Abstract
Afferent fibers expressing the vanilloid receptor 1 (VR1) channel have been implicated in cardiac nociception; however, their role in modulating reflex responses to cardiac stress is not well understood. We evaluated this role in Yorkshire pigs by percutaneous epicardial application of resiniferatoxin (RTX), a toxic activator of the VR1 channel, resulting in the depletion of cardiac VR1-expressing afferents. Hemodynamics, epicardial activation recovery intervals, and in vivo activity of stellate ganglion neurons (SGNs) were recorded in control and RTX-treated animals. Stressors included inferior vena cava or aortic occlusion and rapid right ventricular pacing (RVP) to induce dyssynchrony and ischemia. In the epicardium, stellate ganglia, and dorsal root ganglia, immunostaining for the VR1 channel, calcitonin gene-related peptide, and substance P was significantly diminished by RTX. RTX-treated animals exhibited higher basal systolic blood pressures and contractility than control animals. Reflex responses to epicardial bradykinin and capsaicin were mitigated by RTX. Cardiovascular reflex function, as assessed by inferior vena cava or aortic occlusion, was similar in RTX-treated versus control animals. RTX-treated animals exhibited resistance to hemodynamic collapse induced by RVP. Activation recovery interval shortening during RVP, a marker of cardiac sympathetic outflow, was greater in RTX-treated animals and exhibited significant delay in returning to baseline values after cessation of RVP. The basal firing rate of SGNs and firing rates in response to RVP were also greater in RTX-treated animals, as was the SGN network activity in response to cardiac stressors. These data suggest that elimination of cardiac nociceptive afferents reorganizes the central-peripheral nervous system interaction to enhance cardiac sympathetic outflow.
NEW & NOTEWORTHY Our work demonstrates a role for cardiac vanilloid receptor-1-expressing afferents in reflex processing of cardiovascular stress. Current understanding suggests that elimination of vanilloid receptor-1 afferents would decrease reflex cardiac sympathetic outflow. We found, paradoxically, that sympathetic outflow to the heart is instead enhanced at baseline and during cardiac stress.
Keywords: cardiac afferent nerves, sympathoexcitation, transient receptor potential vanilloid 1, vanilloid receptor 1
INTRODUCTION
Cardiac autonomic regulation is predicated on a series of afferent-efferent feedback loops (peripheral and central) that ensure adequate and timely responses to perturbations in cardiac function (6, 35). The cardiac sympathetic afferent reflex (CSAR), mediated in large part by the vanilloid receptor 1 (VR1) or transient receptor potential vanilloid 1 (TRPV1) channel, responds to a variety of stimuli including noxious heat, bradykinin, acidic milieu, and free radicals (25, 33, 38, 45). Activation of the CSAR induces a pressor and chronotropic response (45). VR1 receptors have also been implicated in other modes of sensory transduction including mechanotransduction (12) and proprioception (20).
In disease states, an important therapeutic role for VR1 afferents is emerging. The CSAR mediated by VR1-expressing afferents is upregulated in congestive heart failure (CHF) (41). Chemical ablation of these afferents by resiniferatoxin (RTX), a toxic activator of the VR1 channel that causes degeneration of cardiac sensory afferents, provides robust protective effects on cardiac function and survival in a chronic myocardial infarction-induced rat model of CHF (40).
Acute blockade of the VR1 channel using iodoresiniferatoxin (28), or chronic elimination of VR1 afferents using RTX, mitigates the pressor responses to bradykinin and capsaicin (45). However, whether VR1 afferents have a tonic modulatory role on cardiac sympathetic reflex control in normal hearts is not known. Importantly, how VR1 afferents modulate central-peripheral interaction in normal hearts is not known.
In the present study, we examined how VR1 afferents modulate central-peripheral feedback responses to cardiac stress. This was done by characterizing hemodynamic, epicardial activation recovery interval (ARI), and in vivo stellate ganglion (SG) neuron (SGN) activity during introduced cardiac stressors in normal swine compared with those 1 mo after percutaneous epicardial RTX application.
METHODS
Animal subjects.
All experimental experiments involving animal subjects (both survival and nonsurvival) were performed in accordance with guidelines set by the University of California Institutional Animal Care and Use Committee and National Institutes of Health Guide for the Care and Use of Laboratory Animals. Control (n = 12) and RTX-treated (n = 9) Yorkshire pigs of either sex (40–50 kg) were used for terminal experiments. Animal subjects sedated with intramuscular Telazol (8–10 mg/kg) and dexmedetomidine (0.05 mg/kg) or ketamine (5 mg/kg) and midazolam (0.25 mg/kg) were intubated and maintained using inhaled isoflurane (1–3%) during terminal surgical procedures.
Percutaneous epicardial RTX instillation.
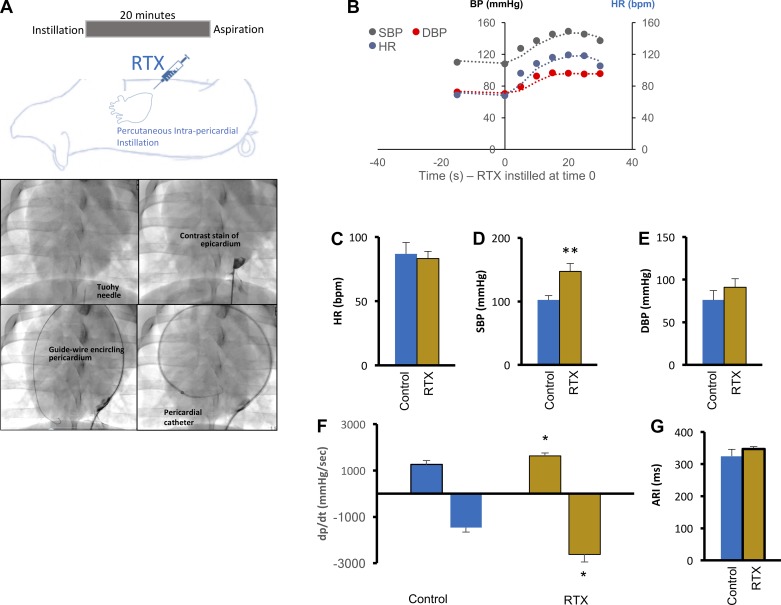
Animals were sedated, intubated, and maintained as described above. In addition, animals were treated within 24 h before RTX infusion with isoflupredone acetate (0.04 mg/kg) and famotidine (0.5 mg/kg) administered intramuscularly. The left sternocostal angle was palpated and anesthetized with 1% lidocaine. A 1-cm incision was made at the apex of the angle. A Tuohy needle was introduced under constant fluoroscopic observation of the heart in the anteroposterior (AP) or left anterior oblique (LAO) view (Fig. 1A). A small amount of radioopaque contrast was injected to ascertain the position of the needle tip. The needle was gradually advanced. When transmitted cardiac pulsations were felt through the needle, firm pressure was applied to perforate the pericardium. Again, contrast was injected to confirm access of the pericardial space. A J-tipped guidewire was advanced through the needle, and the position of the wire was confirmed in the LAO view (care was taken to confirm that the wire is the left heart border forming structure in this view). A 5-Fr soft dilator was advanced over the J-tip wire, and physiological pericardial fluid was aspirated. A multipurpose sheath was advanced over the wire into the pericardial space and positioned in the inferoapical aspect of the pericardium. A 10-ml solution of 25 µg/ml RTX (R8756, Sigma-Aldrich, St. Louis, MO) was infused intrapericardially for 20 min and aspirated in its entirety afterward. To minimize pericardial reaction, 50 mg of methylprednisolone were infused into the pericardial space. Animals were maintained for 1 mo after RTX and compared with age-matched control animals.
Fig. 1.
Percutaneous application of resiniferatoxin (RTX) and baseline hemodynamic indexes in treated subjects. A, top: schematic depiction of the protocol for percutaneous epicardial application of RTX. Bottom, stepwise approach to percutaneously accessing the intrapericardial space and instillation of RTX. B: acute hemodynamic responses to RTX application. C–F: baseline hemodynamic measurements in control and RTX-treated animals 4 wk after percutaneous application. Shown are heart rate [HR; in beats/min (bpm)], systolic and diastolic blood pressures (SBP and DBP, respectively), and contractility (dP/dtmax and dP/dtmin, respectively). G: mean activation recovery interval (ARI) in control and RTX-treated animals. All values shown are means ± SE; n = 6–10 animals/group. *P < 0.05 and **P < 0.01 by Mann-Whitney test.
Terminal experimentation.
Animals were sedated as described above, intubated, and maintained in a surgical plane of anesthesia with inhaled isoflurane (1–3%). Fentanyl was administered intravenously for analgesia before surgery. A midline sternotomy was then performed, and the heart and left and right SG (LSG and RSG, respectively) were exposed. A left ventricular (LV) pressure catheter (SPR350, Millar, Houston, TX) was inserted via the left carotid artery to record LV pressures. Animals were allowed to stabilize for 20–30 min after completion of the surgical preparation, during which time anesthesia was transitioned to alphachloralose (6.25 mg/125 ml, 1 ml/kg bolus over 30–60 min, maintenance dose of 25–35 ml/kg titrated to effect) for the duration of the experimental protocol. Electrocardiographic and hemodynamic indexes were measured along with electrograms (from which ARIs were determined) before, during, and after each stressor. Temperature was maintained at 36–38°C with the use of a heated surgical table and a heated water blanket. The sternotomy site was kept covered to avoid cardiac cooling and insensible losses. Animals received continuous normal saline at 8 ml·kg−1·h−1, and arterial blood gasses were checked hourly or more frequently, as needed. Upon completion of the experimental protocol, animals were euthanized by intravenous pentobarbital sodium (100 mg/kg) or cardiac fibrillation. Paralytic agents were not used.
Stellate ganglion stimulation.
Bipolar electrodes were placed around the RSG (cephalocaudad orientation). RSG stimulation was achieved with repeated square-wave pulses at 4 Hz for 4 ms and a current 10% above the threshold current for 30 s. Threshold was determined as the output current at which blood pressure (BP) and/or heart rate (HR) increased by 10%. Sympathoexcitation was also confirmed by surface T wave changes (32).
Cardiac electrical mapping.
A custom built 64-electrode array (8 × 8, 2-mm interelectrode spacing) was placed on the anterior epicardium of the LV. Electrograms were recorded at baseline and during each stressor (see Experimental protocol below). With the use of custom software (4), local activation time, and repolarization time, ARI, a surrogate for action potential duration (19, 22), was calculated as the difference between the local repolarization and activation times. Electrical maps spatially depicting ARI obtained from all electrodes were generated using publicly available software (Map3D, Scientific Computing and Imaging Institute, University of Utah; http://www.sci.utah.edu/cibc/software/107-map3d.html).
In vivo SGN recording.
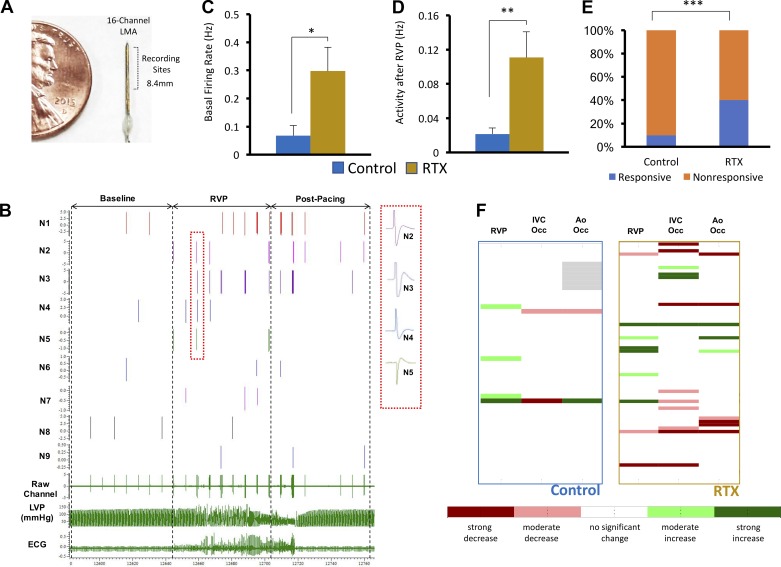
To record in vivo activity of SGNs, a 16-channel linear microelectrode array (Microprobes, Gaithersburg, MD) was deployed into the craniomedial pole of the LSG, similar to previous approaches for other intrathoracic ganglia (8, 31). Each electrode of the array was made of a platinum-iridium electrode, 25 μm in diameter, and impedance characteristics of 0.3–0.5 MΩ at 1 kHz. Signals were acquired using a microelectrode amplifier, with data preamplified via a headstage (model 3600, A-M Systems, Carlsborg, WA). Signals were filtered at 300 Hz to 3 kHz and amplified with a gain of 1,000–2,500. Electrocardiographic, hemodynamic, and neural recording data were input into a data acquisition platform (Power1401, Cambridge Electronic Design, Cambridge, UK) for offline analyses performed in Matlab (MathWorks, Natick, MA). Spike sorting of electrical activity with greater than a 2:1 signal-to-noise ratio after artifact removal to identify the activity of SGNs was performed using Spike2 (Cambridge Electronic Design) as previously described (8, 31).
Experimental protocol.
After equilibration from surgical procedures and transition to α-chloralose, control and RTX-treated animals were exposed to three transient cardiovascular/cardiac stressors: inferior vena cava (IVC) occlusion (IVC occ), aorta occlusion (Ao occ), and rapid decremental right ventricular (RV) pacing (RVP). The order of these stressors was randomized. Occlusion was achieved with the use of a percutaneous endovascular catheter inserted via femoral artery or vein (Atlas Dilation Catheter, 22 mm, Bard, Murray Hill, NJ). The CSAR was tested with epicardial bradykinin (20 µg/ml) and capsaicin (20 µg/ml) applied at the basal anterior aspect of the heart for 30 s. Bradykinin was applied before capsaicin (15–20 min) due to sensory peptide depletion by capsaicin. The epicardium was washed with warm saline after each application. Up to 40 min of recovery was allowed after capsaicin application. Dyssynchronous pacing in the RV was started at 600 ms (100 beats/min) and decreased by 50-ms steps to 300 ms (200 beats/min).
Tissue handling, immunohistochemistry, and imaging.
Tissues were rapidly excised, rinsed in cold 0.1 M PBS to eliminate blood, and placed into freshly prepared, cold 4% paraformaldehyde (15714, Electron Microscopy Sciences, Hatfield, PA) overnight at 4°C. After fixation, tissues were rinsed in PBS (four 20-min washes) and stored in PBS with 0.1% sodium azide for sectioning. Fixed tissues were embedded in 2% agarose-PBS, and 200-µm-thick sections were made using a microtome (VT 1200S, Leica Biosystems, Buffalo Grove, IL). Immunostaining was performed on free-floating sections. Sections were blocked in PBS with 4% horse serum (ab7484, Abcam, Cambridge, MA) and stained with primary antibody for 3 days followed by secondary antibody for 2 days in PBS with 0.1% Triton X-100 (X100, Sigma-Aldrich, St. Louis, MO) at room temperature. Primary and secondary antibodies are shown in Table 1. Imaging was performed with a Zeiss LSM 780 confocal microscope (Carl Zeiss, Oberkochen, Germany). To facilitate comparison, gamma or contrast as well as microscope and laser settings remained consistent across all images. Digitized images were stored for offline analyses using the analytic software Zen (Black edition, Carl Zeiss Microimaging). The evaluation of immunohistochemistry was performed using ImageJ.
Table 1.
List of antibodies used
| Antibody | Concentration | Source |
|---|---|---|
| Primary antibody | ||
| Protein gene product 9.5 | 1:500 | Polyclonal rabbit antibody, ab108986, Abcam (San Francisco, CA) |
| Calcitonin gene-related peptide | 1:500 | Monoclonal mouse antibody, ab81887, Abcam |
| Tyrosine hydroxylase | 1:200 | Polyclonal sheep antibody, AB1542, EMD Millipore (Darmstadt, Germany) |
| Vanilliod receptor 1 | 1:200 | Polyclonal rabbit antibody, NB100-98897, Novus (Littleton, CO) |
| Substance P | 1:1,000 | Polyclonal rabbit antibody, 20064, Immunostar (Hudson, WI) |
| Neurofilament-heavy | 1:1,000 | Polyclonal chicken antibody, AB5539, EMD Millipore |
| Secondary antibody | ||
| Cy3 AffiniPure donkey anti-rabbit IgG | 1:400 | Polyclonal rabbit antibody, 711-165-152, Jackson Immunoresearch Laboratories (West Grove, PA) |
| Alexa Fluor 647 AffiniPure donkey anti-mouse IgG | 1:400 | Polyclonal mouse antibody, 715-605-150, Jackson Immunoresearch Laboratories |
| Alexa Fluor 488 AffiniPure donkey anti-sheep IgG | 1:400 | Polyclonal sheep antibody, 713-545-003, Jackson Immunoresearch Laboratories |
| Cy3 AffiniPure donkey anti-Rabbit IgG | 1:400 | Polyclonal rabbit antibody, 711-165-152, Jackson Immunoresearch Laboratories |
| Cy3 AffiniPure Donkey Anti-Rabbit IgG | 1:400 | Polyclonal rabbit antibody, 711-165-152, Jackson Immunoresearch Laboratories |
| Alexa Fluor 488 AffiniPure Donkey Anti-Chicken IgY | 1:400 | Polyclonal chicken antibody, 703-545-155, Jackson Immunoresearch Laboratories |
Statistical analyses.
Data are reported as means ± SE unless otherwise stated. Paired comparisons were performed with the paired Student t-test for normally distributed data and the Wilcoxon signed-rank test for data not normally distributed. Comparisons of control and RTX-treated animals were performed using the Mann-Whitney test. Data reflecting the effect of ventricular pacing at various paced cycle lengths (600 to 300 ms) between control and RTX-treated animals were compared using a two-way ANOVA with Tukey’s correction for multiple comparisons. Neuronal firing rates were compared between control and RTX-treated animals by a two-tailed unpaired t-test. Statistical significance of changes in firing rates of SGNs from baseline to stressors was determined using a Skellam-based maximum-likelihood decoding, developed for cortical neuronal activity (8, 31, 34). For all comparisons, P values of <0.05 were considered statistically significant. Data were handled and analyzed with Microsoft Excel (Redmond, WA) and GraphPad Prism (La Jolla, CA), respectively.
RESULTS
Cardiac depletion of VR1 afferents enhances systolic BP and contractility.
Using the percutaneous approach shown in Fig. 1A, intrapericardial RTX application induced an acute sympathoexcitatory response, transiently increasing BP and HR (Fig. 1B). Four weeks later, during terminal experimentation, baseline hemodynamic indexes were measured after stabilization of the surgical preparation. Baseline HR was similar between control and RTX-treated animals (86.9 ± 8.8 vs. 83.2 ± 5.6 beats/min, respectively, P = 0.7; Fig. 1C). However, systolic BP (SBP) was significantly greater in RTX-treated animals (147.3 ± 9.4 mmHg) than control animals (102.1 ± 6.9 mmHg, P = 0.009; Fig. 1D), suggesting a tonic contribution of VR1 afferents to BP control. This relationship was not seen for diastolic blood pressure (DBP; 76 ± 5.9 vs. 90.8 ± 10.2 mmHg for control vs. RTX-treated animals, respectivley, P = 0.2; Fig. 1E). Next, we examined whether the increase in SBP was related to cardiac contractile function. As shown in Fig. 1F, dP/dtmax was significantly greater in RTX-treated versus control animals (1,625.5 ± 130 vs. 1,252.5 ± 177 mmHg/s, respectively, P = 0.03), as was dP/dtmin (−2,617 ± 332 vs. −1,468.1 ± 183 mmHg/s, P = 0.02). Baseline ARI (Fig. 1G) was not significantly different between control and RTX-treated subjects (324.6 ± 21.4 vs. 346.9 ± 7.6 ms, P = 0.16).
Structural and functional depletion of cardiac VR1 afferents.
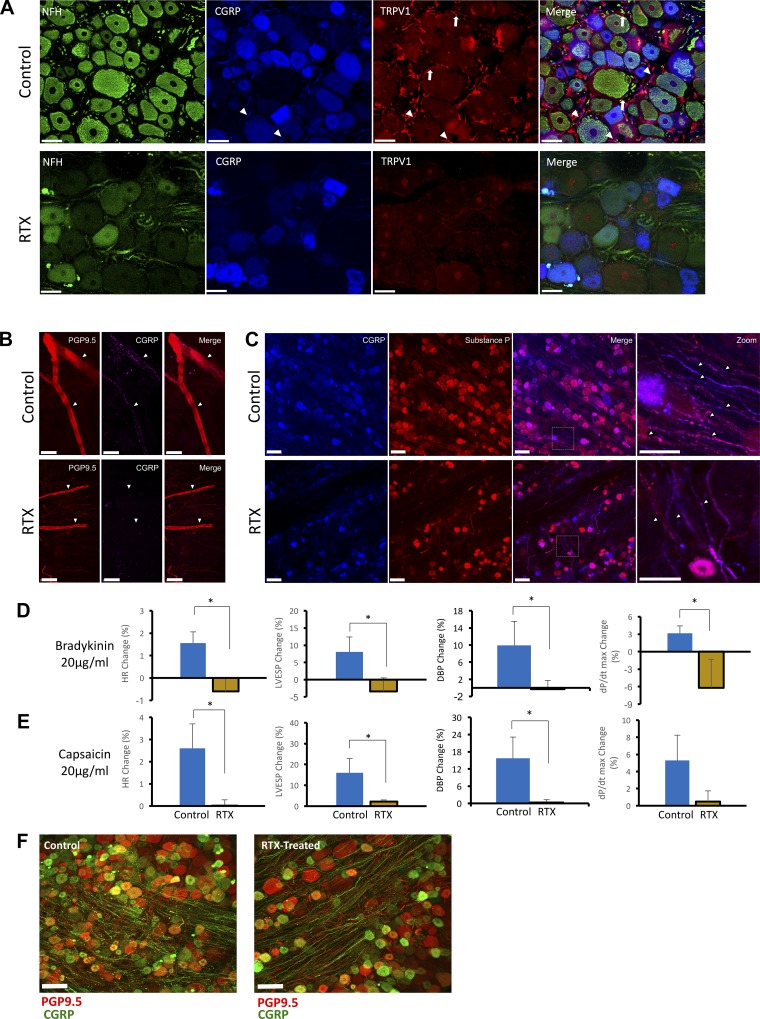
To characterize the VR1 afferent depletion mediated by intrapericardial RTX, we performed immunohistochemical comparisons of the anterior LV epicardium and T1 dorsal root ganglion (DRG) from control and RTX-treated animals. As shown in Fig. 2A, immunoreactivity for the TRPV1 channel was significantly depleted by RTX (1.6 ± 0.4% vs. 4.8 ± 1.5% for TRPV1, P = 0.03, and 17.3 ± 3.8 vs. 41.2 ± 6.7 for neurofilament-heavy fibers, P = 0.013). As shown in Fig. 2B, top, nerve fibers could be identified in the subepicardial layer of the myocardium by protein gene product 9.5 immunoreactivity. Within those nerve bundles, calcitonin gene-related peptide (CGRP)-immunoreactive fibers could be readily seen in control hearts but not in RTX-treated hearts (5.9 ± 0.39% vs. 2.77 ± 0.14%, P = 0.002), confirming depletion of these afferent fibers (Fig. 2B, bottom) in the heart. Given the high degree of colocalization between CGRP, substance P, and the TRPV1 channel (≈50%) (29), CGRP and substance P were used to probe sensory afferent denervation in the DRG. In control animals, fiber tracts positive for CGRP and/or substance P were identified (Fig. 2C, top). In RTX-treated animals, however (Fig. 2C, bottom), there was a significant diminution in the numbers of these fibers compared with control animals (5.8 ± 0.8% vs. 17 ± 1.2% for CGRP, P < 0.001, and 6.3 ± 0.8% vs. 24.31 ± 1.9%, for substance P, P = 0.002).
Fig. 2.
Structural and functional depletion of cardiac afferent fibers by intrapericardial resiniferatoxin (RTX). A: representative images demonstrating depletion of the transient receptor potential vanilloid 1 (TRPV1) channel and calcitonin gene-related peptide (CGRP), a marker of sensory afferent nerves, by RTX in T1 dorsal root ganglia (DRG). TRPV1 immunoreactivity in the cell membrane is indicated by arrows; fibers are shown by arrowheads. The lack of staining for the fibers for neurofilament heavy (NFH) can also be appreciated. TRPV1 and CGRP were significantly diminished in RTX-treated animals. Scale bar = 50 µm. B: left ventricular epicardium showing double immunostaining for protein gene product 9.5 (PGP9.5) and CGRP (top) and in chronic RTX-treated animals (bottom). C: representative images of CGRP and substance P immunoreactivity in DRG from control (top) and RTX-treated (bottom) animals. In both B and C, depletion of afferent nerve fibers can be appreciated. Scale bar = 50 µm. D and E: Hemodynamic responses to epicardial application of 20 μg/ml bradykinin (D) and 20 μg/ml capsaicin (E). Shown are percent change in heart rate [HR; in beats/min (bpm)], left ventricular end-systolic pressure (LVESP), diastolic blood pressure (DBP), and inotropy (dP/dtmax) in response to bradykinin (D) and capsaicin (E) (n = 6–8 animals/group). *P < 0.05 by Mann-Whitney test. F: representative images of CGRP and PGP9.5 immunoreactivity in T1 DRG from control and RTX-treated animals.
To examine the CSAR after RTX treatment, hemodynamic responses to epicardial bradykinin and capsaicin, known activators of cardiac sensory afferents, were studied in control and RTX-treated animals. Bradykinin (20 µg/ml; Fig. 2D) or capsaicin (20 µg/ml; Fig. 2E) elicited chronotropic and pressor responses in control animals, increasing HR, LV end-systolic pressure (LVESP), DBP, and dP/dtmax. In RTX-treated animals, however, hemodynamic responses were lost, confirming functional loss of the CSAR after RTX. The loss of sensory afferents was limited to thoracic DRG, as lumbar DRG did not show significant changes after RTX treatment (Fig. 2F).
VR1 afferent depletion does not interfere with cardiac reflex responses to cardiovascular mechanoreceptor stimuli.
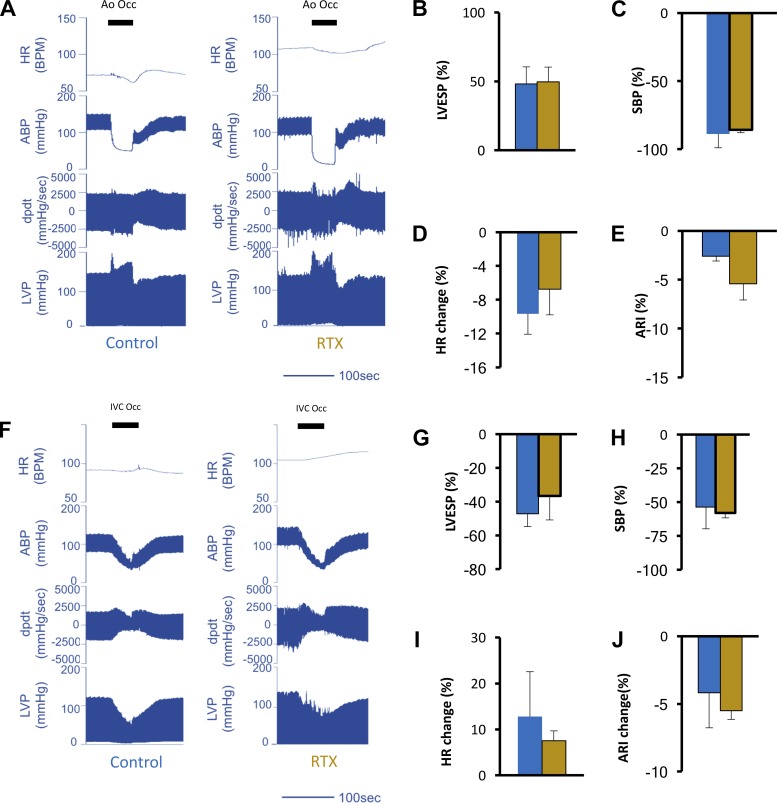
VR1 afferents mediate sensory transduction of a variety of stimuli, including cardiac mechanical perturbations. To investigate how VR1 receptor depletion alters integrated reflex processing of cardiovascular pressure-related inputs, we examined cardiac hemodynamic and electrophysiological responses to increased afterload and decreased preload by occlusion of the aorta just distal to the great vessels and occlusion of the IVC just below the right atrium, respectively. Occlusion of the descending aorta increased LVESP and caused severe hypotension in the arteries distal to the site of the occlusion, as indicated by the arterial BP tracing recorded from the femoral artery (Fig. 3A). The occlusion evoked similar increases in LVESP (48.1 ± 12.4% vs. 49.6 ± 10.7%, P = 0.72) and similar decreases in SBP (−88.8 ± 10% vs. −85.7 ± 2.2%, P = 0.9) downstream from the occlusion point in control and RTX-treated animals, respectively (Fig. 3, B and C), suggesting similar stressor intensity. Reflex bradycardia was not significantly different between control and RTX-treated animals (−9.6 ± 2.4% vs. −6.7.9 ± 3.1%, P = 0.8; Fig. 3D). Similarly, ARI shortening was not significantly different between control and RTX-treated groups (−2.6 ± 0.5% vs. −5.4 ± 1.7%, P = 0.22; Fig. 3E). Representative hemodynamic tracings reflecting IVC occlusion in control and RTX-treated subjects are shown in Fig. 3F. The degree of occlusion and the hemodynamic perturbation created by the intervention were similar between both groups. LVESP dropped by 47.1 ± 7.7% and 36.6 ± 14.2% in control and RTX-treated animals, respectively (P = 0.61), whereas SBP was reduced by 53.7 ± 16% and 58 ± 3.6%, respectively (P = 0.7; Fig. 3, G–H). Reflex tachycardia and ARI shortening mediated by the stress of preload reduction (Fig. 3, I and J) was not different in control and RTX-treated animals (12.8 ± 9.8% vs. 7.5 ± 2.1% for HR, respectively, P = 0.5, and −4.2 ± 2.6% vs. −5.5 ± 0.6% for ARI, respectively, P = 0.09).
Fig. 3.
Vanilloid receptor 1 (VR1) afferent depletion does not modulate reflex responses to cardiovascular mechanoreceptor stimuli. A: representative hemodynamic tracings from control and resiniferatoxin (RTX)-treated animals during increased afterload induced by occlusion of the aorta distal to the great vessels. The duration of aortic occlusion is indicated by the solid horizontal bars. Shown are heart rate [HR; in beats/min (BPM)], arterial blood pressure (ABP), left ventricular contractility (dP/dt), and left ventricular pressure (LVP). During aortic occlusion, ABP recorded in the femoral artery plummeted. The increase in left ventricular end systolic pressure (LVESP; B), decrease in femoral systolic blood pressure (SBP) during occlusion of the aorta (C), percent decrease in HR (reflex bradycardia; D), and activation recovery interval (ARI; E) during aorta occlusion in control and RTX-treated animals are shown. F: representative hemodynamic tracings from control and RTX-treated animals during preload reduction induced by occlusion of the inferior vena cava (IVC) just below its junction with the right atrium. The duration of IVC occlusion is indicated by the solid horizontal bars. G and H: percent decrease in LVESP (G) and percent decrease in femoral SBP (H) during occlusion of the IVC. I and J: percent decrease in HR (I) and ARI (J) during IVC occlusion in control and RTX-treated animals. Data are for n = 5–8 animals/group and were evaluated by a Mann-Whitney test.
VR1 afferent depletion enhances hemodynamic resistance to cardiac stress induced by rapid dyssynchronous RVP.
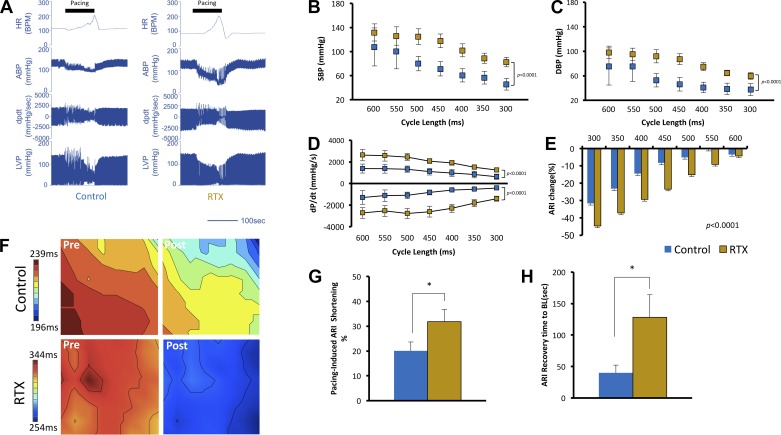
Electrical activation of the ventricles from single point pacing causes electromechanically dyssynchronous contraction of the ventricles (37), reduces stroke work (13), and activates intrinsic cardiac neurons (14, 31). Rapid ventricular pacing also induces ischemia due to poor coronary perfusion (26, 30). We examined the influence of this multimodal stressor on reflex processing in control and RTX-treated animals. Pacing was initiated at a cycle length of 600 ms (100 beats/min) and rapidly accelerated to a cycle length of 300 ms (200 beats/min). Representative hemodynamic tracings during pacing in control and RTX-treated subjects during RVP are shown in Fig. 4A. At a cycle length of 600 ms, there was no significant difference between control and RTX-treated animals in SBP (107.7 ± 31.6 vs. 131.8 ± 14.2 mmHg, respectively, P = 0.6) and DBP (75.2 ± 30.0 vs. 98.2 ± 10.2 mmHg, respectively, P = 0.6). As paced cycle length was gradually decreased (i.e., as HR was increased), SBP and DBP were progressively compromised. However, in RTX-treated compared with control animals, the degree of compromise was significantly lower (P < 0.0001 for both, Fig. 4, B and C), suggesting resistance to the destabilizing influences of RVP. We examined whether this stability was conferred by cardiac contractility during RVP. As shown in Fig. 4D, both inotropy and lusitropy (indicated by dP/dtmax and dP/dtmin) were significantly greater in RTX-treated than control animals during RVP stress (P < 0.0001 for both). With the use of ARI shortening at each paced cycle length as an index of sympathoexcitation (given that HRs were the same), RTX-treated animals showed greater ARI shortening during RVP (550–300 ms, P < 0.0001; Fig. 4E) compared with control animals. To exclude the effect of pacing, the degree of sinus rhythm ARI shortening measured immediately upon cessation of RVP was significantly greater in RTX-treated animals than in control animals (31.8 ± 4.9% vs. 20.1 ± 3.6%, P = 0.049) at similar HRs, further evidence of increased cardiac sympathetic neurotransmission (Fig. 4, F and G). We also examined how rapidly ARI recovered to baseline values after cessation of cardiac pacing, as a marker of recovery from sympathoexcitation induced by RVP. Compared with control animals, RTX-treated animals took significantly longer (128.3 ± 35.7 vs. 40.1 ± 12.2 s, respectively, P = 0.028) to return to baseline ARI values (Fig. 4H), suggesting that the sympathoexcitatory response to stress was sustained longer after VR1 afferent depletion.
Fig. 4.
Transient receptor potential vanilloid 1 (TRPV1) depletion enhances resistance to multimodal stress induced by rapid right ventricular pacing (RVP). A: representative hemodynamic tracings from control and resiniferatoxin (RTX)-treated animals during rapid RVP. The solid horizontal bars indicate the duration of RVP. Shown are heart rate [HR; in beats/min (BPM)], arterial blood pressure (ABP), left ventricular contractility (dP/dt), and left ventricular pressure (LVP). B and C: systolic blood pressure (SBP; B) and diastolic blood pressure (DBP; C) [SBP: P < 0.0001 for treatment, P = 0.0006 for paced cycle length, and P = 0.969 for interaction; DBP: P < 0.0001 for treatment, P = 0.003 for paced cycle length, and P = 0.9 for interaction]. D: contractility [dP/dtmax: P < 0.0001 for treatment, P = 0.006 for paced cycle length, and P = 0.93 for interaction; dP/dtmin: P < 0.0001 for treatment, P = 0.03 for paced cycle length, and P = 0.92 for interaction]. E: activation recovery interval (ARI; E) shortening during rapid cardiac pacing at gradually decreasing cycle lengths from 600 ms (100 BPM) to 300 ms (200 BPM) (P < 0.0001 for treatment, P < 0.0001 for paced cycle length, and P = 0.2 for interaction). F: representative sinus rhythm ARI maps from the anterior left ventricle before and after rapid pacing at 300-ms cycle length in control and RTX-treated animals. Two-way ANOVA with Sidak’s multiple comparison test was used. G: summary data of percent ARI shortening of the sinus rhythm before and after RVP. H: recovery of ARI to baseline values after cessation of cardiac pacing. RTX-treated animals took substantially longer to return to baseline. n = 5–8 animals/group, *P < 0.05 by Mann-Whitney test.
VR1 afferent depletion enhances basal SGN firing rates and responsiveness to cardiac stress.
Neuronal activity recorded from the craniomedial pole of the SG was compared in control and RTX-treated animals. Across groups, 51 and 72 waveforms were examined in control and RTX-treated animals, respectively. The activity of recorded neurons, including sensitivity to BP, was consistent with prior data (7). A representative example of the recording electrode, a raw data channel, and the waveforms comprising the channel are shown in Fig. 5, A and B. As shown in Fig. 5C, mean basal SGN firing frequency was significantly greater in RTX-treated than control animals (0.3 ± 0.1 vs. 0.1 ± 0.03 Hz, P = 0.018). During cardiac stressors, firing frequencies were significantly greater during IVC occ in RTX-treated than control animals (0.1 ± 0.02 vs. 0.02 ± 0.01 Hz, P = 0.032) but not during Ao occ (0.1 ± 0.03 vs. 0.1 ± 0.02 Hz for RTX-treated vs. control animals, respectively, P = 0.43) or RVP (0.1 ± 0.05 vs. 0.03 ± 0.01 Hz for RTX-treated vs. control animals, respectively, P = 0.13). This suggests that the afferent activation induced by Ao occ and RVP significantly increased firing rates in control animals, unlike during IVC occ. Since ARI shortening and hemodynamic responses were sustained in RTX-treated animals relative to control animals after cessation of RVP, we examined SGN firing rates following termination of RVP in RTX-treated and control animals. As shown in Fig. 5D, mean firing rates after termination of RVP were significantly greater in RTX-treated animals (0.11 ± 0.03 Hz) than in control animals (0.02 ± 0.01 Hz, P = 0.006), supporting the thesis that sympathetic tone is sustained after RVP in RTX-treated subjects.
Fig. 5.
Cardiac transient receptor potential vanilloid 1 (TRPV1) depletion increases stellate ganglion neuron (SGN) responsiveness to stress. A: 16-channel linear microelectrode array used for recording SGNs. B: a raw data channel and individual SGNs comprising the channel. Inset: the unique morphology of four SGNs is highlighted. C: mean basal SGN firing frequency in control and resiniferatoxin (RTX)-treated animals. D: mean firing rates of SGNs after termination of rapid right ventricular pacing (RVP) in control and RTX-treated animals. E: responsiveness of recorded SGNs to the stressors instituted. F: summary of changes in activity of SGNs recorded before and during each stressor. *P < 0.05; **P < 0.01; ***P < 0.001. C and D: unpaired t-test; E: χ2-test; F: Skellam-based maximum likelihood decoding.
In addition to the group activity reported above, we also examined reactivity of SG neurons to the cardiac stressors. As shown in Fig. 5, E and F, while 11% of neurons recorded in control animals responded to any cardiac stressor, 67% of neurons in RTX-treated animals responded to the same stressors (P = 0.0002).
RTX treatment does not alter cardiac responses to SG stimulation.
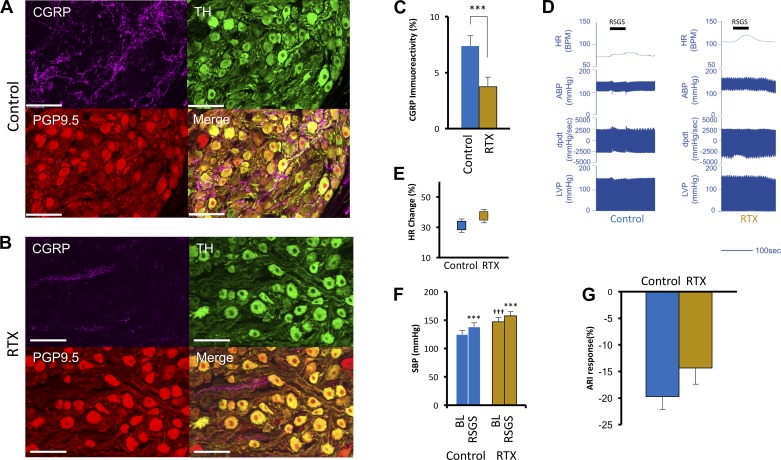
VR1 afferent fibers traverse the sympathetic chain en route to their soma within the DRG. Hence, enhanced sympathetic neurotransmission seen after RTX treatment may be mediated at the level of the SG by cross talk between en pasant afferent fibers and SGNs. As shown in Fig. 6A, these fibers, indicated by CGRP immunostaining, form a mesh-like network around SGNs and are depleted after RTX treatment (Fig. 6B). The percent area of CGRP immunoreactivity decreased from 7.4 ± 0.9% to 3.7 ± 0.9% (P = 0.008) after RTX treatment (Fig. 6C). To examine the integrity of efferent limb of the CSAR between the SG and the heart, cardiac hemodynamic and ARI responses to RSG stimulation were examined. Stimulation amplitudes were not different between control and RTX-treaded animals (2.98 ± 1.89 vs. 2.23 ± 1.03 mA, respectively, P = 0.45). Representative hemodynamic traces in control and RTX-treated animals are shown in Fig. 6D. The mean HR increase during RSGS was 31 ± 4.4% versus 38.3 ± 6.7% (P = 0.35) in control and RTX-treated animals, respectively (Fig. 6E). SBP responses to RSG stimulation (Fig. 6F) were also similar between control and RTX animals (124.7 ± 6.8 vs. 138 ± 7.1 mmHg, for control animals, P < 0.0001, and 146.7 ± 7.7 vs. 157.7 ± 7.2 mmHg for RTX-treated animals, P = 0.0004). Consistent with SBP findings, ARI shortening during RSG stimulation were not different between control and RTX-treated animals (19.7 ± 2.4% vs. 14.3 ± 3.0%, P = 0.12; Fig. 6G). These data indicate that RTX treatment did not disrupt pre- to postganglionic interconnections or the functional manifestations of direct sympathetic projections to cardiomyocytes.
Fig. 6.
Efferent sympathetic function of right stellate ganglia after sensory afferent depletion. Representative images of stellate ganglia stained with calcitonin gene-related peptide (CGRP), tyrosine hydroxylase (TH), and peptide gene product 9.5 (PGP9.5) from control (A) and resiniferatoxin (RTX) (B)-treated animals are shown. Images show individual CGRP, TH, PGP9.5 staining and a merge of all three as indicated. An extensive network of CGRP-positive afferent fibers can be readily identified in A coursing through the stellate ganglion; however, after epicardial RTX application, these fibers were severely depleted, as shown in B. Scale bar = 100 µm. C: quantification of CGRP-positive afferent fiber depletion after RTX treatment. D: representative hemodynamic tracings during right stellate ganglion stimulation (RSGS) in control and RTX-treated animals. The duration of RSGS is indicated by the solid horizontal bars. Shown are heart rate [HR; in beats/min (BPM)], arterial blood pressure (ABP), left ventricular contractility (dP/dt), and left ventricular pressure (LVP). E: the HR response to RSGS was not significantly different between control and RTX-treated animals. F: the increase in mean arterial pressure (MAP) during RSGS was not significantly different between control and RTX-treated animals. G: the activation recovery interval (ARI) shortening in response to RSGS was similar between control and RTX-treated animals. ***P < 0.001 and †††P < 0.001 by two-way ANOVA with Sidak’s multiple comparison test.
DISCUSSION
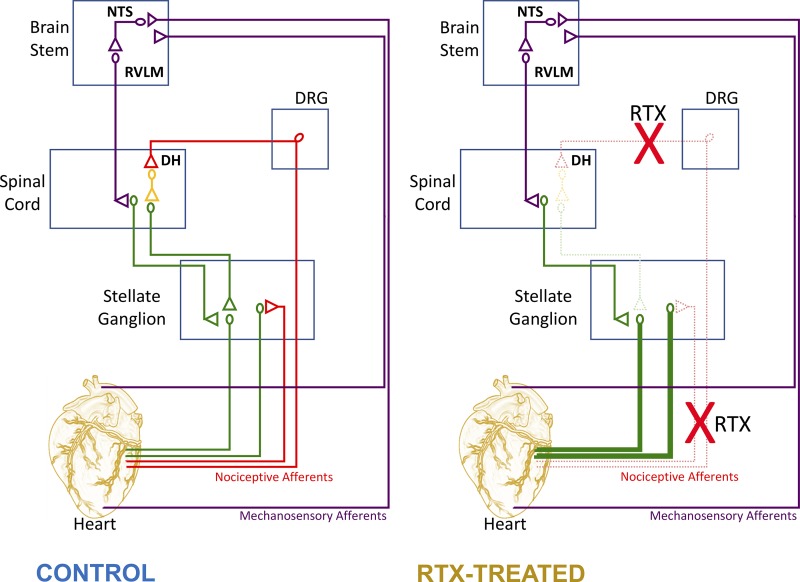
The major findings of the present study are that 1) depletion of cardiac VR1 afferents by percutaneous application of RTX in swine is feasible and effective and 2) VR1 afferent depletion enhances basal cardiac hemodynamic function and resistance to hemodynamic collapse induced by cardiac stress by 3) enhancing basal SGN activity and SGN responsiveness to cardiac stress. These changes may be mediated at the level of the spinal cord (or brain stem) as efferent input from the SG to the heart examined by SG is not altered by RTX treatment. These data suggest that depletion of VR1 afferents reorganizes the central-peripheral interactions (i.e., spinal cord-SG) involved in cardiac control to a more cardiocentric focus, enhancing reflex efferent sympathetic control of the heart to sustain cardiac function (Fig. 7).
Fig. 7.
Schematic representation of the impact of cardiac vanilloid receptor 1 (VR1) depletion on central-peripheral interactions for cardiac control. Illustrations of neural circuits involved in cardiac control are shown. Left: connectivity in control animals. Right: theorized changes suggested by data in the present article. DH, dorsal horn; NTS, nucleus of the solitary tract; RTX, resiniferatoxin; RVLM, rostral ventrolateral medulla.
The cardiac neural hierarchy provides exquisite cardiac control on long and short timescales (6, 35). The net efferent output to the heart (both sympathetic and parasympathetic) is a result of neural processing and interactions occurring across multiple sites within this neural hierarchy. The sympathoexcitatory response to myocardial ischemia is well recognized to be mediated in large part by capsaicin-sensitive nerves present within the heart (28), projecting to the dorsal horn of the spinal cord via the DRG where their soma reside (9). These afferent nerve endings respond to metabolites and free radicals generated within ischemic myocardium (33) via the TRPV1 channel (11, 45). While it is known that these afferent fibers, also known as VR1 afferents, mediate the CSAR, whether they have tonic role in the absence of ischemia or pain remain unknown.
In the present study, we examined this role for cardiac VR1 afferents by depleting them in a large animal model using RTX (36, 40, 45) applied to the epicardium percutaneously. At the doses given, RTX resulted in cell death. This effect of RTX on neurons expressing the TRPV1 channel has been extensively studied, and the elimination of soma and dose dependence have been previously described (18, 27). The dose of RTX used in the present study was selected to eliminate soma. Lower doses of RTX in the 5 μg/ml range eliminate nerve endings, but this is functionally recovered within 10 wk. Higher doses in the 50 μg/ml range result in much more sustained effect, which recovers by 6 mo and is likely related to loss of soma (40).
While that the TRPV1 channel is expressed predominantly in small- and medium-sized neurons, VR1 is also expressed in A-fiber neurons (21), which can mediate afferent neurotransmission (10). At the doses used, we expect that some cardiac sensory neurons within the DRG would undergo cell death. This is not a systemic effect, as lumbar DRG do not show the same soma and fiber loss as the T1 DRG.
Our findings of depleted VR1 fibers in the epicardium and DRG after RTX treatment are also consistent with previous studies (36, 45).
At baseline, RTX-treated swine demonstrated elevated SBP (but not DBP) and increased cardiac contractility, suggesting that VR1 afferents contribute to tonic control of BP. The absence of an elevated DBP suggests that vascular tone is similar in both groups and the elevated SBP is related to enhanced cardiac contractile function. The increase in SBP but not DBP is surprising. It suggests the sympathoexcitation resulting from ablation of epicardial VR1 afferents is greater on the heart than the peripheral vasculature. This can be explained by specific targeting of cardiac afferents and as a result upper thoracic sympathetic ganglia (predominantly SG and middle cervical ganglia). Since sympathetic outflow to the vasculature is mediated via the entirety of the sympathetic chain, it is unlikely that RTX, as administered here, would significantly affect sympathetic outflow to the vasculature to increase resting arterial tone. The enhancing influence exerted by VR1 afferent depletion may occur in part at the level of the spinal cord, as suggested by the sympathoexcitatory effects imparted by T1–T4 dorsal rhizotomy (44). Surgical interruption of spinal afferent input, which includes VR1 afferents, induced basal cardiac sympathoexcitability, as measured by myocardial ARIs, while maintaining direct sympathetic efferent control of the heart (44).
Cardiac and cardiovascular reflex responses to substantial and abrupt decreases in preload or increases in afterload were not impacted by VR1 afferent depletion in RTX-treated animals. This indicates that integrated baroreflex or cardiopulmonary reflex function is not altered by VR1 afferent depletion, likely reflective of minimal effects of RTX on cardiovascular-related mechanotransduction. Dyssynchronous cardiac activation and contraction such as that induced by focal ventricular pacing elicits neural activation (14, 31). RVP not only causes dyssynchrony but also compromises cardiac filling (37) and coronary perfusion and causes ischemia (30). Interestingly, RTX-treated animals demonstrated greater resistivity to the hemodynamic compromise created by RVP, becoming progressively more apparent at faster pacing rates. Contractility, SBP, DBP, and ARI shortening were all greater in RTX-treated animals. Even when the effect of pacing was removed and sinus rhythm ARI was examined, despite similar HRs, percent ARI shortening was greater in RTX-treated animals. Recovery from this excitation was also significantly delayed in RTX-treated compared with control animals. These findings all indicate significantly greater sympathoexcitatory response to cardiac stress in RTX-treated animals and suggest that the residual neural networks for sympathetic control are maintained, and in certain conditions enhanced, after disruptions of cardiac VR1, allowing for effective neural control of the stressed heart. Inotropic and chronotropic response to RSG stimulation was similar between control and RTX-treated animals, suggesting that the efferent limb of the CSAR (pre to post and postganglionic to myocyte) is not functionally affected by RTX. Whether synaptic transmission between pre- and postganglionic sympathetic neurons is altered by depleted fibers of passage remains unclear. Furthermore, it remains unclear whether intrinsic excitability of postganglionic SGNs contribute to enhanced sympathetic tone seen after cardiac-afferent depletion.
These findings in the control state should be considered in the context of what is known in disease states, including chronic CHF, where the CSAR is significantly enhanced (41, 43) and contributes to the pathophysiology of CHF, including adverse remodeling of both the cardiac nervous system and heart (46). VR1 depletion in the setting for chronic myocardial infarction diminished adverse cardiac remodeling and improved cardiovascular function (39, 40). Chronic cardiac dysfunction causes adverse remodeling both in the heart and within its neural hierarchy (1–3, 24, 31, 35). In the setting of chronic disease, the reduction in TRPV1 signaling by RTX is likely reflected in mitigation of the afferent-driven remodeling of the cardiac nervous system and the heart tissues it innervates. Under normal conditions, VR1 afferents are necessary to mediate the sympathetic afferent reflex; however, these afferents may also function to maintain ongoing dynamic cardiocardiac reflexes that control cardiac homeostatic function, loss of which alters normal autonomic function resulting in sympathoexcitation. This is supported by evidence from TRPV1 knockout mice, which have a very high mortality rate after myocardial infarction (16). Although this study attributed the larger infarct size to inflammation, interpretation of this data in light of our data suggests that sympathoexcitation may contribute to this. We posit that ablation of TRPV1 afferents in the heart failure state may be beneficial because afferent signaling is upregulated in heart failure; however, under normal conditions, elimination of such afferents may result in sympathoexcitation as in the present study. Clinically, the findings of this study are highly relevant, as TRPV1-expressing afferents are an important target in a number of conditions such as pain (23); however, how this this may affect patients with normal hearts is not known. Whether sympathoexcitation occurs as an unintended consequence of epidural application of TRPV1 antagonists is not known, The findings of the present study suggest that further study is warranted. The impact of VR1 ablation on sensation of myocardial ischemia, which is a critical alarm signal for a subset of patients with coronary artery disease, and risk of infarction or death is also important to study. It is however likely that such afferents are not completely eliminated and the sensation of ischemia may still be possible.
Limitations.
The present study was conducted while animals were under chloralose anesthesia. Although autonomic reflexes are thought to be preserved under this anesthetic regimen, it is possible that the anesthetic agent modulated cardiac and autonomic reflex function. Parasympathetic afferents also express the VR1 channel and were likely impacted by epicardial RTX treatment. Although parasympathetic function was not examined in the present study, the impact of RTX on cardiac vagal afferents that express TRPV1 and those projecting to the intrinsic cardiac ganglia specifically may have contributed to these findings and should be examined in future studies. Another consideration is the role that volume loading and coronary blood flow may play in modulating cardiac afferent responses (42). This was not assessed in the present study during aortic occlusion. TRPV1 expression in cardiomyocytes has been reported; however, its location and function in myocytes remain highly controversial. It has been reported to be in the cytosol, cell membrane, and mitochondrial membrane in different studies (5, 15, 17). Given this controversy, it is not clear whether the stress responses in our study were affected. Regardless, this is unlikely to have contributed to the findings in the SG and DRG. Finally, SGN activity in the LSG but not RSG was examined. Given the differences in the territory of cardiac innervation by LSG versus RSG, it would be of value to assess neuronal recordings in the RSG, although it should be pointed out that RTX was free flowing in the pericardium after infusion and would have been distributed evenly around the heart. We expect that both SG were equally impacted.
Conclusion.
The findings of the present study suggest that uncoupling central-peripheral interactions involved in cardiac control (by depleting VR1 afferents) enhance efferent cardiac sympathetic control. Depletion of these afferents in normal swine enables a robust sympathoexcitatory reflex response to cardiac stress. These findings improve our understanding of the various roles played by cardiac sympathetic afferents in the control cardiovascular function and continue to suggest the CSAR as a modifiable target in disease states.
GRANTS
This work was supported by a Nihon Kohden/St. Jude Medical Research Fellowship (to K. Yoshie) and National Institutes of Health Grants HL-125730 and DP2-HL-142045 (to O. A. Ajijola), HL-084261 and OT2-OD-023848 (to K. Shivkumar), and 2-P41-RR-0112553-12 (to the Center for Integrative Biomedical Computing, University of Utah).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.Y., J.L.A., K.S., and O.A.A. conceived and designed research; K.Y., P.S.R., L.M., O.K., V.T., and O.A.A. performed experiments; K.Y., P.S.R., L.M., O.K., V.T., S.S., J.L.A., K.S., and O.A.A. analyzed data; P.S.R., S.S., J.L.A., K.S., and O.A.A. interpreted results of experiments; K.Y., L.M., O.K., V.T., S.S., and O.A.A. prepared figures; O.A.A. drafted manuscript; K.Y., P.S.R., O.K., S.S., J.L.A., K.S., and O.A.A. edited and revised manuscript; K.Y., P.S.R., L.M., O.K., V.T., S.S., J.L.A., K.S., and O.A.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Amiksha Gandhi and Dr. Tatsuo Takamiya for excellent technical assistance.
REFERENCES
- 1.Ajijola OA, Lux RL, Khahera A, Kwon O, Aliotta E, Ennis D, Fishbein MC, Ardell JL, Shivkumar K. Sympathetic modulation of electrical activation in normal and infarcted myocardium: implications for arrhythmogenesis. Am J Physiol Heart Circ Physiol 312: H608−H621, 2017. doi: 10.1152/ajpheart.00575.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajijola OA, Shivkumar K. Neural remodeling and myocardial infarction: the stellate ganglion as a double agent. J Am Coll Cardiol 59: 962–964, 2012. doi: 10.1016/j.jacc.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajijola OA, Wisco JJ, Lambert HW, Mahajan A, Stark E, Fishbein MC, Shivkumar K. Extracardiac neural remodeling in humans with cardiomyopathy. Circ Arrhythm Electrophysiol 5: 1010–1116, 2012. doi: 10.1161/CIRCEP.112.972836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol 305: H1031–H1040, 2013. doi: 10.1152/ajpheart.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrei SR, Sinharoy P, Bratz IN, Damron DS. TRPA1 is functionally co-expressed with TRPV1 in cardiac muscle: co-localization at z-discs, costameres and intercalated discs. Channels (Austin) 10: 395–409, 2016. doi: 10.1080/19336950.2016.1185579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardell JL, Andresen MC, Armour JA, Billman GE, Chen PS, Foreman RD, Herring N, O’Leary DS, Sabbah HN, Schultz HD, Sunagawa K, Zucker IH. Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol 594: 3877–3909, 2016. doi: 10.1113/JP271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armour JA. Activity of in situ stellate ganglion neurons of dogs recorded extracellularly. Can J Physiol Pharmacol 64: 101–111, 1986. doi: 10.1139/y86-016. [DOI] [PubMed] [Google Scholar]
- 8.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. doi: 10.1113/jphysiol.2013.259382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blair RW, Weber RN, Foreman RD. Responses of thoracic spinothalamic neurons to intracardiac injection of bradykinin in the monkey. Circ Res 51: 83–94, 1982. doi: 10.1161/01.RES.51.1.83. [DOI] [PubMed] [Google Scholar]
- 10.Brown AM. Excitation of afferent cardiac sympathetic nerve fibres during myocardial ischaemia. J Physiol 190: 35–53, 1967. doi: 10.1113/jphysiol.1967.sp008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 12.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 8: 510–521, 2007. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 13.Hamby RI, Aintablian A. Preload reduction with right ventricular pacing: effects on left ventricular hemodynamics and contractile pattern. Clin Cardiol 3: 169–177, 1980. doi: 10.1002/clc.4960030303. [DOI] [PubMed] [Google Scholar]
- 14.Hamon D, Rajendran PS, Chui RW, Ajijola OA, Irie T, Talebi R, Salavatian S, Vaseghi M, Bradfield JS, Armour JA, Ardell JL, Shivkumar K. Premature ventricular contraction coupling interval variability destabilizes cardiac neuronal and electrophysiological control: insights from simultaneous cardioneural mapping. Circ Arrhythm Electrophysiol 10: e004937, 2017. doi: 10.1161/CIRCEP.116.004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu YL, Mi X, Huang C, Wang HF, Song JR, Shu Q, Ni L, Chen JG, Wang F, Hu ZL. Multiple H+sensors mediate the extracellular acidification-induced [Ca2+]I elevation in cultured rat ventricular cardiomyocytes. Sci Rep 7: 44951, 2017. doi: 10.1038/srep44951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, Rubinstein J, Prieto AR, Thang LV, Wang DH. Transient receptor potential vanilloid gene deletion exacerbates inflammation and atypical cardiac remodeling after myocardial infarction. Hypertension 53: 243–250, 2009. doi: 10.1161/HYPERTENSIONAHA.108.118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurt CM, Lu Y, Stary CM, Piplani H, Small BA, Urban TJ, Qvit N, Gross GJ, Mochly-Rosen D, Gross ER. Transient receptor potential vanilloid 1 regulates mitochondrial membrane potential and myocardial reperfusion injury. J Am Heart Assoc 5: e003774, 2016. doi: 10.1161/JAHA.116.003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 113: 1344–1352, 2004. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kralios FA, Martin L, Burgess MJ, Millar K. Local ventricular repolarization changes due to sympathetic nerve-branch stimulation. Am J Physiol 228: 1621–1626, 1975. doi: 10.1152/ajplegacy.1975.228.5.1621. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Montell C. Forcing open TRP channels: mechanical gating as a unifying activation mechanism. Biochem Biophys Res Commun 460: 22–25, 2015. doi: 10.1016/j.bbrc.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma QP. Expression of capsaicin receptor (VR1) by myelinated primary afferent neurons in rats. Neurosci Lett 319: 87–90, 2002. doi: 10.1016/S0304-3940(01)02537-X. [DOI] [PubMed] [Google Scholar]
- 22.Millar CK, Kralios FA, Lux RL. Correlation between refractory periods and activation-recovery intervals from electrograms: effects of rate and adrenergic interventions. Circulation 72: 1372–1379, 1985. doi: 10.1161/01.CIR.72.6.1372. [DOI] [PubMed] [Google Scholar]
- 23.Moran MM, Szallasi A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol. In press. doi: 10.1111/bph.14044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura K, Ajijola OA, Aliotta E, Armour JA, Ardell JL, Shivkumar K. Pathological effects of chronic myocardial infarction on peripheral neurons mediating cardiac neurotransmission. Auton Neurosci 197: 34–40, 2016. doi: 10.1016/j.autneu.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nerdrum T, Baker DG, Coleridge HM, Coleridge JC. Interaction of bradykinin and prostaglandin E1 on cardiac pressor reflex and sympathetic afferents. Am J Physiol Endocrinol Physiol 250: R815–R822, 1986. doi: 10.1152/ajpregu.1986.250.5.R815. [DOI] [PubMed] [Google Scholar]
- 26.Nikolaidis LA, Hentosz T, Doverspike A, Huerbin R, Stolarski C, Shen Y-T, Shannon RP. Mechanisms whereby rapid RV pacing causes LV dysfunction: perfusion-contraction matching and NO. Am J Physiol Heart Circ Physiol 281: H2270–H2281, 2001. doi: 10.1152/ajpheart.2001.281.6.H2270. [DOI] [PubMed] [Google Scholar]
- 27.Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem 276: 11021–11030, 2001. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]
- 28.Pan HL, Chen SR. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation 110: 1826–1831, 2004. doi: 10.1161/01.CIR.0000142618.20278.7A. [DOI] [PubMed] [Google Scholar]
- 29.Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain 8: 263–272, 2007. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radio SJ, Zucker IH, Chen JS, Caruso RM, Wang W, McManus BM. Ischemic myocardial injury in rapidly paced dogs: contribution to ventricular dysfunction. Cardiovasc Pathol 1: 131–139, 1992. doi: 10.1016/1054-8807(92)90016-H. [DOI] [PubMed] [Google Scholar]
- 31.Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol 594: 321–341, 2016. doi: 10.1113/JP271165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez RJ, Ajijola OA, Zhou W, Holmström B, Lüning H, Laks MM, Shivkumar K, Mahajan A. A new electrocardiographic marker for sympathetic nerve stimulation: modulation of repolarization by stimulation of stellate ganglia. J Electrocardiol 44: 694–699, 2011. doi: 10.1016/j.jelectrocard.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Schultz HD, Ustinova EE. Capsaicin receptors mediate free radical-induced activation of cardiac afferent endings. Cardiovasc Res 38: 348–355, 1998. doi: 10.1016/S0008-6363(98)00031-5. [DOI] [PubMed] [Google Scholar]
- 34.Shin HC, Aggarwal V, Acharya S, Schieber MH, Thakor NV. Neural decoding of finger movements using Skellam-based maximum-likelihood decoding. IEEE Trans Biomed Eng 57: 754–760, 2010. doi: 10.1109/TBME.2009.2020791. [DOI] [PubMed] [Google Scholar]
- 35.Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, Esler M, De Ferrari G, Fishbein MC, Goldberger JJ, Harper RM, Joyner MJ, Khalsa SS, Kumar R, Lane R, Mahajan A, Po S, Schwartz PJ, Somers VK, Valderrabano M, Vaseghi M, Zipes DP. Clinical neurocardiology-defining the value of neuroscience-based cardiovascular therapeutics. J Physiol 594: 3911–3954, 2016. doi: 10.1113/JP271870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 30: 515–520, 1989. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 37.Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol 54: 764–776, 2009. doi: 10.1016/j.jacc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Uchida Y, Murao S. Bradykinin-induced excitation of afferent cardiac sympathetic nerve fibers. Jpn Heart J 15: 84–91, 1974. doi: 10.1536/ihj.15.84. [DOI] [PubMed] [Google Scholar]
- 39.Wang HJ, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent reflex control of cardiac function in normal and chronic heart failure states. J Physiol 595: 2519–2534, 2017. doi: 10.1113/JP273764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 64: 745–755, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Schultz HD, Ma R. Cardiac sympathetic afferent sensitivity is enhanced in heart failure. Am J Physiol Heart Circ Physiol 277: H812–H817, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Schultz HD, Ma R. Volume expansion potentiates cardiac sympathetic afferent reflex in dogs. Am J Physiol Heart Circ Physiol 280: H576–H581, 2001. doi: 10.1152/ajpheart.2001.280.2.H576. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Zucker IH. Cardiac sympathetic afferent reflex in dogs with congestive heart failure. Am J Physiol Regul Integr Comp Physiol 271: R751–R756, 1996. doi: 10.1152/ajpregu.1996.271.3.R751. [DOI] [PubMed] [Google Scholar]
- 44.Yamakawa K, Howard-Quijano K, Zhou W, Rajendran P, Yagishita D, Vaseghi M, Ajijola OA, Armour JA, Shivkumar K, Ardell JL, Mahajan A. Central vs. peripheral neuraxial sympathetic control of porcine ventricular electrophysiology. Am J Physiol Regul Integr Comp Physiol 310: R414–R421, 2016. doi: 10.1152/ajpregu.00252.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol 551: 515–523, 2003. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart Fail Clin 8: 87–99, 2012. doi: 10.1016/j.hfc.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]