Abstract
Objectives
Cognitive dysfunction and dementia are common following ischemic stroke. Endothelial nitric oxide synthase (eNOS) has been found to play an important role in neurological function and cognition. The purpose of the present study was to assess the specific role of eNOS in cognitive performance after stroke.
Design
Male wildtype (WT) and mice lacking eNOS (eNOS−/−) underwent middle cerebral artery occlusion (MCAO) or sham-surgery. Primary outcomes were repeated measures of neurological score, limb asymmetry, sensory/motor function and spatial memory/learning assessed at intervals up to 28-days post-surgery. Group differences in brain microglia activation and infiltration, and levels of interferon-gamma (IFN-γ) were examined.
Results
There was no genotype × surgery interaction effect on the pattern of change in neurological score, limb asymmetry, or sensory motor function across the 28-days post-surgery. In the Morris Water Maze, eNOS−/− MCAO mice displayed learning and memory deficits not evident in WT MCAO mice. Poorer spatial memory and learning in eNOS−/− MCAO mice was associated with a reduction in the number of activated microglia in the striatum on the lesion side and decreased brain tissue levels of IFN-γ.
Conclusions
Our data support a role for eNOS in cognitive performance after stroke. This finding may lead to the development of novel interventions to treat post-stroke cognitive deficits.
Keywords: Stroke, Neuroinflammation, Memory, eNOS
INTRODUCTION
Ischemic stroke is a leading cause of long-term disability in the adult population1. Approximately 30–50% of stroke survivors are functionally disabled2. Post-stroke cognitive impairment is a major disabling symptom following stroke and severely affects long-term recovery. The prevalence of cognitive impairment after stroke is about 25%, and the risk of developing a cognitive deficit increases three-fold after stroke as compared to non-stroke subjects3. The mechanisms underlying post-stroke cognitive dysfunction remain unclear, and there are few effective therapies.
Impaired cerebrovascular perfusion is a risk for cognitive dysfunction4. The cerebral vasculature produces vasoactive mediators that exert local control of blood vessel diameter and consequently tissue perfusion. Nitric oxide (NO) is one of the most important vasoactive factors produced by endothelial cells. Endothelial-derived NO regulates cerebral blood flow by promoting vasodilatation, inhibiting platelet aggregation and leukocyte adherence, and inhibiting vascular smooth muscle cell (VSMC) proliferation5. Endothelial NO synthesis is regulated by the enzyme endothelial nitric oxide synthase (eNOS), which is widely and constitutively expressed within the endothelial lining of the vascular tree. The generation of mice which lack or partially lack eNOS activity has furthered our understanding of endothelial NO function. Mice lacking eNOS develop hypertension6 and have an increased propensity to develop stroke7. In mouse stroke models, eNOS-deficiency results in larger areas of ischemia, worse neurological outcomes and larger infarct volumes than in wild-type (WT) mice7.
ENOS-deficient mice have proven instrumental in defining the role of endothelial function and cerebral blood flow on cognition. Mice lacking or partially lacking eNOS showed age dependent accumulation of cereberal thrombi and amyloid precursor protein (APP) and deficits in spatial memory and learning8–10. In this study, we used mice lacking eNOS and the middle cerebral artery occlusion (MCAO) rodent stroke model to test the hypothesis that eNOS-deficiency impacts cognitive performance post-stroke.
MATERIALS AND METHODS
Mice and Housing
All the animals were approved by the Institutional Animal Care and Use Committee (IACUC). Male wild type (C57BL/6) and eNOS-deficient mice (eNOS−/−; B6.129P2-Nos3tm1Unc/J) that were 10–14 weeks old and 22–29 g, were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Mice were housed in an animal facility (12-hour light/dark cycle) with free access to mouse chow and water. Animals were removed from the study and immediately euthanized based on endpoint criteria including severe infection, seizure and moribund condition.
Transient Middle Cerebral Artery Occlusion
Mice were anesthetized with a 1% to 2% isofluorane in N2O:O2 (70%: 30%) gas mixture via a nose-cone throughout surgery. Body temperature was monitored with a rectal probe and maintained at 37 ± 0.5°C with a heating pad. Cerebral blood flow was monitored in the region of the middle cerebral artery (MCA) by laser doppler flowmetry (PeriFlux System 5000, Perimed, Stockholm, Sweden) during surgery. Occlusion of the MCA was achieved by introducing a 6-0 medium surgical filament through the right external carotid artery until the tip occluded the MCA, causing a cessation of blood flow in the MCA territory. After 60 minutes, the filament was removed and cerebral blood flow restored. Control mice underwent the same procedure without occlusion of the MCA. Mice received intensive peri-surgical care. For analgesia, carprofen (4mg/kg, subcutaneous) was administrated 30 minutes before surgery and every 12 hours for 72 hours after surgery. One drop of 0.5% bupivacaine solution was placed on the cut-down sites. Ampicillin (50 mg/kg s.c) was administered before surgery and every 12 hours for 72 hours after surgery. Mice were provided water gel in the cage until they could freely move to get food and water. Mice were administered normal saline (500 microliter, s.c.) daily for 3 days to prevent dehydration. Mice that developed severe infection, increased seizure activity, or developed a moribund appearance (i.e. limited mobility, hunched posture, ruffled fur) were immediately sacrificed and removed from the study.
Behavioral Tests
Neurological score, cylinder test, and the adhesive removal test were conducted on pre-operative day 1 and post-operative days 1, 3, 7, 14, 21 and 28. The Morris Water Maze test was conducted on post-operative days 21 to 27. The evaluators of behavioral tests were blinded to the group identification. The behavioral testing schedule is outlined in Supp. Figure 2.
Neurological Score (NS)
A four-point score test (Bederson scale) was used to assess global neurological deficits where 0=no deficit, 1=failure to fully extend the forepaw, 2=circling to one side, and 3=no spontaneous walking with depressed level of consciousness.
Cylinder Test
Dissymmetry of forelimb movement was assessed using the cylinder test. Mice were placed into a plexiglass cylinder and the number of times mice used their left, right, or both forepaws to touch the cylinder wall was recorded. Recording stopped after 15 minutes or when the total number of contacts reached 20. The lateralization index was calculated as (the number of right paw touches - number of left paw touches)/total number of touches. Data points were excluded if mice made fewer than 10 contacts in 15 minutes.
Adhesive Removal Test
The adhesive removal test was used to assess sensorimotor deficits. Adhesive tape was placed on both forepaws with equal pressure. The time to contact the adhesive tape and the time to remove the tape from both paws were recorded.
Spatial Learning and Memory
The Morris water maze test (MWM) is a widely used test of rodent spatial learning and memory during which mice learn to use distal cues to navigate a direct path to a hidden submerged platform. Briefly, a large circular tank (LE 820120, Panlab, Harvard apparatus) 120cm in diameter was half filled with water (24–26 degrees Celsius). The tank was divided into four equal quadrants (NE, SE, SW, and NW) and a clear Perspex platform 8cm in diameter was submerged 1.5 cm below the surface of the water in the middle of the NE quadrant. Distal cues were fixed around the tank perimeter and on the testing room walls. Swim performance was recorded by a camera fixed on the ceiling, directly above the center of the pool. Automatic tracking and analysis of each animal’s swim path was performed using the WaterMaze Software (Actimetrics, Inc.). The experimenter was shielded from the animal during swim training by a curtain and blinded to treatment group. The MWM testing protocol was carried out over a 7-day period on post-operative days 21 to 27. Prior to each testing day mice were allowed to acclimate to the testing room in their home cages for at least 30 minutes. On day one mice were placed gently into the water and allowed to swim for 60 seconds after which time they were removed from the tank, dried with a cloth, and returned to their home cage. Learning trials were conducted on days 2 to 6. Mice completed four learning trials each day at 1-hour intervals. For each learning trial mice were carefully placed into the water facing the side of the tank at one of three different starting points (SE, SW, or NW). The starting location for each learning trial was determined randomly, but all start locations were used in a given day. Mice were allowed to swim to find the submerged platform for 60 seconds. The learning trial was terminated and the time taken to find the platform (escape latency) was recorded when the mouse reached the platform and remained on it for 3 seconds. If the mouse failed to reach the platform within 60 sec, the trial was terminated, and the mouse was placed on the platform for 15 sec. Reference memory was assessed on day 7, 24 hours after the final learning trial and with the platform removed. During the probe trial, mice were allowed 60 seconds to attempt to find the hidden platform. The total time that mice spent in the quadrant where the platform was previously located was recorded and used as a measure of reference memory11,12. Because sensorimotor deficits can interfere with performance in the MWM by impacting swimming ability and/or motivation to escape from water we also recorded swim speed (m/s) during the learning trial using the following equation: total distance traveled/time (seconds).
If a mouse failed to swim but rather floated, the experimenter did not intervene to encourage swimming but rather allowed the mouse to swim for 60 seconds before placing on the platform for 15 seconds. In addition, mice that were unable to float and were at risk of becoming submerged were immediately removed from the pool and placed upon the platform for 15 seconds. A mouse that failed to swim or failed to float after the fourth trial of the day was removed from the experiment and termed a “non-performer”.
Immunohistochemistry Staining
Twenty-eight days after surgery, mice were anesthetized and transcardially perfused with normal saline followed by 4% paraformaldehyde (PFA). Whole brain was removed and incubated in 30% sucrose overnight, then frozen at −80°C until sectioning. Before sectioning, brains were embedded in Optimal Cutting Temperature (OCT) compound (Tissue-Tek, Sakura). Twenty-micron coronal sections were cut using a cryostat (Leica CM3050). Six sections from the Bregma (0.74 mm to −1.34 mm with intervals of 400 µm) were selected for immunohistochemistry staining. Sections were incubated in blocking buffer (5% horse serum and 0.4% triton x-100 in 0.01M PBS) at room temperature for 60 minutes. To identify microglial cells, brain sections were incubated with anti-Iba-1 primary antibody (rabbit anti-Iba1,1:200, Abcam) at 4°C for 24 hours. Anti-Iba-1 antibody binds to ionizing calcium-binding adaptor molecule 1 (Iba-1), a protein that is specifically expressed in microglia and up-regulated when these cells are become active. Sections were then washed in 0.01M PBS for 20 minutes before incubation with secondary antibody (Cy3 goat anti-rabbit IgG,1:500, Life technologies) for 2 hours at room temperature in the dark. Finally, sections were washed with 0.01M PBS before mounting in DAPI. Fluorescent images were acquired at 200 × magnification using a Nikon Eclipse TE2000-S fluorescent microscope. The number of Iba1+ cells in the cortex (two fields) and striatum (three fields) in both hemispheres was counted by an observer blinded to the experimental group using the software Image J 1.48u.
Nissl Staining
Nissl staining was performed to delineate the glial scar, which forms in the region of the infarct several days after injury13. Brain sections (50 µm) were mounted on targeting molecule polylysine-coated slides and progressively hydrated by incubating in 100% ethanol for 10 minutes, 95% ethanol for 2 minutes, and distilled water for 2 minutes. Sections were then stained with 0.5% cresyl violet for 10 minutes and then rinsed rapidly in distilled water. Stained sections were then dehydrated 95% in ethyl alcohol for 3 minutes, twice in 100% ethanol for 5 minutes, and finally cleared twice in xylene for 5 minutes. Finally, sections were covered with neutral resins and a cover slip. The glia scar area was visualized using the software Image J 1.48u.
Brain Tissue Extracts
The brain region between 0 mm and −4 mm posterior to the Bregma was dissected from the contra-lateral and infarct hemispheres, flash frozen in liquid nitrogen, and then stored at −80 °C until protein extraction. Approximately 100 mg of frozen brain tissue was homogenized on ice in 1 mL of tissue extraction reagent (50mM Tris, 250mM NaCl, 5 mM EDTA, 2mM Na3VO4, 1mM NaF, 20 mM Na4P2O7, 0.02% NaN3 and complete protease inhibitors. Roche, NJ, USA). Tissue extracts were centrifuged at 10,000 rpm for 5 minutes at 4 °C. The supernatant was collected, and the protein concentration was determined using bicinchoninic acid (BCA) assay. Levels of IFN-γ in brain tissue extracts were measured by ELISA (Sigma-Aldrich, Inc. MO, USA) according to the manufacturer’s instructions.
Statistical Analysis
All statistical evaluations were performed with SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). Kaplan-Meier survival and log-rank tests were used to examine group differences in survival. Chi-square or non-parametric tests were used to examine group differences in performance in the MWM. Two-way analysis of variance (ANOVA) with or without repeated measure was used to determine whether an interaction existed between surgery (MCAO versus no surgery) and genotype (WT versus eNOS−/−) on the functional outcomes tested. Results were expressed as mean ± SD, and statistical significance was accepted at a p value less than 0.05.
3. Results
eNOS-Deficiency and MCAO Survival
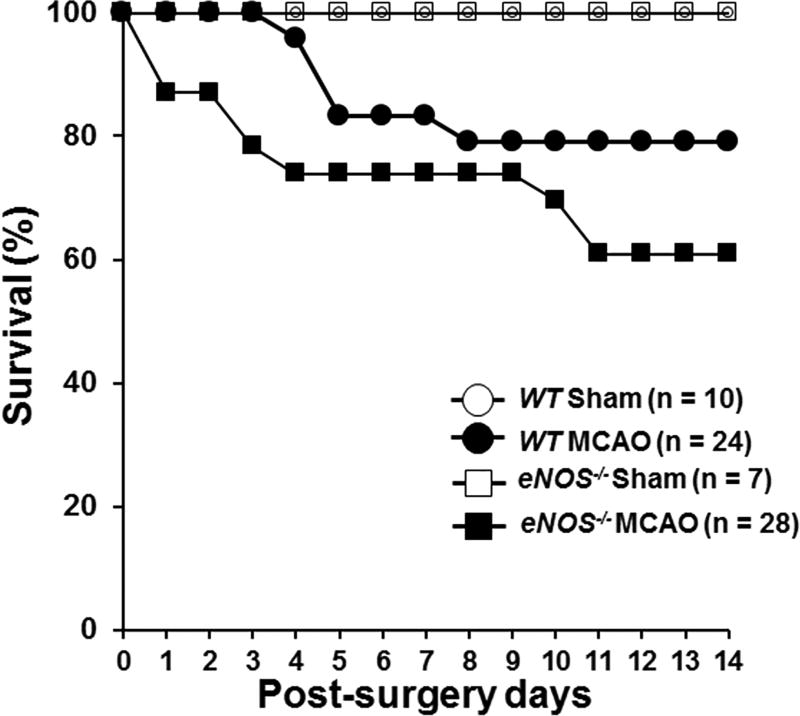
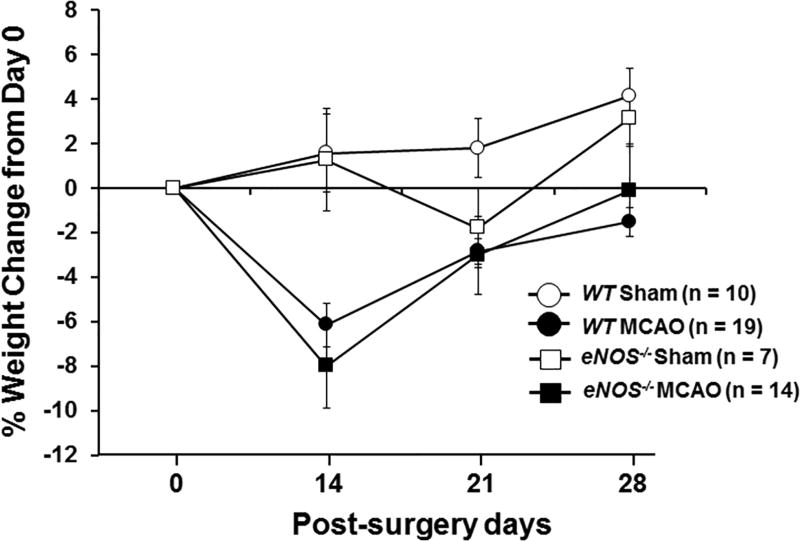
Twenty-four WT mice and 23 eNOS−/− mice underwent MCAO, and 10 WT and 7 eNOS−/− were sham-treated. There was no significant difference in blood flow during occlusion and reperfusion between WT and eNOS−/− mice in the MCAO group (suppl. Figure 1). All mice that underwent the sham procedure survived throughout the 28-day study period. Five of the 24 WT mice and 9 of the 23 eNOS−/− mice met criteria for removal from the study during the 14 days post surgery (See Methods for detailed criteria for removal). The observed mortality rate in WT MCAO mice is consistent with that reported in the literature14. Survival curves shown in Figure 1A revealed no significant difference in survival between WT and eNOS−/− MCAO mice (χ2 = 1.746, df = 1, p =.186). Figure 1B shows body weight change from baseline (Day 0) at 14, 21, and 28 days post surgery in mice that survived the 28-day study period. There was a significant effect of MCAO on weight change over time (F(2.2, 97)=10.202, p<.0001). MCAO mice of both genotypes showed weight loss at 14 days post surgery, but weight returned to pre-surgery levels at 21 and 28 days post surgery. There was no surgery × genotype interaction effect (F(2.2, 97)=.842, p=.444).
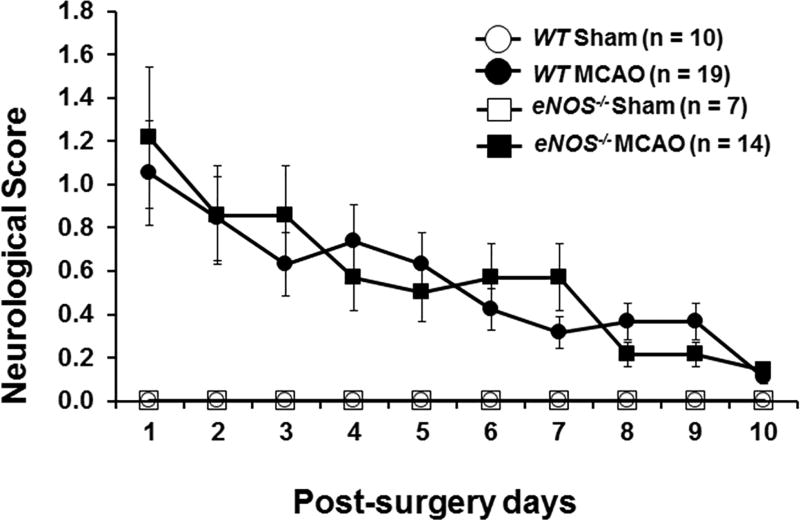
Figure 1.
(A) Kaplan-Meier survival and log-rank tests were used to determine the group differences in survival. (B) The percentage of body weight change from baseline (day 0) at 14, 21, and 28 days post surgery. Shown is the mean percentage of weight change ± SEM in sham and MCAO of WT and eNOS−/− mice that survived the 28-day study period. Means were analyzed by ANOVA with repeated measures. (C). General neurological deficit was assessed using a four-point (0–3) neurological score test: 0=no deficit, 1=failure to fully extend the forepaw, 2=circling to one side, 3=no spontaneous walking with a depressed level of consciousness. The neurological score was evaluated daily on post-MCAO days 1–10 and then once a week until day 28. Data were expressed as the mean ± SEM. Means were analyzed by ANOVA with repeated measures.
Neurological Score (NS)
Sham surgery had no effect on NS in WT (n=10) or eNOS−/− (n=7) mice (Figure 1C). There was a significant effect of MCAO on NS over time (F(9,414)=8.652, p<.0001). MCAO WT (n=19) and MCAO eNOS−/− (n=14) mice displayed mild to moderate deficits (NS score of 1–2) during the first week after surgery, but NS scores improved thereafter. There was no surgery × genotype interaction effect on neurological score over time (F(9,414)=.678, p=.729).
Adhesive Removal Test
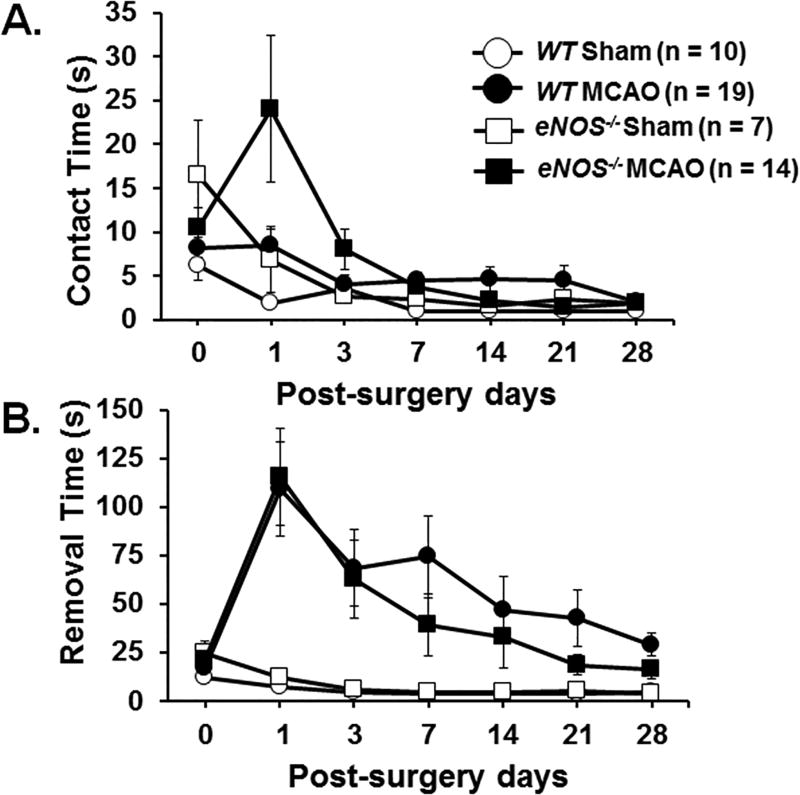
Figure 2 shows time to contact (A) and time to remove (B) the tape on the both forepaws. There was an effect of MCAO on contact time (F(1.9, 84)=3.296, p=.043) and removal time (F(3.8, 155.7)=3.112, p=.006). There was no observed impact of genotype on either contact time (F(3.8, 155.7)=1.362, p=.262) or removal time (F(3.8, 155.7)=.328, p=.922).
Figure 2.
The adhesive removal test measures sensorimotor function. A small piece of adhesive tape (3 mm × 4 mm) was placed on each forepaw, and the time (seconds) needed to contact (Figure 2A) and remove the tape (Figure 2B) from both forepaws was recorded. The adhesive removal test was performed on the day prior to surgery (day 0) and on post-operative days 1, 3, 7, 14, 21, and 28. Data were expressed as mean ± SEM. ANOVA with repeated measures was utilized to perform statistic analysis.
Cylinder Test
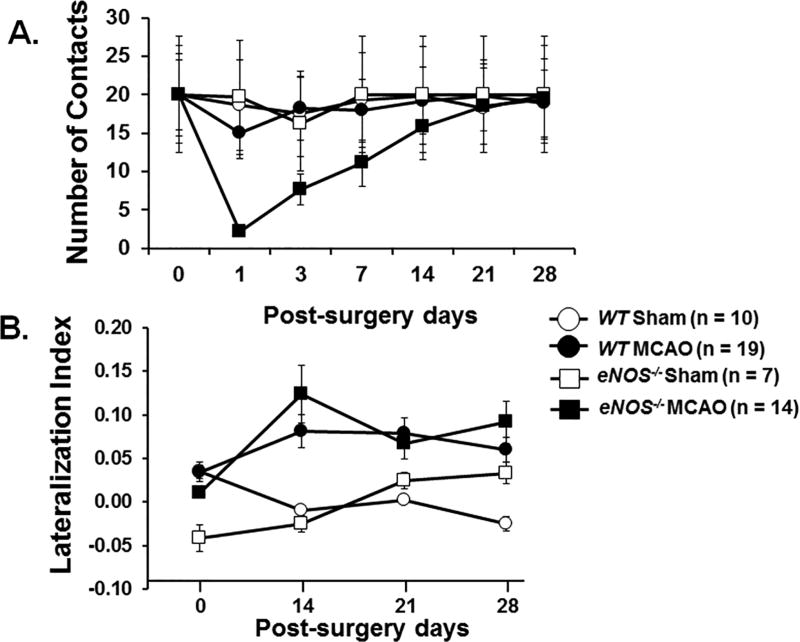
Performance in the cylinder test is shown in Figure 3. There was a significant time × genotype × surgery effect on the total number of paw contacts over time (F(3.5, 148)=4.601, p=.0002). While all of the sham-treated mice and 14/19 WT MCAO mice were able to make >10 paw contacts on post surgery day 1, only 1/14 of the eNOS−/− MCAO mice were able to (Figure 3A). By day 14 post surgery, all of the mice, with the exception of two eNOS−/− MCAO mice, contacted the cylinder walls >10 times, which allowed calculation of the lateralization index for days 14, 21, and 28 (Figure 3B). There was no observed time × surgery × genotype interaction effect for lateralization index over time (F(3,132)=.417, p=.741).
Figure 3.
The asymmetry of forelimb movements was measured using the cylinder test. Recording was stopped after the total number of contacts reached 20 or after 15 minutes. The number of contacts during the 15-minute test is shown in Figure 3A. The lateralization index (Figure 3B) was calculated as (number of right side placement - number of left side placement) / total placement. The test was performed on the day prior to surgery (day 0) and post-surgery days 1, 3, 7, 14, 21, and 28. Data were expressed in mean ± SEM. ANOVA with repeated measures was utilized for the number of contact and lateralization index analysis.
Spatial Learning and Memory
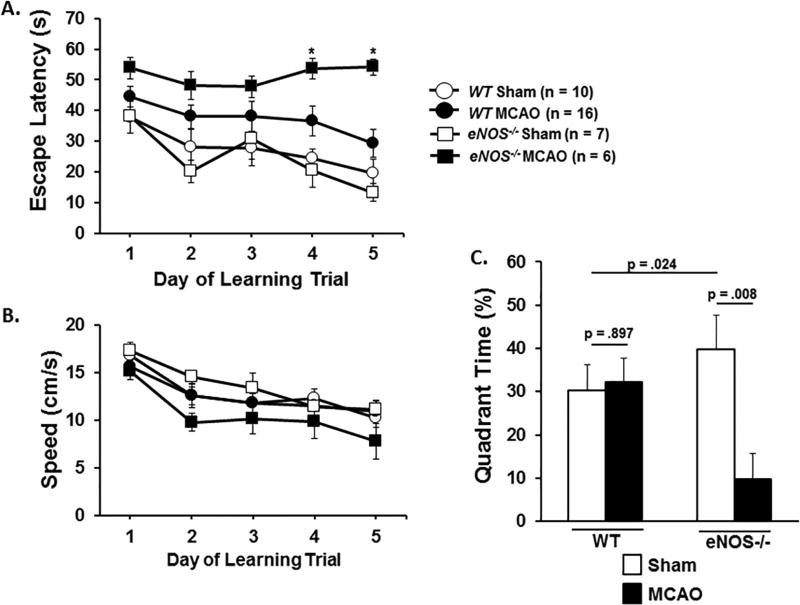
Performance in the Morris Water Maze (MWM) was used as a measure of spatial memory and learning. All of the sham-treated mice (10 WT and 7 eNOS−/− mice) performed in the MWM. One WT MCAO mouse engaged in floating behavior, remaining immobile with its head above water and was labeled a “non-performer” (see Methods for details). Significantly more eNOS−/− MCAO mice (8 out of 14 mice) failed to perform in the MWM (χ2= 9.209, df=1, p=0.002, see Table 1). Rather than engage in floating behavior these mice adopted a twisted posture and displayed uncoordinated movement with both the front and hind limbs when placed in the water. Further analyses were conducted only on performer mice that initiated swimming when placed in the MWM. Figure 4A shows escape latency (average time taken to find the submerged platform) and Fig. 4B shows swim speed on each of the trial days. For escape latency we observed a significant effect of surgery on escape latency (p<.05) but not genotype for each trial day. There was a genotype × surgery effect on escape latency on trial days 4 (F(1,34)=4.357, p =.044) and 5 (F(1,34)=8.977, p = .005). On these days mice in the eNOS−/− MCAO group took significantly longer to find the submerged platform than their sham-treated counterparts. In contrast, there was no significant surgery or genotype effect on swim speed for any of the trial days.
Table 1. Performance of Morris Water Maze.
Morris water maze (MWM) was used to examine deficits in spatial learning and memory. Mice were tested on days 21 to 27 after MCAO or sham-treatment. Sixteen WT and 14 eNOS−/− MCAO mice were tested. Three WT MCAO mice were not tested on post-operative day 21 because of technical issues with the water tank that did not allow testing during that time period. A mouse that failed to swim in the MWM was termed a “non-performer” (See Methods section for details).
| Performer | Non-performer | Chi-Square P | |
|---|---|---|---|
| WT (n=16) | 15 | 1 | 0.002 |
| eNOS−/− (n=14) | 6 | 8 |
Figure 4.
Morris water maze. Figure 4A: Mice were subjected to repeated training trials with the rescue platform in place. The time to find the submerged platform (Escape latency) on learning trial days 1 to 5 was used to assess spatial learning. Figure 4B: Swimming speed (in centimeters per second) during the learning trial in the maze. Figure 4C: Mice were subjected to the probe trial following removal of the rescue platform. Total time spent in the previous hidden platform area was used to assess reference memory. Data are expressed as the mean ± SEM. * denotes a significant difference between WT and eNOS−/− MCAO mice.
In the probe trial conducted on day 7, we found a significant genotype × surgery interaction effect in time spent in the target quadrant (F(3,38)=5.573, p=.024). While there was no treatment effect for time in quadrant for WT mice (F(1,26)=.017, p=.897) eNOS−/− MCAO mice spent significantly less time in the target quadrant compared to sham treated eNOS−/− mice (F(1,11)=10.961, p=.008) (Figure 4c).
Difference in Neuroinflammation after MCAO between WT and eNOS−/− Mice
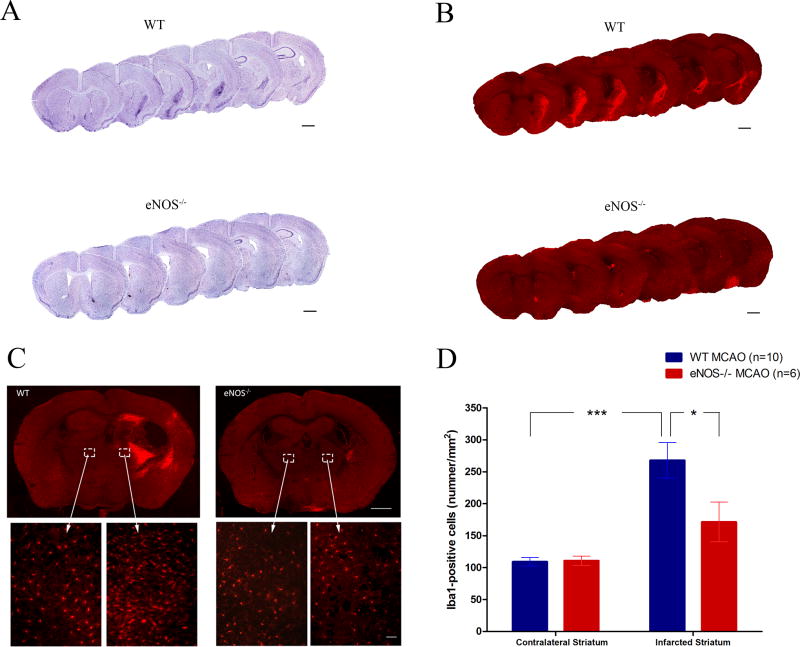
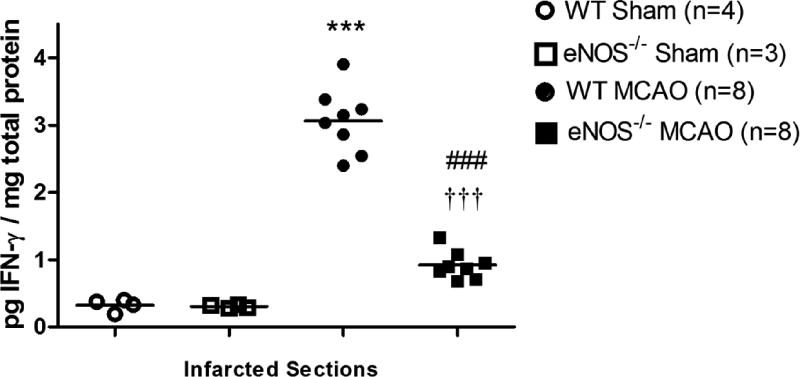
To evaluate differences in the evolvement of neuroinflammation following MCAO in WT and eNOS−/− MCAO mice, we sacrificed mice after the conclusion of behavioral testing on day 28 post-MCAO. Nissl staining was used to label the Nissl body in the cytoplasm of neurons and glia, and also in neuropils a dense network composed of glia, dendrites and unmyelinated axons. While the infarct area was not readily identifiable, we observed a significantly larger cellular aggregation in the infarcted striatum in WT versus eNOS−/− mouse brains (Figure 5A). Immunostaining brain sections with Iba-I, a pan microglia marker that is up-regulated in activated cells, revealed the specific accumulation of activated microglia in these same brain region (Fig 5A–D). Overall the magnitude of the cell and specifically microglial accumulation was dampened in eNOS−/− MCAO mice (Figure 5 A–D). Figure 5E shows levels of IFN-γ in brain lysates isolated from sham WT, sham eNOS−/−, WT MCAO, and eNOS−/− MCAO mice. While levels of IFN-γ in sham WT was similar to those in sham eNOS−/− brain lysates (p=0.631), they were significantly increased in WT MCAO compared to eNOS−/− MCAO brain lysates (p<0.001, Figure 5E). Furthermore, the production of IFN-γ substantially increased in WT MCAO (p<0.001) and eNOS−/− MCAO (p<0.001) compared to sham WT and sham eNOS−/−, respectively (Figure 5E).
Figure 5.
Microglia accumulation and IFN-γ levels in brain after stroke. (A) Representative Nissl-stained brain sections show the accumulation of cells in the ipsilateral striatum in WT MCAO mice and eNOS-deficient MCAO mice. (B) Immunohistochemical staining of Iba1+ cells in the ipsilateral striatum shows microglial hyper-intensity in WT MCAO, but not in eNOS−/− MCAO mice. (C) Representative images of Iba1 staining in bilateral regions in WT MCAO (n = 10) and eNOS−/− MCAO (n = 6) mice. Scale bar represents 0.05mm. (D) Comparison of the density of Iba1+ cells in the bilateral striatum between WT and eNOS−/− MCAO mice. Iba1+ cell numbers in ipsilateral striatum were significantly higher than in contralateral striatum in WT mice. (E): IFN-γ levels in brain extracts of infarcted sides. Protein was extracted from brain tissue harvested from the infarcted sides and levels of IFN-γ levels measured by ELISA. Data are expressed as the mean ± SEM. * indicates difference from sham WT mice (* P<0.05, ***p < 0.001); # indicates difference from WT MCAO mice (###: P<0.001); † indicates difference from sham eNOS−/− mice (†††: p < 0.001), two-tailed Student’s t-test.
Discussion
The present study reveals, for the first time, preliminary evidence of a role for eNOS in cognitive performance post-stroke in mice. Our findings are consistent with prior work implicating eNOS in cognitive performance in mice without stroke. In a study by Tan et al., young mice with partial eNOS deficiency (eNOS+−) developed spontaneous cerebral thromboses, which, increased with age resulting in cognitive deficits that only became evident in older mice >18 months of age8. In the present study, we used young WT and eNOS−/− mice (< 6-months old) because we specifically wanted to examine the impact of eNOS-deficiency on stroke outcomes rather than examine the impact of eNOS-deficiency and increasing age on stroke-related outcomes. Our use of younger mice likely explains why we did not detect any differences in spatial learning and memory between sham-treated WT and eNOS−/− mice. Existing clinical studies support a role for eNOS in cognition. For instance, Polymorphisms in the eNOS gene are associated with Alzheimer’s disease15. Endothelial dysfunction, specifically loss of endothelial NO, has been shown to be associated with the development of vascular dementia, such as Alzheimer’s dementia8. In addition, synaptic activity during long-term potentiation stimulates NO production, suggesting the role of NO in learning and memory16. There is evidence showing that inhibition of NO with non-specific NOS inhibitors may impair learning and/or memory formation17,18; however, there are also studies that failed to show any effect on memory19,20. This inconsistency may be related to poor differentiation between the different NOS isoforms.
Based on the Bederson neurological scoring criteria we did not observe a significant difference in neurological function between WT and eNOS−/− mice after MCAO. This contrasts to the work of Cui and colleagues who reported that eNOS−/− mice had significantly reduced neurological functioning compared to WT mice after MCAO22. This inconsistency might be due to methodological differences in the length of the occlusion and/or the assessment tools used to document neurological deficits between the two studies. In the present study, occlusion lasted for 60 minutes and the traditional Bederson scale was used to calculate neurological score. In contrast Cui and colleagues used the Modified Neurological Severity Score (mNSS). Although the Bederson scoring procedure is easy to perform it is subjective in nature and because it assesses basic neurological deficits it is limited in its ability to detect small deficits in function post-stroke21. The mNSS however, measures a wider range of sensorimotor deficits including motor, sensory, reflex and balance and may be more useful in detecting smaller deficits that persist long-term after stroke22.
The delay in the ability of MCAO eNOS−/− mice to make paw contact with the sides of the cylinder during the 7-days post-surgery suggests greater functional deficits in eNOS−/− mice post-MCAO relative to WTs. While these deficits were not evident 21-days post-MCAO at the time that we conducted the MWM test, greater than 50% of MCAO eNOS-deficient mice were unable to swim when placed into water maze pool. Failure to swim normally may be additional evidence of prolonged motor or somatosensory deficits in MCAO eNOS−/− mice that were not apparent in the other functional tests employed in the present study. Further studies are needed using a larger battery of sensorimoter tests to fully delineate the impact of eNOS-deficiency on post-stroke functional deficits.
Despite the number of non-performers, MCAO eNOS-deficient mice that were capable of swimming showed deficits in spatial learning and reference memory during the MWM test. These mice took significantly longer to find the submerged platform on trial days 4 and 5 compared to their WT counterparts. This delay was not related to genotype differences in cognitive performance since there was no significant difference in time to find the platform between WT and eNOS-deficient mice that underwent sham surgery. The speed at which mice swim during the learning trials of the MWM test is commonly used to determine whether or not mice have underlying sensorimotor deficits. There was no difference in swim speed between MCAO eNOS−/− and WT mice across the 5 training days which suggests that the delay in finding the platform by MCAO eNOS-deficient mice during the learning trials was not due to ongoing sensorimotor deficits but rather to impaired spatial learning.
Mortality rate in MCAO eNOS-deficient mice was approximately 2-fold greater than in WT mice, although this difference was not statistically significant. Our findings are consistent with those of Cui and colleagues who showed that eNOS-deficient mice had a similar increase in mortality in the 7-days post-stroke23. Increased mortality may be related to the development of larger infarcts in eNOS-deficient mice7. We were not able to measure infarct size in the present study because the infarct is normally only visible in the days following stroke, where it can be seen as an unstained area of the brain following Nissl staining. At later time points post-stroke the infarct area is less visible and difficult to discern due to the heavier staining of infiltrating reactive astrocytes, microglia, and other cells, and the deposition of proteoglycans which form the glial scar13. The darker staining that we observed in the area of the infarct at 28-days post MCAO likely represents the glial scar rather than the original infarct area. This Nissl stained area was smaller in eNOS-deficient mice 28-days post-MCAO and further immunostaining with the pan-microglial marker Iba-I showed reduced accumulation of microglia in the lesion striatum in eNOS-deficient mice. Additional studies are needed to determine whether reduced microglia accumulation in damaged brain tissue of eNOS-deficient mice is due to reduced microglial proliferation, migration, or both.
We also demonstrated that levels of IFN-γ were elevated in the infarcted hemisphere compared to the normal hemisphere in WT mice but not in eNOS-deficient mice. Since, microglia are able to produce IFN-γ independently of T cells24 the reduced IFN-gamma levels in the infarcted hemisphere of eNOS-deficient mice may reflect reduced accumulation of microglia in the absence of eNOS.
Given the role of microglia in the tissue repair processes post-stroke it is possible that the long-term functional deficits observed in eNOS-deficient mice post-stroke are due to sub-optimal angiogenesis and neurogenesis. Brain Derived neurotropic Factor (BDNF), a neurotrophin that is abundant in the brain, is up-regulated by endothelium NO and plays an important role in post-stroke neurogenesis and functional recovery25,26,27. Although further investigation is warranted it is intriguing to speculate that the cognitive deficits observed in eNOS-deficient mice in the present study were due to reduced BDNF levels. If this is indeed the case then therapeutic manipulation of BDNF could offer an effective treatment for post-stroke cognitive decline. In a recent study by Harris and colleagues, nano-particle delivery of BDNF reduced infarct size in mice post-stroke and dampened post-stroke depressive behavior in the Tail Suspension Test28. Although these investigators did not examine cognitive deficits post-stroke a similar strategy could be used to determine whether the eNOS-dependent cognitive decline is mediated by BDNF. Additional therapeutic strategies aimed at increasing the production of eNOS have been identified which may prove beneficial at reducing cognitive deficits in stroke survivors in the future. For instance statins like atorvastatin and simvastatin increase eNOS in experimental animals and may prove beneficial at increasing post-stroke function and cognition in the future29.
Supplementary Material
Figure S1: Laser doppler recording of cerebral blood flow during MCAO. The steep drop of blood flow indicates the occlusion of middle cerebral artery. Cerebral blood flow also returns to baseline after removal of the inserted monofilament.
Figure S2: Schema of behavioral tests
Acknowledgments
This work was funded by the National Institutes of Health [grant number HD074668, 2013-2016). The funding organization was not involved in study design, in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. Yao Wang is supported by Shenzhen “Sanming project” (SZSM201610039).
Footnotes
The study was performed at Stroke Biological Recovery Laboratory, Department of Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, the teaching affiliate of Harvard Medical School, Charlestown, MA 02129
Disclosures:
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
An abstract was presented at the annual meeting of American Association of Physiatrists in February 2016.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Skolarus LE, Burke JF, Brown DL, Freedman VA. Understanding stroke survivorship: expanding the concept of poststroke disability. Stroke. 2014;45(1):224–230. doi: 10.1161/STROKEAHA.113.002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke; a journal of cerebral circulation. 2002;33(9):2254–2260. doi: 10.1161/01.str.0000028235.91778.95. [DOI] [PubMed] [Google Scholar]
- 4.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312(1):H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 6.Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377(6546):239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 7.Huang Z, Huang PL, Ma J, et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16(5):981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Tan XL, Xue YQ, Ma T, et al. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol Neurodegener. 2015;10:24. doi: 10.1186/s13024-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin SA, Santhanam AV, Hinton DJ, Choi DS, Katusic ZS. Endothelial nitric oxide deficiency promotes Alzheimer's disease pathology. J Neurochem. 2013;127(5):691–700. doi: 10.1111/jnc.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res. 2010;107(12):1498–1502. doi: 10.1161/CIRCRESAHA.110.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park M, Kim CH, Jo S, et al. Chronic Stress Alters Spatial Representation and Bursting Patterns of Place Cells in Behaving Mice. Sci Rep. 2015;5:16235. doi: 10.1038/srep16235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dilekoz E, Houben T, Eikermann-Haerter K, et al. Migraine mutations impair hippocampal learning despite enhanced long-term potentiation. J Neurosci. 2015;35(8):3397–3402. doi: 10.1523/JNEUROSCI.2630-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zille M, Farr TD, Przesdzing I, et al. Visualizing cell death in experimental focal cerebral ischemia: promises, problems, and perspectives. J Cereb Blood Flow Metab. 2012;32(2):213–231. doi: 10.1038/jcbfm.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang QM, Wei Y, Zheng Y, Waeber C. Efficacy of combined atorvastatin and sildenafil in promoting recovery after ischemic stroke in mice. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2013;92(2):143–150. doi: 10.1097/PHM.0b013e3182643f1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferlazzo N, Gorgone G, Caccamo D, et al. The 894G > T (Glu298Asp) variant in the endothelial NOS gene and MTHFR polymorphisms influence homocysteine levels in patients with cognitive decline. Neuromolecular medicine. 2011;13(3):167–174. doi: 10.1007/s12017-011-8148-8. [DOI] [PubMed] [Google Scholar]
- 16.Haley JE, Wilcox GL, Chapman PF. The role of nitric oxide in hippocampal long-term potentiation. Neuron. 1992;8(2):211–216. doi: 10.1016/0896-6273(92)90288-o. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins RD. NO honey, I don't remember. Neuron. 1996;16(3):465–467. doi: 10.1016/s0896-6273(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 18.de la Torre JC, Aliev G. Inhibition of vascular nitric oxide after rat chronic brain hypoperfusion: spatial memory and immunocytochemical changes. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25(6):663–672. doi: 10.1038/sj.jcbfm.9600057. [DOI] [PubMed] [Google Scholar]
- 19.Tobin JR, Gorman LK, Baxter MG, Traystman RJ. Nitric oxide synthase inhibition does not impair visual or spatial discrimination learning. Brain Res. 1995;694(1–2):177–182. doi: 10.1016/0006-8993(95)00693-k. [DOI] [PubMed] [Google Scholar]
- 20.Knepper BR, Kurylo DD. Effects of nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on spatial and cued leaning. Neuroscience. 1998;83(3):837–841. doi: 10.1016/s0306-4522(97)00457-0. [DOI] [PubMed] [Google Scholar]
- 21.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 22.Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2(1):13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui X, Chopp M, Zacharek A, Zhang C, Roberts C, Chen J. Role of endothelial nitric oxide synthetase in arteriogenesis after stroke in mice. Neuroscience. 2009;159(2):744–750. doi: 10.1016/j.neuroscience.2008.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Suzuki Y. Microglia produce IFN-gamma independently from T cells during acute toxoplasmosis in the brain. J Interferon Cytokine Res. 2007;27(7):599–605. doi: 10.1089/jir.2006.0157. [DOI] [PubMed] [Google Scholar]
- 25.Cui X, Chopp M, Zacharek A, et al. Endothelial nitric oxide synthase regulates white matter changes via the BDNF/TrkB pathway after stroke in mice. PLoS One. 2013;8(11):e80358. doi: 10.1371/journal.pone.0080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Zacharek A, Zhang C, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25(9):2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banoujaafar H, Monnier A, Pernet N, et al. Brain BDNF levels are dependent on cerebrovascular endothelium-derived nitric oxide. Eur J Neurosci. 2016;44(5):2226–2235. doi: 10.1111/ejn.13301. [DOI] [PubMed] [Google Scholar]
- 28.Harris NM, Ritzel R, Mancini N, et al. Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol Biochem Behav. 2016;150–151:48–56. doi: 10.1016/j.pbb.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, Song W, Li L, Fan X. Endothelial nitric oxide synthase: a potential therapeutic target for cerebrovascular diseases. Mol Brain. 2016;9:30. doi: 10.1186/s13041-016-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Laser doppler recording of cerebral blood flow during MCAO. The steep drop of blood flow indicates the occlusion of middle cerebral artery. Cerebral blood flow also returns to baseline after removal of the inserted monofilament.
Figure S2: Schema of behavioral tests