Abstract
We identified a regional dichotomy in murine lymphatic contractile function with regard to vessel location within the periphery or visceral cavity. All vessels isolated from peripheral regions [cervical, popliteal, inguinal, axillary, and internodal inguinal axillary (Ing-Ax)] developed robust contractions with maximal ejection fractions (EFs) of 50–80% in our ex vivo isobaric myograph experiments. Conversely, vessels isolated from the visceral cavity (mesenteric, thoracic duct, and iliac) demonstrated maximal EFs of ≤10%. Using pressure myography, sharp electrode membrane potential recordings, and Ca2+ imaging, we assessed the role of L-type Ca2+ channels in this contractile dichotomy. Ing-Ax membrane potential revealed a ~2-s action potential (AP) cycle (resting −35 mV, spike −5 mV, and plateau −11 mV) with a plateau phase that was significantly lengthened by the L-type Ca2+ channel agonist Bay K8644 (BayK; 100 nM). APs recorded from mesenteric vessels, however, displayed a slower upstroke and an elongated time over threshold. BayK (100 nM) increased the mesenteric AP upstroke velocity and plateau duration but also significantly hyperpolarized the vessel. Contractions of vessels from both regions were preceded by Ca2+ flashes, detected with a smooth muscle-specific endogenous Ca2+ reporter, that typically were coordinated over the length of the vessel. Similar to the membrane potential recordings, Ca2+ flashes in mesenteric vessels were weaker and had a slower rise time but were longer lasting than those in Ing-Ax vessels. BayK (100 nM) significantly increased the Ca2+ transient amplitude and duration in both vessels and decreased time to peak Ca2+ in mesenteric vessels. However, a higher concentration (1 μM) of BayK was required to produce even a modest increase in EF in visceral lymphatics, which remained at <20%.
NEW & NOTEWORTHY Lymphatic collecting vessels isolated from murine peripheral tissues, but not from the visceral cavities, display robust contractile behavior similar to lymphatic vessels from other animal models and humans. These differences are partially explained by L-type Ca2+ channel activity as revealed by the first measurements of murine lymphatic action potentials and contraction-associated Ca2+ transients.
Keywords: lymphatic action potential, lymphatic muscle membrane potential, lymphatic muscle calcium, mouse lymphatic contraction, regional heterogeneity
INTRODUCTION
The spontaneous and coordinated contractions of electrically coupled muscle cells in the walls of collecting lymphatic vessels are critical for the transport of lymph. Contractions of relatively large amplitude (15) and high velocity (62) enable propulsive movement of lymph through the one-way valve system (14) against adverse hydrostatic pressure gradients (15). These contractions are initiated by action potentials (APs) and Ca2+ influx through L-type Ca2+ channels (29, 59, 61). Quantitative analyses of these contractions are difficult to assess in vivo because of the multiple factors that modulate collecting vessel behavior, including neural, hormonal, immunological, and physical forces, in addition to an inability to determine the contribution of all these variables at any given time (51). However, great strides have been made in our understanding of lymphatic collecting vessel physiology across many species through the use of ex vivo preparations in which these factors can be minimized, monitored, and/or controlled individually.
Lymphatic vessel ejection fraction (EF), contraction frequency, and basal tone are modulated by the transmural pressure gradient across the vessel wall as well as by shear stress generated through luminal flow (20, 21), allowing the vessels to respond acutely to changing hydrodynamic conditions (17). Even under ex vivo conditions, lymphatic collecting vessels from the rat display regional heterogeneity in their contractile responses to physical stimuli, suggesting that these vessels adapt to chronic exposure to their respective tissue environments (18). The basic properties of lymphatic collecting vessels have been documented in many species, including the rat (18), bat (24), guinea pig (11), rabbit (52), dog (28), pig (32), cow (37), and sheep (36), and much of that behavior has been confirmed by direct in vivo recordings of lymphatic pressure in the legs of human subjects (44, 45). The latter studies demonstrated that lymph transport in human extremities depends more critically on the intrinsic, pressure-dependent contractions of the lymphatic vessel wall than on passive compressive forces (16), refuting a common misconception that passive forces represent the major determinant of lymph movement in humans.
Missing from the list of lymphatic vessels of the species mentioned above are those from the mouse, the species most commonly used for developmental studies of lymphangiogenesis and morphogenesis because of its amenability to genetic manipulation. The initial reports of murine lymphatic contractile function focused solely on the activity of the mesenteric and iliac lymphatic vessels, where only relatively small-amplitude spontaneous contractile activity, which might have been limited to the inbred DDY mouse strain used in those studies, could be demonstrated (33, 39, 43, 46). The stark contrast in contractile function between these mouse vessels and mesenteric lymphatic vessels from other species elicited a hypothesis “that [all] murine vessels are not highly or consistently contractile” (19). The speculation that the mouse is completely unique in this regard was partially refuted by the recent demonstration of large-amplitude, spontaneous contractions of the murine intermodal inguinal-axillary lymphatic (Ing-Ax) vessel (7) and popliteal afferent lymphatic vessels in situ (1, 27, 31) and ex vivo (49). In the latter study, we recorded EFs of up to 80% in ex vivo popliteal lymphatic collecting vessels from C57BL/6J, endothelial nitric oxide synthase (NOS)-null (eNOS−/−), and inducible NOS-null (iNOS−/−) mice; these values are comparable to those recorded in rat mesenteric collecting lymphatic vessels (15, 50). Thus, under conditions in which transmural pressure and flow are controlled, popliteal afferent lymphatic vessels from the mouse appear to exhibit large-amplitude, propulsive contractions resembling those of other species. This discrepancy in contractile function raises important questions relevant to the physiology of the mouse lymphatic system. Do other lymphatic beds display contractile activity similar to that of the popliteal afferent vessels? Do murine vessels display canonical contractile regulation to both physical and biochemical responses such as pressure and adrenergic/cholinergic stimulation?
To fully address these questions, afferent and/or efferent lymphatic vessels were dissected from seven different regions of the mouse, cleaned of fat and connective tissue, cannulated on micropipettes, and pressurized to different levels to assess their relative degree of contractile function. Thus, contraction parameters, sharp electrode membrane potential recordings, and Ca2+ signaling of the respective vessels could be compared under identical hydrodynamic conditions using a genetically encoded Ca2+ sensor. We used a combination of these approaches to investigate the role of L-type Ca2+ channels, through the use of the L-type Ca2+ channel activator Bay K8644 (BayK), as a possible mechanism to explain the differences in contractile function between vessels isolated from the periphery and those located within the visceral cavity.
METHODS
Mice.
Wild-type male mice (15–25 g body wt) on the C57BL/6J background and GCaMP6f (strain 024105) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and studied at 2–3 mo of age. Prox1-green fluorescent protein (GFP) mice were a gift from Y. K. Hong (University of Southern California). Tamoxifen-inducible MHY11CreERT2 (SMMHC-CreERT2) mice were a gift from Stefan Offermanns (Max Planck Institute, Bad Nauheim, Germany). SMMHC-CreERT2 mice were bred to GCaMP6f mice to express a genetically encoded Ca2+ sensor in all smooth muscles, but only the lymphatic muscle cells expressed GCaMP6f in the isolated lymphatic vessel preparation. As the SMMHC-CreERT2 strain is Y chromosome linked, only male mice were used for these experimental protocols. All animal protocols were approved by the University of Missouri Animal Care and Use Committee and conformed with the United States Public Health Service policy for the humane care and use of laboratory animals (PHS Policy, 1996).
Vessel identification and isolation.
Mice were anesthetized with pentobarbital sodium (Nembutal, 60 mg/kg ip) and placed on a heating pad. Popliteal afferent lymphatic vessels were exposed through a proximal to distal incision in the calf, with the animal facing down and isolated as previously described (49). Popliteal efferent vessels were identified using a similar incision coupled with injection of trypan blue dye (10–20 μl) into the footpad; once dye entered the popliteal node, the fat, muscle, and connective tissue overlying the node were removed, and a segment of the efferent vessel within 5 mm of the node was isolated. Afferent mesenteric lymphatic vessels were identified and isolated after the small intestine was exteriorized through a 2- to 3-cm midline incision and pinned on a Sylgard platform. Inguinal afferent lymphatic vessels were isolated after a midline dorsal incision and lateral retraction of the skin overlying the flank, thereby exposing the multiple afferent lymphatic collectors (each with associated artery-vein pairs) that drained the skin of that region into the inguinal node (57). Axillary afferents were found via injection of trypan blue into the front paw and retraction of the skin from the armpit down to the back of the elbow. The relatively large lymphatic vessel, Ing-Ax, also referred to as the “flank” vessel (7), connecting the inguinal node with the group of axillary nodes (57) was isolated by a midline dorsal incision and retraction of the dorsal skin fold on one side of the animal. This incision exposed the superficial epigastric vein and the Ing-Ax vessel running caudally along the skin outside the thoracic and abdominal cavities. After euthanasia, the abdominal cavity was opened, the major abdominal organs were removed, and the iliac lymphatic vessels were identified and then isolated as previously described elsewhere (33). The superficial cervical lymphatic vessels were exposed by retraction of the skin from the tip of the bottom jaw down toward the top of the thoracic cavity. Retraction of the skin revealed two bilateral pairs of superficial afferent cervical vessels that occasionally anastomosed before entering the node. The thoracic duct (TD) was isolated after removal of the major abdominal organs and dorsal aorta; this clear vessel running parallel to the inferior vena cava in the fat layer on the right side of the spine was then carefully excised. The locations of the respective vessels before they were excised are shown on the accompanying images from Prox1-GFP mice (Fig. 1). In some cases, vessels were isolated from up to four different regions of a single mouse, although care was taken to quickly isolate each vessel without damaging the tissues containing lymphatic vessels to be isolated subsequently. All vessels were studied within 6 h after isolation. Mice were euthanized by pentobarbital sodium overdose after isolation of the target vessels or just before removal of the TD. After removal, each vessel was pinned with short pieces of 40-μm stainless steel wire in a Sylgard chamber filled with Krebs buffer, and the majority of the attached adipose and connective tissue was cleared by microdissection (40). The isolated vessel was transferred to a 3-ml observation chamber, cannulated onto two glass micropipettes (30–80 μm), pressurized to 3 cmH2O, and trimmed of remaining connective tissue and fat at room temperature. Each vessel was cut to a length that contained only a single valve, so that intraluminal pressure was determined only by the pressures in the external reservoirs through the pipettes, with minimal influence from pressure pulses generated by spontaneous contractions (50); the exceptions were vessels used for pump tests, where two valves were required.
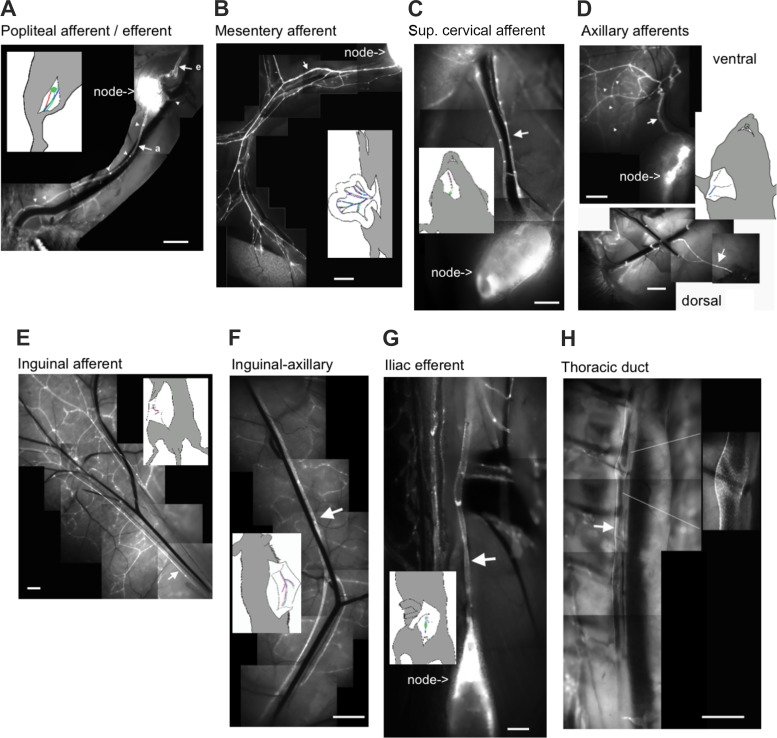
Fig. 1.
Fluorescence microscopy using Prox1-green fluorescent protein (GFP) mice to illustrate the location of each vessel type. Wide-field fluorescence images shows Prox1-GFP lymphatic collecting vessels in the different regions. Bright Prox1-GFP clusters are indicative of lymphatic valves, a few of which are demarcated with white triangles in A. Large white arrows identify afferent (a) and efferent (e) collecting vessels. Insets: anatomic context for the location of the corresponding image. Scale bars = 1 mm.
End-point RT-PCR.
Because of the small size of the vessels, we pooled both Ing-Ax vessels, all 4 popliteal vessels, 1 TD vessel, and ~12–15 mesenteric vessels from single mice for RNA collection. Vessels were snap frozen, and total RNA was extracted using the Arcturus PicoPure RNA isolation kit (ThermoFisher Scientific, Waltham, MA) following the manufacturer’s protocol. On-column DNase (Qiagen, Valencia, CA) treatment was performed, and cDNA was created using the SuperScript III First-Strand Synthesis System (ThermoFisher Scientific). PCR was performed in a reaction mixture containing first-strand cDNA as the template, 2 mM MgCl2, 0.25 μM primers, 0.2 mM deoxynucleotide triphosphates, and GoTaq Flexi DNA polymerase (Promega, Madison, WI). The PCR program comprised an initial denaturation step at 95°C for 4 min followed by 35 repetitions of the following cycle: denaturation (94°C, 30 s), annealing (55°C, 30 s), and extension (72°C, 30 s). This was followed by a final elongation step for 5 min at 72°C. PCR amplification products were separated on a 2% agarose gel by electrophoresis, stained with SYBR-Safe (ThermoFisher Scientific), and visualized by UV transillumination with a gel imaging system (Chemidoc XRS, Bio-Rad). RT-PCR primers were Cav1.2 exon1b sense (5′-ATGGTCAATGAAAACACGAGGATG-3′) (41) and Cav1.2 exon1b antisense (5′-GGAACTGACGGTAGAGATGGTTGC-3′) (6).
Fluorescence-based lymphatic mapping.
Before removal and cannulation of lymphatic collecting vessels, low-magnification epifluorescence microscopy was performed on Prox1-GFP mice to locate the main collecting vessels in each region of the mouse. For each region of an anesthetized mouse, the overlying skin or tissues were removed with careful dissection, and the vessels were located under a Leica DMIL fluorescence dissecting microscope at various magnifications. Images were acquired with a Nikon D3200 camera, and image tiles were stitched together in Adobe Photoshop.
Pressure control.
The cannulated vessel, with micropipette holders, observation chamber, and Burg-style V-track mounting system, was transferred to the stage of an inverted microscope (model ACM modified or IM35, Zeiss). Polyethylene tubing was attached to each micropipette holder and connected to a valve that allowed pressure control to be switched between a manual reservoir and a servo-controlled pump (15). Input and output pressures were set briefly to 10 cmH2O, and the vessel was lengthened to remove axial slack, which minimized lateral bowing and associated diameter-tracking artifacts at the higher pressures. A peristaltic pump maintained constant perfusion of the observation chamber with Krebs buffer at a rate of 0.5 ml/min while the vessel equilibrated at 37°C for 30–60 min with pressures set to 3 cmH2O. Spontaneous contractions typically began within 10 min and stabilized by 30 min. A Windows-based computer was used to digitize the pressure transducer signals and video image of the vessel from a FireWire camera at 30–40 Hz (15). A custom-written LabVIEW program (National Instruments, Austin, TX) detected the inner diameter of the vessel from the video (15).
Pressurized myograph.
To test the response of each vessel to pressure, the input and output pressures were lowered together from 3 to 2, 1, and 0.5 cmH2O and then, in most cases, stepped to 1, 2, 3, 5, 8, and 10 cmH2O. Under these conditions, there was no imposed pressure gradient for forward flow. Spontaneous contractions were recorded at each pressure for 1–10 min, a time interval usually sufficient to obtain at least eight contractions at the lowest pressure of 0.5 cmH2O. In cases where no contractions were recorded in the given timeframe, they were assigned a contraction amplitude and frequency of “0.” To minimize the influence of the previous pressure step or the magnitude of the pressure step, each vessel was allowed to reach a contraction steady state over a period of 30 s before data were collected for analysis after each pressure step. Some cervical and TD vessels were additionally treated with 1 μM norepinephrine (NE) followed by 1 μM acetylcholine (ACh) to assess vasoconstriction/dilation responses. Additionally, after the first pressure step protocol, some mesenteric, TD, Ing-Ax, and popliteal afferent vessels were superfused with Krebs buffer containing 100 nM BayK for 15 min at 3 cmH2O before the pressure step protocol was repeated. After the second step protocol, these mesenteric and TD vessels were further stimulated with 1 μM BayK at a constant pressure of 3 cmH2O in an attempt to elicit a higher EF. All vessels were equilibrated with Ca2+-free Krebs buffer for 20 min and then stepped through the experimental pressure range to obtain the passive diameter at each pressure (15).
Lymphatic pump test.
To test the effectiveness of contractions in lymph propulsion, we performed an output pressure challenge (pump test) on a popliteal afferent and a mesenteric lymphatic vessel segment containing two valves. In this preparation, output pressure was selectively ramped from 1 to 10 cmH2O at 4 cmH2O/min for the popliteal vessel and from 3 to 10 cmH2O at 4 cmH2O/min for the mesenteric vessel to impose an adverse pressure gradient to close the output valve. We chose 3 cmH2O for the mesenteric vessel, as this pressure provided the most consistent contractile activity. Under these conditions, the output valve opens only if the pressure spike generated by the lymphatic contraction in the upstream lymphatic chamber overcomes the output pipette pressure and thus tests the ability of the vessel to pump against physiological loads. During this test, the “output” lymphatic valve position was determined through densitometry at the base of the valve and represented as a binary state (1 = open, 0 = closed). Additionally, a servo-null pipette was inserted into the vessel between the input and output valves to provide real-time intralymphatic pressure measurements to demonstrate the pressure generation associated with lymphatic contractions that allow for positive lymph propulsion against a pressure gradient.
Membrane potential recordings and analysis.
Mouse mesenteric and Ing-Ax lymphatic vessels were isolated and pressurized as described above. Once the vessel had stabilized at a pressure of 3 cmH2O, wortmannin was added and the vessels were incubated for 30–60 min to inhibit myosin light chain kinase and blunt the vessel movement associated with contractions to <5 μm, as assessed by real-time edge tracking of the vessel wall. Lymphatic smooth muscle was impaled using intracellular microelectrodes (300–350 MΩ) filled with 1 M KCl, and membrane potential was sampled at 1 kHz using a NPI SEC-05x amplifier (ALA Instruments, Farmingdale, NY). This allowed simultaneous tracking of diameter and membrane potential in pressurized and contracting vessels. After lymphatic smooth muscle impalement, membrane potential was allowed to stabilize for 15–30 s: in Ing-Ax vessels, the resting membrane potential stabilized at approximately −35 mV, whereas in mesenteric lymphatic vessels, it stabilized at around −40 mV. After multiple contraction cycles had been recorded, 100 nM BayK was added to the bath solution to activate L-type Ca2+ channels. In some cases, the impalement was lost due to the mixing procedure or the enhanced contraction strength elicited by BayK. When an impalement was lost, attempts were made to impale the same cell, if possible, or a second cell in the immediate vicinity of the original cell. Once the recordings had been made, the electrode was retracted and the offset potential was subtracted from the recorded values.
Acquired data files were processed, and APs were automatically measured and characterized using an in-house Python algorithm. Briefly, after resting (baseline) membrane potential was determined and potential recordings were run through a subroutine for peak identification, the number of APs and their corresponding total duration were obtained. Analysis of the depolarization stage for each AP allowed us to identify two different phases: an initial slow depolarization (linear behavior) and a subsequent, exponential-like, rapid depolarization leading to the peak of the AP. These two phases were further analyzed and characterized using a set of first and second derivative-based conditions to determine the membrane potential threshold at which the slow-to-fast depolarization transition occurred. Once determined, the corresponding phase durations and depolarization rates were calculated. For the initial slow-depolarizing phase of the AP, the depolarization rate was obtained from a linear fit and expressed as mV/s. In the case of the fast-depolarizing phase, the time constant (τ; expressed in ms) from an exponential fitting, V(t) = et/τ, was calculated (where V is voltage and t is time). Mean total integrated signal per AP and percent integrated signals for each phase within an AP were computed and compared under control conditions and with 100 nM BayK for each trace consisting of three or more APs per vessel per mouse.
Ca2+ imaging and analysis.
SMMHC-CreERT2;GCaMP6f mice were induced 3 wk before study by one intraperitoneal injection of 100 μl of 10 mg/ml tamoxifen/day for 5 consecutive days with a 2-wk recovery period after the last injection to allow the mouse to metabolize and clear the tamoxifen. This injection protocol was sufficient to induce recombination in >95% of lymphatic muscle cells as assessed with the use of GCaMP6f as a Cre reporter. Use of this genetically encoded Ca2+ sensor eliminated the loading variability and difficulty of isolating Ca2+ signals specifically from muscle cells associated with the use of nonspecific Ca2+ indicator dyes. Cleaned and isolated vessels were cannulated, pressurized, and placed in an observation chamber on an inverted fluorescence microscope (Olympus IX81) equipped with a confocal spinning disk (Yokagawa CSU-X, Andor Technology). After an equilibration period, wortmannin (final concentration: 2 μM) was added to the bath solution to reduce contraction-associated vessel movement during imaging until contractions were below ~3 μm in amplitude; this procedure helped maintain the same focal plane within the vessel. Vessels were then excited with a 488-nm laser and imaged at 20–50 frames/s with an electron-multiplying charge-coupled device camera (Photometrics Cascade II 512). While spontaneous localized intracellular Ca2+ activity was apparent in almost every lymphatic smooth muscle cell, contractions were associated only with high-intensity, L-type-dependent, global Ca2+ events (termed “flashes”) that were nearly synchronous between all muscle cells in the field of view (59, 60). The term “flash” has been used to describe the Ca2+ events linked to APs in smooth muscle driven by voltage-gated Ca2+ (Cav) channels (23). Videos were processed, and the fluorescence from Ca2+ events was automatically characterized and quantified using an in-house Python algorithm that performed a pseudo-line scan over the entire length of video for each pixel column that was normalized to baseline fluorescence and then averaged to provide a ratiometric Ca2+ plot over time. Analysis of Ca2+ recordings allowed us to determine mean amplitude (expressed as a baseline-referenced ratio, F/F0), duration (in s), and total integrated signal per Ca2+ flash before and after 100 nM BayK.
Solutions and chemicals.
Krebs buffer contained (in mM) 146.9 NaCl, 4.7 KCl, 2 CaCl2·H2O, 1.2 MgSO4, 1.2 NaH2PO4·H2O, 3 NaHCO3, 1.5 NaHEPES, and 5 d-glucose (pH 7.4 at 37°C). An identical buffer was prepared with the addition of 0.5% BSA. During cannulation, luminal and abluminal solutions contained Krebs solution with BSA; during the experiment, the abluminal solution was constantly exchanged with plain Krebs solution. For the Ca2+-free Krebs solution, 3 mM EGTA replaced Ca2+. All chemicals except BSA (US Biochemicals, Cleveland, OH) and MgSO4 and NaHEPES (Fisher Scientific, Pittsburgh, PA) were obtained from Sigma (St. Louis, MO). BayK was purchased from Sigma, dissolved in DMSO to 1 mM, separated into aliquots, and stored at −20°C. Tamoxifen was dissolved to 10 mg/ml in a 95% safflower oil-5% ethanol (vol/vol) solution with rocking agitation and stored at −20°C in aliquots.
Myograph data analysis.
After an experiment, custom-written analysis programs (LabVIEW) were used to detect peak end-diastolic diameter (EDD), end-systolic diameter (ESD), and contraction frequency, computed on a contraction-by-contraction basis over time in the 1- to 5-min period corresponding to each pressure level. Data were used to calculate several commonly reported contraction parameters: %tone = [(MaxD – EDD)/MaxD] × 100 and EF = (EDD2 – ESD2)/EDD2, where MaxD is the maximum passive diameter obtained in Ca2+-free Krebs solution at the same pressures at the end of every experiment.
Immunofluorescence and confocal microscopy.
TD, mesenteric, popliteal, and Ing-Ax vessels were isolated from Prox1-GFP mice and cleaned of adipose and remaining tissue. Vessels were cannulated, pressurized, fixed with 4% paraformaldehyde for 15 min at room temperature and then for 45 min at 4°C, washed with PBS, blocked with 5% goat serum for 2 h at 4°C, and incubated overnight in PBS with anti-GFP (catalog no. A-11122, ThermoFisher) and anti-smooth muscle actin (catalog no. A2547, Sigma) primary antibodies. Vessels were then washed with PBS and incubated with goat anti-mouse Alexa Fluor 647 (catalog no. A-21235, ThermoFisher) and goat anti-rabbit Alexa Fluor 488 (catalog no. A-11034, ThermoFisher) secondary antibodies overnight. Thereafter, vessels were subjected to a final wash, cannulated, and pressurized for confocal imaging using the inverted microscope described above and a Hamamatsu Orca Flash4 camera. Images were taken at ×10 magnification with 1-μm steps, and maximum image projections were created using MetaMorph (Molecular Devices) software. Lymphatic smooth muscle coverage was determined by creation of a vessel mask based on the Prox1-GFP signal followed by comparison of the percent coverage of the mask by smooth muscle actin staining.
Statistical tests.
The contraction parameters from the different regions were analyzed by two-way ANOVA with Tukey’s post hoc test using Prism 6 (GraphPad, San Diego, CA). Values are means ± SE. Data for peripheral vessels are shown in Table 1, data for visceral vessels are shown in Table 2, and cervical and TD vessel responses to NE and ACh are shown in Table 3. Changes in contraction frequency, tone, and EF before and after 100 nM BayK were assessed using repeated-measures two-way ANOVA. Responses to 1 μM NE and 1 μM ACh or 1 μM BayK at single pressures (3 cmH2O) were analyzed by repeated-measures ANOVA. Membrane potential and Ca2+-imaging recordings were also assessed by two-way ANOVA with Tukey’s post hoc test and two-way repeated-measures ANOVA with Sidak’s post hoc test, respectively.
Table 1.
Murine peripheral lymphatic vessel contractile parameters
| Pressure, cmH2O | Popliteal Afferent | Popliteal Efferent | Inguinal Afferent | Axillary Afferent | Superficial Cervical | Inguinal Axillary (Efferent) |
|---|---|---|---|---|---|---|
| End-diastolic diameter, μm | ||||||
| 0.5 | 64.9 ± 2.9 | 124 ± 17 | 73.9 ± 5.3 | 51.5 ± 5.9 | 66.0 ± 2.9 | 74.5 ± 6.7 |
| 1 | 67.3 ± 3.4 | 133 ± 15 | 79.3 ± 3.8 | 54.4 ± 6.3 | 69.2 ± 3.3 | 82.6 ± 6.7 |
| 2 | 72.4 ± 3.0 | 138 ± 15 | 84.4 ± 2.6 | 58.9 ± 6.2 | 73.1 ± 2.9 | 87.9 ± 6.9 |
| 3 | 74.7 ± 3.1 | 142 ± 14 | 85.5 ± 2.7 | 60.2 ± 6.1 | 74.6 ± 2.7 | 88.2 ± 7.0 |
| 5 | 75.0 ± 3.0 | 139 ± 15 | 84 ± 3.3 | 61.7 ± 6.0 | 76.9 ± 2.9 | 88.2 ± 7.6 |
| 8 | 74.8 ± 3.1 | 139 ± 15 | 84.3 ± 3 | 61.9 ± 5.6 | 76.6 ± 3.1 | 88.4 ± 8.2 |
| 10 | 75.8 ± 3.2 | 141 ± 15 | 84.7 ± 2.8 | 61.6 ± 5.5 | 76.4 ± 3.4 | 90.8 ± 8.1 |
| Tone, %passive diameter | ||||||
| 0.5 | 10.9 ± 1.8 | 18.4 ± 4.1 | 17.5 ± 4.3 | 18.0 ± 4.8 | 21.7 ± 2.3 | 24.7 ± 3.8 |
| 1 | 11.6 ± 1.8 | 19.2 ± 3.3 | 14.2 ± 3.1 | 18.1 ± 4.3 | 21.5 ± 2.8 | 24.3 ± 3.1 |
| 2 | 11.0 ± 1.0 | 20.7 ± 3.2 | 11.6 ± 2.4 | 14.7 ± 3.0 | 20.2 ± 2.2 | 24.1 ± 3.0 |
| 3 | 10.5 ± 1.2 | 19.4 ± 2.8 | 11 ± 2.5 | 15.0 ± 2.9 | 19.9 ± 2.1 | 25.6 ± 3.1 |
| 5 | 12.1 ± 1.2 | 22.2 ± 3.9 | 11.3 ± 2.8 | 14.8 ± 2.1 | 19.0 ± 2.0 | 27.1 ± 3.5 |
| 8 | 13.7 ± 1.5 | 23.2 ± 4.2 | 11.4 ± 2.5 | 15.0 ± 2.3 | 20.2 ± 1.8 | 28.3 ± 3.8 |
| 10 | 13.7 ± 1.5 | 23 ± 4.0 | 11.5 ± 2.7 | 15.5 ± 2.6 | 20.8 ± 2.3 | 26.9 ± 3.4 |
| Ejection fraction | ||||||
| 0.5 | 0.77 ± 0.02 | 0.50 ± 0.12 | 0.53 ± 0.09 | 0.55 ± 0.07 | 0.84 ± 0.04 | 0.75 ± 0.03 |
| 1 | 0.78 ± 0.02 | 0.53 ± 0.09 | 0.57 ± 0.07 | 0.58 ± 0.06 | 0.72 ± 0.06 | 0.69 ± 0.02 |
| 2 | 0.78 ± 0.02 | 0.49 ± 0.07 | 0.53 ± 0.04 | 0.51 ± 0.05 | 0.65 ± 0.04 | 0.61 ± 0.02 |
| 3 | 0.74 ± 0.02 | 0.41 ± 0.05 | 0.47 ± 0.04 | 0.45 ± 0.04 | 0.58 ± 0.04 | 0.52 ± 0.02 |
| 5 | 0.56 ± 0.03 | 0.26 ± 0.04 | 0.31 ± 0.05 | 0.30 ± 0.02 | 0.44 ± 0.03 | 0.31 ± 0.02 |
| 8 | 0.34 ± 0.04 | 0.13 ± 0.02 | 0.17 ± 0.04 | 0.18 ± 0.03 | 0.29 ± 0.03 | 0.13 ± 0.02 |
| 10 | 0.25 ± 0.03 | 0.09 ± 0.02 | 0.14 ± 0.05 | 0.12 ± 0.02 | 0.20 ± 0.03 | 0.07 ± 0.01 |
| Frequency, contractions/min | ||||||
| 0.5 | 5.2 ± 1.0 | 2.6 ± 1.5 | 6.4 ± 2.8 | 5.4 ± 1.2 | 6.3 ± 1.0 | 10.6 ± 1.3 |
| 1 | 7.5 ± 1.3 | 11.2 ± 3 | 9.1 ± 2.6 | 8.4 ± 0.5 | 11.7 ± 1.4 | 16.3 ± 1.5 |
| 2 | 11.1 ± 1.3 | 14.9 ± 2.2 | 13.2 ± 2.2 | 11.4 ± 1.3 | 16.2 ± 1.0 | 18.3 ± 1.7 |
| 3 | 11.7 ± 1.3 | 17.4 ± 2.6 | 14.4 ± 2.2 | 12.7 ± 1.7 | 17.2 ± 1.6 | 18.6 ± 2.0 |
| 5 | 12.5 ± 1.4 | 18.7 ± 2 | 18 ± 1.9 | 14.6 ± 1.1 | 16.5 ± 1.2 | 19.3 ± 2.2 |
| 8 | 13.3 ± 1.5 | 19.5 ± 2.4 | 19.3 ± 2.3 | 15.7 ± 1.2 | 16.0 ± 1.4 | 21.7 ± 2.8 |
| 10 | 12.9 ± 1.4 | 19.3 ± 2.5 | 19.2 ± 2.6 | 15.8 ± 1.2 | 16.4 ± 1.3 | 23.9 ± 3.2 |
Values are means ± SE; n = 14 popliteal afferent, 6 popliteal efferent, 8 inguinal afferent, 5 axillary afferent, 9 superficial cervical, and 15 inguinal axillary vessels.
Table 2.
Murine visceral lymphatic vessel contractile parameters
| Pressure, cmH2O | Mesentery Afferent | Thoracic Duct | Iliac Efferent |
|---|---|---|---|
| End-diastolic diameter, μm | |||
| 0.5 | 67.3 ± 5.6 | 137 ± 6 | 133 ± 10 |
| 1 | 70.2 ± 5.6 | 152 ± 5 | 146 ± 10 |
| 2 | 70.2 ± 4.4 | 164 ± 4 | 155 ± 11 |
| 3 | 70.2 ± 3.5 | 168 ± 4 | 160 ± 12 |
| 5 | 72.5 ± 3.4 | 174 ± 4 | 165 ± 14 |
| 8 | 80.8 ± 3.5 | 179 ± 3 | 169 ± 14 |
| 10 | 84.7 ± 3.6 | 181 ± 2 | 172 ± 14 |
| Tone, %passive diameter | |||
| 0.5 | 23.6 ± 4.6 | 9.9 ± 1.9 | 9.5 ± 1.7 |
| 1 | 22.6 ± 4.7 | 12.1 ± 2.6 | 8.3 ± 1.4 |
| 2 | 25.3 ± 4.5 | 12.2 ± 2.9 | 8.0 ± 1.6 |
| 3 | 26.6 ± 4.0 | 12.1 ± 2.7 | 7.2 ± 1.6 |
| 5 | 26.4 ± 3.5 | 10.1 ± 2.8 | 8.6 ± 3.4 |
| 8 | 19.2 ± 3.4 | 8.2 ± 2.1 | 8.1 ± 2.8 |
| 10 | 15.5 ± 3.6 | 7.1 ± 2.0 | 7.7 ± 2.5 |
| Ejection fraction | |||
| 0.5 | 0.07 ± 0.02 | 0.03 ± 0.02 | 0.01 ± 0.00 |
| 1 | 0.10 ± 0.02 | 0.02 ± 0.02 | 0.01 ± 0.00 |
| 2 | 0.08 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 |
| 3 | 0.06 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 |
| 5 | 0.04 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 |
| 8 | 0.02 ± 0.01 | <0.01 | <0.01 |
| 10 | 0.02 ± 0.01 | <0.01 | <0.01 |
| Frequency, contractions/min | |||
| 0.5 | 4.4 ± 1.2 | 2.3 ± 0.9 | 7.6 ± 1.4 |
| 1 | 5.6 ± 0.9 | 4.9 ± 2.4 | 7.2 ± 1.0 |
| 2 | 7.8 ± 0.8 | 3.5 ± 2.2 | 10.0 ± 1.3 |
| 3 | 9.1 ± 0.9 | 5.0 ± 3.1 | 6.4 ± 1.6 |
| 5 | 7.9 ± 1.5 | 3.3 ± 3.3 | 6.3 ± 1.6 |
| 8 | 6.5 ± 1.8 | 4.3 ± 4.3 | 5.0 ± 1.7 |
| 10 | 6.7 ± 1.8 | 0 | 5.5 ± 2.0 |
Values are means ± SE; n = 12 mesenteric, 5 iliac, and 11 thoracic duct vessels.
Table 3.
Contractile responses to NE and ACh in superficial cervical and TD vessels at 3 cmH2O pressure
| Krebs | 1 μM NE | 1 μM ACh | |
|---|---|---|---|
| Frequency, contractions/min | |||
| Cervical | 14.5 ± 0.9 | 17.7 ± 1.3 | 7.6 ± 3.3 |
| TD | |||
| Ejection fraction | |||
| Cervical | 0.59 ± 0.07 | 0.55 ± 0.1 | 0.60 ± 0.08 |
| TD | |||
| Tone, %passive diameter | |||
| Cervical | 21.2 ± 1.6 | 28.0 ± 1.4 | 15.9 ± 2.0 |
| TD | 5.8 ± 3.4 | 55.6 ± 4.5 | 21.9 ± 9.1 |
Values are means ± SE; n = 4 thoracic duct (TD) and 5 superior cervical vessels. At 2 min after exposure to 1 μM norepinephrine (NE), vessels were stimulated with 1 μM acetylcholine (ACh).
RESULTS
Lymphatic collecting vessel spatial location.
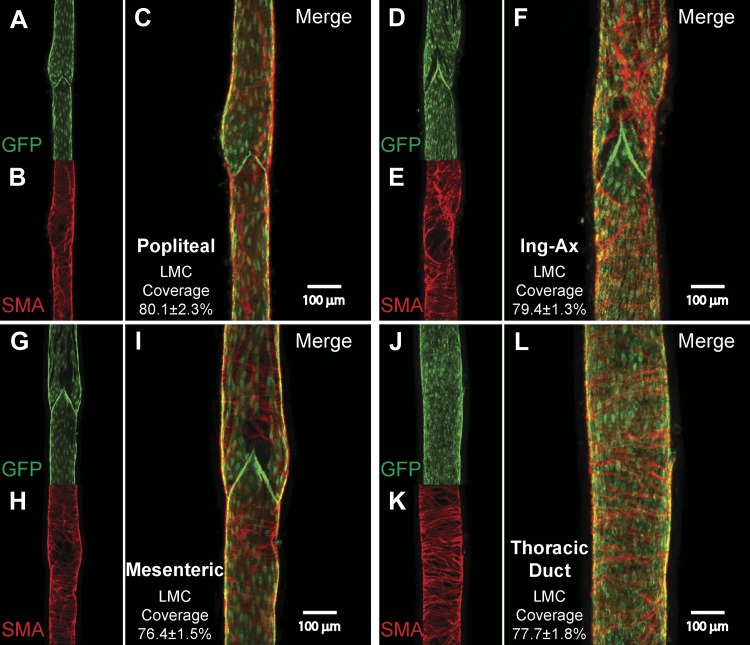
Lymphatic vessel visualization within the tissue is difficult without the use of contrast dyes because of the inherent transparency of nonchylous lymph, thin lymphatic vessel walls, and visual interference by associated matrix and adipose tissue. To aid in their identification and promote further characterization of these vessels, we used the Prox1-GFP lymphatic endothelial reporter mouse with limited surgical manipulation to capture wide-field fluorescence images of each lymphatic collecting vessel bed (Fig. 1). These initial experiments guided isolation of the vessels from subsequent C57BL/6J and SMMHC-CreERT2;GCaMP6f mice. Lymphatic valves were clearly distinguished as bright GFP clusters throughout each of the lymphatic beds. Intervalve distance was variable between different tissue beds, and valve density appeared higher in the precollecting networks. As previously described, we consistently observed two major popliteal collecting vessels running in parallel with the saphenous vein. While anastomoses between these vessels were occasionally observed, each vessel was largely independent up to the popliteal node, at which point the two vessels would often coalesce just before entering the node (Fig. 1A). A single major popliteal efferent collecting vessel was routinely observed (Fig. 1A). The mesenteric lymphatic network has been extensively described in the mouse throughout development, and for this study we isolated one of the larger afferent collecting vessels near the mesenteric node (Fig. 1B). The superficial afferent cervical lymphatic collecting vessels formed a pair of collecting vessels surrounding the left and right external jugular veins (Fig. 1C). In the popliteal and cervical beds, a slight, but noticeable, diameter difference within a pair of vessels was commonly observed, although this was not a discriminating factor for use in subsequent contraction protocols. The axillary afferent and inguinal afferent collecting vessels had extensive upstream precollector networks rich in valves in close proximity to branch points (Fig. 1, D and E). Connecting the inguinal and axillary nodes was the internodal Ing-Ax collecting vessel, which typically coursed in parallel with the superficial epigastric vein/artery (Fig. 1F). The Ing-Ax valves were oriented anterograde to the axillary nodes and retrograde to the inguinal nodes. The Ing-Ax vessel occasionally bifurcated into, or existed as, a pair of vessels. Additionally, smaller collecting vessels originating from the back or abdominal skin were often tributaries directly into this vessel. Within the visceral cavity, the iliac collecting ducts were quite large bilateral collecting vessels, comprising the primary efferent vessels for the iliac lymph nodes (Fig. 1G). The lymphatic TD was consistently observed on the mouse’s right side opposite the aorta and in close proximity to the vena cava (Fig. 1H). During dissection, the TD had a notably flaccid appearance and the vessel wall was quite fragile. Care was taken to avoid branches from the TD that originated from the paravertebral lymphatic plexus within the thoracic wall (not shown). Smooth muscle cell coverage and the presence of valves were assessed to ensure the collector identity of these vessels, which appeared grossly similar in vessels isolated from the visceral cavity and peripheral tissues (Fig. 2). Similar lymphatic smooth muscle density was observed across the four vessels analyzed in the tubular region, with average surface areas of 79.4 ± 1.3% for Ing-Ax (n = 5), 76.4 ± 1.5% for mesenteric (n = 4), 80.1 ± 2.3% for popliteal afferent (n = 5), and 77.7 ± 1.8% for TD (n = 5) vessels. Similar to previously reported rat lymphatic vessel data (5), we observed sparser muscle cell coverage density and altered spatial alignment near the valve sinus regions in all vessels imaged.
Fig. 2.
Immunofluorescence microscopy of isolated, smooth muscle actin (SMA)-stained vessels from Prox1-green fluorescent protein (GFP) mice. Representative images of isolated popliteal (A–C), internodal inguinal axillary (Ing-Ax; D–F), mesenteric (G–I), and thoracic duct (TD, J–L) vessels were stained with anti-GFP (green) and anti-smooth muscle actin (red) to determine the degree of lymphatic muscle cell (LMC) coverage. Images are maximal projections of 1-μm image stacks taken from the outside to the midpoint of the vessel using a ×10 objective. Mean percent lymphatic muscle cell coverage and corresponding SE were quantified from tubular (nonvalve) sections of popliteal (n = 5), Ing-Ax (n = 5), mesenteric (n = 4), and TD (n = 5) vessels only; valve areas were included in the representative images to emphasize that valve areas are also populated with lymphatic muscle cells.
Peripheral, but not visceral, lymphatic collecting vessels have robust contractions.
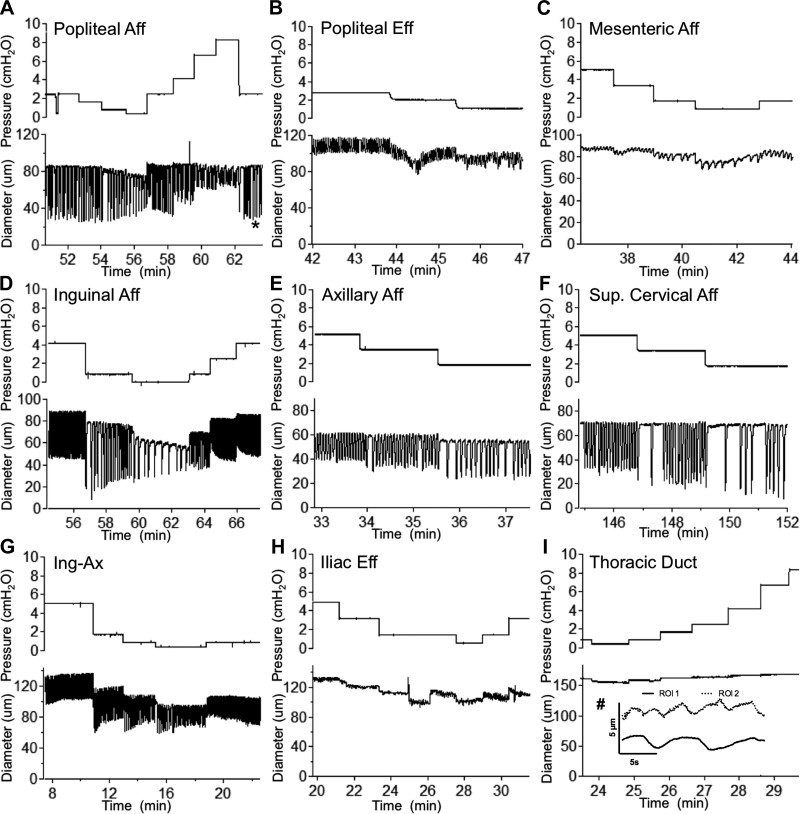
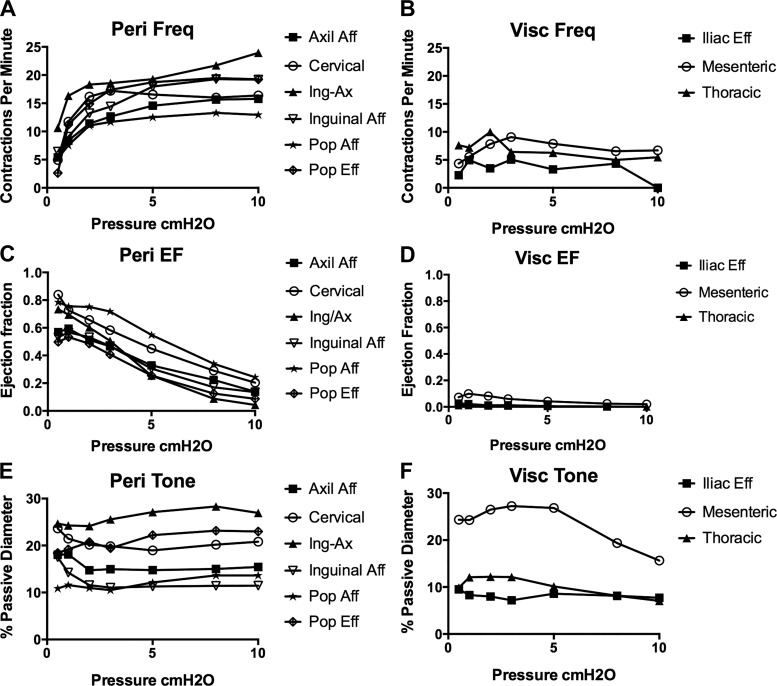
To test the hypothesis that murine lymphatic vessels from multiple regions are contractile, we used a highly controllable ex vivo isobaric method to track lymphatic collecting vessel diameter over a range of pressures (Fig. 3). A stark difference in contractile strength (e.g., EF) was noted between vessels isolated from peripheral regions, originally classified as ”somatic” vessels by Tilney (57), and vessels from the visceral cavities, which included mesenteric afferent lymphatics, iliac efferent lymphatics, and TD vessels. Spontaneous contractions in mesenteric vessels consistently exhibited maximal amplitudes of <10 μm, with the majority of mesenteric vessels (10 of 12 vessels) having maximal amplitudes of <5 μm at any pressure tested; despite the small amplitudes, these contractions were typically coordinated along the entire vessel length (~1 mm). Similarly, weak (<5-μm) contractions were also noted in iliac vessels, where only three of five vessels developed contractions. The TD displayed more of a vasomotion contraction pattern, with an amplitude generally <2 μm, not coordinated between adjacent regions, and with an irregular frequency (Fig. 3I, inset). Conversely, rhythmic, coordinated, and large-amplitude contractions were observed in all vessels isolated from each of the peripheral regions, with consistent and reproducible contractile behavior within each regional cohort. As expected based on a previous study (18), there were regional differences in contraction frequency for the peripheral vessels, although they shared a similar pressure-dependent chronotropy (Fig. 4A) that was significantly different from that in vessels in the visceral beds (Fig. 4B). Furthermore, all peripheral vessels had significantly higher (50–80%) EFs than vessels from any of the visceral lymphatic beds (Fig. 4C). The small contraction amplitudes in the mesenteric, iliac, and TD vessels equated to EFs of <10% for mesenteric and 5% for iliac and TD vessels (Fig. 4D). There were no apparent differences in tone between peripheral and visceral vessels (Fig. 4, E and F).
Fig. 3.
Raw diameter traces of mouse lymphatic collecting vessels isolated from different tissue beds and studied over the experimental pressure range. Isolated lymphatic collecting vessels were exposed to a range of transmural pressures, while automated edge-tracking software allowed for real-time diameter recordings. Traces are representative contractile responses for the different lymphatic collecting vessels. Typical responses are increased contraction frequency and reduced amplitude as pressure is increased. An indication that mouse vessels adapt to changes in pressure with changes in contractility, as do rat vessels, is shown in A (*), where a time-dependent decrease in end-systolic diameter occurred after a step from low to high pressure; this reflects an increase in contractility or, more precisely, a recovery from the reduction in contractility at the lower pressure (15). Lack of coordination of small contractions in the mouse thoracic duct that was set at 2-cmH2O pressure with diameter tracked by two separate regions of interest (ROIs) ~650 μm apart (#) is shown in I. Aff, afferent; Eff, efferent; Sup, superior; Ing-ax, inguinal axillary.
Fig. 4.
Quantification of contractile parameters in response to changes in transmural pressures. Contraction frequency (Freq; A and B), ejection fraction (EF; C and D), and vessel tone (E and F) were calculated for each pressure for peripheral (Peri) and visceral (Visc) lymphatic collecting vessels. Vessels were allowed to reach their contractile “steady state” at each pressure before data were recorded for analysis. Values are means; SEs were omitted for visual clarity and are shown in Tables 1 and 2. Comparison of contraction frequency with EF showed that visceral vessels (mesenteric, thoracic duct, and iliac) were significantly different from all other peripheral vessels (P < 0.05 by two-way ANOVA). Values are means; n = 14 popliteal afferent (Pop Aff), n = 6 popliteal efferent (Pop Eff), n = 12 mesenteric, n = 8 inguinal afferent (inguinal Aff), n = 5 axillary afferent (Axil Aff) and iliac, n = 9 superficial cervical, n = 15 inguinal axillary (Ing-Ax), and n = 11 thoracic duct (thoracic).
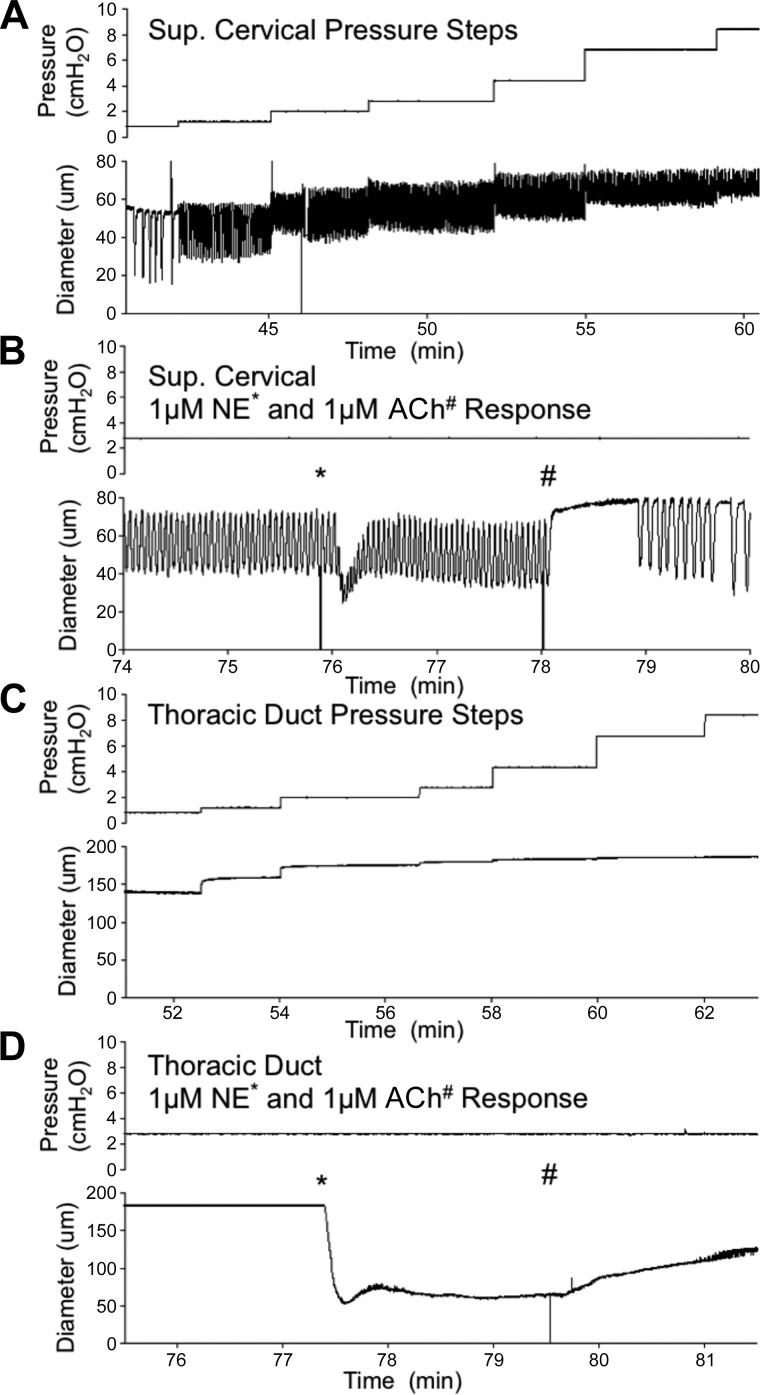
To ensure that the lack of robust contractions in visceral vessels was not due to damage incurred during isolation, we used the TD and superficial cervical vessel as representative vessels of each class and treated each with the classical stimulatory and inhibitory neurotransmitters NE and ACh after a typical pressure-response protocol (Fig. 5). Each TD and superior cervical afferent vessel rapidly constricted to ~50% of maximal passive diameter after stimulation with 1 μM NE; however, this dramatic increase in tone was transient in the superficial cervical vessels, as tone resolved to ~28% after ~30 s (Fig. 5B). The TD maintained ~55% tone (Fig. 5). As such, 1 μM NE significantly increased tone in both vessels, although the increase in tone was significantly higher (P < 0.05) in the TD than the superficial cervical vessel. NE also significantly increased contraction frequency in the superficial cervical vessel, although no contractions were observed in the TD (Fig. 5). Subsequent stimulation with 1 μM ACh caused a rapid and significant relaxation and reduction in contraction frequency in superficial cervical vessels. Application of 1 μM ACh significantly reduced tone in the TD, although this dilation occurred more slowly and diameter did not recover to the preconstriction diameter.
Fig. 5.
Peripheral and visceral collecting lymphatic vessels are responsive to norepinephrine (NE) and acetylcholine (ACh). Representative diameter traces are shown for superficial (Sup) cervical afferents (n = 5; A and B) and thoracic ducts (n = 4; C and D) at different pressures and in response to the classic stimulatory and inhibitory neurotransmitters NE (*) and ACh (#), respectively. Mean and SE for contraction frequency, ejection fraction, and tone after subsequent NE and ACh treatment are shown in Table 3.
Visceral lymphatic collecting vessels are not effective pumps.
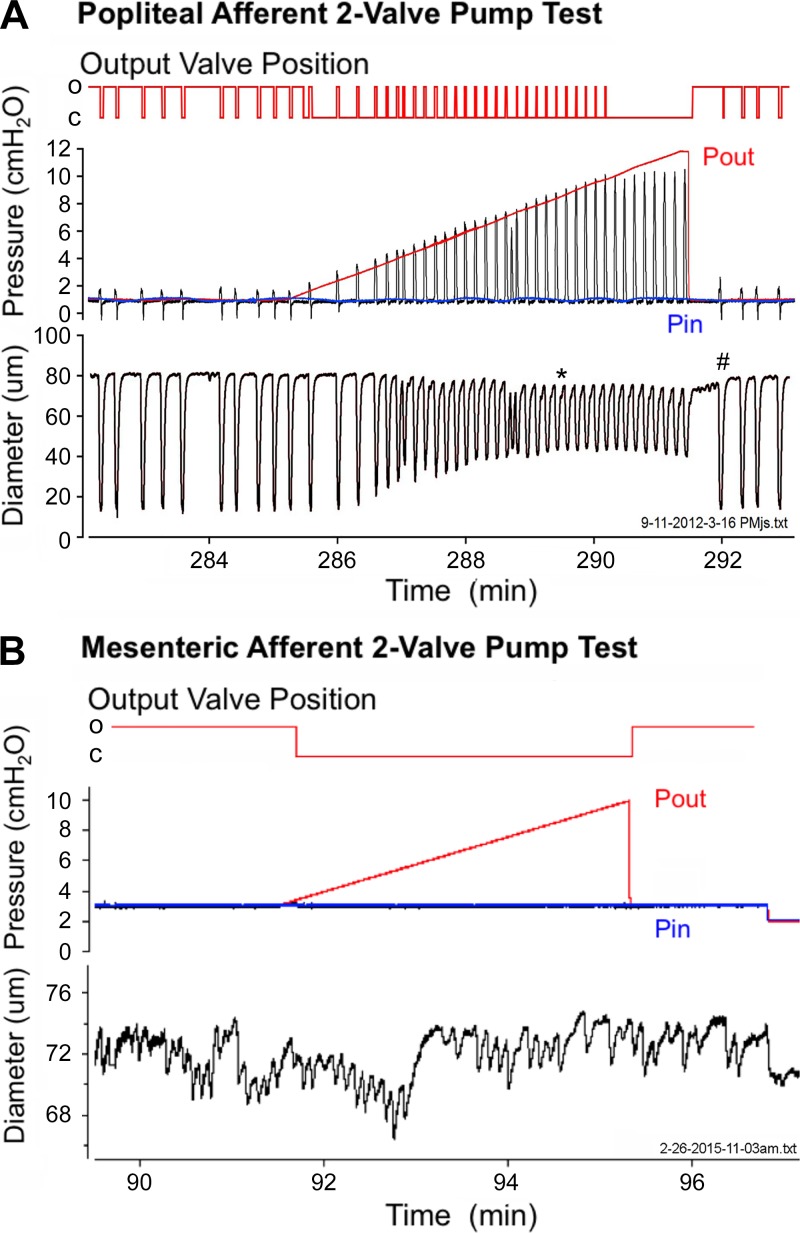
We hypothesized that the small-amplitude contractions in the mesenteric and iliac collecting vessels would be insufficient to pump lymph “uphill.” To test this hypothesis, a slight adverse pressure gradient was applied to a two-valve segment (i.e., lymphangion) of the popliteal afferent (the strongest peripheral vessel) and mesenteric (the strongest visceral vessel) lymphatics. Valves in the popliteal and mesenteric vessels displayed an “open” bias while pressures were equal (3, 14, 51). While inflow pressure was kept constant, the output pipette pressure was raised slowly to close the downstream lymphatic valve, so that any subsequent opening of that valve depended on generation of a transient pressure pulse by the lymphangion chamber during contraction (15). The popliteal afferent vessel had contraction amplitudes of ~40 μm at an inflow pressure of 1 cmH2O and was able to open the valve, despite an adverse pressure gradient of 8 cmH2O (Fig. 6A). In contrast, the mouse mesenteric lymphatic collecting vessel had contraction displacements of only a few micrometers and was unable to overcome even a 1-cmH2O pressure gradient to open the downstream valve and drive lymph flow (Fig. 6B).
Fig. 6.
Pump function in peripheral and visceral lymphatic collecting vessels. A: spontaneous contractions of mouse popliteal afferent (and other peripheral) vessels are sufficiently robust to produce propulsive contractions under an imposed output pressure (Pout) load. These contractions generate internal pressure spikes (black pressure line) that transiently exceed output pressure and open an otherwise closed output valve (top red trace) and partially expel the contents of the lymphangion (15). O, open; C, closed. B: mesenteric lymphatic collecting vessels are unable to overcome even a small adverse pressure gradient (lack of black pressure spikes) to open the output valve and propel lymph. Zoomed diameter scale in B demonstrates the presence of low-amplitude contractions in the mesenteric vessel. In A and B, output valve was tracked and recorded as open or closed throughout the pressure ramp protocol. Blue pressure line refers to input pressure (Pin); red pressure line refers to output pressure. Servo-null pressure was measured between the input and output valves to demonstrate the increase in intralymphatic pressure during the lymphatic contraction (black line). Note time- and pressure-dependent constriction (in end-diastolic diameter; *) as output pressure is gradually raised (50) and rebound in contraction amplitude (#) when output pressure is returned to control level, reflecting an increase in contractility (15).
Contractile responses of peripheral murine lymphatic vessels are comparable to those of rat lymphatic vessels.
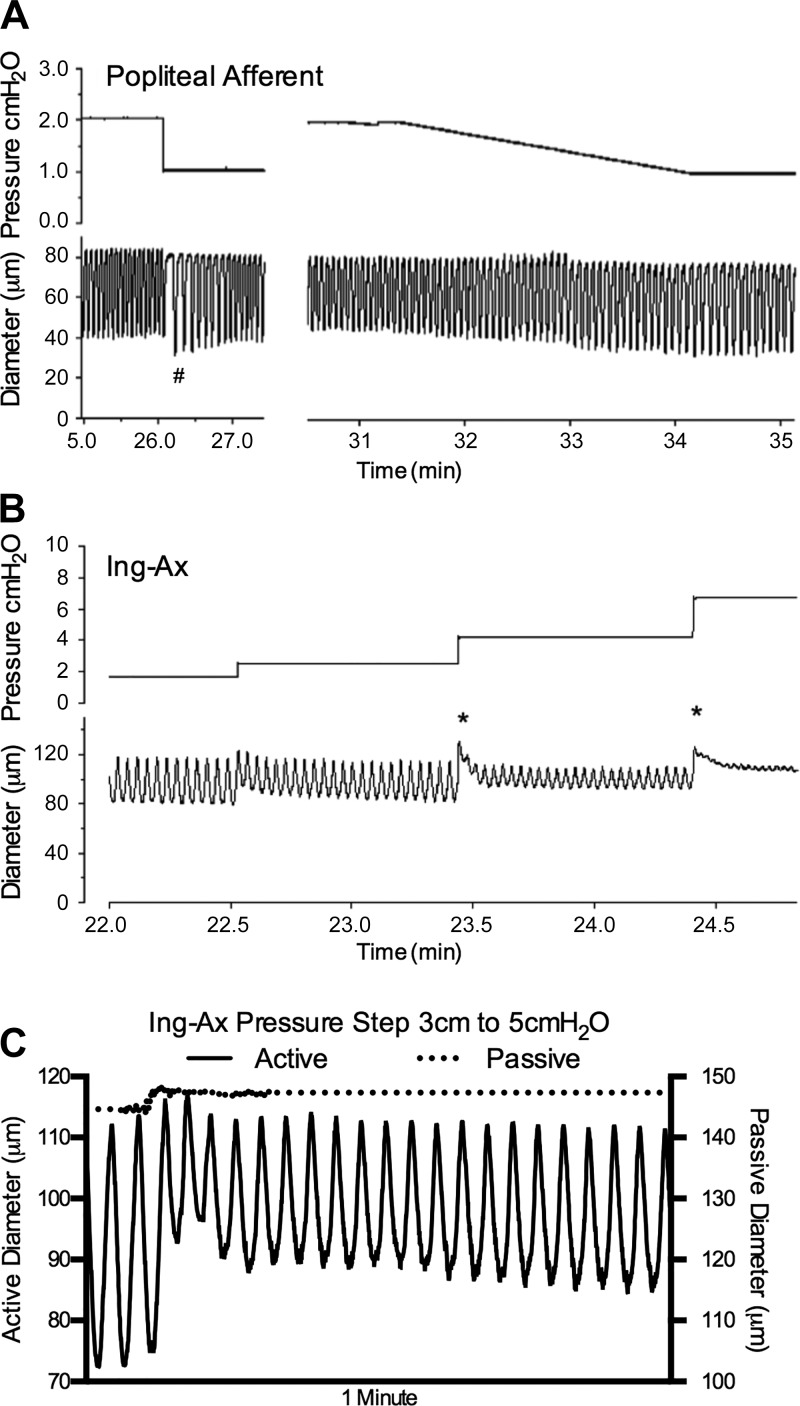
During the pump test in the afferent popliteal collecting vessel, EDD decreased as output pressure increased (Fig. 6A), indicative of a typical lymphatic myogenic response (12). At the culmination of the pump test, a prolonged pause in the contraction pattern was observed when output pressure was returned to the basal level (Fig. 6A); this response is suggestive of rate-sensitive inhibition, as previously reported for rat lymphatics (13). The contraction amplitude was also larger during the first few contractions after normalization of pressure, consistent with a prolonged increase in contractility during and immediately after an elevation in afterload (15). Conversely, the mesenteric lymphatic vessel did not display the typical lymphatic myogenic response (12), nor was rate-sensitive inhibition or change in contractility observed (Fig. 6B). Rate-sensitive inhibition was further demonstrated in the popliteal afferent vessel by the dramatic reduction and subsequent rebound in contraction frequency in response to a downward pressure step (Fig. 7A). In contrast, a ramp-wise reduction in pressure resulted in a constant gradual reduction in contraction frequency that did not undershoot the average contraction frequency for that pressure (Fig. 7A).
Fig. 7.
Peripheral lymphatic collecting vessels replicate the contractile behavior observed in other species. A: rate-sensitive inhibition of contraction frequency (and increase in amplitude) (#) in a popliteal afferent lymphatic when pressure is rapidly stepped down from 2 to 1 cmH2O; initially, contraction frequency slows dramatically and then gradually recovers to a value lower than at 2 cmH2O; in contrast, when pressure is slowly changed over the same range, contraction frequency changes are gradual and do not undershoot. Other examples of rate-sensitive inhibition are evident in response to downward pressure steps in Fig. 2. B: lymphatic myogenic constrictions were evident in the inguinal axillary (Ing-Ax) vessel, as evident by a biphasic decline in end-diastolic diameter in pressure steps above 2 cmH2O that ultimately results in a constant end-diastolic diameter across a 4-fold increase in the pressure steps from 2 to 8 cmH2O. Within the first seconds of each pressure change, the vessels rapidly gain tone (*). C: zoomed-in trace of an Ing-Ax vessel stepped from 3 to 5 cmH2O under active (Krebs buffer) or passive (Ca2+-free) conditions. Note gradual reduction in end-diastolic diameter over time after pressure step in Krebs buffer compared with diameter change under Ca2+-free conditions.
Lymphatic myogenic responses were similar in nature to those reported in the rat mesenteric lymphatic vessels in that they were characterized by a reduction in the EDD measured from the first contraction after a pressure step compared with the steady-state EDD of a given pressure above a certain pressure threshold. The mouse Ing-Ax vessels demonstrated this lymphatic myogenic response in Fig. 7B, as shown by an acute constriction over the first few seconds after each increase in pressure over 2 cmH2O. This acute constriction was followed by a modest time-dependent reduction in EDD until a steady state was reached (Fig. 7C). As in the rat, this lymphatic myogenic behavior occurred after a certain threshold (12) was reached, which appeared to be >2 cmH2O in the mouse. Ultimately, the murine lymphatic myogenic response maintained the EDD constant in the peripheral lymphatic vessels, despite a fourfold pressure increase from 2 to 8 cmH2O (Table 1 and Fig. 7B).
Murine lymphatic vessels express the smooth muscle L-type Ca2+ channel variant Cav1.2b.
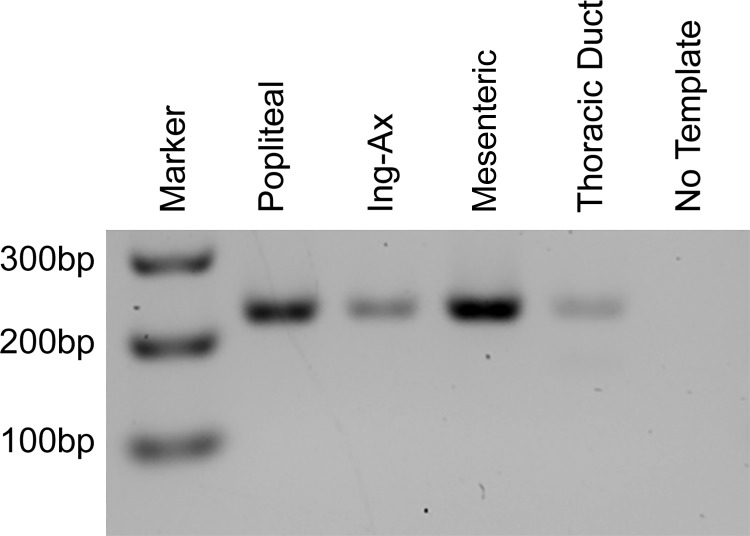
Because L-type Ca2+ channel inhibitors can impair contraction amplitude and, at higher concentrations, block spontaneous lymphatic contractions (2, 29), we hypothesized that the lack of robust contractions in visceral lymphatics of the mouse might be due to lack of expression of Cav1.2. We prepared cDNA from popliteal, Ing-Ax, mesenteric, and TD vessels for end-point RT-PCR with primers designed to amplify the smooth muscle-specific isoform of Cav1.2 that forms the pore-forming Cav α1-subunit that makes up the heterooligomeric L-type Ca2+ channel complex (30). Each vessel type was positive for the smooth muscle Cav1.2 variant as determined by expression of exon 1b (Fig. 8).
Fig. 8.
Visceral and peripheral lymphatic vessel expression of smooth muscle voltage-gated L-type Ca2+ (Cav1.2) channel. Vessels were isolated for RNA collection and end-point PCR to test expression of the smooth muscle isoform of the voltage-gated L-type Ca2+ channel. Primers directed against the Cav1.2 exon1b sequence showed that popliteal, inguinal axillary (Ing-Ax), mesenteric, and thoracic duct vessels were positive for smooth muscle Cav1.2 expression.
Different AP waveforms in visceral and peripheral lymphatic collecting vessels.
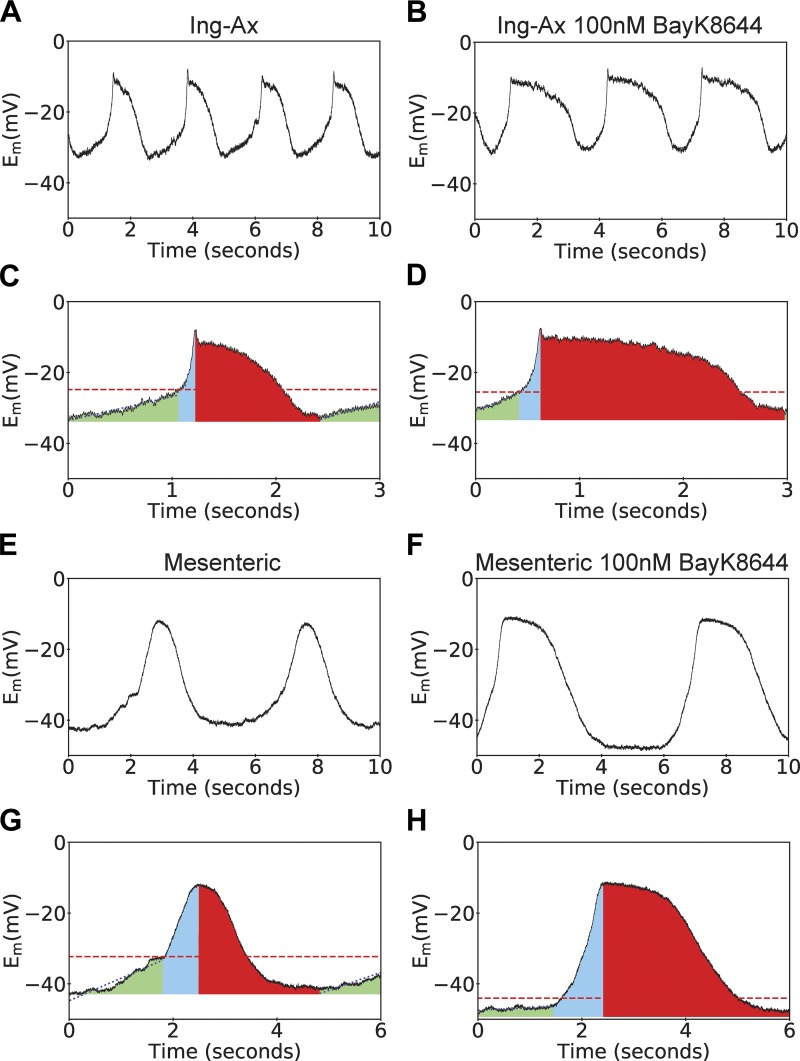
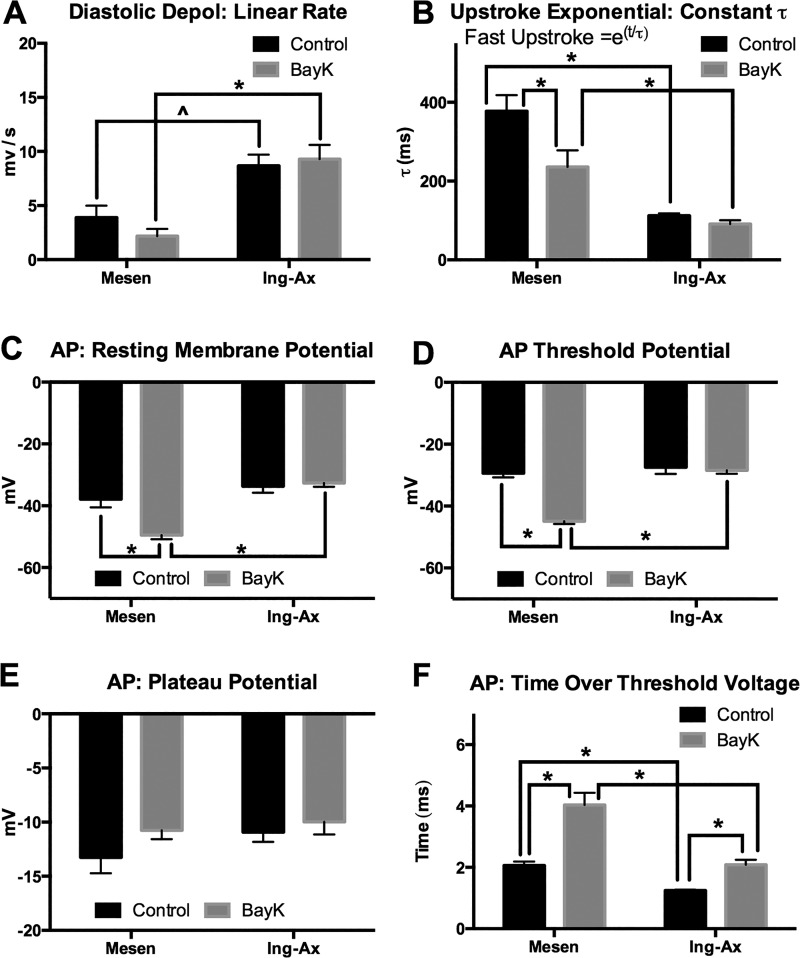
To confirm that peripheral and visceral vessels express functional Cav1.2 channels that mediate APs, we used sharp electrodes to test the effects of the L-type Ca2+ agonist BayK on smooth muscle membrane potential in Ing-Ax and mesenteric vessels (Fig. 9A). Spontaneous APs were observed in both types of vessels. The duration of the Ing-Ax AP was >1 s; it consisted of a diastolic depolarization phase and spike, plateau, and repolarization phases. The diastolic period appeared to have a linear and an exponential component before triggering a rapid upstroke to a spike and plateau that repolarized without a noticeable afterhyperpolarization, even after stimulation with 100 nM BayK (Fig. 9, A and B). In contrast, the mesenteric APs lacked the characteristic fast upstroke phase, and the AP appeared almost symmetrical in shape, without a noticeable plateau phase, until treated with 100 nM BayK (Fig. 9, C and D). Mesenteric lymphatic muscle cells displayed a significantly lower linear diastolic depolarization rate (Fig. 10A) and a slower upstroke rate (i.e., high τ value) than the Ing-Ax (Fig. 10B). No significant differences in resting membrane potential, AP threshold potential, or AP plateau potential between the mesenteric and Ing-Ax APs were observed (Fig. 10, C–E). However, mesenteric APs exhibited a significantly longer time over threshold than the Ing-Ax APs, and this difference persisted in the presence of 100 nM BayK. Stimulation with 100 nM BayK did not significantly affect the parameters of the Ing-Ax AP, other than a significantly increased time over threshold (an elongated plateau phase) that was also observed in the mesenteric vessel (Fig. 10F). Stimulation of mesenteric vessels with 100 nM BayK significantly increased the AP upstroke rate (Fig. 10B), while the linear diastolic depolarization phase was unchanged (Fig. 10A). Similar to the Ing-Ax AP, there was no difference in the mesenteric AP plateau voltage after stimulation with 100 nM BayK (Fig. 10E). The most striking difference in the effect of 100 nM BayK was significant hyperpolarization of the resting membrane potential from approximately −38 to −50 mV (Fig. 10C) and the apparent threshold of activation voltage (Fig. 10D) in mesenteric vessels.
Fig. 9.
Membrane potential (Em) recordings of pressurized inguinal axillary (Ing-Ax) and mesenteric lymphatic vessels. Lymphatic contractions are driven by action potentials (APs) in isolated and pressurized murine lymphatic vessels. A and B: representative membrane potential traces over a 10-s period for one Ing-Ax vessel in Krebs buffer and after treatment with 100 nM Bay K8644 (BayK). C and D: colorized representation of Ing-Ax AP parameters analyzed on a per-AP basis in Krebs buffer and after treatment with 100 nM BayK. Linear diastolic depolarization period (green) reaches a threshold (dotted line), where the fast upstroke (blue area) reaches a peak, usually with a small spike of a few millivolts, followed by a plateau and repolarization phase (red area) to resting membrane potential. Mesenteric APs had a symmetrical, “hill-like” shape, as opposed to the classic AP observed in the Ing-Ax vessel. E–H: 10-s membrane potential traces from a single mesenteric vessel before and after BayK treatment, along with their individual AP analyses.
Fig. 10.
Action potential (AP) parameters from inguinal axillary (Ing-Ax) and mesenteric (Mesen) vessels before and after treatment with 100 nM Bay K8644 (BayK). Membrane potential and AP parameters from Ing-Ax and mesenteric lymphatic collecting vessels were obtained from analysis of ≥3 APs under each condition for each vessel. A and B: analysis of linear diastolic depolarization (Depol) rate and fit of the fast upstroke phase to an exponential equation [y = e(T/τ)] and calculation of the time constant (τ) (where t is time). A higher τ value results in a slower upstroke. C–F: resting membrane potential, threshold potential (transition from linear depolarization to exponential), plateau potential, and time spent over the threshold voltage. Values are means ± SE; n = 4. *P < 0.05; ^P < 0.10.
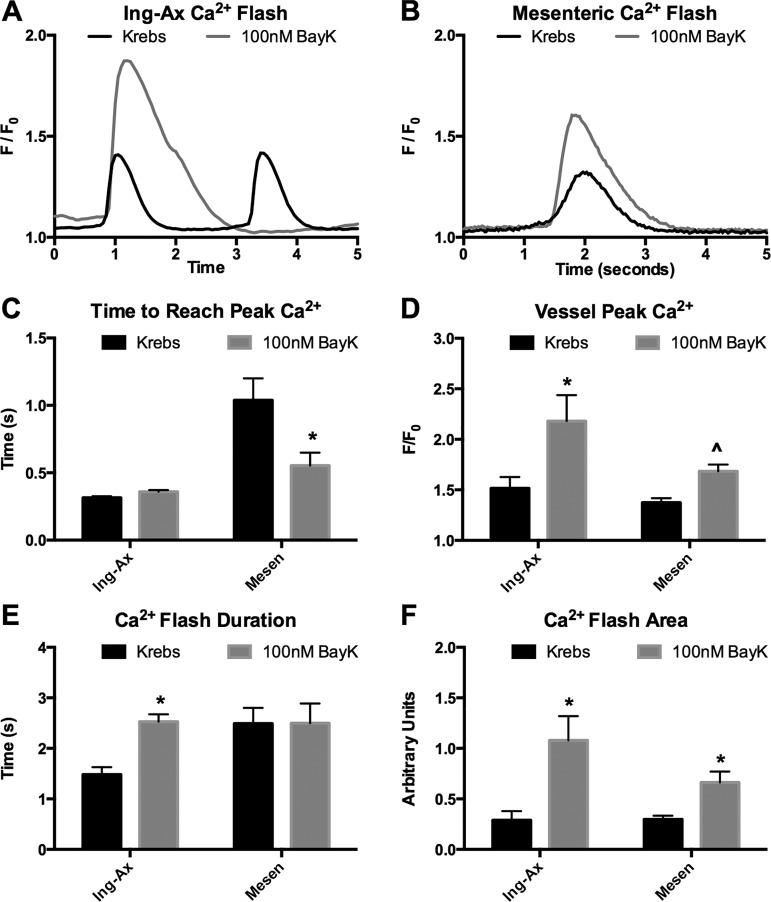
Lymphatic Ca2+ imaging in vessels from SMMHC-CreERT2;GCaMP6f mice.
Lymphatic APs are fundamentally linked to L-type Ca2+ channel activity, and the altered electrical dynamics in the mesenteric APs are likely reflected in AP-mediated Ca2+ influx. To test the hypothesis that Ca2+ influx in visceral lymphatic vessels was impaired during contraction, we used SMMHC-CreERT2 to generate mice expressing the genetically encoded Ca2+ reporter GCaMP6f in all smooth muscle cells. In our isolated vessel preparation, however, GCaMP6f expression was limited to lymphatic muscle cells. The resulting fluorescence was measured by confocal microscopy 2 wk after tamoxifen induction. Representative Ca2+ intensity plots (lasting 5 s) from Ing-Ax and mesenteric vessels are shown in Fig. 11, A and B, respectively. Similar to the slow rise in membrane potential, the elapsed time to reach the peak of the Ca2+ flash was significantly longer for mesenteric (1,000 ms) than for Ing-Ax (315 ms) vessels. Stimulation with 100 nM BayK did not affect the time to peak flash in the Ing-Ax vessel but decreased it by half (to 550 ms) in the mesenteric vessel. Peak Ca2+ intensity, expressed as F/F0, was not significantly different between mesenteric and Ing-Ax vessels, and BayK significantly increased peak Ca2+ intensity in both vessels (Fig. 11D). Interestingly, BayK increased the Ca2+ flash duration in Ing-Ax, but not mesenteric, vessels (Fig. 11E). BayK increased the total Ca2+ flash event in Ing-Ax and mesenteric vessels (Fig. 11F). The Ca2+ flash peak in the Ing-Ax vessel was 1.5 F/F0 (Fig. 11D), and the duration of the flash was 1.5 s (Fig. 11E). Stimulation with 100 nM BayK increased the Ca2+ flash peak in the Ing-Ax vessel to 2.2 F/F0 (Fig. 11D) and increased the duration of the Ca2+ flash to 2.5 s (Fig. 11E). The Ca2+ flash peak in the mesenteric vessel increased from 1.4 F/F0 in Krebs buffer to 1.7 F/F0 in 100 nM BayK (Fig. 11D), although the flash duration was unchanged with BayK (Fig. 11E). There was no significant difference in the Ca2+ flash area between the Ing-Ax and mesenteric vessels in Krebs buffer, but BayK increased the flash area in both vessels (Fig. 11F).
Fig. 11.
Bay K8644 (BayK) potentiates the Ca2+ flash that precedes lymphatic vessel contractions. Ca2+ transients were observed after selective expression of GCaMP6f in >95% of the smooth muscle cells. Global Ca2+ flashes preceded each lymphatic contraction in inguinal axillary (Ing-Ax) and mesenteric lymphatic vessels. A and B: Ca2+ transients from the entire vessel, with or without BayK, for Ing-Ax and mesenteric lymphatic collecting vessels. C and D: BayK decreased time to peak Ca2+ amplitude in mesenteric (Mesen), but not Ing-Ax, vessels and increased peak Ca2+ in Ing-Ax and mesenteric vessels. E and F: BayK also elongated the Ca2+ flash duration in Ing-Ax, but not mesenteric, vessels; however, 100 nM BayK ultimately increased the amplitude of the total Ca2+ event in both vessels. Values are means ± SE; n = 4. *P < 0.05; ^P < 0.10.
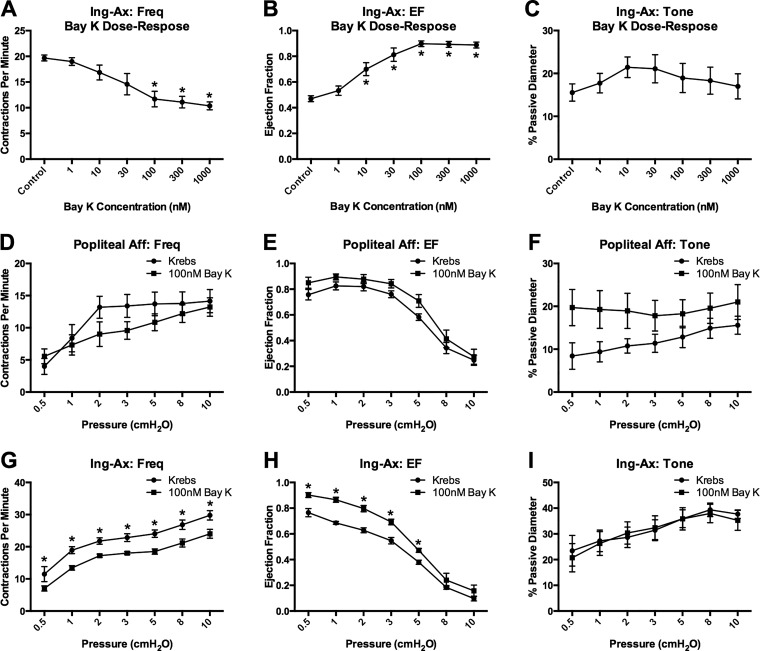
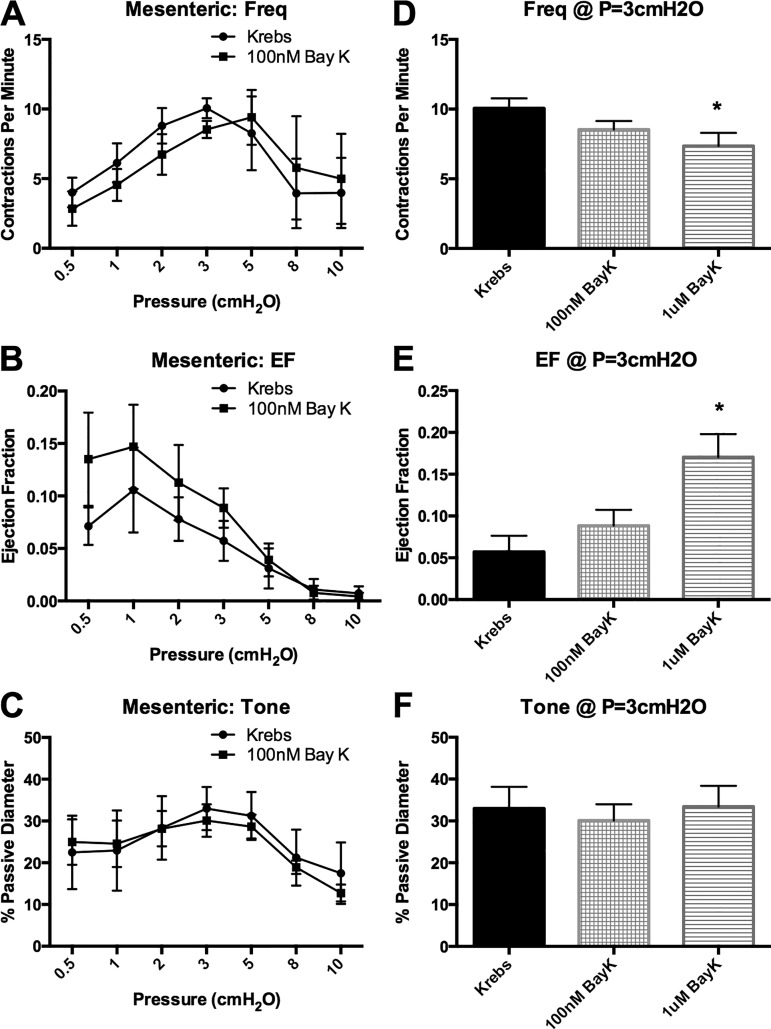
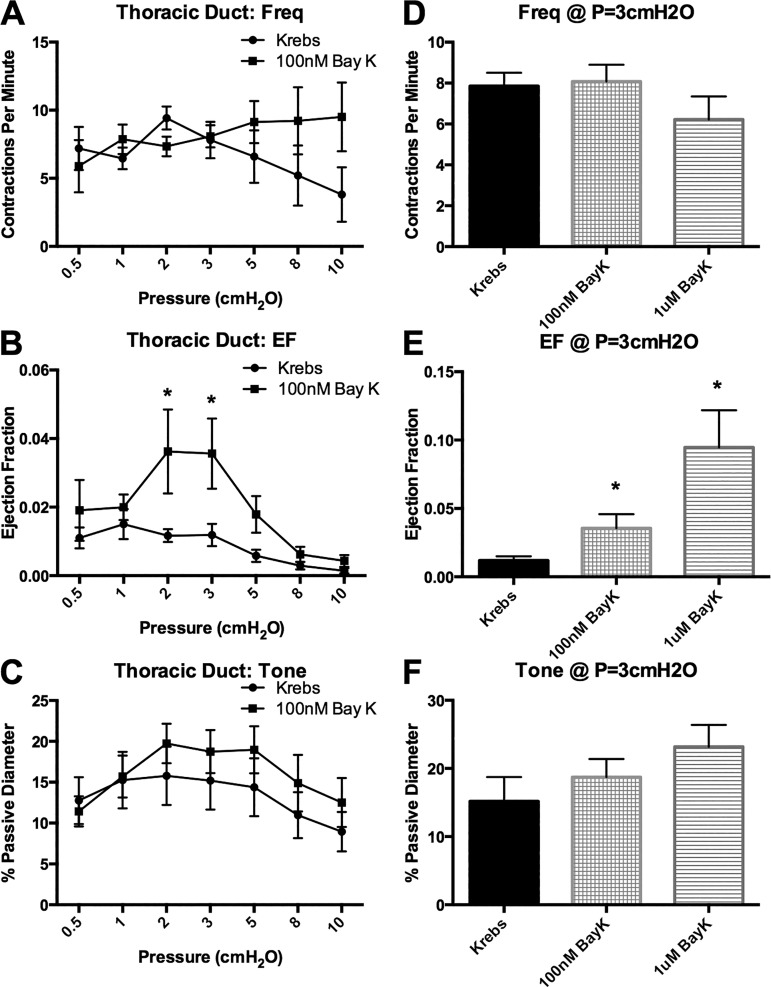
100. nM and 1 μM BayK are insufficient to drive large-amplitude contractions in visceral lymphatics.
Since 100 nM BayK appeared to normalize the AP and increase the Ca2+ flash amplitude in mesenteric vessels, we assessed the ability of BayK to promote large-amplitude contractions in visceral vessels. Mesenteric and TD vessels were used as representative visceral vessels, and popliteal afferent and Ing-Ax vessels were used as representative peripheral vessels. Stimulation with 100 nM BayK did not significantly change contraction frequency, EF, or vessel tone in popliteal afferent vessels but significantly reduced contraction frequency and increased EF at each pressure in the Ing-Ax vessel (Fig. 12). Stimulation with 100 nM BayK did not significantly alter contraction frequency, EF, or vessel tone in the mesenteric vessels (Fig. 13, A–C). However, treatment with 1 μM BayK significantly increased EF in mesenteric vessels from 6% to 17% at 3 cmH2O (Fig. 13E) but did not significantly alter contraction frequency or tone (Fig. 13, D and F). Similarly, 100 nM BayK did not significantly change contraction frequency or tone (Fig. 14, A and C) in the TD, although EF was slightly increased (P = 0.056; Fig. 14, B and E).
Fig. 12.
Popliteal afferent (Aff) and inguinal axillary (Ing-Ax) contractile responses to 100 nM BayK 8644 (BayK). A–C: contractile function [i.e., frequency (Freq), ejection fraction (EF), and tone] in a set of Ing-Ax vessels subjected to a dose-response protocol at 1–1,000 nM BayK. 100 nM BayK was the minimum concentration necessary to induce a maximal response (i.e., increase) in EF (B) and significantly reduce contraction frequency (A). Values are means ± SE; n = 4. *P < 0.05 vs. control. D–I: contractile function in popliteal and Ing-Ax vessels subjected to the pressure step protocol first in Krebs buffer and then, after a recovery period, in the presence of 100 nM BayK. 100 nM BayK did not significantly alter contraction frequency, EF, or tone in the popliteal vessel; 100 nM BayK decreased contraction frequency but increased EF in Ing-Ax vessels without affecting tone. Values are means ± SE; n = 6 popliteal and Ing-Ax. *P < 0.05.
Fig. 13.
Bay K8644 (BayK) is insufficient to drive large-amplitude contractions in mesenteric lymphatic vessels. Mesenteric lymphatic vessels were taken through the pressure step protocol first in Krebs buffer and then, after a recovery period, in the presence of 100 nM BayK. A–C: no differences were found in contraction frequency (Freq), ejection fraction (EF), or tone at any pressure. D–F: exposure to 1 μM BayK significantly increased EF, but not contraction frequency or tone, at 3 cmH2O. However, EF remained small (<20%) even with 1 μM BayK. Values are means ± SE; n = 6. *P < 0.05.
Fig. 14.
Bay K8644 (BayK) is insufficient to drive large-amplitude contractions in lymphatic thoracic duct (TD). Lymphatic TD vessels were taken through the pressure step protocol first in Krebs buffer and then, after a recovery period, in the presence of 100 nM BayK. A and B: no significant difference was found for TD contraction frequency, but EF was significantly increased at 2 and 3 cmH2O. C: 100 nM BayK did not significantly affect tone. D–F: exposure to 1 μM BayK significantly increased EF, but not contraction frequency or tone, at 3 cmH2O. Similar to the mesenteric vessels, EF remained at <15% even with 1 μM BayK. Values are means ± SE; n = 7. *P < 0.05.
DISCUSSION
The initial reports of robust contractile activity in popliteal afferent lymphatics by Liao et al. (31) and confirmed in our own ex vivo studies (49) revealed large-amplitude contractions previously undocumented in the murine model. Evidence for contractile activity in other murine lymphatics has been recently reported (7). However, discrete measurements of phasic contractile activity outside the popliteal vessel bed under defined pressures, a parameter that is critical to the regulation of contractile properties, have not been reported (17). The present study is the first to systematically assess the contractile properties of different murine lymphatic collecting vessels in an isobaric ex vivo preparation across a range of tissue beds. Many, but not all, murine lymphatic vessels have robust spontaneous contractions with EFs of ≥50%, with an apparent dichotomy in the vessel contractile phenotype corresponding to Tilney’s anatomic classification of the vessels as peripheral or visceral (57).
A new lymphatic contractile paradigm in the mouse: peripheral versus visceral.
The majority of the literature on lymphatic contractile function is derived from ex vivo vessel preparations from the mesentery and the TD in a wide range of species, including humans (55), pigs (32), bovines (37), sheep (36), guinea pigs (11), and rats (18). Vessels from these beds are quite commonly used because of the ease of lymphatic vessel identification and accessibility of the tissue. The previous notion that murine lymphatic vessels lacked large-scale phasic contractions stemmed from extrapolations of lymphatic contractile studies that utilized mesenteric lymphatics (46) or other visceral lymphatics from the mouse, where contractions of only a few micrometers and EFs of <15% were observed (39). In contrast to mouse mesenteric vessels, our own studies of rat mesenteric lymphatics routinely demonstrated EFs of up to 80% at lower pressures (15, 50), consistent with a previous report (18). We were not surprised that the low contractile strength and inconsistent contraction patterns in the murine mesenteric, iliac, and TD lymphatics are in line with previous reports using the DDY mouse strain (33, 46). A commonality between these vessel beds is their location within the visceral/abdominal cavities. While not an exhaustive analysis, these weak contractile responses are starkly opposed by the robust contractions consistently observed in peripheral lymphatic vessels located outside the visceral/abdominal cavities, which displayed EFs of >50%.
Propulsion of lymph requires that the intralymphangion pressure generated during lymphatic muscle contraction overcomes the output pressure load to open the output valve. Rat mesenteric lymphatic vessels can overcome an adverse pressure gradient up to ~12 cmH2O (15). Although we measured a somewhat lower pressure gradient (8 cmH2O) for mouse popliteal afferent lymphatics, this value likely exceeds the pressures experienced in the mouse hindlimb. Further work elucidating the intralymphatic pressures in these vessels in vivo is critical for a full appreciation of the physiological relevance of these pump limit estimates. Of the visceral lymphatic vessels tested, the mesenteric vessels had the highest EFs (≤10%), yet these were considerably weaker than those of any peripheral vessels tested and much weaker than corresponding mesenteric vessels in other species (18, 37, 59). When challenged with an adverse pressure gradient, the weak contractions observed in murine mesenteric vessels were insufficient to open the output valve in an isolated two-valve segment, which suggests that the visceral lymphatic vessels rely primarily on extrinsic forces, such as peristalsis, for lymph flow. Future investigations into the in vivo contractile activity and the interstitial and intralymphatic pressures in the mouse visceral cavities are necessary to comprehensively address these issues.
While we took great care in our isolation methods, it is possible that dissection/cannulation trauma may have compromised vessel function ex vivo compared with in vivo. However, we do not expect this to have affected the lymphatic vessels isolated from the visceral cavities more so than the peripheral lymphatic vessels, where large EFs were consistently recorded, despite a very similar dissection/cannulation routine. Furthermore, we examined the response to the classic vasoconstrictor, NE, to ensure that the dissection process did not result in undue damage to the lymphatic muscle cells. We tested the response at a pressure of 3 cmH2O, where the highest degree of spontaneous activity was detected in the TD. Both the TD and superficial cervical vessels had rapid constrictions to ~50% of maximal passive diameter upon NE application, indicating an intact contractile apparatus.
Role for L-type Ca2+ channels in murine lymphatic vessels.
Although an AP precedes every lymphatic contraction (26), there have been surprisingly few studies on the electrical regulation of lymphatic contractile activity and, to our knowledge, none has addressed the murine lymphatic vasculature. The mouse Ing-Ax vessel displayed a classic smooth muscle AP: 1) a diastolic depolarization phase that triggered 2) a fast upstroke to a spike that resolved to 3) a prolonged plateau period that lasted for >1 s before 4) repolarization without a noticeable afterhyperpolarization period. Although the initial AP spike height in the mouse was blunted and the AP plateau potential was slightly more depolarized than the AP observed in the rat mesentery (61) and human TD (56), the overall similarity in AP shape suggests conserved ion channel activity. Our PCR results confirmed that peripheral and visceral mouse lymphatic vessels expressed the smooth muscle isoform of Cav1.2. Interestingly, APs in mesenteric lymphatic vessels did not resemble those recorded in Ing-Ax vessels, aside from a similar resting potential and peak AP potential. The mesenteric AP lacked a sharp upstroke and had an almost symmetrical “hill-like” shape, with the total depolarizing event lasting substantially longer than that of the typical Ing-Ax AP. An elongated cycle time coupled with a significantly lower diastolic depolarization rate fits well with the significantly lower contraction frequency in mesenteric (9 contractions/min at 3 cmH2O) than Ing-Ax (19 contractions/min at 3 cmH2O) vessels in our pressure myograph studies. Furthermore, these differences were also reflected in the GCaMP6f Ca2+-imaging protocols. The time over threshold potential for the Ing-Ax vessels was 1.25 s, corresponding to a 1.5-s Ca2+ flash. Similarly, the time over threshold potential was ~2.1 s for mesenteric vessels, and the average duration of the Ca2+ flash was 2.5 s. The electrical and Ca2+ events each took longer to reach their respective peaks in mesenteric than Ing-Ax vessels, although peak membrane potential and relative Ca2+ intensity were similar between the two vessels. The temporal relationship between the AP and Ca2+ flash underlies the essential and well-documented role for Cav channel activity in the regulation of lymphatic vessel contractile function. Specifically, Cav1.2 channels are required for AP generation, Ca2+ entry (flashes), and lymphatic contraction. In our experiments, BayK significantly increased the rate of the exponential phase of the AP in the mesenteric vessels but did not significantly alter it in the Ing-Ax vessels. The time for the linear diastolic rate to reach threshold evidently was the primary determinant of the contraction frequency, and this was unchanged with the L-type Ca2+ activator BayK. However, L-type Ca2+ activity may play a minor role in determining contraction frequency by regulating the length of the plateau phase and total AP cycle time.
We were surprised that BayK did not reduce contraction frequency in mesenteric vessels to a larger extent, as it significantly hyperpolarized the resting membrane potential without altering the apparent linear diastolic depolarization rate. This was seemingly accomplished by a more hyperpolarized threshold of activation to initiate the upstroke phase. Combined with the hyperpolarized membrane potential, this result is likely indicative of enhanced L-type Ca2+ channel activity, even at the more negative potentials, that may be coupled to Ca2+-activated K+ (KCa) channels. L-type Ca2+ channel coupling with KCa channels has been documented in blood vascular smooth muscle cells (22), although KCa channel expression remains undocumented in murine lymphatic vessels. Finally, BayK increased the upstroke velocity of the mesenteric AP and reduced the time to peak Ca2+, thereby normalizing the shape of the AP and Ca2+ transient, which may suggest a role for reduced L-type Ca2+ channel recruitment within the visceral vessels under basal conditions. These results would suggest that visceral lymphatic vessel muscle cells would have a lower L-type Ca2+ current density (current-to-capacitance ratio, i.e., pA/pF) than cells isolated from peripheral vessels under patch-clamp conditions. Further work combining pharmacology and smooth muscle-specific knockouts of KCa channels is required to address these possibilities. Nonetheless, despite amplification of Ca2+ influx via L-type Ca2+ channels with BayK, visceral lymphatic vessels did not display large-amplitude contractions, suggesting a greater difference in molecular identity between peripheral and visceral vessels.
Lymphatic muscle heterogeneity.
The weak contractile response in visceral vessels was not due to a lack of lymphatic smooth muscle cell coverage, as smooth muscle actin-positive cells with similar morphology and cell density were demonstrated. Also, the vessels could produce sufficient force to develop >50% tone when stimulated with NE. Differences in the origin of lymphatic smooth muscle cells may be the underlying cause of the differential behavior of the peripheral and visceral lymphatic vessels. Recent work on lymphatic endothelial development during embryogenesis has suggested that multiple cell lineages contribute to lymphatic endothelial origin in a tissue-dependent manner (34, 53). Additionally, the totality of blood vascular smooth muscle is a mosaic of up to seven distinct progenitor sources (35), and it is likely that collecting lymphatic vessels have a similar diversity in the origin of their muscle cells, although this issue has not been investigated. The differences in contractile behavior may be due to differential expression and/or regulation of ion channels or contractile machinery. The muscle layer of rat lymphatic collecting vessels expresses contractile proteins found in cardiac, skeletal, and smooth muscle with some degree of regional variation (42), but similar characterizations of the contractile proteins have not been performed in mouse lymphatic muscle. Ultimately, the strong dichotomy in the contractile phenotypes of the vessels, based on their peripheral or visceral classification, strongly suggests differences in lymphatic muscle cell origin or phenotype. Lineage tracing and molecular studies of lymphatic muscle cells in the mouse may further illuminate the phenotypic differences between peripheral and visceral murine collecting lymphatic vessels.
Physiological consequences for lack of murine visceral lymphatic contractions.
The mesenteric vessels are conduits for chylomicron transport during the postprandial state and, as such, play a critical role in the absorption of fat, especially medium- and long-chain fatty acids that are packaged in the chylomicron. Lymph flow typically is elevated fourfold or higher over a period of hours in the postprandial state (4) to facilitate chylomicron uptake (58), but, based on our results, this process is apparently driven by extrinsic factors, such as peristalsis (25, 38), and not intrinsic contractions of the mouse lymphatic muscle. However, it is possible that the milieu of postprandial lymph or the accompanying neural activity in the postprandial state could alter mesenteric lymphatic pump function. The mesenteric lymphatic vessels also transport antigen-rich lymph (8, 9) and various immune cells to the draining mesenteric lymph node (47), and extrinsic forces appear sufficient for lymph transport under both fasted and postprandial states, as the mouse does not develop a lymphedematous bowel, nor does it suffer from immunological deficiencies. Further studies using a combination of in vivo imaging and isolated vessel preparations could explore this possibility. The TD is the terminal lymphatic collecting vessel and the site of lymph return to the bloodstream at the connections between the TD and subclavian vein. The lack of spontaneous contractile activity in the mouse TD raises questions regarding the physiological conditions that regulate the process of lymph entry into the subclavian vein in mice compared with humans, in which TD contractile activity has been well documented (54–56). Similarly, the lack of consistent contractions within the mesenteric network suggests that the mouse may be of limited relevance in the study of digestive diseases where inhibition of lymphatic contractile function and contractility may be significant factors in the etiologies of the diseases (10, 48). While the use of genetic knockouts and other gene manipulation strategies to study lymphatic contractile function in physiological and pathophysiological states presents an exciting research avenue, care must also be taken in understanding the limitations of using lymphatic vessels from within the visceral cavity of the mouse. These results provide a critical framework for studying lymphatic function using murine models and for modeling whole lymph flow patterns in the mouse under both physiological and pathophysiological conditions.
Conclusions and implications for further research.
The results of this study clarify the existing confusion regarding murine lymphatic contractile function. We conclusively demonstrate robust contractile activity in multiple murine lymphatic collecting vessels from the mouse periphery, but not from within the visceral/abdominal cavities; the latter result is in agreement with previous reports in the literature (19). The contractile properties of mouse peripheral lymphatic vessels, including lymphatic myogenic constriction, pressure-sensitive chronotropy, and pressure-dependent contractility and rate sensitivity, resemble those of lymphatic vessels from other species, including humans.
Vessels from both peripheral and visceral regions express message for Cav1.2 channels, although this does not necessarily guarantee similarities in the levels of functional Cav1.2 channels or similar activity of those channels in the smooth muscle layer. However, both types of vessels generate APs and Ca2+ flashes in lymphatic smooth muscle, and those events are modulated to different degrees by the L-type Ca2+ channel agonist BayK. These findings argue that the lack of robust contractions in visceral vessels is not due simply to a lack of Cav1.2 channel expression. Rather, the “hill-shaped” AP in mesenteric vessels could be explained by a lower density of activated Cav1.2 channels, as channel density is highly correlated to AP upstroke velocity. The positive modulation of this AP phase and enhancement of the Ca2+ flash amplitude in the mesenteric vessels by the Cav1.2 activator BayK argue that functional Cav1.2 channels are expressed and that their activity can be enhanced; however, such enhancement does not restore EF of the mesenteric vessels to anywhere near the level of peripheral vessels. Other possibilities for the differences in AP shape between visceral and peripheral vessels include differences in Cav1.2b splice variants, accessory subunits, or the phosphorylation state of the channel and will require further investigation. However, it is unlikely that the lack of robust spontaneous contractions in visceral vessels is explained entirely by L-type Ca2+ channels. For example, the hyperpolarizing effect of BayK on mesenteric lymphatic smooth muscle points to a role for other ion channels, such as KCa channels. Furthermore, differences in contractile machinery or Ca2+-sensitive force production may also underlie the weak spontaneous contractile activity of visceral vessels.
Our results illuminate the need to consider the contractile activity of the particular murine collecting vessels being studied when attempting to model lymph flow patterns. In particular, the mouse may not be an appropriate model to dissect the role of lymphatic contractility in the etiology of diseases within the visceral cavity. However, our experiments are limited to ex vivo observations, and the weak contractile activity in visceral lymphatic vessels reported here should be pursued further with in vivo methodologies. The use of genetic mouse models within the lymphatic contractile field will further our understanding of basic lymphatic biology and how disruption of lymphatic contractions can influence and advance disease.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants R01-HL-120867, R01-HL-122608, and R01-HL-122578 (to M. J. Davis) and U01-HL-123420 and R00-HL-124142 (to J. P. Scallan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.D.Z., J.A.C.-G., and M.J.D. conceived and designed research; S.D.Z., J.A.C.-G., J.S., and M.J.D. performed experiments; S.D.Z., J.A.C.-G., and M.J.D. analyzed data; S.D.Z., J.A.C.-G., and M.J.D. interpreted results of experiments; S.D.Z. and J.A.C.-G. prepared figures; S.D.Z. and J.A.C.-G. drafted manuscript; S.D.Z., J.A.C.-G., J.S., and M.J.D. edited and revised manuscript; S.D.Z., J.A.C.-G., J.S., and M.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for the technical support of Shanyu Ho and Min Li.
REFERENCES
- 1.Aldrich MB, Davies-Venn C, Angermiller B, Robinson H, Chan W, Kwon S, Sevick-Muraca EM. Concentration of indocyanine green does not significantly influence lymphatic function as assessed by near-infrared imaging. Lymphat Res Biol 10: 20–24, 2012. doi: 10.1089/lrb.2011.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azuma T, Ohhashi T, Sakaguchi M. Electrical activity of lymphatic smooth muscles. Proc Soc Exp Biol Med 155: 270–273, 1977. doi: 10.3181/00379727-155-39787. [DOI] [PubMed] [Google Scholar]
- 3.Bertram CD, Macaskill C, Davis MJ, Moore JE Jr. Consequences of intravascular lymphatic valve properties: a study of contraction timing in a multi-lymphangion model. Am J Physiol Heart Circ Physiol 310: H847–H860, 2016. doi: 10.1152/ajpheart.00669.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borgstrom B, Laurell CB. Studies of lymph and lymph-proteins during absorption of fat and saline by rats. Acta Physiol Scand 29: 264–280, 1953. doi: 10.1111/j.1748-1716.1953.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 5.Bridenbaugh EA, Nizamutdinova IT, Jupiter D, Nagai T, Thangaswamy S, Chatterjee V, Gashev AA. Lymphatic muscle cells in rat mesenteric lymphatic vessels of various ages. Lymphat Res Biol 11: 35–42, 2013. doi: 10.1089/lrb.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel CaV1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem 282: 29211–29221, 2007. doi: 10.1074/jbc.M610623200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong C, Scholkmann F, Bachmann SB, Luciani P, Leroux JC, Detmar M, Proulx ST. In vivo visualization and quantification of collecting lymphatic vessel contractility using near-infrared imaging. Sci Rep 6: 22930, 2016. doi: 10.1038/srep22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement CC, Cannizzo ES, Nastke MD, Sahu R, Olszewski W, Miller NE, Stern LJ, Santambrogio L. An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma. PLoS One 5: e9863, 2010. doi: 10.1371/journal.pone.0009863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clement CC, Rotzschke O, Santambrogio L. The lymph as a pool of self-antigens. Trends Immunol 32: 6–11, 2011. doi: 10.1016/j.it.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cromer W, Wang W, Zawieja SD, von der Weid PY, Newell-Rogers MK, Zawieja DC. Colonic insult impairs lymph flow, increases cellular content of the lymph, alters local lymphatic microenvironment, and leads to sustained inflammation in the rat ileum. Inflamm Bowel Dis 21: 1553–1563, 2015. doi: 10.1097/MIB.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowe MJ, von der Weid PY, Brock JA, Van Helden DF. Co-ordination of contractile activity in guinea-pig mesenteric lymphatics. J Physiol 500: 235–244, 1997. doi: 10.1113/jphysiol.1997.sp022013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296: H293–H302, 2009. doi: 10.1152/ajpheart.01040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MJ, Davis AM, Lane MM, Ku CW, Gashev AA. Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol 587: 165–182, 2009. doi: 10.1113/jphysiol.2008.162438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE Jr. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol 301: H48–H60, 2011. doi: 10.1152/ajpheart.00133.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol 303: H795–H808, 2012. doi: 10.1152/ajpheart.01097.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engeset A, Olszewski W, Jaeger PM, Sokolowski J, Theodorsen L. Twenty-four hour variation in flow and composition of leg lymph in normal men. Acta Physiol Scand 99: 140–148, 1977. doi: 10.1111/j.1748-1716.1977.tb10364.x. [DOI] [PubMed] [Google Scholar]
- 17.Gashev AA. Lymphatic vessels: pressure- and flow-dependent regulatory reactions. Ann NY Acad Sci 1131: 100–109, 2008. doi: 10.1196/annals.1413.009. [DOI] [PubMed] [Google Scholar]
- 18.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11: 477–492, 2004. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- 19.Gashev AA, Davis MJ, Gasheva OY, Nepiushchikh ZV, Wang W, Dougherty P, Kelly KA, Cai S, Von Der Weid PY, Muthuchamy M, Meininger CJ, Zawieja DC. Methods for lymphatic vessel culture and gene transfection. Microcirculation 16: 615–628, 2009. doi: 10.1080/10739680903120778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guia A, Wan X, Courtemanche M, Leblanc N. Local Ca2+ entry through L-type Ca2+ channels activates Ca2+-dependent K+ channels in rabbit coronary myocytes. Circ Res 84: 1032–1042, 1999. doi: 10.1161/01.RES.84.9.1032. [DOI] [PubMed] [Google Scholar]
- 23.Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol 3: a004549, 2011. doi: 10.1101/cshperspect.a004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogan RD, Unthank JL. Mechanical control of initial lymphatic contractile behavior in bat’s wing. Am J Physiol Heart Circ Physiol 251: H357–H363, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Kassis T, Kohan AB, Weiler MJ, Nipper ME, Cornelius R, Tso P, Dixon JB. Dual-channel in-situ optical imaging system for quantifying lipid uptake and lymphatic pump function. J Biomed Opt 17: 086005, 2012. doi: 10.1117/1.JBO.17.8.086005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkpatrick CT, McHale NG. Electrical and mechanical activity of isolated lymphatic vessels. J Physiol 272: 33P–34P, 1977. [PubMed] [Google Scholar]
- 27.Kwon S, Agollah GD, Wu G, Sevick-Muraca EM. Spatio-temporal changes of lymphatic contractility and drainage patterns following lymphadenectomy in mice. PLoS One 9: e106034, 2014. doi: 10.1371/journal.pone.0106034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laine GA, Allen SJ, Katz J, Gabel JC, Drake RE. Outflow pressure reduces lymph flow rate from various tissues. Microvasc Res 33: 135–142, 1987. doi: 10.1016/0026-2862(87)90012-4. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Roizes S, von der Weid PY. Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J Physiol 592: 5409–5427, 2014. doi: 10.1113/jphysiol.2014.280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao P, Yong TF, Liang MC, Yue DT, Soong TW. Splicing for alternative structures of Cav1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovasc Res 68: 197–203, 2005. doi: 10.1016/j.cardiores.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D, Padera TP. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA 108: 18784–18789, 2011. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobov GI, Orlov RS. [Electrical and contractile activity of the lymphangions of the mesenteric lymphatic vessels]. Fiziol Zh SSSR Im I M Sechenova 69: 1614–1620, 1983. [PubMed] [Google Scholar]
- 33.Maejima D, Kawai Y, Ajima K, Ohhashi T. Platelet-derived growth factor (PDGF)-BB produces NO-mediated relaxation and PDGF receptor β-dependent tonic contraction in murine iliac lymph vessels. Microcirculation 18: 474–486, 2011. doi: 10.1111/j.1549-8719.2011.00108.x. [DOI] [PubMed] [Google Scholar]
- 34.Mahadevan A, Welsh IC, Sivakumar A, Gludish DW, Shilvock AR, Noden DM, Huss D, Lansford R, Kurpios NA. The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine. Dev Cell 31: 690–706, 2014. doi: 10.1016/j.devcel.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27: 1248–1258, 2007. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 36.McGeown JG, McHale NG, Roddie IC, Thornbury K. Peripheral lymphatic responses to outflow pressure in anaesthetized sheep. J Physiol 383: 527–536, 1987. doi: 10.1113/jphysiol.1987.sp016426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261: 255–269, 1976. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miura S, Sekizuka E, Nagata H, Oshio C, Minamitani H, Suematsu M, Suzuki M, Hamada Y, Kobayashi K, Asakura H, . et al Increased lymphocyte transport by lipid absorption in rat mesenteric lymphatics. Am J Physio Gastrointest Liver Physiol 253: G596–G600, 1987. [DOI] [PubMed] [Google Scholar]
- 39.Mizuno R, Ono N, Ohhashi T. Parathyroid hormone-related protein-(1–34) inhibits intrinsic pump activity of isolated murine lymph vessels. Am J Physiol Heart Circ Physiol 281: H60–H66, 2001. doi: 10.1152/ajpheart.2001.281.1.H60. [DOI] [PubMed] [Google Scholar]
- 40.Mulvany MJ. Procedures for Investigation of Small Vessels Using a Small Vessel Myograph. Aarhus, Denmark: Danish Myo Technology, 2003, p. 1–54. [Google Scholar]
- 41.Murakami M, Ohba T, Takahashi Y, Watanabe H, Miyoshi I, Nakayama S, Ono K, Ito H, Iijima T. Identification of a cardiac isoform of the murine calcium channel α1C (Cav1.2-a) subunit and its preferential binding with the β2 subunit. J Mol Cell Cardiol 41: 115–125, 2006. doi: 10.1016/j.yjmcc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17: 920–922, 2003. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- 43.Nakaya K, Mizuno R, Ohhashi T. B16-BL6 melanoma cells release inhibitory factor(s) of active pump activity in isolated lymph vessels. Am J Physiol Cell Physiol 281: C1812–C1818, 2001. doi: 10.1152/ajpcell.2001.281.6.C1812. [DOI] [PubMed] [Google Scholar]
- 44.Olszewski WL. Contractility patterns of normal and pathologically changed human lymphatics. Ann NY Acad Sci 979: 52–63, 2002. doi: 10.1111/j.1749-6632.2002.tb04867.x. [DOI] [PubMed] [Google Scholar]
- 45.Olszewski WL, Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol Heart Circ Physiol 239: H775–H783, 1980. [DOI] [PubMed] [Google Scholar]
- 46.Ono N, Mizuno R, Nojiri H, Ohhashi T. Development of an experimental apparatus for investigating lymphatic pumping activity of murine mesentery in vivo. Jpn J Physiol 50: 25–31, 2000. doi: 10.2170/jjphysiol.50.25. [DOI] [PubMed] [Google Scholar]
- 47.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 5: 617–628, 2005. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 48.Rehal S, von der Weid PY. Experimental ileitis alters prostaglandin biosynthesis in mesenteric lymphatic and blood vessels. Prostaglandins Other Lipid Mediat 116–117: 37–48, 2015. doi: 10.1016/j.prostaglandins.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol 591: 2139–2156, 2013. doi: 10.1113/jphysiol.2012.250662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA, Davis MJ. Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am J Physiol Heart Circ Physiol 303: H809–H824, 2012. doi: 10.1152/ajpheart.01098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 594: 5749–5768, 2016. doi: 10.1113/JP272088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith RO. Lymphatic contractility: a possible intrinsic mechanism of lymphatic vessels for the transport of lymph. J Exp Med 90: 497–509, 1949. doi: 10.1084/jem.90.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanczuk L, Martinez-Corral I, Ulvmar MH, Zhang Y, Laviña B, Fruttiger M, Adams RH, Saur D, Betsholtz C, Ortega S, Alitalo K, Graupera M, Mäkinen T. cKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep. In press. doi: 10.1016/j.celrep.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 54.Telinius N, Baandrup U, Rumessen J, Pilegaard H, Hjortdal V, Aalkjaer C, Boedtkjer DB. The human thoracic duct is functionally innervated by adrenergic nerves. Am J Physiol Heart Circ Physiol 306: H206–H213, 2014. doi: 10.1152/ajpheart.00517.2013. [DOI] [PubMed] [Google Scholar]
- 55.Telinius N, Drewsen N, Pilegaard H, Kold-Petersen H, de Leval M, Aalkjaer C, Hjortdal V, Boedtkjer DB. Human thoracic duct in vitro: diameter-tension properties, spontaneous and evoked contractile activity. Am J Physiol Heart Circ Physiol 299: H811–H818, 2010. doi: 10.1152/ajpheart.01089.2009. [DOI] [PubMed] [Google Scholar]
- 56.Telinius N, Kim S, Pilegaard H, Pahle E, Nielsen J, Hjortdal V, Aalkjaer C, Boedtkjer DB. The contribution of K+ channels to human thoracic duct contractility. Am J Physiol Heart Circ Physiol 307: H33–H43, 2014. doi: 10.1152/ajpheart.00921.2013. [DOI] [PubMed] [Google Scholar]
- 57.Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. J Anat 109: 369–383, 1971. [PMC free article] [PubMed] [Google Scholar]
- 58.Tso P, Pitts V, Granger DN. Role of lymph flow in intestinal chylomicron transport. Am J Physiol Gastrointest Liver Physiol 249: G21–G28, 1985. [DOI] [PubMed] [Google Scholar]
- 59.Van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol 471: 465–479, 1993. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von der Weid PY. Lymphatic vessel pumping and inflammation—the role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment Pharmacol Ther 15: 1115–1129, 2001. doi: 10.1046/j.1365-2036.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- 61.von der Weid PY, Lee S, Imtiaz MS, Zawieja DC, Davis MJ. Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphat Res Biol 12: 66–75, 2014. doi: 10.1089/lrb.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang R, Taucer AI, Gashev AA, Muthuchamy M, Zawieja DC, Davis MJ. Maximum shortening velocity of lymphatic muscle approaches that of striated muscle. Am J Physiol Heart Circ Physiol 305: H1494–H1507, 2013. doi: 10.1152/ajpheart.00898.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]