx rats via caveolin-1-dependent BMPR2 signaling (95). However, the clinical and hemodynamic effects of these BMPR2-targeting strategies are yet to be determined.
Bardoxolone Methyl
Triterpenoids are a family of electrophilic compounds found in nature (chrysanthemum flower) that covalently modify reactive thiols to signal anti-inflammatory and anticarcinogenic properties and have been used in Asian medicine for disease management (132). Bardoxolone methyl is a synthetic triterpenoid, and it has been shown to inhibit proliferation of PASMCs and exhibit anti-inflammatory effects via activation of Keap1/Nrf2 and downregulation of transcription factor NF-κB (132). While no published preclinical studies of bardoxolone methyl could be found at present, a 16-wk treatment with bardoxolone methyl on one or more background therapies was reported to improve 6MWD in an initial analysis of 24 PAH patients enrolled in an ongoing phase 2 LARIAT trial (NCT02036970) (99). Furthermore, a current phase 3 CATALYST trial (NCT02657356) is recruiting PAH patients associated with connective tissue disease to evaluate the effect of bardoxolone methyl. Other electrophilic compounds, such as nitro-fatty acids (NO2-FAs), have also been shown to exert antiproliferative and anti-inflammatory effects via activation of Keap1/Nrf2 signaling pathway and inhibition of NF-κB (29, 131). NO2-FAs also provide beneficial metabolic effects by serving as partial agonists of peroxisome proliferator-activated receptors (114). NO2-FAs have additionally been shown to improve PH in mice exposed to hypoxia or high-fat diet (65, 71) and may now be tested in human PAH.
CCR5/CCL5
The G protein-coupled receptor CCR5 is a major coreceptor for HIV cell entry, and it has been recently linked to the progression of HIV-associated PH. Elevated CCR5 expression was found in lungs from patients with PAH, in mice with hypoxia-induced PH, and in SIV-infected macaques (6). CCR5 deficiency, as well as pharmacological inactivation of CCR5 by maraviroc, decreased the development of hypoxic PH in mice (6). CCR5 is activated by chemokines including CCL3, CCL4, and CCL5 (also known as regulation upon activation, normal T-cell expressed and secreted). Elevated CCL5 mRNA expression was found in PAECs from patients with PAH (33). However, no clinical trial targeting CCR5/CCL5 in PAH has been planned at present.
Dichloroacetate
It is becoming widely recognized that PASMCs in PAH are glycolytic and are subject to the Warburg effect, similar to transformed malignant cells. These cells become proliferative and resistant to apoptosis. A number of new drugs are designed to target metabolic pathways to reverse this process. One proposed check point controlling the entry of pyruvate into Krebs cycle is pyruvate dehydrogenase, which is necessary for efficient glucose oxidative metabolism. This enzyme is inhibited by PDK, which appears to be upregulated in glycolytic smooth muscle cells from patients and pre-clinical models of PAH. Inhibition of PDK with dichloroacetate (DCA) has been shown to prevent and reverse pulmonary vascular remodeling in rats with PH induced by chronic hypoxia and MCT (91, 93). DCA was shown to increase apoptosis and suppress proliferation by depolarizing mitochondria, which causes the release of H2O2 and cytochrome c, leading to the subsequent restoration of expression and function of Kv channels (91, 93). DCA has also been shown to attenuate PAH through inhibition of nuclear factor of the activated T cells, inactivation of hypoxia-inducible factor-1α, as well as upregulation of Cu/Zn SOD activity and mitochondria-dependent apoptosis in experimental PH models (50, 80, 123). A recently completed phase 1 clinical trial by Michelakis et al. (92) evaluated chronic oral DCA administration on background therapy in 20 patients with PAH. While DCA administration led to an improvement in pulmonary artery pressure, PVR, and functional capacity, DCA resistance has been found in patients with sirtuin-3 (SIRT3) and uncoupling protein 2 variants, both of which predict decreased protein activity and have been linked to the development of PAH (92, 101).
Leukotriene B4
Leukotriene B4 (LTB4) is a leukotriene produced from arachidonic acid metabolism via the 5-lipoxygenase pathway. It has been implicated in several inflammatory diseases, including asthma, atherosclerosis, stroke, and, myocardial infarction (55, 56, 63, 119). A recent study by Tian et al. found high levels of leukotriene A4 hydrolase (LTA4H), the biosynthetic enzyme for LTB4, in Sugen/athymic rat model of severe PH and in patients with PAH (127). In this study, LTB4 was found to induce apoptosis in PAECs through inhibition of the endothelial sphingosine kinase-1 (Sphk1)/eNOS pathway, as well as to stimulate proliferation and hypertrophy of PASMCs. Blocking LTB4, either through bestatin-mediated inhibition of LTB4 biosynthesis or through blocking BLT1, a high-affinity receptor for LTB4, reversed established PH in Sugen/athymic and MCT rats (127). LTB4 has been additionally shown to promote PH pathogenesis through promoting proliferation, migration, and differentiation of adventitial fibroblast via upregulation of p38 MAPK and NADPH oxidase 4 (Nox4) signaling pathways (107). Blocking LTB4 synthesis and inhibition of p38 MAPK by bestatin and SB203580, respectively, reduced fibroblast expansion and Nox4 expression in experimental PH (107). Moreover, it was shown that bleomycin treatment leads to LTB4-meidated macrophage influx and PH. Inhibition of LTB4 formation with zileuton (5-lipoxygenase inhibitor) and SC57461A (LTA4H inhibitor) prevented the development of PH in rat pups exposed to bleomycin (36). Currently, the effect of bestatin (ubenimex) is being examined in a phase 2 clinical trial (LIBERTY Study) in patients with PAH (NCT02664558). Furthermore, Bhat et al. (11) demonstrated that administration of the serotonin receptor antagonist RP5063 reduced LTB4 in SuHx rat model of PAH, suggesting a cross talk between the leukotriene and serotonin pathway in the regulation of PH. Preparations for a phase 2 clinical trial of RP5063 by Reviva Pharmaceuticals for patients with PAH are currently underway.
Mammalian Target of Rapamycin
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase that is involved in the regulation of cell growth, proliferation, and survival. Krymskaya et al. (72) have recently demonstrated that upregulation of mTOR activity and activation of both complexes, the rapamycin-sensitive mTOR complex 1 (mTORC1; mTOR-raptor), which supports cell growth, and the rapamycin-insensitive mTORC2 (mTOR-rictor), which promotes cell survival, are required for the proliferation of PASMCs under chronic hypoxia. Additional studies from the same group further demonstrated that hypoxia-induced activation of NADPH oxidase (Nox4) upregulates mTORC2, which facilitates mTORC1 activation and PASMC proliferation via downregulation of the energy sensor AMPK (47). Treatment with the mTOR kinase inhibitor PP242 reversed pulmonary vascular remodeling in hypoxia-exposed rats and improved RV structure and function in SuHx rats (47, 104). Additionally, attenuation of hypoxia-induced PH has been reported in Akt1-deficient mice, in smooth muscle-specific mTOR conditional and inducible KO mice, and in transgenic mice of phosphatase and tensin homologue deleted on chromosome 10 (PTEN), which is a repressor of Akt activity, underlining the importance of PTEN/Akt1/mTOR signaling pathway in PH (125). Furthermore, molecular and pharmacology-based analyses have uncovered a HIPPO/mTOR pathway in PH as inactivation of HIPPO central component large tumor suppressor 1 (LATS1) and consequent upregulation of its reciprocal effector Yes-associated protein (Yap) are required for AKT/mTOR-mediated proliferation and survival of PASMCs (73). Other work has shown that elevated circulating levels of aldosterone observed in patients of PAH are associated with mTORC1 activation and the subsequent survival and proliferation of PASMCs (1, 89). Combination treatment with Raptor-small interfering RNA and the pharmacological inhibitor spironolactone prevented and reversed MCT and SuHx models of PAH (1). A recent pilot study reported that the mTOR inhibitor, everolimus, improved PVR and 6MWD in 8 out of 10 patients with PAH or CTEPH (115). ABI-009, an albumin-bound mTOR inhibitor, is now being evaluated in patients with severe PAH (NCT02587325).
Peroxisome Proliferator-Activated Receptor-γ
Peroxisome proliferator-activated receptor-γ (PPARγ) is a member of nuclear hormone receptor superfamily and a transcription factor that plays a major beneficial role in cardiovascular homeostasis and glucose metabolism (8, 103). It is highly expressed in endothelial and smooth muscle cells in the lung. Decreased expression levels of PPARγ have been shown in lungs from SuHx- and hypoxia-exposed rats, as well as in lungs from patients with severe PAH and patients with chronic obstructive pulmonary disease (5, 66). Loss of PPARγ in PAECs was reported to reduce PAEC function and survival via decreased apelin expression (2). Mice with targeted deletion of PPARγ in endothelial cells were also found to suffer persisted PH compared with wild-type mice exposed to hypoxia (49). Additionally, Hansmann et al. (52) have shown that vasoprotective PPARγ acts downstream of BMPR2 and that mice with targeted deletion of PPARγ in arterial smooth muscle cells spontaneously developed PAH.
PPARγ agonist rosiglitazone has recently been shown to attenuate hypoxia-induced PH in mice and rats (66, 85, 96). Rosiglitazone was found to decrease ET-1-induced pulmonary vasoconstriction in hypoxic rats (85). Most recently, treatment with rosiglitazone was also reported to reversed PAH through inhibition of TGFβ1-Stat3-FoxO1 pathways in TGFβ1-overexpreesing mice (19). Furthermore, PPARγ activation by rosiglitazone was reported to reverse insulin resistance-associated PH in apolipoprotein E KO mice on a high-fat diet (52). No plans for clinical trials of rosiglitazone for PAH have been reported to date.
FUTURE PERSPECTIVES
In addition to emerging opportunities discussed above, epigenetics is a rapidly expanding filed in PAH. Recently, hypermethylation of BMPR2 promoter in patients with HPAH was discovered, and it was found to inhibit BMPR2 expression (83). Histone deacetylation is linked to downregulation of SOD in PH (67, 97). Several studies have demonstrated that the use of histone deacetylase (HDAC) inhibitors improves established PAH in experimental models (15, 21, 23, 26, 76, 139); however, controversial results were reported as trichostatin A, a pan-HDAC inhibitor, was found ineffective in rats with MCT-, SuHx-, and pulmonary artery banding-induced PAH (31). Moreover, therapies targeting vascular proliferation with the antineoplastic receptor tyrosine kinase inhibitors have been explored. While a multicenter phase 3 trial of the tyrosine kinase inhibitor imatinib demonstrated improvement in 6MWD and PVR, several side effects were observed and the treatment was discontinued in one out of three of the patients. This led the authors to conclude that the use of this therapy should be discouraged, as the side effects outweigh the benefit (39, 57). Lastly, Sphk1 (24), angiotensin-converting enzyme 2 (30, 38, 70, 82), SIRT3 (75, 101), gremlin 1 (Grem1) (18, 28, 40, 46, 133), as well as miRNAs [reviewed in depth by Chun et al. (27)] may present as potential therapeutic targets for PH management in the future.
SUMMARY
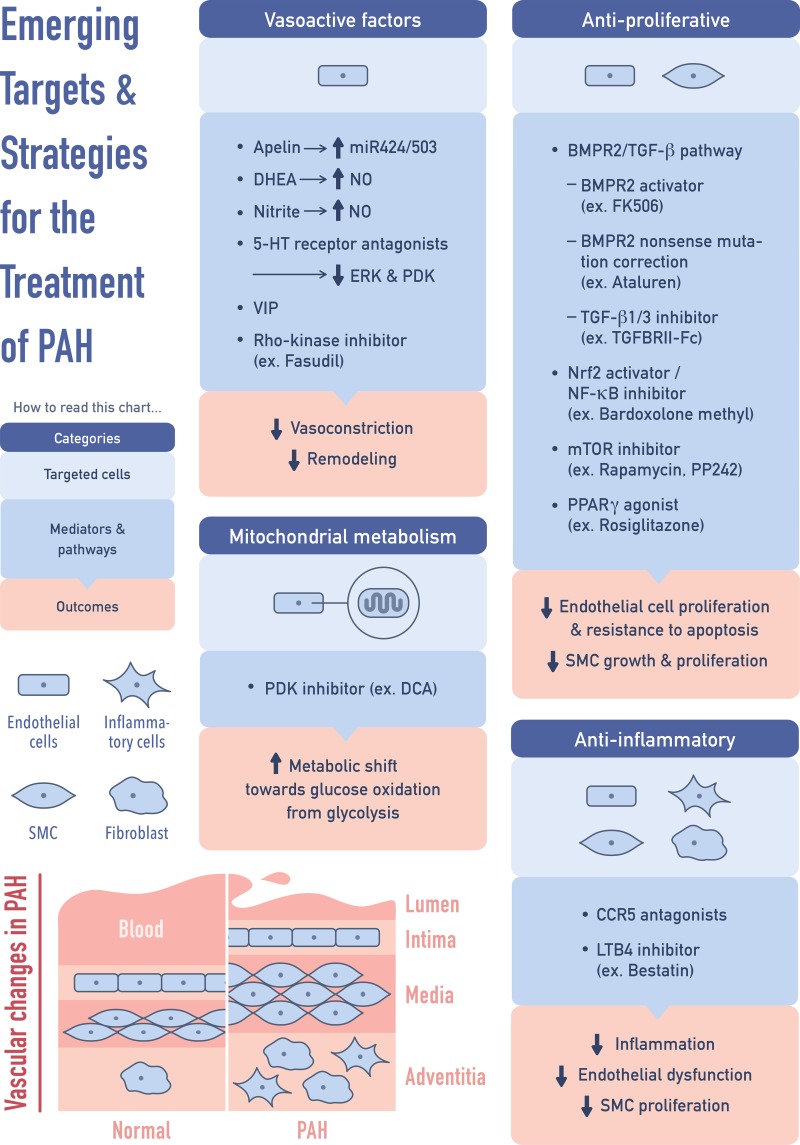
Apart from the current FDA-approved therapies, recent studies have advanced our knowledge and understanding of how inflammation, mitochondrial dysfunction, proliferation, and vasodilation are integrated into the modulation of PAH (Fig. 3). It is noteworthy that some of the new approaches/targets of PAH, such as the use of DHEA, inhaled nitrite, inhalation of VIP, and PPARγ agonists, may provide new insights in the treatment of other forms of PH. Substantial progresses in the understanding of roles of epigenetic modifications and miRNAs in PH also provide potential translational options for the treatment of PH in the future. Finally, as genders and individuals with phenotypic variations are likely to have different response to drug treatments, it is proposed that genotyping with precision medicine may be considered in future studies to maximize beneficial response to specific treatments and to provide significant positive impact on improving outcomes in patients with PH.
Fig. 3.
Emerging targets and therapies for PAH. TGF-β, transforming growth factor-β; SMC, smooth muscle cells; LTB4, leukotriene B4; mTOR, mammalian target of rapamycin.
GRANTS
M. T. Gladwin receives research support from National Heart, Lung, and Blood Institute Grants 2R01-HL-098032, 1R01-HL-125886-01, P01-HL103455, T32-HL-110849, and T32-HL-007563 and Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania. Y. C. Lai receives support from American Heart Association Grant 17SDG33400233.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.K.H., M.T.G., and Y.-C.L. drafted manuscript; M.K.H., A.L., M.T.G., and Y.-C.L. edited and revised manuscript; M.K.H., M.T.G., and Y.-C.L. approved final version of manuscript; Y.-C.L. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. Sergei Snovida for helpful comments on the manuscript and Elfy Chiang for assistance and production of the figures.
REFERENCES
- 1.Aghamohammadzadeh R, Zhang YY, Stephens TE, Arons E, Zaman P, Polach KJ, Matar M, Yung LM, Yu PB, Bowman FP, Opotowsky AR, Waxman AB, Loscalzo J, Leopold JA, Maron BA. Up-regulation of the mammalian target of rapamycin complex 1 subunit Raptor by aldosterone induces abnormal pulmonary artery smooth muscle cell survival patterns to promote pulmonary arterial hypertension. FASEB J 30: 2511–2527, 2016. doi: 10.1096/fj.201500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alastalo TP, Li M, Perez VJ, Pham D, Sawada H, Wang JK, Koskenvuo M, Wang L, Freeman BA, Chang HY, Rabinovitch M. Disruption of PPARγ/β-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J Clin Invest 121: 3735–3746, 2011. doi: 10.1172/JCI43382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldred MA, Vijayakrishnan J, James V, Soubrier F, Gomez-Sanchez MA, Martensson G, Galie N, Manes A, Corris P, Simonneau G, Humbert M, Morrell NW, Trembath RC. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum Mutat 27: 212–213, 2006. doi: 10.1002/humu.9398. [DOI] [PubMed] [Google Scholar]
- 4.Alzoubi A, Toba M, Abe K, O’Neill KD, Rocic P, Fagan KA, McMurtry IF, Oka M. Dehydroepiandrosterone restores right ventricular structure and function in rats with severe pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 304: H1708–H1718, 2013. doi: 10.1152/ajpheart.00746.2012. [DOI] [PubMed] [Google Scholar]
- 5.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 92: 1162–1169, 2003. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 6.Amsellem V, Lipskaia L, Abid S, Poupel L, Houssaini A, Quarck R, Marcos E, Mouraret N, Parpaleix A, Bobe R, Gary-Bobo G, Saker M, Dubois-Randé JL, Gladwin MT, Norris KA, Delcroix M, Combadière C, Adnot S. CCR5 as a treatment target in pulmonary arterial hypertension. Circulation 130: 880–891, 2014. doi: 10.1161/CIRCULATIONAHA.114.010757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badlam JB, Bull TM. Steps forward in the treatment of pulmonary arterial hypertension: latest developments and clinical opportunities. Ther Adv Chronic Dis 8: 47–64, 2017. doi: 10.1177/2040622317693218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks AS, McAllister FE, Camporez JP, Zushin PJ, Jurczak MJ, Laznik-Bogoslavski D, Shulman GI, Gygi SP, Spiegelman BM. An ERK/Cdk5 axis controls the diabetogenic actions of PPARγ. Nature 517: 391–395, 2015. doi: 10.1038/nature13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bei Y, Hua-Huy T, Duong-Quy S, Nguyen VH, Chen W, Nicco C, Batteux F, Dinh-Xuan AT. Long-term treatment with fasudil improves bleomycin-induced pulmonary fibrosis and pulmonary hypertension via inhibition of Smad2/3 phosphorylation. Pulm Pharmacol Ther 26: 635–643, 2013. doi: 10.1016/j.pupt.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Bhat L, Hawkinson J, Cantillon M, Reddy DG, Bhat SR, Laurent CE, Bouchard A, Biernat M, Salvail D. RP5063, a novel, multimodal, serotonin receptor modulator, prevents monocrotaline-induced pulmonary arterial hypertension in rats. Eur J Pharmacol 810: 92–99, 2017. doi: 10.1016/j.ejphar.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 11.Bhat L, Hawkinson J, Cantillon M, Reddy DG, Bhat SR, Laurent CE, Bouchard A, Biernat M, Salvail D. RP5063, a novel, multimodal, serotonin receptor modulator, prevents Sugen 5416-hypoxia-induced pulmonary arterial hypertension in rats. Eur J Pharmacol 810: 83–91, 2017. doi: 10.1016/j.ejphar.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 100: 9488–9493, 2003. doi: 10.1073/pnas.1633724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 66: 1672–1682, 2015. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 14.Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res 119: 880–886, 2016. doi: 10.1161/CIRCRESAHA.116.309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boucherat O, Chabot S, Paulin R, Trinh I, Bourgeois A, Potus F, Lampron MC, Lambert C, Breuils-Bonnet S, Nadeau V, Paradis R, Goncharova EA, Provencher S, Bonnet S. HDAC6: a novel histone deacetylase implicated in pulmonary arterial hypertension. Sci Rep 7: 4546, 2017. doi: 10.1038/s41598-017-04874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brash L, Barnes G, Brewis M, Church C, Gibbs S, Howard L, Johnson M, McGlinchey N, Simpson J, Stirrat C, Thomson S, Watson G, Welsh D, Wilkins M, Newby D, Peacock A. Apelin improves cardiac output in patients with pulmonary arterial hypertension. Eur Respir J 46: PA2107, 2015. [Google Scholar]
- 17.Breitling S, Ravindran K, Goldenberg NM, Kuebler WM. The pathophysiology of pulmonary hypertension in left heart disease. Am J Physiol Lung Cell Mol Physiol 309: L924–L941, 2015. doi: 10.1152/ajplung.00146.2015. [DOI] [PubMed] [Google Scholar]
- 18.Cahill E, Costello CM, Rowan SC, Harkin S, Howell K, Leonard MO, Southwood M, Cummins EP, Fitzpatrick SF, Taylor CT, Morrell NW, Martin F, McLoughlin P. Gremlin plays a key role in the pathogenesis of pulmonary hypertension. Circulation 125: 920–930, 2012. doi: 10.1161/CIRCULATIONAHA.111.038125. [DOI] [PubMed] [Google Scholar]
- 19.Calvier L, Chouvarine P, Legchenko E, Hoffmann N, Geldner J, Borchert P, Jonigk D, Mozes MM, Hansmann G. PPARγ links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab 25: 1118–1134.e7, 2017. doi: 10.1016/j.cmet.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Casey DB, Badejo AM Jr, Dhaliwal JS, Murthy SN, Hyman AL, Nossaman BD, Kadowitz PJ. Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol-sensitive mechanism in the rat. Am J Physiol Heart Circ Physiol 296: H524–H533, 2009. doi: 10.1152/ajpheart.00543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavasin MA, Demos-Davies K, Horn TR, Walker LA, Lemon DD, Birdsey N, Weiser-Evans MC, Harral J, Irwin DC, Anwar A, Yeager ME, Li M, Watson PA, Nemenoff RA, Buttrick PM, Stenmark KR, McKinsey TA. Selective class I histone deacetylase inhibition suppresses hypoxia-induced cardiopulmonary remodeling through an antiproliferative mechanism. Circ Res 110: 739–748, 2012. doi: 10.1161/CIRCRESAHA.111.258426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheli M, Vachiery JL. Controversies in pulmonary hypertension due to left heart disease. F1000Prime Rep 7: 07, 2015. doi: 10.12703/P7-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F, Li X, Aquadro E, Haigh S, Zhou J, Stepp DW, Weintraub NL, Barman SA, Fulton DJ. Inhibition of histone deacetylase reduces transcription of NADPH oxidases and ROS production and ameliorates pulmonary arterial hypertension. Free Radic Biol Med 99: 167–178, 2016. doi: 10.1016/j.freeradbiomed.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, Sammani S, Zhou G, Raj JU, Garcia JG, Berdyshev E, Yuan JX, Natarajan V, Machado RF. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med 190: 1032–1043, 2014. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chettimada S, Gupte R, Rawat D, Gebb SA, McMurtry IF, Gupte SA. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: implication in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L287–L300, 2015. doi: 10.1152/ajplung.00229.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho YK, Eom GH, Kee HJ, Kim HS, Choi WY, Nam KI, Ma JS, Kook H. Sodium valproate, a histone deacetylase inhibitor, but not captopril, prevents right ventricular hypertrophy in rats. Circ J 74: 760–770, 2010. doi: 10.1253/circj.CJ-09-0580. [DOI] [PubMed] [Google Scholar]
- 27.Chun HJ, Bonnet S, Chan SY. Translational advances in the field of pulmonary hypertension. translating MicroRNA biology in pulmonary hypertension. it will take more than “miR” words. Am J Respir Crit Care Med 195: 167–178, 2017. doi: 10.1164/rccm.201604-0886PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciuclan L, Sheppard K, Dong L, Sutton D, Duggan N, Hussey M, Simmons J, Morrell NW, Jarai G, Edwards M, Dubois G, Thomas M, Van Heeke G, England K. Treatment with anti-gremlin 1 antibody ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension in mice. Am J Pathol 183: 1461–1473, 2013. doi: 10.1016/j.ajpath.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J Biol Chem 281: 35686–35698, 2006. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai H, Gong Y, Xiao Z, Guang X, Yin X. Decreased levels of serum angiotensin-(1-7) in patients with pulmonary arterial hypertension due to congenital heart disease. Int J Cardiol 176: 1399–1401, 2014. doi: 10.1016/j.ijcard.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 31.De Raaf MA, Hussaini AA, Gomez-Arroyo J, Kraskaukas D, Farkas D, Happé C, Voelkel NF, Bogaard HJ. Histone deacetylase inhibition with trichostatin A does not reverse severe angioproliferative pulmonary hypertension in rats (2013 Grover Conference series). Pulm Circ 4: 237–243, 2014. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Raaf MA, Kroeze Y, Middelman A, de Man FS, de Jong H, Vonk-Noordegraaf A, de Korte C, Voelkel NF, Homberg J, Bogaard HJ. Serotonin transporter is not required for the development of severe pulmonary hypertension in the Sugen hypoxia rat model. Am J Physiol Lung Cell Mol Physiol 309: L1164–L1173, 2015. doi: 10.1152/ajplung.00127.2015. [DOI] [PubMed] [Google Scholar]
- 33.Dorfmüller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, Capron F, Coulomb-Lherminé A, Marfaing-Koka A, Simonneau G, Emilie D, Humbert M. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 165: 534–539, 2002. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 34.Drake KM, Dunmore BJ, McNelly LN, Morrell NW, Aldred MA. Correction of nonsense BMPR2 and SMAD9 mutations by ataluren in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 49: 403–409, 2013. doi: 10.1165/rcmb.2013-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumas de La Roque E, Savineau JP, Metivier AC, Billes MA, Kraemer JP, Doutreleau S, Jougon J, Marthan R, Moore N, Fayon M, Baulieu EE, Dromer C. Dehydroepiandrosterone (DHEA) improves pulmonary hypertension in chronic obstructive pulmonary disease (COPD): a pilot study. Ann Endocrinol (Paris) 73: 20–25, 2012. doi: 10.1016/j.ando.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Ee MT, Kantores C, Ivanovska J, Wong MJ, Jain A, Jankov RP. Leukotriene B4 mediates macrophage influx and pulmonary hypertension in bleomycin-induced chronic neonatal lung injury. Am J Physiol Lung Cell Mol Physiol 311: L292–L302, 2016. doi: 10.1152/ajplung.00120.2016. [DOI] [PubMed] [Google Scholar]
- 37.Falcão-Pires I, Gonçalves N, Henriques-Coelho T, Moreira-Gonçalves D, Roncon-Albuquerque R Jr, Leite-Moreira AF. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 296: H2007–H2014, 2009. doi: 10.1152/ajpheart.00089.2009. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med 179: 1048–1054, 2009. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost AE, Barst RJ, Hoeper MM, Chang HJ, Frantz RP, Fukumoto Y, Galié N, Hassoun PM, Klose H, Matsubara H, Morrell NW, Peacock AJ, Pfeifer M, Simonneau G, Tapson VF, Torres F, Dario Vizza C, Lawrence D, Yang W, Felser JM, Quinn DA, Ghofrani HA. Long-term safety and efficacy of imatinib in pulmonary arterial hypertension. J Heart Lung Transplant 34: 1366–1375, 2015. doi: 10.1016/j.healun.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Frump AL, Albrecht ME, McClintick JN, Lahm T. Estrogen receptor-dependent attenuation of hypoxia-induced changes in the lung genome of pulmonary hypertension rats. Pulm Circ 7: 232–243, 2017. doi: 10.1177/2045893217702055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita H, Fukumoto Y, Saji K, Sugimura K, Demachi J, Nawata J, Shimokawa H. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels 25: 144–149, 2010. doi: 10.1007/s00380-009-1176-8. [DOI] [PubMed] [Google Scholar]
- 42.Fukumoto Y, Yamada N, Matsubara H, Mizoguchi M, Uchino K, Yao A, Kihara Y, Kawano M, Watanabe H, Takeda Y, Adachi T, Osanai S, Tanabe N, Inoue T, Kubo A, Ota Y, Fukuda K, Nakano T, Shimokawa H. Double-blind, placebo-controlled clinical trial with a rho-kinase inhibitor in pulmonary arterial hypertension. Circ J 77: 2619–2625, 2013. doi: 10.1253/circj.CJ-13-0443. [DOI] [PubMed] [Google Scholar]
- 43.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C; CHEST-1 Study Group . Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 369: 319–329, 2013. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 44.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ; PATENT-1 Study Group . Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 369: 330–340, 2013. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 45.Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 360: 895–900, 2002. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 46.Ghouleh IA, Sahoo S, Meijles DN, Amaral JH, de Jesus DS, Sembrat J, Rojas M, Goncharov DA, Goncharova EA, Pagano PJ. Endothelial Nox1 oxidase assembly in human pulmonary arterial hypertension; driver of Gremlin1-mediated proliferation. Clin Sci (Lond) 131: 2019–2035, 2017. doi: 10.1042/CS20160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 129: 864–874, 2014. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 124: 164–174, 2011. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 49.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-gamma in mice causes PDGF receptor-beta-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol 297: L1082–L1090, 2009. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guignabert C, Tu L, Izikki M, Dewachter L, Zadigue P, Humbert M, Adnot S, Fadel E, Eddahibi S. Dichloroacetate treatment partially regresses established pulmonary hypertension in mice with SM22alpha-targeted overexpression of the serotonin transporter. FASEB J 23: 4135–4147, 2009. doi: 10.1096/fj.09-131664. [DOI] [PubMed] [Google Scholar]
- 51.Gupta N, Rashid J, Nozik-Grayck E, McMurtry IF, Stenmark KR, Ahsan F. Cocktail of superoxide dismutase and fasudil encapsulated in targeted liposomes slows PAH progression at a reduced dosing frequency. Mol Pharm 14: 830–841, 2017. doi: 10.1021/acs.molpharmaceut.6b01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 115: 1275–1284, 2007. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 53.Hatano S, Strasser T. Primary Pulmonary Hypertension: Report on a WHO Meeting. Geneva, Switzerland, 15–17 October 1973. [Google Scholar]
- 54.Helenius MH, Vattulainen S, Orcholski M, Aho J, Komulainen A, Taimen P, Wang L, de Jesus Perez VA, Koskenvuo JW, Alastalo TP. Suppression of endothelial CD39/ENTPD1 is associated with pulmonary vascular remodeling in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 308: L1046–L1057, 2015. doi: 10.1152/ajplung.00340.2014. [DOI] [PubMed] [Google Scholar]
- 55.Helgadottir A, Manolescu A, Helgason A, Thorleifsson G, Thorsteinsdottir U, Gudbjartsson DF, Gretarsdottir S, Magnusson KP, Gudmundsson G, Hicks A, Jonsson T, Grant SFA, Sainz J, O’Brien SJ, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Levey AI, Abramson JL, Reilly MP, Vaccarino V, Wolfe ML, Gudnason V, Quyyumi AA, Topol EJ, Rader DJ, Thorgeirsson G, Gulcher JR, Hakonarson H, Kong A, Stefansson K. A variant of the gene encoding leukotriene A4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nat Genet 38: 68–74, 2006. doi: 10.1038/ng1692. [DOI] [PubMed] [Google Scholar]
- 56.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet 36: 233–239, 2004. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 57.Hoeper MM, Barst RJ, Bourge RC, Feldman J, Frost AE, Galié N, Gómez-Sánchez MA, Grimminger F, Grünig E, Hassoun PM, Morrell NW, Peacock AJ, Satoh T, Simonneau G, Tapson VF, Torres F, Lawrence D, Quinn DA, Ghofrani HA. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation 127: 1128–1138, 2013. doi: 10.1161/CIRCULATIONAHA.112.000765. [DOI] [PubMed] [Google Scholar]
- 58.Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, Jing ZC, Gibbs JS. A global view of pulmonary hypertension. Lancet Respir Med 4: 306–322, 2016. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 59.Homma N, Nagaoka T, Karoor V, Imamura M, Taraseviciene-Stewart L, Walker LA, Fagan KA, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone. Am J Physiol Lung Cell Mol Physiol 295: L71–L78, 2008. doi: 10.1152/ajplung.90251.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 118: 722–730, 2008. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Humbert M, Lau EM, Montani D, Jaïs X, Sitbon O, Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 130: 2189–2208, 2014. doi: 10.1161/CIRCULATIONAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- 62.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med 10: 1122–1127, 2004. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 63.Israel E, Rubin P, Kemp JP, Grossman J, Pierson W, Siegel SC, Tinkelman D, Murray JJ, Busse W, Segal AT, Fish J, Kaiser HB, Ledford D, Wenzel S, Rosenthal R, Cohn J, Lanni C, Pearlman H, Karahalios P, Drazen JM. The effect of inhibition of 5-lipoxygenase by zileuton in mild-to-moderate asthma. Ann Intern Med 119: 1059–1066, 1993. doi: 10.7326/0003-4819-119-11-199312010-00001. [DOI] [PubMed] [Google Scholar]
- 64.Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O, Fujino M. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta 1538: 162–171, 2001. doi: 10.1016/S0167-4889(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 65.Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St Croix CM, Watkins SC, Gor S, Cantu-Medellin N, Weidert ER, Frisbee JC, Gladwin MT, Champion HC, Freeman BA, Khoo NK. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res 101: 352–363, 2014. doi: 10.1093/cvr/cvt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology 15: 659–668, 2010. doi: 10.1111/j.1440-1843.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 67.Kim GH, Ryan JJ, Marsboom G, Archer SL. Epigenetic mechanisms of pulmonary hypertension. Pulm Circ 1: 347–356, 2011. doi: 10.4103/2045-8932.87300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J. Apelin-APJ signaling: a potential therapeutic target for pulmonary arterial hypertension. Mol Cells 37: 196–201, 2014. doi: 10.14348/molcells.2014.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, Erzurum SC, Chun HJ. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med 19: 74–82, 2013. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kleinsasser A, Pircher I, Treml B, Schwienbacher M, Schuster M, Janzek E, Loibner H, Penninger JM, Loeckinger A. Recombinant angiotensin-converting enzyme 2 suppresses pulmonary vasoconstriction in acute hypoxia. Wilderness Environ Med 23: 24–30, 2012. doi: 10.1016/j.wem.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Klinke A, Möller A, Pekarova M, Ravekes T, Friedrichs K, Berlin M, Scheu KM, Kubala L, Kolarova H, Ambrozova G, Schermuly RT, Woodcock SR, Freeman BA, Rosenkranz S, Baldus S, Rudolph V, Rudolph TK. Protective effects of 10-nitro-oleic acid in a hypoxia-induced murine model of pulmonary hypertension. Am J Respir Cell Mol Biol 51: 155–162, 2014. doi: 10.1165/rcmb.2013-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krymskaya VP, Snow J, Cesarone G, Khavin I, Goncharov DA, Lim PN, Veasey SC, Ihida-Stansbury K, Jones PL, Goncharova EA. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J 25: 1922–1933, 2011. doi: 10.1096/fj.10-175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kudryashova TV, Goncharov DA, Pena A, Kelly N, Vanderpool R, Baust J, Kobir A, Shufesky W, Mora AL, Morelli AE, Zhao J, Ihida-Stansbury K, Chang B, DeLisser H, Tuder RM, Kawut SM, Silljé HH, Shapiro S, Zhao Y, Goncharova EA. HIPPO-integrin-linked kinase cross-talk controls self-sustaining proliferation and survival in pulmonary hypertension. Am J Respir Crit Care Med 194: 866–877, 2016. doi: 10.1164/rccm.201510-2003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 115: 115–130, 2014. doi: 10.1161/CIRCRESAHA.115.301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, St Croix CM, Garcia-Ocaña A, Goncharova EA, Tofovic SP, Mora AL, Gladwin MT. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation 133: 717–731, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lan B, Hayama E, Kawaguchi N, Furutani Y, Nakanishi T. Therapeutic efficacy of valproic acid in a combined monocrotaline and chronic hypoxia rat model of severe pulmonary hypertension. PLoS One 10: e0117211, 2015. doi: 10.1371/journal.pone.0117211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leuchte HH, Baezner C, Baumgartner RA, Bevec D, Bacher G, Neurohr C, Behr J. Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur Respir J 32: 1289–1294, 2008. doi: 10.1183/09031936.00050008. [DOI] [PubMed] [Google Scholar]
- 78.Leuchte HH, Prechtl C, Callegari J, Meis T, Haziraj S, Bevec D, Behr J. Augmentation of the effects of vasoactive intestinal peptide aerosol on pulmonary hypertension via coapplication of a neutral endopeptidase 24.11 inhibitor. Am J Physiol Lung Cell Mol Physiol 308: L563–L568, 2015. doi: 10.1152/ajplung.00317.2014. [DOI] [PubMed] [Google Scholar]
- 79.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 116: 1555–1562, 2007. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 80.Li B, Yan J, Shen Y, Liu Y, Ma Z. Dichloroacetate prevents but not reverses the formation of neointimal lesions in a rat model of severe pulmonary arterial hypertension. Mol Med Rep 10: 2144–2152, 2014. doi: 10.3892/mmr.2014.2432. [DOI] [PubMed] [Google Scholar]
- 81.Li F, Xia W, Yuan S, Sun R. Acute inhibition of Rho-kinase attenuates pulmonary hypertension in patients with congenital heart disease. Pediatr Cardiol 30: 363–366, 2009. doi: 10.1007/s00246-008-9315-z. [DOI] [PubMed] [Google Scholar]
- 82.Li G, Liu Y, Zhu Y, Liu A, Xu Y, Li X, Li Z, Su J, Sun L. ACE2 activation confers endothelial protection and attenuates neointimal lesions in prevention of severe pulmonary arterial hypertension in rats. Lung 191: 327–336, 2013. doi: 10.1007/s00408-013-9470-8. [DOI] [PubMed] [Google Scholar]
- 83.Liu D, Yan Y, Chen JW, Yuan P, Wang XJ, Jiang R, Wang L, Zhao QH, Wu WH, Simonneau G, Qu JM, Jing ZC. Hypermethylation of BMPR2 promoter occurs in patients with heritable pulmonary arterial hypertension and inhibits BMPR2 expression. Am J Respir Crit Care Med 196: 925–928, 2017. doi: 10.1164/rccm.201611-2273LE. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Tian HY, Yan XL, Fan FL, Wang WP, Han JL, Zhang JB, Ma Q, Meng Y, Wei F. Serotonin inhibits apoptosis of pulmonary artery smooth muscle cell by pERK1/2 and PDK through 5-HT1B receptors and 5-HT transporters. Cardiovasc Pathol 22: 451–457, 2013. doi: 10.1016/j.carpath.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, Tian XY, Huang Y, Wang N. Rosiglitazone attenuated endothelin-1-induced vasoconstriction of pulmonary arteries in the rat model of pulmonary arterial hypertension via differential regulation of ET-1 receptors. PPAR Res 2014: 374075, 2014. doi: 10.1155/2014/374075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Llobet-Navas D, Rodriguez-Barrueco R, de la Iglesia-Vicente J, Olivan M, Castro V, Saucedo-Cuevas L, Marshall N, Putcha P, Castillo-Martin M, Bardot E, Ezhkova E, Iavarone A, Cordon-Cardo C, Silva JM. The microRNA 424/503 cluster reduces CDC25A expression during cell cycle arrest imposed by transforming growth factor β in mammary epithelial cells. Mol Cell Biol 34: 4216–4231, 2014. doi: 10.1128/MCB.00611-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, Moore SD, Drake KM, Aldred MA, Yu PB, Upton PD, Morrell NW. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 21: 777–785, 2015. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 89.Maron BA, Opotowsky AR, Landzberg MJ, Loscalzo J, Waxman AB, Leopold JA. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: a pilot study. Eur J Heart Fail 15: 277–283, 2013. doi: 10.1093/eurjhf/hfs173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension 38: 1307–1310, 2001. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 91.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 95: 830–840, 2004. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 92.Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, Cupitt J, Paterson I, Thompson RB, Chow K, O’Regan DP, Zhao L, Wharton J, Kiely DG, Kinnaird A, Boukouris AE, White C, Nagendran J, Freed DH, Wort SJ, Gibbs JS, Wilkins MR. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med 9: 9, 2017. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 93.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 105: 244–250, 2002. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 94.Morecroft I, Loughlin L, Nilsen M, Colston J, Dempsie Y, Sheward J, Harmar A, MacLean MR. Functional interactions between 5-hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther 313: 539–548, 2005. doi: 10.1124/jpet.104.081182. [DOI] [PubMed] [Google Scholar]
- 95.Nickel NP, Spiekerkoetter E, Gu M, Li CG, Li H, Kaschwich M, Diebold I, Hennigs JK, Kim KY, Miyagawa K, Wang L, Cao A, Sa S, Jiang X, Stockstill RW, Nicolls MR, Zamanian RT, Bland RD, Rabinovitch M. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med 191: 1273–1286, 2015. doi: 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nozik-Grayck E, Woods C, Stearman RS, Venkataraman S, Ferguson BS, Swain K, Bowler RP, Geraci MW, Ihida-Stansbury K, Stenmark KR, McKinsey TA, Domann FE. Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 311: L124–L134, 2016. doi: 10.1152/ajplung.00263.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Odagiri K, Watanabe H. Effects of the Rho-kinase inhibitor, fasudil, on pulmonary hypertension. Circ J 79: 1213–1214, 2015. doi: 10.1253/circj.CJ-15-0443. [DOI] [PubMed] [Google Scholar]
- 99.Oudiz R, Meyer C, Chin M, Feldman J, Goldsberry A, Mc Connell J, McCullough P, O’Grady M, Tapson V, Torres F, Waxman A, White R. Initial data report from “LARIAT”; a phase 2 study of bardoxolone methyl in PAH patients on stable background therapy. Chest 148: 639A, 2015. doi: 10.1378/chest.2345856. [DOI] [Google Scholar]
- 100.Patel D, Kandhi S, Kelly M, Neo BH, Wolin MS. Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α. Am J Physiol Lung Cell Mol Physiol 306: L383–L391, 2014. doi: 10.1152/ajplung.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, Bowers L, Haromy A, Webster L, Provencher S, Bonnet S, Michelakis ED. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab 20: 827–839, 2014. doi: 10.1016/j.cmet.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 301: H1798–H1809, 2011. doi: 10.1152/ajpheart.00654.2011. [DOI] [PubMed] [Google Scholar]
- 103.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keen HL, Weatherford ET, Faraci FM, Sigmund CD. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARγ and RhoA/Rho-kinase. Cell Metab 16: 462–472, 2012. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pena A, Kobir A, Goncharov D, Goda A, Kudryashova TV, Ray A, Vanderpool R, Baust J, Chang B, Mora AL, Gorcsan J 3rd, Goncharova EA. Pharmacological inhibition of mtor kinase reverses right ventricle remodeling and improves right ventricle structure and function in rats. Am J Respir Cell Mol Biol 57: 615–625, 2017. doi: 10.1165/rcmb.2016-0364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Petkov V, Mosgoeller W, Ziesche R, Raderer M, Stiebellehner L, Vonbank K, Funk GC, Hamilton G, Novotny C, Burian B, Block LH. Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J Clin Invest 111: 1339–1346, 2003. doi: 10.1172/JCI17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension. from cancer biology to new pulmonary arterial hypertension therapeutics. targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med 195: 425–437, 2017. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qian J, Tian W, Jiang X, Tamosiuniene R, Sung YK, Shuffle EM, Tu AB, Valenzuela A, Jiang S, Zamanian RT, Fiorentino DF, Voelkel NF, Peters-Golden M, Stenmark KR, Chung L, Rabinovitch M, Nicolls MR. Leukotriene B4 activates pulmonary artery adventitial fibroblasts in pulmonary hypertension. Hypertension 66: 1227–1239, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313, 2012. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reddy YN, Lewis GD, Shah SJ, LeWinter M, Semigran M, Davila-Roman VG, Anstrom K, Hernandez A, Braunwald E, Redfield MM, Borlaug BA. INDIE-HFpEF (inorganic nitrite delivery to improve exercise capacity in heart failure with preserved ejection fraction): rationale and design. Circ Heart Fail 10: 10, 2017. doi: 10.1161/CIRCHEARTFAILURE.117.003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R; RELAX Trial . Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309: 1268–1277, 2013. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rix PJ, Vick A, Attkins NJ, Barker GE, Bott AW, Alcorn H Jr, Gladwin MT, Shiva S, Bradley S, Hussaini A, Hoye WL, Parsley EL, Masamune H. Pharmacokinetics, pharmacodynamics, safety, and tolerability of nebulized sodium nitrite (AIR001) following repeat-dose inhalation in healthy subjects. Clin Pharmacokinet 54: 261–272, 2015. doi: 10.1007/s40262-014-0201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 115: 1260–1268, 2007. [DOI] [PubMed] [Google Scholar]
- 113.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N, Grimminger F, Seeger W, Schermuly RT, Pullamsetti SS. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med 20: 1289–1300, 2014. doi: 10.1038/nm.3695. [DOI] [PubMed] [Google Scholar]
- 114.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci USA 102: 2340–2345, 2005. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seyfarth HJ, Hammerschmidt S, Halank M, Neuhaus P, Wirtz HR. Everolimus in patients with severe pulmonary hypertension: a safety and efficacy pilot trial. Pulm Circ 3: 632–638, 2013. doi: 10.1086/674311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J Cardiovasc Pharmacol 39: 319–327, 2002. doi: 10.1097/00005344-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Simon MA, Vanderpool RR, Nouraie M, Bachman TN, White PM, Sugahara M, Gorcsan J 3rd, Parsley EL, Gladwin MT. Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI Insight 1: e89620, 2016. doi: 10.1172/jci.insight.89620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D34–D41, 2013. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 119.Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, Moos MP, Kaiser B, Cohnert TU, Wahlers T, Zieske A, Plenz G, Robenek H, Salbach P, Kuhn H, Radmark O, Samuelsson B, Habenicht AJ. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci USA 100: 1238–1243, 2003. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spiekerkoetter E, Sung YK, Sudheendra D, Bill M, Aldred MA, van de Veerdonk MC, Vonk Noordegraaf A, Long-Boyle J, Dash R, Yang PC, Lawrie A, Swift AJ, Rabinovitch M, Zamanian RT. Low-dose FK506 (Tacrolimus) in end-stage pulmonary arterial hypertension. Am J Respir Crit Care Med 192: 254–257, 2015. doi: 10.1164/rccm.201411-2061LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L, de Jesus Perez V, Wang L, Reddy S, Zhao M, Bernstein D, Solow-Cordero DE, Beachy PA, Wandless TJ, Ten Dijke P, Rabinovitch M. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 123: 3600–3613, 2013. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stenmark KR, Orton EC, Reeves JT, Voelkel NF, Crouch EC, Parks WC, Mecham RP. Vascular remodeling in neonatal pulmonary hypertension. Role of the smooth muscle cell. Chest 93, Suppl: 127S–133S, 1988. [PubMed] [Google Scholar]
- 123.Sun XQ, Zhang R, Zhang HD, Yuan P, Wang XJ, Zhao QH, Wang L, Jiang R, Jan Bogaard H, Jing ZC. Reversal of right ventricular remodeling by dichloroacetate is related to inhibition of mitochondria-dependent apoptosis. Hypertens Res 39: 302–311, 2016. doi: 10.1038/hr.2015.153. [DOI] [PubMed] [Google Scholar]
- 124.Taichman DB, Ornelas J, Chung L, Klinger JR, Lewis S, Mandel J, Palevsky HI, Rich S, Sood N, Rosenzweig EB, Trow TK, Yung R, Elliott CG, Badesch DB. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest 146: 449–475, 2014. doi: 10.1378/chest.14-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tang H, Chen J, Fraidenburg DR, Song S, Sysol JR, Drennan AR, Offermanns S, Ye RD, Bonini MG, Minshall RD, Garcia JG, Machado RF, Makino A, Yuan JX. Deficiency of Akt1, but not Akt2, attenuates the development of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L208–L220, 2015. doi: 10.1152/ajplung.00242.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, Granton J, Stewart DJ. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res 98: 209–217, 2006. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
- 127.Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, Inayathullah M, Fridlib M, Rajadas J, Peters-Golden M, Voelkel NF, Nicolls MR. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 5: 200ra117, 2013. doi: 10.1126/scitranslmed.3006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tuder RM, Lee SD, Cool CC. Histopathology of pulmonary hypertension. Chest 114, Suppl: 1S–6S, 1998. doi: 10.1378/chest.114.1_Supplement.1S-a. [DOI] [PubMed] [Google Scholar]
- 129.Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB. Pathology of pulmonary hypertension. Clin Chest Med 34: 639–650, 2013. doi: 10.1016/j.ccm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 130.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, Palmisciano AJ, Krishnan I, Pinder D, Preston IR, Roberts KE, Kawut SM. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med 193: 1168–1175, 2016. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol 293: H770–H776, 2007. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang YY, Yang YX, Zhe H, He ZX, Zhou SF. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: an update on its pharmacokinetic and pharmacodynamic properties. Drug Des Devel Ther 8: 2075–2088, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wellbrock J, Harbaum L, Stamm H, Hennigs JK, Schulz B, Klose H, Bokemeyer C, Fiedler W, Lüneburg N. Intrinsic BMP antagonist gremlin-1 as a novel circulating marker in pulmonary arterial hypertension. Lung 193: 567–570, 2015. doi: 10.1007/s00408-015-9735-5. [DOI] [PubMed] [Google Scholar]
- 134.West JD, Carrier EJ, Bloodworth NC, Schroer AK, Chen P, Ryzhova LM, Gladson S, Shay S, Hutcheson JD, Merryman WD. Serotonin 2B receptor antagonism prevents heritable pulmonary arterial hypertension. PLoS One 11: e0148657, 2016. doi: 10.1371/journal.pone.0148657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao JW, Zhu XY, Wang QG, Zhang DZ, Cui CS, Zhang P, Chen HY, Meng LL. Acute effects of Rho-kinase inhibitor fasudil on pulmonary arterial hypertension in patients with congenital heart defects. Circ J 79: 1342–1348, 2015. doi: 10.1253/circj.CJ-14-1015. [DOI] [PubMed] [Google Scholar]
- 136.Yang P, Read C, Kuc RE, Buonincontri G, Southwood M, Torella R, Upton PD, Crosby A, Sawiak SJ, Carpenter TA, Glen RC, Morrell NW, Maguire JJ, Davenport AP. Elabela/Toddler is an endogenous agonist of the apelin apj receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation 135: 1160–1173, 2017. doi: 10.1161/CIRCULATIONAHA.116.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yin J, Wang L, Yin N, Tabuchi A, Kuppe H, Wolff G, Kuebler WM. Vasodilatory effect of the stable vasoactive intestinal peptide analog RO 25-1553 in murine and rat lungs. PLoS One 8: e75861, 2013. doi: 10.1371/journal.pone.0075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yung LM, Nikolic I, Paskin-Flerlage SD, Pearsall RS, Kumar R, Yu PB. A selective transforming growth factor-β ligand trap attenuates pulmonary hypertension. Am J Respir Crit Care Med 194: 1140–1151, 2016. doi: 10.1164/rccm.201510-1955OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao L, Chen CN, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, Wilkins MR. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation 126: 455–467, 2012. doi: 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zopf DA, das Neves LA, Nikula KJ, Huang J, Senese PB, Gralinski MR. C-122, a novel antagonist of serotonin receptor 5-HT2B, prevents monocrotaline-induced pulmonary arterial hypertension in rats. Eur J Pharmacol 670: 195–203, 2011. doi: 10.1016/j.ejphar.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 141.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation 121: 98–109, 2010. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]