Abstract
Our understanding of platelet function has traditionally focused on their roles in physiological hemostasis and pathological thrombosis, with the latter being causative of vessel occlusion and subsequent ischemic damage to various tissues. In particular, numerous in vivo studies have implicated causative roles for platelets in the pathogenesis of ischemia-reperfusion (I/R) injury to the myocardium. However, platelets clearly have more complex pathophysiological roles, particularly as a result of the heterogeneous nature of biologically active cargo secreted from their granules or contained within released microparticles or exosomes. While some of these released mediators amplify platelet activation and thrombosis through autocrine or paracrine amplification pathways, they can also regulate diverse cellular functions within the localized microenvironment and recruit progenitor cells to the damage site to facilitate repair processes. Notably, there is evidence to support cardioprotective roles for platelet mediators during I/R injury. As such, it is becoming more widely appreciated that platelets fulfill a host of physiological and pathological roles beyond our basic understanding. Therefore, the purpose of this perspective is to consider whether platelets, through their released mediators, can assume a paradoxically beneficial role to promote cardiac recovery after I/R injury.
Keywords: cardiac recovery, myocardial infarction, platelets, secretion
INTRODUCTION
A classical understanding of platelet biology sees these anucleate blood cells as physiological inhibitors of bleeding from healthy blood vessels (hemostasis) but pathological instigators of occlusive events in diseased blood vessels (thrombosis) leading to ischemic damage to multiple tissues, including the heart. Antiplatelet therapies, in particular aspirin and the P2Y12 receptor antagonist clopidogrel, have therefore been highly successful in the primary prevention and secondary management of patients with cardiovascular disease at risk of arterial thrombosis (6, 75). However, more recently, platelets have been functionally implicated in an extensive list of nonclassical roles in the body, ranging from physiological roles in tissue regeneration, lymphangiogenesis, and vascular integrity to pathological roles in tumor angiogenesis and metastasis (22, 42, 63). Therefore, the purpose of this perspective was to explore additional roles that platelets, beyond thrombosis-mediated ischemic damage, may assume within the myocardium after myocardial infarction (MI). Our proposition is that platelets, in particular through the release of bioactive cargo, have the capacity to substantially influence phenotypic responses within infiltrating inflammatory cells and resident cardiac cells: we suggest that this allows platelets to provide paradoxically beneficial roles in cardiac recovery after MI.
PLATELET SECRETION
Key to the functional heterogeneity of platelets is the release of a broad range of biomolecules, including over 300 proteins (comprising growth factors, chemokines, and adhesive ligands), nucleotides, and neurotransmitters. These are stored in three different classes of secretable granules [α-, dense, and lysosomal granules (7)] and are essential for classical platelet biology, acting in autocrine/paracrine manners to amplify platelet aggregation, consolidate thrombus formation, and facilitate clot remodelling. In a broader tissue context, however, these secreted ligands mediate heterocellular cross talk at the site of vascular damage and can also control the homing and differentiation of progenitor cells to facilitate tissue regeneration (56). An additional layer of complexity has been added through platelet microparticles/exosomes (PMP/Es), which represent the most abundant form of extracellular vesicles in blood (27). PMP/Es are released from activated platelets and are loaded with multiple bioactive molecules including proteins, lipids, and small noncoding mRNAs, in particular microRNAs (miRNAs): the latter have been shown to influence gene expression in a variety of different vascular cell types (32, 49).
PLATELETS IN CARDIAC INFLAMMATION AND RESOLUTION
After MI, the heart undergoes a robust inflammatory phase lasting 3–4 days (in mice) involving upregulation of inflammatory genes and immune cell infiltration into the myocardial interstitial space to remove damaged cardiomyocytes and other cardiac cells (47). It is well known that the recruitment, transendothelial migration, and activation of immune cells such as neutrophils and monocytes into the extravascular space are facilitated by secreted platelet cargo [including chemokine (C-X-C motif) ligand 4, chemokine (C-C motif) ligand 5, and histamine] and direct interactions with proteins expressed on the platelet surface [CD62P and glycoprotein (GP)Ibα] (Fig. 1) (15, 50, 52, 62). Importantly, it has been shown that platelets, along with leukocytes, also rapidly accumulate within the infarcted myocardium, which is consistent with their tissue accumulation in other diseases, while recent studies have demonstrated a novel migratory capacity of platelets (18, 33, 37). Additionally, numerous studies demonstrating causative roles for platelets in the pathogenesis of myocardial ischemia-reperfusion (I/R) injury have shown platelet-neutrophil complexes and neutrophil accumulation in the myocardium after MI, and immune cell recruitment during this period is thought to further exacerbate the extent of myocardial damage (12, 30, 68). Indeed, platelets actively contribute to inflammatory diseases, including atherosclerosis and rheumatoid arthritis, and an excessive cardiac inflammatory response in the days after MI would cause additional cellular damage and contractile dysfunction, increasing infarct size and causing aberrant cardiac remodelling (3, 26). On the other hand, an initial, acute period of controlled inflammation is fundamental for restoring tissue homeostasis, and there are numerous studies that have reported cardioprotective and prosurvival benefits of innate immune responses after MI, including a recent study (6) demonstrating worsened cardiac function after neutrophil depletion after MI (14, 24, 65). There are therefore mixed roles for the inflammatory response in damage resolution after MI. After the acute phase, the resolution of the inflammatory cascade is crucial, allowing for an effective switch in resident- and monocyte-derived M1 macrophages toward a reparative M2 macrophage phenotype (47). In this context, it is becoming better understood that platelets actively support inflammation resolution by releasing an abundance of proresolving mediators including lipoxin A4, maresin 1, and annexin A1, which attenuate neutrophil trafficking and enhance their apoptosis (1, 2, 40, 53). The clinical potential of lipoxin A4 and annexin A1 (NH2-terminal derived peptide) has been recently demonstrated in vivo, by restraining inflammatory processes during cerebral I/R injury through engagement of the Fpr2/3 receptor on neutrophils (61). Interestingly, maresin 1 can induce a proresolving phenotype from platelets by suppressing their release of proinflammatory mediators (34). This proresolving capacity of platelets is further evidenced in inflammatory models demonstrating elevated levels of proinflammatory cytokines in the absence of platelets (8, 67). This raises intriguing questions regarding the pro- and anti-inflammatory properties of platelets and how they may potentially regulate inflammatory cell “switching.” For example, are there distinct stimuli that facilitate differential release/secretory patterns from platelets either to activate or suppress inflammation? Undoubtedly, it will be crucial to understand if (and how) platelets, in particular through released factors, influence innate immune responses in cardiac tissue after MI and whether they can act synergistically or independently of other resident cardiac cells to regulate immune cell activation, resolution, and the subsequent transition to a reparative response. Defining these temporal, localized signaling cues within the cardiac microenvironment after MI is undoubtedly challenging but could reveal unsuspected roles for platelets and lead to attractive novel therapeutic targets in the management of cardiac recovery after MI.
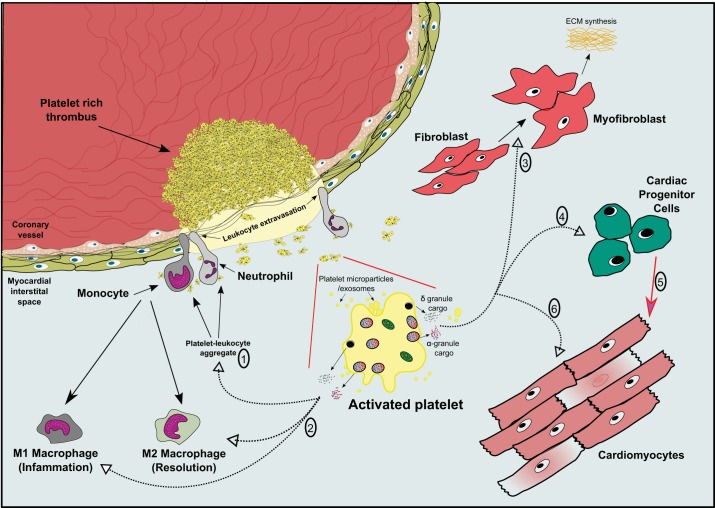
Fig. 1.
Roles for released platelet factors in inflammatory and reparative cell responses in cardiac tissue after myocardial infarction. After coronary thrombosis, activated platelets secrete granule components and release platelet microparticles/exosomes that regulate (1) inflammatory cell extravasation and accumulation in the myocardium and (2) influence the immuno-activatory responses of leukocytes, in particular neutrophils and monocytes/M1 macrophages, while also exerting immune-suppressive effects on these cells allowing M2 macrophage activity necessary for repair. Secreted platelet cargo and released platelet microparticles/exosomes can also modify resident cardiac cell responses, including (3) fibroblast activation and conversion to myofibroblasts to promote extracellular matrix (ECM) synthesis, while specific biomolecules known to be released from activated platelets have demonstrated roles in facilitating (4) cardiac progenitor cell proliferation, mobilization, and differentiation (5) toward cardiomyocytes to promote cardiac remodeling and repair. (6) Cardiomyocytes are also sensitive to released platelet molecules, which may enhance cardiomyocyte inotropic activity (adenine nucleotides and serotonin) but also provide protective/antiapoptotic signals (stromal cell-derived factor-1α/transforming growth factor-β1) during ischemic injury.
PLATELETS AND CARDIAC FIBROSIS
In addition to infiltrating immune cells, there are other critical, resident cardiac cells that respond to the MI insult. Cardiac fibroblasts are traditionally viewed as principally responsible for the laying down of the extracellular matrix after tissue injury, a response that is essential for providing structural and functional integrity to the myocardium (59). However, this leads to the replacement of lost cardiomyocytes with collagenous scar tissue. Numerous mediators of fibroblast activation have been identified, including serotonin, transforming growth factor (TGF)-β1 and platelet-derived growth factor, all of which are highly enriched in platelet granules, which trigger fibroblast expansion and transdifferentiation to myofibroblasts (Fig. 1) (44, 69). Indeed, platelet-derived TGF-β1 has been specifically implicated in cardiac fibrosis and dysfunction after pressure overload, with a corresponding increase in plasma TGF-β1 derived from platelets (39). Prolonged induction of fibroblast activation leads to cardiac fibrosis and adverse remodeling, which can spread into the noninfarcted myocardium. Therefore, knowledge of endogenous inhibitory signals to control fibroblast activity would be of great therapeutic benefit as current antifibrotic strategies target activatory signals that reciprocally facilitate tissue repair (35). Thrombospondin-1 (TSP-1), a protein that is highly abundant in platelet α-granules, has been shown to negatively regulate myofibroblast density and infiltration into noninfarcted areas and also to suppress prolonged post-MI inflammatory responses (17, 66). Considering the high abundance of platelet TSP-1 relative to other tissues (29), it would be interesting to assess the direct contribution of platelet-derived TSP-1 to cardiac fibrosis with conditional knockout mice. Numerous miRNAs have been implicated in myocardial fibrosis, some of which are highly enriched in PMP/Es and can positively (miRNA-21 and miRNA-199) or negatively (miRNA-29 and miRNA-101) regulate fibrotic responses in cardiac tissue (9, 25, 45, 55). Crucially, there is experimental evidence to support the transfer of genetic material (including miRNAs) via microvesicles from other resident and nonresident cardiac cells to influence cardiac fibrosis (21, 70). Considering the abundance of PMP/Es contained by platelets, it is plausible to suggest that activated platelets could exert such heterologous cellular responses. However, given that PMP/Es (and platelet granules) possess both pro- and antifibrotic modulators, there would need to be greater complexity of control to allow fibroblasts to discriminate between opposing signals. A similar paradigm exists within the context of platelet-mediated regulation of angiogenesis and vasculogenesis, where platelets store and secrete an array of both pro- and antiangiogenic proteins from α-granules. At this time, it remains a contentious issue within the platelet field as to whether such functionally opposing proteins are differentially secreted from distinct α-granule populations or whether their release is more a stochastic process influenced by the nature of the stimulus and chemical properties of the protein (28).
CARDIAC PROGENITOR CELL MODULATION BY PLATELETS
It has become widely appreciated that miRNAs play pivotal roles in the cardiovascular system. Endogenously, they regulate numerous vascular cells (endothelial, smooth muscle, and immune cells as well as platelets) but also resident fibroblasts, cardiomyocytes, and cardiac progenitor cells (CPCs) (55). Manipulating miRNA activity or expression is considered an attractive therapeutic target, particularly within the context of cardiac regeneration (23). Targeting CPCs to trigger their differentiation to cardiomyocytes (Fig. 1) or the “reactivation” of proliferation within cardiomyocytes to replace the cells lost during MI are some of the proposed therapeutic avenues, both of which are regulated by miRNAs but also by locally transduced mechanical and biochemical stimuli (38). Platelets also store and release a spectrum of miRNAs, including those where both positive (miRNA-199) and negative (miRNA-29) influences on cardiomyocyte cell cycle reentry have been reported (4, 16). Furthermore, miRNA-1, which is also contained within PMP/Es, has been shown to regulate CPC differentiation toward the cardiomyocyte lineage, whereas chemokine stromal cell-derived factor-1α, which is secreted from platelet α-granules, also facilitates CPC mobilization and transdifferentiation (5, 54, 58). miRNA-126, which was originally believed to be an endothelium-specific miRNA, has been confirmed by several independent groups to be one of the most abundant miRNAs in PMP/Es, and plasma levels of miRNA-126 appear to correlate with platelet activation (10, 57). Notably cardio- and atheroprotective functions have been ascribed to miRNA-126, and it has also been shown to regulate ischemia-induced angiogenesis (46, 51, 60). Currently, there is no evidence supporting PMP/E uptake by cardiac cells, but there are a number of in vitro reports demonstrating functionally relevant miRNA transfer from PMP/Es to endothelial cells and monocytes, with additional evidence supporting their release during MI (20, 32, 49). Undoubtedly, further in vitro and in vivo studies are warranted to determine if (and how) PMP/Es alter resident cardiac cell fate and whether this would confer protective or adverse consequences for cardiac recovery after MI.
MODULATION OF CARDIOMYOCYTE FUNCTION BY PLATELETS
Outlined above are possible routes of communication between platelets and other vascular and cardiac cells. There are, however, several lines of evidence to suggest that platelets and their “secretome” can affect cardiomyocyte function independent of their role in occlusive coronary thrombosis and ischemia. From a pathophysiological context, numerous small signaling molecules that are released from platelet-dense granules (ADP/ATP, histamine, and serotonin) and thromboxane A2 can exert positive inotropic effects on cardiomyocytes (11). There have been a number of reports that have indirectly implicated platelets in exacerbating ischemia-induced ventricular fibrillation, while a more recent publication by Dhanjal et al. (13) provided evidence for a direct role in increasing the incidence of ventricular fibrillation (31). Interestingly, coronary artery ligation in Unc13djinx mice, in which platelets do not secrete dense granule cargo, has no protective effect on infarct size, which argues against a significant role for dense granule secretion in the pathophysiology of myocardial injury after MI (43). On the other hand, several publications have supported a cardioprotective effect of platelet-released factors during myocardial I/R injury, including adenine nucleotides, serotonin, and thromboxane A2, although these effects appear to be indirectly mediated by an intact endothelium (71–73). Similarly, recently published work from our group has further supported this cardioprotective capacity of secreted platelet factors during myocardial I/R injury (64). In this case, the protective effect on ventricular cardiomyocytes during ischemia was directly mediated by cargo actively secreted from platelet α-granules, including stromal cell-derived factor-1α and TGF-β1, and, importantly, this effect was substantially attenuated when platelets were pretreated with a P2Y12 antagonist. Given that P2Y12 antagonists are commonly administered to patients with MI, this observation may have implications for the clinical utility of these drugs during the early recovery phase of MI. It is also well established that platelets, through secreted molecules and PMP/Es, exert both pro- and antiapoptotic effects on different target cells (19). However, similar to the pro- and antiangiogenic capabilities of platelets, the mechanisms differentiating these opposing effects are not well understood but may reflect the relative expression levels of the respective death/survival receptors on target cells.
CONCLUSIONS
Considering the diverse heterogeneity of the platelet secretome and PMP/Es, it is anticipated that future experimental work will uncover additional roles for released platelet factors on the various resident and nonresident cardiac cells in the acute (hours/days) and chronic (weeks) phases after MI. It is our assertion that platelets have the capacity to negate some of the deleterious consequences of coronary thrombosis by providing favorable paracrine mediators to initiate or facilitate cardiac repair processes, as directly evidenced by studies in other tissues including the liver and lungs (19, 36, 48). However, teasing out the relative contribution of platelets in an in vivo context after MI is challenging, as interfering with platelet activation and thus secretion/PMP/E release, would be likely to reduce myocardial damage after coronary thrombosis and therefore skew subsequent interpretations about roles for platelets in cardiac recovery. However, with the current, nonthrombotic model of myocardial I/R injury induced by coronary artery ligation, there have been some reports of comparable infarct rates in mice with markedly reduced platelet activation, including the study using Unc13djinxmice mentioned above (41, 43, 74). While these responses presumably relate to the nature of the model, it would be interesting to follow up longer-term studies in conditional (PF4/GPIbα-Cre) transgenic mice with specific defects in platelet secretion or PMP/E release to monitor the post-MI responses of the various cardiac cells discussed. In conclusion, platelets are continually pushing the boundaries in terms of functional diversity, particularly through the biomolecules they release. While roles for platelets in coronary thrombosis and subsequent cardiac damage are undeniably established, there is sufficient credible evidence, as outlined in this perspective, to imply a “double-edged sword” functionality of platelets promoting cardiac recovery after MI. Furthermore, this raises intriguing questions regarding the efficacy of antiplatelet therapies, as they interfere with the release of platelet paracrine mediators and have the potential, paradoxically, to adversely impact cardiac recovery. Further work will be required to understand these complexities.
GRANTS
This work was funded by British Heart Foundation Grant RG/15/16/31758 (to A. W. Poole).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.G.W. and A.W.P. conceived and designed research; T.G.W. drafted manuscript; T.G.W. and A.W.P. edited and revised manuscript; A.W.P. approved final version of manuscript.
REFERENCES
- 1.Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci USA 111: 16526–16531, 2014. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 16: 51–67, 2016. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327: 580–583, 2010. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao X, Wang J, Wang Z, Du J, Yuan X, Huang W, Meng J, Gu H, Nie Y, Ji B, Hu S, Zheng Z. MicroRNA profiling during rat ventricular maturation: a role for miR-29a in regulating cardiomyocyte cell cycle re-entry. FEBS Lett 587: 1548–1555, 2013. doi: 10.1016/j.febslet.2013.01.075. [DOI] [PubMed] [Google Scholar]
- 5.Chen D, Xia Y, Zuo K, Wang Y, Zhang S, Kuang D, Duan Y, Zhao X, Wang G. Crosstalk between SDF-1/CXCR4 and SDF-1/CXCR7 in cardiac stem cell migration. Sci Rep 5: 16813, 2015. doi: 10.1038/srep16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group . Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 366: 1607–1621, 2005. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 7.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 103: 2096–2104, 2004. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 8.Corken A, Russell S, Dent J, Post SR, Ware J. Platelet glycoprotein Ib-IX as a regulator of systemic inflammation. Arterioscler Thromb Vasc Biol 34: 996–1001, 2014. doi: 10.1161/ATVBAHA.113.303113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Costa Martins PA, Salic K, Gladka MM, Armand AS, Leptidis S, el Azzouzi H, Hansen A, Coenen-de Roo CJ, Bierhuizen MF, van der Nagel R, van Kuik J, de Weger R, de Bruin A, Condorelli G, Arbones ML, Eschenhagen T, De Windt LJ. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol 12: 1220–1227, 2010. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 10.de Boer HC, van Solingen C, Prins J, Duijs JM, Huisman MV, Rabelink TJ, van Zonneveld AJ. Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur Heart J 34: 3451–3457, 2013. doi: 10.1093/eurheartj/eht007. [DOI] [PubMed] [Google Scholar]
- 11.de Jong JS, Dekker LR. Platelets and cardiac arrhythmia. Front Physiol 1: 166, 2010. doi: 10.3389/fphys.2010.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devanathan V, Hagedorn I, Köhler D, Pexa K, Cherpokova D, Kraft P, Singh M, Rosenberger P, Stoll G, Birnbaumer L, Piekorz RP, Beer-Hammer S, Nieswandt B, Nürnberg B. Platelet Gi protein Gαi2 is an essential mediator of thrombo-inflammatory organ damage in mice. Proc Natl Acad Sci USA 112: 6491–6496, 2015. doi: 10.1073/pnas.1505887112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhanjal TS, Medina RA, Leem J, Clark JE, Southworth R, Curtis MJ. Trapped platelets activated in ischemia initiate ventricular fibrillation. Circ Arrhythm Electrophysiol 6: 995–1001, 2013. doi: 10.1161/CIRCEP.113.000591. [DOI] [PubMed] [Google Scholar]
- 14.Dong JW, Vallejo JG, Tzeng HP, Thomas JA, Mann DL. Innate immunity mediates myocardial preconditioning through Toll-like receptor 2 and TIRAP-dependent signaling pathways. Am J Physiol Heart Circ Physiol 298: H1079–H1087, 2010. doi: 10.1152/ajpheart.00306.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M, Idzko M, Bode C, Wagner DD. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 121: 1008–1015, 2013. doi: 10.1182/blood-2012-06-437392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492: 376–381, 2012. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 17.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation 111: 2935–2942, 2005. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 18.Gaertner F, Ahmad Z, Rosenberger G, Fan S, Nicolai L, Busch B, Yavuz G, Luckner M, Ishikawa-Ankerhold H, Hennel R, Benechet A, Lorenz M, Chandraratne S, Schubert I, Helmer S, Striednig B, Stark K, Janko M, Bottcher RT, Verschoor A, Leon C, Gachet C, Gudermann T, Mederos YSM, Pincus Z, Iannacone M, Haas R, Wanner G, Lauber K, Sixt M, Massberg S. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell 171: 1368–1382.e23, 2017. doi: 10.1016/j.cell.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood 122: 2550–2554, 2013. doi: 10.1182/blood-2013-05-468694. [DOI] [PubMed] [Google Scholar]
- 20.Gidlof O, van der Brug M, Ohman J, Gilje P, Olde B, Wahlestedt C, Erlinge D.. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood 121: 3908−3917, S1−S26, 2013. doi: 10.1182/blood-2012-10-461798. [DOI] [PubMed] [Google Scholar]
- 21.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res 116: 255–263, 2015. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho-Tin-Noé B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost 9, Suppl 1: 56–65, 2011. doi: 10.1111/j.1538-7836.2011.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgkinson CP, Kang MH, Dal-Pra S, Mirotsou M, Dzau VJ. MicroRNAs and cardiac regeneration. Circ Res 116: 1700–1711, 2015. doi: 10.1161/CIRCRESAHA.116.304377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 38: 187–197, 2017. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Xiao X, Yang Y, Mishra A, Liang Y, Zeng X, Yang X, Xu D, Blackburn MR, Henke CA, Liu L. MicroRNA-101 attenuates pulmonary fibrosis by inhibiting fibroblast proliferation and activation. J Biol Chem 292: 16420–16439, 2017. doi: 10.1074/jbc.M117.805747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med 9: 61–67, 2003. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 27.Italiano JE Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol 17: 578–584, 2010. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi S, Whiteheart SW. The nuts and bolts of the platelet release reaction. Platelets 28: 129–137, 2017. doi: 10.1080/09537104.2016.1240768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, Thomas JK, Muthusamy B, Leal-Rojas P, Kumar P, Sahasrabuddhe NA, Balakrishnan L, Advani J, George B, Renuse S, Selvan LD, Patil AH, Nanjappa V, Radhakrishnan A, Prasad S, Subbannayya T, Raju R, Kumar M, Sreenivasamurthy SK, Marimuthu A, Sathe GJ, Chavan S, Datta KK, Subbannayya Y, Sahu A, Yelamanchi SD, Jayaram S, Rajagopalan P, Sharma J, Murthy KR, Syed N, Goel R, Khan AA, Ahmad S, Dey G, Mudgal K, Chatterjee A, Huang TC, Zhong J, Wu X, Shaw PG, Freed D, Zahari MS, Mukherjee KK, Shankar S, Mahadevan A, Lam H, Mitchell CJ, Shankar SK, Satishchandra P, Schroeder JT, Sirdeshmukh R, Maitra A, Leach SD, Drake CG, Halushka MK, Prasad TS, Hruban RH, Kerr CL, Bader GD, Iacobuzio-Donahue CA, Gowda H, Pandey A. A draft map of the human proteome. Nature 509: 575–581, 2014. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Köhler D, Straub A, Weissmüller T, Faigle M, Bender S, Lehmann R, Wendel HP, Kurz J, Walter U, Zacharowski K, Rosenberger P. Phosphorylation of vasodilator-stimulated phosphoprotein prevents platelet-neutrophil complex formation and dampens myocardial ischemia-reperfusion injury. Circulation 123: 2579–2590, 2011. doi: 10.1161/CIRCULATIONAHA.110.014555. [DOI] [PubMed] [Google Scholar]
- 31.Koltai M, Tosaki A, Hosford D, Esanu A, Braquet P. Effect of BN 50739, a new platelet activating factor antagonist, on ischaemia induced ventricular arrhythmias in isolated working rat hearts. Cardiovasc Res 25: 391–397, 1991. doi: 10.1093/cvr/25.5.391. [DOI] [PubMed] [Google Scholar]
- 32.Laffont B, Corduan A, Plé H, Duchez AC, Cloutier N, Boilard E, Provost P. Activated platelets can deliver mRNA regulatory Ago2·microRNA complexes to endothelial cells via microparticles. Blood 122: 253–261, 2013. doi: 10.1182/blood-2013-03-492801. [DOI] [PubMed] [Google Scholar]
- 33.Langer HF, Choi EY, Zhou H, Schleicher R, Chung KJ, Tang Z, Göbel K, Bdeir K, Chatzigeorgiou A, Wong C, Bhatia S, Kruhlak MJ, Rose JW, Burns JB, Hill KE, Qu H, Zhang Y, Lehrmann E, Becker KG, Wang Y, Simon DI, Nieswandt B, Lambris JD, Li X, Meuth SG, Kubes P, Chavakis T. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ Res 110: 1202–1210, 2012. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lannan KL, Spinelli SL, Blumberg N, Phipps RP. Maresin 1 induces a novel pro-resolving phenotype in human platelets. J Thromb Haemost 15: 802–813, 2017. doi: 10.1111/jth.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106: 1675–1680, 2010. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 36.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science 312: 104–107, 2006. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Gao XM, Fang L, Jennings NL, Su Y, Q X, Samson AL, Kiriazis H, Wang XF, Shan L, Sturgeon SA, Medcalf RL, Jackson SP, Dart AM, Du XJ. Novel role of platelets in mediating inflammatory responses and ventricular rupture or remodeling following myocardial infarction. Arterioscler Thromb Vasc Biol 31: 834–841, 2011. doi: 10.1161/ATVBAHA.110.220467. [DOI] [PubMed] [Google Scholar]
- 38.Mauretti A, Spaans S, Bax NAM, Sahlgren C, Bouten CVC. Cardiac Progenitor Cells and the Interplay with Their Microenvironment. Stem Cells Int 2017: 7471582, 2017. doi: 10.1155/2017/7471582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer A, Wang W, Qu J, Croft L, Degen JL, Coller BS, Ahamed J. Platelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood 119: 1064–1074, 2012. doi: 10.1182/blood-2011-09-377648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy CT, Peers SH, Forder RA, Flower RJ, Carey F, Westwick J. Evidence for the presence and location of annexins in human platelets. Biochem Biophys Res Commun 189: 1739–1746, 1992. doi: 10.1016/0006-291X(92)90279-T. [DOI] [PubMed] [Google Scholar]
- 41.Nanhwan MK, Ling S, Kodakandla M, Nylander S, Ye Y, Birnbaum Y. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase-2-dependent effect. Arterioscler Thromb Vasc Biol 34: 2078–2085, 2014. doi: 10.1161/ATVBAHA.114.304002. [DOI] [PubMed] [Google Scholar]
- 42.Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59: 1295–1300, 1999. [PubMed] [Google Scholar]
- 43.Pachel C, Mathes D, Arias-Loza AP, Heitzmann W, Nordbeck P, Deppermann C, Lorenz V, Hofmann U, Nieswandt B, Frantz S. Inhibition of platelet GPVI protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 36: 629–635, 2016. doi: 10.1161/ATVBAHA.115.305873. [DOI] [PubMed] [Google Scholar]
- 44.Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, Deuel TF. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol 109: 429–440, 1989. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plé H, Landry P, Benham A, Coarfa C, Gunaratne PH, Provost P. The repertoire and features of human platelet microRNAs. PLoS One 7: e50746, 2012. doi: 10.1371/journal.pone.0050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potus F, Ruffenach G, Dahou A, Thebault C, Breuils-Bonnet S, Tremblay È, Nadeau V, Paradis R, Graydon C, Wong R, Johnson I, Paulin R, Lajoie AC, Perron J, Charbonneau E, Joubert P, Pibarot P, Michelakis ED, Provencher S, Bonnet S. Downregulation of microRNA-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation 132: 932–943, 2015. doi: 10.1161/CIRCULATIONAHA.115.016382. [DOI] [PubMed] [Google Scholar]
- 47.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119: 91–112, 2016. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rafii S, Cao Z, Lis R, Siempos II, Chavez D, Shido K, Rabbany SY, Ding BS. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat Cell Biol 17: 123–136, 2015. doi: 10.1038/ncb3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Risitano A, Beaulieu LM, Vitseva O, Freedman JE. Platelets and platelet-like particles mediate intercellular RNA transfer. Blood 119: 6288–6295, 2012. doi: 10.1182/blood-2011-12-396440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation 106: 1523–1529, 2002. doi: 10.1161/01.CIR.0000028590.02477.6F. [DOI] [PubMed] [Google Scholar]
- 51.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 20: 368–376, 2014. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuhmann MK, Guthmann J, Stoll G, Nieswandt B, Kraft P, Kleinschnitz C. Blocking of platelet glycoprotein receptor Ib reduces “thrombo-inflammation” in mice with acute ischemic stroke. J Neuroinflammation 14: 18, 2017. doi: 10.1186/s12974-017-0792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serhan CN, Sheppard KA. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J Clin Invest 85: 772–780, 1990. doi: 10.1172/JCI114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sluijter JP, van Mil A, van Vliet P, Metz CH, Liu J, Doevendans PA, Goumans MJ. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscler Thromb Vasc Biol 30: 859–868, 2010. doi: 10.1161/ATVBAHA.109.197434. [DOI] [PubMed] [Google Scholar]
- 55.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336–342, 2011. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, Seizer P, Kraemer BF, Siegel-Axel D, May AE, Lindemann S, Gawaz M. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation 117: 206–215, 2008. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 57.Sunderland N, Skroblin P, Barwari T, Huntley RP, Lu R, Joshi A, Lovering RC, Mayr M. MicroRNA Biomarkers and Platelet Reactivity: The Clot Thickens. Circ Res 120: 418–435, 2017. doi: 10.1161/CIRCRESAHA.116.309303. [DOI] [PubMed] [Google Scholar]
- 58.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci USA 105: 1668–1673, 2008. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res 118: 1021–1040, 2016. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Solingen C, Seghers L, Bijkerk R, Duijs JM, Roeten MK, van Oeveren-Rietdijk AM, Baelde HJ, Monge M, Vos JB, de Boer HC, Quax PH, Rabelink TJ, van Zonneveld AJ. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med 13, 8A: 1577–1585, 2009. doi: 10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vital SA, Becker F, Holloway PM, Russell J, Perretti M, Granger DN, Gavins FN. Formyl-peptide receptor 2/3/lipoxin A4 receptor regulates neutrophil-platelet aggregation and attenuates cerebral inflammation: impact for therapy in cardiovascular disease. Circulation 133: 2169–2179, 2016. doi: 10.1161/CIRCULATIONAHA.115.020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res 100: 27–40, 2007. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 63.Walsh TG, Metharom P, Berndt MC. The functional role of platelets in the regulation of angiogenesis. Platelets 26: 199–211, 2015. doi: 10.3109/09537104.2014.909022. [DOI] [PubMed] [Google Scholar]
- 64.Walsh TG, Poole AW. Platelets Protect Cardiomyocytes from Ischaemic Damage. TH Open 1: 24–30, 2017. doi: 10.1055/s-0037-1603928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113: 1004–1012, 2013. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 58: 902–911, 2011. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiang B, Zhang G, Guo L, Li XA, Morris AJ, Daugherty A, Whiteheart SW, Smyth SS, Li Z. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat Commun 4: 2657, 2013. doi: 10.1038/ncomms3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Y, Huo Y, Toufektsian MC, Ramos SI, Ma Y, Tejani AD, French BA, Yang Z. Activated platelets contribute importantly to myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 290: H692–H699, 2006. doi: 10.1152/ajpheart.00634.2005. [DOI] [PubMed] [Google Scholar]
- 69.Yabanoglu S, Akkiki M, Seguelas MH, Mialet-Perez J, Parini A, Pizzinat N. Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J Mol Cell Cardiol 46: 518–525, 2009. doi: 10.1016/j.yjmcc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 70.Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, Tanaka M, Osada-Oka M, Shimada K, Miura K, Yoshiyama M, Iwao H. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol 178: 239–246, 2015. doi: 10.1016/j.ijcard.2014.10.144. [DOI] [PubMed] [Google Scholar]
- 71.Yang BC, Mehta A, Mehta JL. Cardioprotective effects of platelets against ischaemia-reperfusion injury are related in part to platelet glutathione redox cycle. Cardiovasc Res 28: 1586–1593, 1994. doi: 10.1093/cvr/28.10.1586. [DOI] [PubMed] [Google Scholar]
- 72.Yang BC, Mehta JL. Platelet-derived adenosine contributes to the cardioprotective effects of platelets against ischemia-reperfusion injury in isolated rat heart. J Cardiovasc Pharmacol 24: 779–785, 1994. doi: 10.1097/00005344-199424050-00013. [DOI] [PubMed] [Google Scholar]
- 73.Yang BC, Virmani R, Nichols WW, Mehta JL. Platelets protect against myocardial dysfunction and injury induced by ischemia and reperfusion in isolated rat hearts. Circ Res 72: 1181–1190, 1993. doi: 10.1161/01.RES.72.6.1181. [DOI] [PubMed] [Google Scholar]
- 74.Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol 35: 1805–1814, 2015. doi: 10.1161/ATVBAHA.115.305655. [DOI] [PubMed] [Google Scholar]
- 75.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345: 494–502, 2001. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]