Abstract
There are multiple proposed mechanisms for the pathophysiology of heart failure (HF) with preserved ejection fraction (HFpEF). We hypothesized that coronary microvascular dysfunction is common in these patients. In a prospective, observational study, patients undergoing cardiac catheterization with HFpEF [left ventricular (LV) ejection fraction ≥ 50% and with clinical HF] were compared with similar patients without HFpEF. Patients with ≥50% stenosis were excluded, and coronary flow reserve (CFR) and the index of microvascular resistance (IMR) were measured after adenosine administration using a guidewire, with CFR ≤ 2 and IMR ≥ 23 being abnormal. Baseline characteristics and CFR and IMR were compared in 30 HFpEF patients and 14 control subjects. Compared with control subjects, HFpEF patients were older (65.4 ± 9.6 vs. 55.1 ± 3.1 yr, P < 0.01), had higher numbers of comorbidities (4.4 ± 1.5 vs. 2.6 ± 1.9, P = 0.002), had higher median B-type natriuretic peptide [161 (interquartile range: 75–511) pg/dl vs. 37 (interquartile range: 18.5–111) pg/dl, P < 0.01], and had higher LV end-diastolic pressure (17.8 ± 4.2 vs. 8.4 ± 4.2, P < 0.01). HFpEF patients had lower CFR (2.55 ± 1.60 vs. 3.84 ± 1.89, P = 0.024) and higher IMR (26.7 ± 10.3 vs. 19.7 ± 9.7 units, P = 0.037) than control subjects. Most (71.4%) control subjects had normal coronary physiology, whereas 36.7% of HFpEF patients had both abnormal CFR and IMR and another 36.7% had either abnormal CFR or IMR. In conclusion, this is the first study that has reported invasively determined CFR and IMR in HFpEF patients. We demonstrated the presence of four distinct coronary physiology groups in HFpEF patients. Investigation into the potential mechanisms for these findings is needed.
NEW & NOTEWORTHY In this prospective observational study of patients with heart failure with preserved ejection fraction (HFpEF), we found that patients with HFpEF had more abnormalities of coronary flow and resistance than asymptomatic control patients, indicating that coronary microvascular dysfunction may play a role in the HFpEF disease process.
Keywords: heart failure, heart failure with preserved ejection fraction, microvascular
INTRODUCTION
Heart failure (HF) with preserved ejection fraction (HFpEF) is a major public health problem, accounting for 50% of HF admissions (34), with similar mortality and morbidity to HF with reduced ejection fraction (HFrEF) (33). There is a differential etiology between HFpEF and HFrEF, thought to be from the different set of comorbidities associated with HFpEF (16). Paulus and Tschöpe (36) proposed that the comorbidities in HFpEF lead to an inflammatory milieu that ultimately leads to coronary microvascular dysfunction (CMD) and left ventricular (LV) and vascular stiffness. Conditions associated with HFpEF such as obstructive sleep apnea and obesity are linked to systemic inflammation, which, in turn, leads to reduced nitric oxide availability (17, 43). Nitric oxide (NO), through the activation of guanylate cyclase, leads to the production of cGMP, the activator of PKG (44). PKG, in turn, phosphorylates and activates titin, a spring-like protein responsible for myocyte diastolic recoil and distensibility (4, 22). Therefore, reduction in NO through inflammation leads to increased diastolic stiffness through downstream effects on titin. A parallel process of inflammation stimulating the conversion fibroblasts into myofibroblasts, resulting in collagen formation and reduced matrix metallaproteinases, also increases diastolic stiffness in HFpEF (26, 46). Although animal models have confirmed the association between endothelial dysfunction and HFpEF (1, 11) and postmortem studies of HFpEF patients have demonstrated microvascular rarefaction (29), there has been no study to date in alive humans with HFpEF to invasively evaluate the degree of CMD in these patients.
Coronary flow reserve (CFR) and the index of microvascular resistance (IMR) are invasive techniques to measure coronary blood flow (20) and coronary microvascular resistance (9), respectively. In patients with nonobstructive coronary artery disease (CAD), abnormal CFR and IMR is associated with poor outcomes (25). CFR and IMR can therefore be used to determine the presence and severity of CMD in patients without obstructive CAD.
We sought to assess for CMD in HFpEF patients in vivo and compare them with non-HFpEF control subjects using coronary guidewire techniques in the cardiac catheterization laboratory. We predicted that a high percentage of these patients would have decreased flow and increased resistance. Identification of microvascular function of HFpEF patients may provide targets for intervention and improved outcomes.
MATERIALS AND METHODS
Patient population.
This was a prospective, two-center observational study conducted at the University of Chicago Medical Center and Northwestern Memorial Hospital between January 1, 2015, and May 1, 2017. HFpEF patients were selected by data query of inpatient electronic medical records using the following search criteria: diagnosis of HF, B-type natriuretic peptide (BNP) >100 pg/ml, or administration of two or more doses of intravenous diuretics. The electronic medical record was then examined by research staff, and patients were offered inpatient consultation or followup in the respective institutions’ HFpEF clinic if they met all of the following the inclusion criteria: age ≥ 21 yr, LV ejection fraction ≥ 50%, and meeting Framingham criteria for HF (27). Exclusion criteria were prior myocardial infarction, severe valvular disease determined by echocardiogram, cardiac transplantation, patients who previously had significantly reduced LV ejection fraction (<35%), and infiltrative disorders and genetic cardiomyopathies. Patients who met these criteria and who had clinical indication for cardiac catheterization were further screened for inclusion. Further inclusion into the study required absence of obstructive CAD, defined as stenosis ≥ 50% diameter stenosis in any epicardial coronary artery or branch coronary artery >2.0 mm in diameter, determined at the time of catheterization.
Control subjects were recruited if they had no evidence of HF, had normal LV function, and had a clinical indication for cardiac catheterization. Control subjects were excluded if obstructive CAD was present at the time of cardiac catheterization. Eligible patients underwent a coronary physiology study at the time of the cardiac catheterization and echocardiography after the cardiac catheterization if not performed in the prior 3 mo. The study protocol was approved by the University of Chicago Medical Center and Northwestern Memorial Hospital Institutional Review Boards. All enrolled patients provided written informed consent.
Data collection.
Baseline demographic, clinical, laboratory, and medication information was obtained in all subjects through analysis of the electronic medical record at each institution. HFpEF patients underwent right and left heart catheterization and echocardiography analysis. Control subjects underwent left heart catheterization and echocardiography analysis.
Coronary angiography, hemodynamics, and coronary physiology.
Diagnostic angiography was performed using standard procedures outlined in the 2012 ACCF/SCAI Expert Consensus Document of Cardiac Catheterization Laboratory Standards Update (3). LV end-diastolic pressure was measured using either a Judkins right or pigtail catheter with standard fluid-filled pressure transducers. Right heart catheterization was performed using the internal jugular approach and a Swan-Ganz catheter for pressure measurement and cardiac output measurement using both thermodilution and Fick methods. Coronary lesion severity was evaluated at the time of angiography, and if no obstructive CAD was identified, a guide catheter was placed in the left main coronary artery and heparin was administered (50–70 units/kg) for a goal-activated clotting time of 250 s or greater. Intracoronary nitroglycerin (100–200 µg) was injected through the guide catheter, and a 0.014-in. coronary wire (Certus Pressurewire, St. Jude Medical) was calibrated, equalized to the guide catheter pressure with the pressure wire sensor positioned at the tip of the catheter, and then advanced to the distal two-thirds of the left anterior descending coronary artery. CFR, IMR, and fractional flow reserve (FFR) were then measured by previously described methods (9, 38). Briefly, the shaft of the pressure acts as a proximal thermistor by detecting changes in temperature-dependent electrical resistance, while the sensor near the tip of the wire simultaneously measures pressure and temperature. The mean transit time (Tmn) of room temperature saline was determined by thermodilution. Three injections of 3 ml room temperature saline were injected down the left anterior descending coronary artery, and resting Tmn was measured and averaged to determine flow. Care was taken to ensure stability of the guiding catheter into the left main coronary artery, precise equalization of the mean aortic pressure (Pa) and wire pressure, and reproducible shape, size, and Tmn of each thermodilution curve. Intravenous adenosine (140 µg·kg−1·min−1) was administered through the internal jugular venous sheath in the patients with HFpEF and control subjects who underwent right heart catheterization to induce maximum hyperemia, after which three more injections of 3 ml room temperature saline were injected for hyperemic Tmn. Simultaneous measurements were made of Pa within the guide catheter and the mean distal coronary artery pressure (Pd) within the pressure wire at resting and hyperemic states. CFR was calculated using the ratio of hyperemic blood flow (1/Tmn,hyperemia) to resting blood flow (1/Tmn,resting), or simply Tmn,resting/Tmn,hyperemia. IMR was calculated using the ratio of hyperemic Pd to hyperemic flow, or simply Pd × Tmn,hyperemia. Cutoff values (abnormal) of ≤2.0 for CFR and ≥23 units for IMR were used to divide patients into four coronary physiology groups based on the presence or absence of abnormal CFR and IMR (25). Patients were classified as follows: normal CFR and normal IMR (group A), normal CFR and abnormal IMR (group B), abnormal CFR and normal IMR (group C), and abnormal CFR and abnormal IMR (group D).
Echocardiography.
All participants underwent echocardiography (including color and pulse-wave Doppler, tissue Doppler, and strain imaging) within 30 days of cardiac catheterization. Transthoracic, Doppler, and tissue Doppler echocardiography was performed using either Philips iE33 imaging system (Philips Healthcare, Eindhoven, The Netherlands) or General Electric Vivid E (GE Healthcare) machines according to standard American Society of Echocardiography protocol (37). Global strain obtained from speckle-tracking images was obtained using standard parasternal short and apical images obtained at 40–80 frames/s and analyzed offline using two-dimensional Cardiac Performance Analysis 4.5 (TomTec, Munich, Germany) and EchoPAC software (GE Healthcare) (12). Two-dimensional chamber quantification and Doppler parameters were obtained and analyzed using Excelera (Phillips Healthcare) according to echocardiography guidelines for chamber quantification and diastolic dysfunction (24, 30). For left atrial (LA) volume and function, the biplane method of disks was used using apical four- and two- chamber views at the end-systolic frame preceding mitral valve opening in dedicated views to avoid foreshortening. Speckle tracking of the LA was performed by manually tracing the endocardial border, excluding patients with a greater than one segment dropout, missing views, or foreshortening, using the QRS complex as the zero reference, with all longitudinal LA strain values as positive. The components of LA function were defined as follows: LA reservoir strain, peak longitudinal LA strain; LA booster strain, longitudinal LA strain measured between the onset of the P wave and onset of the QRS complex; and LA conduit strain, LA reservoir-booster strain. Strain measurements were calculated by averaging the apical four- and two-chamber strain values.
Statistical analysis.
Baseline clinical, laboratory, echocardiographic, and hemodynamic parameters were compared between HFpEF and control groups using a Kruskal-Wallis test for continuous variables and a Pearson χ2-test for categorical variables. In addition, χ2-analysis and log-rank testing were used to compare the distribution of patients from the HFpEF and control populations into the four coronary physiology groups. As an exploratory analysis, clinical, laboratory, echocardiographic, and hemodynamic variables of coronary physiology groups B−D patients with HFpEF were compared with those of coronary physiology group A patients with HFpEF using descriptive statistics as above. Statistical analysis was performed using Stata 14 (StataCorp, College Station, TX).
RESULTS
Clinical characteristics.
There were 30 patients with HFpEF and 14 control subjects. The average age of the HFpEF group was higher than the control group (65.4 ± 9.6 vs. 55.1 ± 3.1 yr, P < 0.01; Table 1). The distribution of women in the study was similar (63.3% vs. 85.7%, P = not significant). The presence of hypertension was higher in the HFpEF group than in the control group (93.3% vs. 64.3%, P = 0.014). The average comorbidity number was significantly higher in the HFpEF group compared with the control group (4.4 ± 1.5 vs. 2.6 ± 1.9, P = 0.0022). Although blood urea nitrogen was higher in patients with HFpEF compared with control subjects (21.2 ± 10.0 vs. 12.5 ± 4.0 mg/dl, P < 0.01), serum creatinine and the estimated glomerular filtration rate were similar.
Table 1.
Baseline characteristics
| Heart Failure with Preserved Ejection Fraction (n = 30) | Control (n = 14) | P Value | |
|---|---|---|---|
| Age, yr | 65.4 ± 9.6 | 55.1 ± 3.1 | 0.0037 |
| Women | 19 (63.3) | 12 (85.7) | 0.14 |
| Men | 11 (36.7) | 2 (14.3) | |
| Clinical characteristics | |||
| Coronary artery disease | 9 (30.0) | 3 (21.4) | 0.56 |
| Hypertension | 28 (93.3) | 9 (64.3) | 0.014 |
| Chronic kidney disease | 10 (33.3) | 1 (7.1) | 0.064 |
| Hyperlipidemia | 23 (76.7) | 10 (71.4) | 0.72 |
| Chronic obstructive pulmonary disease or asthma | 9 (30.0) | 1 (7.1) | 0.096 |
| Cirrhosis | 2 (6.7) | 0 | 0.33 |
| Diabetes mellitus | 18 (60.0) | 6 (42.9) | 0.30 |
| Atrial fibrillation | 4 (13.3) | 0 | 0.16 |
| Ever smoker | 14 (46.7) | 4 (28.6) | 0.27 |
| New York Heart Association class III−IV | 11 (36.7) | 0 | 0.0081 |
| Average number of comorbidities | 4.4 ± 1.5 | 2.6 ± 1.9 | 0.0022 |
| Physical and laboratory findings | |||
| Weight, kg | 107.2 ± 26.6 (n = 29) | 87.9 ± 28.3 | 0.035 |
| Body mass index, kg/m2 | 38.3 ± 9.3 | 34.2 ± 11.0 | 0.20 |
| Body mass index >30.0, % | 83.3 (n = 30) | 57.1 (n = 14) | 0.064 |
| Systolic blood pressure, mmHg | 132.4 ± 19.6 | 133.0 ± 28.6 | 0.94 |
| Diastolic blood pressure, mmHg | 74.3 ± 18.3 | 73.9 ± 15.0 | 0.95 |
| Heart rate, beats/min | 76.4 ± 15.5 | 77.8 ± 12.2 | 0.76 |
| Serum creatinine, mg/dl | 1.43 ± 2.00 | 0.89 ± 0.17 | 0.32 |
| Blood urea nitrogen, mg/dl | 21.2 ± 10.0 | 12.5 ± 4.0 | 0.0032 |
| Estimated glomerular filtration rate, ml·min−1·1.73 m−2 | 61.6 ± 33.2 | 74.3 ± 30.9 | 0.31 |
| Hemoglobin, g/dl | 12.3 ± 2.0 | 12.9 ± 1.5 | 0.33 |
| Albumin, g/dl | 3.73 ± 0.42 (n = 29) | 3.93 ± 0.32 | 0.13 |
| B-type natriuretic peptide, pg/dl | 161 (75−511) (n = 27) | 37 (18.5−111) (n = 12) | 0.0033 |
| Na+, mEq/l | 140.2 ± 2.7 | 140.8 ± 3.0 | 0.54 |
| Total cholesterol, mg/dl | 156.6 ± 44.6 (n = 28) | 157.8 ± 38.1 (n = 11) | 0.94 |
| High-density lipoprotein, mg/dl | 50.0 ± 15.4 (n = 28) | 53.2 ± 18.5 (n = 11) | 0.59 |
| Low-density lipoprotein, mg/dl | 84.3 ± 35.0 (n = 27) | 88.4 ± 42.8 (n = 11) | 0.76 |
| Triglycerides, mg/dl | 100.0 ± 61.2 (n = 27) | 81.2 ± 59.1 (n = 11) | 0.39 |
| Medications | |||
| ACE inhibitor | 12 (40.0) | 4 (28.6) | 0.47 |
| ARB | 3 (10.0) | 4 (28.6) | 0.12 |
| ACE/ARB | 15 (50.0) | 6 (42.9) | 0.67 |
| β-Adrenergic antagonist | 18 (60.0) | 3 (21.4) | 0.017 |
| Loop diuretic | 25 (83.3) | 4 (28.6) | 0.00020 |
| Ca2+ channel blocker | 10 (33.3) | 4 (28.6) | 0.76 |
| Aspirin | 20 (66.7) | 7 (50.0) | 0.30 |
| Statin | 22 (73.3) | 10 (76.9) (n = 13) | 0.81 |
| Antiplatelet | 2 (6.7) | 2 (14.3) | 0.42 |
| Nitrate | 2 (6.7) | 2 (14.3) | 0.42 |
| Vasodilator | 1 (3.3) | 0 | 0.50 |
| Antiarrhythmic | 4 (13.3) | 0 | 0.16 |
Values are means ± SD, n (%), or medians (interquartile ranges); n = number of subjects. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Echocardiography, hemodynamics, and angiography.
Echocardiograms were obtained in 42 of 44 enrolled patients (Table 2). Patients with HFpEF had greater LV wall thickness (1.19 ± 0.23 vs. 1.01 ± 0.20 cm, P = 0.022), mitral valve inflow early mitral filling velocity (95.2 ± 24.9 vs. 73.5 ± 11.2 cm/s, P = 0.0085), and early mitral filling/early diastolic tissue velocity (12.4 ± 3.7 vs. 9.4 ± 2.3 cm/s, P = 0.017); however, they had similar LV and LA volume indexes compared with control subjects. Right heart catheterization was performed on 29 of 30 patients with HFpEF and 6 of 14 control subjects. Of patients with full hemodynamics, the HFpEF cohort had higher mean right atrial pressure (11.6 ± 3.6 vs. 4.7 ± 3.9 mmHg, P < 0.001), mean right ventricular systolic pressure (46.7 ± 14.3 vs. 25.5 ± 3.6 mmHg, P = 0.0011), mean pulmonary arterial pressure (30.5 ± 9.2 vs. 15.7 ± 2.9 mmHg, P < 0.001), mean pulmonary capillary wedge pressure (19.1 ± 5.6 vs. 8.8 ± 2.7 mmHg, P < 0.001), and mean LV end-diastolic pressure (17.8 ± 4.2 vs. 8.4 ± 4.2, P < 0.001; Table 3). Systemic vascular resistance was lower in the HFpEF group compared with the control group (1,250 ± 430 vs. 1,664 ± 243 dyn·s/cm5, P = 0.046). Cardiac output, cardiac index, and coronary artery stenosis did not differ significantly among patients with HFpEF and control subjects.
Table 2.
Echocardiographic parameters
| Heart Failure with Preserved Ejection Fraction | Control | P Value | |
|---|---|---|---|
| n | 30 | 14 | |
| Two-dimensional measurements | |||
| LV septal wall thickness, cm | 1.19 ± 0.23 | 1.01 ± 0.20 (n = 12) | 0.022 |
| LV end-diastolic dimension, cm | 4.54 ± 0.66 | 4.66 ± 0.39 (n = 12) | 0.57 |
| LV end-diastolic volume index | 52.2 ± 12.4 (n = 27) | 55.2 ± 9.4 (n = 11) | 0.47 |
| LV end-systolic dimension, cm | 2.52 ± 0.78 | 3.01 ± 0.63 (n = 12) | 0.062 |
| LV end-systolic volume index | 21.6 ± 9.2 (n = 28) | 22.1 ± 6.5 (n = 12) | 0.86 |
| LA volume index | 32.8 ± 9.1 (n = 26) | 30.3 ± 5.5 (n = 10) | 0.43 |
| Doppler measurements | |||
| MV E velocity, cm/s | 95.2 ± 24.9 | 73.5 ± 11.2 (n = 11) | 0.0085 |
| MV A velocity, cm/s | 86.4 ± 26.4 (n = 27) | 70.7 ± 24.1 (n = 11) | 0.097 |
| MV E-to-A ratio | 1.22 ± 0.81 (n = 27) | 1.18 ± 0.51 (n = 11) | 0.89 |
| e′ (lateral), cm/s | 9.73 ± 3.20 (n = 28) | 9.40 ± 2.49 (n = 11) | 0.76 |
| e′ (septal), cm/s | 7.00 ± 2.15 (n = 28) | 6.87 ± 1.74 (n = 11) | 0.87 |
| e′ (average), cm/s | 8.17 ± 2.32 (n = 27) | 6.81 ± 3.49 (n = 13) | 0.15 |
| E/e′ (lateral), cm/s | 10.8 ± 3.8 (n = 28) | 8.3 ± 2.6 (n = 11) | 0.055 |
| E/e′ (septal), cm/s | 14.8 ± 4.9 (n = 28) | 11.1 ± 2.6 (n = 11) | 0.026 |
| E/e′ (average), cm/s | 12.4 ± 3.7 (n = 27) | 9.5 ± 2.2 (n = 11) | 0.019 |
| Mitral deceleration time, ms | 207 ± 57 | 222 ± 49 (n = 11) | 0.47 |
| Average isovolumetric relaxation time, ms | 73 ± 27 (n = 27) | 83 ± 31 (n = 10) | 0.36 |
| Average tricuspid regurgitation velocity, m/s | 2.86 ± 0.59 (n = 19) | 2.42 ± 0.12 (n = 5) | 0.12 |
| Diastolic dysfunction grade | 0.90 ± 1.00 (n = 21) | 0.40 (n = 10) | 0.14 |
| Normal | 10 (47.6) | 6 (60.0) | |
| Grade 1 | 4 (19.0) | 4 (40.0) | |
| Grade 2 | 6 (28.6) | 0 (0) | |
| Grade 3 | 1 (4.8) | 0 (0) | |
| Strain measurements | |||
| LV global longitudinal strain, % | −17.6 ± 3.2 (n = 28) | −17.33 ± 3.4 (n = 12) | 0.84 |
| LA booster, % | 14.9 ± 9.2 (n = 22) | 17.7 ± 5.6 (n = 12) | 0.35 |
| LA reservoir, % | 34.9 ± 12.6 (n = 28) | 42.3 ± 12.0 (n = 12) | 0.092 |
Values are means ± SD or n (%); n = number of subjects. LV, left ventricular; LA, left atrial; MV, mitral valve; E, early mitral filling; A, atrial filling; e′, early diastolic tissue velocity.
Table 3.
Hemodynamic and coronary physiology parameters
| Heart Failure With Preserved Ejection Fraction | Control | P Value | |
|---|---|---|---|
| n | 30 | 14 | |
| Procedure indication | |||
| Angina | 11 (36.7) | 8 (57.1) | 0.21 |
| Dyspnea | 30 (100.0) | 4 (28.7) | <0.0001 |
| Abnormal stress test | 4 (13.3) | 8 (57.1) | 0.0018 |
| Preoperative | 0 (0.0) | 2 (14.3) | 0.035 |
| Hemodynamic data | |||
| Mean right atrial pressure, mmHg | 11.6 ± 3.6 (n = 28) | 4.7 ± 3.9 (n = 6) | 0.0002 |
| Right ventricular systolic pressure, mmHg | 46.7 ± 14.3 (n = 29) | 25.5 ± 3.6 (n = 6) | 0.0011 |
| PA systolic pressure, mmHg | 47.9 ± 14.8 (n = 29) | 25.8 ± 4.5 (n = 6) | 0.0011 |
| PA diastolic pressure, mmHg | 22.0 ± 6.8 (n = 29) | 9.2 ± 2.8 (n = 6) | 0.0001 |
| Mean PA pressure, mmHg | 30.5 ± 9.2 (n = 29) | 15.7 ± 2.9 (n = 6) | 0.0005 |
| Mean pulmonary capillary wedge pressure, mmHg | 19.1 ± 5.6 (n = 29) | 8.8 ± 2.7 (n = 6) | 0.0001 |
| Left ventricular end-diastolic pressure, mmHg | 17.8 ± 4.2 (n = 29) | 8.4 ± 4.2 | <0.001 |
| Aortic systolic pressure, mmHg | 133.6 ± 23.8 | 124.9 ± 18.2 | 0.23 |
| Aortic diastolic pressure, mmHg | 70.9 ± 12.8 | 70.9 ± 12.4 | 0.99 |
| Mean arterial pressure, mmHg | 93.7 ± 16.3 | 93.6 ± 18.1 | 0.99 |
| Cardiac output by TD, l/min | 5.8 ± 2.1 (n = 28) | 4.3 ± 0.6 (n = 5) | 0.14 |
| Cardiac output by Fick, l/min | 7.2 ± 1.9 (n = 29) | 5.6 ± 1.3 (n = 6) | 0.055 |
| Cardiac index by TD, l·min−1·m−2 | 2.7 ± 0.8 (n = 28) | 2.5 ± 0.4 (n = 5) | 0.59 |
| Cardiac index by Fick, l·min−1·m−2 | 3.4 ± 0.8 (n = 29) | 3.2 ± 0.9 (n = 6) | 0.66 |
| SVR by TD, dyn⋅s−1·cm−5 | 1,250 ± 430 (n = 28) | 1,664 ± 243 (n = 5) | 0.046 |
| SVR by Fick, dyn⋅s−1·cm−5 | 965 ± 342 (n = 29) | 1,408 ± 513 (n = 6) | 0.012 |
| PVR by TD, dyn⋅s−1·cm−5 | 158 ± 81 (n = 28) | 125 ± 28 (n = 5) | 0.38 |
| PVR by Fick, dyn⋅s−1·cm−5 | 127 ± 69 (n = 29) | 105 ± 35 (n = 6) | 0.44 |
| Coronary artery, %stenosis | |||
| Left main coronary artery | 0 | 0 | — |
| Left anterior descending coronary artery | 15.0 ± 18.5 | 12.1 ± 19.7 | 0.64 |
| Left circumflex coronary artery | 5.3 ± 15.0 | 0 | 0.19 |
| Right coronary artery | 8.7 ± 17.5 | 7.9 ± 13.1 | 0.88 |
| Coronary physiology data | |||
| Resting mean transit time, s | 0.87 ± 0.43 | 1.02 ± 0.61 | 0.35 |
| Hyperemic mean transit time, s | 0.39 ± 0.17 | 0.29 ± 0.15 | 0.054 |
| Hyperemic distal pressure, mmHg | 72.5 ± 19.2 | 71.2 ± 13.4 | 0.82 |
| Fractional flow reserve | 0.91 ± 0.07 (n = 29) | 0.90 ± 0.05 (n = 13) | 0.49 |
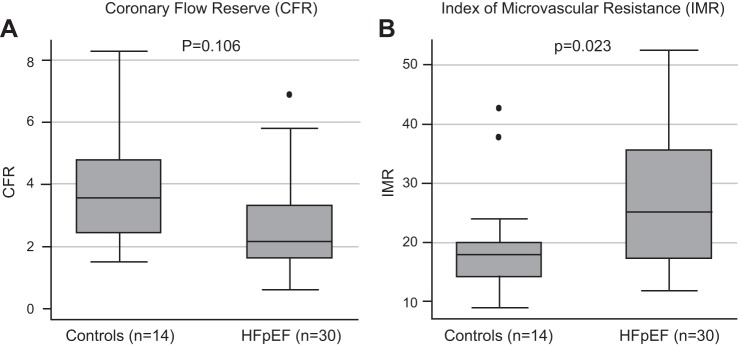
| CFR | 2.55 ± 1.60 | 3.84 ± 1.89 | 0.024 |
| CFR | 2.15 (1.60–3.30) | 3.55 (2.40–4.80) | 0.11 |
| IMR | 26.7 ± 10.3 | 19.7 ± 9.7 | 0.037 |
| IMR | 25.2 (17.0–35.5) | 18.0 (14.0–20.0) | 0.023 |
Values are means ± SD, n (%), or medians (interquartile ranges); n = number of subjects. PA, pulmonary artery; TD, thermodilution; SVR, systemic vascular resistance; PVR, pulmonary vascular resistance; CFR, coronary flow reserve; IMR, index of microvascular resistance.
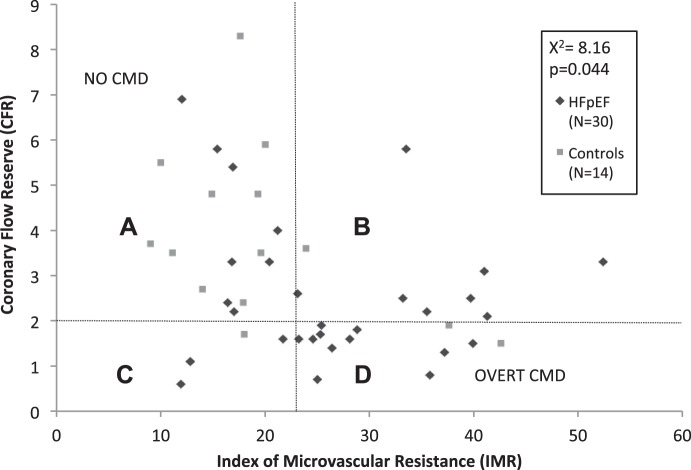
FFR did not differ significantly between the two groups; however, the HFpEF cohort had lower mean CFR (2.55 ± 1.60 vs. 3.84 ± 1.89, P = 0.024) and higher mean IMR (26.7 ± 10.3 vs. 19.7 ± 9.7 units, P = 0.037) compared with the control cohort (Table 3 and Fig. 1). The distribution of patients with HFpEF and control subjects among coronary physiology groups, defined by CFR and IMR, differed significantly (Fig. 2). The majority (71.4%) of control subjects had normal coronary physiology (group A). Of the remaining control subjects, 14.2 had either abnormal CFR or abnormal IMR (7.1% in group B, 7.1% in CP group C, and 14.3% with overt CMD in group D). Of the HFpEF subjects, there were 36.7% with overt CMD (group D), with another 36.7% with either abnormal IMR or abnormal CFR (26.7% in group B and 10.0% in group C) and 26.7% with normal coronary physiology (group A).
Fig. 1.
Markers of coronary microvascular dysfunction. A and B: distribution of coronary flow reserve (CFR; A) and index of microvascular resistance (IMR; B) and measurements within patients with heart failure with preserved ejection fraction (HFpEF) and control subjects. Individual data points are marked with lines, and group averages are marked with circles. See Table 2 for averages ± SD. P values compare medians and mean IMR and CFR values in the HFpEF and control groups.
Fig. 2.
Coronary physiology in patients with heart failure with preserved ejection fraction (HFpEF) patients and control subjects. A–D: descriptions of the four coronary physiology (CP) groups. Subjects were sorted into based on whether they had abnormal (≤2.0) or normal (>2.0) coronary flow reserve (CFR) and abnormal (≥23) or normal (<23) index of microvascular resistance (IMR). A: normal CFR/normal IMR; B: normal CFR/abnormal IMR; C: abnormal CFR/normal IMR; D: abnormal CFR/abnormal IMR. Subjects in group A were considered to have normal coronary physiology, and subjects in group D were considered to have overt coronary microvascular dysfunction. P = 0.044 and χ2 = 8.16.
As an exploratory analysis, the clinical, echocardiographic, and hemodynamic parameters associated with coronary physiology groups B−D were compared with group A within the HFpEF cohort (Table 4). All coronary physiology groups had similar pulmonary capillary wedge pressure; however, groups C and D had higher BNP and right ventricular systolic and mean pulmonary arterial pressures, and groups B−D had higher pulmonary vascular resistance than group A. Group B had the highest resting Tmn across all groups and similar hyperemic Pd and higher hyperemic Tmn compared with group A. Group C had the lowest resting Tmn and Pd, with intermediate hyperemic Tmn. Group D had the highest hyperemic Tmn and intermediate resting Tmn and hyperemic Pd across all groups.
Table 4.
Clinical, echocardiographic, and hemodynamic data by coronary physiology group in patients with heart failure with preserved ejection fraction
|
P Value |
|||||||
|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | Group A vs. group B | Group A vs. group C | Group A vs. group D | |
| n | 8 | 8 | 3 | 11 | |||
| Clinical data | |||||||
| Age, yr | 66.6 ± 9.1 | 66.3 ± 10.6 | 68.7 ± 4.0 | 62.9 ± 10.8 | 0.94 | 0.72 | 0.44 |
| Body mass index | 40.6 ± 11.1 | 32.7 ± 9.0 | 44.8 ± 6.9 | 38.9 ± 7.3 | 0.14 | 0.56 | 0.70 |
| Estimated glomerular filtration rate, ml·min−1·1.73 m−2 | 65.5 ± 55.3 (n = 4) | 67.0 ± 13.6 (n = 6) | 62.7 ± 48.9 | 54.4 ± 30.1 (n = 7) | 0.95 | 0.95 | 0.67 |
| B-type natriuretic peptide, pg/dl | 77 (52–274) (n = 7) | 98 (24–213) (n = 7) | 1,221, (152–3,661) | 359 (161–1,721) (n = 10) | 0.85 | 0.053 | 0.079 |
| Echocardiographic data | |||||||
| LV septal wall thickness, cm | 1.10 ± 0.17 | 1.16 ± 0.26 | 1.17 ± 0.15 | 1.28 ± 0.25 | 0.55 | 0.54 | 0.087 |
| LV end-diastolic dimension, cm | 4.55 ± 0.67 | 4.54 ± 0.82 | 4.63 ± 0.42 | 4.51 ± 0.68 | 0.97 | 0.85 | 0.90 |
| LV end-diastolic volume index | 52.3 ± 8.9 (n = 7) | 50.7 ± 10.8 | 39.3 ± 4.8 | 57.6 ± 15.3 (n = 9) | 0.76 | 0.049 | 0.43 |
| LV end-systolic dimension, cm | 2.75 ± 0.88 | 2.38 ± 0.81 | 2.43 ± 0.49 | 2.47 ± 0.82 | 0.40 | 0.58 | 0.49 |
| LV end-systolic volume index | 20.8 ± 8.6 | 18.6 ± 6.5 | 14.0 ± 1.4 | 27.5 ± 10.7 (n = 9) | 0.57 | 0.23 | 0.18 |
| LA volume index | 30.7 ± 7.5 (n = 7) | 31.6 ± 7.3 (n = 7) | 33.9 ± 3.1 | 34.9 ± 12.9 (n = 9) | 0.82 | 0.51 | 0.47 |
| MV E velocity, cm/s | 86.5 ± 15.2 | 83.8 ± 10.7 | 111.1 ± 18.8 | 105.5 ± 30.1 | 0.77 | 0.050 | 0.12 |
| MV A velocity, cm/s | 93.1 ± 23.9 | 72.3 ± 16.8 | 108.6 ± 17.3 (n = 2) | 88.2 ± 33.3 (n = 9) | 0.063 | 0.42 | 0.73 |
| MV E-to-A ratio | 0.99 ± 0.34 | 1.21 ± 0.37 | 1.00 ± 0.42 (n = 2) | 1.48 ± 1.33 (n = 9) | 0.23 | 0.97 | 0.33 |
| Average e′, cm/s | 8.13 ± 0.80 (n = 6) | 8.48 ± 3.13 | 8.63 ± 2.68 | 7.81 ± 2.39 (n = 10) | 0.80 | 0.66 | 0.76 |
| E/e′ | 10.6 ± 1.3 (n = 6) | 10.6 ± 3.1 | 13.4 ± 3.0 | 14.7 ± 4.1 (n = 10) | 0.97 | 0.090 | 0.038 |
| Mitral deceleration time, ms | 224 ± 60 | 227 ± 50 | 190 ± 44 | 185 ± 61 | 0.93 | 0.40 | 0.19 |
| Average, isovolumetric relaxation time, ms | 75.8 ± 17.2 (n = 6) | 76.7 ± 33.3 | 57 ± 28 | 73 ± 26 (n = 10) | 0.96 | 0.24 | 0.82 |
| LV global longitudinal strain | 19.1 ± 2.1 | 17.9 ± 3.7 (n = 7) | 17.7 ± 6.0 | 16.0 ± 2.2 (n = 10) | 0.45 | 0.57 | 0.0086 |
| LA booster | 20.7 ± 10.2 (n = 7) | 12.8 ± 8.2 (n = 6) | 6.9 ± 3.3 (n = 2) | 13.2 ± 8.2 (n = 7) | 0.15 | 0.11 | 0.16 |
| LA reservoir | 41.5 ± 12.7 (n = 8) | 34.8 ± 18.5 (n = 7) | 28.4 ± 12.0 | 31.5 ± 5.2 (n = 10) | 0.42 | 0.16 | 0.036 |
| Hemodynamic data | |||||||
| Mean right atrial pressure, mmHg | 11.9 ± 3.3 (n = 7) | 10.6 ± 3.3 | 11.3 ± 5.1 | 12.2 ± 3.9 (n = 10) | 0.49 | 0.85 | 0.85 |
| Right ventricular systolic pressure, mmHg | 36.3 ± 4.7 | 43.6 ± 14.2 | 51.7 ± 2.9 | 56.0 ± 15.7 (n = 10) | 0.19 | 0.00050 | 0.0036 |
| Mean pulmonary arterial pressure, mmHg | 23.8 ± 4.2 | 28.9 ± 9.1 | 35.0 ± 4.6 | 35.8 ± 10.0 (n = 10) | 0.17 | 0.0036 | 0.0057 |
| Mean pulmonary capillary wedge pressure, mmHg | 17.1 ± 3.0 | 17.8 ± 6.8 | 20.3 ± 3.8 | 21.4 ± 6.4 (n = 10) | 0.82 | 0.17 | 0.10 |
| LV end-diastolic pressure, mmHg | 16.9 ± 1.6 | 16.1 ± 3.6 | 22.0 ± 4.2 (n = 2) | 18.8 ± 5.3 | 0.60 | 0.015 | 0.33 |
| Mean aortic pressure, mmHg | 93.9 ± 7.5 | 93.6 ± 18.9 | 101.3 ± 23.3 | 89.5 ± 10.0 | 0.97 | 0.41 | 0.32 |
| Aortic systolic pressure, mmHg | 136.6 ± 21.2 | 137 ± 32.3 | 153.0 ± 24.0 | 123.7 ± 15.5 | 0.98 | 0.30 | 0.14 |
| Aortic diastolic pressure, mmHg | 71.5 ± 9.2 | 71.6 ± 16.5 | 73.7 ± 23.5 | 69.1 ± 10.3 | 0.99 | 0.82 | 0.61 |
| Cardiac output by Fick, l/min | 6.9 ± 1.6 | 6.8 ± 2.5 | 8.0 ± 1.7 | 7.7 ± 1.9 (n = 10) | 0.91 | 0.35 | 0.35 |
| Cardiac index by Fick | 3.2 ± 0.7 | 3.2 ± 1.0 | 3.6 ± 0.5 | 3.7 ± 0.9 (n = 10) | 0.93 | 0.44 | 0.28 |
| Systemic vascular resistance by Fick | 1019 ± 316 | 1118 ± 493 | 912 ± 224 | 815 ± 190 (n = 10) | 0.64 | 0.61 | 0.11 |
| Pulmonary vascular resistance by Fick | 77 ± 20 | 136 ± 65 | 144 ± 57 | 156 ± 84 (n = 10) | 0.027 | 0.013 | 0.020 |
| Coronary physiology data | |||||||
| Resting mean transit time, s | 0.78 ± 0.25 | 1.39 ± 0.37 | 0.31 ± 0.21 | 0.70 ± 0.21 | 0.0020 | 0.019 | 0.45 |
| Hyperemic mean transit time, s | 0.20 ± 0.02 | 0.48 ± 0.12 | 0.29 ± 0.10 | 0.50 ± 0.13 | <0.0001 | 0.027 | <0.0001 |
| Hyperemic distal pressure, mmHg | 87.1 ± 8.3 | 79.1 ± 15.3 | 57.0 ± 21.0 | 61.3 ± 18.9 | 0.21 | 0.0055 | 0.0022 |
| Fractional flow reserve | 0.94 ± 0.07 (n = 7) | 0.93 ± 0.06 | 0.85 ± 0.04 | 0.89 ± 0.07 | 0.61 | 0.077 | 0.16 |
| CFR | 4.16 ± 1.70 | 3.01 ± 1.20 | 1.10 ± 0.50 | 1.45 ± 0.38 | 0.14 | 0.015 | 0.0001 |
| IMR | 17.0 ± 2.9 | 37.5 ± 8.4 | 15.5 ± 5.4 | 29.1 ± 5.8 | <0.0001 | 0.54 | <0.0001 |
Values are means ± SD, n (%), or medians (interquartile ranges); n = number of subjects. Groups were as follows: normal coronary flow reserve (CFR) and index of microvascular resistance (IMR) (group A), abnormal IMR and normal CFR (group B), abnormal CFR and normal IMR (group C), and abnormal CFR and abnormal IMR (group D). LV, left ventricular; LA, left atrial; MV, mitral valve; E, early mitral filling; A, atrial filling; e′, early diastolic tissue velocity. Post hoc correction for multiple comparisons was performed using the Bonferroni method, revealing significant differences between the following groups: resting mean transit time (groups B vs. C and groups B vs. D, P < 0.001), hyperemic mean transit time (groups C vs. D, P = 0.036), CFR (groups B vs. D, P = 0.033), and IMR (groups B vs. C, P < 0.001; groups B vs. D, P = 0.036; and groups C vs. D, P = 0.011).
DISCUSSION
In this observational study comparing patients with HFpEF with control subjects, there was an increased incidence of CMD invasively determined by measuring CFR and IMR. Over one-third of the HFpEF cohort exhibited overt CMD (abnormal CFR and IMR), whereas the majority of the control population had normal coronary physiology. Mean CFR was significantly lower and mean IMR was significantly higher in the HFpEF population compared with the control population.
Clinical, echocardiographic, and hemodynamic characteristics of HFpEF.
HFpEF is a heterogeneous disease, with multiple potential mechanisms leading to the clinical syndrome (32). Similar to previous epidemiological studies, our HFpEF population consisted of elderly, mostly female patients, with high rates of hypertension, chronic kidney disease, and other comorbidities compared with control subjects (5, 21). Comorbidities in HFpEF contribute to outcomes (2, 8, 15); however, LV and vascular abnormalities are more severe in patients with HFpEF compared with comorbidity-matched control subjects (28), suggesting a pathophysiology unique to HFpEF that is yet to be defined. When comparing physical and laboratory findings between patients with HFpEF and control subjects, patients with HFpEF had a higher body weight and a higher percentage of obesity. Obesity is a common comorbidity associated with HFpEF, observed in other cohorts, that may drive HFpEF incidence as well as poor outcomes (13, 35).
We found similar LV volumes and ejection fraction in the HFpEF and control groups, characteristic findings in both patients with HFpEF and non-HFpEF control subjects. LV wall thickness, E/e′, tricuspid regurgitation velocities, and mean grade of diastolic dysfunction were higher in the HFpEF group compared with the control group, similar to a detailed echocardiographic analyses in a contemporary HFpEF trial (42). On invasive hemodynamic assessment, we noted higher right- and left-sided filling pressures and similar cardiac index as control subjects. These findings are consistent with other hemodynamic studies of patients with HFpEF (41). The elevated pulmonary arterial pressure compared with control subjects is of particular importance, given that it is found in ~80% of patients with HFpEF, is associated with worse functional class (7), and portends a poor prognosis with higher degrees of elevation (23). The above echocardiographic and hemodynamic findings help confirm that the patients selected for the HFpEF group had elevated filling pressures that contribute to their symptomatology and confirm the diagnosis of HFpEF rather than other forms of dyspnea.
Coronary physiology in HFpEF and its implications.
We chose to use well-established invasive techniques to assess coronary physiology in all patients enrolled in the study. CFR and IMR have been used to characterize CMD in other patient populations such as cardiac syndrome X (14) and acute coronary syndromes (10). A recent study of 313 consecutive patients undergoing invasive coronary angiography, 86% of them for chest pain syndromes, had complete coronary physiology studies including FFR, CFR, and IMR (25). The majority of these patients had both normal CFR and IMR (group A). A minority of patients had both abnormal CFR and IMR (group D). Among patients who had a normal FFR (>0.80), group D had worse outcomes than all other groups (revascularization, myocardial infarction, and death), suggesting that group D is the highest risk group among those with chest pain syndromes. Based on these findings that show a low prevalence of group D patients in primarily a chest pain population and a prior postmortem study (29) showing a high incidence of coronary microvascular dysfunction in patients with HFpEF, we expected a high proportion of patients with HFpEF in group D compared with control subjects. Indeed, 36.7% of patients with HFpEF were in group D and 26.7% were in group A, which was significantly higher than control subjects, where the majority were in group A (71.4%) with only 14.3% in group D. In addition, mean CFR was lower and IMR higher in the HFpEF group compared with the control group. These findings support our hypothesis that CMD may be an important component to the HFpEF phenotype.
We defined overt CMD as having abnormal CFR and IMR and normal microvascular function as having normal CFR and IMR (group D); however, two other CP groups emerged, coronary physiology group B (abnormal IMR and normal CFR) and group C (abnormal CFR and normal IMR). These two groups may represent distinct HFpEF entities, and we explored clinical, echocardiographic, and hemodynamic characteristics in these groups further. For the patients with HFpEF in group B, resting Tmn was the highest of all coronary physiology groups, with similar hyperemic Pd and higher hyperemic Tmn compared with group A, suggesting that resting myocardial blood flow is slow, but the normal CFR implies the precapillary arterioles are able to dilate and provide enough flow when necessary. The elevated hyperemic Pd and therefore high IMR suggest either residual microvascular disease not overcome by arteriolar dilation or incomplete vasodilation of the microvasculature with adenosine. A recent cardiac magnetic resonance imaging study of patients with HFpEF demonstrated high proportions of patients with abnormal extracellular volume fraction as measured by T1 mapping, a marker of diffuse interstitial fibrosis associated with LV stiffness (39). In a recent study of patients with stable and unstable coronary syndromes, IMR was found to correlate to the degree of microvascular obstruction and reduced myocardial perfusion index in the artery of interest (47). Evaluation of extracellular volume may be a better tool for evaluating patients with HFpEF without significant CAD due to its ability to detect diffuse fibrosis. Patients with HFpEF in group B were most similar in BNP levels and pulmonary arterial pressure to group A, which raises the question of whether elevated IMR occurs early in the HFpEF disease process before BNP levels and pulmonary arterial pressures elevate, resulting in patients with a group B profile. Further evaluation of the significance of elevated IMR in group B patients may provide insights into HFpEF pathophysiology. Patients with HFpEF in group C had the lowest resting Tmn and hyperemic Pd of all coronary physiology groups. The low resting Tmn implies that the coronary microvasculature dilation is high or close to maximum as a result of intact microvasculature. This group had the numerically highest body mass index, lowest LV end-diastolic volume index, high LA volume index, highest E wave velocity, numerically lowest LA booster function, and high pulmonary arterial pressures and LV end-diastolic pressure. It is possible that these patients are compensated to the maximum possible at rest due to metabolic demands, and the low CFR is simply a reflection of this and the normal IMR reflects the normal underlying coronary microvascular bed. Resting Pd was not measured in this study; however, it can be approximated as invasively measured mean arterial pressure given the fact that resting Pd/Pa is often close to one in patients without significant CAD (18). A large differential between resting mean arterial pressure and hyperemic Pd likely represents systemic vasodilation without additional augmentation in the already high coronary blood flow. Further characterization of these coronary physiology groups is necessary to fully elucidate the mechanisms behind our findings.
To our knowledge, this is the first study to invasively evaluate for CMD in living patients with HFpEF. Another group has prospectively evaluated 25 patients with HFpEF and compared CFR derived by phase-contrast cine-MRI of the coronary sinus using adenosine as the vasodilator with that of 13 patients with LV hypertrophy without HF and 18 control subjects without HF or risk factors (19). Mean CFR in the HFpEF, LVH, and control groups were 2.21 ± 0.55, 3.05 ± 0.74, and 3.83 ± 0.73, respectively. We sought to expand these findings by measuring both CFR and IMR using the gold-standard wire-based invasive measurements, fully assessing for obstructive CAD by angiography and FFR and evaluating biomarkers, echocardiography, and hemodynamics in patients with HFpEF. Previous HFpEF trials failed to identify a reliable target or biomarker that can represent a predictable signal of therapeutic effects on outcomes in HFpEF, potentially because of the complexity and heterogeneity of patients considered for these trials. Multidomain assessment of a combination of markers may guide downstream research efforts (6). Based on our study and available research, we conclude that CMD may be a precursor to some but not all HFpEF. We found that coronary physiology groups A and B seem to have a milder form of HFpEF, and study of these patients using advanced hemodynamic studies, cardiac MRI, and longitudinal followup may provide the key to understanding the role of CMD in HFpEF pathophysiology. CMD may be a potential target for therapy that, in addition to myocyte and interstitial integrity, may represent important elements of cardiac structure relevant to patients with HFpEF.
Limitations.
The small sample size and lack of age or comorbidity, matching of control subjects in this study preclude firm statistical differentiation of disease entities and the numerous covariates described in materials and methods. In addition, as this was an invasive study, all control subjects underwent coronary angiography for a clinical indication, with >50% with angina or abnormal stress, and there was a relatively high rate of established nonobstructive CAD, diabetes mellitus, hypertension, and hyperlipidemia. It is therefore likely that our control group may not represent a truly normal group of patients without cardiovascular disease or risk factors, which may help explain why nearly 30% of control subjects had abnormal CFR, IMR, or both and there was a lack of important echocardiographic markers of cardiovascular disease such as LA size and function between control subjects and patients with HFpEF. Indeed, although mean LA volume in our cohort was similar to that in a similar HFpEF cohort, our mean control LA volume was larger in our control cohort, and the difference in reservoir strain between HFpEF and control cohorts was less (40). Future studies with larger sample sizes, control subjects without HFpEF risk factors, comorbidity matching, and evaluation for interstitial fibrosis with cardiac magnetic resonance imaging (39) may help ascertain to what degree comorbidities themselves contribute to the CMD found in the HFpEF group and by which potential mechanisms. While the study sought to identify a unifying factor among the HFpEF population to better design therapies for these patients, heterogeneity of microvascular dysfunction in the cohort was found. The use of both CFR and IMR was helpful in describing the underlying coronary physiology of our HFpEF cohort, as demonstrated in the different clinical, laboratory, echocardiographic, and hemodynamic characteristics of the various coronary physiology groups, recognizing that IMR has been demonstrated to be a more reliable measurement of microvascular dysfunction than CFR across various hemodynamic conditions (31). From a methodological standpoint, we used CFR and IMR to determine relative flow reserve and resistance; however, absolute myocardial blood flow was not calculated. Although there are methods to calculate absolute flow (45), commercially available equipment to make these measurements is not yet available in the United States. In addition, we did not test the differential responsiveness to adenosine itself in patients with HFpEF. We administered adenosine centrally through the internal jugular vein and observed drops in blood pressure and/or presence of dyspnea in all patients (data not shown), indicating that the drug was achieving vasodilation.
Conclusions.
Coronary microvascular dysfunction is a potential underlying condition in patients with HFpEF. While these data offer new insights into the pathophysiology of HFpEF, further investigation is necessary to determine the significance of the coronary physiology groups in the present study.
GRANTS
This work was supported in part by American Heart Association Grant 14SDG20380354 and National Institute of Diabetes and Digestive and Kidney Diseases Grant T35-DK-062719-29.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.S. and J.E.B. conceived and designed research; K.D., M.G., M.L., and J.E.A.B. performed experiments; K.D., M.G., N.N., S.J.S., and J.E..B. analyzed data; K.D., M.G., N.N., S.J.S., and J.E.B. interpreted results of experiments; K.D. and J.E.A.B. prepared figures; K.D. and J.E.B. drafted manuscript; K.D., M.G., N.N., M.L., J.P., A.P.S., S.N., J.B., C.J.D., W.F.F., S.J.S., and J.E..B. edited and revised manuscript; K.D., M.G., N.N., M.L., J.P., A.P.S., S.N., J.B., C.J.D., W.F.F., S.J.S., and J.E.B. approved final version of manuscript.
ACKNOWLEDGMENTS
This study is dedicated to the late Dr. Mihai Gheorghiade, without whose mentorship this work would not have been possible.
REFERENCES
- 1.Adams V, Alves M, Fischer T, Rolim N, Werner S, Schütt N, Bowen TS, Linke A, Schuler G, Wisloff U. High-intensity interval training attenuates endothelial dysfunction in a Dahl salt-sensitive rat model of heart failure with preserved ejection fraction. J Appl Physiol 119: 745–752, 2015. doi: 10.1152/japplphysiol.01123.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 59: 998–1005, 2012. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashore TM, Balter S, Barac A, Byrne JG, Cavendish JJ, Chambers CE, Hermiller JB Jr, Kinlay S, Landzberg JS, Laskey WK, McKay CR, Miller JM, Moliterno DJ, Moore JW, Oliver-McNeil SM, Popma JJ, Tommaso CL; ACCF Task Force Members . 2012 American College of Cardiology Foundation/Society for Cardiovascular Angiography and Interventions expert consensus document on cardiac catheterization laboratory standards update: a report of the American College of Cardiology Foundation Task Force on Expert Consensus documents developed in collaboration with the Society of Thoracic Surgeons and Society for Vascular Medicine. J Am Coll Cardiol 59: 2221–2305, 2012. doi: 10.1016/j.jacc.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Borbély A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res 104: 780–786, 2009. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 5.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA 296: 2209–2216, 2006. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 6.Butler J, Hamo CE, Udelson JE, O’Connor C, Sabbah HN, Metra M, Shah SJ, Kitzman DW, Teerlink JR, Bernstein HS, Brooks G, Depre C, DeSouza MM, Dinh W, Donovan M, Frische-Danielson R, Frost RJ, Garza D, Gohring UM, Hellawell J, Hsia J, Ishihara S, Kay-Mugford P, Koglin J, Kozinn M, Larson CJ, Mayo M, Gan LM, Mugnier P, Mushonga S, Roessig L, Russo C, Salsali A, Satler C, Shi V, Ticho B, van der Laan M, Yancy C, Stockbridge N, Gheorghiade M. Reassessing phase II heart failure clinical trials: consensus recommendations. Circ Heart Fail 10: e003800, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalos D, Mascherbauer J, Zotter-Tufaro C, Duca F, Kammerlander AA, Aschauer S, Bonderman D. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol 68: 189–199, 2016. doi: 10.1016/j.jacc.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 8.Edelmann F, Stahrenberg R, Gelbrich G, Durstewitz K, Angermann CE, Düngen HD, Scheffold T, Zugck C, Maisch B, Regitz-Zagrosek V, Hasenfuss G, Pieske BM, Wachter R. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol 100: 755–764, 2011. doi: 10.1007/s00392-011-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation 107: 3129–3132, 2003. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 10.Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, Schnittger I, Lee DP, Vagelos RH, Fitzgerald PJ, Yock PG, Yeung AC. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 51: 560–565, 2008. doi: 10.1016/j.jacc.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 11.Gevaert AB, Shakeri H, Leloup AJ, Van Hove CE, De Meyer GR, Vrints CJ, Lemmens K, Van Craenenbroeck EM. Endothelial senescence contributes to heart failure with preserved ejection fraction in an aging mouse model. Circ Heart Fail 10: e003806, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003806. [DOI] [PubMed] [Google Scholar]
- 12.Gorcsan J 3rd, Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol 58: 1401–1413, 2011. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 4: 324–331, 2011. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasdai D, Holmes DR Jr, Higano ST, Burnett JC Jr, Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc 73: 1133–1140, 1998. doi: 10.4065/73.12.1133. [DOI] [PubMed] [Google Scholar]
- 15.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail 1: 91–97, 2008. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail 9: e003116, 2016. doi: 10.1161/CIRCHEARTFAILURE.115.003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, Le Jemtel TH. Vascular inflammation in obesity and sleep apnea. Circulation 121: 1014–1021, 2010. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeremias A, Maehara A, Généreux P, Asrress KN, Berry C, De Bruyne B, Davies JE, Escaned J, Fearon WF, Gould KL, Johnson NP, Kirtane AJ, Koo BK, Marques KM, Nijjer S, Oldroyd KG, Petraco R, Piek JJ, Pijls NH, Redwood S, Siebes M, Spaan JA, van ’t Veer M, Mintz GS, Stone GW. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol 63: 1253–1261, 2014. doi: 10.1016/j.jacc.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Kimura K, Umemura S. Impairment of coronary flow reserve evaluated by phase contrast cine-magnetic resonance imaging in patients with heart failure with preserved ejection fraction. J Am Heart Assoc 5: e002649, 2016. doi: 10.1161/JAHA.115.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kern MJ. Coronary physiology revisited: practical insights from the cardiac catheterization laboratory. Circulation 101: 1344–1351, 2000. doi: 10.1161/01.CIR.101.11.1344. [DOI] [PubMed] [Google Scholar]
- 21.Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ Jr, Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH; New York Heart Failure Consortium . Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 43: 1432–1438, 2004. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Krüger M, Kötter S, Grützner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res 104: 87–94, 2009. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 23.Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 53: 1119–1126, 2009. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28: 1–39.e14, 2015. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol 67: 1158–1169, 2016. doi: 10.1016/j.jacc.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 26.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation 115: 888–895, 2007. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 27.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 285: 1441–1446, 1971. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 28.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail 5: 710–719, 2012. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131: 550–559, 2015. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29: 277–314, 2016. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation 113: 2054–2061, 2006. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- 32.Oktay AA, Shah SJ. Diagnosis and management of heart failure with preserved ejection fraction: 10 key lessons. Curr Cardiol Rev 11: 42–52, 2015. doi: 10.2174/1573403X09666131117131217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259, 2006. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 34.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis 47: 320–332, 2005. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, Berry JD. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol 69: 1129–1142, 2017. doi: 10.1016/j.jacc.2016.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 37.Picard MH, Adams D, Bierig SM, Dent JM, Douglas PS, Gillam LD, Keller AM, Malenka DJ, Masoudi FA, McCulloch M, Pellikka PA, Peters PJ, Stainback RF, Strachan GM, Zoghbi WA; American Society of Echocardiography . American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr 24: 1–10, 2011. doi: 10.1016/j.echo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Pijls NH, De Bruyne B, Smith L, Aarnoudse W, Barbato E, Bartunek J, Bech GJ, Van De Vosse F. Coronary thermodilution to assess flow reserve: validation in humans. Circulation 105: 2482–2486, 2002. doi: 10.1161/01.CIR.0000017199.09457.3D. [DOI] [PubMed] [Google Scholar]
- 39.Rommel KP, von Roeder M, Latuscynski K, Oberueck C, Blazek S, Fengler K, Besler C, Sandri M, Lücke C, Gutberlet M, Linke A, Schuler G, Lurz P. Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 67: 1815–1825, 2016. doi: 10.1016/j.jacc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, Voors AA, Lefkowitz M, Bransford T, Shi V, Packer M, McMurray JJ, Shah AM, Solomon SD; PARAMOUNT Investigators . Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 16: 1096–1103, 2014. doi: 10.1002/ejhf.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 59: 442–451, 2012. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 42.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD; TOPCAT Investigators . Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail 7: 104–115, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol 302: H2148–H2165, 2012. doi: 10.1152/ajpheart.00907.2011. [DOI] [PubMed] [Google Scholar]
- 44.van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 126: 830–839, 2012. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 45.van ’t Veer M, Adjedj J, Wijnbergen I, Tóth GG, Rutten MC, Barbato E, van Nunen LX, Pijls NH, De Bruyne B. Novel monorail infusion catheter for volumetric coronary blood flow measurement in humans: in vitro validation. EuroIntervention 12: 701–707, 2016. doi: 10.4244/EIJV12I6A114. [DOI] [PubMed] [Google Scholar]
- 46.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschöpe C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4: 44–52, 2011. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 47.Williams RP, de Waard GA, De Silva K, Lumley M, Asrress K, Arri S, Ellis H, Mir A, Clapp B, Chiribiri A, Plein S, Teunissen PF, Hollander MR, Marber M, Redwood S, van Royen N, Perera D. Doppler versus thermodilution-derived coronary microvascular resistance to predict coronary microvascular dysfunction in patients with acute myocardial infarction or stable angina pectoris. Am J Cardiol 121: 1–8, 2018. doi: 10.1016/j.amjcard.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]