Abstract
Acid (HCl) aspiration during anesthesia may lead to acute lung injury. There is no effective therapy. We hypothesized that HCl instilled intratracheally in C57BL/6 mice results in the formation of low-molecular weight hyaluronan (L-HA), which activates RhoA and Rho kinase (ROCK), causing airway hyperresponsiveness (AHR) and increased permeability. Furthermore, instillation of high-molecular weight hyaluronan (H-HA; Yabro) will reverse lung injury. We instilled HCl in C57BL/6 wild-type (WT), myeloperoxidase gene-deficient (MPO−/−) mice, and CD44 gene-deficient (CD44−/−) mice. WT mice were also instilled intranasally with H-HA (Yabro) at 1 and 23 h post-HCl. All measurements were performed at 1, 5, or 24 h post-HCl. Instillation of HCl in WT but not in CD44−/− resulted in increased inflammation, AHR, lung injury, and L-HA in the bronchoalveolar lavage fluid (BALF) 24 h post-HCl; L-HA levels and lung injury were significantly lower in HCl-instilled MPO−/− mice. Isolated perfused lungs of HCl instilled WT but not of CD44−/− mice had elevated values of the filtration coefficient (Kf). Addition of L-HA on the apical surface of human primary bronchial epithelial cell monolayer decreased barrier resistance (RT). H-HA significantly mitigated inflammation, AHR, and pulmonary vascular leakage at 24 h after HCl instillation and mitigated the increase of Kf and RT, as well as ROCK2 phosphorylation. Increased H- and L-HA levels were found in the BALF of mechanically ventilated patients but not in healthy volunteers. HCl instillation-induced lung injury is mediated by the L-HA-CD44-RhoA-ROCK2 signaling pathway, and H-HA is a potential novel therapeutic agent for acid aspiration-induced lung injury.
Keywords: alveolar permeability, filtration coefficient, RhoA, ROCK2, Yabro
INTRODUCTION
Pulmonary aspiration of acid and/or gastric contents is considered to be a direct cause of acute respiratory distress syndrome (3a). Its severity depends on the amount and nature of the aspirated material, the frequency of aspiration, and the host’s response to the aspirated material (42). Up to 15.6% of acute respiratory distress syndrome cases were caused by aspiration of gastric acid (11). Clinical symptoms of pulmonary aspiration are characterized by hypoxemia, bronchospasm, pulmonary edema, and respiratory failure arising from inflammation and airway hyperresponsiveness (AHR). Pathophysiologic changes are characterized by biphasic responses including early direct caustic action of low pH followed by persistent acute inflammation starting at 4–6 h. To date, most studies of pulmonary aspiration have focused on the early phase of lung injury, but little is known about the mechanism of persistent inflammation and lung injury after the initial insult (50, 51).
Acid is the major component of gastric content; a previous study has shown that the pH of gastric content must be <2.5 for the development of aspiration pneumonitis (13). Animal models of intratracheal instillation of hydrochloric acid (HCl) could be used as a lung injury model for patients who aspirated with an empty stomach or patients who were exposed to chlorine gas (Cl2), as HCl is formed by the reaction of Cl2 with water (4). Animal studies have shown that acid aspiration-induced acute lung injury was primarily mediated by neutrophil-dependent mechanisms. Neutralization of interleukin (IL)-8 (major chemotactic factor for recruitment of neutrophils) by anti-IL-8 monoclonal antibodies reduced air space neutrophil counts, as well as IL-8 concentrations and the acid-mediated damage to the blood gas barrier (14, 47). However, the cellular mechanisms responsible for the influx of neutrophils and the initiation of lung injury to pulmonary vasculature following HCl instillation have not been clearly elucidated.
Hyaluronan (HA), a major component of the lung extracellular matrix, is found in high concentrations in mammalian connective tissue (3, 27). Under physiological conditions, HA exists as H-HA, (>1,000 kDa), but in tissue injury or inflammation, there is generation of L-HA, (100–300 kDa) (26, 33). Circulating HA concentrations are elevated (up to 32-fold) in patients with direct lung injury (6, 52). Inhalation of oxidant gases, such as ozone and Cl2, could induce the generation of L-HA fragments. These fragments initiate a series of proinflammatory events by binding to their cognate receptor (CD44) (17, 44), as well as to Toll-like receptor 4 (37), and promote lung inflammation as well as AHR. The effects of L-HA can be antagonized by the naturally occurring H-HA (9, 18, 49). However, the beneficial effects of H-HA administration, post-HCl instillation, have not been studied.
Increased inflammation, lung permeability, and AHR are important pathological events of aspiration-induced lung injury. Herein, it is the first time that we tested the hypothesis that HCl instillation in mice induces L-HA release in the alveolar space from the degradation of extracellular matrix by reactive intermediates produced by inflammatory cells. Furthermore, increased L-HA mediates inflammation, lung edema, and AHR following HCl instillation, and H-HA instillation could serve as a potential treatment for the acid instillation-induced injury.
MATERIALS AND METHODS
Materials.
Antibodies to Rho kinase 1 (ROCK1) and ROCK2 were purchased from Cell Signaling Technology (Beverly, MA). Antibodies to phosphorylated ROCK2 and β-actin were obtained from Sigma-Aldrich (St. Louis, MO) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. DMEM came from GIBCO Laboratories (Gaithersburg, MD). Secondary antibodies (goat anti-rabbit IgGs) conjugated to horseradish peroxidase (HRP), Laemmli sample buffer, PVDF membranes, and 10% TGX gels were from Bio-Rad (Hercules, CA). Chemiluminescent HRP substrate was used for signal visualization (Millipore, Billerica, MA). Unless otherwise noted, all other materials and reagents were obtained from Sigma-Aldrich.
Animals.
Mice were 8- to 12-wk-old males (20–25 g body wt). C57BL/6 wild-type (WT) (40) mice were purchased from Charles River Laboratories (Wilmington, MA). Myeloperoxidase gene-deficient (MPO−/−) mice and CD44 gene-deficient (CD44−/−) mice were purchased from Jackson Laboratories (Chicago, IL). All experimental procedures involving animals were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC-20187).
HCl instillation model.
Mice were anesthetized with isoflurane (2–5%). An incision was made at the neck to expose the trachea, and a 24-G angiocatheter was inserted into the trachea. Either hydrochloric acid (HCl; 0.1 M, pH 1.25, 2 ml/kg) dissolved in sterile normal saline or sterile normal saline (NS) was instilled into the lung through the angiocatheter. The skin incision was closed with 4–0 Ethilon suture, and then, mice woke up and recovered in room air.
H-HA administration.
At 1 and 23 h after HCl or NS instillation, H-HA (3 mg/ml, 50 µl, Yabro; generous donation of IBSA, Lugano, Switzerland; ~1,000 kDa) or PBS was instilled in the external nares of WT mice. The amount and timing of Yabro administration were shown to decrease upper and distal lung injuries in a murine model of chlorine gas inhalation (34).
Lung injury assessment.
Mice were anesthetized with an intraperitoneal injection of ketamine and xylazine (100 and 10 mg/kg body wt, respectively). An incision was made at the neck to expose the trachea, and an 18-G angiocatheter was inserted 3 mm into the trachea. The lungs were lavaged with 1 ml of cold PBS; ~0.7–0.9 ml were recovered in all groups. Recovered aliquots of lavage fluid were kept on ice and centrifuged immediately at 1,000 g for 5 min to pellet the cells. Supernatants were removed and stored on ice for protein analysis using the BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). Cells were suspended in 100 µl of PBS and counted using a hemocytometer (Fisher Scientific, Waltham, MA). Cells were then placed on slides using a Cytospin 4 cytocentrifuge (Thermo Fisher Scientific, Waltham, MA) and stained using a two-stain set consisting of eosin Y and a solution of thiazine dyes (Quik-Stain; Siemens, Washington, DC). Differential counts (specifically macrophages and neutrophils) were then performed on slides via light microscopy.
HA measurement.
HA measurements in cell-free human or mouse bronchoalveolar lavage fluid (BALF) were done by using a commercial ELISA kit (Echelon, San Jose, CA) per manufacturer’s instructions (34, 35). To verify the presence of L-HA, we concentrated the BALF using Amicon ultra-0.5 ml centrifugal filters per manufacturer’s instructions (Millipore, Billerica, MA) and pretreated the recovered BALF with protease/DNase, and BALF was run on 1% agarose (Lonza, Rockland, ME) gels along with commercially available H-HA (Yabro; generous donation of IBSA Institut Biochimique; ~1,000 kDa) and HA ladders as previously described (18, 20, 34). In some cases, BALF was precipitated by incubation with ethanol, centrifuged at 14,000 g for 10 min at room temperature, and suspended in 100 mM ammonium acetate. In some cases, 0.5 ml of BALF from mice at 24 h after HCl instillation were treated with hyaluronidase (10 U/ml; Sigma-Aldrich) overnight at 37°C before agarose gel electrophoresis.
IαI measurement.
IαI levels in mice BALF were determined using a competitive enzyme-linked immunosorbent assay with a monoclonal antibody against human plasma derived inter-α-trypsin inhibitor (IαI; mAb 69.26), as previously described (38). Briefly, 96-well plates were coated with purified IαI (300 ng in 50 pmol/l carbonate buffer, pH 9.6) and incubated overnight at 4°C. Fifty microliters of BALF samples diluted 1:25 in PBS and 50 μl of mAb 69.26 were added to each well, and plates were incubated for 1 h at 37°C. A serial dilution of purified IαI in PBS was used to establish a standard curve. The bound mAb 69.26 was detected by adding human-absorbed, HRP-conjugated goat anti-mouse IgG (EMD Millipore) for 1 h at 37°C and measuring absorbance at 405 nm on an ELISA plate reader (BioTek, Winooski, VT). Each BALF sample was tested in triplicate.
Hyaluronic acid-binding protein staining in mouse lungs.
Paraformaldehyde-fixed, paraffin-embedded sections were prepared at a thickness of 5 µm for detection of HA expression. Tissue sections were deparaffinized in xylenes and rehydrated to 1× Tris-buffered saline plus 0.1% Tween 20 (TBST) through graded ethanol solutions, blocked 1 h in 10% normal donkey serum and 1% BSA in 1× TBST, and then incubated with hyaluronic acid-binding protein (HABP; Calbiochem, EMD Millipore) at a 1:10 dilution for 1 h at room temperature. After being washed in 1× TBST, tissues were incubated with Alexa Fluor 488 Strepavidin and 5 nm colloidal gold conjugate (1:30; Life Technologies) for 1 h and rinsed in 1× PBS, and coverslips were affixed with Prolong Gold plus DAPI (Life Technologies). Images were collected using an Olympus model BX51 epifluoresence microscope fitted with an Olympus DP80 digital camera (Olympus, Center Valley, PA) plus Olympus cellSens software. Scale bar = 100 µm.
Histological analysis and lung injury scoring.
Lung tissues were fixed by instillation of 70% alcoholic formalin at 25 cm H2O for 1 h and removed, continuously soaked in 70% alcoholic formalin for 23 h, and then dehydrated in 70% ethanol before being embedded in paraffin. Paraffin-embedded tissues were cut into 4-µm sections, deparaffinized, and rehydrated using CitriSolv (d-limonene-based solvent) and isopropanol, respectively. The sections were stained with hematoxylin and eosin (H&E) or MPO. MPO-stained slides were prepared as previously described (2). Lung injury was scored using five parameters according to the official American Thoracic Society workshop report (43). The H&E-stained slides were evaluated by scoring for structural changes, and MPO-stained slides were evaluated by scoring for the presence of neutrophils within the alveolar and interstitial spaces. The slides were analyzed by an investigator blinded to the treatments.
Depleting mice neutrophils.
C57BL/6 WT mice were intraperitoneally injected with 395 µg either anti-mouse Ly6G antibody (BioXCell) to deplete neutrophils or rat IgG2a isotype control (BioXCell) at 24 h before HCl aspiration.
Ex vivo lung vascular permeability study.
Mice from different groups were anesthetized, and following sternotomy, the heart and lungs were isolated en bloc at 24 h after HCl or NS instillation and perfused at constant flow with Earl’s buffer at pH 7.4 (37°C). Hemodynamics (pressure of pulmonary artery, vein, and capillary) and the filtration coefficient (Kf) were measured as previously described using zone 3 conditions (25). Kf was calculated as the rate of weight gain obtained 13–15 min after a 7–10 cmH2O increase in pulmonary venous pressure, normalized per grams lung dry weight. Hemodynamic parameters and lung weight gain were determined using ADInstruments PowerLab 8/30 and LabChart pro software. Then lungs wet weight was measured immediately and followed by drying at 80°C for 72 h to obtain dry weight measurements.
Incubation of H-HA with activated neutrophils in vitro.
Mice were anesthetized with ketamine and xylazine as mentioned above and were exsanguinated by cardiac puncture for the collection of blood. Neutrophils were isolated as previously described (48), activated by 5 × 10−8 M phorbol 12-myristate 13-acetate (PMA; P1585; Sigma) for 30 min, incubated with H-HA (1 mg/ml) for 24 h, and then subjected to agarose gel electrophoresis to detect the presence of HA as described above.
Cytochrome c reductase NADPH activity.
NADPH activity measurements, in isolated neutrophils from mice peripheral blood as mentioned above, were measured with a commercial Cytochrome c Reductase Kit (Sigma-Aldrich), and all the instructions were followed, as per manufacturer’s instructions.
RhoA activation assay.
The Rho activity assay was performed and quantified using the RhoA Activation Assay Biochem Kit (Cytoskeleton) based on the Rhotekin pull-down assay as per manufacturer’s instructions. In brief, mice were anesthetized and euthanized. Their lungs were perfused with PBS until they were clear of blood, removed, and homogenized in a radio immunoprecipitation assay lysis buffer (Thermo Fisher Scientific, Rockford, IL) containing protease inhibitors. The samples were then sonicated for 10 s three times on ice in 1.5-ml Eppendorf tubes using an ultrasonic liquid processor and centrifuged at 14,000 g for 20 min at 4°C. Cleared supernatants were used to measure the protein concentration by the BCA assay. Equal amounts of protein were incubated with Rhotekin-RBD beads for 1 h at 4°C. After the beads were washed with wash buffer (Cytoskeketon), proteins were removed from the beads with Laemmli buffer and then subjected to Western blotting.
Quantitative real-time PCR.
RNA was isolated from frozen mice lung tissues using RNeasy (Qiagen, Valencia, CA), and QIAcube (Qiagen). cDNA was synthesized using PrimeScript RT MasterMix (Takara/Clontech, Mountain View, CA). Quantitative real-time PCR was performed using Premix Ex Taq Probe (Takara/Clontech) and Rotor-Gene Q instrument (Qiagen). Primers for 18S (Hs99999901_s1), murine hyaluronan synthase 1 (HAS1) (Mm03048195_m1), HAS2 (Mm00515089_m1), and HAS3 (Mm00515092_m1) were obtained from Applied Biosystems/Thermo Fisher (Foster City, CA). Cycle threshold (55) values were normalized to 18S (ΔCT). Fold changes relative to control were calculated using 2−ΔCT.
Respiratory mechanics.
Mice were mechanically ventilated and challenged with increasing doses of methacholine as described previously (4, 21, 54). Briefly, mice were anesthetized with pentobarbital (50 mg/kg ip; Vortech Pharmaceuticals, Dearborn, MI), paralyzed with pancuronium (4 mg/kg ip; Gensia Sicor Pharmaceuticals, Irvine, CA), intubated, connected to an FX-1 module of the FlexiVent (SCIREQ, Montreal, PQ, Canada), and ventilated at a rate of 160 breaths/min at a tidal volume of 0.2 ml with a positive end-expiratory pressure of 3 cmH2O. Total respiratory system resistance was recorded continuously as previously described (2). Baseline was set via deep inhalation. Increasing doses of methacholine chloride (0–40 mg/ml; Sigma-Aldrich) were administered via aerosolization within an administration time of 10 s. Airway responsiveness was recorded every 15 s for 3 min after each aerosol challenge. Broadband perturbation was used, and impedance was analyzed via a constant phase model.
Western blot analysis.
Mice were anesthetized and euthanized. Their lungs were perfused with PBS until they were clear of blood, removed, and homogenized in a radio immunoprecipitation assay lysis buffer (Thermo Fisher Scientific, Rockford, IL) containing protease inhibitors. The samples were then sonicated for 10 s thrice on ice in 1.5-ml Eppendorf tubes using an ultrasonic liquid processor and centrifuged at 14,000 g for 20 min at 4°C. Cleared supernatants were used to measure the protein concentration by the BCA assay. Equal amounts of protein were loaded in 10% Tris·HCl Criterion precast gels; proteins were transferred to PVDF membranes. Membranes were probed with ROCK1 or phosphorylated (phospho) ROCK2 or ROCK2 antibodies (1:1000 dilution). Bands were detected by the chemiluminescent HRP substrate. Protein loading was normalized by reprobing the membranes with an antibody specific to β-actin.
L-HA generation.
H-HA were exposed to chlorine gas (400 ppm for 30 min), which fragmented HA to produce L-HA (Cl2-L-HA, molecular mass <300 kDa), as previously described (34).
Cell culture.
Human primary bronchial epithelial cells were isolated, characterized, and cultured in DMEM supplemented with 10% FBS and penicillin/streptomycin (generous donation of Dr. Kevin Harrod, Department of Anesthesiology and Perioperative Medicine, University of Alabama at Birmingham).
Transepithelial electric resistance.
Primary human bronchial epithelial cell barrier integrity was measured using an Electric Cell-Substrate Impedance Sensing System (Applied Biophysics, Troy, NY) as described in detail (10). Briefly, primary human bronchial epithelial cells (40 × 103 cells/mm2) were plated onto 8W10E arrays in normal culture medium and used when resistances reached ±900 Ω, usually 2–3 days after seeding. Resistance was taken every 15 min for the duration of the experiments. Baseline resistance was measured for 2 h before addition of L-HA (500 µg/ml) or PBS control (50 µl) in 50 µl media. One hour after that H-HA (500 µg/ml) or PBS control (50 µl) was added to the corresponding group. Epithelial resistance study was conducted in triplicate with a minimum of three independent experiments.
Patient enrollment and collection of BALF samples.
All patients admitted to the Surgical, Neurosurgical, Trauma-Burn and Medical Intensive Care Units at the University of Alabama at Birmingham from June 2016 to August 2017 were eligible. This study was approved by the University of Alabama at Birmingham Institutional Review Board. Inclusion criteria included presence of mechanical ventilation and a BALF culture positive for one of the following microbial species: Pseudomonas aeruginosa, Staphylococcus aureus, or Klebsiella pneumoniae. Mechanically ventilated patients with a negative BALF culture were also included as controls. Both culture-positive and -negative patients were excluded if they were <19 yr old, pregnant, incarcerated, or did not have a surrogate. Due to the nature of the study, investigators had 72 h from the time of known positive BALF culture to obtain informed consent from the patient or an appropriate surrogate.
BALF samples from lung-healthy adults were collected via bronchoscopy after instillation of 50-ml aliquots of normal saline in the right middle lobe for a total volume of 200 ml after informed consent was obtained. Then, BALF supernatant was collected and stored with protease and phosphatase inhibitors to prevent long-term degradation of possibly important components to be measured at a later time. This study was approved by the National Institute of Environmental Health Sciences Institutional Review Board (clinicaltrials.gov identifier NCT01224691). These samples were collected by S. Garantziotis at the National Institute of Environmental Health Sciences and were shipped to University of Alabama at Birmingham with the consent of the Institutional Review Board committees of both institutions.
Statistics.
All data are presented as means ± SE. To eliminate unequal variances, data were log transformed. Statistical analysis among means was performed with ANOVA (one or two way) followed by the Bonferroni post hoc comparisons. Values of *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 were considered significant. Data were graphed using GraphPad Prism 7 for Windows (GraphPad Software, San Diego, CA).
RESULTS
HCl instillation induced lung injury and inflammation.
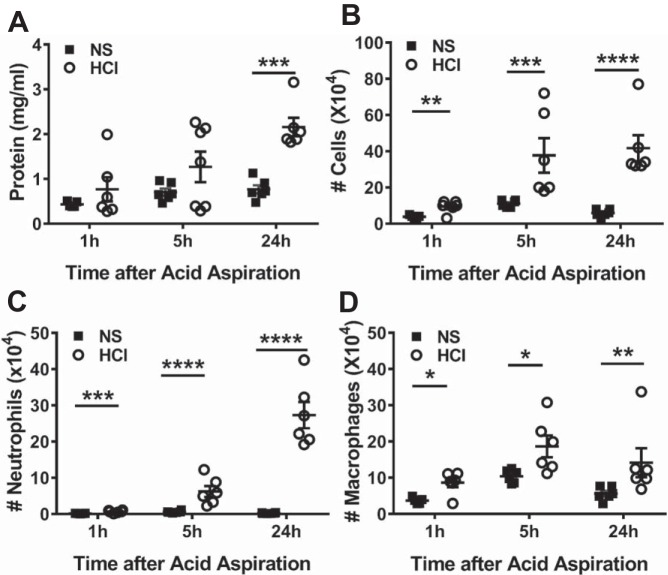
When instilled intratracheally into C57BL/6 mice, HCl resulted in a significant increased level of protein in bronchoalveolar lavage fluid (BALF) 24 h later (Fig. 1A). Instillation of HCl also induced an increased number of inflammatory cells in BALF starting at 1 h and persisting at 24 h postexposure compared with mice received normal saline (NS). More than 70% of BALF inflammatory cells were neutrophils at 24 h post-HCl instillation (Fig. 1, B–D).
Fig. 1.
HCl instillation induces lung injury and inflammation. Wild-type (WT) C57BL6 (40) mice were instilled either with normal saline (NS) or HCl intratracheally; they were euthanized at the indicated time points, and bronchoalveolar lavage fluid (BALF) was performed as described previously (2). A: BALF protein. B: total cells. C: neutrophils. D: macrophages at 1, 5, and 24 h after HCl exposure. Each dot represent a different mouse; means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, compared with corresponding NS control by two-way ANOVA followed by Bonferroni post hoc comparisons; n = 5–6 for each group.
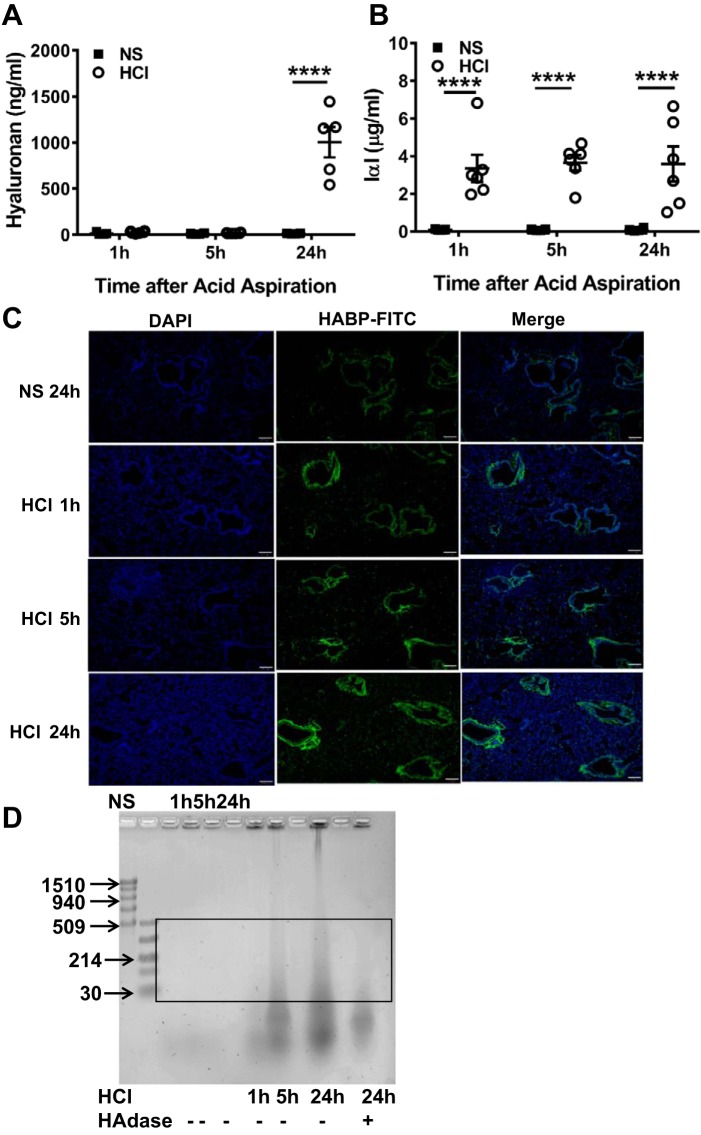
HCl instillation increased L-HA in BALF and lung tissues.
At 24 h post-HCl instillation, there were significant increases of HA in BALF compared with mice received NS (Fig. 2A). Moreover, an elevated level of IαI, which is necessary for crosslinking of HA and binding to CD44, was seen at as early as 1 h after HCl instillation and persisted at 24 h postexposure (Fig. 2B). Furthermore, we incubated lung tissues with fluorescein-labeled HABP to detect HA deposition after HCl instillation using epifluorescence microscopy. The levels of HABP expression in HCl-instilled lung tissue increased gradually compared with those from mice instilled with NS (Fig. 2C). Agarose gel electrophoresis of concentrated BALF from HCl-instilled mic, showed the presence of L-HA fragments at 5 and 24 h post-HCl exposure, the intensity of which increased at 5 and 24 h after HCl instillation; treatment of BALF with hyaluronidase (HAdase), which degrades HA, diminished the intensity of the staining (Fig. 2D). These findings indicated that HCl instillation resulted in the formation of L-HA, which accumulated in the peribronchial space and alveolar/capillary septae.
Fig. 2.
HCl instillation increases low-molecular weight hyaluronan (L-HA) in BALF and lung tissues. BALF hyaluronan (A) and inter-α-trypsin inhibitor (IαI) (B), a hyaluronan-binding partner protein, were measured by ELISA at 1, 5, and 24 h after HCl instillation. Each dot represents a different mouse; means ± SE. ****P < 0.0001, compared with corresponding NS control by two-way ANOVA followed by Bonferroni post hoc comparisons; n = 5–6 for each group. C: FITC-labeled hyaluronic acid-binding proteins (HABPs) were detected in paraformaldehyde-fixed murine lung tissue at 1, 5, and 24 h post-HCl exposure and at 24 h after NS instillation as control. The level of HABP deposition in peribronchial spaces and alveolar/capillary septae increased with increasing time postexposure. Nuclei were highlighted with DAPI stain (blue). Scale bar = 100 µm. Characteristic images which were reproduced in 4 mice in each group. D: agarose gel image of BALF concentrate to detect HA. NS, normal saline; HAdase, hyaluronidase. Box area denotes L-HA; typical image was reproduced 4 times.
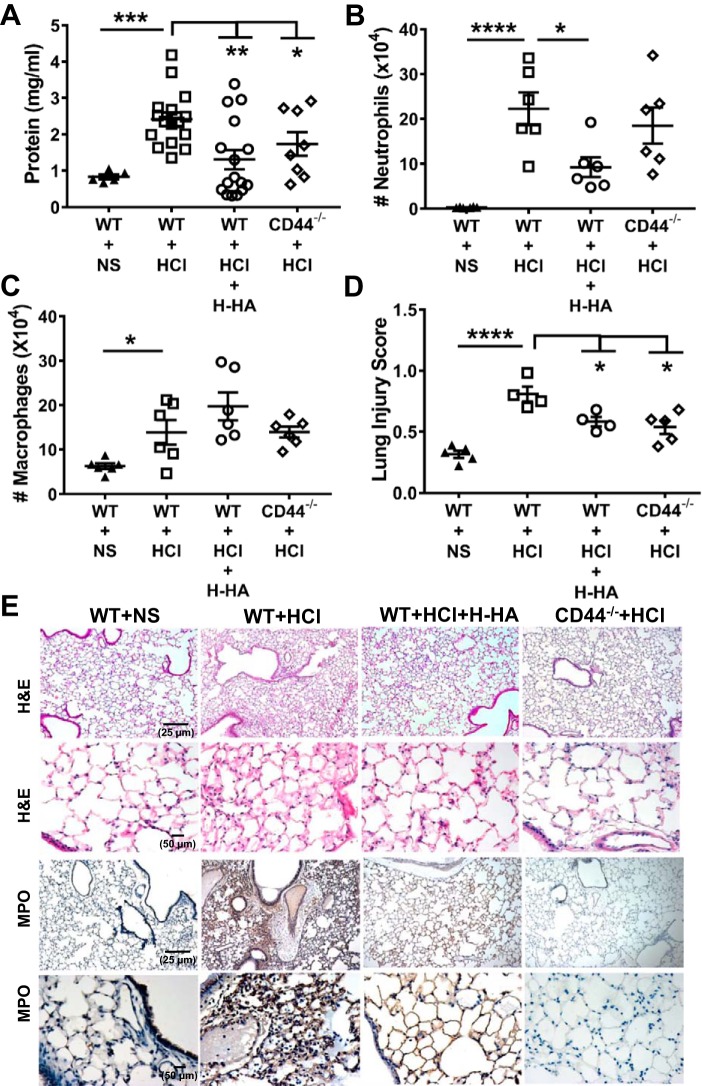
H-HA treatment or lack of CD44 protein attenuated persistent lung injury and inflammation after HCl instillation.
In our next set of experiments, we explored whether intranasal instillation of H-HA, which competes with L-HA for binding to CD44 receptor, would prevent and/or reverse the development of lung injury. C57BL/6 WT (40) or CD44−/− mice were instilled intratracheally with HCl, and at 1 and 23 h later, WT mice were lightly anesthetized and received intranasal administrations of Yabro (50 µl, 3 mg/ml). Our data demonstrated that CD44−/− mice developed significantly less lung injury as compared with WT mice following HCl instillation; furthermore, instillation of Yabro alleviated lung injury in WT mice (Fig. 3A–C).
Fig. 3.
High-molecular weight hyaluronan (H-HA) treatment or lack of CD44 protein attenuates persistent lung injury and inflammation after HCl instillation. BALF protein (A), neutrophils (B), macrophages (C), and lung injury score (D) in WT or CD44−/− mice at 24 h post-HCl or NS; WT mice were also treated with H-HA (Yabro) as described in materials and methods. Each dot represents a different mouse; means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, compared with corresponding WT + HCl by one-way ANOVA followed by Bonferroni post hoc comparisons; n = 6–16 for each group. E: hematoxylin and eosin staining of lung tissue at 24 h after HCl instillation shows the presence of hyaline membranes and protein exudates in alveolar spaces, as well as myeloperoxidase (MPO) staining (3rd row: ×10; 4th row: ×40) in WT mice. HCl instillation-induced injury was decreased by H-HA treatment (3rd column); CD44−/− exhibited less injury, as compared with WT mice, when instilled with HCl (4th column). Typical images were reproduced 4–5 times.
Histological examination of H&E- and MPO (3a)-stained lung sections of WT mice instilled with HCl revealed significant disruption of the airway parenchyma; increased inflammatory cells infiltration, protein, and hyaline membrane accumulation; and thickened alveolar/capillary septae. These changes were much decreased in CD44−/− mice or following instillation of Yabro in WT mice (Fig. 3E). Furthermore, CD44−/− or H-HA-treated mice exhibited significantly lower lung injury scores than WT HCl-instilled mice (Fig. 3D).
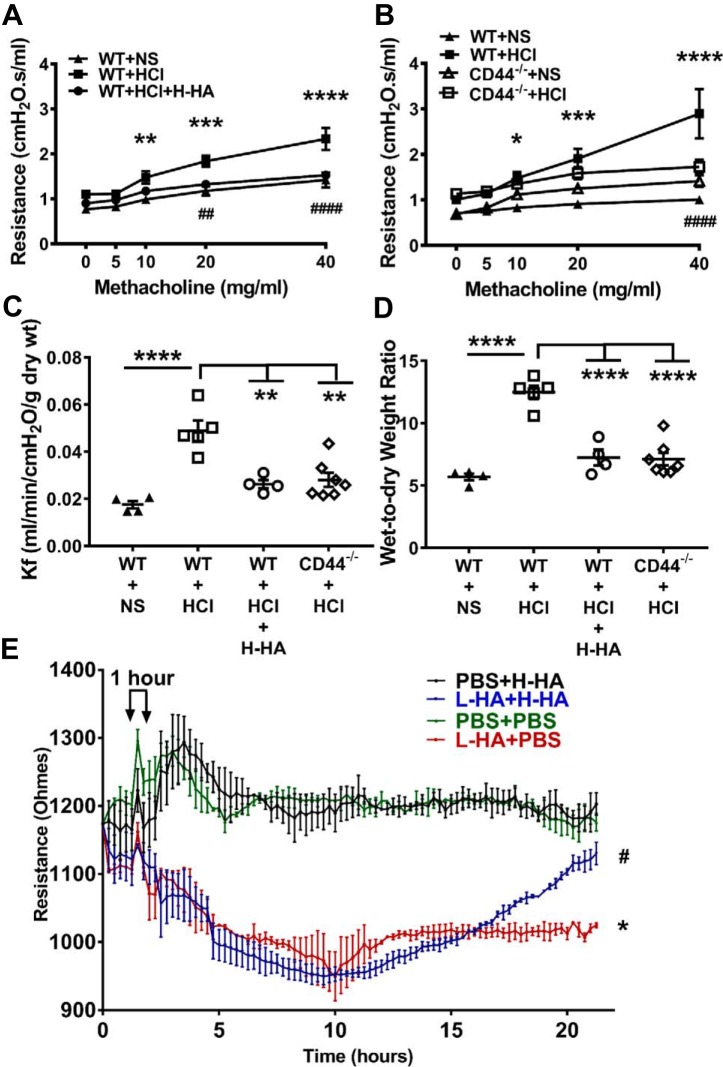
H-HA treatment or lack of CD44 protein rescued AHR and pulmonary permeability after HCl instillation, and H-HA repaired L-HA-induced disruption of the primary human bronchial epithelial cell barrier.
In addition to cellular and histological experiments, we also did functional studies to further investigate the roles of L-HA in mediating HCl instillation-induced lung injury and the potential treatment by H-HA. We first analyzed pulmonary mechanics as expressed by total airway resistance and AHR to methacholine challenge at 24 h after HCl. WT mice showed significantly increased total airway resistance following methacholine challenge 24 h post-HCl, which was decreased significantly following Yabro instillation (Fig. 4A). Furthermore, CD44−/− mice also exhibited significant lower response to methacholine challenge compare with WT mice after HCl exposure (Fig. 4B). These data indicate that the presence of H-HA or lack of CD44 protein ameliorates HCl instillation-induced AHR.
Fig. 4.
Beneficial effects of H-HA following HCl injury in vivo and in vitro. A: airway resistance prior and following methacholine challenge measured by FlexiVent at 24 h in WT C57BL/6 (40) mice after NS, HCl instillation or H-HA treatment post-HCl exposure (means ± SE; n = 5 for NS and n = 6 for HCl or HCl + H-HA group). B: airway resistance prior and following methacholine challenge in WT and CD44−/− mice 24 h after NS or HCl instillation (means ± SE; n = 5 for WT + NS and n = 6 for WT + HCl; n = 4 for CD44−/− + NS and n = 8 for CD44−/− + HCl group). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, WT + HCl compared with corresponding WT + NS control at the same dose of methacholine. ##P < 0.01; ####P < 0.0001, WT + HCl compared with corresponding WT + HCl + H-HA or CD44−/− + HCl at the same dose of methacholine. Data for each dose point were analyzed with one-way ANOVA followed by Bonferroni post hoc comparisons. C: pulmonary filtration coefficients (Kf), measured ex vivo in isolated perfused heart-lung preparations, increased significantly at 24 h after HCl instillation in WT mice; H-HA treatment resulted in normal Kf values; Instillation of HCl in CD44−/− mice did not increase Kf. D: wet-to-dry weigh ratio also significantly increased at 24 h post-HCl exposure in WT but not in CD44−/− mice; the increase of wet-to-dry weight ratio in WT mice was alleviated by H-HA treatment. Each dot represents a different mouse; means ± SE. **P < 0.01; ****P < 0.0001, compared with WT + HCl by one-way ANOVA followed by Bonferroni post hoc comparisons; n = 4–7 for each group. E: electrical resistance values (RT) of confluent primary human bronchial epithelial cell monolayers seeded on Electric Cell-Substrate Impedance Sensing System (ECSIS) arrays before and L-HA or PBS (5) followed H-HA or PBS (5). Notice significant drop in RT in L-HA-treated monolayers and recovery after H-HA. Values are means ± SE. *P < 0.05, L-HA + PBS compared with PBS + PBS; #P < 0.05, L-HA + PBS compared with L-HA + H-HA by one-way ANOVA followed by Bonferroni post hoc comparisons. The experiments were repeated for 3 times and each group had 4 wells on the (ECSIS) array for each experiment.
Subsequent measurements in isolated perfused lungs aimed to determine whether HCl instillation injured the pulmonary vasculature in addition to the alveolar permeability. The filtration coefficient (Kf), a product of endothelial permeability and surface area for exchange, is a sensitive measure of lung endothelial permeability when surface area is fully recruited. Kf increased twofold at 24 h post-HCl in WT but not in CD44−/− mice. In addition, WT mice treated with Yabro had normal values of Kf (Fig. 4C). In parallel with the filtration coefficient studies, we measured the wet-to-dry weight ratios of isolated lungs at the end of perfusion to determine the effect of H-HA treatment or CD44 deficiency on edema formation in HCl-instilled lungs. HCl instillation increased wet-to-dry weight ratio by twofold over controls in WT mice but not in CD44−/− mice and H-HA-treated WT mice abrogated the increase in edema (Fig. 4D). These data indicate that H-HA or HA receptor CD44 deficiency preserves lung surface integrity by decreasing Kf and resolving edema.
We then examined whether L-HA altered epithelial permeability across confluent monolayers of human airway bronchial cell in primary culture. Addition of L-HA resulted in large decreases of electrical resistance across these cells that was reversed by subsequent addition of H-HA (Fig. 4E).
Increased production of L-HA was mediated by neutrophils after HCl instillation.
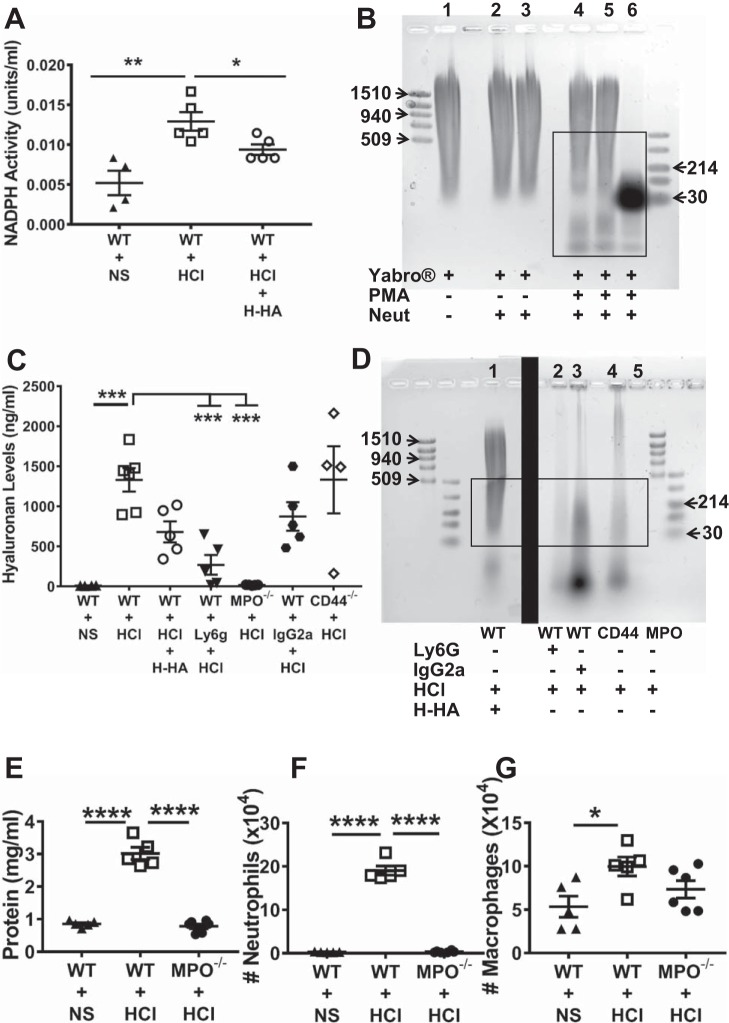
BALF inflammatory cells, 70% of which were neutrophils, had significantly higher NADPH activity 24 h post-HCl exposure while Yabro treatment decreased their activity (Fig. 5A). The next experiment therefore addressed the role of neutrophils in generation of L-HA in lung injury induced by HCl instillation. Neutrophils collected from mice peripheral blood were first incubated with PMA (5 × 10−8 M, 30 min) for activation, and then incubated with media containing Yabro. PMA-activated neutrophils degraded Yabro while inactivated neutrophils did not (Fig. 5B). In vivo studies, we found that BALF HA concentrations (measured by ELISA (34) were significantly decreased in both WT, neutrophil-depleted and MPO−/− mice as compared with WT mice at 24 h after HCl instillation (Fig. 5C). Agarose gel electrophoresis of concentrated BALF showed that neutrophil-depleted mice (following injection of Ly6G antibody), or that lacked MPO activity, exhibited significantly decreased levels of L-HA as compared with their corresponding controls, while BALF of CD44−/− mice showed a similar pattern as the control (following injection of rat IgG2a antibody) mice (Fig. 5D).
Fig. 5.
Contribution of activated neutrophils in H-HA fragmentation A: NADPH activity of neutrophils (106 per sample) isolated from peripheral blood of WT mice 24 h after HCl instillation was significantly increased; treatment of mice with H-HA post HCl resulted in normal levels of NADPH. One NADPH unit will reduce 1 μM of oxidized cytochrome c in the presence of 100 μM of NADPH per minute at pH 7.8 at 25°C. Each dot represents a different mouse; means ± SE. *P < 0.05; **P < 0.01, compared with WT + HCl by one-way ANOVA followed by Bonferroni post hoc comparisons; n = 4–5 for each group. B: Yabro (1 mg/ml) was incubated with either naïve or PMA-activated blood neutrophils (Neut). Notice the appearance of various amounts of L-LA when H-HA was incubated by PMA-activated neutrophils. Box denotes L-HA. This experiment was repeated 3 times. C: BALF HA concentration (measured by ELISA) was significantly decreased in both WT C57BL/6 (40) mice treated with anti-M-Ly6g (depleting neutrophils) and MPO−/− mice as compared with WT mice at 24 h after HCl instillation. However, no significant difference of BALF HA concentration at 24 h after HCl instillation was seen between WT and CD44−/− mice post HCl. Each dot represents a different mouse; means ± SE. ***P < 0.001, compared with WT + HCl by one-way ANOVA followed by Bonferroni post hoc comparisons; n = 4–6 for each group. D: agarose gel image of BALF concentrate in mouse lungs to detect molecular weight of HA: Ly6G, anti-M-Ly6G antibody; IgG2a, rat IgG2a antibody. Box denotes L-HA. Two different gels separated by the solid black box are shown. Individual gels were reproduced 3 times. BALF protein (E), neutrophil counts (F), and macrophage counts (G) in WT and MPO−/− mice 24 h post-HCl or -NS instillation. Each dot represents a different mouse; means ± SE. *P < 0.05; ****P < 0.0001, compared with WT + HCl group by one-way ANOVA followed by Bonferroni post hoc comparisons; n = 5 for WT + NS and WT + HCl; n = 6 for MPO−/− + HCl.
We also instilled HCl intratracheally to MPO−/− mice as well as their WT controls. BALF protein level and neutrophil count were decreased in MPO−/− mice compared with WT mice, again, macrophage count was not changed (Fig. 5, E–G). Taken together, these results suggest that L-HA generation in the lungs after HCl instillation is mediated by activated neutrophils; depletion of neutrophils or inhibition of reactive intermediates production by neutrophils prevented these changes. L-HA injures the alveolar epithelium both directly and by neutrophil dependent mechanisms.
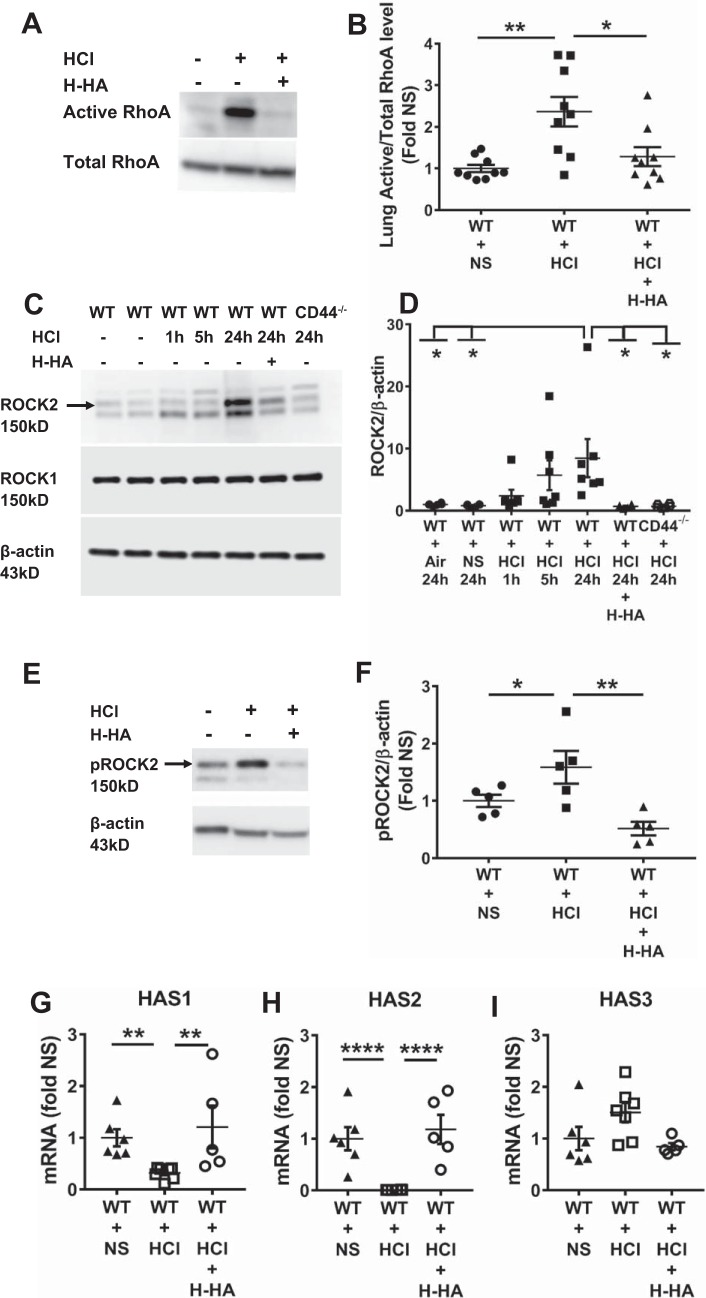
HCl instillation induced activation of RhoA and phospho ROCK2 and ROCK2 and decreased expression of HAS1 and HAS2 mRNAs.
Activation of RhoA and its downstream kinase is a potential mechanism by which HCl instillation may lead to AHR and increased permeability. Data shown in Fig. 6, A–F, indicate that at 24 h post-HCl administration, there was significant activation of lung RhoA and of its downstream kinase ROCK2 and phospho ROCK2 but not of ROCK1 or phospho ROCK1 (data not shown for phospho ROCK1); these changes were not presented in mice treated with Yabro. In addition, ROCK2 expression remained at normal levels in HCl-treated CD44−/− mice (Fig. 6, C and D).
Fig. 6.
HCl instillation activates RhoA and Rho kinase 2 (ROCK2) and decreased the expression of HAS1 and HAS2 mRNAs. A: representative Western blot imaging of active and total RhoA levels in whole lung homogenates from WT mice instilled with HCl or NS 24 h later; WT mice were also treated with or without H-HA at 24 h post-HCl exposure. The blots were reproduced using 2 different sets of mice. B: fold changes of active RhoA/total RhoA in HCl- and HCl + H-HA-treated mice over corresponding groups treated with NS. Each dot represents a different mouse; means ± SE. *P < 0.05; **P < 0.01, compared with WT + HCl group by one-way ANOVA followed by Bonferroni post hoc comparisons; n = 9 for each group. C: representative Western blots of lung ROCK1, ROCK2, and β-actin in WT and CD44−/− for the indicated conditions. The blots were reproduced using 3 different sets of mice. D: quantification of ROCK2/β-actin ratio. Each dot represents a different mouse; means ± SE. *P < 0.05, compared with WT + HCl group by one-way ANOVA followed by Bonferroni post hoc comparisons; n = 6–7 for each group. E: representative Western blot imaging of phosphoROCK2 and β-actin levels in whole lung homogenates from WT mice instilled with HCl or NS 24 h later; WT mice were also treated with or without H-HA at 24 h post-HCl exposure. The blots were reproduced using 2 different sets of mice. F: fold changes of phosphoROCK2/ β-actin in HCl- and HCl + H-HA-treated mice over corresponding groups treated with NS. Each dot represents a different mouse; means ± 1 SE. *P < 0.05; **P < 0.01, compared with WT + HCl group by one-way ANOVA followed by Bonferroni post hoc comparisons; n = 5 for each group. G–I: lung hyaluronan synthase (HAS) mRNAs expression. Each dot represents a different mouse; means ± SE. **P < 0.01; ****P < 0.0001, compared with WT + HCl group by one-way ANOVA followed by Bonferroni post hoc comparisons; n = 5–7 for each group.
To further understand the mechanisms of L-HA generation after HCl instillation, we also analyzed the expression of HAS mRNAs in lung tissues at 24 h post-HCl exposure. We found that HCl instillation caused decreased expression of HAS1 and HAS2 mRNA, which synthesize H-HA, while it did not affect the expression of HAS3, which synthesizes L-HA. H-HA treatment restored HAS1 and HAS2 mRNA expression to control levels compared with HCl instillation mice (Fig. 6, G–I).
Increased HA production in BALF of mechanically ventilated patients.
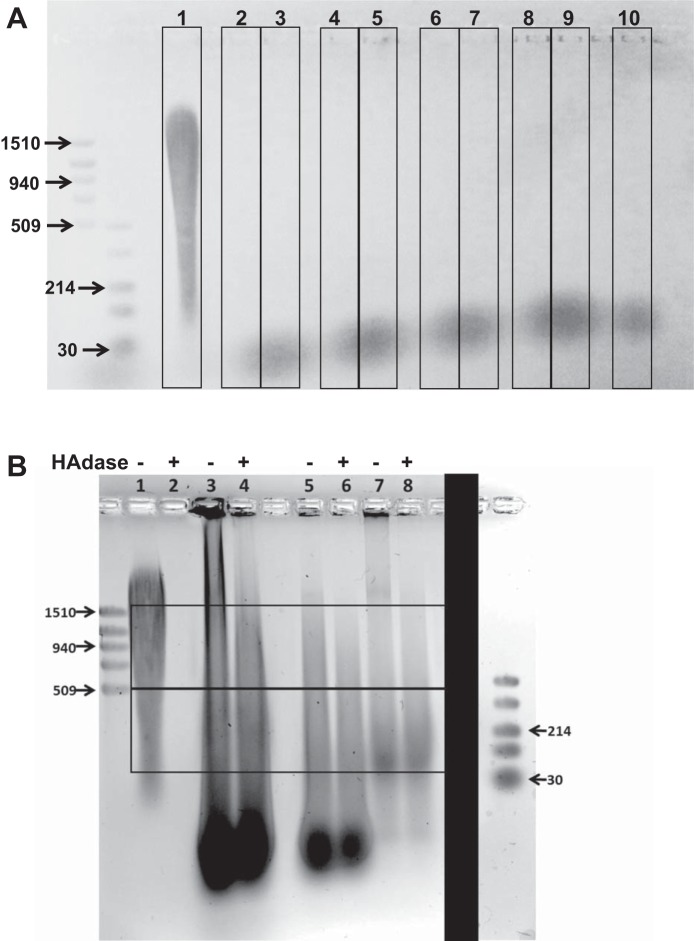
We could not detect any HA in BALF of normal healthy volunteers (Fig. 7A, bottom) black smear represents the loading dye. To determine whether acute inflammation, neutrophil recruitment, and acute lung injury would increase L-HA in patients, we examined the presence of HA from BALF of mechanically ventilated patients with or without pneumonia. There was a continuous smear from higher than 1,510 kDa to below 30 kDa, suggesting that HA might be accounting for the observed staining (Fig. 7B). The mechanically ventilated patient with S. aureus (Fig. 7B, lane 3) or K. pneumoniae (Fig. 7B, lane 5) infection had higher HA levels than a mechanically ventilated patient without pneumonia (Fig. 7B, lane 7). However, in mechanically ventilated patients with or without pneumonia, HA levels in BALF are significantly elevated compared with healthy volunteers. As shown in Fig. 7B, addition of hyaluronidase in human BAL samples had a small but detectable effect on HA levels. This is in contrast to mouse samples in which case hyaluronidase decreased the signal by more than 90% (Fig. 2D). The reason for this discrepancy is not clear.
Fig. 7.
Increased HA levels in mechanically ventilated patient BALF A: agarose gel electrophoresis of precipitated BALF of lane 1: H-HA (Yabro); lanes 2–10: BALF from 9 healthy adults (black smear at bottom represents loading dye). B: agarose gel electrophoresis of concentrated BALF from 3 different mechanical ventilated patients. Lane 1, H-HA (Yabro); lane 2, hyaluronidase treated with H-HA (Yabro); lane 3, patient with Staphylococcus aureus; lane 4, patient with S. aureus (BALF treated with hyaluronidase); lane 5, patient with Klebsiella pneumoniae; lane 6, patient with K. pneumoniae (BALF treated with hyaluronidase); lane 7, patient without pneumonia; lane 8, patient without pneumonia (BALF treated with hyaluronidase). Top box: H-HA. Bottom box: L-HA. Typical gels were reproduced 3 times. The solid black box denotes that the low-molecular weight markers (last left lane) were part of a different gel and added here for clarity.
DISCUSSION
Acid and/or gastric content aspiration is an important risk factor for acute lung injury and its most severe manifestation, acute respiratory distress syndrome (11), especially when patients have altered mental status or neurologic compromise or during induction of general anesthesia. It is generally accepted that inflammation plays an important role in acute respiratory distress syndrome, and numerous clinical trials have been performed to treat it, but only lung-protective ventilation has translated into clinical treatment (1). For this reason, we attempted to uncover the acute pathology (within 24 h of instillation) and potential therapeutic interventions that may be utilized to mitigate human morbidity and mortality associated with gastric aspiration-induced lung injury. The findings we present here emphasize 1) in mechanically ventilated patients with or without pneumonia, HA levels in BALF are significantly elevated compared with healthy volunteers; 2) the important role of L-HA has in HCl instillation-induced airway and distal lung epithelial injury; 3) the L-HA was generated from matrix degradation by reactive intermediates produced by activated neutrophils; 4) depleted neutrophils or MPO−/− or CD44−/− mice showed significantly less injury induced by HCl instillation; and 5) intranasal instillation of exogenous H-HA after HCl exposure prevents lung inflammation, decreases protein extravasation and pulmonary edema, and decreases airway resistance after HCl instillation. This is the first study showing that L-HA plays an important role in the pathophysiology of acid aspiration-induced lung injury and identifying H-HA, administered post-HCl, to be a potential therapeutic agent to compete with L-HA and reverse the lung injury.
Increased L-HA levels have been detected in a number of lung diseases, such as pulmonary fibrosis, chronic obstructive pulmonary disease, and asthma. Furthermore, L-HA is both necessary and sufficient for the development of AHR after exposure to ozone (18, 20) or Cl2 gas (34). On the contrary, H-HA protects the lung from injury induced by ozone exposure (19), bleomycin administration (26), cigarette smoke inhalation (22), sepsis (39), and Cl2 inhalation (34). This allows us to generalize the concept that HA mediates injury by its low-molecular form, L-HA, but its high-molecular form, H-HA, could protect lung against that injury.
HA is synthesized by membrane-bound enzymes. There are at least three mammalian hyaluronan synthases (HAS1, HAS2, and HAS3) (45, 56). HAS1 generally produces larger size HA while HAS3 produces L-HA, whereas HAS2 generates HA with a very large size (average molecular mass of 2,000 kDa) (23, 24). Studies utilizing HAS gene-deficient mice indicate that HAS2 deletion results in lethal defects in cardiac development and vascular abnormalities (7) and HAS2 induction protects mice from bleomycin-induced epithelial apoptosis and fibrosis (26). Our data demonstrate that HAS1 and HAS2 were significantly decreased in mouse lung tissue after HCl instillation; however, H-HA treatment recovered the expression of HAS1 and HAS2. H-HA-producing HAS gene expression was suppressed by HCl instillation, which results in less H-HA to counterbalance the effects of L-HA.
The murine model of HCl instillation-induced ALI has helped elucidate the role of neutrophil accumulation in the lung. Low pH acid instillation is a neutrophil-dependent form of lung injury that is characterized by injury of the airway and alveolar epithelium; moreover, acid instillation also results in injury to the capillary endothelium by a mechanism that appears to require circulating neutrophils (14, 29). Our data demonstrate that, after HCl instillation, L-HA accumulates in lung tissue, especially that located in the peribronchial space and alveolar/capillary septae, where there is also significant accumulation of neutrophils. Activated neutrophils could break down structural HA (directly and indirectly) and contribute to the release of L-HA. Indeed, neutrophil depletion and MPO deficiency led to a significant decrease in L-HA generation after HCl instillation, and MPO−/− mice manifested less severe injury post-HCl exposure. It is thus likely that sustained production of L-HA is achieved through neutrophil influx, which sustains the inflammatory response, barrier disruption and, AHR development.
Recent studies showed that L-HA increases vascular permeability by activating small GTPases (such as RhoA), which in turn induce actin cytoskeletal reorganization; inhibit endothelial cell-cell contacts (36),; activate ROCK, their downstream kinases (28), and Ca2+ channels (such as TRPC); and increase Ca2+ influx from the extracellular milieu to the cytoplasm (46). Moreover, ROCK2+/− mice showed reduced AHR via effects within airway smooth muscle cells in murine asthma model (28). In addition, airway smooth muscle cells and activation of RhoA and ROCKs enhance methacholine-induced contraction via retention of myosin light chain in a phosphorylated state, which favors contraction (28, 31). We found a significant increase in RhoA and ROCK2 activity after HCl instillation, which was ameliorated through blockade of L-HA signaling, either through competitive inhibition by H-HA or by genetic deficiency of HA receptor CD44. Thus our work identifies L-HA as a natural and specific target for pharmacological treatment of HCl instillation-induced AHR. We also report here that L-HA decreases human primary bronchial epithelial cell barrier resistance, while H-HA reverses the resistance back to normal, together with increased wet-to-dry weight ratio and Kf in lung tissue after HCl exposure. We hereby propose that pulmonary edema and barrier disruption due to HCl instillation is mediated by L-HA. Taken together, we postulate that reactive intermediates, which are produced by neutrophils after HCl exposure, lead to breakdown of structural HA and release of L-HA, which in sequence binds to its cognate receptor CD44 and mediates the progression of lung injury via activation of RhoA and ROCK2 (Fig. 8).
Fig. 8.
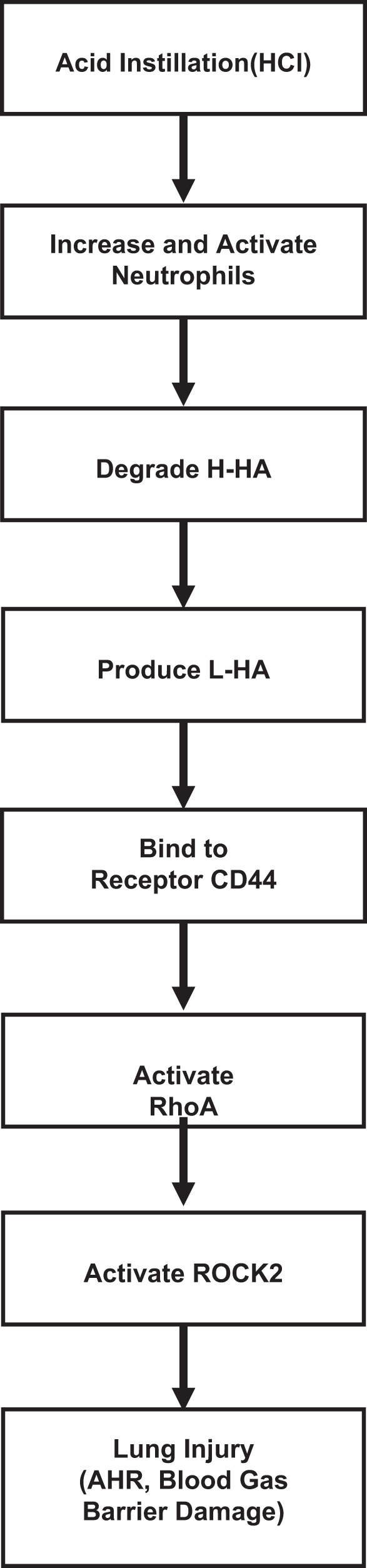
Overall scheme of putative mechanisms contributing to HCl instillation-induced lung injury. AHR, airway hyperresponsiveness.
We also detected HA in BALF from mechanically ventilated patients with or without pneumonia, and patients with bacterial infection have a high level of HA. Similarly, the study also showed elevated levels of BALF HA were associated with worsening lung injury scores (12). Exogenous administration of H-HA has been shown to prevent a variety of diseases, including lung diseases (8, 16, 30, 58) and disorders of vascular barrier disruption (53). Oropharyngeal administration of exogenous H-HA before or after ozone or Cl2 gas exposure significantly attenuates AHR in mice (18, 34), as well as in our work. Thus H-HA emerges as a viable treatment option for the treatment of lung injury. Notably, there are existing H-HA preparations that are available for use in humans: the H-HA formulation we used, Yabro, is approved for use in European countries as a solution for nebulization and inhaled H-HA was shown to improve tolerability of hypertonic saline and increase compliance in cystic fibrosis patients (32, 41). Thus H-HA has substantial potential as adjunct treatment for lung injury.
In conclusion, our work identifies a novel role for L-HA in mediating HCl instillation-induced lung injury. We demonstrate that neutrophils contribute significantly to the sustained inflammatory response by degrading HA to L-HA via reactive intermediates. Furthermore, HCl exposure leads to a downregulation of HAS1 and HAS2 expression and thus H-HA production, further shifting the HA balance toward lower molecular weight forms. L-HA predominance leads to activation of RhoA and ROCK2, and thus to AHR, as well as blood gas barrier damage. However, exogenous H-HA is able to reverse all the observed effects. Thus our work identifies a potential therapeutic adjunct for lung injury that is already available for human use.
GRANTS
This work was supported by grant from Foundation for Anesthesia Education and Research (FAER, 2015) research fellowship (to W. Song) and in part by funds from the Division of Intramural Research, National Institute of Environmental Health Sciences Grants Z01-ES-102605 (to S. Garantziotis) and 5U01-ES-026458 02 and 1U01ES02769701 (to S. Matalon). T. Zhou was funded by the China Scholarship Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.Z., W.S., and S.M. conceived and designed research; T.Z., Z.Y., M.-Y.J., C.S.T., and B.M.W. performed experiments; T.Z., Z.Y., M.-Y.J., C.S.T., B.M.W., S.A., S.G., and S.M. analyzed data; T.Z., M.-Y.J., I.A., C.S.T., B.M.W., S.A., S.G., and S.M. interpreted results of experiments; T.Z., M.-Y.J., C.S.T., B.M.W., S.A., and S.G. prepared figures; T.Z., S.A., S.G., and S.M. drafted manuscript; T.Z., B.M.W., S.A., S.G., W.S., and S.M. edited and revised manuscript; T.Z., Z.Y., M.-Y.J., I.A., C.S.T., B.M.W., J.-F.P., S.A., S.G., and S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Judy Creighton, Changchun Ren, Yilan Liu, and Kevin Harrod for technical support in the generation of the article. We also thank the IBSA, Institut Biochimique, Lugano, Switzerland, which generous donated Yabro for the experiment.
REFERENCES
- 1.Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, Matalon S. Heme Attenuation Ameliorates Irritant Gas Inhalation-Induced Acute Lung Injury. Antioxid Redox Signal 24: 99–112, 2016. doi: 10.1089/ars.2015.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allegra L, Della Patrona S, Petrigni G. Hyaluronic acid : perspectives in lung diseases. Handb Exp Pharmacol 207: 385–401, 2012. doi: 10.1007/978-3-642-23056-1_17. [DOI] [PubMed] [Google Scholar]
- 3a.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA 307: 2526–2533, 2012. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrow CS, Alarie Y, Warrick JC, Stock MF. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch Environ Health 32: 68–76, 1977. doi: 10.1080/00039896.1977.10667258. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Chanez P, Lacoste JY, Enander I, Venge P, Peterson C, Ahlstedt S, Michel FB, Godard P. Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J Allergy Clin Immunol 88: 649–660, 1991. doi: 10.1016/0091-6749(91)90159-L. [DOI] [PubMed] [Google Scholar]
- 7.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med 8: 850–855, 2002. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 8.Cantor JO. Potential therapeutic applications of hyaluronan in the lung. Int J Chron Obstruct Pulmon Dis 2: 283–288, 2007. [PMC free article] [PubMed] [Google Scholar]
- 9.Cantor JO, Cerreta JM, Ochoa M, Ma S, Liu M, Turino GM. Therapeutic effects of hyaluronan on smoke-induced elastic fiber injury: does delayed treatment affect efficacy? Lung 189: 51–56, 2011. doi: 10.1007/s00408-010-9271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creighton J, Jian M, Sayner S, Alexeyev M, Insel PA. Adenosine monophosphate-activated kinase alpha1 promotes endothelial barrier repair. FASEB J 25: 3356–3365, 2011. doi: 10.1096/fj.10-179218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Prost N, Pham T, Carteaux G, Mekontso Dessap A, Brun-Buisson C, Fan E, Bellani G, Laffey J, Mercat A, Brochard L, Maître B; LUNG SAFE Investigators; ESICM Trials Group; REVA Network . Etiologies, diagnostic work-up and outcomes of acute respiratory distress syndrome with no common risk factor: a prospective multicenter study. Ann Intensive Care 7: 69, 2017. doi: 10.1186/s13613-017-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito AJ, Bhatraju PK, Stapleton RD, Wurfel MM, Mikacenic C. Hyaluronic acid is associated with organ dysfunction in acute respiratory distress syndrome. Crit Care 21: 304, 2017. doi: 10.1186/s13054-017-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Exarhos ND, Logan WD Jr, Abbott OA, Hatcher CR Jr. The Importance of Ph and Volume in Tracheobronchial Aspiration. Dis Chest 47: 167–169, 1965. doi: 10.1378/chest.47.2.167. [DOI] [PubMed] [Google Scholar]
- 14.Folkesson HG, Matthay MA, Hébert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 96: 107–116, 1995. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaffney J, Matou-Nasri S, Grau-Olivares M, Slevin M. Therapeutic applications of hyaluronan. Mol Biosyst 6: 437–443, 2010. doi: 10.1039/B910552M. [DOI] [PubMed] [Google Scholar]
- 17.Garantziotis S, Brezina M, Castelnuovo P, Drago L. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am J Physiol Lung Cell Mol Physiol 310: L785–L795, 2016. doi: 10.1152/ajplung.00168.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 19.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 291: 19257–19258, 2016. doi: 10.1074/jbc.A116.802400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med 181: 666–675, 2010. doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 21.Gessner MA, Doran SF, Yu Z, Dunaway CW, Matalon S, Steele C. Chlorine gas exposure increases susceptibility to invasive lung fungal infection. Am J Physiol Lung Cell Mol Physiol 304: L765–L773, 2013. doi: 10.1152/ajplung.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang PM, Syrkina O, Yu L, Dedaj R, Zhao H, Shiedlin A, Liu YY, Garg H, Quinn DA, Hales CA. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology 15: 1131–1139, 2010. doi: 10.1111/j.1440-1843.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 23.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274: 25085–25092, 1999. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson A, Brinck J, Briskin MJ, Spicer AP, Heldin P. Expression of human hyaluronan synthases in response to external stimuli. Biochem J 348: 29–35, 2000. doi: 10.1042/bj3480029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jian MY, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol 305: L844–L855, 2013. doi: 10.1152/ajplung.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 27.Kakehi K, Kinoshita M, Yasueda S. Hyaluronic acid: separation and biological implications. J Chromatogr B Analyt Technol Biomed Life Sci 797: 347–355, 2003. doi: 10.1016/S1570-0232(03)00479-3. [DOI] [PubMed] [Google Scholar]
- 28.Kasahara DI, Mathews JA, Ninin FM, Wurmbrand AP, Liao JK, Shore SA. Role of ROCK2 in CD4+cells in allergic airways responses in mice. Clin Exp Allergy 47: 224–235, 2017. doi: 10.1111/cea.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight PR, Druskovich G, Tait AR, Johnson KJ. The role of neutrophils, oxidants, and proteases in the pathogenesis of acid pulmonary injury. Anesthesiology 77: 772–778, 1992. doi: 10.1097/00000542-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Kogan G, Soltés L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 29: 17–25, 2007. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 31.Kume H. RhoA/Rho-kinase as a therapeutic target in asthma. Curr Med Chem 15: 2876–2885, 2008. doi: 10.2174/092986708786242831. [DOI] [PubMed] [Google Scholar]
- 32.Lamas A, Marshburn J, Stober VP, Donaldson SH, Garantziotis S. Effects of inhaled high-molecular weight hyaluronan in inflammatory airway disease. Respir Res 17: 123, 2016. doi: 10.1186/s12931-016-0442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauer ME, Dweik RA, Garantziotis S, Aronica MA. The rise and fall of hyaluronan in respiratory diseases. Int J Cell Biol 2015: 712507, 2015. doi: 10.1155/2015/712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazrak A, Jurkuvenaite A, Ness EC, Zhang S, Woodworth BA, Muhlebach MS, Stober VP, Lim YP, Garantziotis S, Matalon S. Inter-α-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in ΔF508 mice. Am J Respir Cell Mol Biol 50: 953–962, 2014. doi: 10.1165/rcmb.2013-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennon FE, Singleton PA. Hyaluronan regulation of vascular integrity. Am J Cardiovasc Dis 1: 200–213, 2011. [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Potts-Kant EN, Garantziotis S, Foster WM, Hollingsworth JW. Hyaluronan signaling during ozone-induced lung injury requires TLR4, MyD88, and TIRAP. PLoS One 6: e27137, 2011. doi: 10.1371/journal.pone.0027137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 38.Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis 188: 919–926, 2003. doi: 10.1086/377642. [DOI] [PubMed] [Google Scholar]
- 39.Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H, Sheidlin A, Syrkina O, Huang PM, Garg HG, Hales CA, Quinn DA. High-molecular-weight hyaluronan–a possible new treatment for sepsis-induced lung injury: a preclinical study in mechanically ventilated rats. Crit Care 12: R102, 2008. doi: 10.1186/cc6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Q, Mundy M, Chambers E, Lange T, Newton J, Borgas D, Yao H, Choudhary G, Basak R, Oldham M, Rounds S. Alda-1 protects against acrolein-induced acute lung injury and endothelial barrier dysfunction. Am J Respir Cell Mol Biol 57: 662–673, 2017. doi: 10.1165/rcmb.2016-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macchi A, Castelnuovo P, Terranova P, Digilio E. Effects of sodium hyaluronate in children with recurrent upper respiratory tract infections: results of a randomised controlled study. Int J Immunopathol Pharmacol 26: 127–135, 2013. doi: 10.1177/039463201302600112. [DOI] [PubMed] [Google Scholar]
- 42.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med 344: 665–671, 2001. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 43.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM; Acute Lung Injury in Animals Study Group . An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44: 725–738, 2011. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald B, McAvoy EF, Lam F, Gill V, de la Motte C, Savani RC, Kubes P. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med 205: 915–927, 2008. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald JA, Camenisch TD. Hyaluronan: genetic insights into the complex biology of a simple polysaccharide. Glycoconj J 19: 331–339, 2002. doi: 10.1023/A:1025369004783. [DOI] [PubMed] [Google Scholar]
- 46.Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol 209: 31–41, 2006. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- 47.Modelska K, Pittet JF, Folkesson HG, Courtney Broaddus V, Matthay MA. Acid-induced lung injury. Protective effect of anti-interleukin-8 pretreatment on alveolar epithelial barrier function in rabbits. Am J Respir Crit Care Med 160: 1450–1456, 1999. doi: 10.1164/ajrccm.160.5.9901096. [DOI] [PubMed] [Google Scholar]
- 48.Oh H, Siano B, Diamond S. Neutrophil isolation protocol. J Vis Exp 17: 745, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrigni G, Allegra L. Aerosolised hyaluronic acid prevents exercise-induced bronchoconstriction, suggesting novel hypotheses on the correction of matrix defects in asthma. Pulm Pharmacol Ther 19: 166–171, 2006. doi: 10.1016/j.pupt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Raghavendran K, Davidson BA, Mullan BA, Hutson AD, Russo TA, Manderscheid PA, Woytash JA, Holm BA, Notter RH, Knight PR. Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol 289: L134–L143, 2005. doi: 10.1152/ajplung.00390.2004. [DOI] [PubMed] [Google Scholar]
- 51.Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med 39: 818–826, 2011. doi: 10.1097/CCM.0b013e31820a856b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt EP, Li G, Li L, Fu L, Yang Y, Overdier KH, Douglas IS, Linhardt RJ. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem 289: 8194–8202, 2014. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, Moreno-Vinasco L, Garcia JG. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol 299: L639–L651, 2010. doi: 10.1152/ajplung.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a beta2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol 45: 88–94, 2011. doi: 10.1165/rcmb.2010-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Summerhill EM, Hoyle GW, Jordt SE, Jugg BJ, Martin JG, Matalon S, Patterson SE, Prezant DJ, Sciuto AM, Svendsen ER, White CW, Veress LA; ATS Terrorism and Inhalational Disasters Section of the Environmental, Occupational, and Population Health Assembly . An official American Thoracic Society Workshop Report: chemical inhalational disasters. biology of lung injury, development of novel therapeutics, and medical preparedness. Ann Am Thorac Soc 14: 1060–1072, 2017. doi: 10.1513/AnnalsATS.201704-297WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem 272: 13997–14000, 1997. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 57.Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ 2nd, Patel RP, Matalon S. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol 300: L362–L369, 2011. doi: 10.1152/ajplung.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yadav AK, Mishra P, Agrawal GP. An insight on hyaluronic acid in drug targeting and drug delivery. J Drug Target 16: 91–107, 2008. doi: 10.1080/10611860701794296. [DOI] [PubMed] [Google Scholar]