Abstract
Accumulating evidence indicates that maternal high-fat diet (HFD) is associated with metabolic syndrome and cardiovascular disease in adult offspring. The present study tested the hypothesis that maternal HFD modulates the brain renin-angiotensin system (RAS), oxidative stress, and proinflammatory cytokines that alter angiotensin II (ANG II) and TNF-α actions and sensitize the ANG II-elicited hypertensive response in adult offspring. All offspring were cross fostered by dams on the same or opposite diet to yield the following four groups: offspring from normal-fat control diet-fed dams suckled by control diet-fed dams (OCC group) or by HFD-fed dams (OCH group) and offspring from HFD-fed dams fed a HFD suckled by control diet-fed dams (OHC group) or by HFD-fed dams (OHH group). RT-PCR analyses of the lamina terminalis and paraventricular nucleus indicated upregulation of mRNA expression of several RAS components, NADPH oxidase, and proinflammatory cytokines in 10-wk-old male offspring of dams fed a HFD during either pregnancy, lactation, or both (OHC, OCH, and OHH groups). These offspring also showed decreased cardiac baroreflex sensitivity and increased pressor responses to intracerebroventricular microinjection of either ANG II or TNF-α. Furthermore, chronic systemic infusion of ANG II resulted in enhanced upregulation of mRNA expression of RAS components, NADPH oxidase, and proinflammatory cytokines in the lamina terminalis and paraventricular nucleus and an augmented hypertensive response in the OHC, OCH, and OHH groups compared with the OCC group. The results suggest that maternal HFD blunts cardiac baroreflex function and enhances pressor responses to ANG II or proinflammatory cytokines through upregulation of the brain RAS, oxidative stress, and inflammation.
NEW & NOTEWORTHY The results of our study indicate that a maternal high-fat diet during either pregnancy or lactation is sufficient for perinatal programming of sensitization for hypertension, which is associated with hyperreactivity of central cardiovascular nuclei that, in all likelihood, involves elevated expression of the renin-angiotensin system, NADPH oxidase, and proinflammatory cytokines. The present study demonstrates, for the first time, the central mechanism underlying maternal high-fat diet sensitization of the hypertensive response in adult offspring.
Keywords: blood pressure, cardiac baroreflex function, inflammation, maternal high-fat diet, renin-angiotensin system
INTRODUCTION
As nutritional excess is prevalent worldwide, the adverse effects of maternal obesity and high-fat diet (HFD) intake on offspring health has drawn much attention in recent years (1, 7). Evidence from cohort studies indicates that maternal obesity in humans is associated with increased risk of metabolic disorders and cardiovascular diseases such as hypertension (10, 25). These human data are supported by results from preclinical studies using animal models of obesity. Maternal HFD feeding in mice and rats has shown permanent detrimental effects on body composition and metabolism in the offspring, predisposing them to metabolic syndrome and elevated blood pressure (BP) throughout the lifespan of the offspring (6, 8, 11, 16, 24, 26–28). Although the programming effects of maternal obesity and HFD for offspring are generally well characterized, particularly in these animal models, the exact mechanisms underlying these adverse effects of maternal obesity and HFD on the development of cardiovascular diseases in the offspring remain unclear.
It has been shown that obesity or HFD causes chronic low-grade inflammation, activates the renin-angiotensin system (RAS), and enhances oxidative stress, thereby resulting in sympathetic overactivity and hypertension (12). Similarly, studies on experimental animals have investigated whether there is a causal relationship between these adverse factors and maternal obesity- and HFD-induced cardiovascular and metabolic disorders in the offspring. Desai and colleagues (6, 11) reported that maternal obesity and HFD programmed rat offspring metabolic syndrome and hypertension, which was accompanied by the enhanced expression of several RAS components in the adiposity. Prior et al. (24) demonstrated that the offspring of rabbits fed a HFD had markedly elevated renal sympathetic nerve activity (RSNA) and pressor responses to central leptin and enhanced sympathetic responses to ghrelin compared with offspring from mothers fed a normal-fat control diet. They further observed an exaggerated sympathetic response to acute air jet stress, suggesting that the higher levels of BP and RSNA may be attributable to changes in central pathways regulating sympathetic nervous system activity (24). Recent studies have also indicated that there is an association among maternal HFD, hypothalamic inflammation, and metabolic alterations in the offspring (4, 5).
In the brain, structures associated with the lamina terminalis (LT) and hypothalamic paraventricular nucleus (PVN) have been demonstrated to be sites that sense and process humoral signals related to cardiovascular and metabolic regulation, thereby modulating metabolism, sympathetic activity, and BP (14, 15, 22). Samuelsson et al. (28) reported that the melanocortin-4 receptor in the PVN is required for early life programming of hypertension arising from either maternal obesity or neonatal hyperleptinemia. Early life exposure of the PVN to maternal obesity through postnatal elevation of leptin may have long-term consequences for cardiovascular health (28). Despite evidence showing elevated BP and sympathetic hyperreactivity in the offspring of mothers fed a HFD, there are no studies that have investigated the regulatory roles of the brain RAS, NADPH oxidase, and proinflammatory cytokines in mediating programmed obesity-induced hypertension.
A previous study by Xue et al. (31) showed that maternal hypertension during pregnancy induces enhanced hypertensive response to angiotensin II (ANG II) and is associated with alterations in the brain RAS and proinflammatory cytokines. The present study tested the hypothesis that maternal HFD may also alter brain RAS activity and increase oxidative stress and proinflammatory cytokines contributing altered central ANG II and proinflammatory cytokine sensitivity as well as sensitizing the ANG II-elicited hypertensive response in adult offspring. Regarding the mixed effect of maternal obesity and maternal HFD (26), we investigated the specific influence of maternal HFD on offspring autonomic function, in which female rats were fed a HFD for a limited period before pregnancy and throughout either pregnancy, lactation, or both periods. In conscious adult (10-wk-old) offspring, we investigated cardiac baroreflex function and the autonomic responsiveness of BP and heart rate (HR) to the challenges of acute intracerebroventricular ANG II or TNF-α. Expression of the sensitized hypertensive response to chronic ANG II was also studied in adult offspring while alterations of mRNA expression of RAS components, NADPH oxidase, and proinflammatory cytokines in the LT and PVN were assessed in the offspring as adults before and after systemic ANG II infusion.
METHODS
Animals.
Thirty-two female rats and thirty-two male rats (Sprague-Dawley, 10 wk old) were purchased from Beijing Laboratory Animal Research Center (Beijing, China) and were used for breeding. Parents and offspring were maintained at an animal facility under barrier-sustained conditions with a 12:12-h light-dark cycle under standard ambient conditions (temperature: 23 ± 2°C and relative humidity: 40–80%) and with free access to standard rat chow and water. Beginning 2 wk before mating and continuing until weaning of the offspring at 21 days postpartum, female rats were given either a normal fat control diet (10% calories from lard, 3.85 kcal/g, catalog no. 1032, HFK Bioscience, Beijing, China) or HFD (60% calories from lard, 5.24 kcal/g, catalog no. H10060, HFK Bioscience). Maternal body weights were recorded every 3 days. After parturition, all offspring were cross fostered to dams on either the same or opposite type of diet. This yielded the following four groups: offspring from control diet-fed dams suckled by control diet-fed dams (OCC rats) or by HFD-fed dams (OCH rats) and offspring from HFD-fed dams fed HFD suckled by control diet-fed dams (OHC rats) or by HFD-fed dams (OHH rats). The offspring were weighed and counted at birth, and litter sizes during suckling were then reduced to nine pups. All offspring were weaned onto the control diet at 3 wk of age and weighed 1 time/wk until the experiments began. Each experimental group was composed of individual rats that were randomly selected from different litters. Male offspring were used in all experiments.
At 10 wk of age, male offspring from all groups of dams were deeply anesthetized with isoflurane and then decaptated. Brains were collected for analyses of mRNA expression of RAS components, NADPH oxidase, and proinflammatory cytokines (n = 5 rats/group). The structures lying along the LT [i.e., the subfornical organ (SFO), median preoptic nucleus (MnPO), and organum vasculosum of the LT (OVLT)] and PVN were used for these analyses.
In separate functional experiments, at 10 wk of age, male offspring of all groups were used to determine whether maternal HFD during pregnancy, lactation or both periods had influences on cardiac baroreflex function, the pressor response to acute intracerebroventricular injection of ANG II or TNF-α, and the hypertensive response to chronic systemic infusion of ANG II. Upon completion of the experiments, the offspring were anesthetized with isoflurane and brains were harvested. All tissues were immediately frozen in liquid nitrogen and stored at −80°C. Brains were analyzed for mRNA expression of RAS components, NADPH oxidase, and proinflammatory cytokines in the LT and PVN.
All animal procedures were reviewed and approved by the Hebei North University Institutional Animal Care and Use Committee and conformed with United States National Institutes of Health guidelines.
Evaluation of cardiac baroreflexes.
Male offspring from all groups of dams (n = 6 rats/group) were used to evaluate the cardiac baroreflexes. At 9 wk of age, arterial and venous catheters were chronically implanted into the femoral artery and vein for the measurement of BP and administration of drugs, respectively. After 3 days of recovery, BP was measured with a ML880 BP transducer and continuously recorded using a Powerlab data-acquisition system (Chart version 7.2, AD Instruments) in conscious rats. Cardiac baroreflexes were evoked by increasing BP with ramp infusions of phenylephrine (1.0 mg/ml) and by lowering BP with sodium nitroprusside (SNP; 2.0 mg/ml). Baroreflex sensitivity was estimated by calculating the slope of regression lines relating changes in BP and changes in HR during administration of the vasoactive agents.
Intracerebroventricular cannula implantation and evaluation of the effects of acute microinjection of ANG II or TNF-α.
At 9 wk of age, offspring were anesthetized [pentobarbital sodium (50 mg/kg ip)] and arterial catheters were implanted into the femoral artery for the measurement of BP. At the same time, an intracerebroventricular cannula was also implanted into right lateral ventricle (the coordinates were 1.0 mm caudal, 1.5 mm lateral to the bregma, and 4.5 mm below the skull surface) for acute bolus microinjections of vehicle (saline, 2 µl), ANG II (100 ng), or TNF-α (200 ng).
Telemetry probe and osmotic pump implantations.
In separate functional experiments, male offspring of all groups of dams were used to determine whether maternal HFD during pregnancy, lactation, or both sensitized the hypertensive response to a slow pressor dose of ANG II. At 8 wk of age, the offspring were instrumented with telemetry probes (TA11PA-C40, Data Sciences, St. Paul, MN) through the femoral artery for monitoring of BP and HR and then infused with a slow pressor dose of ANG II for 2 wk (120 ng·kg−1·min−1, model 2002, Alzet). This experiment included the following four groups (n = 6 rats/group): 1) OCC + ANG II, 2) OHC + ANG II, 3) OCH + ANG II, and 4) OHH + ANG II.
Real-time RT-PCR analysis.
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed into cDNA. mRNA levels for RAS components [renin, angiotensinogen (AGT), angiotensin-converting enzyme-1 (ACE1), and ANG II type 1 receptor (AT1R)], NADPH oxidase (NOX2), proinflammatory cytokines (TNF-α, IL-1β, and IL-6), and GAPDH were analyzed with SYRB Green real-time PCR. The sequences for the primers are shown in Table 1. Real-time RT-PCR was performed with the ABI prism 7300 Sequence Detection System (Applied Biosystems, Carlsbad, CA). Values were corrected by GAPDH, and the final concentration of mRNA was calculated using the following formula: x = , where x is the fold difference relative to control and Ct is threshold cycle.
Table 1.
Primer sequences for real-time PCR
| Gene | Forward Primer | Reverse Primer | Product Size, bp |
|---|---|---|---|
| GAPDH | 5′-TGACTCTACCCACGGCAAGTTCAA-3′ | 5′-ACGACATACTCAGCACCAGCATCA-3′ | 141 |
| Renin | 5′-CTGCCACCTTGTTGTGTGAG-3′ | 5′-ACCTGGCTACAGTTCACAACG-3′ | 154 |
| AGT | 5′-TCCCTCGCTCTCTGGACTTA-3′ | 5′-AAGTGAACGTAGGTGTTGAAA-3′ | 209 |
| ACE1 | 5′-GTGTTGTGGAACGAATACGC-3′ | 5′-CCTTCTTTATGATCCGCTTGA-3′ | 187 |
| AT1R, | 5′-CTCAAGCCTGTCTACGAAAATGAG-3′ | 5′-GTGAATGGTCCTTTGGTCGT-3′ | 188 |
| NOX2 | 5′-CAAGATGGAGGTGGGACAGT-3′ | 5′-GCTTATCACAGCCACAAGCA-3′ | 170 |
| IL-1β | 5′-AGCAACGACAAAATCCCT GT-3′ | 5′-GAAGACAAACCGCTTTTCCA-3′ | 209 |
| IL-6 | 5′-GCCTATTGAAAATCTGCTCTGG-3′ | 5′-GGAAGTTGGGGTAGGAAGGA-3′ | 160 |
| TNF-α | 5′-GCCGATTTGCCACTTCATAC-3′ | 5′-AAGTAGACCTGCCCGGACTC-3′ | 209 |
AGT, angiotensinogen; ACE1, angiotensin-converting enzyme 1; AT1R, angiotensin II type 1 receptor; NOX2, NADPH oxidase 2; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Data analysis.
Differences in mean arterial pressure (MAP) and HR were calculated for each animal based on the mean of the baseline subtracted from the mean of MAP or HR after intracerebroventricular microinjection of ANG II (5 min) or TNF-α (10–30 min) or systemic infusion of ANG II (the final 3 days). Two-way ANOVA was then conducted on the means of calculated differences for each of the experimental groups. After a significant ANOVA was established, post hoc analyses were performed with Tukey multiple-comparison tests between pairs of mean changes. The same statistical methods were used to analyze the changes in cardiac baroreflex and differences in maternal and offspring body weights and in the mRNA expression of RAS components, NADPH oxidase, and proinflammatory cytokines in the LT and PVN. All data are expressed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Maternal observations and body weight changes in dams and offspring.
Maternal body weights were the same at the initiation of HFD feeding but were greater at the time of pregnancy and throughout lactation in HFD-fed dams compared with control diet-fed dams (Fig. 1A). HFD feeding during either pregnancy, lactation, or both did not impair reproduction in dams compared with control diet-fed dams. There were no significant differences in litter sizes or birth weights of the pups in all group of dams (OCC group: 12.6 ± 0.4 pups, 6.86 ± 0.0.21 g/pup; OHC group: 11.7 ± 0.7 pups, 7.05 ± 0.23 g/pup; OCH group: 12.0 ± 0.6 pups, 6.76 ± 0.28 g/pup; and OHH group: 12.5 ± 0.7 pups, 6.58 ± 0.24 g/pup, P > 0.05). However, after the week of weaning, offspring weight gain diverged into a rapid weight gain in OCH and OHH groups compared with the OCC group. Offspring in the OHC group tended to gain more weight than offspring in the OCC group, but the differences in mean weight did not reach the level of statistical significance (Fig. 1B).
Fig. 1.
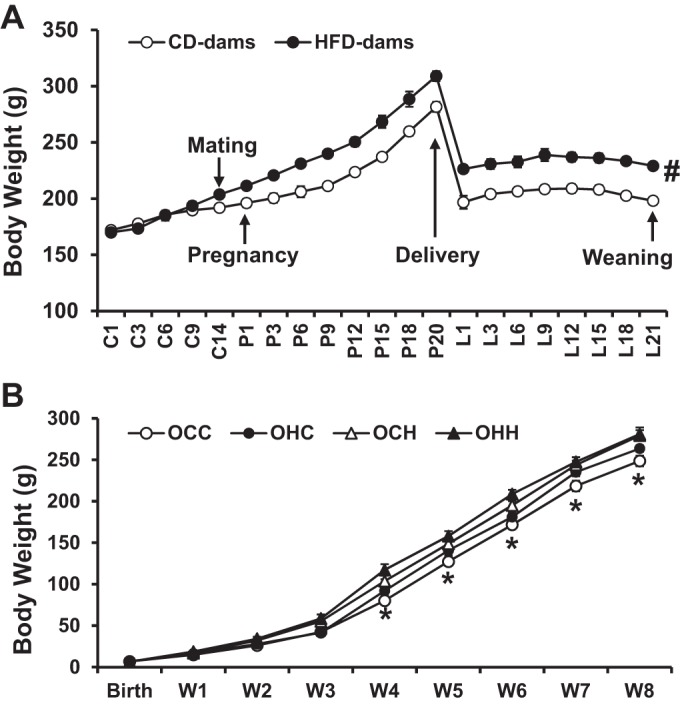
Maternal body weight in control diet (CD)-fed and high-fat diet (HFD)-fed dams during pregnancy and lactation (n = 16 rats/group; A) and their male offspring body weight from birth to 8 wk old (n = 20 rats/group; B). C, control days before mating; P, pregnancy; L, lactation; W, week. Rats were divided into the following groups: offspring from CD-fed dams sucked by CD-fed dams (OCC group), offspring from HFD-fed dams suckled by CD-fed dams (OHC group), offspring from CD-fed dams sucked by HFD-fed dams (OCH group), and offspring from HFD-fed dams suckled by HFD-fed dams (OHH group). *P < 0.05 OCC vs. OCH or OHH; #P < 0.05 vs. CD dams.
mRNA expression of RAS components, NADPH oxidase, and proinflammatory cytokines in the brains of young and adult offspring preceding ANG II treatment.
In brain tissues collected at 10 wk of age, the offspring of HFD dams showed upregulation of mRNA expression of AGT and ACE1 in the LT and AGT, AT1R, and TNF-α in the PVN. Furthermore, greater expression of ACE1 in the LT of the OCH and OHH groups and in the PVN of the OHH group was evident (P < 0.05; Fig. 2, A and B).
Fig. 2.
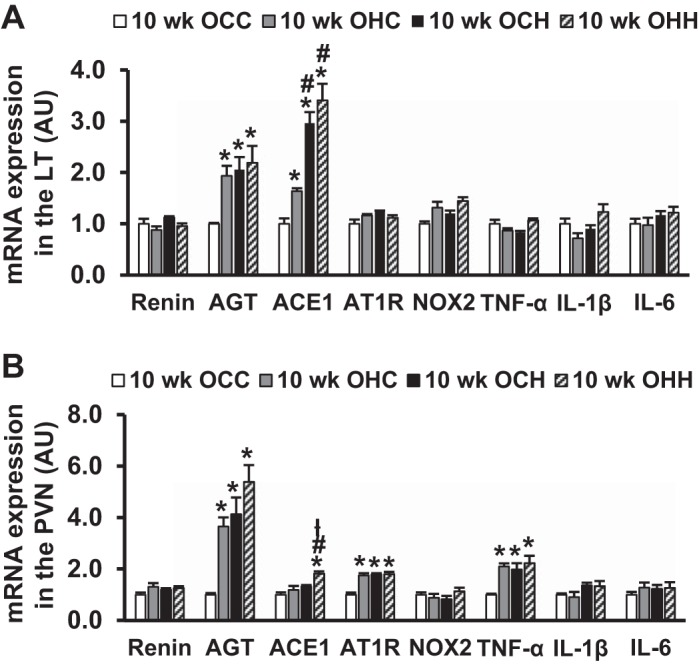
Quantitative comparison of mRNA expression of renin-angiotensin system components, NADPH oxidase (NOX2), and proinflammatory cytokines in the lamina terminalis (LT; A) and paraventricular nucleus (PVN; B) in 10-wk-old male offspring of dams fed a control diet (CD) or high-fat diet (HFD). Rats were divided into the following groups: offspring from CD-fed dams sucked by CD-fed dams (OCC group), offspring from HFD-fed dams suckled by CD-fed dams (OHC group), offspring from CD-fed dams sucked by HFD-fed dams (OCH group), and offspring from HFD-fed dams suckled by HFD-fed dams (OHH group). AGT, angiotensinogen; ACE1, angiotensin-converting enzyme 1; AT1R, angiotensin II type 1 receptor; AU, arbitrary units. n = 5 rats/group. *P < 0.05 vs. the OCC group; #P < 0.05 vs. the OHC group; łP < 0.05 vs. the OCH group.
Effects of maternal HFD feeding on cardiac baroreflex function in adult offspring.
Cardiac baroreflex function was studied using pharmacological challenges. Baroreflex sensitivity was determined from regression line slopes relating changes in HR to changes in BP during phenylephrine (Fig. 3A) or SNP (Fig. 3B) infusion. HFD feeding during either pregnancy, lactation, or both resulted in significant decreases in the slope for bradycardia in the offspring (OHC, OCH, and OHH groups) compared with offspring of dams fed a control diet during both pregnancy and lactation (OCC group, P < 0.05; Table 2). The slopes for tachycardia in the OCH and OHH groups (P < 0.05) but not in the OHC group (P > 0.05; Table 2) were significantly depressed.
Fig. 3.
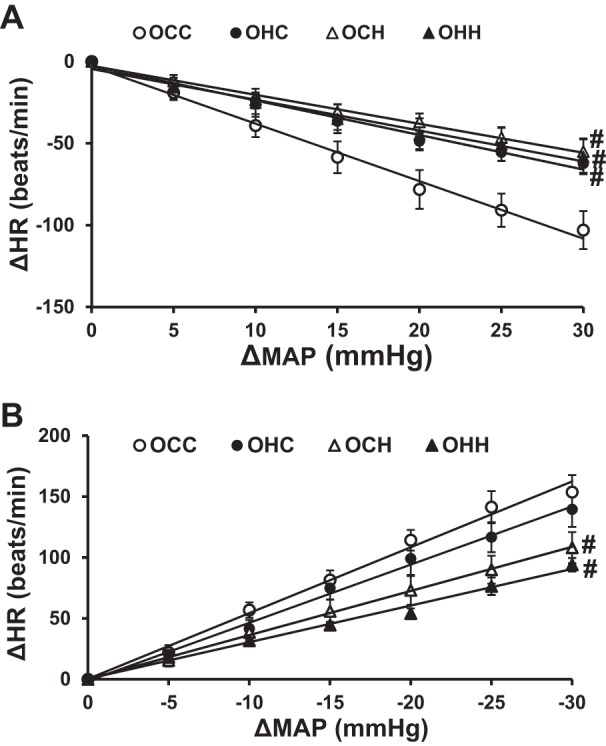
A: comparison of decreases in heart rate (HR; A) to increases in mean arterial pressure (MAP) induced by phenylephrine (PE) in male adult offspring of dams fed a control diet (CD) or high-fat diet (HFD). Solid lines are average representations of the regression lines fit through the data points. B: comparison of increases in HR to decreases in MAP evoked by sodium nitroprusside (SNP). Rats were divided into the following groups: offspring from CD-fed dams sucked by CD-fed dams (OCC group), offspring from HFD-fed dams suckled by CD-fed dams (OHC group), offspring from CD-fed dams sucked by HFD-fed dams (OCH group), and offspring from HFD-fed dams suckled by HFD-fed dams (OHH group). n = 6 rats/group. #P < 0.05 vs. the OCC group.
Table 2.
Slopes of baroreflex function in the offspring of dams treated with normal fat control diet or high-fat diet during pregnancy, lactation, or both
| OCC | OHC | OCH | OHH | |
|---|---|---|---|---|
| Phenylephrine | −3.51 ± 0.39 | −2.11 ± 0.16* | −1.77 ± 0.26* | −1.88 ± 0.28* |
| Sodium nitroprusside | −5.40 ± 0.59 | −4.78 ± 0.51 | −3.63 ± 0.46* | −2.99 ± 0.38* |
Values are means ± SE; n = 6 animals/group. Rats were divided into the following groups: offspring from CD-fed dams sucked by CD-fed dams (OCC group), offspring from HFD-fed dams suckled by CD-fed dams (OHC group), offspring from CD-fed dams sucked by HFD-fed dams (OCH group), and offspring from HFD-fed dams suckled by HFD-fed dams (OHH group).
P < 0.05 vs. the OCC group.
Effect of acute intracerebroventricular administration of ANG II or TNF-α on MAP and HR.
Intracerebroventricular administration of ANG II resulted in significant and comparable increases in MAP in the OCC and OHC groups (12.4 ± 2.3 and 17.3 ± 2.3 mmHg, P < 0.05; Fig. 4, A and B) compared with basal MAP before ANG II injection. In contrast, intracerebroventricular ANG II treatment elicited an augmented pressor response in both the OCH and OHH groups compared with the OCC group but not with the OHC group (20.1 ± 2.5 and 21.5 ± 1.6 mmHg, P < 0.05; Fig. 4, A and B). Intracerebroventricular ANG II administration induced comparable but not significant decreases in HR in all groups (P > 0.05; Fig. 4, C and D).
Fig. 4.
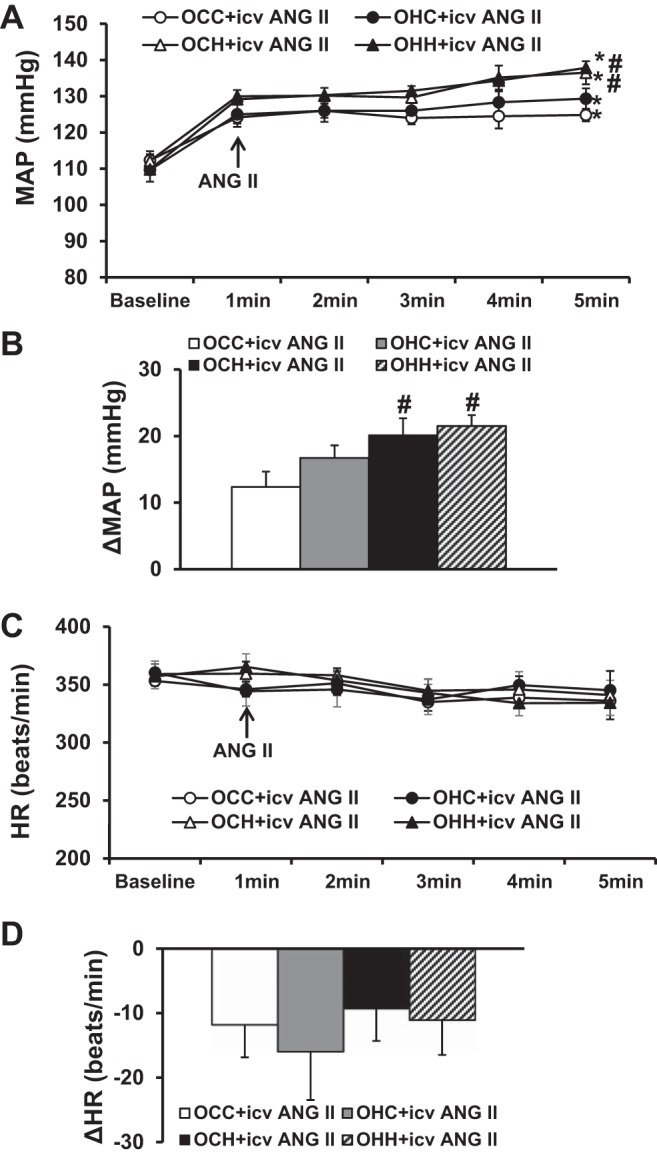
A and B: pressor effects induced by acute intracerebroventricular injections of ANG II in adult offspring of dams fed a control diet (CD) or high-fat diet (HFD). C and D: changes in heart rate (HR) after intracerebroventricular ANG II administration in all groups. Rats were divided into the following groups: offspring from CD-fed dams sucked by CD-fed dams (OCC group), offspring from HFD-fed dams suckled by CD-fed dams (OHC group), offspring from CD-fed dams sucked by HFD-fed dams (OCH group), and offspring from HFD-fed dams suckled by HFD-fed dams (OHH group). MAP, mean arterial pressure. n = 6 rats/group. *P < 0.05 vs. baseline; #P < 0.05 vs. OCC or OHC groups.
Intracerebroventricular administration of TNF-α resulted in slight but significant increases in MAP in the OCC group compared with basal MAP before TNF-α injection (7.5 ± 0.6 mmHg, P < 0.05; Fig. 5, A and B). However, in the offspring of dams fed HFD in either period including the OHC, OCH, and OHH groups, intracerebroventricular TNF-α treatment elicited augmented pressor responses compared with the OCC group with TNF-α administration (19.4 ± 1.9, 18.8 ± 1.5, and 21.4 ± 2.6 mmHg, P < 0.05; Fig. 5, A and B). Intracerebroventricular TNF-α administration induced a slight but significant increase in HR in the OCC group (15.4 ± 2.6 beats/min, P < 0.05; Fig. 5, C and D). However, in the offspring of dams fed HFD during any period (i.e., OHC, OCH, and OHH groups), intracerebroventricular TNF-α treatment elicited augmented tachycardic responses compared with the OCC group (41.6 ± 7.8, 43.8 ± 8.7, and 43.2 ± 6.5 beats/min, P < 0.05; Fig. 5, C and D).
Fig. 5.
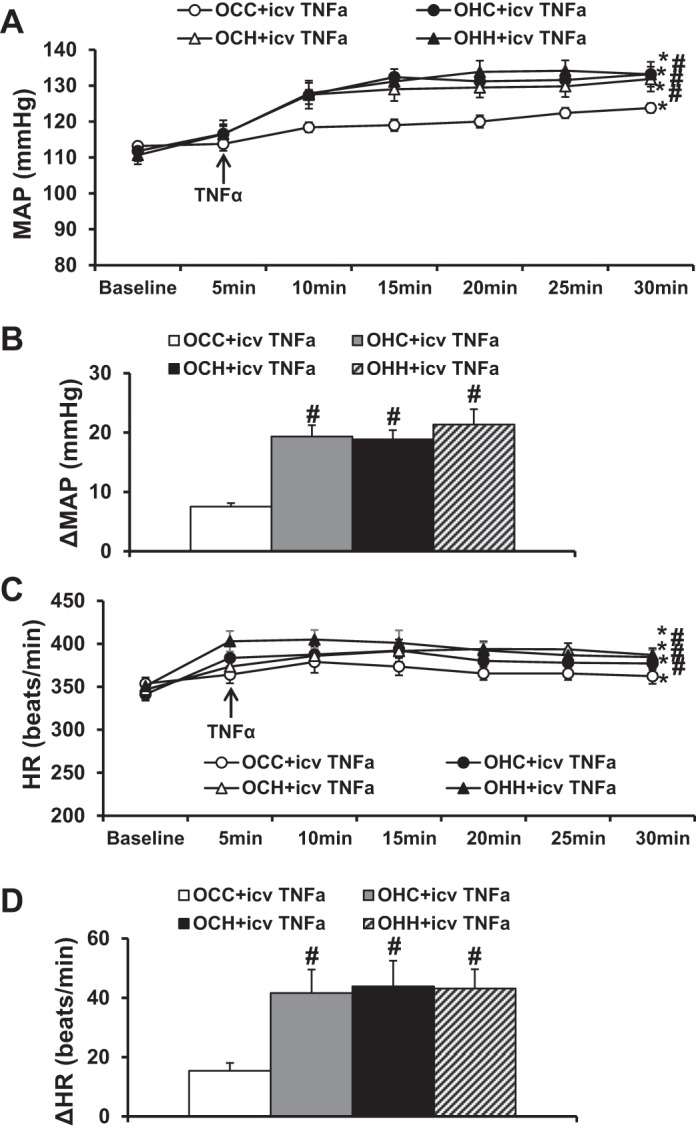
A and B: pressor effects induced by acute intracerebroventricular injections of TNF-α in adult offspring of dams fed a control diet (CD) or high-fat diet (HFD). C and D: tachycardiac effects after intracerebroventricular TNF-α administration in all groups. Rats were divided into the following groups: offspring from CD-fed dams sucked by CD-fed dams (OCC group), offspring from HFD-fed dams suckled by CD-fed dams (OHC group), offspring from CD-fed dams sucked by HFD-fed dams (OCH group), and offspring from HFD-fed dams suckled by HFD-fed dams (OHH group). HR, heart rate; MAP, mean arterial pressure. n = 6 rats/group. *P < 0.05 vs. baseline; #P < 0.05 vs. the OCC group.
Effects of maternal HFD feeding on the chronic ANG II hypertensive response of adult offspring.
At 10 wk of age, there were no significant differences in basal MAP (100.2 ± 1.9, 99.1 ± 1.8, 99.9 ± 2.2, and 100.2 ± 1.2 mmHg) and basal HR (354.4 ± 5.3, 342.8 ± 6.5, 347.5 ± 9.2, and 344.0 ± 9.8 beats/min) among the OCC, OHC, OCH, and OHH groups, respectively. However, during infusion of the slow pressor dose of ANG II, the offspring of dams fed HFD during either pregnancy, lactation, or both showed a significantly enhanced hypertensive response (OHC group: 21.3 ± 3.9 mmHg, OCH group: 25.0 ± 3.8 mmHg, and OHH group: 29.7 ± 2.9 mmHg) compared with male offspring of control diet-fed dams (OCC group: 13.1 ± 2.4 mmHg, P < 0.05; Fig. 6, A and B). The OHH group demonstrated a trend toward having a greater increase in ANG II-induced hypertension than the groups from mothers fed HFD only during pregnancy or during lactation but did not reach statistical significance. ANG II administration did not elicit significant changes in HR in all groups (P > 0.05; Fig. 6, C and D).
Fig. 6.
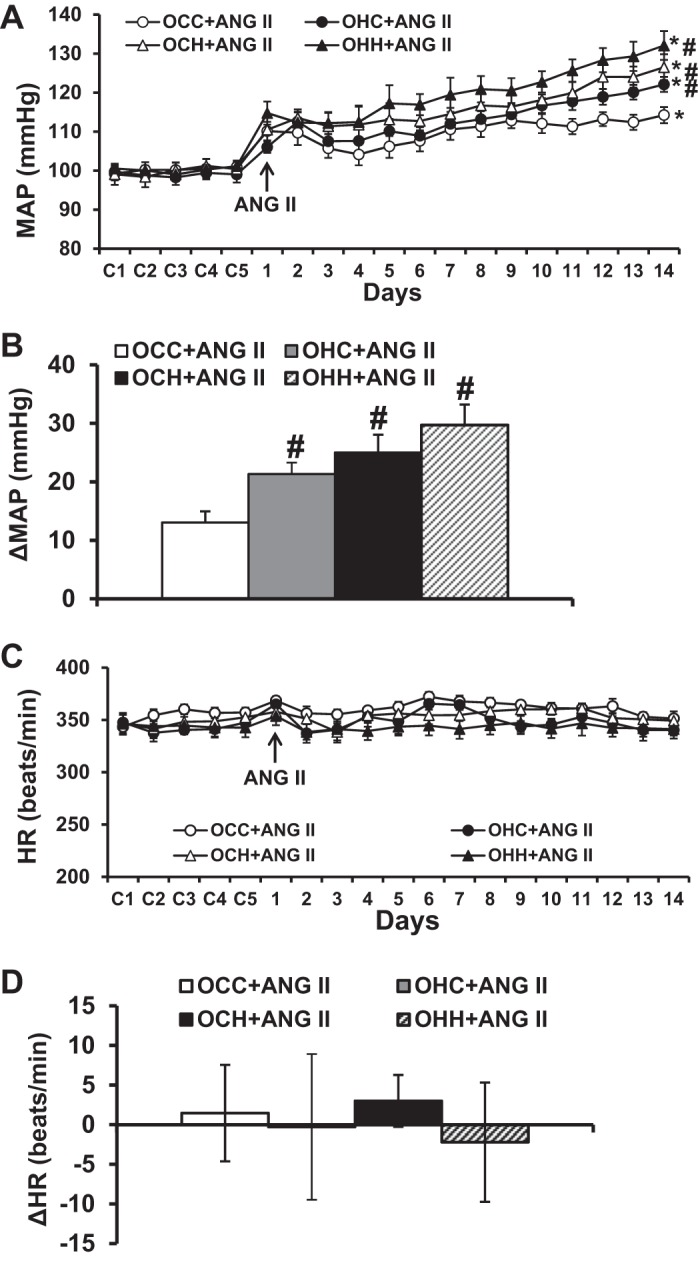
A and B: pressor effects induced by systemic infusion of ANG II in adult offspring of dams fed a control diet (CD) or high-fat diet (HFD). The pressor effect was enhanced in the offspring of dams fed a HFD during either pregnancy, lactation, or both. C and D: daily heart rate (HR) and changes in HR after systemic infusions of ANG II in all groups. Rats were divided into the following groups: offspring from CD-fed dams sucked by CD-fed dams (OCC group), offspring from HFD-fed dams suckled by CD-fed dams (OHC group), offspring from CD-fed dams sucked by HFD-fed dams (OCH group), and offspring from HFD-fed dams suckled by HFD-fed dams (OHH group). MAP, mean arterial pressure. n = 6 rats/group. *P < 0.05 vs. baseline; #P < 0.05 vs. the OCC group.
Effect of maternal HFD on ANG II-induced mRNA expression of RAS components, NADPH oxidase, and proinflammatory cytokines in the brain.
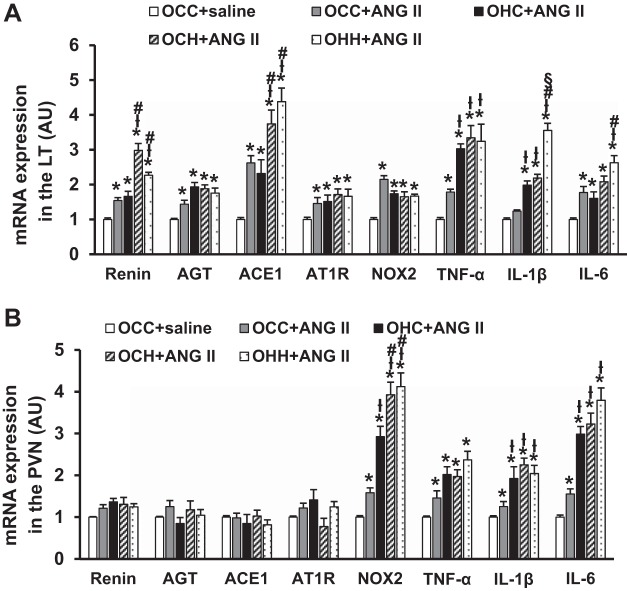
In LT tissues, the slow pressor ANG II infusion resulted in a significant increase in mRNA expression of RAS components (renin, AGT, ACE1, and AT1R), NOX2, and proinflammatory cytokines (TNF-α and IL-6 but not IL-1β) in OCC group compared with the OCC group treated with saline (P < 0.05; Fig. 7A). Compared with the OCC group treated with ANG II, the offspring of dams fed a HFD exhibited enhanced expression of some RAS components and proinflammatory cytokines, which included expression of TNF-α and IL-1β in the OHC group (P < 0.05) and expression of renin, ACE1, TNF-α, and IL-1β in both OCH and OHH groups (P < 0.05) after ANG II treatment. Furthermore, the expression of renin and ACE1 in both OCH and OHH groups and the expression of IL-6 and IL-1β in the OHH group was greater than in the OHC group after ANG II treatment (P < 0.05; Fig. 7A).
Fig. 7.
Quantitative comparison of mRNA expression of renin-angiotensin-aldosterone system components, NADPH oxidase (NOX2), and proinflammatory cytokines in the lamina terminalis (LT; A) and paraventricular nucleus (PVN; B) of adult offspring after ANG II administration. Rats were divided into the following groups: offspring from CD-fed dams sucked by CD-fed dams (OCC group), offspring from HFD-fed dams suckled by CD-fed dams (OHC group), offspring from CD-fed dams sucked by HFD-fed dams (OCH group), and offspring from HFD-fed dams suckled by HFD-fed dams (OHH group). AU, arbitrary units; AGT, angiotensinogen; ACE1, angiotensin-converting enzyme 1; AT1R, ANG II type 1 receptor. n = 6 rats/group. *P < 0.05 vs. OCC + saline; łP < 0.05 vs. OCC + ANG II; #P < 0.05 OHC + ANG II; §P < 0.05 vs. OCH + ANG II.
In PVN tissues, ANG II infusion elicited a significant increase in mRNA expression of NOX2, TNF-α, IL-6, and IL-1β in the OCC group compared with the OCC group treated with saline (P < 0.05; Fig. 7B). mRNA expression of renin, AGT, ACE1, and AT1R was not greater after ANG II (P > 0.05). All of the offspring of HFD-fed dams, including those from OHC, OCH, and OHH groups, showed enhanced mRNA expression of NOX2, IL-1β, and IL-6 after ANG II treatment (P < 0.05). Furthermore, the expression of NOX2 in both OCH and OHH groups was greater than that in the OHC group (P < 0.05; Fig. 7B).
DISCUSSION
The major findings of the present study are that 1) 10-wk-old offspring of HFD dams showed upregulated expression of RAS components, NADPH oxidase, and proinflammatory cytokines in the LT and PVN compared with the offspring of control diet-fed dams; 2) 10-wk-old offspring of HFD dams had blunted cardiac baroreflex function and elevated autonomic sensitivity to central challenges with ANG II or TNF-α; and 3) offspring of dams given a HFD during either pregnancy, lactation, or both periods exhibited normal BP at 10 wk of age. However, these offspring had a significantly greater hypertensive response to a slow pressor dose of ANG II that was accompanied by enhanced upregulation of mRNA expression of RAS components, NADPH oxidase, and proinflammatory cytokines in the LT and PVN. Taken together, these results indicate that maternal HFD during either pregnancy or lactation is sufficient for perinatal programming of sensitization for hypertension, which is associated with hyperreactivity of central cardiovascular nuclei that, in all likelihood, involves elevated expression of RAS components, NADPH oxidase, and proinflammatory cytokines.
Maternal obesity and/or HFD has been demonstrated to have consequences on the health of offspring, altering their responses to environmental challenges and predisposing them to cardiovascular and metabolic disorders in human and experimental animals (1, 7, 10, 25). However, reports on BP levels in the offspring have not always been consistent. The differences in results may have depended on how long the HFD was fed to the nonhuman mothers before pregnancy or when the BP measurements were conducted in the offspring. For example, short-term HFD before pregnancy (10 days to 4 wk) and throughout pregnancy and lactation did not alter basal BP in the offspring until 6 mo of age. However, the alteration of vascular reactivity and epigenetic changes in adipocytokine expression in the adiposity were evident (16, 17, 20, 21). In contrast, mothers fed a long-term HFD including before pregnancy (5–8 wk) and during pregnancy and lactation exhibited significant obesity, while their offspring showed elevated BP from the juvenile period (30 days old) through adulthood (6, 11, 27, 28). These results suggest that there is an additive effect of maternal obesity and maternal HFD on BP regulation. In the present study, female rats were fed a HFD for a limited period before pregnancy (2 wk) and during either pregnancy, lactation, or both. Consistent with previous studies (16, 17, 20, 21), we found no changes in basal BP and HR in the offspring when the parameteres were measured at 10 wk of age. However, the expression of mRNA for RAS components, NADPH oxidase, and proinflammatory cytokine in the brain regions involved in cardiovascular regulation was enhanced. Alterations of RAS components, oxidative stress, and proinflammatory cytokines in peripheral tissues and the brain have been implicated as important mediators in the fetal programming of maternal obesity- and HFD-induced metabolic and cardiovascular dysfunction (1, 2, 4, 7, 11, 18, 19, 23). Our results characterizing gene expression in the LT and PVN may reflect the nature of the central neuroplasticity produced by maternal HFD that mediates sensitization of the hypertensive response. This sensitizing condition may predispose offspring to a greater risk of cardiovascular dysfunction when additional environmental challenges occur.
It is well established that the origin and progression of hypertension are associated with the autonomic nervous system dysfunction involving impaired baroreflex function and an imbalance between sympathetic and parasympathetic arms of the autonomic nervous system (9, 15). Rudyk et al. (26) demonstrated that short-term exposure of rat dams to a diet rich in lard was not associated with an elevation of resting BP but adversely influenced autonomic pathways regulating cardiovascular control, resulting in increased cardiovascular reactivity to acute stress and salt loading in male offspring. Prior et al. (24) reported that the offspring of rabbits fed a HFD had markedly elevated RSNA and pressor responses to central leptin, enhanced sympathetic responses to ghrelin, and an exaggerated sympathetic response to acute air jet stress compared with offspring from mothers fed a normal-fat control diet. Also, the maternal HFD was associated with impaired baroreflex sensitivity and reduced HR variability, implicating abnormalities in autonomic control in the offspring (27). By extending these previous studies, the present experiments showed that short-term maternal HFD resulted in a blunted cardiac baroreflex sensitivity and an elevated acute pressor response to central administration of ANG II or TNF-α. Moreover, the expression of some of the RAS components, NADPH oxidase, and proinflammatory cytokines and the hypertensive response to chronic systemic ANG II were exaggerated in the offspring of dams fed a HFD during either pregnancy, lactation, or both periods, suggesting that the sympathetic nervous system and BP in the offspring of maternal HFD were hyperreactive. These functional data combined with the results of gene expression in the LT and PVN suggest that at central sites the RAS-, NADPH oxidase-, and proinflammatory cytokine-associated mechanisms underlie the transgenerational effects of maternal HFD on the prohypertensive actions in the offspring. The predisposition of the offspring of HFD dams to display enhanced gene expression and a sensitized hypertensive response appears to be similar to sensitization of this response produced by prior mild physiological or dietary challenges that Xue et al. (30–34) have found in adult animals and in the offspring of maternal hypertension during pregnancy.
The adipokine leptin is released in proportion to total fat and primarily acts on the hypothalamus to maintain energy homeostasis and normal body weight. Maternal HFD resulted in greater increases in maternal body weights during pregnancy and lactation that was accompanied by elevated leptin levels (6, 11, 16, 20, 21, 27, 28). Furthermore, the body weights, percent body fat, and plasma leptin levels of their offspring diverged into different body weight gain, which was dependent on maternal HFD given during pregnancy, lactation, or both. As a result, higher body weight and percentage body fat were associated with higher plasma leptin levels in the offspring (6, 11, 16, 20, 21). Consistent with the above studies, we found that maternal body weight was higher in HFD-fed dams than in control diet-fed dams and that the offspring weight gains after weaning in OCH and OHH groups, but not in the OHC group, were greater than those in the OCC group. These results suggest that the levels of plasma leptin would be higher in HFD-fed dams and OCH and OHH offspring, although we did not measure the white adipose mass and plasma leptin levels in the dams and their offspring in the present study.
A recent study (29) has demonstrated that maternal HFD during lactation produced greater leptin sensitivity and played a greater role in determining the metabolic phenotype of the offspring than prenatal HFD exposure. Leptin has been identified as a critical trophic factor that influences the development of the hypothalamic projections (3), while melanocortin-4 receptor in the PVN is required for early life programming of hypertension arising from either maternal obesity or neonatal hyperleptinemia (28). Xue et al. (32) and Hilzendeger et al. (13) have also reported that leptin can interact with the RAS and proinflammatory cytokines to regulate sympathetic activity and sensitize ANG II-induced hypertension. In the present study, OCH and/or OHH groups exhibited greater expression of ACE1 in the LT and PVN compared with the OHC group at 10 wk of age. We did not find a greater increase in the expression of renin and ACE1 in the LT and NOX2 in the PVN after chronic ANG II infusion in the OHC group but did in the OCH and OHH groups. Moreover, maternal HFD during lactation enhanced pressor responses to acute intracerebroventricular administration of ANG II and blunted cardiac baroreflex sensitivity to the SNP test compared with the maternal normal diet. However, maternal HFD during pregnancy alone had no effect. These results combined with the data on the body weights of dams and offspring suggest that leptin-related mechanisms affecting offspring development and cardiovascular system reactivity may account for the different responses between offspring from dams with prenatal HFD and postnatal HFD. These findings warrant further study in the future.
The present study, focusing on the gene expression of RAS, NADPH oxidase, and proinflammatory cytokines in the LT and its primary downstream target, the PVN, may demonstrate an important mechanism underlying maternal HFD-induced cardiac baroreflex dysfunction and sensitization of hypertension in offspring. However, given the roles of hindbrain nuclei such as the rostral ventrolateral medulla and nucleus tractus solitarii in the control of sympathetic tone, baroreceptor reflexes, and BP regulation, the involvement of hindbrain nuclei mediating the cardiac baroreflex dysfunction and the sensitizing effect produced by maternal HFD cannot be excluded. Also, mRNA message results do not always reflect protein expression. A limitation of the present study is that we did not determine protein levels of RAS components, NADPH oxidases, and proinflammatory cytokines as the basis of the changes in the mRNA levels in the offspring. However, in a previous study (31), we found that maternal hypertension upregulated protein expression of ACE1 in the SFO and TNF-α receptor I in the PVN that may mediate the sensitized hypertensive response to chronic infusion of ANG II. Future studies are needed to determine the protein expression of TNF-α and its receptor along with other components of the RAS in brain cardiovascular nuclei of offspring from dams fed a HFD during pregnancy, lactation, or both.
Perspectives and Significance
The present study demonstrated the influence of prenatal and/or postnatal HFD programming on the reactivity of the autonomic nervous system and BP in adult offspring. Such programming was associated with altered gene expression of RAS components, NADPH oxidase, and proinflammatory cytokines in the central nervous system. This study provides evidence that maternal overnutrition during either pregnancy or lactation is sufficient for early life programming of the central hyperreactivity and sensitization that predisposes the next generation to a greater risk of hypertension. However, the mechanisms underlying maternal HFD induction of metabolic and cardiovascular dysfunction in adult offspring are far from being elucidated. As proposed by others, increased rates of hypertension may result from a discrepancy or mismatch between the nutritional environment during fetal/early life and adulthood (8). In the present study, all offspring were weaned onto a normal fat diet regardless of the diet present during pregnancy and lactation. Future studies that provide offspring after weaning with the same food as the mothers need to be conducted to determine if there are potential effects of a food mismatch on sensitization of the hypertensive response.
GRANTS
This work were supported by Funds for Major Program of Hebei North University Grant ZD201316 (to J. D. Li), Natural Fund of Hebei Province Grant H2015405017 (to H. P. Wang), and National Heart, Lung, and Blood Institute Grants HL-14388 (to F.M. Abboud/A.K. Johnson), HL-98207 (to A.K. Johnson) and HL-84027 (to C.D. Sigmund/A.K. Johnson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.P., A.K.J., and B.X. conceived and designed research; Y.-P.Z., Y.-L.H., Z.-Q.F., X.-F.W., J.-D.L., and H.-P.W. performed experiments; Y.-P.Z., Y.-L.H., Z.-Q.F., and X.-F.W. analyzed data; Y.-P.Z., Y.-L.H., Z.-Q.F., W.P., and B.X. interpreted results of experiments; Y.-P.Z., Y.-L.H., and Z.-Q.F. prepared figures; W.P. and B.X. drafted manuscript; W.P., A.K.J., and B.X. edited and revised manuscript; Y.-P.Z., Y.-L.H., Z.-Q.F., X.-F.W., J.-D.L., H.-P.W., W.P., A.K.J., and B.X. approved final version of manuscript.
REFERENCES
- 1.Alexander BT, Dasinger JH, Intapad S. Fetal programming and cardiovascular pathology. Compr Physiol 5: 997–1025, 2015. doi: 10.1002/cphy.c140036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 24: 2104–2115, 2010. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 3.Bouret SG. Neurodevelopmental actions of leptin. Brain Res 1350: 2–9, 2010. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cesar HC, Pisani LP. Fatty-acid-mediated hypothalamic inflammation and epigenetic programming. J Nutr Biochem 42: 1–6, 2017. doi: 10.1016/j.jnutbio.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Deng Y, He X, Chu J, Zhou J, Zhang Q, Guo W, Huang P, Guan X, Tang Y, Wei Y, Zhao S, Zhang X, Wei C, Namaka M, Yi P, Yu J, Li X. Prenatal inflammation-induced NF-κB dyshomeostasis contributes to renin-angiotensin system over-activity resulting in prenatally programmed hypertension in offspring. Sci Rep 6: 21692, 2016. doi: 10.1038/srep21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai M, Jellyman JK, Han G, Beall M, Lane RH, Ross MG. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am J Obstet Gynecol 211: 237.e1−237.e13, 2014. doi: 10.1016/j.ajog.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong M, Zheng Q, Ford SP, Nathanielsz PW, Ren J. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. J Mol Cell Cardiol 55: 111–116, 2013. doi: 10.1016/j.yjmcc.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr 102: 514–519, 2009. doi: 10.1017/S000711450820749X. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez G, Lee JA, Liu LC, Gassler JP. The baroreflex in hypertension. Curr Hypertens Rep 17: 19, 2015. doi: 10.1007/s11906-014-0531-z. [DOI] [PubMed] [Google Scholar]
- 10.Gademan MG, van Eijsden M, Roseboom TJ, van der Post JA, Stronks K, Vrijkotte TG. Maternal prepregnancy body mass index and their children’s blood pressure and resting cardiac autonomic balance at age 5 to 6 years. Hypertension 62: 641–647, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01511. [DOI] [PubMed] [Google Scholar]
- 11.Guberman C, Jellyman JK, Han G, Ross MG, Desai M. Maternal high-fat diet programs rat offspring hypertension and activates the adipose renin-angiotensin system. Am J Obstet Gynecol 209: 262.e1−262.e8, 2013. doi: 10.1016/j.ajog.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 116: 991–1006, 2015. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol 303: H197–H206, 2012. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678–686, 1993. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AK, Zhang Z, Clayton SC, Beltz TG, Hurley SW, Thunhorst RL, Xue B. The roles of sensitization and neuroplasticity in the long-term regulation of blood pressure and hypertension. Am J Physiol Regul Integr Comp Physiol 309: R1309–R1325, 2015. doi: 10.1152/ajpregu.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol 288: R127–R133, 2005. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- 17.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41: 168–175, 2003. doi: 10.1161/01.HYP.0000047511.97879.FC. [DOI] [PubMed] [Google Scholar]
- 18.Kim DW, Glendining KA, Grattan DR, Jasoni CL. Maternal obesity leads to increased proliferation and numbers of astrocytes in the developing fetal and neonatal mouse hypothalamus. Int J Dev Neurosci 53: 18–25, 2016. doi: 10.1016/j.ijdevneu.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Malti N, Merzouk H, Merzouk SA, Loukidi B, Karaouzene N, Malti A, Narce M. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta 35: 411–416, 2014. doi: 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Masuyama H, Hiramatsu Y. Additive effects of maternal high fat diet during lactation on mouse offspring. PLoS One 9: e92805, 2014. doi: 10.1371/journal.pone.0092805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuyama H, Hiramatsu Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology 153: 2823–2830, 2012. doi: 10.1210/en.2011-2161. [DOI] [PubMed] [Google Scholar]
- 22.Mimee A, Smith PM, Ferguson AV. Circumventricular organs: targets for integration of circulating fluid and energy balance signals? Physiol Behav 121: 96–102, 2013. doi: 10.1016/j.physbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Ornellas F, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Combined parental obesity augments single-parent obesity effects on hypothalamus inflammation, leptin signaling (JAK/STAT), hyperphagia, and obesity in the adult mice offspring. Physiol Behav 153: 47–55, 2016. doi: 10.1016/j.physbeh.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Prior LJ, Davern PJ, Burke SL, Lim K, Armitage JA, Head GA. Exposure to a high-fat diet during development alters leptin and ghrelin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 63: 338–345, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02498. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, Sarwar N, Lee AJ, Bhattacharya S, Norman JE. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347: f4539, 2013. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudyk O, Makra P, Jansen E, Shattock MJ, Poston L, Taylor PD. Increased cardiovascular reactivity to acute stress and salt-loading in adult male offspring of fat fed non-obese rats. PLoS One 6: e25250, 2011. doi: 10.1371/journal.pone.0025250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW, Poston L, Taylor PD. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension 55: 76–82, 2010. doi: 10.1161/HYPERTENSIONAHA.109.139402. [DOI] [PubMed] [Google Scholar]
- 28.Samuelsson AS, Mullier A, Maicas N, Oosterhuis NR, Eun Bae S, Novoselova TV, Chan LF, Pombo JM, Taylor PD, Joles JA, Coen CW, Balthasar N, Poston L. Central role for melanocortin-4 receptors in offspring hypertension arising from maternal obesity. Proc Natl Acad Sci USA 113: 12298–12303, 2016. doi: 10.1073/pnas.1607464113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KL. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 61: 2833–2841, 2012. doi: 10.2337/db11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin ii-elicited hypertension. Hypertension 67: 163–170, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue B, Yin H, Guo F, Beltz TG, Thunhorst RL, Johnson AK. Maternal gestational hypertension-induced sensitization of angiotensin II hypertension is reversed by renal denervation or angiotensin-converting enzyme inhibition in rat offspring. Hypertension 69: 669–677, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue B, Yu Y, Zhang Z, Guo F, Beltz TG, Thunhorst RL, Felder RB, Johnson AK. Leptin mediates high-fat diet sensitization of angiotensin II-elicited hypertension by upregulating the brain renin-angiotensin system and inflammation. Hypertension 67: 970–976, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior Ang II treatment. Hypertension 59: 459–466, 2012. doi: 10.1161/HYPERTENSIONAHA.111.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension 60: 1023–1030, 2012. doi: 10.1161/HYPERTENSIONAHA.112.196576. [DOI] [PMC free article] [PubMed] [Google Scholar]