Abstract
Chronic iron overload results in heart and liver diseases and is a common cause of morbidity and mortality in patients with genetic hemochromatosis and secondary iron overload. We investigated the role of tissue inhibitor of metalloproteinase 3 (TIMP3) in iron overload-mediated tissue injury by subjecting male mice lacking Timp3 (Timp3−/−) and wild-type (WT) mice to 12 wk of chronic iron overload. Whereas WT mice with iron overload developed diastolic dysfunction, iron-overloaded Timp3−/− mice showed worsened cardiac dysfunction coupled with systolic dysfunction. In the heart, loss of Timp3 was associated with increased myocardial fibrosis, greater Timp1, matrix metalloproteinase (Mmp)2, and Mmp9 expression, increased active MMP-2 levels, and gelatinase activity. Iron overload in Timp3−/− mice showed twofold higher iron accumulation in the liver compared with WT mice because of constituently lower levels of ferroportin. Loss of Timp3 enhanced the hepatic inflammatory response to iron overload, leading to greater neutrophil and macrophage infiltration and increased hepatic fibrosis. Expression of inflammation-related MMPs (MMP-12 and MMP-13) and inflammatory cytokines (IL-1β and monocyte chemoattractant protein-1) was elevated to a greater extent in iron-overloaded Timp3−/− livers. Gelatin zymography demonstrated equivalent increases in MMP-2 and MMP-9 levels in WT and Timp3−/− iron-overloaded livers. Loss of Timp3 enhanced the susceptibility to iron overload-mediated heart and liver injury, suggesting that Timp3 is a key protective molecule against iron-mediated pathology.
NEW & NOTEWORTHY In mice, loss of tissue inhibitor of metalloproteinase 3 (Timp3) was associated with systolic and diastolic dysfunctions, twofold higher hepatic iron accumulation (attributable to constituently lower levels of ferroportin), and increased hepatic inflammation. Loss of Timp3 enhanced the susceptibility to iron overload-mediated injury, suggesting that Timp3 plays a key protective role against iron-mediated pathology.
Keywords: cardiomyopathy, heart disease, iron overload, liver disease, tissue inhibitor of metalloproteinase 3
INTRODUCTION
Iron is an essential trace element that plays an important role in living systems and is involved in multiple physiological processes, such as oxygen transport, oxidative phosphorylation, and enzymatic function (30, 65). Iron is absorbed primarily via ferroportin (a putative iron exporter on the basolateral surface of duodenal enterocytes) in the gastrointestinal tract. In normal iron homeostasis, the majority of iron in the body is recycled from senescent erythrocytes. The liver plays a crucial role in this process by being the major storage of iron excess and the source of hepcidin, an iron-regulatory hormone, the major suppressor of iron uptake (5). In response to an excess of body iron, the liver increases the production of hepcidin, which, in turn, inhibits iron uptake via ferroportin (6). Disrupted hepcidin expression leads to uncontrolled iron absorption from dietary sources, resulting in the development of iron overload, commonly called genetic hemochromatosis. Impaired iron metabolism can be found in many liver diseases, particularly those with genetic hemochromatosis, secondary iron overload, and alcohol-related liver diseases (1, 39). Hereditary hemochromatosis is associated with loss-of-function mutations mainly in hemochromatosis, hemojuvelin, or hepcidin genes (9, 54, 64). In this condition, the liver produces a negligible amount of hepcidin, leading to uncontrolled iron uptake and iron overload (24, 31, 52, 53, 61). Beside genetic hemochromatosis, secondary iron overload is also prevalent worldwide and arises from frequent therapeutic blood transfusions for congenital anemias (5). Excess accumulation of iron in different organs results in a number of complications, such as heart failure and liver disease (5, 67).
In humans, iron overload cardiomyopathy is characterized by diastolic dysfunction with arrhythmias (29) and progresses from a restrictive phenotype with diastolic dysfunction to an advanced dilated phenotype with reduced systolic function (40, 44). Patients with iron overload may also develop liver injury from excess of iron, leading to end-organ diseases, including liver fibrosis, cirrhosis, and hepatocellular carcinoma (5, 8, 24, 52). Murine models of acquired iron overload and genetic hemochromatosis are able to recapitulate many, but not all, elements of iron overload-induced cardiomyopathy and liver disease (13–15, 46, 47, 56, 58). In the murine heart, iron overload cardiomyopathy is associated with oxidative stress, myocardial fibrosis, and diastolic dysfunction (15, 46, 47). Therefore, a murine model capable of reproducing systolic dysfunction and inflammation is still required to closer recapitulate symptoms of iron overload in humans. The role of tissue inhibitor of metalloproteinase 3 (TIMP3) as a dual regulator of extracellular matrix (ECM) remodeling and tissue inflammation, fibrosis, and repair (20, 21, 37, 38) suggests a potential novel role of TIMP3 in iron overload-mediated tissue injury. We used mice lacking Timp3 (Timp3−/−) that have normal hepatic and cardiac structure and function at baseline and their wild-type (WT) littermates and subjected them to iron overload. We showed that Timp3 is a critical regulator of iron-mediated cardiac and hepatic injury.
MATERIALS AND METHODS
Experimental animals and the iron injection protocol.
All experiments were performed in accordance with University of Alberta institutional guidelines, which conformed with guidelines published by the Canadian Council on Animal Care and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Revised 2011). WT and Timp3−/− male mice of the C57BL6 background were bred in house in the University of Alberta Health Sciences Laboratory Animal Services housing facility and subjected to the iron/placebo injection protocol at 10–12 wk of age. The iron-dextran (effective concentration of iron: 200 mg/kg) or placebo (5% of dextrose with phenol) was injected intraperitoneally 5 days/wk for 4 wk; the dosage was then reduced to 50 mg/kg iron, and injection continued for another 8 wk, as we have previously reported (13–15). Timp3−/− mice were generated as previously described (32, 37).
Echocardiography.
Transthoracic echocardiography was performed in the CVRC core facility with a Vevo2100 high-resolution imaging system equipped with a 30-MHz transducer (Visual Sonic). Mice were anesthetized with 1.5% isoflurane. Both diastolic and systolic cardiac function parameters were measured and analyzed by researchers who were blinded to genotypes and treatments.
Hemodynamic analysis.
Mice were anesthetized using 1.5% isoflurane, and the right common carotid artery was cannulated. A 1.4-Fr Scisense catheter (Scisense) was advanced through the aortic valve and placed into the left ventricular (LV) chamber. Pressure-volume loops were recoded via TCP-500 amplifier (Scisense) and analyzed using LabScribe 2 software (IWorks), as previously described (43, 50).
Histology and immunofluorescence.
Hearts excised from anesthetized mice were arrested in diastole using 1 M KCl, fixed by 10% buffered formalin, and embedded in paraffin. Five-micrometer-thin sections were stained with Prussian blue, picrosirius red (PSR), and trichrome for morphometric analysis (13, 15). Immunofluorescence staining for macrophages and neutrophils was performed using rat anti-mouse F4/80 (MCA497GA, Serotec) and rat anti-mouse Ly-6B.2 alloantigen (MCA771GA, Serotec) primary antibodies, respectively. CY-3-conjugated goat anti-rat (Invitrogen) and Alexa Fluor-594-conjugated goat anti-rat (Invitrogen) secondary antibodies were used for neutrophil and macrophage stainings, respectively.
Tissue iron level quantification.
Twenty milligrams of frozen tissue from LV and liver were subjected to inductive coupled plasma resonance mass spectrometry to quantify the tissue iron level, as we have previously described (Trace Metal Laboratory, London, ON, Canada) (13, 15).
TaqMan real-time PCR.
Total RNA was extracted from flash-frozen tissue using the TRIzol RNA extraction method. One microgram of RNA was subjected to reverse transcription to synthesize cDNA. Suitable dilutions of cDNA unknown (5 µl), standard (5 µl), and TaqMan master mix (includes primers and probes, 8 µl) (13, 15) were loaded on white 384 Light cycler 480 multiwell plates (Roche), run, and analyzed by a Light cycler 480 machine (Roche).
Immunoblot analysis.
Western blot analysis was performed on flash-frozen LV and liver samples as previously described (49, 66). Anti-SLC40A1 (NBP1-21502, Novus Biologicals) was used as primary antibody together with horseradish peroxidase-conjugated secondary antibody. Blots were quantified using ImageQuant LAS 4000 (General Electric).
Gelatin zymography.
Frozen heart and liver samples were analyzed by in vitro gelatin zymography. Protein was extracted after 30 min of incubation in RIPA buffer (1.0% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS in Tris-buffered saline, pH 8.0) containing proteinase inhibitor cocktails (Calbiochem), and concentration was quantified using a DC protein assay kit (Bio-Rad). An equal volume of total protein (25 μg) was subjected to 8% SDS-PAGE gels containing 2 mg/ml gelatin. After incubation in substrate buffer at 37°C for 24 h, gels were stained overnight with Coomassie brilliant blue G250 (0.15%, Bio-Rad). The pro and cleaved forms of matrix metalloproteinase (MMP)-2 and MMP-9 were visualized as negative staining on the gel. Relative densitometry analysis of target bands was performed using Quantity One (Bio-Rad) (32, 37).
Gelatinase activity.
Total gelatinase activity was measured using fluorescence-based activity assays from EnzChek (Molecular Probes), as previously described (32, 33).
Statistical analysis.
All data were statistically analyzed using SPSS Statistics 19 software, and averaged values are presented as means ± SE. A two-tailed t-test with correction for unequal variance was used for simple comparisons, and Tukey’s ANOVA posttest was used for multiple comparisons. P < 0.05 was used as the level of significance.
RESULTS
Iron accumulation in the heart and liver.
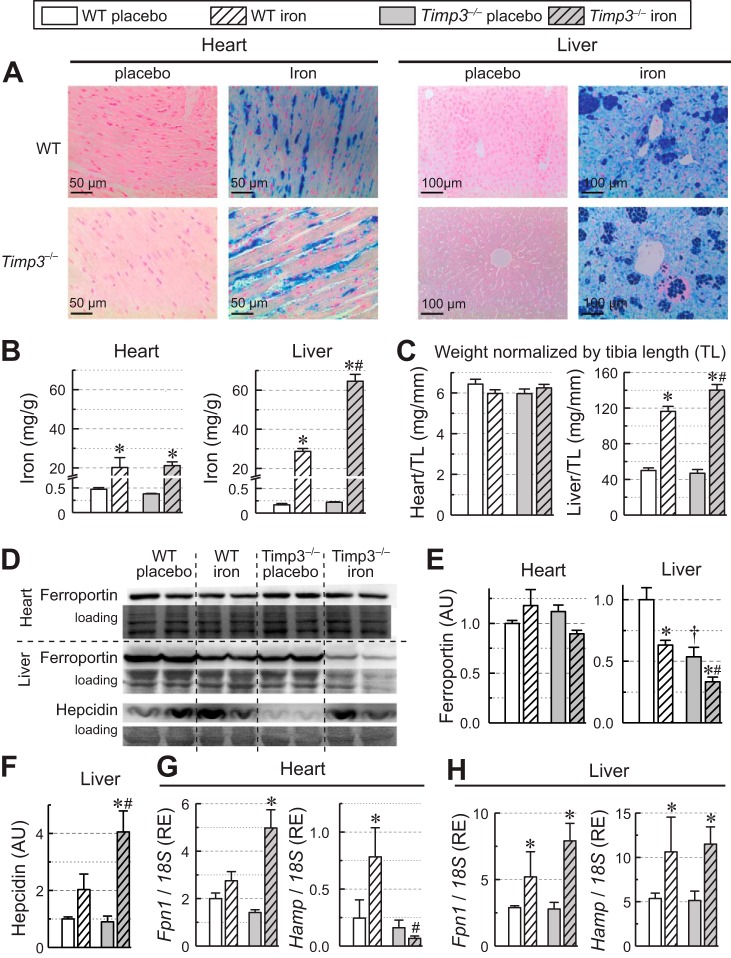
As evident from Prussian blue staining of the heart and liver, WT and Timp3−/− placebo tissue showed no detectable iron deposition, whereas the iron-injected groups had large amounts of iron deposition (Fig. 1A). Direct measurement of iron content using inductive coupled plasma resonance mass spectrometry showed equivalent iron overload in the heart irrespective of genotype, whereas in the liver, markedly higher iron levels were detected in the Timp3−/− group (65 mg/g tissue wt) compared with the WT iron-overloaded group (30 mg/g tissue wt; Fig. 1B), which correlated with the greater hepatomegaly in iron-injected Timp3−/− compared with WT mice (Fig. 1C). To examine the molecular basis for the difference in iron accumulation between tissues, we assessed protein levels of ferroportin in both the heart and liver. In the heart, ferroportin protein levels were largely the same irrespective of genotype or iron load (Fig. 1, D and E), which is consistent with the lack of dependence of iron levels on genotypes. In contrast, protein levels in Timp3−/− livers were dependent on both genotype and iron load (Fig. 1, D and E). Timp3 livers had lower ferroportin levels at baseline, and iron overload reduced ferroportin levels in both genotypes, making the liver a preferred site of iron accumulation over the heart. Hearts regardless of genotype accumulated ~20 mg/g tissue wt in iron overload, whereas WT livers, which lost ferroportin during iron overload, accumulated ~30 mg/g tissue wt. Timp3−/− livers that had lower baseline ferroportin lost more ferroportin during iron overload and accumulated the highest amount of iron (65 mg/g tissue wt). Measurement of hepcidin levels in the liver showed significantly increased hepcidin levels in Timp3−/− + iron livers (Fig. 1F). Such an increase would also favor preferred accumulation of iron in Timp3−/− + iron livers. Expression of iron-related genes in the heart (Fig. 1G) and liver (Fig. 1H) changed differently in response to iron overload. In the heart, ferroportin expression remained mostly unaltered in the WT iron group but increased significantly in the Timp3−/− + iron group (Fig. 1G). Hepcidin, however, showed a different expression profile. It increased significantly in WT + iron than in placebo hearts but failed to increase in Timp3−/− + iron hearts. Hepcidin expression in Timp3−/− iron hearts was much lower than WT + iron hearts. In the liver, expression levels of both ferroportin and hepcidin were higher in the iron-injected group compared with the respective placebo group (Fig. 1H), and the placebo group did not differ in expression levels of both genes. These results demonstrate that loss of Timp3 leads to an increase in hepatic iron overload attributable to reduced ferroportin levels and increased hepcidin levels.
Fig. 1.
Iron accumulation and regulators of iron metabolism in the liver and heart of wild-type (WT) and tissue inhibitor of metalloproteinase 3 knockout (Timp3−/−) mice in response to iron overload. A: representative Prussian blue staining images of myocardial sections (left) and hepatic sections (right). B: heart and liver iron levels measured with inductive coupled plasma resonance mass spectrometry. C: heart and liver weights normalized to tibial length (TL) (n = 10 for the placebo group and n = 15 for the iron overload groups). D: Western blot analysis for ferroportin in the heart and for ferroportin and hepcidin in the liver. E: quantification of Western blots of ferroportin in the heart and liver (n = 6/group). F: quantification of Western blots of hepcidin in the liver (n = 6/group). G: myocardial expression levels of ferroportin (Fnp1) and hepcidin (Hamp) (n = 8/group). H: hepatic expression levels of Fnp1 and Hamp (n = 8/group). AU, arbitrary units; RE, relative expression. *P < 0.05 compared with the respective placebo group; #P < 0.05 compared with the WT + iron group.
Assessment of cardiac function in response to iron overload.
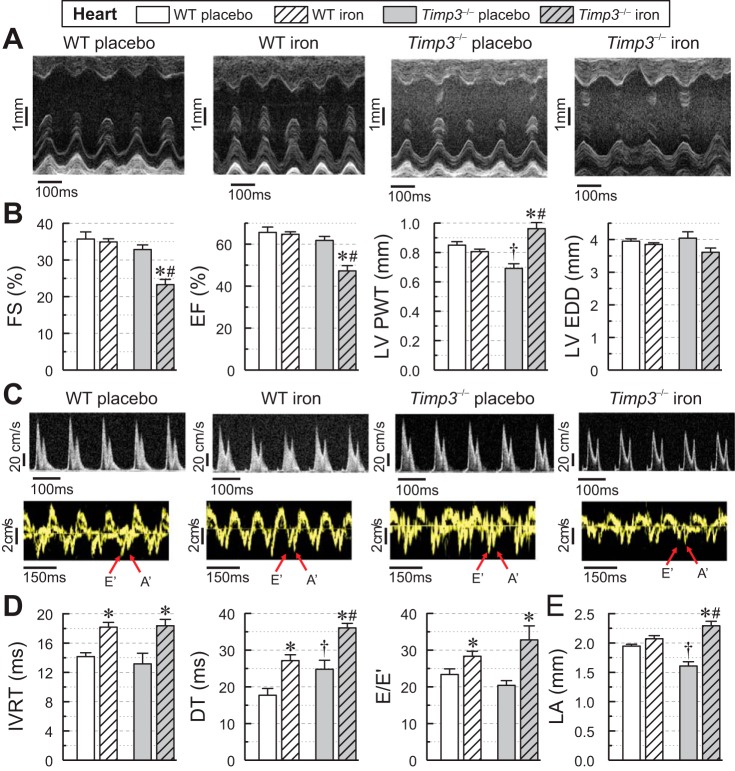
Cardiac function was assessed in four groups using echocardiography. Iron overload resulted in systolic dysfunction in Timp3−/− mice, as indicated by a significant reduction in fractional shortening and ejection fraction observed in iron-overloaded Timp3−/− mice but not in WT mice (Fig. 2, A and B). These changes occurred in the setting of increased ventricular wall thickness without a significant ventricular dilation in the iron-overloaded Timp3−/− group (Fig. 2B). Both genotypes developed diastolic dysfunction in response to iron overload as manifested by transmitral filling pattern and tissue Doppler imaging (Fig. 2C), with a greater increase in isovolumic relaxation time, deceleration time, and the E-to-E′ ratio in iron-overloaded Timp3−/− mice (Fig. 2D). Importantly, only Timp3−/− hearts had an increase in left atrial dimensions, likely driven by the worsened diastolic dysfunction and systolic dysfunction in these hearts (Fig. 2E). Invasive measurement of cardiac function by pressure-volume loops confirmed the development of systolic dysfunction in response to iron overload in Timp3−/− mice but not in WT mice, as evident from reductions in ejection fraction, preload-independent contractility (dP/dtmax/end-diastolic volume), and the end-systolic pressure volume relationship (Table 1). These results demonstrate that loss of Timp3 leads to the development of systolic dysfunction in addition to worsening diastolic dysfunction in response to iron overload.
Fig. 2.
Echocardiographic assessment of cardiac function in wild-type (WT) and tissue inhibitor of metalloproteinase 3 knockout (Timp3−/−) mice in response to iron overload. A: representative M-mode images. B: quantitative assessment of systolic function based on fractional shortening (FS), ejection fraction (EF), left ventricular posterior wall thickness (LVPWT), and left ventricular end-diastolic dimensions (LVEDD) showing systolic dysfunction in iron-overloaded Timp3−/− hearts (n = 10–15/group). C: representative transmitral valve filling profiles (top) and tissue Doppler images (bottom) from WT and Timp3−/− hearts. D: quantitative assessment of diastolic function based on isovolumic relaxation time (IVRT), deceleration time (DT), and peak of E wave (E) to early diastolic tissue Doppler velocity (E′) (E-to-E′ ratio), showing worsening diastolic dysfunction in iron-overloaded Timp3−/− mice (n = 10–15 per group). E: echocardiographic measurements of left atrial size (LA; n = 10–15/group). *P < 0.05 compared with the respective placebo group; #P < 0.05 compared with the WT + iron group; †P < 0.05 compared with the WT + placebo group.
Table 1.
Pressure-volume loop assessment of cardiac function in chronically iron-overloaded male WT and Timp3−/− mice
| Parameters | WT + Placebo | WT + Iron | Timp3−/− + Placebo | Timp3−/− + Iron |
|---|---|---|---|---|
| Number of animals/group | 8 | 8 | 10 | 10 |
| Heart rate, beats/min | 540 ± 9 | 485 ± 14 | 513 ± 18 | 452 ± 22 |
| Left ventricular end-systolic pressure, mmHg | 88.00 ± 1.26 | 96.50 ± 2.05* | 86.90 ± 2.02 | 74.00 ± 2.94*† |
| Left ventricular end-diastolic volume, µl | 24.80 ± 1.08 | 26.00 ± 2.94 | 29.20 ± 2.58 | 49.38 ± 8.88*† |
| Left ventricular end-systolic volume, µl | 6.83 ± 0.77 | 7.10 ± 0.94 | 7.17 ± 1.42 | 21.78 ± 5.21*† |
| Stroke volume, µl | 18.00 ± 0.32 | 18.90 ± 2.50 | 22.00 ± 1.85‡ | 27.60 ± 3.99*† |
| Ejection fraction, % | 72.60 ± 2.00 | 72.70 ± 3.50 | 75.30 ± 3.23 | 55.80 ± 4.61*† |
| dP/dtmax/end-diastolic volume, mmHg·s−1·µl−1 | 357.0 ± 19.5 | 351.0 ± 39.9 | 329.0 ± 33.8 | 162.5 ± 22.8*† |
| End-systolic pressure-volume relation, mmHg/µl | 3.21 ± 0.24 | 3.38 ± 0.52 | 3.62 ± 0.32 | 1.60 ± 0.20*† |
Values are means ± SE. WT, wild-type; Timp3−/−, tissue inhibitor of metalloproteinase 3 knockout.
P < 0.05 compared with the corresponding placebo group;
P < 0.05 between iron groups;
P < 0.05 between placebo groups.
Cardiac iron overload and fibrosis.
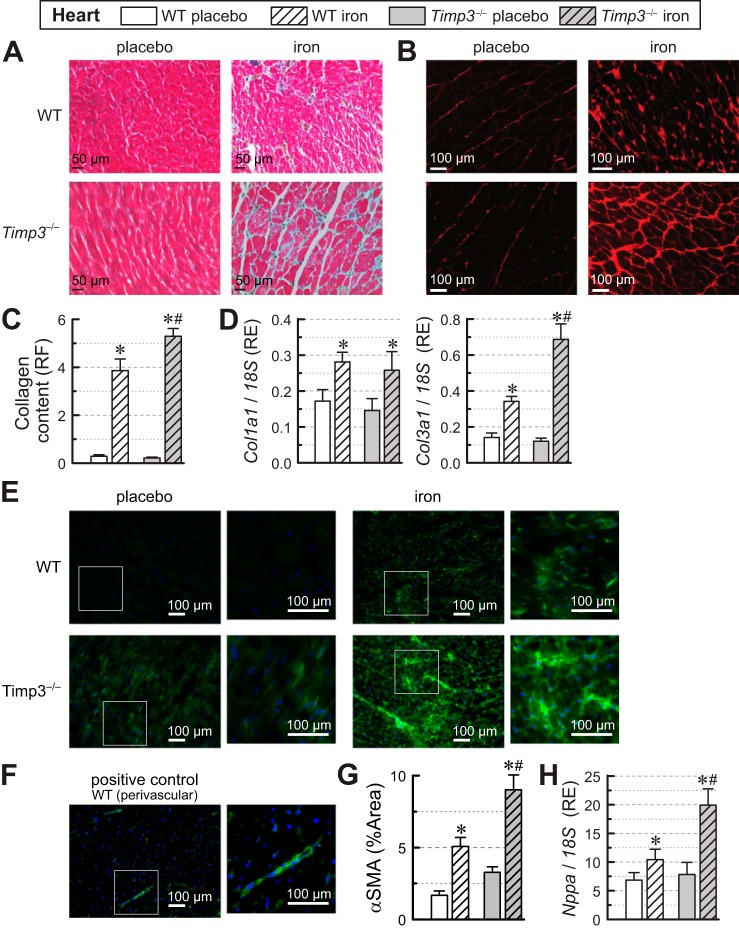
Assessment of myocardial fibrosis using trichrome (Fig. 3A) and PSR (Fig. 3B) stainings of heart sections and quantification of fibrosis from PSR fluorescence (Fig. 3C) revealed elevated levels of interstitial fibrosis in the iron-injected groups with a greater degree of fibrosis for the Timp3−/− + iron group. Myocardial expression of procollagen type I-α1 (Col1a1) was upregulated similarly in the iron-injected groups of both genotypes, whereas procollagen type III-α1 (Col3a1) expression showed significantly greater levels in Timp3−/− + iron hearts (Fig. 3D). We also used α-smooth muscle actin (α-SMA) staining to assess changes in activated fibroblasts attributable to iron overload (Fig. 3E). Perivascular smooth muscle cells were used as a positive control (Fig. 3F). Calculation of the area of α-SMA staining above threshold (Fig. 3G) showed that, in response to iron overload, both WT and Timp3−/− hearts had more activated fibroblasts, and the increase in activated fibroblasts was greater for Timp3−/− hearts than for WT hearts. α-SMA staining showed a pattern similar to Col3a1 expression and PSR staining. Overall, the disease state of the heart was assessed using atrial natriuretic factor (Nppa; Fig. 3H), a heart disease marker. Both iron-overloaded groups had elevated expression of Nppa with Timp3−/− + iron hearts showing clearly greater Nppa levels than WT + iron hearts (Fig. 3H), suggesting more severe heart disease in Timp3−/− + iron hearts than in WT + iron hearts.
Fig. 3.
Fibrosis and expression of fibrotic genes in wild-type (WT) and tissue inhibitor of metalloproteinase 3 knockout (Timp3−/−) hearts in response to iron overload. A: representative images for trichrome staining. B: representative images for picrosirius red (PSR). C: quantification of collagen content from PSR staining expressed as relative fluorescence (RF; n = 12 per group). D: myocardial expression levels of procollagen type I-α1 (Col1a1) and procollagen type III-α1 (Col3a1) in WT and Timp3−/− hearts showing greater profibrotic expression in iron-overloaded Timp3−/− hearts (n = 6–8 per group). RE, relative expression. E: representative images of α-smooth muscle actin (α-SMA) staining of cardiac sections. F: positive control α-SMA staining showing the perivascular area. G: quantification of α-SMA staining expressed as percent area (%Area) with intensity above threshold to exclude background. H: myocardial expression levels of atrial natriuretic factor (Nppa; n = 6–8 per group). *P < 0.05 compared with the respective placebo group; #P < 0.05 compared with the WT + iron group.
Cardiac iron overload and inflammation.
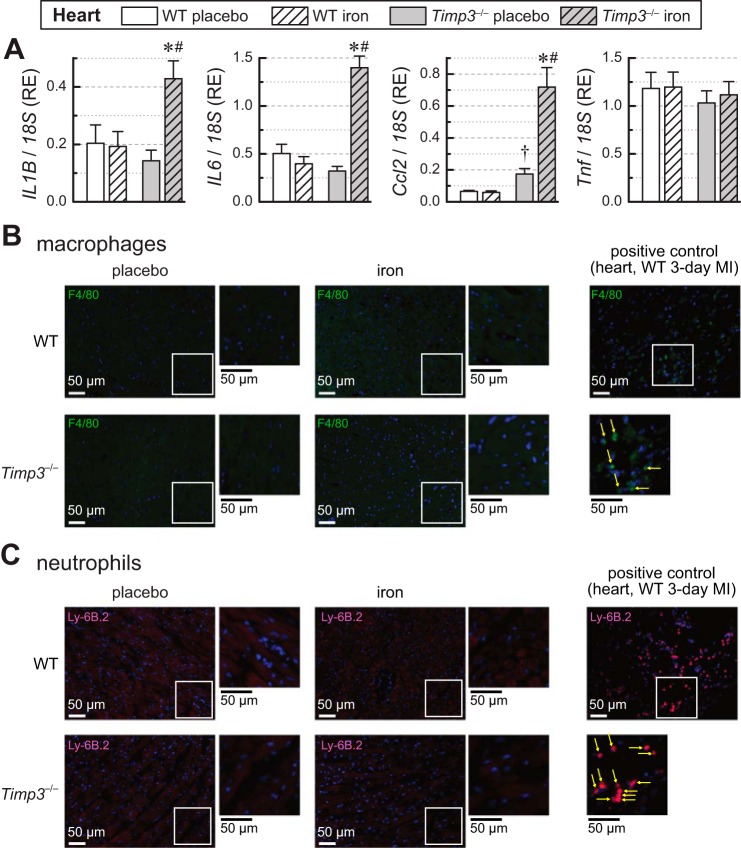
Given the key role of TIMP3 in inflammation (27, 32, 37, 41), we also assessed the myocardial expression of proinflammatory cytokines in response to iron overload (Fig. 4A). Although the expression of cytokines in iron-overloaded WT hearts did not increase, expression of IL-1β (IL1B), IL-6, and monocyte chemoattractant protein-1 [MCP-1; chemokine (C-C motif) ligand 2 (Ccl2)] was markedly increased in Timp3−/− + iron hearts, whereas TNF-α expression (Tnf) remained comparable in all groups (Fig. 4A). Despite this increase in expression of inflammatory markers, infiltration of macrophages and neutrophils in the Timp3−/− + iron myocardium was not detectable (Fig. 4, B and C, for macrophages and neutrophils, respectively, with sections of 3-day myocardial infection used as a positive control).
Fig. 4.
Inflammation and expression of proinflammatory cytokines in wild-type (WT) and tissue inhibitor of metalloproteinase 3 knockout (Timp3−/−) hearts in response to iron overload. A: expression levels of the myocardial inflammatory cytokines IL-1β (IL1B), IL-6 (IL6), monocyte chemotactic protein 1 [MCP-1; chemokine (C-C motif) ligand 2 (Ccl2)], and TNF-α (Tnf) showing greater upregulation of proinflammatory cytokine expression in Timp3−/− iron-overloaded hearts (n = 6–8 per group). RE, relative expression. B: representative images of macrophage (F4/80 + CY-3) staining with positive control images. MI, myocardial infection. C: representative images of neutrophil (Ly-6B.2 +Alexa594) staining with positive control images. *P < 0.05 compared with the respective placebo group; #P < 0.05 compared with the WT + iron group; †P < 0.05 compared with the WT + placebo group.
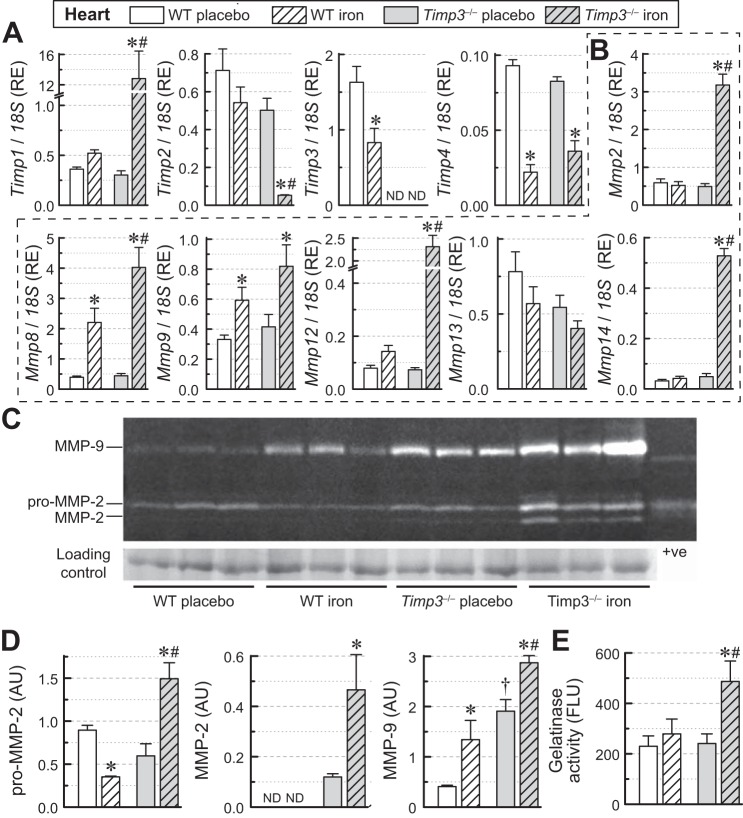
Cardiac iron overload and adverse ECM remodeling.
Remodeling of the myocardial ECM is regulated by a balance between the action of MMPs and TIMPs (21). Iron overload markedly increased the expression of Timp1 and decreased expression of Timp2 in Timp3−/− + iron hearts but not in WT + iron hearts, whereas Timp4 mRNA decreased similarly in both genotypes (Fig. 5A). Importantly, Timp3 levels were lowered in WT iron-overloaded hearts (Fig. 5A). The major MMPs in the heart include MMP-2, MMP-8, MMP-9, MMP-12, MMP-13, and MMP-14 (21). Expression of Mmp2 and Mmp12 was markedly increased in iron-overloaded Timp3−/− hearts but not in WT hearts, whereas expression of Mmp8 and Mmp9 increased in both genotypes, with Mmp8 showing a greater increase in iron-overloaded Timp3−/− hearts (Fig. 5B). In contrast, no significant change was detected for Mmp13, whereas Mmp14 was markedly elevated in iron-overloaded Timp3−/− hearts (Fig. 5B). Gelatin zymography (Fig. 5, C and D) showed that loss of Timp3 resulted in a greater increase in pro-MMP-2, MMP-2, and MMP-9 levels in response to iron overload (Fig. 5, C and D). These changes were in agreement with elevated gelatinase activity in iron-overloaded Timp3−/− hearts (Fig. 5E). Our results demonstrate that Timp3−/− mice display increased and aberrant myocardial fibrosis, increased expression of proinflammatory cytokines, and upregulation of the myocardial MMP axis in response to iron-mediated injury.
Fig. 5.
Changes in myocardial extracellular matrix gene expression, protein levels, and gelatinase activity in wild-type (WT) and tissue inhibitor of metalloproteinase (Timp)3 knockout (Timp3−/−) mice subjected to iron overload. A: mRNA expression levels of TIMPs (Timp1, Timp2, Timp3, and Timp4). RE, relative expression. B: mRNA expression levels of matrix metalloproteinases (MMPs) (Mmp2, Mmp8, Mmp9, Mmp12, Mmp13, and Mmp14; n = 6–8 per group). C: gelatin zymography indicating levels of myocardial pro-MMP-2, MMP-2, and MMP-9. D: quantification of gelatin zymography (C) showing the greater increase in pro-MMP-2, MMP-2, and MMP-9 levels in iron-overloaded Timp3−/− hearts (n = 3/group). ND, not detected; AU, arbitrary units. E: total gelatinase activity showing increased gelatinase activity in iron-overloaded Timp3−/− hearts (n = 6 per group). FLU, fluorescent light units. *P < 0.05 compared with the respective placebo group; #P < 0.05 compared with the WT iron-overloaded group; †P < 0.05 compared with the WT + placebo group.
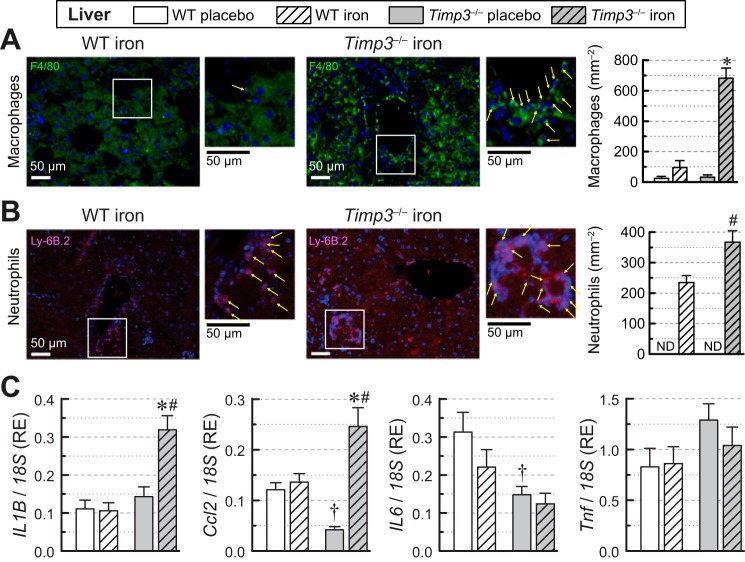
Hepatic iron overload and inflammation.
Immunofluorescence staining for macrophages and neutrophils revealed that hepatic iron overload in Timp3−/− mice resulted in greater infiltration of macrophages with an increase in cell count (Fig. 6A). Similarly, neutrophils were more prevalent in iron-overloaded Timp3−/− livers (Fig. 6B) in the absence of basal inflammation (data not shown). Whereas iron overload did not affect the expression of inflammatory markers in WT livers, IL1B and Ccl2 levels were markedly increased in iron-overloaded Timp3−/− livers (Fig. 6C). Expression of IL6 and Tnf remained unchanged with lowered hepatic IL-6 expression seen in the Timp3−/− genotype (Fig. 6C). These results demonstrate that loss of Timp3 exacerbates the hepatic inflammatory response to iron overload.
Fig. 6.
Inflammation in livers of wild-type (WT) and tissue inhibitor of metalloproteinase 3 knockout (Timp3−/−) mice in response to iron overload. A: immunofluorescence staining of hepatic sections (representative images and quantification) of macrophages (F4/80 + CY-3; n = 4 per group). B: immunofluorescence staining of hepatic sections (representative images and quantification) of neutrophils (Ly-6B.2; n = 4 per group). C: mRNA expression levels of the hepatic inflammatory markers IL-1β (IL1B), monocyte chemoattractant protein 1 [MCP-1; chemokine (C-C motif) ligand 2 (Ccl2)], IL-6 (IL6), and TNF-α (Tnf) showing increased expression of IL-1β and MCP-1 in iron-overloaded Timp3−/− livers (n = 6–8 per group). RE, relative expression. *P < 0.05 compared with the respective placebo group; #P < 0.05 compared with the WT iron-overloaded group; †P < 0.05 compared with the WT + placebo group.
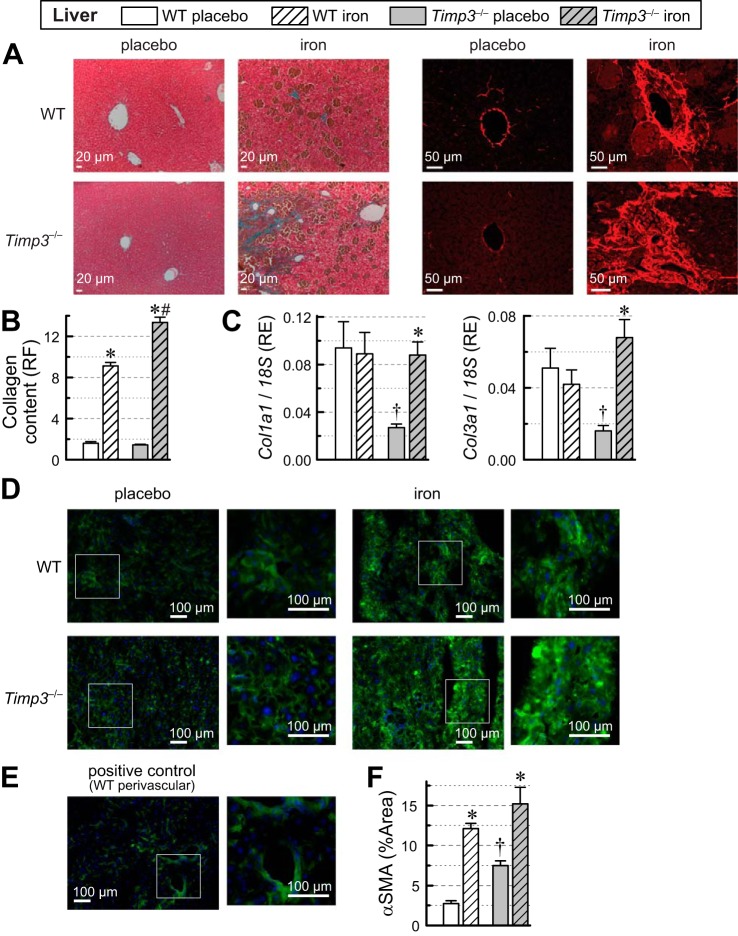
Hepatic iron overload and fibrosis.
Assessment of hepatic fibrosis using trichrome and PSR staining of the liver (Fig. 7A) revealed increased hepatic fibrosis in the iron-overloaded groups with a greater increase in the Timp3−/− iron-overloaded liver (Fig. 7B). The increase in hepatic collagen deposition correlated with an increase in the expression of Col1a1 and Col3a1 in Timp3−/− but not in WT livers in response to iron overload (Fig. 7C). An alternative assessment of fibrosis by α-SMA, a marker of active fibroblasts and smooth muscle cells, showed that Timp3−/− livers had an elevated α-SMA area above threshold at baseline and that the α-SMA area increased in response to iron overload (Fig. 7D), positive control (smooth muscle cells of perivascular area; Fig. 7E), and quantification of α-SMA area above threshold (excluding perivascular areas; Fig. 7F), suggesting higher fibroblast activity in Timp3−/− livers at baseline and increased fibroblast activity in response to iron overload. These results demonstrate that loss of Timp3 exacerbates the hepatic fibrotic response to iron overload.
Fig. 7.
Fibrosis in livers of wild-type (WT) and tissue inhibitor of metalloproteinase 3 knockout (Timp3−/−) mice in response to iron overload. A: representative images for trichrome (left) and picrosirius red (PSR; right) staining of liver sections and quantification of hepatic fibrosis from PSR staining (right) of hepatic sections. B: quantification of collagen content from PSR staining expressed as relative fluorescence (RF; n = 12 per group). C: hepatic expression of procollagen type I-α1 (Col1a1) and procollagen type III-α1 (Col3a1) (n = 6–8 per group). RE, relative expression. D: representative images of α-smooth muscle actin (α-SMA) staining of hepatic sections. E: positive control α-SMA staining showing perivascular area. F: quantification of α-SMA staining expressed as percent area (%Area) with intensity above threshold to exclude background. *P < 0.05 compared with the respective placebo group; #P < 0.05 compared with the WT iron-overloaded group; †P < 0.05 compared with the WT + placebo group.
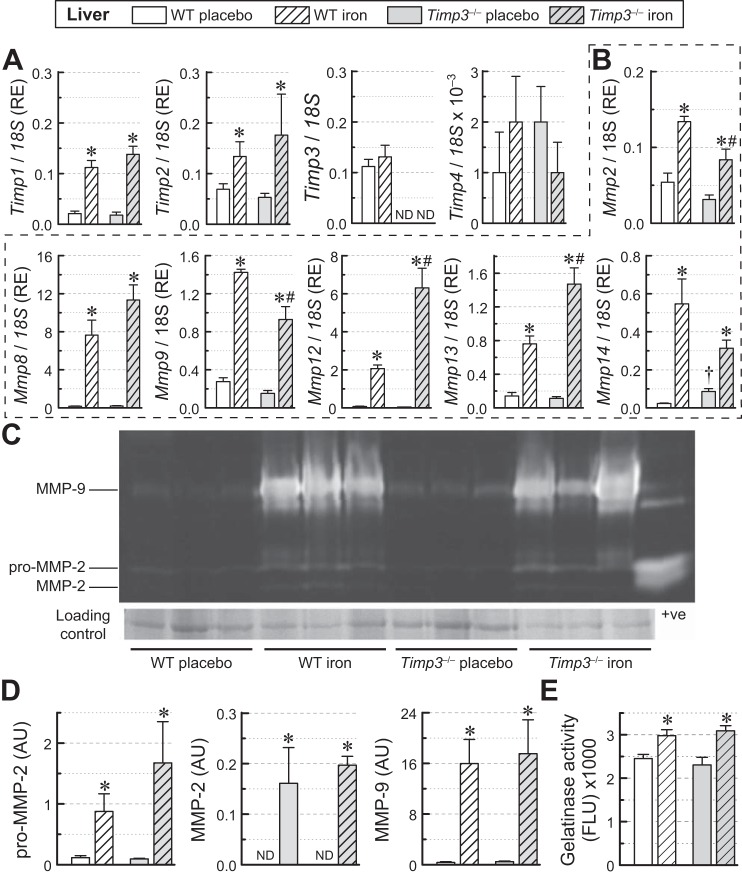
Hepatic iron overload and adverse ECM remodeling.
Iron overload similarly increased Timp1 and Timp2 expression in WT and Timp3−/− livers (Fig. 8A), whereas Timp3 levels were unchanged in WT livers, and Timp4 expression levels were negligible in both genotypes (Fig. 8A). Hepatic MMP expression analysis in response to iron overload showed similar increases in the expression of Mmp8, smaller increases of Mmp2, Mmp9, and Mmp14 expression for Timp3−/− livers, and larger increases of Mmp12 and Mmp13 expression for Timp3−/− livers (Fig. 8B). Gelatin zymography (Fig. 8, C and D) showed that loss of Timp3 resulted in marked but similar increases in pro-MMP-2, MMP-2, and MMP-9 levels in response to iron overload in livers of both genotypes. Total gelatinase activity was not dependent on genotype, but iron-treated groups displayed similarly elevated gelatinase activity (Fig. 8E). Our results indicate that iron overload-mediated differential regulation of hepatic MMPs is associated with increased MMP expression in the absence of Timp3.
Fig. 8.
Changes in hepatic extracellular matrix gene expression and protein levels in wild-type (WT) and tissue inhibitor of metalloproteinase (Timp)3 knockout (Timp3−/−) mice in response to iron overload. A: mRNA expression levels of TIMPs (Timp1, Timp2, Timp3, and Timp4; n = 6–8 per group). RE, relative expression. B: mRNA expression levels of matrix metalloproteinases (MMPs) (Mmp2, Mmp8, Mmp9, Mmp12, Mmp13, and Mmp14; n = 6–8 per group). C: gelatin zymography indicating levels of myocardial pro-MMP-2, MMP-2, and MMP-9. D: quantification of gelatin zymography (C) showing the greater increase in pro-MMP-2, MMP-2, and MMP-9 levels in iron-overloaded Timp3−/− hearts (n = 3 per group). AU, arbitrary units; ND, not detected. E: total gelatinase activity in the liver (n = 6 per group). FLU, fluorescent light units. *P < 0.05 compared with the respective placebo group; #P < 0.05 compared with the WT iron-overloaded group; †P < 0.05 compared with the WT + placebo group.
DISCUSSION
Cardiac and hepatic iron overload in patients.
Iron overload-related mortality attributable to liver disease occurs mostly in the case of hereditary iron overload, whereas heart failure as a cause of death is associated mostly with transfusion-induced iron overload (5, 19, 44). Although cardiomyopathy is a major cause of heart failure in patients with primary hemochromatosis and secondary iron overload (4, 44, 51), the liver accumulates higher levels of iron, and the accumulation happens at earlier stages of iron overload (12, 48). Liver disease resulting from iron overload leads to end-stage cirrhosis and/or hepatocellular carcinoma and contributes to the morbidity and mortality of patients with iron overload (2, 4, 8). Liver disease mortality is mostly associated with hereditary iron overload, whereas heart failure is prevalent in transfusion-induced iron overload (5, 19, 44). Transfusion patients with thalassemia major accumulate more iron in the liver (up to 35 mg/g dry wt) than in the heart (up to 8 mg/g dry wt) (48). Similarly, patients on hemodialysis accumulated iron predominantly in the liver (60).
TIMP3 and cardiac function in iron overload in the mouse model.
In the heart, TIMP3 is expressed in all cell types (cardiomyocytes, fibroblasts, smooth muscle cells, and endothelial cells) (10, 36, 59). Knockout of Timp3 in mice left basal myocardial function unchanged. However, in the settings of iron overload, despite a similar degree of myocardial iron overload between WT and Timp3−/− hearts, cardiac function in Timp3−/− mice was characterized by 1) systolic dysfunction and worsened diastolic function compared with WT mice, 2) a higher degree of cardiac and hepatic fibrosis, 3) overall higher expression and activity of MMPs, and 4) higher expression of the inflammatory cytokines IL-1β, IL-6, and MCP-1 in the absence of overt cellular infiltration. Timp3 deficiency may exacerbate cardiomyopathy because of a number of mechanisms: 1) facilitation of general inflammatory response attributable to lack of moderation of the differentiation of macrophages into proinflammatory (M1) cells by TIMP3 (26, 27), 2) increased shedding of TNF-α in the liver attributable to elevated TNF-α-converting enzyme activity (37, 38, 41), and 3) lack of direct inhibitory action of TIMP3 on MMPs.
Inflammation, in general, is associated with higher proteolytic activity of MMPs (23), which correlates with our observations here (increased expression of inflammatory cytokines and higher expression of multiple MMPs). That increased proteolytic activity can promote fibrosis via activation of transforming growth factor-β (TGF-β); MMP-2 and MMP-14 are known to convert TGF-β to the active form (35, 62), leading to increased fibrosis. Here, we found upregulation of expression of these MMPs and increased activity of MMP-2 in Timp3−/− iron hearts associated with higher fibrosis, which is consistent with activation of TGF-β pathway.
Increased shedding of TNF-α will also promote excessive fibrosis because TNF-α and TNF-α receptor 1 are essential in profibrotic signaling (17, 57), suggesting that a TNF-α-dependent mechanism may exacerbate iron overload-induced fibrosis. Besides that, the excessive release of TNF-α by itself can inhibit myocardial contractility (7, 18), contributing to the development of systolic dysfunction in Timp3−/− + iron mice but not in WT + iron mice.
Lack of a direct inhibitory action of TIMP3 on MMPs could also contribute to increased activities of MMP-2 and MMP-14 in Timp3−/− hearts, which could lead to TGF-β activation and additional fibrosis. In addition, a role of intracellular MMP-2 has been proposed to cleave titin (3) or other contractile proteins (11, 16), contributing to the exacerbated cardiac function in iron-overloaded Timp3−/− hearts.
TIMP3 and hepatic function in iron overload in the mouse model.
In the liver, hepatocytes and stellate cells are the major sources of TIMP3, with hepatocytes supplying TIMP3 under basal conditions and stellate cells being most active during inflammation (45). At baseline, Timp3 deletion was associated with a number of puzzling changes in the liver, including the following: 1) lower protein levels of ferroportin without difference in expression levels, 2) higher fibroblast activity with lower baseline expression of procollagens (Col1a1 and Col3a1), and 3) lower baseline expression of MCP-1 (Ccl2) and IL-6 (IL6). Lower levels of ferroportin at the baseline (Timp3−/− livers) are hard to explain within the present framework of regulation of ferroportin protein levels. We have not found alterations of the hepcidin levels or iron levels at baseline conditions attributable to genotype. Although it is possible that ferroportin undergoes degradation by MMPs, such as MMP-14 that is upregulated in Timp3−/− livers, the substantial upregulation of MMP-14 in Timp3−/− + iron hearts was not associated with a change in ferroportin levels. Lower levels of procollagen expression at baseline may be a result of lower turnover of the collagens in Timp3−/− livers attributable to increased posttranslational stabilization of collagen by matricellular proteins (20, 25). Lower levels of expression of cytokines (Ccl2 and IL6) could be a compensatory decrease in response to higher levels of shedding of TNF-α without changes in TNF-α expression previously observed (41). Timp3 deficiency also altered the response to hepatic iron overload. Ferroportin protein levels in Timp3−/− livers were downregulated to the levels lower than in WT + iron livers. This is consistent with 1) increased protein levels of hepcidin, which is known to promote ferroportin internalization and degradation (55, 63), and 2) higher iron levels that themselves can facilitate binding of hepcidin to ferroportin via Fe3+-dependent mechanism (22). Expression levels of either protein were not dependent on genotype. The inflammatory response was enhanced in Timp3−/− + iron livers, including increased expression of cytokines (IL1B and Ccl2) and infiltration of inflammatory cells above levels observed in WT + iron livers.
Because iron overload promotes an inflammatory response in WT livers (at least in terms of inflammatory cell infiltration), the exacerbated inflammatory response in Timp3−/− livers is in line with the lack of moderation of inflammation by TIMP3 (26, 27). Excessive hepatic fibrosis in Timp3−/− livers is not supported by a parallel increase in procollagen expression or activation of fibroblasts (α-SMA staining) compared with WT + iron livers. There are two possible explanations of such discrepancy. First, if Timp3−/− genotype is associated with enhanced stabilization of collagen attributable to posttranslational modifications by matricellular proteins (20, 25), then a smaller absolute increase in collagen production is sufficient to achieve higher levels of fibrosis. Second, the upregulation of procollagens occurs earlier and transiently in the time course of iron overload injury. Changes in MMP expressions were somewhat mixed. Although all MMPs had been upregulated in Timp3−/− + iron and WT + iron livers compared with respective controls, upregulation of expression was blunted for MMP-2 and MMP-9, was higher for MMP-14, was about the same for MMP-8, and was enhanced for MMP-12 and MMP-13. These changes in expression resulted in overall upregulation of gelatinase activity that was independent of genotype, suggesting that, in the liver, overall MMP activity correlates less with inflammation and fibrosis.
Interplay of TIMP3 with other TIMPs in the settings of iron overload.
In the heart, but not in the liver, Timp3−/− deficiency was associated with changes in TIMP expression in response to iron overload. Timp1 was substantially upregulated and Timp2 was substantially downregulated in Timp3−/− + iron hearts compared with WT + iron hearts. This reduction in Timp2 levels may have contributed to development of hypertrophy (increase in posterior wall thickness) because Timp2 deficiency has been already associated with cardiac hypertrophy in response to angiotensin II infusion (20). What effect excessive Timp1 expression will have on cardiac fibrosis is unclear. The only study that attempted to overexpress Timp1 was done with transplanted embryonic stem cells overexpressing Timp1, and these cells seemingly reduced apoptosis, fibrosis, and hypertrophy (28), none of which were observed in this model.
Timp3−/− mice with iron-dextran injections as an iron toxicity model.
In this model, we have achieved cardiac iron levels of 20 mg/g and hepatic iron levels of 65 mg/g, both of which are considerably higher than observed in patients undergoing transfusion with thalassemia major, who accumulate up to 8 mg/g iron and up to 35 mg/g in the heart and liver, respectively (48). Both genotypes had the same levels of iron overload (20 mg/g), but only Timp3−/− showed systolic dysfunction, making it phenotypically closer to advanced iron overload cardiomyopathy in patients, which also exhibits reduced systolic function (40, 44). The overall cardiac state achieved in Timp3−/− + iron mice can be characterized as an exacerbation of iron overload cardiomyopathy because there was significant impairment in systolic (reduced fractional shortening and ejection fraction) and diastolic (increased deceleration time and left atrial size) function with pathological hypertrophy (increased posterior wall thickness and heart weight) in the absence of overt heart failure. However, because deletion was not tissue specific, systemic effects of Timp3 deficiency and exacerbated hepatic injury may also contribute to deterioration of systolic function via circulating TNF-α or other unknown factors. Liver disease is known to be associated with progressive diastolic dysfunction and the possibility of the development of systolic dysfunction at later stages (34, 42).
Conclusions.
Iron overload leads to well-defined heart and liver diseases. Loss of Timp3 enhances the susceptibility to iron overload-mediated cardiomyopathy and liver disease, suggesting a key role for TIMP3 in control of the ECM, inflammation, and possibly contractility during the pathogenesis in iron overload conditions. Strategies aimed at enhancing TIMP3 function may serve to minimize iron-mediated injury.
Limitations.
Limitations of the present study are as follows: only one time point of data collection was used, which prevented assessing the time course of the changes in collagen turnover and inflammatory cell infiltration. Liver function was not assessed to elucidate effect of Timp3 deficiency and iron overload on the liver and possible systemic influence arising from deficiency in liver function.
GRANTS
This work was supported by Alberta Innovates-Health Solutions, Canadian Institutes of Health Research Operating Grant 285071, and the Heart and Stroke Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.Z., S.K.D., Z.K., and G.Y.O. conceived and designed research; P.Z., S.K.D., R.B., M.S., and V.B.P. performed experiments; P.Z., S.K.D., R.B., M.S., and V.B.P. analyzed data; P.Z., S.K.D., R.B., M.S., and V.B.P. interpreted results of experiments; P.Z., S.K.D., and R.B. prepared figures; P.Z. and S.K.D. drafted manuscript; P.Z. and Z.K. edited and revised manuscript; G.Y.O. approved final version of manuscript.
ACKNOWLEDGMENTS
G. Y. Oudit is the guarantor of this work and, as such, had full access to all data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Abu Rajab M, Guerin L, Lee P, Brown KE. Iron overload secondary to cirrhosis: a mimic of hereditary haemochromatosis? Histopathology 65: 561–569, 2014. doi: 10.1111/his.12417. [DOI] [PubMed] [Google Scholar]
- 2.Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR, Leiendecker-Foster C, Speechley M, Snively BM, Holup JL, Thomson E, Sholinsky P; Hemochromatosis and Iron Overload Screening (HEIRS) Study Research Investigators . Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 352: 1769–1778, 2005. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 3.Ali MA, Cho WJ, Hudson B, Kassiri Z, Granzier H, Schulz R. Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury. Circulation 122: 2039–2047, 2010. doi: 10.1161/CIRCULATIONAHA.109.930222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, McLaren CE, Bahlo M, Nisselle AE, Vulpe CD, Anderson GJ, Southey MC, Giles GG, English DR, Hopper JL, Olynyk JK, Powell LW, Gertig DM. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med 358: 221–230, 2008. doi: 10.1056/NEJMoa073286. [DOI] [PubMed] [Google Scholar]
- 5.Andrews NC. Disorders of iron metabolism. N Engl J Med 341: 1986–1995, 1999. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 6.Andrews NC. Forging a field: the golden age of iron biology. Blood 112: 219–230, 2008. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ao L, Song Y, Fullerton DA, Dinarello CA, Meng X. The interaction between myocardial depressant factors in endotoxemic cardiac dysfunction: role of TNF-alpha in TLR4-mediated ICAM-1 expression. Cytokine 38: 124–129, 2007. doi: 10.1016/j.cyto.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases . Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 54: 328–343, 2011. doi: 10.1002/hep.24330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton JC, Edwards CQ, Acton RT. HFE gene: structure, function, mutations, and associated iron abnormalities. Gene 574: 179–192, 2015. doi: 10.1016/j.gene.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu R, Lee J, Morton JS, Takawale A, Fan D, Kandalam V, Wang X, Davidge ST, Kassiri Z. TIMP3 is the primary TIMP to regulate agonist-induced vascular remodelling and hypertension. Cardiovasc Res 98: 360–371, 2013. doi: 10.1093/cvr/cvt067. [DOI] [PubMed] [Google Scholar]
- 11.Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, Nguyen A, Dahi S, Karliner JS, Lovett DH. Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol 292: H1847–H1860, 2007. doi: 10.1152/ajpheart.00434.2006. [DOI] [PubMed] [Google Scholar]
- 12.Casale M, Meloni A, Filosa A, Cuccia L, Caruso V, Palazzi G, Gamberini MR, Pitrolo L, Putti MC, D’Ascola DG, Casini T, Quarta A, Maggio A, Neri MG, Positano V, Salvatori C, Toia P, Valeri G, Midiri M, Pepe A. Multiparametric cardiac magnetic resonance survey in children with thalassemia major: a multicenter study. Circ Cardiovasc Imaging 8: e003230, 2015. doi: 10.1161/CIRCIMAGING.115.003230. [DOI] [PubMed] [Google Scholar]
- 13.Das SK, DesAulniers J, Dyck JR, Kassiri Z, Oudit GY. Resveratrol mediates therapeutic hepatic effects in acquired and genetic murine models of iron-overload. Liver Int 36: 246–257, 2016. doi: 10.1111/liv.12893. [DOI] [PubMed] [Google Scholar]
- 14.Das SK, Patel VB, Basu R, Wang W, DesAulniers J, Kassiri Z, Oudit GY. Females are protected from iron-overload cardiomyopathy independent of iron metabolism: key role of oxidative stress. J Am Heart Assoc 6: e003456, 2017. doi: 10.1161/JAHA.116.003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das SK, Wang W, Zhabyeyev P, Basu R, McLean B, Fan D, Parajuli N, DesAulniers J, Patel VB, Hajjar RJ, Dyck JR, Kassiri Z, Oudit GY. Iron-overload injury and cardiomyopathy in acquired and genetic models is attenuated by resveratrol therapy. Sci Rep 5: 18132, 2015. doi: 10.1038/srep18132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeCoux A, Lindsey ML, Villarreal F, Garcia RA, Schulz R. Myocardial matrix metalloproteinase-2: inside out and upside down. J Mol Cell Cardiol 77: 64–72, 2014. doi: 10.1016/j.yjmcc.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duerrschmid C, Trial J, Wang Y, Entman ML, Haudek SB. Tumor necrosis factor: a mechanistic link between angiotensin-II-induced cardiac inflammation and fibrosis. Circ Heart Fail 8: 352–361, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan DJ, Hopkins PM, Harrison SM. Negative inotropic effects of tumour necrosis factor-alpha and interleukin-1beta are ameliorated by alfentanil in rat ventricular myocytes. Br J Pharmacol 150: 720–726, 2007. doi: 10.1038/sj.bjp.0707147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med 32: 833–840, 2002. doi: 10.1016/S0891-5849(02)00772-4. [DOI] [PubMed] [Google Scholar]
- 20.Fan D, Takawale A, Basu R, Patel V, Lee J, Kandalam V, Wang X, Oudit GY, Kassiri Z. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc Res 103: 268–280, 2014. doi: 10.1093/cvr/cvu072. [DOI] [PubMed] [Google Scholar]
- 21.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 5: 15, 2012. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farnaud S, Rapisarda C, Bui T, Drake A, Cammack R, Evans RW. Identification of an iron-hepcidin complex. Biochem J 413: 553–557, 2008. doi: 10.1042/BJ20080406. [DOI] [PubMed] [Google Scholar]
- 23.Fingleton B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim Biophys Acta 1864: 2036–2042, 2017. doi: 10.1016/j.bbamcr.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med 366: 348–359, 2012. doi: 10.1056/NEJMra1004967. [DOI] [PubMed] [Google Scholar]
- 25.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 92: 635–688, 2012. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill SE, Gharib SA, Bench EM, Sussman SW, Wang RT, Rims C, Birkland TP, Wang Y, Manicone AM, McGuire JK, Parks WC. Tissue inhibitor of metalloproteinases-3 moderates the proinflammatory status of macrophages. Am J Respir Cell Mol Biol 49: 768–777, 2013. doi: 10.1165/rcmb.2012-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill SE, Huizar I, Bench EM, Sussman SW, Wang Y, Khokha R, Parks WC. Tissue inhibitor of metalloproteinases 3 regulates resolution of inflammation following acute lung injury. Am J Pathol 176: 64–73, 2010. doi: 10.2353/ajpath.2010.090158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glass C, Singla DK. Overexpression of TIMP-1 in embryonic stem cells attenuates adverse cardiac remodeling following myocardial infarction. Cell Transplant 21: 1931–1944, 2012. doi: 10.3727/096368911X627561. [DOI] [PubMed] [Google Scholar]
- 29.Gulati V, Harikrishnan P, Palaniswamy C, Aronow WS, Jain D, Frishman WH. Cardiac involvement in hemochromatosis. Cardiol Rev 22: 56–68, 2014. doi: 10.1097/CRD.0b013e3182a67805. [DOI] [PubMed] [Google Scholar]
- 30.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell 117: 285–297, 2004. doi: 10.1016/S0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 31.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest 115: 2187–2191, 2005. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kandalam V, Basu R, Abraham T, Wang X, Awad A, Wang W, Lopaschuk GD, Maeda N, Oudit GY, Kassiri Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol 299: H1012–H1023, 2010. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kandalam V, Basu R, Moore L, Fan D, Wang X, Jaworski DM, Oudit GY, Kassiri Z. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation 124: 2094–2105, 2011. doi: 10.1161/CIRCULATIONAHA.111.030338. [DOI] [PubMed] [Google Scholar]
- 34.Karagiannakis DS, Papatheodoridis G, Vlachogiannakos J. Recent advances in cirrhotic cardiomyopathy. Dig Dis Sci 60: 1141–1151, 2015. doi: 10.1007/s10620-014-3432-8. [DOI] [PubMed] [Google Scholar]
- 35.Karsdal MA, Larsen L, Engsig MT, Lou H, Ferreras M, Lochter A, Delaissé JM, Foged NT. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem 277: 44061–44067, 2002. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- 36.Kassiri Z, Defamie V, Hariri M, Oudit GY, Anthwal S, Dawood F, Liu P, Khokha R. Simultaneous transforming growth factor beta-tumor necrosis factor activation and cross-talk cause aberrant remodeling response and myocardial fibrosis in Timp3-deficient heart. J Biol Chem 284: 29893–29904, 2009. doi: 10.1074/jbc.M109.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol 20: 1223–1235, 2009. doi: 10.1681/ASN.2008050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kassiri Z, Oudit GY, Sanchez O, Dawood F, Mohammed FF, Nuttall RK, Edwards DR, Liu PP, Backx PH, Khokha R. Combination of tumor necrosis factor-alpha ablation and matrix metalloproteinase inhibition prevents heart failure after pressure overload in tissue inhibitor of metalloproteinase-3 knock-out mice. Circ Res 97: 380–390, 2005. doi: 10.1161/01.RES.0000178789.16929.cf. [DOI] [PubMed] [Google Scholar]
- 39.Ko C, Siddaiah N, Berger J, Gish R, Brandhagen D, Sterling RK, Cotler SJ, Fontana RJ, McCashland TM, Han SH, Gordon FD, Schilsky ML, Kowdley KV. Prevalence of hepatic iron overload and association with hepatocellular cancer in end-stage liver disease: results from the National Hemochromatosis Transplant Registry. Liver Int 27: 1394–1401, 2007. doi: 10.1111/j.1478-3231.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 40.Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation 124: 2253–2263, 2011. doi: 10.1161/CIRCULATIONAHA.111.050773. [DOI] [PubMed] [Google Scholar]
- 41.Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, Khokha R. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet 36: 969–977, 2004. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- 42.Møller S, Wiese S, Halgreen H, Hove JD. Diastolic dysfunction in cirrhosis. Heart Fail Rev 21: 599–610, 2016. doi: 10.1007/s10741-016-9552-9. [DOI] [PubMed] [Google Scholar]
- 43.Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, Desaulniers J, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Angiotensin 1–7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail 7: 327–339, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000672. [DOI] [PubMed] [Google Scholar]
- 44.Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. J Card Fail 16: 888–900, 2010. doi: 10.1016/j.cardfail.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Murthy A, Shao YW, Defamie V, Wedeles C, Smookler D, Khokha R. Stromal TIMP3 regulates liver lymphocyte populations and provides protection against Th1 T cell-driven autoimmune hepatitis. J Immunol 188: 2876–2883, 2012. doi: 10.4049/jimmunol.1102199. [DOI] [PubMed] [Google Scholar]
- 46.Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, Yazdanpanah M, Wilson GJ, Schwartz A, Liu PP, Backx PH. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med 9: 1187–1194, 2003. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 47.Oudit GY, Trivieri MG, Khaper N, Husain T, Wilson GJ, Liu P, Sole MJ, Backx PH. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation 109: 1877–1885, 2004. doi: 10.1161/01.CIR.0000124229.40424.80. [DOI] [PubMed] [Google Scholar]
- 48.Ouederni M, Ben Khaled M, Mellouli F, Ben Fraj E, Dhouib N, Yakoub IB, Abbes S, Mnif N, Bejaoui M. Myocardial and liver iron overload, assessed using T2* magnetic resonance imaging with an Excel spreadsheet for post processing in Tunisian thalassemia major patients. Ann Hematol 96: 133−139, 2017. doi: 10.1007/s00277-016-2841-5. [DOI] [PubMed] [Google Scholar]
- 49.Patel VB, Bodiga S, Basu R, Das SK, Wang W, Wang Z, Lo J, Grant MB, Zhong J, Kassiri Z, Oudit GY. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ Res 110: 1322–1335, 2012. doi: 10.1161/CIRCRESAHA.112.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, Parajuli N, Penninger JM, Grant MB, Lopaschuk GD, Oudit GY. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes 65: 85–95, 2016. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pennell DJ, Udelson JE, Arai AE, Bozkurt B, Cohen AR, Galanello R, Hoffman TM, Kiernan MS, Lerakis S, Piga A, Porter JB, Walker JM, Wood J; American Heart Association Committee on Heart Failure and Transplantation of the Council on Clinical Cardiology and Council on Cardiovascular Radiology and Imaging . Cardiovascular function and treatment in β-thalassemia major: a consensus statement from the American Heart Association. Circulation 128: 281–308, 2013. doi: 10.1161/CIR.0b013e31829b2be6. [DOI] [PubMed] [Google Scholar]
- 52.Pietrangelo A. Hereditary hemochromatosis–a new look at an old disease. N Engl J Med 350: 2383–2397, 2004. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- 53.Pietrangelo A. Non-HFE hemochromatosis. Hepatology 39: 21–29, 2004. doi: 10.1002/hep.20007. [DOI] [PubMed] [Google Scholar]
- 54.Powell LW, Seckington RC, Deugnier Y. Haemochromatosis. Lancet 388: 706–716, 2016. doi: 10.1016/S0140-6736(15)01315-X. [DOI] [PubMed] [Google Scholar]
- 55.Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica 95: 501–504, 2010. doi: 10.3324/haematol.2009.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santos MM, de Sousa M, Rademakers LH, Clevers H, Marx JJ, Schilham MW. Iron overload and heart fibrosis in mice deficient for both beta2-microglobulin and Rag1. Am J Pathol 157: 1883–1892, 2000. doi: 10.1016/S0002-9440(10)64827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sriramula S, Francis J. Tumor necrosis factor-alpha is essential for angiotensin II-induced ventricular remodeling: role for oxidative stress. PLoS One 10: e0138372, 2015. doi: 10.1371/journal.pone.0138372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subramaniam VN, McDonald CJ, Ostini L, Lusby PE, Wockner LF, Ramm GA, Wallace DF. Hepatic iron deposition does not predict extrahepatic iron loading in mouse models of hereditary hemochromatosis. Am J Pathol 181: 1173–1179, 2012. doi: 10.1016/j.ajpath.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 59.Takawale A, Zhang P, Azad A, Wang W, Wang X, Murray AG, Kassiri Z. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis. Am J Physiol Heart Circ Physiol 313: H224–H236, 2017. doi: 10.1152/ajpheart.00108.2017. [DOI] [PubMed] [Google Scholar]
- 60.Tolouian R, Mulla ZD, Diaz J, Aguila J, Ramos-Duran L. Liver and cardiac iron deposition in patients on maintenance hemodialysis by magnetic resonance imaging T2. Iran J Kidney Dis 10: 68–74, 2016. [PubMed] [Google Scholar]
- 61.Trombini P, Paolini V, Pelucchi S, Mariani R, Nemeth E, Ganz T, Piperno A. Hepcidin response to acute iron intake and chronic iron loading in dysmetabolic iron overload syndrome. Liver Int 31: 994–1000, 2011. doi: 10.1111/j.1478-3231.2011.02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol 26: 1503–1509, 2006. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 63.Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta 1823: 1426–1433, 2012. doi: 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhabyeyev P, Oudit GY. Hemochromatosis protein (HFE) knockout mice as a novel model of hemochromatosis: implications for study and management of iron-overload cardiomyopathy. Can J Cardiol 33: 835–837, 2017. doi: 10.1016/j.cjca.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Zhabyeyev P, Oudit GY. Unravelling the molecular basis for cardiac iron metabolism and deficiency in heart failure. Eur Heart J 38: 373−375, 2017. doi: 10.1093/eurheartj/ehw386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 122: 717–728, 2010. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 67.Zurlo MG, De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, Di Gregorio F, Burattini MG, Terzoli S. Survival and causes of death in thalassaemia major. Lancet 2: 27–30, 1989. doi: 10.1016/S0140-6736(89)90264-X. [DOI] [PubMed] [Google Scholar]